Abstract
The actin cytoskeleton plays a key role in the entry of mitosis as well as in cytokinesis. In a previous study, we showed that actin disruption delays mitotic entry at G2/M by sustained activation of extracellular signal-related kinase 1/2 (ERK1/2) in primary cells but not in transformed cancer cell lines. Here, we examined the mechanism of cell cycle delay at G2/M by actin dysfunction in IMR-90 normal human fibroblasts. We observed that de-polymerization of actin with cytochalasin D (CD) constitutively activated ribosomal S6 kinase (RSK) and induced inhibitory phosphorylation of Cdc2 (Tyr 15) in IMR-90 cells. In the presence of an actin defect in IMR-90 cells, activating phosphorylation of Wee1 kinase (Ser 642) and inhibitory phosphorylation of Cdc25C (Ser 216) was also maintained. However, when kinase-dead RSK (DN-RSK) was over-expressed, we observed sustained activation of ERK1/2, but no delay in the G2/M transition, demonstrating that RSK functions downstream of ERK in cell cycle delay by actin dysfunction. In DN-RSK overexpressing IMR-90 cells treated with CD, phosphorylation of Cdc25C (Ser 216) was blocked and phosphorylation of Cdc2 (Tyr 15) was decreased, but the phosphorylation of Wee1 (Ser 642) was maintained, demonstrating that RSK directly controls phosphorylation of Cdc25C (Ser 216), but not the activity of Wee1. These results strongly suggest that actin dysfunction in primary cells activates ERK1/2 to inhibit Cdc2, delaying the cell cycle at G2/M by activating downstream RSK, which phosphorylates and blocks Cdc25C, and by directly activating Wee1.
Keywords: actin dysfunction, Cdc25C and Wee1, cytochalas-in D, extracellular signal-related kinase (ERK), G2/M delay, human normal fibroblast IMR-90, ribosomal S6 kinase (RSK)
INTRODUCTION
The actin cytoskeleton functions by dramatic re-organization during the cell cycle. In addition to the well-established role of actin in cytokinesis where it polymerizes to form the cleavage furrow, actin was also reported to function during early mitosis (for a review, see Kunda and Baum, 2009). Actin filaments present below the plasma membrane, also known as cortical actin, play an important role in centrosome separation and correct spindle positioning during mitotic spindle assembly in mammalian cells (Carreno et al., 2008; Kunda et al., 2008; Rosenblatt et al., 2004). Recently, the role of actin in early mitosis, where actin structure reorganizes to shape the cell from flat to round, has been examined. During the process of rounding in early mitosis, the actin cytoskeleton changes its dynamics to become more stiff and rigid at the cell cortex, providing mechanical strength to correctly separate duplicated centrosomes and position the spindle for proper chromosome segregation (for a review, see Lancaster and Baum, 2014). Moreover, for correct spindle positioning, actin integrity together with actin-related proteins in defined regions of the cell cortex are required (Kaji et al., 2008; Thery et al., 2005; Toyoshima et al., 2007). Several actin regulators such as Rho A and myosin (Maddox and Burridge, 2003), cofilin, and WD repeat-containing protein 1 (Fujibuchi et al., 2005) are involved in the remodeling of actin cytoskeleton, but how changes in actin organization are coupled with mitotic entry is not well-understood.
We previously reported that depolymerization of actin with cytochalasin D (CD) or inhibition of myosin ATPase with butanedione-2-monoxime blocked the cell cycle at G2/M in primary mammalian cells including human normal fibroblast IMR-90 cells (Lee and Song, 2007). We also showed that actin dysfunction induces hyper-activation of extracellular signal-related kinase (ERK1/2) and inhibition of Cdc2 kinase (Lee and Song, 2007). G2/M delay by actin dysfunction was observed in primary cells, but not in transformed cells, as transformed cells are considered to have lost their cell polarity (Lee and Song, 2007).
It is well-known that the Cdc2/Cyclin B complex controls mitotic entry in mammalian cells and its activation is tightly regulated. Cdc25C and Wee1 exert opposing functions to maintain the activation of Cdc2 kinase at proper levels. Cdc25C is a phosphatase that removes the inhibitory phosphorylation (pTyr 15) of Cdc2 and makes it active, whereas Wee1 is a kinase that phosphorylates Cdc2 at Tyr 15, rendering it inactive (for a review, see Perry and Kornbluth, 2007).
Based on the results of our previous study (Lee and Song, 2007), we examined how ERK1/2 activated by actin dysfunction inhibits Cdc2 to cause G2/M delay in human normal fibroblast IMR-90 cells. To control Cdc2 kinase activity in response to actin dysfunction, activated ERK may directly regulate the phosphorylation of Wee1 and Cdc25C or ERK may control these regulators of Cdc2 indirectly by activating its downstream kinase ribosomal S6 kinase (RSK). In this study, we evaluated how ERK1/2 activated by actin dysfunction delays the cell cycle at G2/M and controls the phosphorylation of Wee1 and Cdc25C to inhibit Cdc2 kinase in IMR-90 cells. These findings provide a general mechanism regarding how actin function is coordinated with cell cycle regulation.
MATERIALS AND METHODS
Cell culture and synchronization
IMR-90 human lung fibroblasts (CCL-186) were purchased from the American Type Culture Collection (USA) and the early passages (below 9) of IMR-90 cells were used for experiments. IMR-90 cells were maintained at 37°C in minimum essential medium (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin-streptomycin (Gibco) with 5% CO2.
A stock solution of 0.2 M thymidine (Sigma-Aldrich, USA) in dimethyl sulfoxide (DMSO) was diluted to a final concentration of 2 mM in the cell culture medium. IMR-90 cells were synchronized with 2 mM double thymidine arrest for 18 and 15 h, with 8 h of release between arrests. Cells were washed with 1× phosphate buffered saline (Gibco) and released in fresh MEM containing 10% fetal bovine serum (Gibco) and 1% penicillin-streptomycin (Gibco).
Plasmid and transfection
Dominant-negative RSK (DN-RSK) in pCMV-Tag2B was kindly provided by Dr. Junichi Abe (University of Rochester Medical Center) and used for transfection. After the first thymidine arrest, the cells released for 3–4 h were transfected with 1.5 μg plasmid in 3 μl of 1 mg/ml Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions.
CD treatment
At 5.5–6 h after release from the second thymidine arrest, IMR-90 cells were incubated in media containing 5 μM cytochalasin D (Sigma-Aldrich). A stock solution of CD (10 mM, Sigma) in DMSO was diluted to its final concentration 5 μM in the media.
Immunoblots
Cell extracts were prepared with lysis buffer (50 mM Tris-HCl pH 7.5, 1 mM sodium ethylene-diamine-tetra-acetic acid, 1 mM ethylene glycol tetra-acetic acid, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 10 mM sodium pyrophosphate, 50 mM sodium fluoride, 5 mM beta-glycerophosphate, 0.1% betamercaptoethanol, 0.5 mM sodium vanadate, 1 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride). For immunoblotting, the extracts were resolved by 8% SDS-PAGE and blotted onto nitrocellulose membranes. All primary antibodies were incubated at 4°C overnight at a dilution of 1:2000. Rabbit polyclonal p-Cdc2 (Tyr 15) (ab47594, Abcam, USA), mouse monoclonal anti-cyclin A (4656, Cell Signaling Technology, USA), rabbit polyclonal p-Cdc25C (Ser 216) (9528, Cell Signaling Technology), rabbit monoclonal anti-Cdc25C (ab32444, Abcam), rabbit polyclonal anti-β-actin (4967, Cell Signaling Technology), rabbit monoclonal p-Wee1 (Ser 642) (4910, Cell Signaling Technology), rabbit polyclonal anti-Wee1 (4936, Cell Signaling Technology), rabbit monoclonal anti-FLAG (F7425, Sigma), rabbit polyclonal p-ERK1/2 (Thr 202/Tyr 204) (9101, Cell Signaling), rabbit polyclonal anti-ERK1/2 (9102, Cell Signaling), rabbit monoclonal p-RSK1 (Ser 380) (ab32203, Abcam), rabbit polyclonal anti-RSK1 (9333, Cell Signaling), rabbit monoclonal histone H3 (4499, Cell Signaling Technology), and mouse monoclonal p-H3 (Ser 10) (9706, Cell Signaling Technology) were used in this study.
Immunofluorescence microscopy
Cells were fixed and incubated with antibodies as described previously (Lee and Song, 2007). Human anti-α-tubulin (1:1000, Sigma-Aldrich, #T5168) was used as a primary antibody to detect α-tubulin in the cells. DNA was detected with 0.2 μg/ml 14, 6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich). Coverslips were mounted with Vectashield mounting medium (Vector Laboratories, USA). Immunofluorescence images were collected at 488 nm on an Axio Imager A2 using a 40× 0.75 NA EC Plan-Neofluar objective lens and 10× ocular lens with an AxioCam Hrc CCD camera (all Carl Zeiss). The acquisition parameters and focus were controlled by the AxioVision software.
Flow cytometry
Cells were fixed with 100% cold ethanol, treated with 100 μg/ml RNase A for 4 h at 37°C, and stained with 10 μg/ml propidium iodide (Sigma-Aldrich). Flow cytometry was carried out with a FACS Calibur instrument (BD Biosciences, USA) equipped with Cell Quest software.
Histone extraction
For histone extraction, cells were lysed with lysis buffer (20 mM Tris-HCl pH 7.5, 1 mM sodium ethylene-diamine-tetra-acetic acid, 150 mM sodium chloride, 1 mM ethylene glycol tetra-acetic acid, 0.5% Nonidet P-40, 0.5% sodium dodecyl sulfate, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM sodium vanadate, 1 μg/ml leupeptin, and 1 mM phenylmethylsulphonyl fluoride). The supernatant was collected as the cytosolic fraction. The pellet (containing histones) was extracted in 0.2 M hydrochloric acid overnight at 4°C and neutralized with 1 M NaOH (Shechter et al., 2007).
RESULTS
Actin disruption delays cell cycle at G2/M in normal fibroblast IMR-90 cells
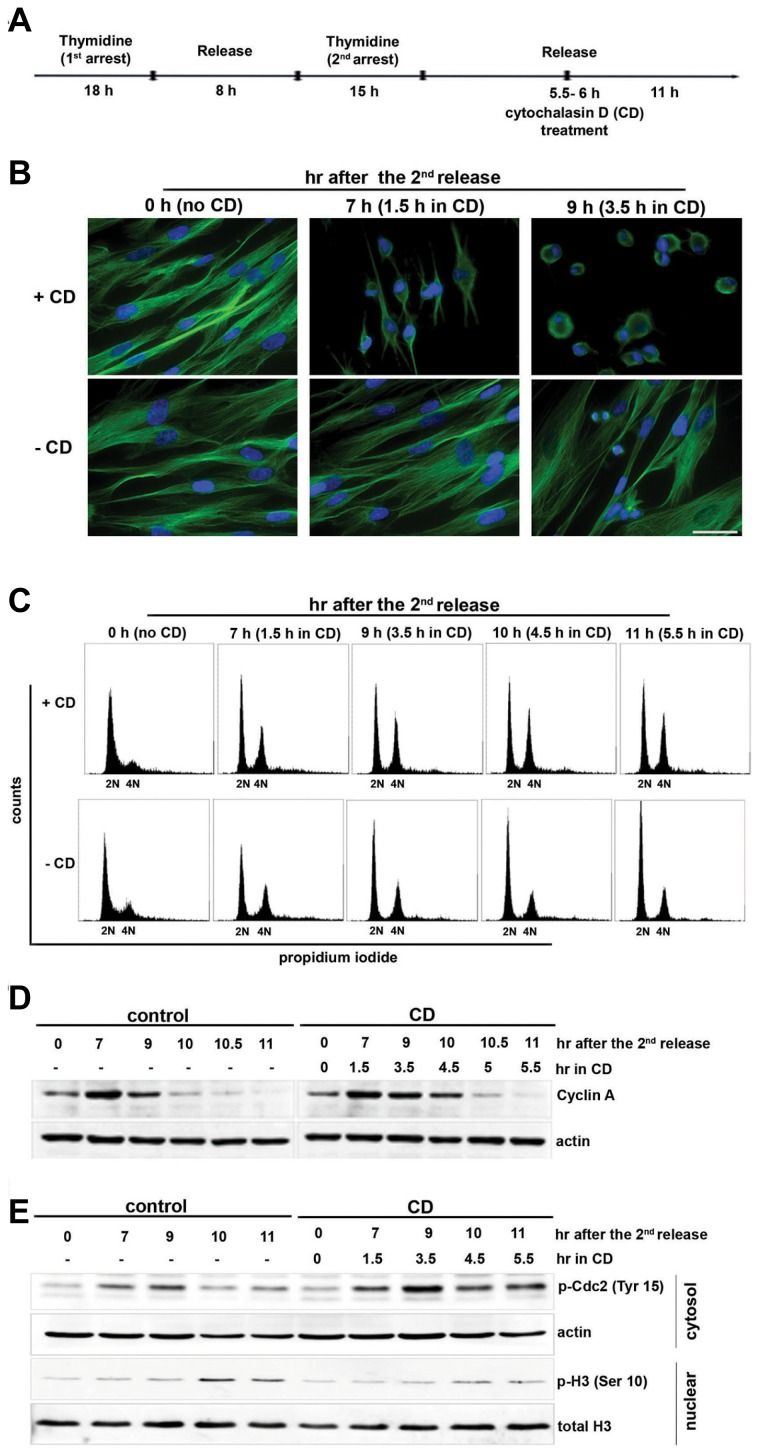
Previously, we reported that actin dysfunction delays the cell cycle at G2/M in IMR-90 normal human lung fibroblasts and mouse embryonic fibroblasts (Lee and Song, 2007). To confirm that actin disruption delays the cell cycle at G2/M in IMR-90 cells, we also examined the shape of cells and analyzed the DNA content by flow cytometry. IMR-90 cells subjected to double thymidine arrest (2 mM) were released and treated with CD (5 μM) at 5.5–6 h after the final release to disrupt the actin structure. When these cells were monitored by fluorescence microscopy, CD-treated IMR-90 cells began to change into a round shape with the nucleus localized on one side near the cell boundary and no mitotic cells were observed, compared to CD-untreated cells in which some cells underwent nuclear division (Fig. 1B). We also examined the cell cycle progression of these cells by flow cytometry and by cyclin A expression levels. When IMR-90 cells were treated with CD, cells containing 4N DNA were accumulated and maintained between 9–11 h, compared to CD-untreated control cells which showed a gradual increase of cells with 2N (Fig. 1C). Moreover, cyclin A degradation was delayed by 30–60 min in these cells compared to in untreated control cells (Fig. 1D). These observations strongly support that there is a delay at the entry of mitosis in CD-treated IMR-90 cells. To confirm the delay in the cell cycle to enter into mitosis in CD-treated IMR-90 cells, we also examined histone H3 phosphorylation, an established marker of mitosis. We observed that histone phosphorylation on Ser 10 increased in CD-untreated IMR-90 control cells at 10–11 h after release, indicating that the cells entered mitosis (Fig. 1E). Consistent with mitotic progression, inhibitory phosphorylation of Cdc2 at Tyr 15 was diminished at 10–11 h in these cells (Fig. 1E). In contrast, in CD-treated IMR-90 cells, histone phosphorylation on Ser 10 was not as prominent as in control cells at 10–11 h, supporting the G2/M delay of the cell cycle (Fig. 1E). In these cells, inhibitory phosphorylation of Cdc2 at Tyr 15 was also maintained (Fig. 1E). Taken together, these observations strongly indicate that G2/M delay occurs in normal IMR-90 cells when the actin structure is disorganized by CD.
Fig. 1. Actin disruption delays cell cycle at G2/M transition in normal IMR-90 cells.
(A) The layout shows how the experiment was designed and performed. IMR-90 cells were synchronized with 2 mM double thymidine arrest. The cells were treated with 5 μM cytochalasin D (CD) or the solvent DMSO as a control at 5.5–6 h after the second release and were collected at each indicated time point after the second release. (B) CD-treated and CD-untreated control cells were immunostained with an antibody against α-tubulin as described in the Materials and Methods. Nuclei were counterstained with DAPI. Scale bar, 50 μm. (C) Cells were fixed, stained with PI (10 μg/ml), and processed for flow cytometry. For each analysis, 10,000 cells were counted, and each fraction of cells with 2N and 4N DNA content is shown. (D) The cell lysate was resolved by 8% SDS-PAGE and blotted with anti-cyclin A and reprobed with anti-actin. (E) Histone extraction was performed as described in the “Materials and Methods”. The cytosolic fraction and histone fraction was resolved by 12% SDS-PAGE and blotted with p-Cdc2 (Tyr 15) and p-H3 Ser 10, respectively. Actin and histone H3 were used as loading controls.
Actin dysfunction along with sustained ERK-RSK activation inhibits Cdc25C and activates Wee1 for cell cycle delay in IMR-90 cells
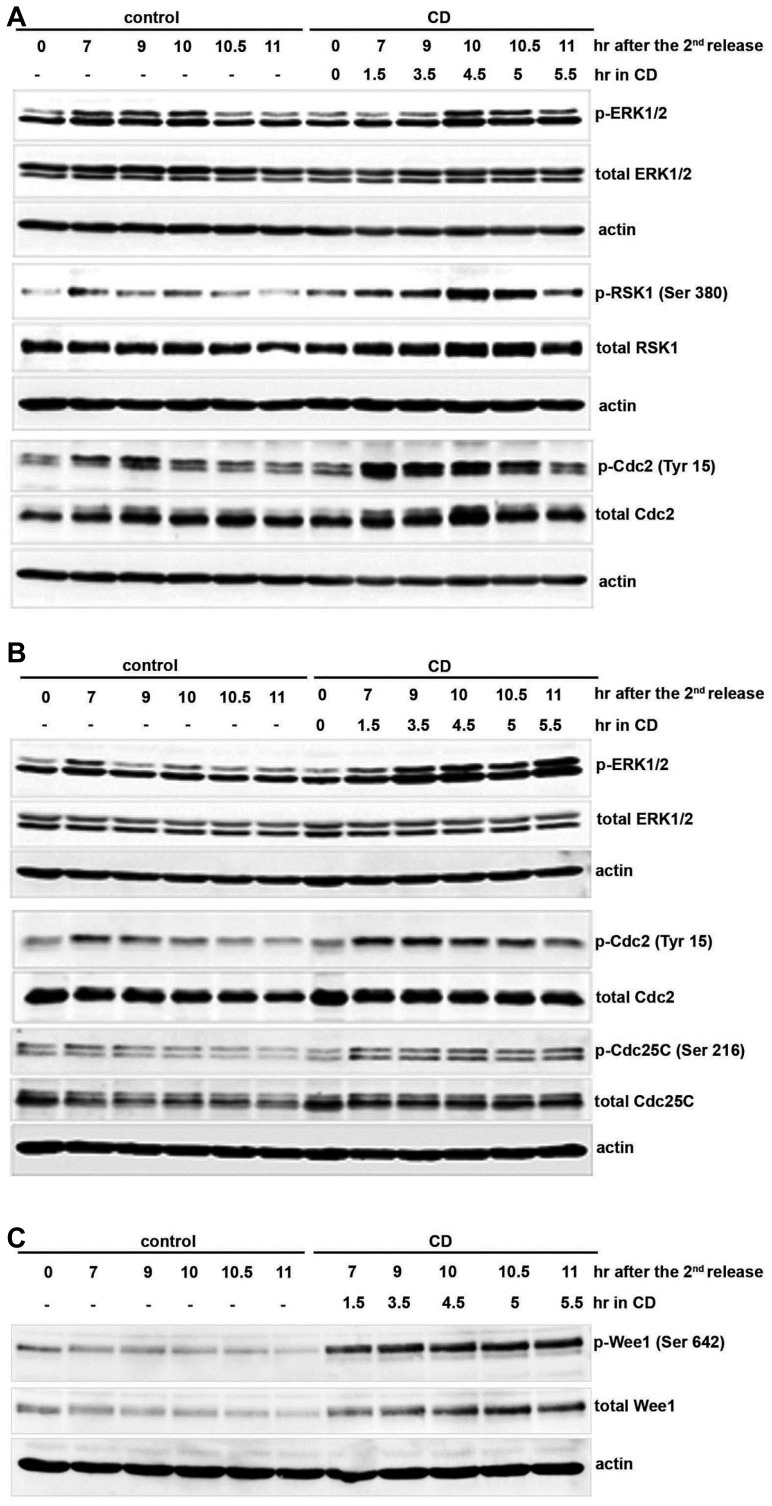
In our previous study, we found that actin dysfunction induces sustained activation of ERK1/2 to delay the cell cycle at G2/M in IMR-90 normal human lung fibroblasts and mouse embryonic fibroblasts (Lee and Song, 2007). p90RSK is well-known to be activated by p42MAPK (a human homologue of ERK kinase) and to arrest the cell cycle at G2/M in cycling Xenopus egg extracts (Chun et al., 2005). We then questioned whether ERK activation by actin disruption activates RSK downstream of ERK1/2 in IMR-90 cells, leading to Cdc2 inhibition to cause G2/M delay. First, we examined the activation of RSK downstream of ERK1/2 by actin dysfunction in IMR-90 cells. The expression levels of ERK1/2, RSK1, and Cdc2 were similar in both CD-treated and untreated IMR-90 cells (Figs. 2A and 2B). As reported by Lee and Song (2007), ERK activation was sustained for 30–60 min in CD-treated cells (Figs. 2A and 2B). Consistent with sustained ERK activation, continued activation of RSK1 was observed in IMR-90 cells treated with CD (Fig. 2A). In addition, inhibitory phosphorylation of Cdc2 (Tyr 15) was maintained until 10.5 h after the release in CD-treated IMR-90 cells, while it started to decline between 9–9.5 h in CD-untreated control cells, supporting G2/M delay of the cell cycle (Figs. 2A and 2B). Taken together, these observations demonstrate that actin dysfunction sustains RSK1 activation concomitantly with ERK activation and delays the cell cycle at G2/M by inhibiting Cdc2 kinase in normal IMR-90 cells.
Fig. 2. Actin dysfunction sustains RSK activation and Cdc2 inactivation in IMR-90 cells.
As denoted in Fig. 1A, IMR-90 cells were synchronized with 2 mM double thymidine arrest, incubated with 5 μM cytochalasin D or the solvent DMSO as a control at 5.5–6 h after the second release, and collected at each indicated time point after the second release. Cell lysates were resolved by 8% SDS-PAGE and blotted. Blots were probed with (A) p-ERK1/2 and p-RSK1 (Ser 380) and re-probed with anti-ERK1/2 and anti-RSK1 to observe the total amount of each protein, (B) p-ERK1/2 and p-Cdc25C (Ser 216), and re-probed with anti-ERK1/2 and anti-Cdc25C. (A, B) Cell cycle progression at G2/M was monitored by detecting p-Cdc2 (Tyr 15) followed by re-probing with anti-Cdc2 to detect the total amount of Cdc2. (C) The same samples from (A) and (B) were blotted with p-Wee1 (Ser 642) and re-probed with anti-Wee1. Each blot was re-probed with anti-actin as a loading control.
In CD-treated IMR-90 cells, we observed that the inhibitory phosphorylation of Cdc2 (Tyr 15) was maintained until 10.5 h after release (Figs. 2A and 2B). It is well-known that Wee1 inactivates Cdc2 kinase by phosphorylating Tyr 15, which is removed by Cdc25C phosphatase to activate Cdc2. Thus, we examined how actin dysfunction by CD controls Cdc25C and Wee1 to inhibit the kinase activity of Cdc2 to cause G2/M delay. Cdc25C activity is controlled by inhibitory phosphorylation at Ser 216, which is mainly detected during interphase (Peng et al., 1997). Once the cell enters mitosis, Ser 216 of Cdc25C is dephosphorylated and activating phosphorylation of Cdc25C at Ser 214 is detected during mitosis (Bulavin et al., 2003; Peng et al., 1997). Inhibitory phosphorylation of Cdc25C at Ser 216 in CD-treated IMR-90 cells was maintained until 11 h after the thymidine release, while it started to decrease after 9 h in CD-untreated control cells (Fig. 2B). We also examined the activation of Wee1 in response to actin dysfunction in CD-treated IMR-90 cells. Wee1 is activated during interphase by phosphorylation at Ser 642 (Rajeshkumar et al., 2011), but its hyper-phosphorylation at other sites is correlated with its inactivation at the entry of mitosis (Watanabe et al., 1995). In addition to being inactivated by hyper-phosphorylation at the G2/M transition, Wee1 is proteolytically degraded and its levels are decreased during mitosis (for a review, see Perry and Kornbluth, 2007). Activating phosphorylation of Wee1 at Ser 642 as well as total Wee1 protein was maintained until 11 h after the second release in CD-treated IMR-90 cells, while it started to decrease after 9 h in CD-untreated control cells (Fig. 2C). These results suggest that actin disruption delays the cell cycle at G2/M by activating Wee1 and inactivating Cdc25C, which maintains the inhibitory phosphorylation of Cdc2 at Tyr 15 in IMR-90 cells.
ERK and RSK control activation of Wee1 and Cdc25C to cause G2/M delay by actin dysfunction in IMR-90 cells
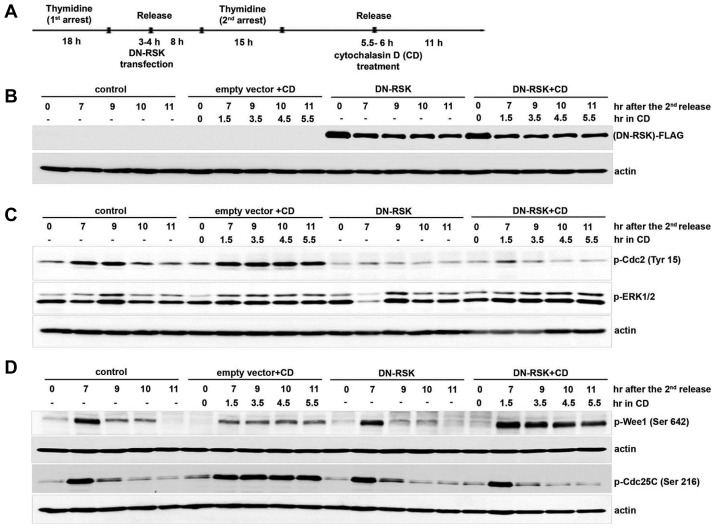
Our results showed that actin dysfunction activates ERK and its downstream RSK to maintain the inhibitory phosphorylation of Cdc2 by regulating the phosphorylation of Wee1 and Cdc25C to delay the cell cycle at G2/M. We then examined how phosphorylation of Wee1 and Cdc25C are regulated by ERK1 and RSK1. We overexpressed the kinase-dead mutant RSK (DN-RSK) in IMR-90 cells and examined the phosphorylation of Wee1 and Cdc25C after the cells were treated with CD. IMR-90 cells transfected with a vector containing DN-RSK or control empty vector at 3–4 h after the first thymidine release were arrested by the second thymidine treatment and released. Cells were treated with CD at 5.5–6 h after the second thymidine release and cell cycle progression was monitored, as summarized in Fig. 3A. The expression of DN-RSK in these cells was confirmed with anti-FLAG antibody (Fig. 3B). We first compared the activation of ERK in DN-RSK-overexpressing cells and in cells transfected with an empty vector, after the cells were treated with CD to induce actin dysfunction. ERK showed sustained activation in cells overexpressing DN-RSK, which was similar as the results in cells transfected with an empty vector and treated with CD, while ERK activation was not sustained in CD-untreated cells of untransfected control and DN-RSK-overexpressing cells (Fig. 3C). These results confirm that ERK sustains its activation by actin dysfunction. We then compared the inhibitory phosphorylation of Cdc2 at Tyr 15 in CD-treated IMR-90 cells of DN-RSK-overexpressing and empty vector-transfected with that in CD-untreated control and DN-RSK-overexpressing cells. Inhibitory phosphorylation of Cdc2 at Tyr 15 was not sustained in DN-RSK-overexpressing cells treated with CD compared to in DN-RSK-overexpressing cells without CD treatment, while cells transfected with empty vector showed the sustained inhibitory phosphorylation of Cdc2 by CD treatment (Fig. 3C). In response to actin dysfunction by CD, DN-RSK-overexpressing cells did not maintain inhibitory phosphorylation of Cdc2, although ERK activation was sustained (Fig. 3C). These results strongly suggest that RSK plays a key role downstream of ERK to control Cdc2 activity for cell cycle delay in response to actin dysfunction.
Fig. 3. Overexpression of dominant-negative RSK partially releases the cell cycle delay by actin dysfunction.
(A) The layout shows how the experiment was performed. IMR-90 cells were synchronized with 2 mM thymidine and transfected with DN-RSK or empty vector as described in the Materials and Methods. Cells were treated with 5 μM CD or only DMSO as a control at 5.5–6 h after the second release and collected at each indicated time point after the second release. Each cell lysate was resolved by 8% SDS-PAGE and blotted with (B) anti-FLAG and anti-actin, (C) p-ERK1/2 and p-Cdc2 (Tyr 15), (D) p-Cdc25C (Ser 216), and p-Wee1 (Ser 642). Each membrane was reprobed with anti-actin as a loading control.
In order to determine whether RSK is directly responsible for the phosphorylation of Wee1 and Cdc25C by actin dysfunction, we examined the phosphorylation of Cdc25C and Wee1 in DN-RSK-overexpressing cells with activated ERK by CD treatment. Inhibitory phosphorylation of Cdc25C at Ser 216 began decreasing at 9 h after the release in both CD-treated and CD-untreated DN-RSK-overexpressing cells as in CD-untreated control cells, while it was maintained until 11h after release in empty vector-transfected cells treated with CD (Fig. 3D). These results strongly suggest that RSK mainly regulates the inhibitory phosphorylation of Cdc25C. We also compared the activating phosphorylation of Wee1 kinase at Ser 642 in DN-RSK-overexpressing IMR-90 cells with and without CD treatment. In response to CD treatment, DN-RSK-overexpressing cells maintained the activating phosphorylation of Wee1 until 11 h after the release, as in empty vector-transfected cells (Fig. 3D). In contrast, activating phosphorylation of Wee1 kinase at Ser 642 was not maintained in CD-untreated DN-RSK-expressing cells (Fig. 3D). These observations strongly suggest that ERK and not RSK controls the activating phosphorylation of Wee1 to delay the cell cycle by actin dysfunction in IMR-90 cells.
DISCUSSION
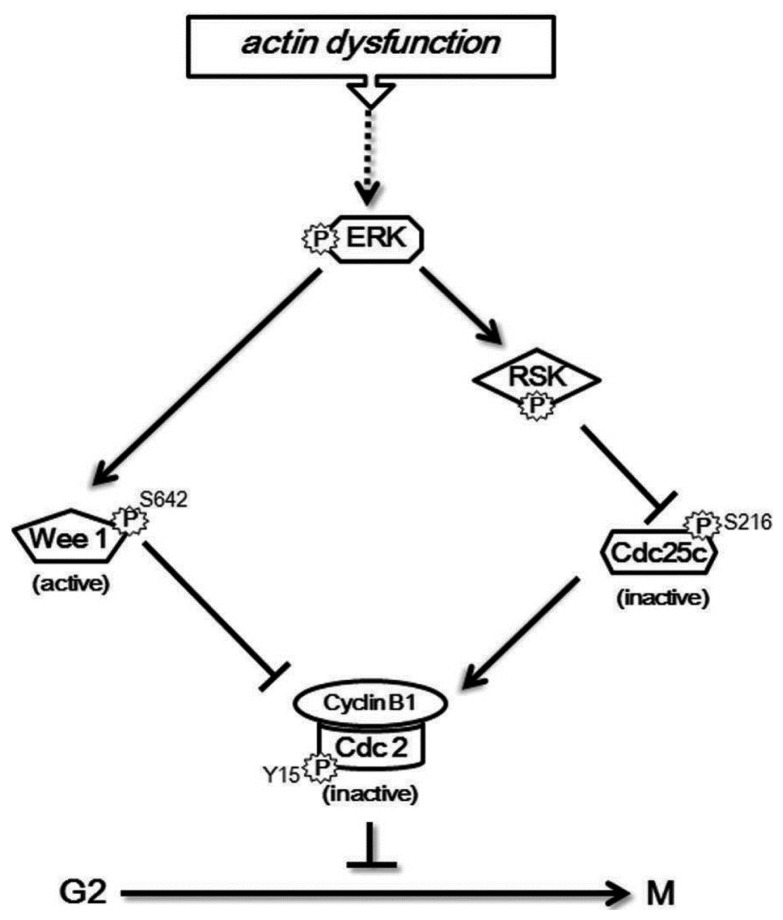
Our previous study showed that actin dysfunction induces sustained ERK activation to delay the cell cycle at G2/M by accumulating inhibitory phosphorylation of Cdc2 at Tyr 15 in normal human fibroblast IMR-90 cells (Lee and Song, 2007). In this study, we investigated how ERK controls the inhibitory phosphorylation of Cdc2 to delay the cell cycle in response to actin dysfunction in IMR-90 cells. We showed that sustained activation of ERK by actin defect induces continued activation of downstream RSK and both ERK and RSK control the inhibitory phosphorylation of Cdc2 to delay the cell cycle at G2/M: ERK is responsible for the activating phosphorylation of Wee1, while RSK controls the inhibitory phosphorylation of Cdc25C. These results are summarized as a model in Fig. 4.
Fig. 4.
A schematic model representing the mechanism to delay the cell cycle at G2/M by actin disruption.
It is well-known that DNA damage delays G2/M by activating Chk1, which inhibits Cdc25C by phosphorylation at Ser 216 (Sanchez et al., 1997). We also examined whether Chk1 is involved in the G2/M delay in response to actin dysfunction, but we observed no Chk1 activation by phosphorylation in actin-disrupted IMR-90 cells (data not shown). Walter et al. (1997) reported that in cycling Xenopus egg extracts, G2/M arrest is induced by sustained activation of p42MAPK during interphase. Chun et al. showed that p42MAPK in Xenopus eggs activates p90RSK, which negatively regulates Cdc25C via phosphorylation at Ser 287 (which corresponds to Ser 216 in mammalian cells), inactivating the Cdc2/cyclin complex to delay mitotic entry in Xenopus eggs (Chun et al., 2005). Thus, we examined RSK as a major downstream kinase of ERK in delaying the cell cycle at G2/M in response to actin defects. As expected, RSK activation was sustained after CD treatment in normal IMR-90 cells (Fig. 2A).
ERK has been reported to directly interact with actin-binding proteins such as palladin and calponin (Asano et al., 2011; Leinweber et al., 1999). An intact actin structure is also known to be important for translocalization of ERK to the nucleus, where it phosphorylates Elk-1 to initiate cell cycle progression and cell growth (Alphine et al., 2001). Moreover, an intact actin structure is needed to dephosphorylate ERK by the C3G and PP2A complex to suppress the malignant transformation of NIH3T3 cells (Martin-Encabo et al., 2007). These reports strongly suggest a close functional relationship between the actin cytoskeleton and ERK. In addition, RSK was also shown to play an important role in regulating the actin cytoskeleton. RSK1 is specifically required for cleavage furrow formation and ingression during cytokinesis (Nam et al., 2014). RSK has been shown to phosphorylate and activate filamin, an actin-binding protein, which forms a platform for the activation of Cdc2 by Cdc25C (Cukier et al., 2007; Otha et al., 1996; Telles et al., 2011; Woo et al., 2004). These studies strongly suggest a functional link between the actin cytoskeleton and ERK and RSK pathway to coordinate cell cycle progression and the actin cytoskeleton.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Junichi Abe (University of Rochester Medical Center) for generously providing dominant-negative RSK (DN-RSK) in pCMV-Tag2B. This work was supported by the National Research Foundation of Korea (NRF) funded to K. Song (No. NRF-2017R1A2B40097). D. Shrestha was supported in part by the BK21 and BK21 PLUS programs.
REFERENCES
- Alphine A.E., Stewart S.A., Assoian R.K., Juliano R.L. Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J Cell Biol. 2001;153:273–282. doi: 10.1083/jcb.153.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E., Maeda M., Hasegawa H., Ito S., Hyodo T., Yuan H., Takahashi M., Hamaguchi M., Senga T. Role of palladin phosphorylation by extracellular signal-regulated kinase in cell migration. PloS One. 2011;6:e29338. doi: 10.1371/journal.pone.0029338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulavin D.V., Higashimoto Y., Demidenko Z.N., Meek S., Graves P., Phillips C., Zhao H., Moody S.A., Appella E., Piwnica-Worms H., Fornace A.J., et al. Dual phosphorylation controls Cdc25 phosphatases and mitotic entry. Nat Cell Biol. 2003;5:545–551. doi: 10.1038/ncb994. [DOI] [PubMed] [Google Scholar]
- Carreno S., Kouranti I., Glusman E.S., Fuller M.T., Echard A., Payre F. Moesin and its activating kinase Slik are required for cortical stability and microtubule organization in mitotic cells. J Cell Biol. 2008;180:739–746. doi: 10.1083/jcb.200709161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J., Chau A.S., Maingat F.G., Edmonds S.D., Ostergaard H.L., Shibuya E.K. Phosphorylation of Cdc25C by p90RSK contributes to the G2 cell cycle arrest in Xenopus Cycling Egg extracts. Cell Cycle. 2005;4:148–154. doi: 10.4161/cc.4.1.1323. [DOI] [PubMed] [Google Scholar]
- Cukier I.H., Li Y., Lee J.M. Cyclin B1/Cdk1 binds and phosphorylates Filamin A and regulates its ability to cross-link actin. FEBS Lett. 2007;581:1661–1672. doi: 10.1016/j.febslet.2007.03.041. [DOI] [PubMed] [Google Scholar]
- Fujibuchi T., Abe Y., Takeuchi T., Imai Y., Kamei Y., Murase R., Ueda N., Shigemoto K., Yamamoto H., Kito K., et al. AIP1/WDR1 supports mitotic cell rounding. Biochem Biophys Res Commun. 2005;327:268–275. doi: 10.1016/j.bbrc.2004.11.156. [DOI] [PubMed] [Google Scholar]
- Kaji N., Muramoto A., Mizuno K. LIM-kinase-mediated cofilin phosphorylation during mitosis is required for precise spindle positioning. J Biol Chem. 2008;283:4983–4992. doi: 10.1074/jbc.M708644200. [DOI] [PubMed] [Google Scholar]
- Kunda P., Baum B. The actin cytoskeleton in spindle assembly and positioning. Trends Cell Biol. 2009;19:174–179. doi: 10.1016/j.tcb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Kunda P., Pelling A.E., Liu T., Baum B. Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr Biol. 2008;18:91–101. doi: 10.1016/j.cub.2007.12.051. [DOI] [PubMed] [Google Scholar]
- Lancaster O.M., Baum B. Shaping up to divide: coordinating actin and microtubule cytoskeletal remodelling during mitosis. Semin Cell Dev Biol. 2014;34:109–115. doi: 10.1016/j.semcdb.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Lee K., Song K. Actin dysfunction activates ERK1/2 and delays entry into mitosis in mammalian cells. Cell Cycle. 2007;6:1487–1495. [PubMed] [Google Scholar]
- Leinweber B.D., Leavis P.C., Grabarek Z., Wang C.L., Morgan K.G. Extracellular regulated kinase (ERK) interaction with actin and the calponin homology (CH) domain of actin-binding proteins. Biochem J. 1999;344:117–123. [PMC free article] [PubMed] [Google Scholar]
- Maddox A.S., Burridge K. RhoA is required for cortical retraction and rigidity during mitotic cell rounding. J Cell Biol. 2003;160:255–265. doi: 10.1083/jcb.200207130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Encabo S., Santos E., Guerrero C. C3G mediated suppression of malignant transformation involves activation of PP2A phosphatases at the subcortical actin cytoskeleton. Exp Cell Res. 2007;313:3881–3891. doi: 10.1016/j.yexcr.2007.07.036. [DOI] [PubMed] [Google Scholar]
- Nam H.J., Lee I.J., Jang S., Bae C.D., Kwak S.J., Lee J.H. p90 ribosomal S6 kinase 1 (RSK1) isoenzyme specifically regulates cytokinesis progression. Cell Signal. 2014;26:208–219. doi: 10.1016/j.cellsig.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Hartwig J.H. Phosphorylation of actin-binding protein 280 by growth factors is mediated by p90 ribosomal protein S6 kinase. J Biol Chem. 1996;271:11858–11864. doi: 10.1074/jbc.271.20.11858. [DOI] [PubMed] [Google Scholar]
- Peng C.Y., Graves P.R., Thomas R.S., Wu Z., Shaw A.S., Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Perry J.A., Kornbluth S. Cdc25 and Wee1: analogous opposites? Cell Div. 2007;2:12. doi: 10.1186/1747-1028-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeshkumar N.V., De Oliveira E., Ottenhof N., Watters J., Brooks D., Demuth T., Shumway S.D., Mizuarai S., Hirai H., Maitra A., et al. MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. Clin Cancer Res. 2011;17:2799–2806. doi: 10.1158/1078-0432.CCR-10-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J., Cramer L.P., Baum B., McGee K.M. Myosin II dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell. 2004;117:361–372. doi: 10.1016/s0092-8674(04)00341-1. [DOI] [PubMed] [Google Scholar]
- Sanchez Y., Wong C., Thomas R.S., Richman R., Wu Z., Piwnica-Worms H., Elledge S.J. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- Shechter D., Dormann H.L., Allis C.D., Hake S.B. Extraction, purification and analysis of histones. Nat Protoc. 2007;2:1445–1457. doi: 10.1038/nprot.2007.202. [DOI] [PubMed] [Google Scholar]
- Telles E., Gurjar M., Ganti K., Gupta D., Dalala S.N. Filamin A stimulates Cdc25C function and promotes entry into mitosis. Cell Cycle. 2011;10:776–782. doi: 10.4161/cc.10.5.14954. [DOI] [PubMed] [Google Scholar]
- Thery M., Racine V., Pepin A., Piel M., Chen Y., Sibarita J.B., Bornens M. The extracellular matrix guides the orientation of the cell division axis. Nat Cell Biol. 2005;7:947–953. doi: 10.1038/ncb1307. [DOI] [PubMed] [Google Scholar]
- Toyoshima F., Nishida E. Integrin-mediated adhesion orients the spindle parallel to the substratum in an EB1- and myosin X-dependent. EMBO J. 2007;26:1487–1498. doi: 10.1038/sj.emboj.7601599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S.A., Guadagno T.M., Ferrell J.E. Induction of a G2-phase arrest in Xenopus egg extracts by activation of p42 mitogen-activated protein kinase. Mol Biol Cell. 1997;8:2157–2169. doi: 10.1091/mbc.8.11.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Broome M., Hunter T. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 1995;14:1878–1891. doi: 10.1002/j.1460-2075.1995.tb07180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo M.S., Ohta Y., Rabinovitz I., Stossel T.P., Blenis J. Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin A on an important regulatory site. Mol Cell Biol. 2004;24:3025–3035. doi: 10.1128/MCB.24.7.3025-3035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]