Abstract
Parental environmental factors including diet, body composition, metabolism and stress affect the health and chronic disease risk of people throughout their lives, as captured in the ‘Developmental Origins of Health and Disease’ (DOHaD) concept. Research across epidemiological, clinical and basic science fields has identified the period around conception as being critical in the processes mediating parental influences on the next generation’s health. During this time, from the maturation of gametes through to early embryonic development, parental lifestyle can adversely influence long-term risks of offspring cardiovascular, metabolic, immune and neurological morbidities, often termed ‘developmental programming’. We review ‘periconceptional’ induction of disease risk from four broad exposures: maternal overnutrition and obesity; maternal undernutrition; related paternal factors; and from the use of assisted reproductive treatment. Human studies and animal models demonstrate the underlying biological mechanisms, including epigenetic, cellular, physiological and metabolic processes. A novel meta-analysis of mouse paternal and maternal protein undernutrition indicate distinct parental periconceptional contributions to postnatal outcomes. We propose that the evidence for periconceptional effects on lifetime health is now so compelling that it calls for new guidance on parental preparation for pregnancy, beginning before conception, to protect the health of offspring.
Introduction
The notion that maternal physiology, body composition, diet and lifestyle during pregnancy have profound and enduring effects on offspring long-term health and disease risk into adulthood has received strong evidential support across epidemiological, medical and basic science fields1–3. Thus, the ‘Developmental Origins of Health and Disease’ (DOHaD) concept has emerged, proposing that poor developmental experience can provoke increased risk of non-communicable disease in later life, particularly cardiovascular and metabolic comorbidities such as hypertension, obesity and type-2 diabetes, atopic conditions and some forms of cancer, as well as neurological impairment. A recent focus in DOHaD research has been to probe when during pregnancy the conceptus is most vulnerable to such adverse influences, thereby informing targeted protection and possible intervention. Increasing evidence points to the importance of the time around conception (=periconceptional period).
Periconceptional developmental conditioning
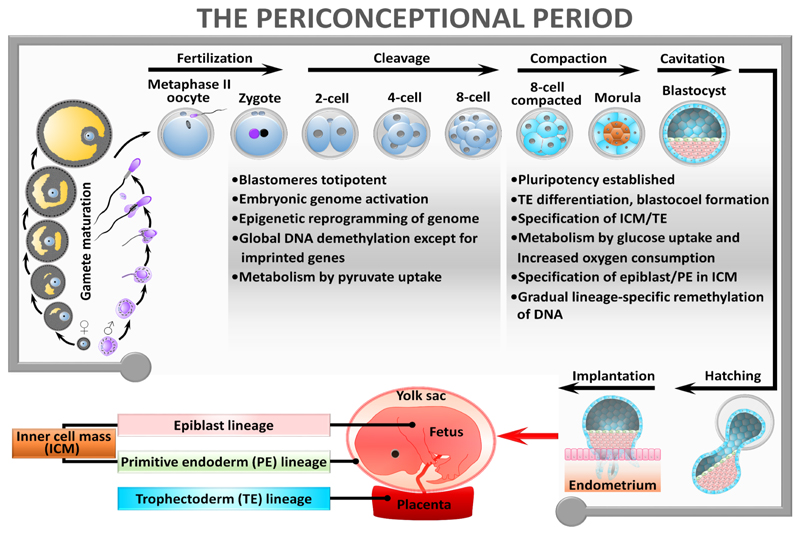
The periconceptional period has been variously defined, but for DOHaD processes the key events broadly cover the completion of meiotic maturation of oocytes, differentiation of spermatozoa, fertilisation and resumption of mitotic cell cycles in the zygote, marking the transition from parental to embryonic genomes4 and the onset of morphogenesis up to implantation5. This represents a period of a few weeks, dependent upon mammalian species, and is characterised by extensive change in morphology (emergence of distinct embryonic and placental cell lineages); genomic re-organisation (epigenetic modifications such as DNA methylation to regulate lineage-specific gene expression in the conceptus); and changes in metabolism (setting homeostatic regulators for growth and energy supply). See Figure 1 for a resumé of key events. It is however recognised that influences at every stage from earliest childhood can shape preconception health and thereby influence eventual pregnancy and birth outcomes.
Figure 1. Biological events underpinning periconceptional conditioning.
The periconceptional period is one of extensive cellular change comprising the completion of meiotic maturation of oocytes, differentiation of spermatozoa, fertilisation and resumption of mitotic cell cycles in the zygote, marking the transition from parental to embryonic genomes 4 and the onset of morphogenesis 5. Periconceptional biology is indeed ‘busy’ – the morphological and cellular changes occurring during the switch from parental to embryonic generations leading to blastocyst formation are driven by pronounced sub-cellular and molecular processes. These include global restructuring of the epigenome (mainly DNA methylation and histone modifications that control gene expression), such that expression from the new embryonic genome is distinct from the parental genomes99. Epigenetic reorganisation allows the embryo to first exhibit totipotency, a naïve cellular state conferring the ability to construct both true embryonic (future fetal) cell lineages and the extra-embryonic (placental) lineages that become evident in the blastocyst. Subsequently, epigenetic modifications underpin embryo pluripotency, the capacity to generate all three germ layers (ectoderm, mesoderm, endoderm) once gastrulation has taken place. Morphogenesis of the blastocyst is followed by embryo hatching from the zona pellucida coat and implantation mediated through adhesion of the outer trophectoderm layer of the blastocyst to the uterine endometrium and subsequent invasion and decidualisation. Activation of the new embryonic genome before implantation not only permits de novo gene expression distinct from parental genomes but also involves establishment of the embryo’s metabolism that matures over time100.
Adverse developmental processes around the time of conception have been demonstrated in human and animal models in response to diverse environmental situations. In vivo, the quality of a mother’s diet, both overnutrition and obesity6 or undernutrition7, and/or other aspects of her physiological status including hyperglycemia/lipidemia8, may affect embryo potential with consequences for offspring disease risk over the lifetime. Paternal lifestyle and phenotype can similarly influence long-term offspring health, mediated either through the sperm or seminal plasma9. Periconceptional parental influences may have particular and differing effects on male and female offspring10. In addition, more babies are being born as a result of assisted reproductive treatments (ART) some of which involve embryo culture and exposure to potentially inappropriate environmental factors, which may alter offspring phenotype10,12. Long-term outcomes are consistent with the DOHaD concept, including cardiometabolic, immunological and neurological non-communicable disorders.
To some the concept of ‘periconceptional’ origins of lifetime health may not be intuitive. Why should this short window at the very start of development have such profound consequences for the rest of our lives? Critically, the essential steps in reproduction over this period occur when the few cells involved are fully exposed to environmental conditions, making them vulnerable to disturbance of epigenetic mechanisms and an altered profile of embryonic gene expression that persists through subsequent cell cycles and drives an altered developmental programme. Metabolic and cellular homeostatic characteristics of the embryo, including mitochondrial activity, can also change in response to nutrient availability. Conversely, periconceptional sensitivity to environmental cues also raises the possibility that this window is one of opportunity, providing the embryo with capacity to respond to prevailing conditions and to optimise development to best suit survival and fitness7. Thus, periconceptional developmental plasticity (induction of different phenotypes from a single genotype) may facilitate setting of suitable growth and metabolic parameters to match the perceived environment but which, if environmental conditions change, may become maladaptive and lead to later disease3.
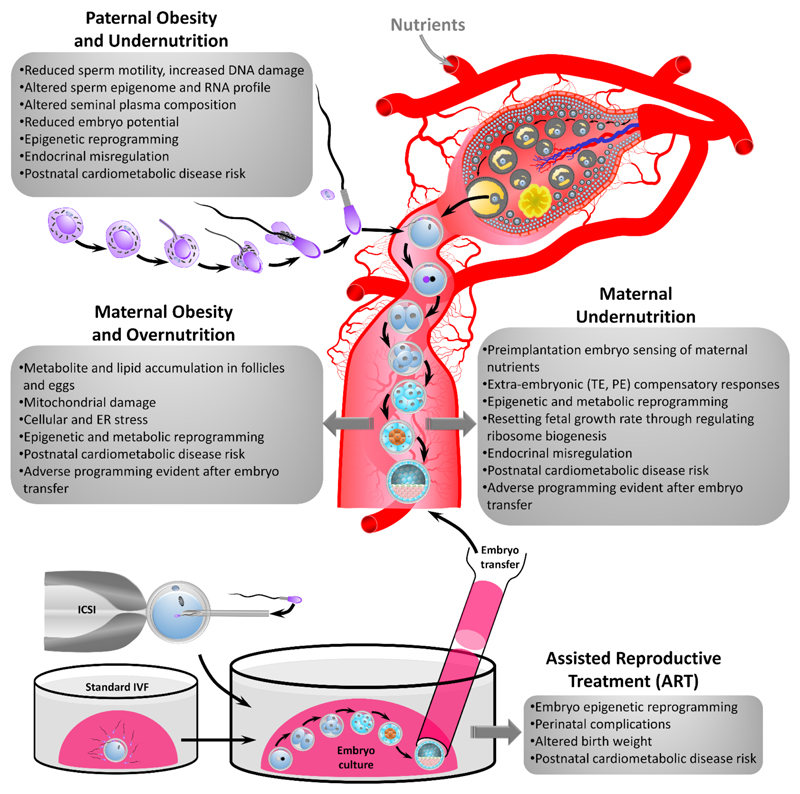
This article focuses on four broad periconceptional environmental exposures shown to induce adverse effects in humans and animal models (Figure 2), and discusses mechanistic causes and consequences. We also report new data on the relative contributions of maternal and paternal influences to long-term periconceptional influences in an established low protein diet model of parental undernutrition.
Figure 2. Summary of periconceptional developmental conditioning from the four areas reviewed with main mechanisms highlighted in the progression of disease risk. ICSI = intracytoplasmic sperm injection, IVF = in vitro fertilization.
Periconceptional developmental conditioning through maternal overnutrition and obesity
The global rise in maternal obesity is associated with reduced female fertility and heightened risk of obesity in the offspring2. Adverse effects of high maternal body mass index (BMI) on the offspring may reflect elevated maternal glucose and insulin concentrations driving fetal growth and adiposity, resulting in increased birth and childhood weight, but may also include shared lifestyle factors within families6. Impaired offspring metabolism may also be associated with increased risk of allergic and atopic conditions, revealing the complexity in phenotype2. Maternal obesity models in animals have confirmed the link with offspring cardiovascular and metabolic disease risk6,13.
Why might the periconceptional period be causal for obesity-related conditioning? Obese women have higher circulating concentrations of inflammatory cytokines14, and of hormones and metabolites which accumulate within the ovarian follicular fluid and can affect oocyte maturation and potential adversely. Thus, maternal BMI is positively associated with increased follicular fluid insulin, lactate, triglycerides, leptin and other metabolic regulators15. This rich follicular fluid compromises the developmental competence of exposed animal oocytes in experimental models, reducing embryo quality16. Moreover, oocytes from obese women are smaller and produce blastocysts with increased triglycerides and reduced glucose consumption, markers of poorer potential17.
In addition to metabolite overexposure, maternal obesity in mice induces defects in the mitochondrial phenotype of eggs, including abnormal morphology and cristae structure18, altered membrane potential and distribution18 and increased mitochondrial DNA content18,19, all markers of disturbed mitochondrial function and energy homeostasis. Oocytes from obese dams also exhibit increased oxidative stress and spindle abnormalities suggesting increased risk of aneuploidy18,19.
These mitochondrial defects in oocytes may derive from the elevated lipid content and inherent insulin resistance caused by high maternal adiposity. Oocyte hyperlipidaemia in turn leads to impaired metabolic regulation and endoplasmic reticulum stress in mice16, a condition where proteins misfold during biosynthesis and which contributes to metabolic and cardiovascular disease. Bovine and murine in vitro oocyte maturation models demonstrate that elevated fatty acid concentrations perturb follicular physiology, reduce oocyte developmental competence, including altered transcriptome and epigenome profiles in blastocysts, and lead to early embryos with compromised metabolism and lower potential12.
The combination of metabolic, mitochondrial and chromosomal alterations in oocytes and embryos from obese mothers has important implications for subsequent development. In mice, obese mothers have smaller fetuses and pups which develop overgrowth, adiposity and glucose intolerance after birth20. Transfer of mouse blastocysts from obese mothers to normal recipients produces similarly growth-restricted fetuses with associated malformations despite the absence of gestational maternal obesity18. Similarly, in sheep, female offspring from embryos of obese natural mothers transferred to non-obese mothers exhibit increased adiposity, with dysregulation in liver and muscle insulin signalling and hepatic fatty acid oxidation21. These changes are associated with epigenetic perturbations in the liver, including upregulation of microRNAs regulating insulin signalling21. Similarly, mouse embryos transferred from diabetic mothers to control recipients exhibit fetal growth retardation and congenital anomalies resembling natural diabetic pregnancies8; such structural changes are in keeping with clinical practice, in which pre/periconceptional folic acid supplementation and improved diabetes control reduce the incidence of anomalies.
The periconceptional effects of maternal obesity are also apparent in ART pregnancies. Fertility declines with increasing BMI in women receiving donor oocytes, as in non-donated pregnancies, suggesting reduced uterine receptivity22. However, in other studies, recipient BMI had no effect on donor oocyte pregnancy success, whilst donor BMI was negatively associated23, indicating that pre-conception oocyte quality is influenced by maternal adiposity.
Periconceptional developmental conditioning through maternal undernutrition
Human studies
Poor nutrition in utero and low birth weight remain highly prevalent in low and middle income countries and are associated with increased risks of chronic diseases in later life across diverse human populations, particularly if followed by accelerated weight gain during infancy1,3. Similar human cardiometabolic and neurological consequences arise from maternal exposure to famine, e.g. the Dutch Hunger Winter of 1944/45. In human studies it is difficult to pinpoint gestational windows when heightened sensitivity to maternal undernutrition occurs, but the Dutch famine analyses suggest a poorer prognosis for those offspring conceived during the famine rather than experiencing it later in gestation24. Similarly, individuals exposed in utero, particularly during the first trimester, to the Chinese Great Famine (1959-61) have increased risk of hypertension in adulthood25. Exposure during the periconceptional period of the Dutch famine is reported to cause epigenetic dysregulation resulting in reduced DNA methylation of the imprinted growth-regulating IGF2 gene persisting into adulthood, along with differential methylation in the regulatory regions of genes affecting growth and metabolism24.
In another important human study, dramatic seasonal variation in maternal nutrient consumption in The Gambia affected perinatal outcomes including birth weight, adult health and mortality26. By studying genomic regions where methylation patterns are highly correlated across tissues derived from all three germ lines it has been possible to demonstrate that maternal nutrition at conception alters the epigenome prior to gastrulation, with the effects persisting, at minimum, well into childhood and adolescence27. This periconceptional legacy coincided with seasonal changes in maternal plasma methyl-donor biomarkers which, along with BMI, are also predictive of childhood methylation patterns28. So far, significant deviations in the methylation patterns of loci predictive of immune function, tumour suppression29 and obesity30 have been noted.
Animal models
Animal models have been essential for investigating mechanisms involved in the multistep processes linking periconceptional maternal undernutrition with later-life disease risk. In rodents, feeding a low protein diet (LPD) - specifically during the periconceptional period, either exclusively during the final 3 days of oocyte maturation31 or the 3-4 day window of preimplantation embryo development (Emb-LPD)32,33, with normal nutrition at all other times - is sufficient to induce an altered growth trajectory and cardiovascular, metabolic and neuro-behavioural dysfunction in adulthood. Such targeted dietary models commonly show hypertension in adult offspring, coupled with increased adiposity7,31–33. Similar findings have been reported in sheep34.
Rodent and sheep models of maternal periconceptional undernutrition suggest that impaired regulation of fetal development may underlie co-morbidities. For example, studies in sheep have shown that the late gestation fetal cardiovascular response to hypoglycaemia is modified by prior peri-implantation undernutrition35. Moreover, peri-implantation and late gestation maternal undernutrition affect fetal sheep skeletal muscle development differentially36, and maternal undernutrition in early gestation alters gestation length and fetal and postnatal growth37.
Induction and response mechanisms
The mouse embryonic period low protein diet (Emb-LPD) model has helped reveal how periconceptional maternal undernutrition may initiate adverse effects during early embryogenesis7. Emb-LPD reduces circulating maternal insulin and amino acid concentrations, including reduced branched-chain amino acids (BCAAs) within the uterine luminal fluid that bathes early embryos before implantation38. BCAAs act as targets for embryo nutrient sensors, enabling nutrient status to be sensed by blastocysts via the mammalian target of rapamycin complex 1 (mTORC1) growth-regulating signalling pathway, inducing an altered growth trajectory from before implantation38 (see below), and shown by embryo transfer to be induced within the blastocyst33. Altered induction by Emb-LPD in mice activates compensatory responses that are distinct between extra-embryonic (trophectoderm; primitive endoderm) and embryonic (epiblast) lineages of the blastocyst (Figure 1). The Emb-LPD trophectoderm becomes more proliferative, adopts a more invasive migratory phenotype at implantation, and activates increased endocytosis of maternal uterine luminal fluid proteins as an alternative source of nutrients, leading to a placenta that is more efficient in nutrient transfer to the fetus38–40. Similarly, the primitive endoderm activates compensatory responses to enhance nutrient delivery via the yolk sac placenta, mediated through epigenetic mechanisms40,41.
In response to Emb-LPD, changes in embryonic lineages may help set the embryonic and fetal growth trajectory to match prevailing nutrient availability. The embryonic lineages utilise preimplantation nutrient sensing to regulate growth across somatic organs (e.g., liver and kidney) through adaptations in the rate of ribosome biogenesis42. In essence, rRNA expression is suppressed during periods of maternal dietary restriction but is increased, beyond that of the control rate, when the dietary challenge is removed. This mechanism modulates the level of DNA methylation at the rDNA promoter, thereby mediating RNA polymerase I interaction with the promoter to regulate ribosome biogenesis and growth42,43. Interestingly, rDNA has also been found to be a genomic target for growth regulation in models of maternal high-fat or obesogenic diets43. This exquisite lifetime mechanism, activated in the preimplantation embryo, is likely to be responsive to uterine luminal fluid nutrient concentrations and appears to utilise a nutrient-sensing ribosome factor, Rrn3, to mediate the rDNA responses42. The growth-regulating role of the embryonic lineages is critical since perinatal weight associates with adult disease risk33.
Paternal origin of periconceptional developmental programming
Whilst the connection between a mother’s diet and the long-term health of her offspring has been studied in detail, our understanding of how a father’s diet impacts his offspring remains limited. However, links are now emerging between paternal lifestyle, sperm quality and impaired offspring health9. Here, both direct (sperm quality, epigenetic status, DNA integrity) and indirect (seminal fluid composition) paternal mechanisms have been identified, with the potential to affect mouse offspring development across multiple generations44.
Mirroring female reproductive fitness, male fertility is closely linked to nutrition and body composition. In humans and rodents, elevated BMI is associated with reduced sperm motility45, increased sperm abnormality46, increased sperm reactive oxygen species levels, reduced serum testosterone and increased oestradiol concentrations47. Consumption of a ‘Western-style’ diet high in sugar, fat and processed food associates with reduced sperm motility in men48, while consumption of energy-dense diets in men and rodents is associated with poor sperm motility, morphology and DNA integrity49. Reduced sperm DNA integrity, as occurs in obesity and diabetes, correlates with reduced human embryonic development and decreased pregnancy rates50. In men undergoing IVF treatment, obesity is associated with reduced blastocyst development and live birth rates51. In rodents, paternal obesity induced by high-fat diet increases sperm DNA damage52, reduces blastocyst development and implantation rates53 and causes sub-fertility in male and female offspring for up to two generations54. Interestingly, these negative effects on offspring development can be prevented through paternal dietary and exercise interventions in mice55, indicating that sperm-mediated effects may be transient and even reversible. In rats, a paternal high-fat diet for 10 weeks before mating affected female (but not male) offspring pancreatic β-cell function and increased body weight, glucose intolerance and impaired insulin secretion56. Offspring of male mice over-nourished during neonatal life demonstrate glucose intolerance, fasting hyperglycaemia and insulin resistance, mirroring the metabolic disturbance seen in their fathers57.
Similar to the impacts of paternal obesity, paternal LPD in mice induces the expression of genes involved in offspring hepatic lipid and cholesterol biosynthesis58. Analysis of offspring hepatic epigenetic status revealed genome-wide changes in DNA methylation, including the key lipid regulator PPARα. In adulthood, offspring from male mice fed LPD have higher birth weight, a reduced male:female offspring ratio, increased adult adiposity, hypotension, glucose intolerance and elevated serum TNF-α levels59. Furthermore, paternal LPD also affects blastocyst AMPK gene expression, placental size, fetal growth and skeletal development60.
As for maternal periconceptional nutrition models, epigenetic mechanisms are likely mediators of effects of paternal phenotype and exposures on offspring development61. Changes in patterns of sperm histone modifications (methylation, acetylation), DNA methylation and/or RNA content are prime candidates for such paternal periconceptional programming. Sperm from infertile men display significant changes in histone populations62, with enrichment of active histone marks (i.e. H3K27me3) at key developmental and pluripotency genes in human and mouse sperm62. Studies have also revealed that sperm-derived histones are transferred into the oocyte and incorporate into zygotic chromatin following human fertilisation63. However, whether any of the 2-15% histones retained within the mammalian sperm contribute directly to zygotic gene expression regulation is unknown. Human sperm also contain several thousand coding RNA transcripts64 and altered expression is linked with infertility65. Recent studies have shown that levels of sperm tRNA-derived small RNAs (tsRNAs) are altered by paternal diet in mice66. Interestingly, offspring generated by injecting zygotes with sperm tsRNA taken from male mice fed a HFD showed impaired glucose tolerance and insulin secretion66. While such studies highlight the role of RNA populations in intergenerational programming67, the significance of these sperm-derived RNA molecules remains to be elucidated.
Apart from sperm-specific mechanisms of developmental programming, seminal plasma composition, (e.g. granulocyte-macrophage colony-stimulating factor) influences mouse embryonic, placental and offspring development68 and initiates maternal reproductive tract immunological responses, essential in the establishment and maintenance of human pregnancy69. In mice, paternal seminal fluid impacts on the maternal uterine environment, altering blastocyst development, placental size and adult offspring glucose tolerance, adiposity and blood pressure70.
Defining the parental contribution to periconceptional developmental effects
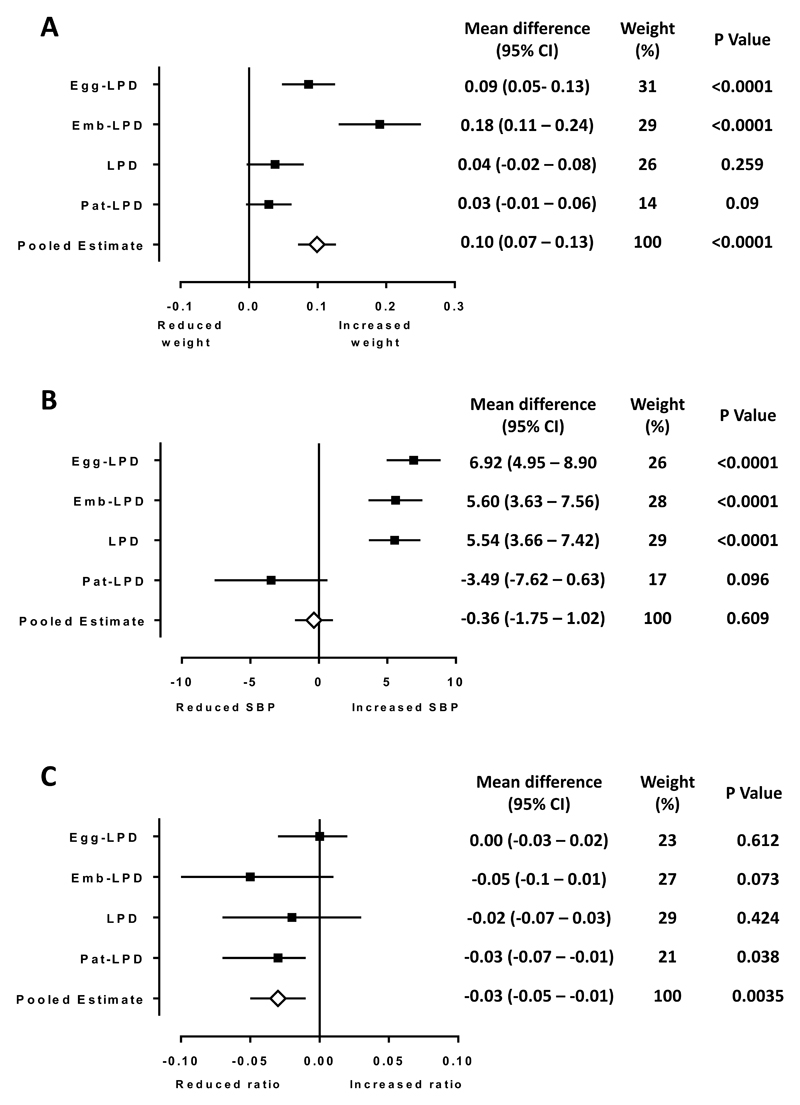
Shared maternal and paternal dietary and other lifestyle influences may potentially combine for greater impact on periconceptional development. However, most research models to date are uniparental in design and the combined effects of both parents are unknown. Whether the impact of poor paternal diet on offspring development and wellbeing is of equivalent significance to that of poor maternal diet is also unknown. As a first step, Box 2 and Figure 3 show a meta-analysis of our mouse maternal and paternal LPD diet models using published data for offspring weight at birth, adult systolic blood pressure (SBP) and adult heart:body weight ratio (a measure of heart capacity) including datasets covering maternal intervention restricted to the periods of oocyte maturation, preimplantation development or the entirety of gestation31,33,59. The use of the same robust, statistical random effects regression analysis across each of these studies strengthens our comparison of parental effects in the current analysis. However, such rigorous statistical approached are rarely adopted, especially in animal model studies, and so we have restricted our analysis to data from these three studies alone. Offspring birth weight was increased in response to maternal LPD during the terminal stages of oocyte development (Egg-LPD) and during preimplantation preimplantation development (Emb-LPD) (Figure 3a). Overall, the pooled estimate demonstrated parental LPD increased offspring birth weight. Our second analysis explored the impact of parental LPD on adult offspring SBP. Here, all maternal challenges resulted in offspring hypertension (Figure 3b), while paternal LPD resulted in a trend towards lower blood pressure in the adult offspring. Our final analysis examined the impact of parental diet on adult heart:body weight ratio (Figure 3c). Only paternal LPD had a significant effect, reducing offspring heart:body weight ratio. These new data demonstrate differential effects from paternal and maternal periconceptional developmental exposures on offspring phenotype. It is essential that further studies define the precise impacts and underlying mechanisms by which parental diet regimes affect offspring development and wellbeing. Studies examining concurrent paternal and maternal interventions on shared offspring outcomes are also warranted.
Box 2. Analysis of parental contribution effect.
Data for offspring phenotype were taken from Watkins et al 2008a31, 2008b33 and 201459. Each study used the same NPD and LPD formulation fed to either female or male mice for distinct periconceptional durations.
All three studies employed the same rigorous random effects regression analysis to account for the hierarchical nature of the studies in the statistical analyses.
Raw data on individual offspring weight at birth, adult tail-cuff systolic blood pressure measurement and adult heart:body weight ratio for all groups were used for the analyses.
Raw mean differences between experimental and study-specific control group (normalised to a value of 0) offspring were calculated (Δ = µ1 - µ2) for birth weight, systolic blood pressure (SBP) and heart:body weight ratio parameters.
Weight (%) refers to the individual contribution (by number of animals) of each study to the total Pooled Estimate. Heterogeneity (i.e. variation in outcomes between studies) was assessed using χ2 test on Cochran’s Q-statistic and by calculating I2 (i.e. percentage of variation across studies attributed to heterogeneity rather than chance). As heterogeneity was significant for all analyses, pooled estimates were calculated by the random effects (Mantel-Haenszel) method.
The largest effect on offspring birth weight was in response to maternal preimplantation (Emb-LPD) diet (raw mean difference: 0.18g, 95% CI 0.11 – 0.24; P<0.0001) (Figure 3a). Maternal LPD restricted to the terminal stages of oocyte maturation (Egg-LPD) also resulted in increased birth weight (raw mean difference: 0.09g, 95% CI 0.05 – 0.13; P<0.0001). However, maternal LPD throughout gestation had no impact (raw mean difference: 0.04g, P=0.26) on offspring birth weight (likely reflecting fetal growth regulation during gestation, discussed above), as did paternal LPD (raw mean difference 0.03g, P=0.09). Overall we observe a significant pooled estimate effect of parental LPD on offspring weight at birth (raw mean difference: 0.1g, 95% CI 0.07 – 0.13; P<0.0001) representing an increase in LPD offspring weight of 7.8%.
Analysis of offspring SBP revealed all maternal LPD groups had elevated SBP (raw mean difference: Egg-LPD 6.92mmHg, 95% CI 4.95 – 8.90; P<0.0001; Emb-LPD 5.60mmHg, 95% CI 3.63 – 7.56; P<0.001; LPD 5.54mmHg, 95% CI 3.66 – 7.42; P<0.0001) (Figure 3b). In contrast, paternal LPD resulted in a trend towards the programming of lower offspring blood pressure (raw mean difference: -3.49mmHg, 95% CI -7.62 – 0.63; P=0.096). The differential parental effect on offspring SBP meant the pooled estimate showed no overall difference (raw mean difference: -0.36mmHg, 95% CI -1.75 – 1.02; P=0.61).
Our final analysis examined the impact of parental diet on adult heart:body weight ratio. All groups displayed either a negative impact or no effect (Figure 3c). The largest size effect was observed in response to maternal Emb-LPD (raw mean difference: -0.05, 95% CI -0.1 – 0.01 P=0.073). Only the paternal LPD offspring heart:body weight ratio reached significance (raw mean difference: -0.03, 95% CI -0.07 – -0.01; P=0.038) (Figure 3c). Overall, the pooled effects indicated a reduction in adult heart:body weight ratio following parental, both maternal and paternal, LPD (raw mean difference: -0.03, 95% CI -0.05 – -0.01; P=0.0035).
Figure 3. Defining the relative influence of maternal and paternal factors during periconceptional conditioning in mice following parental low protein diet (LPD; 9 % casein).
The effect of parental LPD on (A) offspring weight at birth, (B) adult offspring systolic blood pressure (SBP), and (C) adult offspring heart:body weight ratio are shown when compared with offspring from normal protein diet (NPD; 18% casein) fed parents. Analysis of 4 studies involving female MF1 mice being fed LPD exclusively during the terminal stages of oocyte maturation (3.5 days prior to mating; Egg-LPD), exclusively during preimplantation embryo development (Emb-LPD) or throughout gestation (LPD). Forest plots also include offspring data in response to a paternal low protein (Pat-LPD) fed to C57BL6 males prior to mating. For Egg-NPD n = 189–80 from 19 litters; Egg-LPD n = 201-67 from 19 litters; NPD n = 131-76 from 19 litters; LPD n = 116-85 from 19 litters; Emb-LPD n = 134-78 from 19 litters; Pat-NPD n = 85-76 from 16 litters; Pat-LPD n = 73-62 from 16 litters. A. Plots present differences between means (± 95% CI) of birth weight (grams) to study specific NPD group. Data combining all LPD and all NPD treatment groups is used to determine the Pooled Estimate. Heterogeneity (χ2) between studies = 1.96 (3 df), I2 = 33%. B. Plots present differences between means (± 95% CI) of adult SBP (mmHg) to study specific NPD group. Data combining all LPD and all NPD treatment groups is used to determine the Pooled Estimate. Heterogeneity (χ2) between studies = 1.05 (4 df), I2 = 39%. C. Plots present differences between means (± 95% CI) of heart:body weight ratio to study specific NPD group. Data combining all LPD and all NPD treatment groups is used to determine the Pooled Estimate. heterogeneity (χ2) between studies = 1.86 (3 df), I2 = 61%.
Periconceptional developmental programming and ART
Direct evidence for human periconceptional effects comes from assisted reproductive treatments (ART) in which mature gametes and the preimplantation embryo are exposed to precisely timed in vitro manipulations. Several million apparently healthy ART children have now been born worldwide, but relatively little is known about the possible impact of the technology-associated exposures during conception and very early development on their health status during childhood and later life. The spectrum of human demographic confounders (including parental infertility), changes and improvements in ART techniques over time, and the relative sample sizes used make analyses complex and the reported outcomes need to be interpreted with caution. Nevertheless, it is well established that singleton ART pregnancies have increased risk of low birth weight, congenital abnormalities and higher mortality rate, although disentangling confounding by parental infertility is difficult71. Human embryo culture media have changed over time and the predominant current practice is to use commercially sourced media of proprietary (unspecified) composition (discussed in12). Comparison of perinatal outcome following use of different commercial media, including a multicentre randomised controlled trial, has indicated that birth weight is significantly affected72, with effects on growth still manifest at age 2 years73.
Compared with naturally conceived offspring, the cardiovascular phenotype of IVF children and adolescents reveals increased risk of high blood pressure11,74, vascular dysfunction with abnormal blood flow and vessel thickness75 and evidence of cardiovascular remodelling during development in utero affecting heart shape and chamber size74. Metabolic consequences include increased fasting glucose and peripheral insulin resistance11,76, raised plasma lipids, and obesity76. A systematic review found no difference in cognitive outcomes among children conceived with conventional IVF and those conceived naturally, but did identify conflicting findings that require clarification among studies of children conceived with intracytoplasmic sperm injection77.
Collectively, current evidence suggests that ART, like the in vivo nutritional models discussed above, may alter the development and growth trajectory of human embryos, and increase the risk of postnatal chronic cardiometabolic dysfunction. This legacy is unlikely to be due to parental infertility in isolation since controls in some studies comprise those naturally conceived offspring from sub-fertile parents11,75. Moreover, ART animal models demonstrate similar long-term consequences to human studies, despite normal parental fertility78. Thus, IVF embryo culture and transfer in mice results in offspring with altered growth trajectory, relative hypertension, cardiovascular abnormalities and glucose/insulin dysfunction78.
ART-associated adverse effects on long-term health appear to have an epigenetic origin induced during the periconceptional period. ART children have an increased risk of rare imprinting disorders associated with DNA methylation errors on imprinted genes79 and aberrant methylation of imprinted H19 gene has been reported in human cultured embryos80. In mouse models, embryo culture may cause imprinted genes to lose their allele-specific expression (particularly at the growth regulating H19/IGF2 locus) together with aberrant methylation patterning in embryos, placental and fetal tissues81. ART-induced aberrant epigenetic profiles may also be propagated during human pregnancy in fetal and placental tissues and persist into childhood affecting genes regulating growth such as the IGF2/H19 locus82. Media composition, particularly albumin or serum components or ammonium ion accumulation from amino acid catabolism, may contribute to altered mouse epigenetic status83. Importantly, even a very limited culture period is sufficient to induce epigenetic changes81. Embryo culture exposure also modifies expression and methylation of non-imprinted genes in mice and alters expression of DNA methyltransferases84. For example, in mouse models ART affects the endothelial nitric oxide synthase (eNOS) gene implicated in vascular dysfunction and modification of culture media composition may prevent this effect85. Although provocative, more studies in both animal models and humans are required in order to replicate findings to date.
Diversity and commonality in periconceptional effects
The evidence reviewed above reveals that periconceptional experience can induce lifelong changes in phenotype, affecting disease risk. Beyond these nutritional and ART conditions, studies in rodents show broader examples of periconceptional effects, such as from maternal stress86. Moreover, maternal alcohol consumption exclusively around conception induced metabolic dysfunction in rat adult offspring with evidence of epigenetic disturbance87. In the case of mouse maternal systemic inflammation at conception, whilst not affecting cardiometabolic health, suppressed adult offspring innate immunity after challenge, possibly to protect ‘self’ in a predicted pathogenic postnatal environment88. In addition, mouse embryo transfer experiments suggest that advanced maternal age may adversely affect offspring cardiometabolic health89, but the mechanisms underlying this age-associated effect are unknown.
The diversity of periconceptional induction conditions identified across mammalian species, coupled with clear evidence of both maternal and paternal pathways, implicates an early window when environmental exposures, combined with an inherent capacity for developmental plasticity, may confer advantage when the offspring are exposed to a similar environment postnatally. During the periconceptional period there is rapid and radical molecular, cellular and morphogenetic restructuring; the signalling pathways that control these processes are sensitive to multiple molecules and other factors within the cellular environment and may provide a mechanistic underpinning for this concept90. However, as we have described, the periconceptional setting of metabolic homeostasis may become maladaptive if conditions change or if nutrient levels induce perturbations in metabolism, generating the circumstances underlying adverse health risk. A consistent mechanism identified across conditions and species has been epigenetic variation, a plausible pathway to ‘biological embedding’ of early life exposures and transmission of phenotypic effects throughout life. This has been demonstrated directly through manipulation of maternal one-carbon (1-C) metabolism during early embryogenesis, potentially reducing the availability of methyl donor groups necessary for DNA and histone methylation91, but such epigenetic changes are not necessarily linked directly with changes in gene expression92. Thus, a periconceptional maternal diet deficient in 1-C metabolite substrates and cofactors (vitamin B12, folate, methionine) in sheep modified offspring DNA methylation and led to adverse cardiometabolic and immune dysfunction93. Similarly, folate addition to rodent maternal LPD can rescue normal expression and DNA methylation of metabolic regulators in offspring which underlie cardiovascular dysfunction94. A mouse paternal low folate diet altered sperm DNA methylation profile, changed the placental transcriptome and resulted in offspring with craniofacial and musculoskeletal malformations95. Moreover, the negative impact of mouse paternal undernutrition on sperm quality, testicular oxidative stress, fertility and offspring fat accumulation and dyslipidaemia are reversed through vitamin and antioxidant supplementation96. As with ART, additional studies are warranted to define the critical window(s) and pathways linking perinatal one-carbon metabolism, epigenetic variation and programming of later offspring health.
Conclusion: Protecting health of the next generation and the way forward
We propose there is now sufficient evidence from human and animal research that the periconceptional period is a key window during which poor maternal and paternal physiology, body composition, metabolism and diet can induce increased risk of chronic disease in offspring, a lifetime legacy and major driver of health burden in the 21st century. The evidence that similar consequences can result from ART practices sharpens the focus on this window. Environmental factors may perturb gametes or early embryos, affecting homeostatic mechanisms, or may induce adaptations to developmental environmental signals with consequences persisting into adulthood.
This evidence calls for a major re-examination of public health policy to protect against future disease risk through societal advice on, and greater provision of, preconception care97 as also promoted in the two accompanying reviews in this series (Stephenson et al, submitted; Barker et al, submitted). Whilst a preconception focus on parental risk factors such as smoking and excess alcohol intake is wise and well established, new drives to prepare nutritionally for pregnancy are critical, including healthy body composition, physical activity and diet for both parents98. Further definition of the underlying epigenetic, cellular, metabolic and/or physiological mechanisms and the exposures that drive them, is an important research agenda that is pivotal to the characterisation of more specific recommendations for preconception health.
Box 1. Key messages.
Whilst evidence for developmental origins of later disease can be found throughout gestation and beyond, there is a growing consensus from both human and animal studies that a critical period is around conception and hence merits particular attention.
As we review, preconception maternal overnutrition and obesity, maternal undernutrition, related paternal factors, and assisted reproductive treatments all may change the phenotype and potential of gametes and early embryos, with enduring consequences across the lifespan.
Our new data reveal that suboptimal maternal and paternal nutrition around conception have similar effects on offspring weight, but differing effects on offspring blood pressure.
These critical influences on lifetime health occurring so early in development may reflect perturbations or adaptations in epigenetic, cellular, metabolic and/or physiological mechanisms. Defining these mechanisms and the exposures that drive them is critical to the characterisation of more specific recommendations for preconception health.
This emerging knowledge has significant societal and medical implications. In particular, it provides the basis for a new emphasis on preparation for pregnancy, before conception, to safeguard public health and as a means of disease prevention.
Acknowledgements
The idea for this series was conceived by Judith Stephenson and developed during a four day symposium, led by Mary Barker and Judith Stephenson and funded by The Rank Prize Funds, on ‘Developmental Programming for Human Disease: Preconception Nutrition and Lifelong Health’ in Grasmere, UK, February 2016. We are grateful for research funding from BBSRC (BB/1001840/1; BB/F007450/1), European Union FP7 (Epihealth, 278418; EpiHealthNet, 317146) and Rosetrees Trust to TPF. KMG is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (as an NIHR Senior Investigator (NF-SI-0515-10042) and through the NIHR Southampton Biomedical Research Centre and by the European Union's Erasmus+ Capacity-Building ENeASEA Project and Seventh Framework Programme (FP7/2007-2013), projects EarlyNutrition and ODIN under grant agreement numbers 289346 and 613977. AJW is supported by an Aston Research Centre for Healthy Ageing fellowship from Aston University. AMP is supported by the UK Medical Research Council (MRC; grant no MC-A760-5QX00) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement. MAH is supported by the British Heart Foundation. RS is funded by a National Health and Medical Research Fellowship and the Victorian Government operational infrastructure support scheme (Australia). JCM’s research is supported by the MRC and BBSRC through the Centre for Ageing & Vitality (MR/L016354/1).
Footnotes
Conflict of interest statement
KMG reports other from Nestle Nutrition Institute, grants from Abbott Nutrition and Nestec, outside the submitted work. The other authors have nothing to disclose.
Contributors
The manuscript was drafted by Tom Fleming, Adam Watkins, Miguel Velazquez and Keith Godfrey. All authors provided input into the manuscript and approved the final version of the manuscript.
References
- 1.Barker DJ, Thornburg KL. The obstetric origins of health for a lifetime. Clinical obstetrics and gynecology. 2013;56(3):511–9. doi: 10.1097/GRF.0b013e31829cb9ca. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. The lancet Diabetes & endocrinology. 2017;5(1):53–64. doi: 10.1016/S2213-8587(16)30107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev. 2014;94:1027–76. doi: 10.1152/physrev.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L, Lu X, Dean J. The maternal to zygotic transition in mammals. Molecular aspects of medicine. 2013;34(5):919–38. doi: 10.1016/j.mam.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedzhov I, Graham SJ, Leung CY, Zernicka-Goetz M. Developmental plasticity, cell fate specification and morphogenesis in the early mouse embryo. Philos Trans R Soc Lond B Biol Sci. 2014;369(1657) doi: 10.1098/rstb.2013.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholas LM, Morrison JL, Rattanatray L, Zhang S, Ozanne SE, McMillen IC. The early origins of obesity and insulin resistance: timing, programming and mechanisms. Int J Obes (Lond) 2016;40(2):229–38. doi: 10.1038/ijo.2015.178. [DOI] [PubMed] [Google Scholar]
- 7.Fleming TP, Watkins AJ, Sun C, Velazquez MA, Smyth NR, Eckert JJ. Do little embryos make big decisions? How maternal dietary protein restriction can permanently change an embryo. Reprod Fertil Dev. 2015 doi: 10.1071/RD14455. [DOI] [PubMed] [Google Scholar]
- 8.Wyman A, Pinto AB, Sheridan R, Moley KH. One-cell zygote transfer from diabetic to nondiabetic mouse results in congenital malformations and growth retardation in offspring. Endocrinology. 2008;149(2):466–9. doi: 10.1210/en.2007-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinclair KD, Watkins AJ. Parental diet, pregnancy outcomes and offspring health: metabolic determinants in developing oocytes and embryos. Reprod Fertil Dev. 2013;26(1):99–114. doi: 10.1071/RD13290. [DOI] [PubMed] [Google Scholar]
- 10.Hansen PJ, Dobbs KB, Denicol AC, Siqueira LG. Sex and the preimplantation embryo: implications of sexual dimorphism in the preimplantation period for maternal programming of embryonic development. Cell and tissue research. 2016;363(1):237–47. doi: 10.1007/s00441-015-2287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ceelen M, van Weissenbruch MM, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA. Cardiometabolic differences in children born after in vitro fertilization: follow-up study. J Clin Endocrinol Metab. 2008;93(5):1682–8. doi: 10.1210/jc.2007-2432. [DOI] [PubMed] [Google Scholar]
- 12.Sunde A, Brison D, Dumoulin J, Harper J, Lundin K, Magli MC, et al. Time to take human embryo culture seriously. Hum Reprod. 2016;31(10):2174–82. doi: 10.1093/humrep/dew157. [DOI] [PubMed] [Google Scholar]
- 13.Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51(2):383–92. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 14.Ruebel ML, Cotter M, Sims CR, Moutos DM, Badger TM, Cleves MA, Shankar K, Andres A. Obesity modulates inflammation and lipid metabolism oocyte gene expression: a single cell transcriptome perspective. J Clin Endocrinol Metab. 2017;102:2029–2038. doi: 10.1210/jc.2016-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robker RL, Akison LK, Bennett BD, Thrupp PN, Chura LR, Russell DL, et al. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. The Journal of clinical endocrinology and metabolism. 2009;94(5):1533–40. doi: 10.1210/jc.2008-2648. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Wu LL, Chura LR, Liang X, Lane M, Norman RJ, et al. Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertil Steril. 2012;97(6):1438–43. doi: 10.1016/j.fertnstert.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 17.Leary C, Leese HJ, Sturmey RG. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod. 2015;30(1):122–32. doi: 10.1093/humrep/deu276. [DOI] [PubMed] [Google Scholar]
- 18.Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, et al. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One. 2012;7(11):e49217. doi: 10.1371/journal.pone.0049217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, et al. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One. 2010;5(4):e10074. doi: 10.1371/journal.pone.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151(8):4039–46. doi: 10.1210/en.2010-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholas LM, Rattanatray L, MacLaughlin SM, Ozanne SE, Kleemann DO, Walker SK, et al. Differential effects of maternal obesity and weight loss in the periconceptional period on the epigenetic regulation of hepatic insulin-signaling pathways in the offspring. FASEB J. 2013;27(9):3786–96. doi: 10.1096/fj.13-227918. [DOI] [PubMed] [Google Scholar]
- 22.Bellver J, Pellicer A, Garcia-Velasco JA, Ballesteros A, Remohi J, Meseguer M. Obesity reduces uterine receptivity: clinical experience from 9,587 first cycles of ovum donation with normal weight donors. Fertil Steril. 2013;100(4):1050–8. doi: 10.1016/j.fertnstert.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Cardozo ER, Karmon AE, Gold J, Petrozza JC, Styer AK. Reproductive outcomes in oocyte donation cycles are associated with donor BMI. Hum Reprod. 2016;31(2):385–92. doi: 10.1093/humrep/dev298. [DOI] [PubMed] [Google Scholar]
- 24.Tobi EW, Goeman JJ, Monajemi R, Gu H, Putter H, Zhang Y, et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nature communications. 2014;5:5592. doi: 10.1038/ncomms6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang PX, Wang JJ, Lei YX, Xiao L, Luo ZC. Impact of fetal and infant exposure to the Chinese Great Famine on the risk of hypertension in adulthood. PLoS One. 2012;7(11):e49720. doi: 10.1371/journal.pone.0049720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rayco-Solon P, Fulford AJ, Prentice AM. Differential effects of seasonality on preterm birth and intrauterine growth restriction in rural Africans. Am J Clin Nutr. 2005;81(1):134–9. doi: 10.1093/ajcn/81.1.134. [DOI] [PubMed] [Google Scholar]
- 27.Waterland RA, Kellermayer R, Laritsky E, Rayco-Solon P, Harris RA, Travisano M, et al. Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles. PLoS genetics. 2010;6(12):e1001252. doi: 10.1371/journal.pgen.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dominguez-Salas P, Moore SE, Baker MS, Bergen AW, Cox SE, Dyer RA, et al. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nature communications. 2014;5:3746. doi: 10.1038/ncomms4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silver MJ, Kessler NJ, Hennig BJ, Dominguez-Salas P, Laritsky E, Baker MS, Coarfa C, Hernandez-Vargas H, Castelino JM, Routledge MN, Gong YY, et al. Independent genomewide screens identify the tumor suppressor VTRNA2-1 as a human epiallele responsive to periconceptional environment. Genome Biol. 2015;16:118. doi: 10.1186/s13059-015-0660-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kühnen P, et al. Interindividual Variation in DNA Methylation at a Putative POMC Metastable Epiallele Is Associated with Obesity. Cell Metab. 2016;24:502–9. doi: 10.1016/j.cmet.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Watkins AJ, Wilkins A, Cunningham C, Perry VH, Seet MJ, Osmond C, et al. Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring. J Physiol. 2008;586(8):2231–44. doi: 10.1113/jphysiol.2007.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127(19):4195–202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- 33.Watkins AJ, Ursell E, Panton R, Papenbrock T, Hollis L, Cunningham C, et al. Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol Reprod. 2008;78(2):299–306. doi: 10.1095/biolreprod.107.064220. [DOI] [PubMed] [Google Scholar]
- 34.Torrens C, Snelling TH, Chau R, Shanmuganathan M, Cleal JK, Poore KR, et al. Effects of pre- and periconceptional undernutrition on arterial function in adult female sheep are vascular bed dependent. Exp Physiol. 2009;94(9):1024–33. doi: 10.1113/expphysiol.2009.047340. [DOI] [PubMed] [Google Scholar]
- 35.Burrage D, Braddick L, Cleal J, Costello P, Noakes D, Hanson M, Green L. The late gestation fetal cardiovascular response to hypoglycaemia is modified by prior peri-implantation undernutrition in sheep. J Physiol. 2009;587:611. doi: 10.1113/jphysiol.2008.165944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costello PM, Rowlerson A, Astaman NA, Anthony FE, Sayer AA, Cooper C, Hanson MA, Green LR. Peri-implantation and late gestation maternal undernutrition differentially affect fetal sheep skeletal muscle development. J Physiol. 2008;586:2371–9. doi: 10.1113/jphysiol.2008.150987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cleal JK, Poore KR, Newman JP, Noakes DE, Hanson MA, Green LR. The effect of maternal undernutrition in early gestation on gestation length and fetal and postnatal growth in sheep. Ped Res. 2007;62:422–7. doi: 10.1203/PDR.0b013e31813cbe60. [DOI] [PubMed] [Google Scholar]
- 38.Eckert JJ, Porter R, Watkins AJ, Burt E, Brooks S, Leese HJ, et al. Metabolic induction and early responses of mouse blastocyst developmental programming following maternal low protein diet affecting life-long health. PLoS One. 2012;7(12):e52791. doi: 10.1371/journal.pone.0052791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watkins AJ, Lucas ES, Marfy-Smith S, Bates N, Kimber SJ, Fleming TP. Maternal nutrition modifies trophoblast giant cell phenotype and fetal growth in mice. Reproduction. 2015;149(6):563–75. doi: 10.1530/REP-14-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun C, Velazquez MA, Marfy-Smith S, Sheth B, Cox A, Johnston DA, et al. Mouse early extra-embryonic lineages activate compensatory endocytosis in response to poor maternal nutrition. Development. 2014;141(5):1140–50. doi: 10.1242/dev.103952. [DOI] [PubMed] [Google Scholar]
- 41.Sun C, Denisenko O, Sheth B, Cox A, Lucas ES, Smyth NR, et al. Epigenetic regulation of histone modifications and Gata6 gene expression induced by maternal diet in mouse embryoid bodies in a model of developmental programming. BMC developmental biology. 2015;15(1):3. doi: 10.1186/s12861-015-0053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denisenko O, Lucas ES, Sun C, Watkins AJ, Mar D, Bomsztyk K, et al. Regulation of ribosomal RNA expression across the lifespan is fine-tuned by maternal diet before implantation. Biochimica et biophysica acta. 2016;1859(7):906–13. doi: 10.1016/j.bbagrm.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holland ML, Lowe R, Caton PW, Gemma C, Carbajosa G, Danson AF, Carpenter AA, Loche E, Ozanne SE, Rakyan VK. Early-life nutrition modulates the epigenetic state of specific rDNA genetic variants in mice. Science. 2016;353:495–8. doi: 10.1126/science.aaf7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cropley JE, Eaton SA, Aiken A, Young PE, Giannoulatou E, Ho JW, et al. Male-lineage transmission of an acquired metabolic phenotype induced by grand-paternal obesity. Molecular metabolism. 2016;5(8):699–708. doi: 10.1016/j.molmet.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammoud AO, Gibson M, Stanford J, White G, Carrell DT, Peterson M. In vitro fertilization availability and utilization in the United States: a study of demographic, social, and economic factors. Fertil Steril. 2009;91(5):1630–5. doi: 10.1016/j.fertnstert.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 46.Kort HI, Massey JB, Elsner CW, Mitchell-Leef D, Shapiro DB, Witt MA, et al. Impact of body mass index values on sperm quantity and quality. Journal of andrology. 2006;27(3):450–2. doi: 10.2164/jandrol.05124. [DOI] [PubMed] [Google Scholar]
- 47.Tunc O, Bakos HW, Tremellen K. Impact of body mass index on seminal oxidative stress. Andrologia. 2011;43(2):121–8. doi: 10.1111/j.1439-0272.2009.01032.x. [DOI] [PubMed] [Google Scholar]
- 48.Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27(10):2899–907. doi: 10.1093/humrep/des298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agbaje IM, Rogers DA, McVicar CM, McClure N, Atkinson AB, Mallidis C, et al. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007;22(7):1871–7. doi: 10.1093/humrep/dem077. [DOI] [PubMed] [Google Scholar]
- 50.Seli E, Gardner DK, Schoolcraft WB, Moffatt O, Sakkas D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil Steril. 2004;82(2):378–83. doi: 10.1016/j.fertnstert.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 51.Bakos HW, Henshaw RC, Mitchell M, Lane M. Paternal body mass index is associated with decreased blastocyst development and reduced live birth rates following assisted reproductive technology. Fertility and sterility. 2011;95(5):1700–4. doi: 10.1016/j.fertnstert.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 52.Bakos HW, Mitchell M, Setchell BP, Lane M. The effect of paternal diet-induced obesity on sperm function and fertilization in a mouse model. Int J Androl. 2011;34(5 Pt 1):402–10. doi: 10.1111/j.1365-2605.2010.01092.x. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell M, Bakos HW, Lane M. Paternal diet-induced obesity impairs embryo development and implantation in the mouse. Fertil Steril. 2011;95(4):1349–53. doi: 10.1016/j.fertnstert.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 54.Fullston T, Ohlsson Teague EM, Palmer NO, DeBlasio MJ, Mitchell M, Corbett M, et al. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27(10):4226–43. doi: 10.1096/fj.12-224048. [DOI] [PubMed] [Google Scholar]
- 55.Palmer NO, Bakos HW, Owens JA, Setchell BP, Lane M. Diet and exercise in an obese mouse fed a high-fat diet improve metabolic health and reverse perturbed sperm function. Am J Physiol Endocrinol Metab. 2012;302(7):E768–80. doi: 10.1152/ajpendo.00401.2011. [DOI] [PubMed] [Google Scholar]
- 56.Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature. 2010;467(7318):963–6. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 57.Pentinat T, Ramon-Krauel M, Cebria J, Diaz R, Jimenez-Chillaron JC. Transgenerational inheritance of glucose intolerance in a mouse model of neonatal overnutrition. Endocrinology. 2010;151(12):5617–23. doi: 10.1210/en.2010-0684. [DOI] [PubMed] [Google Scholar]
- 58.Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143(7):1084–96. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watkins AJ, Sinclair KD. Paternal low protein diet affects adult offspring cardiovascular and metabolic function in mice. American journal of physiology Heart and circulatory physiology. 2014;306(10):H1444–52. doi: 10.1152/ajpheart.00981.2013. [DOI] [PubMed] [Google Scholar]
- 60.Watkins AJ, Sirovica S, Stokes B, Isaacs M, Addison O, Martin RA. Paternal low protein diet programs preimplantation embryo gene expression, fetal growth and skeletal development in mice. Biochim Biophys Acta. 2017;1863(6):1371–81. doi: 10.1016/j.bbadis.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 61.Zeybel M, Hardy T, Wong YK, Mathers JC, Fox CR, Gackowska A, Oakley F, Burt AD, Wilson CL, Anstee QM, Barter MJ, et al. Multigenerational epigenetic adaptation of the hepatic wound-healing response. Nat Med. 2012;18:1369–77. doi: 10.1038/nm.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hammoud SS, Nix DA, Hammoud AO, Gibson M, Cairns BR, Carrell DT. Genome-wide analysis identifies changes in histone retention and epigenetic modifications at developmental and imprinted gene loci in the sperm of infertile men. Human reproduction. 2011;26(9):2558–69. doi: 10.1093/humrep/der192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Heijden GW, Ramos L, Baart EB, van den Berg IM, Derijck AA, van der Vlag J, et al. Sperm-derived histones contribute to zygotic chromatin in humans. BMC developmental biology. 2008;8:34. doi: 10.1186/1471-213X-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ostermeier GC, Dix DJ, Miller D, Khatri P, Krawetz SA. Spermatozoal RNA profiles of normal fertile men. Lancet. 2002;360(9335):772–7. doi: 10.1016/S0140-6736(02)09899-9. [DOI] [PubMed] [Google Scholar]
- 65.Jodar M, Kalko S, Castillo J, Ballesca JL, Oliva R. Differential RNAs in the sperm cells of asthenozoospermic patients. Hum Reprod. 2012;27(5):1431–8. doi: 10.1093/humrep/des021. [DOI] [PubMed] [Google Scholar]
- 66.Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351(6271):397–400. doi: 10.1126/science.aad7977. [DOI] [PubMed] [Google Scholar]
- 67.Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, Belleannee C, Kucukural A, Serra RW, Sun F, Song L, et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2016 Jan 22;351(6271):391–6. doi: 10.1126/science.aad6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sjoblom C, Roberts CT, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor alleviates adverse consequences of embryo culture on fetal growth trajectory and placental morphogenesis. Endocrinology. 2005;146(5):2142–53. doi: 10.1210/en.2004-1260. [DOI] [PubMed] [Google Scholar]
- 69.Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Molecular human reproduction. 2007;13(7):491–501. doi: 10.1093/molehr/gam028. [DOI] [PubMed] [Google Scholar]
- 70.Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ, Robertson SA. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(6):2200–5. doi: 10.1073/pnas.1305609111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin JB, Sheng XQ, Wu D, Gao SY, You YP, Yang TB, et al. Worldwide prevalence of adverse pregnancy outcomes among singleton pregnancies after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Archives of gynecology and obstetrics. 2017;295(2):285–301. doi: 10.1007/s00404-016-4250-3. [DOI] [PubMed] [Google Scholar]
- 72.Kleijkers SH, Mantikou E, Slappendel E, Consten D, van Echten-Arends J, Wetzels AM, et al. Influence of embryo culture medium (G5 and HTF) on pregnancy and perinatal outcome after IVF: a multicenter RCT. Hum Reprod. 2016;31(10):2219–30. doi: 10.1093/humrep/dew156. [DOI] [PubMed] [Google Scholar]
- 73.Kleijkers SH, van Montfoort AP, Smits LJ, Viechtbauer W, Roseboom TJ, Nelissen EC, et al. IVF culture medium affects post-natal weight in humans during the first 2 years of life. Hum Reprod. 2014;29(4):661–9. doi: 10.1093/humrep/deu025. [DOI] [PubMed] [Google Scholar]
- 74.Valenzuela-Alcaraz B, Crispi F, Bijnens B, Cruz-Lemini M, Creus M, Sitges M, et al. Assisted reproductive technologies are associated with cardiovascular remodeling in utero that persists postnatally. Circulation. 2013;128(13):1442–50. doi: 10.1161/CIRCULATIONAHA.113.002428. [DOI] [PubMed] [Google Scholar]
- 75.Scherrer U, Rimoldi SF, Rexhaj E, Stuber T, Duplain H, Garcin S, et al. Systemic and pulmonary vascular dysfunction in children conceived by assisted reproductive technologies. Circulation. 2012;125(15):1890–6. doi: 10.1161/CIRCULATIONAHA.111.071183. [DOI] [PubMed] [Google Scholar]
- 76.Gkourogianni A, Kosteria I, Telonis AG, Margeli A, Mantzou E, Konsta M, et al. Plasma metabolomic profiling suggests early indications for predisposition to latent insulin resistance in children conceived by ICSI. PLoS One. 2014;9(4):e94001. doi: 10.1371/journal.pone.0094001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rumbold AR, Moore VM, Whitrow MJ, Oswald TK, Moran LJ, Fernandez RC, Barnhart KT, Davies MJ. The impact of specific fertility treatments on cognitive development in childhood and adolescence: a systematic review. Hum Reprod. 2017 May 4;:1–19. doi: 10.1093/humrep/dex085. [DOI] [PubMed] [Google Scholar]
- 78.Watkins AJ, Platt D, Papenbrock T, Wilkins A, Eckert JJ, Kwong WY, et al. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc Natl Acad Sci U S A. 2007;104(13):5449–54. doi: 10.1073/pnas.0610317104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lazaraviciute G, Kauser M, Bhattacharya S, Haggarty P. A systematic review and meta-analysis of DNA methylation levels and imprinting disorders in children conceived by IVF/ICSI compared with children conceived spontaneously. Hum Reprod Update. 2014;20(6):840–52. doi: 10.1093/humupd/dmu033. [DOI] [PubMed] [Google Scholar]
- 80.Chen SL, Shi XY, Zheng HY, Wu FR, Luo C. Aberrant DNA methylation of imprinted H19 gene in human preimplantation embryos. Fertil Steril. 2010;94(6):2356–8. doi: 10.1016/j.fertnstert.2010.01.120. 8 e1. [DOI] [PubMed] [Google Scholar]
- 81.Rivera RM, Stein P, Weaver JR, Mager J, Schultz RM, Bartolomei MS. Manipulations of mouse embryos prior to implantation result in aberrant expression of imprinted genes on day 9.5 of development. Human molecular genetics. 2008;17(1):1–14. doi: 10.1093/hmg/ddm280. [DOI] [PubMed] [Google Scholar]
- 82.Turan N, Katari S, Gerson LF, Chalian R, Foster MW, Gaughan JP, et al. Inter- and intra- individual variation in allele-specific DNA methylation and gene expression in children conceived using assisted reproductive technology. PLoS genetics. 2010;6(7):e1001033. doi: 10.1371/journal.pgen.1001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khosla S, Dean W, Brown D, Reik W, Feil R. Culture of preimplantation mouse embryos affects fetal development and the expression of imprinted genes. Biol Reprod. 2001;64(3):918–26. doi: 10.1095/biolreprod64.3.918. [DOI] [PubMed] [Google Scholar]
- 84.Horii T, Suetake I, Yanagisawa E, Morita S, Kimura M, Nagao Y, et al. The Dnmt3b splice variant is specifically expressed in in vitro-manipulated blastocysts and their derivative ES cells. J Reprod Dev. 2011;57(5):579–85. doi: 10.1262/jrd.10-194a. [DOI] [PubMed] [Google Scholar]
- 85.Rexhaj E, Pireva A, Paoloni-Giacobino A, Allemann Y, Cerny D, Dessen P, et al. Prevention of vascular dysfunction and arterial hypertension in mice generated by assisted reproductive technologies by addition of melatonin to culture media. American journal of physiology Heart and circulatory physiology. 2015;309(7):H1151–6. doi: 10.1152/ajpheart.00621.2014. [DOI] [PubMed] [Google Scholar]
- 86.Prasad S, Tiwari M, Pandey AN, Shrivastav TG, Chaube SK. Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci. 2016;23:36. doi: 10.1186/s12929-016-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gårdebjer EM, Anderson ST, Pantaleon M, Wlodek ME, Moritz KM. Maternal alcohol intake around the time of conception causes glucose intolerance and insulin insensitivity in rat offspring, which is exacerbated by a postnatal high-fat diet. FASEB J. 2015;29(7):2690–701. doi: 10.1096/fj.14-268979. [DOI] [PubMed] [Google Scholar]
- 88.Williams CL, Teeling JL, Perry VH, Fleming TP. Mouse maternal systemic inflammation at the zygote stage causes blunted cytokine responsiveness in lipopolysaccharide-challenged adult offspring. BMC Biol. 2011;9:49. doi: 10.1186/1741-7007-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Velazquez MA, Smith CG, Smyth NR, Osmond C, Fleming TP. Advanced maternal age causes adverse programming of mouse blastocysts leading to altered growth and impaired cardiometabolic health in post-natal life. Hum Reprod. 2016;31(9):1970–80. doi: 10.1093/humrep/dew177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Basson MA. Signaling in cell differentiation and morphogenesis. Cold Spring Harb Perspect Biol. 2012;4:1–21. doi: 10.1101/cshperspect.a008151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Steegers-Theunissen RP, Twigt J, Pestinger V, Sinclair KD. The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Hum Reprod Update. 2013;19(6):640–55. doi: 10.1093/humupd/dmt041. [DOI] [PubMed] [Google Scholar]
- 92.McKay JA, Adriaens M, Evelo CT, Ford D, Mathers JC. Gene promoter DNA methylation patterns have a limited role in orchestrating transcriptional changes in the fetal liver in response to maternal folate depletion during pregnancy. Mol Nutr Food Res. 2016;60:2031–42. doi: 10.1002/mnfr.201600079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(49):19351–6. doi: 10.1073/pnas.0707258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. The Journal of nutrition. 2005;135(6):1382–6. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 95.Lambrot R, Xu C, Saint-Phar S, Chountalos G, Cohen T, Paquet M, et al. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nature communications. 2013;4:2889. doi: 10.1038/ncomms3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McPherson NO, Fullston T, Kang WX, Sandeman LY, Corbett MA, Owens JA, et al. Paternal under-nutrition programs metabolic syndrome in offspring which can be reversed by antioxidant/vitamin food fortification in fathers. Scientific reports. 2016;6 doi: 10.1038/srep27010. 27010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barker D, Barker M, Fleming T, Lampl M. Developmental biology: Support mothers to secure future public health. Nature. 2013;504(7479):209–11. doi: 10.1038/504209a. [DOI] [PubMed] [Google Scholar]
- 98.Hanson M, Godfrey K, Poston L, Bustreo F, Stephenson J, Preconception health . In: Annual Report of the Chief Medical Officer 2014, The Health of the 51%: Women. Davies SC, editor. London: Department of Health; 2015. pp. 53–66. Chapter 5. [Google Scholar]
- 99.Messerschmidt DM, Knowles BB, Solter D. DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes & development. 2014;28(8):812–28. doi: 10.1101/gad.234294.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gardner DK, Harvey AJ. Blastocyst metabolism. Reprod Fertil Dev. 2015;27(4):638–54. doi: 10.1071/RD14421. [DOI] [PubMed] [Google Scholar]