Purpose and Scope of the Guidance
This AASLD 2018 Hepatitis B Guidance is intended to complement the AASLD 2016 Practice Guidelines for Treatment of Chronic Hepatitis B (1) and update the previous hepatitis B virus (HBV) guidelines from 2009. The 2018 updated guidance on chronic hepatitis B (CHB) includes (i) updates on treatment since the 2016 HBV guideline (notably the use of tenofovir alafenamide) and guidance on (ii) screening, counseling, and prevention; (iii) specialized virologic and serologic tests; (iv) monitoring of untreated patients; and (v) treatment of hepatitis B in special populations, including persons with viral coinfections, acute hepatitis B, recipients of immunosuppressive therapy, and transplant recipients.
The AASLD 2018 Hepatitis B Guidance provides a data-supported approach to screening, prevention, diagnosis, and clinical management of patients with hepatitis B. It differs from the published 2016 AASLD guideline which conducted systematic reviews and used a multidisciplinary panel of experts to rate the quality (level) of the evidence and the strength of each recommendation using the Grading of Recommendations Assessment, Development and Evaluation system in support of guideline recommendations (1–4). In contrast, this guidance document was developed by consensus of an expert panel, without formal systematic review or use of the Grading of Recommendations Assessment, Development, and Evaluation system. The 2018 guidance is based upon the following: (i) formal review and analysis of published literature on the topics; (ii) World Health Organization guidance on prevention, care, and treatment of persons with CHB (5); and (iii) the authors’ experience in acute hepatitis B and CHB.
Intended for use by health care providers, this guidance identifies preferred approaches to the diagnostic, therapeutic, and preventive aspects of care for patients with CHB. As with clinical practice guidelines, it provides general guidance to optimize the care of the majority of patients and should not replace clinical judgement for a unique patient. This guidance does not seek to dictate a “one size fits all” approach for the management of CHB. Clinical considerations may justify a course of action that differs from this guidance.
Interim Data Relevant to the AASLD 2018 Hepatitis B Guidance
Since the publication of the 2016 AASLD Hepatitis B Guideline, tenofovir alafenamide (TAF) has been approved for treatment of CHB in adults. Tenofovir alafenamide joins the list of preferred HBV therapies, along with entecavir, tenofovir disoproxil fumarate (TDF), and peginterferon (peg-IFN) (Tables 1 and 2) (6–16) (section: Updated Recommendations on the Treatment of Patients With Chronic Hepatitis B).
Table 1.
Approved Antiviral Therapies in Adults and Children
| Drug | Dose in Adults1 | Use in Children1 | Pregnancy Category2 | Potential Side Effects2 | Monitoring on Treatment3 |
|---|---|---|---|---|---|
| Preferred | |||||
|
Peg-IFN-α-2a (adult) IFN-α-2b (children) |
180 mcg weekly | ≥1 year dose: 6 million IU/m2 three times weekly4 |
C | Flu-like symptoms, fatigue, mood disturbances, cytopenia, autoimmune disorders in adults, anorexia and weight loss in children | Complete blood count (monthly to every 3 months) TSH (every 3 months) Clinical monitoring for autoimmune, ischemic, neuropsychiatric, and infectious complications |
| Entecavir | 0.5 mg daily5 | ≥2 years dose: weight-based to 10–30 kg; above 30 kg: 0.5 mg daily5 |
C | Lactic acidosis (decompensated cirrhosis only) | Lactic acid levels if there is clinical concern Test for HIV prior to treatment initiation |
| Tenofovir dipovoxil fumarate | 300 mg daily | ≥12 years | B | Nephropathy, Fanconi syndrome, osteomalacia, lactic acidosis | Creatinine clearance at baseline If at risk for renal impairment, creatinine clearance, serum phosphate, urine glucose and protein at least annually Consider bone density study at baseline and during treatment in patients with history of fracture or risks for osteopenia Lactic acid levels if there is clinical concern Test for HIV prior to treatment initiation |
| Tenofovir alafenamide | 25 mg daily | -- | There are insufficient human data on use during pregnancy to inform a drug-associated risk of birth defects and miscarriage | Lactic acidosis | Lactic acid levels if clinical concern Assess serum creatinine, serum phosphorus, creatinine clearance, urine glucose, and urine protein prior to initiating and during therapy in all patients as clinically appropriate Test for HIV prior to treatment initiation |
| Non-Preferred | |||||
| Lamivudine | 100 mg daily | ≥2 years dose: 3 mg/kg daily to max 100 mg |
C | Pancreatitis Lactic acidosis |
Amylase if symptoms are present Lactic acid levels if there is clinical concern Test for HIV prior to treatment initiation |
| Adefovir | 10 mg daily | ≥12 years | C | Acute renal failure Fanconi syndrome Lactic acidosis |
Creatinine clearance at baseline If at risk for renal impairment, creatinine clearance, serum phosphate, urine glucose, and urine protein at least annually Consider bone density study at baseline and during treatment in patients with history of fracture or risks for osteopenia Lactic acid levels if clinical concern |
| Telbivudine | 600 mg daily | -- | B | Creatine kinase elevations and myopathy Peripheral neuropathy Lactic acidosis |
Creatine kinase if symptoms are present Clinical evaluation if symptoms are present Lactic acid levels if there is clinical concern |
Dose adjustments are needed in patients with renal dysfunction.
In 2015, the US Food and Drug Administration replaced the pregnancy risk designation by letters A, B, C, D, and X with more specific language on pregnancy and lactation. This new labeling is being phased in gradually, and to date only TAF includes these additional data.
Per package insert.
Peg-IFN-α-2a is not approved for children with chronic hepatitis B but is approved for treatment of chronic hepatitis C. Providers may consider using this drug for children with chronic HBV. The duration of treatment indicated in adults is 48 weeks.
Entecavir dose is 1 mg daily if the patient is lamivudine experienced or if they have decompensated cirrhosis.
Table 2.
Efficacy of Approved First-Line Antiviral Therapies in Adults with Treatment-Naïve Chronic Hepatitis B and Immune-Active Disease (Not Head-to-Head Comparisons)
| HBeAg Positive | Peg-IFN1 | Entecavir2 | Tenofovir Disoproxil Fumarate2 | Tenofovir Alafenamide3 |
|---|---|---|---|---|
| % HBV DNA suppression (cutoff to define HBV DNA suppression)4 | 30–42 (<2000–40,000 IU/mL) 8–14 (<80 IU/mL) |
61 (<50–60 IU/mL) | 76 (<60 IU/mL) | 73 (<29 IU/mL) |
| % HBeAg loss | 32–36 | 22–25 | -- | 22 |
| % HBeAg seroconversion | 29–36 | 21–22 | 21 | 18 |
| % Normalization ALT | 34–52 | 68–81 | 68 | -- |
| % HBsAg loss | 2–7 11 (at 3 years posttreatment) |
2–3 4–5 (2 years) |
3 8 (3 years) |
1 (2 years) |
| HBeAg Negative | Peg-IFN | Entecavir | Tenofovir Disoproxil Fumarate2 | Tenofovir Alafenamide3 |
| % HBV DNA suppression (cutoff to define HBV DNA suppression)5 | 43 (<4000 IU/mL) 19 (<80 IU/mL) |
90–91 (<50–60 IU/mL) | 93 (<60 U/mL) | 90 (<29 IU/mL) |
| % Normalization ALT6 | 59 | 78–88 | 76 | 81 |
| % HBsAg loss | 4 6 (at 3 years posttreatment) |
0–1 | 0 | <1 |
Assessed 6 months after completion of 12 months of therapy
Assessed after 3 years of continuous therapy
Assessed after 2 years of continuous therapy
HBV DNA <2000–40,000 IU/mL for peginterferon; <60 IU/mL for entecavir and tenofovir disoproxil fumarate; <29 IU/mL for tenofovir alafenamide
HBV DNA <20,000 IU/mL for peginterferon; <60 IU/mL for entecavir and tenofovir disoproxil fumarate; <29 IU/mL for tenofovir alafenamide
ALT normalization defined by laboratory normal rather than ≤35 and ≤25 U/L for males and females
to TDF being elevated to the level of preferred therapy in this setting (section 1C of Screening, Counseling, and Prevention of Hepatitis B).
Tenofovir alafenamide, like TDF, is a nucleotide analogue that inhibits reverse transcription of pregenomic RNA to HBV DNA. Tenofovir alafenomide is more stable than TDF in plasma and delivers the active metabolite to hepatocytes more efficiently, allowing a lower dose to be used with similar antiviral activity, less systemic exposure, and thus decreased renal and bone toxicity.
The phase 3 trial of 873 hepatitis B e antigen (HBeAg)-positive patients (26% with prior nucleos(t)ide analogue [NA] therapy) randomized to TAF 25 mg daily or TDF 300 mg daily in a 2:1 ratio found similar 48-week responses, with serum HBV DNA <29 IU/mL in 64% vs 67%, alanine aminotransferase (ALT) normalization in 72% vs 67%, HBeAg loss in 14% vs 12%, and hepatitis B surface antigen (HBsAg) loss in 1% vs 0.3% in the TAF and TDF groups, respectively (17). Week 96 follow-up results likewise showed that 73% and 75% had serum HBV DNA <29 IU/mL, 22% and 18% lost HBeAg, and 1% and 1% lost HBsAg in TAF and TDF patients, respectively (6).
Analogously, a phase 3 trial of 426 HBeAg-negative patients (21% with prior NA therapy) randomized to TAF 25 mg daily or TDF 300 mg daily in a 2:1 ratio found comparable 48-normalization in 83% vs 75% in the TAF and TDF groups, respectively. However, no patient in either group lost HBsAg (18). Week 96 follow-up results also showed serum HBV DNA <29 IU/mL in 90% of TAF patients and 91% of TDF patients, with 1 TAF-treated patient losing HBsAg (7). The approved dose of TAF is 25 mg orally once daily, with no dose adjustment needed unless creatinine clearance is <15 mL/min.
In these phase 3 studies, TAF had significantly less decline than TDF in bone density and renal function at 48 weeks of treatment. In HBeAg-positive patients, the mean decline in the estimated glomerular filtration rate was −0.6 mL/min for TAF patients, whereas the decline was −5.4 mL/min in TDF patients (P < .0001). In HBeAg-negative patients, the mean decline in the estimated glomerular filtration rate was −1.8 mL/min in TAF patients, whereas the decline for TDF patients was −4.8 mL/min (P = .004) (17, 18). In hip and spine bone mineral density measurements, the adjusted percentage difference in spine bone mineral density for TAF vs TDF was 1.88% (95% confidence interval 1.44 to 2.31, P < .0001) for HBeAg-positive patients and 1.64% (95% confidence interval 1.01 to 2.27, P < .0001) in HBeAg-negative patients (17, 18). In human immunodeficiency virus (HIV)–infected patients, TAF (N = 300) vs TDF (N = 333) containing antiretroviral therapy (ARVT) for up to 144 weeks also showed that TAF had a less negative impact on bone mineral density and renal biomarkers, with fewer patients on TAF vs TDF developing proximal tubulopathy (0 vs 4) or requiring treatment discontinuation because of renal complications (0 vs 12, P < .001) (19). While longer-term data in HBV-monoinfected patients are lacking, particularly with respect to the impact on clinical outcomes such as renal disease and fracture risk, the current safety profile of TAF combined with evidence of similar antiviral efficacy led to its inclusion among the preferred HBV therapies for those patients requiring treatment.
Most studies of switching from TDF to TAF come from the HIV literature. In studies of up to 96 weeks, a switch to TAF vs continued TDF treatment (as part of an antiretroviral regimen) was associated with improvements in proteinuria, albuminuria, proximal renal tubular function (mostly within the first 24 weeks), and bone mineral density (20). Collectively, these studies suggest TAF has a better safety profile than TDF and similar antiviral efficacy in studies of up to 2 years’ duration.
1. Screening, Counseling, and Prevention of Hepatitis B
1A. Screening
The presence of HBsAg establishes the diagnosis of hepatitis B. Chronic vs acute infection is defined by the presence of HBsAg for at least 6 months. The prevalence of HBsAg varies greatly across countries, with high prevalence of HBsAg-positive persons defined as ≥8%, intermediate as 2% to 7%, and low as <2% (21, 22). In developed countries, the prevalence is higher among those who immigrated from high- or intermediate-prevalence countries and in those with high-risk behaviors (22, 23).
Hepatitis B virus is transmitted by perinatal, percutaneous, and sexual exposure and by close person-to-person contact (presumably by open cuts and sores, especially among children in hyperendemic areas) (24, 25). In most countries where HBV is endemic, perinatal transmission remains the most important cause of chronic infection. Perinatal transmission also occurs in nonendemic countries (including the United States), mostly in children of HBV-infected mothers who do not receive appropriate HBV immunoprophylaxis at birth. The majority of children and adults with CHB in the United States are immigrants, have immigrant parents, or became exposed through other close household contacts (26, 27).
HBV can survive outside the body for prolonged periods (28). The risk of developing chronic HBV infection after acute exposure ranges from 90% in newborns of HBeAg-positive mothers to 25% to 30% in infants and children under 5 to less than 5% in adults (29–33). In addition, immunosuppressed persons are more likely to develop chronic HBV infection after acute infection (34).
Table 3 displays those at risk for CHB who should be screened for HBV infection and immunized if seronegative (23, 35, 36). HBsAg and antibody to hepatitis B surface antigen (anti-HBs) should be used for screening (Table 4). Alternatively, antibody to hepatitis B core antigen (anti-HBc) can be utilized for screening as long as those who test positive are further tested for both HBsAg and anti-HBs to differentiate current infection from previous HBV exposure. HBV vaccination does not lead to anti-HBc positivity.
Table 3.
Groups at High Risk for HBV Infection Who Should Be Screened
| ▪ | Persons born in regions of high or intermediate HBV endemicity (HBsAg prevalence of ≥2%) | |
| Africa | All countries | |
| North, Southeast, East Asia | All countries | |
| Australia and South Pacific | All countries except Australia and New Zealand | |
| Middle East | All countries except Cyprus and Israel | |
| Eastern Europe | All countries except Hungary | |
| Western Europe | Malta, Spain, and indigenous populations of Greenland | |
| North America | Alaskan natives and indigenous populations of Northern Canada | |
| Mexico and Central America | Guatemala and Honduras | |
| South America | Ecuador, Guyana, Suriname, Venezuela, and Amazonian areas | |
| Caribbean | Antigua-Barbuda, Dominica, Grenada, Haiti, Jamaica, Saint Kitts and Nevis, Saint Lucia, and Turks and Caicos Islands | |
| ▪ | US-born persons not vaccinated as an infant whose parents were born in regions with high HBV endemicity (≥8%)* | |
| ▪ | Persons who have ever injected drugs* | |
| ▪ | Men who have sex with men* | |
| ▪ | Persons needing immunosuppressive therapy, including chemotherapy, immunosuppression related to organ transplantation, and immunosuppression for rheumatologic or gastroenterologic disorders | |
| ▪ | Individuals with elevated ALT or AST of unknown etiology* | |
| ▪ | Donors of blood, plasma, organs, tissues, or semen | |
| ▪ | Persons with end-stage renal disease, including predialysis, hemodialysis, peritoneal dialysis, and home dialysis patients* | |
| ▪ | All pregnant women | |
| ▪ | Infants born to HBsAg-positive mothers* | |
| ▪ | Persons with chronic liver disease, eg, HCV* | |
| ▪ | Persons with HIV* | |
| ▪ | Household, needle-sharing and sexual contacts of HBsAg-positive persons* | |
| ▪ | Persons who are not in a long-term, mutually monogamous relationship (eg >1 sex partner during the previous 6 months)* | |
| ▪ | Persons seeking evaluation or treatment for a sexually transmitted disease* | |
| ▪ | Health care and public safety workers at risk for occupational exposure to blood or blood-contaminated body fluids* | |
| ▪ | Residents and staff of facilities for developmentally disabled persons* | |
| ▪ | Travelers to countries with intermediate or high prevalence of HBV infection* | |
| ▪ | Persons who are the source of blood or body fluid exposures that might require post-exposure prophylaxis | |
| ▪ | Inmates of correctional facilities* | |
| ▪ | Unvaccinated persons with diabetes who are aged 19 through 59 years (discretion of clinician for unvaccinated adults with diabetes who are aged ≥60 years)* | |
Table 4.
Interpretation of Screening Tests for HBV Infection
| Screening Test Results | Interpretation | Management | Vaccinate? | ||
|---|---|---|---|---|---|
| HBsAg | Anti-HBc | Anti-HBs | |||
| + | + | − | Chronic hepatitis B | Additional testing and management needed | No |
| − | + | + | Prior HBV infection, resolved | No further management unless immunocompromised or undergoing chemotherapy or immunosuppressive therapy | No |
| − | + | − | Prior HBV infection, resolved or false-positive | HBV DNA testing if immunocompromised patient | Yes, if not from area of intermediate or high endemicity |
| − | − | + | Immune | No further testing | No |
| − | − | − | Uninfected and not immune | No further testing | Yes |
Some persons may test positive for anti-HBc but not HBsAg; they may or may not also have anti-HBs, with the prevalence depending on local endemicity or the risk group (37, 38). The finding of isolated anti-HBc (anti-HBc positive but negative for HBsAg and anti-HBs) can occur for a variety of reasons.
Among intermediate- to high-risk populations, the most common reason is previous exposure to HBV infection; the majority of these persons recovered from acute HBV infection earlier in life and anti-HBs titers have waned to undetectable levels, but some had been chronically infected with HBV for decades before clearing HBsAg. In the former case, the risk of hepatocellular carcinoma (HCC) or cirrhosis due to HBV is minimal. In the latter, these persons are still at risk of developing HCC, with an incidence rate that appears to be similar to those with inactive chronic HBV with undetectable HBV DNA levels (39–41). These individuals usually have low HBV DNA levels (20–200 IU/mL, more commonly if they are anti-HBs negative than if they are anti-HBs positive) and are typically born in regions with high prevalence of HBV infection or have HIV or hepatitis C virus (HCV) infection (37, 42–44).
Much less commonly with new, more specific anti-HBc tests, anti-HBc may be a false-positive test result, particularly in persons from low-prevalence areas with no risk factors for HBV infection. Earlier anti-HBc enzyme immunoassay and radioimmunoassay tests were less specific, more frequently yielding false positive results (45).
Anti-HBc may be the only marker of HBV infection during the window phase of acute hepatitis B; these persons should test positive for anti-HBc immunoglobulin M (37, 38).
Lastly, reports exist of HBsAg mutations leading to false-negative HBsAg results (37).
Because of the risk for HBV transmission, screening for anti-HBc occurs routinely in blood donors and, if feasible, in organ donors (37). Since the original anti-HBc studies, the specificity of anti-HBc tests has improved to 99.88% in blood donors and 96.85% in non-HBV medical conditions (46, 47). Individuals with HIV infection or those about to undergo HCV or immunosuppressive therapy are at risk for potential reactivation if they have preexisting HBV and should be screened for anti-HBc (37, 48).
The majority of individuals positive for anti-HBc do not have detectable HBV DNA (37), especially with older, less sensitive assays. For anti-HBc–positive individuals, additional tests to detect prior or current infection include immunoglobulin M anti-HBc, antibody to hepatitis B e antigen (anti-HBe), and HBV DNA with a sensitive assay. Detectable HBV DNA documents infectivity, but a negative HBV DNA result does not rule out low levels of HBV DNA. Additionally repeat anti-HBc testing can be performed over time, particularly in blood donors in whom subsequent anti-HBc negativity suggests an initial false-positive result (37, 48). Although reports vary depending on the sensitivity and specificity of the anti-HBc test used and HBV prevalence in the study population, the minority of patients have an anamnestic response to HBV vaccination, with the majority having a primary antibody response to hepatitis B vaccination similar to persons without any HBV seromarkers (23, 49). Thus, vaccination could be considered reasonable for all screening indications in Table 3. Anti-HBc–positive HIV-infected individuals should receive HBV vaccination (ideally when CD4 counts exceed 200/μL) because most have primary responses to HBV vaccination, with ~60% to 80% developing anti-HBs levels ≥10 mIU/mL after 3 or 4 vaccinations (50, 51). Thus, limited data suggest that vaccination may be considered (48, 52, 53). When considering the benefit of using an anti-HBc–positive donor organ with possible occult HBV infection, the harm of hepatitis B transmission must be weighed against the clinical condition of the recipient patient.
While persons who are positive for anti-HBc but negative for HBsAg are at very low risk of HBV reactivation, the risk can be substantial when chemotherapeutic or immunosuppressive drugs are administered singly or in combination (see Screening, Counseling, and Prevention of Hepatitis B, section 6D). Thus, all persons who are positive for anti-HBc (with or without anti-HBs) should be considered potentially at risk for HBV reactivation in this setting.
Guidance Statements on Screening for Hepatitis B Infection.
Screening should be performed using both HBsAg and anti-HBs.
Screening is recommended in all persons born in countries with a HBsAg seroprevalence of ≥2%, US-born persons not vaccinated as infants whose parents were born in regions with high HBV endemicity (≥8%), pregnant women, persons needing immunosuppressive therapy, and the at-risk groups listed in Table 3.
Anti-HBs–negative screened persons should be vaccinated.
Screening for anti-HBc to determine prior exposure is not routinely recommended but is an important test in patients who have HIV infection, who are about to undergo HCV or anticancer and other immunosuppressive therapies or renal dialysis, and in donated blood (or, if feasible, organs) (see Screening, Counseling, and Prevention of Hepatitis B, section 6D).
1B. Counseling Patients With Chronic Hepatitis B, Including Prevention of Transmission to Others
Patients with chronic HBV infection should be counseled regarding lifestyle modifications and prevention of transmission as well as the importance of lifelong monitoring. No specific dietary measures have been shown to have any effect on the progression of CHB per se, but metabolic syndrome and fatty liver contribute to liver-related morbidity (54, 55). Ingestion of more than 7 drinks per week for women and more than 14 drinks per week for men are associated with increased risk of cirrhosis and HCC (56, 57). Studies evaluating the risk of lesser amounts of alcohol intake are sparse (58), but the conservative approach is to recommend abstinence or minimal alcohol ingestion (59, 60). Individuals with CHB should be immunized against hepatitis A if not already immune (61).
HBsAg-positive persons should be counseled regarding transmission to others (see Table 5). Because of increased risk of acquiring HBV infection, household members and sexual partners should be vaccinated if they test negative for HBV serologic markers. For casual sex partners or steady partners who have not been tested or have not completed the full immunization series, barrier protection methods should be employed. Transmission of HBV from infected health care workers to patients has been shown to occur in rare instances (62). For persons with CHB who are health care workers, the Centers for Disease Control and Prevention recommends that those who perform exposure-prone procedures should seek counseling and advice from an expert review panel (63). If serum HBV DNA exceeds 1,000 IU/mL, antiviral therapy is recommended, and performance of exposure-prone procedures is permitted if serum HBV DNA is suppressed to <1,000 IU/mL and maintained below that cutoff (63). Since 2013, the US Department of Justice has ruled that it is unlawful for medical and dental schools to exclude applicants who are HBsAg positive. No special arrangements need to be made for HBV-infected children in the community other than practicing universal precautions in daycare centers, schools, sports clubs, and camps.
Table 5.
Recommendations for Infected Persons Regarding Prevention of Transmission of HBV to Others
Persons Who Are HBsAg Positive Should:
|
Children and Adults Who Are HBsAg Positive:
|
Guidance Statements on Counseling of Persons Who Are HBsAg Positive.
HBsAg-positive persons should be counseled regarding prevention of transmission of HBV to others (Table 4).
-
For health care workers and students who are HBsAg positive:
They should not be excluded from training or practice because they have hepatitis B.
Only HBsAg-positive health care workers and students whose job requires performance of exposure-prone procedures are recommended to seek counseling and advice from an expert review panel at their institution. They should not perform exposure-prone procedures if their serum HBV DNA level exceeds 1,000 IU/mL but may resume these procedures if their HBV DNA level is reduced and maintained below 1,000 IU/mL.
No special arrangements are indicated for HBV-infected children in the community other than practicing universal precautions in daycare centers, schools, sports clubs, and camps.
Abstinence or only limited use of alcohol is recommended in HBV-infected persons.
Optimization of body weight and treatment of metabolic complications, including control of diabetes and dyslipidemia, are recommended to prevent concurrent development of metabolic syndrome and fatty liver.
Guidance Statements on Counseling of Persons Who Are HBsAg Negative and anti-HBc Positive (With or Without anti-HBs).
Screening for anti-HBc is not routinely recommended except in patients who have HIV infection or who are about to undergo HCV therapy or immunosuppressive treatment.
Persons who are anti-HBc positive without HBsAg are not at risk of transmission of HBV, either sexually or to close personal contacts.
Persons who are positive only for anti-HBc and who are from an area with low endemicity with no risk factors for HBV should be given the full series of hepatitis B vaccine.
Persons who are positive only for anti-HBc and have risk factors for hepatitis B (Table 3) are not recommended for vaccination unless they are HIV positive or immunocompromised.
1C. Counseling of HBsAg-Positive Women in Pregnancy and Postpartum
All pregnant women should be screened for HBsAg. Pregnant women with CHB should be encouraged to discuss with their obstetrician and/or pediatrician the prevention of mother-to-child transmission. Hepatitis B immune globulin (HBIG) and HBV vaccine should be administered to their newborn immediately after delivery (64). Antiviral therapy in the third trimester is recommended for pregnant women with serum HBV DNA >200,000 IU/mL (1, 4).
A proportion of women (about 25%) have hepatitis flares with or without HBeAg seroconversion within the first months after delivery (65). Seroconversion rates of up to 17% have been described. It has been postulated that the rapid decrease in cortisol levels characteristic of the postpartum state is analogous to the steroid withdrawal therapy that has been used to elicit seroconversion. Although the flares are often mild and resolve spontaneously, cases of acute liver failure have been described in the peripartum period (66–68). Extending third trimester antiviral therapy from 2 to 12 weeks postpartum did not protect against postpartum flares in one study (68), supporting the AASLD guideline recommendation that antiviral therapy given for prevention of mother-to-child transmission be discontinued at the time of delivery or up to 4 weeks postpartum (1).
Prior systematic review of any antiviral therapy in the third trimester showed a significant reduction in perinatal transmission of HBV (4) with lamivudine, telbivudine, or TDF, but TDF is the preferred choice owing to its antiviral potency and concerns for resistance with the other antiviral agents. Two recent randomized control trials of TDF vs no antiviral treatment in the third trimester confirmed significant reductions in risk of mother-to-child transmission of hepatitis B with TDF in women with a high level of HBV DNA (69, 70). Elevated maternal creatine kinase levels were more frequent in TDF-treated vs untreated women in one study, though none were assessed as clinically significant (69). Both studies found no difference in the rates of prematurity, congenital malformations, or Apgar scores. Additional data on infant safety (including bone growth) from studies of pregnant women receiving antiretroviral therapy found no increase in adverse events among TDF-exposed vs unexposed infants (71–73). Although a prior study of HIV-infected pregnant mothers found TDF-exposed infants to have 12% lower whole-body bone mineral content than unexposed infants (74), the follow-up study showed no differences at 2 years of age (71).
Whether invasive procedures during pregnancy, such as amniocentesis, increase the risk of HBV infection in the infants is unclear. Two studies including 21 and 47 HBsAg mother-infant pairs respectively concluded that the risk of HBV transmission by amniocentesis is low (75). However, more recently, the risk of mother-to-child transmission of HBV was significantly higher in women with a high HBV DNA level (≥7 log copies/mL) who underwent amniocentesis compared with those who did not (50% vs 4.5%, odds ratio 21.3, 95% confidence interval 2.96 to 153) (75, 76). Therefore, the risk of mother-to-child transmission must be considered when assessing the potential benefit of amniocentesis in highly viremic women.
Although antiviral drug labels do not recommend breastfeeding when taking these drugs, clinical studies support the safety of these drugs during breastfeeding (77, 78).
Vaccination against HBV is both safe and efficacious during pregnancy (79). In addition, titers of the passively transferred maternal antibody to newborns wane over time, as would be expected without the addition of active vaccination (80). An accelerated vaccination schedule has been shown to be feasible and efficacious in high-risk pregnant women (81). Chronic HBV infection does not usually affect the outcome of pregnancy unless the mother has cirrhosis or advanced liver disease. However, extra care is necessary to evaluate the mother and to ensure that the infant receives hepatitis B immune globulin and a birth dose of HBV vaccine.
Guidance Statements on Counseling of Women in Pregnancy.
HBV vaccination is safe in pregnancy, and pregnant women who are not immune to or infected with HBV should receive this vaccine series.
Women identified as HBsAg positive during pregnancy should be linked to care for additional testing (ALT, HBV DNA, imaging for HCC surveillance if indicated) and determination of need for antiviral therapy.
Women who meet standard indications for HBV therapy should be treated. Women without standard indications but who have HBV DNA >200,000 IU/mL in the second trimester should consider treatment to prevent mother-to-child transmission (1).
HBV-infected pregnant women who are not on antiviral therapy as well as those who stop antiviral at or early after delivery should be monitored closely for up to 6 months after delivery for hepatitis flares and seroconversion. Long-term follow up should be continued to assess need for future therapy.
The potential risk of mother-to-child transmission of HBV with amniocentesis should be included in the risk of harms vs benefits discussion in HBsAg-positive mothers with high level viremia.
HBV-infected pregnant women with cirrhosis should be managed in high-risk obstetrical practices and treated with TDF to prevent decompensation.
Sexual partners of women identified as HBV-infected during pregnancy should be assessed for HBV infection or immunity and receive HBV vaccine if appropriate.
Breastfeeding is not prohibited.
1D. Vaccination, Follow-up Testing, and Boosters
Recommendations for vaccination are outlined in the Centers for Disease Control and Prevention and Advisory Committee on Immunization Practices guidelines (35, 82). Follow-up testing is recommended for those who remain at risk of infection, such as health care workers, infants of HBsAg-positive mothers, sexual partners of persons with CHB, chronic hemodialysis patients, and immunocompromised persons, including those with HIV. Furthermore, annual testing of hemodialysis patients is recommended since immunity wanes rapidly in these individuals who are at a high risk of continued exposure to HBV. Booster doses are not indicated in immunocompetent individuals if the primary vaccination series is completed, as long-term follow-up studies indicated that immune memory persists despite declining anti-HBs levels (83). For individuals undergoing postvaccination serologic testing, especially immunocompromised patients (such as persons on dialysis or with chronic inflammatory conditions, including HIV), a booster injection is advised when the anti-HBs titer falls below 10 mIU/mL.
For those who are nonresponders to the initial vaccination series, a second series of 0-, 1-, and 6-month vaccination is recommended (84). For those who are immunocompromised, including those with HIV, on dialysis, or with cirrhosis, use of a double dose of vaccine has been shown to increase the percentage of patients achieving protective antibody titers, the level of anti-HBs achieved, and/or the duration of protection (85–87). HBV vaccine with or without HBIG is also recommended for postexposure immunoprophylaxis of unimmunized individuals who have percutaneous, mucosal, or sexual exposure to HBsAg-positive or HBsAg-unknown sources. This includes bites, needlesticks, sexual contacts, and sexual assaults. Immunoprophylaxis should be administered within 24 hours of exposure. Studies are limited on the maximum interval after exposure during which postexposure prophylaxis is effective, but the interval is unlikely to exceed 7 days for percutaneous exposures and 14 days for sexual exposures. The Centers for Disease Control and Prevention has updated guidelines for vaccination and postexposure prophylaxis for health care workers (HCW) (88).
Infants born to women whose HBsAg status is unknown should also receive prompt initiation of vaccination at birth. Because low-birth-weight infants (<2,000g) may have suboptimal vaccine responses, low-birth-weight infants of HBsAg-positive women should receive HBIG and HBV vaccine within 12 hours of birth followed by the usual 3-dose vaccination schedule, and premature or low-birth-weight infants born to HBsAg-negative women should be vaccinated prior to 1 month of age or at hospital discharge (89). Only monovalent HBV vaccine should be used for preterm or term infants younger than 6 weeks.
Guidance Statements for Prevention of Transmission of Hepatitis B From Individuals With Chronic HBV Infection.
HBV vaccines have an excellent safety record and are given as a 3-dose series at 0, 1, and 6 months (with or without hepatitis A vaccine). An alternate 4-dose schedule given at 0, 7, and 21 to 30 days followed by a dose at 12 months can be used for the combination hepatitis A and B vaccine (Twinrix®) for adults (90). Recently, a 2-dose series given at 0 and 1 months has been approved for adults (HEPLISAV-B®).
Sexual and household contacts of HBV-infected persons who are negative for HBsAg and anti-HBs should receive HBV vaccination.
Newborns of HBV-infected mothers should receive HBIG and HBV vaccine at delivery and complete the recommended vaccination series. Infants of HBsAg-positive mothers should undergo postvaccination testing at 9 to 15 months of age.
Health care workers, sexual partners of persons with chronic HBV infection, chronic hemodialysis patients, and immunocompromised persons (including those with HIV) should be tested for their response to the vaccination 1 to 2 months after the last dose of vaccine.
For nonresponders to the initial vaccine series, a repeat 3-dose vaccination series is recommended, with a double dose used for immunocompromised patients, including those with cirrhosis (91).
Follow-up testing of vaccine responders is recommended annually for chronic hemodialysis patients.
Booster doses are not recommended except for individuals who are immunocompromised.
2. Definitions and Phases of Chronic Hepatitis B Infection
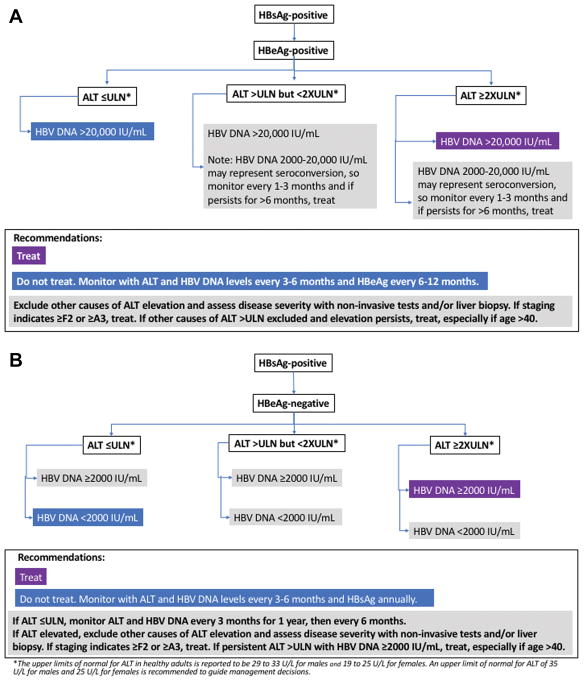
The diagnostic criteria for CHB and clinical terms relating to HBV infection are summarized in Table 6. The presence of HBsAg for at least 6 months establishes the chronicity of infection. As HBV is not directly cytopathic, host responses to the virus-infected hepatocytes are believed to mediate liver cell injury and, with long-term chronic liver inflammation and ineffective immune-mediated viral clearance, contribute to the development of cirrhosis and liver cancer (92), (93). Importantly, CHB is a dynamic disease and individuals with CHB can transition through different clinical phases with variable levels of serum ALT activity, HBV DNA, and HBV antigens. The levels of serum ALT and HBV DNA as well as liver fibrosis are important predictors of long-term outcome that inform decisions for treatment initiation as well as treatment response. Therefore, serial testing of ALT and HBV DNA levels are needed to guide treatment decisions (Figure 1). Additionally, staging of liver disease severity using liver biopsy or noninvasive tests such as elastography are important in guiding surveillance and assisting with treatment decisions.
Table 6.
Diagnostic Criteria and Definitions for Chronic Hepatitis B
Chronic Hepatitis B (CHB)
|
Immune-Tolerant CHB
|
Immune-Active CHB
|
Inactive CHB
|
Other Definitions
|
Figure 1.
The upper limits of normal (ULN) for ALT in healthy adults are reported to be 29 to 33 U/L for males and 19 to 25 U/L for females (94–96). For purposes of guiding management of CHB, an upper limit of normal for ALT of 35 U/L for males and 25 U/L for females is recommended (Figure 1), though differences in repeat testing of the same sample have been described (97, 98). This might prompt clinicians to repeat testing when a single ALT elevation is near the cutoff for treatment. Interpretation of ALT elevations in the context of treatment decisions requires consideration that the ALT elevation may be due to causes other than CHB, such as drug-induced liver injury, alcohol-associated liver disease, or fatty liver.
3. Selected Diagnostic Tests Used in Management of Chronic Hepatitis B
3A. HBV DNA Quantitation
Quantification of serum HBV DNA is a crucial component in the evaluation of patients with CHB and in the assessment of the efficacy of antiviral treatment. Most HBV DNA assays used in clinical practice utilize real-time polymerase chain reaction technology with a sensitivity of 5 to 10 IU/mL and a dynamic range up to 7 log10 IU/mL (99). Some patients with CHB have widely fluctuating HBV DNA levels that may vary from undetectable to >2,000,000 IU/mL (100). Thus, serial monitoring of HBV DNA levels is more important than any single arbitrary cutoff value in prognostication and in determining the need for treatment.
Generally, patients with inactive CHB have HBV DNA levels <2,000 IU/mL and those with immune-active CHB have HBV DNA levels >20,000 IU/mL, with levels lower in those with HBeAg-negative CHB than in HBeAg-positive CHB. The 20,000-IU/mL cutoff is an arbitrary value (101), which reflects the detection limit of historical non–polymerase chain reaction assays. However, chronic hepatitis, cirrhosis, and HCC have been found in patients with lower HBV DNA levels (102), highlighting the importance of interpreting HBV DNA levels in the context of other host factors (including age, duration of infection, ALT elevation, and stage of disease) when making treatment decisions (1).
3B. HBV Genotypes
Ten genotypes of HBV have been identified labeled A through J (103, 104). The prevalence of HBV genotypes varies geographically. HBV genotypes A through H have been found in the United States, with genotypes A, B, and C being most prevalent (26). HBV genotypes may play an important role in the progression of HBV-related liver disease as well as response to interferon (IFN) therapy (103, 105). Genotype A (vs B–D) is associated with significantly higher rates of HBeAg and HBsAg loss with IFN therapy (105, 106). Studies from Asia found that HBV genotype B is associated with HBeAg seroconversion at an earlier age, more sustained remission after HBeAg seroconversion, less active hepatic necroinflammation, a slower rate of progression to cirrhosis, and a lower rate of HCC development compared with genotype C (103). Studies from Alaska also show that HBeAg seroconversion occurs on average 2 decades later in persons infected with HBV genotype C than in those infected with HBV genotypes A, B, D, or F (107). In addition, a significantly higher incidence of HCC has been reported in persons infected with genotypes C or F in Alaska compared with the others (108).
3C. Quantitative Hepatitis B Surface Antigen (qHBsAg)
The desire to assess covalently closed circular DNA (cccDNA) inside hepatocytes led to development of reproducible, automated, and standardized (IU/mL) assays (Architect QT assay [Abbott], Elecsys HBsAg III Quant [Roche], Liaison XL [DiaSorin]) to quantify hepatitis B surface antigen (109). Although qHBsAg reflects covalently closed circular DNA and intrahepatic DNA levels, it also measures HBsAg that arises from integrated DNA, thereby reducing its specificity as a biomarker for viral replication. qHBsAg levels vary by genotype (higher in A) and by presence of preS/S mutants or host immune control (inverse correlation with both) (109).
The levels of HBsAg are generally higher in HBeAg-positive patients than HBeAg-negative patients (109–111). In HBeAg-negative patients, low qHBsAg (<1,000 IU/mL) and low HBV DNA (≤2,000 IU/mL) suggest inactive CHB. A qHBsAg <100 IU/mL increases the specificity of identifying those with inactive CHB but reduces sensitivity to 35% (112). Higher qHBsAg levels have been associated with progression to cirrhosis and HCC. qHBsAg <1,000 IU/mL predicts spontaneous HBsAg clearance in HBeAg-negative patients with a low viral load (113).
For peg-IFN treatment of HBeAg-positive patients, qHBsAg helps predict response and provides a stopping rule. A qHBsAg <1500 IU/mL at week 12 resulted in likelihoods of 57% for HBeAg seroconversion and 18% for HBsAg loss. Similarly, the absence of any decline at week 12 suggested that HBeAg loss or HBV DNA <2,000 IU/mL 24 weeks after treatment were unlikely (109). In particular, none of the patients with genotype B and C who had HBsAg >20,000 IU/mL at week 12 and 24 achieved HBeAg seroconversion (109). For peg-IFN treatment of HBeAg-negative patients, none of the genotype D patients who had no HBsAg decline and <2 log decline of HBV DNA at week 12 had a treatment response, as defined by a sustained HBV DNA level <2,000 IU/mL off treatment (109). For NA treatment of HBeAg-negative patients, a >1 log decline in qHBsAg predicted increased loss of HBsAg, and qHBsAg level <100 IU/mL were associated with a sustainable off-treatment response following 3 years or more of consolidation therapy (109).
3D. Viral Resistance Testing
Hepatitis B antiviral drug resistance mutations in treatment-naïve patients are rare (114). For patients on antiviral therapy, the first manifestation of antiviral resistance is virologic breakthrough, which is defined as a 1 log10 (10-fold) increase in serum HBV DNA from nadir during treatment in a patient who had an initial virologic response. Most antiviral-resistant mutants have decreased replication fitness compared with wild-type HBV. However, compensatory mutations that can restore replication fitness frequently emerge during continued treatment, leading to a progressive increase in serum HBV DNA that may exceed pretreatment levels. Genotypic resistance, measured with commercially available assays, evaluate sequence variations in specific positions in the polymerase. The current diagnostic methods include restriction fragment length polymorphism analysis, hybridization, and sequencing (115). Current assays typically require an HBV DNA level >1,000 IU/mL.
Guidance Statements on Use of Selected Serologic and Virologic Assays.
Quantitative HBV DNA testing is essential to guide treatment decisions, including initiation of treatment and evaluation of a patient’s response to antiviral treatment.
HBsAg quantitation can be useful in managing patients receiving peg-IFN therapy. HBsAg quantitation is not recommended for the routine testing or follow-up of patients with CHB.
HBV genotyping can be useful in patients being considered for peg-IFN therapy, as genotypes A and B are associated with higher rates of HBeAg and/or HBsAg loss than genotype C and D, but it is not otherwise recommended for routine testing or follow-up of patients with CHB.
Testing for viral resistance in treatment-naïve patients is not recommended. Resistance testing can be useful in patients with prior treatment experience, those with persistent viremia on NA therapy, or those who experience virologic breakthrough during treatment.
4. Follow-up of Patients Not Currently on Antiviral Treatment
Patients not meeting criteria for antiviral therapy require regular monitoring to assess the need for future therapy per the AASLD 2016 HBV Guideline (1).
4A. HBeAg-Positive Patients With High Serum HBV DNA but Normal ALT (Immune-Tolerant CHB)
These patients should be monitored at 3 to 6 month intervals (Figure 1). More frequent monitoring should be performed when ALT levels become elevated (116–119). Patients with compensated liver disease who remain HBeAg positive with HBV DNA levels greater than 20,000 IU/mL after a 3 to 6 month period of elevated ALT levels greater than 2 times the upper limit of normal (>50 U/L for women and >70 U/L for men) should be considered for antiviral treatment (1). Liver biopsy should be considered in patients with persistent borderline normal or slightly elevated ALT levels, particularly in patients over age 40 who have been infected with HBV from a young age (120). Patients with moderate to severe inflammation (A3 or higher) and/or fibrosis (F2 or higher) can be considered for antiviral therapy (1). Noninvasive methods may be used in lieu of liver biopsies to assess for severity of fibrosis and/or inflammation (121, 122). Liver stiffness measurements are more accurate than serum fibrosis panels in predicting significant or advanced fibrosis (AST to platelet ratio index and FIB-4) (123, 124). Noninvasive methods overestimate fibrosis if high levels of necroinflammation, as reflected by elevated ALT, are present (122).
4B. HBeAg-Negative, Anti-HBe–Positive Patients With Normal ALT and HBV DNA <2,000 IU/mL (Inactive CHB)
These patients should be monitored with ALT determination every 3 months during the first year to verify that they are truly in the “inactive phase” and then every 6 to 12 months (100, 125). If the ALT level becomes elevated, monitoring should occur more frequently. In addition, for persistent or recurrent ALT elevation, additional evaluation for causes (eg, HBV DNA tests) should be initiated (Figure 1). Studies suggest that a 1-time qHBsAg test combined with HBV DNA may help differentiate HBeAg-negative patients in the “grey zone,” in which HBV DNA or ALT levels are borderline between inactive CHB and immune-active, HBeAg-negative CHB (126, 127) (Figure 1). In one study, qHBsAg <1,000 IU/mL and HBV DNA <2,000 IU/mL differentiated inactive CHB from HBeAg-negative, immune-active CHB with a sensitivity and specificity of 71% and 85%, respectively (127), but more validation of the specific cutoff is needed.
4C. Patients Who Have Achieved HBsAg Loss Spontaneously or With Therapy (Resolved CHB or Functional Cure)
Spontaneous HBsAg loss has been reported to occur at the rate of roughly 1% per year, but this rare event does not occur at a linear rate (128, 129). In a study of 1076 patients with CHB in Taiwan, cumulative probabilities of spontaneous HBsAg loss were 8.1% after 10 years and increased to 44.7% after 25 years (129). HBsAg loss can also occur in response to antiviral therapy, being more common with IFN than with NAs. Although progression of liver disease to cirrhosis or hepatic decompensation generally stops when patients lose HBsAg unless other causes of liver injury are present (eg, heavy alcohol consumption or nonalcoholic fatty liver), the risk of HCC persists, particularly if HBsAg loss occurred in patients older than 50 years or in those with cirrhosis or coinfection with HCV or hepatitis D virus (HDV) (128, 130–132). Loss of HBsAg with acquisition of anti-HBs has been termed functional cure. This is distinguished from true cure, in which HBsAg and covalently closed circular DNA are eliminated.
Guidance Statements for Monitoring Patients With Chronic HBV Infection Who Are Not Currently on Treatment.
As CHB is a dynamic disease, persons who are not receiving treatment should be assessed regularly to determine if an indication for treatment has developed.
HBeAg-positive patients with persistently normal ALT should be tested for ALT at 3- to 6-month intervals. If ALT levels increase above upper limits of normal, ALT along with HBV DNA should be tested more frequently. HBeAg status should be checked every 6 to 12 months.
-
Patients who are HBeAg positive with HBV DNA levels >20,000 IU/mL and ALT levels less than 2 times the ULN (<50 U/L for females, <70 U/L for males) should undergo testing to evaluate histologic disease severity, especially those >40 years old and who were infected at young age (ie, long duration of infection).
Liver biopsy offers the only means of assessing both fibrosis and inflammation. If the biopsy specimen shows moderate or severe inflammation (A2 or A3) or significant fibrosis (≥F2), treatment is recommended.
Alternative methods to assess fibrosis are elastography (preferred) and liver fibrosis biomarkers (eg, FIB-4 or FibroTest®). If these noninvasive tests indicate significant fibrosis (≥F2), treatment is recommended.
-
Patients who are HBeAg negative with HBV DNA levels >2,000 IU/mL and elevated ALT levels less than 2 times the ULN should undergo testing to evaluate disease severity, especially those who are >40 years old and who were infected at a young age (ie, long duration of infection).
Liver biopsy offers the only means of assessing both fibrosis and inflammation. If the biopsy specimen shows moderate or severe inflammation (A2 or A3) or significant fibrosis (≥F2), treatment is recommended.
Alternative methods to assess fibrosis are elastography (preferred) and liver fibrosis markers (eg, FIB-4 or FibroTest®). If these noninvasive tests indicate significant fibrosis (≥F2), treatment is recommended.
Patients who are HBeAg negative with normal ALT (≤25 U/L women, ≤35 U/L men) and HBV DNA <2,000 IU/mL should be tested for ALT and HBV DNA every 3 months during the first year to confirm they have inactive CHB. Thereafter their ALT and HBV DNA levels should be tested at 6- to 12-month intervals. If costs are a concern, ALT monitoring alone can be used. When ALT levels increase above the normal limit, ALT along with HBV DNA should be tested more frequently (every 3–6 months).
In persons with HBV DNA <2,000 IU/mL but elevated ALT levels, other causes of liver disease should be investigated, including but not limited to HCV or HDV, drug toxicity, nonalcoholic fatty liver, alcohol, or autoimmune liver disease.
Persons with inactive CHB should be evaluated for loss of HBsAg annually.
In persons who achieve sustained HBsAg seroclearance, routine ALT and HBV DNA monitoring are no longer required. HCC surveillance should continue if the person has cirrhosis, a first-degree family member with HCC, or a long duration of infection (>40 years for males and >50 years for females who have been infected with HBV from a young age).
5. Screening for HCC
The AASLD 2018 Practice Guideline on HCC has been published (133). Of the 2 tests prospectively evaluated as screening tools for HCC, alpha-fetoprotein (AFP) and ultrasonography (US), the sensitivity, specificity, and diagnostic accuracy of US are higher than those of AFP. The guideline for HCC recommends surveillance of persons at high risk of HCC with US every 6 months. There was insufficient evidence for or against the addition of AFP every 6 months to screening algorithms. AFP alone is not recommended except in those circumstances where US is unavailable or cost is an issue. HCC surveillance is considered cost-effective if the annual risk of HCC is ≥0.2% per year (134). Using this principle, all patients with cirrhosis warrant screening. For noncirrhotic patients, age, sex, race, and family history determine when surveillance should begin (134, 135). Other subgroups with a higher risk of HCC include persons with HCV, HDV, or HIV coinfections and those with fatty liver (55, 136–139). At this time, there is insufficient evidence to recommend HCC surveillance in children except in children with cirrhosis or with a first-degree family member with HCC.
Guidance Statements for HCC Screening in HBsAg-Positive Persons.
All HBsAg-positive patients with cirrhosis should be screened with US examination with or without alpha-fetoprotein every 6 months.
HBsAg-positive adults at high risk for HCC (including Asian or African American men over 40 years and Asian women over 50 years of age), persons with a first-degree family member with a history of HCC, or persons with HDV should be screened with US examination with or without alpha-fetoprotein every 6 months.
There are insufficient data to identify high-risk groups for HCC in children. However, it is reasonable to screen HBsAg-positive children and adolescents with advanced fibrosis (F3) or cirrhosis and those with a first-degree family member with HCC using US examination with or without alpha-fetoprotein every 6 months.
For HBsAg-positive persons at high risk for HCC who are living in areas where US is not readily available, screening with AFP every 6 months should be performed.
6. Management of Chronic HBV in Special Populations
6A. Coinfection With HCV
As with any patient with CHB, the treatment goals are to reduce risk of progression to cirrhosis and liver-related complications, including HCC. In HBV-HCV coinfected patients, the viral activity responsible for liver disease can be determined by measuring HCV RNA and HBV DNA levels. If HCV RNA is detectable, treatment of HCV should be undertaken (140). If HBV DNA is detectable, treatment is determined by the HBV DNA and ALT levels (Figure 1) (1). Importantly, treatment of one virus may lead to changes in the activity of the other virus, and thus monitoring during and after treatment is necessary to assess for viral activity.
In the IFN era, the treatment of choice for patients coinfected with HBV and HCV infections was peg-IFN and ribavirin for 24 to 48 weeks, depending on the HCV genotype. Moderate to high rates of HCV eradication and HBV suppression were reported with this combination (141, 142). However, a rebound in serum HBV DNA after an initial decline and increased HBV replication in patients with undetectable HBV DNA prior to treatment have been reported with peg-IFN and ribavirin (141, 143, 144). Similarly, direct-acting antiviral (DAA) HCV therapy has been reported to increase HBV DNA levels in HBsAg-positive patients (145) and to elevate ALT concurrently with HBV reactivation, leading to liver decompensation (146), though the frequency of liver failure is very low (145, 147). The majority of reported reactivation events (elevated ALT with elevated HBV DNA) occurred between 4 to 12 weeks of DAA treatment (148).
In those HBV-HCV coinfected patients with cirrhosis or those meeting recommended criteria for HBV treatment (Figure 1), HBV antiviral therapy should be started concurrently with DAA therapy (140). Entecavir, TDF, or TAF are the preferred antivirals. For HBsAg-negative, anti-HBc-positive patients with chronic HCV infection, monitoring ALT levels is reasonable, with testing for HBsAg and HBV DNA recommended if ALT levels fail to normalize or increase despite declining or undetectable HCV RNA levels. HBV antiviral therapy should be initiated if there is evidence of HBV reactivation (increase in HBV DNA from baseline—see section 6D1). There are no known interactions between HBV antivirals (entecavir, TDF, TAF) and approved HCV DAAs. For triply infected patients with HIV, HBV, and HCV, more opportunities for drug interactions exist, and careful review of antiretroviral therapy before initiation of HCV or HBV therapy is recommended (Screening, Counseling, and Prevention of Hepatitis B, section 6C).
Guidance Statements for Treatment of Patients with HBV and HCV Coinfection.
All HBsAg-positive patients should be tested for HCV infection using the anti-HCV test.
HCV treatment is indicated for patients with HCV viremia (113).
HBV treatment is determined by HBV DNA and ALT levels as per the AASLD HBV guidelines for monoinfected patients (1).
HBsAg-positive patients are at risk of HBV DNA and ALT flares with HCV DAA therapy, and monitoring of HBV DNA levels every 4 to 8 weeks during treatment and for 3 months posttreatment is indicated in those who do not meet treatment criteria for monoinfected patients (per AASLD–Infectious Diseases Society of America HCV Guidance).
HBsAg-negative, anti-HBc positive patients with HCV are at very low risk of reactivation with HCV DAA therapy. ALT levels should be monitored at baseline, at the end of treatment, and during follow-up, with HBV DNA and HBsAg testing reserved for those whose ALT levels increase or fail to normalize during treatment or posttreatment.
6B. Hepatitis D Infection
The AASLD 2016 HBV Guideline recommends testing of HBsAg-positive persons at risk for HDV, including those with HIV infection, persons who inject drugs, men who have sex with men, and immigrants from areas of high HDV endemicity (149, 150) (Table 7) Additionally, HBsAg-positive patients with low or undetectable HBV DNA but high ALT levels should be considered for HDV testing. Given the importance of HDV to the long-term management of the HBsAg-positive patient, if there is any uncertainty regarding the need to test, HDV screening is recommended. The recommended screening test is anti-HDV, and if this test result is positive, it should be followed by HDV RNA testing to diagnose active HDV infection. A high degree of heterogeneity in sensitivity and specificity has been identified across HDV assays (151), and the availability of the first international external quality control for HDV quantification via the World Health Organization has led to improvements in HDV diagnostics.
Table 7.
HBsAg-Positive Persons at High Risk of HDV Infection Who Should Be Screened
| ▪ | Persons born in regions with reported high HDV endemicity* | |
| Africa | West Africa, horn of Africa | |
| Asia | Central and Northern Asia, Vietnam, Mongolia, Pakistan, Japan, Taiwan, | |
| Pacific Islands | Kiribati, Nauru | |
| Middle East | All countries | |
| Eastern Europe | Eastern Mediterranean regions, Turkey | |
| South America | Amazonian basin | |
| Other | Greenland | |
| ▪ | Persons who have ever injected drugs | |
| ▪ | Men who have sex with men | |
| ▪ | Individuals infected with HCV or HIV | |
| ▪ | Persons with multiple sexual partners or any history of sexually transmitted disease | |
| ▪ | Individuals with elevated ALT or AST with low or undetectable HBV DNA | |
The primary endpoint of treatment is the suppression of HDV replication, which is usually accompanied by normalization of ALT levels and a decrease in necroinflammatory activity on a liver biopsy specimen. For patients with elevated ALT levels, measurement of HBV DNA and HDV RNA will allow determination of the need for NA alone, peg-IFN alone, or combination therapy. The presence of underlying cirrhosis may further modify treatment decisions, as is the case in HBV monotherapy. Because NAs have no efficacy against HDV infection, they are not recommended in patients with suppressed or low HBV replication except patients with cirrhosis. HBV DNA levels may change over time, including during treatment of HDV infection, and if the HBV DNA levels become elevated, treatment with preferred NAs (entecavir, TDF, TAF) is recommended. Long-term suppression of active HBV infection may be expected to reduce quantitative HBsAg levels, which should have a beneficial effect on HDV coinfection.
The only approved treatment of chronic hepatitis D is IFN-α. Peg-IFN is the drug of choice without clear differences in efficacy between peg-IFN alpha-2a (180 μg weekly) or 2b (1.5 μg/kg weekly) (152). Treatment success, defined as undetectable HDV RNA 24 weeks after completing treatment, ranges from 23% to 57% (152–154). ALT normalization typically parallels the virologic responses. The combination of NA with peg-IFN does not increase the likelihood of an off-treatment virologic response (153). Late relapses can occur with longer follow-up, leading to very low rates of sustained HDV RNA undetectability. In the multicenter HIDIT-1 study of peg-IFN for 48 weeks with or without adefovir, 40% of patients achieved an undetectable HDV RNA level 24 weeks after completing therapy (153), but at a mean follow-up 4.3 years later, only 12% remained undetectable (155). A complete virologic response, defined as loss of HBsAg plus sustained suppression of HDV RNA, is a more desirable endpoint of therapy, but this occurs rarely with 1 year of treatment. Longer treatment duration may increase HBsAg loss, eg, peg-IFN for up to 5 years resulted in HBsAg loss in 3 of 13 patients (23%) (156).
An early virologic response, defined by loss of HDV RNA after 24 weeks of treatment, was associated with a higher likelihood of a sustained off-treatment response, whereas a failure to achieve at least a 2-log copies/mL decline by this same time point was associated with a <5% chance of sustained off-treatment response (157). The benefits of peg-IFN on disease progression and clinical outcomes have been most closely associated with undetectability of HDV RNA during follow-up.
Given the poor response to current peg-IFN therapy, new drug therapies are urgently needed for HDV-infected persons. Phase 2 studies of prenylation inhibitors and entry inhibitors offer hope for new treatment options in the future (158, 159).
Guidance Statements for Management of Patients With HDV Infection.
Anti-HDV screening is recommended in HIV-positive persons, persons who inject drugs, men who have sex with men, those at risk for sexually transmitted diseases, and immigrants from areas of high HDV endemicity. Patients with low HBV DNA levels and elevated ALT levels may be considered for HDV screening. If there is any uncertainty regarding the need to test, an initial anti-HDV test is recommended.
For those at risk for HDV acquisition, periodic retesting is recommended.
Anti-HDV–positive patients should have periodic assessment of HDV RNA and HBV DNA.
Peg-IFN-α for 12 months is the recommended therapy for those with elevated HDV RNA levels and ALT elevation.
If HBV DNA levels are elevated, concurrent therapy with NA using preferred drugs (entecavir, TDF, or TAF) is indicated.
Assessment of HDV relapse is warranted if ALT elevation occurs following treatment because of the high rates of relapse.
Given the limited efficacy of current therapies, it is reasonable to refer patients to specialized centers that offer access to experimental therapies for HDV.
6C. Coinfection With HIV
Lamivudine, emtricitabine, and tenofovir are NAs with activity against both HIV and HBV (160, 161). However, the rate of HBV resistance to lamivudine monotherapy in HBV and HIV coinfected patients reaches 90% at 4 years (162). All patients with HBV and HIV coinfection should receive ARVT that includes 2 drugs with activity against HBV: specifically, tenofovir (TAF or TDF) plus lamivudine or emtricitabine (163). In the setting of confirmed lamivudine resistance in patients already receiving ARVT therapy, adding tenofovir is generally preferred. Tenofovir alafenamide is approved for HIV in combination with emtricitabine with or without other HIV drugs and is preferred to tenofovir disoproxil fumarate because of its improved safety profile (20, 164–166).
Because entecavir has been shown to decrease serum HIV RNA levels in lamivudine-experienced and lamivudine-naïve patients and result in the selection of M184V mutation (167), entecavir should only be used in HBV and HIV coinfected patients receiving a fully suppressive antiretroviral regimen (163). Telbivudine and adefovir are not recommended (163) because adefovir has no activity against HIV and telbivudine results in the selection of M204I mutation in the YMDD motif.
Hepatitis flares may occur during the first few weeks of treatment from immune reconstitution (168) or when drugs with HBV activity are discontinued, particularly in the absence of HBeAg seroconversion. Thus, when ARVT regimens are altered, drugs that are effective against HBV should not be discontinued without substituting another drug that has activity against HBV. Elevation in ALT can also be due to hepatotoxicity of HIV drugs or HIV-related opportunistic infections (169, 170). HBV treatment should be continued indefinitely with monitoring of virologic response and adverse events.
Guidance Statements for Treatment of Patients With HBV and HIV Coinfection.
All patients with HBV and HIV coinfection should initiate ARVT, regardless of CD4 count. The ARVT regimen should include 2 drugs with activity against HBV. Specifically, the backbone of the ARVT regimen should be TDF or TAF plus lamivudine or emtricitabine.
Patients who are already receiving effective ARVT that does not include a drug with antiviral activity against HBV should have treatment changed to include TDF or TAF with emtricitabine or lamivudine. Alternatively, entecavir is reasonable if patients are receiving a fully suppressive ARVT.
When ARVT regimens are altered, drugs that are effective against HBV should not be discontinued without substituting another drug that has activity against HBV.
TDF-emtricitabine–inclusive regimens require dose adjustment if creatinine clearance is <50 mL/min, and TAF-emtricitabine–inclusive regimens are not recommended in patients with a creatinine clearance of <30 mL/min.
6D. Patients Who Receive Immunosuppressive or Cytotoxic Therapy
6D.1 Definitions for HBV Reactivation and Associated Outcomes
HBV reactivation reflects the loss of HBV immune control in HBsAg-positive, anti-HBc–positive or HBsAg-negative, anti-HBc–positive patients receiving immunosuppressive therapy for a concomitant medical condition. The criteria for HBV reactivation (171–178) include the following: (i) a rise in HBV DNA compared to baseline (or an absolute level of HBV DNA when a baseline is unavailable) and (ii) reverse seroconversion (seroreversion) from HBsAg negative to HBsAg positive for HBsAg-negative and anti-HBc–positive patients. Following HBV reactivation, a hepatitis flare demonstrated by ALT elevation can occur. Many previous studies were retrospective and thus lacked the data to fully describe the incidence of HBV-associated hepatitis, liver failure (manifested by impaired synthetic function, ascites, or encephalopathy), or liver-associated death. However, one systematic review reported liver failure rates among HBsAg-positive, anti-HBc–positive patients receiving anticancer therapy to be 13.9% (pooled estimate: range 8.6%–20.3%) (177). Because of the heterogeneity of definitions for HBV reactivation and its associated outcomes, we recommend using uniform criteria and propose coupling HBV reactivation with a hepatitis flare to define HBV-associated hepatitis. The AASLD-recommended criteria for HBV-associated hepatitis and associated clinical outcomes are as follows:
-
a
HBV-Associated Hepatitis (HBV Reactivation Plus Hepatitis Flare)
HBV reactivation in HBsAg-positive, anti-HBc–positive patients is reasonably defined as 1 of the following: (i) a ≥2 log (100-fold) increase in HBV DNA compared to the baseline level, (ii) HBV DNA ≥3 log (1,000) IU/mL in a patient with previously undetectable level (since HBV DNA levels fluctuate), or (iii) HBV DNA ≥4 log (10,000) IU/mL if the baseline level is not available. For HBsAg-negative, anti-HBc–positive patients, the following criteria are reasonable for HBV reactivation: (i) HBV DNA is detectable or (ii) reverse HBsAg seroconversion occurs (reappearance of HBsAg). A hepatitis flare is reasonably defined as an ALT increase to ≥3 times the baseline level and >100 U/L.
-
b
Clinical Outcomes of HBV-Associated Hepatitis
HBV-associated liver failure is reasonably defined as 1 of the following: (i) impaired synthetic function (total bilirubin >3 mg/dL or international normalized ratio >1.5), (ii) ascites, (iii) encephalopathy, or (iv) death following HBV-associated liver failure due to HBV reactivation.
6D.2 Screening Recommendations in the Setting of Immunosuppressive or Cytotoxic Drugs
Previous studies showed that HBV reactivation from anticancer therapies occurred in 41% to 53% (179) of HBsAg-positive, anti-HBc–positive patients and 8% to 18% (180) of HBsAg-negative, anti-HBc–positive patients. The rate of HBV reactivation from antirheumatic therapies has been reported to be 12.3% (181) in HBsAg-positive, anti-HBc–positive patients and 1.7% (182) in HBsAg-negative, anti-HBc–positive patients. As such, both the HBsAg and anti-HBc (total or immunoglobulin G) tests should be used for HBV screening. The role for anti-HBs in screening prior to immunosuppressive therapy has not yet been established. The presence of anti-HBs does not prevent HBV reactivation, but anti-HBs may be useful for detecting prior infection in HBsAg negative, anti-HBc positive patients, and in surveillance as the loss of anti-HBs may be a predictor of HBV reactivation (183–185).
In regions of the world where HBV prevalence is moderate to high, universal HBV testing prior to the initiation of immunosuppressive therapy is recommended (186, 187). In the United States, some medical centers have established universal HBV testing procedures that are aligned with the CDC recommendation (23). Among patients with cancer, HBV testing rates based on risk factors have been reported to be low (19% to 55%) (188–190), while the prevalence of HBV risk factors among patients with cancer may be high (191). This supports universal HBV testing as a reasonable option to reduce the risk of missing persons with HBV infection prior to the initiation of anticancer therapies, especially in centers where widespread, systematic, risk-based HBV testing does not occur.
6D.3 Antiviral Prophylaxis vs On-Demand Therapy
Although many immunosuppressive and immune-modulating drugs have been associated with HBV reactivation (192–194), it is difficult to discern the risk caused by specific drugs or drug regimens because of the lack of systemically collected data. HBsAg-positive patients are at high risk of HBV reactivation, especially if their HBV DNA levels are elevated (195, 196), and they should receive anti-HBV prophylaxis prior to the initiation of immunosuppressive or cytotoxic therapy, which is supported by 3 randomized controlled trials of HBsAg-positive, anti-HBc–positive patients receiving anticancer therapy (174, 179, 197).
HBsAg-negative, anti-HBc–positive patients are at lower risk of HBV reactivation than HBsAg-positive patients, and depending on their clinical situation and feasibility of close monitoring, they could be initiated on anti-HBV prophylaxis or monitored with the intent of on-demand anti-HBV therapy initiation at the first sign of HBV reactivation. HBsAg-negative, anti-HBc–positive patients with rheumatologic conditions receiving biologic therapies (198–200), inflammatory bowel disease treated with TNF inhibitors (201), and patients with psoriasis treated with biologics or conventional immunosuppressive therapies (202) were successfully monitored without anti-HBV prophylaxis. While HBsAg-negative, anti-HBc–positive lymphoma patients have been reported to have been successfully monitored with close, on-demand antiviral therapy while receiving rituximab (180, 203, 204) or conventional anticancer therapy (204) without adverse liver outcomes, we recommend that HBsAg-negative, anti-HBc–positive patients on drugs that target B lymphocytes such as rituximab be given prophylaxis.
6D.4. Preferred Antivirals and Duration of Therapy
Regardless of baseline serum HBV DNA level, prophylactic antiviral therapy should be administered to patients with CHB before (ie, most often in the literature, antivirals were given 7 days prior to) the onset of anticancer therapy or a finite course of immunosuppressive therapy (205). Because of their higher potency and high resistance barrier, prophylactic first-line NAs (eg, entecavir or tenofovir) should be preferred over other NAs, as multiple meta-analyses have demonstrated reduced reactivation, hepatitis, mortality, and anticancer therapy interruption (192, 205–207). When monitoring at-risk patients without prophylaxis, the preferred antivirals for on-demand treatment remain first-line preferred NAs, although the evidence base is far weaker (192). The most commonly studied and recommended duration of prophylactic antiviral therapy is 6 to 12 months (205) after discontinuation of anticancer therapy or immunosuppression. Reactivation beyond 12 months has been reported, so further monitoring should be considered, particularly for patients who received anti-CD20 antibody therapy (208–210). Much less is known about the optimal duration of prophylaxis in patients receiving chronic immunosuppression, eg, transplantation and biologic therapy (182, 211–214).
Guidance Statements for Patients Undergoing Immunosuppressive and Cytotoxic Therapy.
HBsAg and anti-HBc (total or immunoglobulin G) testing should be performed in all persons prior to initiation of any immunosuppressive, cytotoxic, or immunomodulatory therapy.
HBsAg-positive, anti-HBc–positive patients should initiate anti-HBV prophylaxis before immunosuppressive or cytotoxic therapy.
HBsAg-negative, anti-HBc–positive patients could be carefully monitored with ALT, HBV DNA, and HBsAg with the intent for on-demand therapy, except for patients receiving anti-CD20 antibody therapy (eg, rituximab) or undergoing stem cell transplantation, for whom anti-HBV prophylaxis is recommended.
When indicated, anti-HBV prophylaxis should be initiated as soon as possible before or, at the latest, simultaneously with the onset of immunosuppressive therapy. Once started, anti-HBV prophylaxis should continue during immunosuppressive therapy and for at least 6 months (or for at least 12 months for patients receiving anti-CD20 therapies) after completion of immunosuppressive therapy.
Anti-HBV drugs with a high resistance barrier (entecavir, TDF, or TAF) should be preferred over low-barrier agents.
For patients being monitored without prophylaxis, HBV DNA levels should be obtained every 1 to 3 months. Patients should be monitored for up to 12 months after cessation of anti-HBV therapy.
6E. Symptomatic Acute Hepatitis B Infection
Antiviral therapy is generally not necessary in patients with symptomatic acute hepatitis B because >95% of immunocompetent adults with acute hepatitis B recover spontaneously. Small case series with or without comparisons to historical untreated controls have reported that lamivudine improves survival in patients with severe infection or acute liver failure (215, 216). In the largest randomized controlled trial of lamivudine vs placebo, 71 patients with acute symptomatic acute hepatitis B were studied, with over half of the patients having severe acute hepatitis B as defined by 2 of the following 3 criteria: hepatic encephalopathy, serum bilirubin >10.0 mg/dL, or international normalized ratio >1.6 (217). Although the group treated with lamivudine had a significantly greater reduction of HBV DNA at week 4, there was no difference in the rate of biochemical improvement for all patients and in the subgroup with severe hepatitis. Nor did the rate of loss of HBsAg differ at month 12: 93.5% with lamivudine vs 96.7% with placebo. Other studies of smaller size were underpowered to assess for benefits (216, 218).
Despite the above lack of observed benefit, treating all patients with acute liver failure due to HBV using an NA may be reasonable given its safety and the ultimate need for liver transplantation in many of these patients, for whom lower HBV DNA levels are desirable to reduce the risk of recurrent hepatitis B after transplant. At the 2006 National Institutes of Health HBV Meeting, it was also proposed that patients with protracted, severe, acute hepatitis B (increase in international normalized ratio and deep jaundice persisting for >4 weeks) be treated (219). Entecavir, TAF, or TDF are preferred antivirals in this setting. IFN-α is contraindicated because of the risks of worsening hepatitis and the frequent adverse effects.
Guidance Statements for Treatment of Patients With Acute Symptomatic Hepatitis B.
Antiviral treatment is indicated for only those patients with acute hepatitis B who have acute liver failure or who have a protracted, severe course, as indicated by total bilirubin >3 mg/dL (or direct bilirubin >1.5 mg/dL), international normalized ratio > 1.5, encephalopathy, or ascites.
-
Entecavir, TDF, or TAF are the preferred antiviral drugs.
Treatment should be continued until HBsAg clearance is confirmed or indefinitely in those who undergo liver transplantation.
Peg-IFN is contraindicated.
For those diagnosed with CHB by failing to clear HBsAg after 6 to 12 months, ongoing management should follow the chronic HBV guideline (1).
6F. Treatment of Patients With Virologic Failure on NA Therapy
A major concern with long-term NA treatment is the selection of antiviral resistance mutations. The rate at which resistance variants are selected is related to the pretreatment serum HBV DNA level, rapidity of viral suppression, duration of treatment, prior exposure to NA therapies, and most importantly, the NA’s genetic barrier to drug resistance. Among the preferred NA therapies for CHB, entecavir, TDF, and TAF have very low rates of drug resistance in NA-naïve patients, and tenofovir (TDF or TAF) has very low rates of drug resistance in NA-experienced patients (17, 18, 220, 221).
Virologic breakthroughs, defined as a >1 log10 (10-fold) increase in serum HBV DNA from nadir after initial virologic response, may be related to medication nonadherence, so adherence should be ascertained before testing for genotypic resistance (222). Virologic breakthrough is usually followed by biochemical breakthrough, defined as ALT elevation during treatment in a patient who had achieved an initial biochemical response. Emergence of antiviral resistance mutations can lead to negation of the initial response and in some cases hepatitis flares and hepatic decompensation. Antiviral resistance mutations may also result in cross-resistance with other NAs, thus reducing future treatment options.
Resistance to entecavir appears to occur through a 2-hit mechanism, with initial selection of the lamivudine resistance M204V or M204I mutation followed by amino acid substitutions at rtT184, rtS202, or rtM250. In vitro studies showed that the mutations at positions 184, 202, or 250 on their own have a minimal effect on susceptibility to entecavir, but susceptibility to entecavir is decreased by 10- to 250-fold when one of these mutations accompanies a M204V or M204I mutation and by >500-fold when 2 or more of them are present with a M204V or M204I mutation. Thus, although entecavir monotherapy has a low rate of drug resistance in NA-naïve patients (approximately 1% after 5 years of treatment) (223), it has a high rate of resistance in lamivudine-refractory patients (approximately 50% after 5 years of treatment) (223). Use of entecavir at high doses (1 mg vs 0.5 mg daily) reduces the rate of resistance but is inferior to combination therapy of lamivudine plus adefovir or tenofovir monotherapy (224–229). Resistance to tenofovir (at position rtA194T) was reported in 2 patients with HBV and HIV coinfection (230), but this finding has not been confirmed by other studies. In phase III clinical trials of TDF, there was no evidence of TDF resistance among 641 NA-naïve patients who received TDF for up to 8 years, and most cases of virologic breakthrough were attributed to nonadherence (221). Similarly, in another study of 280 patients with lamivudine resistance who received TDF alone or in combination with emtricitabine for up to 240 weeks, TDF resistance was not found (231). Although long-term data on risk of resistance with TAF are lacking, no resistance has been reported in clinical trials with 2-year follow-up (6, 221).
To prevent emergence of resistance, NAs with the lowest rate of genotypic resistance should be administered and adherence reinforced in treatment-naïve patients. De novo combination therapy is unnecessary when NAs with a high barrier to resistance (entecavir, TDF, or TAF) are used. Tenofovir disoproxil fumarate monotherapy has been shown to be effective in patients with lamivudine-, adefovir-, or entecavir- resistant HBV (231–233) and is the preferred salvage therapy, particularly in patients in whom the history of prior NA therapy is unclear (Table 8). Entecavir may be used in patients with adefovir or tenofovir-resistant HBV, though confirmed cases of tenofovir resistance are notably extremely rare (Table 8). Entecavir should not be used in patients with lamivudine or telbivudine resistance, as the risk of subsequent entecavir resistance is high. In vitro studies showed that susceptibility of adefovir-resistant HBV with a single N236T, A181V/T mutation to TDF is minimally changed compared with wild-type HBV, but susceptibility is lower when both mutations are present. Clinically, most studies have found that TDF is effective in suppressing adefovir-resistant HBV without any additional benefit from emtricitabine (231–233).
Table 8.
Antiviral Options for Management of Antiviral Resistance
| Antiviral Resistance by Genotypic Testing | Switch Strategy (Preferred) | Add Strategy: 2 Drugs Without Cross-Resistance |
|---|---|---|
| Lamivudine resistance | Tenofovir* (TDF or TAF) | Continue lamivudine; add tenofovir (TDF or TAF) (or alternative emtricitabine-tenofovir) |
| Telbivudine resistance | Tenofovir* (TDF or TAF) | Continue telbivudine; add tenofovir (TDF or TAF) |
| Adefovir resistance | Entecavir or Tenofovir* (TDF or TAF) | Continue adefovir; add entecavir |
| Entecavir resistance | Tenofovir* (TDF or TAF) | Continue entecavir; add tenofovir (TDF or TAF) or alternative emtricitabine-tenofovir |
| Tenofovir resistance | Entecavir* | Continue tenofovir (TDF or TAF) and add entecavir |
| Multidrug resistance | Tenofovir | Combined tenofovir (TDF or TAF) and entecavir* |
Efficacy appears similar with switching to an antiviral with high genetic barrier to resistance and without cross-resistance versus combination therapy with follow-up periods to 5 years. Thus, switching is the preferred strategy except if HBV is multidrug resistant.
Guidance Statements for Management of Persons With Persistent Low-Level Viremia on NA Therapy (See Updated Recommendations on the Treatment of Patients with Chronic Hepatitis B, Section 6B)
6G. Decompensated Cirrhosis
Patients with decompensated cirrhosis should be referred for consideration of liver transplantation. Concurrently, antiviral therapy should be started. Antiviral therapy has been shown to improve outcomes in decompensated cirrhosis, especially with early treatment initiation (234). Both improved liver function and increased survival have been reported in recent meta-analyses (2, 234, 235). Transplant-free survival has been shown to exceed 80% in patients who have been treated (2, 235, 236), with 1 study removing 34% of treated patients from the liver transplantation waiting list (234). Survival depended on antiviral response and was significantly better in responders (234). Indefinite therapy is recommended in those with decompensated cirrhosis (1). Despite successful treatment with antivirals, this group remains at high risk for HCC and should continue long-term HCC surveillance (237–239).
Peg-IFN is contraindicated in this patient group because of safety concerns (240). Entecavir or TDF are recommended as preferred first-line agents in patients with decompensated cirrhosis (1). Both have been shown to be effective and well tolerated (241–247). Tenofovir alafenamide has not been studied in patients with decompensated cirrhosis, but use of TAF would be reasonable in patients when TDF adverse effects are a concern and entecavir is not an option. Among 112 patients with decompensated cirrhosis randomized to TDF, TDF with emtricitabine, or entecavir, the proportion with HBV DNA <69 IU/mL and normal ALT was similar at 48 weeks in all 3 groups (248). In a prospective study of 70 entecavir-treated patients with decompensated cirrhosis, the 1-year transplant-free survival was 87.1%, with improved Model for End-Stage Liver Disease and Child-Turcotte-Pugh scores (236). In a prospective study of 96 patients, TDF treatment for 24 months significantly improved hepatic function and reversed decompensation (233), and in a prospective study of 57 patients with decompensated cirrhosis treated with TDF for 12 months, 49% improved their Child-Turcotte-Pugh score by 2 points (249). In this study, confirmed 0.5-mg/dL increases in creatinine occurred in 7% of decompensated patients and 2.5% of compensated patients. In another retrospective study that included 52 patients with decompensated cirrhosis, TDF was shown to have similar renal safety to that of ETV over a 2-year period of time (250).
Despite an overall high safety profile, lactic acidosis remains a rare but serious side effect with use of any NA and is likely a higher risk in patients with decompensated cirrhosis. In a single-center series, 5 of 16 patients with decompensated cirrhosis and Model for End-Stage Liver Disease scores ≥20 developed lactic acidosis (251). One case was fatal, and the other cases resolved after discontinuing antiviral therapy. No patient with a Model for End-Stage Liver Disease score below 18 developed lactic acidosis in this study. For this reason, close monitoring of patients with decompensated cirrhosis receiving antiviral therapy is advised regardless of Model for End-Stage Liver Disease score.
Guidance Statements for Patients With Decompensated Cirrhosis (See Updated Recommendations on the Treatment of Patients With Chronic Hepatitis B, Section 7B)
6H. Liver Transplant Recipients
The prevention of HBV reinfection by using antiviral therapy pretransplant and continuing antiviral therapy with or without HBIG posttransplant has reduced the HBV reinfection rate to less than 10% (252). Antiviral therapy should be started in all patients with decompensated cirrhosis and detectable serum HBV DNA. Entecavir, TDF, and TAF are preferred antivirals because of their high potency and low rate of drug resistance. Although TAF is not FDA-approved for use in patients with decompensated cirrhosis, it is a reasonable option for patients needing tenofovir therapy (eg, patients who are lamivudine resistant) who have or are at risk for bone or renal diseases that might be complicated by the use of TDF. Therapy should be continued posttransplant indefinitely, regardless of HBeAg or HBV DNA status.
While many transplant centers use HBIG in addition to NAs during the early posttransplant period, transplant centers vary in the dose and duration of HBIG beyond the immediate posttransplant period. In patients at low risk for recurrence, either no HBIG or HBIG for only 5 to 7 days combined with NAs long-term has been highly effective (253, 254). In 42 consecutive HBsAg-positive patients with HBV DNA levels <100 IU/mL at the time of transplant, prophylaxis using HBIG (5,000 IU daily) in the anhepatic phase and for 5 days postoperatively in conjunction with long-term NA therapy prevented HBV recurrence in 97% at 3 years, with the only treatment failure being a patient with recurrent HCC (HBsAg detectable but HBV DNA undetectable) (210). The Hong Kong group has shown that HBIG-free prophylaxis using entecavir alone can prevent HBV recurrence (defined by HBsAg positivity) in the majority of patients. In 265 recipients treated with entecavir monotherapy post–liver transplant, 85%, 88%, 87%, and 92% remained HBsAg negative after 1, 3, 5, and 8 years of follow-up, respectively, and 100% maintained undetectable HBV DNA (255). Thus, 5 to 7 days of HBIG or no HBIG can be used in combination with long-term NAs as prophylaxis, but it is important to use NAs with a high barrier to resistance with long-term use.
Patients with HIV and HDV coinfection or those with questionable medication adherence warrant combination HBIG and NA therapy for prophylaxis (Table 9) because of the limited rescue therapies available if HBV recurs. Persistence of circulating HBsAg even in low concentrations may increase the risk of HDV infection. Hepatitis B virus and HIV coinfected patients frequently have intermittent low-level HBV DNA on NA therapy post–liver transplant (256), suggesting an important role for HBIG to minimize virological breakthrough. For patients maintained on HBIG, subcutaneous and intramuscular routes achieve comparable success in preventing HBV recurrence and offer a more convenient mode of HBIG administration (257, 258).
Table 9.
Factors Influencing the Choice of Prophylaxis of HBsAg-Positive Liver Transplant Recipients
| Long-term HBIG Plus Indefinite NAs | Perioperative Only or No HBIG Plus Indefinite NAs | |
|---|---|---|
| Patient Factors | Questionable adherence | Adherent High share of cost for medications |
| Virologic Factors | Presence of drug resistance or HBV DNA detectable at time of LT HIV coinfection HDV coinfection |
No drug-resistant variants Undetectable to low (<100 IU/mL) HBV DNA at time of LT Absence of HIV and HDV coinfection |
| Other | Access to HBIG Lack access to entecavir or tenofovir (TDF or TAF) |
Access to entecavir or tenofovir (TDF or TAF) |
NA: nucleos(t)ide analogues; HBIG: hepatitis B immune globulin; TDF: Tenofovir dipovoxil fumarate; TAF: tenofovir alafenamide; LT: liver transplant; HIV: human immunodeficiency virus; HDV: hepatitis D virus
For HBsAg-negative liver transplant recipients who receive a HBsAg-negative, anti-HBc–positive graft, the reported risk of HBV transmission is as high as 75% but varies with the HBV immune status of the recipient. Risk is lower for recipients who are anti-HBs-positive and highest in those without anti-HBc or anti-HBs (259). Antiviral therapy has been shown to be effective in preventing infection and should be started as soon as possible postsurgery. Hepatitis B immune globulin is not required for prophylaxis (260). Though lamivudine has been used widely because of the lower rate of replication risk (260), use of antivirals such as entecavir, TDF, or TAF would be predicted to have the lowest risk for resistance with long-term use. Tenofovir alafenamide or entecavir are preferred in patients who are at higher risk of renal disease (261).
Guidance Statements for Treatment of Liver Transplant Recipients With Hepatitis B.
-
All HBsAg-positive patients undergoing liver transplantation should receive prophylactic therapy with NAs with or without HBIG posttransplantation regardless of HBeAg status or HBV DNA level pretransplant.
HBIG monotherapy should not be used.
Entecavir, TDF, and TAF are preferred antiviral drugs because of their low rate of resistance with long-term use.
An individualized approach to use of HBIG is recommended (Table 9). HBIG for 5 to 7 days or no HBIG is reasonable in low-risk patients. Combination antiviral therapy and HBIG may be the best strategy for those at highest risk of progressive disease posttransplantation, such as HDV and HIV coinfected patients. Nonadherent patients may benefit from combination prophylaxis with HBIG plus antivirals.
All HBsAg-negative patients who receive HBsAg-negative but anti-HBc–positive grafts should receive long-term antiviral therapy to prevent viral reactivation. Although lamivudine has been used successfully in this scenario, entecavir, TDF, and TAF are preferred choices.
Prophylactic therapy should be lifelong.
6I. Nonliver Solid Organ Transplant Recipients
All patients evaluated for nonliver solid organ transplantation should be tested for HBsAg, anti-HBc, and anti-HBs. Patients who are HBsAg-positive should have ALT and HBV DNA measurements and undergo staging with biopsy or elastography to determine whether advanced fibrosis or cirrhosis is present. Though previously felt to be a contraindication, in the current era of antiviral therapies, patients with compensated cirrhosis without portal hypertension may be considered for nonhepatic solid organ transplantation, with the largest clinical experience in kidney transplantation. Patients with decompensated cirrhosis and those with compensated cirrhosis and portal hypertension should be considered for combined liver and kidney, heart, and/or lung transplantation.
Compared with non–HBV-infected recipients, untreated HBsAg-positive nonliver transplant recipients have a higher mortality rate, with liver-related complications as a major cause of death (262, 263). Antiviral therapy, however, can mitigate\this mortality risk (262, 264, 265). To effectively prevent reactivation, therapy should begin prior to or at the time of surgery, regardless of ALT and HBV DNA status, since these parameters prior to transplantation have only a limited ability to predict HBV reactivation after transplantation. Entecavir, TDF, and TAF are preferred antivirals because of the low rate of resistance with long-term use.
The subset of patients who are anti-HBc positive and HBsAg negative are at low risk of reactivation posttransplantation, although the risk likely varies with the potency of induction and subsequent immunosuppression. While there is insufficient evidence to recommend long-term antiviral therapy, a limited duration of prophylaxis for 6 to 12 months and during periods of intensified immunosuppression may be a reasonable strategy. When prophylaxis is stopped, these patients should be monitored using ALT levels every 3 months followed by HBV DNA levels if ALT rises.
HBsAg-negative nonliver transplant recipients (kidneys, lungs, heart) who receive an organ from an HBsAg-negative, anti-HBc–positive donor have a very low risk of HBV acquisition (259, 266, 267). In a systematic review of studies that included 1385 kidney recipients with organs from donors that were HBsAg-negative but anti-HBc positive, 0.3% became HBsAg positive and 2.3% became anti-HBc positive (267). The presence of anti-HBc and/or anti-HBs in the recipients is associated with protection against HBV seroconversion (268). However, to reduce this small risk of HBV infection further, antiviral therapy should be administered to prevent de novo HBV infection (269). While the optimal duration of prophylactic therapy in these nonliver transplant recipients has not been determined, a limited duration (such as 6 to 12 months) may be sufficient. Vaccination of the recipients is recommended in those with levels of anti-HBs <10 mIU/mL.
Guidance Statements for Management of Hepatitis B in Nonliver Solid Organ Transplant Recipients.
All transplant recipients of extrahepatic organs should be evaluated for HBV infection and immunity with HBsAg, anti-HBc, and anti-HBs. Patients without anti-HBs should receive hepatitis B vaccination pretransplant.
All HBsAg-positive organ transplant recipients should receive lifelong antiviral therapy to prevent or treat reactivation of HBV after transplantation.
Tenofovir (TAF, TDF) and entecavir are preferred antiviral drugs because of the low rate of resistance with long-term use.
HBsAg-negative, anti-HBc–positive nonliver recipients should be monitored for reactivation without prophylactic therapy. Alternatively, antiviral therapy for the first 6 to 12 months, the period of maximal immunosuppression, may be considered.
HBsAg-negative, anti-HBc–positive nonliver recipients who received anti-HBc–positive grafts should be monitored for HBV infection without prophylactic therapy.
Any untreated nonliver recipient undergoing monitoring for reactivation should have ALT and HBV DNA measurements every 3 months for the first year posttransplant and after receipt of T cell–depleting therapies, such as antithymocyte globulin.
Updated Recommendations on the Treatment of Patients With Chronic Hepatitis B
The 2016 HBV treatment guideline recommendations and technical remarks are reproduced here, with the new content presented in italics within the Guidance boxes. Note that rigorous systematic reviews were used to inform the quality of the evidence and the strength (Grading of Recommendations Assessment, Development, and Evaluation) of each 2016 Guideline recommendation, but the new Guidance content used a comprehensive review of the literature, including studies published after the release of the Guideline and expert opinion.
Treatment of Persons With Immune-Active Disease
1A. The AASLD recommends antiviral therapy for adults with immune-active CHB (HBeAg negative or HBeAg positive) to decrease the risk of liver-related complications
Quality and Certainty of Evidence: Moderate
Strength of Recommendation: Strong
1B. The AASLD recommends peg-IFN, entecavir, or tenofovir (TDF) as preferred initial therapy for adults with immune-active CHB
Quality and Certainty of Evidence: Low
Strength of Recommendation: Strong
Guidance: TAF is also a preferred initial therapy for adults with immune-active CHB.
Consider TAF or entecavir in patients with or at risk for renal dysfunction or bone disease.
TAF is not recommended in patients with creatinine clearance <15 mL/min or those on dialysis.
Technical Remarks
Immune-active CHB is defined by an elevation of ALT ≥2 the ULN or evidence of significant histologic disease plus elevated HBV DNA above 2,000 IU/mL (HBeAg negative) or above 20,000 IU/mL (HBeAg positive).
- Guidance: The upper limits of normal for ALT in healthy adults is reported to be 29 to 33 U/L for males and 19 to 25 U/L for females. An upper limit of normal for ALT of 35 U/L for males and 25 U/L for females is recommended to guide management decisions.
There is insufficient evidence for or against use of ALT criterion other than ALT ≥2 the ULN. The decision to treat patients with ALT above the upper limits of normal but <2 the ULN requires consideration of the severity of liver disease (defined by biopsy or noninvasive testing). Therapy is recommended for persons with immune-active CHB and cirrhosis if HBV DNA is >2,000 IU/mL, regardless of ALT level.
-
Additional factors included in the decision to treat persons with immune-active CHB but ALT <2 the ULN and HBV DNA below thresholds (≤2,000 IU/mL if HBeAg negative or ≤20,000 IU/mL if HBeAg positive) are as follows:
Age: older age (>40 years) is associated with a higher likelihood of significant histologic disease.
Family history of cirrhosis or HCC.
-
Previous treatment history.
Serologic and virologic benefits of peg-IFN occur after treatment discontinuation (delayed).
Prior NA exposure is a risk for drug resistance.
Presence of extrahepatic manifestations: indication for treatment independent of liver disease severity.
Presence of cirrhosis.
The level of HBV DNA should be compatible with immune-active disease and the cutoffs recommended should be viewed as a sufficient but not absolute requirement for treatment.
-
Head-to-head comparisons of antiviral therapies fail to show superiority of one therapy over another in achieving risk reduction in liver-related complications. However, in recommending peg-IFN, tenofovir, and entecavir as preferred therapies, the most important factor considered was the lack resistance with long-term use. Patient-specific factors that need to be considered in choosing between peg-IFN, entecavir, and tenofovir include the following:
Desire for finite therapy (see below).
Anticipated tolerability of treatment side effects.
Comorbidities: peg-IFN is contraindicated in persons with autoimmune disease, uncontrolled psychiatric disease, cytopenia, severe cardiac disease, uncontrolled seizures, and decompensated cirrhosis.
Previous history of lamivudine resistance (entecavir is not preferred in this setting).
Family planning: finite therapy with peg-FN prepregnancy or use of an oral antiviral agent that is safe in pregnancy (preferably TDF) is best.
HBV genotype: A and B genotypes are more likely to achieve HBeAg and HBsAg loss with peg-IFN than non-A or non-B genotypes.
Medication costs.
Peg-IFN is preferred over nonpegylated forms for simplicity.
For patients treated with peg-IFN, 48 weeks’ duration is used in most studies and is preferred. This treatment duration yields HBeAg seroconversion rates of 20% to 31% and sustained off-treatment HBV DNA suppression of <2,000 IU/mL in 65% of persons who achieve HBeAg to anti-HBe seroconversion. The combination of peg-IFN and NAs has not yielded higher rates of off-treatment serological or virological responses and is not recommended.
Duration of therapy for NA-based therapy is variable and influenced by HBeAg status, duration of HBV DNA suppression, and presence of cirrhosis and/or decompensation. All NAs except TAF require dose adjustment in persons with creatinine clearance <50 mL/min.
Evaluation for cirrhosis using noninvasive methods or a liver biopsy is useful to guide treatment decisions, including duration of therapy.
Treatment with antivirals does not eliminate the risk of HCC, and surveillance for HCC should continue in persons who are at risk.
Treatment of Immune-Tolerant Adults With Chronic Hepatitis B
2A. The AASLD recommends against antiviral therapy for adults with immune-tolerant CHB
Quality and Certainty of Evidence: Moderate
Strength of Recommendation: Strong
Technical Remarks
Guidance: Immune-tolerant status should be defined by ALT levels, utilizing 35 U/L for men and 25 U/L for women as ULN rather than local laboratory ULN.
2B. The AASLD suggests that ALT levels be tested at least every 6 months for adults with immune tolerant CHB to monitor for potential transition to immune-active or immune-inactive CHB
Quality and Certainty of Evidence: Very Low
Strength of Recommendation: Conditional
2C. The AASLD suggests antiviral therapy in the select group of adults >40 years of age with normal ALT and elevated HBV DNA (1,000,000 IU/mL) and liver biopsy specimen showing significant necroinflammation or fibrosis
Quality and Certainty of Evidence: Very Low
Strength of Recommendation: Conditional
Technical Remark
Moderate-to-severe necroinflammation or fibrosis on a liver biopsy specimen is a reason to consider initiation of antiviral therapy if other causes of liver disease are excluded.
Treatment of HBeAg-Positive, Immune-Active Persons With Chronic Hepatitis Who Seroconvert to Anti-HBe on NA Therapy
3A. The AASLD suggests that HBeAg-positive adults without cirrhosis but with CHB who seroconvert to anti-HBe on therapy discontinue NAs after a period of treatment consolidation
Quality and Certainty of Evidence: Very Low
Strength of Recommendation: Conditional
Technical Remarks
The period of consolidation therapy generally involves treatment of persistently normal ALT levels and undetectable serum HBV DNA levels for at least 12 months.
It is not currently known whether a longer duration of consolidation would further reduce rates of virological relapse. Thus, an alternative approach is to treat until HBsAg loss.
Decisions regarding treatment duration and length of consolidation before treatment discontinuation require careful consideration of risks and benefits for health outcomes, including the following: (i) risk for virological relapse, hepatic decompensation, liver cancer, and death; (ii) burden of continued antiviral therapy, financial concerns associated with medication costs and long-term monitoring, adherence, and potential for drug resistance with treatment interruptions; and (iii) patient and provider preferences. These considerations apply for both HBeAg-positive adults without and with cirrhosis who seroconvert to anti-HBe on therapy.
Persons who stop antiviral therapy should be monitored every 3 months for at least 1 year for recurrent viremia, ALT flares, seroconversion, and clinical decompensation.
3B. The AASLD suggests indefinite antiviral therapy for HBeAg-positive adults with cirrhosis with CHB who seroconvert to anti-HBe on NA therapy, based on concerns for potential clinical decompensation and death, unless there is a strong competing rationale for treatment discontinuation
Quality and Certainty of Evidence: Very Low
Strength of Recommendation: Conditional
Technical Remarks
Persons with cirrhosis who stop antiviral therapy should be monitored closely (eg, monthly for the first 6 months, then every 3 months) for recurrent viremia, ALT flares, seroreversion, and clinical decompensation.
Treatment discontinuation may be considered in persons who have demonstrated loss of HBsAg. However, there is currently insufficient evidence to definitively guide treatment decisions for such persons.
Duration of Treatment in Persons With HBeAg-Negative, Immune-Active CHB
4. The AASLD suggests indefinite antiviral therapy for adults with HBeAg-negative, immune-active CHB unless there is a compelling rationale for treatment discontinuation
Quality and Certainty of Evidence: Low
Strength of Recommendation: Conditional
Technical Remarks
A decision to discontinue therapy for HBeAg-negative adults without cirrhosis requires careful consideration of risks and benefits for health outcomes, including the following: (i) risk for virological relapse, hepatic decompensation, liver cancer, and death; (ii) burden of continued antiviral therapy, financial concerns associated with medication costs and long-term monitoring, adherence, and potential for drug resistance with treatment interruptions; and (iii) patient and provider preferences.
Treatment discontinuation in persons with cirrhosis is not recommended owing to the potential for decompensation and death, although data are limited.
Treatment discontinuation may be considered in persons who have demonstrated loss of HBsAg. However, there is currently insufficient evidence to definitively guide treatment decisions for such persons.
Persons who stop antiviral therapy should be monitored every 3 months for at least 1 year for recurrent viremia, ALT flares, and clinical decompensation.
Antiviral therapy is not recommended for persons without cirrhosis who are HBeAg negative with normal ALT activity and low-level viremia (<2,000 U/mL; “inactive chronic hepatitis B”).
Renal and Bone Disease in Persons on NA Therapy
Quality and Certainty of Evidence: Very Low (Bone); Low (Renal)
Strength of Recommendation: Conditional
5. The AASLD suggest no preference between entecavir or tenofovir (TDF) regarding potential long-term risks of renal and bone complications
Guidance: TAF is associated with lower rates of bone and renal abnormalities than TDF.
Technical Remarks
The existing studies do not show significant differences in renal dysfunction, hypophosphatemia, or bone mineral density between HBV-infected persons treated with tenofovir (TDF) or entecavir. However, renal events, such as acute renal failure or hypophosphatemia, have been reported in TDF-treated persons.
In persons on TDF, renal safety monitoring with serum creatinine, phosphorus, urine glucose, and urine protein should be assessed before treatment initiation and periodically thereafter (eg, at least annually and more frequently if the patient is at high risk for renal dysfunction or has a preexisting renal dysfunction).
In the absence of other risk factors for osteoporosis or osteomalacia, there is insufficient evidence for or against monitoring of bone mineral density in HBV-infected persons on TDF.
- Guidance: In cases of suspected TDF-associated renal dysfunction and/or bone disease, TDF should be discontinued and substituted with TAF or entecavir, with consideration for any previously known drug resistance.
Dosage of NAs should be adjusted based on renal function and creatinine clearance, as recommended by manufacturers.
Management of Persons With Persistent Low-Level Viremia on NA Therapy
6A. The AASLD suggests that persons with persistent low-level viremia (<2,000 IU/mL) on entecavir or tenofovir monotherapy continue monotherapy, regardless of ALT
Quality and Certainty of Evidence: Very Low
Strength of Recommendation: Conditional
Guidance: Persons on TAF with persistent low-level viremia (<2,000 IU/mL) should continue monotherapy, regardless of ALT.
6B. The AASLD suggests 1 of 2 strategies in persons with virological breakthrough on entecavir or tenofovir monotherapy: either switch to another antiviral monotherapy with a high barrier to resistance or add a second antiviral drug that lacks cross-resistance (Table 8)
Quality and Certainty of Evidence: Very Low
Strength of Recommendation: Conditional
Technical Remarks
Counseling patients about medication adherence is important, especially in those with persistent viremia on antiviral therapy.
-
Persistent viremia has traditionally been defined as detectable HBV DNA after 48 weeks of treatment. This time point was defined by outcomes of virological response in clinical trials and reflects an era of antiviral therapy with drugs of lower antiviral potency and higher rates of resistance.
Guidance: With the current preferred therapies of entecavir, TDF, and TAF, persistent viremia is defined as a plateau in the decline of HBV DNA and/or failure to achieve an undetectable HBV DNA level after 96 weeks of therapy. There is insufficient comparative evidence to advocate for adding a second drug or switching to another drug in lieu of continuing monotherapy.Resistance testing in this setting may not be technically possible if the viral level is low. Medical providers should ensure patient adherence to therapy.
-
Viral breakthrough is defined by an increase in HBV DNA by >1 log compared to nadir or an HBV DNA level of 100 IU/mL or higher in persons on NA therapy with a previously undetectable level (<10 IU/mL). Confirmatory testing should be obtained before making a therapy change. Resistance testing may assist with decisions regarding subsequent therapy. A confirmed virological breakthrough constitutes a rationale for switching to another antiviral monotherapy with a high genetic barrier to resistance or adding a second antiviral with a complementary resistance profile (Table 8).
Guidance:- For patients on entecavir with virological breakthrough, change to or add TDF or TAF.
- For patients on TDF or TAF with virological breakthrough, changing to or adding entecavir is preferred, depending upon prior NA experience.
There is insufficient long-term comparative evidence to advocate one approach over another. Based upon virological principles, the risk of viral resistance is predicted to be lower with combination antiviral therapy compared with monotherapy. Comparative evidence with follow-up to 5 years suggests monotherapy achieves rates of HBV DNA suppression comparable to those of combination therapy when antivirals such as tenofovir are used (231, 232).
Although the optimal frequency of HBV DNA monitoring has not been fully evaluated, monitoring of HBV DNA levels every 3 months until HBV DNA is undetectable and then every 3 to 6 months thereafter allows for detection of persistent viremia and virological breakthrough.
-
For persons on treatment with NAs other than tenofovir or entecavir, viral breakthrough warrants a switch to another antiviral monotherapy with a high genetic barrier to resistance or the addition of a second antiviral with a complementary resistance profile (Table 8).
Guidance.
- For patients on the nonpreferred antivirals lamivudine or telbivudine who develop virologic breakthrough, change to or add TAF or TDF.
- For patients on the nonpreferred antiviral adefovir who develop virologic breakthrough, change to or add entecavir, TAF, TDF.
Management of Adults With Cirrhosis and Low-Level Viremia
7A. The AASLD suggests that adults with compensated cirrhosis and low level viremia (<2,000 IU/mL) be treated with antiviral therapy to reduce the risk of decompensation, regardless of ALT level
Quality and Certainty of Evidence: Very Low
Strength of Recommendation: Conditional
Technical Remarks
-
Tenofovir and entecavir are preferred because of their potency and minimal risk of resistance, decompensation, and serious side effects. Antivirals with a low genetic barrier to resistance are not recommended because the emergence of resistance can lead to decompensation.
Guidance: TAF should be considered an additional preferred antiviral drug. Peg-IFN is not contraindicated in persons with compensated cirrhosis, but NAs are safer.
If treatment is not offered to persons with compensated cirrhosis and low levels of viremia, they must be closely monitored (every 3–6 months) for a rise in HBV DNA and/or clinical decompensation. Treatment should be initiated if either occurs.
The ALT level in these persons is typically normal or less than 2 times the ULN. Higher ALT levels (>2 times the ULN) warrant consideration of other causes for ALT elevation and, if none are found, they are a stronger indication for antiviral therapy.
Current evidence does not provide an optimal length of treatment. If therapy were discontinued, close monitoring (at least every 3 months for at least 1 year) would allow for early detection of viral rebound that could lead to decompensation.
Persons with compensated cirrhosis and high HBV DNA level (>2,000 U/mL) are treated per recommendations for HBeAg-positive and HBeAg-negative immune-active CHB (recommendation 1A and 1B).
Treatment with antivirals does not eliminate the risk of HCC and surveillance for HCC should continue.
7B. The AASLD recommends that HBsAg-positive adults with decompensated cirrhosis be treated with antiviral therapy indefinitely regardless of HBV DNA level, HBeAg status, or ALT level to decrease risk of worsening liver-related complications
Quality and Certainty of Evidence: Moderate
Strength of Recommendation: Strong
Technical Remarks
-
Entecavir and tenofovir (TDF) are recommended drugs.
Guidance: TAF has not been studied in patients with decompensated cirrhosis, thus limiting recommendations to use TAF in these patients. However, TAF or entecavir should be considered in patients with decompensated cirrhosis who have renal dysfunction and/or bone disease. Peg-IFN is contraindicated in patients with decompensated cirrhosis because of safety concerns.
Concurrent consideration for liver transplantation is indicated in eligible persons.
Patients should be monitored closely for the development of adverse effects of antiviral therapy, such as renal insufficiency and lactic acidosis.
Treatment with antivirals does not eliminate the risk of HCC and surveillance for HCC should continue.
Management of Chronic Hepatitis B in Pregnancy
8A. The AASLD suggests antiviral therapy to reduce the risk of perinatal transmission of HBV in HBsAg-positive pregnant women with an HBV DNA level >200,000 IU/mL
Quality and Certainty of Evidence: Low
Strength of Recommendation: Conditional
Technical Remarks
The infants of all HBsAg-positive women should receive immunoprophylaxis (HBV vaccination with or without hepatitis B immunoglobulin, per World Heath Organization and Centers for Disease Control and Prevention recommendations).
-
The only antivirals studied in pregnant women are lamivudine, telbivudine, and TDF. Of these 3 options, TDF is preferred to minimize the risk of emergence of viral resistance during treatment. Interim studies show high efficacy of TDF in preventing mother-to-child transmission.
Guidance: TAF has not been studied in pregnant women and no data have been reported to the antiretroviral registry regarding the safety of TAF in pregnancy. Thus, there are insufficient data to recommend use of TAF in pregnancy. Antiviral therapy was started at 28 to 32 weeks of gestation in most of the studies.
Antiviral therapy was discontinued at birth to 3 months postpartum in most of the studies. With discontinuation of treatment, women should be monitored for ALT flares every 3 months for 6 months.
There are limited data on the level of HBV DNA for which antiviral therapy is routinely recommended. The level of >200,000 IU/mL is a conservative recommendation.
For pregnant women with immune-active hepatitis, treatment should be based on recommendations for nonpregnant women.
Breastfeeding is not contraindicated. These antivirals are minimally excreted in breast milk and are unlikely to cause significant toxicity. The unknown risk of low-level exposure to the infant should be discussed with mothers.
There are insufficient long-term safety data in infants born to mothers who took antiviral agents during pregnancy and while breastfeeding.
C-section is not indicated owing to insufficient data to support its benefit.
8B. The AASLD recommends against the use of antiviral therapy to reduce the risk of perinatal transmission of HBV in HBsAg-positive pregnant women with an HBV DNA level ≤200,000 IU/mL
Quality and Certainty of Evidence: Low
Strength of Recommendation: Strong
Treatment of CHB in Children
9A. The AASLD suggests antiviral therapy in HBeAg-positive children (ages 2 to <18 years) with both elevated ALT and measurable HBV DNA levels, with the goal of achieving sustained HBeAg seroconversion
Quality and Certainty of Evidence: Moderate
Strength of Recommendation: Conditional
Technical Remarks
-
Most studies required ALT elevation (>1.3 times the ULN) for at least 6 months with HBV DNA elevations for inclusion. Given that HBV DNA levels are typically very high during childhood (>106 IU/mL), there is no basis for a recommendation for a lower limit value with respect to treatment. However, if a level <104 IU/mL is observed, therapy might be deferred until other causes of liver disease and spontaneous HBeAg seroconversion are excluded.
Guidance: The upper limits of normal for ALT in healthy children are not firmly established and appear to vary not only by sex but age, pubertal stage, and BMI (270). Reports suggest cutoff values from 22 to 31 U/L for girls and 25 to 38 U/L for boys after infancy (271–273), although not all studies carefully excluded overweight children. For CHB purposes and for consistency with recommendations in adults, an upper limit of normal for ALT of 35 U/L for males and 25 U/L for females is suggested to guide management decisions. IFN-α-2b is approved for children 1 year of age and older, whereas lamivudine and entecavir are approved for children 2 years of age and older. Peg-IFN-α-2a (180 mcg/1.73 m2 body surface area to maximum 180 mcg once weekly) is not approved for children with CHB but is approved for treatment of chronic hepatitis C for children 5 years of age or older. Providers may consider using this drug for children with chronic HBV.
Treatment with entecavir is associated with a lower risk of viral resistance compared with lamivudine.
-
TDF is approved for children 12 years of age and older.
Guidance: TAF has not been studied in children. Thus, there are insufficient data to recommend use of TAF in children 12 years of age and older. Duration of treatment with IFN-α-2b is 24 weeks.
The duration of treatment with oral antivirals that has been studied is 1 to 4 years. It may be prudent to use HBeAg seroconversion as a therapeutic endpoint when oral antivirals are used and continue treatment for an additional 12 months of consolidation, as recommended in adults. It is currently unknown whether a longer duration of consolidation would reduce rates of virological relapse.
Children who stop antiviral therapy should be monitored every 3 months for at least 1 year for recurrent viremia, ALT flares, and clinical decompensation.
9B. The AASLD recommends against the use of antiviral therapy in HBeAg-positive children (ages 2 to <18 years) with persistently normal ALT, regardless of HBV DNA level
Quality and Certainty of Evidence: Very Low
Strength of Recommendation: Strong
Technical Remarks
-
Normal ALT in children has not been clearly defined.
Guidance: An upper limit of normal for ALT of 35 U/L for males and 25 U/L for females is suggested to guide management decisions. Although some studies of IFN included children with normal ALT values, studies of oral antiviral agents did not include children with normal ALT values.
Acknowledgments
This Practice Guidance was developed under the direction of the AASLD Practice Guidelines Committee, which approved the scope of the guidance and provided the peer review. Members of the Committee include Tram T. Tran, MD, FAASLD (Chair), Michael W. Fried, MD, FAASLD (Board Liaison), Joseph Ahn, MD, Alfred Sidney Barritt IV, MD, MSCR, James R Burton, Jr., MD, Udeme Ekong, MD, MD, George Ioannou, MD, FAASLD, Whitney E. Jackson, MD, Patrick S Kamath, MD, David G Koch, MD, Raphael B. Merriman, MD, FACP, FRCPI, David J. Reich, MD, FACS, Amit G. Singal, MD, (Vice-Chair), James R. Spivey, MD, Helen S. Te, MD, FAASLD, and Michael Volk, MD.
FUNDING
The funding for the development of this Practice Guidance was provided by the American Association for the Study of Liver Diseases.
Abbreviations
- AFP
alpha-fetoprotein
- ALT
alanine transaminase
- anti-HBc
antibody to hepatitis B core antigen
- anti-HBe
antibody to hepatitis B e antigen
- anti-HBs
antibody to hepatitis B surface antigen
- ARVT
antiretroviral therapy
- CHB
chronic hepatitis B
- DAA
direct-acting antiviral
- HBeAg
hepatitis B e antigen
- HBIG
hepatitis B immune globulin
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HDV
hepatitis D virus
- HIV
human immunodeficiency virus
- IFN
interferon
- IFN-α
interferon-alfa
- NA
nucleos(t)ide analogue
- peg-IFN
peginterferon
- peg-IFN-α
pegylated interferon-alfa
- qHBsAg
quantitative hepatitis B surface antigen
- TAF
tenofovir alafenamide
- TDF
tenofovir disoproxil fumarate
- ULN
upper limits of normal
- US
ultrasonography
Footnotes
AASLD APPROVAL
This practice guidance was approved by the American Association for the Study of Liver Diseases on December 4, 2017.
Contributor Information
NA Terrault, Division of Gastroenterology/Hepatology, University of California San Francisco, San Francisco, CA.
AS Lok, Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, MI.
BJ McMahon, Liver Diseases and Hepatitis Program, Alaska NativeTribal Health Consortium, Anchorage, AK.
KM Chang, Division of Gastroenterology, Corporal Michael J. Crescenz VA Medical Center & University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
JP Hwang, Department of General Internal Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX.
MM Jonas, Division of Gastroenterology, Hepatology and Nutrition, Boston Children’s Hospital, Boston, MA.
RS Brown, Jr, Division of Gastroenterology and Hepatology, Weill Cornell Medical College, New York, NY.
NH Bzowej, Ochsner Medical Center, New Orleans, LA.
JB Wong, Division of Clinical Decision Making, Tufts Medical Center, Tufts University School of Medicine, Boston, MA.
References
- 1.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–283. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lok AS, McMahon BJ, Brown RS, Jr, Wong JB, Ahmed AT, Farah W, Almasri J, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology. 2016;63:284–306. doi: 10.1002/hep.28280. [DOI] [PubMed] [Google Scholar]
- 3.Jonas MM, Lok AS, McMahon BJ, Brown RS, Jr, Wong JB, Ahmed AT, Farah W, et al. Antiviral therapy in management of chronic hepatitis B viral infection in children: A systematic review and meta-analysis. Hepatology. 2016;63:307–318. doi: 10.1002/hep.28278. [DOI] [PubMed] [Google Scholar]
- 4.Brown RS, Jr, McMahon BJ, Lok AS, Wong JB, Ahmed AT, Mouchli MA, Wang Z, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: A systematic review and meta-analysis. Hepatology. 2016;63:319–333. doi: 10.1002/hep.28302. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. 2015 [PubMed] [Google Scholar]
- 6.Agarwal K, Fung S, Seto WK, Lim Y, Gane E, Janssen H, Sharma M, et al. A phase 3 study comparing tenofovir alafenamide (TAF) to tenofovir disoproxil fumarate (TDF) in patients with HBeAg-positive, chronic hepatitis B: efficacy and safety results at week 96. Journal of Hepatology. 2017;66(suppl 1):S478. [Google Scholar]
- 7.Brunetto M, Lim YS, Gane E, Seto WK, Osipenko M, Ahn SH, Janssen HS, et al. A Phase 3 Study Comparing Tenofovir Alafenamide (TAF) to Tenofovir Disoproxil Fumarate (TDF) in Patients With HBeAg-Negative, Chronic Hepatitis B (CHB): Efficacy and Safety Results at Week 96. Journal of Hepatology. 2017;152(suppl 1):S1086. [Google Scholar]
- 8.Heathcote EJ, Marcellin P, Buti M, Gane E, De Man RA, Krastev Z, Germanidis G, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140:132–143. doi: 10.1053/j.gastro.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 10.Liaw YF, Jia JD, Chan HL, Han KH, Tanwandee T, Chuang WL, Tan DM, et al. Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology. 2011;54:1591–1599. doi: 10.1002/hep.24555. [DOI] [PubMed] [Google Scholar]
- 11.Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, Simon C, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123–129. doi: 10.1016/S0140-6736(05)17701-0. [DOI] [PubMed] [Google Scholar]
- 12.Buster EH, Flink HJ, Cakaloglu Y, Simon K, Trojan J, Tabak F, So TM, et al. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology. 2008;135:459–467. doi: 10.1053/j.gastro.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 13.Lok AS, Trinh H, Carosi G, Akarca US, Gadano A, Habersetzer F, Sievert W, et al. Efficacy of entecavir with or without tenofovir disoproxil fumarate for nucleos(t)ide-naive patients with chronic hepatitis B. Gastroenterology. 2012;143:619–628. e611. doi: 10.1053/j.gastro.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 14.Gish RG, Lok AS, Chang TT, de Man RA, Gadano A, Sollano J, Han KH, et al. Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2007;133:1437–1444. doi: 10.1053/j.gastro.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–2455. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 16.Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 17.Chan HL, Fung S, Seto WK, Chuang WL, Chen CY, Kim HJ, Hui AJ, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:185–195. doi: 10.1016/S2468-1253(16)30024-3. [DOI] [PubMed] [Google Scholar]
- 18.Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, Hui AJ, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:196–206. doi: 10.1016/S2468-1253(16)30107-8. [DOI] [PubMed] [Google Scholar]
- 19.Arribas JR, Thompson M, Sax PE, Haas B, McDonald C, Wohl DA, DeJesus E, et al. Brief Report: Randomized, Double-Blind Comparison of Tenofovir Alafenamide (TAF) vs Tenofovir Disoproxil Fumarate (TDF), Each Coformulated With Elvitegravir, Cobicistat, and Emtricitabine (E/C/F) for Initial HIV-1 Treatment: Week 144 Results. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2017;75:211–218. doi: 10.1097/QAI.0000000000001350. [DOI] [PubMed] [Google Scholar]
- 20.Raffi F, Orkin C, Clarke A, Slama L, Gallant J, Daar E, Henry K, et al. Long-term (96-week) Efficacy and Safety After Switching from Tenofovir Disoproxil Fumarate (TDF) to Tenofovir Alafenamide (TAF) in HIV-infected, Virologically Suppressed Adults. J Acquir Immune Defic Syndr. 2017 doi: 10.1097/QAI.0000000000001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 22.Nelson NP, Easterbrook PJ, McMahon BJ. Epidemiology of Hepatitis B Virus Infection and Impact of Vaccination on Disease. Clin Liver Dis. 2016;20:607–628. doi: 10.1016/j.cld.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinbaum CM, IW, Mast EE, Wang SA, Finelli L, Wasley A, Neitzel SM, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57(RR-8):1–20. [PubMed] [Google Scholar]
- 24.Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, Moyer LA, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54:1–31. [PubMed] [Google Scholar]
- 25.Petersen NJ, Barrett DH, Bond WW, Berquist KR, Favero MS, Bender TR, Maynard JE. Hepatitis B surface antigen in saliva, impetiginous lesions, and the environment in two remote Alaskan villages. Appl Environ Microbiol. 1976;32:572–574. doi: 10.1128/aem.32.4.572-574.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghany MG, Perrillo R, Li R, Belle SH, Janssen HL, Terrault NA, Shuhart MC, et al. Characteristics of adults in the hepatitis B research network in North America reflect their country of origin and hepatitis B virus genotype. Clin Gastroenterol Hepatol. 2015;13:183–192. doi: 10.1016/j.cgh.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarz KB, Cloonan YK, Ling SC, Murray KF, Rodriguez-Baez N, Schwarzenberg SJ, Teckman J, et al. Children with Chronic Hepatitis B in the United States and Canada. J Pediatr. 2015;167:1287–1294. e1282. doi: 10.1016/j.jpeds.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bond WW, Favero MS, Petersen NJ, Gravelle CR, Ebert JW, Maynard JE. Survival of hepatitis B virus after drying and storage for one week. Lancet. 1981;1:550–551. doi: 10.1016/s0140-6736(81)92877-4. [DOI] [PubMed] [Google Scholar]
- 29.Beasley RP, Huang LY. Postnatal infectivity of hepatitis B surface antigen-carrier mothers. J Infect Dis. 1983;147:185–190. doi: 10.1093/infdis/147.2.185. [DOI] [PubMed] [Google Scholar]
- 30.Beasley RP, Hwang LY, Lin CC, Leu ML, Stevens CE, Szmuness W, Chen KP. Incidence of hepatitis B virus infections in preschool children in Taiwan. J Infect Dis. 1982;146:198–204. doi: 10.1093/infdis/146.2.198. [DOI] [PubMed] [Google Scholar]
- 31.Coursaget P, Yvonnet B, Chotard J, Vincelot P, Sarr M, Diouf C, Chiron JP, et al. Age- and sex-related study of hepatitis B virus chronic carrier state in infants from an endemic area (Senegal) J Med Virol. 1987;22:1–5. doi: 10.1002/jmv.1890220102. [DOI] [PubMed] [Google Scholar]
- 32.McMahon BJ, Alward WL, Hall DB, Heyward WL, Bender TR, Francis DP, Maynard JE. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985;151:599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- 33.Tassopoulos NC, Papaevangelou GJ, Roumeliotou-Karayannis A, Ticehurst JR, Feinstone SM, Purcell RH. Detection of hepatitis B virus DNA in asymptomatic hepatitis B surface antigen carriers: relation to sexual transmission. American Journal of Epidemiology. 1987;126:587–591. doi: 10.1093/oxfordjournals.aje.a114698. [DOI] [PubMed] [Google Scholar]
- 34.Bodsworth N, Cooper D, Donovan B. The influence of human immunodeficiency virus type 1 infection on the development of the hepatitis B virus carrier state. J Infect Dis. 1991;163:1138–1140. doi: 10.1093/infdis/163.5.1138. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease C, Prevention. Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2011;60:1709–1711. [PubMed] [Google Scholar]
- 36.Division of Viral Hepatitis and National Center for HIV/AIDS Viral Hepatitis STD and TB Prevention. Hepatitis B FAQs for Health Professionals. Atlanta, GA: Centers for Disease Control and Prevention; 2016. [Google Scholar]
- 37.Raimondo G, Pollicino T, Cacciola I, Squadrito G. Occult hepatitis B virus infection. Journal of Hepatology. 2007;46:160–170. doi: 10.1016/j.jhep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 39.Gounder PP, Bulkow LR, McMahon BJ. Letter: hepatitis B surface seroclearance does reduce the risk of hepatocellular carcinoma - authors’ reply. Aliment Pharmacol Ther. 2016;44:650–651. doi: 10.1111/apt.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Yang HI, Lee MH, Lu SN, Jen CL, Batrla-Utermann R, Wang LY, et al. Spontaneous seroclearance of hepatitis B seromarkers and subsequent risk of hepatocellular carcinoma. Gut. 2014;63:1648–1657. doi: 10.1136/gutjnl-2013-305785. [DOI] [PubMed] [Google Scholar]
- 41.Nathanson MH, Terrault N. Hepatitis B surface antigen loss: Not all that we hoped it would be. Hepatology. 2016;64:328–329. doi: 10.1002/hep.28640. [DOI] [PubMed] [Google Scholar]
- 42.Gandhi RT, Wurcel A, McGovern B, Lee H, Shopis J, Corcoran CP, Toner S, et al. Low prevalence of ongoing hepatitis B viremia in HIV-positive individuals with isolated antibody to hepatitis B core antigen. J Acquir Immune Defic Syndr. 2003;34:439–441. doi: 10.1097/00126334-200312010-00013. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda K, Marusawa H, Osaki Y, Nakamura T, Kitajima N, Yamashita Y, Kudo M, et al. Antibody to hepatitis B core antigen and risk for hepatitis C-related hepatocellular carcinoma: a prospective study. Ann Intern Med. 2007;146:649–656. doi: 10.7326/0003-4819-146-9-200705010-00008. [DOI] [PubMed] [Google Scholar]
- 44.Lok AS, Lai CL, Wu PC. Prevalence of isolated antibody to hepatitis B core antigen in an area endemic for hepatitis B virus infection: implications in hepatitis B vaccination programs. Hepatology. 1988;8:766–770. doi: 10.1002/hep.1840080411. [DOI] [PubMed] [Google Scholar]
- 45.McMahon BJ, Parkinson AJ, Helminiak C, Wainwright RB, Bulkow L, Kellerman-Douglas A, Schoenberg S, et al. Response to hepatitis B vaccine of persons positive for antibody to hepatitis B core antigen. Gastroenterology. 1992;103:590–594. doi: 10.1016/0016-5085(92)90851-o. [DOI] [PubMed] [Google Scholar]
- 46.Abbot Laboratories. Hepatitis B Virus Core Antigen (E. coli, Recombinant) Food and Drug Administration; 2002. [Google Scholar]
- 47.U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research. Guidance for Industry. Rockville, MD: Office of Communication, Outreach and Development; 2010. Requalification Method for Reentry of Blood Donors Deferred Because of Reactive Test Results for Antibody to Hepatitis B Core Antigen (Anti-HBc) [Google Scholar]
- 48.Paul S, Dickstein A, Saxena A, Terrin N, Viveiros K, Balk EM, Wong JB. Role of surface antibody in hepatitis B reactivation in patients with resolved infection and hematologic malignancy: A meta-analysis. Hepatology. 2017;66:379–388. doi: 10.1002/hep.29082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lok AS, Lai CL, Wu PC, Leung EK. Long-term follow-up in a randomised controlled trial of recombinant alpha 2-interferon in Chinese patients with chronic hepatitis B infection. Lancet. 1988;2:298–302. doi: 10.1016/s0140-6736(88)92355-0. [DOI] [PubMed] [Google Scholar]
- 50.Gandhi RT, Wurcel A, Lee H, McGovern B, Shopis J, Geary M, Sivamurthy R, et al. Response to hepatitis B vaccine in HIV-1-positive subjects who test positive for isolated antibody to hepatitis B core antigen: implications for hepatitis B vaccine strategies. Journal of Infectious Diseases. 2005;191:1435–1441. doi: 10.1086/429302. [DOI] [PubMed] [Google Scholar]
- 51.Piroth L, Launay O, Michel ML, Bourredjem A, Miailhes P, Ajana F, Chirouze C, et al. Vaccination Against Hepatitis B Virus (HBV) in HIV-1-Infected Patients With Isolated Anti-HBV Core Antibody: The ANRS HB EP03 CISOVAC Prospective Study. Journal of Infectious Diseases. 2016;213:1735–1742. doi: 10.1093/infdis/jiw011. [DOI] [PubMed] [Google Scholar]
- 52.Onozawa M, Hashino S, Darmanin S, Okada K, Morita R, Takahata M, Shigematsu A, et al. HB vaccination in the prevention of viral reactivation in allogeneic hematopoietic stem cell transplantation recipients with previous HBV infection. Biology of Blood & Marrow Transplantation. 14:1226–1230. doi: 10.1016/j.bbmt.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Takahata M, Hashino S, Onozawa M, Shigematsu A, Sugita J, Fujimoto K, Endo T, et al. Hepatitis B virus (HBV) reverse seroconversion (RS) can be prevented even in non-responders to hepatitis B vaccine after allogeneic stem cell transplantation: long-term analysis of intervention in RS with vaccine for patients with previous HBV infection. Transplant Infectious Disease. 2014;16:797–801. doi: 10.1111/tid.12283. [DOI] [PubMed] [Google Scholar]
- 54.Wong GL, Chan HL, Yu Z, Chan AW, Choi PC, Chim AM, Chan HY, et al. Coincidental metabolic syndrome increases the risk of liver fibrosis progression in patients with chronic hepatitis B--a prospective cohort study with paired transient elastography examinations. Aliment Pharmacol Ther. 2014;39:883–893. doi: 10.1111/apt.12658. [DOI] [PubMed] [Google Scholar]
- 55.Chan AW, Wong GL, Chan HY, Tong JH, Yu YH, Choi PC, Chan HL, et al. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J Gastroenterol Hepatol. 2017;32:667–676. doi: 10.1111/jgh.13536. [DOI] [PubMed] [Google Scholar]
- 56.Villa E, Rubbiani L, Barchi T, Ferretti I, Grisendi A, De Palma M, Bellentani S, et al. Susceptibility of chronic symptomless HBsAg carriers to ethanol-induced hepatic damage. Lancet. 1982;2(8310):1243–1244. doi: 10.1016/s0140-6736(82)90104-0. [DOI] [PubMed] [Google Scholar]
- 57.Chevillotte G, Durbec JP, Gerolami A, Berthezene P, Bidart JM, Camatte R. Interaction between hepatitis b virus and alcohol consumption in liver cirrhosis. An epidemiologic study. Gastroenterology. 1983;85:141–145. [PubMed] [Google Scholar]
- 58.Tanaka K, Hirohata T, Takeshita S, Hirohata I, Koga S, Sugimachi K, Kanematsu T, et al. Hepatitis B virus, cigarette smoking and alcohol consumption in the development of hepatocellular carcinoma: a case-control study in Fukuoka, Japan. Int J Cancer. 1992;51:509–514. doi: 10.1002/ijc.2910510402. [DOI] [PubMed] [Google Scholar]
- 59.Austin H, Delzell E, Grufferman S, Levine R, Morrison AS, Stolley PD, Cole P. A case-control study of hepatocellular carcinoma and the hepatitis B virus, cigarette smoking, and alcohol consumption. Cancer Research. 1986;46:962–966. [PubMed] [Google Scholar]
- 60.Villa E, Rubbiani L, Barchi T, Ferretti I, Grisendi A, De Palma M, Bellentani S, et al. Susceptibility of chronic symptomless HBsAg carriers to ethanol-induced hepatic damage. Lancet. 1982;2:1243–1244. doi: 10.1016/s0140-6736(82)90104-0. [DOI] [PubMed] [Google Scholar]
- 61.Advisory Committee on Immunization P. Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–23. [PubMed] [Google Scholar]
- 62.Harpaz R, Seidlein LV, Averhoff FM, Tormey MP, Sinha SD, Kotsopoulou K, Lambert SB, et al. Transmission of hepatitis B to multiple patients from a surgeon without evidence of inadequate infection control. New England Journal of Medicine. 1996;334:549–554. doi: 10.1056/NEJM199602293340901. [DOI] [PubMed] [Google Scholar]
- 63.Centers for Disease C, Prevention. Updated CDC recommendations for the management of hepatitis B virus-infected health-care providers and students. MMWR Recomm Rep. 2012;61:1–12. [PubMed] [Google Scholar]
- 64.Mast EE, Weinbaum CM, Fiore AE, Alter MJ, Bell BP, Finelli L, Rodewald LE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006;55:1–33. quiz CE31–34. [PubMed] [Google Scholar]
- 65.Samadi Kochaksaraei G, Castillo E, Osman M, Simmonds K, Scott AN, Oshiomogho JI, Lee SS, et al. Clinical course of 161 untreated and tenofovir-treated chronic hepatitis B pregnant patients in a low hepatitis B virus endemic region. Journal of Viral Hepatitis. 2016;23:15–22. doi: 10.1111/jvh.12436. [DOI] [PubMed] [Google Scholar]
- 66.Chang CY, Aziz N, Poongkunran M, Javaid A, Trinh HN, Lau D, Nguyen MH. Serum Alanine Aminotransferase and Hepatitis B DNA Flares in Pregnant and Postpartum Women with Chronic Hepatitis B. Am J Gastroenterol. 2016;111:1410–1415. doi: 10.1038/ajg.2016.296. [DOI] [PubMed] [Google Scholar]
- 67.Chang CY, Aziz N, Poongkunran M, Javaid A, Trinh HN, Lau DT, Nguyen MH. Serum Aminotransferase Flares in Pregnant and Postpartum Women With Current or Prior Treatment for Chronic Hepatitis B. J Clin Gastroenterol. 2017 doi: 10.1097/MCG.0000000000000822. [DOI] [PubMed] [Google Scholar]
- 68.Nguyen V, Tan PK, Greenup AJ, Glass A, Davison S, Samarasinghe D, Holdaway S, et al. Anti-viral therapy for prevention of perinatal HBV transmission: extending therapy beyond birth does not protect against post-partum flare. Aliment Pharmacol Ther. 2014;39:1225–1234. doi: 10.1111/apt.12726. [DOI] [PubMed] [Google Scholar]
- 69.Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, Zhang H, et al. Tenofovir to Prevent Hepatitis B Transmission in Mothers with High Viral Load. N Engl J Med. 2016;374:2324–2334. doi: 10.1056/NEJMoa1508660. [DOI] [PubMed] [Google Scholar]
- 70.Chen HL, Lee CN, Chang CH, Ni YH, Shyu MK, Chen SM, Hu JJ, et al. Efficacy of maternal tenofovir disoproxil fumarate in interrupting mother-to-infant transmission of hepatitis B virus. Hepatology. 2015;62:375–386. doi: 10.1002/hep.27837. [DOI] [PubMed] [Google Scholar]
- 71.Jacobson DL, Patel K, Williams PL, Geffner ME, Siberry GK, DiMeglio LA, Crain MJ, et al. Growth at 2 Years of Age in HIV-exposed Uninfected Children in the United States by Trimester of Maternal Antiretroviral Initiation. Pediatr Infect Dis J. 2017;36:189–197. doi: 10.1097/INF.0000000000001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jao J, Abrams EJ, Phillips T, Petro G, Zerbe A, Myer L. In Utero Tenofovir Exposure Is not Associated With Fetal Long Bone Growth. Clin Infect Dis. 2016;62:1604–1609. doi: 10.1093/cid/ciw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nachega JB, Uthman OA, Mofenson LM, Anderson JR, Kanters S, Renaud F, Ford N, et al. Safety of Tenofovir Disoproxil Fumarate-Based Antiretroviral Therapy Regimens in Pregnancy for HIV-Infected Women and Their Infants: A Systematic Review and Meta-Analysis. J Acquir Immune Defic Syndr. 2017;76:1–12. doi: 10.1097/QAI.0000000000001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siberry GK, Jacobson DL, Kalkwarf HJ, Wu JW, DiMeglio LA, Yogev R, Knapp KM, et al. Lower Newborn Bone Mineral Content Associated With Maternal Use of Tenofovir Disoproxil Fumarate During Pregnancy. Clin Infect Dis. 2015;61:996–1003. doi: 10.1093/cid/civ437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alexander JM, Ramus R, Jackson G, Sercely B, Wendel GD., Jr Risk of hepatitis B transmission after amniocentesis in chronic hepatitis B carriers. Infect Dis Obstet Gynecol. 1999;7:283–286. doi: 10.1002/(SICI)1098-0997(1999)7:6<283::AID-IDOG6>3.0.CO;2-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yi W, Pan CQ, Hao J, Hu Y, Liu M, Li L, Liang D. Risk of vertical transmission of hepatitis B after amniocentesis in HBs antigen-positive mothers. J Hepatol. 2014;60:523–529. doi: 10.1016/j.jhep.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 77.Benaboud S, Pruvost A, Coffie PA, Ekouevi DK, Urien S, Arrive E, Blanche S, et al. Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Cote d’Ivoire, in the ANRS 12109 TEmAA Study, Step 2. Antimicrob Agents Chemother. 2011;55:1315–1317. doi: 10.1128/AAC.00514-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mirochnick M, Taha T, Kreitchmann R, Nielsen-Saines K, Kumwenda N, Joao E, Pinto J, et al. Pharmacokinetics and safety of tenofovir in HIV-infected women during labor and their infants during the first week of life. J Acquir Immune Defic Syndr. 2014;65:33–41. doi: 10.1097/QAI.0b013e3182a921eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gupta I, Ratho RK. Immunogenicity and safety of two schedules of Hepatitis B vaccination during pregnancy. Journal of Obstetrics & Gynaecology Research. 2003;29:84–86. doi: 10.1046/j.1341-8076.2002.00076.x. [DOI] [PubMed] [Google Scholar]
- 80.Levy M, Koren G. Hepatitis B vaccine in pregnancy: maternal and fetal safety. American Journal of Perinatology. 1991;8:227–232. doi: 10.1055/s-2007-999384. [DOI] [PubMed] [Google Scholar]
- 81.Sheffield JS, Hickman A, Tang J, Moss K, Kourosh A, Crawford NM, Wendel GD., Jr Efficacy of an accelerated hepatitis B vaccination program during pregnancy. Obstetrics & Gynecology. 2011;117:1130–1135. doi: 10.1097/AOG.0b013e3182148efe. [DOI] [PubMed] [Google Scholar]
- 82.Kim DK, Riley LE, Harriman KH, Hunter P, Bridges CB. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Adults Aged 19 Years or Older - United States, 2017. Am J Transplant. 2017;17:1132–1135. doi: 10.15585/mmwr.mm6605e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.FitzSimons D, Hendrickx G, Vorsters A, Van Damme P. Vaccine; Hepatitis B vaccination: a completed schedule enough to control HBV lifelong?; Milan, Italy. 17–18 November 2011; 2013. pp. 584–590. [DOI] [PubMed] [Google Scholar]
- 84.Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. In: Hamborsky J, Kroger A, Wolfe S, editors. The Pink Book. 13. Washington DC: Public Health Foundation; 2015. pp. 157–174. [Google Scholar]
- 85.Aggeletopoulou I, Davoulou P, Konstantakis C, Thomopoulos K, Triantos C. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev Med Virol. 2017;27:1–8. doi: 10.1002/rmv.1942. [DOI] [PubMed] [Google Scholar]
- 86.Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, Bousvaros A, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:309–318. doi: 10.1093/cid/cit816. [DOI] [PubMed] [Google Scholar]
- 87.Rey D, Piroth L, Wendling MJ, Miailhes P, Michel ML, Dufour C, Haour G, et al. Safety and immunogenicity of double-dose versus standard-dose hepatitis B revaccination in non-responding adults with HIV-1 (ANRS HB04 B-BOOST): a multicentre, open-label, randomised controlled trial. Lancet Infect Dis. 2015;15:1283–1291. doi: 10.1016/S1473-3099(15)00220-0. [DOI] [PubMed] [Google Scholar]
- 88.Schillie SF, Murphy TV. Seroprotection after recombinant hepatitis B vaccination among newborn infants: a review. Vaccine. 2013;31:2506–2516. doi: 10.1016/j.vaccine.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 89.American Academy of Pediatrics. Hepatitis B, Special Considerations. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Red Book 2015: Report of the Committee on Infectious Diseases. 30. Elk Grove Village, IL: American Academy of Pediatrics; 2015. pp. 416–418. [Google Scholar]
- 90.Center for Disease Control and Prevention. Notice to Readers: FDA Approval of an Alternate Dosing Schedule for a Combined Hepatitis A and B Vaccine (Twinrix®) MMWR Morb Mortal Wkly Rep. 2007;56:1057. [Google Scholar]
- 91.Leise MD, Talwalkar JA. Immunizations in chronic liver disease: what should be done and what is the evidence. Curr Gastroenterol Rep. 2013;15:300. doi: 10.1007/s11894-012-0300-6. [DOI] [PubMed] [Google Scholar]
- 92.Tan A, Koh S, Bertoletti A. Immune Response in Hepatitis B Virus Infection. Cold Spring Harb Perspect Med. 2015;5:a021428. doi: 10.1101/cshperspect.a021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bengsch B, Chang KM. Evolution in Our Understanding of Hepatitis B Virus Virology and Immunology. Clin Liver Dis. 2016;20:629–644. doi: 10.1016/j.cld.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 94.Lee JK, Shim JH, Lee HC, Lee SH, Kim KM, Lim YS, Chung YH, et al. Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatology. 2010;51:1577–1583. doi: 10.1002/hep.23505. [DOI] [PubMed] [Google Scholar]
- 95.Ruhl CE, Everhart JE. Upper limits of normal for alanine aminotransferase activity in the United States population. Hepatology. 2012;55:447–454. doi: 10.1002/hep.24725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 97.Dutta A, Saha C, Johnson CS, Chalasani N. Variability in the upper limit of normal for serum alanine aminotransferase levels: a statewide study. Hepatology. 2009;50:1957–1962. doi: 10.1002/hep.23200. [DOI] [PubMed] [Google Scholar]
- 98.Neuschwander-Tetri BA, Unalp A, Creer MH Nonalcoholic Steatohepatitis Clinical Research N. Influence of local reference populations on upper limits of normal for serum alanine aminotransferase levels. Arch Intern Med. 2008;168:663–666. doi: 10.1001/archinternmed.2007.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allice T, Cerutti F, Pittaluga F, Varetto S, Gabella S, Marzano A, Franchello A, et al. COBAS AmpliPrep-COBAS TaqMan hepatitis B virus (HBV) test: a novel automated real-time PCR assay for quantification of HBV DNA in plasma. J Clin Microbiol. 2007;45:828–834. doi: 10.1128/JCM.00914-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chu CJ, Hussain M, Lok AS. Quantitative serum HBV DNA levels during different stages of chronic hepatitis B infection. Hepatology. 2002;36:1408–1415. doi: 10.1053/jhep.2002.36949. [DOI] [PubMed] [Google Scholar]
- 101.Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007;45:1056–1075. doi: 10.1002/hep.21627. [DOI] [PubMed] [Google Scholar]
- 102.Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang L, Su J, et al. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010;138(5):1747–54. doi: 10.1053/j.gastro.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 103.Lin CL, Kao JH. Natural history of acute and chronic hepatitis B: The role of HBV genotypes and mutants. Best Pract Res Clin Gastroenterol. 2017;31:249–255. doi: 10.1016/j.bpg.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 104.Shi W, Zhang Z, Ling C, Zheng W, Zhu C, Carr MJ, Higgins DG. Hepatitis B virus subgenotyping: history, effects of recombination, misclassifications, and corrections. Infect Genet Evol. 2013;16:355–61. doi: 10.1016/j.meegid.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 105.Marcellin P, Ahn SH, Ma X, Caruntu FA, Tak WY, Elkashab M, Chuang WL, et al. Combination of Tenofovir Disoproxil Fumarate and Peginterferon alpha-2a Increases Loss of Hepatitis B Surface Antigen in Patients With Chronic Hepatitis B. Gastroenterology. 2016;150:134–144. e110. doi: 10.1053/j.gastro.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 106.Buster EH, Hansen BE, Lau GK, Piratvisuth T, Zeuzem S, Steyerberg EW, Janssen HL. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology. 2009;137:2002–2009. doi: 10.1053/j.gastro.2009.08.061. [DOI] [PubMed] [Google Scholar]
- 107.Livingston SE, Simonetti JP, McMahon BJ, Bulkow LR, Hurlburt KJ, Homan CE, Snowball MM, et al. Hepatitis B virus genotypes in Alaska Native people with hepatocellular carcinoma: preponderance of genotype F. J Infect Dis. 2007;195:5–11. doi: 10.1086/509894. [DOI] [PubMed] [Google Scholar]
- 108.Ching LK, Gounder PP, Bulkow L, Spradling PR, Bruce MG, Negus S, Snowball M, et al. Incidence of hepatocellular carcinoma according to hepatitis B virus genotype in Alaska Native people. Liver Int. 2016;36:1507–1515. doi: 10.1111/liv.13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cornberg M, Wong VW, Locarnini S, Brunetto M, Janssen HL, Chan HL. The role of quantitative hepatitis B surface antigen revisited. J Hepatol. 2017;66:398–411. doi: 10.1016/j.jhep.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 110.Zeng DW, Zhang JM, Liu YR, Dong J, Jiang JJ, Zhu YY. A Retrospective Study on the Significance of Liver Biopsy and Hepatitis B Surface Antigen in Chronic Hepatitis B Infection. Medicine (Baltimore) 2016;95:e2503. doi: 10.1097/MD.0000000000002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang L, Zou ZQ, Wang K, Yu JG, Liu XZ. Role of serum hepatitis B virus marker quantitation to differentiate natural history phases of HBV infection. Hepatol Int. 2016;10:133–138. doi: 10.1007/s12072-015-9657-6. [DOI] [PubMed] [Google Scholar]
- 112.Brouwer WP, Chan HL, Brunetto MR, Martinot-Peignoux M, Arends P, Cornberg M, Cherubini B, et al. Repeated Measurements of Hepatitis B Surface Antigen Identify Carriers of Inactive HBV During Long-term Follow-up. Clin Gastroenterol Hepatol. 2016;14:1481–1489. e1485. doi: 10.1016/j.cgh.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 113.Tseng TC, Kao JH. Clinical utility of quantitative HBsAg in natural history and nucleos(t)ide analogue treatment of chronic hepatitis B: new trick of old dog. J Gastroenterol. 2013;48:13–21. doi: 10.1007/s00535-012-0668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lok AS, Ganova-Raeva L, Cloonan Y, Punkova L, Lin HS, Lee WM, Ghany MG, et al. Prevalence of hepatitis B antiviral drug resistance variants in North American patients with chronic hepatitis B not receiving antiviral treatment. J Viral Hepat. 2017;24:1032–1042. doi: 10.1111/jvh.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim JH, Park YK, Park ES, Kim KH. Molecular diagnosis and treatment of drug-resistant hepatitis B virus. World J Gastroenterol. 2014;20:5708–5720. doi: 10.3748/wjg.v20.i19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liaw YF, Chu CM, Su IJ, Huang MJ, Lin DY, Chang-Chien CS. Clinical and histological events preceding hepatitis B e antigen seroconversion in chronic type B hepatitis. Gastroenterology. 1983;84:216–219. [PubMed] [Google Scholar]
- 117.Liaw YF, Pao CC, Chu CM, Sheen IS, Huang MJ. Changes of serum hepatitis B virus DNA in two types of clinical events preceding spontaneous hepatitis B e antigen seroconversion in chronic type B hepatitis. Hepatology. 1987;7:1–3. doi: 10.1002/hep.1840070102. [DOI] [PubMed] [Google Scholar]
- 118.Lok AS, Lai CL, Wu PC, Leung EK, Lam TS. Spontaneous hepatitis B e antigen to antibody seroconversion and reversion in Chinese patients with chronic hepatitis B virus infection. Gastroenterology. 1987;92:1839–1843. doi: 10.1016/0016-5085(87)90613-5. [DOI] [PubMed] [Google Scholar]
- 119.Lok AS, Lai CL. Acute exacerbations in Chinese patients with chronic hepatitis B virus (HBV) infection. Incidence, predisposing factors and etiology. J Hepatol. 1990;10:29–34. doi: 10.1016/0168-8278(90)90069-4. [DOI] [PubMed] [Google Scholar]
- 120.Chu CM, Liaw YF. Chronic hepatitis B virus infection acquired in childhood: special emphasis on prognostic and therapeutic implication of delayed HBeAg seroconversion. J Viral Hepat. 2007;14:147–152. doi: 10.1111/j.1365-2893.2006.00810.x. [DOI] [PubMed] [Google Scholar]
- 121.Cheng J, Hou J, Ding H, Chen G, Xie Q, Wang Y, Zeng M, et al. Validation of Ten Noninvasive Diagnostic Models for Prediction of Liver Fibrosis in Patients with Chronic Hepatitis B. PLoS One. 2015;10:e0144425. doi: 10.1371/journal.pone.0144425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jia J, Hou J, Ding H, Chen G, Xie Q, Wang Y, Zeng M, et al. Transient elastography compared to serum markers to predict liver fibrosis in a cohort of Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2015;30:756–762. doi: 10.1111/jgh.12840. [DOI] [PubMed] [Google Scholar]
- 123.Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute Technical Review on the Role of Elastography in Chronic Liver Diseases. Gastroenterology. 2017;152:1544–1577. doi: 10.1053/j.gastro.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 124.Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. 2015;61:292–302. doi: 10.1002/hep.27382. [DOI] [PubMed] [Google Scholar]
- 125.Brunetto MR, Oliveri F, Coco B, Leandro G, Colombatto P, Gorin JM, Bonino F. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long term cohort study. Journal of Hepatology. 2002;36:263–270. doi: 10.1016/s0168-8278(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 126.Chan HL, Thompson A, Martinot-Peignoux M, Piratvisuth T, Cornberg M, Brunetto MR, Tillmann HL, et al. Hepatitis B surface antigen quantification: why and how to use it in 2011 - a core group report. J Hepatol. 2011;55:1121–1131. doi: 10.1016/j.jhep.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 127.Liu J, Yang HI, Lee MH, Jen CL, Batrla-Utermann R, Lu SN, Wang LY, et al. Serum Levels of Hepatitis B Surface Antigen and DNA Can Predict Inactive Carriers With Low Risk of Disease Progression. Hepatology. 2016;64:381–389. doi: 10.1002/hep.28552. [DOI] [PubMed] [Google Scholar]
- 128.Liaw YF, Sheen IS, Chen TJ, Chu CM, Pao CC. Incidence, determinants and significance of delayed clearance of serum HBsAg in chronic hepatitis B virus infection: a prospective study. Hepatology. 1991;13:627–631. [PubMed] [Google Scholar]
- 129.Chu CM, Liaw YF. HBsAg seroclearance in asymptomatic carriers of high endemic areas: appreciably high rates during a long-term follow-up. Hepatology. 2007;45:1187–1192. doi: 10.1002/hep.21612. [DOI] [PubMed] [Google Scholar]
- 130.Yip TC, Chan HL, Wong VW, Tse YK, Lam KL, Wong GL. Impact of age and gender on risk of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J Hepatol. 2017;67:902–908. doi: 10.1016/j.jhep.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 131.Chen YC, Sheen IS, Chu CM, Liaw YF. Prognosis following spontaneous HBsAg seroclearance in chronic hepatitis B patients with or without concurrent infection. Gastroenterology. 2002;123:1084–1089. doi: 10.1053/gast.2002.36026. [DOI] [PubMed] [Google Scholar]
- 132.Ahn SH, Park YN, Park JY, Chang HY, Lee JM, Shin JE, Han KH, et al. Long-term clinical and histological outcomes in patients with spontaneous hepatitis B surface antigen seroclearance. J Hepatol. 2005;42:188–194. doi: 10.1016/j.jhep.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 133.Heimbach J, Kulik LM, Finn R, Sirlin CB, Abecassis M, Roberts LR, Zhu A, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–38. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 134.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 135.Chayanupatkul M, Omino R, Mittal S, Kramer JR, Richardson P, Thrift AP, El-Serag HB, et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol. 2017;66:355–362. doi: 10.1016/j.jhep.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 136.Yu MW, Lin CL, Liu CJ, Yang SH, Tseng YL, Wu CF. Influence of Metabolic Risk Factors on Risk of Hepatocellular Carcinoma and Liver-Related Death in Men With Chronic Hepatitis B: A Large Cohort Study. Gastroenterology. 2017;153:1006–1017. e1005. doi: 10.1053/j.gastro.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 137.Huang YT, Yang HI, Liu J, Lee MH, Freeman JR, Chen CJ. Mediation Analysis of Hepatitis B and C in Relation to Hepatocellular Carcinoma Risk. Epidemiology. 2016;27:14–20. doi: 10.1097/EDE.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 138.Ioannou GN, Bryson CL, Weiss NS, Miller R, Scott JD, Boyko EJ. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology. 2013;57:249–257. doi: 10.1002/hep.25800. [DOI] [PubMed] [Google Scholar]
- 139.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 140.American Association for the Study of Liver Diseases, America IDSo. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Updated April 27, 2017. [Google Scholar]
- 141.Kim YJ, Lee JW, Kim YS, Jeong SH, Kim YS, Yim HJ, Kim BH, et al. Clinical features and treatment efficacy of peginterferon alfa plus ribavirin in chronic hepatitis C patients coinfected with hepatitis B virus. Korean J Hepatol. 2011;17:199–205. doi: 10.3350/kjhep.2011.17.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Uyanikoglu A, Akyuz F, Baran B, Simsek BP, Ermis F, Demir K, Gulluoglu M, et al. Co-infection with hepatitis B does not alter treatment response in chronic hepatitis C. Clin Res Hepatol Gastroenterol. 2013;37:485–490. doi: 10.1016/j.clinre.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 143.Liu CJ, Chuang WL, Lee CM, Yu ML, Lu SN, Wu SS, Liao LY, et al. Peginterferon Alfa-2a Plus Ribavirin for the Treatment of Dual Chronic Infection With Hepatitis B and C Viruses. Gastroenterology. 2008;136:496–504. e3. doi: 10.1053/j.gastro.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 144.Potthoff A, Wedemeyer H, Boecher WO, Berg T, Zeuzem S, Arnold J, Spengler U, et al. The HEP-NET B/C co-infection trial: A prospective multicenter study to investigate the efficacy of pegylated interferon-alpha2b and ribavirin in patients with HBV/HCV co-infection. J Hepatol. 2008;49:688–694. doi: 10.1016/j.jhep.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 145.Wang C, Ji D, Chen J, Shao Q, Li B, Liu J, Wu V, et al. Hepatitis due to Reactivation of Hepatitis B Virus in Endemic Areas Among Patients With Hepatitis C Treated With Direct-acting Antiviral Agents. Clin Gastroenterol Hepatol. 2017;15:132–136. doi: 10.1016/j.cgh.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 146.Bersoff-Matcha SJ, Cao K, Jason M, Ajao A, Jones SC, Meyer T, Brinker A. Hepatitis B Virus Reactivation Associated With Direct-Acting Antiviral Therapy for Chronic Hepatitis C Virus: A Review of Cases Reported to the U.S. Food and Drug Administration Adverse Event Reporting System. Ann Intern Med. 2017;166:792–798. doi: 10.7326/M17-0377. [DOI] [PubMed] [Google Scholar]
- 147.Belperio PS, Shahoumian TA, Mole LA, Backus LI. Evaluation of hepatitis B reactivation among 62,920 veterans treated with oral hepatitis C antivirals. Hepatology. 2017;66:27–36. doi: 10.1002/hep.29135. [DOI] [PubMed] [Google Scholar]
- 148.Chen G, Wang C, Chen J, Ji D, Wang Y, Wu V, Karlberg J, et al. Hepatitis B reactivation in hepatitis B and C coinfected patients treated with antiviral agents: A systematic review and meta-analysis. Hepatology. 2017;66:13–26. doi: 10.1002/hep.29109. [DOI] [PubMed] [Google Scholar]
- 149.Abbas Z, Jafri W, Raza S. Hepatitis D: Scenario in the Asia-Pacific region. World Journal of Gastroenterology. 2010;16:554–562. doi: 10.3748/wjg.v16.i5.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.World Health Organization. Hepatitis D: Fact Sheet. Media Centre; Geneva: 2017. [Google Scholar]
- 151.Chow SK, Atienza EE, Cook L, Prince H, Slev P, Lape-Nixon M, Jerome KR. Comparison of Enzyme Immunoassays for Detection of Antibodies to Hepatitis D Virus in Serum. Clin Vaccine Immunol. 2016;23:732–734. doi: 10.1128/CVI.00028-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Castelnau C, Le Gal F, Ripault M, EG, Martinot-Peignoux M, Boyer N, Pham B, et al. Efficacy of peginterferon alfa-2b in chronic delta hepatitis. Relevance of quantitative RT-PCR for follow-up. Hepatology. 2006;44:728–735. doi: 10.1002/hep.21325. [DOI] [PubMed] [Google Scholar]
- 153.Wedemeyer H, Yurdaydin C, Dalekos GN, Erhardt A, Cakaloglu Y, Degertekin H, Gurel S, et al. Peginterferon plus adefovir versus either drug alone for hepatitis delta. New England Journal of Medicine. 2011;364:322–331. doi: 10.1056/NEJMoa0912696. [DOI] [PubMed] [Google Scholar]
- 154.Abbas Z, Memon MS, Mithani H, Jafri W, Hamid S. Treatment of chronic hepatitis D patients with pegylated interferon: a real-world experience. Antivir Ther. 2014;19:463–468. doi: 10.3851/IMP2728. [DOI] [PubMed] [Google Scholar]
- 155.Heidrich B, Yurdaydin C, Kabacam G, Ratsch BA, Zachou K, Bremer B, Dalekos GN, et al. Late HDV RNA relapse after peginterferon alpha-based therapy of chronic hepatitis delta. Hepatology. 2014;60:87–97. doi: 10.1002/hep.27102. [DOI] [PubMed] [Google Scholar]
- 156.Heller T, Rotman Y, Koh C, Clark S, Haynes-Williams V, Chang R, McBurney R, et al. Long-term therapy of chronic delta hepatitis with peginterferon alfa. Aliment Pharmacol Ther. 2014;40:93–104. doi: 10.1111/apt.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Keskin O, Wedemeyer H, Tuzun A, Zachou K, Deda X, Dalekos GN, Heidrich B, et al. Association Between Level of Hepatitis D Virus RNA at Week 24 of Pegylated Interferon Therapy and Outcome. Clin Gastroenterol Hepatol. 2015;13:2342–2349. e2341–2342. doi: 10.1016/j.cgh.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 158.Bogomolov P, Alexandrov A, Voronkova N, Macievich M, Kokina K, Petrachenkova M, Lehr T, et al. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: First results of a phase Ib/IIa study. J Hepatol. 2016;65:490–498. doi: 10.1016/j.jhep.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 159.Koh C, Canini L, Dahari H, Zhao X, Uprichard SL, Haynes-Williams V, Winters MA, et al. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis. 2015;15:1167–1174. doi: 10.1016/S1473-3099(15)00074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dore GJ, Cooper DA, Pozniak AL, DeJesus E, Zhong L, Miller MD, Lu B, et al. Efficacy of tenofovir disoproxil fumarate in antiretroviral therapy-naive and -experienced patients coinfected with HIV-1 and hepatitis B virus. J Infect Dis. 2004;189:1185–1192. doi: 10.1086/380398. [DOI] [PubMed] [Google Scholar]
- 161.Hoff J, Bani-Sadr F, Gassin M, Raffi F. Evaluation of chronic hepatitis B virus (HBV) infection in coinfected patients receiving lamivudine as a component of anti-human immunodeficiency virus regimens. Clin Infect Dis. 2001;32:963–969. doi: 10.1086/319368. [DOI] [PubMed] [Google Scholar]
- 162.Benhamou Y, Bochet M, Thibault V, Di Martino V, Caumes E, Bricaire F, Opolon P, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology. 1999;30:1302–1306. doi: 10.1002/hep.510300525. [DOI] [PubMed] [Google Scholar]
- 163.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; 2016. [Google Scholar]
- 164.Gallant J, Brunetta J, Crofoot G, Benson P, Mills A, Brinson C, Oka S, et al. Brief Report: Efficacy and Safety of Switching to a Single-Tablet Regimen of Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Alafenamide in HIV-1/Hepatitis B-Coinfected Adults. J Acquir Immune Defic Syndr. 2016;73:294–298. doi: 10.1097/QAI.0000000000001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gallant JE, Daar ES, Raffi F, Brinson C, Ruane P, DeJesus E, Johnson M, et al. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed-dose combinations containing emtricitabine as backbones for treatment of HIV-1 infection in virologically suppressed adults: a randomised, double-blind, active-controlled phase 3 trial. Lancet HIV. 2016;3:e158–165. doi: 10.1016/S2352-3018(16)00024-2. [DOI] [PubMed] [Google Scholar]
- 166.Huhn GD, Tebas P, Gallant J, Wilkin T, Cheng A, Yan M, Zhong L, et al. A Randomized, Open-Label Trial to Evaluate Switching to Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Alafenamide Plus Darunavir in Treatment-Experienced HIV-1-Infected Adults. J Acquir Immune Defic Syndr. 2017;74:193–200. doi: 10.1097/QAI.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.McMahon MA, Jilek BL, Brennan TP, Shen L, Zhou Y, Wind-Rotolo M, Xing S, et al. The HBV drug entecavir - effects on HIV-1 replication and resistance. N Engl J Med. 2007;356:2614–2621. doi: 10.1056/NEJMoa067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Manegold C, Hannoun C, Wywiol A, Dietrich M, Polywka S, Chiwakata CB, Gunther S. Reactivation of hepatitis B virus replication accompanied by acute hepatitis in patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2001;32:144–148. doi: 10.1086/317535. [DOI] [PubMed] [Google Scholar]
- 169.Sulkowski MS, Thomas D, Chaisson R, Moore R. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immumodeficiency virus and the role of hepatitis C and B virus infection. JAMA. 2000;283:74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- 170.den Brinker M, Wit FW, Wertheim-van Dillen PM, Jurriaans S, Weel J, van Leeuwen R, Pakker NG, et al. Hepatitis B and C virus co-infection and the risk for hepatotoxicity of highly active antiretroviral therapy in HIV-1 infection. Aids. 2000;14:2895–2902. doi: 10.1097/00002030-200012220-00011. [DOI] [PubMed] [Google Scholar]
- 171.Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991;100:182–188. doi: 10.1016/0016-5085(91)90599-g. [DOI] [PubMed] [Google Scholar]
- 172.Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, Hui P, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. Journal of Medical Virology. 2000;62:299–307. doi: 10.1002/1096-9071(200011)62:3<299::aid-jmv1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 173.Yeo W, Lam KC, Zee B, Chan PS, Mo FK, Ho WM, Wong WL, et al. Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systemic chemotherapy. Ann Oncol. 2004;15:1661–1666. doi: 10.1093/annonc/mdh430. [DOI] [PubMed] [Google Scholar]
- 174.Jang JW, Choi JY, Bae SH, Yoon SK, Chang UI, Kim CW, Cho SH, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology. 2006;43:233–240. doi: 10.1002/hep.21024. [DOI] [PubMed] [Google Scholar]
- 175.Hui CK, Cheung WW, Zhang HY, Au WY, Yueng YH, Leung AY, Leung N, et al. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology. 2006;131:59–68. doi: 10.1053/j.gastro.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 176.Hsiao L-T, Chiou T-J, Liu J-H, Chu C-J, Lin Y-C, Chao T-C, Wang W-S, et al. Extended lamivudine therapy against hepatitis B virus infection in hematopoietic stem cell transplant recipients. Biology of Blood and Marrow Transplantation. 2006;12:84–94. doi: 10.1016/j.bbmt.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 177.Loomba R, Rowley A, Wesley R, Liang TJ, Hoofnagle JH, Pucino F, Csako G. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148:519–528. doi: 10.7326/0003-4819-148-7-200804010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Paul S, Saxena A, Terrin N, Viveiros K, Balk EM, Wong JB. Hepatitis B Virus Reactivation and Prophylaxis During Solid Tumor Chemotherapy: A Systematic Review and Meta-analysis. Ann Intern Med. 2016;164:30–40. doi: 10.7326/M15-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, Cheung M, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125:1742–1749. doi: 10.1053/j.gastro.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 180.Huang YH, Hsiao LT, Hong YC, Chiou TJ, Yu YB, Gau JP, Liu CY, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013;31:2765–2772. doi: 10.1200/JCO.2012.48.5938. [DOI] [PubMed] [Google Scholar]
- 181.Lee YH, Bae SC, Song GG. Hepatitis B virus reactivation in HBsAg-positive patients with rheumatic diseases undergoing anti-tumor necrosis factor therapy or DMARDs. International Journal of Rheumatic Diseases. 2013;16:527–531. doi: 10.1111/1756-185X.12154. [DOI] [PubMed] [Google Scholar]
- 182.Mori S, Fujiyama S. Hepatitis B virus reactivation associated with antirheumatic therapy: Risk and prophylaxis recommendations. World Journal of Gastroenterology. 2015;21:10274–10289. doi: 10.3748/wjg.v21.i36.10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kanaan N, Kabamba B, Maréchal C, Pirson Y, Beguin C, Goffin E, Hassoun Z. Significant rate of hepatitis B reactivation following kidney transplantation in patients with resolved infection. Journal of Clinical Virology. 2012;55:233–238. doi: 10.1016/j.jcv.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 184.Onozawa M, Hashino S, Izumiyama K, Kahata K, Chuma M, Mori A, Kondo T, et al. Progressive disappearance of anti-hepatitis B surface antigen antibody and reverse seroconversion after allogeneic hematopoietic stem cell transplantation in patients with previous hepatitis B virus infection. Transplantation. 2005;79:616–619. doi: 10.1097/01.tp.0000151661.52601.fb. [DOI] [PubMed] [Google Scholar]
- 185.Hammond SP, Borchelt AM, Ukomadu C, Ho VT, Baden LR, Marty FM. Hepatitis B virus reactivation following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:1049–1059. doi: 10.1016/j.bbmt.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 186.European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 187.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hwang JP, Fisch MJ, Zhang H, Kallen MA, Routbort MJ, Lal LS, Vierling JM, et al. Low rates of hepatitis B virus screening at the onset of chemotherapy. Journal of Oncology Practice. 2012;8:e32–e39. doi: 10.1200/JOP.2011.000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hwang JP, Fisch MJ, Lok AS, Zhang H, Vierling JM, Suarez-Almazor ME. Trends in hepatitis B virus screening at the onset of chemotherapy in a large US cancer center. BMC Cancer. 2013;13:534. doi: 10.1186/1471-2407-13-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Visram A, Chan KK, McGee P, Boro J, Hicks LK, Feld JJ. Poor recognition of risk factors for hepatitis B by physicians prescribing immunosuppressive therapy: a call for universal rather than risk-based screening. PLoS One. 2015;10:e0120749. doi: 10.1371/journal.pone.0120749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hwang JP, Lok A, Fisch MJ, Cantor SB, Barbo AG, Lin HY, Foreman JT, et al. Models to Predict Hepatitis B Virus Infection among Patients with Cancer Undergoing Systemic Anti-cancer Therapy: A Prospective Cohort Study. Society of General Internal Medicine Annual Meeting; Washington, D.C. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:221–244. e223. doi: 10.1053/j.gastro.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 193.Voican CS, Mir O, Loulergue P, Dhooge M, Brezault C, Dréanic J, Chaussade S, et al. Hepatitis B virus reactivation in patients with solid tumors receiving systemic anticancer treatment. Annals of Oncology. 2016;27:2172–2184. doi: 10.1093/annonc/mdw414. [DOI] [PubMed] [Google Scholar]
- 194.Keam B, Lee JH, Im SA, Yoon JH. Why, when, and how to prevent hepatitis B virus reactivation in cancer patients undergoing chemotherapy. Journal of the National Comprehensive Cancer Network. 2011;9:465–477. doi: 10.6004/jnccn.2011.0045. [DOI] [PubMed] [Google Scholar]
- 195.Yeo W, Zee B, Zhong S, Chan PK, Wong WL, Ho WM, Lam KC, et al. Comprehensive analysis of risk factors associating with hepatitis B virus (HBV) reactivation in cancer patients undergoing cytotoxic chemotherapy. Br J Cancer. 2004;90:1306–1311. doi: 10.1038/sj.bjc.6601699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lau GK, Leung YH, Fong DY, Au WY, Kwong YL, Lie A, Hou JL, et al. High hepatitis B virus (HBV) DNA viral load as the most important risk factor for HBV reactivation in patients positive for HBV surface antigen undergoing autologous hematopoietic cell transplantation. Blood. 2002;99:2324–2330. doi: 10.1182/blood.v99.7.2324. [DOI] [PubMed] [Google Scholar]
- 197.Hsu C, Hsiung CA, Su IJ, Hwang WS, Wang MC, Lin SF, Lin TH, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin’s lymphoma: a randomized trial. Hepatology. 2008;47:844–853. doi: 10.1002/hep.22106. [DOI] [PubMed] [Google Scholar]
- 198.Barone M, Notarnicola A, Lopalco G, Viggiani MT, Sebastiani F, Covelli M, Iannone F, et al. Safety of long-term biologic therapy in rheumatologic patients with a previously resolved hepatitis B viral infection. Hepatology. 2015;62:40–46. doi: 10.1002/hep.27716. [DOI] [PubMed] [Google Scholar]
- 199.Varisco V, Vigano M, Batticciotto A, Lampertico P, Marchesoni A, Gibertini P, Pellerito R, et al. Low Risk of Hepatitis B Virus Reactivation in HBsAg-negative/Anti-HBc-positive Carriers Receiving Rituximab for Rheumatoid Arthritis: A Retrospective Multicenter Italian Study. Journal of Rheumatology. 2016;43:869–874. doi: 10.3899/jrheum.151105. [DOI] [PubMed] [Google Scholar]
- 200.Tamori A, Koike T, Goto H, Wakitani S, Tada M, Morikawa H, Enomoto M, et al. Prospective study of reactivation of hepatitis B virus in patients with rheumatoid arthritis who received immunosuppressive therapy: evaluation of both HBsAg-positive and HBsAg-negative cohorts. Journal of Gastroenterology. 2011;46:556–564. doi: 10.1007/s00535-010-0367-5. [DOI] [PubMed] [Google Scholar]
- 201.Papa A, Felice C, Marzo M, Andrisani G, Armuzzi A, Covino M, Mocci G, et al. Prevalence and natural history of hepatitis B and C infections in a large population of IBD patients treated with anti-tumor necrosis factor-alpha agents. Journal of Crohn’s & colitis. 2013;7:113–119. doi: 10.1016/j.crohns.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 202.Morisco F, Guarino M, La Bella S, Di Costanzo L, Caporaso N, Ayala F, Balato N. Lack of evidence of viral reactivation in HBsAg-negative HBcAb-positive and HCV patients undergoing immunosuppressive therapy for psoriasis. BMC Gastroenterology. 2014;14:214. doi: 10.1186/s12876-014-0214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Seto WK, Chan TS, Hwang YY, Wong DK, Fung J, Liu KS, Gill H, et al. Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. J Clin Oncol. 2014;32:3736–3743. doi: 10.1200/JCO.2014.56.7081. [DOI] [PubMed] [Google Scholar]
- 204.Masarone M, De Renzo A, La Mura V, Sasso FC, Romano M, Signoriello G, Rosato V, et al. Management of the HBV reactivation in isolated HBcAb positive patients affected with Non Hodgkin Lymphoma. BMC Gastroenterology. 2014;14:31. doi: 10.1186/1471-230X-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang MY, Zhu GQ, Shi KQ, Zheng JN, Cheng Z, Zou ZL, Huang HH, et al. Systematic review with network meta-analysis: Comparative efficacy of oral nucleos(t)ide analogues for the prevention of chemotherapy-induced hepatitis B virus reactivation. Oncotarget. 2016;7:30642–30658. doi: 10.18632/oncotarget.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yang C, Qin B, Yuan Z, Chen L, Zhou HY. Meta-analysis of prophylactic entecavir or lamivudine against hepatitis B virus reactivation. Annals of Hepatology. 2016;15:501–511. [PubMed] [Google Scholar]
- 207.Yu S, Luo H, Pan M, Luis AP, Xiong Z, Shuai P, Zhang Z. Comparison of entecavir and lamivudine in preventing HBV reactivation in lymphoma patients undergoing chemotherapy: a meta-analysis. International Journal of Clinical Pharmacy. 2016;38:1035–1043. doi: 10.1007/s11096-016-0358-6. [DOI] [PubMed] [Google Scholar]
- 208.Cerva C, Colagrossi L, Maffongelli G, Salpini R, Di Carlo D, Malagnino V, Battisti A, et al. Persistent risk of HBV reactivation despite extensive lamivudine prophylaxis in haematopoietic stem cell transplant recipients who are anti-HBc-positive or HBV-negative recipients with an anti-HBc-positive donor. Clinical Microbiology & Infection. 2016;22:946.e941–946.e948. doi: 10.1016/j.cmi.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 209.Liu WP, Wang XP, Zheng W, Ping LY, Zhang C, Wang GQ, Song YQ, et al. Hepatitis B virus reactivation after withdrawal of prophylactic antiviral therapy in patients with diffuse large B cell lymphoma. Leukemia & Lymphoma. 2016;57:1355–1362. doi: 10.3109/10428194.2015.1116121. [DOI] [PubMed] [Google Scholar]
- 210.Nakaya A, Fujita S, Satake A, Nakanishi T, Azuma Y, Tsubokura Y, Hotta M, et al. Delayed HBV reactivation in rituximab-containing chemotherapy: How long should we continue anti-virus prophylaxis or monitoring HBV-DNA? Leukemia Research. 2016;50:46–49. doi: 10.1016/j.leukres.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 211.Lenci I, Tisone G, Di Paolo D, Marcuccilli F, Tariciotti L, Ciotti M, Svicher V, et al. Safety of complete and sustained prophylaxis withdrawal in patients liver-transplanted for HBV-related cirrhosis at low risk of HBV recurrence. J Hepatol. 2011;55:587–593. doi: 10.1016/j.jhep.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 212.Morisco F, Castiglione F, Rispo A, Stroffolini T, Vitale R, Sansone S, Granata R, et al. Hepatitis B virus infection and immunosuppressive therapy in patients with inflammatory bowel disease. Digestive & Liver Disease. 2011;43(Suppl 1):S40–48. doi: 10.1016/S1590-8658(10)60691-3. [DOI] [PubMed] [Google Scholar]
- 213.Cho JH, Lim JH, Park GY, Kim JS, Kang YJ, Kwon O, Choi JY, et al. Successful withdrawal of antiviral treatment in kidney transplant recipients with chronic hepatitis B viral infection. Transplant Infectious Disease. 2014;16:295–303. doi: 10.1111/tid.12202. [DOI] [PubMed] [Google Scholar]
- 214.Hwang JP, Lok AS. Management of patients with hepatitis B who require immunosuppressive therapy. Nature Reviews Gastroenterology & Hepatology. 2014;11:209–219. doi: 10.1038/nrgastro.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kondili LA, Osman H, Mutimer D. The use of lamivudine for patients with acute hepatitis B (a series of cases) J Viral Hepat. 2004;11:427–431. doi: 10.1111/j.1365-2893.2004.00504.x. [DOI] [PubMed] [Google Scholar]
- 216.Tillmann HL, Hadem J, Leifeld L, Zachou K, Canbay A, Eisenbach C, Graziadei I, et al. Safety and efficacy of lamivudine in patients with severe acute or fulminant hepatitis B, a multicenter experience. J Viral Hepat. 2006;13:256–263. doi: 10.1111/j.1365-2893.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 217.Kumar M, Satapathy S, Monga R, Das K, Hissar S, Pande C, Sharma BC, et al. A randomized controlled trial of lamivudine to treat acute hepatitis B. Hepatology. 2007;45:97–101. doi: 10.1002/hep.21486. [DOI] [PubMed] [Google Scholar]
- 218.Schmilovitz-Weiss H, Melzer E, Tur-Kaspa R, Ben-Ari Z. Excellent outcome of Lamivudine treatment in patients with chronic renal failure and hepatitis B virus infection. J Clin Gastroenterol. 2003;37:64–67. doi: 10.1097/00004836-200307000-00016. [DOI] [PubMed] [Google Scholar]
- 219.Lok AS, Heattcote EJ, Hoofnagle JH. Management of hepatitis B: 2000 - Summary of a Workshop. Gastroenterology. 2001;120:1828–1853. doi: 10.1053/gast.2001.24839. [DOI] [PubMed] [Google Scholar]
- 220.Chang TT, Chao YC, Gorbakov VV, Han KH, Gish RG, de Man R, Cheinquer H, et al. Results of up to 2 years of entecavir vs lamivudine therapy in nucleoside-naive HBeAg-positive patients with chronic hepatitis B. J Viral Hepat. 2009;16:784–789. doi: 10.1111/j.1365-2893.2009.01142.x. [DOI] [PubMed] [Google Scholar]
- 221.Liu Y, Miller MD, Kitrinos KM. Tenofovir alafenamide demonstrates broad cross-genotype activity against wild-type HBV clinical isolates and maintains susceptibility to drug-resistant HBV isolates in vitro. Antiviral Res. 2017;139:25–31. doi: 10.1016/j.antiviral.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 222.Sheppard-Law S, Zablotska-Manos I, Kermeen M, Holdaway S, Lee A, Zekry A, Dore GJ, et al. Factors associated with HBV virological breakthrough. Antivir Ther. 2017;22:53–60. doi: 10.3851/IMP3087. [DOI] [PubMed] [Google Scholar]
- 223.Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 224.Chung GE, Kim W, Lee KL, Hwang SY, Lee JH, Kim HY, Jung YJ, et al. Add-on adefovir is superior to a switch to entecavir as rescue therapy for Lamivudine-resistant chronic hepatitis B. Digestive Diseases & Sciences. 2011;56:2130–2136. doi: 10.1007/s10620-010-1561-2. Erratum appears in Dig Dis Sci 2011 Aug;56(8):2509. [DOI] [PubMed] [Google Scholar]
- 225.Huang ZB, Zhao SS, Huang Y, Dai XH, Zhou RR, Yi PP, Chen RC, et al. Comparison of the efficacy of Lamivudine plus adefovir versus entecavir in the treatment of Lamivudine-resistant chronic hepatitis B: a systematic review and meta-analysis. Clinical Therapeutics. 2013;35:1997–2006. doi: 10.1016/j.clinthera.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 226.Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, Jeon WK, Kim BI. Rescue therapy for lamivudine-resistant chronic hepatitis B: comparison between entecavir 1. 0 mg monotherapy, adefovir monotherapy and adefovir add-on lamivudine combination therapy. Journal of Gastroenterology & Hepatology. 2010;25:1374–1380. doi: 10.1111/j.1440-1746.2010.06381.x. [DOI] [PubMed] [Google Scholar]
- 227.Park JH, Jung SW, Park NH, Park BR, Kim MH, Kim CJ, Lee BU, et al. Efficacy of Tenofovir-based Rescue Therapy in Lamivudine-resistant Chronic Hepatitis B Patients With Failure of Lamivudine and Adefovir Combination. Clinical Therapeutics. 2015;37:1433–1442. doi: 10.1016/j.clinthera.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 228.Suzuki Y, Suzuki F, Kawamura Y, Yatsuji H, Sezaki H, Hosaka T, Akuta N, et al. Efficacy of entecavir treatment for lamivudine-resistant hepatitis B over 3 years: histological improvement or entecavir resistance? Journal of Gastroenterology & Hepatology. 2009;24:429–435. doi: 10.1111/j.1440-1746.2008.05760.x. [DOI] [PubMed] [Google Scholar]
- 229.Yim HJ, Seo YS, Yoon EL, Kim CW, Lee CD, Park SH, Lee MS, et al. Adding adefovir vs. switching to entecavir for lamivudine-resistant chronic hepatitis B (ACE study): a 2-year follow-up randomized controlled trial. Liver International. 2013;33:244–254. doi: 10.1111/liv.12036. [DOI] [PubMed] [Google Scholar]
- 230.Sheldon J, Camino N, Rodes B, Bartholomeusz A, Kuiper M, Tacke F, Nunez M, et al. Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovir. Antivir Ther. 2005;10:727–734. [PubMed] [Google Scholar]
- 231.Fung S, Kwan P, Fabri M, Horban A, Pelemis M, Hann HW, Gurel S, et al. Tenofovir disoproxil fumarate (TDF) vs. emtricitabine (FTC)/TDF in lamivudine resistant hepatitis B: A 5-year randomised study. J Hepatol. 2017;66:11–18. doi: 10.1016/j.jhep.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 232.Berg T, Zoulim F, Moeller B, Trinh H, Marcellin P, Chan S, Kitrinos KM, et al. Long-term efficacy and safety of emtricitabine plus tenofovir DF vs. tenofovir DF monotherapy in adefovir-experienced chronic hepatitis B patients. J Hepatol. 2014;60:715–722. doi: 10.1016/j.jhep.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 233.van Bömmel F, de Man R, Wedemeyer H, Deterding K, Petersen J, Buggisch P, Erhardt A, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51:73–80. doi: 10.1002/hep.23246. [DOI] [PubMed] [Google Scholar]
- 234.Jang JW, Choi JY, Kim YS, Woo HY, Choi SK, Lee CH, Kim TY, et al. Long-term effect of antiviral therapy on disease course after decompensation in patients with hepatitis B virus-related cirrhosis. Hepatology. 2015;61:1809–1820. doi: 10.1002/hep.27723. [DOI] [PubMed] [Google Scholar]
- 235.Peng CY, Chien RN, Liaw YF. Hepatitis B virus-related decompensated liver cirrhosis: benefits of antiviral therapy. J Hepatol. 2012;57:442–450. doi: 10.1016/j.jhep.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 236.Shim JH, Lee HC, Kim KM, Lim YS, Chung YH, Lee YS, Suh DJ. Efficacy of entecavir in treatment-naive patients with hepatitis B virus-related decompensated cirrhosis. J Hepatol. 2010;52:176–182. doi: 10.1016/j.jhep.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 237.Kim SS, Hwang JC, Lim SG, Ahn SJ, Cheong JY, Cho SW. Effect of virological response to entecavir on the development of hepatocellular carcinoma in hepatitis B viral cirrhotic patients: comparison between compensated and decompensated cirrhosis. Am J Gastroenterol. 2014;109:1223–1233. doi: 10.1038/ajg.2014.145. [DOI] [PubMed] [Google Scholar]
- 238.Papatheodoridis GV, Dalekos GN, Yurdaydin C, Buti M, Goulis J, Arends P, Sypsa V, et al. Incidence and predictors of hepatocellular carcinoma in Caucasian chronic hepatitis B patients receiving entecavir or tenofovir. J Hepatol. 2015;62:363–370. doi: 10.1016/j.jhep.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 239.Singal AK, Salameh H, Kuo YF, Fontana RJ. Meta-analysis: the impact of oral anti-viral agents on the incidence of hepatocellular carcinoma in chronic hepatitis B. Aliment Pharmacol Ther. 2013;38:98–106. doi: 10.1111/apt.12344. [DOI] [PubMed] [Google Scholar]
- 240.Iacobellis A, Andriulli A. Antiviral therapy in compensated and decompensated cirrhotic patients with chronic HCV infection. Expert Opin Pharmacother. 2009;10:1929–1938. doi: 10.1517/14656560903066811. [DOI] [PubMed] [Google Scholar]
- 241.Wang FY, Li B, Li Y, Liu H, Qu WD, Xu HW, Qi JN, et al. Entecavir for Patients with Hepatitis B Decompensated Cirrhosis in China: a meta-analysis. Sci Rep. 2016;6:32722. doi: 10.1038/srep32722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhang X, Liu L, Zhang M, Gao S, Du Y, An Y, Chen S. The efficacy and safety of entecavir in patients with chronic hepatitis B- associated liver failure: a meta-analysis. Ann Hepatol. 2015;14:150–160. [PubMed] [Google Scholar]
- 243.Miquel M, Nunez O, Trapero-Marugan M, Diaz-Sanchez A, Jimenez M, Arenas J, Canos AP. Efficacy and safety of entecavir and/or tenofovir in hepatitis B compensated and decompensated cirrhotic patients in clinical practice. Ann Hepatol. 2013;12:205–212. [PubMed] [Google Scholar]
- 244.Ye XG, Su QM. Effects of entecavir and lamivudine for hepatitis B decompensated cirrhosis: meta-analysis. World J Gastroenterol. 2013;19:6665–6678. doi: 10.3748/wjg.v19.i39.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Cholongitas E, Papatheodoridis GV, Goulis J, Vlachogiannakos J, Karatapanis S, Ketikoglou J, Vasiliadis T, et al. The impact of newer nucleos(t)ide analogues on patients with hepatitis B decompensated cirrhosis. Ann Gastroenterol. 2015;28:109–117. [PMC free article] [PubMed] [Google Scholar]
- 246.Yue-Meng W, Li YH, Wu HM, Yang J, Xu Y, Yang LH, Yang JH. Telbivudine versus lamivudine and entecavir for treatment-naive decompensated hepatitis B virus-related cirrhosis. Clin Exp Med. 2017;17:233–241. doi: 10.1007/s10238-016-0420-7. [DOI] [PubMed] [Google Scholar]
- 247.Singal AK, Fontana RJ. Meta-analysis: oral anti-viral agents in adults with decompensated hepatitis B virus cirrhosis. Aliment Pharmacol Ther. 2012;35:674–689. doi: 10.1111/j.1365-2036.2011.04990.x. [DOI] [PubMed] [Google Scholar]
- 248.Liaw YF, Sheen IS, Lee CM, Akarca US, Papatheodoridis GV, Suet-Hing Wong F, Chang TT, et al. Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology. 2011;53:62–72. doi: 10.1002/hep.23952. [DOI] [PubMed] [Google Scholar]
- 249.Lee SK, Song MJ, Kim SH, Lee BS, Lee TH, Kang YW, Kim SB, et al. Safety and efficacy of tenofovir in chronic hepatitis B-related decompensated cirrhosis. World J Gastroenterol. 2017;23:2396–2403. doi: 10.3748/wjg.v23.i13.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Park J, Jung KS, Lee HW, Kim BK, Kim SU, Kim DY, Ahn SH, et al. Effects of Entecavir and Tenofovir on Renal Function in Patients with Hepatitis B Virus-Related Compensated and Decompensated Cirrhosis. Gut Liver. 2017;11:828–834. doi: 10.5009/gnl16484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lange CM, Bojunga J, Hofmann WP, Wunder K, Mihm U, Zeuzem S, Sarrazin C. Severe lactic acidosis during treatment of chronic hepatitis B with entecavir in patients with impaired liver function. Hepatology. 2009;50:2001–2006. doi: 10.1002/hep.23346. [DOI] [PubMed] [Google Scholar]
- 252.Fox AN, Terrault NA. Individualizing hepatitis B infection prophylaxis in liver transplant recipients. Journal of Hepatology. 2011;55:507–509. doi: 10.1016/j.jhep.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 253.Radhakrishnan K, Chi A, Quan D, Roberts J, Terrault N. Short Course of Post-Operative Hepatitis B Immunoglobulin plus Antivirals Prevents Reinfection of Liver Transplant Recipients. Transplantation. 2017;101:2079–2082. doi: 10.1097/TP.0000000000001786. [DOI] [PubMed] [Google Scholar]
- 254.Teperman L, Spivey J, Poordad F, Schiano T, Bzowej N, Martin P, Coombs D, et al. Randomized Trial of Emtricitabine/Tenofovir DF Plus/Minus HBIG Withdrawal in Prevention of Chronic Hepatitis B Recurrence Post-Liver Transplantation: 48 Week Results. American Journal of Transplantation. 2011;11(suppl 2):48. [Google Scholar]
- 255.Fung J, Wong T, Chok K, Chan A, Cheung TT, Dai J, Sin SL, et al. Long Term Outcomes of Entecavir Monotherapy for Chronic Hepatitis B after Liver Transplantation: Results up to 8 years. Hepatology. 2017;66:1036–1044. doi: 10.1002/hep.29191. [DOI] [PubMed] [Google Scholar]
- 256.Coffin CS, Stock PG, Dove LM, Berg CL, Nissen NN, Curry MP, Ragni M, et al. Virologic and clinical outcomes of hepatitis B virus infection in HIV-HBV coinfected transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10:1268–1275. doi: 10.1111/j.1600-6143.2010.03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.De Simone P, Romagnoli R, Tandoi F, Carrai P, Ercolani G, Peri E, Zamboni F, et al. Early Introduction of Subcutaneous Hepatitis B Immunoglobulin Following Liver Transplantation for Hepatitis B Virus Infection: A Prospective, Multicenter Study. Transplantation. 2016;100:1507–1512. doi: 10.1097/TP.0000000000001171. [DOI] [PubMed] [Google Scholar]
- 258.Yao FY, Osorio RW, Roberts JP, Poordad FF, Briceno MN, Garcia-Kennedy R, Gish RR. Intramuscular hepatitis B immune globulin combined with lamivudine for prophylaxis against hepatitis B recurrence after liver transplantation. Liver Transplantation & Surgery. 1999;5:491–496. doi: 10.1002/lt.500050605. [DOI] [PubMed] [Google Scholar]
- 259.Cholongitas E, Papatheodoridis GV, Burroughs AK. Liver grafts from anti-hepatitis B core positive donors: a systematic review. J Hepatol. 2010;52:272–279. doi: 10.1016/j.jhep.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 260.Perrillo R. Hepatitis B virus prevention strategies for antibody to hepatitis B core antigen-positive liver donation: a survey of North American, European, and Asian-Pacific transplant programs. Liver Transpl. 2009;15:223–232. doi: 10.1002/lt.21675. [DOI] [PubMed] [Google Scholar]
- 261.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 262.Lee J, Cho JH, Lee JS, Ahn DW, Kim CD, Ahn C, Jung IM, et al. Pretransplant Hepatitis B Viral Infection Increases Risk of Death After Kidney Transplantation: A Multicenter Cohort Study in Korea. Medicine (Baltimore) 2016;95:e3671. doi: 10.1097/MD.0000000000003671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Fabrizi F, Martin P, Dixit V, Kanwal F, Dulai G. HBsAg seropositive status and survival after renal transplantation: meta-analysis of observational studies. Am J Transplant. 2005;5:2913–2921. doi: 10.1111/j.1600-6143.2005.01113.x. [DOI] [PubMed] [Google Scholar]
- 264.Chan TM, Fang GX, Tang CS, Cheng IK, Lai KN, Ho SK. Preemptive lamivudine therapy based on HBV DNA level in HBsAg-positive kidney allograft recipients. Hepatology. 2002;36:1246–1252. doi: 10.1053/jhep.2002.36156. [DOI] [PubMed] [Google Scholar]
- 265.Yap DY, Tang CS, Yung S, Choy BY, Yuen MF, Chan TM. Long-term outcome of renal transplant recipients with chronic hepatitis B infection-impact of antiviral treatments. Transplantation. 2010;90:325–330. doi: 10.1097/TP.0b013e3181e5b811. [DOI] [PubMed] [Google Scholar]
- 266.Wachs ME, Amend WJ, Ascher NL, Bretan PN, Emond J, Lake JR, Melzer JS, et al. The risk of transmission of hepatitis B from HBsAg(−), HBcAb(+), HBIgM(−) organ donors. Transplantation. 1995;59:230–234. [PubMed] [Google Scholar]
- 267.Mahboobi N, Tabatabaei SV, Blum HE, Alavian SM. Renal grafts from anti-hepatitis B core-positive donors: a quantitative review of the literature. Transpl Infect Dis. 2012;14:445–451. doi: 10.1111/j.1399-3062.2012.00782.x. [DOI] [PubMed] [Google Scholar]
- 268.Satterthwaite R, Ozgu I, Shidban H, Aswad S, Sunga V, Zapanta R, Jr, Asai P, et al. Risks of transplanting kidneys from hepatitis B surface antigen-negative, hepatitis B core antibody-positive donors. Transplantation. 1997;64:432–435. doi: 10.1097/00007890-199708150-00011. [DOI] [PubMed] [Google Scholar]
- 269.Ouseph R, Eng M, Ravindra K, Brock GN, Buell JF, Marvin MR. Review of the use of hepatitis B core antibody-positive kidney donors. Transplant Rev (Orlando) 2010;24:167–171. doi: 10.1016/j.trre.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 270.Bussler S, Vogel M, Pietzner D, Harms K, Buzek T, Penke M, Handel N, et al. New pediatric percentiles of liver enzyme serum levels (ALT, AST, GGT): Effects of age, sex, BMI and pubertal stage. Hepatology. 2017 doi: 10.1002/hep.29542. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 271.Poustchi H, George J, Esmaili S, Esna-Ashari F, Ardalan G, Sepanlou SG, Alavian SM. Gender differences in healthy ranges for serum alanine aminotransferase levels in adolescence. PLoS One. 2011;6:e21178. doi: 10.1371/journal.pone.0021178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Schwimmer JB, Dunn W, Norman GJ, Pardee PE, Middleton MS, Kerkar N, Sirlin CB. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology. 2010;138:1357–1364. 1364.e1–2. doi: 10.1053/j.gastro.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.England K, Thorne C, Pembrey L, Tovo PA, Newell ML. Age- and sex-related reference ranges of alanine aminotransferase levels in children: European paediatric HCV network. J Pediatr Gastroenterol Nutr. 2009;49:71–77. doi: 10.1097/MPG.0b013e31818fc63b. [DOI] [PubMed] [Google Scholar]