Abstract
Long-term heavy cigarette smoking is a well-known high-risk factor for carcinogenesis in various organs such as the head and neck, lungs, and urinary bladder. Furthermore, cigarette smoking can systemically accelerate aging, and as the result, promoting carcinogenesis via changing the host microenvironment. Various inflammatory factors, hormones, and chemical mediators induced by smoking mediate carcinoma-related molecules and induce carcinogenesis. MicroRNAs (miRNAs) are a family of short noncoding RNA molecules that bind to mRNAs and inhibit their expression. Cigarette smoke induces the expression of various miRNAs, many of which are known to function in the post-transcriptional silencing of anticancer molecules, thereby leading to smoking-induced carcinogenesis. Analysis of expression profiles of smoking-induced miRNAs can help identify biomarkers for the diagnosis and prognosis of smoking-related cancers and prediction of therapeutic responses, as well as revealing promising therapeutic targets. Here, we introduce the most recent and useful findings of miRNA analyses focused on lung cancer and urinary bladder cancer, which are strongly associated with cigarette smoking, and discuss the utility of miRNAs as clinical biomarkers.
Keywords: microRNA, smoking, carcinogenesis, lung carcinoma, bladder carcinoma
1. Introduction
Cigarette smoking has been proposed as the cause of various cancers, including lung, urinary system and bladder, head and neck, liver, esophagus, pancreas, and oral cancer [1,2]. Smoking substantially increases the risk of carcinogenesis and development of cancer in various types of organs [2]. The number of cigarettes smoked per day is positively correlated with clinical outcomes, such as the onset of various carcinomas [1].
The main adverse effect of smoking is that carcinogens derived from cigarettes trigger inflammatory reactions, which lead to damage of tumor-related genes, misrepair, and neoplastic transformations, including autonomy. Aging is also a factor. Immune function is gradually impaired with age, while production of inflammatory cytokines increases. The consequence can be the induction of genetic abnormalities. Smoking-related carcinogenesis is closely associated with aging due to chronic persistent inflammation [3]. The myriad of carcinogens in cigarette smoke includes tobacco nitrosoamine, which may affect tumorigenesis in diverse organs. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is a tobacco-specific metabolite that is a potent carcinogen in experimental animal models of lung adenoma and carcinoma [4,5,6,7,8].
MicroRNAs (miRNAs) are short, single-stranded, noncoding RNAs that participate in the post-transcriptional regulation of gene expression [9]. Their primary function is to reduce the expression of their target genes by binding to the 3′ untranslated region (3′-UTR) of the messenger RNA (mRNA), which leads to the degradation of the mRNA or other translational repressive mechanisms. miRNAs are among the most abundant classes of gene regulatory molecules, and thousands of distinct miRNAs have been identified in mammals including humans [10]. The majority of mammalian genes may be under miRNA regulation. At least 45,000 miRNA target sites within human 3′-UTRs are conserved above background levels, and more than 60% of human protein-coding genes are under selective pressure to maintain pairing to miRNAs [11]. miRNAs contribute to biological processes, including the development, maturation, metabolism, aging, and carcinogenesis of various organs. A central goal for understanding the function of all these small regulatory RNAs has been to determine which messages are targeted for repression.
In addition to lung carcinoma, urothelial carcinoma in the urinary tract is among the most common malignancies in individuals with a history of heavy smoking. However, even though smoking is considered to be a common risk factor of urothelial and lung carcinomas, it remains unclear how smoking and its related molecular signals can initiate or promote carcinogenic processes in the urinary tract as compared with lung carcinoma. To date, reviews have focused only on comparisons between urothelial and lung carcinoma relative to cigarette smoking. Previous studies revealed that cigarette smoking regulates key miRNAs involved in the expression and regulation of target genes in lung carcinoma [12,13,14,15,16]. In addition, molecular markers associated with cigarette smoking have been found in urothelial carcinomas [17,18,19]. These reports have raised the possibility that cigarette smoking may contribute to oncogenic or antioncogenic gene expression by regulating miRNAs in urinary tract cancer.
In this review, we summarize the evidence concerning the smoking-dependent regulation of miRNA and its target genes in lung and urinary tract carcinomas. We also propose available methods or evidence to elucidate the mechanisms of tumorigenesis and progression of urothelial carcinomas.
2. Correlation among Smoking, Cancer, and Expression of miRNAs
Environmental factors, including diet, smoking, alcohol consumption, stress, infectious agents, and environmental carcinogens, are important in the pathogenesis of cancers through epigenetic modifications [20]. Increasing epidemiological evidence links cigarette smoking with cancer. Cigarette smoking and infectious agents are the major causes of cancer in Japan [21]. Evidence of the association between cigarette smoking and cancers of the respiratory system (including lung), digestive tract, and urinary system has accumulated over time [1]. A comprehensive meta-analysis quantified much of the existing evidence linking smoking with well-known anatomic cancer sites, such as the respiratory, upper digestive, and urinary tracts [1].
Carcinogenesis is a multistep process that includes the initiation, promotion, progression, malignant conversion, and finally the formation of a fully developed tumor in which genetic changes, including some kinds of molecular function failure based on DNA breaks, play critical roles [22]. At variable steps of DNA breaks, the aberrant expression patterns of miRNAs can be oncogenic or antioncogenic factors [23] and are associated with cellular dysfunction and onset of disease [12]. Altered miRNA expression causes neoplastic transformation [24]. In non-small cell lung carcinoma (NSCLC), miR-224 promotes cell migration, invasion, and proliferation. miR-224 directly targets tumor necrosis factor-alpha-induced protein 1 and SMAD4, and the expression of miR-224 is regulated by extracellular signal-regulated kinase signaling through the binding of c-Jun binding to the miR-224 promoter region in lung carcinoma [25]. Immunoglobulin binding protein 1 (IGBP1) is commonly expressed in lung adenocarcinoma, including the early stage of lung adenocarcinogenesis. miR-3941 is a tumor-suppressive miRNA that directly targets and regulates IGBP1. Overexpression of miR-3941 and suppression of IGBP1 induces apoptosis by events that include increased cleavage of caspase-3 and poly (ADP-ribose) polymerase [26]. Alterations in the expression of miR-125b, a tumor-suppressing miRNA, trigger an early event in prostate carcinoma. miR-125b directly targets ErbB2/B3 and MET, and loss of miR-125b results in enhanced signaling via the MET-regulated phosphoinositol-3-kinase/protein kinase B and RAS/pMEK pathways [27].
Aging manifests biologically as changes in genome and protein instability, telomeric shortening, nutrient-sensitive abnormalities, mitochondrial dysfunction, cellular senescence, stem cell depletion, and intercellular signal transduction abnormalities [28,29,30]. These molecular dysfunctions mediate apoptosis, cell senescence, and/or autophagy or carcinogenesis by aberrant genomic expression. Various carcinogens produced by smoking accelerate aging accompanied with genomic and molecular changes through genetic modifications or mutations [31,32,33,34]. Oncogenic miRNAs inhibit the expression of some tumor suppressor molecules and promote the acceleration of aging with associated carcinogenesis; in contrast, suppression of these miRNAs promotes cellular senescence and decreases cell proliferation [2,11,35].
Some miRNAs play an important role in tumor invasion and metastasis [36]. The epithelial-mesenchymal transition (EMT) is a crucial mechanism for tumor invasion and metastasis. We previously demonstrated that the miR-331-3p and syndecan-1 axis may contribute to the progression of prostate cancer through the regulation of nucleus accumbens-associated protein 1 and neuropilin 2, and the promotion of EMT [37]. The overexpression of miR-331-3p in asbestos-related lung cancer has been described [38]. The collective evidence demonstrates that miR-331-3p is a tumor-promoting miRNA because it enhances the EMT. Upon binding of programmed cell death protein 1 (PD-1), a cell surface receptor present on T cells and pro-B cells, to its ligands PD-L1 and PD-L2, an immunosuppressive effect is triggered, which allows tumor cells to escape immune detection and destruction [39]. PD-L1 is highly expressed in multiple intracarcinoma cell types that may play an effective role in CD8+ T-cell suppression. This has profound therapeutic effects in advanced NSCLC, such as the disturbance of interactions between PD-1, which is a coinhibitory receptor, and PD-L1, which is a ligand [40]. miR-200 acts as the target of the EMT-inducing transcription factor zinc finger E-box-binding homeobox 1 (ZEB1) in the regulation of gene expression of PD-L1. miR-200 also controls cytotoxic CD8p tumor-infiltrating lymphocytes, and the miR-200/ZEB1 axis also regulates the expression of PD-L1. Thus, an EMT regulatory program for the exhaustion of CD8p tumor-infiltrating lymphocytes and immunosuppression of PD-L1 via the miR-200/ZEB1 axis is critical to tumor metastasis. Likewise, miR-155 functions as an immune system activator by blocking autophagy, which promotes the upregulation of CD4+ and infiltration of FoxP3+ tumor-infiltrating lymphocytes, which results in increased lung carcinoma chemosensitivity [41].
2.1. Lung
Lung cancer is a major neoplasia. There are two main distinct histological types: small cell and non-small cell carcinomas (NSCLC) [42]. Small cell carcinoma is completely different from NSCLC from the perspectives of pathogenesis, cellular origin, molecular alteration, and histopathological and clinical features [43,44]. On the basis of molecular pathology, lung carcinoma occurs after multiple processes including genomic instability, mutations, deletions, insertions, modifications (such as methylation and sialylation), and gene silencing by miRNAs [13,45]. During the early steps in the oncogenesis of lung carcinoma, mutations of tumor suppressor or enhancer genes have been identified. Once the repair of DNA damage fails, these intrinsic mutations may be important carcinogens underlying genomic instability and may accelerate cancer progression [46].
As well as genetic modification, epigenetic coregulation of miRNAs and their target molecules is an important step. miRNA-486-5p is a tumor suppressor miRNA that is often downregulated, and which correlated with ankyrin 1 (ANK1) expression in NSCLC. The ANK1 promoter CpG island is unmethylated in normal lungs, but is methylated in the lungs of individuals with adenocarcinoma, and more so than in those with squamous cell carcinoma [47]. Among exogenous carcinogens, cigarette smoking is the most important risk factor for the onset and development of lung cancer. Both smokers and those exposed to secondhand smoke in households or workspaces are at a higher risk of lung carcinoma than nonsmokers [46,48]. Exposure of more than pack-years increased the risk of lung cancer among nonsmokers by approximately 30% [48,49]. ANK1 methylation is significantly more prevalent in adenocarcinoma compared to squamous cell carcinoma [47]. E-cadherin expression levels in smokers with non-small cell lung cell carcinoma are reportedly lower than those in people with the disease who had never smoked. Cigarette smoking induces the repression of E-cadherin by regulating the expression of transcription factors lymphoid enhancer-binding factor 1 and Slug, which leads to EMT [8]. Cigarette smoking for many decades leads to genetic mutations or deletions of bronchial epithelial cells, which can result in dysplasia and subsequent carcinoma. Smoking affects the expression of miRNAs in the bronchial epithelium. Gene mutations most closely related to carcinogenesis occur due to exposure to some cigarette carcinogens. A spectrum of TP53 mutations have been documented in cancers arising in active as well as former smokers [50,51], with greater prevalence in women with a history of heavy smoking. In addition, the significance of the genetic background for lung carcinoma has been noted, with miRNA-related molecular signals mainly involved in malignant transformation. For example, we introduced miR-126, which plays a crucial role as a cancer-suppressive miRNA in the liver, pancreas, lung, colon, and stomach [52,53,54,55,56,57]. In lung carcinoma cells, including cell lines, miR-126 expression is suppressed, leading to the upregulation of the target gene encoding vascular endothelial growth factor [58]. Significant changes have been found in the expression of 133 miRNAs in the lungs of rats exposed to cigarette smoke; some of these miRNAs may participate in the carcinogenic process [59]. In vitro experimental model systems for miRNA alterations mediated by cigarette smoking in normal human bronchial epithelial and lung cancer cells derived from smokers and nonsmokers have demonstrated the repression of miR-487b, which leads to the upregulation of suppressor of zest 12 (SUZ12), B cell-specific Moloney murine leukemia virus insertion site 1 (BMI1), wingless-type family member 5A (WNT5A), MYC, and KRAS, thereby increasing cell proliferation, invasion, tumorigenicity, and metastatic potential of lung cancer cells in the cigarette-smoking group [14]. In smokers, the expression of antioncogenic let-7 family members is inversely associated with the number of cigarettes smoked per day by females, but not by males [13]. Expression of oncogenic miR-21 correlates with the number of cigarettes smoked per day in squamous cell carcinoma, but not in adenocarcinoma [13]. Interestingly, in one study, no significant differences were observed in smoking histories, such as current versus former smoking status, years of smoking, age at smoking initiation, or exposure to passive smoking [13]. The microarray analysis of RNA in bronchial epithelial cells revealed a significant difference between smokers and nonsmokers in the expression of 28 miRNAs [15]. Interestingly, in females, smokers have a higher risk for epidermal growth factor receptor (EGFR) mutations (exons 18, 19, and 21) compared to nonsmokers. These findings suggest that the histological type is closely associated with the mechanism of action of smoking-related carcinogens. There are two mechanisms: One is smoking-related and -unrelated carcinogenesis and cancer development integrated by miRNA. The other is EGFR mutations based on the DNA modification and mutations in lung cancer. Moreover, detailed information of the smoking history allows us to select biomarkers and the most useful cancer therapy.
2.2. Urinary Tract
Various risk factors for urothelial carcinoma have been reported. They include cigarette smoking and occupational exposure to aromatic amines, polycyclic aromatic hydrocarbons, or dyes; alcohol or tea consumption; treatment with medical compounds; and genetic susceptibility [19]. Cigarette smoke contains more than 4000 identified chemical constituents that are associated with a high risk for cancer throughout the urinary tract [19,60]. Cigarette smoke contains polycyclic aromatic hydrocarbons, aromatic amines, and N-nitroso compounds [61,62]. These carcinogens induce the formation of DNA adducts and cause tumorigenesis of the bladder. Therefore, the level of DNA adducts is a useful biomarker to understand the degree of exposure to cigarette smoke-related carcinogens. Case-control studies have reported the higher incidence of renal pelvis and ureter cancers compared to bladder cancer, because exposure to carcinogens may be more concentrated in the renal pelvis and ureter than the bladder, where dilution or degradation of carcinogens may occur [19].
Some mechanisms of carcinogenesis in the urinary tract have been suggested. For example, DNA methylation retains cell proliferation in the normal urothelium [63,64,65,66]. One group of gene clusters was estimated taking into account the important role of miRNAs. miR-99a was found to be overexpressed in tumors with muscle invasion of the bladder. Marked downregulation of miR-99a and miR-205 was observed in smokers with bladder cancer [67]. Additionally, a significant association was detected between smoking and muscle invasion of the bladder [59].
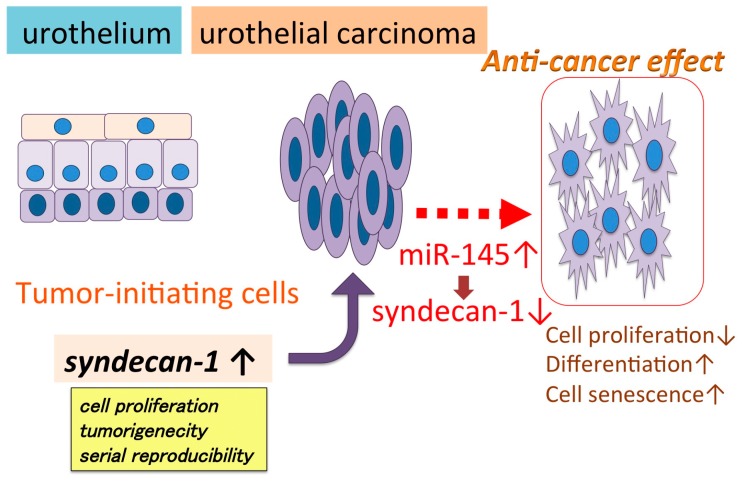
Many reports [68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105] have indicated the key role of miRNAs in neoplastic transformation by propagating cell proliferation and tumor invasion and migration (Table 1, Figure 1). Our previous study indicated that miR-145 suppresses syndecan-1, upregulates stem cell factors, and induces cell senescence and differentiation, and increased squamous, glandular, and neuroendocrine markers. These results suggest that miR-145 utilizes syndecan-1 to modulate cell proliferation, reprogramming, and differentiation in urothelial carcinoma [86] (Figure 1). However, we have limited data on the interaction of miRNA with high-risk compounds, such as cigarette smoke, for carcinogenesis. Cigarette smoking greatly contributes to urothelial carcinoma, and it is reported that cigarette smoking cessation decreases the risk of urothelial carcinoma and may contribute to preventing recurrence and progression. Smoking-induced carcinogenesis may be different from the miRNA-related tumor extension mechanism that has been reported. Thus, miRNA expression associated with smoking-related carcinogenesis may be different from the mechanism of progression-related miRNA expression. If the miRNAs associated with carcinogenesis related to smoking can be determined, it could help in the elucidation of more detailed carcinogenetic mechanisms and detection of early stages of urothelial carcinomas. New findings of miRNAs involved in molecular abnormalities caused by smoking may lead to the development of liquid biopsy techniques using blood and urine for the diagnosis of cigarette-smoking related tumors, including lung and urothelial carcinomas.
Table 1.
Relationship between the microRNA (miRNA) and mRNA of target molecules.
| miRNA | Target Molecules | Function | Reference |
|---|---|---|---|
| miR-99a-5p | mTOR | Decreased phosphorylation of mTOR and AKT | [68] |
| miR-29a | DNMT3A, DNMT3B, MAT2A, SMS | Involvement in the cysteine and methionine metabolism | [69] |
| miR-210 | HIF-1α | Promoting upper tract urothelial carcinoma carcinogenesis | [70,71] |
| miR-429 | CDKN2B | Promoted cell growth and decreased apoptosis | [72] |
| miR-30a-5p | claudin-5 | Suppressed cell proliferation, migration and EMT | [73] |
| miR-32-5p, -224-5p, -412-3p, -203a-3p, -205-5p | Cancer specific survival, tumor progression, EMT | [74] | |
| miR-21-5p | Novel biomarker of urothelial carcinoma in urine | [75] | |
| miR-193b | ETS1, Cyclin D1 | Inhibited cell migration activity, arrested cell at G1 phase; sensitized CDDP treatment | [76] |
| miR-3713 | MMP9 | Control of cell invasiveness | [77] |
| miR-451 | c-Myc | Suppressed cell migration and invasion | [78] |
| miR-497 | E2F3 | Inhibited cell proliferation, migration and invasion | [79] |
| miR-877-3p | p16 | Increased the expression of p16, inhibited cell proliferation and tumorigenicity | [80] |
| miR-130b | NF-κB | Persistent activation of NF-κB; promote the malignant progression of urothelial carcinoma | [81] |
| miR-133b | Novel biomarker of urothelial carcinoma in the tissue | [82] | |
| miR-146a-5p | Novel biomarker of urothelial carcinoma in urine | [83] | |
| miR-429 | E-cadherin | Decreased cell migration and invasion through reducing ZEB1 and β-catenin | [84] |
| miR-30a | Notch1 | Decreased cell proliferation and migration, activated cell cycle arrest | [85] |
| miR-145 | syndecan-1 | Suppressed cell proliferation, induced cell senescence, differentiation | [86] |
| miR-24 | CARMA3 | Inhibited cell proliferation, invasion and EMT | [87] |
| miR-148a | DNMT1 | Reduced cell viability through apoptosis | [88] |
| miR-182 | Novel biomarker of urothelial carcinoma in urine | [89] | |
| miR-9 | CBX7 | Decreased cell invasion ability | [90] |
| miR-34a | S100P | Decreased cell invasion ability | [91] |
| miR-100 | BAZ2A, mTOR, SMARCA5 | Increased cell proliferation, anti-apoptosis | [92] |
| miR-99a, -100 | FGFR3, FOXA1 | Associated with regional hypomethylation | [93] |
| miR-1 | UCA1 | Decreased cell proliferation and motility, induced apoptosis | [94] |
| miR-29c | BCL-2, MCL-1 | Induced apoptosis | [95] |
| miR-101 | Novel biomarker of urothelial carcinoma in the tissue | [96] | |
| miR-126 | ADAM9 | Decreased cell invasion | [97] |
| miR27a | AGGF1 | Regulation of hypoxia-induced apoptosis | [98] |
| miR-320a | ITGB3. | Decreased cell invasion ability | [99] |
| miR-23b | Zeb1 | Inhibited cell proliferation, induced G0/G1 cell cycle arreset | [100] |
| miR-96 | FOXO1 | Tumorigenesis, control cell apoptosis | [101] |
| miR-34a | Notch1 | Decreased cell invasion and migration | [102] |
| miR-143 | cyclooxygenase-2 | Decreased cell proliferation and motility | [103] |
| miR-125b | E2F3 | Regulate G1/S transition through the E2F3-cyclin A2 signaling pathway | [104] |
| miR-101 | EZH2 | Inhibited cell proliferation and colony formation | [105] |
Figure 1.
miR-145 regulates cell proliferation, differentiation, and senescence by regulating syndecan-1 in urothelial carcinoma cells. miR-145 and syndecan-1, a putative direct target of miR-145, control the expression of cell differentiation markers [70].
3. Relationship between Smoking and DNA Breaks
Cigarette smoke is a rich source of polycyclic aromatic hydrocarbons, aromatic amines, heterocyclic amines, and N-nitroso compounds, and these compounds may lead to DNA damage. Additionally, cigarette smoke is also a rich source of reactive oxygen species, which can induce damage to DNA and can accumulate in the urinary bladder as metabolic products of the chemical carcinogens of cigarette smoke [31,32]. The constituents of cigarette smoke can cause DNA double-strand breaks, leading to tumorigenesis. Nibrin (NBS1), a component of the MRE-RAD50-NBS1 (MRN) gene, plays an important role in the DNA double-strand break repair pathway [22]. NBS1 has been found to increase NBS1 gene expression and associated NBS1 polymorphisms in smoking-related cancers, such as those of the lungs, liver, esophagus, head, and neck [106,107].
These findings support the hypothesis that genetic variation plays a critical role in smoking-related carcinogenesis.
4. Smoking and Aging
During aging, activation of various steps of the carcinogenic process occurs. These include mutation, induction, and cell proliferation. Cigarette smoking has cancer-promoting effects that include the induction of mutations. Cigarette smoking induces many steps of carcinogenesis. If cigarette smoking affects all carcinogenic processes in the same proportion to aging, the risk prediction in current and former smokers can be an efficient epidemiological cancer model. The duration of cigarette smoking is a more powerful predictor of lung carcinoma mortality than cigarette smoking intensity, regardless of age and sex [108].
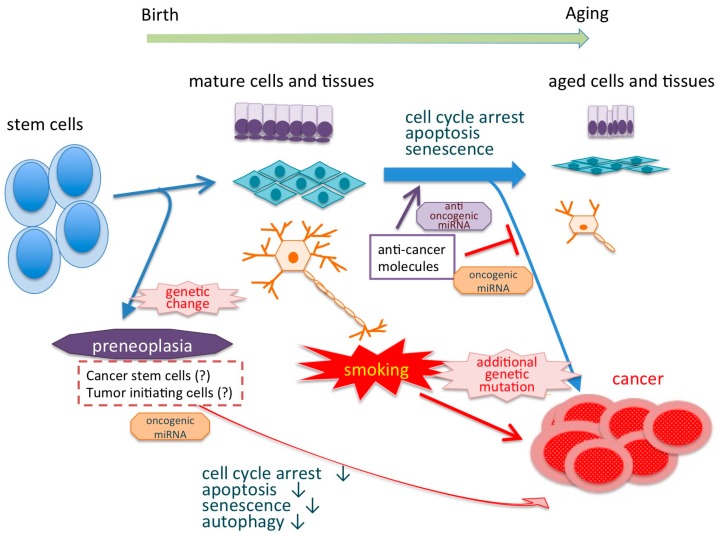
Regardless of smoking, most cancers occur in adults older than 70 years of age. However, many cancers, including lung, gastrointestinal, and urothelial carcinoma, occur in younger adults because of cigarette smoking. Aging has various genomic and epigenomic features, such as increased genomic instability, shortened telomeres, global DNA hypomethylation, complex histone modification, and deregulation of miRNA expression. Features of genetic change, cellular dysfunction, and carcinogenesis in aging cells are biologically similar to the characteristics observed during the carcinogenesis arising from cigarette smoking [28] (Figure 2). For example, autophagy and cell senescence have a dual role in carcinogenesis. They both have tumor growth-promoting and tumor-suppressive effects. Autophagy is downregulated in precancerous lesions and cancers. However, autophagy is initiated to respond to smoking-induced oxidative damage, which can have a protective effect in the early stages of carcinogenesis [109]. In our study, miR-331-3p upregulated autophagy via increasing LC3 concomitantly with a reduction of cell proliferation in urothelial cell lines (unpublished data). Determination of the miRNAs controlling the biological pathways that greatly contribute to aging, such as autophagy and cell senescence, may reveal the mechanism underlying smoking-related carcinogenesis.
Figure 2.
Life time from stem cells to aging and/or carcinogenesis. Genetic changes such as mutations induce carcinogenesis by suppressing the production of anticancer molecules via activating oncogenic miRNAs.
5. MiRNA Detection
Blood- and urine-based biomarkers for circulating miRNAs can be detected using several tools, such as quantitative reverse transcription PCR (qRT-PCR) [110], microarray [111], deep sequencing [112], and next-generation sequencing (NGS) [112] in various tumors [113]. miRNAs are stably expressed in human blood, and circulating miRNAs may serve as novel molecular markers for tumors [114,115,116]. An analysis of the expression of miRNAs in plasma from ALK-positive NSCLC patients identified mir-660-5p and miR-362-5p as potential predictors for the response to crizotinib treatment [114]. The detection of circulating miRNAs in plasma and other body cavity fluids is a more useful tool for noninvasive biomarkers than other tissue or cytology specimens for the evaluating of the therapeutic response or to predict outcome [114,115,116].
High-quality nucleic acid extraction is necessary to obtain better sensitivity and specificity in the detection of tumor-specific RNA. The liquid-based cytology and tissue specimens treated with formalin-based fixation are unsuitable for extraction of DNA and/or RNA. We previously reported that the quantity of nucleic acid derived from cancer cells fixed using formaldehyde-containing fixatives is significantly lower compared to alcohol-based fixatives [117]. Since liquid biopsy specimens do not require formalin fixation, high-quality nucleic acid can be extracted. It is important to choose the method based on the detection-related purpose of the extracted nucleic acid extraction to the detection tool.
6. Conclusions
Various genes have been associated with the neoplastic transformation of respiratory epithelium by cigarette smoking. miRNAs play an important role in the regulation of genes involved in carcinogenesis and cancer progression, such as those involved in autophagy and cell senescence. In addition, a number of reports have shown that miRNAs contribute to the genetic change caused by cigarette smoking. Thus, miRNAs appear to be a mainstay of smoking-induced cancer development in the lung. Similar mechanisms involving aberrant expression and mutations of genes due to smoking, leading to urothelial carcinoma in the urinary tract, have been reported (Table 1). However, the relationship between the cigarette smoking-related regulation of miRNA and carcinogenesis remains unknown. Chemical compounds produced by smoking cause biological influences via aberrant expression and mutation of miRNAs. Therefore, cancer-related molecules controlled by miRNA are involved in carcinogenesis and cancer progression and could be the leading cause of smoking-related carcinogenesis. miRNA and downstream molecules could be considered as biomarkers, and it is a major future goal to consider these biomarkers in the development of molecular target therapy. Smoking cessation is expected to improve the prognosis or prevent the recurrence or (temporally heterogeneous) multiple occurrences of urinary tract cancers.
The molecular mechanisms of smoking-related carcinogenesis in relation to miRNA have been well established in lung cancer, but many unknown issues and problems need to be addressed in urothelial carcinomas because smoking is a high-risk factor for these types of cancers. We need to examine the association between urothelial carcinoma and smoking. The duration of cigarette smoking is associated with biological genetic features and carcinogenesis-related changes similar to aging. The promotion of the aging effect by cigarette smoking is responsible for the occurrence of carcinogenesis in adolescence or young adults (Figure 2). Estimation of miRNAs and their target molecules associated with smoking provides useful information to develop a novel cancer prevention tool, diagnostic markers, and molecular target therapy.
Author Contributions
K.S., T.N., C.O. and T.F. prepared the manuscript; C.O. critically revised the manuscript. All authors approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gandini S., Botteri E., Iodice S., Boniol M., Lowenfels A.B., Maisonneuve P., Boyle P. Tobacco smoking and cancer: A meta-analysis. Int. J. Cancer. 2008;122:155–164. doi: 10.1002/ijc.23033. [DOI] [PubMed] [Google Scholar]
- 2.Momi N., Kaur S., Rachagani S., Ganti A.K., Batra S.K. Smoking and microRNA dysregulation: A cancerous combination. Trends Mol. Med. 2014;20:36–47. doi: 10.1016/j.molmed.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franceschi C., Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69(Suppl. 1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 4.Hecht S.S., Hoffmann D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis. 1988;9:875–884. doi: 10.1093/carcin/9.6.875. [DOI] [PubMed] [Google Scholar]
- 5.Lee H.L., Hsueh Y.M., Chung C.J., Pu Y.S., Chang L.W., Hsieh D.P., Liou S.H., Lin P. Correlation between the urine profile of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone metabolites and N7-methylguanine in urothelial carcinoma patients. Cancer Epidemiol. Biomark. Prev. 2008;17:3390–3395. doi: 10.1158/1055-9965.EPI-08-0761. [DOI] [PubMed] [Google Scholar]
- 6.Hecht S.S. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann D., Brunnemann K.D., Prokopczyk B., Djordjevic M.V. Tobacco-specific N-nitrosamines and Areca-derived N-nitrosamines: Chemistry, biochemistry, carcinogenicity, and relevance to humans. J. Toxicol. Environ. Health. 1994;41:1–52. doi: 10.1080/15287399409531825. [DOI] [PubMed] [Google Scholar]
- 8.Nagathihalli N.S., Massion P.P., Gonzalez A.L., Lu P., Datta P.K. Smoking induces epithelial-to-mesenchymal transition in non-small cell lung cancer through HDAC-mediated downregulation of E-cadherin. Mol. Cancer Ther. 2012;11:2362–2372. doi: 10.1158/1535-7163.MCT-12-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Driscoll L. The emerging world of microRNAs. Anticancer Res. 2006;26:4271–4278. [PubMed] [Google Scholar]
- 10.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 11.Friedman R.C., Farh K.K., Burge C.B., Bartel D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee A., Luettich K. MicroRNAs as potential biomarkers of smoking-related diseases. Biomark. Med. 2012;6:671–684. doi: 10.2217/bmm.12.50. [DOI] [PubMed] [Google Scholar]
- 13.Landi M.T., Zhao Y., Rotunno M., Koshiol J., Liu H., Bergen A.W., Rubagotti M., Goldstein A.M., Linnoila I., Marincola F.M., et al. MicroRNA expression differentiates histology and predicts survival of lung cancer. Clin. Cancer Res. 2010;16:430–441. doi: 10.1158/1078-0432.CCR-09-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xi S., Xu H., Shan J., Tao Y., Hong J.A., Inchauste S., Zhang M., Kunst T.F., Mercedes L., Schrump D.S. Cigarette smoke mediates epigenetic repression of miR-487b during pulmonary carcinogenesis. J. Clin. Investig. 2013;123:1241–1261. doi: 10.1172/JCI61271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schembri F., Sridhar S., Perdomo C., Gustafson A.M., Zhang X., Ergun A., Lu J., Liu G., Zhang X., Bowers J., et al. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc. Natl. Acad. Sci. USA. 2009;106:2319–2324. doi: 10.1073/pnas.0806383106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inamura K., Ishikawa Y. MicroRNA in Lung Cancer: Novel Biomarkers and Potential Tools for Treatment. J. Clin. Med. 2016;5:36. doi: 10.3390/jcm5030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brennan P., Bogillot O., Cordier S., Greiser E., Schill W., Vineis P., Lopez-Abente G., Tzonou A., Chang-Claude J., Bolm-Audorff U., et al. Cigarette smoking and bladder cancer in men: A pooled analysis of 11 case-control studies. Int. J. Cancer. 2000;86:289–294. doi: 10.1002/(SICI)1097-0215(20000415)86:2<289::AID-IJC21>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 18.Strope S.A., Montie J.E. The causal role of cigarette smoking in bladder cancer initiation and progression, and the role of urologists in smoking cessation. J. Urol. 2008;180:31–37. doi: 10.1016/j.juro.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin J.K., Silverman D.T., Hsing A.W., Ross R.K., Schoenberg J.B., Yu M.C., Stemhagen A., Lynch C.F., Blot W.J., Fraumeni J.F., Jr. Cigarette smoking and cancers of the renal pelvis and ureter. Cancer Res. 1992;52:254–257. [PubMed] [Google Scholar]
- 20.Mathers J.C., Strathdee G., Relton C.L. Induction of epigenetic alterations by dietary and other environmental factors. Adv. Genet. 2010;71:3–39. doi: 10.1016/B978-0-12-380864-6.00001-8. [DOI] [PubMed] [Google Scholar]
- 21.Inoue M., Sawada N., Matsuda T., Iwasaki M., Sasazuki S., Shimazu T., Shibuya K., Tsugane S. Attributable causes of cancer in Japan in 2005—Systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann. Oncol. 2012;23:1362–1369. doi: 10.1093/annonc/mdr437. [DOI] [PubMed] [Google Scholar]
- 22.Park S.L., Bastani D., Goldstein B.Y., Chang S.C., Cozen W., Cai L., Cordon-Cardo C., Ding B., Greenland S., He N., et al. Associations between NBS1 polymorphisms, haplotypes and smoking-related cancers. Carcinogenesis. 2010;31:1264–1271. doi: 10.1093/carcin/bgq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lema C., Cunningham M.J. MicroRNAs and their implications in toxicological research. Toxicol. Lett. 2010;198:100–105. doi: 10.1016/j.toxlet.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Stanitz E., Juhasz K., Gombos K., Gocze K., Toth C., Kiss I. Alteration of miRNA expression correlates with lifestyle, social and environmental determinants in esophageal carcinoma. Anticancer Res. 2015;35:1091–1097. [PubMed] [Google Scholar]
- 25.Cui R., Meng W., Sun H.L., Kim T., Ye Z., Fassan M., Jeon Y.J., Li B., Vicentini C., Peng Y., et al. MicroRNA-224 promotes tumor progression in non-small cell lung cancer. Proc. Natl. Acad. Sci. USA. 2015;112:E4288–E4297. doi: 10.1073/pnas.1502068112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato T., Shiba-Ishii A., Kim Y., Dai T., Husni R.E., Hong J., Kano J., Sakashita S., Iijima T., Noguchi M. miR-3941: A novel microRNA that controls IGBP1 expression and is associated with malignant progression of lung adenocarcinoma. Cancer Sci. 2017;108:536–542. doi: 10.1111/cas.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budd W.T., Seashols-Williams S.J., Clark G.C., Weaver D., Calvert V., Petricoin E., Dragoescu E.A., O’Hanlon K., Zehner Z.E. Dual action of miR-125b as a tumor suppressor and oncomiR-22 promotes prostate cancer tumorigenesis. PLoS ONE. 2015;10:e0142373. doi: 10.1371/journal.pone.0142373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aunan J.R., Cho W.C., Soreide K. The biology of aging and cancer: A brief overview of shared and divergent molecular hallmarks. Aging Dis. 2017;8:628–642. doi: 10.14336/AD.2017.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernardes de Jesus B., Blasco M.A. Telomerase at the intersection of cancer and aging. Trends Genet. 2013;29:513–520. doi: 10.1016/j.tig.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe M. Smoking: Additional burden on aging and death. Genes Environ. 2016;38 doi: 10.1186/s41021-016-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stern M.C., Lin J., Figueroa J.D., Kelsey K.T., Kiltie A.E., Yuan J.M., Matullo G., Fletcher T., Benhamou S., Taylor J.A., et al. Polymorphisms in DNA repair genes, smoking, and bladder cancer risk: Findings from the international consortium of bladder cancer. Cancer Res. 2009;69:6857–6864. doi: 10.1158/0008-5472.CAN-09-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vineis P., Talaska G., Malaveille C., Bartsch H., Martone T., Sithisarankul P., Strickland P. DNA adducts in urothelial cells: Relationship with biomarkers of exposure to arylamines and polycyclic aromatic hydrocarbons from tobacco smoke. Int. J. Cancer. 1996;65:314–316. doi: 10.1002/(SICI)1097-0215(19960126)65:3<314::AID-IJC6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 33.Turesky R.J., Freeman J.P., Holland R.D., Nestorick D.M., Miller D.W., Ratnasinghe D.L., Kadlubar F.F. Identification of aminobiphenyl derivatives in commercial hair dyes. Chem. Res. Toxicol. 2003;16:1162–1173. doi: 10.1021/tx030029r. [DOI] [PubMed] [Google Scholar]
- 34.Pryor W.A., Hales B.J., Premovic P.I., Church D.F. The radicals in cigarette tar: Their nature and suggested physiological implications. Science. 1983;220:425–427. doi: 10.1126/science.6301009. [DOI] [PubMed] [Google Scholar]
- 35.Liz J., Esteller M. lncRNAs and microRNAs with a role in cancer development. Biochim. Biophys. Acta. 2016;1859:169–176. doi: 10.1016/j.bbagrm.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Ruan K., Fang X., Ouyang G. MicroRNAs: Novel regulators in the hallmarks of human cancer. Cancer Lett. 2009;285:116–126. doi: 10.1016/j.canlet.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 37.Fujii T., Shimada K., Tatsumi Y., Tanaka N., Fujimoto K., Konishi N. Syndecan-1 up-regulates microRNA-331-3p and mediates epithelial-to-mesenchymal transition in prostate cancer. Mol. Carcinog. 2016;55:1378–1386. doi: 10.1002/mc.22381. [DOI] [PubMed] [Google Scholar]
- 38.Nymark P., Guled M., Borze I., Faisal A., Lahti L., Salmenkivi K., Kettunen E., Anttila S., Knuutila S. Integrative analysis of microRNA, mRNA and aCGH data reveals asbestos- and histology-related changes in lung cancer. Genes Chromosomes Cancer. 2011;50:585–597. doi: 10.1002/gcc.20880. [DOI] [PubMed] [Google Scholar]
- 39.Meng X., Huang Z., Teng F., Xing L., Yu J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat. Rev. 2015;41:868–876. doi: 10.1016/j.ctrv.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K., et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarogoulidis P., Petanidis S., Domvri K., Kioseoglou E., Anestakis D., Freitag L., Zarogoulidis K., Hohenforst-Schmidt W., Eberhardt W. Autophagy inhibition upregulates CD4(+) tumor infiltrating lymphocyte expression via miR-155 regulation and TRAIL activation. Mol. Oncol. 2016;10:1516–1531. doi: 10.1016/j.molonc.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffman P.C., Mauer A.M., Vokes E.E. Lung cancer. Lancet. 2000;355:479–485. doi: 10.1016/S0140-6736(00)82038-3. [DOI] [PubMed] [Google Scholar]
- 43.Megiorni F., Pizzuti A., Frati L. Clinical significance of microRNA expression profiles and polymorphisms in lung cancer development and management. Pathol. Res. Int. 2011;2011:780652. doi: 10.4061/2011/780652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Massion P.P., Carbone D.P. The molecular basis of lung cancer: Molecular abnormalities and therapeutic implications. Respir. Res. 2003;4:12. doi: 10.1186/1465-9921-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angulo M., Lecuona E., Sznajder J.I. Role of microRNAs in lung disease. Arch. Bronconeumol. 2012;48:325–330. doi: 10.1016/j.arbres.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brownson R.C., Alavanja M.C., Hock E.T., Loy T.S. Passive smoking and lung cancer in nonsmoking women. Am. J. Public Health. 1992;82:1525–1530. doi: 10.2105/AJPH.82.11.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tessema M., Yingling C.M., Picchi M.A., Wu G., Ryba T., Lin Y., Bungum A.O., Edell E.S., Spira A., Belinsky S.A. ANK1 Methylation regulates expression of MicroRNA-486-5p and discriminates lung tumors by histology and smoking status. Cancer Lett. 2017;410:191–200. doi: 10.1016/j.canlet.2017.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung G.T., Sundaresan V., Hasleton P., Rudd R., Taylor R., Rabbitts P.H. Sequential molecular genetic changes in lung cancer development. Oncogene. 1995;11:2591–2598. [PubMed] [Google Scholar]
- 49.Toyooka S., Matsuo K., Shigematsu H., Kosaka T., Tokumo M., Yatabe Y., Ichihara S., Inukai M., Suehisa H., Soh J., et al. The impact of sex and smoking status on the mutational spectrum of epidermal growth factor receptor gene in non-small cell lung cancer. Clin. Cancer Res. 2007;13:5763–5768. doi: 10.1158/1078-0432.CCR-07-0216. [DOI] [PubMed] [Google Scholar]
- 50.Toyooka S., Tsuda T., Gazdar A.F. The TP53 gene, tobacco exposure, and lung cancer. Hum. Mutat. 2003;21:229–239. doi: 10.1002/humu.10177. [DOI] [PubMed] [Google Scholar]
- 51.Vahakangas K.H., Bennett W.P., Castren K., Welsh J.A., Khan M.A., Blomeke B., Alavanja M.C., Harris C.C. p53 and K-ras mutations in lung cancers from former and never-smoking women. Cancer Res. 2001;61:4350–4356. [PubMed] [Google Scholar]
- 52.Zhai X., Xu W. Long noncoding RNA ATB promotes proliferation, migration and invasion in bladder cancer by suppressing microRNA-126. Oncol. Res. 2018 doi: 10.3727/096504018X15152072098476. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Li Q., Wang G., Wang H. MiR-126 functions as a tumor suppressor by targeting SRPK1 in human gastric cancer. Oncol. Res. 2018 doi: 10.3727/096504018X15180508535835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jing B.Q., Ou Y., Zhao L., Xie Q., Zhang Y.X. Experimental study on the prevention of liver cancer angiogenesis via miR-126. Eur. Rev. Med. Pharmacol. Sci. 2017;21:5096–5100. doi: 10.26355/eurrev_201711_13825. [DOI] [PubMed] [Google Scholar]
- 55.Grimolizzi F., Monaco F., Leoni F., Bracci M., Staffolani S., Bersaglieri C., Gaetani S., Valentino M., Amati M., Rubini C., et al. Exosomal miR-126 as a circulating biomarker in non-small-cell lung cancer regulating cancer progression. Sci. Rep. 2017;7:15277. doi: 10.1038/s41598-017-15475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng W., Zhou Y., Lu J., Xu H., Lei L., Chen C., Zhao J., Xu L. The prognostic value of miR-126 expression in non-small-cell lung cancer: A meta-analysis. Cancer Cell Int. 2017;17:71. doi: 10.1186/s12935-017-0440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fiala O., Pitule P., Hosek P., Liska V., Sorejs O., Bruha J., Vycital O., Buchler T., Poprach A., Topolcan O., et al. The association of miR-126-3p, miR-126-5p and miR-664-3p expression profiles with outcomes of patients with metastatic colorectal cancer treated with bevacizumab. Tumour Biol. 2017;39:1010428317709283. doi: 10.1177/1010428317709283. [DOI] [PubMed] [Google Scholar]
- 58.Liu B., Peng X.C., Zheng X.L., Wang J., Qin Y.W. MiR-126 restoration down-regulate VEGF and inhibit the growth of lung cancer cell lines in vitro and in vivo. Lung Cancer. 2009;66:169–175. doi: 10.1016/j.lungcan.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 59.Izzotti A., Calin G.A., Arrigo P., Steele V.E., Croce C.M., De Flora S. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J. 2009;23:806–812. doi: 10.1096/fj.08-121384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang C.H., Liu C.S., Liu H.J., Huang C.P., Huang C.Y., Hsu H.T., Liou S.H., Chung C.J. Association between levels of urinary heavy metals and increased risk of urothelial carcinoma. Int. J. Urol. 2016;23:233–239. doi: 10.1111/iju.13024. [DOI] [PubMed] [Google Scholar]
- 61.Stellman S.D., Djordjevic M.V. Monitoring the tobacco use epidemic II: The agent: Current and emerging tobacco products. Prev. Med. 2009;48(Suppl. 1):S11–S15. doi: 10.1016/j.ypmed.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burger M., Catto J.W., Dalbagni G., Grossman H.B., Herr H., Karakiewicz P., Kassouf W., Kiemeney L.A., La Vecchia C., Shariat S., et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 63.Schulz W.A., Goering W. DNA methylation in urothelial carcinoma. Epigenomics. 2016;8:1415–1428. doi: 10.2217/epi-2016-0064. [DOI] [PubMed] [Google Scholar]
- 64.Wang S.C., Huang C.C., Shen C.H., Lin L.C., Zhao P.W., Chen S.Y., Deng Y.C., Liu Y.W. Gene expression and DNA methylation status of glutathione S-transferase Mu1 and Mu5 in urothelial carcinoma. PLoS ONE. 2016;11:e0159102. doi: 10.1371/journal.pone.0159102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brait M., Munari E., LeBron C., Noordhuis M.G., Begum S., Michailidi C., Gonzalez-Roibon N., Maldonado L., Sen T., Guerrero-Preston R., et al. Genome-wide methylation profiling and the PI3K-AKT pathway analysis associated with smoking in urothelial cell carcinoma. Cell Cycle. 2013;12:1058–1070. doi: 10.4161/cc.24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chung C.J., Lee H.L., Yang H.Y., Lin P., Pu Y.S., Shiue H.S., Su C.T., Hsueh Y.M. Low ratio of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol-glucuronides (NNAL-Gluc)/free NNAL increases urothelial carcinoma risk. Sci. Total Environ. 2011;409:1638–1642. doi: 10.1016/j.scitotenv.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 67.Ganji S.M., Saidijam M., Amini R., Mousavi-Bahar S.H., Shabab N., Seyedabadi S., Mahdavinezhad A. Evaluation of microRNA-99a and microRNA-205 expression levels in bladder cancer. Int. J. Mol. Cell. Med. 2017;6:87–95. doi: 10.22088/acadpub.BUMS.6.2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsai T.F., Lin J.F., Chou K.Y., Lin Y.C., Chen H.E., Hwang T.I. miR-99a-5p acts as tumor suppressor via targeting to mTOR and enhances RAD001-induced apoptosis in human urinary bladder urothelial carcinoma cells. OncoTargets Ther. 2018;11:239–252. doi: 10.2147/OTT.S114276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao J., Li H., Liu L., Song L., Lv Y., Han Y. Identification and functional analysis of risk-related microRNAs for the prognosis of patients with bladder urothelial carcinoma. Oncol. Lett. 2017;14:7297–7303. doi: 10.3892/ol.2017.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geva G.A., Gielchinsky I., Aviv N., Max K.E.A., Gofrit O.N., Gur-Wahnon D., Ben-Dov I.Z. Urine cell-free microRNA as biomarkers for transitional cell carcinoma. BMC Res. Notes. 2017;10:641. doi: 10.1186/s13104-017-2950-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ke H.L., Li W.M., Lin H.H., Hsu W.C., Hsu Y.L., Chang L.L., Huang C.N., Li C.C., Chang H.P., Yeh H.C., et al. Hypoxia-regulated microRNA-210 overexpression is associated with tumor development and progression in upper tract urothelial carcinoma. Int. J. Med. Sci. 2017;14:578–584. doi: 10.7150/ijms.15699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang J., Liu Y., He A., Liu Y., Wu J., Liao X., Lv Z., Wang F., Mei H. Hsa-miR-429 promotes bladder cancer cell proliferation via inhibiting CDKN2B. Oncotarget. 2017;8:68721–68729. doi: 10.18632/oncotarget.19878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chung Y.H., Li S.C., Kao Y.H., Luo H.L., Cheng Y.T., Lin P.R., Tai M.H., Chiang P.H. MiR-30a-5p inhibits epithelial-to-mesenchymal transition and upregulates expression of tight junction protein Claudin-5 in human upper tract urothelial carcinoma cells. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18081826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lenherr S.M., Tsai S., Silva Neto B., Sullivan T.B., Cimmino C.B., Logvinenko T., Gee J., Huang W., Libertino J.A., Summerhayes I.C., et al. MicroRNA expression profile identifies high grade, non-muscle-invasive bladder tumors at elevated risk to progress to an invasive phenotype. Genes. 2017;8 doi: 10.3390/genes8020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsuzaki K., Fujita K., Jingushi K., Kawashima A., Ujike T., Nagahara A., Ueda Y., Tanigawa G., Yoshioka I., Ueda K., et al. MiR-21-5p in urinary extracellular vesicles is a novel biomarker of urothelial carcinoma. Oncotarget. 2017;8:24668–24678. doi: 10.18632/oncotarget.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin S.R., Yeh H.C., Wang W.J., Ke H.L., Lin H.H., Hsu W.C., Chao S.Y., Hour T.C., Wu W.J., Pu Y.S., et al. MiR-193b mediates CEBPD-induced cisplatin sensitization through targeting ETS1 and Cyclin D1 in human urothelial carcinoma cells. J. Cell. Biochem. 2017;118:1563–1573. doi: 10.1002/jcb.25818. [DOI] [PubMed] [Google Scholar]
- 77.Wu W.B., Wang W., Du Y.H., Li H., Xia S.J., Liu H.T. MicroRNA-3713 regulates bladder cell invasion via MMP9. Sci. Rep. 2016;6:32374. doi: 10.1038/srep32374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J., Zhao X., Shi J., Pan Y., Chen Q., Leng P., Wang Y. miR-451 suppresses bladder cancer cell migration and invasion via directly targeting c-Myc. Oncol. Rep. 2016;36:2049–2058. doi: 10.3892/or.2016.5040. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y., Zhang Z., Li Z., Gong D., Zhan B., Man X., Kong C. MicroRNA-497 inhibits the proliferation, migration and invasion of human bladder transitional cell carcinoma cells by targeting E2F3. Oncol. Rep. 2016;36:1293–1300. doi: 10.3892/or.2016.4923. [DOI] [PubMed] [Google Scholar]
- 80.Li S., Zhu Y., Liang Z., Wang X., Meng S., Xu X., Xu X., Wu J., Ji A., Hu Z., et al. Up-regulation of p16 by miR-877-3p inhibits proliferation of bladder cancer. Oncotarget. 2016;7:51773–51783. doi: 10.18632/oncotarget.10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cui X., Kong C., Zhu Y., Zeng Y., Zhang Z., Liu X., Zhan B., Piao C., Jiang Z. miR-130b, an onco-miRNA in bladder cancer, is directly regulated by NF-κB and sustains NF-κB activation by decreasing Cylindromatosis expression. Oncotarget. 2016;7:48547–48561. doi: 10.18632/oncotarget.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen X., Wu B., Xu Z., Li S., Tan S., Liu X., Wang K. Downregulation of miR-133b predict progression and poor prognosis in patients with urothelial carcinoma of bladder. Cancer Med. 2016;5:1856–1862. doi: 10.1002/cam4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sasaki H., Yoshiike M., Nozawa S., Usuba W., Katsuoka Y., Aida K., Kitajima K., Kudo H., Hoshikawa M., Yoshioka Y., et al. Expression level of urinary MicroRNA-146a-5p is increased in patients with bladder cancer and decreased in those after transurethral resection. Clin. Genitourin. Cancer. 2016;14:e493–e499. doi: 10.1016/j.clgc.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 84.Wu C.L., Ho J.Y., Chou S.C., Yu D.S. MiR-429 reverses epithelial-mesenchymal transition by restoring E-cadherin expression in bladder cancer. Oncotarget. 2016;7:26593–26603. doi: 10.18632/oncotarget.8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang C., Ma X., Du J., Yao Z., Shi T., Ai Q., Chen X., Zhang Z., Zhang X., Yao X. MicroRNA-30a as a prognostic factor in urothelial carcinoma of bladder inhibits cellular malignancy by antagonising Notch1. BJU Int. 2016;118:578–589. doi: 10.1111/bju.13407. [DOI] [PubMed] [Google Scholar]
- 86.Fujii T., Shimada K., Tatsumi Y., Hatakeyama K., Obayashi C., Fujimoto K., Konishi N. microRNA-145 promotes differentiation in human urothelial carcinoma through down-regulation of syndecan-1. BMC Cancer. 2015;15:818. doi: 10.1186/s12885-015-1846-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang S., Zhang C., Liu W., Zheng W., Zhang Y., Wang S., Huang D., Liu X., Bai Z. MicroRNA-24 upregulation inhibits proliferation, metastasis and induces apoptosis in bladder cancer cells by targeting CARMA3. Int. J. Oncol. 2015;47:1351–1360. doi: 10.3892/ijo.2015.3117. [DOI] [PubMed] [Google Scholar]
- 88.Lombard A.P., Mooso B.A., Libertini S.J., Lim R.M., Nakagawa R.M., Vidallo K.D., Costanzo N.C., Ghosh P.M., Mudryj M. miR-148a dependent apoptosis of bladder cancer cells is mediated in part by the epigenetic modifier DNMT1. Mol. Carcinog. 2016;55:757–767. doi: 10.1002/mc.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wei S., Bing Z., Yao Y., Master S.R., Gupta P. Higher expression of miR-182 in cytology specimens of high-grade urothelial cell carcinoma: A potential diagnostic marker. Acta Cytol. 2015;59:109–112. doi: 10.1159/000371507. [DOI] [PubMed] [Google Scholar]
- 90.Xie D., Shang C., Zhang H., Guo Y., Tong X. Up-regulation of miR-9 target CBX7 to regulate invasion ability of bladder transitional cell carcinoma. Med. Sci. Monit. 2015;21:225–230. doi: 10.12659/MSM.893232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andrew A.S., Marsit C.J., Schned A.R., Seigne J.D., Kelsey K.T., Moore J.H., Perreard L., Karagas M.R., Sempere L.F. Expression of tumor suppressive microRNA-34a is associated with a reduced risk of bladder cancer recurrence. Int. J. Cancer. 2015;137:1158–1166. doi: 10.1002/ijc.29413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morais D.R., Reis S.T., Viana N., Piantino C.B., Massoco C., Moura C., Dip N., Silva I.A., Srougi M., Leite K.R. The involvement of miR-100 in bladder urothelial carcinogenesis changing the expression levels of mRNA and proteins of genes related to cell proliferation, survival, apoptosis and chromosomal stability. Cancer Cell Int. 2014;14:119. doi: 10.1186/s12935-014-0119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Drayton R.M., Peter S., Myers K., Miah S., Dudziec E., Bryant H.E., Catto J.W. MicroRNA-99a and 100 mediated upregulation of FOXA1 in bladder cancer. Oncotarget. 2014;5:6375–6386. doi: 10.18632/oncotarget.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang T., Yuan J., Feng N., Li Y., Lin Z., Jiang Z., Gui Y. Hsa-miR-1 downregulates long non-coding RNA urothelial cancer associated 1 in bladder cancer. Tumour Biol. 2014;35:10075–10084. doi: 10.1007/s13277-014-2321-2. [DOI] [PubMed] [Google Scholar]
- 95.Xu X.D., Wu X.H., Fan Y.R., Tan B., Quan Z., Luo C.L. Exosome-derived microRNA-29c induces apoptosis of BIU-87 cells by down regulating BCL-2 and MCL-1. Asian Pac. J. Cancer Prev. 2014;15:3471–3476. doi: 10.7314/APJCP.2014.15.8.3471. [DOI] [PubMed] [Google Scholar]
- 96.Zhang H., Qi F., Cao Y., Chen M., Zu X. Down-regulated microRNA-101 in bladder transitional cell carcinoma is associated with poor prognosis. Med. Sci. Monit. 2014;20:812–817. doi: 10.12659/MSM.890300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jia A.Y., Castillo-Martin M., Bonal D.M., Sanchez-Carbayo M., Silva J.M., Cordon-Cardo C. MicroRNA-126 inhibits invasion in bladder cancer via regulation of ADAM9. Br. J. Cancer. 2014;110:2945–2954. doi: 10.1038/bjc.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu Y., Zhou M., Wang J., Zhao Y., Li S., Zhou B., Su Z., Xu C., Xia Y., Qian H., et al. Role of microRNA-27a in down-regulation of angiogenic factor AGGF1 under hypoxia associated with high-grade bladder urothelial carcinoma. Biochim. Biophys. Acta. 2014;1842:712–725. doi: 10.1016/j.bbadis.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 99.Shang C., Zhang H., Guo Y., Hong Y., Liu Y., Xue Y. MiR-320a down-regulation mediates bladder carcinoma invasion by targeting ITGB3. Mol. Biol. Rep. 2014;41:2521–2527. doi: 10.1007/s11033-014-3110-0. [DOI] [PubMed] [Google Scholar]
- 100.Majid S., Dar A.A., Saini S., Deng G., Chang I., Greene K., Tanaka Y., Dahiya R., Yamamura S. MicroRNA-23b functions as a tumor suppressor by regulating Zeb1 in bladder cancer. PLoS ONE. 2013;8:e67686. doi: 10.1371/journal.pone.0067686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guo Y., Liu H., Zhang H., Shang C., Song Y. miR-96 regulates FOXO1-mediated cell apoptosis in bladder cancer. Oncol. Lett. 2012;4:561–565. doi: 10.3892/ol.2012.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang C., Yao Z., Zhu M., Ma X., Shi T., Li H., Wang B., Ouyang J., Zhang X. Inhibitory effects of microRNA-34a on cell migration and invasion of invasive urothelial bladder carcinoma by targeting Notch1. J. Huazhong Univ. Sci. Technol. Med. Sci. 2012;32:375–382. doi: 10.1007/s11596-012-0065-z. [DOI] [PubMed] [Google Scholar]
- 103.Song T., Zhang X., Wang C., Wu Y., Dong J., Gao J., Cai W., Hong B. Expression of miR-143 reduces growth and migration of human bladder carcinoma cells by targeting cyclooxygenase-2. Asian Pac. J. Cancer Prev. 2011;12:929–933. [PubMed] [Google Scholar]
- 104.Huang L., Luo J., Cai Q., Pan Q., Zeng H., Guo Z., Dong W., Huang J., Lin T. MicroRNA-125b suppresses the development of bladder cancer by targeting E2F3. Int. J. Cancer. 2011;128:1758–1769. doi: 10.1002/ijc.25509. [DOI] [PubMed] [Google Scholar]
- 105.Friedman J.M., Liang G., Liu C.C., Wolff E.M., Tsai Y.C., Ye W., Zhou X., Jones P.A. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 2009;69:2623–2629. doi: 10.1158/0008-5472.CAN-08-3114. [DOI] [PubMed] [Google Scholar]
- 106.Yang M.H., Chiang W.C., Chou T.Y., Chang S.Y., Chen P.M., Teng S.C., Wu K.J. Increased NBS1 expression is a marker of aggressive head and neck cancer and overexpression of NBS1 contributes to transformation. Clin. Cancer Res. 2006;12:507–515. doi: 10.1158/1078-0432.CCR-05-1231. [DOI] [PubMed] [Google Scholar]
- 107.Chen Y.C., Su Y.N., Chou P.C., Chiang W.C., Chang M.C., Wang L.S., Teng S.C., Wu K.J. Overexpression of NBS1 contributes to transformation through the activation of phosphatidylinositol 3-kinase/Akt. J. Biol. Chem. 2005;280:32505–32511. doi: 10.1074/jbc.M501449200. [DOI] [PubMed] [Google Scholar]
- 108.Flanders W.D., Lally C.A., Zhu B.P., Henley S.J., Thun M.J. Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: Results from Cancer Prevention Study II. Cancer Res. 2003;63:6556–6562. [PubMed] [Google Scholar]
- 109.Kim H.P., Wang X., Chen Z.H., Lee S.J., Huang M.H., Wang Y., Ryter S.W., Choi A.M. Autophagic proteins regulate cigarette smoke-induced apoptosis: Protective role of heme oxygenase-1. Autophagy. 2008;4:887–895. doi: 10.4161/auto.6767. [DOI] [PubMed] [Google Scholar]
- 110.Chen C., Ridzon D.A., Broomer A.J., Zhou Z., Lee D.H., Nguyen J.T., Barbisin M., Xu N.L., Mahuvakar V.R., Andersen M.R., et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Castoldi M., Schmidt S., Benes V., Noerholm M., Kulozik A.E., Hentze M.W., Muckenthaler M.U. A sensitive array for microRNA expression profiling (miChip) based on locked nucleic acids (LNA) RNA. 2006;12:913–920. doi: 10.1261/rna.2332406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schulte J.H., Marschall T., Martin M., Rosenstiel P., Mestdagh P., Schlierf S., Thor T., Vandesompele J., Eggert A., Schreiber S., et al. Deep sequencing reveals differential expression of microRNAs in favorable versus unfavorable neuroblastoma. Nucleic Acids Res. 2010;38:5919–5928. doi: 10.1093/nar/gkq342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mensah M., Borzi C., Verri C., Suatoni P., Conte D., Pastorino U., Orazio F., Sozzi G., Boeri M. MicroRNA based liquid biopsy: The experience of the plasma miRNA signature classifier (MSC) for lung cancer screening. J. Vis. Exp. 2017;128 doi: 10.3791/56326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li L.L., Qu L.L., Fu H.J., Zheng X.F., Tang C.H., Li X.Y., Chen J., Wang W.X., Yang S.X., Wang L., et al. Circulating microRNAs as novel biomarkers of ALK-positive non-small cell lung cancer and predictors of response to crizotinib therapy. Oncotarget. 2017;8:45399–45414. doi: 10.18632/oncotarget.17535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen X., Ba Y., Ma L., Cai X., Yin Y., Wang K., Guo J., Zhang Y., Chen J., Guo X., et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 116.Mitchell P.S., Parkin R.K., Kroh E.M., Fritz B.R., Wyman S.K., Pogosova-Agadjanyan E.L., Peterson A., Noteboom J., O’Briant K.C., Allen A., et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fujii T., Asano A., Shimada K., Tatsumi Y., Obayashi C., Konishi N. Evaluation of RNA and DNA extraction from liquid-based cytology specimens. Diagn. Cytopathol. 2016;44:833–840. doi: 10.1002/dc.23524. [DOI] [PubMed] [Google Scholar]