Abstract
In unfolded proteins, peptide bonds involving Pro residues exist in an equilibrium between the minor cis and major trans conformations. Folded proteins predominantly contain trans-Pro bonds, and slow cis-trans Pro isomerization in the unfolded state is often found to be a rate limiting step in protein folding. Moreover, kinases and phosphatases that act upon Ser/Thr-Pro motifs exhibit preferential recognition of either the cis- or trans-Pro conformer. NMR spectra obtained at both atmospheric and high pressures indicate that the population of cis-Pro falls well below previous estimates, an effect attributed to the use of short peptides with charged termini in most prior model studies. For the intrinsically disordered protein α-synuclein, cis-Pro populations at all of its five X-Pro bonds are less than 5%, with only modest ionic strength dependence and no detectable effect of the previously demonstrated interaction between the N- and C-terminal halves of the protein. Comparison to small peptides with the same amino acid sequence indicates that peptides, particularly those with unblocked, oppositely charged amino and carboxyl end groups, strongly overestimate the amount of cis-Pro.
Keywords: α-synuclein, cis-trans proline isomerization, protein folding, NMR spectroscopy, high pressure
Quantification of cis-Pro in unfolded proteins
Multi-dimensional NMR was used to quantify cis-Pro peptide bond fractions in three unfolded proteins. Systematically lower cis values than in corresponding short peptides are attributed to reduced conformational entropy in the antiparallel segments preceding and following cis Pro peptide bonds, and to electrostatic attraction between oppositely charged termini in unblocked peptides.

Within proteins, the vast majority (>99.5%) of peptide bonds not involving proline exist in the trans conformation, in which the dihedral angle Ω is 180°. The lowly populated cis conformer requires a 180° rotation about the planar CO(i−1)–N(i) peptide bond (Ω = 0°), but such a rotation induces a steric clash between the Cα(i−1) and Cα(i) atoms. This creates a free energy difference between the trans and cis conformations of ca. 2–6 kcal/mol in non-Pro peptide bonds, and a high energy barrier to rotation of the partial double bond (ca. 20 kcal/mol) overwhelmingly favors the trans state.[1–3] However, in peptide bonds between any amino acid (X) and proline (X-Pro), the trans and cis conformers have a substantially lower energy difference, owing to the cyclic nature of the proline side-chain. Thus, cis-peptidyl-prolyl (cis-Pro) conformations in unfolded polypeptide chains are populated to significantly higher levels, with values that range from 5 to 80% in model peptides,[4–13] depending on the precise amino acid composition, with virtually no detectable dependence on temperature.[11,14] In folded proteins, local interactions around X-Pro bonds typically induce 100% population of either the cis or trans conformation.[15–17]
Cis-Pro bonds and their slow isomerization to the trans state, ca. 10−3 to 10−2 s−1 at room temperature, depending on the types of adjacent residues,[12] can be the rate-limiting step in protein folding,[2] as most non-native cis-Pro bonds in the unfolded protein require isomerization to the native trans conformations in order for folding to proceed. Indeed, a class of molecular chaperones has evolved to catalyze cis-trans proline isomerization in nascent polypeptides,[18] and in vitro refolding studies have demonstrated that such peptidyl-prolyl isomerases, including the ribosome-localized trigger factor,[19] enhance the rate of protein folding.[20]
The human proteome contains 6.3% proline residues,[21] and intrinsically disordered proteins (IDPs) generally contain primary sequences that are enriched in proline by nearly 2-fold over folded proteins.[22] Biologically relevant post-translational modifications occur at numerous Ser-/Thr-Pro motifs, with some kinases and phosphatases specifically recognizing either the cis- or trans-proline conformation.[23] Moreover, in chemically denatured proteins used for in vitro folding studies, native trans-Pro bonds will isomerize to the cis state upon denaturation and equilibration. For proteins with multiple Pro residues, knowledge of the fraction of cis-Pro at each X-Pro bond is critical for the resultant analysis of refolding, which typically contains multiple phases. However, the quantification of cis-Pro propensity in intact, denatured proteins has remained limited,[8,14,24] with most studies instead opting to quantify cis-Pro populations in small, model peptides.[4–7,9–13]
Despite the often low abundance of cis-Pro conformers, NMR is well suited to characterize these states, as cis-trans Pro isomerization is in the slow exchange regime on the NMR timescale. Therefore, separate resonances for the minor cis states are observable. Multi-dimensional NMR provides a spectroscopic probe at every backbone amide moiety, thereby enabling accurate, quantitative analysis of cis-Pro populations from multiple residues affected by the same Pro, as well as the structural impact of this isomerization. Here, we have employed two- and three-dimensional solution-state NMR to accurately quantify the fractions of cis-Pro at 15 different X-Pro bonds in three unfolded proteins, an IDP (α-synuclein) and two pressure-denatured proteins (α-crystallin domain of HSP27 (cHSP27) and ubiquitin V17A/V26A). Comparison of these fractions to those measured for small peptides of identical sequence reveal that peptides tend to overestimate the fraction of cis-Pro, in particular when the peptides contain oppositely charged N-terminal amino and C-terminal carboxyl groups. The relatively uniform impact of cis-Pro on the chemical shifts of nearby residues will facilitate identification of cis-Pro conformers in future NMR studies of IDPs and intrinsically disordered regions (IDRs) of proteins.
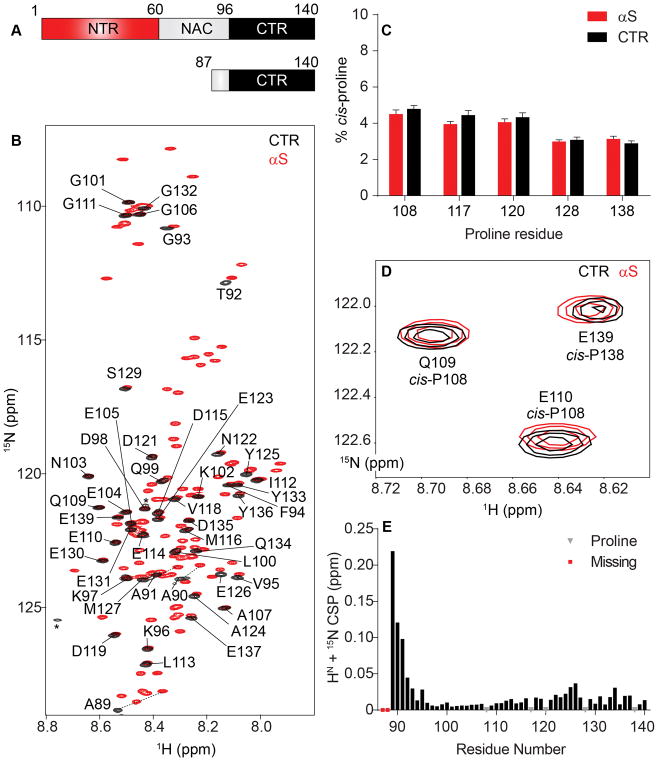
IDPs typically yield a single set of intense cross-peaks in the two-dimensional (2D) 1H-15N HSQC spectrum. The HSQC spectrum of α-synuclein (αS) exemplifies such behavior, but weak signals from alternate conformers are observable at low contour levels (Figure 1; Figure S1). There are five Pro residues in the acidic C-terminal region of αS, and the minor signals corresponding to cis-Pro bonds cluster near resonances of residues that are close in sequence to Pro. To assign the chemical shifts from the cis-Pro conformers in αS, triple resonance NMR spectra were acquired on a 0.9 mM [U-13C,15N]-labeled sample. High signal-to-noise ratios in these spectra afforded unambiguous assignment of the residues in the vicinity of all cis-Pro conformers (P108, P117, P120, P128, P138), despite the low population of these states (3–4.5%, i.e., 27–40 μM effective sample concentration).
Figure 1.
Identification of cis-Pro bonds in αS. (A) 2D 1H-15N HSQC of 0.9 mM 13C,15N-αS at pH 6, 288 K. Resonances impacted by the cis/trans state of nearby Pro residues are colored (P108, red; P117, P120, orange; P128, green; P138, blue). (B–E) Zoomed-in regions corresponding to the boxed areas from (A) are shown at a lower contour level. The major trans- and minor cis-Pro peaks are indicated with their assignments. See Figure S1 for the full, low-contour spectrum
In the cis-Pro state, the i−1 residue with respect to Pro shows significant upfield changes in chemical shifts for 1HN (ca. 0.2–0.4 ppm) and 15N (ca. 2–4 ppm), with a smaller and usually upfield shift for 13Cα (Figure S2). The small, downfield shift of E137 13Cα, in close proximity to the C-terminus, is an exception to this rule. Another diagnostic chemical shift change of the cis state is seen for the i−2 13Cα resonance, which exhibits a substantial upfield change (ca. 0.4–1 ppm) that is similar in sign and magnitude to the 13Cα chemical shift change of the isomerizing Pro. Residues in the i+1 and i+2 positions show downfield 1HN chemical shift changes in the cis state (up to 0.2 ppm), but variable 15N chemical shift differences. The chemical shift differences typically become vanishingly small for residues in the i±4 positions and beyond.
Using the peak intensities from resonances impacted by the cis and trans states, the cis-Pro population was calculated as [cis]/([cis] + [trans]). In αS, the fraction of cis-Pro ranged from 3% to 4.5%, significantly lower than values generally reported in the literature (e.g. 10–20%).[13] The fraction of cis-Pro is highly consistent among the various impacted residues near a given Pro, and the signal-to-noise ratio for each cis-Pro-impacted cross peak was near ca. 80:1, indicating the high precision of these measurements.
To compare the cis-Pro values in αS to values of cis-Pro in small peptides of identical sequence, natural abundance NMR spectra were acquired on a set of tetra-peptides (Ac-XXPX-NH2) that were N-terminally acetylated and C-terminally amidated. All of the blocked, tetra-peptides, except that corresponding to P120, displayed a significantly higher population of cis-Pro than full-length αS (Figure 2), indicating that longer-range interactions, presumably steric clashing or repulsive electrostatic interactions of the chains, are unfavorable to the formation of the cis state at P108, P117, P128, and P138. The highly negatively charged nature of the carboxy-terminal tail of αS (net charge of −14 for the last 40 residues) is expected to enhance the electrostatic contribution. Indeed, at very high ionic strength (1.1 M NaCl), increased cis fractions are observed, albeit remaining below those of the blocked tetra-peptides (Figure 2).
Figure 2.
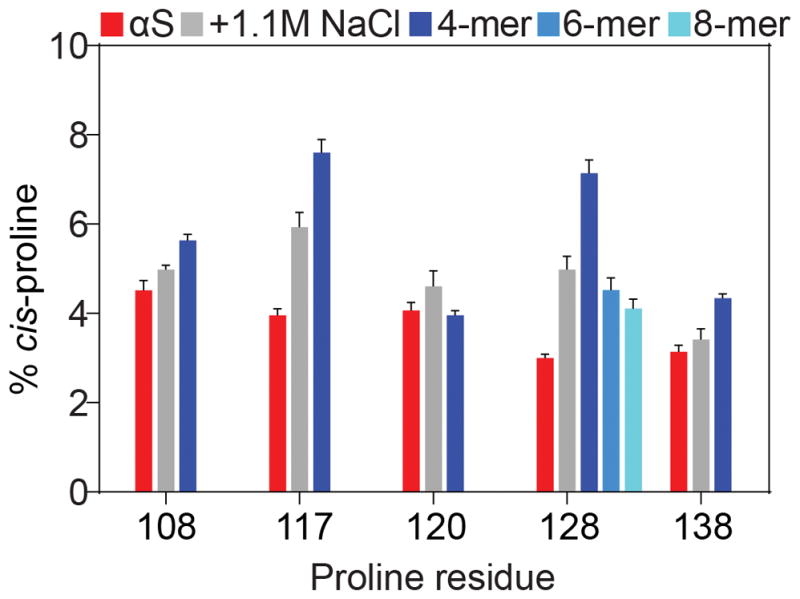
Fractions of cis-Pro bonds in αS and comparison to oligo-peptides. All samples were at pH 6, 288 K. The fraction of cis-Pro is shown for each Pro residue in αS (red), with error bars representing one standard deviation from the mean. Upon the addition of 1160 mM NaCl, the fraction of cis-Pro increases (grey), indicative of decreased electrostatic repulsion in the acidic C-terminal region of αS. Blocked, tetra-peptides (blue) in general contain elevated levels of cis-Pro, whereas hexa- (teal) and octa-peptides (cyan) approach the values seen in full length αS.
High fractions of cis-Pro previously have been reported for peptides with an aromatic residue in the i−1 position; [25] however, these peptides mostly were studied with unblocked, charged terminal residues. For example, a high cis fraction was previously reported for the FPA tri-peptide.[7] However, charge neutralization by N-terminal acetylation and C-terminal amidation reduces the cis-Pro fraction by more than one-fifth (Figure S3). Analogously, the addition of 1.1 M NaCl to the unblocked peptide decreases cis-Pro by nearly the same fraction (Figure S3). In the tri-peptide MPS, we observe even larger reductions in the cis fraction upon the addition of blocking groups at the termini or 1.1 M NaCl, which decrease the cis-Pro fraction by about a third and a quarter, respectively (Figure S3). Similar results were obtained for the peptide APQ, which displayed fractional changes in cis content of 43% and 17% upon terminal blockage or the addition of 1.1 M NaCl (Figure S3). Therefore, electrostatic attraction between oppositely charged termini enhances the cis-Pro fraction in oligo-peptides.
As P128 showed the largest discrepancy between values obtained for full-length αS and its corresponding blocked tetra-peptide, the impact of longer range interactions on cis-Pro was assessed by comparing the fraction of cis-Pro at P128 as a function of peptide length. Upon increasing the length of the peptide from four to six to eight residues (Figure 2), a progressive decrease in the fraction of cis-Pro was observed. However, even though the blocked octa-peptide exhibited a cis-Pro fraction that approached that of full-length αS, the remaining significant difference of 1.5% must result from weak interactions that extend beyond the ±4 residue range.
We therefore also evaluated the impact on cis-Pro formation in full-length αS from potential inter-domain contacts. Previous NMR measurements have indicated that the oppositely charged N- and C-terminal regions of αS transiently contact each other,[26,27] an effect held responsible for the compaction of the radius of hydration of this molecule relative to that of a fully random coil, and decreased amyloidogenicity relative to sequences that lack the C-terminal tail.[29] Moreover, the N- and C-termini have been observed to make transiently intermolecular contacts.[30] Depending on the nature of such contacts, they could either restrict or favor cis-Pro formation. However, a construct comprising residues 87–140 of αS[31] showed no detectable differences in the populations of cis-Pro between the full-length and truncated proteins (Figure 3), indicating that the N-terminal region does not measurably impact cis-Pro propensities in the C-terminal region. Nevertheless, the small chemical shift differences between the full length protein and this C-terminal 54-residue peptide (Figure 3E) are consistent with the previously noted interactions between the two domains, but are too weak to cause a detectable impact on the fraction of cis-Pro. To compare the amount of cis-Pro in αS with other unfolded proteins, we used high pressure NMR, which was previously shown to have no significant effect on the structure and dynamics of αS.[32] In order to verify that hydrostatic pressure does not impact the cis-Pro content in an unfolded protein, we also measured the fraction of cis-Pro in αS at 2.5 kbar. Indeed, these values were found to be unchanged with respect to their 1 bar counterparts (Figure S4).
Figure 3.
The isolated C-terminal region of αS retains its native cis-Pro fractions. (A) Depiction of αS and its three regions: the amphipathic N-terminal region (NTR), the hydrophobic NAC region, and the acidic C-terminal region (CTR). The isolated CTR (bottom) contains an S87C mutation. (B) 2D 1H-15N HSQC spectra of full-length αS (red) and the isolated CTR (black). Buffer conditions were as in Figure 1, except that 5 mM BME was added to the isolated CTR to prevent disulfide formation. The peak indicated with an asterisk arises from an impurity. (C) Quantification of the cis-Pro fractions in both full-length αS and its isolated CTR. (D) Zoomed-in region from panel (B) showing resonances affected by cis-Pro. (E) Combined and weighted HN and 15N chemical shift perturbations (CSPs) between full-length αS and its isolated CTR.
Two proteins were denatured at 2.5 kbar and their NMR signals corresponding to residues impacted by cis-Pro were assigned. The α-crystallin domain of HSP27 (cHSP27), a 2×10 kDa non-covalent dimer, contains seven Pro residues per subunit that all adopt the trans conformation in the native state.[33] cHSP27 readily unfolds at high pressures (Figure 4A), and 3D NMR spectra were recorded at 2.5 kbar in order to assign the chemical shifts of the pressure-denatured state at pH 7 and 288 K. Previous data indicated that cis-Pro populations were independent of pH for all X-Pro bonds excluding His-Pro and Tyr-Pro,[13] neither of which exist in cHSP27. At least 32 out of 86 1H-15N correlations in cHSP27 are visibly impacted by cis-Pro formation (Figure 4B). Interestingly, cHSP27 contains Pro clustering, with a P145-P146 pair close to P150, and P168 near P170. As a result, nearby residues are affected by cis-trans isomerization from multiple X-Pro bonds. For example, four signals are observed for G147: all trans, cis-P145, cis-P146, and cis-P150 (Figure 4C), with sequential assignments providing unambiguous identification of each X-Pro bond of interest. As compared to αS, the amount of cis-Pro in pressure-denatured cHSP27 varied widely, from 5% to 10% across the seven X-Pro bonds (Figure 4D).
Figure 4.
Quantification of cis-Pro bonds in two pressure-denatured proteins. (A) 1.6 mM 13C,15N-labeled α-crystallin domain of HSP27 (cHSP27) at 2.5 kbar in 30 mM sodium phosphate, pH 7, 2 mM EDTA, 2 mM benzamidine, 288 K. (B) 0.75 mM 2H,13C,15N-labeled ubiquitin-(V17A/V26A) at 2.5 kbar in 20 mM potassium phosphate buffer, pH 6.4, 2 mM benzamidine, 288 K. In both (A) and (B), colored resonances have distinguishable assignments for the trans and cis conformations. (C) Boxed region of (A) at a lower contour level; c and t denote cis and trans, respectively. (D,E) Average cis-Pro fraction at each Pro residue for the samples in (A) and (B).
Ubiquitin contains three Pro residues, but the wild-type protein does not easily unfold at high pressure. Therefore, a cavity-containing double mutant (V17A/V26A)[33] was prepared in order to facilitate pressure-induced unfolding. These mutations lowered the mid-point of pressure denaturation from >5 kbar to ca. 1.4 kbar, enabling acquisition of a set of 3D NMR spectra to assign the minor signals corresponding to cis-Pro (Figure 4B). The cis-Pro fraction varied by a factor of three across the Pro residues, from as low as 2.5% at P38, which follows P37, to 8% at P19 (Figure 4E). A hepta-peptide of the same amino acid composition centered around P19 exhibited the same fraction of cis-Pro as full-length, pressure-unfolded ubiquitin (Figure 4E). The P37–P38 bond in ubiquitin-(V17A/V26A) displays the lowest cis-Pro value (2.5%) across all 15 X-Pro bonds. However, the chemically similar P145–P146 bond in cHSP27 contains ca. 6% cis-Pro, highlighting the importance of residues remote from the Pro-Pro bond.
Our results indicate that populations of cis-Pro conformers in unfolded proteins are considerably lower than for corresponding short peptides, previously used to estimate these fractions. The presence of cis-Pro is often considered a hurdle in protein folding, and with multiple Pro residues per chain, the fraction of all-trans X-Pro chains can be strongly impacted by even a modest change in the cis-Pro ratios. We attribute the lower cis-Pro fractions in the longer polypeptides to increased steric clashing between the segments of the chain preceding and following the cis peptide bond, which effectively reverses the chain direction and reduces conformational space available to the two segments. Electrostatic repulsion between these segments, as applies in αS, can enhance this effect. The utility of high-pressure NMR to accurately quantify cis-Pro populations in proteins is key to on-going studies of protein folding by pressure-jump NMR,[33–35] and the reported cis/trans chemical shift differences serve as convenient guides for identifying cis-Pro related resonances in NMR-based studies of IDPs and denatured proteins.
Antibody data have implicated elevated cis-Pro levels in toxic fibril formation of the intrinsically disordered Tau protein, associated with traumatic brain injury,[36] but NMR data refuted this conclusion.[14] In context of αS and its formation of amyloid fibrils, mutation of its Pro residues to Ala accelerates fibril formation.[37] Considering that the C-terminal region does not become embedded in the fibril core,[38] this points to a possible inhibitory role of cis-Pro in fibril elongation, where a low cis fraction would suffice for such a role. NMR spectra of αS obtained in living bacterial and mammalian cells[39–41] indicate that αS retains its predominantly trans-Pro conformation, implying that in vivo interactions do not drastically alter the inherent cis-Pro propensities of αS.
Experimental Section
Isotopically labelled proteins were expressed in E. coli and purified as described in the Supporting Information. All NMR spectra were acquired on Bruker Avance III spectrometers (700 and 800 MHz) equipped with cryogenically cooled probes. The amino acid sequences of oligo-peptides and proteins used in this study are listed in Table S1 and Table S2, respectively. Additional experimental details can be found in the Supporting Information.
Supplementary Material
Acknowledgments
We thank J.L. Baber, Y. Shen, and D. Garrett for technical support; J. Roche, Robert Best, and D.A. Torchia for insightful discussions; Zhiping Jiang and Jennifer C. Lee (NHLBI) for the plasmid coding for the C-terminal region of αS; Justin Benesch, Andrew Baldwin, and Heidi Gastall (University of Oxford) for the plasmid encoding cHSP27; and Galina Abdoulaeva (CBER/FDA) for peptide synthesis. This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases and by the Intramural Antiviral Target Program of the Office of the Director, NIH.
References
- 1.Weiss MS, Jabs A, Hilgenfeld R. Nat Struct Mol Biol. 1998;5:676. doi: 10.1038/1368. [DOI] [PubMed] [Google Scholar]
- 2.Wedemeyer WJ, Welker E, Scheraga HA. Biochemistry. 2002;41:14637–14644. doi: 10.1021/bi020574b. [DOI] [PubMed] [Google Scholar]
- 3.Dugave C, Demange L. Chem Rev. 2003;103:2475–2532. doi: 10.1021/cr0104375. [DOI] [PubMed] [Google Scholar]
- 4.Deber CM, Torchia DA, Wong SC, Blout ER. Proc Natl Acad Sci U S A. 1972;69:1825–9. doi: 10.1073/pnas.69.7.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torchia DA. Biochemistry. 1972;11:1462–8. doi: 10.1021/bi00758a021. [DOI] [PubMed] [Google Scholar]
- 6.Grathwohl C, Wuthrich K. Biopolymers. 1976;15:2043–2057. doi: 10.1002/bip.1976.360151013. [DOI] [PubMed] [Google Scholar]
- 7.Grathwohl C, Wuthrich K. Biopolymers. 1981;20:2623–2633. [Google Scholar]
- 8.Sarkar SK, Young PE, Sullivan CE, Torchia DA. Proc Natl Acad Sci U S A. 1984;81:4800–3. doi: 10.1073/pnas.81.15.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dyson HJ, Rance M, Houghten RA, Lerner RA, Wright PE. J Mol Biol. 1988;201:161–200. doi: 10.1016/0022-2836(88)90446-9. [DOI] [PubMed] [Google Scholar]
- 10.Yao J, Feher VA, Espejo BF, Reymond MT, Wright PE, Dyson HJ. J Mol Biol. 1994;243:736–53. doi: 10.1016/0022-2836(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 11.Raleigh DP, Evans PA, Pitkeathly M, Dobson CM. J Mol Biol. 1992;228:338–342. doi: 10.1016/0022-2836(92)90822-2. [DOI] [PubMed] [Google Scholar]
- 12.Reimer U, Scherer G, Drewello M, Kruber S, Schutkowski M, Fischer G. J Mol Biol. 1998;279:449–460. doi: 10.1006/jmbi.1998.1770. [DOI] [PubMed] [Google Scholar]
- 13.Osvath S, Gruebele M. Biophys J. 2003;85:1215–1222. doi: 10.1016/S0006-3495(03)74557-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahuja P, Cantrelle FX, Huvent I, Hanoulle X, Lopez J, Smet C, Wieruszeski JM, Landrieu I, Lippens G. J Mol Biol. 2016;428:79–91. doi: 10.1016/j.jmb.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Capaldi AP, Ferguson SJ, Radford SE. J Mol Biol. 1999;286:1621–1632. doi: 10.1006/jmbi.1998.2588. [DOI] [PubMed] [Google Scholar]
- 16.Sarkar P, Reichman C, Saleh T, Birge RB, Kalodimos CG. Mol Cell. 2007;25:413–26. doi: 10.1016/j.molcel.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu KP, Finn G, Lee TH, Nicholson LK. Nat Chem Biol. 2007;3:619–629. doi: 10.1038/nchembio.2007.35. [DOI] [PubMed] [Google Scholar]
- 18.Schmid FX. Annu Rev Biophys Biomol Struct. 1993;22:123–143. doi: 10.1146/annurev.bb.22.060193.001011. [DOI] [PubMed] [Google Scholar]
- 19.Hesterkamp T, Hauser S, Lütcke H, Bukau B. Proc Natl Acad Sci U S A. 1996;93:4437–41. doi: 10.1073/pnas.93.9.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang K, Schmid FX, Fischer G. Nature. 1987;329:268–70. doi: 10.1038/329268a0. [DOI] [PubMed] [Google Scholar]
- 21.Morgan AA, Rubenstein E, Hosack D, Yang J, Gao W. PLoS One. 2013;8:e53785. doi: 10.1371/journal.pone.0053785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theillet FX, Kalmar L, Tompa P, Han KH, Selenko P, Dunker AK, Daughdrill GW, Uversky VN. Intrinsically Disord Proteins. 2014;1:e24360. doi: 10.4161/idp.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werner-Allen JW, Lee CJ, Liu P, Nicely NI, Wang S, Greenleaf AL, Zhou P. J Biol Chem. 2011;286:5717–5726. doi: 10.1074/jbc.M110.197129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alderson TR, Benesch JLP, Baldwin AJ. Cell Stress Chaperones. 2017;22:639–651. doi: 10.1007/s12192-017-0791-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas KM, Naduthambi D, Zondlo NJ. J Am Chem Soc. 2006;128:2216–2217. doi: 10.1021/ja057901y. [DOI] [PubMed] [Google Scholar]
- 26.Bertoncini CW, Jung YS, Fernandez CO, Hoyer W, Griesinger C, Jovin TM, Zweckstetter M. Proc Natl Acad Sci. 2005;102:1430–1435. doi: 10.1073/pnas.0407146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rospigliosi CC, McClendon S, Schmid AW, Ramlall TF, Barré P, Lashuel HA, Eliezer D. J Mol Biol. 2009;388:1022–32. doi: 10.1016/j.jmb.2009.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uversky VN, Li J, Fink AL. J Biol Chem. 2001;276:10737–44. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- 29.Wu KP, Baum J. J Am Chem Soc. 2010;132:5546–5547. doi: 10.1021/ja9105495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang Z, Heinrich F, McGlinchey RP, Gruschus JM, Lee JC. J Phys Chem Lett. 2017;8:29–34. doi: 10.1021/acs.jpclett.6b02304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roche J, Ying J, Maltsev AS, Bax A. ChemBioChem. 2013;14:1754–1761. doi: 10.1002/cbic.201300244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hochberg GK, Ecroyd H, Liu C, Cox D, Cascio D, Sawaya MR, Collier MP, Stroud J, Carver JA, Baldwin AJ, Robinson CV, Eisenberg DS, Benesch JL, Laganowsky A. Proc Natl Acad Sci U S A. 2014;111:E1562–E1570. doi: 10.1073/pnas.1322673111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alderson TR, Charlier C, Torchia DA, Anfinrud P, Bax A. J Am Chem Soc. 2017;139:11036–11039. doi: 10.1021/jacs.7b06676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kremer W, Arnold M, Munte CE, Hartl R, Erlach MB, Koehler J, Meier A, Kalbitzer HR. J Am Chem Soc. 2011;133:13646–13651. doi: 10.1021/ja2050698. [DOI] [PubMed] [Google Scholar]
- 35.Roche J, Dellarole M, Caro JA, Norberto DR, Garcia AE, Garcia-Moreno B, Roumestand C, Royer CA. J Am Chem Soc. 2013;135:14610–14618. doi: 10.1021/ja406682e. [DOI] [PubMed] [Google Scholar]
- 36.Kondo A, Shahpasand K, Mannix R, Qiu J, Moncaster J, Chen C-H, Yao Y, Lin Y-M, Driver JA, Sun Y, Wie S, Luo M-L, Albayram O, Huang P, Rotenberg A, Ryo A, Goldstein LE, AP-L, McKee AC, Meehan W, Zhou XZ, Lu KP. Nature. 2015;523:431–436. doi: 10.1038/nature14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meuvis J, Gerard M, Desender L, Baekelandt V, Engelborghs Y. Biochemistry. 2010;49:9345–9352. doi: 10.1021/bi1010927. [DOI] [PubMed] [Google Scholar]
- 38.Tuttle MD, Comellas G, Nieuwkoop AJ, Covell DJ, Berthold DA, Kloepper KD, Courtney JM, Kim JK, Barclay AM, Kendall A, Wan W, Stubbs G, Schwieters CD, Lee VM, George JM, Rienstra CM. Nat Struct Mol Biol. 2016;23:409–415. doi: 10.1038/nsmb.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Binolfi A, Theillet FX, Selenko P. Biochem Soc Trans. 2012;40:950–954. doi: 10.1042/BST20120096. [DOI] [PubMed] [Google Scholar]
- 40.Binolfi A, Limatola A, Verzini S, Kosten J, Theillet FX, Rose HM, Bekei B, Stuiver M, van Rossum M, Selenko P. Nat Commun. 2016;7:10251. doi: 10.1038/ncomms10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theillet FX, Binolfi A, Bekei B, Martorana A, Rose HM, Stuiver M, Verzini S, Lorenz D, van Rossum M, Goldfarb D, Selenko P. Nature. 2016;530:45–50. doi: 10.1038/nature16531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.