Abstract
The lack of α2–6-linkage specific sialidases limits the structural and functional studies of sialic acid-containing molecules. Photobacterium damselae α2–6-sialyltransferase (Pd2,6ST) was shown previously to have α2–6-specific, but weak, sialidase activity. Here we develop a high-throughput blue-white colony screening method to identify Pd2,6ST mutants with improved α2–6-sialidase activity from mutant libraries generated by sequential saturation mutagenesis. A triple mutant (Pd2,6ST S232L/T356S/W361F) has been identified with 100-fold improved activity, high α2–6-sialyl linkage selectivity, and ability in cleaving two common sialic acid forms N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc). It is a valuable tool for sialoglycan structural analysis and functional characterization. The sequential saturation mutagenesis and screening strategy developed here can be explored to evolve other linkage-specific neoglycosidases from the corresponding glycosyltransferases.
Keywords: carbohydrate, enzyme engineering, sialic acid, sialidase, sialyltransferase
Graphical abstract

Sialidases are crucial tools for the structural and functional characterization of sialic acid-containing carbohydrates and glycoconjugates, including those presented in cellular extracts and physiological fluids,1 on cellular surfaces,2–4 and in tissues.5–8 Sialidase treatment provides a convenient method for determining the presence of sialic acids,9 and it is mild enough to be useful for the functional evaluation of sialic acids on sensitive biological samples.10 For example, glycoproteins treated with a sialidase were rapidly cleared to the liver upon intravenous injection in rabbits, leading to the discovery that terminal sialic acids are critically important to the serum half-life of circulating therapeutic glycoproteins.11 Similarly, α2–3-selective sialidase treatment of lymphoid organ samples eliminated binding of mouse lymphocytes to the peripheral lymph node high endothelial venules, providing the first evidence that the endogenous ligands of L-selectin contained terminal α2–3-linked sialic acid.12 Sialidase treatment has also been used to enhance the immunogenicity of conjugated vaccines prepared from group B Streptococcus type V capsular polysaccharide, producing robust protection against lethal challenge by live group B Streptococcus in neonatal mice.13
Although powerful and broadly useful for the study or modification of carbohydrates, known sialidases possess either specificity towards α2–3-linked sialic acid or a broad promiscuity towards sialic acid with α2–3-, α2–6-, and α2–8-linkages.14 For example, commercially available sialidases from Arthrobacter ureafaciens, Clostridium perfringens, and Vibrio cholerae, as well as recombinant human cytosolic sialidase hNEU2, Streptococcus pneumoniae SpNanA, and Bifidobacterium infantis sialidase BiNanH2 can catalyze the cleavage of α2–3/6/8-linked sialic acid. While commercially available sialidases from Salmonella typhimurium and Streptococcus pneumoniae SpNanB, and the sialidase activity of multifunctional Pasteurella multocida α2–3-sialyltransferase PmST1 are selectively towards α2–3-linked sialic acid. All of these sialidases can cleave N-acetylneuraminic acid (Neu5Ac, the most common sialic acid form),15 N-glycolylneuraminic acid (Neu5Gc, a non-human sialic acid form),15 and some of their C-9, C-5, and C-7 derivatives.16–22 The lack of α2–6-linkage specific sialidases in the toolbox limits the functional studies of sialic acid-containing biomolecules. We aim to obtain a highly active α2–6-linkage-specific sialidase with promiscuity in cleaving various sialic acid forms.
Previously we have shown that several bacterial sialyltransferases including those in the Carbohydrate Active Enzyme (CAZy)23 glycosyltransferase GT8024–26 and GT5427 families display linkage-specific sialidase and donor hydrolysis activities, although such activities were much lower than their glycosyltransferase activities. Recently, Withers et al. showed that these types of sialidase activities require cytidine 5’-monophosphate (CMP) and suggested a two-step mechanism beginning with the cleavage of the sialosidic linkage in the presence of CMP by a reverse sialyltransferase reaction to form CMP-sialic acid, followed by a forward sialyltransferase reaction using water as the acceptor substrate to form CMP and sialic acid (donor hydrolysis26).28 Here, we use enzyme engineering to improve this “neosialidase” activity of Photobacterium damselae α2–6-sialyltransferase (Pd2,6ST) to useful rates while retaining its sialyl-linkage specificity.
RESULTS AND DISCUSSION
Development of a Blue-white Membrane-blot High-throughput Screening Method
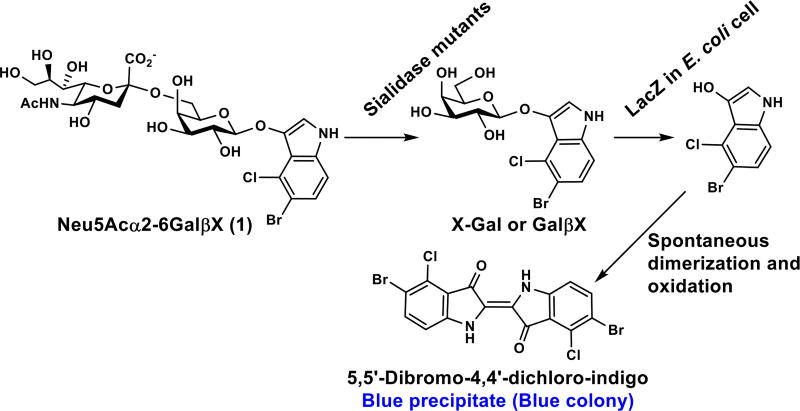
To allow easy identification of mutants with improved α2–6-sialidase activity, a novel blue-white membrane-blot high-throughput screening method was developed. To do this, a 5-bromo-4-chloro-3-indolyl-D-galactopyranoside (X-Gal or GalβX)-like α2–6-sialoside probe Neu5Acα2–6GalβX (1) (Scheme 1) was designed and synthesized. The screening works similarly to a plate-based high-throughput method for linkage-specific sialidase substrate specificity studies using para-nitrophenyl sialyl galactosides (Siaα2–3/6/8GalβpNP) as substrates.16, 20 Enzymatic cleavage of the α2–6-linked sialic acid on the Neu5Acα2–6GalβX probe by active Pd2,6ST mutants expressed in E. coli BL21(DE3) cells forms GalβX (or X-Gal). The terminal galactose (Gal) residue is then rapidly hydrolyzed by endogenous β-galactosidase expressed in E. coli BL21(DE3) cells to yield the indole aglycone. This aglycone spontaneously dimerizes and forms a bright blue precipitate (Scheme 1). To avoid potential problems with membrane impermeability of the probe and minimize the amount of the probe used, colonies are not screened directly on agar plates but are instead lifted onto nitrocellulose filters, induced to express the mutant proteins, lysed over chloroform vapors, and screened by soaking the nitrocellulose filter in the Neu5Acα2–6GalβX solution. The ease and high throughput of this assay allow mutant libraries to be screened as quickly as they can be generated. Therefore, each round of mutagenesis ends upon identification of an improved variant, and further mutagenesis is performed on the improved variant to provide libraries for the next round of saturation mutagenesis.
Scheme 1.
High-throughput α2–6-sialidase activity screening for LacZ-containing E. coli cells expressing sialidase mutants.
Selection of Mutation Sites Based on Crystal Structures of Sialyltransferases
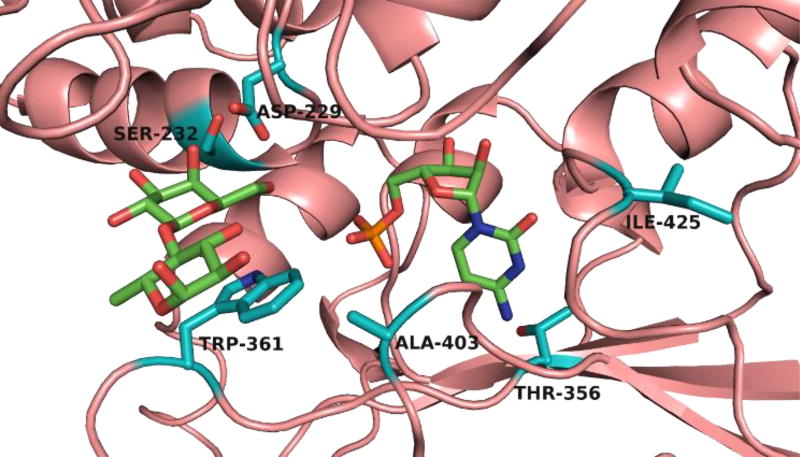
Considerable structural information is available for GT80 sialyltransferases, including the binary complex structure (PDB ID: 4R84) of Δ15Pd2,6ST(N) with CMP-3F(a)Neu5Ac,29 the ternary complex structure (PDB ID: 2Z4T) of Photobacterium sp. JT-ISH-224 α2–6-sialyltransferase (or Δ16Psp2,6ST) with CMP and acceptor lactose,30 and the ternary complex structure (PDB ID: 2IHZ) of Pasteurella multocida sialyltransferase 1 (Δ24PmST1) with donor analog CMP-3F(a)Neu5Ac and lactose.31 Analysis of these structures identified four (Asp229, Ser232, Trp361, and Ala403 in the substrate binding site) of the six residues ultimately chosen for mutagenesis (Figure 1). Thr356 and Ile425 were also chosen based on previously described mutants of PmST1 and Pd2,6ST, respectively, with increased sialyltransferase activity.32
Figure 1.
The substrate binding site of Δ15Pd2,6ST(N) structure modelled based on the co-crystal structure of Δ16Psp2,6ST (PDB ID: 2Z4T) with CMP and lactose (represented with green-colored carbons). Structural modelling was performed with SWISS-MODEL. The six sites chosen for mutagenesis are represented with teal-colored carbons.
The first two residues targeted for mutagenesis were Asp229, the catalytic aspartate, and Trp361, a tryptophan sitting underneath the lactose and hydrogen bonded to the 7-OH of CMP-3F(a)Neu5Ac in PmST1 (PDB ID: 2IHZ). Mutating Asp229 was a test of the proposed mechanism, as any detectable sialidase activity from mutants at this position would be evidence that the proposed catalytic function of Asp229 was incorrect. No improved variants were found from this library. In comparison, several colonies from the W361X library became noticeably blue after approximately 2 hours (Figure S1). All of these colonies were found to have the same W361F mutation.
From the W361F mutant, libraries S232X and A403X were generated. Mutations of Ser232 and the homologous residue in related enzymes have been shown to affect a wide variety of properties including donor hydrolysis and sialidase activities, donor specificity, and acceptor specificity.26, 33 Ala403 aligns with PmST1 residue Arg313, which has been found to affect sialidase activity.34 From the A403X library, the colonies that turned blue first were those retaining Ala403. However, in the S232X library, several colonies turned noticeably blue after only 20 minutes (Figure S1). These colonies were sequenced and all were found to have the S232L mutation.
From the S232L/W361F mutant, the next library screened was T356X. Mutations at this site were previously found to improve the sialyltransferase activity of PmST1.32 Interestingly, this site is positioned near the nucleotide binding region of the active site and does not interact with any part of the sialoside. This library was screened at pH 7.0 and with no supplemented CMP in order to slow the reaction down and improve visual detection of the fastest color development. Two colonies turned light blue with overnight incubation and were found to encode the T356S mutation (Figure S1). From the S232L/T356S/W361F mutant, the I425X library was generated. This site was found to also improve sialyltransferase activity of Pd2,6ST in the same work that identified the importance of Thr356.32 However, no improved α2–6-neosialidase variants were found from this library.
The Effect of CMP and Observation of CMP-Neu5Ac formation
The effect of CMP concentration on Pd2,6ST S232L/T356S/W361F triple mutant α2–6-neosialidase activity (Figure S2) indicated that the presence of 0.5 mM CMP was close to optimum. At this CMP concentration, the formation of CMP-Neu5Ac as an intermediate during the cleavage of Neu5Acα2–6LacβMU was detected by high resolution mass spectrometry (Figure S3). This provided additional evidence for the two-step, reverse sialylation followed by CMP-sialic acid hydrolysis process proposed for the sialidase activity of GT80 family multifunctional sialyltransferases.28
The pH Profile of Pd2,6ST S232L/T356S/W361F Neosialidase
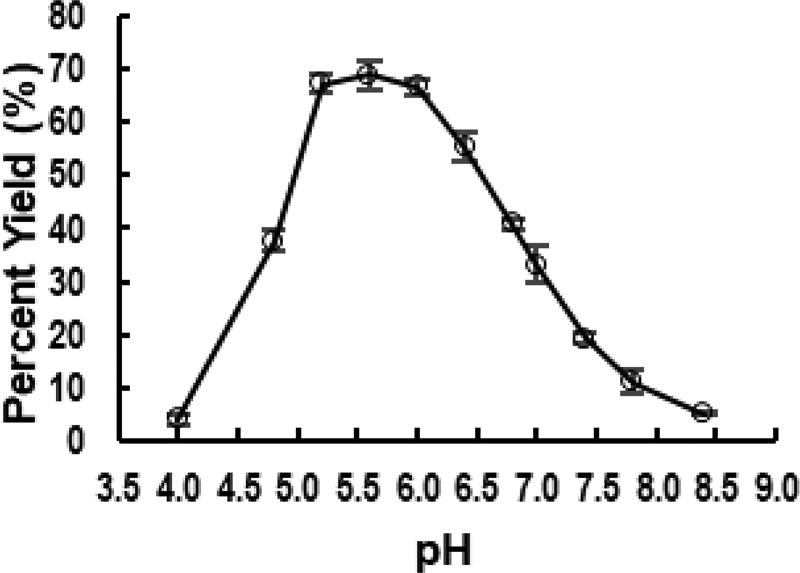
The pH profile study of the neosialidase activity of the Pd2,6ST S232L/T356S/W361F mutant was carried out using Neu5Acα2–6LacβMU as the substrate (Figure 2). The optimal pH was found to be between 5.2 and 6.0. This agreed with previous pH profiles of sialyltransferase-catalyzed sialidase activity,24 suggesting that the engineering process did not significantly alter the optimal pH.
Figure 2.
The pH-profile of Pd2,6ST S232L/T356S/W361F neosialidase.
Kinetics Studies
Three Pd2,6ST mutants including W361F, S232L/W361F, and S232L/T356S/W361F were kinetically characterized for neosialidase activity using Neu5Acα2–6LacβMU as the substrate (Table 1). The use of this probe with a different aglycon was a precaution to avoid mistaking improved recognition of the indole in 1 for improved neosialidase activity.36 Gratifyingly, the Pd2,6ST S232L/T356S/W361F triple mutant displayed 100-fold improved α2–6-sialidase activity compared to the wild-type enzyme. The high activity of the Pd2,6ST triple mutant was due to almost entirely an increase in kcat. However, the kinetic constants for the intermediate mutants show that each mutation had a greatly different effect on kcat and KM. The W361F mutation resulted in a 2.35-fold increase in sialidase activity via a decrease in kcat but a larger decrease in KM. Addition of the S232L mutation had little effect on KM but greatly enhanced kcat and provided the largest single-round gain in activity. The additional T356S mutation provided another large gain for kcat but also increased the KM nearly to that of the wild-type enzyme. Relative to the activity of human NEU2 (hNEU2),17, 20 an α2–3/6/8-sialidase, the Pd2,6ST S232L/T356S/W361F neosialidase displayed nearly 22-fold higher activity on a similar Neu5Acα2–6-containing probe. The donor hydrolysis activity of the triple mutant was found to have increased 341-fold from the wild-type (Table 2).
Table 1.
Kinetic parameters for Pd2,6ST mutant neosialidase activity in the presence of 0.5 mM CMP.
Table 2.
Kinetic parameters for the CMP-Neu5Ac hydrolysis activities of Pd2,6ST and Pd2,6ST S232L/T356S/W361F neosialidase.
| Enzymes and mutants | kcat(min−1) | KM(mM) |
kcat/KM (min−1mM−1) |
|---|---|---|---|
| Pd2,6ST[a] | (4.0±0.9)×102 | 45±14 | 8.8 |
| Pd2,6ST S232L/T356S/W361F | (1.1±0.01)×104 | 3.7±0.1 | 3.0 × 103 |
Reported previously.35
The Substrate Specificity of Pd2,6ST S232L/T356S/W361F Neosialidase
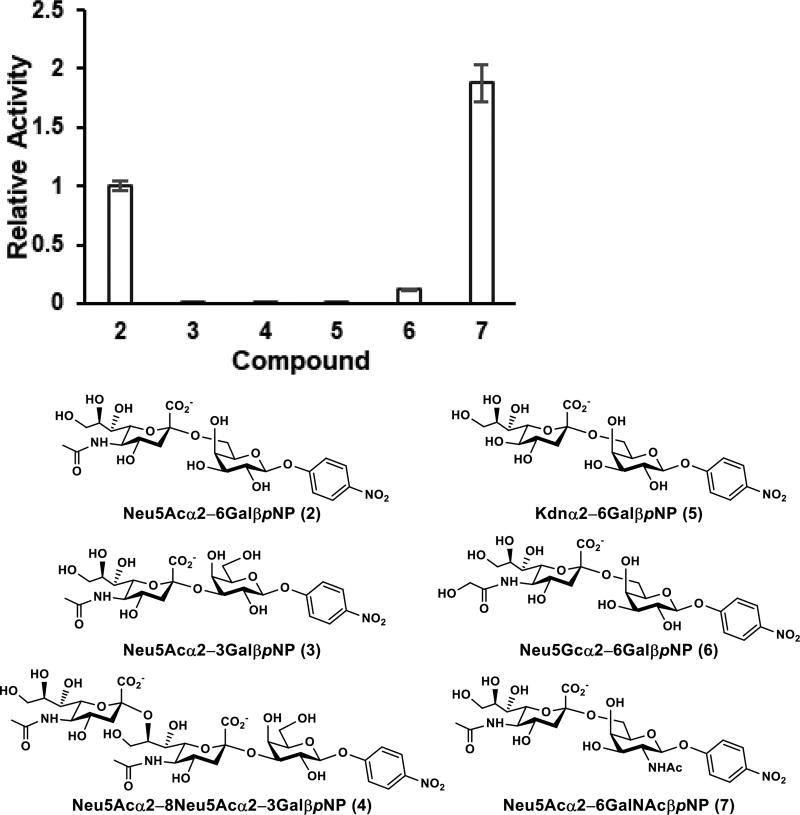
The sialidase substrate specificity of the Pd2,6ST S232L/T356S/W361F triple mutant was investigated by high-performance liquid chromatography (HPLC) analysis using probes containing varied sialyl linkages (α2–3/6/8), different sialic acid forms including Neu5Ac, Neu5Gc, and 2-keto-3-deoxy-D-glycero-D-galacto-nononic acid (Kdn), and various internal glycans (GalβpNP and GalNAcβpNP). The Pd2,6ST S232L/T356S/W361F triple mutant was selective towards α2–6-linked sialic acid while retaining some promiscuity to the sialic acid form and internal glycan (Figure 3). For example, among Neu5Acα2–3/6GalβpNP (compounds 2 and 3) and Neu5Acα2–8Neu5Acα2–3GalβpNP (compound 4) tested, only Neu5Acα2–6GalβpNP (2) was a suitable sialidase substrate for the Pd2,6ST S232L/T356S/W361F. Activities towards Neu5Acα2–3GalβpNP (3) and Neu5Acα2–8Neu5Acα2–3GalβpNP (4) were 400-fold and 303-fold, respectively, lower than Neu5Acα2–6GalβpNP (2) (Table S1), suggesting the high selectivity of the Pd2,6ST-derived neosialidase towards α2–6-sialyl linkage. The triple mutant was able to cleave α2–6-linked Neu5Gc in Neu5Gcα2–6GalβpNP (6) at 12% of the activity of Neu5Acα2–6GalβpNP (2), although its sialidase activity towards Kdnα2–6GalβpNP (5) containing an α2–6-linked Kdn was 1,020-fold lower than 2. Quite interestingly, the neosialidase activity of the Pd2,6ST S232L/T356S/W361F triple mutant was 1.9-folder higher towards Neu5Acα2–6GalNAcβpNP (7) than for Neu5Acα2–6GalβpNP (2).
Figure 3.
Relative activities of Pd2,6ST S232L/T356S/W361F towards sialosides (2–7) with various sialic acid forms, internal glycans, and sialyl linkages. Error bars represent standard deviations from duplicated assay results.
Recognition of Egg Yolk Sialoglycopeptide
To demonstrate the utility of Pd2,6ST S232L/T356S/W361F towards more complex glycoconjugates, the neosialidase was tested against egg yolk sialoglycopeptide, a hexapeptide with a biantennary complex-type N-linked glycan containing α2–6-linked sialic acid on each antenna. Detection of the desialylated glycopeptide by HPLC (Figure S4) and high resolution mass spectrometry (Figure S5) confirmed that the engineered neosialidase can recognize and cleave α2–6-linked sialic acid from complex sialylated glycoconjugates.
Concluding Remarks
The reprogramming of natural enzymes for non-natural functions is an important area of interest for enzyme engineering.37 By exploiting the reversibility of glycosyltransferase activity and the evolvability of glycosyltransferase substrate promiscuity, we have demonstrated that glycosyltransferases can be conveniently engineered into efficient neoglycosidases with specificities not known to exist in nature. This strategy will likely provide a valuable source of new enzymes to supplement known exoglycosidases, particularly for the selective cleavage of sugar residues from natural product glycosides or complex carbohydrates.
The Pd2,6ST-derived neosialidase developed here catalyzes the removal of sialic acid with high selectivity towards α2–6-linkages and promiscuity towards both Neu5Ac and Neu5Gc via a mechanism different from all known sialidase mechanisms. The engineered mutant will be a valuable addition to glycobiology, assisting in the elucidation of sialoglycan structure and function.
We are pleasantly surprised to discover three beneficial mutations across just six investigated residues within the active site of Pd2,6ST. This implies that the sialyltransferase activity of the enzyme is quite robust towards active site mutations and that the discrimination of nucleophilic water is quite sensitive to mutations. However, the Pd2,6ST triple mutant did not display the expected α2–6-sialidase activity towards Kdn even though Pd2,6ST was efficient in synthesizing Kdnα2–6-containing sialosides in high yield.38 This data suggests that the mutations that improve neosialidase towards Neu5Ac-containing probe Neu5Acα2–6GalβX (1) may have also altered the substrate specificity towards Kdn-containing compound Kdnα2–6GalβpNP (5).
The blue-white screening method used for the neosialidase engineering can be easily modified for the engineering of other neoglycosidases using suitable X-based probes. The throughput and simplicity of this method make the engineering of neoglycosidases practical for non-specialists in the absence of expensive equipment such as automated liquid handling systems and microplate reader spectrophotometers. It is particularly convenient that the disaccharide-X probes can be synthesized from commercially available monosaccharide-X building blocks using wild-type glycosyltransferase activity.
In conclusion, Pd2,6ST S232L/T356S/W361F neosialidase has been generated by sequential saturation mutagenesis and screening using a high-throughput blue-white colony assay. This triple mutant displays over 100-fold improved sialidase catalytic efficiency relative to the wild-type enzyme while retaining linkage selectivity of the wild-type sialyltransferase activity. The mutant can catalyze the cleave of α2–6-linked sialic acid in egg yolk sialoglycopeptide efficiently. This enzyme is a useful new tool for studying the structure and function of sialoglycans, and the engineering strategy may be proven useful to researchers interested in obtaining enzymes with glycosidase specificities not already known to exist in nature.
METHODS
Materials
Chemicals were purchased and used as received. NMR spectra were recorded in the NMR facility of the University of California, Davis, on a Bruker Avance-800 NMR spectrometer (800 MHz for 1H, 200 MHz for 13C). Chemical shifts are reported in parts per million (ppm) on the δ scale. High resolution (HR) electrospray ionization (ESI) mass spectra were obtained using a Thermo Electron LTQ-Orbitrap Hybrid MS at the Mass Spectrometry Facility in the University of California, Davis. N-Acetylneuraminic acid (Neu5Ac) was from Inalco (Italy). Cytosine 5’-triphosphate (CTP) was purchased from Hangzhou Meiya Pharmaceutical Co. Ltd. X-Gal was purchased from Sigma. Egg yolk sialoglycopeptide was purchased from TCI America. Neu5Acα2–6GalβpNP (2), Neu5Acα2–3GalβpNP (3),16 Kdnα2–6GalβpNP (5),16 Neu5Gcα2–6GalβpNP (6),16 and Neu5Acα2–6GalNAcβpNP (7),16 Neu5Acα2–8Neu5Acα2–3GalβpNP (4),20 and Neu5Acα2–6LacβMU24 were synthesized as described previously. Neisseria meningitidis CMP-sialic acid synthetase (NmCSS)39 and Photobacterium species α2–6-sialyltransferase (Psp2,6ST)40 were expressed and purified as reported previously.
Mutagenesis
Pd2,6ST libraries were constructed using either the Q5 Mutagenesis Kit (D229X and W361X) or the QuikChange II Site Directed Mutagenesis kit using the following primers: D229X_f: NNKGGTTCTTCTGAATATGTAAGTTTATATCAATGG, D229X_r: ATCATACAAACTAATATGAGAAATTTTCACCTTCTCG, S232X_f: 5' AATTTCTCATATTAGTTTGTATGATGATGGTTCTNNKGAATATGTAAGTTTATATCAATGGAAAGATACAC 3', S232X_r: 5' GTGTATCTTTCCATTGATATAAACTTACATATTCMNNAGAACCATCATCATACAAACTAATATGAGAAATT 3', T356X_f: 5' ACAATATTCACAATCCCCACTACCAAACTTTATTTTTNNKGGCACAACAACTTTTGCTG 3', T356X_r: 5' CAGCAAAAGTTGTTGTGCCMNNAAAAATAAAGTTTGGTAGTGGGGATTGTGAATATTGT 3', W361X_f: 5' NNKGCTGGGGGGGAAACG 3', W361X_r: 5' AGTTGTTGTGCCGGTAAAAATAAAGTTTGG 3', A403X_f: 5' GACTACGATCTATTTTTCAAGGGGCATCCTNNKGGTGGCGTTATTAACG 3', A403X_r: 5' CGTTAATAACGCCACCMNNAGGATGCCCCTTGAAAAATAGATCGTAGTC 3', I425X_f: 5' TGATATGATCAATATTCCAGCCAAGNNKTCATTTGAGGTCTTGATGATGACGG 3', and I425X_r: 5' CCGTCATCATCAAGACCTCAAATGAMNNCTTGGCTGGAATATTGATCATATCA 3'. The assembled DNA was transformed into E. coli 10 G electrocompetent cells (Lucigen). Ten percent of the transformed cells were plated on LB agar plates supplemented with ampicillin in order to determine the number of total transformants. The remaining transformed cells were diluted into fresh LB media (10 g L−1 tryptone, 5 g L−1 yeast extract, and 10 g L−1 NaCl) supplemented with ampicillin, grown overnight at 37 °C 250 rpm, and the plasmid DNA was isolated. This DNA was transformed into homemade chemically competent E. coli BL21(DE3) cells.
One-pot Two-enzyme synthesis of Neu5Acα2–6GalβX (1)
A reaction mixture in a total volume of 20 mL containing Tris-HCl buffer (100 mM, pH 8.5), 5-bromo-4-chloro-3-indolyl-β-D-galactopyranosides (X-Gal, 50 mg, 0.122 mmol), Neu5Ac (57 mg, 0.184 mmol), CTP (97 mg, 0.184 mmol), DMF (7%), MgCl2 (20 mM), NmCSS39 (2.5 mg), and Psp2,6ST40 (4.0 mg) were incubated in a shaker at 30 °C for 18 h. The reaction was stopped by adding 20 mL of 95% ethanol followed by incubation at 4 °C for 30 minutes. After centrifugation, the supernatant was concentrated and purified using a C18 column on a CombiFlash Rf 200i system eluted with a gradient of 0–100% acetonitrile in water for 20 minutes and a 30 mL min−1 flow rate. The fractions containing the desired product were collected and dried to give Neu5Acα2–6GalβX as a white powder (81 mg, 92%). 1H NMR (800 MHz, MeOD) δ 7.18 (d, J = 8.8 Hz, 1 H), 7.14 (bs, 1 H), 7.04 (d, J = 8.8 Hz, 1 H), 4.58 (d, J = 8.0 Hz, 1 H), 3.93–3.40 (m, 11 H), 2.77 (d, J = 12.8 and 4.8 Hz, 1 H), 1.92 (s, 3 H), 1.53 (t, J = 12.0 Hz, 1 H); 13C NMR (200 MHz, MeOD) δ 173.96, 173.13, 136.65, 133.36, 125.39, 123.78, 117.98, 113.36, 111.81, 111.11, 104.08, 100.40, 73.96, 73.37, 72.93, 71.52, 71.17, 68.84, 68.49, 68.08, 62.98, 62.42, 52.66, 41.18, 21.14. HRMS (ESI) m/z calcd for C25H31BrClN2O14 [M−H]− 697.0653, found 697.0609.
Library Screening
Mutant libraries were transformed to BL21(DE3) chemically competent cells and plated on LB-agar plates supplemented with ampicillin. Following overnight incubation at 37 °C, colonies were lifted onto 0.45 µm 47 mm Mixed Cellulose Esters Surfactant-Free Membrane Filters (Millipore). These nitrocellulose filters were carefully placed colony-side up on LB-agar plates supplemented with ampicillin and 0.1 mM IPTG, and these plates were incubated for 3 hours at 37 °C. Meanwhile, the original LB-agar plates were incubated for 3–5 hours at 37 °C until the colonies regrew and then stored at 4 °C as master plates. The filters were then suspended over chloroform vapors for 10 minutes, briefly air dried, and were placed colony-side up on 55 mm Whatman filter paper soaked with 0.5 mL of the assay solution. For the first two rounds, the assay solution contained 3 mM Neu5Acα2–6GalβX, 0.5 mM CMP, 100 mM MES pH 5.5, and MgCl2 (10 mM). For the third and fourth rounds, the assay solution contained Neu5Acα2–6GalβX (3 mM), Tris-HCl (pH 7.0, 100 mM), and MgCl2 (10 mM). Reactions were conducted at 37 °C with regular examination of the filters for the development of blue color.
Overexpression and Purification
Flasks containing 1 L of autoclaved LB media supplemented with ampicillin (100 µg mL−1) were inoculated with 1 mL of overnight cultured E. coli BL21(DE3) cells harboring the mutant plasmids. The 1 L cultures were grown at 37 °C until OD600 nm reached 0.6 to 1.0, then expression was induced with isopropyl β-D-1-thiogalactoside (IPTG) to a final concentration of 0.1 mM and the cells shaken at 20 °C overnight. Cells were harvested in a Sorvall Legend RT centrifuge at 4000 rpm for 30 minutes, resuspended in 20 mL of Tris-HCl (pH 7.5, 100 mM) and lysed by sonication with the following method: amplitude at 65%, 10 s pulse on and 20 s pulse off for 18 cycles. The lysate was collected after centrifugation at 8000 pm for 30 minutes and then loaded onto a Ni2+-NTA affinity column at 4 °C that was pre-equilibrated with 6 column volumes of binding buffer (50 mM Tris-HCl buffer, pH 7.5, 10 mM imidazole, 0.5 M NaCl). The column was washed with 10 column volumes of binding buffer and 10 column volumes of washing buffer (50 mM of Tris-HCl buffer, pH 7.5, 50 mM of imidazole, 0.5 M of NaCl) sequentially to wash away the nonspecific binding protein. The target protein was eluted using Tris-HCl buffer (50 mM, pH 7.5) containing 200 mM of imidazole and 0.5 M NaCl. Fractions containing the purified protein were combined and dialyzed against Tris-HCl buffer (20 mM, pH 7.5) supplemented with 10% glycerol. The enzyme solutions were aliquoted, flash frozen in liquid N2, and stored at −20 °C.
Neosialidase Kinetics
Reactions were performed in duplicate at 37 °C for 10 to 30 minutes with Tris-HCl (100 mM, pH 6.0), MgCl2 (10 mM), CMP (0.5 mM), enzyme (7.0 µM Pd2,6ST W361F, 0.32 µM Pd2,6ST S232L/W361F, 0.070 µM Pd2,6ST S232L/T356S/W361F), and varying concentrations (0.5, 1.0, 2.0, and 5.0 mM) of Neu5Acα2–6LacβMU. Reactions were stopped by adding an equal volume of pre-chilled methanol. The mixtures were incubated on ice for 30 minutes and centrifuged at 13,000 rpm for 5 minutes. Supernatants were analyzed with a P/ACE™ MDQ capillary electrophoresis (CE) system equipped with a UV-Vis detector (Beckman Coulter, Fullerton, CA). The CE procedure utilized a 75 µm i.d. capillary, 25 KV/80 µÅ, 5 s vacuum injections, was monitored at 315 nm, and used sodium tetraborate (25 mM, pH 9.4) buffer as the running buffer. The apparent kinetic parameters were obtained by fitting the experimental data from duplicate assays into the Michaelis-Menten equation using Grafit 5.0.
Donor Hydrolysis Kinetics
Reactions were performed in duplicate at 37 °C for 10 to 30 minutes with Tris-HCl (100 mM, pH 8.5), MgCl2 (10 mM), enzyme (0.030 µM Pd2,6ST S232L/T356S/W361F), and varying concentrations (2.0, 5.0, 10.0, and 20.0 mM) of CMP-Neu5Ac. Reactions were stopped by adding an equal volume of pre-chilled methanol. The mixtures were incubated on ice for 30 minutes and centrifuged at 13,000 rpm for 5 minutes. Supernatants were analyzed with a P/ACE MDQ capillary electrophoresis (CE) system equipped with a UV-Vis detector (Beckman Coulter, Fullerton, CA). The CE procedure utilized a 75 µm i.d. capillary, 25 KV/80 µÅ, 5 s vacuum injections, was monitored at 254 nm, and used sodium tetraborate (25 mM, pH 9.4) buffer as the running buffer. The apparent kinetic parameters were obtained by fitting the experimental data from duplicate assays into the Michaelis–Menten equation using Grafit 5.0.
pH Profile
Reactions were performed in duplicate at 37 °C for 30 minutes with a suitable buffer (100 mM MES from pH 4 to 6 or 100 mM Tris-HCl from pH 6.5 to 8.5), MgCl2 (10 mM), Neu5Acα2–6LacβMU (1 mM), and CMP (0.5 mM). Reactions were stopped by adding an equal volume of pre-chilled methanol. The mixtures were incubated on ice for 30 minutes and centrifuged at 13,000 rpm for 5 minutes. Supernatants were analyzed with an Infinity 1290-II HPLC equipped with a UV-Vis detector (Agilent Technologies, CA). The HPLC procedure utilized a ZORBAX Eclipse Plus C18 Rapid Resolution HD 1.8 µm particle 2.1 × 50 mm column (Agilent Technologies, CA), an isocratic flow of 1 mL min−1 for a 9% acetonitrile and 91% aqueous solution containing 0.1% TFA, and an injection volume of 2 µL. The 4-methylumbelliferone absorbance signal was monitored at 315 nm.
CMP Concentration Effect Assays
Reactions were performed in duplicate at 37 °C for 30 minutes in MES buffer (100 mM, pH 6.0) containing MgCl2 (10 mM), Neu5Acα2–6LacβMU (1 mM), CMP with a concentration varying from 0.1 mM to 25.0 mM (0.1, 0.2, 0.5, 1.0, 2.0, 5.0, 10.0, and 25.0 mM), and Pd2,6ST S232L/T356S/W361F (0.130 µM). Reactions were stopped by adding an equal volume of pre-chilled methanol. The mixtures were incubated on ice for 30 minutes and centrifuged at 13,000 rpm for 5 minutes. Supernatants were analyzed using an Infinity 1290-II HPLC equipped with a UV-Vis detector (Agilent Technologies, CA). The HPLC procedure utilized a ZORBAX Eclipse Plus C18 Rapid Resolution HD 1.8 µm particle 2.1 × 50 mm column (Agilent Technologies, CA), an isocratic flow of 1 mL min−1 for a 9% acetonitrile and 91% aqueous solution containing 0.1% TFA, and an injection volume of 2 µL. The 4-methylumbelliferone absorbance signal was monitored at 315 nm.
Desialylation of Egg Yolk Sialoglycopeptide
Reactions were performed at 37 °C for 60 minutes with MES buffer (100 mM, pH 6.0), MgCl2 (10 mM), CMP (0.5 mM), Pd2,6ST S232L/T356S/W361F (0.0 or 13.0 µM), and egg yolk sialoglycopeptide (1 mM). Reactions were stopped by thermal denaturation of the enzyme at 60 °C for 10 minutes. The mixtures were incubated on ice for 30 minutes and centrifuged at 13,000 rpm for 5 minutes. Chromatographic separation and detection were achieved with an Infinity 1290-II HPLC equipped with a UV-Vis detector (Agilent Technologies, CA). The HPLC procedure utilized a ZORBAX Bonus-RP Rapid Resolution HD 1.8 µm particle 2.1 × 100 mm column (Agilent Technologies, CA), a gradient flow of 0.7 mL min−1 of 0.3 to 8% acetonitrile over 6 minutes in aqueous solution containing 0.1% TFA, and an injection volume of 1 µL. The peptide bond absorbance signal was monitored at 214 nm. High resolution (HR) electrospray ionization (ESI) mass spectra were obtained using a Thermo Electron LTQ-Orbitrap Hybrid MS at the Mass Spectrometry Facility in the University of California, Davis.
Linkage Specificity Assays for the Pd2,6ST Mutants
Reactions were performed in duplicate at 37 °C for 30 minutes in MES buffer (100 mM, pH 6.0), MgCl2 (10 mM), CMP (0.5 mM), and 1 mM substrate. Enzyme concentrations were 0.030 µM for 2, 0.30 µM for 7, 3.0 µM 6, and 30.0 µM for 3–5. These conditions provided testing at initial rates (1.2–24% yield) for each substrate. Reactions were stopped by adding an equal volume of pre-chilled methanol. The mixtures were incubated on ice for 30 minutes and centrifuged at 13,000 rpm for 5 minutes. Supernatants were analyzed with an Infinity 1290-II HPLC equipped with a UV-Vis detector (Agilent Technologies, CA). The HPLC procedure utilized a ZORBAX Eclipse Plus C18 Rapid Resolution HD 1.8 µm particle 2.1 × 50 mm column (Agilent Technologies, CA), an isocratic flow of 1 mL min−1 for a 9% acetonitrile and 91% aqueous solution containing 0.1% TFA, and an injection volume of 2 µL. The para-nitrophenyl absorbance signal was monitored at 315 nm.
Supplementary Material
Acknowledgments
This work was partially supported by National Institutes of Health (NIH) grants R01HD065122 and R01AI130684.
ABBREVIATIONS
- CE
capillary electrophoresis
- CMP
cytidine 5’-monophosphate
- CTP
cytosine 5’-triphosphate
- ESI
electrospray ionization
- Kdn
2-keto-3-deoxy-D-glycero-D-galacto-nononic acid
- HPLC
high-performance liquid chromatography
- HRMS
hig resolution mass spectrometry
- IPTG
isopropyl β-D-1-thiogalactoside
- Neu5Ac
N-acetylneuraminic acid
- Neu5Gc
N-glycolylneuraminic acid
- NmCSS
Neisseria meningitidis CMP-sialic acid synthetase
- Pd2,6ST
Photobacterium damselae α2–6-sialyltransferase
- PmST1
Pasteurella multocida sialyltransferase 1 (Δ24PmST1)
- pNP
para-nitrophenyl
- NMR
nuclear magnetic resonance
- ppm
parts per million
- Psp2,6ST
Photobacterium species α2–6-sialyltransferase
- Sia
sialic acid
- X-Gal
5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside
Footnotes
ASSOCIATED CONTENT
Supporting Information. Supplemental table and figures, and NMR spectra for Neu5Acα2–6GalβX (1). This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
References
- 1.Morelle W, Michalski JC. Analysis of protein glycosylation by mass spectrometry. Nat. Protoc. 2007;2:1585–1602. doi: 10.1038/nprot.2007.227. [DOI] [PubMed] [Google Scholar]
- 2.Sadler JE, Paulson JC, Hill RL. The role of sialic acid in the expression of human MN blood group antigens. J. Biol. Chem. 1979;254:2112–2119. [PubMed] [Google Scholar]
- 3.Belisle JA, Horibata S, Jennifer GA, Petrie S, Kapur A, Andre S, Gabius HJ, Rancourt C, Connor J, Paulson JC, Patankar MS. Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Mol. Cancer. 2010;9:118. doi: 10.1186/1476-4598-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razi N, Varki A. Cryptic sialic acid binding lectins on human blood leukocytes can be unmasked by sialidase treatment or cellular activation. Glycobiology. 1999;9:1225–1234. doi: 10.1093/glycob/9.11.1225. [DOI] [PubMed] [Google Scholar]
- 5.Chan RW, Karamanska R, Van Poucke S, Van Reeth K, Chan IW, Chan MC, Dell A, Peiris JS, Haslam SM, Guan Y, Nicholls JM. Infection of swine ex vivo tissues with avian viruses including H7N9 and correlation with glycomic analysis. Influenza Other Respir. Viruses. 2013;7:1269–1282. doi: 10.1111/irv.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito N, Nishi K, Nakajima M, Okamura Y, Hirota T. Histochemical analysis of the chemical structure of blood group-related carbohydrate chains in serous cells of human submandibular glands using lectin staining and glycosidase digestion. J. Histochem. Cytochem. 1989;37:1115–1124. doi: 10.1177/37.7.2499620. [DOI] [PubMed] [Google Scholar]
- 7.Filipe MI, Sandey A, Carapeti EA. Goblet cell mucin in human foetal colon, its composition and susceptibility to enzyme degradation: a histochemical study. Symp. Soc. Exp. Bio. 1989;43:249–258. [PubMed] [Google Scholar]
- 8.Truong LD, Phung VT, Yoshikawa Y, Mattioli CA. Glycoconjugates in normal human kidney. A histochemical study using 13 biotinylated lectins. Histochemistry. 1988;90:51–60. doi: 10.1007/BF00495707. [DOI] [PubMed] [Google Scholar]
- 9.Szigeti M, Guttman A. Automated N-glycosylation sequencing of biopharmaceuticals by capillary electrophoresis. Sci. Rep. 2017;7:11663. doi: 10.1038/s41598-017-11493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pretzlaff RK, Xue VW, Rowin ME. Sialidase treatment exposes the beta1-integrin active ligand binding site on HL60 cells and increases binding to fibronectin. Cell Adhes. Commun. 2000;7:491–500. doi: 10.3109/15419060009040306. [DOI] [PubMed] [Google Scholar]
- 11.Van Den Hamer CJ, Morell AG, Scheinberg IH, Hickman J, Ashwell G. Physical and chemical studies on ceruloplasmin. IX. The role of galactosyl residues in the clearance of ceruloplasmin from the circulation. J. Biol. Chem. 1970;245:4397–4402. [PubMed] [Google Scholar]
- 12.Rosen SD, Singer MS, Yednock TA, Stoolman LM. Involvement of sialic acid on endothelial cells in organ-specific lymphocyte recirculation. Science. 1985;228:1005–1007. doi: 10.1126/science.4001928. [DOI] [PubMed] [Google Scholar]
- 13.Guttormsen HK, Paoletti LC, Mansfield KG, Jachymek W, Jennings HJ, Kasper DL. Rational chemical design of the carbohydrate in a glycoconjugate vaccine enhances IgM-to-IgG switching. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5903–5908. doi: 10.1073/pnas.0710799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juge N, Tailford L, Owen CD. Sialidases from gut bacteria: a mini-review. Biochem. Soc. Trans. 2016;44:166–175. doi: 10.1042/BST20150226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Varki A. Advances in the biology and chemistry of sialic acids. ACS Chem. Biol. 2010;5:163–176. doi: 10.1021/cb900266r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chokhawala HA, Yu H, Chen X. High-throughput substrate specificity studies of sialidases by using chemoenzymatically synthesized sialoside libraries. Chembiochem. 2007;8:194–201. doi: 10.1002/cbic.200600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Cao H, Yu H, Chen Y, Lau K, Qu J, Thon V, Sugiarto G, Chen X. Identifying selective inhibitors against the human cytosolic sialidase NEU2 by substrate specificity studies. Mol. BioSyst. 2011;7:1060–1072. doi: 10.1039/c0mb00244e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khedri Z, Muthana MM, Li Y, Muthana SM, Yu H, Cao H, Chen X. Probe sialidase substrate specificity using chemoenzymatically synthesized sialosides containing C9-modified sialic acid. Chem. Commun. 2012;48:3357–3359. doi: 10.1039/c2cc17393j. [DOI] [PubMed] [Google Scholar]
- 19.Khedri Z, Li Y, Muthana S, Muthana MM, Hsiao CW, Yu H, Chen X. Chemoenzymatic synthesis of sialosides containing C7-modified sialic acids and their application in sialidase substrate specificity studies. Carbohydr. Res. 2014;389:100–111. doi: 10.1016/j.carres.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tasnima N, Yu H, Li Y, Santra A, Chen X. Chemoenzymatic synthesis of para-nitrophenol (pNP)-tagged alpha2–8-sialosides and high-throughput substrate specificity studies of alpha2–8-sialidases. Org. Biomol. Chem. 2016;15:160–167. doi: 10.1039/c6ob02240e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sela DA, Li Y, Lerno L, Wu S, Marcobal AM, German JB, Chen X, Lebrilla CB, Mills DA. An infant-associated bacterial commensal utilizes breast milk sialyloligosaccharides. J. Biol. Chem. 2011;286:11909–11918. doi: 10.1074/jbc.M110.193359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khedri Z, Xiao A, Yu H, Landig CS, Li W, Diaz S, Wasik BR, Parrish CR, Wang LP, Varki A, Chen X. A chemical biology solution to problems with studying biologically important but unstable 9-O-acetyl sialic acids. ACS Chem. Biol. 2017;12:214–224. doi: 10.1021/acschembio.6b00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:D490–495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng J, Huang S, Yu H, Li Y, Lau K, Chen X. Trans-sialidase activity of Photobacterium damsela alpha2,6-sialyltransferase and its application in the synthesis of sialosides. Glycobiology. 2010;20:260–268. doi: 10.1093/glycob/cwp172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu H, Chokhawala H, Karpel R, Yu H, Wu B, Zhang J, Zhang Y, Jia Q, Chen X. A multifunctional Pasteurella multocida sialyltransferase: a powerful tool for the synthesis of sialoside libraries. J. Am. Chem. Soc. 2005;127:17618–17619. doi: 10.1021/ja0561690. [DOI] [PubMed] [Google Scholar]
- 26.Sugiarto G, Lau K, Qu J, Li Y, Lim S, Mu S, Ames JB, Fisher AJ, Chen X. A sialyltransferase mutant with decreased donor hydrolysis and reduced sialidase activities for directly sialylating LewisX. ACS Chem. Biol. 2012;7:1232–1240. doi: 10.1021/cb300125k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng J, Yu H, Lau K, Huang S, Chokhawala HA, Li Y, Tiwari VK, Chen X. Multifunctionality of Campylobacter jejuni sialyltransferase CstII: characterization of GD3/GT3 oligosaccharide synthase, GD3 oligosaccharide sialidase, and trans-sialidase activities. Glycobiology. 2008;18:686–697. doi: 10.1093/glycob/cwn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehr K, Withers SG. Mechanisms of the sialidase and trans-sialidase activities of bacterial sialyltransferases from glycosyltransferase family 80. Glycobiology. 2016;26:353–359. doi: 10.1093/glycob/cwv105. [DOI] [PubMed] [Google Scholar]
- 29.Huynh N, Li Y, Yu H, Huang S, Lau K, Chen X, Fisher AJ. Crystal structures of sialyltransferase from Photobacterium damselae. FEBS Lett. 2014;588:4720–4729. doi: 10.1016/j.febslet.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kakuta Y, Okino N, Kajiwara H, Ichikawa M, Takakura Y, Ito M, Yamamoto T. Crystal structure of Vibrionaceae Photobacterium sp. JT-ISH-224 alpha2,6-sialyltransferase in a ternary complex with donor product CMP and acceptor substrate lactose: catalytic mechanism and substrate recognition. Glycobiology. 2008;18:66–73. doi: 10.1093/glycob/cwm119. [DOI] [PubMed] [Google Scholar]
- 31.Ni L, Chokhawala HA, Cao H, Henning R, Ng L, Huang S, Yu H, Chen X, Fisher AJ. Crystal structures of Pasteurella multocida sialyltransferase complexes with acceptor and donor analogues reveal substrate binding sites and catalytic mechanism. Biochemistry. 2007;46:6288–6298. doi: 10.1021/bi700346w. [DOI] [PubMed] [Google Scholar]
- 32.Choi YH, Kim JH, Park JH, Lee N, Kim DH, Jang KS, Park IH, Kim BG. Protein engineering of alpha2,3/2,6-sialyltransferase to improve the yield and productivity of in vitro sialyllactose synthesis. Glycobiology. 2014;24:159–169. doi: 10.1093/glycob/cwt092. [DOI] [PubMed] [Google Scholar]
- 33.Watson DC, Wakarchuk WW, Leclerc S, Schur MJ, Schoenhofen IC, Young NM, Gilbert M. Sialyltransferases with enhanced legionaminic acid transferase activity for the preparation of analogs of sialoglycoconjugates. Glycobiology. 2015;25:767–773. doi: 10.1093/glycob/cwv017. [DOI] [PubMed] [Google Scholar]
- 34.Sugiarto G, Lau K, Li Y, Khedri Z, Yu H, Le DT, Chen X. Decreasing the sialidase activity of multifunctional Pasteurella multocida alpha2–3-sialyltransferase 1 (PmST1) by site-directed mutagenesis. Mol. BioSyst. 2011;7:3021–3027. doi: 10.1039/c1mb05182b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McArthur JB, Yu H, Zeng J, Chen X. Converting Pasteurella multocida alpha2–3-sialyltransferase 1 (PmST1) to a regioselective alpha2–6-sialyltransferase by saturation mutagenesis and regioselective screening. Org. Biomol. Chem. 2017;15:1700–1709. doi: 10.1039/c6ob02702d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aharoni A, Thieme K, Chiu CP, Buchini S, Lairson LL, Chen H, Strynadka NC, Wakarchuk WW, Withers SG. High-throughput screening methodology for the directed evolution of glycosyltransferases. Nat. Methods. 2006;3:609–614. doi: 10.1038/nmeth899. [DOI] [PubMed] [Google Scholar]
- 37.Renata H, Wang ZJ, Arnold FH. Expanding the enzyme universe: accessing non-natural reactions by mechanism-guided directed evolution. Angew. Chem. Int. Ed. 2015;54:3351–3367. doi: 10.1002/anie.201409470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu H, Huang S, Chokhawala H, Sun M, Zheng H, Chen X. Highly efficient chemoenzymatic synthesis of naturally occurring and non-natural alpha-2,6-linked sialosides: a P. damsela alpha-2,6-sialyltransferase with extremely flexible donor-substrate specificity. Angew. Chem. Int. Ed. 2006;45:3938–3944. doi: 10.1002/anie.200600572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu H, Yu H, Karpel R, Chen X. Chemoenzymatic synthesis of CMP-sialic acid derivatives by a one-pot two-enzyme system: comparison of substrate flexibility of three microbial CMP-sialic acid synthetases. Bioorg. Med. Chem. 2004;12:6427–6435. doi: 10.1016/j.bmc.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 40.Ding L, Yu H, Lau K, Li Y, Muthana S, Wang J, Chen X. Efficient chemoenzymatic synthesis of sialyl Tn-antigens and derivatives. Chem. Commun. 2011;47:8691–869. doi: 10.1039/c1cc12732b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.