Abstract
Worldwide, drought affects crop yields; therefore, understanding plants’ strategies to adapt to drought is critical. Chloroplasts are key regulators of plant responses, and signals from chloroplasts also regulate nuclear gene expression during drought. However, the interactions between chloroplast-initiated retrograde signals and ion channels under stress are still not clear. In this review, we summarise the retrograde signals that participate in regulating plant stress tolerance. We compare chloroplastic transporters that modulate retrograde signalling through retrograde biosynthesis or as critical components in retrograde signalling. We also discuss the roles of important plasma membrane and tonoplast ion transporters that are involved in regulating stomatal movement. We propose how retrograde signals interact with ion transporters under stress.
Keywords: retrograde signalling, ion channels, pumps, cotransporters, stress responsive genes
1. Introduction
The world will need to feed more than 9 billion people by 2050 [1], thus food production needs to increase by at least 70% [2]. However, because of the decrease of land available for agriculture and of climate change, this will be a substantial challenge [3]. To meet this challenge, a better understanding of crop agronomy and physiology is needed together with the development of new germplasm allowing sustainable crop production under adverse environmental conditions such as drought. About half of earth’s land area is susceptible to drought [4], which is regarded as a sunstantial threat to global food security [5]. During evolution, plants have adopted many mechanisms to counteract drought. One significant mechanism is stomatal regulation, and highly responsive stomata have been suggested as key factors for the success of grasses in adverse environments [6]. During drought plants close their stomata, thereby reducing water uptake, which affects their normal physiological functioning and nutrient uptake from the soil and reduces their growth and yield [7]. Plants protect themselves in the short term by closing stomata [8] and in the long term by increasing the root/shoot ratio [9], root hydraulic conductance [10], thickness of leaf cuticle [11], stomatal development [12], and cuticular wax [13]. If these drought avoidance mechanisms are not successful, mechanisms to tolerate dehydration may be switched on. These mechanisms include ways of maintaining cell water content through ion accumulation [8], cell wall stiffening [14], production of protective compounds [15], metabolic changes, and detoxification of reactive oxygen species (ROS) [16]. However, to respond to drought, a plant must first perceive the stress and then transduce the related recognition events via signalling networks. As a result, the transcription of specific drought stress response genes occurs, leading to changes in physiological processes and systemically transducing further signals throughout the plant.
Apart from the dominant function of photosynthesis, chloroplasts also function as sensors of environmental stimuli such as drought stress and initiate signals that induce nuclear gene expression (NGE) across a range of evolutionarily important plant species [16]. The endosymbiotic theory suggests that a plant cell is the result of hundreds of millions of years of co-evolution of cyanobacteria and early eukaryotic cells, i.e., the host [17]. From this stable endosymbiotic relationship, cyanobacteria evolved into modern chloroplasts, and large numbers of cyanobacterial genes were transferred to the host nucleus during evolution [18]. However, around 100 genes involved in photosynthesis have been retained in chloroplasts [19]. Therefore, the expression of nuclear and chloroplast genes must be combined and coordinated for the cells to function efficiently [20]. Nucleus to plastid signalling is termed anterograde signals [21], and plastid-derived signals that target the regulation of NGE are called retrograde signals [22]. In addition to coordinating chloroplast functioning, there is evidence that retrograde signalling has a role in adaptation to stress. The progenitors of plastids would have contained functions helping plants respond to the environment through the expression of stress response genes [23].
Stomata evolved from early land plant species like mosses, function in regulating water potential and CO2 fixation and regulate drought tolerance [24]. A chloroplast-initiated retrograde signalling pathway has been identified as having a significant role in regulating stomatal movement, which greatly affects plant drought tolerance [25,26]. Plants have a complex signalling system to control stomatal opening that is driven by the uptake and intracellular generation of solutes, which decrease guard cell water potential and create a driving force for water uptake into guard cells. In contrast, during stomatal closure, a reduction of solute contents regulated by membrane transport systems in the guard cells leads to cell deflation and a narrowing of the stomatal aperture [27]. Stomata have evolved an abscisic acid (ABA)-dependent network from the last common ancestor of mosses and vascular plants for drought response [28,29]. The ABA signal transduction system consists of PYR/PYL/RCAR-type ABA receptors, group A 2C-type protein phosphatases (PP2C), and SNF1-related protein kinase 2 (SnRK2) family of proteins, which are key negative regulators of ABA signalling [30]. Once bound to ABA, the receptor complex inactivates PP2C, thereby activating protein kinase SnRK2 [31], which induces the production of ROS [32] and nitric oxide (NO) [33]. Hydrogen peroxide (H2O2) can activate Ca2+ channels in the plasma membrane (PM) of Arabidopsis guard cells and inhibit inward K+ channels [34,35]. Nitric oxide has also been identified to regulate K+ and Cl− channels through a subset of ABA-evoked signalling pathways in guard cells [33]. Guard cell anion channels are activated by Ca2+ and become permeable, allowing the efflux of anions [36], and K+ outwardly rectifying channels (GORK) are activated by K+ loss [37], leading to stomatal closure.
Despite the advancements in understanding retrograde signals and plant membrane transport [22,27], the interactions between retrograde signalling pathways and ion transport across the plasma membrane, tonoplast, and chloroplast membranes are poorly understood. Chloroplasts are also involved in other signalling networks such as sulphate metabolism and signalling. For instance, sulphate transporters play important roles in plant drought and salinity tolerance [38]. Accumulation of sulphate in the leaves enhances ABA biosynthesis in the leaves, ABA sensitivity causing stomatal closure [39]. Newly synthesised ABA is then transported to the roots for signal transduction and the expression of drought stress-responsive genes [40]. Therefore, sulphate and its transporters are suggested as important components in long-distance signalling under drought [38,39]. A compound associated with sulphate metabolism, 3′-phosphoadnenosine 5′-phosphate (PAP), is thought to act as a typical retrograde signal. PAP is produced in chloroplasts under drought stress to induce the expression of nuclear-encoded stress response genes, leading to stomatal closure [25,26]. Whether the PAP signal joins the ABA signalling pathway or whether PAP is part of a separate pathway from ABA is still unclear. In this review, we summarise the progress towards a better understanding of the relationships between retrograde signals and ion transport in adverse environments. We illustrate the possible roles for retrograde signalling in plant stress response using the recently discovered PAP signalling pathway. We also propose a potential interaction whereby chloroplasts sense drought and produce signals (e.g., PAP) which regulate stomatal movement to maintain water potential in plant cells and to guarantee a stable photosynthesis rate under drought.
2. Typical Retrograde Signals in Chloroplasts
Retrograde signalling refers to communications from organelles to the nucleus. Chloroplasts, whose primary function is photosynthesis, are also critical for other aspects of plant development and physiology, including the synthesis of amino acids, nucleotides, fatty acids, phytohormones, and the assimilation of sulphur [16]. Several chloroplastic secondary metabolites also function as retrograde signals that are responsible for plant defence against pathogens and for plant adaptation to heat, drought, and high light [20]. Some abiotic stress responses in plants are likely to share common signalling mechanisms or components [41]. For example, 69% of drought-induced genes are also induced by high-light stress, suggesting a strong interconnection between the responses to these two types of stress [42]. This makes abiotic stress defence systems more efficient in plants because genes induced by one type of stress would be efficient also in response to other stress signals. Tremendous progress has been made in identifying retrograde signalling components and their corresponding pathways in chloroplasts. Retrograde molecules include carotenoid oxidation products [43], ROS such as H2O2 [44], tetrapyrroles [45], phosphoadenosines [25], carbohydrate metabolites [46,47], and isoprenoid precursor methylerythritol cyclodiphosphate (MEcPP) [23]. Retrograde signals include metabolite by-products [48], transcription factors [49], and thylakoid redox state [50] (Table 1).
Table 1.
Source of typical retrograde signals in plants.
2.1. Reactive Oxygen Species
Plastid ROS molecules can regulate specific proteins and plastid redox-associated nuclear genes (PRANGs) [16] and usually cause oxidation of biomolecules and act as signal molecules in plants [43]. ROS occur as singlet oxygen (1O2), superoxide anions (O2−), H2O2, or hydroxyl radicals (OH−), and excessive ROS can lead to programmed cell death (PCD). Plants have developed efficient and versatile scavenging systems to keep ROS under control. Compared with other ROS, H2O2 appears most likely to be a retrograde signal, because of its small size, lower toxicity, longer half-life, relatively high concentration in cells, and ability to cross cell membranes and move between cell compartments [54].
During plant evolution, ROS have developed many functions associated with plant development and stress tolerance. For instance, H2O2 is involved in ABA-induced stomatal closure by regulating Ca2+, K+, and Cl− channels [36,55]. H2O2 regulates a mitogen-activated protein kinase-like enzyme (MAPK) in Arabidopsis thaliana [44], indicating a role for H2O2 in retrograde signalling. The MAPK gene family has been found to co-operate with ABA in plant abiotic stress responses [56]. Experimental evidence suggests a role for 1O2 in addition to H2O2 in retrograde signalling. The transient nature of 1O2and its localised production suggest that it may act via more stable second messengers [57]. Accumulation of 1O2 occurred in the fluorescent (flu) mutant of Arabidopsis during a shift from dark to light, resulting the differential expression of 70 nuclear genes [57]. FLU is a negative regulator of tetrapyrrole metabolism which over-accumulates the photosensitiser protochlorophyllide in the dark and consequently generates 1O2 under light [57]. Over-accumulation of singlet oxygen leads to PCD in flu leaves but does not occur in double-mutant ex1 flu (execute 1) [58]. Interestingly, 1O2 still over-accumulates in ex1 flu but stops PCD in leaves, indicating that 1O2 and EX1 have roles in a retrograde pathway that regulate programmed cell death.
2.2. Tetrapyrrole and Mg-Protoporphyrin
With different structures, along with a variety of ring substituents, tetrapyrroles show different functions [59]. In Arabidopsis, the role of tetrapyrroles in retrograde signalling was first identified in studies of genomes uncoupled (gun) mutants [60]. Tetrapyrrole-controlled gene expression is evolutionarily conserved in many plant species [51]. GUN1 is located in chloroplasts and functions to impair plastid gene expression [60]. Mg-protoporphyrin (Mg-ProtoIX) acts between the chloroplast and the nucleus in the tetrapyrrole signalling pathway [45]. Accumulation of Mg-ProtoIX regulates the expression of many nuclear genes encoding photosynthesis-associated chloroplast proteins, such as heat shock protein HSP81-2 [45]. The heat shock protein gene HSP70 can be induced by either exogenous hemin (an oxidized form of heme that is reduced to heme in vivo) and Mg-ProtoIX treatment or light incubation, suggesting that these chemicals may converge in the same pathway [61]. Further evidence shows that heme is produced by plastid ferrochelatase 1 (FC1) in chloroplasts from which heme is exported to regulate gene expression of photosynthesis-related nuclear genes (PhANGs) [48,61].
2.3. Transcription Factors
Transcription factors also have roles in retrograde signalling. The APETALA 2/ethylene-responsive element binding protein (AP2/EREBP) family of transcription factors has been implicated in hormone, sugar, and redox signalling in relation to abiotic stresses [62]. Members of the Ethylene-Responsive Factor (ERF) subfamily, the largest group of transcription factors among the AP2/EREBP family, were first identified through their regulation of ethylene responses [63]. Among the ERF group, the AP2-like transcription factor Abscisic Acid Insensitive 4 (ABI4) functions in three retrograde signalling processes [64]: tetapyrrole synthesis [45], plastid gene expression (PGE) [65], and photosynthesis electron transfer chain (PET), affecting both photosynthesis- and stress-related genes [65,66]. The pathways associated with ABI4 also involve PHD-type transcription factor with transmembrane domains (PTM), which is located in the chloroplast envelope. In response to retrograde signals, the accumulated mature form of PTM modifies histones, thereby regulating ABI4 transcription [53,67]. Moreover, AP2/EREBP family has also been implicated in retrograde signalling by interacting with redox signalling in abiotic stresses such as cold and drought [49]. A typical example is plastid redox-insensitive 2 (PRIN2), which is a chloroplast component located in the nucleus and which functions in redox-mediated retrograde signalling, specifically by interacting with plastid-encoded RNA polymerase (PEP). PEP functions as a retrograde signal synchronizing nuclear and plastid genomes for the expression of photosynthesis-associated nuclear genes (PhANGs) [51].
WHIRLY (WHY) proteins are activators of nuclear gene transcription [68] and are required for plastid genome stability [69]. WHY1 proteins with the same molecular weight have been found in both chloroplasts and nucleus of the same cell [70]. This suggests that the mature forms of these proteins are intracellularly mobile [52]. Salicylic acid (SA) is a phenolic compound that is involved in plant responses to stresses [71]. WHY1 in Arabidopsis participates in both SA-dependent disease resistance and SA-induced expression of systemic acquired resistance (SAR) responsive genes [72], which suggests that WHY1 may act as a retrograde signal. It was proposed that WHY1 may be sensitive to the redox state of the chloroplast, which may cause changes in the polymerisation of WHY1 allowing monomers to translocate to the nucleus to trigger NGE [73].
2.4. 3′-Phosphoadenosine 5′-Phosphate (PAP)
PAP is produced in secondary sulphur assimilation as a by-product of the transfer of sulphate from 3′-phosphoadenosine 5′-phosphosulfate (PAPS) to acceptor molecules in a reaction catalysed by sulphotransferases (SOTs) [74]. It was reported that there is a 20-fold increase of PAP levels in Arabidopsis under drought stress, and a PAP-accumulating mutant, altered expression of APX2 (alx8) shows considerably improved drought tolerance [75]. Contrasting results were shown concerning the location of the phosphatase SAL1, which functions in dephosphorylating PAP to AMP (Adenosine Monophosphate) to reduce PAP concentrations [25]. Cellular SAL1 has been localized to the chloroplast [76], cytosol [77], and nucleus [78]; however, detailed and more convincing data has confirmed that SAL1 accumulates in the mitochondria and chloroplasts and that PAP is present in chloroplasts [25], which suggests that PAP is transported to the cytosol by a thylakoid ADP/ATP (Adenosine diphosphate/Adenosine triphosphate) carrier such as PAPST1 [79]. Once in the nucleus, PAP regulates drought stress-responsive gene expression (such as the expression of APX2, ZAT10, DREB2A) [25]. All this evidence suggests there is a SAL1–PAP retrograde pathway that alters NGE during drought stress.
2.5. Methylerythritol Cyclodiphosphate (MEcPP)
In plants, isoprenoids such as MEcPP are synthesised via two different pathways, i.e., the cytosolic mevalonate pathway and the 2-C-methyl-d-erythritol 4-phosphate pathway [80], and regulate a specific set of stress-responsive nuclear-encoded plastidial proteins [23]. Hydroperoxide lyase (HPL) is a stress-inducible nuclear gene encoding a plastid-localised protein in the oxylipin pathway that produces compounds for plants’ response to biotic and abiotic stresses [81]. SA has broad roles in regulating important plant physiological processes such as photosynthesis [82], antioxidant defence [83], and water maintenance under drought stress [71]. MEcPP causes high levels of SA and HPL, so that plants under stress have higher levels of MEcPP. Thus, MEcPP acts as a retrograde signalling molecule that induces expression of stress-related genes [23].
3. Linking Retrograde Signals to Chloroplastic Ion Transporters under Stress
Since chloroplast-initiated retrograde signals are involved in transducing various environmental stresses, and chloroplastic ion transporters are significant in regulating chloroplast status [84], do chloroplast ion transporters affect the generation of retrograde signals? Three general categories of proteins have been classified as ions transporters, i.e., channels/porins, primary transporters/pumps, and secondary transporters [85]; these have been shown to have important roles in photosynthesis. Here, we focus on the chloroplastic ion transporters that are involved in retrograde signalling. For details of channels or transporters related to photosynthesis, readers are directed to two excellent reviews [84,86].
3.1. Ion Transport, Retrograde Signalling and ROS-Regulated Photosynthesis in Chloroplasts
Chloroplastic ion transporters may participate in retrograde signalling via the synthesis of retrograde signals or of intermediates such as ROS and chloroplasts are the source and target of cellular redox regulation [87]. Many chloroplastic transporters affect chloroplast redox status and, therefore, determine the photosynthetic rate. ROS production from chloroplasts has been regarded as a key retrograde signal, affecting NGE [16,88,89]. It was suggested that accumulation of H2O2 specifically in the chloroplasts induces the expression of nuclear-encoded genes, such as cytoplasmic ascorbate peroxidase 2 (APX2) [90]. Silencing a thylakoid membrane-bound APX (tAPX) gene regulates H2O2, resulting in increased levels of oxidized proteins in chloroplasts. However, the expression of ROS-responsive genes was negatively regulated in a tAPX silenced mutant, which suggests H2O2 triggered retrograde signalling from chloroplasts under stress [91]. Indeed, photosynthetic redox state and ROS production have been proposed to be modulated by the status of plastoquinol (PQH2) and plastoquinone (PQ) in chloroplasts [92] as well as by chloroplastic ion transporters [84].
The natural light environment of plants changes rapidly and has driven to the evolution of sensing mechanisms that allow the efficient acclimation of plants to light conditions [93]. In chloroplasts, thylakoids are packed to occupy a small volume with a large surface area, and thylakoid membranes also have the highest known protein-to-lipid ratio among all membranes. They contain six major protein complexes, namely, light-harvesting complexes I (LHCI) and II (LHCII), photosystems I (PSI) and II (PSII), Cytochrome b6f, and ATP synthase CF0F1 [94]. Electron transport should be balanced among these two photosystems (PSI and PSII) for NADPH and ATP production; an imbalance in electron flow can occur during stress conditions. This imbalance can lead to excess electron excitation, photoinhibition, and elevation of ROS. Under high light, induced adaptive structural changes, such as the swelling of thylakoids and an increase in the partition gaps between the thylakoids, can occur [95]. Therefore, the chloroplastic ion channels and transporters located in thylakoids become important, as these transporters are responsible for preventing membrane depolarization or hyperpolarization due to the excessive accumulation of cations and anions via ion transporters [96]. Light energizes H+-ATPase in thylakoid membrane to generate the H+-motive force necessary for ion and solute transport during photosynthesis [97]. However, the H+ concentration in the different compartments of chloroplasts needs to be well regulated; for example, stromal pH is maintained at pH 8.0 while the luminal pH is around pH 5.5–6.2 [98]. The luminal acidification is necessary to activate non-photochemical quenching (NPQ), which alters LHCII and allows part of the excitation energy to be dissipated to prevent ROS production [84]. All these processes are likely to require ion and solute homeostasis regulated by ion channels, pumps, and co-transporters to maintain the balance between chloroplasts and the rest of the cell (Figure 1).
Figure 1.
A schematic diagram of typical retrograde signalling pathways in plant cells. High-light stress could induce 1O2 accumulation which causes the accumulation of β-cyclocitral in the chloroplast. β-cyclocitral is exported to the nucleus to regulate expression of defense genes [43]. Elements in the tetrapyrrole pathways act as retrograde signals. Mg-ProtoIX and heme can both be regulated by FC1 and then transported from the chloroplasts to the nucleus to regulate photosynthesis-related genes [48,61]. Methylerythritol 4-phosphate (MEP) pathways also participate in retrograde signalling pathways, and high light could also induce methylerythritol cyclodiphosphate (MEcPP) production in chloroplasts and then regulate nuclear HPL gene expression [23]. PAP (3′-phosphoadnenosine 5′-phosphate), induced by drought and high light, could be transferred from the chloroplasts to the nucleus and regulate the expression of a set of genes [25]. Abbreviations: Protop IX, Protoporphyrin IX; FC1, ferrochelatase 1; TPK3, Tandem-pore K+ selective channel3; KEA 3, Cation/proton antiporter 3; CLC, anion channel of Cl− channel (CLC) family; ROS, reactive oxygen species; PSI and PSII, Reaction centres of photosystem I and II; HMA, P-type ATPase of Arabidopsis/Heavy-metal-associated; bf6, cytochrome b6f complex; PC, plastocyanin; LHCII, Light harvesting complex; SULTR, phloem-localized sulphate transporter; ATPs, ATP sulphurylase; APS, Adenosine 5′-phosphosulfate; APK, APS kinase; PAPS, 3′phosphoadnosine 5′-phosphosulfate; SOT, Sulfotransferase; PAP, 3′-Phosphoadnenosine 5′phosphate; APX, Ascorbate peroxidase 2; DREB2A, Drought responsive element binding 2A; ZAT10, Salt tolerance Zinc Finger.
3.2. Direct Modulation of Chloroplastic Retrograde Signalling by Ion Transporters
Some ion transporters have roles in regulating ion homeostasis and balancing ROS production, but their direct interaction with retrograde signals is still elusive. In Arabidopsis, a thylakoid membrane two-pore potassium channel, i.e., TPK3, was shown to regulate H+ concentration through ion counterbalancing [99]. TPK3-silenced plants display impaired CO2 assimilation and reduced non-photochemical dissipation of excessively absorbed light [99]. The K+/H+ and antiporter KEA3 is responsible for the recuperation of luminal K+ concentration during the night [100] and for optimizing photosynthesis [101]. A member of the Cl− channel (CLCs) family, CLCe, has been identified in thylakoid membranes for ion counterbalancing in chloroplasts during light-driven proton transfer across the thylakoid [102]. However, under environmental stresses such as drought, the balance is disrupted, resulting in high ROS production in chloroplasts. Photosynthetic electron transport-generated redox signals in chloroplast also control PhANGs [103] (Figure 1).
3.3. Indirect Regulation of Chloroplastic Retrograde Signalling by Ion Transporters
Other chloroplast-located ion transporters responsible for transporting specific ions for the biosynthesis of retrograde signals have been identified by proteomic studies [104]. Since Cu is critical for electron transport and ROS scavenging in chloroplasts, undoubtedly, chloroplast-located Cu transporters play critical roles in reducing ROS production. Copper in chloroplasts has two forms: reduced and oxidized. Cu2+ is the redox cofactor of plastocyanin (PC), the protein required for transferring electrons from the cytochrome b6f complex to PSI [105], leading to lower ROS production in chloroplasts. Cu is also one of the components of Cu/Zn superoxide dismutase (Cu/Zn-SOD), which functions in scavenging ROS produced during photosynthesis under stress conditions [100]. In Arabidopsis, three proteins (HMA1, HMA6, and HMA8) are involved in Cu homeostasis. HMA1 and HMA6 are located in the chloroplast envelope and are involved in importing Cu into the chloroplast for Cu/Zn-SOD synthesis [106,107], while HMA8 is found in the non-appressed fractions of thylakoid membrane required for PC biosynthesis [108] (Figure 1). The Arabidopsis mutant hma1 has lower chloroplast copper content and a diminution of the total chloroplast SOD activity, which is essential for ROS reduction under stress [106].
Being a component involved in all photosystems and an important redox-active metal ion critical for photosynthetic electron flow, iron is involved in various chelation and oxidation/reduction steps that affect ROS production [109]. However, Fe homeostasis must be fine-tuned because excessive free Fe promotes the formation of free radicals via the Fenton reaction in plants. Ferritins are ion-storage proteins, responsible for either sequestering or releasing iron upon demand [110]. Transgenic plants overexpressing the wheat ferritin gene TaFER-5B exhibited enhanced temperature, drought, oxidative, and iron stress tolerance associated with ROS scavenging [111]. Most importantly, chloroplastic Fe transporters may participate in retrograde signalling by importing Fe into chloroplasts for the biosynthesis of heme, a key retrograde signaling molecule [48,61] (Table 1 and Figure 1). Biosynthesis of heme needs ferrochelatse I (FC I), which catalyses the insertion of Fe2+ into protoporphyrin IX (ProtoP IX) to form heme [112]. The Arabidopsis Permease Chloroplasts 1 (PIC1), which contains four predicted α-helices targeted to the inner envelope, is involved in iron transport in chloroplast [113]. Moreover, Multiple Antibiotic Resistance 1 (MAR1), which is a homolog of the ferroportin efflux transporters, was also identified as mediator of the transport of Fe or Fe-chelating polyamines such as nicotianamine into chloroplasts [114].
Magnesium (Mg) is another element essential for heme retrograde signalling via Mg2+ insertion into ProtoP IX by Mg-chelatase from Mg-protoporphyrin, the precursor of chlorophyll and heme biosynthesis [61]. In Arabidopsis, a putative Mg2+ transporter, MRS2-11, is located in the chloroplast envelop and is responsible for Mg transport [115]. Mg-protoporphyrin can also be regarded as a retrograde signaling molecule, regulating the expression of PhANGs [116]. Moreover, sulphate transporters are also likely to participate in the biosynthesis of retrograde signals by importing ions into chloroplasts. The sulphate transporter SULTR3;1 is located in the chloroplast membrane and is responsible for sulphate uptake into chloroplasts [117]. For instance, another retrograde signal PAP is synthesised from sulphate [74,118] and is capable of moving between chloroplasts and cytosol to upregulate APX2 and drought-responsive element binding protein 2A (DREB2A) under high-light and drought stress [25,41,75].
In summary, chloroplastic ion transporters are coordinated to regulate ion fluxes and electron transport between all the photosystems in chloroplasts. These transporters may also participate in retrograde signalling pathways either through affecting ROS production or through delivering the components required for the synthesis and regulation of the retrograde molecules. As a balanced electron flux needs to be maintained in chloroplasts, we propose that chloroplast-derived retrograde signals are potential feedback signals that regulate chloroplastic ion transporters. However, few studies have addressed whether retrograde signals like ROS regulate ion transporters in chloroplast membranes. Patch clamp measurements on chloroplasts and membrane patches will be essential to explore the interactions between retrograde signals and membrane transport.
4. Linking Retrograde Signals to Ion Transport for Stomatal Regulation
The retrograde signal PAP regulates stomatal closure, enhancing drought tolerance [25,26]. In addition, drought tolerance is partially controlled by the activity of pumps, ion channels, and cotransporters located in the plasma membrane and tonoplast of guard cells, which generate ion gradients, regulating stomatal opening and closure [6,12] (Figure 2). Are there any links between retrograde signals and these membrane transporters? Published reports showing direct links between retrograde signals and ion transporters at the plasma membrane and tonoplast are still limited. Here, we focus on a key component of drought tolerance, i.e., membrane transporters that regulate stomatal opening and closure. We illustrate the potential interactions between retrograde signals and ion transporters in the context of stomatal guard cells and drought tolerance.
Figure 2.
A schematic diagram of chloroplast-located ion transporters and retrograde signal molecule 3′-phosphoadnenosine 5′-phosphate (PAP), and their roles in stomatal regulation. PHOT1 and PHOT2 sense blue light, which activates plasma membrane proton pump AHAs, and this leads to the efflux of H+ from cytosol [119]. The accumulated electrons on the cytosolic side lead to activation of plasma membrane-located potassium inward-rectifying channels [120], leading to K+ influx. However, these potassium inward-rectifying channels can be inhibited by cytosolic Ca2+ accumulation [121]. CNGCs and CAXs are responsible for cytosolic Ca2+ accumulation [36], and CAX can be inactivated by ABA, which increases cytosolic Ca2+ accumulation [122]. ABA also inhibits blue light-induced H+-ATPase activation, which leads to stomatal closure [123]. Sulphate can be transported into chloroplasts by SULTR for the biosynthesis of PAP [117,118]. PAP is degraded by SAL1/ALX8 to AMP [25]. Under drought stress, ROS production in chloroplasts reduces SAL1 activity, which leads to PAP accumulation in the protoplast [25]. PAP is then transported into the cytosol by PAP transporter, PAPST1 [79], from where it moves to the nucleus to bind to the stress response genes XRNs, which potentially leads to CDPKs expression [26]. CDPKs activate SLAC1 channels, which leads to anion efflux [26]. Cytosolic Ca2+ also has a role in regulating CDPKs [124]. Besides, CDPKs and protein 14-3-3 have a role in regulating vacuole potassium channels activity [125,126]. ABA-induced stomatal closure depends on OST1 activity. OST1 has a role in activating anion efflux and inhibits water aquaporin channel PIP2;1 activity [127,128], which leads to stomatal closure. Abbreviations: PHOT, phototropins; AHA, Plasma membrane H+-ATPase; ATP, adenosine triphosphate; ADP, Adenosine diphosphate; KAT1, K+ channel 1 in Arabidopsis; KAT2, K+ channel in Arabidopsis 2; AKT, Arabidopsis Thaliana Rectifying channel ; ACA, Ca2+-ATPase; CNGC, Arabidopsis Cyclic nucleotide-gated ion channels; NRT1.1, Nitrate Transporter 1.1; STP1, Sugar Transporter 1; ABA, Abscisic acid; ALMT, Aluminium-activated malate transporter; VHA, vacuolar H+-ATPase; AVP, vacuolar H+/K+-PPase; TIPs, Tonoplast Intrinsic Proteins; CAX, Cation Exchanger; CLCa, Chloride Channel a; NHX, Na+,K+/H+ antiporters; AMP, Adenosine Monophosphate; SAL1, Altered expression of APX2; PAP, 3′-phosphoadnenosine 5′-phosphate; SULTR, phloem-localized sulphate transporter; PAPST1, 3′-Phosphoadenosine 5′-Phosphosulfate Transporter 1;ABI, ABA Insensitive; OST1, Open Stomata 1; TPC, Two-pore Ca2+ channel; TPK, Two-pore K+ channel; CDPKs, Ca2+ dependent protein kinases; SLAC1, Slow Anion channel-associated 1; PIP2;1, Plasma Membrane Intrinsic Protein 2; GORK, Guard Cell Outwardly Rectifying K+ channel.
5. Plasma Membrane Transport in Stomatal Guard Cells
5.1. Plasma Membrane Pumps
The plasma membrane H+-ATPase (AHA) protein family has many members in different plant species [6], and these proteins are responsible for H+ movement by coupling with ATP hydrolysis, which is the primary motive force for stomatal movement [129]. In guard cells, blue light, ABA, auxin, and exogenous Ca2+ play roles in H+-ATPase regulation [130,131,132]. For instance, the blue light receptor phototropins (PHOT1 and PHOT2) [133] sense blue light and activate plasma membrane H+-ATPases, which results in an efflux of H+ from the cytosol [119]. The activated H+-ATPase induces hyperpolarization which in turn induces K+ uptake via inward-rectifying K+ channels [120]. Conversely, ABA strongly inhibits blue light-induced H+-ATPases activation, which leads to stomatal closure. ABA-induced Ca2+ accumulation in the cytosol also inhibits H+ pumping and ATP hydrolysis in guard cells [123]. For example, Mg-chelatase H subunit (CHLH), which was found to mediate chlorophyll biosynthesis, regulates stomatal closure in part through dephosphorylating and inhibiting guard cell H+-ATPase [134]. Ca2+-ATPases (ACAs) regulate Ca2+ homeostasis on different membranes in a range of plant cell types in response to stress [135,136,137,138]. Ca2+-ATPases ACA8 and ACA10 were found to be targeted to guard cells in Arabidopsis. The expression of ACA8 was found to be upregulated, while that of ACA10 was downregulated by cold treatment, and the promoter of ACA8 has been shown to contain cold-responsive C-repeat/dehydration-responsive element motif [136]. BONZAI1 (BON1) interacts with the autoinhibitory domains of ACA10, ACA8, and ACA10/8, and it functions in the generation of cytosol calcium signatures that are critical for stomatal movement [137].
5.2. Plasma Membrane Ion Channels
Potassium (K) participates in plant growth and development [139] and also affects the homeostasis of other processes [140]. Potassium channels located in guard cell plasma membranes play a critical role in K+ uptake and release, thus modulating guard cell turgor and volume [141]. In Arabidopsis, inward K+ channels (e.g., KAT1, KAT2, AKT1, AKT2) and outward K+ channels (e.g., GORK) were identified as responsible for the K+ fluxes during stomatal movement [142]. For example, the Arabidopsis mutant gork, that has a non-functional gated outward-rectifying K+ channel, showed impaired stomatal closure [37]. In addition, overexpressing KAT1 impaired stomatal movement in Arabidopsis [143], but stomatal behaviour of a knockout mutant kat1 was little affected [144]. Guard cell K+ channels can be regulated by ABA, cytosolic Ca2+, pH, protein kinases, and phosphatases [121,142,145]. All these results suggest that potassium channels are downstream in guard cell signalling pathways and directly affect stomatal movement by changing cell turgor.
Ca2+ accumulation in the cytosol is one of the most important processes in ABA-induced stomatal closure, and many voltage-dependent Ca2+-permeable channels were identified in the plasma membrane of different types of plant cells [6,146]. Cyclic nucleotide-gated ion channels (CNGC)-mediated cytosolic Ca2+ rise contributes to the dynamic regulation of guard cell anion channels and stomatal closure [36,147]. Arabidopsis CNGC5 and CNGC6 have been identified as plasma membrane Ca2+ channels that are highly expressed in guard cells. Guard cells in the cngc5cngc6 double mutant exhibited dramatically impaired cGMP-activated currents. Moreover, the guard cells of the double mutant exhibited functional ABA-activated hyperpolarization-dependent Ca2+-permeable cation channel currents, intact ABA-induced stomatal closing responses, and whole-plant stomatal conductance responses to darkness and changes in CO2 concentration [148]. It has been reported that Ca2+ functions downstream of ROS, and, for instance, a Ca2+ current is activated by ROS in ABA-induced stomatal closure [149].
There are two major types of anion channels located in the plasma membrane: rapid (R-type) and slow (S-type) anion channels [6,147,150]. An anion efflux is essential for stomatal closure through both R-type [151] and S-type [152,153] anion channels. Both types of anion channels can be activated by cytosolic Ca2+ and are permeable to a range of anions, including Cl−, malate2−, and NO3− [27]. Plasma membrane Aluminium-activated malate transporter (ALMT12) is highly expressed in Arabidopsis guard cells, and plants lacking ALMT12 are impaired in ABA-induced stomatal closure. ALMT12 is capable of transiently depolarising guard cells to trigger membrane potential oscillations and initiates long-term anion and K+ efflux via slow anion channel 1 (SLAC1) and GORK, respectively [151].
Water channels, or aquaporins, have varied functions in stomatal regulation and transport of H2O, CO2, and H2O2 [154]. For instance, knocking out the plasma membrane Intrinsic Protein (PIP) PIP2;1 in Arabidopsis leads to a defect in ABA-induced stomatal closure in pip2;1 plants. In addition, work using Xenopus laevis oocytes has shown that PIP2;1 water transport activity is increased when open stomata 1 (OST1) phosphorylates a cytosolic PIP2;1 peptide at Ser-121. ABA-triggered stomatal closure requires an increase in guard cell permeability to water and possibly H2O2, through OST1-dependent phosphorylation of PIP2;1 [128].
5.3. Plasma Membrane Cotransporters
Many plasma membrane cotransporters regulate stomatal movement and play a role in stress tolerance [6,155]. Here, we only present three key examples of cotransporters. The dual-affinity nitrate transporter gene, NRT1.1/CHL1, is expressed in Arabidopsis guard cells, and the chl1 mutant shows enhanced drought tolerance. It was reported that chl1 mutants showed reduced nitrate accumulation in guard cells during stomatal opening and failed to show nitrate-induced depolarization of guard cells [156]. The guard cells of several plant species were shown to accumulate sucrose as an osmoticum that drives water influx to increase stomatal aperture, and an Arabidopsis H+-monosaccharide symporter, STP1, was identified in guard cells. A transient increase in STP1 expression correlates in time with the described guard cell-specific accumulation of sucrose, and a role for STP1 in monosaccharide import into guard cells has been reported [157]. Moreover, the ATP-binding cassette (ABC) transporter gene MRP4 is highly expressed in stomata and MRP4 is localized to the plasma membrane in Arabidopsis. Stomatal aperture in three independent mrp4 mutant was larger than in wild-type plants, indicating the involvement of MRP4 in the complex regulation of stomatal aperture [158].
5.4. Tonoplast Transport in Stomatal Guard Cells
5.4.1. Tonoplast Pumps
Vacuolar acidification requires the combined activity of vacuolar type H+-ATPase (V-ATPase) and tonoplast inorganic pyrophosphatase (V-PPase), both of which are key determinants for stomatal regulation and stress response [147,159,160]. V-ATPase maintains a proton electrochemical gradient across endomembrane compartments, including the vacuole [147,161]. V-ATPase is highly abundant, representing 6.5–35% of the total tonoplast proteins in different species [162]. This enzyme is composed of several polypeptide subunits that are located in two major domains, a membrane peripheral domain (V1) and a membrane integral domain (V0) [162]. The expression of all V-ATPase subunits can be increased in response to salt stress [162], and expressing Arabidopsis VHA-C in Hordeum vulgare improved plant performance under saline conditions [163]. Further evidence showed that ABA significantly increases V-ATPase H+-transport activity [164]. This suggests an important role of V-ATPase in regulating plant stress tolerance. V-PPase activity in guard cells is involved in stomatal regulation [165]. Guard cell protoplasts of Vicia faba exhibited hydrolytic activity characteristic of tonoplast-localized V-PPase. The activity was inhibited by a specific V-PPase inhibitor and by cytosolic Ca2+ and stimulated by K+. V-PPase AVP1 controls auxin transport and, consequently, auxin-dependent development [159,166]. Expression of an Arabidopsis AVP1 in cotton improves drought and salt tolerance [167]; however, the role of Ca2+-ATPases in stomatal regulation is still elusive.
5.4.2. Tonoplast Ion Channels
Stomatal closure requires the release of large amounts of K+ from guard cells, mostly from the vacuoles [168]. Therefore, vacuolar K+ channels are key components in regulating stomatal closure. The Arabidopsis genome contains five genes that encode two-pore K+ channels (TPK), and TPK1 is located in vacuolar membranes where it mediates K+-selective currents between the cytosol and the vacuolar compartments. TPK1 plays a role in intracellular K+ homeostasis, slows stomatal closure kinetics [169], and is activated by 14-3-3 proteins [125] and calcium-dependent protein kinases (CDPKs) [126]. In vacuoles, the Arabidopsis two-pore channel 1 gene, TPC1, encodes a slow vacuolar channel with high affinity for Ca2+ permeation [170]. A tpc1 knockout mutant was shown to lack functional slow vacuolar channel activity and to be defective in ABA-induced stomatal closure because of a poor Ca2+ efflux from guard cell vacuoles, which suggests a critical role for intracellular Ca2+-release channels in the physiological processes of plants [171,172]. Aluminum-activated malate transporters (ALMTs) are malate channels involved in vacuolar malate accumulation and in tolerance to aluminum [173,174]. In Arabidopsis, ALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening [175], whereas ABA-induced stomatal closure involves the phosphorylation-dependent vacuolar anion channel ALMT4 [176] and the vacuolar malate channel ALMT6 in guard cells, both of which are subject to multiple regulation processes [177]. Moreover, the Arabidopsis nitrate transporter CLCa is localized in the tonoplast and is able to accumulate nitrate in the vacuole to regulate stomatal movement [178]. While much effort has substantiated the importance of water channel PIPs, comparably little is known about the function of intracellular aquaporins, such as tonoplast intrinsic proteins (TIPs) [179]. For instance, sunflower SunTIP7 and SunTIP20 are guard cell-localized aquaporins, and their expression in Xenopus oocytes caused a marked increase in water permeability. The transcript levels of SunTIP7 were markedly and systematically increased during drought-induced stomatal closure, suggesting that SunTIP7 regulates guard cell volume and stomatal aperture [180].
5.4.3. Tonplast Cotransporters
Many vacuolar cotransporters regulate stomatal movement and play a role in stress tolerance [6,181,182]. Here, we only present a couple of key examples: Na+, K+/H+ antiporters (NHXs) and vacuolar cation exchangers. NHXs are involved in K+ homeostasis, pH regulation, and salt tolerance [182]. Tonoplast-localized NHX1 and NHX2 are highly expressed in guard cells, but nhx1/nhx2 mutant plants showed defective stomatal function and had reduced ability to maintain the vacuolar K+ pools. Thus, NHX proteins are essential for active K+ uptake into the tonoplast, for turgor regulation, and for stomatal function [183,184]. Vacuolar cation exchangers CAX1 and CAX3 are involved in mediating calcium transport from the cytosol to the vacuoles using the proton gradient across the tonoplast [122]. Inhibition of ABA-induced stomatal closure by indole-3-acetic acid (IAA) is impaired in the cax1/cax3 double mutant. The cax1/cax3 mutant exhibited constitutive hyperpolarisation of the plasma membrane with a higher apoplastic pH than the wild-type plant. Lower extracellular pH fully restored IAA inhibition of ABA-induced stomatal closure in the cax1/cax3 mutant [185].
6. Retrograde Signals and Ion Transport in Drought-Induced Stomatal Closure
Stomatal guard cell turgor is regulated by cell solute concentration, thus ion channels or transporters in cells determine stomatal movement. Each membrane is equipped with a unique set of ion transporters that enables transport of nutrients, solutes, and metabolites [84]. Furthermore, retrograde signals are regulators that enable plants to survive adverse environments. The substantial knowledge of ion transport in stomatal guard cells and the deep understanding of many retrograde signals summarised above have enabled the dissection of these two types of processes and allowed the identification of their complex interactions (e.g., ABA signalling). Here, we present some emerging evidence of these potential interactions.
In a recent report, chloroplast-derived PAP accumulation induced stomatal closure in Arabidopsis [26]. PAP increased K+ and Cl− efflux from stomatal guard cells, suggesting a role for potassium and anion channels in PAP-induced stomatal closure [26]. Therefore, potential interactions may occur between a drought-related retrograde signal PAP and plasma membrane-located ion transporters. The SAL1–PAP signalling pathway has been identified as a typical retrograde signal with multiple roles, such as regulating programmed cell death [186], and also functions in drought and high-light signalling [25]. Drought-induced ROS production in chloroplasts inhibits SAL1 activity [187], which leads to PAP accumulation and transport to the nucleus. PAP accumulation could activate downstream signalling through binding to nuclear exoribonucleases (XRNs), transcriptionally up-regulating multiple signalling proteins. These proteins, including four CDPKs, activate SLAC1 anion channel activity for stomatal closure under drought [26] (Figure 2). This discovery opens the door to future research on retrograde signals and membrane transport in plant stress tolerance.
7. Concluding Remarks and Future Perspectives
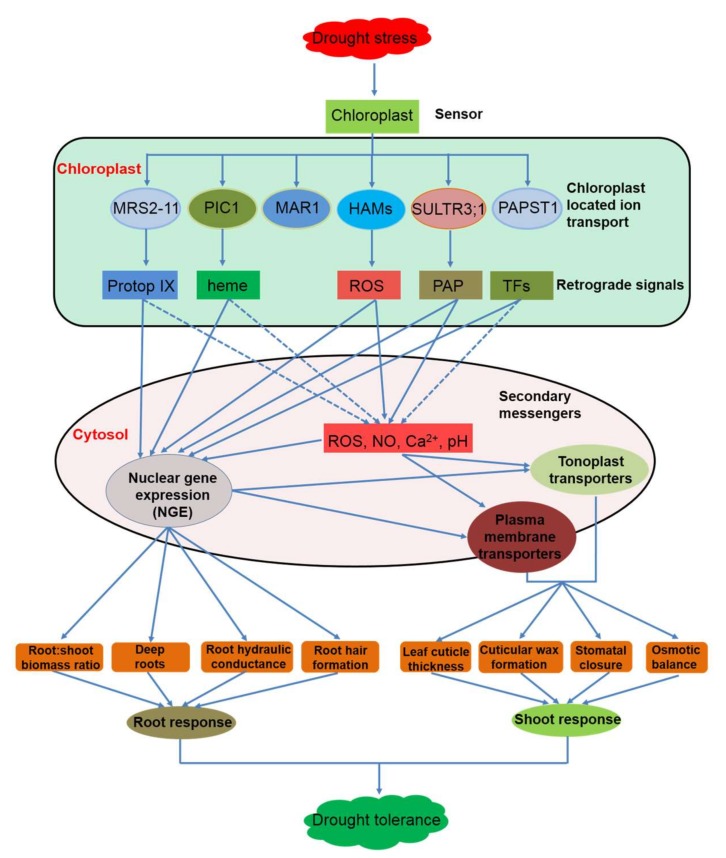
In this review, we summarised some retrograde signals that participate in the regulation of plant stress tolerance (Figure 1 and Table 1). We compared the chloroplastic transporters that modulate retrograde signalling through retrograde biosynthesis or as critical components in retrograde signalling (Figure 1). We also discussed the roles of important plasma membrane and tonoplast ion transporters that are involved in regulating stomatal movement (Figure 2). Moreover, we illustrated that chloroplast retrograde molecules and plasma membrane- or tonoplast-located ion transporters may interact to regulate plant drought tolerance (Figure 3).
Figure 3.
A schematic diagram of retrograde signals and potential mechanisms in regulating plant drought tolerance. Drought can be perceived by chloroplasts. Chloroplast-located ion channels participate in biosynthetic processes of retrograde signals, such as Mg-Protop IX [45], heme [48], ROS [100], and PAP [117], which target either nuclear genes expression (NGE) [48,54,116] or secondary messengers [55]. Secondary messengers and NGE regulate plasma membrane or tonoplast ion transporters [171], triggering the root and shoot responses to drought. Dotted blue arrows: potential interactions. Abbreviations: see legends of Figure 1 and Figure 2.
This review highlights some significant questions that need to be addressed. Do chloroplast-initiated retrograde signals and chloroplastic ion transporters regulate each other, and if so, how? How do plant cells establish the interactions between retrograde signals and ion transporters at the plasma membrane and tonoplast? Research is obviously required to identify additional proteins located in the three chloroplast membranes and to study the effects of retrograde signals on transporters in these membranes.
Acknowledgments
We thank Barry Pogson (Australian National University) for his generous support on the projects and Shengguan Cai and Michelle Mak for their technical support. Z.H.C is supported by an Australian Research Council (ARC) Discovery Early Career Researcher Award (DE1401011143), Horticulture Innovation Australia, and Australia–India strategic Research Fund.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Evans A. The Feeding of the Nine Billion. [(accessed on 15 March 2018)]; Available online: http://www.wfp.org/stories/feeding-ten-billion-global-food-security-21st-century.
- 2.FAO How to Feed the World in 2050. [(accessed on 15 March 2018)]; Available online: http://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf.
- 3.Zhang X., Cai X. Climate change impacts on global agricultural land availability. Environ. Res. Lett. 2011;6:014014. doi: 10.1088/1748-9326/6/1/014014. [DOI] [Google Scholar]
- 4.Kogan F.N. Global drought watch from space. Bull. Am. Meteorol. Soc. 1997;78:621–636. doi: 10.1175/1520-0477(1997)078<0621:GDWFS>2.0.CO;2. [DOI] [Google Scholar]
- 5.Schmidhuber J., Tubiello F.N. Global food security under climate change. Proc. Natl. Acad. Sci. USA. 2007;104:19703–19708. doi: 10.1073/pnas.0701976104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z.H., Chen G., Dai F., Wang Y., Hills A., Ruan Y.L., Zhang G., Franks P.J., Nevo E., Blatt M.R. Molecular evolution of grass stomata. Trends Plant Sci. 2016;22:124–139. doi: 10.1016/j.tplants.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Hetherington A.M., Woodward F.I. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- 8.Mak M., Babla M., Xu S.C., O’Carrigan A., Liu X.H., Gong Y.M., Holford P., Chen Z.H. Leaf mesophyll K+, H+ and Ca2+ fluxes are involved in drought-induced decrease in photosynthesis and stomatal closure in soybean. Environ. Exp. Bot. 2014;98:1–12. doi: 10.1016/j.envexpbot.2013.10.003. [DOI] [Google Scholar]
- 9.Sharp R.E., Poroyko V., Hejlek L.G., Spollen W.G., Springer G.K., Bohnert H.J., Nguyen H.T. Root growth maintenance during water deficits: Physiology to functional genomics. J. Exp. Bot. 2004;55:2343–2351. doi: 10.1093/jxb/erh276. [DOI] [PubMed] [Google Scholar]
- 10.Kudoyarova G., Veselova S., Hartung W., Farhutdinov R., Veselov D., Sharipova G. Involvement of root ABA and hydraulic conductivity in the control of water relations in wheat plants exposed to increased evaporative demand. Planta. 2011;233:87–94. doi: 10.1007/s00425-010-1286-7. [DOI] [PubMed] [Google Scholar]
- 11.Kosma D.K., Bourdenx B., Bernard A., Parsons E.P., Lü S., Joubès J., Jenks M.A. The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol. 2009;151:1918–1929. doi: 10.1104/pp.109.141911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai S., Papanatsiou M., Blatt M.R., Chen Z.H. Speedy grass stomata: emerging molecular and evolutionary features. Mol. Plant. 2017;7:912–914. doi: 10.1016/j.molp.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Xue D., Zhang X., Lu X., Chen G., Chen Z.H. Molecular and evolutionary mechanisms of cuticular wax for plant drought tolerance. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tenhaken R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015;5:771. doi: 10.3389/fpls.2014.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Streeter J., Lohnes D., Fioritto R. Patterns of pinitol accumulation in soybean plants and relationships to drought tolerance. Plant Cell Environ. 2001;24:429–438. doi: 10.1046/j.1365-3040.2001.00690.x. [DOI] [Google Scholar]
- 16.Chan K.X., Phua S.Y., Crisp P., McQuinn R., Pogson B.J. Learning the languages of the chloroplast: Retrograde signaling and beyond. Annu. Rev. Plant Biol. 2016;67:25–53. doi: 10.1146/annurev-arplant-043015-111854. [DOI] [PubMed] [Google Scholar]
- 17.Martin W.F., Garg S., Zimorski V. Endosymbiotic theories for eukaryote origin. Philos. Trans. R. Soc. B Biol. Sci. 2015;370:20140330. doi: 10.1098/rstb.2014.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McFadden G.I. Chloroplast origin and integration. Plant Physiol. 2001;125:50–53. doi: 10.1104/pp.125.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raven J.A., Allen J.F. Genomics and chloroplast evolution: What did cyanobacteria do for plants? Genome Biol. 2003;4:209. doi: 10.1186/gb-2003-4-3-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bobik K., Burch-Smith T.M. Chloroplast signaling within, between and beyond cells. Front. Plant Sci. 2015;6 doi: 10.3389/fpls.2015.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bräutigam K., Dietzel L., Pfannschmidt T. Cell and Molecular Biology of Plastids. Springer; Berlin/Heidelberg, Germany: 2007. Plastid-nucleus communication: Anterograde and retrograde signalling in the development and function of plastids; pp. 409–455. [Google Scholar]
- 22.Chi W., Sun X., Zhang L. Intracellular signaling from plastid to nucleus. Annu. Rev. Plant Biol. 2013;64:559–582. doi: 10.1146/annurev-arplant-050312-120147. [DOI] [PubMed] [Google Scholar]
- 23.Xiao Y., Savchenko T., Baidoo E.E., Chehab W.E., Hayden D.M., Tolstikov V., Corwin J.A., Kliebenstein D.J., Keasling J.D., Dehesh K. Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell. 2012;149:1525–1535. doi: 10.1016/j.cell.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 24.Raven J.A. Selection pressures on stomatal evolution. New Phytol. 2002;153:371–386. doi: 10.1046/j.0028-646X.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 25.Estavillo G.M., Crisp P.A., Pornsiriwong W., Wirtz M., Collinge D., Carrie C., Giraud E., Whelan J., David P., Javot H., et al. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell. 2011;23:3992–4012. doi: 10.1105/tpc.111.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pornsiriwong W., Estavillo G.M., Chan K.X., Tee E.E., Ganguly D., Crisp P.A., Phua S.Y., Zhao C., Qiu J., Park J. A chloroplast retrograde signal, 3′-phosphoadenosine 5′-phosphate, acts as a secondary messenger in abscisic acid signaling in stomatal closure and germination. eLife. 2017;6:e23361. doi: 10.7554/eLife.23361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jezek M., Blatt M.R. The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol. 2017;174:487–519. doi: 10.1104/pp.16.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lind C., Dreyer I., López-Sanjurjo E.J., von Meyer K., Ishizaki K., Kohchi T., Lang D., Zhao Y., Kreuzer I., Al-Rasheid K.A. Stomatal guard cells co-opted an ancient ABA-dependent desiccation survival system to regulate stomatal closure. Curr. Biol. 2015;25:928–935. doi: 10.1016/j.cub.2015.01.067. [DOI] [PubMed] [Google Scholar]
- 29.Cai S., Chen G., Wang Y., Huang Y., Marchant D.B., Wang Y., Yang Q., Dai F., Hills A., Franks P.J. Evolutionary conservation of ABA signaling for stomatal closure. Plant Physiol. 2017;174:732–747. doi: 10.1104/pp.16.01848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umezawa T., Nakashima K., Miyakawa T., Kuromori T., Tanokura M., Shinozaki K., Yamaguchi-Shinozaki K. Molecular basis of the core regulatory network in ABA responses: Sensing, signaling and transport. Plant Cell Physiol. 2010;51:1821–1839. doi: 10.1093/pcp/pcq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cutler S.R., Rodriguez P.L., Finkelstein R.R., Abrams S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant. Biol. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X., Zhang L., Dong F., Gao J., Galbraith D.W., Song C.P. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 2001;126:1438–1448. doi: 10.1104/pp.126.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Mata C., Gay R., Sokolovski S., Hills A., Lamattina L., Blatt M.R. Nitric oxide regulates K+ and Cl− channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc. Natl. Acad. Sci. USA. 2003;100:11116–11121. doi: 10.1073/pnas.1434381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Köhler B., Hills A., Blatt M.R. Control of guard cell ion channels by hydrogen peroxide and abscisic acid indicates their action through alternate signaling pathways. Plant Physiol. 2003;131:385–388. doi: 10.1104/pp.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwak J.M., Mori I.C., Pei Z.M., Leonhardt N., Torres M.A., Dangl J.L., Bloom R.E., Bodde S., Jones J.D., Schroeder J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Z.H., Hills A., Lim C.K., Blatt M.R. Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. Plant J. 2010;61:816–825. doi: 10.1111/j.1365-313X.2009.04108.x. [DOI] [PubMed] [Google Scholar]
- 37.Hosy E., Vavasseur A., Mouline K., Dreyer I., Gaymard F., Porée F., Boucherez J., Lebaudy A., Bouchez D., Véry A.A. The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. USA. 2003;100:5549–5554. doi: 10.1073/pnas.0733970100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallardo K., Courty P.E., Le Signor C., Wipf D., Vernoud V. Sulfate transporters in the plant’s response to drought and salinity: Regulation and possible functions. Front. Plant Sci. 2014;5:580. doi: 10.3389/fpls.2014.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ernst L., Goodger J.Q., Alvarez S., Marsh E.L., Berla B., Lockhart E., Jung J., Li P., Bohnert H.J., Schachtman D.P. Sulphate as a xylem-borne chemical signal precedes the expression of ABA biosynthetic genes in maize roots. J. Exp. Bot. 2010;61:3395–3405. doi: 10.1093/jxb/erq160. [DOI] [PubMed] [Google Scholar]
- 40.Christmann A., Grill E., Huang J. Hydraulic signals in long-distance signaling. Curr. Opin. Plant Biol. 2013;16:293–300. doi: 10.1016/j.pbi.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Rossel J.B., Walter P.B., Hendrickson L., Chow W.S., Poole A., Mullineaux P.M., Pogson B.J. A mutation affecting ASCORBATE PEROXIDASE 2 gene expression reveals a link between responses to high light and drought tolerance. Plant Cell Environ. 2006;29:269–281. doi: 10.1111/j.1365-3040.2005.01419.x. [DOI] [PubMed] [Google Scholar]
- 42.Kimura M., Yamamoto Y.Y., Seki M., Sakurai T., Sato M., Abe T., Yoshida S., Manabe K., Shinozaki K., Matsui M. Identification of Arabidopsis genes regulated by high light-stress using cDNA microarray. J. Photochem. Photobiol. B. 2003;77:226–233. doi: 10.1562/0031-8655(2003)077<0226:ioagrb>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 43.Ramel F., Birtic S., Ginies C., Soubigou-Taconnat L., Triantaphylidès C., Havaux M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA. 2012;109:5535–5540. doi: 10.1073/pnas.1115982109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desikan R., Clarke A., Hancock J.T., Neill S.J. H2O2 activates a MAP kinase-like enzyme in Arabidopsis thaliana suspension cultures. J. Exp. Bot. 1999;50:1863–1866. doi: 10.1093/jxb/50.341.1863. [DOI] [Google Scholar]
- 45.Strand Å., Asami T., Alonso J., Ecker J.R., Chory J. Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrinIX. Nature. 2003;421:79–83. doi: 10.1038/nature01204. [DOI] [PubMed] [Google Scholar]
- 46.Häusler R.E., Heinrichs L., Schmitz J., Flügge U.I. How sugars might coordinate chloroplast and nuclear gene expression during acclimation to high light intensities. Mol. Plant. 2014;7:1121–1137. doi: 10.1093/mp/ssu064. [DOI] [PubMed] [Google Scholar]
- 47.Vogel M.O., Moore M., König K., Pecher P., Alsharafa K., Lee J., Dietz K.J. Fast retrograde signaling in response to high light involves metabolite export, MITOGEN-ACVIATED PROTEIN KINASE6, and AP2/ERF transcription factors in Arabidopsis. Plant Cell. 2014;26:1151–1165. doi: 10.1105/tpc.113.121061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woodson J.D., Perez-Ruiz J.M., Chory J. Heme synthesis by plastid Ferrochelatase I regulates nuclear gene expression in plants. Curr. Biol. 2011;21:897–903. doi: 10.1016/j.cub.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dietz K.J., Vogel M.O., Viehhauser A. AP2/EREBP transcription factors are part of gene regulatory networks and integrate metabolic, hormonal and environmental signals in stress acclimation and retrograde signalling. Protoplasma. 2010;245:3–14. doi: 10.1007/s00709-010-0142-8. [DOI] [PubMed] [Google Scholar]
- 50.Apel K., Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 51.Kindgren P., Eriksson M.J., Benedict C., Mohapatra A., Gough S.P., Hansson M., Kieselbach T., Strand A. A novel proteomic approach reveals a role for Mg-protoporphyrin IX in response to oxidative stress. Physiol. Plant. 2011;141:310–320. doi: 10.1111/j.1399-3054.2010.01440.x. [DOI] [PubMed] [Google Scholar]
- 52.Isemer R., Mulisch M., Schafer A., Kirchner S., Koop H.U., Krupinska K. Recombinant Whirly1 translocates from transplastomic chloroplasts to the nucleus. FEBS Lett. 2012;586:85–88. doi: 10.1016/j.febslet.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 53.Sun X., Feng P., Xu X., Guo H., Ma J., Chi W., Lin R., Lu C., Zhang L. A chloroplast envelope-bound PHD transcription factor mediates chloroplast signals to the nucleus. Nat. Commun. 2011;2:477. doi: 10.1038/ncomms1486. [DOI] [PubMed] [Google Scholar]
- 54.Møller I.M., Sweetlove L.J. ROS signalling-specificity is required. Trends Plant Sci. 2010;15:370–374. doi: 10.1016/j.tplants.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y., Chen Z.H., Zhang B., Hills A., Blatt M.R. PYR/PYL/RCAR abscisic acid receptors regulate K+ and Cl− channels through reactive oxygen species-Mediated activation of Ca2+ channels at the plasma membrane of intact Arabidopsis guard cells. Plant Physiol. 2013;163:566–577. doi: 10.1104/pp.113.219758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Danquah A., de Zelicourt A., Colcombet J., Hirt H. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol. Adv. 2014;32:40–52. doi: 10.1016/j.biotechadv.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Op den Camp R.G., Przybyla D., Ochsenbein C., Laloi C., Kim C., Danon A., Wagner D., Hideg É., Göbel C., Feussner I. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell. 2003;15:2320–2332. doi: 10.1105/tpc.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wagner D., Przybyla D., op den Camp R., Kim C., Landgraf F., Lee K.P., Würsch M., Laloi C., Nater M., Hideg E. The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science. 2004;306:1183–1185. doi: 10.1126/science.1103178. [DOI] [PubMed] [Google Scholar]
- 59.Larkin R.M. Tetrapyrrole signaling in plants. Front. Plant Sci. 2016;7:1586. doi: 10.3389/fpls.2016.01586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Susek R.E., Ausubel F.M., Chory J. Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell. 1993;74:787–799. doi: 10.1016/0092-8674(93)90459-4. [DOI] [PubMed] [Google Scholar]
- 61.Von Gromoff E.D., Alawady A., Meinecke L., Grimm B., Beck C.F. Heme, a plastid-derived regulator of nuclear gene expression in Chlamydomonas. Plant Cell. 2008;20:552–567. doi: 10.1105/tpc.107.054650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feng J.X., Liu D., Pan Y., Gong W., Ma L.G., Luo J.C., Deng X.W., Zhu Y.X. An annotation update via cDNA sequence analysis and comprehensive profiling of developmental, hormonal or environmental responsiveness of the Arabidopsis AP2/EREBP transcription factor gene family. Plant Mol. Biol. 2005;59:853–868. doi: 10.1007/s11103-005-1511-0. [DOI] [PubMed] [Google Scholar]
- 63.Fujimoto S.Y., Ohta M., Usui A., Shinshi H., Ohme-Takagi M. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell. 2000;12:393–404. doi: 10.1105/tpc.12.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koussevitzky S., Nott A., Mockler T.C., Hong F., Sachetto-Martins G., Surpin M., Lim J., Mittler R., Chory J. Multiple signals from damaged chloroplasts converge on a common pathway to regulate nuclear gene expression. Science. 2007;316:715–719. doi: 10.1126/science.1140516. [DOI] [PubMed] [Google Scholar]
- 65.León P., Gregorio J., Cordoba E. ABI4 and its role in chloroplast retrograde communication. Front. Plant Sci. 2013;3:304. doi: 10.3389/fpls.2012.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kerchev P.I., Pellny T.K., Vivancos P.D., Kiddle G., Hedden P., Driscoll S., Vanacker H., Verrier P., Hancock R.D., Foyer C.H. The transcription factor ABI4 is required for the ascorbic acid-dependent regulation of growth and regulation of jasmonate-dependent defense signaling pathways in Arabidopsis. Plant Cell. 2011;23:3319–3334. doi: 10.1105/tpc.111.090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Dios Barajas-López J., Blanco N.E., Strand Å. Plastid-to-nucleus communication, signals controlling the running of the plant cell. Biochim. Biophys. Acta Mol. Cell Res. 2013;1833:425–437. doi: 10.1016/j.bbamcr.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 68.Desveaux D., Allard J., Brisson N., Sygusch J. A new family of plant transcription factors displays a novel ssDNA-binding surface. Nat. Struct. Mol. Biol. 2002;9:512–517. doi: 10.1038/nsb814. [DOI] [PubMed] [Google Scholar]
- 69.Maréchal A., Parent J.-S., Véronneau-Lafortune F., Joyeux A., Lang B.F., Brisson N. Whirly proteins maintain plastid genome stability in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2009;106:14693–14698. doi: 10.1073/pnas.0901710106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grabowski E., Miao Y., Mulisch M., Krupinska K. Single-stranded DNA-binding protein Whirly1 in barley leaves is located in plastids and the nucleus of the same cell. Plant Physiol. 2008;147:1800–1804. doi: 10.1104/pp.108.122796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miura K., Tada Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014;5:4. doi: 10.3389/fpls.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Desveaux D., Subramaniam R., Després C., Mess J.N., Lévesque C., Fobert P.R., Dangl J.L., Brisson N. A “Whirly” transcription factor is required for salicylic acid-dependent disease resistance in Arabidopsis. Dev. Cell. 2004;6:229–240. doi: 10.1016/S1534-5807(04)00028-0. [DOI] [PubMed] [Google Scholar]
- 73.Foyer C.H., Karpinska B., Krupinska K. The functions of WHIRLY1 and REDOX-RESPONSIVE TRANSCRIPTION FACTOR 1 in cross tolerance responses in plants: A hypothesis. Philos. Trans. R. Soc. B. 2014;369:20130226. doi: 10.1098/rstb.2013.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klein M., Papenbrock J. The multi-protein family of Arabidopsis sulphotransferases and their relatives in other plant species. J. Exp. Bot. 2004;55:1809–1820. doi: 10.1093/jxb/erh183. [DOI] [PubMed] [Google Scholar]
- 75.Wilson P.B., Estavillo G.M., Field K.J., Pornsiriwong W., Carroll A.J., Howell K.A., Woo N.S., Lake J.A., Smith S.M., Harvey Millar A., et al. The nucleotidase/phosphatase SAL1 is a negative regulator of drought tolerance in Arabidopsis. Plant J. 2009;58:299–317. doi: 10.1111/j.1365-313X.2008.03780.x. [DOI] [PubMed] [Google Scholar]
- 76.Rodríguez V.M., Chételat A., Majcherczyk P., Farmer E.E. Chloroplastic phosphoadenosine phosphosulfate metabolism regulates basal levels of the prohormone jasmonic acid in Arabidopsis Leaves. Plant Physiol. 2010;152:1335–1345. doi: 10.1104/pp.109.150474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang J., Vanneste S., Brewer P.B., Michniewicz M., Grones P., Kleine-Vehn J., Löfke C., Teichmann T., Bielach A., Cannoot B. Inositol trisphosphate-Induced Ca2+ signaling modulates auxin transport and PIN polarity. Dev. Cell. 2011;20:855–866. doi: 10.1016/j.devcel.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 78.Kim B.H., Von Arnim A.G. FIERY1 regulates light-mediated repression of cell elongation and flowering time via its 3′(2′),5′-bisphosphate nucleotidase activity. Plant J. 2009;58:208–219. doi: 10.1111/j.1365-313X.2008.03770.x. [DOI] [PubMed] [Google Scholar]
- 79.Gigolashvili T., Geier M., Ashykhmina N., Frerigmann H., Wulfert S., Krueger S., Mugford S.G., Kopriva S., Haferkamp I., Flügge U.I. The Arabidopsis thylakoid ADP/ATP carrier TAAC has an additional role in supplying plastidic phosphoadenosine 5-phosphosulfate to the cytosol. Plant Cell. 2012;24:4187–4204. doi: 10.1105/tpc.112.101964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chang W.C., Song H., Liu H.W., Liu P. Current development in isoprenoid precursor biosynthesis and regulation. Curr. Opin. Chem. Biol. 2013;17:571–579. doi: 10.1016/j.cbpa.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma M., Laxmi A. Jasmonates: Emerging players in controlling temperature stress tolerance. Front. Plant Sci. 2016;6:1129. doi: 10.3389/fpls.2015.01129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Janda T., Gondor O.K., Yordanova R., Szalai G., Pál M. Salicylic acid and photosynthesis: Signalling and effects. Acta Physiol. Plant. 2014;36:2537–2546. doi: 10.1007/s11738-014-1620-y. [DOI] [Google Scholar]
- 83.Khan N.A., Syeed S., Masood A., Nazar R., Iqbal N. Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. Int. J. Plant Biol. 2010;1:1–8. doi: 10.4081/pb.2010.e1. [DOI] [Google Scholar]
- 84.Pottosin I., Shabala S. Transport across chloroplast membranes: Optimizing photosynthesis for adverse environmental conditions. Mol. Plant. 2016;9:356–370. doi: 10.1016/j.molp.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 85.Saier M.H., Yen M.R., Noto K., Tamang D.G., Elkan C. The transporter classification database: Recent advances. Nucleic Acids Res. 2009;37:D274–D278. doi: 10.1093/nar/gkn862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carraretto L., Teardo E., Checchetto V., Finazzi G., Uozumi N., Szabo I. Ion channels in plant bioenergetic organelles, chloroplasts and mitochondria: From molecular identification to function. Mol. Plant. 2016;9:371–395. doi: 10.1016/j.molp.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 87.Jung H.S., Crisp P.A., Estavillo G.M., Cole B., Hong F., Mockler T.C., Pogson B.J., Chory J. Subset of heat-shock transcription factors required for the early response of Arabidopsis to excess light. Proc. Natl. Acad. Sci. USA. 2013;110:14474–14479. doi: 10.1073/pnas.1311632110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kopczewski T., Kuźniak E. Redox signals as a language of interorganellar communication in plant cells. Open Life Sci. 2013;8 doi: 10.2478/s11535-013-0243-4. [DOI] [Google Scholar]
- 89.Chan K.X., Crisp P.A., Estavillo G.M., Pogson B.J. Chloroplast-to-nucleus communication: Current knowledge, experimental strategies and relationship to drought stress signaling. Plant Signal. Behav. 2010;5:1575–1582. doi: 10.4161/psb.5.12.13758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yabuta Y., Maruta T., Yoshimura K., Ishikawa T., Shigeoka S. Two distinct redox signaling pathways for cytosolic APX induction under photooxidative stress. Plant Cell Physiol. 2004;45:1586–1594. doi: 10.1093/pcp/pch181. [DOI] [PubMed] [Google Scholar]
- 91.Maruta T., Noshi M., Tanouchi A., Tamoi M., Yabuta Y., Yoshimura K., Ishikawa T., Shigeoka S. H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J. Biol. Chem. 2012;287:11717–11729. doi: 10.1074/jbc.M111.292847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bräutigam K., Dietzel L., Kleine T., Ströher E., Wormuth D., Dietz K.J., Radke D., Wirtz M., Hell R., Dörmann P. Dynamic plastid redox signals integrate gene expression and metabolism to induce distinct metabolic states in photosynthetic acclimation in Arabidopsis. Plant Cell. 2009;21:2715–2732. doi: 10.1105/tpc.108.062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dietz K.J. Efficient high light acclimation involves rapid processes at multiple mechanistic levels. J. Exp. Bot. 2015;66:2401–2414. doi: 10.1093/jxb/eru505. [DOI] [PubMed] [Google Scholar]
- 94.Pribil M., Labs M., Leister D. Structure and dynamics of thylakoids in land plants. J. Exp. Bot. 2014;65:1955–1972. doi: 10.1093/jxb/eru090. [DOI] [PubMed] [Google Scholar]
- 95.Yoshioka-Nishimura M., Nanba D., Takaki T., Ohba C., Tsumura N., Morita N., Sakamoto H., Murata K., Yamamoto Y. Quality control of photosystem II: Direct imaging of the changes in the thylakoid structure and distribution of ftsh proteases in spinach chloroplasts under light stress. Plant Cell Physiol. 2014;55:1255–1265. doi: 10.1093/pcp/pcu079. [DOI] [PubMed] [Google Scholar]
- 96.Spetea C., Schoefs B. Solute transporters in plant thylakoid membranes: Key players during photosynthesis and light stress. Commun. Integr. Biol. 2010;3:122–129. doi: 10.4161/cib.3.2.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Merchant S., Sawaya M.R. The light reactions: A guide to recent acquisitions for the picture gallery. Plant Cell. 2005;17:648–663. doi: 10.1105/tpc.105.030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takizawa K., Cruz J.A., Kanazawa A., Kramer D.M. The thylakoid proton motive force in vivo. Quantitative, non-invasive probes, energetics, and regulatory consequences of light-induced pmf. Biochim. Biophys. Acta Bioenergy. 2007;1767:1233–1244. doi: 10.1016/j.bbabio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 99.Carraretto L., Formentin E., Teardo E., Checchetto V., Tomizioli M., Morosinotto T., Giacometti G.M., Finazzi G., Szabó I. A thylakoid-located two-pore K+ channel controls photosynthetic light utilization in plants. Science. 2013;342:114–118. doi: 10.1126/science.1242113. [DOI] [PubMed] [Google Scholar]
- 100.Finazzi G., Petroutsos D., Tomizioli M., Flori S., Sautron E., Villanova V., Rolland N., Seigneurin-Berny D. Ions channels/transporters and chloroplast regulation. Cell Calcium. 2015;58:86–97. doi: 10.1016/j.ceca.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 101.Wang C., Yamamoto H., Narumiya F., Munekage Y.N., Finazzi G., Szabo I., Shikanai T. Fine-tuned regulation of the K+/H+ antiporter KEA3 is required to optimize photosynthesis during induction. Plant J. 2017;89:540–553. doi: 10.1111/tpj.13405. [DOI] [PubMed] [Google Scholar]
- 102.Marmagne A., Vinauger-Douard M., Monachello D., De Longevialle A.F., Charon C., Allot M., Rappaport F., Wollman F.A., Barbier-Brygoo H., Ephritikhine G. Two members of the Arabidopsis CLC (chloride channel) family, AtCLCe and AtCLCf, are associated with thylakoid and Golgi membranes, respectively. J. Exp. Bot. 2007;58:3385–3393. doi: 10.1093/jxb/erm187. [DOI] [PubMed] [Google Scholar]
- 103.Surpin M., Larkin R.M., Chory J. Signal transduction between the chloroplast and the nucleus. Plant Cell. 2002;14:S327–S338. doi: 10.1105/tpc.010446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Froehlich J.E., Wilkerson C.G., Ray W.K., McAndrew R.S., Osteryoung K.W., Gage D.A., Phinney B.S. Proteomic study of the Arabidopsis thaliana chloroplastic envelope membrane utilizing alternatives to traditional two-dimensional electrophoresis. J. Proteome Res. 2003;2:413–425. doi: 10.1021/pr034025j. [DOI] [PubMed] [Google Scholar]
- 105.Redinbo M.R., Yeates T.O., Merchant S. Plastocyanin: Structural and functional analysis. J. Bioenergy Biomembr. 1994;26:49–66. doi: 10.1007/BF00763219. [DOI] [PubMed] [Google Scholar]
- 106.Seigneurin-Berny D., Gravot A., Auroy P., Mazard C., Kraut A., Finazzi G., Grunwald D., Rappaport F., Vavasseur A., Joyard J. HMA1, a NEW Cu-ATPase of the chloroplast envelope, is essential for growth under adverse light conditions. J. Biol. Chem. 2006;281:2882–2892. doi: 10.1074/jbc.M508333200. [DOI] [PubMed] [Google Scholar]
- 107.Boutigny S., Sautron E., Finazzi G., Rivasseau C., Frelet-Barrand A., Pilon M., Rolland N., Seigneurin-Berny D. HMA1 and PAA1, two chloroplast-envelope PIB-ATPases, play distinct roles in chloroplast copper homeostasis. J. Exp. Bot. 2014;65:1529–1540. doi: 10.1093/jxb/eru020. [DOI] [PubMed] [Google Scholar]
- 108.Tomizioli M., Lazar C., Brugière S., Burger T., Salvi D., Gatto L., Moyet L., Breckels L.M., Hesse A.M., Lilley K.S. Deciphering thylakoid SUB-compartments using a mass spectrometry-based approach. Mol. Cell Proteom. 2014;13:2147–2167. doi: 10.1074/mcp.M114.040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Briat J.F., Curie C., Gaymard F. Iron utilization and metabolism in plants. Curr. Opin. Plant Biol. 2007;10:276–282. doi: 10.1016/j.pbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 110.Theil E.C. Ferritin: Structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu. Rev. Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- 111.Zang X., Geng X., Wang F., Liu Z., Zhang L., Zhao Y., Tian X., Ni Z., Yao Y., Xin M. Overexpression of wheat ferritin gene TAFER-5B enhances tolerance to heat stress and other abiotic stresses associated with the ROS scavenging. BMC Plant Biol. 2017;17:14. doi: 10.1186/s12870-016-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith A.G., Santana M.A., Wallace-Cook A., Roper J.M., Labbe-Bois R. Isolation of a cDNA encoding chloroplast ferrochelatase from Arabidopsis thaliana by functional complementation of a yeast mutant. J. Biol. Chem. 1994;269:13405–13413. [PubMed] [Google Scholar]
- 113.Duy D., Wanner G., Meda A.R., von Wirén N., Soll J., Philippar K. PIC1, an ancient permease in Arabidopsis chloroplasts, mediates iron transport. Plant Cell. 2007;19:986–1006. doi: 10.1105/tpc.106.047407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Conte S., Stevenson D., Furner I., Lloyd A. Multiple antibiotic resistance in Arabidopsis is conferred by mutations in a chloroplast-localized transport protein. Plant Physiol. 2009;151:559–573. doi: 10.1104/pp.109.143487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Drummond R., Tutone A., Li Y.C., Gardner R. A putative magnesium transporter AtMRS2-11 is localized to the plant chloroplast envelope membrane system. Plant Sci. 2006;170:78–89. doi: 10.1016/j.plantsci.2005.08.018. [DOI] [Google Scholar]
- 116.Zhang Z.W., Yuan S., Feng H., Xu F., Cheng J., Shang J., Zhang D.W., Lin H.H. Transient accumulation of Mg-protoporphyrin IX regulates expression of PhANGs—New evidence for the signaling role of tetrapyrroles in mature Arabidopsis plants. J. Plant Physiol. 2011;168:714–721. doi: 10.1016/j.jplph.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 117.Cao M.J., Wang Z., Wirtz M., Hell R., Oliver D.J., Xiang C.B. SULTR3; 1 is a chloroplast-localized sulfate transporter in Arabidopsis thaliana. Plant J. 2013;73:607–616. doi: 10.1111/tpj.12059. [DOI] [PubMed] [Google Scholar]
- 118.Mugford S.G., Yoshimoto N., Reichelt M., Wirtz M., Hill L., Mugford S.T., Nakazato Y., Noji M., Takahashi H., Kramell R. Disruption of Adenosine-5′-Phosphosulfate kinase in Arabidopsis reduces levels of sulfated secondary metabolites. Plant Cell. 2009;21:910–927. doi: 10.1105/tpc.109.065581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kinoshita T., Doi M., Suetsugu N., Kagawa T., Wada M., Shimazaki K.I. Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature. 2001;414:656–660. doi: 10.1038/414656a. [DOI] [PubMed] [Google Scholar]
- 120.Kwak J.M., Murata Y., Baizabal-Aguirre V.M., Merrill J., Wang M., Kemper A., Hawke S.D., Tallman G., Schroeder J.I. Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and Light-Induced stomatal opening in Arabidopsis. Plant Physiol. 2001;127:473–485. doi: 10.1104/pp.010428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blatt M.R. Ca2+ signalling and control of guard-cell volume in stomatal movements. Curr. Opin. Plant Biol. 2000;3:196–204. doi: 10.1016/S1369-5266(00)00064-9. [DOI] [PubMed] [Google Scholar]
- 122.Conn S.J., Gilliham M., Athman A., Schreiber A.W., Baumann U., Moller I., Cheng N.H., Stancombe M.A., Hirschi K.D., Webb A.A. Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. Plant Cell. 2011;23:240–257. doi: 10.1105/tpc.109.072769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kinoshita T., Nishimura M., Shimazaki K. Cytosolic concentration of Ca2+ regulates the plasma membrane H+-ATPase in guard Cells of fava bean. Plant Cell. 1995;7:1333–1342. doi: 10.1105/tpc.7.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim T.H., Bohmer M., Hu H., Nishimura N., Schroeder J.I. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Latz A., Becker D., Hekman M., Müller T., Beyhl D., Marten I., Eing C., Fischer A., Dunkel M., Bertl A. TPK1, a Ca2+-regulated Arabidopsis vacuole two-pore K+ channel is activated by 14-3-3 proteins. Plant J. 2007;52:449–459. doi: 10.1111/j.1365-313X.2007.03255.x. [DOI] [PubMed] [Google Scholar]
- 126.Latz A., Mehlmer N., Zapf S., Mueller T.D., Wurzinger B., Pfister B., Csaszar E., Hedrich R., Teige M., Becker D. Salt stress triggers phosphorylation of the Arabidopsis vacuolar K+ channel TPK1 by calcium-dependent protein kinases (CDPKs) Mol. Plant. 2013;6:1274–1289. doi: 10.1093/mp/sss158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Geiger D., Scherzer S., Mumm P., Stange A., Marten I., Bauer H., Ache P., Matschi S., Liese A., Al-Rasheid K.A. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc. Natl. Acad. Sci. USA. 2009;106:21425–21430. doi: 10.1073/pnas.0912021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Grondin A., Rodrigues O., Verdoucq L., Merlot S., Leonhardt N., Maurel C. Aquaporins Contribute to ABA-Triggered stomatal closure through OST1-Mediated phosphorylation. Plant Cell. 2015;27:1945–1954. doi: 10.1105/tpc.15.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Falhof J., Pedersen J.T., Fuglsang A.T., Palmgren M. Plasma membrane H+-ATPase regulation in the center of plant physiology. Mol. Plant. 2016;9:323–337. doi: 10.1016/j.molp.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 130.Kinoshita T., Shimazaki K.I. Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J. 1999;18:5548–5558. doi: 10.1093/emboj/18.20.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Planes M.D., Niñoles R., Rubio L., Bissoli G., Bueso E., García-Sánchez M.J., Alejandro S., Gonzalez-Guzmán M., Hedrich R., Rodriguez P.L. A mechanism of growth inhibition by abscisic acid in germinating seeds of Arabidopsis thaliana based on inhibition of plasma membrane H+-ATPase and decreased cytosolic pH, K+, and anions. J. Exp. Bot. 2014;66:813–825. doi: 10.1093/jxb/eru442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Takahashi K., Hayashi K.I., Kinoshita T. Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol. 2012;159:632–641. doi: 10.1104/pp.112.196428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Christie J.M. Phototropin blue-light receptors. Annu. Rev. Plant Biol. 2007;58:21–45. doi: 10.1146/annurev.arplant.58.032806.103951. [DOI] [PubMed] [Google Scholar]
- 134.Tsuzuki T., Takahashi K., Tomiyama M., Inoue S., Kinoshita T. Overexpression of the Mg-chelatase H subunit in guard cells confers drought tolerance via promotion of stomatal closure in Arabidopsis thaliana. Front. Plant Sci. 2013;4:440. doi: 10.3389/fpls.2013.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang F., Chen Z.H., Liu X., Colmer T.D., Zhou M., Shabala S. Tissue-specific root ion profiling reveals essential roles of the CAX and ACA calcium transport systems in response to hypoxia in Arabidopsis. J. Exp. Bot. 2016;67:3747–3762. doi: 10.1093/jxb/erw034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schiøtt M., Palmgren M.G. Two plant Ca2+ pumps expressed in stomatal guard cells show opposite expression patterns during cold stress. Physiol. Plant. 2005;124:278–283. doi: 10.1111/j.1399-3054.2005.00512.x. [DOI] [Google Scholar]
- 137.Yang D.L., Shi Z., Bao Y., Yan J., Yang Z., Yu H., Li Y., Gou M., Wang S., Zou B. Calcium pumps and interacting BON1 protein modulate calcium signature, stomatal closure, and plant immunity. Plant Physiol. 2017;175:424–437. doi: 10.1104/pp.17.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schiøtt M., Romanowsky S.M., Bækgaard L., Jakobsen M.K., Palmgren M.G., Harper J.F. A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc. Natl. Acad. Sci. USA. 2004;101:9502–9507. doi: 10.1073/pnas.0401542101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen Z.H., Wang Y., Wang J.W., Babla M., Zhao C., García-Mata C., Sani E., Differ C., Mak M., Hills A. Nitrate reductase mutation alters potassium nutrition as well as nitric oxide-mediated control of guard cell ion channels in Arabidopsis. New Phytol. 2016;209:1456–1469. doi: 10.1111/nph.13714. [DOI] [PubMed] [Google Scholar]
- 140.Chen Z., Pottosin I.I., Cuin T.A., Fuglsang A.T., Tester M., Jha D., Zepeda-Jazo I., Zhou M., Palmgren M.G., Newman I.A. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol. 2007;145:1714–1725. doi: 10.1104/pp.107.110262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Véry A.A., Sentenac H. Molecular mechanisms and regulation of K+ transport in higher plants. Annu. Rev. Plant Biol. 2003;54:575–603. doi: 10.1146/annurev.arplant.54.031902.134831. [DOI] [PubMed] [Google Scholar]
- 142.Pilot G., Pratelli R., Gaymard F., Meyer Y., Sentenac H. Five-group distribution of the Shaker-like K+ channel family in higher plants. J. Mol. Evol. 2003;56:418–434. doi: 10.1007/s00239-002-2413-2. [DOI] [PubMed] [Google Scholar]
- 143.Ichida A.M., Pei Z.M., Baizabal-Aguirre V.M., Turner K.J., Schroeder J.I. Expression of a Cs+-Resistant guard cell K+ channel confers Cs+-Resistant, light-induced stomatal opening in transgenic Arabidopsis. Plant Cell. 1997;9:1843–1857. doi: 10.1105/tpc.9.10.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Szyroki A., Ivashikina N., Dietrich P., Roelfsema M.R.G., Ache P., Reintanz B., Deeken R., Godde M., Felle H., Steinmeyer R. KAT1 is not essential for stomatal opening. Proc. Natl. Acad. Sci. USA. 2001;98:2917–2921. doi: 10.1073/pnas.051616698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ivashikina N., Deeken R., Fischer S., Ache P., Hedrich R. AKT2/3 subunits render guard cell K+ channels Ca2+ sensitive. J. Gen. Physiol. 2005;125:483–492. doi: 10.1085/jgp.200409211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.White P.J., Bowen H.C., Demidchik V., Nichols C., Davies J.M. Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochim. Biophys. Acta Biomembr. 2002;1564:299–309. doi: 10.1016/S0005-2736(02)00509-6. [DOI] [PubMed] [Google Scholar]
- 147.Chen Z.H., Hills A., Bätz U., Amtmann A., Lew V.L., Blatt M.R. Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiol. 2012;159:1235–1251. doi: 10.1104/pp.112.197350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang Y.F., Munemasa S., Nishimura N., Ren H.M., Robert N., Han M., Puzõrjova I., Kollist H., Lee S., Mori I. Identification of cyclic GMP-activated nonselective Ca2+-Permeable cation channels and associated CNGC5 and CNGC6 genes in Arabidopsis guard cells. Plant Physiol. 2013;163:578–590. doi: 10.1104/pp.113.225045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hamilton D.W., Hills A., Köhler B., Blatt M.R. Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc. Natl. Acad. Sci. USA. 2000;97:4967–4972. doi: 10.1073/pnas.080068897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Roberts S.K. Plasma membrane anion channels in higher plants and their putative functions in roots. New Phytol. 2006;169:647–666. doi: 10.1111/j.1469-8137.2006.01639.x. [DOI] [PubMed] [Google Scholar]
- 151.Meyer S., Mumm P., Imes D., Endler A., Weder B., Al-Rasheid K.A., Geiger D., Marten I., Martinoia E., Hedrich R. AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J. 2010;63:1054–1062. doi: 10.1111/j.1365-313X.2010.04302.x. [DOI] [PubMed] [Google Scholar]
- 152.Vahisalu T., Kollist H., Wang Y.F., Nishimura N., Chan W.Y., Valerio G., Lamminmaki A., Brosche M., Moldau H., Desikan R., et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature. 2008;452:487–491. doi: 10.1038/nature06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Negi J., Matsuda O., Nagasawa T., Oba Y., Takahashi H., Kawai-Yamada M., Uchimiya H., Hashimoto M., Iba K. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature. 2008;452:483–486. doi: 10.1038/nature06720. [DOI] [PubMed] [Google Scholar]
- 154.Maurel C., Verdoucq L., Rodrigues O. Aquaporins and plant transpiration. Plant Cell Environ. 2016;39:2580–2587. doi: 10.1111/pce.12814. [DOI] [PubMed] [Google Scholar]
- 155.Amtmann A., Blatt M.R. Regulation of macronutrient transport. New Phytol. 2009;181:35–52. doi: 10.1111/j.1469-8137.2008.02666.x. [DOI] [PubMed] [Google Scholar]
- 156.Guo F.Q., Young J., Crawford N.M. The nitrate transporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in Arabidopsis. Plant Cell. 2003;15:107–117. doi: 10.1105/tpc.006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stadler R., Büttner M., Ache P., Hedrich R., Ivashikina N., Melzer M., Shearson S.M., Smith S.M., Sauer N. Diurnal and light-regulated expression of AtSTP1 in guard cells of Arabidopsis. Plant Physiol. 2003;133:528–537. doi: 10.1104/pp.103.024240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Klein M., Geisler M., Suh S.J., Kolukisaoglu H.Ü., Azevedo L., Plaza S., Curtis M.D., Richter A., Weder B., Schulz B. Disruption of AtMRP4, a guard cell plasma membrane ABCC-type ABC transporter, leads to deregulation of stomatal opening and increased drought susceptibility. Plant J. 2004;39:219–236. doi: 10.1111/j.1365-313X.2004.02125.x. [DOI] [PubMed] [Google Scholar]
- 159.Rea P.A., Poole R.J. Vacuolar H+-translocating pyrophosphatase. Annu. Rev. Plant Biol. 1993;44:157–180. doi: 10.1146/annurev.pp.44.060193.001105. [DOI] [Google Scholar]
- 160.Kriegel A., Andrés Z., Medzihradszky A., Krüger F., Scholl S., Delang S., Patir-Nebioglu M.G., Gute G., Yang H., Murphy A.S. Job sharing in the endomembrane system: Vacuolar acidification requires the combined activity of V-ATPase and V-PPase. Plant Cell. 2015;27:3383–3396. doi: 10.1105/tpc.15.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dettmer J., Hong-Hermesdorf A., Stierhof Y.D., Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006;18:715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ratajczak R. Structure, function and regulation of the plant vacuolar H+-translocating ATPase. Biochim. Biophys. Acta Biomembr. 2000;1465:17–36. doi: 10.1016/S0005-2736(00)00129-2. [DOI] [PubMed] [Google Scholar]
- 163.Adem G.D., Roy S.J., Huang Y., Chen Z.H., Wang F., Zhou M., Bowman J.P., Holford P., Shabala S. Expressing Arabidopsis thaliana V-ATPase subunit C in barley (Hordeum vulgare) improves plant performance under saline condition by enabling better osmotic adjustment. Funct. Plant Biol. 2017;44:1147–1159. doi: 10.1071/FP17133. [DOI] [PubMed] [Google Scholar]
- 164.Kasai M., Yamamoto Y., Maeshima M., Matsumoto H. Effects of in vivo treatment with abscisic acid and/or cytokinin on activities of vacuolar H+ pumps of tonoplast-enriched membrane vesicles prepared from barley roots. Plant Cell Physiol. 1993;34:1107–1115. doi: 10.1093/oxfordjournals.pcp.a078525. [DOI] [Google Scholar]
- 165.Darley C.P., Skiera L.A., Northrop F.D., Sanders D., Davies J.M. Tonoplast inorganic pyrophosphatase in Vicia faba guard cells. Planta. 1998;206:272–277. doi: 10.1007/s004250050400. [DOI] [Google Scholar]
- 166.Li J., Yang H., Peer W.A., Richter G., Blakeslee J., Bandyopadhyay A., Titapiwantakun B., Undurraga S., Khodakovskaya M., Richards E.L. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science. 2005;310:121–125. doi: 10.1126/science.1115711. [DOI] [PubMed] [Google Scholar]
- 167.Pasapula V., Shen G., Kuppu S., Paez-Valencia J., Mendoza M., Hou P., Chen J., Qiu X., Zhu L., Zhang X. Expression of an Arabidopsis vacuolar H+-pyrophosphatase gene (AVP1) in cotton improves drought-and salt tolerance and increases fibre yield in the field conditions. Plant Biotechnol. J. 2011;9:88–99. doi: 10.1111/j.1467-7652.2010.00535.x. [DOI] [PubMed] [Google Scholar]
- 168.Ward J.M., Schroeder J.I. Calcium-activated K+ channels and calcium-Induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell. 1994;6:669–683. doi: 10.1105/tpc.6.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gobert A., Isayenkov S., Voelker C., Czempinski K., Maathuis F.J. The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc. Natl. Acad. Sci. USA. 2007;104:10726–10731. doi: 10.1073/pnas.0702595104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Furuichi T., Cunningham K.W., Muto S. A Putative two pore channel AtTPC1 mediates Ca2+ flux in Arabidopsis leaf cells. Plant Cell Physiol. 2001;42:900–905. doi: 10.1093/pcp/pce145. [DOI] [PubMed] [Google Scholar]
- 171.Peiter E., Maathuis F.J., Mills L.N., Knight H., Pelloux J., Hetherington A.M., Sanders D. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005;434:404–408. doi: 10.1038/nature03381. [DOI] [PubMed] [Google Scholar]
- 172.Guo J., Zeng W., Chen Q., Lee C., Chen L., Yang Y., Cang C., Ren D., Jiang Y. Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana. Nature. 2016;531:196–201. doi: 10.1038/nature16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu J., Magalhaes J.V., Shaff J., Kochian L.V. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009;57:389–399. doi: 10.1111/j.1365-313X.2008.03696.x. [DOI] [PubMed] [Google Scholar]
- 174.Eisenach C., De Angeli A. Ion transport at the vacuole during stomatal movements. Plant Physiol. 2017;174:520–530. doi: 10.1104/pp.17.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.De Angeli A., Zhang J., Meyer S., Martinoia E. AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat. Commun. 2013;4:1804. doi: 10.1038/ncomms2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Eisenach C., Baetz U., Huck N.V., Zhang J., De Angeli A., Beckers G., Martinoia E. ABA-Induced stomatal closure involves ALMT4, a phosphorylation-dependent vacuolar anion channel of Arabidopsis. Plant Cell. 2017;29:2552–2569. doi: 10.1105/tpc.17.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Meyer S., Scholz-Starke J., De Angeli A., Kovermann P., Burla B., Gambale F., Martinoia E. Malate transport by the vacuolar AtALMT6 channel in guard cells is subject to multiple regulation. Plant J. 2011;67:247–257. doi: 10.1111/j.1365-313X.2011.04587.x. [DOI] [PubMed] [Google Scholar]
- 178.De Angeli A., Monachello D., Ephritikhine G., Frachisse J., Thomine S., Gambale F., Barbier-Brygoo H. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature. 2006;442:939–942. doi: 10.1038/nature05013. [DOI] [PubMed] [Google Scholar]
- 179.Wudick M.M., Luu D.T., Maurel C. A look inside: Localization patterns and functions of intracellular plant aquaporins. New Phytol. 2009;184:289–302. doi: 10.1111/j.1469-8137.2009.02985.x. [DOI] [PubMed] [Google Scholar]
- 180.Sarda X., Tousch D., Ferrare K., Legrand E., Dupuis J., Casse-Delbart F., Lamaze T. Two TIP-like genes encoding aquaporins are expressed in sunflower guard cells. Plant J. 1997;12:1103–1111. doi: 10.1046/j.1365-313X.1997.12051103.x. [DOI] [PubMed] [Google Scholar]
- 181.Maeshima M. Tonoplast transporters: Organization and function. Annu. Rev. Plant. Biol. 2001;52:469–497. doi: 10.1146/annurev.arplant.52.1.469. [DOI] [PubMed] [Google Scholar]
- 182.Reguera M., Bassil E., Blumwald E. Intracellular NHX-Type Cation/H+ antiporters in plants. Mol. Plant. 2014;7:261–263. doi: 10.1093/mp/sst091. [DOI] [PubMed] [Google Scholar]
- 183.Barragán V., Leidi E.O., Andrés Z., Rubio L., De Luca A., Fernández J.A., Cubero B., Pardo J.M. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell. 2012;24:1127–1142. doi: 10.1105/tpc.111.095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Andrés Z., Pérez-Hormaeche J., Leidi E.O., Schlücking K., Steinhorst L., McLachlan D.H., Schumacher K., Hetherington A.M., Kudla J., Cubero B. Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proc. Natl. Acad. Sci. USA. 2014;111:E1806–E1814. doi: 10.1073/pnas.1320421111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cho D., Villiers F., Kroniewicz L., Lee S., Seo Y.J., Hirschi K.D., Leonhardt N., Kwak J.M. Vacuolar CAX1 and CAX3 influence auxin transport in guard cells via regulation of apoplastic pH. Plant Physiol. 2012;160:1293–1302. doi: 10.1104/pp.112.201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bruggeman Q., Mazubert C., Prunier F., Lugan R., Chan K.X., Phua S.Y., Pogson B.J., Krieger-Liszkay A., Delarue M., Benhamed M., et al. Chloroplast activity and 3′phosphadenosine 5′phosphate signaling regulate programmed cell death in Arabidopsis. Plant Physiol. 2016;170:1745–1756. doi: 10.1104/pp.15.01872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chan K.X., Wirtz M., Phua S.Y., Estavillo G.M., Pogson B.J. Balancing metabolites in drought: The sulfur assimilation conundrum. Trends Plant Sci. 2013;18:18–29. doi: 10.1016/j.tplants.2012.07.005. [DOI] [PubMed] [Google Scholar]