Abstract
The current interest of the scientific community for research in the field of calcium sensing in general and on the calcium-sensing Receptor (CaR) in particular is demonstrated by the still increasing number of papers published on this topic. The extracellular calcium-sensing receptor is the best-known G-protein-coupled receptor (GPCR) able to sense external Ca2+ changes. Widely recognized as a fundamental player in systemic Ca2+ homeostasis, the CaR is ubiquitously expressed in the human body where it activates multiple signalling pathways. In this review, old and new notions regarding the mechanisms by which extracellular Ca2+ microdomains are created and the tools available to measure them are analyzed. After a survey of the main signalling pathways triggered by the CaR, a special attention is reserved for the emerging concepts regarding CaR function in the heart, CaR trafficking and pharmacology. Finally, an overview on other Ca2+ sensors is provided.
Keywords: Ca2+-signalling, microelectrode, fluorophore, exocytosis, cAMP signalling, parathyroid extracellular-Ca2+-sensing receptor (CaR), cancer, apoptosis, ischemia/reperfusion, hypertrophy, heart, cardiomyocytes
1. Introduction
Over the years, the intracellular Ca2+ signal has emerged as the most intensely studied second messenger pathway [1]. Ca2+ is the most versatile intracellular messenger, not only in the cytoplasm but also in diverse subcellular compartments, such as mitochondria [2], nuclei [3], lysosomes [4], and the endoplasmic reticulum [5]. The cloning of the extracellular Calcium-sensing receptor (CaR) 25 years ago by Brown et al. [6] paved the way for a heightened understanding of the mechanisms used by Ca2+ to signal in the microenvironments outside the cell. Thus, Ca2+ acts as a multipurpose messenger, exerting effects at the cellular, subcellular, and extracellular levels. A very interesting and comprehensive list of reviews on the CaR has been recently published by leading experts in the field of extracellular Calcium-sensing and signalling [7]. Other key topics, such as the structure-function relationships [8] and role of the CaR in cancer [9], have also been very recently covered elsewhere and will be named here in the corresponding paragraphs. The readers are thus referred to these fine reviews for a complete understanding of the CaR’s function in cell physiology and pathology [10].
The present review will examine the potential conditions under which extracellular Ca2+ concentration ([Ca2+]) may change in the local microenvironment outside the cell, with a particular emphasis on the experimental approaches utilized to measure ex vivo and in vitro such Ca2+ fluctuations. These extracellular Ca2+ changes are sensed by the CaR (and other Ca2+ sensors) and transduced by means of different signalling pathways that modulate organ-specific physiological processes. Over the last few years, new concepts have emerged regarding the CaR’s role in cancer and cardiac physiology and pathology. Also, exciting news have come into view in the fields of CaR signalling, trafficking, and pharmacology. In addition, it is now clear that a number of different Ca2+ sensors share important tasks with the CaR. Therefore, the aim of this review is to summarize for the reader of International Journal of Molecular Science these new and exciting notions.
2. Extracellular Ca2+ Microdomains
Serum-ionized Ca2+ concentration (~1.4 mM) is usually precisely regulated by a number of cooperative organs, including parathyroid glands, bones, kidney, and intestine [11]. However, this tight modulation does not prevent local changes in extracellular Ca2+, as those have been identified in the microscopic volume of interstitial fluid surrounding cells of many tissues [12]. This scenario has become increasingly important because the amplitude and the shape of these extracellular Ca2+ fluctuations represent an autocrine/paracrine cell-to-cell communication form based on the activity of extracellular Ca2+ sensors.
Many factors have been proposed in the onset of such significant extracellular Ca2+ changes. At first, we will evaluate how the activity of the cells, in terms of intracellular Ca2+ signalling events, can be rapidly translated in asymmetrical Ca2+ changes. Then, we will analyze how the exocytosis of Ca2+-loaded vesicles and the synchronous opening of voltage-gated Ca2+ conductances perturb Ca2+ levels in the close proximity of a cell.
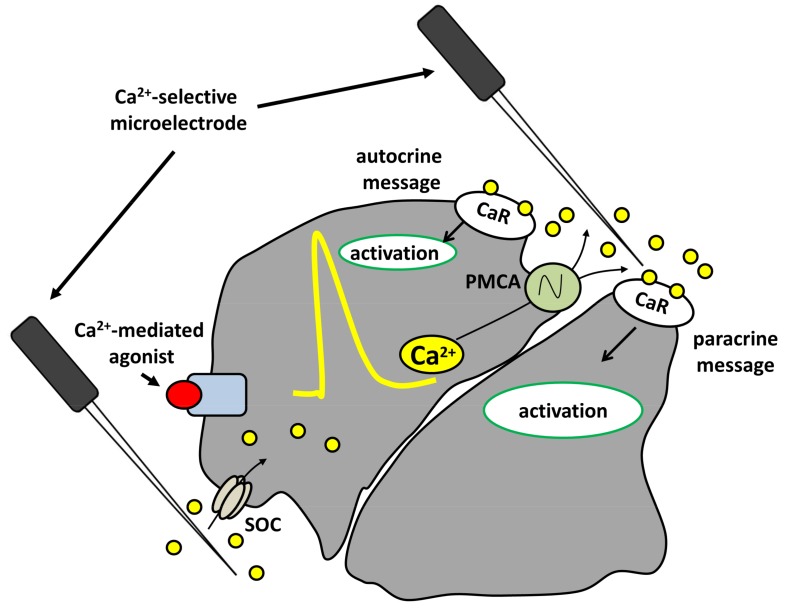
When closely packed to form a functional tissue, cells delimit small diffusion spaces. Within this context, cells themselves represent major sources and/or sinks for Ca2+ during intracellular Ca2+ signalling events as a result of the activation of Ca2+ efflux (e.g., by the plasma membrane Ca2+-ATPase and/or Na+/Ca2+ exchanger) and Ca2+ influx (e.g., by store-operated channels, SOCs) pathways at the plasma membrane, respectively. Differential dynamics or a polarized distribution of these Ca2+ influx/efflux mechanisms [13,14,15,16] are required for the onset of significant extracellular Ca2+ microdomains. Our group showed that stimulation with carbachol, a Ca2+-mediated agonist, resulted in asymmetrical fluctuations in local extracellular Ca2+ levels in a polarized epithelium.
Using Ca2+-selective microelectrodes, we measured a substantial plasma membrane Ca2+-ATPase (PMCA)-dependent local increase in the extracellular Ca2+ concentration at the apical face and a similar store-operated channel (SOC)-dependent depletion at the basolateral side of amphibian acid-secreting cells (Figure 1). Thus, in intact epithelia, asymmetrical changes in extracellular Ca2+ reflect the polarized distribution of Ca2+-handling mechanisms in the plasma membrane [17]. Of note, PMCA is highly expressed at the apical membrane of these cells where it shares a strategic localization with the extracellular CaR [17]. In fact, these carbachol-induced changes in extracellular Ca2+ act as paracrine/autocrine messages sufficient and essential to strictly regulate tissue-specific functions (e.g., alkaline secretion, pepsinogen secretion, and water flux) independently of intracellular [Ca2+] changes [18,19].
Figure 1.
Schematic representation of intercellular communication mediated by the calcium-sensing Receptor (CaR) in polarized epithelial cells. When a Ca2+-mediated agonist (red circle) binds a specific receptor, Ca2+ concentration (yellow line) increases within the cytosol. Extrusion of Ca2+ via the plasma membrane Ca2+-ATPase (PMCA), localized at the apical membrane, might activate the CaR expressed on cells in close proximity (paracrine message) or on the same cell (autocrine message). The asymmetrical changes in extracellular Ca2+ are the result of the polarized localization of PMCA and store-operated channels (SOCs) at the apical and basolateral membranes, respectively. These fluctuations can be recorded with Ca2+-sensitive microelectrodes. Modified from [29].
Secretory granules are characterized by very high intraluminal Ca2+ concentrations [20,21,22]. Among others, insulin granules of rat insulinoma contain ionized Ca2+ in a range between 60 and 120 mM [23]. Thus, it does not come as a surprise that exocytotic events can significantly change extracellular Ca2+ in the microenvironment immediately surrounding the cells. Recently, we showed using Ca2+-microelectrodes that stimulation of insulin secretion by high glucose and other secretagogues (glibenclamide, forskolin/IBMX, and ATP) induced a delayed elevation of extracellular Ca2+ within rat insulinoma (INS-1E) β-cell pseudoislets [24]. The exocytotic extrusion of Ca2+ through Ca2+-loaded vesicles released in the extracellular environment has also been demonstrated in different cell types, such as salivary gland cells [25], bovine adrenal medullary cells [26], neurohypophyseal nerve endings [27], and sea urchin eggs [28].
In addition to the above-mentioned mechanisms, excitable cells express a different kind of voltage-gated Ca2+ conductances that, during their physiological activity, might influence extracellular Ca2+ levels [30,31,32]. It has been demonstrated that, in the central nervous system, the synchronized opening of voltage-gated Ca2+ channels (VGCCs) elicited significant reductions in extracellular Ca2+ levels [33,34]. We and others have shown that in mouse islets of Langerhans and INS-1E pseudoislets, glucose stimulation depleted the concentration of Ca2+ in the extracellular milieu (of about 500 μM) as a consequence of Ca2+ influx through a VGCC at the plasma membrane [24,35,36]. Transient reductions were also recorded in rat neocortex [33] and in the cardiac muscle [37].
3. Measuring Fluctuations in Extracellular Ca2+ Levels
Real-time measurement of extracellular Ca2+ changes in the proximity of the plasma membrane (e.g., intercellular spaces) is a technically challenging experimental maneuver. In fact, researchers must deal on the one side with the lack of proper tools to directly access these restricted compartments, and on the other side with the quantification of small Ca2+ changes (few hundreds micromolar) over a high extracellular Ca2+ background (physiologically around 1.1–1.4 mM). Over the years, many different experimental approaches to measure extracellular Ca2+ fluctuations have been proposed in a number of different tissue preparations, although each one of them has its own drawbacks in terms of either sensitivity or spatial resolution.
Microelectrode-based electrophysiological approaches have been applied to diverse cell and tissue types to monitor extracellular Ca2+ changes. Early studies directly measured changes in extracellular Ca2+ concentration in the rat cerebellum using double-barreled Ca2+-selective micropipettes. During repetitive stimulation of the central nervous system, extracellular Ca2+ was significantly reduced, indicating that Ca2+ modulation in brain microenvironments may be a significant parameter in the behavior of neuronal ensembles [38].
The vibrating probe technique uses a low-resistance Ca2+-sensitive electrode vibrating in proximity of the surface of cells or tissues in order to measure small extracellular Ca2+ gradients (for more technical details refer to [39,40]). Self-referencing, low-resistance vibrating Ca2+ microelectrodes were shown to non-invasively quantify, in real time, local extracellular ion gradients with high sensitivity and square micron spatial resolution. Ca2+-selective self-referencing electrodes have been used extensively to show Ca2+ flux at different regions of hair cells, cells of the zona pellucida, and the central nervous system [41,42,43,44].
As described in the previous section, we extensively used Ca2+-selective microelectrodes to directly record the profile of agonist-induced changes in extracellular [Ca2+] in the restricted domain of both the intact amphibian gastric mucosa [17] and in the microenvironment immediately surrounding INS-1E cells organized as pancreatic pseudoislets [24].
Finally, Mupanomunda and colleagues used in situ microdialysis techniques in anesthetized Wistar rats to monitor extracellular [Ca2+] in the sub-epithelial spaces of the intestine [45] and kidney [46]. In these studies, an increased load of [Ca2+] in the gut lumen or perturbations in whole-animal Ca2+ homeostasis were shown to cause significant changes in the external [Ca2+] underlying the epithelium of these tissues.
Ca2+-sensitive dyes have shown extensive utility in the study of the spatio-temporal dynamics of Ca2+ fluctuations. These tools, compared with electrophysiological techniques, are more sensitive and provide for a better time resolution and more reliable access to limited spaces. However, these approaches are performed in free extracellular Ca2+ (thus, non-physiological conditions) in order to avoid saturation of the fluorophores.
Ca2+-sensitive metallochromic indicators (antipyrylazo III and tetramethylmurexide) were used to estimate extracellular Ca2+ dynamics in intact beating hearts. These indicators revealed significant depletion of external Ca2+ following the synchronous opening of a Ca2+ influx pathway during contraction [37,47,48]. Later on, Tepikin and Petersen introduced the droplet technique [49,50,51], an elegant approach to measure extracellular Ca2+ changes elicited by the activity of the PMCA. This method employs Fluo-3 under Ca2+-free media conditions to quantify extracellular Ca2+ changes in small clusters of exocrine gland cells contained in a small droplet of solution (20–50 times the volume of the cells). Extracellular Ca2+ was also measured in cell clusters digested from exocrine glands (pancreas, submandibular gland) and placed in a small experimental chamber containing Ca2+ green-1 bound to high molecular weight dextran beads in Ca2+-free solution [25,52].
Ca2+-sensitive probes conjugated to a hydrophobic tail have been created to cross the external leaflet of the cell membrane and directly measure extracellular Ca2+ levels. A classic example is Ca2+-Green-C-18, the fluorophore conjugated with a lipid derivative that has been successfully used to record Ca2+ extrusion and fluctuations 2+inside the T-tubules [53]. In smooth muscle cells, the Ca2+-sensitive indicator Fura-C18 was used to measure extracellular Ca2+ changes near the plasma membrane. The same dye was used to compare the extracellular Ca2+ changes recorded with signals measured from the bulk cytosol [54,55]. In addition, Fura-C18 has been used to directly measure near-membrane extracellular [Ca2+] changes in HEK-293 cells, showing that Ca2+ extruded to the extracellular space by PMCA, as a consequence of intracellular Ca2+ events, activates a positive feedback through the CaR [56].
4. The Extracellular Calcium-Sensing Receptor (CaR)
4.1. Discovery and Cloning of the Extracellular Calcium-Sensing Receptor
The first hints about the existence of an extracellular Ca2+ sensor come from a very early study (1966) correlating serum Ca2+ concentration and parathyroid hormone (PTH) secretion in whole animals [57,58]. About 10 years later, further evidences corroborated such a correlation in isolated glands and dispersed parathyroid cells [11,59,60,61,62,63,64].
The direct link between extracellular [Ca2+] changes, PTH secretion, and intracellular Ca2+ signals was finally demonstrated by Nemeth and Scarpa [65], while the actual molecular identification of the Ca2+ sensor had to wait until 1993, when Brown and colleagues cloned a peculiar G protein coupled receptor (GPCR) from the bovine parathyroid glands [6] and named it “extracellular Calcium-sensing receptor”. This receptor, here named CaR, is also referred to as CaSR, after its gene name, or more recently CaS, as recommended by NC-IUPHAR [66].
4.2. Structural Features of the CaR
The extracellular calcium-sensing receptor belongs to class C (or 3) of the GPCR superfamily, which consists of eight metabotropic glutamate receptors (mGlu1-8), two type B metabotropic-aminobutyric acid receptors (GABAB1 and GABAB2), three taste receptors (T1R1-3), a promiscuous l-α-amino acid (GPRC6A) receptor [67], and seven orphan receptors [68]. The primary structure of human CaR consists of four main parts: a large N-terminal extracellular domain (ECD) (612 amino acids) [69], a cysteine-rich domain, a seven-transmembrane domain (TMD, 250 amino acids), and a 216-residue carboxyterminal (C)-tail [70].
The ECD contains a large bilobed, nutrient-binding Venus Flytrap (VFT) domain related to bacterial periplasmic binding proteins which has been crystallized in several members of GPCR class C (mGlu1 [71], mGlu3 and mGlu7 [72], and the GABAB2 subunit [73]). It seems that upon Ca2+ (or other agonists) binding, the open cleft of the VFT closes inducing, in turn, conformational changes in both the TMD and the intracellular domains that are believed to initiate signal transduction [74,75].
The functional, highly glycosilated CaR proteins reside on the cell membrane as homodimers [76,77] or hetero-dimers with other class C receptors (mGluR [78] or GABAB [79]). While non-covalent interactions across the dimer interface [80] are essential for dimer formation, the intermolecular disulfide between two conserved cysteines of the VFTs (C129 and C131 [81,82,83]) seems not to influence the formation of dimer but to stabilize it [84]. Critical for CaR expression and activity seem to be other three intra-molecular disulfides formed by cysteines in the ECD of the CaR, including C60–C101, C358–C395, and C437–C449 [85]. More recently, Brauner-Osborn showed that one allosteric site per CaR dimer is sufficient for achieving the modulatory effect of positive allosteric modulators, while prevention of activation in both 7TM domains is needed in order to obtain complete inhibition by negative allosteric modulators [86].
The nine-cysteine structure domain (found in all members of the GPCR family C but the GABA B receptor) serves as a linker between the ECD and TMD and is essential for the CaR signalling capacity as demonstrated by the consequences of its removal [87]. The intracellular domain of the CaR shows a low degree of conservation among species [88] except for residues located at the beginning of the C-tail (863–925) that are crucial for surface expression [89] and those (960–984) involved in the interaction with downstream proteins [8,90,91]. As for other GPCRs, CaR activity can be regulated via phosphorylation by different protein kinases, such as PKC [76], which plays a significant role in the negative regulation of the CaR’s activity and the generation of CaR-induced intracellular Ca2+ oscillations. On the other hand, PKA [92] is involved in the intracellular retention of the receptor [93]. For detailed reviews on the structure of the CaR and new insights on ligand binding pockets, see [8,70,94]. The structural requirements for processes such as bias and allosteric modulation of the CaR have been analyzed by recent works [95]. In addition, a number of papers have highlighted the importance of the potential Ca2+ binding sites and their relevance for associated diseases [8,96,97,98,99,100,101,102]. Recently, the first high-resolution crystal structure of the ECD of the human CaR bound with Mg2+ has been proposed [102]. In this work, the authors found a high-affinity tryptophan derivative in the crystal structure of the CaR, which seems to play a role in potentiating the function of the receptor. The proposed CaR co-activation operated by extracellular Ca2+ and amino acids might have a wide impact for the regulation of the receptor in districts of our body that are constantly exposed to both CaR agonists, such as the intestine. An exhaustive review about the recent insights regarding the structure and functional cooperativity of the CaR (and other members of cGPCRs) in health and disease has been published by the group of Jenny Yang [103].
4.3. CaR Promiscuity: Many Orthosteric Agonists and Allosteric Modulators Concur in Modulating CaR Function at Multiple Sites
Although, as the name states, the main ligands of the CaR are extracellular Ca2+ ions, which bind to the receptor with a high positive cooperativity, the CaR is also able to respond to other extracellular stimuli (see Table 1), such as inorganic di- and tri-valent cations, including Sr2+, Ba2+, Gd3+, Al3+ [11,104], various organic polycations, such as the polyamines spermine and spermidine [105], and basic polypeptides, including polylysine, polyarginine [11,106], and amyloid β-peptides [107]. The CaR has also been shown to be activated by some aminoglycoside antibiotics, such as neomycin and gentamycin [108,109]. These ligands, referred to as type I calcimimetics or orthosteric agonists, can stimulate the receptor also in the absence of Ca2+ or any other ligands.
Table 1.
Orthosteric agonists and allosteric modulators of the calcium-sensing receptor (modified from [110]).
| Orthosteric Agonists (Type I Calcimimetics) | Class Members |
| A. Cations | High potency: Gd3+; Eu3+; Tb3+ |
| Intermediate potency: Zn2+; Ni2+; Cd2+; Pb2+; Co2+; Fe2+ | |
| Low potency: Ca2+; Mg2+; Ba2+; Sr2+; Mn2+ | |
| B. Polyamines | spermine, spermidine, putrescine |
| C. Aminoglycoside antibiotics | neomycin, gentamycin, tobramycin, poromomycin, kanamycin, ribostamycin |
| D. Basic polypeptides | Poly-L-arginine, poly-L-lysine, protamine, amyloid β-peptides |
| Allosteric Modulators (Type II Calcimimetics) | |
| A. L-amino acids | phenylalanine, tryptophan, tyrosine, histidine |
| B. Glutathione analogs | γ-glutamyl-tripeptides: glutathione, S-methylglutathione, S-propylglutathione, γ-glutamyl-tripeptides (γ-Glu-Ala, γ-Glu-Cys) |
| C. Small molecule calcimimetics | The first generation: NPS R-568, NPS R-467, AMG 073, AMG 416 |
| The second generation: cinacalcet | |
| The third generation: dibenzylamine calcimimetics, R,R-calcimimetic B, AC-265347 | |
| D. Small molecule calcilytics | NPS 2143, Calhex 231, ATF396, AXT914, ronacaleret, NPSP795, SB-423557, SB-423562 |
The other class of CaR agonists is represented by type II calcimimetics (or allosteric modulators), composed by ligands which bind to a site different from that of the orthosteric agonists, thus altering the receptor conformation and, as a consequence, the affinity and/or the signaling capacity of the orthosteric agonist. This action can be exerted in a positive (calcimimetics) or a negative direction (calcilytics).
Belonging to this class of CaR agonists are natural l-amino acids, especially aromatic [111,112,113] and glutathione analogs [114,115], which synergize with extracellular Ca2+. The receptor’s activity is also negatively modulated by low extracellular pH [116] and high ionic strength [117].
A detailed review on the binding sites of orthosteric and allosteric agonists of the CaR has been recently published by Zhang and colleagues [8].
Among the allosteric modulators of the CaR, there are drugs with a high therapeutical potential: first, second, and third generation calcimimetics [118,119,120,121].
After the first generation of calcimimetics, which is represented by phenylalkylamine derivatives, such as NPS R-568 and NPS R-467 [122], the second generation reached the clinic: cinacalcet is the only CaR modulator currently used in clinical practice to reduce parathyroid glands activity in patients under dialysis for end-stage renal disease [123,124,125]. The third generation calcimimetics, which comprise the dibenzylamine calcimimetic, R,R-calcimimetic B, and the structurally distinct calcimimetic AC-265347, appear to have enhanced tissue-selective effects on PTH and calcitonin release in rats [126,127] most probably via biased allosteric modulation of the CaR’s activity [128] (see below).
Allosteric antagonists or calcilitics, such as NPS-2143 and Calhex 231, antagonize the stimulatory effects of extracellular Ca2+ and are currently in clinical trials for treating osteoporosis [129].
In addition to affecting the signaling capacity of the CaR, allosteric CaR modulators have been shown more recently to act as pharmacochaperones that rescue cell surface expression of mutant CaRs that are retained intracellularly (see the paragraphs below).
4.4. Trimeric G Proteins and Signalling Pathways Activated by the CaR
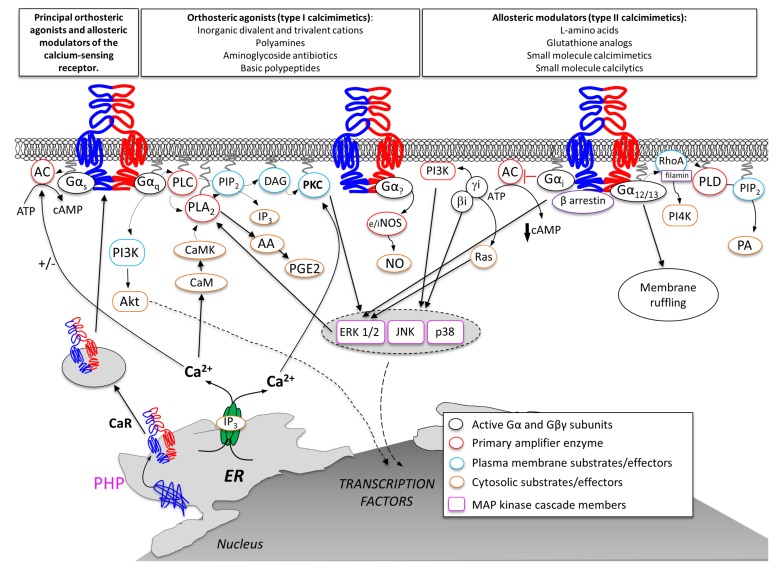
Besides its ability to sense changes in multiple physiological parameters via interaction with different ligands, the CaR has also been shown to activate a wide array of different intracellular pathways (Figure 2) (for comprehensive reviews see [130,131]).
Figure 2.
Schematic illustration of intracellular transduction cascades activated by orthosteric agonists or allosteric modulators on the CaR. Modified from [110].
4.4.1. Gq/G11
Most of the studies available in the literature on CaR physiopathological functions in diverse tissues of the body have demonstrated that this receptor, as shown in parathyroids [61,132], mainly interacts with the Gq/11 heterotrimeric G protein, inducing in turn the activation of phospholipase C (PLC)/inositol-1,4,5-trisphosphate (InsP3)/Ca2+ mobilization and activation of PKC and mitogen-activated protein kinase (MAPK) pathways [133]. These intracellular events cause a decrease in parathyroid hormone (PTH) secretion and reduction in renal tubular Ca2+ reabsorption [133].
PKC has been described to phosphorylate specific CaR residues and attenuate signalling and/or activate the mitogen-activated protein kinase (MAPK) cascade (ERK1/2, p38 MAPK, JNK), ultimately causing phosphorylation and activation of ERK1/2 [134]. CaR-induced and Gq-mediated activation of phospholipase A2 and phospholipase D have also been reported [135]. Also, activation of the Akt pathway by Gq (via phosphatidylinositol 3-kinase, PI3K) has been described as a determinant in CaR-mediated cell migration [136] and metalloproteases production [137]. The activation of the intracellular Ca2+ signalling pathway is also responsible for a Gi/o-independent inhibition of cyclic AMP synthesis mediated by Ca2+-induced inhibition of adenylyl cyclase isoforms 5, 6, and/or 9 [138,139,140]. For a recent review see also [141].
4.4.2. Gi/o
Early studies have led to the suggestion that the CaR can interact with the pertussis-toxin-sensitive inhibitory G protein, Gαi/o, causing inhibitory control of PTH secretion [142,143] although the matter is still debated [144]. More recent work has clearly demonstrated CaR-mediated inhibition of adenylyl cyclase (AC) by a pertussis toxin (PTX)-sensitive mechanism. In gastric mucosa, CaR activation led to PTX-dependent alkaline and pepsinogen secretion [18]. In CaR-expressing HEK-293 cells, CaR stimulation quickly reversed or significantly prevented the agonist-induced rise in cytosolic cAMP via a dual mechanism involving pertussis-toxin-sensitive Gi and the CaR-stimulated increase in intracellular Ca2+ level [139]. The beta gamma subunits of the Gi/o protein have also been described as mediators of CaR -induced ERK1/2 phosphorylation via Ras and MAPK activation [134]. More recently, the role of beta-arrestin in Gi/o protein-dependent activation of (ERK) and c-Jun N-terminal kinases (JNK) has been directly demonstrated [145]. Gi activation by the CaR has also been reported to induce activation of phosphatidylinositol 4-kinase (PI4K) [146,147].
4.4.3. G12/13
G12/13 pathways have been described in different cell types to differentially modulate CaR-mediated intracellular Ca2+ kinetics.
Both early and recent studies have described the capacity of aromatic amino acids to induce intracellular Ca2+ oscillations via a G12/13 /Rho/ filamin-A pathway, which in turn induce Ca2+ influx from the extracellular fluid via the TRPC1 channel opening [148,149,150]. On the contrary, in pre-osteoblasts, a G12-dependent dephosphorylation of CaR residue T888 by phosphatase PP2A [151,152] seems to be involved in the appearance of the sustained Ca2+ increases necessary to activate chemotaxis [153]. In bone, CaR-mediated G12/13 activation positively modulates osteoblast differentiation [154] and negatively modulates osteoclastogenesis [155]. Importantly, CaR-dependent G12/13 signalling seems to be also implicated in the spreading of breast and prostate cancer cells [156].
4.4.4. Gs
In some cell contexts, such as human breast cancer [157] and pituitary cells [158], the CaR has been shown to modulate hormone secretion by activating Gs. Interestingly, a CaR-induced Gs-independent mechanism for the increase of cyclic AMP synthesis has been recently identified in human fetal lung [159].
Finally, a role for nitric oxide (NO) in mediating CaR action on ion channels has been recently demonstrated in the vasculature [160,161,162,163,164].
4.5. Physiopathological Significance of CaR: Old and New Aspects
As the name suggests, the main physiological function of the CaR is to sense subtle changes in serum Ca2+ and maintain systemic Ca2+ homeostasis within a narrow range via the oppositely directed changes in secretion of PTH, which in turn modulate the equilibrium between Ca2+ uptake and conservation via a direct action on kidney, bone, and thyroid C cells [165] and indirect actions on intestine. Also, the CaR controls calcitonin secretion from thyroidal C-cells upon detection of variations in [Ca2+]ext (for a review, see [11]).
When the extracellular Ca2+ concentration is reduced, CaR-induced parathyroid hormone (PTH) secretion is elicited by the parathyroid glands. Consequences of PTH release are: (a) reduced excretion of Ca2+ by the kidney; (b) increased intestinal absorption of Ca2+, which is mediated by PTH-induced synthesis of the active vitamin D metabolite 1,25-dihydroxyvitamin D; and (c) net release of skeletal Ca2+ under sustained PTH secretion. All these tasks cooperate to increase serum Ca2+ back to physiological levels. On the other hand, when serum Ca2+ levels are increased over the physiological range, the receptor is activated inhibiting PTH synthesis and secretion [166].
The physiological significance of the CaR in tissues involved in systemic Ca2+ homeostasis is well-highlighted by the identification of mutations of the CaR gene that cause several inherited disorders of extracellular calcium sensing (for a review, see [167]).
Familial hypocalciuric hypercalcemia (FHH) and neonatal severe hyperparathyroidism (NSHPT) [168] are caused by loss-of-function mutations of the CaR gene, whereas autosomal dominant hypocalcemia (ADH) and Bartter Syndrome type V [169] occur from gain-of-function mutations of the CaR [170,171,172].
Besides its expression in tissues involved in Ca2+ homeostasis, such as thyroid glands, kidneys, and bone, the CaR has been identified in an astonishing number of diverse cell types (reviewed in [11] and [173]), i.e., brain [174], pancreas [175], stomach [89], liver [176], and heart [177,178]. In these tissues, the CaR has been shown to regulate a variety of cellular processes, such as secretion, differentiation, and gene expression. For recent reviews on this topic see also [74,179].
4.5.1. CaR Involvement in Cancer
The massive impact of the CaR in such a wide array of cellular processes, including proliferation, differentiation, migration, adhesion, and apoptosis foresees a role for this receptor in many aspects (incidence, progression, recurrence, and lethality) of various cancers (e.g., parathyroid, pancreatic, prostate, breast, colorectal, ovarian, gastric, skin, and neuroblastoma) [9,180]. However, mutations of the CaR are not early events in tumorigenesis. Still, an increasing amount of literature is concordant on CaR participation in various aspects of the neoplastic progression. In this line, a number of papers evaluating the role of CaR polymorphisms in the risk, incidence, recurrence, or lethality of different kinds of cancer has been published [9].
The CaR is associated with both anti- and pro-proliferative signals. Thus, it can either prevent or promote tumorigenesis depending on the cellular context and the type of cancer [180]. Tissue-specific alterations of CaR expression level, functional activity, or coupling to signaling pathways might explain such opposing behavior.
In tissues such as parathyroid or colon, CaR inhibits cell proliferation and induces terminal differentiation and apoptosis. In tumors of these organs (and more recently also of the gastric mucosa), the observed and progressive reduction in CaR expression (e.g., from normal tissue to carcinomas) grants malignant potential, indicating for the receptor and the physiological significance of its activation a putative role as tumor suppressor [181,182,183,184,185]. Accordingly, when taking also CaR functional activity into consideration, inactivating mutations of the CaR or calcilytics might induce hyperplasia, while calcimimetics have the opposite outcome [186,187]. This scenario is extremely important assuming that the CaR is a novel target for the prevention/treatment of these tumors. However, as reported throughout this review, the incredible number of CaR interactors (both extracellular and intracellular) increases the complexity associated with this kind of pharmacological approach.
Indeed, some of the first evidence connecting extracellular Ca2+ and tumors was the inverse correlation between dietary Ca2+ level and risk of colorectal cancer [188]. This effect seemed to be mediated by the CaR and potentiated by the active vitamin D metabolite calcitriol [189]. Since CaR transcription is strongly upregulated by vitamin D [190], a Ca2+-containing diet could protect against colon cancer partly via upregulation of CaR expression. The work by the group of Enikö Kallay provided clear evidence about the role of dietary Ca2+ and dissected the reciprocal interplay between extracellular Ca2+/CaR and the Vitamin D system (for an exhaustive review refer to [189]). Significant reduction in CaR expression level has also been observed in unfavorable neuroblastomas when compared with benign, differentiated neuroblastic tumors. However, information about the mechanisms underlying the loss of CaR expression is still scattered and thus under intense investigation. Yet, in colon cancer and neuroblastomas, genetic or epigenetic mechanisms, such as hypermethylation and histone deacetylation of CaR promoter or monosomy of the chromosome 3, have been proposed [9,191].
On the other hand, in prostate and breast tumors, the expression level of the CaR is increased [192,193]. This experimental evidence is in line with an oncogene-like behavior of the CaR (e.g., increasing proliferation and inhibiting cell death), which seems strictly related to the Gαs/cAMP-dependent production of the parathyroid hormone-related protein (PTHrP). Strikingly, in normal mammary epithelial cells, during lactation, the CaR inhibits PTHrP synthesis and secretion through a Gαi, clearly indicating the ability of the receptor to bind and activate opposing G proteins in normal and breast cancer cells [157]. In addition, in breast cancer cells, CaR signalling through Gα12/13 affects cell migration via rho-dependent actin filament formation facilitating the metastatic spread of the tumor [194]. These tumors often lead to bone metastasis, since bone provides homing signals (such as PTHrP-induced high extracellular Ca2+) to several cancers, such as breast, prostate, lung, and kidney cancer.
4.5.2. The CaR in the Heart
Another field of emerging interest for its implications on human health concerns CaR function in cardiovascular physiology and pathology.
The CaR has been demonstrated to exert a fundamental role in the vasculature and to affect blood pressure in different experimental models [160,195,196,197,198]. Nice reviews on this topic have been recently published by experts in the field [199,200,201,202].
Hence, the focus of this section is on the specific role of the CaR in the heart, a topic which is still quite controversial and, in our opinion, needs further study in the near future.
The first paper describing the functional expression of the CaR in the heart was published 15 years ago [177]. In that first report, the authors identified CaR mRNA and protein both in atrial and ventricular tissues from adult rats. Also, a tentative imaging approach was performed by using high extracellular Ca2+ concentrations, spermine (1–10 mM), and gadolinium on isolated adult ventricular cardiomyocytes. Although the interpretation of data collected with high extracellular Ca2+ on Fura-2-loaded cells could be hampered by a protocol in which the cardiomyocytes were first bathed in 0 Ca2+, thus activating a serious Ca2+ overload upon Ca2+ re-addition, the authors provided evidence of effects of extracellular calcium, spermine, and gadolinium on changes of Inositol Phosphate (IP) levels, thus implicating the PLC/InsP3 signalling pathway in CaR activation.
The existence of a functional CaR was next clearly demonstrated in neonatal rat ventricular cardiomyocytes by Tfelt-Hansen and collaborators [178]. Importantly, in this study the calcimimetic AMG 073 was used to activate the CaR and proven to be effective both in IPs accumulation and ERK1/2 activation. Also, the involvement of CaR in IPs modulation was straightforwardly demonstrated by the significant inhibition of Ca2+-induced IP accumulation upon infection of neonatal cardiac myocytes with adeno-associated viruses containing the dominant negative CaR R185Q.
Starting from 2006, a conspicuous number of papers has been published by researchers from the Harbin University, where the first paper on the CaR in cardiac tissues was published [177]. The results collected during the last decade by this group depict a general scenario in which CaR activation is mostly causative of cardiac diseases.
On the contrary, other groups have provided evidence of a cardioprotective role of the CaR. Various experimental models and approaches have been used to inquire into the CaR function in cardiac apoptosis induced by different drugs or by ischemia/reperfusion, hypertrophy and fibrosis, and diabetic cardiomyopathy. The role of the CaR in pre- and post-conditioning has also been investigated.
Role of the CaR in Cardiac Apoptosis
A pro-apototic effect of GdCl3, used as the only CaR agonist (in the presence of NiCl2 and CdCl2 to block L-type Ca2+ channels and Na+/Ca2+ exchangers, respectively) was reported by Sun and colleagues in neonatal cardiac myocytes [203]. CaR pro-apoptotic action was suggested to be mediated by intracellular Ca2+-induced activation of extracellular signal-regulated protein kinase (ERK), c-Jun NH2-terminal protein kinases (JNK), and p38. GdCl3 was also shown to activate caspase 9 under these experimental conditions.
In parallel, another work was published on isolated rat hearts subjected to an ischemia/reperfusion protocol [204]. By using GdCl3 as CaR agonist, the authors suggested that an increased CaR expression is involved in the induction of cardiomyocytes apoptosis via Ca2+ overload-induced cytochrome C/caspase 3 axis activation. Later, Sun and colleagues suggested, by using the same experimental protocol, that one of the molecular players involved in CaR-mediated apoptosis in neonatal cardiomyocytes is the Ca2+ channel TRPC6 [205], whose expression was found, like the CaR, to be increased after Gd3+ stimulation. Similarly, an increase of TRPC3 expression was described in neonatal rat cardiomyocytes in a following paper, although no evaluation of apoptosis was performed in this study [206].
The effects of known proapoptotic substances were also investigated. Ciclosporin A (CsA) was described to exert its adverse effects (also) by increasing CaR expression both in vitro [207] and in vivo [208].
In neonatal rat cardiomyocytes [207], the proapoptotic role of the CaR in CsA-induced apoptosis was investigated by using Gd3+ and NPS-2390 as CaR agonist and inhibitor, respectively. While in the neonatal cell model the CsA-induced CaR-mediated cell death was attributed to Ca2+ overload [207], in a similar work performed two years later on H9c2 cardiomyoblasts the involvement of the MAP kinases pathway was suggested [209]. In the same year, the CaR was also implicated in lipopolysaccaride (LPS)-induced apoptosis via its increased expression and consequent Ca2+ overload and TNF alpha and IL-6 release [210].
Role of the CaR in Apoptotic Pathways Activated by Ischemia/Reperfusion
During the years 2006–2011, some of the same researchers from Harbin University made a considerable effort to disclose the involvement of the CaR in the apoptotic pathway activated by ischemia/reperfusion (I/R) or hypoxia/reoxigenation protocols both on in vivo and in vitro models, i.e., neonatal rat cardiomyocytes [211,212,213,214], isolated adult rat cardiomyocytes [204,215], and adult rat hearts [212,215]. Altogether, the collected results suggest that I/R induces a significant increase of CaR expression. Subsequent CaR-mediated signalling pathways activate apoptosis. The involvement of mitochondria in such an effect was suggested to be mediated by Ca2+ overload via inter-organelle signalling [213], ER stress [212], and PKC delta [216]. Activation of the C-Jun NH2 terminal protein kinase signalling pathway was also described as one of the mechanisms relevant for CaR-mediated injury during ischemia/reperfusion in adult rats [215].
Later, another group at the Nanjing University confirmed the work of Jiang et al. [211,217] and implicated a downregulation of CaR expression and signalling in the anti-apoptotic effect of hepatocyte growth factor in neonatal cardiomyocytes subjected to simulated ischemia/reperfusion [218].
Role of CaR Inhibition in Post-Conditioning
Almost in the same years, Zhang et al. [219] suggested on isolated rat hearts subjected to an ischemia/reperfusion protocol followed by post-conditioning (PC) that PKCε is involved in the protective effect of the PC maneuver via its negative feedback on the CaR and the subsequent reduction of intracellular Ca2+. Also, in these studies GdCl3 + NiCl2 + CdCl2 was the only experimental maneuver used to activate the CaR.
Similar results were obtained some years later on the neonatal rat cardiomyocytes model, this time using also the calcilytic NPS 2390 and implicating the attenuation of CaR-induced sarcoplasmic reticulum–mitochondria crosstalk in the protective effects of PKCε during post-conditioning [220].
In 2012, in a work performed in vivo and on isolated adult rat cardiomyocytes subjected to a hypoxia post-conditioning protocol, the authors suggested that the pro-apoptotic action of CaR exerted via ER stress activation was counteracted during post-conditioning by a reduction of CaR protein expression [221]. Very recently, Zhang and colleagues confirmed that ischemic post-conditioning and KATP channel agonists diazoxide and pinacidil exert a protective effect against ischemia/reperfusion injury through a mitigation of Ca2+ overload induced by downregulation of the CaR [222].
Given the relevance of CaR expression levels for the rationale of these studies, it is worth mentioning a recent short communication from the group of Schluter [223] focused on the assessment of CaR expression levels after ischemia reperfusion and cardiac pre- and post-conditioning. This report pointed out that different CaR regulation patterns seem to exist between left and right ventricular tissues and that also the size of infarcts could be a determinant in regulating CaR expression [223].
Role of the CaR in Pre-Conditioning
In opposition to the theory of a deleterious action of the CaR in the heart, there was already in 2010 the work by Sun and Murphy in which the authors suggested a cardioprotective role for the CaR, strategically localized in caveolae, during ischemic pre-conditioning (IPC) [224].
In this study, the perfusion of adult rat hearts with the CaR antagonist NPS 2143 during the application of the ischemic pre-conditioning protocol was indeed able to attenuate cardioprotection of IPC, as assessed by measuring the levels of the cardioprotective phospho ERK1/2, AKT, and GSK3β kinases, infarct size, and functional recovery. The suggestive speculation of the authors depicts a scenario in which the changes in pH and ionic strength imaginable at the particular microdomains of caveolae may activate the CaR, which could thus “sense” and “mediate” IPC.
Another controversial topic is the role of CaR activation in the progress of diabetic cardiomyopathy [225,226].
On the one hand, Bai et al. suggested that CaR expression is reduced in diabetic rat hearts and could contribute to the progress of diabetic cardiomyopathy via impaired intracellular Ca2+ signalling in adult cardiac cells [225]. On the other hand, Qi and colleagues showed that CaR expression is rather increased in diabetic rat hearts and by using Gd3+, NPS 2390, and a siRNA to silence CaR protein, suggested that CaR signalling contributes to apoptosis of neonatal cardiomyocytes exposed to high concentrations of glucose in vitro via Ca2+ overload, reduction of the Bcl2/Bax ratio protein expression, and modulation of the MAP kinase pathway [226]. The apparent discrepancy of results on CaR expression was explained by invoking a difference in the experimental models which, according to Qi et al., were representative of type 2 diabetes in the first work [225] and type 1 diabetes in the latter [226].
Role of the CaR in Hypertrophy and Heart Failure
A role for the CaR in cardiac hypertrophy has been postulated since 2006, when Tfelt-Hansen and collaborators [178] observed a CaR-induced decrease in DNA synthesis in neonatal cardiomyocytes. Considering that this parameter increases in cells undergoing hypertrophy [227], the authors supposed a protective role for the CaR against cardiac hypertrophy.
In opposition to this theory, in a study performed by Wang and colleagues [228] on an in vitro model of hypertrophy, an increased expression of the CaR was found and exacerbating effects of Gd3+ on Ca2+ signalling and hypertrophic markers were argued to be mediated by the CaR.
Later, Zhong et al. suggested a role for the CaR in the Gd3+-induced increase of nuclear Ca2+ spikes’ frequency and consequent activation of the Calcineurin/NFAT pathway [229], a well-known player in cardiac hypertrophy [230].
The role of the CaR in cardiac hypertrophy and heart failure was further investigated in a successive work [231], in which Calindol and Calhex 231, as agonist and inhibitor of the CaR, respectively, were subcutaneously injected in isoproterenol-treated Wistar rats to assess the role of the CaR in ER stress in vivo. CaR expression was found to significantly increase during heart failure induced by isoproterenol and Chalex 231 significantly reduced the cross-sectional diameter of hypertrophic cardiomyocytes. In the same study, the CaR was suggested to play a role in cardiac hypertrophy and heart failure also in mice subjected to thoracic aorta constriction (TAC) [231]. In this model, Calindol was found to induce significant cardiac hypertrophy.
The involvement of the CaR in ER stress-mediated apoptosis in failing hearts was also suggested by the observation that Calindol and Chalex 231 were able to increase and decrease, respectively, ER stress protein expression and apoptosis in both the rat and mouse models. Finally, a CaR-induced IP3-dependent Ca+ overload of mitochondria and subsequent initiation of apoptosis were suggested in rat hearts and neonatal rat cardiomyocytes incubated for 48 h with isoproterenol. On these bases, the authors proposed that the CaR could represent a putative target for pharmacological intervention in heart failure.
Very recently, Liu et al. suggested the possibility to ameliorate isoproterenol-induced cardiac hypertrophy through suppression of CaR signalling with the CaR inhibitor Chalex 231 both in vivo and in vitro [232]. In this paper, the authors suggested that the anti-hypertrophic effect and amelioration of cardiac function by Chalex 231 may be mediated through inhibition of autophagy and the CaMKK-AMPK-mTOR signalling pathway.
A recent in vitro and in vivo study also suggested a role for the CaR in promoting cardiac fibrosis [233].
In opposition to a maladaptive pro-hypertrophic effect of the CaR, which is the common denominator of the above-described studies, there are recent contributions from the group of Schluter [223] which sustain the theory of a cardioprotective effect of the CaR in a pharmacological model of hypertension in adult rats. Interestingly, these authors showed that upon NO-synthase inhibition an increased expression of endothelin A and/or B1 receptors and CaR was measurable. This upregulation of CaR, most probably mediated by a paracrine action of endothelin 1, was suggestive of a CaR-induced adaptive hypertrophy. Indeed, CaR inhibition or destabilization by NPS 2390 and siRNA against RAMP, respectively, decreased load-free cell shortening, which was used as a read-out of cardiac function.
Role of the CaR on the Electrical Properties of Cardiac Cells
Notwithstanding the abrupt proliferation of literature on the role of the CaR in the heart in the last 15 years, very few data were available, until recently, on the effect of CaR agonists and modulators on the electrical properties of cardiac cells.
In very recent times, the group of Schluter [234] and Shieh [235] have published interesting data on this topic on adult rat hearts and guinea pig cardiomyocytes, respectively, by using different physiologically relevant experimental approaches.
First, Schreckenberg and colleagues examined the effects of CaR acute stimulation on the cardiac physiology of adult male rats [234]. The experimental approaches were diverse: the contractile response of ventricular cardiomyocytes was recorded with a cell-edge-detection system in parallel with Fura-2 measurements of cytosolic Ca2+, while cardiac performance in response to acute CaR stimulation or inhibition was assessed on left ventricle muscle stripes and whole hearts by the Langendorff system.
Putrescine and Gd3+ were used as CaR agonists, while NPS-2390 (10/100 µM) and downregulation of CaR by siRNA were used to assess the specificity of the responses.
The data collected, while confirming the expression of CaR by adult rat ventricular cardiomyocytes, demonstrated that CaR action is relevant for basal cell shortening at physiological extracellular Ca2+ concentrations and that additional activation of the CaR speeds up cardiomyocytes relaxation and augments cell shortening, supposedly via increased Ca2+ transients induced by activation of the Gq/PLC/IP3 pathway.
Very interestingly, Liu et al. clearly demonstrated that activation of the CaR in guinea pig cardiomyocytes by the allosteric agonist NPS R 568 activates both the PLC and phsphatidylinositol-4 kinase (PI4) Kinase pathways and that the PI4 Kinase pathway predominates [235]. The net effect is an increase in PIP2 at the plasma membrane, which in turn determines a significant increase in currents through inward rectifier K+ channels. This action could thus exert a stabilization of the resting membrane potential of cardiomyocytes and contribute to a re-evaluation of old data on the stabilizing and cardioprotective effects of polyamines [236].
Finally, interesting hints on the CaR’s role in the heart come from a paper focused on the function of the CaR in regulating blood vessel tone and blood pressure [237].
Since the experimental model of CaR KO mice (SM22αCaSRΔflox/ΔfloxKO mice) used in this work also display a deletion of the CaR in cardiac cells, the authors tested the hearts of KO mice by perfusion in the Langendorff mode. Given the significantly reduced basal heart rate found in KO mice versus wild-type (WT) animals, the authors suggested that CaR deletion from heart directly affects chronotropy. Also, ex vivo lung slice preparations from KO mice showed a reduced rate of spontaneous activity, suggesting that the decreased chronotropy in KO mice could be due to decreased pacemaker activity.
In summary, notwithstanding the numerous results collected on physio-pathological roles of the CaR in the heart, the current findings are far from providing a coherent picture.
Considering CaR promiscuity and pleiotropicity and the emerging concept of biased signalling at the CaR (see below), an interesting field of future investigation would be the accurate pharmacological assessment of the effects exerted by panels of CaR agonists and modulators on different signalling pathways in physiologically relevant experimental models.
Given the actual and potential use of calcimimetics and calcilytics in the clinic, these studies would be highly significant for their implication for human health.
4.6. Novel Trafficking/Signaling Modes of the CaR
Although GPCR have often been seen as cell surface proteins able to go through a simple on-off switch, research of the past few years has drawn a much more complex scenario, in which receptors exist in multiple conformations and, in dependence of the different ligands, can be stabilized in different conformational states, in turn activating multiple, yet specifically selected, downstream pathways whose identity depends on the combination of ligands and the specific signalling toolkit of the cell where they are expressed [238,239,240,241,242].
The development of new technologies, such as microscopy techniques and probes to follow receptor trafficking and subcellular signal dynamics (such as FRET and single molecule microscopy) as well as BRET-based high throughput assays for biased signalling [243], have been essential to demonstrate that these proteins are capable of trafficking among a variety of subcellular compartments, initiating specific signalling pathways at different locations: nuclear [244], mitochondrial [245], and endosomal–Golgi membrane [246,247].
As stated in the paragraph regarding the structural features of the CaR, functional receptors localized at the plasma membrane are dimers of the glycosilated CaR which are formed intracellularly [248] and are stabilized by ligand binding at the cell surface [249]. As for other GPCRs, the expression level of CaR is the result of a fine regulation of the dynamic equilibrium between mechanisms that precede its biosynthesis (transcription/mRNA level, translation efficiency, epigenetic control [250,251]) and regulate its anterograde trafficking and those controlling its retrograde trafficking, including degradation. In fact, it has been known since a long time that the fine balance between maturation, internalization, recycling, and degradation can influence the net level of cell surface receptor and thus represent a mechanism for the cell to regulate receptor desensitization and modulate strength of signal transduction [252]. Thus, the strength of signaling depends upon the level of GPCRs expression at the plasma membrane and the consequent receptor availability for agonists and modulators. This is also the case for CaR signalling as recently established by Conigrave’s group [253].
A significant contribution to the field of CaR trafficking has come in the last ten years from different groups who have focused on the elucidation of the key steps involved in CaR biosynthesis and trafficking from the ER membrane to the Golgi membrane and then to the plasma membrane (see [254] for a recent review). A fundamental role in this field is also played by research focused on the CaR-interacting proteins, which finely modulate anterograde and retrograde trafficking of the CaR (for comprehensive reviews see [10,133,254,255,256]).
The Agonist-Driven Insertional Signalling (ADIS)
Both early and recent studies have revealed two peculiar characteristics of the CaR: a minimal functional desensitization (both by phosphorylation and/or β-arrestins) and a significant “reservoir” of CaR proteins in intracellular membrane compartments. Western blotting or immunohistochemistry analyses [11,76,257] have clearly showed, since early studies, that CaR immunoreactivity is predominantly represented by an intracellular, core-glycosilated form. Recent data are making increasingly clear that such an observation is not an artifact but is strictly related to the complex interaction between CaR trafficking and signalling. In fact, both the hallmarks of the CaR, minimal desensitization and high levels of intracellular proteins, can be explained by a novel signalling mode described by the group of Dr. Breitwieser and termed Agonist-driven insertional signalling (ADIS) [250,258].
In this model, CaR signalling is regulated by an agonist-dependent modulation of the net amount of receptor proteins at the plasma membrane. In particular, the level of CaR cell-surface expression results from the balance between the rate of trafficking from a large, pre-plasma membrane pool located at the Golgi/trans-Golgi/post-Golgi vesicles (which depends upon the concentration of CaR agonists and/or allosteric modulators) and the process of constitutive endocytosis of the receptor already at the plasma membrane. The sensitivity of parathyroid and renal tubular cells to extracellular Ca2+, for example, is likely influenced by the balance between ADIS, which enhances anterograde trafficking of newly synthesized CaR proteins to the plasma membrane [258] and clathrin-mediated endocytosis and retrograde trafficking of cell-surface CaR receptors [10].
4.7. Synthetic Allosteric Modulators of the CaR Can Function as Pharmacoperones
Strictly related to the research in the field of CaR trafficking is the observation that CaR allosteric modulators (see Paragraph 4.3) can work as pharmacoperones. Pharmacoperones are permeant substances (which could be agonists, antagonists, or allosteric modulators) that can reach a misfolded protein at its intracellular location (most commonly the endoplasmic reticulum), stabilize it, and rescue the receptor to the cell membrane surface.
A relevant effort on the capacity of CaR allosteric modulators to work as pharmacoperones comes from Breitwieser’s group [259,260,261]. These authors firstly demonstrated that the positive allosteric modulator NPS-R-568 was able to rescue almost fifty percent of the loss-of-function mutant CaRs analyzed [259,260]. In parallel, another group demonstrated the same ability of NPS-R-568 to rescue intracellular signalling in four mutants (out of seven) although the plasma membrane localization of the receptors was not assessed [262].
In the last five years, relevant findings in the field of pharmacoperone action of type II calcimimetics and calcilytics have been provided by studies from the group of Dr. Leach. These authors, while confirming data on NPS-R-568, showed the ability of cinacalcet, the only CaR modulator approved in the clinic, to effectively rescue trafficking and signalling of CaR mutants with loss of cell surface expression. [128,263]. They also showed that NPS-2143, a negative allosteric modulator, is able to positively modulate CaR trafficking to the cell membrane while negatively modulating CaR signalling in the presence of the orthosteric substrate [144,263], underlining a striking example of positive bias (see also above) toward trafficking by a CaR allosteric modulator.
Nonetheless, both early and recent studies suggest that the pharmacoperone action of NPS R-568 [264] and NPS-2143 [259,264] is mutant-specific.
Importantly, the effectiveness of old and new calcilytics on gain-of-function mutations of the CaR was further explored in vitro [265,266], demonstrating that a novel treatment option for patients with activating CaR mutations could be represented by the new quinazolinone-derived calcilytics, which have been shown to be effective in attenuating enhanced cytosolic Ca2+ signalling in four known mutations causing Bartter syndrome (BS) type 5 and autosomal dominant hypocalcemia (ADH). Very interestingly, recent in vivo works involving a mouse model harboring an ADH gain-of-function CaR mutation demonstrated the effectiveness of both old (i.e., NPS 2143) [267] and new (i.e., JTT-305/MK-5442) [268] calcilytics in normalizing the defect and improving the associated hypocalcemia.
4.8. Biased Signalling
Biased signalling (also termed biased agonism or ligand-directed signalling or stimulus bias) represents a recently described, yet quite general, modality of the signalling characteristic of diverse GPCRs [240].
It concerns the capability of different ligands to stabilize diverse conformations of the receptor and thus preferentially head GPCR signalling towards a specific transduction cascade.
This new concept, which substitutes the idea of one receptor/one GPCR conformation/one-uniform activation of signaling pathway(s), greatly complicates the scenario of GPCRs signalling, but opens new perspectives in the design of smart and tissue-specific drugs that avoid off-target actions or on-target effects on multiple tissues [269].
Given the promiscuity and pleiotropicity of the CaR and the wide expression of this receptor in tissues involved or not in the regulation of systemic Ca2+, it is not surprising that fundamental insights into this new ligand- and tissue-specific pharmacology come from the study of biased signalling at the CaR.
In fact, the existence of ligand- and tissue-specific effects in the signalling pathways activated by the CaR, although not precisely quantified/measured, are traceable in a vast number of papers published throughout the years.
It could also be hypothesized that the experimental approaches used in the recent past could have contributed to an underestimation of the phenomenon of biased signalling at the CaR. Most of the work on CaR signalling is in fact performed by testing only a small number of CaR agonists and modulators on a limited array of signal transduction cascades (most frequently cytosolic Ca2+ dynamics). A striking example of biased signalling at the CaR comes from an acquired hypocalciuric hypocalcemia autoantibody, which was shown to increase the effects of external Ca2+ on Gq signalling to the detriment of the Gi-induced signalling pathway [270]. As far as the differential effects of CaR agonists and modulators are concerned, both early studies by Riccardi’s group on rat pancreas [271] and more recent work by Ziegelstein and colleagues on human aortic endothelial cells showed that only some CaR ligands were able to induce intracellular Ca2+ release, while others were ineffective [160]. Also, at the level of physiological functions, Tfelt-Hansen’s group demonstrated that while cinacalcet was able to induce vasodilatation of rat aorta, the neomycin and Gd3+ were ineffective [161].
In the last years, a large number of findings has highlighted the physiopathological significance of biased signalling.
A major contribution in this area has come from Bräuner-Osborne’s group [145,272,273]. Specifically, these authors explored the action of a panel of well-known orthosteric CaR agonists on the Gq/11 protein, Gi/o protein, and extracellular signal-regulated kinases 1 and 2 (ERK1/2) pathways. Thomsen and colleagues [145] revealed that in HEK-293 cells stably transfected with rat CaR, the natural agonist Ca2+ is biased toward cAMP inhibition and IP3 accumulation over ERK1/2 phosphorylation. Differently, a significant bias toward ERK1/2 phosphorylation was shown for the polyamine spermine.
Interestingly, while two different groups showed that strontium ranelate, a drug used as treatment for osteoporosis, is a biased agonist of the CaR toward the ERK1/2 pathway and another cascade independent of Gq/11 signalling [273,274], different results were reported by others [275]. In another study, while both Sr2+ and Ca2+ were shown to stimulate PLC and NF-kB, only strontium ranelate induced apoptosis via PKC activation [276].
In recent years, the possibility of exploiting and “inducing” stimulus bias at the CaR has been deeply explored also by the group of Christopoulos and Leach [95,263,277,278] (reviewed in [144,269]). The first quantitative evidence that an allosteric modulator used in clinical practice exhibits stimulus bias was provided a few years ago by Davey and colleagues [277], which straightforwardly proved that allosteric modulators are biased toward intracellular Ca2+ signalling in a HEK293-TREx c-myc-CaR cell line.
Next, the same group analyzed in detail the effect of CaR allosteric modulators on naturally occurring CaR mutations [278], demonstrating that mutations can alter signalling bias by stabilizing receptor conformations that differentially couple to downstream signalling pathways. Importantly, and as stated above, in a subsequent paper, both Cinacalcet and the calcylitic NPS-2143 were demonstrated to have a bias toward Ca2+ signalling and rescue CaR mutants to the cell membrane [263]. The therapeutic implications of such data are enormous.
Just as an example, the calcimimetic cinacalcet has been used in clinic for the treatment of patients carrying loss-of-function CaR mutations only in the cases of end-stage renal disease. This is because of its hypocalcemic properties, most probably due to the Ca2+-mediated increase of calcitonin secretion [272]. It is thus clear that designing a novel drug able to act on parathyroid glands without affecting calcitonin secretion from parafollicular cells would be the best therapeutic option.
Remarkably, in a very recent paper, the search for a calcimimetic able to suppress PTH levels at concentrations with low/no effect on serum calcitonin seems to have found good candidates among the third generation of calcimimetics [128]. Altogether, these findings highlight the importance of a deeper comprehension of the mechanisms of action of different orthosteric agonists and modulators in the different tissues for the better design of drugs that are devoid of side effects and are able to activate specific pathways in a cell-selective fashion [144,269].
It is worthwhile noting that an even higher level of complexity in the scenario of biased agonism and pharmacoperones action of CaR ligands is the observation that GPCRs have been recently shown to sustain G (Gs or Gi) protein signalling at different subcellular sites even after internalization of ligand–receptor complexes in endosomes (for a recent review see [242]).
5. Old and New Extracellular Ca2+ Sensors
Even though the CaR is so far the best-studied extracellular Ca2+ sensor, other receptors (either membrane proteins or channels) able to change their function following significant fluctuations in extracellular Ca2+ level have been identified [279]. Of note, those of the same family of the CaR have been the focus of intense studies based on a high degree of structural homologies, conservation of specific domains, and a common functional role as nutrient/salinity sensor. This family includes eight metabotropic glutamate receptors (mGluR1–8), two gamma-aminobutyric acid (GABA) receptors, three taste receptors (T1-T3), the recently “deorphanized” GPRC6A, and seven orphan receptors [280].
5.1. Metabotropic Glutamate Receptors
Metabotropic glutamate receptors (mGluRs) are neurotransmitters expressed mainly in the central nervous system, where they modulate key functions in a variety of different neurological processes (e.g., memory, learning, pain, and synaptic plasticity) [281]. mGluRs (especially mGluR1 and -R5) possess significant sensitivity to extracellular Ca2+ [282,283]; however, it is still unclear whether they exist in nature as proper Ca2+ sensors [282] or whether extracellular Ca2+ just sensitizes the receptor to its principal ligand, the excitatory amino acid l-glutamate [284,285]. For example, Ca2+ depletion in the synaptic cleft during burst firing induces significant inhibition of postsynaptic mGluR1 function [286]. In addition, Gerber et al. showed that increasing concentrations of extracellular Ca2+ might enhance the potency/efficacy of glutamate action on mGluRs1 [287]. Recently, a novel potential extracellular Ca2+-binding site was identified within the extracellular domain of the mGluR1 adjacent to the known l-Glu-binding site [288,289]. When both l-Glu and Ca2+ are bound to their partially overlapping binding sites, they synergistically modulate mGluR1 activation. Strikingly, a significant reduction in the sensitivity of mGluR1 to extracellular Ca2+ and, in some cases, to l-Glu is induced by a mutation in the predicted Ca2+-binding residue [290]. Furthermore, this Ca2+-binding site is also adjacent to the interaction sites of orthosteric agonists and antagonists; thus, extracellular Ca2+ might modulate the sensitivity of mGluR1 not only to orthosteric agonists and antagonists but also to allosteric modulators [291].
5.2. The GABAB Receptor
The gamma-aminobutyric acid subtype B (GABAB) receptor plays a key role in regulating membrane excitability and synaptic transmission in the brain. The functional receptor consists of a heterodimer of GABAB-R1 and GABAB-R2 [292]. As for the CaR and mGluRs, the GABAB receptor has the ability to sense extracellular Ca2+ (0.001–1 mM) but not other divalent and trivalent cations. Wise et al. showed that an addition of 1 mM extracellular Ca2+ potentiated GABA stimulation (EC50 was reduced from 72 to 7.7 μM) in membrane preparations from both CHO cells (that stably express the GABAB-R1/R2 heterodimer) and rat brain cortex. Of note, even though Ca2+ does not act as a direct agonist of the receptor, extracellular Ca2+ may allosterically enhance the GABA-induced responses of the GABAB receptor [293].
5.3. Taste Receptors
T1R1 and T1R3 belong to the T1R family and form an heterodimer that is considered a broadly tuned l-amino acid sensor. Silve et al. reported a Ca2+-binding pocket for T1R3. Of note, this receptor subunit is characterized by Ca2+-binding residues that are conserved in the Venus fly trap region of the CaR [294]. Recently, in vivo taste studies have suggested the involvement of T1R3 in Ca2+-Mg2+ taste in mice and humans [295,296]. These studies have also supposed that T1R3 might form a dimer with another receptor able to sense extracellular Ca2+, such as the CaR.
5.4. The G Protein-Coupled Receptor Family C Group 6 Member A (GPRC6A)
The G protein-coupled receptor family C group 6 member A (GPRC6A) displays the closest mammalian homology to the CaR. This receptor, cloned in 2004, is extensively expressed at the tissue level [297] while limited information is available at the sub-tissue level. So far, experiments with KO mice for the receptor have shown GPCR6 involvement in the regulation of biological processes such as inflammation, metabolism, and hormonal functions. GPCR6 is activated by a large number of basic and small aliphatic l-α-amino acids (being l-arginine, l-lysine, and l-ornithine the most potent compounds). In addition, high extracellular Ca2+ levels (5–10 mM) either directly activate or positively modulate the receptor. This range of extracellular Ca2+ concentration can be physiological only in a tissue, such as the bone, where the receptor seems to be involved in bone remodelling processes [298]. However, it remains unclear which of the proposed agonists are the most important physiologically. Furthermore, CaR allosteric modulators, such as NPS568, Calindol, and NPS2143 (IC50~10 μM), were shown to be effective also on GPRC6A [67,298,299].
5.5. Notch Receptor
The epidermal growth factor (EGF)-like domain contains a Ca2+-binding domain with a wide range of Ca2+ affinities [300]. This type of module is probably the most prevalent extracellular Ca2+-binding site. Among the proteins containing Ca2+-binding EGF-like domains, there are proteins involved in blood coagulation, fibrinolysis and its complement system, matrix proteins, and the developmentally important cell surface receptors Notch [300]. Left–right asymmetry in all vertebrates is established during embryo development by several different molecular events. Raya et al., using a combination of modelling and experimental procedures, showed that, in chick embryos, asymmetric patterns of gene expression were the result of an extracellular Ca2+ gradient toward the left margin of the developing embryo. Here, extracellular Ca2+-induced activation of Notch results in the asymmetric secretion of Nodal, a specific signalling molecule [301]. Of note is that the perturbed establishment of the left–right patterning in embryo maturation was induced by the disruption of the extracellular Ca2+ gradient.
5.6. Cadherins
Cadherins are a family of Ca2+-dependent adherent receptors involved in development, morphogenesis, synaptogenesis, differentiation, and carcinogenesis. Most of the members are single membrane-spanning proteins possessing Ca2+-binding extracellular domains with a wide range of Ca2+ affinities (Kd from μM to mM) [302]. The cadherin extracellular domains act as a Ca2+-activated “mechanical switch” through which extracellular Ca2+ fluctuations are detected and integrated to control their mechanical integrity and mechano-transduction capability.
5.7. Stromal Interaction Molecule 1 (STIM1)
Ca2+-binding proteins containing EF-hand domains are present in the extracellular environment or matrix. These proteins do also localize at the plasma membrane, with the EF-hand domain facing the extracellular medium [303,304]. An important example of this class of proteins is the single transmembrane-spanning Ca2+-binding protein, STIM1, generally known as the endoplasmic reticulum Ca2+ sensor, which provides the trigger for store-operated channels (SOCs) activation. STIM1 at the cell surface may represent a fundamental pharmacological target to finely tune Ca2+-regulated functions that are mediated by SOCs.
5.8. Hemichannels
Gap junctions are constituted by the juxtaposition of two membrane-spanning connexons (hemichannels) positioned end-to-end. However, functionally active hemichannels exist at the plasma membrane where they allow the rapid bi-directional movement of ions, second messengers, and metabolites between the cytosol and the extracellular space and vice-versa [305]. Opening of most hemichannels depends critically on decreases in extracellular Ca2+ levels (of about ~200 μM) around the physiological Ca2+ levels [306,307]. The vestibule of Connexin 32 has a Ca2+-binding site involved in the regulatory effect of external Ca2+. Of note is that it has been shown that the complete Ca2+ deregulation of connexin 32 hemichannel activity is induced by a mutation causing an inherited peripheral neuropathy. This evidence led to the assumption that the pathogenesis of the neuropathy may be strictly related to this dysfunction [308]. The pore of connexin 43 hemichannels might be in an open or closed conformation, with the open probability significantly dependent on external Ca2+ levels (from 1.8 to 1.0 mM) [309].
5.9. Ion Channels
Dynamic changes in extracellular Ca2+ are known to impact on a number of ion channels, especially those located in the brain.
Ca2+ homeostasis modulator 1 (CALHM1) is an ion channel expressed at the cell membrane of cortical neurons and type II taste bud cells of the tongue [310]. This peculiar channel, closed at resting membrane potential and modulated by significant membrane depolarizations, is also able to sense a decrease in extracellular Ca2+ that thus works, in synergy with voltage, to allosterically increase the CALHM channel gating open probability.
Interestingly, Ca2+ and other cations, anions, and ATP are able to permeate through the non-specific pore of CALHM1 [310].
Acid-sensing ion channels (ASICs) are trimeric cationic channels activated by extracellular protons and primarily expressed in the nervous system. These channels are permeable to Na+ and Ca2+ and “sense” extracellular Ca2+, which modulates their open probability by competing with protons [311,312,313]. In addition, two negatively charged residues near the entrance of the channel pore have been shown to be critical for the direct Ca2+ blockade of ASIC1a currents [313]. Of note is that these channels seem to be also modulated by small fluctuations in extracellular Zn2+, Mg2+, and spermine [311,314].
Another class of ion channels modulated by dynamic changes of extracellular [Ca2+] are the voltage-gated Human ether-à-go-go-related gene (HERG) encoded K+ channels, which have been shown to be implicated in a variety of physiopathological mechanisms, such as neuronal spike frequency adaptation and tumor cell growth [315]. Johnson et al. showed that small changes in extracellular Ca2+ under physiological conditions shift the voltage dependence of channel activation to more positive membrane potentials, allowing for fine-tuned cell repolarization [316]. Under this scenario, HERG channels seem to be responsible for a delayed rectifier K+ current functional to cellular repolarization of the cardiac contractile cycle in the heart [317].
Finally, the Transient receptor potential cation channel, subfamily M, member 7 (TRPM7) was shown to sense a reduction of extracellular Ca2+ in hippocampal and pyramidal neurons and aid an inward cation current [318,319,320]. These 36-pS channels (originally named Ca2+-sensing nonselective cation channels, IcsNSC), which are almost silent at the 2.0 mM extracellular Ca2+ level, become robustly activated by small decreases in extracellular Ca2+ [321].
The history of research on the CaR’s role in physiology and pathological states is a perfect example of the relevance of basic research for ameliorating human health. It is in fact clear that none of the clinically relevant discoveries in the pharmacological field today could have ever taken place without a deep knowledge of the specific mechanisms underlying the functionality of the tissues and organs on whom CaR mutations have their more striking effects.
It is also clear that the novel methodological tools available to researchers in the field of GPCRs and signal transduction will be of paramount importance to further improve the knowledge of basic signalling modes, a step that is indispensable for the design of smart drugs able to rescue GPCR defects in human diseases.
Acknowledgments
The authors gratefully acknowledge the contribution and support of all the colleagues and students who participated in the studies described in the present article.
Abbreviations
| AA | arachidonic acid |
| AC | adenylate cyclase |
| ATP | adenosine triphosphate |
| CaM | Calmodulin |
| CaMK | Ca2+/calmodulin-dependent protein kinase |
| cAMP | cyclic AMP |
| DAG | Diacylglycerol |
| eNOS | endothelial nitric oxide synthase |
| ER | endoplasmic reticulum |
| ERK 1/2 | extracellullar-signal regulated kinase |
| iNOS | inducible nitric oxide synthase |
| InsP3 | inositol-1,4,5-trisphosphate |
| JNK | Jun amino-terminal kinase |
| MAPK | mitogen-activated protein kinase |
| MEK | MAPK kinase |
| NO | nitric oxide |
| p38 | p38 mitogen-activated protein kinase |
| PA | phosphatidic acid |
| PHP | Pharmacoperones |
| PI3K | phosphatidylinositol 3-kinase |
| PI4K | phosphatidylinositol 4-kinase |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| PKC | protein kinase C |
| PLA2 | phospholipase A2 |
| PLC | phospholipase C |
| PLD | phospholipase D |
| RhoA | Ras homolog gene family, member A |
| SOC | store-operated Ca2+ channel |
Author Contributions
Matilde Colella and Andrea Gerbino contributed equally to this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Rizzuto R., Bernardi P., Pozzan T. Mitochondria as all-round players of the calcium game. Pt 1J. Physiol. 2000;529:37–47. doi: 10.1111/j.1469-7793.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bootman M.D., Fearnley C., Smyrnias I., MacDonald F., Roderick H.L. An update on nuclear calcium signalling. J. Cell Sci. 2009;122:2337–2350. doi: 10.1242/jcs.028100. [DOI] [PubMed] [Google Scholar]
- 4.Medina D.L., Di Paola S., Peluso I., Armani A., De Stefani D., Venditti R., Montefusco S., Scotto-Rosato A., Prezioso C., Forrester A., et al. Lysosomal calcium signalling regulates autophagy through calcineurin and tfeb. Nat. Cell Biol. 2015;17:288–299. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbett E.F., Michalak M. Calcium, a signaling molecule in the endoplasmic reticulum? Trends Biochem. Sci. 2000;25:307–311. doi: 10.1016/S0968-0004(00)01588-7. [DOI] [PubMed] [Google Scholar]
- 6.Brown E.M., Gamba G., Riccardi D., Lombardi M., Butters R., Kifor O., Sun A., Hediger M.A., Lytton J., Hebert S.C. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 7.Brown E.M., Conigrave A.D. Preface. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27:283–284. doi: 10.1016/j.beem.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Zhang C., Miller C.L., Brown E.M., Yang J.J. The calcium sensing receptor: From calcium sensing to signaling. Sci. China Life Sci. 2015;58:14–27. doi: 10.1007/s11427-014-4779-y. [DOI] [PubMed] [Google Scholar]
- 9.Tennakoon S., Aggarwal A., Kallay E. The calcium-sensing receptor and the hallmarks of cancer. Biochim. Biophys. Acta. 2016;1863:1398–1407. doi: 10.1016/j.bbamcr.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Hannan F.M., Olesen M.K., Thakker R.V. Calcimimetic and calcilytic therapies for inherited disorders of the calcium-sensing receptor signalling pathway. Br. J. Pharmacol. 2017 doi: 10.1111/bph.14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown E.M., MacLeod R.J. Extracellular calcium sensing and extracellular calcium signaling. Physiol. Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 12.Hofer A.M. Another dimension to calcium signaling: A look at extracellular calcium. J. Cell Sci. 2005;118:855–862. doi: 10.1242/jcs.01705. [DOI] [PubMed] [Google Scholar]
- 13.Ashby M.C., Tepikin A.V. Polarized calcium and calmodulin signaling in secretory epithelia. Physiol. Rev. 2002;82:701–734. doi: 10.1152/physrev.00006.2002. [DOI] [PubMed] [Google Scholar]
- 14.Belan P., Gerasimenko O., Petersen O.H., Tepikin A.V. Distribution of Ca2+ extrusion sites on the mouse pancreatic acinar cell surface. Cell Calcium. 1997;22:5–10. doi: 10.1016/S0143-4160(97)90084-1. [DOI] [PubMed] [Google Scholar]
- 15.Peng J.B., Brown E.M., Hediger M.A. Apical entry channels in calcium-transporting epithelia. News Physiol. Sci. 2003;18:158–163. doi: 10.1152/nips.01440.2003. [DOI] [PubMed] [Google Scholar]
- 16.Petersen O.H. Localization and regulation of Ca2+ entry and exit pathways in exocrine gland cells. Cell Calcium. 2003;33:337–344. doi: 10.1016/S0143-4160(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 17.Caroppo R., Gerbino A., Debellis L., Kifor O., Soybel D.I., Brown E.M., Hofer A.M., Curci S. Asymmetrical, agonist-induced fluctuations in local extracellular [Ca2+] in intact polarized epithelia. EMBO J. 2001;20:6316–6326. doi: 10.1093/emboj/20.22.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caroppo R., Gerbino A., Fistetto G., Colella M., Debellis L., Hofer A.M., Curci S. Extracellular calcium acts as a “Third messenger” To regulate enzyme and alkaline secretion. J. Cell Biol. 2004;166:111–119. doi: 10.1083/jcb.200310145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerbino A., Fistetto G., Colella M., Hofer A.M., Debellis L., Caroppo R., Curci S. Real time measurements of water flow in amphibian gastric glands: Modulation via the extracellular Ca2+-sensing receptor. J. Biol. Chem. 2007;282:13477–13486. doi: 10.1074/jbc.M610585200. [DOI] [PubMed] [Google Scholar]
- 20.Andersson T., Berggren P.O., Gylfe E., Hellman B. Amounts and distribution of intracellular magnesium and calcium in pancreatic beta-cells. Acta Physiol. Scand. 1982;114:235–241. doi: 10.1111/j.1748-1716.1982.tb06977.x. [DOI] [PubMed] [Google Scholar]
- 21.Nicaise G., Maggio K., Thirion S., Horoyan M., Keicher E. The calcium loading of secretory granules. A possible key event in stimulus-secretion coupling. Biol. Cell. 1992;75:89–99. doi: 10.1016/0248-4900(92)90128-N. [DOI] [PubMed] [Google Scholar]
- 22.Gillot I., Ciapa B., Payan P., Sardet C. The calcium content of cortical granules and the loss of calcium from sea urchin eggs at fertilization. Dev. Biol. 1991;146:396–405. doi: 10.1016/0012-1606(91)90241-T. [DOI] [PubMed] [Google Scholar]
- 23.Hutton J.C., Penn E.J., Peshavaria M. Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem. J. 1983;210:297–305. doi: 10.1042/bj2100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerbino A., Maiellaro I., Carmone C., Caroppo R., Debellis L., Barile M., Busco G., Colella M. Glucose increases extracellular [Ca2+] in rat insulinoma (ins-1e) pseudoislets as measured with Ca2+-sensitive microelectrodes. Cell Calcium. 2012;51:393–401. doi: 10.1016/j.ceca.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Belan P., Gardner J., Gerasimenko O., Gerasimenko J., Mills C.L., Petersen O.H., Tepikin A.V. Isoproterenol evokes extracellular Ca2+ spikes due to secretory events in salivary gland cells. J. Biol. Chem. 1998;273:4106–4111. doi: 10.1074/jbc.273.7.4106. [DOI] [PubMed] [Google Scholar]
- 26.Von Grafenstein H.R., Powis D.A. Calcium is released by exocytosis together with catecholamines from bovine adrenal medullary cells. J. Neurochem. 1989;53:428–435. doi: 10.1111/j.1471-4159.1989.tb07352.x. [DOI] [PubMed] [Google Scholar]
- 27.Thirion S., Stuenkel E.L., Nicaise G. Calcium loading of secretory granules in stimulated neurohypophysial nerve endings. Neuroscience. 1995;64:125–137. doi: 10.1016/0306-4522(94)00414-Z. [DOI] [PubMed] [Google Scholar]
- 28.Kuhtreiber W.M., Gillot I., Sardet C., Jaffe L.F. Net calcium and acid release at fertilization in eggs of sea urchins and ascidians. Cell Calcium. 1993;14:73–86. doi: 10.1016/0143-4160(93)90020-7. [DOI] [PubMed] [Google Scholar]
- 29.Hofer A.M., Gerbino A., Caroppo R., Curci S. The extracellular calcium-sensing receptor and cell-cell signaling in epithelia. Cell Calcium. 2004;35:297–306. doi: 10.1016/j.ceca.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Cohen J.E., Fields R.D. Extracellular calcium depletion in synaptic transmission. Neuroscientist. 2004;10:12–17. doi: 10.1177/1073858403259440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egelman D.M., Montague P.R. Calcium dynamics in the extracellular space of mammalian neural tissue. Biophys. J. 1999;76:1856–1867. doi: 10.1016/S0006-3495(99)77345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vassilev P.M., Mitchel J., Vassilev M., Kanazirska M., Brown E.M. Assessment of frequency-dependent alterations in the level of extracellular Ca2+ in the synaptic cleft. Biophys. J. 1997;72:2103–2116. doi: 10.1016/S0006-3495(97)78853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pumain R., Heinemann U. Stimulus- and amino acid-induced calcium and potassium changes in rat neocortex. J. Neurophysiol. 1985;53:1–16. doi: 10.1152/jn.1985.53.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Rusakov D.A., Fine A. Extracellular Ca2+ depletion contributes to fast activity-dependent modulation of synaptic transmission in the brain. Neuron. 2003;37:287–297. doi: 10.1016/S0896-6273(03)00025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moura A.S. Membrane potential and intercellular calcium during glucose challenge in mouse islet of langerhans. Biochem. Biophys. Res. Commun. 1995;214:798–802. doi: 10.1006/bbrc.1995.2357. [DOI] [PubMed] [Google Scholar]
- 36.Perez-Armendariz E., Atwater I. Glucose-evoked changes in [K+] and [Ca2+] in the intercellular spaces of the mouse islet of langerhans. Adv. Exp. Med. Biol. 1986;211:31–51. doi: 10.1007/978-1-4684-5314-0_3. [DOI] [PubMed] [Google Scholar]
- 37.Cleemann L., Pizarro G., Morad M. Optical measurements of extracellular calcium depletion during a single heartbeat. Science. 1984;226:174–177. doi: 10.1126/science.6091269. [DOI] [PubMed] [Google Scholar]
- 38.Nicholson C., Bruggencate G.T., Steinberg R., Stöckle H. Calcium modulation in brain extracellular microenvironment demonstrated with ion-selective micropipette. Proc. Natl. Acad. Sci. USA. 1977;74:1287–1290. doi: 10.1073/pnas.74.3.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaffe L.F., Nuccitelli R. An ultrasensitive vibrating probe for measuring steady extracellular currents. J. Cell Biol. 1974;63:614–628. doi: 10.1083/jcb.63.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuhtreiber W.M., Jaffe L.F. Detection of extracellular calcium gradients with a calcium-specific vibrating electrode. J. Cell Biol. 1990;110:1565–1573. doi: 10.1083/jcb.110.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamoah E.N., Lumpkin E.A., Dumont R.A., Smith P.J., Hudspeth A.J., Gillespie P.G. Plasma membrane Ca2+-atpase extrudes Ca2+ from hair cell stereocilia. J. Neurosci. 1998;18:610–624. doi: 10.1523/JNEUROSCI.18-02-00610.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pepperell J.R., Kommineni K., Buradagunta S., Smith P.J., Keefe D.L. Transmembrane regulation of intracellular calcium by a plasma membrane sodium/calcium exchanger in mouse ova. Biol. Reprod. 1999;60:1137–1143. doi: 10.1095/biolreprod60.5.1137. [DOI] [PubMed] [Google Scholar]
- 43.Knox R.J., Jonas E.A., Kao L.S., Smith P.J., Connor J.A., Kaczmarek L.K. Ca2+ influx and activation of a cation current are coupled to intracellular Ca2+ release in peptidergic neurons of aplysia californica. Pt 3J. Physiol. 1996;494:627–639. doi: 10.1113/jphysiol.1996.sp021520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith P.J., Hammar K., Porterfield D.M., Sanger R.H., Trimarchi J.R. Self-referencing, non-invasive, ion selective electrode for single cell detection of trans-plasma membrane calcium flux. Microsc. Res. Tech. 1999;46:398–417. doi: 10.1002/(SICI)1097-0029(19990915)46:6<398::AID-JEMT8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 45.Mupanomunda M.M., Ishioka N., Bukoski R.D. Interstitial Ca2+ undergoes dynamic changes sufficient to stimulate nerve-dependent Ca2+-induced relaxation. Am. J. Physiol. 1999;276:H1035–H1042. doi: 10.1152/ajpheart.1999.276.3.H1035. [DOI] [PubMed] [Google Scholar]
- 46.Mupanomunda M.M., Tian B., Ishioka N., Bukoski R.D. Renal interstitial Ca2+ Am. J. Physiol. Renal Physiol. 2000;278:F644–F649. doi: 10.1152/ajprenal.2000.278.4.F644. [DOI] [PubMed] [Google Scholar]
- 47.Hilgemann D.W. Extracellular calcium transients at single excitations in rabbit atrium measured with tetramethylmurexide. J. Gen. Physiol. 1986;87:707–735. doi: 10.1085/jgp.87.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hilgemann D.W., Langer G.A. Transsarcolemmal calcium movements in arterially perfused rabbit right ventricle measured with extracellular calcium-sensitive dyes. Circ. Res. 1984;54:461–467. doi: 10.1161/01.RES.54.4.461. [DOI] [PubMed] [Google Scholar]
- 49.Tepikin A.V., Llopis J., Snitsarev V.A., Gallacher D.V., Petersen O.H. The droplet technique: Measurement of calcium extrusion from single isolated mammalian cells. Pflugers Arch. 1994;428:664–670. doi: 10.1007/BF00374591. [DOI] [PubMed] [Google Scholar]
- 50.Tepikin A.V., Voronina S.G., Gallacher D.V., Petersen O.H. Pulsatile Ca2+ extrusion from single pancreatic acinar cells during receptor-activated cytosolic Ca2+ spiking. J. Biol. Chem. 1992;267:14073–14076. [PubMed] [Google Scholar]
- 51.Tepikin A.V., Voronina S.G., Gallacher D.V., Petersen O.H. Acetylcholine-evoked increase in the cytoplasmic Ca2+ concentration and Ca2+ extrusion measured simultaneously in single mouse pancreatic acinar cells. J. Biol. Chem. 1992;267:3569–3572. [PubMed] [Google Scholar]
- 52.Belan P.V., Gerasimenko O.V., Tepikin A.V., Petersen O.H. Localization of Ca2+ extrusion sites in pancreatic acinar cells. J. Biol. Chem. 1996;271:7615–7619. doi: 10.1074/jbc.271.13.7615. [DOI] [PubMed] [Google Scholar]
- 53.Blatter L.A., Niggli E. Confocal near-membrane detection of calcium in cardiac myocytes. Cell Calcium. 1998;23:269–279. doi: 10.1016/S0143-4160(98)90023-9. [DOI] [PubMed] [Google Scholar]
- 54.Etter E.F., Kuhn M.A., Fay F.S. Detection of changes in near-membrane Ca2+ concentration using a novel membrane-associated Ca2+ indicator. J. Biol. Chem. 1994;269:10141–10149. [PubMed] [Google Scholar]
- 55.Etter E.F., Minta A., Poenie M., Fay F.S. Near-membrane [Ca2+] transients resolved using the ca2+ indicator ffp18. Proc. Natl. Acad. Sci. USA. 1996;93:5368–5373. doi: 10.1073/pnas.93.11.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Luisi A., Hofer A.M. Evidence that Ca2+ cycling by the plasma membrane Ca2+-atpase increases the ‘excitability’ of the extracellular Ca2+-sensing receptor. J. Cell Sci. 2003;116:1527–1538. doi: 10.1242/jcs.00368. [DOI] [PubMed] [Google Scholar]
- 57.Care A.D., Sherwood L.M., Potts J.T., Jr., Aurbach G.D. Perfusion of the isolated parathyroid gland of the goat and sheep. Nature. 1966;209:55–57. doi: 10.1038/209055a0. [DOI] [PubMed] [Google Scholar]
- 58.Sherwood L.M., Potts J.T., Jr., Care A.D., Mayer G.P., Aurbach G.D. Evaluation by radioimmunoassay of factors controlling the secretion of parathyroid hormone. Nature. 1966;209:52–55. doi: 10.1038/209052a0. [DOI] [PubMed] [Google Scholar]
- 59.Lopez-Barneo J., Armstrong C.M. Depolarizing response of rat parathyroid cells to divalent cations. J. Gen. Physiol. 1983;82:269–294. doi: 10.1085/jgp.82.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shoback D., Thatcher J., Leombruno R., Brown E. Effects of extracellular Ca2+ and Mg2+ on cytosolic Ca2+ and pth release in dispersed bovine parathyroid cells. Endocrinology. 1983;113:424–426. doi: 10.1210/endo-113-1-424. [DOI] [PubMed] [Google Scholar]
- 61.Brown E., Enyedi P., LeBoff M., Rotberg J., Preston J., Chen C. High extracellular Ca2+ and Mg2+ stimulate accumulation of inositol phosphates in bovine parathyroid cells. FEBS Lett. 1987;218:113–118. doi: 10.1016/0014-5793(87)81029-3. [DOI] [PubMed] [Google Scholar]
- 62.Kifor O., Brown E.M. Relationship between diacylglycerol levels and extracellular Ca2+ in dispersed bovine parathyroid cells. Endocrinology. 1988;123:2723–2729. doi: 10.1210/endo-123-6-2723. [DOI] [PubMed] [Google Scholar]
- 63.Shoback D.M., McGhee J.M. Fluoride stimulates the accumulation of inositol phosphates, increases intracellular free calcium, and inhibits parathyroid hormone release in dispersed bovine parathyroid cells. Endocrinology. 1988;122:2833–2839. doi: 10.1210/endo-122-6-2833. [DOI] [PubMed] [Google Scholar]
- 64.Shoback D.M., Membreno L.A., McGhee J.G. High calcium and other divalent cations increase inositol trisphosphate in bovine parathyroid cells. Endocrinology. 1988;123:382–389. doi: 10.1210/endo-123-1-382. [DOI] [PubMed] [Google Scholar]
- 65.Nemeth E.F., Scarpa A. Are changes in intracellular free calcium necessary for regulating secretion in parathyroid cells? Ann. N. Y. Acad. Sci. 1987;493:542–551. doi: 10.1111/j.1749-6632.1987.tb27239.x. [DOI] [PubMed] [Google Scholar]
- 66.Foord S.M., Bonner T.I., Neubig R.R., Rosser E.M., Pin J.P., Davenport A.P., Spedding M., Harmar A.J. International union of pharmacology. Xlvi. G protein-coupled receptor list. Pharmacol. Rev. 2005;57:279–288. doi: 10.1124/pr.57.2.5. [DOI] [PubMed] [Google Scholar]
- 67.Clemmensen C., Smajilovic S., Wellendorph P., Brauner-Osborne H. The GPCR, class c, group 6, subtype a (GPRC6A) receptor: From cloning to physiological function. Br. J. Pharmacol. 2014;171:1129–1141. doi: 10.1111/bph.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norskov-Lauritsen L., Brauner-Osborne H. Role of post-translational modifications on structure, function and pharmacology of class c g protein-coupled receptors. Eur. J. Pharmacol. 2016;763:233–240. doi: 10.1016/j.ejphar.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 69.Garrett J.E., Capuano I.V., Hammerland L.G., Hung B.C., Brown E.M., Hebert S.C., Nemeth E.F., Fuller F. Molecular cloning and functional expression of human parathyroid calcium receptor cdnas. J. Biol. Chem. 1995;270:12919–12925. doi: 10.1074/jbc.270.21.12919. [DOI] [PubMed] [Google Scholar]
- 70.Hu J., Spiegel A.M. Structure and function of the human calcium-sensing receptor: Insights from natural and engineered mutations and allosteric modulators. J. Cell. Mol. Med. 2007;11:908–922. doi: 10.1111/j.1582-4934.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kunishima N., Shimada Y., Tsuji Y., Sato T., Yamamoto M., Kumasaka T., Nakanishi S., Jingami H., Morikawa K. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407:971–977. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- 72.Muto T., Tsuchiya D., Morikawa K., Jingami H. Structures of the extracellular regions of the group ii/iii metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA. 2007;104:3759–3764. doi: 10.1073/pnas.0611577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geng Y., Xiong D., Mosyak L., Malito D.L., Kniazeff J., Chen Y., Burmakina S., Quick M., Bush M., Javitch J.A., et al. Structure and functional interaction of the extracellular domain of human GABAB receptor GBR2. Nat. Neurosci. 2012;15:970–978. doi: 10.1038/nn.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chakravarti B., Chattopadhyay N., Brown E.M. Signaling through the extracellular calcium-sensing receptor (CASR) Adv. Exp. Med. Biol. 2012;740:103–142. doi: 10.1007/978-94-007-2888-2_5. [DOI] [PubMed] [Google Scholar]
- 75.Hu J., Reyes-Cruz G., Goldsmith P.K., Gantt N.M., Miller J.L., Spiegel A.M. Functional effects of monoclonal antibodies to the purified amino-terminal extracellular domain of the human Ca2+ receptor. J. Bone Miner Res. 2007;22:601–608. doi: 10.1359/jbmr.070111. [DOI] [PubMed] [Google Scholar]
- 76.Bai M., Trivedi S., Brown E.M. Dimerization of the extracellular calcium-sensing receptor (CAR) on the cell surface of car-transfected hek293 cells. J. Biol. Chem. 1998;273:23605–23610. doi: 10.1074/jbc.273.36.23605. [DOI] [PubMed] [Google Scholar]
- 77.Bai M., Trivedi S., Kifor O., Quinn S.J., Brown E.M. Intermolecular interactions between dimeric calcium-sensing receptor monomers are important for its normal function. Proc. Natl. Acad. Sci. USA. 1999;96:2834–2839. doi: 10.1073/pnas.96.6.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gama L., Wilt S.G., Breitwieser G.E. Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons. J. Biol. Chem. 2001;276:39053–39059. doi: 10.1074/jbc.M105662200. [DOI] [PubMed] [Google Scholar]
- 79.Chang W., Tu C., Cheng Z., Rodriguez L., Chen T.H., Gassmann M., Bettler B., Margeta M., Jan L.Y., Shoback D. Complex formation with the type b gamma-aminobutyric acid receptor affects the expression and signal transduction of the extracellular calcium-sensing receptor. Studies with HEK-293 cells and neurons. J. Biol. Chem. 2007;282:25030–25040. doi: 10.1074/jbc.M700924200. [DOI] [PubMed] [Google Scholar]
- 80.Jiang Y., Minet E., Zhang Z., Silver P.A., Bai M. Modulation of interprotomer relationships is important for activation of dimeric calcium-sensing receptor. J. Biol. Chem. 2004;279:14147–14156. doi: 10.1074/jbc.M307422200. [DOI] [PubMed] [Google Scholar]
- 81.Ray K., Hauschild B.C., Steinbach P.J., Goldsmith P.K., Hauache O., Spiegel A.M. Identification of the cysteine residues in the amino-terminal extracellular domain of the human Ca2+ receptor critical for dimerization. Implications for function of monomeric Ca2+ receptor. J. Biol. Chem. 1999;274:27642–27650. doi: 10.1074/jbc.274.39.27642. [DOI] [PubMed] [Google Scholar]
- 82.Reyes-Cruz G., Hu J., Goldsmith P.K., Steinbach P.J., Spiegel A.M. Human Ca2+ receptor extracellular domain. Analysis of function of lobe i loop deletion mutants. J. Biol. Chem. 2001;276:32145–32151. doi: 10.1074/jbc.M102977200. [DOI] [PubMed] [Google Scholar]
- 83.Pace A.J., Gama L., Breitwieser G.E. Dimerization of the calcium-sensing receptor occurs within the extracellular domain and is eliminated by cys → ser mutations at cys101 and cys236. J. Biol. Chem. 1999;274:11629–11634. doi: 10.1074/jbc.274.17.11629. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Z., Sun S., Quinn S.J., Brown E.M., Bai M. The extracellular calcium-sensing receptor dimerizes through multiple types of intermolecular interactions. J. Biol. Chem. 2001;276:5316–5322. doi: 10.1074/jbc.M005958200. [DOI] [PubMed] [Google Scholar]
- 85.Fan G.F., Ray K., Zhao X.M., Goldsmith P.K., Spiegel A.M. Mutational analysis of the cysteines in the extracellular domain of the human Ca2+ receptor: Effects on cell surface expression, dimerization and signal transduction. FEBS Lett. 1998;436:353–356. doi: 10.1016/S0014-5793(98)01165-X. [DOI] [PubMed] [Google Scholar]
- 86.Jacobsen S.E., Gether U., Brauner-Osborne H. Investigating the molecular mechanism of positive and negative allosteric modulators in the calcium-sensing receptor dimer. Sci. Rep. 2018;7:46355. doi: 10.1038/srep46355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu J., Hauache O., Spiegel A.M. Human Ca2+ receptor cysteine-rich domain. Analysis of function of mutant and chimeric receptors. J. Biol. Chem. 2000;275:16382–16389. doi: 10.1074/jbc.M000277200. [DOI] [PubMed] [Google Scholar]
- 88.Pin J.P., Galvez T., Prezeau L. Evolution, structure, and activation mechanism of family 3/c G-protein-coupled receptors. Pharmacol. Ther. 2003;98:325–354. doi: 10.1016/S0163-7258(03)00038-X. [DOI] [PubMed] [Google Scholar]
- 89.Ray J.M., Squires P.E., Curtis S.B., Meloche M.R., Buchan A.M. Expression of the calcium-sensing receptor on human antral gastrin cells in culture. J. Clin. Invest. 1997;99:2328–2333. doi: 10.1172/JCI119413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Awata H., Huang C., Handlogten M.E., Miller R.T. Interaction of the calcium-sensing receptor and filamin, a potential scaffolding protein. J. Biol. Chem. 2001;276:34871–34879. doi: 10.1074/jbc.M100775200. [DOI] [PubMed] [Google Scholar]
- 91.Hjalm G., MacLeod R.J., Kifor O., Chattopadhyay N., Brown E.M. Filamin-a binds to the carboxyl-terminal tail of the calcium-sensing receptor, an interaction that participates in car-mediated activation of mitogen-activated protein kinase. J. Biol. Chem. 2001;276:34880–34887. doi: 10.1074/jbc.M100784200. [DOI] [PubMed] [Google Scholar]
- 92.Bosel J., John M., Freichel M., Blind E. Signaling of the human calcium-sensing receptor expressed in HEK293-cells is modulated by protein kinases a and c. Exp. Clin. Endocrinol. Diabetes. 2003;111:21–26. doi: 10.1055/s-2003-37496. [DOI] [PubMed] [Google Scholar]
- 93.Stepanchick A., McKenna J., McGovern O., Huang Y., Breitwieser G.E. Calcium sensing receptor mutations implicated in pancreatitis and idiopathic epilepsy syndrome disrupt an arginine-rich retention motif. Cell. Physiol. Biochem. 2010;26:363–374. doi: 10.1159/000320560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hendy G.N., Canaff L., Cole D.E. The casr gene: Alternative splicing and transcriptional control, and calcium-sensing receptor (CASR) protein: Structure and ligand binding sites. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27:285–301. doi: 10.1016/j.beem.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 95.Leach K., Gregory K.J., Kufareva I., Khajehali E., Cook A.E., Abagyan R., Conigrave A.D., Sexton P.M., Christopoulos A. Towards a structural understanding of allosteric drugs at the human calcium-sensing receptor. Cell Res. 2016;26:574–592. doi: 10.1038/cr.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang Y., Zhou Y., Yang W., Butters R., Lee H.W., Li S., Castiblanco A., Brown E.M., Yang J.J. Identification and dissection of Ca2+-binding sites in the extracellular domain of Ca2+-sensing receptor. J. Biol. Chem. 2007;282:19000–19010. doi: 10.1074/jbc.M701096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang Y., Zhou Y., Castiblanco A., Yang W., Brown E.M., Yang J.J. Multiple Ca2+-binding sites in the extracellular domain of the Ca2+-sensing receptor corresponding to cooperative Ca2+ response. Biochemistry. 2009;48:388–398. doi: 10.1021/bi8014604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang Y., Zhou Y., Wong H.C., Castiblanco A., Chen Y., Brown E.M., Yang J.J. Calmodulin regulates Ca2+-sensing receptor-mediated Ca2+ signaling and its cell surface expression. J. Biol. Chem. 2010;285:35919–35931. doi: 10.1074/jbc.M110.147918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang C., Zhuo Y., Moniz H.A., Wang S., Moremen K.W., Prestegard J.H., Brown E.M., Yang J.J. Direct determination of multiple ligand interactions with the extracellular domain of the calcium-sensing receptor. J. Biol. Chem. 2014;289:33529–33542. doi: 10.1074/jbc.M114.604652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang C., Huang Y., Jiang Y., Mulpuri N., Wei L., Hamelberg D., Brown E.M., Yang J.J. Identification of an L-phenylalanine binding site enhancing the cooperative responses of the calcium-sensing receptor to calcium. J. Biol. Chem. 2014;289:5296–5309. doi: 10.1074/jbc.M113.537357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang C., Mulpuri N., Hannan F.M., Nesbit M.A., Thakker R.V., Hamelberg D., Brown E.M., Yang J.J. Role of Ca2+ and l-phe in regulating functional cooperativity of disease-associated “Toggle” Calcium-sensing receptor mutations. PLoS ONE. 2014;9:e113622. doi: 10.1371/journal.pone.0113622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang C., Zhang T., Zou J., Miller C.L., Gorkhali R., Yang J.Y., Schilmiller A., Wang S., Huang K., Brown E.M., et al. Structural basis for regulation of human calcium-sensing receptor by magnesium ions and an unexpected tryptophan derivative co-agonist. Sci. Adv. 2016;2:e1600241. doi: 10.1126/sciadv.1600241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang C., Miller C.L., Gorkhali R., Zou J., Huang K., Brown E.M., Yang J.J. Molecular basis of the extracellular ligands mediated signaling by the calcium sensing receptor. Front. Physiol. 2016;7:441. doi: 10.3389/fphys.2016.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brown E.M., Fuleihan G.E.-H., Chen C.J., Kifor O. A comparison of the effects of divalent and trivalent cations on parathyroid hormone release, 3′,5′-cyclic-adenosine monophosphate accumulation, and the levels of inositol phosphates in bovine parathyroid cells. Endocrinology. 1990;127:1064–1071. doi: 10.1210/endo-127-3-1064. [DOI] [PubMed] [Google Scholar]
- 105.Quinn S.J., Ye C.P., Diaz R., Kifor O., Bai M., Vassilev P., Brown E. The Ca2+-sensing receptor: A target for polyamines. Am. J. Physiol. 1997;273:C1315–C1323. doi: 10.1152/ajpcell.1997.273.4.C1315. [DOI] [PubMed] [Google Scholar]
- 106.Brown E.M., Katz C., Butters R., Kifor O. Polyarginine, polylysine, and protamine mimic the effects of high extracellular calcium concentrations on dispersed bovine parathyroid cells. J. Bone Miner Res. 1991;6:1217–1225. doi: 10.1002/jbmr.5650061112. [DOI] [PubMed] [Google Scholar]
- 107.Ye C., Ho-Pao C.L., Kanazirska M., Quinn S., Rogers K., Seidman C.E., Seidman J.G., Brown E.M., Vassilev P.M. Amyloid-beta proteins activate Ca2+-permeable channels through calcium-sensing receptors. J. Neurosci. Res. 1997;47:547–554. doi: 10.1002/(SICI)1097-4547(19970301)47:5<547::AID-JNR10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 108.Brown E.M., Butters R., Katz C., Kifor O. Neomycin mimics the effects of high extracellular calcium concentrations on parathyroid function in dispersed bovine parathyroid cells. Endocrinology. 1991;128:3047–3054. doi: 10.1210/endo-128-6-3047. [DOI] [PubMed] [Google Scholar]
- 109.Nemeth E.F. Regulation of cytosolic calcium by extracellular divalent cations in c-cells and parathyroid cells. Cell Calcium. 1990;11:323–327. doi: 10.1016/0143-4160(90)90033-Q. [DOI] [PubMed] [Google Scholar]
- 110.Colella M., Gerbino A., Hofer A.M., Curci S. Recent advances in understanding the extracellular calcium-sensing receptor. F1000Res. 2016;5 doi: 10.12688/f1000research.8963.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Conigrave A.D., Quinn S.J., Brown E.M. L-amino acid sensing by the extracellular Ca2+-sensing receptor. Proc. Natl. Acad. Sci. USA. 2000;97:4814–4819. doi: 10.1073/pnas.97.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Conigrave A.D., Mun H.C., Lok H.C. Aromatic l-amino acids activate the calcium-sensing receptor. J. Nutr. 2007;137:1524S–1527S; discussion 1548S. doi: 10.1093/jn/137.6.1524S. [DOI] [PubMed] [Google Scholar]
- 113.Conigrave A.D., Hampson D.R. Broad-spectrum amino acid-sensing class c G-protein coupled receptors: Molecular mechanisms, physiological significance and options for drug development. Pharmacol. Ther. 2010;127:252–260. doi: 10.1016/j.pharmthera.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 114.Wang M., Yao Y., Kuang D., Hampson D.R. Activation of family c G-protein-coupled receptors by the tripeptide glutathione. J. Biol. Chem. 2006;281:8864–8870. doi: 10.1074/jbc.M512865200. [DOI] [PubMed] [Google Scholar]
- 115.Broadhead G.K., Mun H.C., Avlani V.A., Jourdon O., Church W.B., Christopoulos A., Delbridge L., Conigrave A.D. Allosteric modulation of the calcium-sensing receptor by gamma-glutamyl peptides: Inhibition of pth secretion, suppression of intracellular camp levels, and a common mechanism of action with l-amino acids. J. Biol. Chem. 2011;286:8786–8797. doi: 10.1074/jbc.M110.149724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Quinn S.J., Bai M., Brown E.M. Ph sensing by the calcium-sensing receptor. J. Biol. Chem. 2004;279:37241–37249. doi: 10.1074/jbc.M404520200. [DOI] [PubMed] [Google Scholar]
- 117.Quinn S.J., Kifor O., Trivedi S., Diaz R., Vassilev P., Brown E. Sodium and ionic strength sensing by the calcium receptor. J. Biol. Chem. 1998;273:19579–19586. doi: 10.1074/jbc.273.31.19579. [DOI] [PubMed] [Google Scholar]
- 118.Nemeth E.F., Steffey M.E., Hammerland L.G., Hung B.C., van Wagenen B.C., DelMar E.G., Balandrin M.F. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc. Natl. Acad. Sci. USA. 1998;95:4040–4045. doi: 10.1073/pnas.95.7.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kiefer L., Leiris S., Dodd R.H. Novel calcium sensing receptor ligands: A patent survey. Expert Opin. Ther. Pat. 2011;21:681–698. doi: 10.1517/13543776.2011.568479. [DOI] [PubMed] [Google Scholar]
- 120.Kiefer L., Beaumard F., Gorojankina T., Faure H., Ruat M., Dodd R.H. Design and synthesis of calindol derivatives as potent and selective calcium sensing receptor agonists. Bioorg. Med. Chem. 2015;24:554–569. doi: 10.1016/j.bmc.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 121.Nemeth E.F., Goodman W.G. Calcimimetic and calcilytic drugs: Feats, flops, and futures. Calcif. Tissue Int. 2016;98:341–358. doi: 10.1007/s00223-015-0052-z. [DOI] [PubMed] [Google Scholar]
- 122.Nemeth E.F. Calcimimetic and calcilytic drugs: Just for parathyroid cells? Cell Calcium. 2004;35:283–289. doi: 10.1016/j.ceca.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 123.Lindberg J.S., Culleton B., Wong G., Borah M.F., Clark R.V., Shapiro W.B., Roger S.D., Husserl F.E., Klassen P.S., Guo M.D., et al. Cinacalcet hcl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: A randomized, double-blind, multicenter study. J. Am. Soc. Nephrol. 2005;16:800–807. doi: 10.1681/ASN.2004060512. [DOI] [PubMed] [Google Scholar]
- 124.Hebert S.C. Therapeutic use of calcimimetics. Annu. Rev. Med. 2006;57:349–364. doi: 10.1146/annurev.med.57.121304.131328. [DOI] [PubMed] [Google Scholar]
- 125.Brown E.M. Clinical utility of calcimimetics targeting the extracellular calcium-sensing receptor (casr) Biochem. Pharmacol. 2010;80:297–307. doi: 10.1016/j.bcp.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 126.Henley C., 3rd, Yang Y., Davis J., Lu J.Y., Morony S., Fan W., Florio M., Sun B., Shatzen E., Pretorius J.K., et al. Discovery of a calcimimetic with differential effects on parathyroid hormone and calcitonin secretion. J. Pharmacol. Exp. Ther. 2011;337:681–691. doi: 10.1124/jpet.110.178681. [DOI] [PubMed] [Google Scholar]
- 127.Ma J.N., Owens M., Gustafsson M., Jensen J., Tabatabaei A., Schmelzer K., Olsson R., Burstein E.S. Characterization of highly efficacious allosteric agonists of the human calcium-sensing receptor. J. Pharmacol. Exp. Ther. 2011;337:275–284. doi: 10.1124/jpet.110.178194. [DOI] [PubMed] [Google Scholar]
- 128.Cook A.E., Mistry S.N., Gregory K.J., Furness S.G., Sexton P.M., Scammells P.J., Conigrave A.D., Christopoulos A., Leach K. Biased allosteric modulation at the cas receptor engendered by structurally diverse calcimimetics. Br. J. Pharmacol. 2015;172:185–200. doi: 10.1111/bph.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nemeth E.F. The search for calcium receptor antagonists (calcilytics) J. Mol. Endocrinol. 2002;29:15–21. doi: 10.1677/jme.0.0290015. [DOI] [PubMed] [Google Scholar]
- 130.Magno A.L., Ward B.K., Ratajczak T. The calcium-sensing receptor: A molecular perspective. Endocr. Rev. 2011;32:3–30. doi: 10.1210/er.2009-0043. [DOI] [PubMed] [Google Scholar]
- 131.Conigrave A.D., Ward D.T. Calcium-sensing receptor (CASR): Pharmacological properties and signaling pathways. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27:315–331. doi: 10.1016/j.beem.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 132.Nemeth E.F., Scarpa A. Rapid mobilization of cellular Ca2+ in bovine parathyroid cells evoked by extracellular divalent cations. Evidence for a cell surface calcium receptor. J. Biol. Chem. 1987;262:5188–5196. [PubMed] [Google Scholar]
- 133.Hannan F.M., Babinsky V.N., Thakker R.V. Disorders of the calcium-sensing receptor and partner proteins: Insights into the molecular basis of calcium homeostasis. J. Mol. Endocrinol. 2016;57:R127–R142. doi: 10.1530/JME-16-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kifor O., MacLeod R.J., Diaz R., Bai M., Yamaguchi T., Yao T., Kifor I., Brown E.M. Regulation of map kinase by calcium-sensing receptor in bovine parathyroid and car-transfected hek293 cells. Am. J. Physiol. Renal Physiol. 2001;280:F291–F302. doi: 10.1152/ajprenal.2001.280.2.F291. [DOI] [PubMed] [Google Scholar]
- 135.Kifor O., Diaz R., Butters R., Brown E.M. The Ca2+-sensing receptor (CAR) activates phospholipases c, a2, and d in bovine parathyroid and car-transfected, human embryonic kidney (HEK293) cells. J. Bone Miner Res. 1997;12:715–725. doi: 10.1359/jbmr.1997.12.5.715. [DOI] [PubMed] [Google Scholar]
- 136.Boudot C., Saidak Z., Boulanouar A.K., Petit L., Gouilleux F., Massy Z., Brazier M., Mentaverri R., Kamel S. Implication of the calcium sensing receptor and the phosphoinositide 3-kinase/AKT pathway in the extracellular calcium-mediated migration of raw 264.7 osteoclast precursor cells. Bone. 2010;46:1416–1423. doi: 10.1016/j.bone.2010.01.383. [DOI] [PubMed] [Google Scholar]
- 137.Li H.X., Kong F.J., Bai S.Z., He W., Xing W.J., Xi Y.H., Li G.W., Guo J., Li H.Z., Wu L.Y., et al. Involvement of calcium-sensing receptor in oxldl-induced MMP-2 production in vascular smooth muscle cells via PI3K/AKT pathway. Mol. Cell. Biochem. 2012;362:115–122. doi: 10.1007/s11010-011-1133-6. [DOI] [PubMed] [Google Scholar]
- 138.de Jesus Ferreira M.C., Helies-Toussaint C., Imbert-Teboul M., Bailly C., Verbavatz J.M., Bellanger A.C., Chabardes D. Co-expression of a Ca2+-inhibitable adenylyl cyclase and of a Ca2+-sensing receptor in the cortical thick ascending limb cell of the rat kidney. Inhibition of hormone-dependent camp accumulation by extracellular Ca2+ J. Biol. Chem. 1998;273:15192–15202. doi: 10.1074/jbc.273.24.15192. [DOI] [PubMed] [Google Scholar]
- 139.Gerbino A., Ruder W.C., Curci S., Pozzan T., Zaccolo M., Hofer A.M. Termination of camp signals by Ca2+ and Gαi via extracellular Ca2+ sensors: A link to intracellular Ca2+ oscillations. J. Cell Biol. 2005;171:303–312. doi: 10.1083/jcb.200507054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beierwaltes W.H. The role of calcium in the regulation of renin secretion. Am. J. Physiol. Renal Physiol. 2010;298:F1–F11. doi: 10.1152/ajprenal.00143.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ward B.K., Magno A.L., Walsh J.P., Ratajczak T. The role of the calcium-sensing receptor in human disease. Clin. Biochem. 2012;45:943–953. doi: 10.1016/j.clinbiochem.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 142.Fitzpatrick L.A., Brandi M.L., Aurbach G.D. Prostaglandin F2 alpha and alpha-adrenergic agonists regulate parathyroid cell function via the inhibitory guanine nucleotide regulatory protein. Endocrinology. 1986;118:2115–2119. doi: 10.1210/endo-118-5-2115. [DOI] [PubMed] [Google Scholar]
- 143.Chen C.J., Barnett J.V., Congo D.A., Brown E.M. Divalent cations suppress 3′,5′-adenosine monophosphate accumulation by stimulating a pertussis toxin-sensitive guanine nucleotide-binding protein in cultured bovine parathyroid cells. Endocrinology. 1989;124:233–239. doi: 10.1210/endo-124-1-233. [DOI] [PubMed] [Google Scholar]
- 144.Leach K., Sexton P.M., Christopoulos A., Conigrave A.D. Engendering biased signalling from the calcium-sensing receptor for the pharmacotherapy of diverse disorders. Br. J. Pharmacol. 2014;171:1142–1155. doi: 10.1111/bph.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thomsen A.R.B., Hvidtfeldt M., Bräuner-Osborne H. Biased agonism of the calcium-sensing receptor. Cell Calcium. 2012;51:107–116. doi: 10.1016/j.ceca.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 146.Huang C., Handlogten M.E., Miller R.T. Parallel activation of phosphatidylinositol 4-kinase and phospholipase c by the extracellular calcium-sensing receptor. J. Biol. Chem. 2002;277:20293–20300. doi: 10.1074/jbc.M200831200. [DOI] [PubMed] [Google Scholar]
- 147.Avlani V.A., Ma W., Mun H.C., Leach K., Delbridge L., Christopoulos A., Conigrave A.D. Calcium-sensing receptor-dependent activation of CREB phosphorylation in HEK293 cells and human parathyroid cells. Am. J. Physiol. Endocrinol. Metab. 2013;304:E1097–E1104. doi: 10.1152/ajpendo.00054.2013. [DOI] [PubMed] [Google Scholar]
- 148.Rey O., Young S.H., Yuan J., Slice L., Rozengurt E. Amino acid-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor are mediated by a phospholipase c/inositol 1,4,5-trisphosphate-independent pathway that requires g12, rho, filamin-a, and the actin cytoskeleton. J. Biol. Chem. 2005;280:22875–22882. doi: 10.1074/jbc.M503455200. [DOI] [PubMed] [Google Scholar]
- 149.Rey O., Young S.H., Papazyan R., Shapiro M.S., Rozengurt E. Requirement of the trpc1 cation channel in the generation of transient Ca2+ oscillations by the calcium-sensing receptor. J. Biol. Chem. 2006;281:38730–38737. doi: 10.1074/jbc.M605956200. [DOI] [PubMed] [Google Scholar]
- 150.Rey O., Young S.H., Jacamo R., Moyer M.P., Rozengurt E. Extracellular calcium sensing receptor stimulation in human colonic epithelial cells induces intracellular calcium oscillations and proliferation inhibition. J. Cell. Physiol. 2010;225:73–83. doi: 10.1002/jcp.22198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhu D., Kosik K.S., Meigs T.E., Yanamadala V., Denker B.M. Galpha12 directly interacts with pp2a: Evidence for Gα12-stimulated PP2A phosphatase activity and dephosphorylation of microtubule-associated protein, tau. J. Biol. Chem. 2004;279:54983–54986. doi: 10.1074/jbc.C400508200. [DOI] [PubMed] [Google Scholar]
- 152.Zhu D., Tate R.I., Ruediger R., Meigs T.E., Denker B.M. Domains necessary for galpha12 binding and stimulation of protein phosphatase-2a (PP2A): Is galpha12 a novel regulatory subunit of PP2A? Mol. Pharmacol. 2007;71:1268–1276. doi: 10.1124/mol.106.033555. [DOI] [PubMed] [Google Scholar]
- 153.McCormick W.D., Atkinson-Dell R., Campion K.L., Mun H.C., Conigrave A.D., Ward D.T. Increased receptor stimulation elicits differential calcium-sensing receptor(T888) dephosphorylation. J. Biol. Chem. 2010;285:14170–14177. doi: 10.1074/jbc.M109.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rybchyn M.S., Slater M., Conigrave A.D., Mason R.S. An AKT-dependent increase in canonical wnt signaling and a decrease in sclerostin protein levels are involved in strontium ranelate-induced osteogenic effects in human osteoblasts. J. Biol. Chem. 2011;286:23771–23779. doi: 10.1074/jbc.M111.251116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Brennan T.C., Rybchyn M.S., Green W., Atwa S., Conigrave A.D., Mason R.S. Osteoblasts play key roles in the mechanisms of action of strontium ranelate. Br. J. Pharmacol. 2009;157:1291–1300. doi: 10.1111/j.1476-5381.2009.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brennan S.C., Thiem U., Roth S., Aggarwal A., Fetahu I., Tennakoon S., Gomes A.R., Brandi M.L., Bruggeman F., Mentaverri R., et al. Calcium sensing receptor signalling in physiology and cancer. Biochim. Biophys. Acta. 2013;1833:1732–1744. doi: 10.1016/j.bbamcr.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 157.Mamillapalli R., VanHouten J., Zawalich W., Wysolmerski J. Switching of G-protein usage by the calcium-sensing receptor reverses its effect on parathyroid hormone-related protein secretion in normal versus malignant breast cells. J. Biol. Chem. 2008;283:24435–24447. doi: 10.1074/jbc.M801738200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mamillapalli R., Wysolmerski J. The calcium-sensing receptor couples to Galpha(s) and regulates pthrp and acth secretion in pituitary cells. J. Endocrinol. 2010;204:287–297. doi: 10.1677/JOE-09-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Brennan S.C., Wilkinson W.J., Tseng H.E., Finney B., Monk B., Dibble H., Quilliam S., Warburton D., Galietta L.J., Kemp P.J., et al. The extracellular calcium-sensing receptor regulates human fetal lung development via cftr. Sci. Rep. 2016;6:21975. doi: 10.1038/srep21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ziegelstein R.C., Xiong Y., He C., Hu Q. Expression of a functional extracellular calcium-sensing receptor in human aortic endothelial cells. Biochem. Biophys. Res. Commun. 2006;342:153–163. doi: 10.1016/j.bbrc.2006.01.135. [DOI] [PubMed] [Google Scholar]
- 161.Smajilovic S., Sheykhzade M., Holmegard H.N., Haunso S., Tfelt-Hansen J. Calcimimetic, AMG 073, induces relaxation on isolated rat aorta. Vascul. Pharmacol. 2007;47:222–228. doi: 10.1016/j.vph.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 162.Greenberg H.Z.E., Shi J., Jahan K.S., Martinucci M.C., Gilbert S.J., Vanessa Ho W.S., Albert A.P. Stimulation of calcium-sensing receptors induces endothelium-dependent vasorelaxations via nitric oxide production and activation of ikca channels. Vascul. Pharmacol. 2016;80:75–84. doi: 10.1016/j.vph.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Qu Y.Y., Wang L.M., Zhong H., Liu Y.M., Tang N., Zhu L.P., He F., Hu Q.H. TRPC1 stimulates calciumsensing receptorinduced storeoperated Ca2+ entry and nitric oxide production in endothelial cells. Mol. Med. Rep. 2017;16:4613–4619. doi: 10.3892/mmr.2017.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Greenberg H.Z.E., Carlton-Carew S.R.E., Khan D.M., Zargaran A.K., Jahan K.S., Vanessa Ho W.S., Albert A.P. Heteromeric TRPV4/TRPC1 channels mediate calcium-sensing receptor-induced nitric oxide production and vasorelaxation in rabbit mesenteric arteries. Vascul. Pharmacol. 2017;96–98:53–62. doi: 10.1016/j.vph.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Garrett J.E., Tamir H., Kifor O., Simin R.T., Rogers K.V., Mithal A., Gagel R.F., Brown E.M. Calcitonin-secreting cells of the thyroid express an extracellular calcium receptor gene. Endocrinology. 1995;136:5202–5211. doi: 10.1210/endo.136.11.7588259. [DOI] [PubMed] [Google Scholar]
- 166.Hofer A.M., Brown E.M. Extracellular calcium sensing and signalling. Nat. Rev. Mol. Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 167.Brown E.M. Mutations in the calcium-sensing receptor and their clinical implications. Horm. Res. 1997;48:199–208. doi: 10.1159/000185516. [DOI] [PubMed] [Google Scholar]
- 168.Pollak M.R., Brown E.M., Chou Y.H., Hebert S.C., Marx S.J., Steinmann B., Levi T., Seidman C.E., Seidman J.G. Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell. 1993;75:1297–1303. doi: 10.1016/0092-8674(93)90617-Y. [DOI] [PubMed] [Google Scholar]
- 169.Watanabe S., Fukumoto S., Chang H., Takeuchi Y., Hasegawa Y., Okazaki R., Chikatsu N., Fujita T. Association between activating mutations of calcium-sensing receptor and Bartter’s syndrome. Lancet. 2002;360:692–694. doi: 10.1016/S0140-6736(02)09842-2. [DOI] [PubMed] [Google Scholar]
- 170.Pollak M.R., Brown E.M., Estep H.L., McLaine P.N., Kifor O., Park J., Hebert S.C., Seidman C.E., Seidman J.G. Autosomal dominant hypocalcaemia caused by a Ca2+-sensing receptor gene mutation. Nat. Genet. 1994;8:303–307. doi: 10.1038/ng1194-303. [DOI] [PubMed] [Google Scholar]
- 171.Zhao X.M., Hauache O., Goldsmith P.K., Collins R., Spiegel A.M. A missense mutation in the seventh transmembrane domain constitutively activates the human Ca2+ receptor. FEBS Lett. 1999;448:180–184. doi: 10.1016/S0014-5793(99)00368-3. [DOI] [PubMed] [Google Scholar]
- 172.Carmosino M., Gerbino A., Hendy G.N., Torretta S., Rizzo F., Debellis L., Procino G., Svelto M. NKCC2 activity is inhibited by the Bartter’s syndrome type 5 gain-of-function CAR-A843E mutant in renal cells. Biol. Cell. 2015;107:98–110. doi: 10.1111/boc.201400069. [DOI] [PubMed] [Google Scholar]
- 173.Tfelt-Hansen J., Brown E.M. The calcium-sensing receptor in normal physiology and pathophysiology: A review. Crit. Rev. Clin. Lab. Sci. 2005;42:35–70. doi: 10.1080/10408360590886606. [DOI] [PubMed] [Google Scholar]
- 174.Ruat M., Molliver M.E., Snowman A.M., Snyder S.H. Calcium sensing receptor: Molecular cloning in rat and localization to nerve terminals. Proc. Natl. Acad. Sci. USA. 1995;92:3161–3165. doi: 10.1073/pnas.92.8.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Squires P.E. Non-Ca2+-homeostatic functions of the extracellular Ca2+-sensing receptor (CAR) in endocrine tissues. J. Endocrinol. 2000;165:173–177. doi: 10.1677/joe.0.1650173. [DOI] [PubMed] [Google Scholar]
- 176.Canaff L., Petit J.L., Kisiel M., Watson P.H., Gascon-Barre M., Hendy G.N. Extracellular calcium-sensing receptor is expressed in rat hepatocytes. Coupling to intracellular calcium mobilization and stimulation of bile flow. J. Biol. Chem. 2001;276:4070–4079. doi: 10.1074/jbc.M009317200. [DOI] [PubMed] [Google Scholar]
- 177.Wang R., Xu C., Zhao W., Zhang J., Cao K., Yang B., Wu L. Calcium and polyamine regulated calcium-sensing receptors in cardiac tissues. Eur. J. Biochem. 2003;270:2680–2688. doi: 10.1046/j.1432-1033.2003.03645.x. [DOI] [PubMed] [Google Scholar]
- 178.Tfelt-Hansen J., Hansen J.L., Smajilovic S., Terwilliger E.F., Haunso S., Sheikh S.P. Calcium receptor is functionally expressed in rat neonatal ventricular cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1165–H1171. doi: 10.1152/ajpheart.00821.2005. [DOI] [PubMed] [Google Scholar]
- 179.Alfadda T.I., Saleh A.M., Houillier P., Geibel J.P. Calcium-sensing receptor 20 years later. Am. J. Physiol. Cell Physiol. 2014;307:C221–C231. doi: 10.1152/ajpcell.00139.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Saidak Z., Mentaverri R., Brown E.M. The role of the calcium-sensing receptor in the development and progression of cancer. Endocr. Rev. 2009;30:178–195. doi: 10.1210/er.2008-0041. [DOI] [PubMed] [Google Scholar]
- 181.Corbetta S., Mantovani G., Lania A., Borgato S., Vicentini L., Beretta E., Faglia G., di Blasio A.M., Spada A. Calcium-sensing receptor expression and signalling in human parathyroid adenomas and primary hyperplasia. Clin. Endocrinol. 2000;52:339–348. doi: 10.1046/j.1365-2265.2000.00933.x. [DOI] [PubMed] [Google Scholar]
- 182.Gogusev J., Duchambon P., Hory B., Giovannini M., Goureau Y., Sarfati E., Drueke T.B. Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int. 1997;51:328–336. doi: 10.1038/ki.1997.41. [DOI] [PubMed] [Google Scholar]
- 183.Farnebo F., Enberg U., Grimelius L., Backdahl M., Schalling M., Larsson C., Farnebo L.O. Tumor-specific decreased expression of calcium sensing receptor messenger ribonucleic acid in sporadic primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 1997;82:3481–3486. doi: 10.1210/jc.82.10.3481. [DOI] [PubMed] [Google Scholar]
- 184.Sheinin Y., Kallay E., Wrba F., Kriwanek S., Peterlik M., Cross H.S. Immunocytochemical localization of the extracellular calcium-sensing receptor in normal and malignant human large intestinal mucosa. J. Histochem. Cytochem. 2000;48:595–602. doi: 10.1177/002215540004800503. [DOI] [PubMed] [Google Scholar]
- 185.Chakrabarty S., Wang H., Canaff L., Hendy G.N., Appelman H., Varani J. Calcium sensing receptor in human colon carcinoma: Interaction with Ca2+ and 1,25-dihydroxyvitamin D3. Cancer Res. 2005;65:493–498. [PubMed] [Google Scholar]
- 186.Miller G., Davis J., Shatzen E., Colloton M., Martin D., Henley C.M. Cinacalcet hcl prevents development of parathyroid gland hyperplasia and reverses established parathyroid gland hyperplasia in a rodent model of ckd. Nephrol. Dial. Transplant. 2012;27:2198–2205. doi: 10.1093/ndt/gfr589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mizobuchi M., Hatamura I., Ogata H., Saji F., Uda S., Shiizaki K., Sakaguchi T., Negi S., Kinugasa E., Koshikawa S., et al. Calcimimetic compound upregulates decreased calcium-sensing receptor expression level in parathyroid glands of rats with chronic renal insufficiency. J. Am. Soc. Nephrol. 2004;15:2579–2587. doi: 10.1097/01.ASN.0000141016.20133.33. [DOI] [PubMed] [Google Scholar]
- 188.Garland C., Shekelle R.B., Barrett-Connor E., Criqui M.H., Rossof A.H., Paul O. Dietary vitamin d and calcium and risk of colorectal cancer: A 19-year prospective study in men. Lancet. 1985;1:307–309. doi: 10.1016/S0140-6736(85)91082-7. [DOI] [PubMed] [Google Scholar]
- 189.Aggarwal A., Hobaus J., Tennakoon S., Prinz-Wohlgenannt M., Graca J., Price S.A., Heffeter P., Berger W., Baumgartner-Parzer S., Kallay E. Active vitamin d potentiates the anti-neoplastic effects of calcium in the colon: A cross talk through the calcium-sensing receptor. J. Steroid Biochem. Mol. Biol. 2016;155:231–238. doi: 10.1016/j.jsbmb.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 190.Canaff L., Hendy G.N. Human calcium-sensing receptor gene. Vitamin d response elements in promoters P1 and P2 confer transcriptional responsiveness to 1,25-dihydroxyvitamin D. J. Biol. Chem. 2002;277:30337–30350. doi: 10.1074/jbc.M201804200. [DOI] [PubMed] [Google Scholar]
- 191.Hendy G.N., Canaff L. Calcium-sensing receptor gene: Regulation of expression. Front. Physiol. 2016;7:394. doi: 10.3389/fphys.2016.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mihai R., Stevens J., McKinney C., Ibrahim N.B. Expression of the calcium receptor in human breast cancer—A potential new marker predicting the risk of bone metastases. Eur. J. Surg. Oncol. 2006;32:511–515. doi: 10.1016/j.ejso.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 193.Sanders J.L., Chattopadhyay N., Kifor O., Yamaguchi T., Brown E.M. Ca2+-sensing receptor expression and pthrp secretion in pc-3 human prostate cancer cells. Am. J. Physiol. Endocrinol. Metab. 2001;281:E1267–E1274. doi: 10.1152/ajpendo.2001.281.6.E1267. [DOI] [PubMed] [Google Scholar]
- 194.Ward D.T., Riccardi D. New concepts in calcium-sensing receptor pharmacology and signalling. Br. J. Pharmacol. 2012;165:35–48. doi: 10.1111/j.1476-5381.2011.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Weston A.H., Absi M., Ward D.T., Ohanian J., Dodd R.H., Dauban P., Petrel C., Ruat M., Edwards G. Evidence in favor of a calcium-sensing receptor in arterial endothelial cells: Studies with calindol and calhex 231. Circ. Res. 2005;97:391–398. doi: 10.1161/01.RES.0000178787.59594.a0. [DOI] [PubMed] [Google Scholar]
- 196.Smajilovic S., Hansen J.L., Christoffersen T.E., Lewin E., Sheikh S.P., Terwilliger E.F., Brown E.M., Haunso S., Tfelt-Hansen J. Extracellular calcium sensing in rat aortic vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2006;348:1215–1223. doi: 10.1016/j.bbrc.2006.07.192. [DOI] [PubMed] [Google Scholar]
- 197.Berra Romani R., Raqeeb A., Laforenza U., Scaffino M.F., Moccia F., Avelino-Cruz J.E., Oldani A., Coltrini D., Milesi V., Taglietti V., et al. Cardiac microvascular endothelial cells express a functional Ca 2+-sensing receptor. J. Vascul. Res. 2009;46:73–82. doi: 10.1159/000140677. [DOI] [PubMed] [Google Scholar]
- 198.Weston A.H., Absi M., Harno E., Geraghty A.R., Ward D.T., Ruat M., Dodd R.H., Dauban P., Edwards G. The expression and function of Ca2+-sensing receptors in rat mesenteric artery; comparative studies using a model of type ii diabetes. Br. J. Pharmacol. 2008;154:652–662. doi: 10.1038/bjp.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Smajilovic S., Tfelt-Hansen J. Calcium acts as a first messenger through the calcium-sensing receptor in the cardiovascular system. Cardiovasc. Res. 2007;75:457–467. doi: 10.1016/j.cardiores.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 200.Weston A.H., Geraghty A., Egner I., Edwards G. The vascular extracellular calcium-sensing receptor: An update. Acta Physiol. 2011;203:127–137. doi: 10.1111/j.1748-1716.2010.02249.x. [DOI] [PubMed] [Google Scholar]
- 201.Smajilovic S., Yano S., Jabbari R., Tfelt-Hansen J. The calcium-sensing receptor and calcimimetics in blood pressure modulation. Br. J. Pharmacol. 2011;164:884–893. doi: 10.1111/j.1476-5381.2011.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Schreckenberg R., Schluter K.D. Calcium sensing receptor expression and signalling in cardiovascular physiology and disease. Vascul. Pharmacol. 2018 doi: 10.1016/j.vph.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 203.Sun Y.H., Liu M.N., Li H., Shi S., Zhao Y.J., Wang R., Xu C.Q. Calcium-sensing receptor induces rat neonatal ventricular cardiomyocyte apoptosis. Biochem. Biophys. Res. Commun. 2006;350:942–948. doi: 10.1016/j.bbrc.2006.09.142. [DOI] [PubMed] [Google Scholar]
- 204.Zhang W.H., Fu S.B., Lu F.H., Wu B., Gong D.M., Pan Z.W., Lv Y.J., Zhao Y.J., Li Q.F., Wang R., et al. Involvement of calcium-sensing receptor in ischemia/reperfusion-induced apoptosis in rat cardiomyocytes. Biochem. Biophys. Res. Commun. 2006;347:872–881. doi: 10.1016/j.bbrc.2006.06.176. [DOI] [PubMed] [Google Scholar]
- 205.Sun Y.H., Li Y.Q., Feng S.L., Li B.X., Pan Z.W., Xu C.Q., Li T.T., Yang B.F. Calcium-sensing receptor activation contributed to apoptosis stimulates trpc6 channel in rat neonatal ventricular myocytes. Biochem. Biophys. Res. Commun. 2010;394:955–961. doi: 10.1016/j.bbrc.2010.03.096. [DOI] [PubMed] [Google Scholar]
- 206.Feng S.L., Sun M.R., Li T.T., Yin X., Xu C.Q., Sun Y.H. Activation of calcium-sensing receptor increases TRPC3 expression in rat cardiomyocytes. Biochem. Biophys. Res. Commun. 2011;406:278–284. doi: 10.1016/j.bbrc.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 207.Tang J., Wang G., Liu Y., Fu Y., Chi J., Zhu Y., Zhao Y., Yin X. Cyclosporin a induces cardiomyocyte injury through calcium-sensing receptor-mediated calcium overload. Pharmazie. 2011;66:52–57. [PubMed] [Google Scholar]
- 208.Zhao Y., Hou G., Zhang Y., Chi J., Zhang L., Zou X., Tang J., Liu Y., Fu Y., Yin X. Involvement of the calcium-sensing receptor in cyclosporin a-induced cardiomyocyte apoptosis in rats. Pharmazie. 2011;66:968–974. [PubMed] [Google Scholar]
- 209.Chi J., Zhu Y., Fu Y., Liu Y., Zhang X., Han L., Yin X., Zhao D. Cyclosporin a induces apoptosis in H9C2 cardiomyoblast cells through calcium-sensing receptor-mediated activation of the erk MAPK and p38 MAPK pathways. Mol. Cell. Biochem. 2013;367:227–236. doi: 10.1007/s11010-012-1336-5. [DOI] [PubMed] [Google Scholar]
- 210.Wang H.-Y., Liu X.-Y., Han G., Wang Z.-Y., Li X.-X., Jiang Z.-M., Jiang C.-M. LPS induces cardiomyocyte injury through calcium-sensing receptor. Mol. Cell. Biochem. 2013;379:153–159. doi: 10.1007/s11010-013-1637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jiang C.M., Han L.P., Li H.Z., Qu Y.B., Zhang Z.R., Wang R., Xu C.Q., Li W.M. Calcium-sensing receptors induce apoptosis in cultured neonatal rat ventricular cardiomyocytes during simulated ischemia/reperfusion. Cell Biol. Int. 2008;32:792–800. doi: 10.1016/j.cellbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 212.Lu F., Tian Z., Zhang W., Zhao Y., Bai S., Ren H., Chen H., Yu X., Wang J., Wang L., et al. Calcium-sensing receptors induce apoptosis in rat cardiomyocytes via the Endo (sarco) plasmic reticulum pathway during hypoxia/reoxygenation. Basic Clin. Pharmacol. Toxicol. 2010;106:396–405. doi: 10.1111/j.1742-7843.2009.00502.x. [DOI] [PubMed] [Google Scholar]
- 213.Lu F.H., Tian Z., Zhang W.H., Zhao Y.J., Li H.L., Ren H., Zheng H.S., Liu C., Hu G.X., Tian Y., et al. Calcium-sensing receptors regulate cardiomyocyte Ca2+ signaling via the sarcoplasmic reticulum-mitochondrion interface during hypoxia/reoxygenation. J. Biomed. Sci. 2010;17:50. doi: 10.1186/1423-0127-17-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Guo J., Li H.Z., Zhang W.H., Wang L.C., Wang L.N., Zhang L., Li G.W., Li H.X., Yang B.F., Wu L., et al. Increased expression of calcium-sensing receptors induced by ox-LDL amplifies apoptosis of cardiomyocytes during simulated ischaemia-reperfusion. Clin. Exp. Pharmacol. Physiol. 2010;37:e128–e135. doi: 10.1111/j.1440-1681.2010.05345.x. [DOI] [PubMed] [Google Scholar]
- 215.Jiang C.M., Xu C.Q., Mi Y., Li H.Z., Wang R., Li. W.-M. Calcium-sensing receptor induced myocardial ischemia/reperfusion injury via the c-jun nh2-terminal protein kinase pathway. Acta Cardiol. Sin. 2010;26:102–110. [Google Scholar]
- 216.Zheng H.S., Liu J., Liu C., Lu F.H., Zhao Y.J., Jin Z.F., Ren H., Leng X.N., Jia J., Hu G.X., et al. Calcium-sensing receptor activating phosphorylation of PKC delta translocation on mitochondria to induce cardiomyocyte apoptosis during ischemia/reperfusion. Mol. Cell. Biochem. 2011;358:335–343. doi: 10.1007/s11010-011-0984-1. [DOI] [PubMed] [Google Scholar]
- 217.Yan L., Zhu T., Sun T., Wang L., Pan S., Tao Z., Yang Z., Cao K. Activation of calcium-sensing receptors is associated with apoptosis in a model of simulated cardiomyocytes ischemia/reperfusion. J. Biomed. Res. 2010;24:301–307. doi: 10.1016/S1674-8301(10)60042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yan L., Zhu T.B., Wang L.S., Pan S.Y., Tao Z.X., Yang Z., Cao K., Huang J. Inhibitory effect of hepatocyte growth factor on cardiomyocytes apoptosis is partly related to reduced calcium sensing receptor expression during a model of simulated ischemia/reperfusion. Mol. Biol. Rep. 2011;38:2695–2701. doi: 10.1007/s11033-010-0412-8. [DOI] [PubMed] [Google Scholar]
- 219.Zhang W.H., Lu F.H., Zhao Y.J., Wang L.N., Tian Y., Pan Z.W., Lv Y.J., Wang Y.L., Du L.J., Sun Z.R., et al. Post-conditioning protects rat cardiomyocytes via pkcepsilon-mediated calcium-sensing receptors. Biochem. Biophys. Res. Commun. 2007;361:659–664. doi: 10.1016/j.bbrc.2007.07.077. [DOI] [PubMed] [Google Scholar]
- 220.Dong S., Teng Z., Lu F.H., Zhao Y.J., Li H., Ren H., Chen H., Pan Z.W., Lv Y.J., Yang B.F., et al. Post-conditioning protects cardiomyocytes from apoptosis via PKC (epsilon)-interacting with calcium-sensing receptors to inhibit Endo (sarco) plasmic reticulum-mitochondria crosstalk. Mol. Cell. Biochem. 2010;341:195–206. doi: 10.1007/s11010-010-0450-5. [DOI] [PubMed] [Google Scholar]
- 221.Gan R.T., Hu G.X., Zhao Y.J., Li H.L., Jin Z.F., Ren H., Dong S.Y., Zhong X., Li H.Z., Yang B.F., et al. Post-conditioning protecting rat cardiomyocytes from apoptosis via attenuating calcium-sensing receptor-induced Endo (sarco) plasmic reticulum stress. Mol. Cell. Biochem. 2012;361:123–134. doi: 10.1007/s11010-011-1096-7. [DOI] [PubMed] [Google Scholar]
- 222.Zhang L., Cao S., Deng S., Yao G., Yu T. Ischemic postconditioning and pinacidil suppress calcium overload in anoxia-reoxygenation cardiomyocytes via down-regulation of the calcium-sensing receptor. PeerJ. 2016;4:e2612. doi: 10.7717/peerj.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dyukova E., Schreckenberg R., Sitdikova G., Schlüter K.-D. Influence of ischemic pre- and post-conditioning on cardiac expression of calcium-sensing receptor. BioNanoScience. 2017;7:112–114. doi: 10.1007/s12668-016-0316-8. [DOI] [Google Scholar]
- 224.Sun J., Murphy E. Calcium sensing receptor: A sensor and mediator of ischemic preconditioning in the hearts. Am. J. Physiol. Heart Circ. Physiol. 2010;299 doi: 10.1152/ajpheart.00373.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bai S.Z., Sun J., Wu H., Zhang N., Li H.X., Li G.W., Li H.Z., He W., Zhang W.H., Zhao Y.J., et al. Decrease in calcium-sensing receptor in the progress of diabetic cardiomyopathy. Diabetes Res. Clin. Pract. 2012;95:378–385. doi: 10.1016/j.diabres.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 226.Qi H., Cao Y., Huang W., Liu Y., Wang Y., Li L., Liu L., Ji Z., Sun H. Crucial role of calcium-sensing receptor activation in cardiac injury of diabetic rats. PLoS ONE. 2013;8:e65147. doi: 10.1371/journal.pone.0065147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Matturri L., Milei J., Grana D.R., Lavezzi A.M. Characterization of myocardial hypertrophy by DNA content, pcna expression and apoptotic index. Int. J. Cardiol. 2002;82:33–39. doi: 10.1016/S0167-5273(01)00578-2. [DOI] [PubMed] [Google Scholar]
- 228.Wang L.N., Wang C., Lin Y., Xi Y.H., Zhang W.H., Zhao Y.J., Li H.Z., Tian Y., Lv Y.J., Yang B.F., et al. Involvement of calcium-sensing receptor in cardiac hypertrophy-induced by angiotensinii through calcineurin pathway in cultured neonatal rat cardiomyocytes. Biochem. Biophys. Res. Commun. 2008;369:584–589. doi: 10.1016/j.bbrc.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 229.Zhong X.L.J., Lu F., Wang Y., Zhao Y., Dong S., Leng X., Jia J., Ren H., Xu C., Zhang W. Calcium sensing receptor regulates cardiomyocyte function through nuclear calcium. Cell Biol. Int. 2012;36:937–943. doi: 10.1042/CBI20110594. [DOI] [PubMed] [Google Scholar]
- 230.Colella M., Pozzan T. Cardiac cell hypertrophy in vitro: Role of calcineurin/nfat as Ca2+ signal integrators. Ann. N. Y. Acad. Sci. 2008;1123:64–68. doi: 10.1196/annals.1420.008. [DOI] [PubMed] [Google Scholar]
- 231.Lu F.H., Fu S.B., Leng X., Zhang X., Dong S., Zhao Y.J., Ren H., Li H., Zhong X., Xu C.Q., et al. Role of the calcium-sensing receptor in cardiomyocyte apoptosis via the sarcoplasmic reticulum and mitochondrial death pathway in cardiac hypertrophy and heart failure. Cell. Physiol. Biochem. 2013;31:728–743. doi: 10.1159/000350091. [DOI] [PubMed] [Google Scholar]
- 232.Liu L., Wang C., Lin Y., Xi Y., Li H., Shi S., Li H., Zhang W., Zhao Y., Tian Y., et al. Suppression of calcium-sensing receptor ameliorates cardiac hypertrophy through inhibition of autophagy. Mol. Med. Rep. 2016;14:111–120. doi: 10.3892/mmr.2016.5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhang X., Zhang T., Wu J., Yu X., Zheng D., Yang F., Li T., Wang L., Zhao Y., Dong S., et al. Calcium sensing receptor promotes cardiac fibroblast proliferation and extracellular matrix secretion. Cell. Physiol. Biochem. 2014;33:557–568. doi: 10.1159/000358634. [DOI] [PubMed] [Google Scholar]
- 234.Schreckenberg R., Dyukova E., Sitdikova G., Abdallah Y., Schluter K.D. Mechanisms by which calcium receptor stimulation modifies electromechanical coupling in isolated ventricular cardiomyocytes. Pflugers Arch. 2015;467:379–388. doi: 10.1007/s00424-014-1498-y. [DOI] [PubMed] [Google Scholar]
- 235.Liu C.-H., Chang H.-K., Lee S.-P., Shieh R.-C. Activation of the Ca2+-sensing receptors increases currents through inward rectifier k+ channels via activation of phosphatidylinositol 4-kinase. Pflugers Arch. 2017;468:1931–1943. doi: 10.1007/s00424-016-1901-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tagliavini S., Genedani S., Bertolini A., Bazzani C. Ischemia- and reperfusion-induced arrhythmias are prevented by putrescine. Eur. J. Pharmacol. 1991;194:7–10. doi: 10.1016/0014-2999(91)90116-8. [DOI] [PubMed] [Google Scholar]
- 237.Schepelmann M., Yarova P.L., Lopez-Fernandez I., Davies T.S., Brennan S.C., Edwards P.J., Aggarwal A., Graca J., Rietdorf K., Matchkov V., et al. The vascular Ca2+-sensing receptor regulates blood vessel tone and blood pressure. Am. J. Physiol. Cell Physiol. 2016;310:C193–C204. doi: 10.1152/ajpcell.00248.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wess J. G-protein-coupled receptors: Molecular mechanisms involved in receptor activation and selectivity of g-protein recognition. FASEB J. 1997;11:346–354. doi: 10.1096/fasebj.11.5.9141501. [DOI] [PubMed] [Google Scholar]
- 239.Lefkowitz R.J. Historical review: A brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol. Sci. 2004;25:413–422. doi: 10.1016/j.tips.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 240.Rajagopal S., Ahn S., Rominger D.H., Gowen-MacDonald W., Lam C.M., Dewire S.M., Violin J.D., Lefkowitz R.J. Quantifying ligand bias at seven-transmembrane receptors. Mol. Pharmacol. 2010;80:367–377. doi: 10.1124/mol.111.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lohse M.J., Hofmann K.P. Spatial and temporal aspects of signaling by G-protein-coupled receptors. Mol. Pharmacol. 2015;88:572–578. doi: 10.1124/mol.115.100248. [DOI] [PubMed] [Google Scholar]
- 242.Pupo A.S., Duarte D.A., Lima V., Teixeira L.B., Parreiras E.S.L.T., Costa-Neto C.M. Recent updates on gpcr biased agonism. Pharmacol. Res. 2016;112:49–57. doi: 10.1016/j.phrs.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 243.Calebiro D., Sungkaworn T., Maiellaro I. Real-time monitoring of GPCR/CAMP signalling by fret and single-molecule microscopy. Horm. Metab. Res. 2014;46:827–832. doi: 10.1055/s-0034-1384523. [DOI] [PubMed] [Google Scholar]
- 244.Ibarra C., Vicencio J.M., Estrada M., Lin Y., Rocco P., Rebellato P., Munoz J.P., Garcia-Prieto J., Quest A.F., Chiong M., et al. Local control of nuclear calcium signaling in cardiac myocytes by perinuclear microdomains of sarcolemmal insulin-like growth factor 1 receptors. Circ. Res. 2013;112:236–245. doi: 10.1161/CIRCRESAHA.112.273839. [DOI] [PubMed] [Google Scholar]
- 245.Benard G., Massa F., Puente N., Lourenco J., Bellocchio L., Soria-Gomez E., Matias I., Delamarre A., Metna-Laurent M., Cannich A., et al. Mitochondrial cb1 receptors regulate neuronal energy metabolism. Nat. Neurosci. 2012;15:558–564. doi: 10.1038/nn.3053. [DOI] [PubMed] [Google Scholar]
- 246.Calebiro D., Nikolaev V.O., Gagliani M.C., de Filippis T., Dees C., Tacchetti C., Persani L., Lohse M.J. Persistent camp-signals triggered by internalized g-protein coupled receptors. PLoS Biol. 2009;7:e1000172. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Irannejad R., Tomshine J.C., Tomshine J.R., Chevalier M., Mahoney J.P., Steyaert J., Rasmussen S.G., Sunahara R.K., El-Samad H., Huang B., et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Pidasheva S., Grant M., Canaff L., Ercan O., Kumar U., Hendy G.N. Calcium-sensing receptor dimerizes in the endoplasmic reticulum: Biochemical and biophysical characterization of casr mutants retained intracellularly. Hum. Mol. Genet. 2006;15:2200–2209. doi: 10.1093/hmg/ddl145. [DOI] [PubMed] [Google Scholar]
- 249.Ward D.T., Brown E.M., Harris H.W. Disulfide bonds in the extracellular calcium-polyvalent cation-sensing receptor correlate with dimer formation and its response to divalent cations in vitro. J. Biol. Chem. 1998;273:14476–14483. doi: 10.1074/jbc.273.23.14476. [DOI] [PubMed] [Google Scholar]
- 250.Breitwieser G.E. Minireview: The intimate link between calcium sensing receptor trafficking and signaling: Implications for disorders of calcium homeostasis. Mol. Endocrinol. 2012;26:1482–1495. doi: 10.1210/me.2011-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Breitwieser G.E. Pharmacoperones and the calcium sensing receptor: Exogenous and endogenous regulators. Pharmacol. Res. 2014;83:30–37. doi: 10.1016/j.phrs.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 252.Holtback U., Brismar H., DiBona G.F., Fu M., Greengard P., Aperia A. Receptor recruitment: A mechanism for interactions between g protein-coupled receptors. Proc. Natl. Acad. Sci. USA. 1999;96:7271–7275. doi: 10.1073/pnas.96.13.7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Brennan S.C., Mun H.C., Leach K., Kuchel P.W., Christopoulos A., Conigrave A.D. Receptor expression modulates calcium-sensing receptor mediated intracellular Ca2+ mobilization. Endocrinology. 2015;156:1330–1342. doi: 10.1210/en.2014-1771. [DOI] [PubMed] [Google Scholar]
- 254.Breitwieser G.E. The calcium sensing receptor life cycle: Trafficking, cell surface expression, and degradation. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27:303–313. doi: 10.1016/j.beem.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 255.Ray K. Calcium-sensing receptor: Trafficking, endocytosis, recycling, and importance of interacting proteins. Prog. Mol. Biol. Transl. Sci. 2015;132:127–150. doi: 10.1016/bs.pmbts.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 256.Huang C., Miller R.T. The calcium-sensing receptor and its interacting proteins. J. Cell Mol. Med. 2007;11:923–934. doi: 10.1111/j.1582-4934.2007.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bai M., Quinn S., Trivedi S., Kifor O., Pearce S.H., Pollak M.R., Krapcho K., Hebert S.C., Brown E.M. Expression and characterization of inactivating and activating mutations in the human Ca2+ o-sensing receptor. J. Biol. Chem. 1996;271:19537–19545. doi: 10.1074/jbc.271.32.19537. [DOI] [PubMed] [Google Scholar]
- 258.Grant M.P., Stepanchick A., Cavanaugh A., Breitwieser G.E. Agonist-driven maturation and plasma membrane insertion of calcium-sensing receptors dynamically control signal amplitude. Sci. Signal. 2011;4:ra78. doi: 10.1126/scisignal.2002208. [DOI] [PubMed] [Google Scholar]
- 259.Huang Y., Breitwieser G.E. Rescue of calcium-sensing receptor mutants by allosteric modulators reveals a conformational checkpoint in receptor biogenesis. J. Biol. Chem. 2007;282:9517–9525. doi: 10.1074/jbc.M609045200. [DOI] [PubMed] [Google Scholar]
- 260.White E., McKenna J., Cavanaugh A., Breitwieser G.E. Pharmacochaperone-mediated rescue of calcium-sensing receptor loss-of-function mutants. Mol. Endocrinol. 2009;23:1115–1123. doi: 10.1210/me.2009-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Cavanaugh A., McKenna J., Stepanchick A., Breitwieser G.E. Calcium-sensing receptor biosynthesis includes a cotranslational conformational checkpoint and endoplasmic reticulum retention. J. Biol. Chem. 2010;285:19854–19864. doi: 10.1074/jbc.M110.124792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Rus R., Haag C., Bumke-Vogt C., Bahr V., Mayr B., Mohlig M., Schulze E., Frank-Raue K., Raue F., Schofl C. Novel inactivating mutations of the calcium-sensing receptor: The calcimimetic nps r-568 improves signal transduction of mutant receptors. J. Clin. Endocrinol. Metab. 2008;93:4797–4803. doi: 10.1210/jc.2008-1076. [DOI] [PubMed] [Google Scholar]
- 263.Leach K., Wen A., Cook A.E., Sexton P.M., Conigrave A.D., Christopoulos A. Impact of clinically relevant mutations on the pharmacoregulation and signaling bias of the calcium-sensing receptor by positive and negative allosteric modulators. Endocrinology. 2013;154:1105–1116. doi: 10.1210/en.2012-1887. [DOI] [PubMed] [Google Scholar]
- 264.Nakamura A., Hotsubo T., Kobayashi K., Mochizuki H., Ishizu K., Tajima T. Loss-of-function and gain-of-function mutations of calcium-sensing receptor: Functional analysis and the effect of allosteric modulators NPS R-568 and NPS 2143. J. Clin. Endocrinol. Metab. 2013;98:E1692–E1701. doi: 10.1210/jc.2013-1974. [DOI] [PubMed] [Google Scholar]
- 265.Letz S., Rus R., Haag C., Dorr H.G., Schnabel D., Mohlig M., Schulze E., Frank-Raue K., Raue F., Mayr B., et al. Novel activating mutations of the calcium-sensing receptor: The calcilytic NPS-2143 mitigates excessive signal transduction of mutant receptors. J. Clin. Endocrinol. Metab. 2010;95:E229–E233. doi: 10.1210/jc.2010-0651. [DOI] [PubMed] [Google Scholar]
- 266.Letz S., Haag C., Schulze E., Frank-Raue K., Raue F., Hofner B., Mayr B., Schofl C. Amino alcohol- (NPS-2143) and quinazolinone-derived calcilytics (ATF936 and AXT914) differentially mitigate excessive signalling of calcium-sensing receptor mutants causing bartter syndrome type 5 and autosomal dominant hypocalcemia. PLoS ONE. 2014;9:e115178. doi: 10.1371/journal.pone.0115178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hannan F.M., Walls G.V., Babinsky V.N., Nesbit M.A., Kallay E., Hough T.A., Fraser W.D., Cox R.D., Hu J., Spiegel A.M., et al. The calcilytic agent NPS 2143 rectifies hypocalcemia in a mouse model with an activating calcium-sensing receptor (CASR) mutation: Relevance to autosomal dominant hypocalcemia type 1 (ADH1) Endocrinology. 2015;156:3114–3121. doi: 10.1210/en.2015-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Dong B., Endo I., Ohnishi Y., Kondo T., Hasegawa T., Amizuka N., Kiyonari H., Shioi G., Abe M., Fukumoto S., et al. Calcilytic ameliorates abnormalities of mutant calcium-sensing receptor (CASR) knock-in mice mimicking autosomal dominant hypocalcemia (ADH) J. Bone Miner. Res. 2015;30:1980–1993. doi: 10.1002/jbmr.2551. [DOI] [PubMed] [Google Scholar]
- 269.Leach K., Conigrave A.D., Sexton P.M., Christopoulos A. Towards tissue-specific pharmacology: Insights from the calcium-sensing receptor as a paradigm for GPCR (patho)physiological bias. Trends Pharmacol. Sci. 2015;36:215–225. doi: 10.1016/j.tips.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 270.Makita N., Sato J., Manaka K., Shoji Y., Oishi A., Hashimoto M., Fujita T., Iiri T. An acquired hypocalciuric hypercalcemia autoantibody induces allosteric transition among active human ca-sensing receptor conformations. Proc. Natl. Acad. Sci. USA. 2007;104:5443–5448. doi: 10.1073/pnas.0701290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Bruce J.I., Yang X., Ferguson C.J., Elliott A.C., Steward M.C., Case R.M., Riccardi D. Molecular and functional identification of a Ca2+ (polyvalent cation)-sensing receptor in rat pancreas. J. Biol. Chem. 1999;274:20561–20568. doi: 10.1074/jbc.274.29.20561. [DOI] [PubMed] [Google Scholar]
- 272.Thomsen A.R., Smajilovic S., Brauner-Osborn H. Novel strategies in drug discovery of the calcium-sensing receptor based on biased signaling. Curr. Drug. Targets. 2012;13:1324–1335. doi: 10.2174/138945012802429642. [DOI] [PubMed] [Google Scholar]
- 273.Thomsen A.R., Worm J., Jacobsen S.E., Stahlhut M., Latta M., Brauner-Osborne H. Strontium is a biased agonist of the calcium-sensing receptor in rat medullary thyroid carcinoma 6-23 cells. J. Pharmacol. Exp. Ther. 2012;343:638–649. doi: 10.1124/jpet.112.197210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Chattopadhyay N., Quinn S.J., Kifor O., Ye C., Brown E.M. The calcium-sensing receptor (CAR) is involved in strontium ranelate-induced osteoblast proliferation. Biochem. Pharmacol. 2007;74:438–447. doi: 10.1016/j.bcp.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 275.Coulombe J., Faure H., Robin B., Ruat M. In vitro effects of strontium ranelate on the extracellular calcium-sensing receptor. Biochem. Biophys. Res. Commun. 2004;323:1184–1190. doi: 10.1016/j.bbrc.2004.08.209. [DOI] [PubMed] [Google Scholar]
- 276.Hurtel-Lemaire A.S., Mentaverri R., Caudrillier A., Cournarie F., Wattel A., Kamel S., Terwilliger E.F., Brown E.M., Brazier M. The calcium-sensing receptor is involved in strontium ranelate-induced osteoclast apoptosis: New insights into the associated signaling pathways. J. Biol. Chem. 2009;284:575–584. doi: 10.1074/jbc.M801668200. [DOI] [PubMed] [Google Scholar]
- 277.Davey A.E., Leach K., Valant C., Conigrave A.D., Sexton P.M., Christopoulos A. Positive and negative allosteric modulators promote biased signaling at the calcium-sensing receptor. Endocrinology. 2012;153:1232–1241. doi: 10.1210/en.2011-1426. [DOI] [PubMed] [Google Scholar]
- 278.Leach K., Wen A., Davey A.E., Sexton P.M., Conigrave A.D., Christopoulos A. Identification of molecular phenotypes and biased signaling induced by naturally occurring mutations of the human calcium-sensing receptor. Endocrinology. 2012;153:4304–4316. doi: 10.1210/en.2012-1449. [DOI] [PubMed] [Google Scholar]
- 279.Hofer A.M., Lefkimmiatis K. Extracellular calcium and cAMP: Second messengers as “Third messengers”? Physiology. 2007;22:320–327. doi: 10.1152/physiol.00019.2007. [DOI] [PubMed] [Google Scholar]
- 280.Wellendorph P., Johansen L.D., Brauner-Osborne H. Molecular pharmacology of promiscuous seven transmembrane receptors sensing organic nutrients. Mol. Pharmacol. 2009;76:453–465. doi: 10.1124/mol.109.055244. [DOI] [PubMed] [Google Scholar]
- 281.Kano M., Kato M. Quisqualate receptors are specifically involved in cerebellar synaptic plasticity. Nature. 1987;325:276–279. doi: 10.1038/325276a0. [DOI] [PubMed] [Google Scholar]
- 282.Francesconi A., Duvoisin R.M. Divalent cations modulate the activity of metabotropic glutamate receptors. J. Neurosci. Res. 2004;75:472–479. doi: 10.1002/jnr.10853. [DOI] [PubMed] [Google Scholar]
- 283.Kubo Y., Miyashita T., Murata Y. Structural basis for a Ca2+-sensing function of the metabotropic glutamate receptors. Science. 1998;279:1722–1725. doi: 10.1126/science.279.5357.1722. [DOI] [PubMed] [Google Scholar]
- 284.Nash M.S., Saunders R., Young K.W., Challiss R.A., Nahorski S.R. Reassessment of the Ca2+ sensing property of a type i metabotropic glutamate receptor by simultaneous measurement of inositol 1,4,5-trisphosphate and Ca2+ in single cells. J. Biol. Chem. 2001;276:19286–19293. doi: 10.1074/jbc.M007600200. [DOI] [PubMed] [Google Scholar]
- 285.Tabata T., Kano M. Calcium dependence of native metabotropic glutamate receptor signaling in central neurons. Mol. Neurobiol. 2004;29:261–270. doi: 10.1385/MN:29:3:261. [DOI] [PubMed] [Google Scholar]
- 286.Hardingham N.R., Bannister N.J., Read J.C., Fox K.D., Hardingham G.E., Jack J.J. Extracellular calcium regulates postsynaptic efficacy through group 1 metabotropic glutamate receptors. J. Neurosci. 2006;26:6337–6345. doi: 10.1523/JNEUROSCI.5128-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Gerber U., Gee C.E., Benquet P. Metabotropic glutamate receptors: Intracellular signaling pathways. Curr. Opin. Pharmacol. 2007;7:56–61. doi: 10.1016/j.coph.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 288.Wang X., Kirberger M., Qiu F., Chen G., Yang J.J. Towards predicting Ca2+-binding sites with different coordination numbers in proteins with atomic resolution. Proteins. 2009;75:787–798. doi: 10.1002/prot.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wang X., Zhao K., Kirberger M., Wong H., Chen G., Yang J.J. Analysis and prediction of calcium-binding pockets from apo-protein structures exhibiting calcium-induced localized conformational changes. Protein Sci. 2010;19:1180–1190. doi: 10.1002/pro.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Jiang Y., Huang Y., Wong H.C., Zhou Y., Wang X., Yang J., Hall R.A., Brown E.M., Yang J.J. Elucidation of a novel extracellular calcium-binding site on metabotropic glutamate receptor 1α (mGluR1α) that controls receptor activation. J. Biol. Chem. 2010;285:33463–33474. doi: 10.1074/jbc.M110.147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Jiang J.Y., Nagaraju M., Meyer R.C., Zhang L., Hamelberg D., Hall R.A., Brown E.M., Conn P.J., Yang J.J. Extracellular calcium modulates actions of orthosteric and allosteric ligands on metabotropic glutamate receptor 1α. J. Biol. Chem. 2014;289:1649–1661. doi: 10.1074/jbc.M113.507665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Jones K.A., Borowsky B., Tamm J.A., Craig D.A., Durkin M.M., Dai M., Yao W.J., Johnson M., Gunwaldsen C., Huang L.Y., et al. Gaba(b) receptors function as a heteromeric assembly of the subunits GABAbR1 and GABAbR2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 293.Wise A., Green A., Main M.J., Wilson R., Fraser N., Marshall F.H. Calcium sensing properties of the GABAb receptor. Neuropharmacology. 1999;38:1647–1656. doi: 10.1016/S0028-3908(99)00119-7. [DOI] [PubMed] [Google Scholar]
- 294.Silve C., Petrel C., Leroy C., Bruel H., Mallet E., Rognan D., Ruat M. Delineating a Ca2+ binding pocket within the venus flytrap module of the human calcium-sensing receptor. J. Biol. Chem. 2005;280:37917–37923. doi: 10.1074/jbc.M506263200. [DOI] [PubMed] [Google Scholar]
- 295.Tordoff M.G., Shao H., Alarcón L.K., Margolskee R.F., Mosinger B., Bachmanov A.A., Reed D.R., McCaughey S. Involvement of t1r3 in calcium-magnesium taste. Physiol. Genomics. 2008;34:338–348. doi: 10.1152/physiolgenomics.90200.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Tordoff M.G., Alarcón L.K., Valmeki S., Jiang P. T1R3: A human calcium taste receptor. Sci. Rep. 2012;2:496. doi: 10.1038/srep00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Wellendorph P., Bräuner-Osborne H. Molecular cloning, expression, and sequence analysis of gprc6a, a novel family c G-protein-coupled receptor. Gene. 2004;335:37–46. doi: 10.1016/j.gene.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 298.Pi M., Quarles L.D. Osteoblast calcium-sensing receptor has characteristics of ANF/7TM receptors. J. Cell Biochem. 2005;95:1081–1092. doi: 10.1002/jcb.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Faure H., Gorojankina T., Rice N., Dauban P., Dodd R.H., Bräuner-Osborne H., Rognan D., Ruat M. Molecular determinants of non-competitive antagonist binding to the mouse gprc6a receptor. Cell Calcium. 2009;46:323–332. doi: 10.1016/j.ceca.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 300.Stenflo J., Stenberg Y., Muranyi A. Calcium-binding EGF-like modules in coagulation proteinases: Function of the calcium ion in module interactions. Biochim. Biophys. Acta. 2000;1477:51–63. doi: 10.1016/S0167-4838(99)00262-9. [DOI] [PubMed] [Google Scholar]
- 301.Raya A., Kawakami Y., Rodríguez-Esteban C., Ibañes M., Rasskin-Gutman D., Rodríguez-León J., Büscher D., Feijó J.A., Izpisúa Belmonte J.C. Notch activity acts as a sensor for extracellular calcium during vertebrate left-right determination. Nature. 2004;427:121–128. doi: 10.1038/nature02190. [DOI] [PubMed] [Google Scholar]
- 302.Halbleib J.M., Nelson W.J. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 303.Spassova M.A., Soboloff J., He L.P., Xu W., Dziadek M.A., Gill D.L. Stim1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc. Natl. Acad. Sci. USA. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Jardin I., López J.J., Redondo P.C., Salido G.M., Rosado J.A. Store-operated Ca2+ entry is sensitive to the extracellular Ca2+ concentration through plasma membrane stim1. Biochim. Biophys. Acta. 2009;1793:1614–1622. doi: 10.1016/j.bbamcr.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 305.Fasciani I., Temperán A., Pérez-Atencio L.F., Escudero A., Martínez-Montero P., Molano J., Gómez-Hernández J.M., Paino C.L., González-Nieto D., Barrio L.C. Regulation of connexin hemichannel activity by membrane potential and the extracellular calcium in health and disease. Neuropharmacology. 2013;75:479–490. doi: 10.1016/j.neuropharm.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 306.Ebihara L., Liu X., Pal J.D. Effect of external magnesium and calcium on human connexin46 hemichannels. Biophys. J. 2003;84:277–286. doi: 10.1016/S0006-3495(03)74848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Quist A.P., Rhee S.K., Lin H., Lal R. Physiological role of Gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J. Cell Biol. 2000;148:1063–1074. doi: 10.1083/jcb.148.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Gomez-Hernandez J.M., de Miguel M., Larrosa B., Gonzalez D., Barrio L.C. Molecular basis of calcium regulation in connexin-32 hemichannels. Proc. Natl. Acad. Sci. USA. 2003;100:16030–16035. doi: 10.1073/pnas.2530348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Thimm J., Mechler A., Lin H., Rhee S., Lal R. Calcium-dependent open/closed conformations and interfacial energy maps of reconstituted hemichannels. J. Biol. Chem. 2005;280:10646–10654. doi: 10.1074/jbc.M412749200. [DOI] [PubMed] [Google Scholar]
- 310.Ma Z., Tanis J.E., Taruno A., Foskett J.K. Calcium homeostasis modulator (CALHM) ion channels. Pflugers Arch. 2016;468:395–403. doi: 10.1007/s00424-015-1757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Babini E., Paukert M., Geisler H.S., Grunder S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1) J. Biol. Chem. 2002;277:41597–41603. doi: 10.1074/jbc.M205877200. [DOI] [PubMed] [Google Scholar]
- 312.Immke D.C., McCleskey E.W. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron. 2003;37:75–84. doi: 10.1016/S0896-6273(02)01130-3. [DOI] [PubMed] [Google Scholar]
- 313.Paukert M., Babini E., Pusch M., Gründer S. Identification of the Ca2+ blocking site of acid-sensing ion channel (ASIC) 1: Implications for channel gating. J. Gen. Physiol. 2004;124:383–394. doi: 10.1085/jgp.200308973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Jiang Q., Inoue K., Wu X., Papasian C.J., Wang J.Q., Xiong Z.G., Chu X.P. Cysteine 149 in the extracellular finger domain of acid-sensing ion channel 1b subunit is critical for zinc-mediated inhibition. Neuroscience. 2011;193:89–99. doi: 10.1016/j.neuroscience.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Johnson J.P., Mullins F.M., Bennett P.B. Human ether-à-go-go-related gene k+ channel gating probed with extracellular Ca2+. Evidence for two distinct voltage sensors. J. Gen. Physiol. 1999;113:565–580. doi: 10.1085/jgp.113.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Johnson J.P., Balser J.R., Bennett P.B. A novel extracellular calcium sensing mechanism in voltage-gated potassium ion channels. J. Neurosci. 2001;21:4143–4153. doi: 10.1523/JNEUROSCI.21-12-04143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Sanguinetti M.C., Jiang C., Curran M.E., Keating M.T. A mechanistic link between an inherited and an acquired cardiac arrhythmia: Herg encodes the ikr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 318.Xiong Z.G., Chu X.P., MacDonald J.F. Effect of lamotrigine on the Ca2+-sensing cation current in cultured hippocampal neurons. J. Neurophysiol. 2001;86:2520–2526. doi: 10.1152/jn.2001.86.5.2520. [DOI] [PubMed] [Google Scholar]
- 319.Xiong Z., Lu W., MacDonald J.F. Extracellular calcium sensed by a novel cation channel in hippocampal neurons. Proc. Natl. Acad. Sci. USA. 1997;94:7012–7017. doi: 10.1073/pnas.94.13.7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Burgo A., Carmignoto G., Pizzo P., Pozzan T., Fasolato C. Paradoxical Ca2+ rises induced by low external Ca2+ in rat hippocampal neurones. J. Physiol. 2003;549:537–552. doi: 10.1113/jphysiol.2003.041871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Wei W.L., Sun H.S., Olah M.E., Sun X., Czerwinska E., Czerwinski W., Mori Y., Orser B.A., Xiong Z.G., Jackson M.F., et al. Trpm7 channels in hippocampal neurons detect levels of extracellular divalent cations. Proc. Natl. Acad. Sci. USA. 2007;104:16323–16328. doi: 10.1073/pnas.0701149104. [DOI] [PMC free article] [PubMed] [Google Scholar]