Abstract
Soluble N-ethylmaleimide-sensitive fusion attachment protein receptor (SNARE)-mediated fusion of synaptic vesicles with the presynaptic-plasma membrane is essential for communication between neurons. Disassembly of the SNARE complex requires the ATPase N-ethylmaleimide-sensitive fusion protein (NSF). To determine where in the synaptic-vesicle cycle NSF functions, we have undertaken a genetic analysis of comatose (dNSF-1) in Drosophila. Characterization of 16 comatose mutations demonstrates that NSF mediates disassembly of SNARE complexes after synaptic-vesicle fusion. Hypomorphic mutations in NSF cause temperature-sensitive paralysis, whereas null mutations result in lethality. Genetic-interaction studies with para demonstrate that blocking evoked fusion delays the accumulation of assembled SNARE complexes and behavioral paralysis that normally occurs in comatose mutants, indicating NSF activity is not required in the absence of vesicle fusion. In addition, the entire vesicle pool can be depleted in shibire comatose double mutants, demonstrating that NSF activity is not required for the fusion step itself. Multiple rounds of vesicle fusion in the absence of NSF activity poisons neurotransmission by trapping SNAREs into cis-complexes. These data indicate that NSF normally dissociates and recycles SNARE proteins during the interval between exocytosis and endocytosis. In the absence of NSF activity, there are sufficient fusion-competent SNAREs to exocytose both the readily released and the reserve pool of synaptic vesicles.
Keywords: neurotransmitter release, exocytosis, synapse, Drosophila
The core components of the vesicle-fusion apparatus consist of the plasma-membrane soluble N-ethylmaleimide-sensitive fusion (NSF) attachment protein (SNAP) receptors (SNAREs) syntaxin and soluble NSF attachment protein of 25 kDa (SNAP-25) and the vesicle SNARE synaptobrevin/vesicle-associated membrane protein. Neuronal SNAREs assemble into highly stable SDS-resistant 7S SNARE complexes (1). The ATPase NSF has been implicated in numerous membrane-trafficking steps, including regulated exocytosis (2–8). NSF is recruited to the 7S complex by means of the adapter proteins α/β- and γ-SNAP to form a larger 20S complex that is subsequently disassembled by the ATPase activity of NSF (1). Both assembly and disassembly of the SNARE complex are essential for synaptic-vesicle trafficking in vivo (6–10). NSF belongs to the AAA family of ATPases (ATPases with diverse cellular activities) and forms a hollow cylindrical hexamer when viewed with high-resolution electron microscopy (11, 12). The structure of the NSF hexamer depends on the presence or absence of bound ATP or ADP and undergoes substantial conformational changes upon ATP hydrolysis (11). Each monomer consists of three domains (13): an N-terminal domain essential for interaction with the SNAP/SNARE complex, and two tandem ATPase domains termed D1 and D2. The D2 domain is required for hexamerization of NSF (11, 14–16). The D1 domain contains a high-affinity ATP-binding and hydrolysis site that accounts for the majority of the ATPase activity in NSF (15, 17). Recruitment of NSF to SNARE complexes via the SNAP adapters results in the specific stimulation of D1 ATPase activity and disassembly of the SNARE complex (17–20).
Molecular analysis in Drosophila has revealed two closely related NSF genes, dNSF-1 and dNSF-2 (21–23). NSF-1 and NSF-2 largely show a developmental and spatially restricted expression, suggesting nonredundant roles for the two proteins (21, 22). For example, NSF-2 is not expressed in the optic lobe (22), whereas NSF-1 is absolutely required for synaptic transmission in the fly eye (6). Mutations in NSF-1 have been shown to correspond to the temperature-sensitive paralytic mutation comatose (comt; ref. 23), whereas mutations in NSF-2 cause embryonic lethality (24). Tissue-specific rescue experiments suggest an essential role for NSF-1 in the nervous system, whereas NSF-2 is required in the mesoderm (24). Experimental analysis has not yet resolved where in the synaptic-vesicle cycle NSF functions. SNARE disassembly could be required for vesicle recruitment to active zones, for vesicle docking and priming, for fusion, or for vesicle endocytosis. Here, we provide evidence that the disassembly of SNARE complexes by NSF-1 occurs after synaptic-vesicle fusion. In the absence of NSF activity, multiple rounds of fusion of the vesicle pool can occur, with the consequent accumulation of cis-SNARE pairs (7S complexes present on the vesicle or plasma membrane). Only then is synaptic transmission disrupted in comt mutants, secondary to an inability to form trans-SNARE pairs that are capable of bridging the synaptic vesicle and plasma membrane.
Materials and Methods
Fly Strains.
Drosophila melanogaster were cultured on standard medium at 23°C. Lethal comt alleles were generated by feeding 1- to 2-day-old g sd f males ethyl methanesulfonate (EMS) or by exposure to 5,000 rad and crossing them to homozygous ST53 virgin females. Approximately 8,000–10,000 heterozygous F1 females were collected from these crosses and tested for temperature-sensitive (TS) paralysis in a 38°C water bath. Extra conditional comt mutations were generated by feeding Canton S males with EMS and mating to attached X females. Conditional paralytic mutations were maintained and tested for failure of complementation with known comt alleles.
Sequence Analysis of comt Mutations.
Genomic DNA of lethal comt alleles was isolated from comtlethal/comtST53 heterozygotes, and the genomic region spanning the ORF was amplified by PCR. The presence of a silent base-pair polymorphism distinguished the mutagenized g sd f and the ST53-bearing chromosomes, resulting in the creation of an Sau3A site at base pair 1266 of the comt locus carried by the g sd f chromosome. This polymorphism allowed DNA from the allele of interest to be unambiguously identified from the heterozygous comtlethal/comtST53 flies. An Sau3A-positive, g sd f-derived insert then was sequenced with a series of 10 overlapping primers. Mutations detected in these inserts were confirmed by sequencing the region of interest in an independently isolated g sd f insert. Genomic DNA of homozygous viable comt alleles was amplified directly and sequenced.
Preparation of 7S Complexes and Head Homogenates.
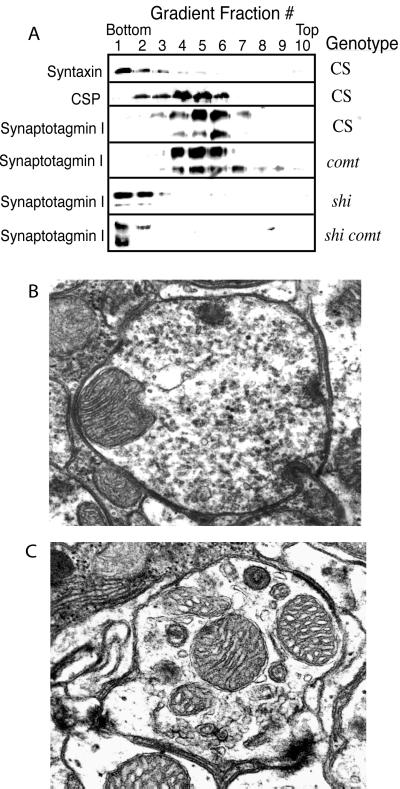
Native 7S complexes were prepared as described (6). Briefly, flies of the indicated genotype were frozen in liquid nitrogen, vortexed, and 10 heads were homogenized in 50 μl of SDS sample buffer. The supernatant (20 μl) was resuspended in 30 μl of fresh SDS sample buffer, and 10 μl of samples were loaded onto discontinuous SDS/9%-15% PAGE gels without boiling. The gels were immunoblotted with antisyntaxin monoclonal antibody 8C3 at 1:1,000 dilution. Immunoreactive bands were visualized by using enhanced chemiluminescence. Separation of subcellular fractions as shown in Fig. 3 was carried out with 15 ml of flies of the indicated genotypes maintained at room temperature or given a 20-min 38°C heat pulse. Flies were frozen in liquid nitrogen, and heads were obtained by sieving and grinding to a powder in liquid nitrogen. The powder was then homogenized on ice in 5 mM Hepes buffer (pH 7.4) containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride/2 μg/ml aprotinin/1 μg/ml leupeptin/1 mM EDTA/1 μg/ml pepstatin). Cell debris and cuticle were removed by centrifugation at 3,000 × g for 5 min. Velocity sedimentation was done with a 10–30% sucrose gradient centrifuged for 1 h at 50,000 RPM in a VTi65 rotor (Beckman Coulter). One milliliter of protein fractions was obtained beginning from the bottom of the gradient to the top and, subsequently, run out on SDS/PAGE gels, transferred to nitrocellulose, and probed for the specified antigens.
Figure 3.
NSF is not required for vesicle fusion until a pool of free SNAREs are depleted. (A) SDS/PAGE gels were run with fractions collected from sucrose-gradient velocity sedimentation of Drosophila head membranes from Canton S, comtST17, shiTS1, or comtST17 shiTS1 adults that were given a 20-min 38°C heat shock. Proteins were transferred to nitrocellulose and probed with antibodies to the indicated proteins. Plasma-membrane proteins such as syntaxin and the sodium pump migrate to the bottom of the gradient in the last few fractions. In contrast, synaptic-vesicle proteins migrate in fractions three to six. In shiTS1 or comtST17 shiTS1 mutants, synaptic-vesicle proteins accumulate in the plasma-membrane fractions, indicating that vesicles are capable of fusing with the redistribution of vesicle antigens to the plasma membrane. Ultrastructural analysis of comtST17 (B) or comtST17 shiTS1 (C) synapses from 1- to 3-day-old adult flies given a 10-min 38°C heat pulse under constant light stimulation support the biochemical results. Representative cross-sectioned retinal axon terminals are shown. In comt mutants, the terminals are filled with synaptic vesicles that have not fused with the membrane. In contrast, cross-sectioned retinal terminals of shiTS1 (not shown) or comtST17 shiTS1 mutants are devoid of synaptic vesicles and show an increase in larger vacuolar-type structures, as previously documented in shi mutants. Similar results were obtained from three to seven individuals of each genotype.
Electroretinograms and Transmission Electron Microscopy (TEM) Analysis.
Electroretinograms and TEM analysis were performed as described (6). Flies for TEM were maintained at 38°C for 10 min under constant light stimulation to drive vesicle cycling in photoreceptors before fixation.
Results and Discussion
To characterize further the role of NSF-1 in synaptic transmission, we isolated a series of new comt mutations through two approaches. Our first approach was to screen for X-linked temperature-sensitive paralytic mutants. Approximately 150,000 males containing an ethyl methanesulfonate (EMS)-mutagenized X-chromosome were tested for reversible temperature-dependent paralysis at 38°C. Three mutations failed to complement comt and thus represented new alleles. Three additional temperature-sensitive alleles of comt were identified in independent screens by Satpal Singh (personal communication). Together with three previously known comt mutations (ST17, TP7, and ST53), we characterized nine conditional alleles of NSF-1. A second screen that permitted isolation of new comt alleles—even if they were associated with a lethal phenotype—also was carried out. This screen was based on the observation that flies heterozygous for a conditional comt allele and a deletion that removes the gene are viable and manifest temperature-sensitive paralysis. Males were mutagenized with either EMS or γ-rays and crossed to homozygous comtST53 virgin females. Approximately 10,000 heterozygous F1 females were collected from these crosses and tested for temperature-sensitive paralysis at 38°C. Four new lethal alleles and one viable allele of comt were identified in this screen. Two additional recessive lethal comt alleles identified by B. Baker and K. S. Krishnan (personal communication) were included in our analysis.
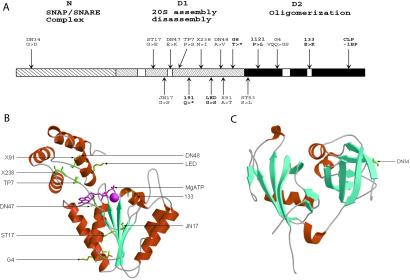
To determine the underlying amino acid changes that cause the comt mutant phenotypes, we sequenced the ORF of each allele (Fig. 1A). All but one of the temperature-sensitive paralytic mutants altered highly conserved amino acids within the D1 domain, a region required for NSF-mediated disassembly of the SNARE complex. comt alleles in this category include ST17 (G274E), JN17 (G387S), DN47 (E394K), TP7 (P398S), X238 (N418I), DN48 (A447V), X91 (A448T), and ST53 (S483L). One lethal mutation, LED, maps to the D1 domain and causes a G to S substitution at amino acid 443. The remaining comt temperature-sensitive paralytic mutation we identified was DN34, which alters a highly conserved Gly residue in the N-domain of NSF (G58D), a region required for SNAP-mediated binding to the SNARE complex (25). Analysis of the crystal structure of the N terminus of NSF reveals that Gly-58 lies in the first loop of the double-ψ β-barrel (DPBB) motif (Fig. 1C; refs. 25–27). The DPBB motif is common in many proteins and is often important for substrate binding (28). A third class of comt alleles alters the D2 domain of NSF, a region required for assembly of NSF into functional hexamers. Three lethal comt mutants lie in this domain, including 1121 (P494L), 133 (E606K), and CLP, which has a 1-bp deletion that shifts the reading frame 36 aa upstream of the carboxyl terminus and leads to a premature stop codon. One viable allele, comtG4, also disrupts the D2 domain and results in a 3-aa deletion/2-aa substitution (VQQ to GS at amino acids 525–527) near the ATP-binding site in the D2 domain. comtG4 mutants are only weakly temperature sensitive, but show a substantial reduction in lifespan with progressive weakness and lack of coordination. Because the three-dimensional structures of the D1 and D2 domains of NSF are likely to be highly conserved (14), we modeled the sites of the comt D1 and D2 mutants on the crystal structure of NSF-D2 (Fig. 1B). A large number of residues mutated in the temperature-sensitive mutants reside in a loop region that has been postulated to serve as a lever that imparts force to the SNARE complex during ATP hydrolysis. This region of the AAA domain also has been shown to undergo conformational shifts during the ATP-binding/hydrolysis cycle in the NSF-related protein, p97 (29, 30). The remaining conditional alleles cluster near the ATP-binding/hydrolysis site. Of the D2 domain alleles, comt133 alters a conserved glutamate in the ATP-hydrolysis domain, indicating that this mutation is likely to abolish hexamerization of NSF.
Figure 1.
Structural alteration of NSF in comt mutants. (A) The amino acid alterations in the NSF-1 protein relative to the previously defined functional domains are depicted diagrammatically. Lethal alleles are indicated in bold. (B) The locations of the mutations found in the D1 or D2 domain are modeled onto the crystal structure reported for the D2 domain. (C) The location of the G58D change in the DN34 allele is modeled onto the crystal structure reported for the N-terminal domain of NSF.
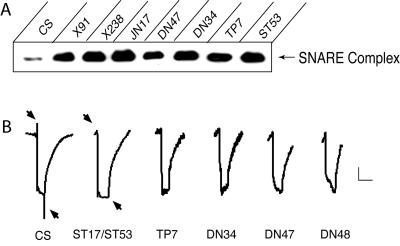
To characterize further the requirements for NSF-1 in vesicle trafficking, we analyzed both the temperature-sensitive and lethal comt mutants in more detail. Most comt temperature-sensitive mutations paralyze within several minutes at 38°C, similar to alleles described (6). Although the comtDN34 allele displays temperature-sensitive paralysis at 38°C, it requires a longer incubation time (6–7 min) and shows residual locomoter activity during longer 38°C incubations. The delayed paralysis in comtDN34 mutants correlates with its unique molecular defect in the N-terminal domain. All temperature-sensitive comt alleles showed a dramatic accumulation of SDS-resistant SNARE complexes compared with wild type, suggesting an essential role for NSF in the disassembly of SNARE complexes (Fig. 2A). Each comt temperature-sensitive mutant also blocked vesicle-trafficking in Drosophila photoreceptors at 38°C, as indicated by a loss of the on/off synaptic events in electroretinogram recordings from the retina (Fig. 2B). In contrast to the temperature-sensitive paralysis of the conditional alleles, complete removal of NSF-1 resulted in lethality. Two lethal alleles we identified result in premature stop codons that cause truncations of NSF-1. comt191 truncates in the middle of the D1 domain (Q405*) and is likely to be a null allele. comtG8 truncates at the end of D1 (T464*; Fig. 1). These mutations can be rescued with a free duplication carrying a wild-type comt allele or with a heat-shock driven NSF-1 transgene, indicating the lethal phenotypes are specifically associated with loss of NSF-1 activity. Null mutants died during embryonic and larval stages, although a small percentage survived to pupae.
Figure 2.
Temperature-sensitive comt alleles accumulate SNARE complexes and block synaptic transmission in the retina. (A) 7S complexes from wild type or the specified comt allele were isolated after a 20-min 38°C heat shock. Syntaxin was present in a 35-kDa monomeric form and in a 73-kDa complex with SNAP-25 and synaptobrevin. The 73-kDa complex is shown in A. All comt conditional alleles showed a dramatic accumulation of the 7S complex after exposure to 38°C. (B) Electroretinograms were recorded from wild type and comt temperature-sensitive paralytic mutations. Flies were rapidly heated from 20°C to 38°C and maintained at 38°C for 3–10 min. Note the loss of on- and off-transients (arrows) with a normal photoreceptor depolarization in comt flies. Similar losses of on/off transients were observed in X91, JN17, and X238 (not shown). Three to seven individuals of each genotype aged from 1 to 5 days were tested, with results similar to those shown. Scale bar is a 2 mV/division (vertical) and a 2 sec/division (horizontal).
The accumulation of SNARE complexes in the temperature-sensitive paralytic mutants suggests these mutations disrupt the ATPase activity or subsequent force generation required for SNARE-mediated disassembly (Fig. 2A). Consistent with this model, the altered NSF protein in comtST17 mutants has been biochemically demonstrated to have a temperature-dependent block in SNARE disassembly in vitro (31). However, it is unknown where NSF-mediated SNARE disassembly is required during the synaptic-vesicle cycle: before docking, after docking, during fusion, or after exocytosis. To determine the stage in the synaptic-vesicle cycle where NSF is required, we choose to study the comtST17 mutation in more detail. We took advantage of two other mutations that allow us to block specific stages in vesicle trafficking. shiTS1 is a temperature-sensitive mutation in dynamin that blocks synaptic-vesicle endocytosis (32, 33). paraTS1 is a temperature-sensitive mutation in the α-subunit of the sodium channel that blocks action potential propagation and subsequent evoked-vesicle fusion (34). We generated double mutants containing shiTS1 comtST17 or comtST17 paraTS1 by recombination. If NSF is required for exocytosis of the nerve terminal pool of synaptic vesicles, we would expect that NSF should be epistatic to dynamin in nerve terminals and lead to a block in vesicle fusion before the terminals are depleted of vesicles. If the inactivation of NSF does not block a step on the exocytotic side of the synaptic-vesicle pathway, then all of the vesicles would be capable of translocating to active zones, and of docking, priming, and fusing. Then, we would expect to see a depletion of synaptic vesicles in shiTS1 comtST17 double mutants similar to what is observed in shiTS1 mutants. In shiTS1 mutants incubated at 38°C, synaptic-vesicle proteins such as synaptotagmin, CSP, and synaptobrevin redistribute to the plasma-membrane fraction at 38°C when assayed on sucrose velocity sedimentation gradients (Fig. 3A; refs. 35 and 36). In wild type or comtST17 mutations incubated at 38°C, there was no shift in the distribution of synaptic-vesicle proteins. However, in shiTS1 comtST17 double mutants and in shiTS1 mutants incubated at 38°C (Fig. 3A), synaptic-vesicle proteins redistributed to the plasma membrane, indicating the synaptic-vesicle pool is capable of completing all stages of exocytosis, including fusion in comt mutants. To extend our biochemical analysis, we performed an ultrastructural analysis on photoreceptor nerve terminals. As reported, comtST17 mutants accumulate docked and undocked synaptic vesicles when incubated for 10 min at 38°C under constant light stimulation (Fig. 3B; refs. 6 and 8). In contrast, shiTS1 photoreceptor terminals are depleted of synaptic vesicles. Photoreceptor terminals of shiTS1 comtST17 double mutants show a depletion of synaptic vesicles similar to shiTS1 (Fig. 3C), confirming that both the docked and reserve pool of vesicles can fuse in comt mutants.
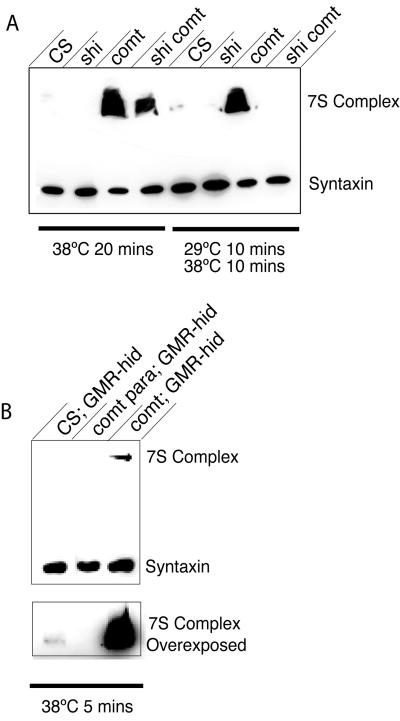
Next, we investigated how SNARE-complex accumulation in comtST17 mutants was affected by blocking vesicle recycling in shiTS1 comtST17 double mutants. If the large accumulation of SNARE complexes represents those complexes that assembled during one round of vesicle fusion at nerve terminals, SNARE-complex accumulation in shiTS1 comtST17 double mutants should be the same as in comtST17 alone. However, if multiple rounds of exocytosis of the vesicle pool can occur in the absence of NSF activity, and if these rounds of fusion are necessary to generate the large excess of unresolved SNARE complexes, we would expect to see a decrease in 7S accumulation in the double mutant, because shiTS1 blocks multiple rounds of vesicular cycling. SNARE complexes were isolated from comtST17 and shiTS1 comtST17 mutants. As shown in Fig. 4A, SNARE-complex accumulation was greatly reduced in the double mutant compared with comtST17 mutants alone, suggesting that multiple rounds of vesicle cycling do occur in the absence of NSF. As expected, there is still more SNARE complex present in the double mutant than in wild type, which is consistent with the accumulation of unresolved SNARE complexes formed during a single round of exocytosis. If endocytosis could be blocked without disrupting NSF activity, we reasoned that the accumulation of SNARE complexes should not occur in shiTS1 comtST17 mutants. We were able to test this possibility by taking advantage of the differential sensitivity of shiTS1 and comtST17 to temperature. Whereas shiTS1 mutants become paralyzed at 29°C, comtST17 mutants do not paralyze until 35°C. Therefore, we incubated double mutants at 29°C for 10 min to block endocytosis and to deplete the vesicle pool with NSF still active and then shifted to 38°C for 10 min to block NSF activity. Analysis of SNARE-complex accumulation with this protocol demonstrated that there was no SNARE-complex accumulation in the double mutant when the vesicle pool was depleted with NSF still active (Fig. 4A). These results suggest that NSF disassembles cis-SNARE complexes that reside in the plasma membrane as a consequence of fusion. In addition, free SNAREs that are acted upon by NSF and that are trapped in the plasma membrane in shi mutants do not spontaneously reassemble into cis-SNARE complexes once NSF activity is blocked.
Figure 4.
(A) shi reduces the accumulation of SNARE complexes in comt mutants. 7S complexes from 10 Canton S, comtST17, shiTS1, or comtST17 shiTS1 flies that were given a 20-min 38°C heat pulse were isolated. A reduction in the 7S complex was found in shi flies after exposure to 38°C, whereas comt mutants show a dramatic accumulation of 7S complex. This result is in marked contrast with comtST17 shiTS1 double mutants, which show a less dramatic accumulation of 7S complex after exposure to 38°C. 7S complexes also were isolated from 10 flies of the indicated genotypes after a 10-min heat pulse at 29°C, which was followed by a 10-min heat pulse at 38°C. Inactivation of dynamin before the inactivation of NSF prevents the buildup of 7S complex in shi comt double mutants. (B) Vesicle fusion is required for SNARE-complex accumulation in comt mutants. 7S complexes were isolated from 10 Canton S, comtST17 paraTS1, or comtST17 flies after a 5-min 38°C heat pulse. Each strain also expressed a GMR-hid transgene to eliminate the contribution of photoreceptor synapses to the 7S assay. para reduced the amount of 7S complex present (compared with both controls and comt mutants) and eliminated 7S accumulation in comt mutants.
A prediction of our model for NSF function is that SNARE complexes accumulate in comt as a direct consequence of vesicle fusion and a subsequent failure in disassembly of the complex postfusion. Manipulations that block release would be predicted to block the accumulation of SNARE complexes. We tested this hypothesis by using comtST17 paraTS1 double mutants. We have noted that paraTS1 does not block synaptic transmission in the fly retina (photoreceptors do not use para-dependent action potentials; ref. 6). Because the eye constitutes a significant portion of the Drosophila head and would, therefore, compromise our assays in comtST17 paraTS1 double mutants, we eliminated the eye and the contribution of photoreceptor synapses to our SNARE-complex assays by expression of GMR-hid (which drives the cell-death gene hid in photoreceptor primordial cells). To avoid the cumulative effect of spontaneous fusion events contributing to the build up of SNARE complexes in our assay, we assayed SNARE accumulation at 38°C at 5 min. In flies lacking photoreceptors, paraTS1 suppressed the accumulation of SNARE complex that otherwise would occur in comt mutations incubated at 38°C (Fig. 4B). This result confirms that SNARE complexes accumulate in comt mutants as a consequence of vesicle fusion. In addition, paraTS1 also eliminated the pool of SNARE complexes normally present in wild-type flies, suggesting that assembly of SNARE complexes requires nerve activity. This finding argues against stable SDS-resistant SNARE complexes forming during synaptic-vesicle docking or priming, because paraTS1 specifically blocks action potentials. An alternative explanation for the experimental findings in comt double mutants is that loss of NSF is not rapid at the restrictive temperature but occurs slowly. Several lines of evidence argue against this interpretation. First, eight conditional comt paralytic mutants alter distinct amino acids in the D1 domain, yet all show delayed paralysis, suggesting that this result is a general feature of the comt phenotype. Most convincingly, however, is the behavior of comt para double mutants (37). Double mutants exposed to 38°C for 5 min show a rapid recovery like para mutants alone when returned to the permissive temperature. After rapid recovery and normal behavior, these flies then suddenly reparalyze several minutes later at the permissive temperature. Because comt mutants would be completely paralyzed after 5 min at 38°C and require greater than 30 min to recover at permissive temperature, we conclude that NSF would be inactivated in the double mutant at 38°C.
Together, these results suggest that NSF does not function in fusion, recruitment of vesicles to active zones, docking, or priming. Rather, we propose that NSF specifically disassembles SNARE complexes after fusion and before endocytosis. In the absence of synaptic transmission in comtST17 paraTS1 mutants, SNARE complexes do not accumulate, and behavioral paralysis induced by loss of NSF function is delayed. Thus, in the absence of vesicle fusion, NSF is not required to prime or maintain synaptic vesicles in a fusion-competent state. Once NSF has been inactivated, vesicle release and activity continues for several minutes before paralysis occurs. In shi comt double mutants, inactivating both dynamin and NSF results in a shi phenotype, with synapses devoid of synaptic vesicles, confirming that vesicles continue to fuse in the absence of NSF activity. Thus, a large pool of excess v-SNAREs are present and can support fusion in the absence of NSF. After these pools are exhausted, a postdocking defect in fusion occurs because of an inability to form trans-SNARE pairs. Our findings complement and extend the models of NSF function provided by previous analyses of comt mutants in Drosophila (6–8, 24, 37, 38). Dorsal longitudinal flight muscle recordings from temperature-sensitive comt mutants have demonstrated a reduction in synaptic current during repetitive stimulation that is accompanied by an increase in the number of docked synaptic vesicles (8). In the absence of repetitive stimulation, no defects in fusion were detected, confirming that vesicle fusion itself does not require NSF activity. Rather, NSF is required to restore the pool of vesicles that are readily released, likely through its ability to break apart cis-SNARE complexes and reactivate individual SNAREs for additional rounds of fusion. Thus, multiple lines of evidence support the conclusion that several rounds of vesicle fusion can continue in comt mutants before synaptic transmission is finally poisoned by a lack of fusion-competent SNAREs. A similar postfusion role for the yeast NSF homolog, SEC1, also has been proposed (39).
Additional studies will be required to determine the pool size of fusion-competent SNAREs maintained by NSF activity at different synapses. This pool size will have important implications for presynaptic plasticity, as it would determine whether modulation of SNARE assembly/disassembly rates plays a role in controlling presynaptic output during stimulation. In fly photoreceptors and central synapses, we estimate that this pool size is sufficient to mediate the fusion of several rounds of the entire nerve-terminal synaptic-vesicle population. We base this conclusion on our observation that in shi comt double mutants, there is a substantial reduction (>2-fold) in the amount of SNARE complexes that accumulate, compared with comt mutants alone. This observation suggests the large increase in assembled SNARE complexes in comt mutants results from recycling of the vesicle pool more than once. At adult Drosophila neuromuscular junctions, the pool of fusion-competent SNAREs seems to be smaller, because nerve-stimulation rates as low as 5 Hz can rapidly decrease exocytosis in comt conditional mutants (8). Thus, the size of the fusion-competent pool of SNAREs can be differentially controlled at distinct synapses. Although it is unknown how many SNARE complexes are required for the fusion of a synaptic vesicle, our results argue that there are sufficient v-SNAREs present on each vesicle to suffice for several rounds of exocytosis without being regenerated through NSF action. Likewise, there seems to be a large pool of synaptic t-SNAREs that can function in many cycles of fusion without requiring regeneration through NSF action. Overexpression of the NSF adapter α-SNAP at crayfish neuromuscular junctions (40) or at the squid giant synapse (41) results in an increase in the pool of synaptic vesicles readily released. These findings suggest that modification of the functional pool size of fusion-competent SNAREs over time can alter synaptic function and may be an important mechanism for modulating synaptic plasticity. The use of fast FM dyes recently has defined an additional synaptic-vesicle pathway of vesicle fusion and immediate reuse (42). In such cases, the ability to rapidly dissociate SNARE complexes may be essential, and NSF activity may be rate-limiting in this rapid reuse pathway. The recent finding that patients with schizophrenia show a substantial reduction in NSF expression in the prefrontal cortex (43) suggests that disruption of SNARE-mediated disassembly rates also may be a contributing factor in psychiatric dysfunction. Further studies into the mechanisms that maintain fusion-competent SNAREs and accelerate NSF activity should provide insights into presynaptic plasticity and the machinery that modulates synaptic-vesicle fusion.
Acknowledgments
We thank Robert Kreber for help with mutagenesis, Martin Garment and Stanley Carlson for help with electron microscopy preparation, Seymour Benzer for generous gifts of antibodies, and S. Singh, K. S. Krishnan, B. Baker, and the Bloomington Stock Center for fly strains. This work was supported by National Institutes of Health Grants GM 56827-01, GM43100, NS40296-01, and NS15390.
Abbreviations
- NSF
N-ethylmaleimide-sensitive fusion
- SNAP
soluble NSF attachment protein
- SNARE
SNAP receptor
- TS
temperature sensitive
References
- 1.Sollner T, Whiteheart S W, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman J E. Nature (London) 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 2.Block M R, Glick B S, Wilcox C A, Wieland F T, Rothman J E. Proc Natl Acad Sci USA. 1988;85:7852–7856. doi: 10.1073/pnas.85.21.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckers C J, Block M R, Glick B S, Rothman J E, Balch W E. Nature (London) 1989;339:397–398. doi: 10.1038/339397a0. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee A, Barry V A, DasGupta B R, Martin T F. J Biol Chem. 1996;271:20223–20226. doi: 10.1074/jbc.271.34.20223. [DOI] [PubMed] [Google Scholar]
- 5.Wilson D W, Wilcox C A, Flynn G C, Chen E, Kuang W, Henzel W J, Block M R, Ullrich A, Rothman J E. Nature (London) 1989;339:355–359. doi: 10.1038/339355a0. [DOI] [PubMed] [Google Scholar]
- 6.Littleton J T, Chapman E R, Kreber R, Garment M B, Carlson S D, Ganetzky B. Neuron. 1998;21:401–413. doi: 10.1016/s0896-6273(00)80549-8. [DOI] [PubMed] [Google Scholar]
- 7.Tolar L A, Pallanck L. J Neurosci. 1998;18:10250–10256. doi: 10.1523/JNEUROSCI.18-24-10250.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawasaki F, Mattiuz A M, Ordway R W. J Neurosci. 1998;18:10241–10249. doi: 10.1523/JNEUROSCI.18-24-10241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y A, Scales S J, Patel S M, Doung Y C, Scheller R H. Cell. 1999;97:165–174. doi: 10.1016/s0092-8674(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 10.Xu T, Rammner B, Margittai M, Artalejo A R, Neher E, Jahn R. Cell. 1999;99:713–722. doi: 10.1016/s0092-8674(00)81669-4. [DOI] [PubMed] [Google Scholar]
- 11.Hanson P I, Roth R, Morisaki H, Jahn R, Heuser J E. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- 12.Hohl T M, Parlati F, Wimmer C, Rothman J E, Sollner T H, Engelhardt H. Mol Cell. 1998;2:539–548. doi: 10.1016/s1097-2765(00)80153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tagaya M, Wilson D W, Brunner M, Arango N, Rothman J E. J Biol Chem. 1993;268:2662–2666. [PubMed] [Google Scholar]
- 14.Lenzen C, Steinmann D, Whiteheart S, Weis W. Cell. 1998;94:525–536. doi: 10.1016/s0092-8674(00)81593-7. [DOI] [PubMed] [Google Scholar]
- 15.Whiteheart S W, Rossnagel K, Buhrow S A, Brunner M, Jaenicke R, Rothman J E. J Cell Biol. 1994;126:945–954. doi: 10.1083/jcb.126.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagiec E B, Bernstein A, Whiteheart S W. J Biol Chem. 1995;270:29182–29188. doi: 10.1074/jbc.270.49.29182. [DOI] [PubMed] [Google Scholar]
- 17.Matveeva E A, He P, Whiteheart W S. J Biol Chem. 1997;272:26413–26418. doi: 10.1074/jbc.272.42.26413. [DOI] [PubMed] [Google Scholar]
- 18.Barnard R J, Morgan A, Burgoyne R. J Cell Biol. 1997;139:875–883. doi: 10.1083/jcb.139.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnard R J, Morgan A, Burgoyne R D. Mol Biol Cell. 1996;7:693–701. doi: 10.1091/mbc.7.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steel G H, Morgan A. FEBS Lett. 1998;423:113–116. doi: 10.1016/s0014-5793(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 21.Ordway R W, Pallanck L, Ganetzky B. Proc Natl Acad Sci USA. 1994;91:5715–5719. doi: 10.1073/pnas.91.12.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulianne G L, Trimble W S. Proc Natl Acad Sci USA. 1995;92:7095–7099. doi: 10.1073/pnas.92.15.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pallanck L, Ordway R W, Ganetzky B. Nature (London) 1995;376:25. doi: 10.1038/376025a0. [DOI] [PubMed] [Google Scholar]
- 24.Golby J A, Tolar L A, Pallanck L. Genetics. 2001;158:265–278. doi: 10.1093/genetics/158.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babor S M, Fass D. Proc Natl Acad Sci USA. 1999;96:14759–14764. doi: 10.1073/pnas.96.26.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu R C, Jahn R, Brunger A T. Mol Cell. 1999;4:97–107. doi: 10.1016/s1097-2765(00)80191-4. [DOI] [PubMed] [Google Scholar]
- 27.May A P, Misura K M S, Whiteheart S W, Weis W I. Nat Cell Biol. 1999;1:175–182. doi: 10.1038/11097. [DOI] [PubMed] [Google Scholar]
- 28.Castillo R M, Mizuguchi K, Dhanaraj V, Albert A, Blundell T L, Murzin A G. Structure (London) 1999;7:227–236. doi: 10.1016/s0969-2126(99)80028-8. [DOI] [PubMed] [Google Scholar]
- 29.Rouiller I, Butel V, Latterich M, Milligan R, Wilson-Kubalek E. Mol Cell. 2000;6:1485–1490. doi: 10.1016/s1097-2765(00)00144-1. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Shaw A, Bates P, Newman R, Gowen B, Orlova E, Gorman M, Kondo H, Dokurno P, Lally J, et al. Mol Cell. 2000;1:1473–1485. doi: 10.1016/s1097-2765(00)00143-x. [DOI] [PubMed] [Google Scholar]
- 31.Muller J M, Rabouille C, Newman R, Shorter J, Freemont P, Schiavo G, Warren G, Shima D T. Nat Cell Biol. 1999;1:335–340. doi: 10.1038/14025. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda K, Ozawa S, Hagiwara S. Nature (London) 1976;259:489–491. doi: 10.1038/259489a0. [DOI] [PubMed] [Google Scholar]
- 33.Koenig J H, Ikeda K. J Neurosci. 1989;9:3844–3860. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loughney K, Kreber R, Ganetzky B. Cell. 1989;58:1143–1154. doi: 10.1016/0092-8674(89)90512-6. [DOI] [PubMed] [Google Scholar]
- 35.van de Goor J, Ramaswami M, Kelly R. Proc Natl Acad Sci USA. 1995;92:5739–5743. doi: 10.1073/pnas.92.12.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Littleton J T, Serano T L, Rubin G M, Ganetzky B, Chapman E R. Nature (London) 1999;400:757–760. doi: 10.1038/23462. [DOI] [PubMed] [Google Scholar]
- 37.Sanyal S, Basole A, Krishnan K, S. J Neurosci. 1999;19:RC47. doi: 10.1523/JNEUROSCI.19-24-j0005.1999. , 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawasaki F, Ordway R W. J Neurophysiol. 1999;82:123–130. doi: 10.1152/jn.1999.82.1.123. [DOI] [PubMed] [Google Scholar]
- 39.Grote E, Carr C, Novick P. J Cell Biol. 2000;151:439–451. doi: 10.1083/jcb.151.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He P, Southard R C, Chen D, Whiteheart S W, Cooper R L. J Neurophysiol. 1999;82:3406–3416. doi: 10.1152/jn.1999.82.6.3406. [DOI] [PubMed] [Google Scholar]
- 41.DeBello W M, O'Connor V, Dresbach T, Whiteheart S W, Wang S S, Schweizer F E, Betz H, Rothman J E, Augustine G J. Nature (London) 1995;373:626–630. doi: 10.1038/373626a0. [DOI] [PubMed] [Google Scholar]
- 42.Pyle J L, Kavalal E T, Piedras-Rentería E S, Tsien R W. Neuron. 2000;28:221–231. doi: 10.1016/s0896-6273(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 43.Mirnic K, Middleton F, Marquez A, Lewis D, Levitt P. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]