Summary
Trafficking and recruitment of immune cells to the site of inflammation with spatial and temporal synchronization is crucial for the development of allergic airway inflammation. Particularly, chemokines are known to be key players in these processes. Previous studies revealed that the CXCL12/CXCR4 axis plays an important role in regulating allergic airway inflammation. However, the role of CXCR7, a recently discovered second receptor for CXCL12, in regulating airway inflammation has not been explored. Initially, CXCR7 was considered as a decoy receptor; however, numerous subsequent studies revealed that engagement of CXCR7 triggered its own signalling or modulated CXCR4‐mediated signalling. In the present study, we detected the expression of CXCR7 in airway epithelial cells. Use of a lentiviral delivery system to knock down the expression of CXCR7 in the lung of sensitized mice abrogated the cardinal features of asthma, indicating that CXCR7 plays a role in regulating allergic airway inflammation. The activation of mitogen‐activated protein kinase and Akt signalling in response to CXCL12 in the mouse epithelial cell line MLE‐12 was reduced when CXCR7 expression was knocked down. However, either knockdown or overexpression of CXCR7 in MLE‐12 did not affect CXCL12‐mediated calcium influx, indicating that CXCR7 does not modulate CXCR4‐mediated signalling, and that it functions as a signalling receptor rather than a decoy receptor. Finally, we found that the expression of chemokine CCL2 is regulated by CXCR7/CXCL12‐mediated signalling through β‐arrestin in airway epithelial cells. Hence, regulating the expression of CCL2 in airway epithelial cells may be one mechanism by which CXCR7 participates in regulating allergic airway inflammation.
Keywords: airway epithelial cells, allergic airway inflammation, CXCR7
Introduction
Allergic airway inflammation is a disorder in which sensitized individuals develop eosinophilic airway inflammation, mucus hyper‐secretion and airway hyper‐responsiveness in response to inhaled aeroallergens. These features are orchestrated by dendritic cells, T helper type 2 (Th2) cells, eosinophils, mast cells and airway structure cells, such as epithelial cells. Trafficking and recruitment of leucocytes with spatial and temporal synchronization are important for establishing allergic airway inflammation. Chemokines, small chemotactic proteins, are key players in these processes and allow leucocytes with a corresponding receptor to migrate from the bloodstream into interstitial spaces and contribute to the inflammation process.
CXCL12, also known as stromal‐derived factor‐1, was originally identified as a pre‐B‐cell growth factor.1 Despite its roles in haematopoiesis and inflammation, CXCL12 also has important functions in various biological processes, including cardiac and neuronal development, stem cell motility, neovascularization, angiogenesis, apoptosis, tumorigenesis and metastasis.2, 3, 4, 5, 6 CXCR4 has long been thought to be the only receptor for CXCL12, as CXCR4‐deficient and CXCL12‐deficient mice have similar phenotypes.7, 8 Evidence from human and animal studies revealed that CXCL12/CXCR4 plays an important role in allergic airway inflammation.9, 10, 11, 12 Using a CXCR4 or CXCL12 neutralization antibody or the CXCR4 antagonist AMD3100, the CXCL12/CXCR4 axis has been demonstrated to play a pivotal role in airway inflammation and airway hyper‐responsiveness in an allergic airway‐inflammation mouse model.9, 10 Indeed, in patients with asthma, a high concentration of CXCL12 was observed in the bronchoalveolar lavage fluid (BALF), and levels of the chemokine were correlated with the number of leucocytes, including lymphocytes, macrophages and eosinophils, indicating that CXCL12 contributes to their recruitment into the airway.11
Recently, the orphan receptor RDC‐1, now known as CXCR7, was demonstrated to be an additional receptor for CXCL12.13, 14 Its binding affinity to CXCL12 is fivefold to tenfold higher than that of CXCR4 to CXCL12.13, 15 It is well accepted that CXCR7 is an atypical chemokine receptor, as engagement of CXCR7 does not trigger typical chemokine receptor signalling, such as calcium mobilization and chemotaxis.14, 16 Therefore, CXCR7 has been suggested to function as a decoy receptor that sequesters/scavenges CXCL12 from the environment, thereby shaping the availability of the chemokine for the signalling receptor CXCR4.17, 18, 19, 20, 21 However, studies of different cells and pathophysiological conditions have revealed a broad range of CXCR7 functions, showing that CXCL12 engagement to CXCR7 can induce a range of cellular responses, such as activation of the mitogen‐activated protein kinase (MAPK) and Akt pathways,22 receptor internalization,13, 17 cell survival,14, 22, 23, 24 proliferation25 and adhesion.22, 23, 26 Indeed, overexpression studies showed that when heterodimerized with CXCR4, CXCR7 can influence CXCR4‐dependent G protein‐mediated cell signalling, therefore affecting the role of CXCR4 in cellular physiology.16, 26, 27, 28 Recently, other studies showed that β‐arrestin‐2, other than G protein‐mediated signalling, is triggered by the engagement of CXCR7.27, 29, 30, 31
As the second and newly identified receptor for CXCL12, the role of CXCR7 in allergic airway inflammation has not been evaluated. In the present study, we examined whether CXCR7 is involved in regulating allergic airway inflammation. Our results suggest that epithelial CXCR7 may be involved in regulating the cardinal features of asthma by functioning as a signalling receptor rather than a decoy receptor, without affecting CXCR4‐mediated signalling. One of the possible mechanisms by which epithelial CXCR7 regulates allergic airway inflammation is through regulating the expression of CCL2 during inflammation.
Materials and methods
Animals and cells
Female BALB/c mice (6–8 weeks old) were purchased from the National Laboratory Animal Centre of Taiwan. All experiments conducted in this study were in accordance with the Institutional Animal Care and Use Committee of China Medical University according to Care of the Animals and Surgical Procedures of China Medical University Protocols (Approval protocol number: 102‐183‐N). MLE‐12, a mouse respiratory epithelial cell line obtained from ATCC (Manassas, VA), was cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum.
Immunofluorescence staining of CXCR7 and CXCR4
Lungs were inflated with a mixture of 1 : 1 OCT/PBS and snap‐frozen in optimum cutting temperature (OCT) on dry ice. Frozen sections (10 μm) were prepared, fixed with cold acetone for 3 min, dried and washed. The tissue sections were blocked using 1% bovine serum albumin inPBS containing 0·05% Tween‐20 and stained with CXCR7 (11G8) (R&D Systems, Minneapolis, MN) or CXCR4 (H‐118) (Santa Cruz Biotechnology, Dallas, TX) antibodies. The tissue sections were then incubated with secondary antibodies, Alexa Fluor 488‐conjugated chicken anti‐rabbit IgG and Alexa Fluor 594‐conjugated chicken anti‐rabbit IgG (Thermo Fisher Scientific, Waltham, MA), respectively. Nuclei were counterstained with DAPI (Sigma‐Aldrich, St Louis, MO). Protein expression was observed using a fluorescence microscope ECLIPSE 80i (Nikon Instruments, Inc., Tokyo, Japan).
Murine model of allergic airway inflammation
The protocol has been described previously.32 Briefly, the scheme of animal sensitization, treatment and challenge is shown in Fig. 1. Cytospin preparations from BALF, obtained by flushing the lungs with PBS, were stained with Pappenheim stain for cell differential analysis. Serum obtained by cardiac puncture was used to measure ovalbumin (OVA) ‐specific IgE. Cells from mediastinal lymph nodes were prepared for the T‐cell re‐stimulation assay.32 The concentrations of cytokines in culture supernatants after 48 hr of culture were measured using a Duo Set ELISA kit (R&D Systems). Whole lung lobes were dissected for histological and biochemical analyses.
Figure 1.
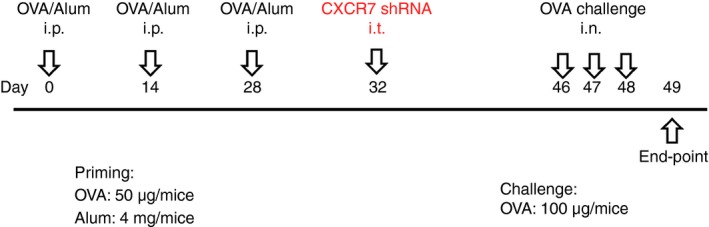
Brief scheme of animal sensitization, treatment and challenge. Abbreviations: i.p., intraperitoneal; i.t., intratracheal; i.n., intranasal; OVA, ovalbumin; Alum, Imject Alum (containing 40 mg/mL aluminum hydroxide and 40 mg/mL magnesium hydroxide)(Pierce, Rockford, IL, USA). [Colour figure can be viewed at http://wileyonlinelibrary.com]
Assay of airway hyper‐responsiveness
Airway resistance was assessed by invasive body plethysmography (flexiVent, SCIPEQ Scientific Respiratory Equipment Inc., Montreal, Quebec, Canada). The detailed protocol was described previously.32
Western blot analysis
Total cellular protein was prepared by lysing cells with SDS sample buffer. The proteins were separated on a 10% SDS–polyacrylamide gel and transferred onto a polyvinylidene fluoride membrane. Membranes were blocked with PBS containing 5% bovine serum albumin and incubated with the indicated antibodies. The blots were further incubated with the appropriate horseradish peroxidase‐conjugated secondary antibodies and developed using an enhanced chemiluminescence detection system (PerkinElmer, Waltham, MA) according to the manufacturer's instructions. The intensity of each band was quantified and analysed using image J software (National Institutes of Health, Bethesda, MD).
Lentivirus preparation and stable silencing of target genes
Lentivirus bearing target gene short hairpin RNA (shRNA) was prepared according to the protocols provided by the National Core Facility for Manipulation of Gene Function by RNAi, miRNA, miRNA sponges and CRISPR/Genomic Research Centre at Academia Sinica in Taiwan.
The target sequences for mouse CXCR7 were:
shRNA#1: 5′‐GCCTGGCAACTACTCTGACAT‐3′ (TRCN0000026660)
shRNA#2: 5′‐GCCTTCATCTTCAAGTACTCG‐3′.24
The target sequences for mouse β‐arrestin‐2 were:
5′‐CCTCATCGAATTCGATACCAA‐3′ (TRCN0000287639)
MLE‐12 cells (5 × 105) were transfected with lentiviral expression vector containing shRNA in six‐well culture plates and selected in medium containing 0·75 μg/ml puromycin 1 day later for stable silencing of the target gene.
Calcium mobilization assay
Calcium mobilization responses were measured using Fluo3 according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). MLE‐12 wild‐type cells were loaded with 10 nm/ml Fluo3 and 0·02% F127 for 30 min at 37° in a 5% CO2 incubator. The cells were then treated with or without 250 ng/ml CXCL‐12 (R&D Systems). Fluo‐3 was excited at 488 nm and emitted fluorescence was measured with a 515‐nm long‐pass filter. The relative fluorescence unit of calcium binding was measured using a Synergy H1 Hybrid Multi‐Mode Microplate Reader (BioTek, Winooski, VT).
Statistical analysis
The results were compared using one‐way analysis of variance (anova) with the software program graphpad prism 5 (GraphPad, Inc., La Jolla, CA). The data are presented as the mean ± standard error of mean (SEM). Probability values (P) of < 0·05 were considered statistically significant.
Results
Identification of CXCR7 expression in airway epithelial cells
To examine whether CXCR7 is expressed in the lung, Western blotting analysis was performed using the protein prepared from lungs of naive mice or allergen‐induced airway‐inflamed mice. As shown in Fig. 2(a), the expression of CXCR7 was increased in the lungs of inflamed mice. Furthermore, immunofluorescence staining of lung tissue from inflamed mice indicated that CXCR7 was predominantly expressed in epithelial cells. In contrast to CXCR4, which was expressed in airway epithelial cells, the expression of CXCR7 could be further detected in vessel endothelial cells (Fig. 2b).
Figure 2.

Expression of CXCR7 in the lung. (a) The expression level of CXCR7 in the lung was analysed by Western blot analysis. The protein extract was prepared from the lungs of three naive and three ovalbumin (OVA) ‐sensitized and challenged inflamed BALB/c mice. Band intensity was quantified and analysed with image J software after normalizing the expression levels of protein to β‐actin. Numbers represent the mean ± SEM of relative expression level to naive‐1. *P < 0·001 compared with naive. Statistical significance was determined using Student's t‐test. (b) Frozen mouse lung sections derived from inflamed BALB/c mice were evaluated for the expression of CXCR7 (green) and CXCR4 (red) using immunofluorescence staining.
Knockdown of CXCR7 in the lung reduced allergic airway inflammation
Because CXCR7 knockout mice show early postnatal lethality,16, 33 to examine whether CXCR7 plays a role in regulating Th2‐mediated allergic airway inflammation, we carried out an experiment using a murine asthma model in which CXCR7 was knocked‐down by intratracheally delivering lentivirus carrying CXCR7 shRNA into sensitized mice before challenge (Fig. 1). To investigate the effect of CXCR7 on airway inflammation, total and differential cell counts in BALF were analysed after the mice were challenged with the allergen OVA. The relative expression levels of CXCR7 in the lung of challenged mice are shown in the Supplementary material (Fig. S1). When CXCR7 expression was knocked down before challenge, less cell infiltration was observed in the lungs of challenged mice (Fig. 3a). Histological examination of fixed lung tissue prepared from these mice revealed that the extent of peribronchiolar cell inflammation and mucus secretion was lower in mice that received lentivirus carrying CXCR7 shRNA than in mice that received lentivirus carrying the control vector alone (Fig. 3b).
Figure 3.
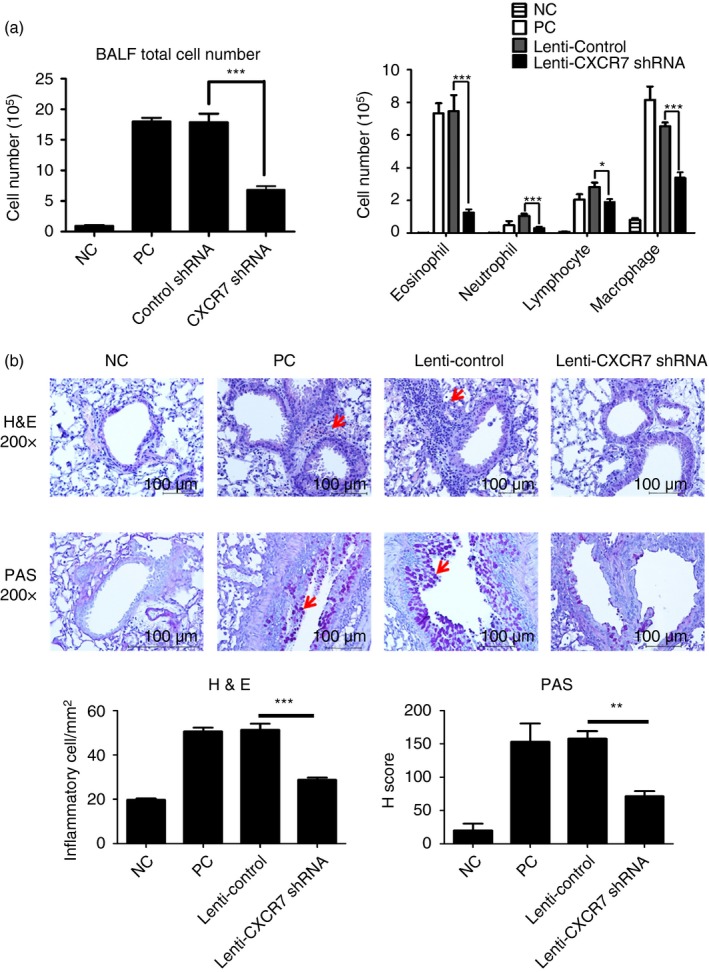
Reduced airway inflammation following CXCR7 knockdown (a) Bronchoalveolar lavage fluid (BALF) total cell number (left) and differential cell counts (right) following CXCR7 knockdown virus delivery and three ovalbumin (OVA) challenges were assessed by Pappenheim stain. Numbers represent the mean ± SEM of cell number (n ≥ 4). *P < 0·05; ***P < 0·001 compared with Lenti‐Control. Statistical significance was determined using one‐way anova. Data shown are representative of three independent experiments. (b) Representative lung sections stained with haematoxylin and eosin (H&E; ×200) or periodic acid‐Schiff (PAS; ×200). Arrows indicate areas of airway infiltrate (H&E) or presence of mucous substance (PAS). Numbers for H&E staining represent the mean ± SEM of infiltrating inflammatory cells in subepithelial and subendothelial area (mm2) in lung section (n = 3). Numbers for PAS staining represent the mean ± SEM of H‐Score (n = 3). **P < 0·01; ***P < 0·001 compared with Lenti‐Control. Statistical significance was determined by one‐way anova. NC: negative control, mice sensitized and challenged with PBS. PC: positive control, mice sensitized and challenged with OVA. Lenti‐Control: lentivirus carrying control vector was intratracheally administered to sensitized mice 2 weeks before challenge. Lenti‐CXCR7 shRNA: lentivirus carrying CXCR7 short hairpin RNA was intratracheally administered to sensitized mice 2 weeks before challenge.
Knockdown of CXCR7 in the lung reduced serum allergen‐specific IgE production and T‐cell cytokine production
Elevated levels of IgE in patients with asthma are also an indicator of allergy. Therefore, we examined whether knockdown of CXCR7 in the lung could reduce the level of OVA‐specific IgE in the serum. We found that OVA‐specific IgE levels were significantly reduced (Fig. 4a).
Figure 4.
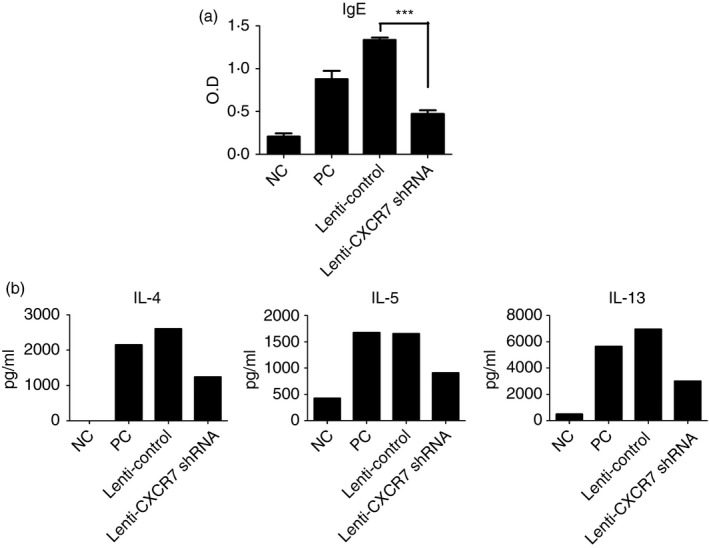
Serum ovalbumin (OVA) ‐specific IgE and cytokine production was reduced in CXCR7 knockdown mice. (a) OVA‐specific IgE in the serum was measured by ELISA. Numbers represent the mean ± SEM of optical density (OD; n = 4). ***P < 0·001 compared with Lenti‐Control. Statistical significance was determined by one‐way anova. (b) Cytokine production by mediastinal lymph node (LN) cells isolated from mice following re‐stimulation with antigen‐presenting cells and OVA were analysed by ELISA. In each experiment, mediastinal LN cells in the same group were pooled for culturing. Data shown are representative of three independent experiments. Data for an additional two additional experiments are shown in the Supplementary material (Fig. S2). NC: negative control, mice sensitized and challenged with PBS. PC: positive control, mice sensitized and challenged with OVA. Lenti‐Control: lentivirus carrying control vector was intratracheally administered to sensitized mice 2 weeks before challenge. Lenti‐CXCR7 shRNA: lentivirus carrying CXCR7 short hairpin RNA was intratracheally administered to sensitized mice 2 weeks before challenge.
Th2 cells play a central role in the disease process, contributing to IgE production by B cells, growth and differentiation of eosinophils and mast cells, and the development of bronchial hyperactivity and goblet cell hyperplasia. Hence, to further establish whether the T‐cell response was defective, cytokine production by mediastinal lymph node cells isolated from mice following re‐stimulation with antigen‐presenting cells and OVA were analysed by ELISA. As shown in Fig. 4(b) and the Supplementary material (Fig. S2), Th2‐related cytokine production was diminished when CXCR7 expression was knocked down in the lung. Because CXCR7 was knocked‐down after sensitization, the function and numbers of existing effector and memory T cells were the same as in the control, indicating that the re‐activation/expansion of effector/memory cells was defective during challenge when CXCR7 expression was knocked down.
Development of airway hyper‐responsiveness was reduced when CXCR7 was knocked down in the lung
We subsequently examined whether knockdown of CXCR7 expression in the lung suppressed the development of airway hyper‐responsiveness. As shown in Fig. 5, CXCR7‐knockdown mice showed significant decreases in pulmonary resistance compared with the control.
Figure 5.
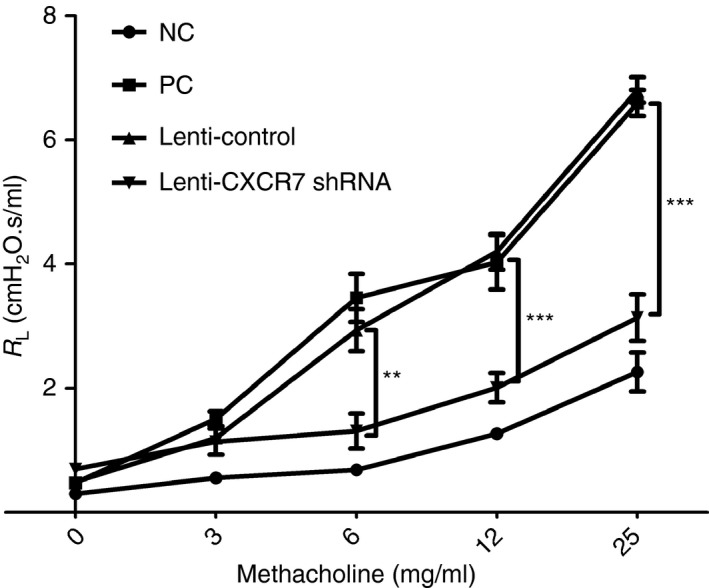
Reduced airway hyper‐responsiveness following CXCR7 knockdown. Airway resistance was measured 48 hr after the final challenge by invasive body plethysmography. Data are expressed as the mean ± SEM of pulmonary resistance (RL) (n = 3). **P < 0.01; ***P < 0·001. Statistical significance was determined by one‐way anova. NC: negative control, mice sensitized and challenged with PBS. PC: positive control, mice sensitized and challenged with ovalbumin (OVA). Lenti‐Control: lentivirus carrying control vector was intratracheally administered to sensitized mice 2 weeks before challenge. Lenti‐CXCR7 shRNA: lentivirus carrying CXCR7 short hairpin RNA was intratracheally administered to sensitized mice 2 weeks before challenge.
CXCR7 functions as a signalling receptor rather than a decoy receptor in lung epithelial cells
Depending on the cell type, tissue and pathophysiological state, CXCR7 can function as a decoy receptor or signalling receptor. As shown in Fig. 2(b), both CXCR7 and CXCR4 were expressed in airway epithelial cells; so it is unlikely that CXCR7 functions as a decoy receptor that sequesters CXCR12 from the environment to shape the availability for the signalling receptor CXCR4. As we delivered lentivirus carrying CXCR7 shRNA into mice intratracheally, the cell types infected were predominantly epithelial cells. To confirm whether engagement of CXCR7 participates in CXCL‐12‐mediated lung epithelial signalling, MLE‐12 cells, a mouse lung epithelial cell line, and their derived control (bearing control vector), as well as a CXCR7‐knockdown cell line (bearing CXCR7 shRNA expression construct) were stimulated with CXCL12, after which the activation of MAPK (ERK, JNK, and P38) and Akt was analysed. Knockdown efficacy is shown in the Supplementary material (Fig. S3). Although CXCL12 activated MAPK and Akt signalling in wild‐type and control MLE‐12 cells, the knockdown of CXCR7 attenuated the intensity of the CXCL12‐MAPK and CXCL12‐Akt signals (Fig. 6a and the Supplementary material, Fig. S4), indicating that CXCR7 participates in CXCL‐12‐mediated signalling.
Figure 6.
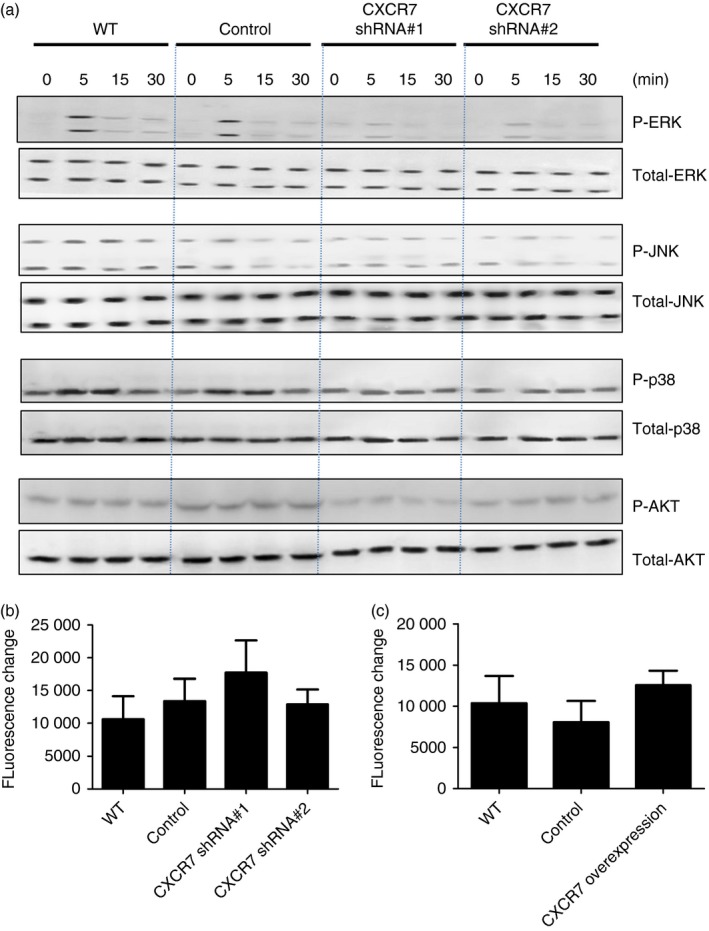
CXCR7 participates in CXCL12‐mediated signalling in lung epithelial cells. (a) Protein extract was isolated from MLE‐12 cells, their derived control (bearing control vector, control), and CXCR7 knockdown cell line (bearing CXCR7 shRNA expression construct, CXCR7 short hairpin RNA) following stimulation with 100 ng/ml CXCL12 for the indicated time. Western blotting was performed using the indicated antibodies. Quantitative data are shown in the Supplementary material (Fig. S2). (b, c) Indicated cell lines were loaded with 10 nm/ml Fluo3 and stimulated with 250 ng/ml CXCL12. The changes in relative fluorescence intensity due to calcium influx were assessed. Data are expressed as the mean ± SEM of fluorescence change. Statistical significance was determined by one‐way anova. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Although the engagement of CXCR7 does not trigger typical G protein coupled receptor‐dependent signalling, it has been proposed that CXCR7 might serve as a co‐receptor for CXCR4 and influence CXCL12/CXCR4‐mediated G‐protein signalling.16, 26, 28 CXCR7 knockdown does not alter the expression of CXCR4 in airway epithelial cells (see Supplementary material, Fig. S3); however, to clarify whether CXCR7 might influence CXCL12/CXCR4‐mediated G‐protein signalling, calcium influx, a typical Gαi‐coupling‐mediated signalling, induced by CXCL12 was examined. No significant difference was observed between wild‐type airway epithelial cells and CXCR7‐knockdown cells (Fig. 6b) or cells overexpressing CXCR7 (Fig. 6c), indicating that engagement of CXCR7 by CXCL12 in airway epithelial cells triggers signalling and does not interfere with CXCR4/CXCL‐12‐mediated signalling.
CXCR7 signal elevates the expression levels of CCL2 in airway epithelial cells
To identify the potential mediators induced by CXCR7 engagement that participate in regulating allergic airway inflammation, a cytokine protein array was used to examine the cytokine profile in the BALF of challenged mice. We found that the concentration of monocyte chemotactic protein‐1, also known as CCL2, was also reduced (Fig. 7a). CCL2 can be produced by lung epithelial cells and monocytes and is known to recruit CCR2+ monocytes to the site of inflammation and plays an important role in asthmatic responses.34, 35, 36, 37 To confirm that airway epithelial cells can produce CCL2 in response to CXCL‐12, MLE‐12 was stimulated with CXCL‐12. As shown in Fig. 7(b), CXCL‐12 can induce airway epithelial cells to produce CCL2. To confirm that airway epithelial cells can produce CCL2 through the CXCL12/CXCR7‐mediated pathway, MLE‐12 was stimulated with CXCL‐12. Indeed, when CXCR7 was overexpressed in airway epithelial cells, the production of CCL2 by these cells was augmented (Fig. 7c). This indicates that CXCR7 could regulate the production of CCL2 in airway epithelial cells. To examine whether CXCL12/CXCR7‐induced CCL2 expression bypasses the CXCL12/CXCR4‐mediated signalling pathway, AMD3100, a CXCR4 antagonist was used. As shown in Fig. 7(b), in response to CXCL12, the production of CCL2 was not altered when CXCR4‐mediated signalling was blocked by AMD3100, indicating that CXCL12‐induced expression of CCL2 is not through CXCR4. It is known that β‐arrestin‐2, other than G protein‐mediated signalling, is triggered by the engagement of CXCR7.27, 29, 30, 31 To examine whether CXCL12/CXCR7‐induced CCL2 expression is through β‐arrestin, β‐arrestin was knocked down in MLE‐12 cells and the production of CCL2 in response to CXCL12 was examined. As shown in Fig. 7(d), the production of CCL2 was decreased when β‐arrestin was knocked down in MLE‐12 cells. These data indicate that CXCL12/CXCR7 induced CCL2 expression through β‐arrestin, without bypassing the CXCL12/CXCR4‐mediated signalling pathway.
Figure 7.
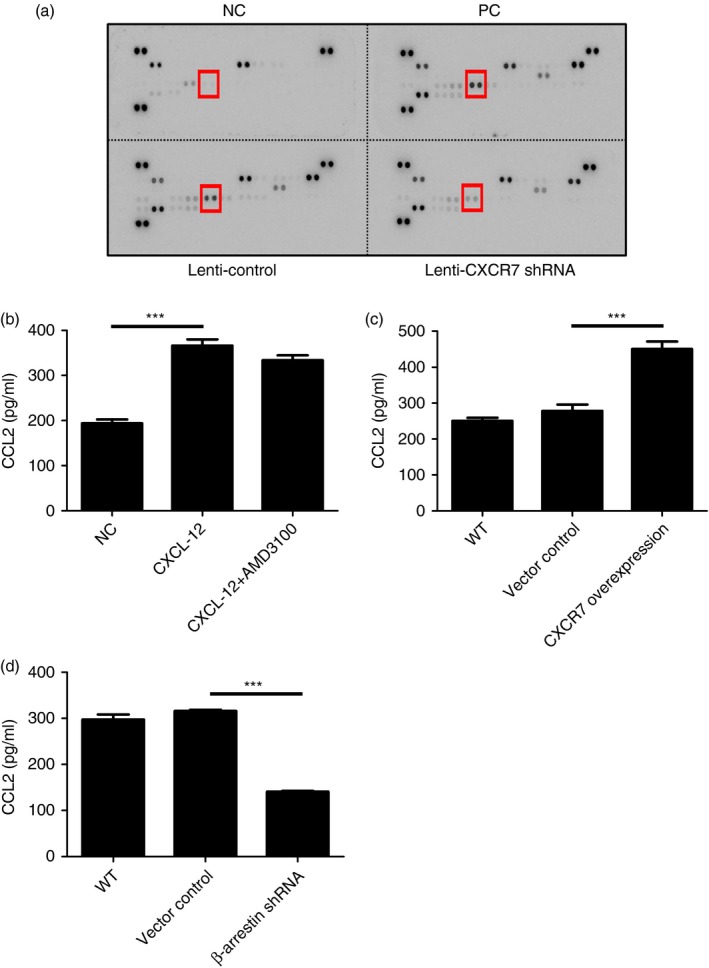
Production of CCL2 by airway epithelial cells was regulated by engagement of CXCR7. (a) The Mouse Cytokine Array Panel A purchased from R&D Systems was used to analyse the levels of cytokines in the bronchoalveolar fluid collected from indicated mice. Indicated cytokine is CCL2. (b) Concentrations of CCL2 in cell culture supernatant collected from MLE‐12 cell line stimulated with 100 ng/ml CXCL12 with or without 10 μm AMD3100 for 15 hr were determined by ELISA using a Duo Set ELISA kit (R&D Systems). (c) Concentrations of CCL2 in the cell culture supernatant collected from MLE‐12 cells, their derived control (bearing control vector, p), and CXCR7 overexpression cell line (bearing CXCR7 cDNA expression construct) stimulated with 100 ng/ml CXCL12 for 15 hr were analysed by ELISA. (d) Concentrations of CCL2 in the cell culture supernatant collected from MLE‐12, their derived control (bearing control vector), and β‐arrestin 2 knockdown cell line (bearing β‐arrestin 2 short hairpin RNA expression construct) following stimulation with 100 ng/ml CXCL12 for 15 hr were analysed by ELISA. Data were expressed as the mean ± SEM of protein concentration. ***P < 0·001. Statistical significance was determined by one‐way anova. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Discussion
Our data indicate that CXCR7 functions as a signalling receptor and is expressed in the airway epithelium. Its expression was further augmented under inflamed conditions. Engagement of CXCR7 is involved in regulating the cardinal features of asthma, as knockdown of CXCR7 in the lung of sensitized mice using a lentiviral delivery system abrogated the characteristic features of asthma, such as inflammation, mucus secretion, serum IgE concentration and airway hyper‐responsiveness. We further found that these defective features may have resulted in part from lower production of CCL2 in the lung.
CCL2 (monocyte chemotactic protein/CCL2) is expressed by many different cell types, including epithelial cells. CCL2 is a potent chemoattractant not only for macrophages, but also for basophils and activated and memory T cells.38, 39, 40, 41 Several studies have suggested that CCL2/CCR2 play important roles in the pathogenesis of asthma.42 Elevated CCL2 levels are present in the BALF of individuals with asthma.37 Significant release of CCL2 into the airway after endobronchial challenge has also been observed in patients with asthma.36 Polymorphisms in the CCL2 gene are associated with asthma in children and adults.43 When CCL2 activity was blocked with neutralization antibodies, the levels of airway inflammation and hyper‐responsiveness were attenuated.34, 35, 42 Additionally, in CCR2‐deficient mice, airway hyper‐responsiveness was significantly reduced.34 Furthermore, CCL2 was also shown to influence T‐cell differentiation towards Th2 cells and enhance T cells to produce interleukin‐4.44, 45
In this study, we observed defective recall T‐cell responses in the lymph node (Fig. 4b and Supplementary material, Fig. S1). Because CXCR7 was knocked‐down before challenge, the function and numbers of existing effector and memory T cells before challenge were expected to be the same between control and experimental groups. It is likely that the re‐activation/expansion of effector/memory cells was defective, resulting in a reduced number of effector T cells in the mediastinal lymph nodes, and so fewer activated effector T cells in the lymph node were available for re‐stimulation.
Dendritic cells (DCs) are known to be important in inducing T helper cell immunity in the lymph node.46, 47 When CD11c+ cells were conditionally depleted locally from the lung immediately before the antigen (OVA) challenge in OVA‐sensitized mice, van Rijt LS et al. observed that in the absence of DCs, the cardinal features of asthma failed to develop and that ex vivo isolated CD4 T cells produced greatly diminished amounts of Th2‐related cytokines,46 indicating that DCs are key cells in the development of secondary immune responses. DCs in the lung are comprised of heterogeneous populations including conventional CD11b+ DCs, CD103+ DCs, plasmacytoid DCs and monocyte‐derived DCs. Among these cells, only conventional CD11b+ CD64‐MAR‐1– DCs and CD11b+ CD64+ MAR‐1+ monocyte‐derived DCs are principle subsets for inducing Th2 cell‐mediated immunity.47 Upon inhalation of an antigen, lung epithelial cells produce CCL2 and CCL7, which induce the release of CCR2+ monocytes from the bone marrow. When the monocytes reach the airway, they give rise to not only monocyte‐derived macrophages, but also monocyte‐derived DCs. Interestingly, we found that the concentrations of CCL2 in the BALF of CXCR7‐knockdown inflamed mice were reduced, which may explain why we observed fewer macrophages in the BALF (Fig. 3a). We further demonstrated that CCL2 expression was regulated by CXCL12/CXCR7‐mediated signalling in lung epithelial cells. Epithelial cell‐derived products clearly have the potential to modulate the lung microenvironment and DC functional maturation. Hence, one of the mechanisms by which CXCR7 regulates allergic airway inflammation may be through regulating the expression of epithelial cell‐derived proteins such as CCL2, which may influence DC functional maturation or regulate the recruitment of monocytes. This subsequently gives rise to DCs, so increasing the number of antigen‐bearing DCs migrating to the draining lymph node. It would be of interest to examine the function and number of DCs that migrate into mediastinal lymph nodes.
In conclusion, the present study demonstrated that expression of CXCR7 in the lung is up‐regulated during inflamed conditions and that engagement of CXCR7 with CXCL12 triggers signal transduction and regulates gene expression. CCL2, an important mediator of allergic immune responses, is one of the molecules regulated by CXCR7‐mediated signalling, implicating the critical role of CXCR7 in regulating allergic airway inflammation. Hence, CXCR7 could be a potential therapeutic target for allergic airway diseases.
Authors' Contribution
Conception and design: H‐C Chen, J Lu; acquisition of data: H‐C Chang, P‐H Huang, F‐S Syu, S L‐Y Chang; analysis and interpretation: H‐C Chen, J Lu, H‐C Chang, P‐H Huang, F‐S Syu, C‐H Hsieh; writing the manuscript for important intellectual content: H‐C Chen, H‐C Chang.
Disclosures
The authors declare no conflict of interest.
Supporting information
Figure S1. Expression level of CXCR7 in the lung of CXCR7 knockdown mice.
Figure S2. Cytokine production was reduced in CXCR7 knockdown mice.
Figure S3. Knockdown efficacy of CXCR7 short hairpin RNA in MLE‐12 cells.
Figure S4. Quantitative analysis of Western blotting in Fig. 6(a).
Acknowledgements
This work was supported by the Ministry of Science and Technology, Republic of China (Taiwan) (NSC 102‐2320‐B‐039‐041) and China Medical University (CMU101‐S‐37, CMU102‐S‐06, CMU103‐S‐27 and CMU 104‐TC‐05). Experiments and data analysis were performed in part through the use of the Medical Research Core Facilities Centre, Office of Research & Development at China Medical University, Taichung, Taiwan. Florescence microscopy, Nikon ECLIPSE 80i was provided by the Centre for Molecular Medicine, China Medical University Hospital. We thank Mark Penfold from ChemoCentryx for the gift of CXCR7 antibody.
Contributor Information
Jean Lu, Email: jeanlu@gate.sinica.edu.tw.
Hui‐Chen Chen, Email: hcchen725@mail.cmu.edu.tw.
References
- 1. Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre‐B‐cell growth‐stimulating factor. Proc Natl Acad Sci U S A 1994; 91:2305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atluri P, Woo YJ. Pro‐angiogenic cytokines as cardiovascular therapeutics: assessing the potential. BioDrugs 2008; 22:209–22. [DOI] [PubMed] [Google Scholar]
- 3. Chen M, Xie HQ, Deng L, Li XQ, Wang Y, Zhi W et al Stromal cell‐derived factor‐1 promotes bone marrow‐derived cells differentiation to cardiomyocyte phenotypes in vitro . Cell Prolif 2008; 41:336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hernandez‐Lopez C, Valencia J, Hidalgo L, Martinez VG, Zapata AG, Sacedon R et al CXCL12/CXCR4 signaling promotes human thymic dendritic cell survival regulating the Bcl‐2/Bax ratio. Immunol Lett 2008; 120:72–8. [DOI] [PubMed] [Google Scholar]
- 5. Schonemeier B, Kolodziej A, Schulz S, Jacobs S, Hoellt V, Stumm R. Regional and cellular localization of the CXCl12/SDF‐1 chemokine receptor CXCR7 in the developing and adult rat brain. J Comp Neurol 2008; 510:207–20. [DOI] [PubMed] [Google Scholar]
- 6. Zhang S, Qi L, Li M, Zhang D, Xu S, Wang N et al Chemokine CXCL12 and its receptor CXCR4 expression are associated with perineural invasion of prostate cancer. J Exp Clin Cancer Res 2008; 27:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 1998; 393:595–9. [DOI] [PubMed] [Google Scholar]
- 8. Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T et al Impaired B‐lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4‐ and SDF‐1‐deficient mice. Proc Natl Acad Sci U S A 1998; 95:9448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gonzalo JA, Lloyd CM, Peled A, Delaney T, Coyle AJ, Gutierrez‐Ramos JC. Critical involvement of the chemotactic axis CXCR4/stromal cell‐derived factor‐1α in the inflammatory component of allergic airway disease. J Immunol 2000; 165:499–508. [DOI] [PubMed] [Google Scholar]
- 10. Lukacs NW, Berlin A, Schols D, Skerlj RT, Bridger GJ. AMD3100, a CxCR4 antagonist, attenuates allergic lung inflammation and airway hyperreactivity. Am J Pathol 2002; 160:1353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Negrete‐Garcia MC, Velazquez JR, Popoca‐Coyotl A, Montes‐Vizuet AR, Juarez‐Carvajal E, Teran LM. Chemokine (C‐X‐C motif) ligand 12/stromal cell‐derived factor‐1 is associated with leukocyte recruitment in asthma. Chest 2010; 138:100–6. [DOI] [PubMed] [Google Scholar]
- 12. Hachet‐Haas M, Balabanian K, Rohmer F, Pons F, Franchet C, Lecat S et al Small neutralizing molecules to inhibit actions of the chemokine CXCL12. J Biol Chem 2008; 283:23189–99. [DOI] [PubMed] [Google Scholar]
- 13. Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B et al The chemokine SDF‐1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem 2005; 280:35760–6. [DOI] [PubMed] [Google Scholar]
- 14. Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z et al A novel chemokine receptor for SDF‐1 and I‐TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med 2006; 203:2201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crump MP, Gong JH, Loetscher P, Rajarathnam K, Amara A, Arenzana‐Seisdedos F et al Solution structure and basis for functional activity of stromal cell‐derived factor‐1; dissociation of CXCR4 activation from binding and inhibition of HIV‐1. EMBO J 1997; 16:6996–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sierro F, Biben C, Martinez‐Munoz L, Mellado M, Ransohoff RM, Li M et al Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF‐1 receptor, CXCR7. Proc Natl Acad Sci U S A 2007; 104:14759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boldajipour B, Mahabaleshwar H, Kardash E, Reichman‐Fried M, Blaser H, Minina S et al Control of chemokine‐guided cell migration by ligand sequestration. Cell 2008; 132:463–73. [DOI] [PubMed] [Google Scholar]
- 18. Cruz‐Orengo L, Holman DW, Dorsey D, Zhou L, Zhang P, Wright M et al CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. J Exp Med 2011; 208:327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanchez‐Alcaniz JA, Haege S, Mueller W, Pla R, Mackay F, Schulz S et al Cxcr7 controls neuronal migration by regulating chemokine responsiveness. Neuron 2011; 69:77–90. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Li G, Stanco A, Long JE, Crawford D, Potter GB et al CXCR4 and CXCR7 have distinct functions in regulating interneuron migration. Neuron 2011; 69:61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dambly‐Chaudiere C, Cubedo N, Ghysen A. Control of cell migration in the development of the posterior lateral line: antagonistic interactions between the chemokine receptors CXCR4 and CXCR7/RDC1. BMC Dev Biol 2007; 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang J, Shiozawa Y, Wang Y, Jung Y, Pienta KJ, Mehra R et al The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF‐1 in prostate cancer. J Biol Chem 2008; 283:4283–94. [DOI] [PubMed] [Google Scholar]
- 23. Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML et al Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med 2008; 205:479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miao Z, Luker KE, Summers BC, Berahovich R, Bhojani MS, Rehemtulla A et al CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor‐associated vasculature. Proc Natl Acad Sci U S A 2007; 104:15735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meijer J, Ogink J, Roos E. Effect of the chemokine receptor CXCR7 on proliferation of carcinoma cells in vitro and in vivo . Br J Cancer 2008; 99:1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26. Hartmann TN, Grabovsky V, Pasvolsky R, Shulman Z, Buss EC, Spiegel A et al A crosstalk between intracellular CXCR7 and CXCR4 involved in rapid CXCL12‐triggered integrin activation but not in chemokine‐triggered motility of human T lymphocytes and CD34+ cells. J Leukoc Biol 2008; 84:1130–40. [DOI] [PubMed] [Google Scholar]
- 27. Decaillot FM, Kazmi MA, Lin Y, Ray‐Saha S, Sakmar TP, Sachdev P. CXCR7/CXCR4 heterodimer constitutively recruits β‐arrestin to enhance cell migration. J Biol Chem 2011; 286:32188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12‐mediated G protein signaling. Blood 2009; 113:6085–93. [DOI] [PubMed] [Google Scholar]
- 29. Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP et al β‐arrestin‐ but not G protein‐mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci U S A 2010; 107:628–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luker KE, Gupta M, Steele JM, Foerster BR, Luker GD. Imaging ligand‐dependent activation of CXCR7. Neoplasia 2009; 11:1022–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of β‐arrestin‐biased agonism at seven‐transmembrane receptors. Annu Rev Pharmacol Toxicol 2011; 52:179–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen JH, Huang PH, Lee CC, Chen PY, Chen HC. A bovine whey protein extract can induce the generation of regulatory T cells and shows potential to alleviate asthma symptoms in a murine asthma model. Br J Nutr 2013; 109:1813–20. [DOI] [PubMed] [Google Scholar]
- 33. Gerrits H, van Ingen Schenau DS, Bakker NE, van Disseldorp AJ, Strik A, Hermens LS et al Early postnatal lethality and cardiovascular defects in CXCR7‐deficient mice. Genesis 2008; 46:235–45. [DOI] [PubMed] [Google Scholar]
- 34. Campbell EM, Charo IF, Kunkel SL, Strieter RM, Boring L, Gosling J et al Monocyte chemoattractant protein‐1 mediates cockroach allergen‐induced bronchial hyperreactivity in normal but not CCR2–/– mice: the role of mast cells. J Immunol 1999; 163:2160–7. [PubMed] [Google Scholar]
- 35. Gonzalo JA, Lloyd CM, Wen D, Albar JP, Wells TN, Proudfoot A et al The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med 1998; 188:157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holgate ST, Bodey KS, Janezic A, Frew AJ, Kaplan AP, Teran LM. Release of RANTES, MIP‐1 alpha, and MCP‐1 into asthmatic airways following endobronchial allergen challenge. Am J Respir Crit Care Med 1997; 156:1377–83. [DOI] [PubMed] [Google Scholar]
- 37. Sousa AR, Lane SJ, Nakhosteen JA, Yoshimura T, Lee TH, Poston RN. Increased expression of the monocyte chemoattractant protein‐1 in bronchial tissue from asthmatic subjects. Am J Respir Cell Mol Biol 1994; 10:142–7. [DOI] [PubMed] [Google Scholar]
- 38. Conti P, Boucher W, Letourneau R, Feliciani C, Reale M, Barbacane RC et al Monocyte chemotactic protein‐1 provokes mast cell aggregation and [3H]5‐HT release. Immunology 1995; 86:434–40. [PMC free article] [PubMed] [Google Scholar]
- 39. Bischoff SC, Krieger M, Brunner T, Dahinden CA. Monocyte chemotactic protein 1 is a potent activator of human basophils. J Exp Med 1992; 175:1271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bonecchi R, Bianchi G, Bordignon PP, D'Ambrosio D, Lang R, Borsatti A et al Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med 1998; 187:129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med 1998; 187:875–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lukacs NW, Strieter RM, Warmington K, Lincoln P, Chensue SW, Kunkel SL. Differential recruitment of leukocyte populations and alteration of airway hyperreactivity by C‐C family chemokines in allergic airway inflammation. J Immunol 1997; 158:4398–404. [PubMed] [Google Scholar]
- 43. Szalai C, Kozma GT, Nagy A, Bojszko A, Krikovszky D, Szabo T et al Polymorphism in the gene regulatory region of MCP‐1 is associated with asthma susceptibility and severity. J Allergy Clin Immunol 2001; 108:375–81. [DOI] [PubMed] [Google Scholar]
- 44. Chensue SW, Warmington KS, Ruth JH, Sanghi PS, Lincoln P, Kunkel SL. Role of monocyte chemoattractant protein‐1 (MCP‐1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen‐induced granuloma formation: relationship to local inflammation, Th cell expression, and IL‐12 production. J Immunol 1996; 157:4602–8. [PubMed] [Google Scholar]
- 45. Karpus WJ, Lukacs NW, Kennedy KJ, Smith WS, Hurst SD, Barrett TA. Differential CC chemokine‐induced enhancement of T helper cell cytokine production. J Immunol 1997; 158:4129–36. [PubMed] [Google Scholar]
- 46. van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C et al In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med 2005; 201:981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco‐Madeira F, Toussaint W et al Conventional and monocyte‐derived CD11b+ dendritic cells initiate and maintain T helper 2 cell‐mediated immunity to house dust mite allergen. Immunity 2013; 38:322–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Expression level of CXCR7 in the lung of CXCR7 knockdown mice.
Figure S2. Cytokine production was reduced in CXCR7 knockdown mice.
Figure S3. Knockdown efficacy of CXCR7 short hairpin RNA in MLE‐12 cells.
Figure S4. Quantitative analysis of Western blotting in Fig. 6(a).


