Abstract
Background
The original equine sepsis score provided a method of identifying foals with sepsis. New variables associated with sepsis have been evaluated, but the sepsis score has not been updated.
Objectives
To evaluate the sensitivity and specificity of 2 updated sepsis scores and the systemic inflammatory response syndrome (SIRS) criteria in regard to detecting sepsis in foals.
Animals
Two‐hundred and seventy‐three ill foals and 25 healthy control foals.
Methods
Historical, physical examination, and clinicopathologic findings were used to calculate the original sepsis score and 2 updated sepsis scores. SIRS criteria were also evaluated. Sepsis scores and positive SIRS scores were statistically compared to foals with sepsis.
Results
One‐hundred and twenty‐six foals were septic and 147 sick‐nonseptic. The original and updated sepsis scores were significantly higher in septic foals as compared to sick‐nonseptic and healthy foals. The sensitivity and specificity of the updated sepsis scores to predict sepsis were not significantly better than those of the original sepsis score. One‐hundred and twenty‐seven of 273 (46.5%) foals met the original SIRS criteria and 88/273 (32%) foals met the equine neonatal SIRS criteria. The original SIRS criteria had similar sensitivity and specificity for predicting sepsis as did the 3 sepsis scores in our study.
Conclusions and Clinical Importance
The updated sepsis scores did not provide improved ability in predicting sepsis. Fulfilling the original SIRS criteria provided similar sensitivity and specificity in predicting sepsis as the modified sepsis score and might serve as a diagnostic aid in identifying foals at risk for sepsis.
Keywords: foal, infection, septic, SIRS
Abbreviations
- AUC
area under the curve
- CI
confidence interval
- OR
odds ratio
- ROC
receiver operating characteristic
- SIRS
systemic inflammatory response syndrome
1. INTRODUCTION
Sepsis remains a leading cause of morbidity and mortality in neonatal foals despite the improved survival that has been reported over the last 25 years.1, 2, 3 A vital component in improving outcome in human adult septic patients is early detection of bacteremia as well as rapid implementation of antimicrobial therapy.4 Blood culture remains the gold standard to confirm sepsis and provides valuable antimicrobial susceptibility information.5 However, blood culture techniques have several limitations including temporal delay in result reporting (48–72 hours), low chance of isolation of some pathogens and only fair sensitivity.6, 7, 8, 9 Furthermore, positive blood cultures have been documented in healthy neonatal foals or can be positive in association with other disease processes that allow bacterial translocation, both of which might cause transient bacteremia, but not reflect true infection.10 False negative blood culture results also might occur from low numbers of circulating bacteria, collection of low volumes of blood for culture, and prior antimicrobial administration.11 Multiplex PCR techniques have been used to help improve the sensitivity of detecting blood pathogens in some intensive care units in human medicine, but these techniques are not widely used in equine medicine.9, 11
The original equine sepsis score was developed in the 1980s as an adjunctive method to blood culture to provide clinicians a rapid tool to predict the likelihood of sepsis in foals.12, 13 This scoring system uses subjective clinical criteria and objective clinicopathological data and assigns a number for each criterion.12, 13 Points are summated and compared with a cut‐point (initially >11 was considered predictive of sepsis).12, 13 A modified sepsis score subsequently was developed that eliminated some variables (eg, metabolic acidosis, PaO2) and was more clinically practical.13 Retrospective analysis of the modified sepsis score demonstrated good sensitivity (94%) and specificity (86%), but more recent evaluations have yielded lower sensitivity (56%‐67%) and specificity (73%‐76%), suggesting that score cut‐points might be hospital‐dependent.13, 14, 15
Since the introduction of the modified equine sepsis score, more recent studies have identified updated or additional variables that have potential association with neonatal sepsis.2, 15, 16, 17, 18 For example, the median l‐lactate concentration in septic foals (4.8 mmol/L) was significantly higher than that of nonseptic foals (3.3 mmol/L) in a large prospective study.16 In another study, serum creatinine concentration and lymphocyte count were evaluated in a multivariable model that correctly classified 62% of septic cases but in that study, the modified sepsis score was better at predicting sepsis when compared with the model.15 A different study also described an association between hypoglycemia and sepsis in foals.17 In addition, the concept of the systemic inflammatory response syndrome (SIRS) was introduced in 1991 to describe the pro‐inflammatory response, primarily observed with infection, in people and criteria were developed that would aid in patient classification for research studies (subsequently referred to as the original SIRS criteria in our study).19 Although a variety of criteria have been used to describe SIRS in horses, a recent review outlined specific criteria that expand upon the original definition used in people to potentially improve specificity in foals (subsequently referred to as the equine neonatal SIRS criteria in our study).20 Several studies in horses have evaluated the relationship between blood glucose or l‐lactate concentrations and SIRS, but none have directly correlated SIRS with sepsis,17, 18 in part because of the variability in definitions for sepsis, some of which are dependent on a positive sepsis score or positive blood culture. Thus, validation of SIRS criteria is lacking in equine neonates.
Our objectives were to evaluate the sensitivity and specificity of the modified sepsis score as well as updated sepsis scores (to include lymphocyte count, and blood l‐lactate and serum creatinine concentrations) using the original SIRS criteria and an updated sepsis score using the proposed equine neonatal SIRS criteria in a large multicenter population of regionally diverse hospitalized neonatal foals. A secondary objective was to determine the association between the original SIRS criteria as well as the proposed equine neonatal SIRS criteria with sepsis and survival.
2. MATERIALS AND METHODS
2.1. Animals
The study was a concurrent and prospective study involving neonatal foals (≤30 days of age) from the 2016 foaling season presented to Iowa State University (Ames, IA), Ohio State University (Columbus, OH), Rood & Riddle Equine Hospital (Lexington Kentucky), University of Florida (Gainesville, FL), University of California (Davis, CA), Cornell University (Ithaca, NY), and Hagyard Equine Medical Institute (Lexington, KY). As part of the initial routine diagnostic evaluation of a sick foal, blood culture samples were collected from all foals presented to the intensive care units of the internal medicine services at the respective hospitals. To be included in the study, foals must have had recorded results of a blood culture performed during hospitalization, documentation of either discharge from the hospital or nonsurvival (death or euthanasia), and availability of criteria to generate a modified and updated sepsis score. In addition, 25 university‐owned healthy neonatal foals (11 from Iowa State University; 14 from Ohio State University) were used as control foals. The study was approved by Iowa State University's Animal Care and Use Committee.
2.2. Classification
Foals were classified as septic if they fulfilled any or all of the following criteria: (1) positive blood culture, (2) > 1 site of infection based on cytology, bacterial culture or histopathology results, or (3) postmortem evidence of > 1 septic process as evidenced by positive bacterial culture, identification of bacteria on histopathology, or microscopic evidence of infection and inflammation in multiple synovial structures.15 Foals that did not meet any of the above criteria were classified as sick‐nonseptic. Foals that were discharged from the hospital were classified as survivors whereas foals that died or were euthanized were classified as nonsurvivors. Control foals were determined to be healthy based on normal physical examination and CBC results as well as adequate passive transfer of maternal antibodies.
2.3. Updated sepsis score
The foundation of the updated sepsis score was the modified equine neonatal sepsis score as presented previously (Score 1).12, 13 This standardized semiquantitative score was updated (updated sepsis scores) based on data available since the modified sepsis score was implemented. Specifically, the scoring system for blood glucose concentration was slightly modified based on another study.17 In the updated sepsis score, a numeric score of 1 was assigned if the blood glucose concentration was 50–75 mg/dL as compared with the modified sepsis score, which used 50–80 mg/dL.17 Blood glucose concentration was considered acceptable if it was > 75 mg/dL in the updated sepsis score as compared with > 80 mg/dL in the modified sepsis score. New criteria incorporated into the updated sepsis score included blood l‐lactate concentration, serum creatinine concentration, lymphocyte count, and the presence of SIRS. Serum creatinine concentration and lymphocyte count cut‐points were based on calculations performed on published data3 using a specificity of at least 85% for detection of sepsis by receiver operator characteristic (ROC) analysis (S. Giguere, personal communication). The numeric score for blood l‐lactate concentration was based on data from previous studies that documented normal blood l‐lactate concentration in healthy 24‐hour old foals (mean, 2.1 mmol/L) as well as median (7.65 mmol/L) and range (0.9–24 mmol/L) of blood l‐lactate concentrations in septic foals.18, 21 Lastly, in the updated sepsis scores, fever was not evaluated as a sole criterion, but rather the rectal temperature was incorporated into the proposed equine neonatal SIRS criteria (Score 2), which is detailed in Figure 1.20 In addition to the equine neonatal SIRS criteria, the original SIRS criteria also were evaluated in an updated sepsis score (Score 3). Score 3 was identical to Score 2 with the exception that the original SIRS criteria were used. In Score 3, fever was not evaluated as a sole criterion as the rectal temperature was evaluated within the SIRS criteria; a foal was positive for the original SIRS definition if it had ≥ 2 of the following criteria: rectal temperature > 102.6°F or < 99.0°F, tachycardia (using age‐specific values noted in Figure 1), respiratory rate > 56 breaths/min, or leukocytosis or leucopenia (using age‐specific values noted in Figure 1). Thus, each foal included in the study had 3 scores calculated using data from the time of admission: Score 1 (modified sepsis score),12, 13 Score 2 (updated sepsis score with equine neonatal SIRS criteria),20 and Score 3 (updated sepsis score with original SIRS criteria).19
Figure 1.
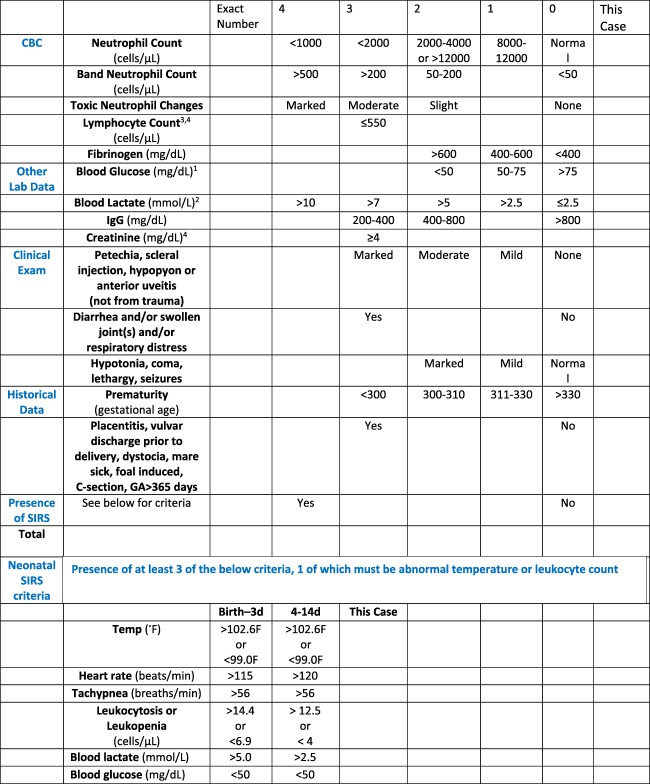
Updated sepsis score using the equine neonatal SIRS criteria (score 2)
2.3.1. Sampling and blood processing
Each facility processed blood samples for bacterial culture at the respective institution and each had slight variations in blood culture methods. In general, 5–10 mL of blood was collected from all foals at admission using sterile technique directly from the jugular or cephalic vein or through a jugular catheter immediately after catheter placement. Blood was placed in two commercial blood culture bottles (eg, enriched soybean‐casein digest broth medium with resins for antimicrobial neutralization) for incubation in aerobic and anaerobic environments for a maximum of 7 days in an incubator at 37°C. Blood culture bottles were sub‐cultured onto various culture media (laboratory dependent) 2–3 times over the 7‐day period. Bacterial colonies grown on media were identified using standard microbiology methods including biochemical identification or matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry (MALDI‐TOF) identification. Morphologic assessment of leukocytes (ie, toxic neutrophils) was evaluated within 24 hours of presentation because some institutions did not examine blood smears after‐hours.
2.3.2. Data collection and statistical evaluation
None of the data was normally distributed when tested for normality by the Shapiro‐Wilk statistic. Medians and ranges were calculated for continuous variables. The Mann‐Whitney‐U test was used to compare sepsis scores between survivors and non‐survivors. The Kruskal‐Wallis statistic was used to compare sepsis scores, white blood cell counts, blood glucose concentrations, l‐lactate concentrations, temperature, and heart and respiratory rates between foal groups. Associations between positive blood culture and categorical variables were analyzed using contingency tables and chi‐square analysis. Univariate logistic regression was applied to examine the odds ratios (OR) for sepsis and non‐survival in foals presented with the equine neonatal SIRS and original SIRS. The Hosmer and Lemeshow Goodness‐of‐Fit test indicated that the data fit the model (P = .71).
Blood culture results were consolidated and indicated if the culture was positive or negative, and, if positive, what specific bacteria were identified. The overall performance of the three sepsis scores for predicting sepsis was assessed by use of ROC curve analysis. The area under the ROC curve is a summary statistic of the overall diagnostic performance of a test. The difference between the area under the curve (AUC) of ROC curves for the 3 sepsis scores was assessed using the Hanley and McNeli method as previously described.22 Blood culture has historically been considered the reference standard for detection of bacteremia. However, sensitivity, specificity, accuracy, and predictive values were calculated by defining sepsis as fulfilling 1 of the 3 aforementioned criteria (positive blood culture, multifocal sites of infection, or postmortem evidence of disseminated septic processes).15
3. RESULTS
3.1. Study population
A total of 273 sick foals with a median age at presentation of 12 hours was included in the study; 158 were colts, 109 were fillies, 1 was a hermaphrodite and in 5 foals, sex was not recorded. Of the foals that met the inclusion criteria, the number of foals admitted to each hospital was as follows: Rood & Riddle Equine Hospital‐109; Hagyard Equine Medical Institute‐53; Ohio State University‐35; Iowa State University‐26; University of California‐17; Cornell University‐17; and, University of Florida‐16. One‐hundred and twelve foals were classified as septic based on bacterial growth on culture of blood samples (112/273; 41%) with 135 bacterial isolates identified (Table 1). An additional 14 foals were classified as septic based on antemortem presence of multiple sites of infection in an individual patient or postmortem examination, yielding a total 126/273 (46%) classified as septic and 147/273 (54%) as sick‐nonseptic. The median scores for each of the 3 sepsis scores were significantly higher in the sick‐nonseptic and septic groups compared with healthy foals (Table 2). In addition, the modified and updated sepsis scores were significantly higher in the septic foals when compared with sick‐nonseptic foals. The median value for each sepsis score is presented in Table 2.
Table 1.
Blood culture results from 273 neonatal foals presented to 7 referral hospitals during the 2016 foaling season
| Gram‐negative organisms | Number of positive results | Percent of Gram‐negative | Percent total positive | Gram‐positive organisms | Number of positive results | Percent of gram‐positive | Percent total positive |
|---|---|---|---|---|---|---|---|
| Escherichia coli | 15 | 21.1 | 11.2 | Staphylococcus spp | 12 | 19 | 9 |
| Pantoea agglomerans | 12 | 16.9 | 9 | Enterococcus spp | 6 | 9.5 | 4.5 |
| Actinobacillus spp | 9 | 12.7 | 6.7 | Staphylococcus spp (coag. neg) | 6 | 9.5 | 4.5 |
| Enterobacter spp | 7 | 9.9 | 5.2 | Streptococcus (α‐hemolytic) | 6 | 9.5 | 4.5 |
| Klebsiella pneumonia | 7 | 9.9 | 5.2 | Bacillus spp | 5 | 7.9 | 3.7 |
| Agrobacterium spp | 4 | 5.6 | 3 | Diptheroids | 5 | 7.9 | 3.7 |
| Acintobacter spp | 3 | 4.2 | 2.2 | Streptococcus Group A | 4 | 6.3 | 3 |
| Gram‐Negative rod | 3 | 4.2 | 2.2 | Streptococcus spp | 4 | 6.3 | 3 |
| Pseudomonas spp. | 3 | 4.2 | 2.2 | Corynebacterium spp | 3 | 4.8 | 2.2 |
| Salmonella spp | 3 | 4.2 | 2.2 | Gram‐Positive rod | 3 | 4.8 | 2.2 |
| Gram‐Negative bacilli | 2 | 2.8 | 1.5 | Leifsonia aquatic | 3 | 4.8 | 2.2 |
| Aeromonas | 1 | 1.4 | 0.7 | Clostridium spp | 2 | 3.2 | 1.5 |
| Campylobacter fetus | 1 | 1.4 | 0.7 | Curtobacterium flaccumfaciens | 1 | 1.6 | 0.7 |
| Neisseria | 1 | 1.4 | 0.7 | Gemella morbillorum | 1 | 1.6 | 0.7 |
| Saccharomyces cerevisiae a | 1 | – | – | Kytococcus sedantarius | 1 | 1.6 | 0.7 |
| Okibacterium fritillariae | 1 | 1.6 | 0.7 | ||||
| Total | 71 | 100 | 53 | 63 | 100 | 47 |
aCategorized as a yeast (neither Gram‐negative nor Gram‐positive); not factored into percentages.
A total of 135 organisms from 112 foals were recorded.
Table 2.
Median (and range) scores for sick‐nonseptic, septic and healthy foals using the modified sepsis score (score 1), updated sepsis score with the equine neonatal SIRS criteria (scores 2), and the updated sepsis score with the original SIRS criteria (score 3)
| Sick‐nonseptic | Septic | Healthy | |
|---|---|---|---|
| N = 147 | N = 126 | N = 25 | |
| Sepsis Score 1 | 6 (0–19) b , ** | 9 (1–24) a , b , ** | 1 (0–5) |
| Sepsis Score 2 | 9 (0–29) b , ** | 13 (0–34) a , b , ** | 1 (0–5) |
| Sepsis Score 3 | 10 (0–29) b , ** | 13 (3–34) a , b , ** | 1 (0–5) |
aSignificantly different when compared with sick‐nonseptic foal group.
bSignificantly different when compared with healthy foal group.
**P < .01.
Area under the ROC curve and cutoff values for the modified and updated sepsis scores to predict sepsis in hospitalized foals were calculated. Optimal cut‐off value was defined as the point where the numeric values of sensitivity and specificity were maximized. Evaluating Score 2 using a cut‐off of 12, the sensitivity and specificity to predict sepsis were 60% and 61%, respectively, whereas using a cut‐off of 10, the sensitivity and specificity were 73% and 40%, respectively. The sensitivity and specificity of Score 2 were not significantly improved when compared with the sensitivity and specificity of Score 1, which was 62% and 64%, respectively, when using a cut‐off of 8. A cut‐off value of 11 for Score 3 maximized sensitivity (62%) and specificity (67%) to predict sepsis in hospitalized foals but was not significantly better in predicting sepsis when compared with Score 1. The AUC and ROC curves for Scores 1, 2, and 3 were 0.73 (0.67‐0.8 [95% confidence interval (CI)]), 0.71 (0.6‐0.77 [95% CI]) and 0.73 (0.67‐0.79 [95% CI]), respectively (P > .05; Figure 2).
Figure 2.
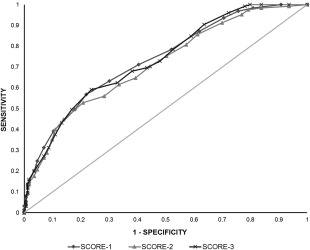
Receiver operating characteristic (ROC) curve for 3 sepsis scores to predict sepsis. Score 1: diamond; Score 2: triangle; Score 3: cross
There were 127/273 foals (46.5%) that met the original SIRS criteria and 88/273 foals (32%) that met the equine neonatal SIRS criteria. Of those foals that met the original SIRS criteria, 75/127 (59%) were considered septic based on the previously noted criteria. In contrast, 52/88 (60%) foals that met the equine neonatal SIRS criteria were septic. No statistically significant difference in positive bacterial culture was found between the equine neonatal SIRS and original SIRS criteria. In contrast, 52/127 (41%) sick‐nonseptic foals met the original SIRS criteria and 36/88 (40%) sick‐nonseptic foals met the equine neonatal SIRS criteria. The sensitivity and specificity for predicting sepsis when being SIRS positive using the original SIRS criteria were 60% and 69%, respectively. In comparison, sensitivity was lower (42%) and specificity higher (76%) when using the equine neonatal SIRS criteria (Table 4). The original and equine neonatal SIRS criteria had a significant association with predicting sepsis and non‐survival (Table 3).
Table 4.
Median (and range) scores for survivors and nonsurvivors for all foals in this study (overall survival) and for foals categorized as septic based on study criteria (septic foal survival)
| Overall survival | Septic foal survival | |||
|---|---|---|---|---|
| Survival | Nonsurvival | Survival | Nonsurvival | |
| n = 217 | n = 56 | n = 92 | n = 34 | |
| Sepsis Score 1 | 7 (0–20) | 10.5 (2–24)** | 8 (1–20) | 11 (4–24)** |
| Sepsis Score 2 | 9 (0–29) | 14 (3–34)** | 11 (0–29) | 16.5 (0–34)** |
| Sepsis Score 3 | 10 (0–29) | 15 (4–34)** | 13 (3–29) | 16.5 (5–34)** |
**Significantly different between survival and nonsurvival; P < .01.
Table 3.
Descriptive statistics of the original SIRS criteria and the equine neonatal SIRS criteria for predicting sepsis and survival
| Sensitivity predicting sepsis (%) | Specificity predicting sepsis (%) | Positive predictive value (%) | Negative predictive value (%) | OR if SIRS + to predict sepsis | OR if SIRS + to predict nonsurvival | |
|---|---|---|---|---|---|---|
| Original SIRS positive | 60 | 69 | 59 | 70 | 3.6** | 4.8** |
| Eq neonatal SIRS positive | 42 | 76 | 60 | 61 | 2.3* | 3.45** |
*P < .05.
**P < .01.
Abbreviation: SIRS, systemic inflammatory response syndrome.
Descriptive statistics of various physical examination and laboratory variables that were used in the equine neonatal SIRS criteria are reported for septic, sick‐nonseptic and healthy foals (Table 5). Notable differences in these variables included a significantly lower WBC count in septic foals when compared with sick‐nonseptic and healthy foals and a significantly lower rectal temperature and significantly higher blood l‐lactate concentration in the septic and sick‐nonseptic foals when compared with healthy foals. Overall survival to discharge was 79.5% (217 of 273 foals) whereas survival to discharge of septic foals was 73% (92 of 126). The median score for all sick foals (sick‐nonseptic and septic groups combined) was significantly higher in nonsurvivors compared with survivors, regardless of sepsis score used. The median score for only the septic foal group was significantly higher in nonsurvivors compared with survivors, regardless of sepsis score used (Table 4).
Table 5.
Descriptive statistics of various physical examination and laboratory parameters in septic, sick‐nonseptic and healthy foals
| Variable | Sick‐nonseptic | Septic | Healthy |
|---|---|---|---|
| Glucose (mg/dL) | 120 (17–227) a , ** | 122 (18–388) a , ** | 153 (108–194) |
| l‐Lactate (mmol/L) | 4.5 (0.7–26) a , ** | 4.7 (0.3–25) a , ** | 1.2 (0.7‐3.7) |
| WBC (×103 cells/L) | 7.35 (1.1–24.14) | 5.5 (1.4–23.6) a , b , ** | 8.2 (2.9–13.14) |
| Resp Rate (breaths/min) | 36 (18–160) a , * | 38 (12–124) a , * | 64 (32–110) |
| Heart Rate (beats/min) | 112 (50–200) | 108 (52–200) | 102 (78–128) |
| Temperature (°F) | 100.4 (91.9–103.2) a , * | 100.5 (91.7–103.9) a , * | 101 (99.8–102.5) |
aSignificantly different when compared with healthy foals.
bSignificantly different when compared with sick‐nonseptic.
*P < .05.
**P < .01.
Results reported as median and range.
4. DISCUSSION
The intent of the modified sepsis score, the updated sepsis scores, and the SIRS concept was to provide a rapid ancillary aid in detecting septic equine patients based on subjective clinical criteria as well as objective vital parameters and common clinicopathologic measurements. Historically, a common definition of sepsis in neonatal foals was a positive blood culture along with ≥ 2 original SIRS criteria. However, this definition of sepsis could not be used in our study because the study aimed to test the SIRS concept. The original and modified sepsis scores were developed in the 1980s to identify foals at high risk of sepsis, but more recent variables associated with sepsis have not been incorporated into an updated sepsis score to determine if higher sensitivity and specificity can be achieved. In our study, slight changes to the current modified sepsis score of some variables (eg, blood glucose concentration) as well as the addition of new variables (eg, blood l‐lactate concentration, serum creatinine concentration, lymphocyte count, SIRS) did not improve sensitivity or specificity when compared with the modified sepsis score.12, 13 Although these additional variables have been significantly associated with sepsis, their addition to the sepsis score failed to improve the diagnostic quality of the scoring system.2, 15, 16, 17, 18 The sensitivity and specificity of the modified sepsis score in this foal population (62% and 64%, respectively) were similar to what was found in previous studies that evaluated the modified sepsis score in 2015 (56.4% and 73.4%, respectively) and 2003 (67% and 76%, respectively), but were lower than results of a 1984 report (92.8% and 87.5%, respectively).13, 14, 15 In the past, different geographical locations and hospital populations have been proposed reasons for the discrepancies among various studies, but our study was a multi‐institutional study from various parts of the United States, thus suggesting that regional or population differences might not importantly impact the sepsis score. In our study, the overall performance of the three sepsis scores was relatively weak, thus the clinician should use the sepsis scores with caution and in conjunction with clinical assessment and judgment along with blood culture results. Interestingly, in our study, the optimal cut‐point for the modified sepsis score, based on area under the ROC curve was > 8, rather than the originally recommended cut‐point of 11.13 This finding is similar to another recent study evaluating the sepsis score, which suggested a cut‐point of > 7 to optimize the sensitivity of the sepsis score, thereby avoiding the potential consequences of untreated sepsis in equine neonates.15
Akin to the sepsis score, the purpose for establishing the SIRS concept in people was to help rapidly identify patients with a likelihood of sepsis in order to administer innovative therapies in sepsis.19 The authors of the consensus statement made the SIRS inclusion criteria broad to detect the highest possible number of cases and facilitate investigation of pathologic mechanisms involved in sepsis and the inflammatory response.19 Thus, the original definition lacked specificity from its inception, and this lack of specificity has been confirmed by several studies.23, 24, 25 Because of this, the SIRS concept has remained a source of debate among clinicians in regard to its usefulness.26, 27 The original SIRS definition in human medicine was intended for adults and designed to categorize human patients into similar clinically affected populations for research studies, relating patients to illness severity but not necessarily to sepsis. The concept of SIRS was developed as an investigational tool and not as a clinical diagnostic aid. In addition, recognizing that pediatric patients have different physiologic variables at different ages, another group of international experts participated in the International Pediatric Sepsis Consensus Conference to develop SIRS criteria for newborns to young adults (<18 years of age).28 An important difference between the pediatric and adult definitions of SIRS is that pediatric SIRS requires that either temperature or leukocyte abnormalities be present to fulfill the pediatric SIRS criteria with the basis of this recommendation stemming from the fact that several disease processes in pediatric patients present with tachycardia and tachypnea.28 In our study, the equine neonatal SIRS definition was evaluated using variables similar to the pediatric SIRS criteria, but the original SIRS criteria for adults also were examined.19, 28 In the foals of our study, 32% met the equine neonatal SIRS criteria, whereas 46.5% were SIRS positive using the original criteria, which is similar to another previous study in foals that also used the original SIRS criteria (44%).18 Positive SIRS status using the original SIRS criteria yielded comparable sensitivity and specificity (60% and 69%, respectively) for predicting sepsis as the sepsis scores evaluated in our study. Although SIRS criteria are much simpler and faster to use when compared with the sepsis scores and might serve as a more rapid screening tool for sepsis in neonatal foals, the clinician must be cognizant of the fact that SIRS can be caused by a number of causes other than infection such as trauma, burns, pancreatitis, ischemia, hemorrhage, and anaphylaxis.19 As expected, foals in our study that fulfilled the equine neonatal or original SIRS criteria had higher OR of being septic and increased odds of nonsurvival, but the clinical utility of this information is limited. Interestingly, in the recent Third International Consensus definitions for sepsis in adult people, sepsis was redefined as life‐threatening organ dysfunction caused by a dysregulated host response to infection with updated diagnostic criteria for sepsis based on the sequential organ failure assessment scoring system.29 Moreover, SIRS criteria were unanimously considered by the task force to be unhelpful in defining sepsis and were eliminated because of the fact that SIRS results from various disease processes and is not specific for sepsis.29 The basis of this decision included the fact that many hospitalized patients fulfill the SIRS criteria but do not have evidence of infection or experience adverse outcomes.29 The true clinical utility of the SIRS criteria in neonatal foals remains to be determined.
In our study, 41% (112 of 273) of foals had bacterial growth on blood culture with the percentage of Gram‐negative and Gram‐positive microorganisms being 53% and 47%, respectively. In previous studies of large populations of foals, microorganisms were isolated from 25% to 44% of blood cultures submitted with 54%‐66% of cultures yielding Gram‐negative organisms and 27%‐43% of cultures yielding Gram‐positive organisms.30, 31, 32 A slightly higher percentage of Gram‐positive organisms was isolated when compared with previous studies, supporting the notion that blood cultures from neonatal foals have shown a slow upward trend in the presence of Gram‐positive organisms.30, 31, 33 Overall survival rate of all sick foals in our study (79.5%) was similar to that previously reported in other studies, as was survival of septic foals (73%).15, 31, 34, 35
Individual variables that were statistically different in foals in the septic and sick‐nonseptic groups as compared with healthy foals included low blood glucose concentration, increased blood l‐lactate concentration, lower respiratory rate, and lower rectal temperature. Some of these differences have been reported previously in other studies. For example, low blood glucose concentration at admission was associated with sepsis, positive blood culture, and SIRS in a previous study in sick neonatal foals and numerous other studies have documented the association between hyperlactatemia and sepsis or SIRS.17, 18, 21 Lower rectal temperature has been associated with non‐survival in sick neonatal foals,36 however lower respiratory rate had not previously been associated with illness in foals. The reason for this difference between groups is not known, but the higher respiratory rate in healthy foals could be a consequence of excitement or anxiety in response to restraint and physical examination. The only variable that was statistically different between the septic group and the sick‐nonseptic and healthy groups in our study was a lower WBC count in the septic group, previously associated with nonsurvival in neonatal foals.3, 36
Our study had a number of limitations. First, although the gold standard of defining sepsis is positive growth on blood culture, blood culture suffers from relatively poor sensitivity, thus making it difficult or impossible to identify the true number of septic foals and could have resulted in misclassification of some foals as nonseptic if culture negative and septic if culture positive.6, 8, 10 This limitation commonly affects research projects such ours because the “gold standard” is inadequate and imprecise. Better diagnostic methods to identify bacteremia are necessary to improve the quality of such research. Additionally, consideration of the fact that some healthy neonatal foals are bacteremic, but not septic, should be given.10 Although it is unlikely that the septic foals in our study were falsely identified as septic (ie, positive blood culture but sick‐nonseptic or healthy) because all foals were admitted to referral hospitals and were considered sick, it is possible that some merely had transient bacteremia with no adverse consequences. A further limitation is reliance on recorded historical and physical examination information, which, on occasion, can be missing or recorded inaccurately.
In summary, the purpose of the sepsis score and SIRS criteria is to facilitate identification of patients that are likely septic. In our study, the addition of sepsis‐associated variables did not enhance the performance of the modified sepsis score. In addition, the original SIRS concept might serve as a rapid adjunctive tool in categorizing and identifying septic foals with modest sensitivity and specificity, but the clinician must remain aware of other noninfectious conditions that can result in SIRS. Despite the potential utility of these scoring systems, clinical acumen and experience in which a clinician believes a patient “looks septic” still play a vital role in the treatment of ill foals.37
CONFLICT OF INTEREST DECLARATION
The authors declare that they have no conflict of interest with the contents of this article.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
ACKNOWLEDGMENTS
The authors thank Andrea Whittle for her help in collecting data from Rood and Riddle Equine Hospital.
Wong DM, Ruby RE, Dembek KA, et al. Evaluation of updated sepsis scoring systems and systemic inflammatory response syndrome criteria and their association with sepsis in equine neonates. J Vet Intern Med. 2018;32:1185–1193. https://doi.org/10.1111/jvim.15087
REFERENCES
- 1. Cohen ND. Causes of and farm management factors associated with disease and death in foals. J Am Vet Med Assoc. 1994;204:1644–1651. [PubMed] [Google Scholar]
- 2. Borchers A, Wilkins PA, Marsh PM. Association of admission L‐lactate concentration in hospitalized equine neonates with presenting complaint, periparturient events, clinical diagnosis and outcome: a prospective multicenter study. Equine Vet J. 2012;44:57–63. [DOI] [PubMed] [Google Scholar]
- 3. Giguere S, Weber EJ, Sanchez LC. Factors associated with outcome and gradual improvement in survival over time in 1065 equine neonates admitted to an intensive care unit. Equine Vet J. 2017;49:45–50. [DOI] [PubMed] [Google Scholar]
- 4. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Intensive Care Med. 2013;39:165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Troger B, Hartel C, Buer J, et al. Clinical relevance of pathogens detected by multiplex PCR in blood of very‐low‐birth weight infants with suspected sepsis‐multicenter study of the German Neonatal Network. PLoS One. 2016;11:e0159821–e0159810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bates DW, Cook EF, Goldman L, et al. Predicting bacteremia in hospitalized patients. A prospectively validated model. Ann Intern Med. 1990;113:495–500. [DOI] [PubMed] [Google Scholar]
- 7. Weinstein MP, Towns ML, Quartey SM, et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24:584–602. [DOI] [PubMed] [Google Scholar]
- 8. Venkatesh M, Flores A, Luna RA, Versalovic J. Molecular microbiological methods in the diagnosis of neonatal sepsis. Expert Rev Anti Infect Ther. 2010;8:1037–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Book M, Lehmann LE, Zhang XH, Stüber F. Monitoring infection: from blood culture to polymerase chain reaction (PCR). Best Pract Res Clin Anaesthesiol. 2013;27:279–288. [DOI] [PubMed] [Google Scholar]
- 10. Hackett ES, Lunn DP, Ferris RA, Horohov DW, Lappin MR, McCue PM. Detection of bacteremia and host response in healthy neonatal foals. Equine Vet J. 2015;47:405–409. [DOI] [PubMed] [Google Scholar]
- 11. Pusterla N, Mapes S, Byrne BA, Magdesian KG. Detection of bloodstream infection in neonatal foals with suspected sepsis using real‐time PCR. Vet Rec. 2009;165:114–117. [DOI] [PubMed] [Google Scholar]
- 12. Brewer BD, Koterba AM. Development of a scoring system for the early diagnosis of equine neonatal sepsis. Equine Vet J. 1988;20:18–22. [DOI] [PubMed] [Google Scholar]
- 13. Brewer BD, Koterba AM, Carter RL, Rowe ED. Comparison of empirically developed sepsis score with a computer generated and weighted scoring system for the identification of sepsis in the equine neonate. Equine Vet J. 1988;20:23–24. [DOI] [PubMed] [Google Scholar]
- 14. Corley KTT, Furr MO. Evaluation of a score designed to predict sepsis in foals. J Vet Emerg Crit Care. 2003;13:149–155. [Google Scholar]
- 15. Weber EJ, Sanchez LC, Giguere S. Re‐evaluation of the sepsis score in equine neonates. Equine Vet J. 2015;47:275–278. [DOI] [PubMed] [Google Scholar]
- 16. Borchers A, Wilkins PA, Marsh PM, et al. Sequential L‐lactate concentration in hospitalized equine neonates: a prospective multicenter study. Equine Vet J. 2013;45:2–7. [DOI] [PubMed] [Google Scholar]
- 17. Hollis AR, Furr MO, Magdesian KG, et al. Blood glucose concentrations in critically ill neonatal foals. J Vet Intern Med. 2008;22:1223–1227. [DOI] [PubMed] [Google Scholar]
- 18. Corley KTT, Donaldson LL, Furr MO. Arterial lactate concentration, hospital survival, sepsis and SIRS in critically ill neonatal foals. Equine Vet J. 2010;37:53–59. [DOI] [PubMed] [Google Scholar]
- 19. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The AACP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. [DOI] [PubMed] [Google Scholar]
- 20. Wong DM, Wilkins PA. Defining the systemic inflammatory response syndrome in equine neonates. Vet Clin Equine. 2015;31:463–481. [DOI] [PubMed] [Google Scholar]
- 21. Castagnetti C, Pirrone A, Mariella J, Mari G. Venous blood lactate evaluation in equine neonatal intensive care. Theriogenology. 2010;73:343–357. [DOI] [PubMed] [Google Scholar]
- 22. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- 23. Rangel‐Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). JAMA 1995;273:117–123. [PubMed] [Google Scholar]
- 24. Muckart DJ, Bhagwanjee S. American College of Chest Physician/Society of Critical Care Medicine Consensus Conference definitions of the systemic inflammatory response syndrome and allied disorder in relation to critically injured patients. Crit Care Med. 1997;25:1789–1795. [DOI] [PubMed] [Google Scholar]
- 25. Jaimes F, Garcés J, Cuervo J, et al. The systemic inflammatory response syndrome (SIRS) to identify infected patients in the emergency room. Intensive Care Med. 2003;29:1368–1371. [DOI] [PubMed] [Google Scholar]
- 26. Vincent JL. Dear SIRS, I'm sorry to say that I don't like you. Crit Care Med. 1997;25:372–374. [DOI] [PubMed] [Google Scholar]
- 27. Bone RC. Sir Isaac Newton, sepsis, SIRS and CARS. Crit Care Med. 1996;24:1125–1128. [DOI] [PubMed] [Google Scholar]
- 28. Goldstein B, Giroir B, Randolph A. International Pediatric Sepsis Consensus Conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. [DOI] [PubMed] [Google Scholar]
- 29. Singer M, Deutschman CS, Seymour C, et al. The Third International consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA 2016;315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marsh PS, Palmer JE. Bacterial isolates from blood and their susceptibility patterns in critically ill foals; 543 cases (1991–1998). J Am Vet Med Assoc. 2001;218:1608–1610. [DOI] [PubMed] [Google Scholar]
- 31. Russell CM, Axon JE, Blishen A, et al. Blood culture isolates and antimicrobial sensitivities from 427 critically ill neonatal foals. Aust Vet J. 2008;86:266–271. [DOI] [PubMed] [Google Scholar]
- 32. Hytychová T, Bezděková B. Retrospective evaluation of blood culture isolates and sepsis survival rate in foals in the Czech Republic: 50 cases (2011–2013). J Vet Emerg Crit Care. 2015;25:660–666. [DOI] [PubMed] [Google Scholar]
- 33. Wilson WD, Madigan JE. Comparison of bacteriologic culture of blood and necropsy specimens for determining the cause of foal septicemia: 47 cases (1978–1987). J Am Vet Med Assoc. 1989;195:1759–1763. [PubMed] [Google Scholar]
- 34. Toth B, Slovis NM, Constable PD, Taylor SD. Plasma adrenomedullin concentrations in critically ill neonatal foals. J Vet Intern Med. 2014;28:1294–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zabrecky KA, Slovis NM, Constable PD, Taylor SD. Plasma C‐reactive protein and haptoglobin concentrations in critically ill neonatal foals. J Vet Intern Med. 2015;29:673–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dembek KA, Hurcombe SD, Frazer ML, et al. Development of a likelihood of survival scoring system for hospitalized equine neonates using generalized boosted regression modeling. PLos One. 2014;9:e109212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rohrbach BW, Buchanan BR, Drake JM, et al. Use of multivariable model to estimate the probability of discharge in hospitalized foals that are 7 days of age or less. J Am Vet Med Assoc. 2006;228:1748–1756. [DOI] [PubMed] [Google Scholar]


