Abstract
Objective
To determine whether identification of previously undiagnosed high cholesterol, hypertension, and/or diabetes during an in‐home assessment impacts care seeking among Medicare beneficiaries.
Data Sources/Study Setting
Data from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study, which recruited African American and white participants across the continental United States from 2003–2007, were linked to Medicare claims.
Study Design
We used panel data models to analyze changes in doctor visits for evaluation and management of conditions after participants were assessed, utilizing the study's rolling recruitment to control for secular trends.
Data Extraction Methods
We extracted Medicare claims for the 24 months before through 24 months after assessment via REGARDS for 5,884 participants.
Principal Findings
Semi‐annual doctor visits for previously undiagnosed conditions increased by 22 percentage points (95 percent confidence interval: 16–28) 2 years following assessment. The effect was similar by gender, race, region, and Medicaid, but it may have been lower among participants who lacked a usual health care provider.
Conclusions
In‐home assessment of cholesterol, blood pressure, and blood glucose can increase doctor visits for individuals with previously undiagnosed conditions. However, biomarker assessment may have more limited impact among individuals with low access to care.
Keywords: Medicare, screening, diabetes, hypertension, high cholesterol
High cholesterol, hypertension, and diabetes are important contributors to premature death and ill health in the United States (Danaei et al. 2009; U.S. Burden of Disease Collaborators 2013; Patel et al. 2015). However, many people with these conditions are unaware of them because, in early stages, these conditions are asymptomatic. About one‐fifth of cases of high cholesterol, hypertension, and diabetes are undiagnosed among U.S. adults (Cowie et al. 2009; Egan, Zhao, and Axon 2010; Ford et al. 2010; Centers for Disease Control and Prevention 2014). Lengthy gaps in diagnosis and treatment can lead to negative health consequences (Bindman et al. 1995; D'Agostino et al. 2008; American Diabetes Association 2014; Bressler et al. 2014; James et al. 2014; Stone et al. 2014). Therefore, increasing screening for these conditions is an important avenue to increase treatment of undiagnosed conditions and thereby improve population health (Farley et al. 2010; Maciosek et al. 2010).
The prevalence of undiagnosed high cholesterol, hypertension, and diabetes is particularly high in the Medicare population (McDonald et al. 2009). Policy efforts to increase screening of Medicare beneficiaries have expanded in recent years. Medicare offered new beneficiaries a “Welcome to Medicare” wellness visit without cost sharing starting in 2005, but uptake of this benefit seems to have been incomplete (Sloan et al. 2012). Subsequently, the Affordable Care Act (ACA) has eliminated cost sharing for annual wellness visits for all Medicare beneficiaries and eliminated cost sharing for high cholesterol, hypertension, and diabetes screening for patients at sufficient risk according to U.S. Preventive Services Task Force guidelines (Burke and Simmons 2014; Healthcare.gov 2014; U.S. Preventive Services Task Force 2016). The ACA also created the Center for Medicare and Medicaid Innovation, which has funded two demonstration projects designed to encourage provider‐to‐patient outreach related to screening. Accountable Care Organizations lose their shared savings payments if they fail to achieve targets for blood pressure screening rates and other quality metrics, and Accountable Health Communities are charged with implementing community outreach to promote awareness of clinical delivery services (Center for Medicare and Medicaid Services 2015; Alley et al. 2016). As more Medicare beneficiaries receive care under these new payment models, outreach to encourage screening may become increasingly common.
Although outreach has intuitive appeal as a strategy to increase screening among hard‐to‐reach Medicare beneficiaries, it is not clear how many beneficiaries who are screened as a result of outreach would visit a doctor to evaluate and initiate management of previously undiagnosed conditions. Studies of changes in self‐reported treatment have shown small or nonsignificant effects of screening with telephone outreach among Medicare beneficiaries, but we are not aware of studies that track health care utilization with claims data rather than self‐reported data (Edwards 2013). In addition, it is not clear which individuals are most likely to seek care for previously undiagnosed conditions. Screening interventions in vulnerable populations showed high rates of loss to follow‐up, particularly among minority women and women with lower levels of education (Finkelstein, Khavjou, and Will 2006; Homan, McBride, and Yun 2014).
This study addresses these gaps in the literature by using Medicare claims data to test whether in‐home biomarker assessment after telephone outreach translates to doctor visits for evaluation and management of previously undiagnosed conditions among Medicare beneficiaries. We used data from a geographically and demographically diverse sample of Medicare beneficiaries and separately track the impact for high‐priority groups such as women, African Americans, beneficiaries who are dually eligible for Medicaid, beneficiaries without a usual health care provider, beneficiaries with less than high school education and beneficiaries living in a Health Professional Shortage Area. In particular, we utilized an epidemiological study (the REasons for Geographic And Racial Differences in Stroke study, or REGARDS) that recruited participants from across the continental United States using residential telephone calls and an in‐home evaluation for an assessment of biomarkers related to high cholesterol, hypertension, and diabetes (Howard et al. 2005). We compared doctor visits for evaluation and management of these conditions before and after each participant was evaluated by REGARDS, using the rolling recruitment into the study to tease out the impact of biomarker evaluation from the impact of secular trends.
Methods
Data
Study Population
The REGARDS study is a longitudinal, population‐based cohort study designed to answer questions about racial and geographic differences in risks for stroke and stroke mortality. Recruitment was conducted on a rolling basis over 2003–2007 and was accomplished through the use of commercially available lists of residential phone numbers in the 48 contiguous United States. Sampling was stratified across African Americans and whites and three regions: the stroke belt (Alabama, Arkansas, Mississippi, Tennessee, and noncoastal North Carolina, South Carolina, and Georgia), stroke buckle (coastal plains of North Carolina, South Carolina, and Georgia), and elsewhere. Individuals who did not identify as either African American or white, were non‐English speaking, under 45 years of age, undergoing cancer treatment, or on a waiting list for a nursing home were excluded from the REGARDS study (Howard et al. 2005). Appendix Figure S1 shows the geographic distribution of participants by race (Howard et al. 2011). Data from the REGARDS study have been linked to Medicare claims. Details of the linking process are described elsewhere (Muntner et al. 2014). The REGARDS participants with merged Medicare data have been shown to resemble a national 5 percent sample of fee‐for‐service Medicare beneficiaries (Xie et al. 2016).
We limited the analysis to REGARDS study participants who (1) were aged 67 or older at the time or REGARDS enrollment, (2) had Medicare linked data, (3) were enrolled in Medicare fee‐for‐service insurance coverage (Parts A and B but not Medicare Advantage or Part C) throughout the 24 months before through 24 months after their enrollment, and (4) had one or more of our three conditions of interest, as defined in the study procedures section below. Of the 30,239 REGARDS participants, 5,884 met all inclusion criteria. Appendix Table S1 details the stepwise exclusion of participants.
Study Procedures
Participants first answered questions by phone, including whether they had been diagnosed with high cholesterol, hypertension, or diabetes by a health professional, and questions about their age, race, sex, income, education, self‐reported health, smoking status, and number of alcoholic drinks per week. During the interview, participants also completed a short memory test to assess their cognitive functioning and the Short Form 12 (SF‐12) questionnaire to assess their physical and mental health.
Participants were instructed to fast for an in‐home visit. During the in‐home visit, trained health professionals measured participants’ blood pressure and collected blood samples that were shipped on ice packs overnight to a central laboratory. Blood pressure was measured twice using an aneroid sphygmomanometer, after the participant was seated with both feet on the floor for 5 minutes. The two blood pressure measurements were averaged for the analysis. Serum glucose, triglycerides, and total and high‐density lipoprotein cholesterol were measured from blood samples using colorimetric reflectance spectrophotometry with the Ortho Vitros 950 IRC Clinical Analyzer (Johnson and Johnson Clinical Diagnostics). Low‐density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation (Friedewald, Levy, and Fredrickson 1972). Participants were compensated $30 for their time. They were notified of their results and advised to seek medical care for abnormal results using telephone calls, as well as letters and cards with standard text reprinted in Appendix Figure S2. Participants also completed and mailed back a questionnaire that included the following question about prior health care use: “Do you have a primary clinic, doctor, nurse, or physician's assistant who provides your usual medical care?” The study received IRB approval, and all participants signed an informed consent form (Howard et al. 2005).
We complemented the REGARDS biomarker and survey data with participants’ Medicare claims to identify doctor visits for evaluation and management of high cholesterol, hypertension, or diabetes in each 6‐month interval during a given participant's 48‐month window of observation. Doctor visits for high cholesterol were defined by a claim in the carrier or outpatient files with a code for this condition in any position linked to an evaluation and management code. Similar definitions were used to define doctor visits for hypertension and diabetes (see Appendix Table S2). We also used Medicare data to identify participants who were dually eligible for Medicaid, and extracted Medicare claims data on hospitalizations during each 6‐month interval.
We identified participants with high cholesterol, hypertension, and/or diabetes and classified each condition as diagnosed or undiagnosed using self‐reported data, claims data, and biomarker data. Participants were classified as diagnosed if they responded positively to the question “Has a doctor or other health professional ever told you that you have…” specific to high blood pressure, diabetes or high blood sugar, or high cholesterol without a positive response to the question “Was this only when you were pregnant?” in the case of diabetes or hypertension. To correct for underreporting of diagnosis in self‐reported data, we used the Medicare claims data to identify additional diagnosed conditions (Meyer, Mok, and Sullivan 2015). In particular, biomarker‐identified cases of high cholesterol, hypertension, and diabetes were categorized as diagnosed if the participant's claims data met Chronic Conditions Warehouse definitions for the condition, that is, the participant had two or more claims coded as relevant to the condition within the past 2 years. (The Chronic Conditions Warehouse definitions were designed to identify chronic conditions using claims data and correctly identify 69 percent of true diabetes cases in validation tests (Gorina and Kramarow 2011). In our analysis, the use of this additional criterion increased the prevalence of diagnosed conditions by 4 percent for hypertension, and by 2 percent for high cholesterol and diabetes.) Biomarker‐identified cases of high cholesterol, hypertension, and diabetes that failed to meet either of these criteria were classified as undiagnosed. We used biomarker cutoffs that took participants’ fasting status into account, as detailed in Appendix Table S3. We allowed cholesterol control cutoffs to vary by 10‐year estimated risk category per national recommendations, as detailed in Appendix Table S4 (National Cholesterol Education Expert Panel 2002; American Diabetes Association 2014; James et al. 2014).
Outcome of Interest
In our main analysis, the outcome of interest was a binary variable indicating whether participants with prevalent high cholesterol, hypertension, or diabetes received any doctor visits for evaluation and management of these conditions in a given 6‐month interval. This outcome was measured on the condition level (so that participants with multiple conditions were entered into the data multiple times) and was tracked for each 6‐month period of the participant's 48‐month period of observation. (A single doctor visit could be coded as addressing multiple conditions in the Medicare data.) We also analyzed the number of doctor visits for evaluation and management of each condition in each 6‐month interval.
Predictors of Interest
The key predictors of interest were (1) whether the participant's biomarkers had already been assessed via REGARDS and (2) whether each prevalent condition was diagnosed or undiagnosed prior to REGARDS participation.
Control Variables Used in Multivariate Modeling
Control variables were selected to address two possible biases. First, we expected that secular trends would contribute to observed changes in doctor visits after biomarker assessment via REGARDS. For example, all participants were older after enrollment in REGARDS than before enrollment in REGARDS, and policy changes were implemented during our period of observation. These secular trends could have biased our estimates if not controlled for in the model. Two aspects of our data make it possible to control for secular trends: (1) the rolling recruitment into the REGARDS study and (2) the availability of panel data for all participants over the 24 months prior to participation. Figure 1 demonstrates this point using a graphical example: When analyzing data from the hypothetical participants in Figure 1, we could separate the effect of screening on person A in 2003 from the effect of secular trends in 2003 using the data from person B and person C in 2003. A similar graphic could be drawn to show how we were able to identify and control for the effects of aging.
Figure 1.
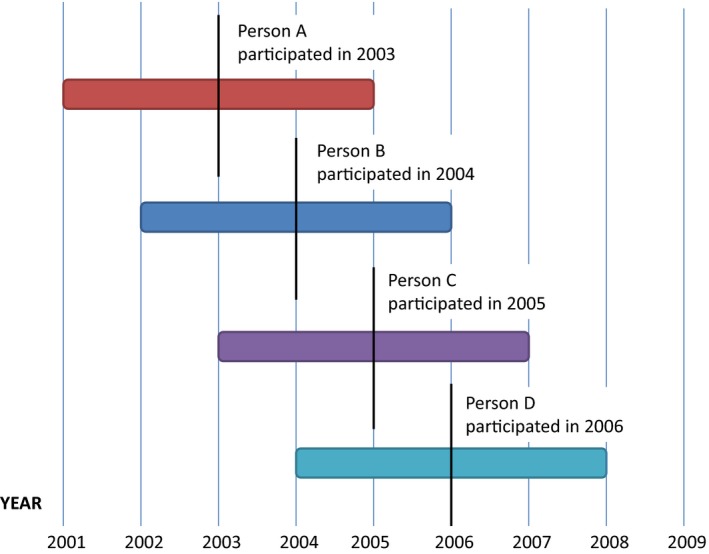
Illustration Showing the Months of Observation for Four Hypothetical REGARDS Participants
Notes. This figure shows an example of the periods of observation for four hypothetical individuals in the Medicare panel data who were recruited on January 1, 2003, January 1, 2004, January 1, 2005, and January 1, 2006. The center of each horizontal bar indicates the date that the individual enrolled in REGARDS. The length of the horizontal bar indicates the window of time over which we track each individual's Medicare claims, that is, 24 months prior to their enrollment in REGARDS through 24 months after their enrollment in REGARDS. [Color figure can be viewed at http://wileyonlinelibrary.com].
Second, our results might be biased if the type of individual willing to participate in REGARDS changed over time. This would be problematic because, as noted above, not‐yet‐screened individuals were compared with recently screened individuals to control for secular trends. We addressed this concern by controlling for a number of observable characteristics in the models and, in some specifications, controlling for all time‐invariant individual‐level characteristics using fixed effects. (We also compared the measured and self‐reported health of participants in our sample with the measured and self‐reported health of participants in a national biomarker survey, the National Health and Nutrition Examination Survey.)
To this end, we included two main groups of control variables. Control variables that varied over time included year dummies, interactions between region and year, and individual age. Age was binned into eight categories of equal size based on quintiles of the sample distribution to allow for a nonlinear relationship between age and doctor visits. Control variables that were only measured once, at the time of REGARDS enrollment, included physical health measures and demographic and health‐related characteristics inquired about in the REGARDS survey. These control variables included waist size in centimeters, BMI, glucose, lipid panel (total cholesterol, triglycerides, LDL and HDL cholesterol), the average of two blood pressure measures (both systolic and diastolic), reported physical health from the SF‐12, type of condition (high cholesterol, hypertension, or diabetes), race (African American or white), sex (male or female), income (less than $20,000, $20,000‐<$35,000, $35,000‐$75,000, and over $75,000), education (less than high school education, high school, some college education, or graduated from college), fair or poor self‐reported health, usual health care provider at the time of the interview (self‐reported having versus not having a usual health care provider), self‐reported smoking status (current smoker, past smoker, or nonsmoker), number of alcoholic drinks per week, fasting status at the time of the interview (fasting or not), cognitive status according to a short memory test (impaired or not), Medicaid dual eligibility in 2008 (eligible or not), status of county as a primary care Health Professional Shortage Area (all, part, or none of the county is a Health Professional Shortage Area), and the fraction of residents in poverty in the participant's county of residence. The continuous variables were binned into four categories of equal size based on quartiles of the sample distribution to allow nonlinearity in the relationship between these variables and doctor visits.
Analytic Plan
The unit of analysis was a person, condition (high cholesterol, hypertension, or diabetes), and 6‐month interval. As such, people with multiple conditions were entered into the data multiple times. We analyzed changes in doctor visits for evaluation and management of previously diagnosed versus previously undiagnosed high cholesterol, hypertension, and diabetes after enrollment in REGARDS using multivariate panel data models of the following form:
where i indexes individual, j indexes condition (either high cholesterol, hypertension, or diabetes), t indexes a given 6‐month time interval in individual i's 48‐month period of observation, and s indicates the average time since individual i's enrollment in REGARDS during interval t.
δ 1, δ 2, γ 1, and γ 2 were the coefficients of interest, indicating the changes in levels and trends in doctor visits after assessment via REGARDS for undiagnosed and diagnosed conditions, respectively. We modeled changes in levels and trends of the outcome of interest separately to examine whether changes in doctor visits occurred immediately, developed over time, or both. U ij was a binary variable that took the value 1 if individual i's condition j was undiagnosed prior to biomarker assessment via REGARDS, and 0 otherwise. T it was a binary variable indicating whether individual i had already been assessed via REGARDS at 6‐month interval t (i.e., this variable took the value 1 for all 6‐month intervals where s > 0 and 0 for intervals where s < 0). X ijt included the control variables listed above and all relevant lower‐order interaction terms. The α ij term captured the correlation across measures of the same person and condition over time and was modeled as a random effect in the basic specification. We modeled ε ijt using heteroskedasticity‐robust standard errors clustered on the individual level, to account for the heteroskedasticity that arose due to the use of a binary outcome variable and account for the fact that some participants had multiple conditions (Stock and Watson 2008).
To additionally control for secular trends and any changes in the composition of REGARDS participants over time, we used 6 different regression specifications that adjusted for participants’ time‐invariant characteristics and predicted health trajectories in a progressively stricter fashion. In particular, we ran models with and without (1) controlling for participants’ hospitalizations in the current 6‐month interval, (2) allowing background trends in doctor visits to vary with participants’ biomarkers at the time of REGARDS enrollment (i.e., interacting s with biomarkers), and (3) controlling for time‐invariant characteristics using person‐by‐condition fixed effects (i.e., modeling α ij as a fixed rather than random effect). To illuminate whether timing of REGARDS enrollment was related to time‐invariant individual‐level characteristics, we conducted a Hausman test to compare the models using random versus fixed effects.
In the main analysis, we pooled high cholesterol, hypertension, and diabetes together. In additional analyses, we restricted the data to examine changes in doctor visits for high cholesterol, hypertension, and diabetes separately.
To check whether observed changes in doctor visits after biomarker assessment via REGARDS could have been produced by nonlinearity in the trends prior to REGARDS enrollment, we ran placebo regressions. In the placebo regressions, we restricted the sample to only include data from prior to REGARDS enrollment and compared participants’ doctor visits 2 years before enrollment versus 1 year before enrollment. In two additional robustness checks, we coded all participants with abnormal biomarkers who reported no prior diagnosis as undiagnosed regardless of their claims data, and excluded people with only diagnosed conditions from the data.
We also investigated the predictors of doctor visits for previously undiagnosed conditions by interacting the changes in levels and trends in doctor visits after biomarker assessment via REGARDS (the quantities with coefficients δ 1, δ 2, γ 1, and γ 2) with characteristics of participants. These characteristics included gender, race, Medicaid dual eligibility, low income (<$20,000 per year), marital status, fair or poor self‐reported health, region of residence (stroke belt vs. other), health care use in the 12 months prior to enrollment in REGARDS, having a usual health care provider, having multiple chronic conditions, having less than a high school education, living in a high‐poverty county (>25 percent poverty), and living in a county that is a primary care Health Professional Shortage Area. We examined one of these variables at a time. In all cases, the relevant lower‐order interaction terms were included in the regressions.
Results
Complete panel data on doctor visits were available for 6,571 participants. Appendix Figure S3 shows that these participants resembled the National Health and Nutrition Survey, a nationally representative biomarker survey, on measured and self‐reported health in similar years when the REGARDS inclusion criteria were applied.
Among the 6,571 participants with complete panel data, 5,884 had one or more of our conditions of interest and were therefore included in the analysis. In total, 4,268 participants had high cholesterol, including 726 participants with undiagnosed high cholesterol; 4,502 participants had hypertension, including 332 with undiagnosed hypertension; and 1,309 participants had diabetes, including 117 with undiagnosed diabetes. Because participants with multiple conditions were entered into the dataset multiple times, our final dataset comprised a panel of 10,079 prevalent conditions, including 1,175 previously undiagnosed conditions.
Table 1 compares the characteristics of participants with only diagnosed conditions versus participants with one or more undiagnosed conditions. Participants with undiagnosed conditions had higher blood pressure and fasting blood glucose, higher total and LDL cholesterol, and lower HDL cholesterol than participants with only diagnosed conditions. Participants with undiagnosed conditions were more likely to be male and more likely to lack a usual health care provider than participants with only diagnosed conditions; they were less likely than participants with only diagnosed conditions to have seen a doctor for evaluation and management of any condition in the prior year.
Table 1.
(A) Characteristics of Participants Meeting All Inclusion Criteria, by Diagnosis Status at the Time of REGARDS Enrollment. (B) Doctor Visits for Diabetes, Hypertension, and High Cholesterol 0–6 Months Prior to REGARDS Enrollment, by Diagnosis Status
| (A) | |||
|---|---|---|---|
| Participants w/Only Diagnosed Conditions | Participants w/Undiagnosed Conditions | p‐Value of the Difference | |
| Mean (SE) | Mean (SE) | ||
| Age | 74.1 (0.1) | 74.9 (0.2) | <.01 |
| Systolic blood pressure | 130.3 (0.2) | 138.1 (0.5) | <.01 |
| Diastolic blood pressure | 74.6 (0.1) | 78.1 (0.3) | <.01 |
| Fasting glucose | 100.6 (0.4) | 109 (1.2) | <.01 |
| Total cholesterol | 185.2 (0.7) | 201.2 (1.2) | <.01 |
| Triglycerides | 130.9 (1.2) | 139.2 (3.9) | <.01 |
| LDL cholesterol | 106.5 (0.6) | 125.8 (1) | <.01 |
| HDL cholesterol | 52.3 (0.3) | 48.1 (0.5) | <.01 |
| N (%) | N (%) | ||
|---|---|---|---|
| Total | 4,562 | 1,322 | |
| Male | 2,116 (46) | 810 (61) | <0.01 |
| Any doctor visits the year before participation | 4,461 (98) | 964 (73) | <0.01 |
| Had a usual health care provider | 4,101 (90) | 964 (73) | <0.01 |
| Current smoker | 324 (7) | 114 (9) | 0.06 |
| African American | 1,367 (30) | 398 (30) | 0.91 |
| Lives in stroke belt state | 1,583 (35) | 476 (36) | 0.37 |
| Lives in stroke buckle state | 1,104 (24) | 302 (23) | 0.32 |
| Married | 2,580 (57) | 784 (59) | 0.07 |
| (B) | ||
|---|---|---|
| Condition Was Diagnosed | Condition Was Undiagnosed | |
| Number of Doctor Visits for the Condition | Mean (SE) | Mean (SE) |
| Diabetes | 2.6 (0.1) | 0.0 (0.0) |
| High cholesterol | 0.8 (0.0) | 0.0 (0.0) |
| Hypertension | 1.7 (0.0) | 0.1 (0.0) |
| All conditions pooled | 1.5 (0.0) | 0.0 (0.0) |
| Any Doctor Visits for the Condition | Percent | Percent |
|---|---|---|
| Diabetes | 82% | 3% |
| High cholesterol | 50% | 4% |
| Hypertension | 70% | 8% |
| All conditions pooled | 63% | 5% |
HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SE, standard error of the mean. In this chart, glucose and lipid measurements are included only from participants who were fasting.
Table 2 shows results from the 6 model specifications used to test for impacts of biomarker assessment on the fraction of high cholesterol, hypertension, and diabetes cases that were seen by a doctor for evaluation and management per 6 months. The results were highly similar across the six specifications. Overall, we found no change in doctor visits for diagnosed conditions after biomarker assessment via REGARDS, but did find changes in doctor visits for previously undiagnosed conditions after biomarker assessment via REGARDS. This evidence is consistent with a hypothesis that assessment changed participants’ care use patterns by informing participants about previously undiagnosed conditions. In the most conservative model, the fraction of previously undiagnosed conditions that received a semi‐annual doctor visit for evaluation and management increased by 15 percentage points (95 percent confidence interval: 11–19) by 1 year after assessment and by 22 percentage points (95 percent confidence interval: 16–28) by 2 years after assessment. Findings were qualitatively similar when we examined the number of visits; see Appendix Table S5. The raw data showed a similar trend; see Figure 2.
Table 2.
Percentage Point Change in Any Semi‐annual Doctor Visits for Evaluation and Management of Previously Diagnosed versus Undiagnosed Conditions One and Two Years after REGARDS Enrollment (Average Marginal Effects from Regression)
| (1) | (2) | (3) | (4) | (5) | (6) | |
|---|---|---|---|---|---|---|
| Diagnosed conditions | ||||||
| Change after 1 year | −1 | −1 | −1 | −1 | −1 | −1 |
| (−4 to 1) | (−4 to 1) | (−4 to 1) | (−4 to 1) | (−4 to 1) | (−4 to 1) | |
| Change after 2 years | −3 | −3 | −3 | −2 | −3 | −3 |
| (−6 to 1) | (−7 to 1) | (−7 to 1) | (−6 to 2) | (−7 to 1) | (−7 to 1) | |
| Undiagnosed conditions | ||||||
| Change after 1 year | 16*** | 15*** | 15*** | 16*** | 15*** | 15*** |
| (12 to 20) | (11 to 19) | (11 to 19) | (12 to 20) | (11 to 19) | (11 to 19) | |
| Change after 2 years | 23*** | 22*** | 22*** | 23*** | 22*** | 22*** |
| (17 to 29) | (16 to 28) | (16 to 28) | (17 to 29) | (16 to 28) | (16 to 28) | |
| Fixed effects | N | N | N | Y | Y | Y |
| Control for hospitalizations | N | Y | Y | N | Y | Y |
| Background trends vary by biomarkers | N | N | Y | N | N | Y |
Notes. 95% confidence intervals in parentheses.
The rows of the table include marginal effects from a multivariate panel data regression on the condition level indicating changes in health care utilization 1 and 2 years after REGARDS enrollment. The first rows indicate change in doctor visits for diagnosed conditions, and the latter rows indicate change in doctor visits for undiagnosed conditions. The columns indicate 6 regression specifications. All specifications include the control variables noted in the text. In columns 2, 3, 5, and 6, estimates are adjusted for hospitalizations. In columns 4 through 6, estimates are adjusted for time‐invariant individual characteristics using individual‐by‐condition fixed effects. In columns 3 and 6, background trends in doctor visits are allowed to vary with participants’ biomarkers measured at the time of REGARDS enrollment.
***p < .01, **p < .05, *p < .1.
Figure 2.
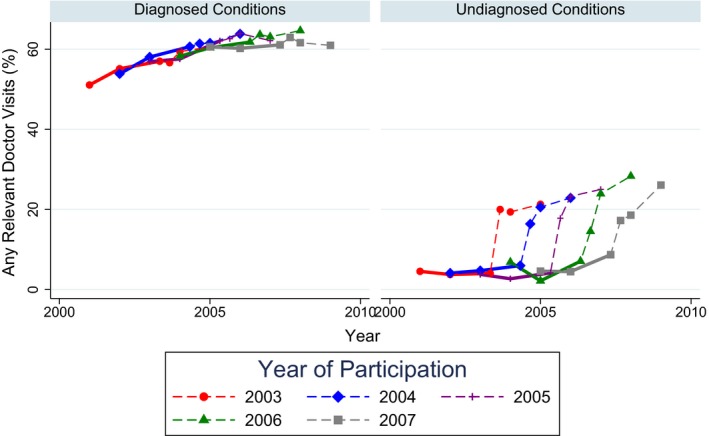
Assessment of Biomarkers via REGARDS and Doctor Visits for Previously Diagnosed and Previously Undiagnosed Conditions in the Raw Data
Notes. Solid lines indicate individuals who have not yet had their biomarkers assessed via REGARDS, and dashed lines indicate individuals who have recently had their biomarkers assessed via REGARDS. The year of REGARDS enrollment is split into time points before versus after REGARDS enrollment. [Color figure can be viewed at http://wileyonlinelibrary.com].
A Hausman test between the first and fourth models in Table 2, which were identical except for the use of fixed versus random effects to model the correlation of measures over time in the panel data, failed to reject the null hypothesis that both were consistent, assuming the specification of the model was correct (F(54) = 37.77, p = .954). The Hausman test therefore provided no evidence that the use of a fixed effects model was necessary.
Running the models separately by condition, we found that doctor visits increased for all three conditions. Over the 2 years after participation in REGARDS, semi‐annual evaluation and management visits increased by 45 percentage points for previously undiagnosed diabetes (95 percent confidence interval: 30–60), 19 percentage points for previously undiagnosed high cholesterol (95 percent confidence interval: 12–26), and 20 percentage points for previously undiagnosed hypertension (95 percent confidence interval: 8–31). Results were similar when we examined the number of doctor visits: Visits per 6‐month interval increased by 1.1 for previously undiagnosed diabetes (95 percent confidence interval: 0.5–1.7), 0.3 for previously undiagnosed high cholesterol (95 percent confidence interval: 0.2–0.5), and 0.4 for previously undiagnosed hypertension (95 percent confidence interval: 0.2–0.7). The raw data showed a similar pattern, as shown in Appendix Table S6.
The results disappeared as expected in the placebo regressions, which used only data from prior to REGARDS enrollment and tested for changes in levels and trends in doctor visits the year prior to REGARDS enrollment; see Appendix Table S7 and Appendix Figure S4. In addition, our findings remained qualitatively similar when we coded all patients with abnormal biomarkers who reported no prior diagnosis as undiagnosed regardless of their claims data, or when we excluded data from people with only diagnosed conditions. See Appendix Table S8.
Finally, we examined which participants were most likely to seek care for previously undiagnosed conditions. Table 3 shows the impact of biomarker assessment on semi‐annual doctor visits for previously undiagnosed conditions 2 years after assessment by participant characteristics. We found no significant differences in rates of follow‐up for previously undiagnosed conditions by gender, race, Medicaid dual eligibility, low income, marital status, fair or poor self‐reported health, region of residence (stroke belt vs. other), having multiple chronic conditions, having less than a high school education, living in a high‐poverty county (>25 percent poverty) or a county that is a primary care Health Professional Shortage Area, doctor visits the year before participation, or failing a cognitive test. However, participants who self‐reported having no usual health care provider at the time of enrollment in REGARDS may have been 11 percentage points less likely to seek care for a newly diagnosed condition (95 percent confidence interval: 0–23, two‐sided p‐value: .05) on a semi‐annual basis than participants who reported having a usual health care provider at the time of enrollment in REGARDS.
Table 3.
Percentage Point Change in Any Semi‐annual Doctor Visits for Evaluation and Management of Previously Undiagnosed Conditions Two Years after Enrollment in REGARDS, by Participant Characteristics (Average Marginal Effects from Regression)
| Average Marginal Effect If in Group | Average Marginal Effect If Not in Group | p‐Value of the Difference | |
|---|---|---|---|
| Mean (SE) | Mean (SE) | ||
| Had usual health care provider | 25 (4) | 14 (5) | .05 |
| Any doctor visits the year before enrollment in REGARDS | 23 (3) | 24 (8) | .88 |
| Male | 20 (4) | 27 (4) | .21 |
| African American | 18 (5) | 25 (4) | .29 |
| Medicaid dual eligible | 21 (8) | 23 (3) | .78 |
| Income < $20,000 | 25 (7) | 23 (3) | .80 |
| Married | 25 (4) | 20 (4) | .39 |
| Fair or poor self‐reported health | 24 (8) | 23 (3) | .85 |
| Lives in a stroke belt state | 25 (4) | 20 (5) | .45 |
| Has multiple chronic conditions | 23 (4) | 22 (5) | .85 |
| Less than high school education | 21 (7) | 23 (3) | .72 |
| County of residence has >25% residents in poverty | 28 (11) | 23 (3) | .63 |
| County of residence is primary care Health Professional Shortage Area | 25 (8) | 23 (3) | .80 |
| Failed cognitive test | 14 (6) | 24 (3) | .16 |
Note. The Bonferroni cutoff for statistical significance for 14 hypothesis tests is p = .004.
Discussion
In our national sample of Medicare beneficiaries, 12 percent of cases of high cholesterol, hypertension, and diabetes were undiagnosed and 20 percent of participants were undiagnosed for at least one of these conditions. In this national observational study, in‐home biomarker assessment after telephone enrollment increased use of semi‐annual doctor visits for previously undiagnosed conditions by 22 percentage points after 2 years. The impact of assessment on doctor visits for previously undiagnosed high cholesterol, hypertension, and diabetes was statistically similar for a wide variety of Medicare beneficiaries and did not vary by factors such as gender, race, living in the stroke belt, individual‐ or area‐level poverty, or living in a Health Professional Shortage Area. Beneficiaries who reported lacking a usual health care provider showed increases in semi‐annual doctor visits that were 11 percentage points lower than participants with a usual health care provider. However, this difference was at the margin of significance at the 0.05 level and did not meet stricter significance cutoffs used to account for multiple hypothesis testing. Beneficiaries who reported lacking a usual health care provider accounted for about one‐quarter of beneficiaries with undiagnosed conditions in our sample.
Our analysis builds on previous investigations of the relationship between health beliefs and health care seeking behaviors and provides several methodological advantages with respect to studying Medicare beneficiaries (Janz and Becker 1984; Carpenter 2010; Edwards 2013). First, due to the merge of REGARDS data with Medicare claims, we were able to track participants’ awareness of health conditions and health care utilization in the months directly before and after assessment, and to measure health care utilization prospectively using Medicare claims rather than retrospectively using self‐reported data. Second, the REGARDS study recruited participants from across the continental United States using random phone calls. This recruitment procedure produced a sample that resembled a national 5 percent sample of fee‐for‐service Medicare beneficiaries on a variety of characteristics (Xie et al. 2016). Third, due to the random variation in the timing of participants’ recruitment into the REGARDS study, we were able to tease apart the impact of biomarker assessment on doctor visits for high cholesterol, hypertension, and diabetes from the impact of aging or secular trends. In this way, our analysis addresses concerns about time‐varying confounders that are important in studies with before–after designs. Fourth, we incorporated a number of control variables to address possible remaining confounders. The results did not change when we controlled for all time‐invariant individual‐level characteristics using fixed effects, although the results of our Hausman test indicate that these additional control variables were not required to produce an unbiased estimate. This result follows logically from the random, rolling nature of recruitment into the REGARDS study.
We exploited the fact that one individual can have multiple conditions to provide evidence that REGARDS affected participants’ health care use by changing their health beliefs. If biomarker assessment via REGARDS chiefly affected participants’ health care use by providing information about previously undiagnosed conditions, then enrollment in REGARDS should not increase care of already diagnosed conditions. Accordingly, we found that biomarker assessment via REGARDS was not associated with an increase in visits for already diagnosed conditions. This finding was unchanged when we restricted the sample to only include participants with at least one undiagnosed condition.
The results of our study should be interpreted with the relevant limitations in mind. Because we lack data from individuals who declined to participate in this longitudinal cohort study and receive an assessment, we could not calculate the impact of being offered assessment (i.e., the intent to treat effect). Instead, we calculated the impact of assessment for individuals who were willing to be enrolled in this long‐term, observational study (i.e., the treatment on the treated effect) (Heckman and Vytlacil 2005). We cannot be certain how this impact would differ from the impact of recruitment for a shorter‐term observational study or a one‐time screening in a doctor's office. In addition, we cannot say whether our results will generalize beyond the group of REGARDS participants with available Medicare claims, namely African American and white adults who were enrolled in fee‐for‐service Medicare and not Medicare Advantage. Finally, although the REGARDS study only informed participants of their biomarker results, the final number of health care visits for a newly diagnosed condition is decided jointly by each patient and his or her doctor.
Our findings have implications for new models of care being tested by the Center for Medicare and Medicaid Innovation. In care models such as Accountable Care Organizations and Accountable Health Communities, health care providers are incentivized to reach out to individuals who have not recently been screened. Based on our findings, outreach to encourage screening may be unlikely to exacerbate existing disparities in chronic condition care by gender, race, region, or Medicaid dual eligibility because uptake of doctor visits after biomarker assessment did not vary by these factors. However, we found that the hardest‐to‐reach individuals—those who lacked a usual health care provider—may have had lower uptake of doctor visits for previously undiagnosed conditions. In such a case, multipronged efforts to support and engage hard‐to‐reach individuals, as in the Accountable Health Communities model, could become increasingly important to chronic condition care as more people become diagnosed.
Supporting information
Appendix SA1: Author Matrix.
Appendix SA2:
Figure S1: Location of REGARDS Participants.
Figure S2: Text from the Card and Letter Given to REGARDS Participants Informing them About their Blood Pressure and the Results of their Lab Tests.
Table S1: Participants’ Cascade.
Table S2: Chronic Conditions Warehouse ICD‐9 Codes Related to Diabetes, Hypertension, and High Cholesterol.
Table S3: Definitions used for Diabetes, Hypertension, and High Cholesterol.
Table S4: Cholesterol Levels used as Definition of High Cholesterol Based on Target Values Recommended by the ATP III Guideline.
Figure S3: Comparison of the REGARDS Sample with the NHANES Sample Year by Year, Using Comparable Sample Restrictions and Sample Weights.
Table S5: Change in Number of Semi‐Annual Doctor Visits for Evaluation and Management of Previously Diagnosed Versus Undiagnosed Conditions One and Two Years after REGARDS Enrollment (Average Marginal Effects from Regression).
Table S6: Tabulations of Raw Medicare Claims Data: Fraction of Previously Undiagnosed Diabetes, High Cholesterol, or Hypertension that Receives a Relevant Evaluation and Management Visit from a Doctor Each 6 Months.
Table S7: Placebo Models: Percentage Point Change in Any Semi‐Annual Doctor Visits for Evaluation and Management of Previously Diagnosed Versus Undiagnosed Conditions One Year Before REGARDS Enrollment, Using Only Data from Prior to REGARDS Enrollment (Average Marginal Effects from Regression).
Figure S4: Biomarker Assessment via REGARDS and Any Doctor Visits for Undiagnosed and Diagnosed Conditions in the Raw Data.
Table S8: Change in Number of Semi‐Annual Doctor Visits for Evaluation and Management of Previously Undiagnosed vs. Previously Diagnosed Conditions One and Two Years After REGARDS Enrollment: Re‐Estimated in Two Different Ways.
Acknowledgements
Joint Acknowledgment/Disclosure Statement: This research project was supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service, as well as grants R01 HL080477 and K24 HL111154 from the National Heart, Lung, and Blood Institute, grant R36HS023964‐01 from the Agency for Healthcare Research and Quality, and grants K24 DK105340 and P30 DK092949 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org. Dr. Safford receives funding from Amgen to support investigator initiated research. No other disclosures.
Disclaimer: None.
References
- Alley, D. E. , Asomugha C. N., Conway P. H., and Sanghavi D. M.. 2016. “Accountable Health Communities—Addressing Social Needs through Medicare and Medicaid.” New England Journal of Medicine 374 (1): 8–11. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association . 2014. “Standards of Medical Care in Diabetes—2015.” Diabetes Care 37 (Supplement 1): S14–80. [DOI] [PubMed] [Google Scholar]
- Bindman, A. , Grumbach K., Osmond D., Komaromy M., Vranizan K., Lurie N., Billings J., and Stewart A.. 1995. “Preventable Hospitalizations and Access to Health Care.” JAMA: The Journal of the American Medical Association 274 (4): 305–11. [PubMed] [Google Scholar]
- Bressler, N. M. , Varma R., Doan Q. V., Gleeson M., Danese M., Bower J. K., Selvin E., Dolan C., Fine J., Colman S., Turpcu A.. 2014. “Underuse of the Health Care System by Persons with Diabetes Mellitus and Diabetic Macular Edema in the United States.” JAMA Ophthalmology 132 (2): 168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke, A. , and Simmons A.. 2014. “Increased Coverage of Preventive Services with Zero Cost Sharing under the Affordable Care Act.” Department of Health and Human Services [accessed on December 6, 2016]. Available at http://aspe.hhs.gov/sites/default/files/pdf/76901/ib_PreventiveServices.pdf
- Carpenter, C. J. 2010. “A Meta‐Analysis of the Effectiveness of Health Belief Model Variables in Predicting Behavior.” Health Communication 25 (8): 661–9. [DOI] [PubMed] [Google Scholar]
- Center for Medicare and Medicaid Services . 2015. “ACO Shared Savings Program Quality Measures” [accessed on December 6, 2016]. Available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Downloads/ACO-Shared-Savings-Program-Quality-Measures.pdf
- Centers for Disease Control and Prevention . 2014. “National Diabetes Statistics Report, 2014.” Atlanta, GA [accessed on December 6, 2016]. Available at http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf
- Cowie, C. , Rust K., Ford E., Eberhardt M., Byrd‐Holt D., Li C., Williams D., Gregg E., Bainbridge K., Saydah S., and Geiss L.. 2009. “Full Accounting of Diabetes and Pre‐Diabetes in the US Population in 1988‐1994 and 2005‐2006.” Diabetes Care 32 (2): 0–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino, R. B. , Vasan R. S., Pencina M. J., Wolf P. A., Cobain M., Massaro J. M., and Kannel W. B.. 2008. “General Cardiovascular Risk Profile for Use in Primary Care: The Framingham Heart Study.” Circulation 117 (6): 743–53. [DOI] [PubMed] [Google Scholar]
- Danaei, G. , Ding E. L., Mozaffarian D., Taylor B., Rehm J., Murray C. J. L., and Ezzati M.. 2009. “The Preventable Causes of Death in the United States: Comparative Risk Assessment of Dietary, Lifestyle, and Metabolic Risk Factors.” PLoS Medicine 6 (4): e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, R. D. . 2013. “If My Blood Pressure Is High, Do I Take It to Heart? Behavioral Impacts of Biomarker Collection in the Health and Retirement Study” [accessed on December 6, 2016]. Available at http://www.nber.org/papers/w19311 [DOI] [PubMed]
- Egan, B. M. , Zhao Y., and Axon R. N.. 2010. “US Trends in Prevalence, Awareness, Treatment, and Control of Hypertension, 1988–2008.” JAMA: The Journal of the American Medical Association 303 (20): 2043–50. [DOI] [PubMed] [Google Scholar]
- Farley, T. , Dalal M., Mostashari F., and Frieden T.. 2010. “Deaths Preventable in the U.S. by Improvements in Use of Clinical Preventive Services.” American Journal of Preventive Medicine 38 (6): 600–9. [DOI] [PubMed] [Google Scholar]
- Finkelstein, E. A. , Khavjou O., and Will J. C.. 2006. “Cost‐Effectiveness of WISEWOMAN, a Program Aimed at Reducing Heart Disease Risk among Low‐Income Women.” Journal of Women's Health 15 (4): 379–89. [DOI] [PubMed] [Google Scholar]
- Ford, E. S. , Li C., Pearson W. S., Zhao G., and Mokdad A. H.. 2010. “Trends in Hypercholesterolemia, Treatment and Control among United States Adults.” International Journal of Cardiology 140 (2): 226–35. [DOI] [PubMed] [Google Scholar]
- Friedewald, W. T. , Levy R. I., and Fredrickson D. S.. 1972. “Estimation of the Concentration of Low‐Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge.” Clinical Chemistry, 18: 499–502. [PubMed] [Google Scholar]
- Gorina, Y. , and Kramarow E. A.. 2011. “Identifying Chronic Conditions in Medicare Claims Data: Evaluating the Chronic Condition Data Warehouse Algorithm.” Health Services Research 46 (5): 1610–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healthcare.gov . 2014. Preventive Services Covered Under the Affordable Care Act. [accessed on December 6, 2016]. Available at http://www.hhs.gov/healthcare/facts/factsheets/2010/07/preventive-services-list.html
- Heckman, J. J. , and Vytlacil E.. 2005. “Structural Equations, Treatment Effects, and Econometric Policy Evaluation1.” Econometrica 73 (3): 669–738. [Google Scholar]
- Homan, S. G. , McBride D. G., and Yun S.. 2014. “The Effect of the Missouri WISEWOMAN Program on Control of Hypertension, Hypercholesterolemia, and Elevated Blood Glucose among Low‐Income Women.” Preventing Chronic Disease 11: E74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, V. , Cushman M., Pulley L. V., Gomez C., Go R., Prineas R., Graham A., Moy C., and Howard G.. 2005. “The Reasons for Geographic and Racial Differences in Stroke Study: Objectives and Design.” Neuroepidemiology 25: 135–43. [DOI] [PubMed] [Google Scholar]
- Howard, V. J. , Kleindorfer D. O., Judd S. E., McClure L. A., Safford M. M., Rhodes J. D., Cushman M., Moy C. S., Soliman E. Z., Kissela B. M., and Howard G.. 2011. “Disparities in Stroke Incidence Contributing to Disparities in Stroke Mortality.” Annals of Neurology 69 (4): 619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, P. , Oparil S., Carter B., Cushman W., Dennison‐Himmelfarb C., Handler J., Lackland D., LeFevre, M. , MacKenzie T., Ogedegbe O., Smith S. Jr, Svetkey L., Taler S., Townsend R., Wright J. Jr, Narva A., and Ortiz E.. 2014. “2014 Evidence‐Based Guideline for the Management of High Blood Pressure in Adults: Report from the Panel Members Appointed to the Eighth Joint National Committee (JNC 8).” JAMA: The Journal of the American Medical Association 311 (5): 507–20. [DOI] [PubMed] [Google Scholar]
- Janz, N. K. , and Becker M. H.. 1984. “The Health Belief Model: A Decade Later.” Health Education & Behavior 11 (1): 1–47. [DOI] [PubMed] [Google Scholar]
- Maciosek, M. V. , Coffield A. B., Flottemesch T. J., Edwards N. M., and Solberg L. I.. 2010. “Greater Use Of Preventive Services In U.S. Health Care Could Save Lives at Little or No Cost.” Health Affairs 29 (9): 1656–60. [DOI] [PubMed] [Google Scholar]
- McDonald, M. , Hertz R. P., Unger A. N., and Lustik M. B.. 2009. “Prevalence, Awareness, and Management of Hypertension, Dyslipidemia, and Diabetes among United States Adults Aged 65 and Older.” Journals of Gerontology ‐ Series A Biological Sciences and Medical Sciences 64 (2): 256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, B. , Mok W., and Sullivan J.. 2015. “Household Surveys in Crisis.” Journal of Economic Perspectives 29 (4): 1–29. [Google Scholar]
- Muntner, P. , Colantonio L. D., Cushman M., Goff D. C., Howard G., Howard V. J., Kissela B., Levitan E. B., Lloyd‐Jones D. M., and Safford M. M.. 2014. “Validation of the Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Equations.” JAMA: The Journal of the American Medical Association 311 (14): 1406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) . 2002. “Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).” Circulation 106 (25): 3143. [PubMed] [Google Scholar]
- Patel, S. , Winkel M., Mohammed Ali K. M., Narayan V., and Mehta N.. 2015. “Cardiovascular Mortality Associated with 5 Leading Risk Factors: National and State Preventable Fractions Estimated from Survey Data.” Annals of Internal Medicine 163 (4): 245–53. [DOI] [PubMed] [Google Scholar]
- Sloan, F. , Acquah K., Lee P., and Sangvai D.. 2012. “Despite ‘Welcome to Medicare’ Benefit, One in Eight Enrollees Delay First Use of Part B Services for at Least Two Years.” Health Affairs 31 (6): 1260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock, J. H. , and Watson M. W.. 2008. “Heteroskedasticity‐Robust Standard Errors for Fixed Effects Panel Data Regression.” Econometrica 76 (1): 155–74. [Google Scholar]
- Stone, N. , Robinson J., Lichtenstein A., Noel Bairey Mertz C., Blum C., Eckel R., Goldberg A., Gordon D., Levy D., Lloyd‐Jones D., McBride P., Schwartz J., Shero S., Smith S., Watson K., and Wilson P.. 2014. “2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.” Journal of the American College of Cardiology 63 (25 Pt B): 2889–934. [DOI] [PubMed] [Google Scholar]
- U.S. Burden of Disease Collaborators . 2013. “The State of US Health, 1990‐2010: Burden of Diseases, Injuries, and Risk Factors.” JAMA: The Journal of the American Medical Association 310 (6): 591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force . 2016. “US Preventive Services Task Force Grade A and B Recommendations” [accessed on December 6, 2016]. Available at http://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/
- Xie, F. , Colantonio L. D., Curtis J. R., Safford M. M., Levitan E. B., Howard G., and Muntner P.. 2016. “Linkage of a Population‐Based Cohort with Primary Data Collection to Medicare Claims: The Reasons for Geographic and Racial Differences in Stroke Study.” American Journal of Epidemiology 184 (7): 532–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Appendix SA2:
Figure S1: Location of REGARDS Participants.
Figure S2: Text from the Card and Letter Given to REGARDS Participants Informing them About their Blood Pressure and the Results of their Lab Tests.
Table S1: Participants’ Cascade.
Table S2: Chronic Conditions Warehouse ICD‐9 Codes Related to Diabetes, Hypertension, and High Cholesterol.
Table S3: Definitions used for Diabetes, Hypertension, and High Cholesterol.
Table S4: Cholesterol Levels used as Definition of High Cholesterol Based on Target Values Recommended by the ATP III Guideline.
Figure S3: Comparison of the REGARDS Sample with the NHANES Sample Year by Year, Using Comparable Sample Restrictions and Sample Weights.
Table S5: Change in Number of Semi‐Annual Doctor Visits for Evaluation and Management of Previously Diagnosed Versus Undiagnosed Conditions One and Two Years after REGARDS Enrollment (Average Marginal Effects from Regression).
Table S6: Tabulations of Raw Medicare Claims Data: Fraction of Previously Undiagnosed Diabetes, High Cholesterol, or Hypertension that Receives a Relevant Evaluation and Management Visit from a Doctor Each 6 Months.
Table S7: Placebo Models: Percentage Point Change in Any Semi‐Annual Doctor Visits for Evaluation and Management of Previously Diagnosed Versus Undiagnosed Conditions One Year Before REGARDS Enrollment, Using Only Data from Prior to REGARDS Enrollment (Average Marginal Effects from Regression).
Figure S4: Biomarker Assessment via REGARDS and Any Doctor Visits for Undiagnosed and Diagnosed Conditions in the Raw Data.
Table S8: Change in Number of Semi‐Annual Doctor Visits for Evaluation and Management of Previously Undiagnosed vs. Previously Diagnosed Conditions One and Two Years After REGARDS Enrollment: Re‐Estimated in Two Different Ways.


