Abstract
Tumor‐associated macrophages (TAMs) contribute to tumor progression, but it is not clear how they are recruited to tumor sites. Here we showed that periostin (POSTN) was present at high levels in ovarian cancer ascetic fluids and was correlated with CD163+ TAMs. The high POSTN level and macrophage infiltration were inversely associated with relapse‐free survival for ovarian cancer patients. In vitro studies showed that coculture with macrophages significantly increased POSTN production in ovarian cancer cells. Further investigation found that POSTN production in ovarian cancer cells was promoted by transforming growth factor‐β generated by macrophages. Moreover, siRNA of POSTN and POSTN neutralizing antibody treatment showed that ovarian cancer cell‐derived POSTN promoted the recruitment of macrophages and modulated their cytokine secretion profile. Collectively, these data indicated that POSTN was an important factor for macrophage recruitment in the tumor microenvironment and is involved in the interactions between macrophages and ovarian cancer cells.
Keywords: macrophages, ovarian cancer, periostin, recruitment, relapse‐free survival
1. INTRODUCTION
Ovarian carcinoma is the most common cause of gynecologic cancer death worldwide.1 Although diverse clinical treatments have been improved, mortality rates have failed to decrease significantly.2 Accumulating evidence indicates that the potential recurring and metastatic behavior of ovarian cancer is attributable to the characteristics of tumor associated macrophages (TAMs).3, 4 It is generally accepted that TAMs represent a distinct type of M2 macrophages and show mostly pro‐tumoral functions, including the promotion of tumor cell proliferation, invasion, and metastasis.5, 6 Much clinical data show a close association of M2 TAMs with poor outcome for ovarian cancer patients.7, 8 However, the detailed mechanisms by which macrophage recruitment occurs during tumor progression are poorly understood.
Periostin (POSTN), a unique, evolutionarily conserved ECM protein, was first identified in a mouse osteoblastic cell line as a cell adhesion protein for pre‐osteoblasts.9 Recent studies have reported that POSTN is involved in promoting tumor progression in many cancers.10, 11, 12, 13 It has also been shown to recruit macrophages through integrin ανβ3.14 Interestingly, POSTN is negligibly expressed in normal ovaries, and elevated expression of POSTN is observed in ovarian cancer.15 Moreover, POSTN accumulates in ascites of ovarian cancer and is absent or very low in ascetic fluids from non‐ovarian cancer patients.16 The above observations suggest the possibility that ovarian cancer cell‐derived POSTN might contribute to macrophage recruitment into ascites of ovarian cancer patients.
In this study, we found a positive correlation between POSTN and CD163+ TAMs in ovarian ascetic fluids. The high POSTN level and macrophage infiltration suggest to be inversely correlated with relapse‐free survival (RFS) for ovarian cancer patients. In vitro studies show that macrophages enhance POSTN production in ovarian cancer cells through transforming growth factor‐β (TGF‐β). More importantly, ovarian cancer cell‐derived POSTN contributes to macrophage recruitment.
2. MATERIALS AND METHODS
2.1. Antibodies and reagents
Phorbol 12‐myristate 13‐acetate was obtained from Sigma‐Aldrich (St. Louis, MO, USA). The FITC‐labeled monoclonal anti‐human CD163 and anti‐POSTN neutralizing and isotype‐matched antibodies were obtained from R&D Systems (Abingdon, UK). Phosphorylated (p‐)Smad2, Smad2, and β‐actin for Western blot assays were purchased from Cell Signaling Technology (Danvers, MA, USA).
2.2. Patient samples
The malignant ascites were collected from 25 ovarian cancer patients undergoing primary surgery at Qingdao Central Hospital (Qingdao, China) during the period September 2014–March 2016. Informed consent for our study was obtained from all patients and the protocol was approved by our Institutional Review Board. Tumor stage was based on FIGO criteria, and grade was determined following WHO standards. Patient clinical characteristics are presented in Table S1.
2.3. Flow cytometric analysis
Mononuclear cells were isolated from leukocyte‐enriched buffy coats from malignant ascites using density gradient centrifugation and were positively selected by MACS CD14 microbeads (Miltenyi Biotec, Auburn, CA, USA). After MACS isolation, CD14+ cells were stained for FITC‐labeled mAb against CD163 and subsequently analyzed through flow cytometry.
2.4. Cell culture and experimental treatments
To generate THP‐1‐derived macrophages, THP‐1 cells were seeded into complete growth medium supplemented with 100 ng/mL PMA for 48 hours. CD14+ monocytes were differentiated into macrophages in RPMI‐1640 medium using 10% FBS containing 100 ng/mL recombinant human macrophage colony‐stimulating factor (M‐CSF) for 7 days.
For Transwell chamber cocultures, THP‐1‐derived macrophages were seeded into each well of a 6‐well plate. A2780 cells were seeded into the upper well. After the coculture for 24 hours, the coculture medium, A2780 cells, and macrophages were collected. As in other studies, macrophages and A2780 cells were separated and cultured alone in 2 mL fresh medium for another 24 hours. The conditioned mediums (CMs) were then collected.
For experiments using TGF‐β receptor inhibitor, A2780 cells were pre‐incubated with 10 μmol/L LY2109761 for 120 minutes. Subsequently, A2780 cells were cocultured with THP‐1‐derived macrophages.
2.5. Quantitative RT‐PCR
Total RNA from THP‐1‐derived macrophages and A2780 cells was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized by reverse transcription. Relative gene expression levels were determined by quantitative RT‐PCR using the SYBR Green I methods; the primers are shown in Table S2. Relative mRNA expression levels were calculated for each gene and each time point after normalization against GAPDH using the ΔΔCt method.
2.6. RNA interference
Macrophages were transiently transfected with 100 nmol/L control siRNA or POSTN siRNA (Dharmacon, Lafayette, CO, USA) using a Lipofectamine 2000 transfection reagent (Invitrogen Life Technologies, Gaithersburg, MD, USA) at 2:1 (lipid:siRNA) ratio according to the manufacturer's instructions for siRNA transfection. Forty‐eight hours later, the transfected cells were collected and cocultured with A2780 cells.
2.7. Cytokine measurement
Malignant ascites, the CM of A2780 cells, and THP‐1‐derived macrophages were collected. Transforming growth factor‐β, POSTN, interleukin (IL) ‐10, IL‐6, M‐CSF, and IL‐8 concentration were determined by ELISA kits according to the manufacturer's instructions (R&D Systems).
2.8. Western blot analysis
A2780 cells were lysed in RIPA buffer containing a protease inhibitor cocktail. Cell lysates were electrophoresed in 10% SDS‐PAGE and then transferred onto a nitrocellulose membrane, which was blocked with TBST (Tris‐buffered saline, 0.1% Tween‐20) containing 5% non‐fat dried milk. The membrane was incubated with the indicated antibodies, including anti‐β‐actin, anti‐Smad2, and anti‐p‐Smad2 overnight at 4°C, followed by washing with TBST and incubation with peroxidase‐conjugated second antibody for 1 hour. The protein bands were detected with enhanced chemiluminescence.
2.9. Transwell assay
THP‐1‐derived macrophages or M‐CSF‐induced macrophages were added into the upper chamber of the inserts in 200 μL serum‐free medium. Then 600 μL RPMI‐1640 medium containing 10% FBS, CMs of A2780 cells, and malignant ascites were added into the lower chamber. In order to detect the function of POSTN in regulating the recruitment of macrophages, 2.5 μg/mL anti‐POSTN neutralizing antibody (NAb) was added into CMs before the Transwell assay. Cells were incubated at 37°C in 5% CO2 for 24 hours. The migrated cells on the lower side of the filter were fixed by methanol and stained with Giemsa. Five random fields of each well were photographed and cell numbers were counted.
2.10. Statistical analysis
The statistical analysis was carried out using GraphPad Prism software (version 6). Data are presented as mean ± SD based on triplet experiments. The data were analyzed using ANOVA and Student's t‐test (two tailed). Spearman's test was used for the correlation analysis. Kaplan–Meier analysis was used and P‐values were determined by the log–rank test. Statistical significance was considered as P < .05.
3. RESULTS
3.1. Correlation of CD163+ TAMs with ascites cytokine levels
In order to analyze macrophage infiltration in ovarian carcinoma, malignant ascites from 25 patients with serious ovarian carcinoma were collected. Tumor‐associated macrophages were purified by MACS as CD14+ mononuclear cells from malignant ascites and analyzed by FACS for CD163, a marker of M2 macrophages (Figures 1A, S1A).17, 18, 19 We also determined the concentrations of several key mediators (POSTN, IL‐10, IL‐6, M‐CSF, TGF‐β, and IL‐8) involved in the expansion, recruitment, and activation of macrophages (Figure 1A). Periostin was present at high levels (>200 ng/mL) in patient ascites. Interestingly, we observed a significant correlation between POSTN concentration and the percentage of CD163+ TAMs in malignant ascites (Figure 1B). None of the other cytokines tested was significantly associated with CD163+ TAMs.
Figure 1.
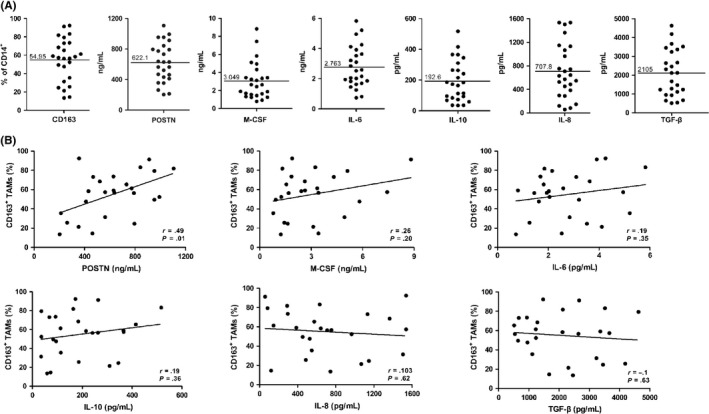
Percentages of CD163+ tumor‐associated macrophages (TAMs) and cytokine levels in ascites from ovarian cancer patients. A, Expression of CD163 on CD14+ cells and distribution of periostin (POSTN), interleukin (IL)‐10, IL‐6, macrophage colony‐stimulating factor (M‐CSF), IL‐8, and transforming growth factor‐β (TGF‐β) levels in ascites. B, Correlation of CD163 expression with ascites levels of POSTN, IL‐10, IL‐6, M‐CSF, IL‐8, and TGF‐β
3.2. CD163+ macrophage and POSTN concentrations in ascites are associated with RFS of ovarian cancer patients
We investigated potential relationships between CD163 expression and cytokine levels in ascites with clinical progression of ovarian cancer patients. Patients were divided into high or low subgroups for CD163 expression and each cytokine, based on the respective median as a cut‐off point. We found that the CD163 low group had a significant advantage in RFS compared with the CD163 high group (Figure 2A). This RFS advantage was also shown in the POSTN low group (Figure 2B). In contrast, other cytokines showed no association with RFS (Figure 2C‐G).
Figure 2.
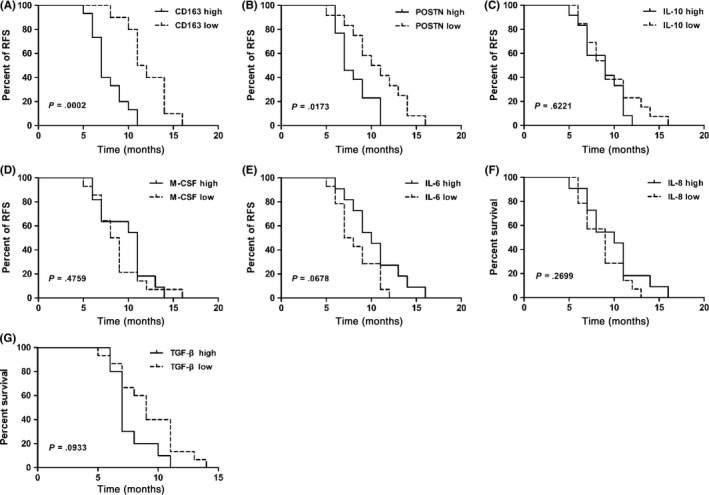
CD163+ macrophages and periostin (POSTN) concentration in ascites were associated with relapsed‐free survival (RFS) of ovarian cancer patients. Kaplan–Meier plots showing the correlation between RFS and high or low levels (median as cut‐off) of CD163+ macrophages (A), POSTN (B), interleukin (IL)‐10 (C), macrophage colony‐stimulating factor (M‐CSF) (D), IL‐6 (E), IL‐8 (F) and transforming growth factor‐β (TGF‐β) (G) concentrations in ascites from ovarian cancer patients
3.3. Coculture increased POSTN production in ovarian carcinoma cells
To explore the expression and function of POSTN in the tumor microenvironment, we used a two‐chamber coculture system to mimic the interaction between macrophages and ovarian cancer cells. The A2780 ovarian cancer cell line secreted a high level of POSTN, whereas the monocyte cell line, THP‐1‐derived macrophages, produced less POSTN (Figure 3A). Interestingly, POSTN was significantly increased in supernatant of the coculture of the two cell lines (Figure 3A). To identify the sources of increased POSTN production in the coculture system, we measured POSTN mRNA expression in THP‐1‐derived macrophages and A2780 cells at different time points of coculture. As shown in Figure 3B, C, POSTN mRNA expression was significantly upregulated in A2780 cells compared to THP‐1‐derived macrophages. A2780 cells and THP‐1‐derived macrophages were separated after coculture and cultured alone in fresh medium for another 24 hours. Periostin production was also significantly upregulated in A2780 cell CM 24 hours after coculture (Figure 3D). Another ovarian cancer cell line, OVCAR‐3, did not produce POSTN, and the production of POSTN was not detected after coculture (data not shown).
Figure 3.
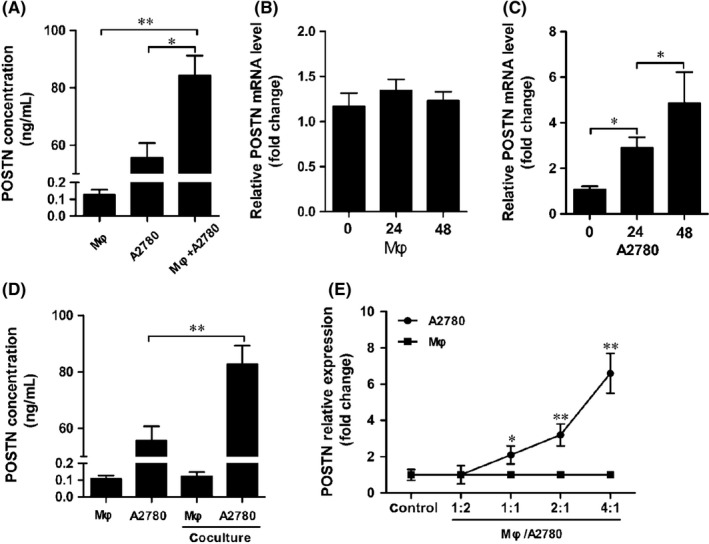
Coculture with THP‐1‐derived macrophages (Mφ) increased periostin (POSTN) production in A2780 ovarian cancer cells. A, A2780 cells were cocultured with THP‐1‐derived macrophages for 24 h. POSTN concentration in THP‐1‐derived macrophages, A2780 cells, and coculture medium was analyzed by ELISA. B,C, POSTN mRNA expression in THP‐1‐derived macrophages and A2780 cells at different time points of coculture. D, A2780 cells and THP‐1‐derived macrophages were separated after coculture and cultured alone in fresh medium for another 24 h. E, A2780 cells were cocultured with THP‐1‐derived macrophages at different ratios. POSTN mRNA expression in A2780 cells and THP‐1‐derived macrophages was determined by quantitative RT‐PCR
To determine whether the upregulation of POSTN was correlated with the numbers of ovarian cancer cells and macrophages, A2780 cells were cocultured with increased numbers of THP‐1‐derived macrophages. As shown in Figure 3E, POSTN expression started to increase at a 1:1 ratio of macrophages to A2780 cells and reached its peak at a 4:1 ratio.
3.4. Macrophages promoted POSTN expression in ovarian cancer cells through TGF‐β
Transforming growth factor‐β is an inflammatory cytokine highly produced by tumor‐associated macrophages.20, 21 Based on a recent finding that TGF‐β significantly induces POSTN expression,22 we investigated the possibility that macrophage‐derived TGF‐β increased POSTN production in ovarian cancer cells. First, we found that coculture with A2780 significantly increased TGF‐β mRNA expression in THP‐1‐derived macrophages (Figure 4A). Transforming growth factor‐β production was also significantly increased in CM of THP‐1‐derived macrophages 24 hours after coculture (Figure 4B). In order to verify whether TGF‐β modulated POSTN production in A2780 cells, TGF‐β NAb was added to the coculture system. The expression and secretion of POSTN in A2780 cells was decreased by addition of TGF‐β NAb, whereas it was not significantly altered in THP‐1‐derived macrophages (Figure 4C,D).
Figure 4.
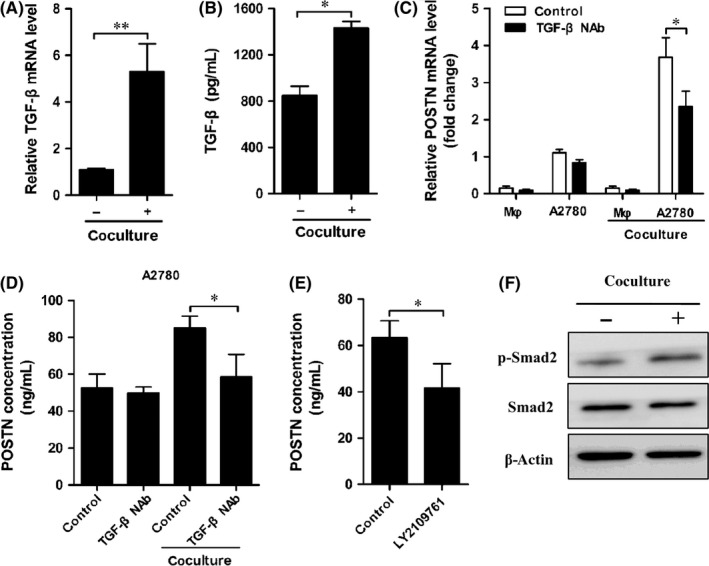
THP‐1‐derived macrophages promoted periostin (POSTN) expression in ovarian carcinoma cells via the transforming growth factor‐β (TGF‐β) signaling pathway. A, Coculture of A2780 cells with THP‐1‐derived macrophages significantly increased TGF‐β mRNA expression in THP‐1‐derived macrophages. B, THP‐1‐derived macrophages and A2780 cells were separated after coculture and cultured alone in fresh medium for another 24 h. Then conditioned medium (CM) of THP‐1‐derived macrophages was collected and TGF‐β concentration was measured. C, THP‐1‐derived macrophages (Mφ) were cocultured with A2780 cells with/without TGF‐β neutralizing antibody (NAb) for 24 h. Cells were collected and POSTN mRNA expression was determined. D, Cocultured A2780 cells were cultured alone for a further 24 h. POSTN concentration in CM of A2780 cells was determined. E, A2780 cells were pretreated with 10 μmol/L LY2109761, and then were cocultured with THP‐1‐derived macrophages for 24 h. Cocultured A2780 cells were cultured alone for another 24 h. POSTN concentration in CM of A2780 cells was determined. F, A2780 cells were cocultured with THP‐1‐derived macrophages for 24 h. The phosphorylation (p‐) of Smad2 in A2780 cells was determined through Western blot analysis
To further confirm that TGF‐β increased the expression of POSTN, A2780 cells were pretreated with TGF‐β receptor inhibitor LY2109761 and then were cocultured with THP‐1‐derived macrophages. The result indicated that the positive effect of coculture on POSTN production was completely blocked by LY2109761 (Figure 4E). In addition, Western blot analyses revealed that coculture with THP‐1‐derived macrophages significantly increased the phosphorylation of Smad2 in A2780 cells (Figure 4F). Taken together, these results suggest that TGF‐β production by macrophages is critical for POSTN expression in ovarian carcinoma cells.
3.5. Ovarian carcinoma cell‐derived POSTN is responsible for macrophage recruitment
To determine whether POSTN produced by ovarian cancer cells was capable of attracting macrophages, ovarian cancer cell‐derived CMs were analyzed for the recruitment of THP‐1‐derived macrophages or M‐CSF‐induced macrophages by Transwell assay. Remarkably increased numbers of THP‐1‐derived macrophages migrated towards cocultured A2780 CM in the lower chamber compared with A2780 CM alone (Figure 5A). In contrast, cocultured OVCAR‐3 CM did not significantly increase the recruitment of THP‐1‐derived macrophages compared with OVCAR‐3 CM alone. To verify whether POSTN was involved in macrophage recruitment, POSTN siRNA was transfected into A2780 cells before the Transwell assay. The results showed that targeting POSTN with siPOSTN in A2780 cells significantly reduced the recruitment of THP‐1‐derived macrophages or M‐CSF‐induced macrophages towards A2780 CM compared with scramble treated cells (Figures 5B, S1B). To further verify that POSTN produced by A2780 cells was involved in the recruitment of THP‐1‐derived macrophages, POSTN NAb was added to A2780 CM before the Transwell assay. The results indicated that POSTN NAb significantly decreased the number of THP‐1‐derived macrophages or M‐CSF‐induced macrophages recruited by A2780 CM (Figures 5C, S1B). These data strongly indicate that ovarian cancer cells might enhance macrophage recruitment in a paracrine manner that is partly mediated by POSTN.
Figure 5.
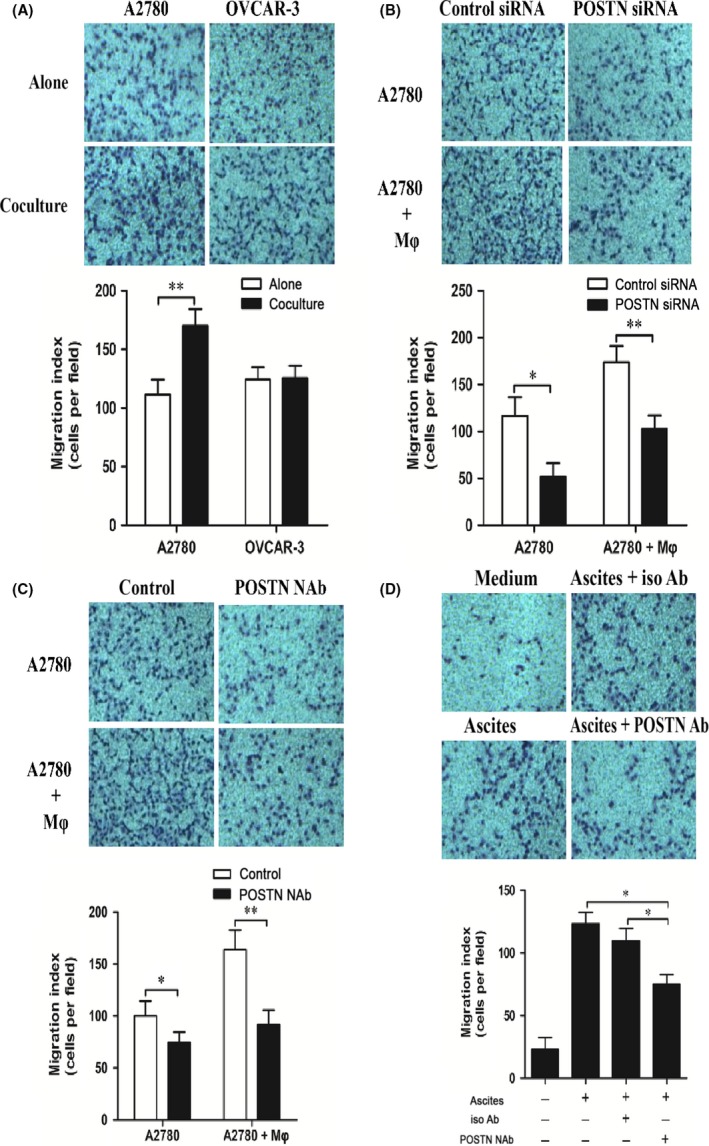
THP‐1‐derived macrophages were recruited by periostin (POSTN) by A2780 cell conditioned medium (CM). A, Representative images of THP‐1‐derived macrophages (Mφ) migrating to A2780 cell‐derived CM. OVCAR‐3 cells were used as a negative control for ovarian cancer‐derived POSTN. B, CM of A2780 cells transfected by control or POSTN siRNA were used for Transwell assay. C, Representative images of THP‐1‐derived macrophages migrating to A2780 cell‐derived CM pretreated with or without POSTN neutralizing antibody (NAb). D, Malignant ascites (n = 3) were pretreated with 2.5 μg/mL POSTN NAb or isotype (iso) Ab. Ascites were applied to the Transwell assay and the number of THP‐1‐derived macrophages recruited by ascetic fluids was counted
To determine whether macrophage recruitment was linked with POSTN present in ovarian cancer patients, we added POSTN NAb into malignant ascites before the Transwell assay. The results showed that POSTN NAb decreased the number of THP‐1‐derived macrophages or M‐CSF‐induced macrophages recruited by malignant ascites (Figures 5D, S1B).
3.6. Blockade of POSTN re‐educate cytokine profile of THP‐1‐derived macrophages
Periostin is involved in regulating the expression of inflammatory mediators.23, 24 We aimed to determine whether A2780‐derived POSTN affected the expression of cell surface markers and cytokines associated with M1/M2 macrophages. M1 macrophages express high levels of IL‐1β, IL‐6, and TNF‐α and lower levels of CD163, CD206, arginase‐1, TGF‐β, CC chemokine ligand (CCL)17, CCL18, and IL‐10 compared with M2 macrophages.25, 26 THP‐1‐derived macrophages were cocultured with A2780 cells, and the expression of these genes was measured. We found a dramatic downregulation of anti‐inflammatory cytokine and chemokine TGF‐β and CCL17 but a substantial upregulation of pro‐inflammatory cytokine IL‐1β in THP‐1‐derived macrophages, when cocultured with siPOSTN A2780 cells (Figure 6A). However, no significant differences in cell surface markers of M1/M2 macrophages were observed (Figure 6A). When POSTN NAb was added into the coculture system, similar results were observed (Figure 6B).
Figure 6.
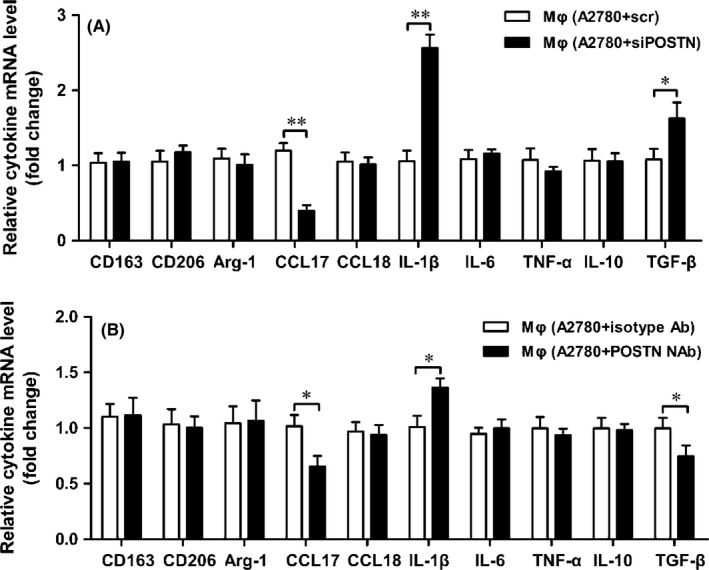
Periostin (POSTN) re‐educated the cytokine profiles of THP‐1‐derived macrophages (Mφ). A, THP‐1‐derived macrophages were cocultured with A2780 cells transfected by control and siPOSTN for 24 h, then the transcription of cytokines of THP‐1‐derived macrophages was measured by quantitative RT‐PCR. B, POSTN neutralizing antibody (NAb) or isotype antibodies were added to the conditioned medium of the coculture system for 24 h, then the transcription of cytokines of THP‐1‐derived macrophages was measured by quantitative RT‐PCR. Arg‐1, arginase‐1; CCL, CC chemokine ligand; IL, interleukin; scr, scrambled; TGF‐β, transforming growth factor‐β; TNF‐α, tumor necrosis factor‐α
4. DISCUSSION
Malignant ascites often contain a large number of TAMs, which are correlated with poor prognosis in ovarian cancer patients.8, 27 However, the mechanism by which TAMs accumulate in the tumor microenvironment remains unclear. In the present study, we found that high levels of POSTN existed in malignant ascites and were correlated with CD163+ TAMs. The in vitro studies suggested that macrophages promoted POSTN expression in ovarian cancer cells though TGF‐β. Subsequently, ovarian cancer cell‐derived POSTN enhanced macrophage recruitment. Together, these studies revealed the interplay between tumor cells and macrophages through POSTN (Figure 7).
Figure 7.
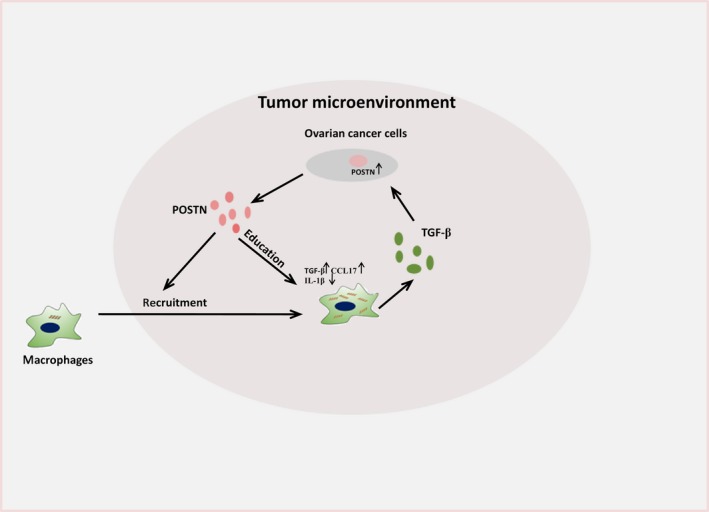
Schematic representation of interactions between ovarian cancer cells and macrophages through periostin (POSTN). CCL, CC chemokine ligand; IL, interleukin; TGF‐β, transforming growth factor‐β
Advanced ovarian cancer disseminates into the peritoneal cavity and causes the formation of ascetic fluids containing a large number of macrophages and cytokines.28, 29 In our study, we found high CD163+ macrophage infiltration in ascites from ovarian cancer patients, and high levels of POSTN were also detected. Many studies have reported that macrophages in ascites are skewed toward an M2 phenotype and play an essential role in tumor progression.30, 31 Our results indicated that high CD163+ macrophage infiltration and POSTN production in ascites were inversely associated with RFS of ovarian cancer patients. Interestingly, POSTN was associated with the percentage of CD163+ TAMs, indicating that POSTN might be involved in the recruitment and/or polarization of macrophages in malignant ascites.
Increasing evidence has shown that the interactions between tumor cells and infiltrated macrophages create a tumor‐supportive microenvironment.32, 33 High levels of POSTN accumulate in ascites from ovarian cancer patients. We explored the mechanism by which high levels of POSTN in the tumor microenvironment were formed. Our results showed that the secretion of POSTN from A2780 cells was significantly promoted after coculture with THP‐1‐derived macrophages. In addition, the higher density of THP‐1‐derived macrophages resulted in the marked upregulation of POSTN expression in A2780 cells. These results supported that the interactions between cancer cells and macrophages produced high levels of POSTN in the tumor microenvironment. The mechanism regulating POSTN production was further investigated. We found that addition of TGF‐β NAb into coculture medium significantly reduced POSTN production in A2780 cells. Pretreatment of A2780 cells with TGF‐β receptor inhibitors also showed similar results. These results suggested that TGF‐β is involved in POSTN generated by A2780 cells.
A number of studies have reported that POSTN is associated with the promotion of tumor cell migration and invasion.34, 35, 36 Recent studies have also found that POSTN shows a potent capacity to recruit macrophages.14 Consistent with these findings, our results showed that ovarian cancer cell‐derived POSTN was involved in macrophage recruitment. In addition, in vitro studies showed that POSTN blocking decreased macrophage migration toward malignant ascetic fluids. To determine further biological effects of POSTN downregulation in ovarian cancer cells, we determined the impact of targeting POSTN on cell surface markers and secretion activities of macrophages. We found that ovarian cell‐derived POSTN increased the expression of TGF‐β and CCL17, which were highly expressed in M2 macrophages compared with M1 macrophages; however, there was no significant effect on cell surface markers of M1/M2 macrophages. These results suggest that ovarian cancer cell‐derived POSTN promoted the recruitment of macrophages and re‐educated tumor‐infiltrated macrophages.
In conclusion, our results showed that POSTN was a key mediator in the interplay between ovarian cancer cells and TAMs. Periostin secreted by ovarian cancer cells recruited macrophages to tumor sites. Then macrophages promoted a higher production of POSTN in ovarian cancer cells through production of TGF‐β, which formed the high levels of POSTN in the tumor microenvironment. Therefore, POSTN might constitute a potential target for antitumor strategies aimed at reducing the recruitment of TAMs.
CONFLICT OF INTEREST
The authors have no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China to Peng Zhao (No. 81501422) and Qingdao Outstanding Health Professional Development Fund to Peng Zhao (2017).
Tang M, Liu B, Bu X, Zhao P. Cross‐talk between ovarian cancer cells and macrophages through periostin promotes macrophage recruitment. Cancer Sci. 2018;109:1309–1318. https://doi.org/10.1111/cas.13567
REFERENCES
- 1. Karnezis AN, Cho KR, Gilks CB, Pearce CL, Huntsman DG. The disparate origins of ovarian cancers: pathogenesis and prevention strategies. Nat Rev Cancer. 2017;17:65‐74. [DOI] [PubMed] [Google Scholar]
- 2. Rustin G, Vergote I, Micha JP, et al. A multicenter, open‐label, expanded phase 2 study to evaluate the safety and efficacy of etirinotecan pegol, a polymer conjugate of irinotecan, in women with recurrent platinum‐resistant or refractory ovarian cancer. Gynecol Oncol. 2017;147:276‐282. [DOI] [PubMed] [Google Scholar]
- 3. Yin M, Li X, Tan S, et al. Tumor‐associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Invest. 2016;126:4157‐4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cortes M, Sanchez‐Moral L, De Barrios O, et al. Tumor‐associated macrophages (TAMs) depend on ZEB1 for their cancer‐promoting roles. EMBO J. 2017;36:3336‐3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pollard JW. Tumour‐educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71‐78. [DOI] [PubMed] [Google Scholar]
- 6. Liu Y, Cao X. The origin and function of tumor‐associated macrophages. Cell Mol Immunol. 2015;12:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yuan X, Zhang J, Li D, et al. Prognostic significance of tumor‐associated macrophages in ovarian cancer: a meta‐analysis. Gynecol Oncol. 2017;147:181‐187. [DOI] [PubMed] [Google Scholar]
- 8. Reinartz S, Schumann T, Finkernagel F, et al. Mixed‐polarization phenotype of ascites‐associated macrophages in human ovarian carcinoma: correlation of CD163 expression, cytokine levels and early relapse. Int J Cancer. 2014;134:32‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horiuchi K, Amizuka N, Takeshita S, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239‐1249. [DOI] [PubMed] [Google Scholar]
- 10. Guo X, Xue H, Shao Q, et al. Hypoxia promotes glioma‐associated macrophage infiltration via periostin and subsequent M2 polarization by upregulating TGF‐beta and M‐CSFR. Oncotarget. 2016;7:80521‐80542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baril P, Gangeswaran R, Mahon PC, et al. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia‐induced cell death: role of the beta4 integrin and the PI3k pathway. Oncogene. 2007;26:2082‐2094. [DOI] [PubMed] [Google Scholar]
- 12. Kanno A, Satoh K, Masamune A, et al. Periostin, secreted from stromal cells, has biphasic effect on cell migration and correlates with the epithelial to mesenchymal transition of human pancreatic cancer cells. Int J Cancer. 2008;122:2707‐2718. [DOI] [PubMed] [Google Scholar]
- 13. Erkan M, Kleeff J, Gorbachevski A, et al. Periostin creates a tumor‐supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132:1447‐1464. [DOI] [PubMed] [Google Scholar]
- 14. Zhou W, Ke SQ, Huang Z, et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour‐associated macrophages and promotes malignant growth. Nat Cell Biol. 2015;17:170‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu M, Fejzo MS, Anderson L, et al. Periostin promotes ovarian cancer angiogenesis and metastasis. Gynecol Oncol. 2010;119:337‐344. [DOI] [PubMed] [Google Scholar]
- 16. Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002;62:5358‐5364. [PubMed] [Google Scholar]
- 17. Jager NA, Teteloshvili N, Zeebregts CJ, Westra J, Bijl M. Macrophage folate receptor‐beta (FR‐beta) expression in auto‐immune inflammatory rheumatic diseases: a forthcoming marker for cardiovascular risk? Autoimmun Rev. 2012;11:621‐626. [DOI] [PubMed] [Google Scholar]
- 18. Saha B, Momen‐Heravi F, Furi I, et al. Extracellular vesicles from mice with alcoholic liver disease carry a distinct protein cargo and induce macrophage activation via Hsp90. Hepatology. 2017. [Epub ahead of print]. https://doi.org/10.1002/hep.29732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hollander L, Guo X, Velazquez H, et al. Renalase expression by melanoma and tumor‐associated macrophages promotes tumor growth through a STAT3‐mediated mechanism. Cancer Res. 2016;76:3884‐3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sideras K, Braat H, Kwekkeboom J, et al. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat Rev. 2014;40:513‐522. [DOI] [PubMed] [Google Scholar]
- 21. Szondy Z, Sarang Z, Kiss B, Garabuczi E, Koroskenyi K. Anti‐inflammatory mechanisms triggered by apoptotic cells during their clearance. Front Immunol. 2017;8:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen G, Nakamura I, Dhanasekaran R, et al. Transcriptional induction of periostin by a sulfatase 2‐TGFbeta1‐SMAD signaling axis mediates tumor angiogenesis in hepatocellular carcinoma. Cancer Res. 2017;77:632‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li W, Gao P, Zhi Y, et al. Periostin: its role in asthma and its potential as a diagnostic or therapeutic target. Respir Res. 2015;16:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prakoura N, Kavvadas P, Kormann R, Dussaule JC, Chadjichristos CE, Chatziantoniou C. NFkappaB‐induced periostin activates integrin‐beta3 signaling to promote renal injury in GN. J Am Soc Nephrol. 2017;28:1475‐1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tjiu JW, Chen JS, Shun CT, et al. Tumor‐associated macrophage‐induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase‐2 induction. J Invest Dermatol. 2009;129:1016‐1025. [DOI] [PubMed] [Google Scholar]
- 26. Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23‐35. [DOI] [PubMed] [Google Scholar]
- 27. Adhikary T, Wortmann A, Finkernagel F, et al. Interferon signaling in ascites‐associated macrophages is linked to a favorable clinical outcome in a subgroup of ovarian carcinoma patients. BMC Genom. 2017;18:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luo T, Sun J, Zhu S, et al. Ultrasound‐mediated destruction of oxygen and paclitaxel loaded dual‐targeting microbubbles for intraperitoneal treatment of ovarian cancer xenografts. Cancer Lett. 2017;391:1‐11. [DOI] [PubMed] [Google Scholar]
- 29. Duluc D, Delneste Y, Tan F, et al. Tumor‐associated leukemia inhibitory factor and IL‐6 skew monocyte differentiation into tumor‐associated macrophage‐like cells. Blood. 2007;110:4319‐4330. [DOI] [PubMed] [Google Scholar]
- 30. Finkernagel F, Reinartz S, Lieber S, et al. The transcriptional signature of human ovarian carcinoma macrophages is associated with extracellular matrix reorganization. Oncotarget. 2016;7:75339‐75352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takaishi K, Komohara Y, Tashiro H, et al. Involvement of M2‐polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via Stat3 activation. Cancer Sci. 2010;101:2128‐2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang X, Zhao X, Wang K, Wu L, Duan T. Interaction of monocytes/macrophages with ovarian cancer cells promotes angiogenesis in vitro. Cancer Sci. 2013;104:516‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yigit R, Massuger LF, Figdor CG, Torensma R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol Oncol. 2010;117:366‐372. [DOI] [PubMed] [Google Scholar]
- 34. Wang Z, Xiong S, Mao Y, et al. Periostin promotes immunosuppressive premetastatic niche formation to facilitate breast tumour metastasis. J Pathol. 2016;239:484‐495. [DOI] [PubMed] [Google Scholar]
- 35. Mikheev AM, Mikheeva SA, Trister AD, et al. Periostin is a novel therapeutic target that predicts and regulates glioma malignancy. Neuro Oncol. 2015;17:372‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ruan K, Bao S, Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci. 2009;66:2219‐2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


