Abstract
Estrogens are powerful modulators of neuronal physiology and in humans may affect a broad range of functions, including reproductive, emotional, and cognitive behaviors. We studied the contribution of estrogen receptors (ERs) in modulation of emotional processes and analyzed the effects of deleting ERα or ERβ in mice. Behavior consistent with increased anxiety was observed principally in ERβ mutant females and was associated with a reduced threshold for the induction of synaptic plasticity in the basolateral amygdala. Local increase of 5-hydroxytryptamine 1a receptor expression in medial amygdala may contribute to these changes. Our data show that, particularly in females, there is an important role for ERβ-mediated estrogen signaling in the processing of emotional behavior.
There is a strong link between estrogen and emotional disturbances in humans. Mood fluctuations, depression, irritability, and anxiety have often been associated with low levels of estradiol in postmenopausal women (1, 2), whereas estrogen replacement therapy ameliorates these psychological conditions (1–3). Reduced estrogen signaling in rodents leads to behavior indicative of increased anxiety (4, §). Little is known, however, about either the mechanisms or sites of estrogen actions in these modulatory processes, or the neurotransmitter systems involved in these regulations.
In the nervous system, estrogen signals are transduced by both nuclear estrogen receptors (ERα and ERβ), which act as transcription factors, and by a nongenomic pathway, which has yet to be identified. Both receptors show similar patterns of expression and are found at abundance in medial amygdala, bed nucleus of stria terminalis and preoptic area, whereas in hippocampus, ERα is the predominant ER isotype in mouse (5). Recent analysis of reproductive and aggressive behavior in ERα and ERβ null mutant mice provided the first clear evidence for a role for ERs in brain functions (6–8).
High levels of estrogens have been shown to increase dendrite growth and synaptic plasticity in the rat hippocampus in vitro and in vivo (9, 10). Furthermore, the in vivo experiments revealed that estrogen enhances neuronal excitability in the hippocampus (11). In the basolateral amygdala, a structure involved in fear and anxiety, estrogens have quite the opposite effect and reduce neuronal excitability (11, 12). Although the exact mode of action by which estrogen exerts its diverse effects is not clear, it is almost certain that the inhibitory neurotransmitter γ-aminobutyric acid (GABA) is involved (13, 14, ¶). GABA is implicated in fear and anxiety, on the evidence of both pharmacological (15, 16) and gene-targeting (17, 18) experiments. Regulation of the expression of the GABA synthesizing enzyme glutamic acid decarboxylase (GAD) by ERs (13,¶, 19) may be crucial to modulation of GABA type A activity and, therefore, to fear and anxiety. Moreover, GABAergic tone in amygdala can be affected by serotonergic signaling (20, 21), which in turn can be down-regulated by estrogen's effects on the 5-hydroxytryptamine 1a (5-HT1a) receptor (22, 23). This receptor has been directly implicated in pathological depression and anxiety (24, 25) and thus provides another possible mechanism of estrogen action in the amygdala.
In the present study, we have assessed the role of ERs in emotional behavior by analyzing mice carrying null mutations in the corresponding genes. We also have established that observations of enhanced anxiety in mutant females coincides with changes in synaptic plasticity in the basolateral amygdala. The data indicate that changes in serotonergic signaling may underlie the observed behavioral and physiological abnormalities.
Materials and Methods
Behavioral Testing.
Erα and ERβ mutant mice were generated as described (26) and were in a 25% 129/Sv, 75% C57/B6 genetic background at the time of testing. All animals were housed in cohorts of 5–10 mice per cage in a 12-h light/dark cycle with freely available food and water. Behavioral testing was conducted between 13:00 and 17:00. For the open field (OF) test, each animal was placed in the middle of a brightly illuminated 80 × 80-cm enclosure with transparent walls and a floor marked with 16 (20 × 20 cm) squares. To begin each trial, a mouse was placed in the center of the arena, and the time taken to move to the edge at the beginning of the test, the total number of squares crossed (locomotion), and the number of times each mouse reared on its hind legs and tail were scored for 15 min/day. The number of forward head extensions (stretching activity) was recorded during the time before each animal moved from the center at the beginning of the test. Thigmotaxis was calculated as the number of squares crossed along the walls and expressed as the percent of total square crosses during the first 5 min after an animal reached the wall of the box at the beginning of the test. OF testing was conducted on 3 consecutive days. For the elevated plus maze, mice were placed in the central part of the maze, and the percentage of time spent in the open arm and the number of times an animal stretched its head to look down were recorded during 5 min. The rotarod test was performed on 7–8 mice for each group. Each mouse was positioned on the rubber-covered rod for 30 s, after which time the rod began to rotate. Each mouse was tested three times, and the longest time the animal stayed on the rotating rod was used for calculations. For each behavioral paradigm, results were analyzed by using ANOVA and Tukey–Kramer post hoc tests.
Electrophysiology.
Coronal sections of 400 μm were prepared in ice-cold artificial cerebrospinal fluid composed of 126 mM NaCl/2.5 mM KCl/2.5 mM CaCl2/1.3 mM MgS04/1 mM NaHPO4/26 mM NaHCO3/11 mM glucose (which only during dissection contained 1 mM kynurenic acid) and were incubated in artificial cerebrospinal fluid at room temperature at least 1 h before testing. Recordings were made in artificial cerebrospinal fluid in a submersion chamber at 30°C by stimulating lateral amygdala or Schaffer collaterals and recording in basolateral amygdala or striatum radiatum of hippocampal CA1, respectively. To induce long-term potentiation (LTP) in amygdala, we used five trains of 10 high-frequency theta-like bursts at 5 Hz, either at baseline stimulus intensity (weak tetanus) or at double the baseline stimulus intensity (strong tetanus). To induce LTP in hippocampus, three trains of 10 high-frequency theta bursts at 5 Hz were delivered at baseline stimulus intensity. Repeated measures (across time) analysis of variance was conducted on the mean values for amplitude or slope in 5-min time epochs before and after LTP induction. The Tukey–Kramer test was used for post hoc analysis.
Immunohistochemistry.
Frozen, 14-μm-thick sections from 8-month-old females were collected on Superfrost plus slides and fixed for 10 min in Zamboni fixative at room temperature. After 30 min blocking with 10% normal goat serum in the PBS/0.05% Tween-20 buffer, the staining was performed at 4°C overnight with one of the following antibodies: guinea pig anti-5-HT1a (1:2,000, Chemicon), rabbit anti-serotonin reuptake transporter (1:5,000, gift from P. Gaspar, INSERM, France), or rabbit anti-GAD65/67 (1:2,000, Sigma). The 1-h incubations with CY3-conjugated secondary antibodies against guinea pig or rabbit (1:500, The Jackson Laboratory) and three 5-min washes after each incubation were performed in PBS/0.05% Tween-20 buffer at room temperature. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI), which was contained in the final mounting medium Vectashield (Vector Laboratories).
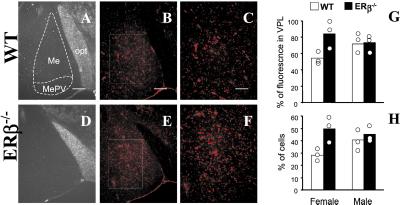
The quantification of the fluorescence and cell counts were done at magnification of ×500 (see Fig. 4 C and F). Briefly, in each slice medial amygdala (Me) and ventral posterolateral thalamus (VPL) regions were identified by comparing the overall morphology to the DAPI staining, and the total fluorescence was scored in both regions by using the image analyses software IMAGE-PRO PLUS (Media Cybernetics, Silver Spring, MD). For the cell counts, the number of DAPI-stained nuclei was considered as the total number of cells, and all of the cells in the same region labeled with anti-5-HT1A antibody were counted as 5-HT1A-expressing cells.
Figure 4.
Immunohistochemical detection of 5-HT1a receptors in the medial amygdala. The regions of selected brain sections are presented for histological identification (A and D). Sections from WT (B and C) and ERβ−/− (E and F) females were stained for the 5-HT1a receptor. Fluorescence in the medial amygdala was represented as the percent of the fluorescence in VPL (G). All of the cells labeled for 5-HT1a in medial amygdala were represented as the percent of total cell number in this region as determined by DAPI staining (H). Me, medial amygdala; MePV, medical amygdala posteroventral; opt, optic tract. (Bar: 100 μm in A, B, D, and E; 50 μm in C and F.)
Steroid Measurements.
Blood samples were collected from mice between 8 and 9 a.m. immediately after removing the mice from their cages. For evaluating corticosterone levels in stress conditions, mice were subjected to an open field with background noise for 20 min, 10 min before blood sampling. Plasma preparations were kept frozen until progesterone and corticosterone levels were measured with RIA kits (ICN).
Results
Behavioral Analysis of ER Mutant Mice.
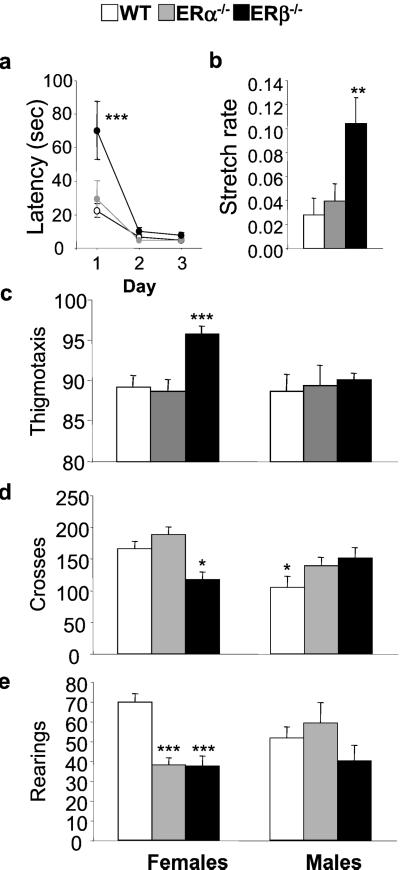
Adult (>7 months old) ERα−/− and ERβ−/− mice and their wild-type (WT) littermate controls were tested in the OF and the elevated plus maze (Figs. 1 and 2), two paradigms in which rodents display anxiety (27, 28). In the OF, more anxious animals exhibit a range of behaviors that include increased latency to move from the center of the field to the wall at the beginning of the test, increased thigmotaxis (wall hugging), and a reduction in overall locomotion and rearing (29, 30). A three-way ANOVA comparing genotype and sex over 3 testing days revealed a significant interaction of genotype by day for latency to move to the edge [F(4,128) = 3.5, P < 0.02]. Subsequent post hoc analysis indicated that ERβ−/− mice showed a significantly longer latency to move to the edge of the OF on first being placed in the arena (70 ± 17 s for ERβ−/− mice compared with 29 ± 11 and 22 ± 5 s for ERα−/− and WT groups respectively, P < 0.001; Fig. 1a). During this initial time spent in the center of the OF, but not later, mutant mice also showed more head and neck stretching behavior than WT mice [F(2,34) = 5.6, P < 0.003], another indication of enhanced anxiety (31). The rate of stretching, calculated as the number of stretches divided by the amount of time spent in the center before moving to the edge, was significantly higher for ERβ−/− (0.1 ± 0.02 stretches per s) than ERα−/− mice (0.04 ± 0.01) or WT littermates (0.03 ± 0.01; Fig. 1b). For all of the other measures including thigmotaxis [F(2,65) = 3.2, P < 0.05], rearings [F(2,65) = 3.2, P < 0.05], and locomotion [F(2,65) = 3.4, P < 0.05], two-way ANOVA showed significant interactions of genotype by sex, indicating that these aspects of OF behavior were affected by the mutation in a gender-dependent manner. Further, post hoc analysis showed that the significant increase in thigmotaxis was apparent only in ERβ−/− females. During the first 5 min after reaching the edge of the OF, 96 ± 1% of the locomotor activity of ERβ−/− females was in the outermost squares (i.e., along the wall) compared with 89 ± 1.2% or 87 ± 1.3% in WT or ERα−/− females (Fig. 1c). No difference was observed in male groups (Fig. 1c). Total locomotion (117 ± 11) was also significantly reduced in ERβ mutant females compared with ERα−/− and WT females (188 ± 12 and 166 ± 11, respectively; Fig. 1d), whereas rearing was significantly reduced in both ERα−/− and ERβ−/− (38 ± 3.6 and 37 ± 4.9) females as compared with WT controls (69 ± 4; Fig. 1e). It is likely that the reduction in ERα−/− rearing is because of obesity, as the ERα−/− females weighed on average 37 ± 2 g, which was significantly more (P < 0.001) than WT or ERβ−/− females (28 ± 1 and 29 ± 1 g, respectively; see also ref. 44). In contrast to female behavior, ERα−/− and ERβ−/− males showed a trend toward increased locomotion when compared with their WT controls (Fig. 1d; P <0.07 and P < 0.06, respectively), which although not statistically significant, agrees with observations reported by Ogawa et al. (6, 7).
Figure 1.
Behavioral analysis of ERα and ERβ null mutant mice in the open field. The latency to move to the edge of the OF after being placed in the center at the beginning of the test was scored (a), and the rate of stretching during this period on the first day of testing was expressed as stretches per s (b). Thygmotaxis (c), locomotion (d), and rearing (e) all were recorded in WT (n = 26, n = 24), ERα−/− (n = 9, n = 7), and ERβ−/− (n = 11, n = 15) females and males, respectively, and were expressed as means ± SEM. ***, P < 0.001; **, P < 0.01; *, P < 0.05 with respect to WT female group.
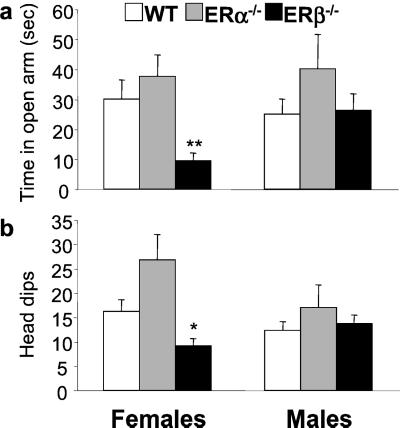
Figure 2.
Behavioral evaluation of ERα−/− and ERβ−/− mice in the elevated plus maze. The percent of time mice spent in the open arm (a) and the number of head dips (b) were expressed as means ± SEM. ***, P < 0.001; **, P < 0.01; *, P < 0.05 with respect to WT female group.
Increased anxiety in ERβ mutant females was also evident in the elevated plus maze. Two-way ANOVA revealed a significant main effect of genotype [F(2,71) = 8, P < 0.005; F(2,71) = 12, P < 0.001] and a significant interaction of genotype by sex for time spent in the open arm [F(2,71) = 3.2, P < 0.05] and head dips; i.e., the number of times each mouse looked down from the maze [F(2,71) = 4.5, P < 0.02]. Post hoc analysis showed that female ERβ−/− mice were significantly different from ERα−/− and WT females in spending less time in the open arm (10 ± 3% vs. 37 ± 6% and 40 ± 7%, respectively; Fig. 2a) and head dipping less frequently (9 ± 2% vs. 26 ± 5% and 16 ± 2%, respectively; Fig. 2b). WT, ERα, and ERβ mutant males were not significantly different on either of these measures (Fig. 2).
Female ERβ−/− was therefore the only group that showed consistent behavioral abnormalities across all tests that are sensitive to enhanced anxiety. ERβ, however, is expressed in the hypothalamic-pituitary axis and in brain structures implicated in motor control and coordination (for review see refs. 14 and 32). Thus, the abnormal behavior of ERβ−/− mice could result from either locomotor deficits or abnormal stress hormone responses. The former is unlikely for two reasons: (i) WT and ERβ−/− females or males performed equally well on the rotarod test for motor functions and remained on the rotating rod (5 revolutions/min) for 160 ± 19 and 173 ± 4 s or 155 ± 15 and 151 ± 17 s, respectively; (ii) the scores for latency to move were not significantly different between mutant and WT males and females on the second day of testing (Fig. 1a), indicating that these mice can move as easily as their controls. Elevated stress hormone levels were equally unlikely to account for behavioral differences, as corticosterone levels did not differ between WT and ERβ−/− females, either in unstressed mice [F(1,11) = 0.4, P < 0.6; 42 ± 15 and 31 ± 6 mg/ml] or stressed mice [F(1,9) = 4.2, P < 0.07; 893 ± 17 and 740 ± 62 mg/ml, respectively].
Electrophysiological Evaluation of Amygdala Functions.
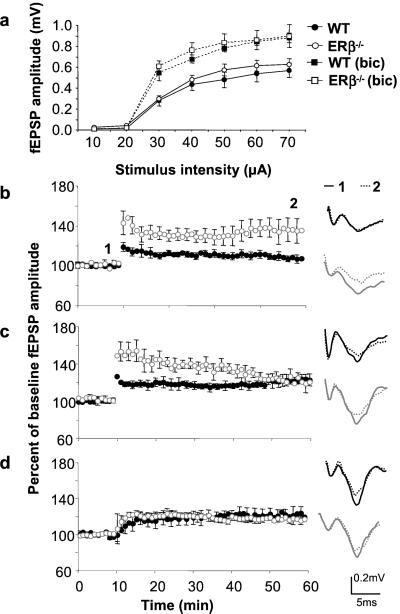
Using brain slice preparations, we analyzed synaptic plasticity in the amygdala. Before tetanic stimulation, field excitatory postsynaptic potentials (fEPSP) in basolateral amygdala (BLA) were recorded in response to a range of stimulus intensities delivered to the lateral amygdala. We found no significant differences in responses of WT and ERβ−/− females at any stimulus intensity (Fig. 3a). However, significant sex-dependent genotype differences [F(1,32) = 5.6, P < 0.02] were found in LTP. Post hoc analysis revealed that LTP was induced by weak tetanic stimulus delivered at half the intensity of typical tetanic stimulation in BLA of ERβ−/− (136 ± 12% of baseline amplitude or 170 ± 30% of baseline slope values), but not WT females (107 ± 4% or 113 ± 5%) or WT (107 ± 3% or 100 ± 8%) and ERβ−/− (104 ± 1% or 98 ± 7%) male mice (P < 0.01, 40 min after potentiation in Fig. 3b). Stronger tetanic stimulation was sufficient to induce LTP in WT amygdala (124 ± 3% or 115 ± 4%) that was not different from ERβ−/− (124 ± 6% or 124 ± 13%), 40 min after tetanus in females or WT (124 ± 3% or 113 ± 8%) and ERβ−/− (121 ± 3% or 113 ± 1%) males, although short-term potentiation was still greater in the slices from mutant females than controls (P < 0.01) 5 min after potentiation (Fig. 3c). In contrast to the amygdala, hippocampal synaptic function was apparently not affected in ERβ mutant mice. Field excitatory postsynaptic potentials recorded in the striatum radiatum of ERβ−/− and WT mice were comparable across a range of different stimulus intensities activating Schaffer collaterals (not shown). We also found that hippocampal synaptic plasticity in the CA1 region, a site of estrogen action in rat (33), was not altered in the absence of ERβ. Theta-burst stimulation induced LTP 50 min after tetanus in slices from both mutant (150 ± 2%) and WT (146 ± 1%) mice.
Figure 3.
Effects of ERβ deletion on synaptic physiology in the amygdala. Input–output curves recorded in the absence or presence of 3 μM bicuculline (a), induction of LTP by weak tetanus (b), strong tetanus (c), and weak tetanus in the presence of 3 μM bicuculline (d) were collected from seven ERβ−/− and eight WT females except in the bicuculline experiment, where three ERβ mutant and four WT females were used. Data were expressed as means ± SEM.
GABAergic signaling is one of the principle modulators of amygdala activity. We therefore tested the hypothesis that reduced GABAergic inhibition could lead to reduced thresholds for synaptic plasticity in the amygdala. Weak tetanic stimulation delivered in the presence of the GABAA antagonist bicuculline (3 μM) resulted in potentiation of synaptic responses in BLA of both WT (122 ± 8) and ERβ−/− (115 ± 4%) females (Fig. 3d). The low level of potentiation and lack of significant difference between the groups may reflect the observation that baseline responses were larger in both groups after bicuculline administration (Fig. 3a).
Effects of ER Inactivation on GABAergic and Serotonergic Neurotransmission.
Although it is possible that estrogen-mediated changes in GABAergic function are responsible for both the reduction of LTP induction threshold and the changes in behavior, it is unlikely that any drastic changes in GABA receptor distribution or GABA metabolism could be at the origin of these alterations. The distribution of the GABA type A–benzodiazepine receptor complex and the expression of GAD were unchanged in mutant females when tested by in situ binding with 2 nM benzodiazepine ligand [3H]RO 15-1788 and by GAD65/67 immunohistochemistry (data not shown).
We looked, therefore, at serotonin (5-HT), a strong modulator of GABAergic neurotransmission in amygdala. Our focus was on the 5-HT1a receptor, one of the principal 5-HT receptors involved in emotional processing (24, 25) and target for estrogen modulation (23, 34) that has recently been shown to be an important regulator of GABA type A functions (20, 35, 36). Our immunohistochemical analysis revealed a significant increase in the expression of 5-HT1a in amygdala of mutant females (Fig. 4 A–F). In contrast, its expression was unchanged in other parts of the brain, including BLA and VPL, a structure positioned close to the amygdaloid complex. Within the amygdala, the increase of 5-HT1a was particularly marked in the Me and reached 84% when compared with the VPL as the intraslice reference in mutant females (P = 0.05, Fig. 4G). The number of labeled cells was also significantly higher in mutants and ranged from 28% to 50% of the total number of cells in the Me of WT and mutant females, respectively (P < 0.05, Fig. 4H). Statistical analyses indicate that the changes in 5-HT1A expression were, indeed, greater in females, as the ANOVA revealed a significant interaction of genotype by sex for both measures [F(1,8) = 5.4, P < 0.05 for percent of cells fluorescing in the amygdala compared with VPL and F(1,8) = 5.4, P < 0.05 for the percent of Me cells that were fluorescing]. There is a possibility that these changes could have been caused by local changes in serotonergic innervation (37). However, the distribution of the serotonergic terminals was not changed when visualized by immunohistochemical detection of 5-HT reuptake transporter (not shown). Furthermore, the reduction of 5-HT1a transcript and/or protein expression in the Me of estrogen-treated rats (23) along with the strong expression of ERβ in this region (5, 38) suggests that alteration of 5-HT1a expression is at least in part at the transcriptional level. Finally, it was also possible that the changes in synaptic plasticity threshold could be attributed to changes in progesterone concentration (11, 12). However, we found no differences in progesterone plasma concentrations of WT and ERβ−/− females as measured during diestrous (2.3 ± 0.6 and 2.1 ± 0.6 ng/ml, respectively).
Discussion
There is an established link between depression, anxiety, and low levels of estradiol in women (1, 2, §). We propose that estrogen nuclear receptors may be key mediators of these regulatory functions of estrogen. Moreover, ERβ may be the principle receptor implicated in these regulatory processes because, with the exception of rearing behaviors, ERα null mutant mice were comparable to their WT littermates and did not display abnormal anxiety. ERβ functions may also be implicated in the control of some aspects of male fear and anxiety, as mutant males and females were equally affected in the measures of latency and stretching rate (Fig. 1 a and b). It is significant that the enhanced anxiety is associated with a reduced threshold for synaptic plasticity in the amygdala, principally in adult female mice. A large body of evidence indicates that the amygdala is important to anxiety and fear for both sexes in rodents (16, 39, 40) and humans (41). A reduced threshold for synaptic enhancement in this structure could result in exaggerated responses to normally innocuous stimuli or environments, a characteristic feature of fear and anxiety disorders. Of the many possible mechanisms for this phenomenon, our data suggest that regulation of inhibitory GABAergic neurotransmission may play a critical role. Whether reduction of GABA responses in the lateral amygdala–BLA complex is the mechanism by which anxiety is increased in ERβ mutant mice remains to be tested, but our evidence indicates that pharmacological manipulation of GABA type A receptors in WT mice can mimic some of the effects of ERβ null mutation. The reduced GABAergic inhibition in BLA could be at the origin of changes in synaptic plasticity and increased anxiety in ERβ mutant females. Although the number of benzodiazepine-sensitive GABA receptors and the expression of GAD are unchanged in knockout mice, it is possible that the loss of ERβ affects transcription and/or post-translational modifications of subunits of GABA type A receptors. This could lead to functional changes in GABA type A receptor-mediated transmission that would not affect expression levels or binding. Regulation of transmembrane chloride gradient may be another mechanism by which ERβ could modulate GABA signaling. Differential expression of ion cotransporters, which was recently suggested to account for sexually dimorphic GABA responses in postnatal brain (42), may be a powerful means of regulating neuronal functions by ERβ. The effects of ERβ signaling on GABAergic tone may also be mediated by serotonergic neurotransmission. It is reasonable to propose that increased presynaptic 5-HT1A activation may reduce GABA release in the amygdaloid complex including BLA, an observation suggested also by in vitro studies on dissociated BLA neurons (35, 36). However, further investigations will be necessary to understand the functional relationships between different subnuclei of amygdala and the relevance of serotonergic and GABAergic signaling in these processes. In the view of ERβ expression in other structures, particularly the dorsal raphe and bed nucleus stria terminalis (5, 43), it is possible that abnormalities in ERβ modulation of the functions of these and related structures may also contribute to present behavioral alterations. Finally, the present study suggests also that ERβ may be critically involved in the expression of anxiety disorders to which women may be particularly susceptible.
Acknowledgments
We thank M. Good for critical comments on the manuscript. This work was funded by Human Frontiers (to W.K.), the Medical Research Council and the European Commission (to P.F.C.), and Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Collège de France, and the Hôpital Universitaire de Strasbourg (to S.D., A.K., and P.C.).
Abbreviations
- ER
estrogen receptor
- WT
wild type
- OF
open field
- BLA
basolateral amygdala
- LTP
long-term potentiation
- GAD
glutamic acid decarboxylase
- DAPI
4′,6-diamidino-2-phenylindole
- Me
medial amygdala
- VPL
ventral posterolateral thalamus
- 5-HT
5-hydroxytryptamine
- GABA
γ-aminobutyric acid
Footnotes
Koss, W. A., Sajdyk, T.J., Morin, S. M., Shekhar, A. & Gehlert, D.R. (2000) Meeting of the Society for Neuroscience, New Orleans, Nov. 2000, abstr. 659.3.
Rudick, C. N. & Woolley, C. S. (2000) Meeting of the Society for Neuroscience, New Orleans, Nov. 2000, abstr. 431.12.
References
- 1.Genazzani A R, Spinetti A, Gallo R, Bernardi F. Maturitas. 1999;31:103–110. doi: 10.1016/s0378-5122(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 2.Joffe H, Cohen L S. Biol Psychiatry. 1998;44:798–811. doi: 10.1016/s0006-3223(98)00169-3. [DOI] [PubMed] [Google Scholar]
- 3.Soares C N, Almeida O P, Joffe H, Cohen L S. Arch Gen Psychiatry. 2001;58:529–534. doi: 10.1001/archpsyc.58.6.529. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R R, Sen S, Diepenhorst L L, Rudick C N, Maren S. Brain Res. 2001;888:356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- 5.Shughrue P J, Lane M V, Merchenthaler I. Endocrinology. 1999;140:2613–2620. doi: 10.1210/endo.140.6.6876. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa S, Lubahn D B, Korach K S, Pfaff D W. Proc Natl Acad Sci USA. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa S, Chan J, Chester A E, Gustafsson J A, Korach K S, Pfaff D W. Proc Natl Acad Sci USA. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogawa S, Chester A E, Hewitt S C, Walker V R, Gustafsson J A, Smithies O, Korach K S, Pfaff D W. Proc Natl Acad Sci USA. 2000;97:14737–14741. doi: 10.1073/pnas.250473597. . (First Published December 12, 2000, 10.1073/pnas.250473597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Good M, Day M, Muir J L. Eur J Neurosci. 1999;11:4476–4480. doi: 10.1046/j.1460-9568.1999.00920.x. [DOI] [PubMed] [Google Scholar]
- 10.Foy M R, Xu J, Xie X, Brinton R D, Thompson R F, Berger T W. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 11.Terasawa E, Timiras P S. Endocrinology. 1968;83:207–216. doi: 10.1210/endo-83-2-207. [DOI] [PubMed] [Google Scholar]
- 12.Edwards H E, Burnham W M, Mendonca A, Bowlby D A, MacLusky N J. Brain Res. 1999;838:136–150. doi: 10.1016/s0006-8993(99)01619-4. [DOI] [PubMed] [Google Scholar]
- 13.Murphy D D, Cole N B, Greenberger V, Segal M. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEwen B S, Alves S E. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 15.Sanders S K, Morzorati S L, Shekhar A. Brain Res. 1995;699:250–259. doi: 10.1016/0006-8993(95)00915-d. [DOI] [PubMed] [Google Scholar]
- 16.File S E, Gonzalez L E, Gallant R. Neuropsychopharmacology. 1998;19:397–405. doi: 10.1016/S0893-133X(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 17.Rudolph U, Crestani F, Benke D, Brunig I, Benson J A, Fritschy J M, Martin J R, Bluethmann H, Mohler H. Nature (London) 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 18.McKernan R M, Rosahl T W, Reynolds D S, Sur C, Wafford K A, Atack J R, Farrar S, Myers J, Cook G, Ferris P, et al. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy M M, Kaufman L C, Brooks P J, Pfaff D W, Schwartz-Giblin S. J Comp Neurol. 1995;360:685–697. doi: 10.1002/cne.903600412. [DOI] [PubMed] [Google Scholar]
- 20.Sibille E, Pavlides C, Benke D, Toth M. J Neurosci. 2000;20:2758–2765. doi: 10.1523/JNEUROSCI.20-08-02758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stutzmann G E, LeDoux J E. J Neurosci. 1999;19:RC8. doi: 10.1523/JNEUROSCI.19-11-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gundlah C, Pecins-Thompson M, Schutzer W E, Bethea C L. Brain Res Mol Brain Res. 1999;63:325–339. doi: 10.1016/s0169-328x(98)00295-2. [DOI] [PubMed] [Google Scholar]
- 23.Osterlund M K, Halldin C, Hurd Y L. Synapse. 2000;35:39–44. doi: 10.1002/(SICI)1098-2396(200001)35:1<39::AID-SYN5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 24.Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Biol Psychiatry. 2000;48:1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- 25.Parks C L, Robinson P S, Sibille E, Shenk T, Toth M. Proc Natl Acad Sci USA. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Development (Cambridge, UK) 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 27.Crawley J N. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- 28.Rodgers R J, Cole J C. Physiol Behav. 1993;54:729–736. doi: 10.1016/0031-9384(93)90084-s. [DOI] [PubMed] [Google Scholar]
- 29.Liebsch G, Montkowski A, Holsboer F, Landgraf R. Behav Brain Res. 1998;94:301–310. doi: 10.1016/s0166-4328(97)00198-8. [DOI] [PubMed] [Google Scholar]
- 30.Ramos A, Mellerin Y, Mormede P, Chaouloff F. Behav Brain Res. 1998;96:195–205. doi: 10.1016/s0166-4328(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 31.Rodgers R J, Cole J C, Davies A. Pharmacol Biochem Behav. 1994;48:959–963. doi: 10.1016/0091-3057(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 32.Couse J F, Korach K S. Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 33.Woolley C S, Weiland N G, McEwen B S, Schwartzkroin P A. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osterlund M K, Hurd Y L. Brain Res Mol Brain Res. 1998;55:169–172. doi: 10.1016/s0169-328x(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 35.Koyama S, Kubo C, Rhee J S, Akaike N. J Physiol (London) 1999;518:525–538. doi: 10.1111/j.1469-7793.1999.0525p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kishimoto K, Koyama S, Akaike N. Eur J Pharmacol. 2000;407:257–265. doi: 10.1016/s0014-2999(00)00673-7. [DOI] [PubMed] [Google Scholar]
- 37.Frankfurt M, Mendelson S D, McKittrick C R, McEwen B S. Brain Res. 1993;601:349–352. doi: 10.1016/0006-8993(93)91735-b. [DOI] [PubMed] [Google Scholar]
- 38.Shughrue P J, Lane M V, Merchenthaler I. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 39.Davis M. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 40.LeDoux J E. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 41.Davidson R J, Abercrombie H, Nitschke J B, Putnam K. Curr Opin Neurobiol. 1999;9:228–234. doi: 10.1016/s0959-4388(99)80032-4. [DOI] [PubMed] [Google Scholar]
- 42.Auger A P, Perrot-Sinal T S, McCarthy M M. Proc Natl Acad Sci USA. 2001;98:8059–8064. doi: 10.1073/pnas.131016298. . (First Published June 26, 2001; 10.1073/pnas.131016298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alves S E, McEwen B S, Hayashi S, Korach K S, Pfaff D W, Ogawa S. J Comp Neurol. 2000;427:185–195. doi: 10.1002/1096-9861(20001113)427:2<185::aid-cne2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 44.Heine P A, Taylor J A, Iwamoto G A, Lubahn D B, Cooke P S. Proc Natl Acad Sci USA. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]