Abstract
Background and Purpose
The Gi‐coupled, ADP‐activated P2Y12 receptor is well characterized as playing a key role in platelet activation via crosstalk with the P2Y1 receptor in ADP‐evoked intracellular Ca2+ responses. However, there is limited knowledge on the role of P2Y12 receptors in ADP‐evoked Ca2+ responses in other blood cells. Here, we investigated the role of P2Y12 receptor activation in the modulation of ADP‐evoked Ca2+ responses in human THP‐1 monocytic cells.
Experimental Approach
A combination of intracellular Ca2+ measurements, RT‐PCR, immunocytochemistry, leukocyte isolation and siRNA‐mediated gene knockdown were used to identify the role of P2Y12 receptor activation.
Key Results
ADP‐evoked intracellular Ca2+ responses (EC50 2.7 μM) in THP‐1 cells were abolished by inhibition of PLC (U73122) or sarco/endoplasmic reticulum Ca2+‐ATPase (thapsigargin). Loss of ADP‐evoked Ca2+ responses following treatment with MRS2578 (IC50 200 nM) revealed a major role for P2Y6 receptors in mediating ADP‐evoked Ca2+ responses. ADP‐evoked responses were attenuated either with pertussis toxin treatment, or P2Y12 receptor inhibition with two chemically distinct antagonists (ticagrelor, IC50 5.3 μM; PSB‐0739, IC50 5.6 μM). ADP‐evoked responses were suppressed following siRNA‐mediated P2Y12 gene knockdown. The inhibitory effects of P2Y12 antagonists were fully reversed following adenylate cyclase inhibition (SQ22536). P2Y12 receptor expression was confirmed in freshly isolated human CD14+ monocytes.
Conclusions and Implications
Taken together, these data suggest that P2Y12 receptor activation positively regulates P2Y6 receptor‐mediated intracellular Ca2+ signalling through suppression of adenylate cyclase activity in human monocytic cells.
Abbreviations
- IP3
inositol 1,4,5‐triphosphate
- PIP2
phosphatidylinositol 4,5‐bisphosphate
- UDP
uridine diphosphate
Introduction
The adenine nucleotide http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1712 serves as an important signalling molecule that is implicated in processes such as platelet aggregation (Dorsam and Kunapuli, 2004) and immune modulation (Ben Addi et al., 2010). ADP binds to http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=323, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=326, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=328 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=329 receptors, which are members of the G protein‐coupled P2Y receptor family. Activation of Gq‐coupled P2Y1 and P2Y6 receptors causes downstream inositol 1,4,5‐triphosphate (IP3) generation via PLC‐mediated breakdown of phosphatidylinositol 4,5‐bisphosphate (PIP2) and subsequent calcium ion (Ca2+) release through endoplasmic reticulum IP3 receptors (Erb and Weisman, 2012). Conversely, P2Y12 and P2Y13 receptors are Gi‐coupled and hence inhibit production of cAMP and stimulate PI3K when activated (Erb and Weisman, 2012).
Platelet aggregation in response to ADP is one of the earliest examples of extracellular nucleotide signalling. It is firmly established that ADP activates platelets via the P2Y1 and P2Y12 receptors, and the functionality of both receptors is required for normal platelet aggregation (Jin and Kunapuli, 1998). The emergence of the P2Y12 receptor as a vital component in ADP‐evoked platelet activation has led to the development of anti‐platelet drugs targeting this receptor, such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1765 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7150. The rise in cytosolic Ca2+ in response to ADP is deemed responsible for the ADP‐mediated activation of platelets (Dorsam and Kunapuli, 2004). Although the P2Y12 receptor is Gi‐coupled and should therefore not cause intracellular Ca2+ mobilization, outcomes from a previous study suggest that the P2Y12 receptor potentiates the Gq P2Y1 receptor‐mediated Ca2+ response to ADP in a crosstalk mechanism (Hardy et al., 2004). Hardy et al. (2004) proposed that P2Y12 receptors mediate inhibition of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=257#1279 and activation of PI3K, which collectively positively modulates the Ca2+ signal induced by ADP. The same P2Y1‐P2Y12 crosstalk mechanism described in platelets by Hardy et al. (2004) was also suggested to be present in glioma C6 cells (Suplat et al., 2007).
The role of P2Y12 receptors in cells other than platelets is not well described, although recent evidence is promising. Findings by West et al. (2014) indicate a role for vessel wall P2Y12 receptors in early atherogenesis, rather than platelet P2Y12 receptors. In addition to cardiovascular disease, the P2Y12 receptors has potentially been implicated in immune responses to ADP in macrophages (Zhang et al., 2018) and dendritic cells (Ben Addi et al., 2010) in functions such as antigen uptake and chemotaxis. Therefore, non‐platelet roles for P2Y12 receptors have been suggested and should be further investigated.
Monocytes are essential immune cells that, together with their progeny, facilitate innate immune defence via phagocytosis and cytokine production, but also activate the adaptive immune system through antigen uptake and presentation (Ziegler‐Heitbrock, 2006). In this study, we applied the THP‐1 monocytic cell line as an experimental model to investigate the expression of P2Y12 receptors and the contribution of the receptor in ADP‐evoked Ca2+ responses, exploring the signal transduction mechanisms involved. As there have been no publications reporting a role for P2Y12 receptors in monocytes, this investigation reveals a new role for P2Y12 receptors in non‐platelet Ca2+ responses and contributes to our understanding of how monocytes function in health and disease.
Methods
Isolation of CD14+ human monocytes
Peripheral blood mononucleated cells (PBMCs) were isolated from the blood of human volunteers using Histopaque‐1077 (Sigma‐Aldrich, Haverhill, UK). CD14+ monocytes were magnetically labelled from a PBMC suspension using MACS CD14 MicroBeads (Miltenyi Biotec, Germany) and positively selected for via the MACS Cell Separation Column (Miltenyi Biotec, Germany) together with the QuadroMACS Separator (Miltenyi Biotec, Germany).
Intracellular Ca2+ measurements and drug treatments
THP‐1 cells, 1 × 106 ·mL−1 were loaded for 1 h with 2 μM fura‐2 AM in SBS buffer plus 0.01% (w.v‐1) pluronic acid at 37°C. Cells were then pelleted and washed using SBS and plated at a density of 2 × 105 cells per well. The plated cells were allowed to settle for 1 h at 37°C, during which stage antagonists were added 30 min into the hour, unless otherwise stated. Measurements were taken at 37°C using the FlexStation 3 instrument (Molecular Devices, Wokingham, UK) measuring fura‐2 fluorescence (340 nm excitation when Ca2+‐bound; 380 nm excitation when unbound; 510 nm emission) at intervals of 2 s giving. For all Ca2+ experiments, the signal reported is ‘F ratio’, the ratio between Fura‐2 emission at 510 when excited at 340 and 380 nm. For http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=890‐treated ADP stocks, 1 mM ADP in SBS solution was treated for 1 h at 37°C with 3 U·mL−1 hexokinase from Saccharomyces cerevisiae (Sigma, Haverhill, UK) prior to agonist application.
Cell culture
Cells from the THP‐1 cell line were cultured at 37°C, 5% CO2 in RPMI 1640 medium containing 2 mM L‐glutamine and supplemented with 10% (v.v‐1) FBS, 50 IU·mL−1 penicillin and 50 μg·mL−1 streptomycin. Cells were maintained at a density between 1 × 105 and 1 × 106 cells ·mL−1.
siRNA‐mediated gene knockdown
THP‐1 cells (2 × 105 final amount) were incubated overnight in complete RPMI (10% FBS) without antibiotic before cells were transfected using Dharmacon siRNA (25 nM final concentration) via DharmaFECT 2 transfection reagent (obtained from Dharmacon Research, Inc., Cambridge, UK) using the manufacturer's protocol in 96‐well format.
RNA extraction and RT‐PCR
Total RNA was extracted from THP‐1 cells and CD14+ monocytes using Tri reagent (Sigma Aldrich, Haverhill, UK) with a subsequent DNase I treatment (Ambion). Complementary DNA was synthesized from 1 μg of total RNA using Superscript II reverse transcriptase (Invitrogen, Waltham, USA). PCR was performed using a Taq polymerase readymix (Sigma Aldrich, Haverhill, UK) using primer pairs designed using the following sequences (accession numbers): P2RY1 ‐ GTTCAATTTGGCTCTGGCCG (5′‐3′), TTTTGTTTTTGCGGACCCCG (3′‐5′) (NM_002563); P2RY6 ‐ GCTCTCACTGTCATCGGCTT (5′‐3′), TCTGCCATTTGGCTGTGAGT (3′‐5′) (NM_176798); P2RY12 ‐ ACTGGGAACAGGACCACTGA (5′‐3′), CAGAATTGGGGCACTTCAGC (3′‐5′) (NM_022788); P2RY13 ‐ TTCCCAGCCCTCTACACAGT (5′‐3′), GGCCCCTTTAAGGAAGCACA (3′‐5′) (NM_176894).
Immunocytochemistry
THP‐1 cells adhered to glass coverslips were washed twice in PBS followed by fixative with 4% (w.v‐1) paraformaldehyde. Cells were permeabilized with 0.25% (v.v‐1) triton X‐100 for 10 min followed by blocking with 1% (w.v‐1) BSA for 30 min at room temperature. Primary and secondary antibodies were diluted in PBS containing 1% (w.v‐1) BSA and incubated with cells overnight a 4°C and for 1 h at room temperature respectively. Cells were mounted in Vectashield containing nucleus counterstain (DAPI). Goat polyclonal anti‐P2Y12 (Santa Cruz Biotechnology, Texas, USA) was used with Alexa 488‐conjugated rabbit anti‐goat (Abcam, Cambridge, UK). Rabbit polyclonal anti‐P2Y1, anti‐P2Y6 and anti‐P2Y13 (Alomone, Jerusalem, Israel) were used with Alexa 488‐conjugated goat anti‐rabbit (Invitrogen, Waltham, USA). Cell imaging was performed using a laser‐scanning Zeiss LSM510 Meta confocal microscope.
Transmigration assays
Transwell migration assay was performed as previously described (Sivaramakrishnan et al., 2012; Campwala et al., 2014). Briefly, assays were performed in 24‐well plates using polyethylene terephthalate membrane transwell inserts with 3 μm pores. THP‐1 cells, 1 × 106 in RPMI (no serum) with vehicle or drug treatment were added to the upper chamber, and 3 μM ADP or vehicle added to the lower chamber. Assays were performed for 2 h at 37°C, and migrated cells counted on the underside of the transwell support using crystal violet staining. Chemotaxic index was calculated as the ratio of cells that migrated to ADP over vehicle control.
Data analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Data analysis was performed using Origin Pro 9.0 software (Origin Lab, USA). Dose–response curves were fitted assuming a Hill coefficient of 1, with the Hill equation used to determine the degree of ligand–receptor cooperation. Figure data points represent mean values ± SEM (error bars). Statistical significance was determined using Student's paired t‐tests. Each point in the dose–response plots represents the average of the peak Ca2+ response. A confidence interval of 5% (P < 0.05) is used throughout for statement of significance.
Dose–response relationships were fitted using the Hill equation:
where n H represents the Hill slope.
Chemicals and reagents
Unless otherwise stated, all chemicals and reagents were purchased from Sigma‐Aldrich (Haverhill, UK), with the exception of pertussis toxin and SQ22536 (Tocris, Bristol, UK). All chemicals (agonists and antagonists) were diluted using physiological saline (SBS buffer) (containing (mM): NaCl, 130; KCl, 5; MgCl2, 1.2; CaCl2, 1.5; D‐glucose, 8; HEPES, 10; pH 7.4) with the exception of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=771 [SBS buffer containing 1% (w.v‐1) BSA], http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1753, SQ22536 and ticagrelor [SBS buffer containing 1% (v.v‐1) DMSO].
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b).
Results
ADP‐evoked intracellular Ca2+ responses are mediated by P2Y6 receptor activation and modulated by Gi‐dependent signalling
ADP evoked concentration‐dependent increases in intracellular Ca2+ with an EC50 of 2.7 ± 0.3 μM (n = 5) (Figure 1A, B). Responses to maximal ADP concentrations were abolished following either PLC inhibitor treatment (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5283) (Figure 1C) or calcium depletion of the ER store (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5351) (Figure 1D). A study by Mahaut‐Smith et al. (2000) identified that commercially available ADP contains some ATP. To exclude any contribution of trace ATP to ADP‐evoked Ca2+ responses, ADP stocks were treated with hexokinase. The Ca2+ responses evoked by treated ADP were similar to untreated ADP, with an EC50 of 2.2 ± 0.5 μM (n = 5) (P > 0.05 vs. untreated ADP) (See Supporting Information Figure S1).
Figure 1.
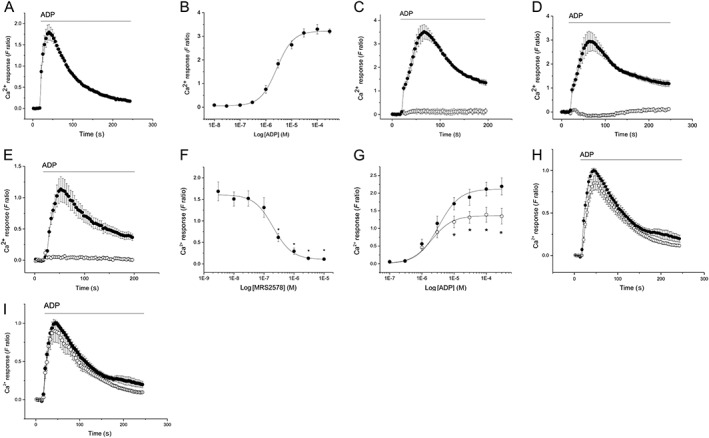
P2Y6 receptor mediates ADP‐evoked intracellular Ca2+ responses in THP‐1 cells. (A) Averaged (n = 5) intracellular Ca2+ response evoked by ADP (30 μM). (B) Concentration‐dependency of ADP‐evoked responses (EC50 2.7 ± 0.3 μM; n = 5). Abolition of responses evoked by 30 μM ADP in control conditions (closed circles) or following pre‐incubation (open circles) with 5 μM U73122 (C) or 1 μM thapsigargin (D); n = 5 for both. (E) ADP concentration‐response curve in the absence (closed circles) and presence (open circles) of 300 nM MRS2578 (n = 5). (F) Concentration‐inhibition curve for P2Y6 antagonist MRS2578 on intracellular Ca2+ response evoked by ADP (3 μM; n = 5). (G) ADP concentration‐response curve in the absence (closed circles) and presence (open circles) of 300 nM MRS2578 (n = 5). (H and I) Averaged (n = 5) intracellular Ca2+ responses evoked by 3 μM ADP in the presence of vehicle (closed circles) or in the presence of P2Y1 antagonist 1 μM MRS2500 (H; open circles) or P2Y13 antagonist 10 μM MRS2211 (I; open circles). For all experiments, F ratio is the ratiometric measurement of intracellular Ca2+ using fura‐2. *P < 0.05.
P2Y6 antagonism by MRS2578 inhibited the ADP response almost completely with an EC50 of 200 ± 20 nM (n = 3) (Figure 1E, F). MRS2578 inhibited the ADP response in a non‐competitive fashion (Figure 1G), consistent with the reported mode of antagonism at P2Y6 receptors (Mamedova et al., 2004) and with previous findings (Sivaramakrishnan et al., 2012; Campwala et al., 2014). Selective inhibition of P2Y1 receptors with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1724 (tested up to 10 μM) (Figure 1H) or P2Y13 receptors with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1777 (tested up to 10 μM) (Figure 1I) had no significant effect on ADP‐ evoked Ca2+ responses. In addition to a dependence on PLC activity and release of ER Ca2+ stores, we investigated the effect of Gi‐dependent signalling by using Bordetella pertussis toxin (PTx). PTx treatment (5 nM, 3 h) caused a significant attenuation of ADP‐evoked intracellular Ca2+ responses (Figure 2A), significantly suppressing the maximal response by approximately 30% and the EC50 for ADP (EC50 1.9 ± 0.3 μM vs. 2.9 ± 0.2 μM with PTx; P < 0.05, n = 5) (Figure 2B). In control experiments, CCL2‐evoked Ca2+ responses were abolished by PTx treatment (Figure 2C), in agreement with our previous observations. Together, these findings suggest that ADP‐evoked Ca2+ responses are mediated via P2Y6 receptor activation and release of ER Ca2+ and that Gi‐dependent signalling either constitutively or following ADP activation, positively modulates the ADP‐evoked response.
Figure 2.
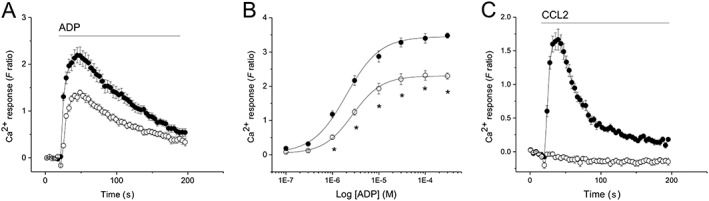
Pertussis toxin attenuates ADP‐evoked intracellular Ca2+ responses in THP‐1 cells. (A) Averaged (n = 5) intracellular Ca2+ response evoked by ADP (3 μM) in the absence (closed circles) and presence (open circles) of 5 nM pertussis toxin (3 h pre‐incubation). (B) ADP concentration–response relationship in absence and presence of 5 nM pertussis toxin (n = 5). (C) Positive control showing abolition of control CCL2‐evoked response by 5 nM pertussis toxin (n = 5). Responses evoked by 50 ng·mL−1 CCL2. For all experiments, F ratio is the ratiometric measurement of intracellular Ca2+ using fura‐2. *P < 0.05.
We next determined the expression of ADP receptors in THP‐1 cells in an effort to probe further the molecular basis of ADP‐evoked Ca2+ responses and identify receptors that may modulate them. RT‐PCR analysis of P2Y receptors activated by ADP (Communi et al., 1996; Erb and Weisman, 2012) in THP‐1 cells revealed the expression of P2Y1, P2Y6, P2Y12 and P2Y13 receptors (Figure 3A). P2Y12 receptor mRNA expression was confirmed in freshly isolated CD14+ monocytes (Figure 3B). P2Y1, P2Y6, P2Y12 and P2Y13 receptor protein expression was confirmed by immunocytochemistry and confocal microscopy (Figure 3C).
Figure 3.
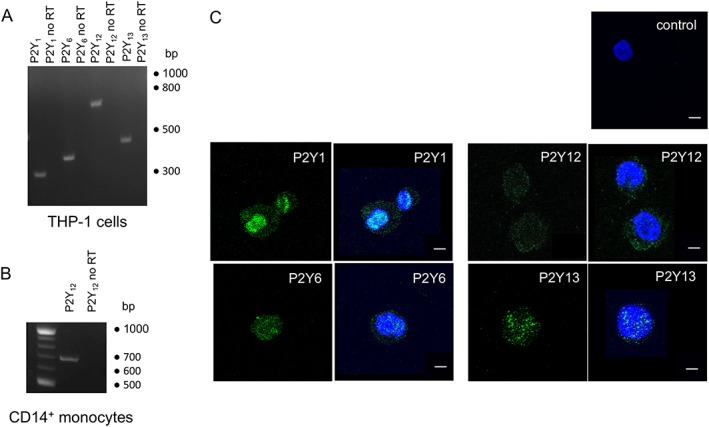
Expression of ADP‐activated P2Y receptors in THP‐1 cells. (A) RT‐PCR analysis of P2Y1 (326 bp), P2Y6 (391 bp), P2Y12 (698 bp) and P2Y13 (461 bp) receptor expression in THP‐1 monocytes. RT‐PCR analysis of P2Y12 receptors (698) expression in freshly isolated CD14+ monocytes from human peripheral blood. For (A) and (B): predicted PCR ampilicon size given in parentheses; no RT (no reverse transcriptase) denotes negative control experiments for genomic DNA contamination. (C) Representative confocal microscopy images showing P2Y primary antibody immunoreactivity in fixed THP‐1 cells. Cells are labelled with polyclonal antibodies against P2Y receptor subunits and fluorescence (green) visualized by using a AF488‐conjugated secondary antibody (lefthand panels). The “control” is representative of an experiment where primary antibodies have been omitted. Cells are counterstained with diamidino‐2‐phenylindole to identify nuclei (blue; in overlay in righthand panel). Scale bar is 5 μm. Experiments are representative of at least three independent experiments.
Effect of P2Y12 receptor antagonism and knockdown on ADP‐evoked Ca2+ responses in THP‐1 cells
Of the ADP receptors expressed by THP‐1 monocytes, the P2Y12 receptor is a known Gi‐coupled receptor. The role of P2Y12 receptor activation during ADP challenge was investigated using http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5904, a high affinity competitive antagonist (Baqi et al., 2009), and ticagrelor, a P2Y12 antagonist used clinically as an anti‐thrombotic agent (Husted et al., 2006). Ticagrelor inhibited ADP‐evoked Ca2+ responses in THP‐1 cells in a concentration‐dependent fashion (Figure 4A). Ticagrelor inhibited ADP‐evoked Ca2+ responses with an EC50 of 4.7 ± 1.8 μM (n = 5) and a non‐competitive mode of action (Figure 4B, C). This is consistent with the observations of van Giezen et al. (2009) where ticagrelor binds to P2Y12 at a site distinct from that of ADP, producing a non‐competitive inhibition of ADP‐induced aggregation in human platelets. PSB‐0739 inhibited ADP‐evoked Ca2+ responses with an EC50 of 5.4 ± 1.8 μM (n = 5) (Figure 4D, E) and caused a rightward parallel shift in the ADP concentration–response curve (Figure 4F). Inhibition of the ADP response was approximately 80% at the highest concentrations of PSB‐0739 tested. Several studies have suggested that ticagrelor produces cardiovascular benefit through a pleiotropic inhibition of the equilibrative nucleoside transporter 1 (ENT‐1) (Aungraheeta et al., 2016), and a consequent elevation in extracellular adenosine. To rule out this mechanism of action in THP‐1 cells, we inhibited ENT‐1 using 3 μM 6‐S‐[(4‐nitrophenyl)methyl]‐6‐thioinosine (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=summary&ligandId=4512), but observed no effect on ADP‐evoked Ca2+ responses (data not shown). Next, we undertook a gene knockdown strategy to support our observations with P2Y12 receptor antagonists. siRNA targeting of P2Y12 receptors or control gene GADPH achieved approximately 40% mRNA knockdown versus scrambled siRNA control cells. ADP‐evoked Ca2+ responses were attenuated by approximately 30% in cells with P2Y12 knockdown versus either GADPH knockdown or scrambled control cells (Figure 4G, H). To investigate the biological relevance of P2Y12 receptor inhibition in THP‐1 cells, we assayed transmigration in response to ADP. In these experiments, ADP stimulated significant THP‐1 cell transmigration over a 2 h period compared to vehicle (Figure 4I). The addition of either ticagrelor or PSB‐0739 attenuated cell migration to ADP (Figure 4I). Together, these data reveal that P2Y12 receptor activity is functionally important for the generation of ADP‐evoked intracellular Ca2+ responses and migration in THP‐1 cells.
Figure 4.
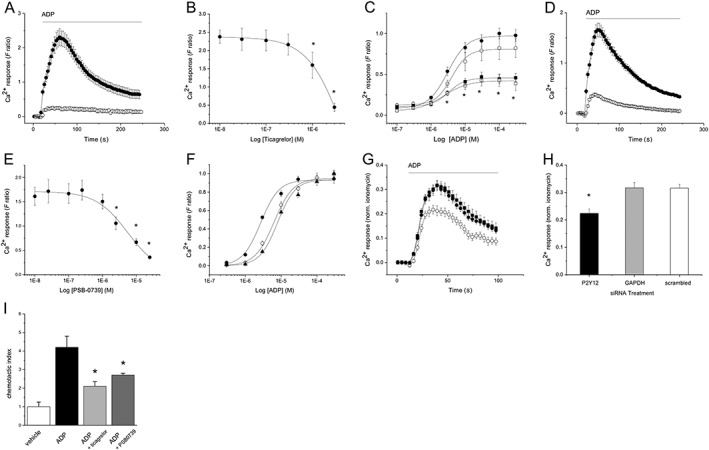
Effect of P2Y12 receptor antagonism and gene knockdown on ADP‐evoked intracellular Ca2+ responses in THP‐1 cells. (A) Averaged (n = 5) ADP‐evoked Ca2+ response in the absence (closed circles) and presence (open circles) of 3 μM ticagrelor. (B) Ticagrelor concentration‐inhibition curve (IC50 4.7 ± 1.8 μM; n = 5) for responses evoked by 3 μM ADP. (C) ADP concentration–response curve (n = 5) in the presence of vehicle (closed circles) or 0.1 (open circles), 1 (closed squares) and 5 μM ticagrelor (open squares). (D) Averaged (n = 5) ADP‐evoked Ca2+ response in the absence (closed circles) and presence (open circles) of 25 μM PSB‐0739. (E) PSB‐0739 concentration‐inhibition curve (IC50 5.4 ± 1.8 μM; n = 5) for responses evoked by 3 μM ADP. (F) ADP concentration response curve (n = 4) in the presence of vehicle (closed circles) or 5 (open circles) and 10 μM PSB‐0739 (triangles). (G) Effect of siRNA‐mediated silencing of P2Y12 receptors on ADP‐evoked Ca2+ responses. Averaged (n = 5) Ca2+ responses evoked by 3 μM ADP in THP‐1 cells following siRNA‐mediated mRNA knockdown of P2Y12 (open circles) or GAPDH (closed squares) compared with cells transfected with scrambled siRNA (closed circles) (n = 5). (H) Bar chart showing effect of different siRNA treatment on peak Ca2+ responses evoked by 3 μM ADP (n = 5). Responses in panels G and H are normalized to the magnitude of Ca2+ response elicited by 100 μM ionomycin to control for cell number. For all experiments, F ratio is the ratiometric measurement of intracellular Ca2+ using fura‐2. *P < 0.05. (I) P2Y12 antagonists attenuate THP‐1 transwell migration towards ADP. Chemotactic indexes comparing cell movement over 2 h in control conditions (vehicle), 3 μM ADP alone or in the presence of either ticagrelor (3 μM) or PSB‐0739 (25 μM) (*P < 0.05 vs. ADP alone; n = 5).
Adenylate cyclase inhibition reverses antagonistic effect of ticagrelor
We hypothesized that the molecular mechanism underlying the positive contribution of the P2Y12 receptor to ADP‐evoked Ca2+ responses could be due to a number of possibilities. Firstly, that the P2Y12 receptor is solely Gi‐coupled and its activity positively regulates P2Y6 receptor‐mediated Ca2+ signalling through classical suppression of adenylate cyclase, or secondly, that the P2Y12 receptor can directly elicit a Ca2+ response, either through promiscuous Gq‐coupling or via Gi‐coupling that directly elicits Ca2+ signalling through activation of atypical PLC, for signalling on the CCL2‐http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=59 axis (Myers et al., 1995). To explore this further, we investigated the requirement of adenylate cyclase activity for mediating the inhibitory action of ticagrelor. Adenylate cyclase inhibition with 300 μM SQ22536 had no effect on ADP‐evoked Ca2+ responses (Figure 5A). However, SQ22536 could reverse the effect of ticagrelor, restoring the ADP concentration–response relationship and maximum response (F max = 3.32 ± 0.26 for ADP + 5 μM ticagrelor + 300 μM SQ22536; F max = 2.64 ± 0.21, ADP + 5 μM ticagrelor; F max = 1.06 ± 0.10; n = 5. The F max for ADP + 5 μM ticagrelor vs. ADP + vehicle control is significantly different, P < 0.05) (Figure 5B, C).
Figure 5.
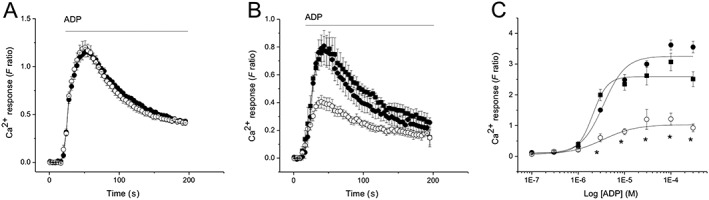
Inhibition of adenylate cyclase reverses antagonistic action of ticagrelor on ADP‐evoked Ca2+ responses in THP‐1 cells. (A) Averaged (n = 5) 3 μM ADP‐evoked Ca2+ in the presence of vehicle (closed circles) or following adenylate cyclase inhibition with 300 μM SQ22536. (B) Averaged (n = 5) 3 μM ADP‐evoked Ca2+ in the presence of vehicle, 5 μM ticagrelor (open circles) or 5 μM ticagrelor plus 300 μM SQ22536 (closed squares). (C) ADP concentration–response curve (n = 5) in the presence of vehicle (closed circles), 5 μM ticagrelor (open circles) or 5 μM ticagrelor plus 300 μM SQ22536 (closed squares). For all experiments, F ratio is the ratiometric measurement of intracellular Ca2+ using fura‐2. *P < 0.05.
Discussion
In this study, we demonstrated novel findings identifying a role for P2Y12 receptors in regulating intracellular Ca2+ signalling in non‐platelet cells. We utilized THP‐1 cells, a model used extensively to investigate human monocyte function, to demonstrate a functional role for the P2Y12 receptor. In addition, we identified P2Y12 receptor expression in human CD14+ monocytes. There have been no published reports on the expression of P2Y12 receptors in monocytes thus far; however, the involvement of P2Y12 receptors in the function of macrophages was investigated by Kronlage et al. (2010). Stimulation of P2Y12 receptors in macrophages was found to induce cell spreading with formation of lamellipodia and inhibition of multiple purine receptors, including P2Y12, attenuated chemotaxis (Kronlage et al., 2010). The signal transduction mechanisms downstream of the P2Y12 receptor were not investigated in this study. P2Y12 receptor expression has also been reported in glial cells, smooth muscle and endothelium (Cattaneo, 2007).
In this study, we demonstrated that ADP evokes intracellular Ca2+ signalling via P2Y6 receptor activation. The P2Y6 receptor is often considered as the metabotropic receptor for uridine diphosphate (UDP). Although UDP is a more potent agonist at the human P2Y6 receptor by several orders of magnitude, ADP is a full agonist with a half‐maximal concentration for IP3 production in the micromolar range (Communi et al., 1996). ADP is an important signalling cue in monocyte/macrophage biology. For example, in the same cell‐line used in this investigation, THP‐1, ADP stimulation caused release of the cytokine TNF‐α (Mattana et al., 2002). TNF‐α release causes an inflammatory innate immune response including immune cell recruitment. In addition, ADP has been shown in this investigation to induce calcium signals in THP‐1 cells, and calcium signalling in circulating monocytes has in fact been suggested to result in the development of mature dendritic cells (Czerniecki et al., 1997). Such examples indicate that ADP contributes to monocyte function, with specific reference to immune responses and differentiation. ADP released from Escherichia coli‐infected mice, and from macrophages exposed to LPS, protected mice from E. coli‐induced peritonitis via macrophage recruitment (Zhang et al., 2018). Additionally, ADP caused production of CCL2, a crucial chemokine in immune cell recruitment, which accordingly attracted more macrophages in a transwell assay (Zhang et al., 2018). Inhibition of downstream P2Y12 receptor signalling, or macrophage P2Y12 receptor deficiency, blocked immune responses to ADP, in turn allowing more bacteria to persist in infected mice (Zhang et al., 2018). This investigation by Zhang et al. revealed that upon sensing danger, macrophages release ADP, which binds to receptors such as P2Y12, mediating actions such as chemokine release and consequent recruitment of immune cells. Such findings may implicate ADP and P2Y12 receptors as being crucial in forming a comprehensive immune response to infectious disease.
It was discovered in platelets by Hardy et al. (2004) that the selective pharmacological P2Y12 receptor inhibitor AR‐C69931MX partially blocked the calcium response to 10 μM ADP, which was also shown to be completely abolished by selective inhibition the P2Y1 receptor. This suggested that the P2Y12 receptor is able to positively modulate the P2Y1 receptor‐mediated calcium response to ADP in platelets. Subsequently, Hardy et al. identified that the PI3K inhibitor LY294002 (10 μM) partially inhibited the P2Y12 receptor‐dependent part of the calcium response to ADP. Moreover, the adenylate cyclase inhibitor SQ22536 partially restored ADP‐evoked calcium responses in the presence of the P2Y12 receptor inhibitor AR‐C69931MX (Hardy et al., 2004). Taken together, these findings by Hardy et al. indicated that in platelets the P2Y12 receptor regulates P2Y1 receptor‐mediated calcium responses to ADP through activation of PI3K and inhibition of adenylate cyclase. The findings in this investigation suggest that, of the ADP‐activated P2Y receptors, ADP‐induced calcium responses in THP‐1 cells are dependent on P2Y12 and P2Y6 receptor activation, but not dependent on P2Y1 or P2Y13 receptors. Therefore, the basic principle identified and reported by Hardy et al. is supported here for THP‐1 monocytic cells, only with the P2Y6 receptor acting as the equivalent of the P2Y1 receptor.
An inhibitory action of SQ22536 on ADP‐evoked Ca2+ signals is not observed in the absence of P2Y12 antagonism. These data suggest adenylate cyclase activity does not exert a suppressive effect when P2Y6 and P2Y12 receptors are concurrently activated by ADP. There are mechanistic explanations that could explain this observation. For example, there may be no net change in adenylate cyclase activity upon ADP challenge due to co‐activation of a Gi‐dependent pathway, mediated by P2Y12 receptors and a Gs‐dependent pathway mediated by another ADP receptor or possible adenosine receptor. A predominance of Gs signalling could be revealed following P2Y12 receptor inhibition and increased adenylate cyclase activity suppresses ADP‐evoked Ca2+ signalling. Previous reports (Communi et al., 1997) have highlighted promiscuity for Gs‐ and Gq‐coupling by P2Y11 receptors that is expressed by THP‐1 cells (unpublished data). However, the P2Y11 receptor is activated by ATP not by ADP at concentrations used in this study (Communi et al., 1997).
How might the P2Y12 receptor regulate P2Y6 receptor‐dependent signalling via adenylate cyclase? Firstly, this is unlikely to involve PKA‐dependent phosphorylation of the P2Y6 receptor or receptor desensitization. Compared with other P2Y receptors, such as P2Y4, the P2Y6 receptor displays limited reduction in cell surface number even in the presence of maximal agonist concentrations (Brinson and Harden, 2000). There are no consensus PKA phosphorylation sites in the cytoplasmic loops of P2Y6, receptors, although this cannot discount the possibility of PKA phosphorylation of an auxiliary protein that positively regulates P2Y6 receptor activity.
There is currently much interest in the biological effects of P2Y12 antagonists beyond platelets (Nylander and Schulz, 2016). Although further work is required to investigate the role of the P2Y12 receptor in freshly isolated human monocytes, this work suggests that physiological and pharmacological modulation of P2Y12 receptors can influence ADP‐evoked intracellular Ca2+ signalling in THP‐1 cells and perhaps monocytes, which will likely influence key monocyte functions such as migration, adhesion and cytokine production.
Author contributions
J.J.M. and J.A.L. carried out the experiments and analysed the data. J.J.M., J.A.L. and S.J.F. designed the experiments and co‐wrote the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 Ca2+ response in THP‐1 cells evoked by hexokinase‐treated ADP. (A) Average (N = 5) intracellular Ca2+ response evoked by ADP (20 μM). Concentration‐response curve of ADP‐evoked responses (EC50 2.2±0.5 μM; N = 5).
Acknowledgement
This work was supported by the Biotechnology and Biological Sciences Research Council.
Micklewright, J. J. , Layhadi, J. A. , and Fountain, S. J. (2018) P2Y12 receptor modulation of ADP‐evoked intracellular Ca2+ signalling in THP‐1 human monocytic cells. British Journal of Pharmacology, 175: 2483–2491. doi: 10.1111/bph.14218.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aungraheeta R, Conibear A, Butler M, Kelly E, Nylander S, Mumford A et al (2016). Inverse agonism at the P2Y12 receptor and ENT1 transporter blockade contribute to platelet inhibition by ticagrelor. Blood 128: 2717–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baqi Y, Atzler K, Köse M, Glänzel M, Müller C (2009). High‐affinity, non‐nucleotide‐derived competitive antagonists of platelet P2Y12 receptors. J Med Chem 52: 3784–3793. [DOI] [PubMed] [Google Scholar]
- Ben Addi A, Cammarata D, Conley P, Boeynaems J, Robaye B (2010). Role of the P2Y12 receptor in the modulation of murine dendritic cell function by ADP. J Immunol 185: 5900–5906. [DOI] [PubMed] [Google Scholar]
- Brinson A, Harden T (2000). Differential regulation of the uridine nucleotide‐activated P2Y4 and P2Y6 receptors. J Biol Chem 276: 11939–11948. [DOI] [PubMed] [Google Scholar]
- Campwala H, Sexton D, Crossman D, Fountain SJ (2014). P2Y6 receptor inhibition perturbs CCL2‐evoked signalling in human monocytic and peripheral blood mononuclear cells. J Cell Sci 127: 4964–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo M (2007). The platelet P2 receptors In: Platelets, pp. 201–220. [Google Scholar]
- Communi D, Govaerts C, Parmentier M, Boeynaems J (1997). Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J Biol Chem 272: 31969–31973. [DOI] [PubMed] [Google Scholar]
- Communi D, Parmentier M, Boeynaems J (1996). Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochem Biophys Res Commun 222: 303–308. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniecki B, Carter C, Rivoltini L, Koski G, Kim H, Weng D et al (1997). Calcium ionophore‐treated peripheral blood monocytes and dendritic cells rapidly display characteristics of activated dendritic cells. J Immunol 159: 3823–3837. [PubMed] [Google Scholar]
- Dorsam R, Kunapuli S (2004). Central role of the P2Y12 receptor in platelet activation. J Clin Investig 113: 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb L, Weisman G (2012). Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdiscip Rev Membr Transp Signal 1: 789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy A, Jones M, Mundell S, Poole A (2004). Reciprocal cross‐talk between P2Y1 and P2Y12 receptors at the level of calcium signaling in human platelets. Blood 104: 1745–1752. [DOI] [PubMed] [Google Scholar]
- Husted S, Emanuelsson H, Heptinstall S, Sandset P, Wickens M, Peters G (2006). Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double‐blind comparison to clopidogrel with aspirin. Eur Heart J 27: 1038–1047. [DOI] [PubMed] [Google Scholar]
- Jin J, Kunapuli S (1998). Coactivation of two different G protein‐coupled receptors is essential for ADP‐induced platelet aggregation. Proc Natl Acad Sci 95: 8070–8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronlage M, Song J, Sorokin L, Isfort K, Schwerdtle T, Leipziger J et al (2010). Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal 3 ra55‐ra55. [DOI] [PubMed] [Google Scholar]
- Mahaut‐Smith MP, Ennion SJ, Rolf MG, Evans RJ (2000). ADP is not an agonist at P2X1 receptors: evidence for separate receptors stimulated by ATP and ADP on human platelets. Br J Pharmacol 131: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamedova L, Joshi B, Gao Z, von Kügelgen I, Jacobson K (2004). Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem Pharmacol 67: 1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattana A, Cappai V, Alberti L, Serra C, Fiori P, Cappuccinelli P (2002). ADP and other metabolites released from Acanthamoeba castellanii lead to human monocytic cell death through apoptosis and stimulate the secretion of proinflammatory cytokines. Infect Immun 70: 4424–4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers S, Wong L, Charo I (1995). Signal transduction and ligand specificity of the human monocyte chemoattractant protein‐1 receptor in transfected embryonic kidney cells. J Biol Chem 270: 5786–5792. [DOI] [PubMed] [Google Scholar]
- Nylander S, Schulz R (2016). Effects of P2Y12 receptor antagonists beyond platelet inhibition–comparison of ticagrelor with thienopyridines. Br J Pharmacol 173: 1163–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan V, Bidula S, Campwala H, Katikaneni D, Fountain SJ (2012). Constitutive lysosome exocytosis releases ATP and engages P2Y receptors in human monocytes. J Cell Sci 125: 4567–4575. [DOI] [PubMed] [Google Scholar]
- Suplat D, Krzemiński P, Pomorski P, Barańska J (2007). P2Y1 and P2Y12 receptor cross‐talk in calcium signalling: evidence from nonstarved and long‐term serum‐deprived glioma C6 cells. Purinergic Signal 3: 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Giezen J, Nilsson L, Berntsson P, Wissing B, Giordanetto F, Tomlinson W et al (2009). Ticagrelor binds to human P2Y12independently from ADP but antagonizes ADP‐induced receptor signaling and platelet aggregation. J Thromb Haemost 7: 1556–1565. [DOI] [PubMed] [Google Scholar]
- West L, Steiner T, Judge H, Francis S, Storey R (2014). Vessel wall, not platelet, P2Y12 potentiates early atherogenesis. Cardiovasc Res 102: 429–435. [DOI] [PubMed] [Google Scholar]
- Zhang X, Qin J, Zou J, Lv Z, Tan B, Shi J et al (2018). Extracellular ADP facilitates monocyte recruitment in bacterial infection via ERK signaling. Cell Mol Immunol 15: 58–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler‐Heitbrock L (2006). The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 81: 584–592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Ca2+ response in THP‐1 cells evoked by hexokinase‐treated ADP. (A) Average (N = 5) intracellular Ca2+ response evoked by ADP (20 μM). Concentration‐response curve of ADP‐evoked responses (EC50 2.2±0.5 μM; N = 5).


