Abstract
The blood-brain barrier (BBB) is the tightest endothelial barrier in humans. Characterized by the presence of tight endothelial junctions and adherens junctions, the primary function of the BBB is to maintain brain homeostasis through the control of solute transit across the barrier. The specific features of this barrier make for unique modes of transport of solutes, nanoparticles, and cells across the BBB. Understanding the different routes of traffic adopted by each of these is therefore critical in the development of targeted therapies. In an attempt to move towards controlled experimental assays, multiple groups are now opting for the use of microfluidic systems. A comprehensive understanding of bio-transport processes across the BBB in microfluidic devices is therefore necessary to develop targeted and efficient therapies for a host of diseases ranging from neurological disorders to the spread of metastases in the brain.
I. INTRODUCTION
The blood-brain barrier (BBB) constitutes the interface between microvasculature and brain parenchyma, and features unique characteristics. Described as the tightest endothelial barrier in the body, the BBB is composed of an endothelial monolayer, pericytes enclosed within the endothelial basal lamina, and astrocytes touching their end-feet to the abluminal side of the brain vessels.1 Maturation and function of endothelial cells (ECs) are largely dependent on their tight junction (TJ) and adherens junction (AJ) protein expression, upregulated by intercellular interactions between the brain stromal cells and the endothelial cells themselves.2
These junctions regulate the exchange of ions, molecules, particles, cells, and fluids between the blood circulation and the brain parenchyma, and limit the paracellular permeation of hydrophilic agents,3 forcing much traffic across the BBB to take a transcellular route.1 The astrocytic end-feet surrounding the BBB regulate barrier permeability, and dictate the localization of transporters.4 These unique attributes of the BBB microvasculature govern molecular trafficking across the barrier and significantly affect drug delivery to the brain.
Brain disorders are some of the most debilitating and life-threatening ones, affecting numerous functions in the human body.5 Brain diseases are prevalent with up to 40% of cancer patients showing metastatic lesions to the brain and an estimated 5.4 millions of Americans suffering from Alzheimer's disease in 2016, a statistic that is expected to double by the mid-century.6 By 2040, it is expected that 700 000 people will be affected by Parkinson's disease in the United States alone.7 In light of these statistics, the need for successful and targeted drug delivery across the BBB is of utmost importance.8 Yet, the characteristic features of this barrier act as an obstacle for the development of drugs capable of reaching the brain tissue.
Despite its tight endothelial junctions, the BBB is comprised of transport molecules whose role is to regulate the traffic of essential molecules between the brain and the blood in both directions. Consequently, multiple endogenous mechanisms for biomolecule transport can be exploited for enhancing drug transfer to the brain, such as active transport with carriers and vesicular transcytosis.9
In this context, the focus has been directed towards the development of effective in vitro models for the study of drug transport across the BBB.10 For years, in vivo trials in animal model have been considered the most reliable experimental platform to study BBB permeability and are still regarded as the gold standard.11 However, crucial genetic, molecular, immunologic, and cellular differences exist between humans and mice, decreasing the ability of animal models to be used as an effective platform to develop drugs for the treatment of human pathologies,12,13 as evidenced by exceedingly high failure rates in clinical trials of candidate drugs validated in animal models (>80% of drugs fail clinical tests).14 Coupled with the fact that in vivo animal tests are labor-intensive, ethically contentious, and expensive, several groups are turning towards the use of in vitro models of human physiology to enhance the effectiveness of pharmaceutical research in the development of effective drugs for humans.3,15,16
In this review article, we examine the biological characteristics of the BBB giving rise to unique bio-transport processes across this barrier, and present the current state-of-the-art in vitro models of the BBB, with a particular attention on the typical characterizations performed in such models and on the main outcomes deriving from their use. This work provides a comprehensive understanding of transport processes across the BBB in microfluidic devices, crucial to the development of targeted and efficient therapies for a host of diseases ranging from neurological disorders to the spread of metastases in the brain.
II. PHYSICAL BARRIER AT THE BLOOD-BRAIN INTERFACE
The unique properties of the BBB can be attributed to tight junctions (TJs) and adherens junctions (AJs), elaborate junctional complexes formed between adjacent brain endothelial cells.16
A. Tight junctions
Both brain astrocytes and brain pericytes have been shown to secrete factors playing a role in BBB maturation and TJ formation. While not all factors have been identified, it has been shown that pericytes primarily secrete transforming growth factor β1 which in turn activates Smad2 and Smad3 leading to the formation of TJ proteins.17 Astrocytes, the star-shaped glial cells of the nervous system, also play a role in BBB formation and integrity through their secretion of Sonic HedgeHog (Shh), which increases BBB transendothelial electrical resistance (TEER) and decreases permeability.18 Astrocytes secrete of α-dystrobrevin as well, contributing to scaffold formation in astrocytic end-feet and regulation of TJ proteins in the endothelium.19,20 These interactions between brain stromal cells and brain endothelial cells are responsible for barrier tightening and for the expression of polarized transport systems to maintain the homeostasis of the central nervous system (CNS).21 While astrocytes are found only in the brain and spinal cord, pericytes can be found outside of the brain tissue, in the kidneys, the retina, the cardiac muscle, and several other organs. Pericytes from different tissues have been found to adopt different behaviors when it comes to barrier tightening. While the loss of cardiac pericytes has been observed to increase interstitial permeability, their role in barrier function was found to be insignificant at the retina and kidneys.22–25
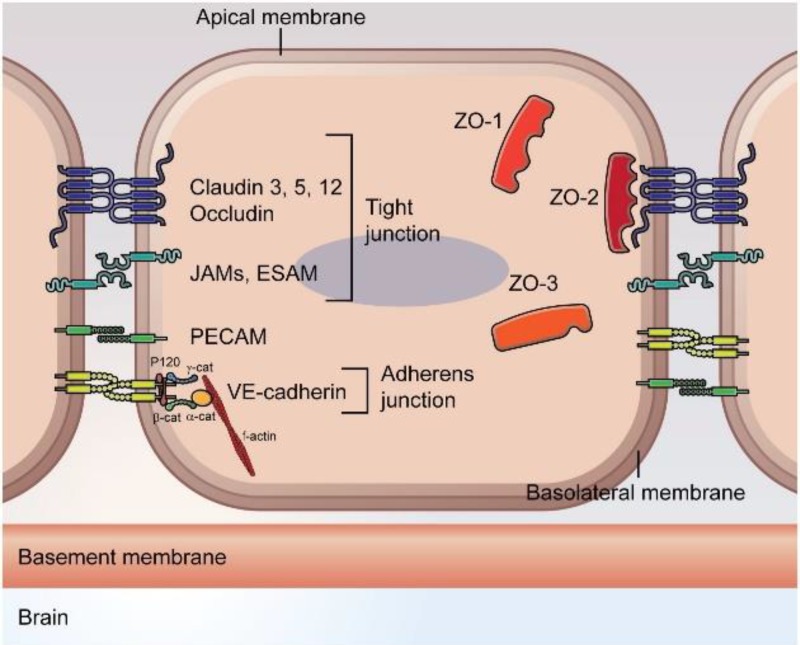
Transport mechanisms at the blood-brain interface rely mostly on the presence of TJ proteins, leading to the development of a tight physical barrier between brain vasculature and parenchyma. TJ proteins consist of transmembrane proteins, such as occludin, claudins, and junction adhesion molecules (JAMs), which rely on the support of cytoplasmic proteins zonula occludens (ZOs), cingulin, and 7H6 phosphoprotein, as well as heterotrimeric G-proteins.16,26 Occludin, claudins and JAMs are expressed at the interface between adjacent endothelial cells while zonula occludens and other submembranous proteins are found in their cytoplasm. These TJ proteins are found in other organs, such as the skin epidermis, the kidneys, and the respiratory tract.27–29 However, the ones found at the blood-brain interface tend to be more highly expressed between adjacent endothelial cells, and are found to restrict ion and fluid transport at the brain significantly more than TJs found in other tissues. Brain pericytes and astrocytes have also been shown to play a role in tightening the endothelial barrier by upregulating the expression of several genes involved in the development of TJ proteins, such as claudins-3 and -5.21,31 Moreover, TJ proteins were found to reduce the number of fenestrations between adjacent endothelial cells significantly more at the brain than at other sites. These “tight” TJ proteins at the brain force most solute traffic through a transendothelial route.30 It has been shown that occludin contributes to the redox-sensitive processes of TJ assembly, while claudins support TJ integrity and permeability via their capacity of homodimerization with claudins on adjacent endothelial cells.32 JAMs facilitate the assembly of TJ components and the recruitment of the polarity complex to TJs.21,33 TJ expression and integrity is sustained by interactions between transmembrane proteins and heterotrimeric G-proteins, as well as the PDZ and SH3 domains of zonula occludens.21 For instance, PDZ domains on ZO-1 bind to claudins and JAMs, while other ZO regions bind to occludin.34 Other cytoplasmic proteins such as cingulin connect to zonula occludens, JAM proteins, and myosin to create a scaffold between transmembrane proteins and the cytoskeleton. Cingulin and 7H6 phosphoprotein have also been shown to play a role in permeability regulation and act as barriers to the transport of ions and large molecules.26,35
B. Adherens junctions
While TJs primarily regulate the passage of ions and solutes, AJs have been shown to initiate cell-to-cell contacts and promote maturation, maintenance, and plasticity of endothelial cells.36 Hence, AJs arise at early stages of junction formation and have been reported to influence TJ organization.37 At TJs, cell-cell adhesion is mainly promoted by claudins, occludin, and JAMs as described above. At AJs, on the other hand, adhesion is mediated by members of the cadherin family, such as vascular endothelial (VE)-cadherin expressed by all types of vessels, platelet endothelial cell adhesion molecule-1 (PECAM-1), and N-cadherin which regulates angiogenesis, in part, by controlling VE-cadherin expression.38
Both VE- and N-cadherins bind to catenins, particularly p120 and β-catenin, which, via interaction with α-catenin and afadin, promote actin bundling.36 This link with the actin cytoskeleton is essential in regulating adhesion, cellular morphology, and signal transduction.39 PECAM-1 also interacts with β-catenin to modulate endothelial permeability and promote leukocyte transmigration across the barrier.40 AJs and TJs are the backbone of transport mechanisms across the BBB and play a crucial role in maintaining vascular integrity and modulating vessel permeability. Table I summarizes the characteristics and major roles of the TJ and AJ proteins at the BBB. A schematic of the different TJ and AJ proteins at the BBB is presented in Fig. 1.
TABLE I.
Localizations and major roles of different TJ and AJ proteins at the BBB.31,36–39
| Type of junction | Junction protein | Localization in brain endothelial cells | Role |
|---|---|---|---|
| Tight junctions | Occludin | Membrane | Promote redox-sensitive processes of TJ assembly |
| Claudins | Membrane | Homodimerize with claudins on adjacent endothelial cells | |
| JAMs | Membrane | Facilitate assembly of TJ components and recruit polarity complex | |
| ZOs | Cytoplasm | Bind to claudins and JAMs via PDZ domain and to occluding via other regions to sustain TJ integrity | |
| Cingulin | Cytoplasm | Connect to ZOs, JAMs and myosin to create scaffold between transmembrane proteins and cytoskeleton | |
| 7H6 phosphoprotein | Cytoplasm | Act as barrier to transport of ions and large molecules | |
| Heterotrimeric G-protein | Cytoplasm | Continuously interact with transmembrane proteins to promote TJ integrity | |
| Adherens junction | VE-cadherin | Membrane | Promote actin bundling |
| N-cadherin | Membrane | Regulate angiogenesis | |
| PECAM-1 (CD31) | Membrane | Interact with VE-cadherin to initiate intercellular contacts |
FIG. 1.
Schematic of endothelial TJ and AJ proteins at the blood-brain barrier. Reproduced with permission from Troletti et al., Biochim. Biophys. Acta 1862, 452–460 (2016). Copyright 2016 Elsevier.41
III. TRANSPORT ROUTES ACROSS THE BLOOD-BRAIN BARRIER
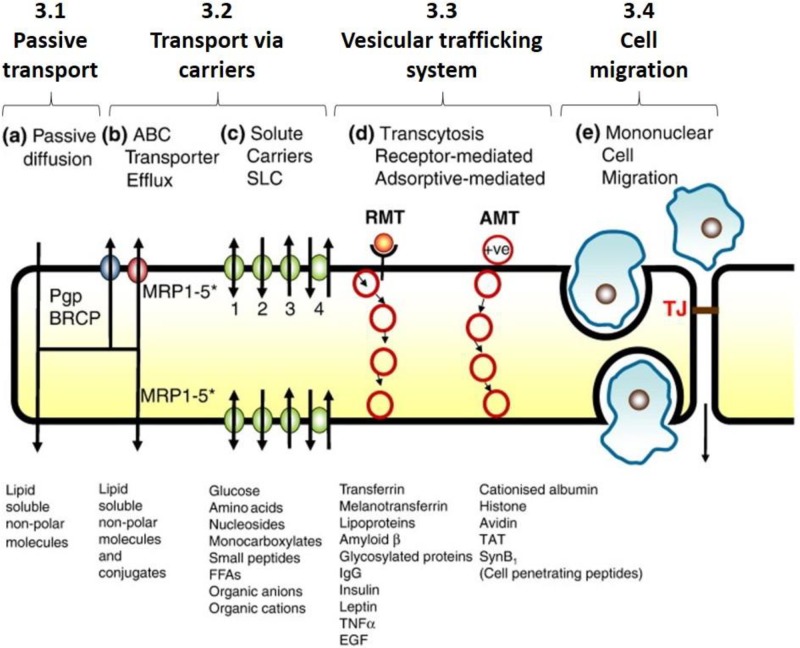
There are several distinguishable routes of transport of solutes across the BBB. While similar transport routes are adopted across many endothelial or epithelial barriers other than the BBB, capillaries at the brain have a smaller number of fenestra, the small “windows” between adjacent endothelial cells that allow small and large molecules, as well as cells to move easily across the endothelium. The flow of solutes across the BBB is thus more regulated than across general capillaries, and larger molecules are prevented from crossing the barrier, unless they are recognized by specific proteins allowing them to be carried into and out of the brain tissue.42,43 Depending on both the solute type and physicochemical properties, and the biological features of the blood vessel wall, the solute will permeate across the barrier through a particular path that involves a specific set of mechanisms.44 Molecular trafficking across the BBB can be grouped into four different categories outlined in Fig. 2.
FIG. 2.
Four different modes of transport across the BBB. Reproduced with permission from Abbott et al., Neurobiol. Dis. 37, 13–25 (2010). Copyright 2010 Elsevier.1
A. Passive transport
Passive transport consists of two main pathways, namely paracellular diffusion, through the intercellular space between the endothelial cells lining the BBB, and transcellular diffusion, through the lipid bilayer and cytosol of the endothelial cells.45
Diffusion through the intercellular cleft, or paracellular diffusion, is mainly adopted by small hydrophilic entities. However, the presence of TJs renders this space extremely narrow, thus physically hindering this mode of transport across the BBB.45 Thus, molecular diffusion at the brain predominantly adopts transcellular pathways.46
In contrast to paracellular diffusion, transcellular passive diffusion through the BBB relies on the solute melding into the cell membrane. On a molecular level, the primary diffusion barrier characterizing the BBB consists of a lipid bilayer, constituent of cell membranes.47 This bilayer is described as a macroreceptor that interacts simultaneously with multiple ligands, thus providing a large surface of exchange between solutes and cell membrane. Once contact is established, the movement of the fatty acid side chains in the lipid membrane leads to the formation of “holes” or “kinks” through which solutes can diffuse.45 Given the mechanism underlying transcellular passive diffusion, solutes with high degrees of lipid solubility (lipophilic solutes) and low molecular weights have been shown to adopt this transport route.44 The absence of rotatable bonds, as well as the low affinity of binding to plasma proteins also promote passive diffusion through a transcellular route.1 In light of these findings, the pharmaceutical industry has developed a set of rules based on solute molecular size, presence of hydrogen-bon acceptors/donors, and lipophilicity, for the development of therapeutics able to efficiently cross the BBB through transcellular passive diffusion.48
B. Active transport via carriers
Tight junctions in brain endothelial cells act as a physical barrier to paracellular diffusion. As a result, multiple nutrients needed for brain function and metabolism find their entry to the brain parenchyma prevented. A number of specific solute carriers are then recruited as active transporters across the BBB through transcellular routes.1 These carriers are found on several other endothelial barriers, but their expression is more significant in the brain where paracellular transport is restricted due to the relative lack of fenestra.49,50 If substances need to be transported through the barrier down a concentration gradient, no additional energy is required for these processes and the transports are established via solute carriers. On the other hand, if compounds need to be moved against a concentration gradient, adenosine triphosphate (ATP) may provide the energy to facilitate the process through efflux pumps or ABC transporters.16
To cross the BBB transcellularly, solutes can bind to protein carriers on one side of the membrane. This triggers a conformational change in the protein, resulting in the influx of the solute to the other side of the membrane down its concentration gradient, without any additional energy.16 The majority of these carriers are encoded by genes in the solute carrier (SLC) family, and are thus often called SLC transporters.2 Among these transporters, we distinguish GLUT1 (coded by SLC2A1), a glucose carrier located both inside the vasculature and in the extracellular matrix surrounding brain capillaries, as well as LAT1 (coded by SLC7A5) which transport neutral amino acids from the blood to the brain and vice-versa.51
To transport compounds against a concentration gradient, energy is required. This involves the solute binding to an ATP-binding cassette (ABC) transporter in the BBB which acts as an active efflux pump consuming ATP and releasing energy to pump the solute across the barrier, against a concentration gradient.16 Efflux carriers, such as ABC transporters, have the opposite effect to influx transporters, such as SLC carriers, since the former transports solutes against a concentration gradient, while the latter transports them down the concentration gradient.44 As a result, ABC transporters are often responsible for decreasing the uptake rate of potential drugs crossing the BBB, as well as enhancing the barrier properties of the BBB.52 The most common ABC transporters are P-glycoprotein, responsible for the poor penetration of large (>400 Da) hydrophobic drugs in the brain, the breast cancer resistance protein (BCRP), conferring resistance to non-chemotherapeutic drugs and xenobiotics, and several members of the multidrug resistance-associated protein family.52–54
C. Vesicular trafficking system
Due to their size and the presence of TJs at the BBB, macromolecules generally cannot cross the barrier passively or through carriers. To bypass this obstacle, they rely instead on transcytosis via endocytotic mechanisms.1 Large molecular weight solutes, such as proteins and peptides, are internalized by brain endothelial cells and carried across the barrier through an internal membrane system which shuttles the macromolecules between chemically distinct membrane-enclosed vesicles.55 These vesicular trafficking mechanisms involve either specific, receptor-mediated transcytosis (RMT), or non-specific, adsorptive-mediated transcytosis (AMT).
In RMT, macromolecules bind their ligands to specific, cognate receptors on the cell surface, triggering the endocytotic event.1 The receptors on the cell surface and their bound ligands from the macromolecules cluster together, causing caveolae (membrane invaginations) to form, which pinch off intracellular transport vesicles. These vesicles are actively translocated via microtubules into the opposite pole of the endothelial cells where they are released.56 The receptor-ligand complexes are dissociated during cellular transit or during the exocytotic event. Most macromolecules, such as iron-transferrin, insulin, leptin, and epidermal growth factor (EGF), are transported from the blood to brain; however, a few compounds, such as low-density lipoprotein cholesterol (LDL-1 and 2) and amyloid β, can cross the barrier bi-directionally.1
AMT requires an excess of positive charges on the molecule for it to interact with the cell surfaces that are negatively charged. These non-specific electrostatic interactions allow macromolecules to bind to cell surfaces, thus inducing endocytosis.57 Several positively charged substances, such as cationic lipids, polymers, dendrimers, and albumin, are known to cross the BBB through AMT.2
This understanding of RMT and AMT, similarly to the findings related to passive diffusion, has promoted the development of neuropharmaceuticals with specific physicochemical properties allowing them to undergo transcytosis and cross the BBB.55 In fact, several groups have functionalized the surface of nanoparticle (NP) drug carriers with specific ligands promoting their binding to receptors on the surface of brain endothelial cells (RMTs). Alternatively, modulating the surface charge of drugs has been of interest to stimulate AMT across the BBB.58,59
D. Cell transmigration
In the normal BBB and other endothelial barriers, resident mononuclear cells, such as leukocytes, monocytes, and macrophages, can undergo diapedesis and cross the barrier via both paracellular and transcellular routes. During inflammation, trafficking of mononuclear cells across the BBB is highly upregulated. Leukocytes have been observed to cross the blood-brain interface via a transcellular route through the endothelial cells during autoimmune encephalomyelitis, thus leaving TJs and barrier function undisrupted.1,60 However, CNS inflammation is also characterized by the opening of TJs due to the action of certain cytokines, such as interleukin (IL)-8 and matrix metalloprotease (MMP)-9.61 This in turn can cause mononuclear cells to also enter the brain parenchyma using a paracellular route.62,63
Mononuclear cells are not the only ones crossing endothelia, including the BBB. During metastasis, tumor cells from various primary sites can extravasate from within capillaries to colonize the secondary organ. This process is prominent at the brain, where 20%–40% of cancer patients develop metastases.8,64 Most tumor cell transmigration observed occurred through the paracellular pathway.65,66 Evidence suggests that tumor cells can release enzymes that induce the digestion of VE-cadherin known to tighten vascular barriers. This results in VE-cadherin cleavage, thus increasing vascular leakage and tumor cell extravasation.37,67
IV. MICROFLUIDIC MODELS OF TRANSPORT ACROSS THE BBB
In order to observe and study the cellular, physical, and biochemical characteristics of a specific microenvironment, several groups have shifted their work towards using in vitro microfluidic assays rather than in vivo animal models or other in vitro models relying on cells cultured in Petri dishes.68 The shift towards microfluidics has been significant in studies pertaining to the brain microenvironment in health and disease.
For several years, animal models have been used extensively to study transport across the BBB. These in vivo models provide the advantage of studying the BBB in its natural microenvironment, where cellular interactions are fully represented and physiological responses can be observed at the scale of the entire organism.69 Moreover, in vivo models allow for the investigation of specific genes, proteins, and carriers involved in transport across the BBB, which might be limited in vitro models that do not fully recapitulate the in vivo microenvironment when it comes to protein expression.70 Yet, despite these advantages, significant challenges remain when it comes to translating experimental results obtained in animal brains to conclusive outcomes for human brains. Genetic, molecular, and cellular differences prevent animal models from achieving high physiological relevance when it comes to studies of transport across the BBB. In addition, in vivo models offer a low-throughput and low-resolution platform of study when compared to in vitro models.64 Coupled with the costs associated with animal models, several groups have shifted their focus towards the use of in vitro models.
As described, most BBB models in the current literature rely on either in vivo animal models or in vitro monolayers of brain cells, be it endothelial cells or brain stromal cells.71 In the past few years, microfluidics have become a more popular platform to conduct more physiologically relevant and controlled experiments.68 However, they have been rarely used for brain microenvironment applications, where in vivo animal models and in vitro 2D cell culture models predominate.72–74
In the context of in vitro BBB models, several groups rely on the use of primary human brain microvascular endothelial cells (HBMECs) isolated from the human brain tissue, as well as primary human pericytes, astrocytes, neurons, microglia, and oligodendrocytes.75,76 These primary cells offer the advantage of being directly isolated from the relevant brain tissue, and can be well-characterized by their cell-specific protein expression.77 One of the drawbacks of primary human brain cells lies in the lack of well-defined long-term behavior of the individual cells cultured in 2D, as well as the lack of freezing protocols for extended use. For these reasons, immortalized cell lines have been developed, particularly for brain endothelial cells and microglia. While these cells have the advantage of being able to be cultured for extended periods of time without loss of specific phenotype, their values of barrier permeability when cultured alone and with brain stromal cells remain significantly higher than the permeabilities observed with primary HBMECs. In the process of immortalization, these cells experienced a loss in barrier integrity, thus preventing them from accurately capturing the unique properties of the BBB.78 To address these issues and to develop patient-specific models, several groups have shifted towards the use of human induced-pluripotent stem cells (iPSCs) and human embryonic stem cells (ESCs). These cells can be cultured in 2D for extended periods of time, with defined cell freezing protocols. In addition, these cells can be employed in the development of patient-specific models since they are derived from the skin or blood of individual patients. Their exact behavior and phenotype when differentiated into the different brain cell types remains to be fully characterized. However, several groups have been able to differentiate these pluripotent cells into brain-specific endothelial cells, astrocytes, and neurons, thus developing BBB models on a chip with greater accuracy and physiological relevance.79–82
A. 2D monolayers in microfluidic devices with physiologically relevant conditions
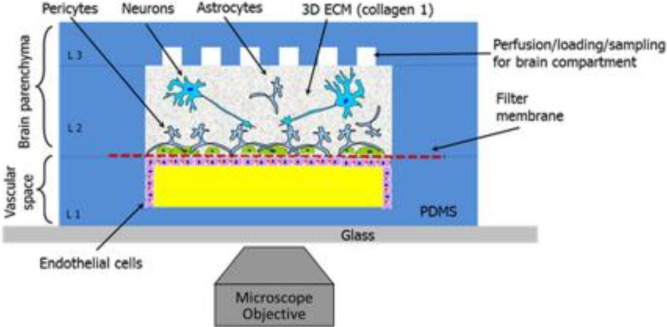
Moving away from in vivo animal models in order to create more physiologically relevant models, the most common in vitro platform is created using transwells with one, two or more cell types used, such as brain endothelial cells, with the addition of astrocytes or pericytes on the abluminal side of the membrane.73 This system, as opposed to simple cell culture in Petri dishes, allows for the addition of various cell types on different layers, for the application of various physical parameters to the culture such as flow and shear stresses, and for the measurement of relevant properties characteristic of the BBB, such as permeability and transendothelial electric resistance (TEER) values.72,83
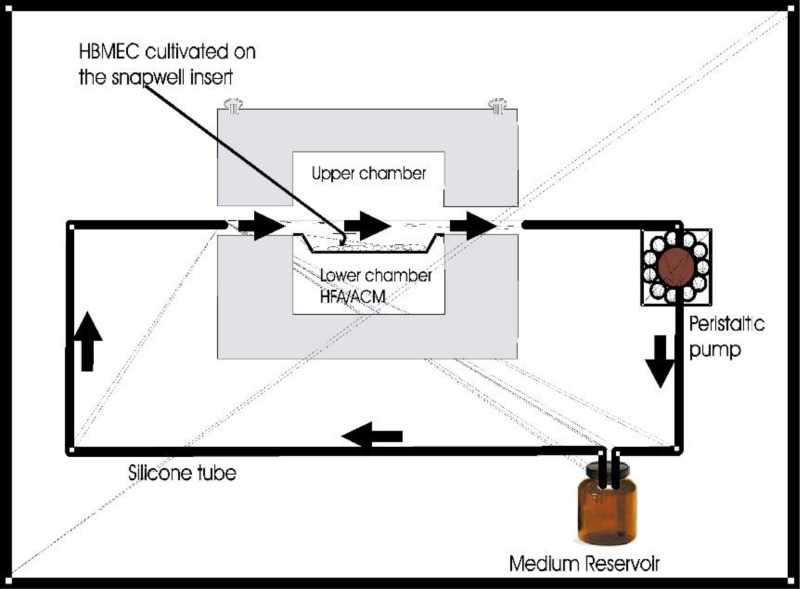
For instance, Siddharthan et al. cultured HBMECs in a snapwell insert which they then placed in a flow chamber within a temperature-controlled CO2 incubator (Fig. 3). This enabled coculture of HBMECs with human fetal astrocytes (HFAs) while applying shear stress on the cells. Their experiments suggest that coculture with astrocytes, the application of shear stress and their combination decrease the permeability of the monolayer while increasing its TEER, thus enhancing the properties of HBMECs and yielding a more physiologically relevant BBB model.75
FIG. 3.
Siddharthan et al. cultured HBMECs in a flow chamber with human fetal astrocytes and showed that flow and coculture with astrocytes decreases permeability and increases TEER. Reproduced with permission from Siddharthan et al., Brain Res. 1147, 39–50 (2007). Copyright 2007 Elsevier.75
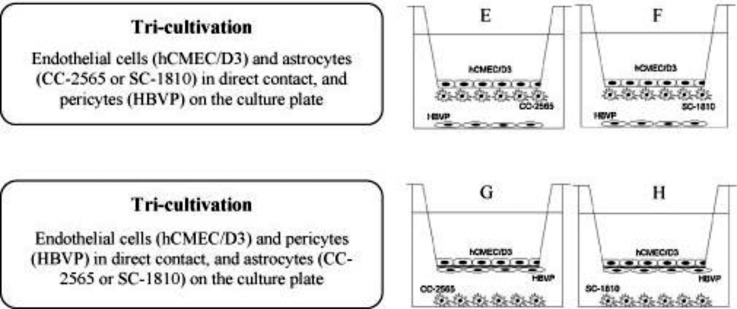
Expanding on this idea, Hatherell et al. conducted a comparative analysis of mono-, co-, and tri-cultures of human brain endothelial cells, pericytes, and astrocytes in transwell assays, to study the effect of brain stromal cells on BBB properties. Due to the geometry of transwells, the group was not able to perform adjacent tri-cultures but instead resorted to placing brain endothelial cells on the insert mask, brain pericytes or brain astrocytes on the abluminal side and brain astrocytes or brain pericytes, respectively, at the bottom of the dish (Fig. 4).76
FIG. 4.
Different tri-culture models employed by Hatherell et al. to study the effect of brain pericytes and brain astrocytes on brain endothelial cell permeability. Reproduced with permission from Hatherell et al., J. Neurosci. Methods 199, 223–229 (2011). Copyright 2011 Elsevier.76
Their results showed significant increase in TEER and barrier tightening in their co-culture cases where brain endothelial cells were in contact with either pericytes or astrocytes. Although their tri-culture results showed significantly increased TEER compared to the mono-culture case, the TEER values were lower than the ones obtained for the co-culture cases. They concluded that cellular contact communications from pericytes and astrocytes are needed to induce proper barrier function in brain endothelial cells.71,76,83
Since primary BMECs may lose some of their BBB characteristics in culture, Wang et al. have utilized human brain endothelial cells derived from human iPSCs via a biased spontaneous differentiation approach derived by Shusta and Palecek groups. They co-cultured these brain endothelial cells with rat primary astrocytes in their pumpless microfluidic device to apply physiologically relevant shear stresses matching the blood residence time in the human brain. They reported sustained high TEER levels above 2000 Ω cm2 for up to 10 days.84
D. Assembled 3D monolayers in microfluidic devices
To circumvent some of the limitations of 2D monolayer models and to create more physiologically relevant BBB systems, several groups have adopted more geometrically relevant assembled 3D monolayer models. Brown et al. constructed a 3D BBB model in a microfluidic chip consisting of a monolayer of HBMECs coating a rectangular vascular chamber, and primary human brain pericytes, and astrocytes, as well as neurons differentiated from human iPSCs, in an adjacent brain parenchyma chamber (Fig. 5). With this microfluidic model, they were able to test for diffusion of dextran to measure for permeability, and perform TEER measurements with a custom-built impedance analyzer.85
FIG. 5.
Assembled 3D monolayer of HBMECs in contact with human brain pericytes and astrocytes, as well as neurons differentiated from human iPSCs. Reproduced with permission from Brown et al., Biomicrofluidics 9, 054124 (2015); Copyright 2015 Authors, licensed under a Creative Commons Attribution 3.0 License.85
Adriani et al. have also developed a similar platform using both human umbilical vein endothelial cells (HUVECs) and human cerebral microvascular endothelial cells (hCMEC/D3) in a rectangular channel adjacent to channels containing primary rat astrocytes and neurons in a collagen gel. With this platform, they were able to obtain measurements of barrier permeability using dextran for both HUVECs and hCMEC/D3, with astrocytes and neurons.86
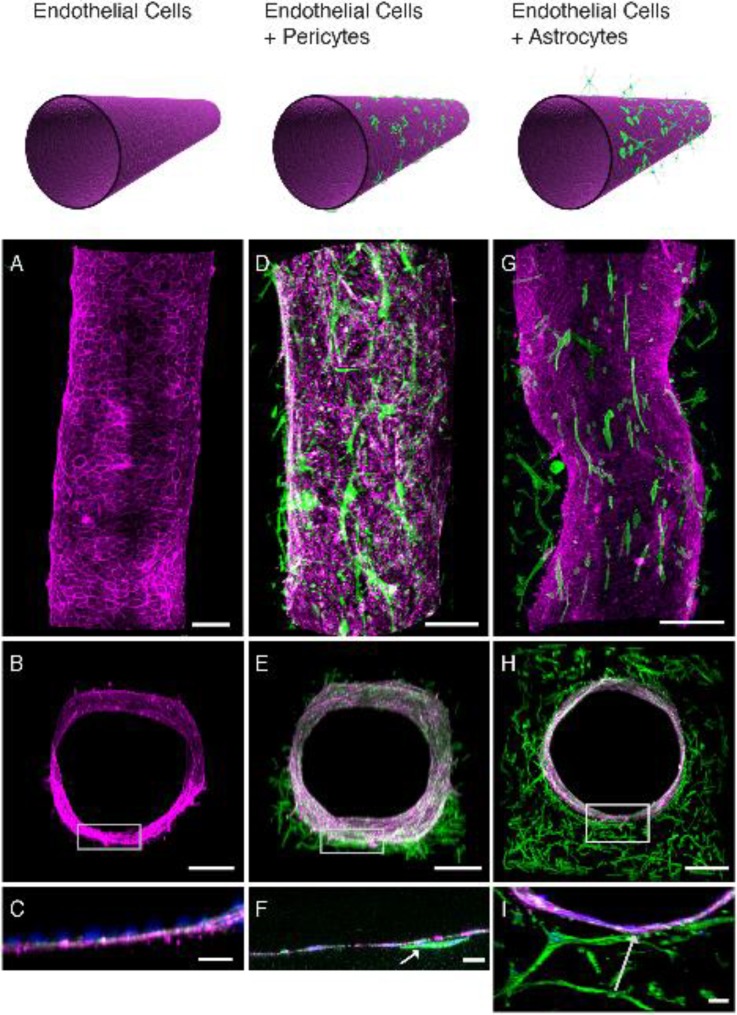
Herland et al. developed an assembled 3D monolayer BBB model using HBMECs, primary brain pericytes, and primary brain astrocytes. However, instead of a rectangular geometry with one plane of contact with brain stromal cells, their platform consists of a circular hollow lumen inside a collagen gel where the HBMECs are coated with pericytes or astrocytes on the abluminal side of the assembled endothelial cell vessel (Fig. 6). This design allows for a more physiologically relevant geometry and cell-cell contact for the study of permeability and response to inflammation.87
FIG. 6.
Co-culture of human brain microvascular endothelial cells and pericytes or astrocytes in the circular 3D BBB chip. Reproduced with permission from Herland et al., PLoS One 11, e0150360 (2016); Copyright 2016 Authors, licensed under a Creative Commons Attribution 4.0 License.87
C. Vascular networks in a 3D gel
While assembled 3D monolayers in microfluidic chips allow for greater control of the vessel geometry and area of contact between brain endothelial cells and stromal cells, they may not fully replicate self-assembled vascular networks in terms of branching, diameter sizes, and barrier tightness. Very recently, Bang et al. have developed a BBB model in a microfluidic chip using perfusable vascular networks composed of HUVECs in a separate channel, adjacent to astrocytes, neurons, and normal human lung fibroblasts. This platform allowed for a higher degree of neurovascular interfacing and low vascular permeabilities, characteristic of the BBB.88 While this type of model is promising in terms of network morphology, cell-cell interactions, and physiological barrier tightness, it is still very recent in terms of its application to the brain and remains to be further studied.
D. Fluid flow stimuli in BBB models
Several groups have observed that exposure of brain endothelial cells and stromal cells to fluid flow stimuli, such as shear stress from blood flow, changes the expression of TJ proteins and decreases the permeability of the barrier.75,85,87 Physiologically relevant shear stress values tested in the literature for media flow on brain endothelial cells range from a few mPa to ∼600 mPa.89,90 In Siddharthan et al., a pump circulates media exposing the HBMEC monolayer to fluid shear stresses of ∼100–200 mPa and leading to significantly reduced permeability values.75
Shear stress from media flow is not the only fluid flow stimulus endothelial cells may experience. Transmural pressure gradients can also generate flows that can convect solutes and molecules across the barrier. In fact, many permeability tests impose a pressure gradient during the introduction of a specific tracer, such as fluorescently labeled dextran, propidium iodide (PI) or Evans Blue (EB) dye. The flux rate at which the dye crosses the cellular barrier is correlated with barrier tightness and permeability.72,75,87
V. PERMEABILITY MEASUREMENTS IN BBB MODELS
In order to evaluate barrier function, most groups rely on permeability measurements using fluorescent solute diffusion or TEER. Key features and relevant values of each measurement technique are outlined below.
A. Fluorescent solute diffusion
Several groups have relied on the use of fluorescent solute diffusion across the BBB to measure permeability and assess barrier tightness.10 The general protocol consists in filling one side of the monolayer or the lumen of the vessel with a solution of fluorescent dye and creating a pressure gradient with the opposite compartment on the abluminal side of the endothelial cells. This allows the dye to diffuse across the barrier. Fluorescent intensity is recorded at different timepoints in both compartments to assess the rate of solute exchange across the barrier. Permeability is proportional to the flux of the dye, which depends on its Stokes radius.72,75 Considering the principle of mass conservation, the mass transfer rate (J) that crosses the endothelial barrier is expressed by
where As is the surface area of the endothelial barrier, is the concentration difference, and Pv is the permeability coefficient of the vasculature. The fluorescent solute concentration is assumed to be proportional to the fluorescent intensity (Iv for the vessels and IT for the tissue outside the vessels at two different time points), V is the tissue volume (or gel region), and Δt is the time between two measurements. The permeability coefficient of the vasculature is given by
While the general experimental protocol for dye diffusion is relatively similar across all studies, multiple parameters are left at to the discretion of each group performing the measurement, such as the type of solute (dextran, Evans Blue, propidium iodide, etc.), its size, its lipophilicity, and its propensity to bind to tissue and proteins.91–93 This lack of standardization and consensus among researchers often limits comparison and validation of BBB-on-chip models. Several reviews have promoted the need for commonly accepted standards in terms of permeability and TEER measurements.10,92
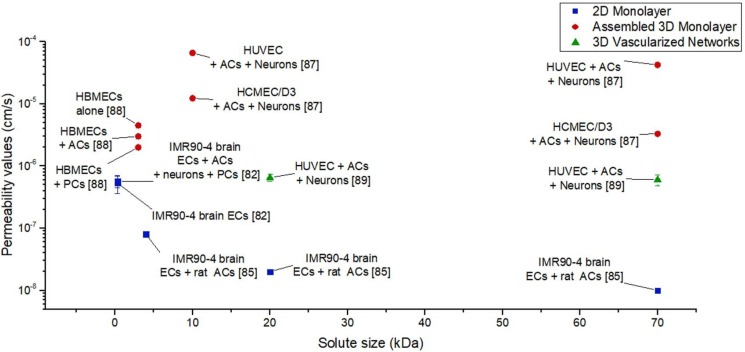
While a universal protocol for permeability measurement, independent of the microfluidic device design, is still needed, a summary of most reported measures of permeability for human in vitro BBB models is shown in Fig. 7. In light of the considerable variability in recorded permeability values for human BBB models employing similar cell types and microfluidic platforms, a need exists for a standardized protocol for choosing fluorescent solutes and performing permeability tests. Nevertheless, several trends are evident, namely that vascularized networks and brain endothelial cells derived from iPSCs tend to yield lower permeability measures (∼10−7–10−8 cm/s).
FIG. 7.
Permeability values for human in vitro BBB models for different fluorescent solute sizes and different types of microfluidic models (references included in brackets for data obtained from Lippmann et al.,82 Herland et al.,87 Wang et al.,84 Bang et al.,88 and Adriani et al.86).
B. TEER
Several groups have relied on transendothelial electrical resistance (TEER) as a measure of the tightness of barrier function, based on measuring ohmic resistance or impedance to the passage of small ions through the BBB over a range of frequencies.94,95 These measurements are made using two electrodes placed on either side of a membrane in which the cells are cultured.26 Typically, TEER is measured in 2D transwell membrane systems by applying an alternating current (AC) voltage signal with a square waveform, as direct current could damage the cells.94 Subtracting the resistance of the semipermeable membrane RBLANK from the measured total resistance RTOTAL, the tissue resistance RTISSUE is given by
With resistance being inversely proportional to area, TEER values are computed as:
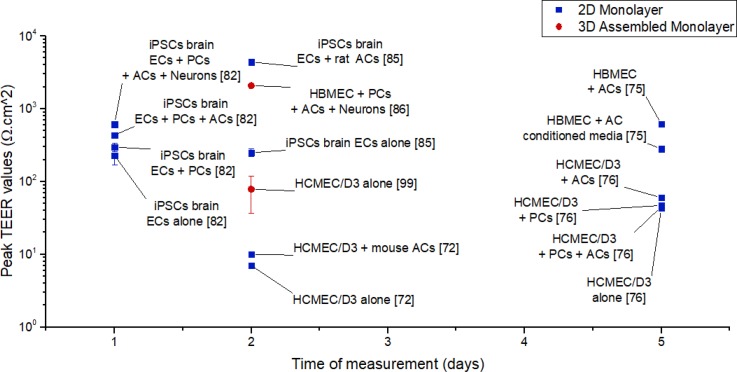
Most groups have reported TEER values below 600 Ω cm2, indicative of poor barrier function in vitro (Fig. 8). However, Wang et al. managed to measure TEER values above 4000 Ω cm2, thanks to a microfluidic device allowing for oxygen and nutrient transportation, as well as waste product clearance.84
FIG. 8.
TEER values for human in vitro BBB models at different times and for different types of microfluidic models (references included in brackets for data obtained from Siddharthan et al.,75 Hatherell et al.,76 Griep et al.,96 Czupalla et al.,72 Lippmann et al.,82 Wang et al.,84 and Brown et al.85).
Due to the lack of in vivo permeability and TEER measurements in humans, researchers often compare measurements in in vitro human models to measurements in in vivo animal models, that typically range from 1500 to 8000 Ω cm2; most in vitro values reported for human models fall well beneath this range.71 However, the comprehensive assembled 3D monolayer developed by Brown et al. with HBMECs, primary pericytes, and astrocytes, as well as neurons derived from iPSCs yielded TEER values of ∼2080 Ω cm2, within the in vivo range obtained for animal models.85 Moreover, 2D monolayer model of Wang et al. with brain endothelial cells derived from iPSCs, co-cultured with rat astrocytes, resulted in TEER values peaking at 4399 Ω cm2 at day 2, and remaining nearly constant at 2000 Ω cm2 until day 10 in culture.84 Although caution must be used in making a direct comparison between human in vitro models and animal in vivo models, the two models developed by Brown et al. and Wang et al. resulted in the largest TEER values recorded in microfluidic systems, shedding some light on the role of brain stromal cells and neurons in barrier tightness, and adding to the argument that brain primary endothelial cells might lose some of their BBB characteristics in culture.84,97
VI. SIGNALING PATHWAYS, GENE, AND PROTEIN EXPRESSIONS IN BBB MODELS
The expression and activity of signaling molecules are typically similar for both 3D microfluidic systems and traditional 2D models, although 3D models offer a more functional and relevant organ-like context. Most protocols rely on the use of conditioned media and cell lysates collected from fluidic or gel channel regions to perform Western Blot and polymerase chain reaction analyses, molecular detection, and mass spectroscopy.98 Due to the small size of most microfluidic models, an adequate scale-up of the device might be required in order to get a proportionally functional amount of proteins and RNA.99 In order to understand how signaling pathways, gene, and protein expressions are affected in BBB models, fundamental questions should be addressed, namely (i) how cytokines and chemokines modulate barrier functions;100 (ii) how microenvironmental cues and cellular interactions regulate BBB differentiation4 and genetic expression;101 (iii) how BBB function may be altered by physical stimuli102 or drug transport effects,103 as well as inflammation or infection that may occur in the human brain.
A. Cytokine profiling
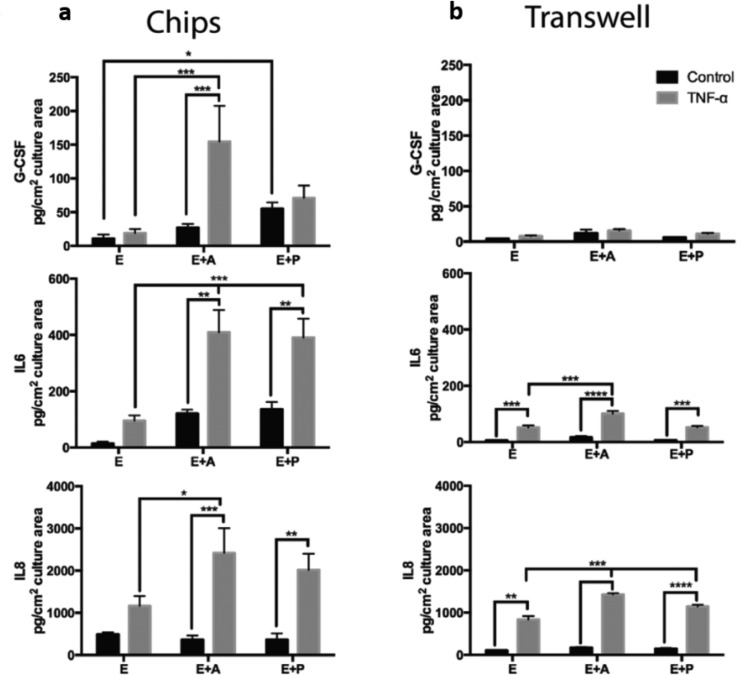
Cytokine expression at the blood-brain interface modulates CNS function100 and the activity of membrane transporters.104 The BBB is comprised of several receptors that selectively transport cytokines, such as interleukins (ILs) and tumor necrosis factor (TNF)-α implicated in various inflammatory responses,105 or leptin involved in neuroendocrine regulation.106 Some of these physiological responses, such as the neuroinflammatory response of the CNS, are associated with several neurodegenerative diseases, including meningitis105 and Alzheimer's disease.107 In these situations, elevated TNF-α levels have been measured in the brain, mainly secreted by astrocytes, microglia, and injured neurons, in response to inflammation (Fig. 9).105
FIG. 9.
Comparison of inflammatory cytokine profiling of (a) a human 3D BBB model on a chip and (b) a transwell membrane model for granulocyte colony-stimulating factor (G-CSF), interleukin-6 (IL-6), and interleukin-8 (IL-8). Reproduced with permission from Herland et al., PLoS One 11, e0150360 (2016); Copyright 2016 Authors, licensed under a Creative Commons Attribution 4.0 License.87
Previously, cytokine release has been studied using static 2D transwell membranes108 and complex in vitro systems.109 Recently, microfluidic chips have permitted the reproduction of complex and physiologically relevant conditions with stimulation of TNF-α under fluid flow.87 Importantly, a substantial amount of granulocyte colony-stimulating factor (G-CSF) and interleukin-6 (IL-6), functioning as neuro-protectors in vivo, were detected in the 3D BBB model on a chip, significantly higher than in the static transwell membranes. Interestingly, pericytes and astrocytes were associated with this increase in cytokine release in the 3D BBB model on a chip. Microfluidic platforms have provided significant advantages in cytokine profiling studies during neuroinflammation and other related brain diseases.87
B. Protein and gene expression in BBB models
Genomic and proteomic studies have provided tremendously useful information on BBB function and revealed the genes and proteins responsible for inducing a BBB phenotype.110 Studies in 2D culture and 3D systems have shown that protein and gene expressions of membrane transporters and efflux pump increase during fluid flow stimuli in microfluidic BBB models compared to static cultures.89 However, thus far, variation in gene expression has been evaluated only in 2D transwell membranes,111,112 or in iPSC-derived BBB models.113 Gene expression of membrane transporters has been measured in complex 3D assembled monolayer systems that recreate hollow lumens. Flow was observed to up-regulate the expression of most junctional genes, responsible for barrier tightening through TJs and AJs, and drug transporter genes, responsible for transport of molecules across the BBB, studied. This indicates the importance of physiological fluid flow in the development of proper blood-brain barrier function.109 The analysis of gene expression in self-vascularized BBB microfluidic models should be performed to validate the 3D assembled monolayer results;109 particularly, gene expression of TJ proteins and membrane transporters by the brain endothelial cells should increase during fluid flow experiments or in co-culture with pericytes or astrocytes.114
VII. DRUG DELIVERY STUDIES IN BBB IN VITRO MODELS
To study drug delivery into the brain parenchyma, several groups have based their investigations on initial in vitro BBB models, followed by in vivo animal models.68
A. In vitro BBB models for drug transport screening
As animal models fail to replicate the human BBB microenvironment, new in vitro models of human pathological and healthy BBB are under development115 with the aim to study and predict drug transport. Optimal in vitro BBB models should satisfy different requirements: simplicity, low cost, resemblance to in vivo conditions, and capabilities for high throughput screening.3 Particularly, they should display similar permeability values to human BBB to be exploited in studies for drug targeting validation and/or toxicological evaluations. The relevance of in vitro BBB models can be understood by comparing in vitro results with data obtained using in vivo methodologies.3
As discussed below, 2D transwell membrane models have been widely used for BBB in vitro modeling. Such models have been mainly generated from human primary and immortalized cells on a 2D porous membrane with or without the co-culture of astrocytes and pericytes in the lower chamber or in the upside-down membrane.111,116,117 Such in vitro BBB models, when based on animal cells, have shown low correlations with respect to in vivo results.118,119 This has been mainly attributed to a low expression of L-amino-acid transporter (LAT), suggesting that the model was not completely functional. Hence, beside the establishment of TJs, other parameters affect the performance of BBB models, for example the expression of transporter molecules.
Another human BBB model using transwell membrane has been developed to assess functional activity of P-GP, BCRP, and multidrug resistance-associated proteins using hCMEC/D3 cells.111 Several compounds such as inulin, lucifer yellow, sucrose, propranolol, morphine, and midazolam have been investigated in the model. The measured permeability values were consistent with previous data120 and lower than those measured using rat cell lines.121 However, several issues limit the use of transwell models, such as constraints in availability of human neural cells, as well as the lack of expression of those genes and TJ proteins present in the human immortalized BBB cells. On the other hand, recently, two different transwell platforms using iPSCs120 in combination with neural multipotent stem cells (NMSCs) have been established, with excellent potential to generate a more reliable BBB model.113 Appelt-Menzel et al. have differentiated human iPSCs and NMSCs into endothelial cells (ECs), astrocytes (ACs) and neurons with BBB characteristics within a few weeks. Then, transport studies using small molecules able to permeate the BBB via the transcellular route were performed. Additionally, in this BBB model, paracellular transport was characterized and compared to in vivo observations testing drugs and other small hydrophilic compounds, such as caffeine, diazepam, ibuprofen, and diclofenac.122 As expected, permeation was significantly lower in the quadruple culture of ECs, pericytes, ACs, and neurons compared to the mono-culture of ECs, indicating an even more in vivo-like phenotype. These results were in agreement with other in vitro data obtained from BBB models based on rat primary cells. Comparison with in vivo measurements showed that the human iPSC-derived BBB model was effective in predicting the permeability of drugs.
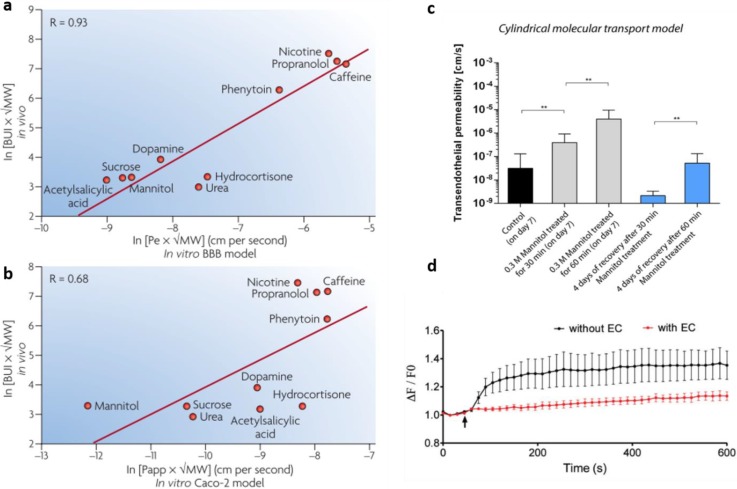
One advantage of the transwell models for drug studies is that they all provide significant barrier integrity. However, care should be taken when interpreting results derived from correlations between in vitro and in vivo models, because any misleading interpretation of data could generate inaccurate results and failure in the next clinical trials3 [Figs. 10(a) and 10(b)]. For these reasons, in vitro models of the BBB should reflect as many relevant properties of the in vivo brain endothelium as possible, to serve as reliable tools for predictions of human conditions and consequent effective drug development.
FIG. 10.
Brain uptake index (BUI) correlation between in vivo and in vitro. (a) In vitro model using human brain capillary endothelial cells and (b) Caco-2 cell line (colon-epithelial cell line). Reproduced with permission from Kim et al., Biomicrofluidics 9, 1–15 (2015).Copyright 2015 AIP publishing.123 (c) Vascular permeability measurement after mannitol disruption and (d) average normalized variation in fluorescence intensities (ΔF/F0) of X-Rhod-1 that measures calcium concentrations and consequently reduced glutamate transport across the endothelial barrier. Reproduced with permission from Adriani et al., Lab Chip 17, 448–459 (2017). Copyright 2016 Royal Society of Chemistry.86
B. Microfluidic platforms and organs-on-chips for drug studies
Organ-on-a-chip technology is being used to develop cost-effective in vitro models that can more reliably predict the efficacy, toxicity, and pharmacokinetics of drug compounds in humans, using novel phenotypic screening assays.98,124 To evaluate drug efficacy, organs-on-chips provide many advantages over 2D in vitro models, such as improved representation of complex pathophysiology in a 3D microenvironment with possibility of physiologically relevant fluid-mechanical stimuli. 3D tissue structures with barrier function more accurately simulate and predict delivery and penetration of drug compounds in vivo than 2D cell monolayers in conventional culture models. Several drug delivery studies have been developed including 3D microfluidic platforms. In particular, some groups have estimated drugs and compounds transport permeabilities through the EC layers by evaluating TEER.109
Cucullo et al. have performed drug transport studies in a 3D assembled monolayer BBB model with a hollow cylindrical geometry, operating under a laminar flow regime.89,109 Under the effect of shear stress, brain endothelial cells redistributed their actin fibers and changed their morphology. This model predicted a lower permeability for mannitol and morphine by measuring TEER compared to the case without treatment. However, such a platform is incompatible with high-throughput pharmaceutical screening because of the large number of cells and high-handed technical skills needed to set up the model, as well as the lack of a real-time monitoring of cell behavior.
In addition, Booth et al. have developed a microfluidic BBB model on a dual layer of mouse brain endothelial cells and astrocytes in multi polydimethylsiloxane (PDMS) glass layers including electrodes to measure TEER and fluid flow. They have found a drop and recovery of TEER compared to the original value when exposed to histamine, indicating the robustness of the model for repeated long-term testing purposes.125
Kim et al. have also engineered a brain microvascular structure by using a 3D printed frame where microneedles were inserted. Then, a collagen mixture was applied on the frame, microneedles were removed, allowing the mouse brain endothelial cells to form hollow tubular microvessels.123 The BBB model was temporarily disrupted by the use of mannitol.123 Interestingly vasculature permeability already decreased at a low concentration of mannitol after 60 min of treatment [Fig. 10(b)].
Moreover, Adriani et al. have assessed the possibility of using a microfluidic BBB model comprising a 3D assembled monolayer of hCMEC/D3, with rat astrocytes and neurons for compound screening.86 Their model served as a functional transport assay of monosodium glutamate, a potent neurotransmitter whose presence is highly regulated by the BBB. The 3D monolayer model prevented the monosodium glutamate added into the endothelial cell channel to pass through the barrier and activate the neurons cultured on the opposite side of the channel. To this purpose, they measured the neuronal activity by calcium imaging and c-Fos expression. A higher concentration of calcium was measured in neurons when the endothelial barrier was not present, compared to the 3D monolayer model [Fig. 10(c)]. Hence, the presence of the endothelial barrier was shown to restrict the passage of glutamate and the consequent increase in calcium in neurons.
A new modeling approach has come to light a few years ago based on the in vitro culture of brain spheroids and organoids.126 These are usually self-organized multicellular structures grown in low attachment conditions and showing a similar organization as the human brain. This emerging approach has been used for the screening of drug transport127 and could be potentially integrated with microfluidic devices including microvascular networks.
VIII. FUTURE PERSPECTIVES FOR BBB MICROFLUIDIC MODELS
Microfluidic devices for in vitro BBB modeling have the potential to make basic research more effective in the process of drug development for treating neurodegenerative diseases and brain cancers. The optimal BBB model should provide a valuable tool for studying the mechanistic aspects of drug transport and the biological outcomes of drug delivery in the presence of pathological processes affecting the BBB. However, before in vitro BBB models can be used for transport studies and other applications, they need to fulfill a number of requirements, namely provide reproducible permeability values for reference compounds, as well as express TJ proteins, BBB phenotypic transporters, and transcytotic activity.10
Additionally, the model should be able to mimic physiological and pathological states as well as recreate stress responses, such as the effects of ischemia, regional hypoxia, and nutritional deprivation. Microfluidic technologies could be combined with patient-derived primary or iPS cells115 from different genetic subpopulations or engineered with gene editing technologies (e.g., CRISPR) to obtain patient-specific disease models that facilitate drug discovery targeted to specific subpopulations.128 Microfluidic platforms are promising for the investigation of novel drug candidates, including drug bioactivation, drug clearance, and drug-drug interactions through induction/inhibition pathways.129
On the other hand, certain limitations need to be overcome before microfluidic models can be widely used in research laboratories. For example, performing long-term experiments (on the order of months) is not currently possible in microfluidic systems. A number of research studies on dose-dependent species-specific drug interactions need long-term treatment. Moreover, drug toxicity response might emerge a few weeks or months after the start of the treatment. Thus far, such experiments cannot be replicated in more complex and physiologically relevant microfluidic in vitro models, but only in 2D culture and in in vivo models. This is mainly caused by cell overgrowth and/or extracellular matrix (ECM) matrix breakdown when model complexity is increased in microfluidic systems.98,124 Additional limitations in microfluidic models arise from the use of PDMS in the micro-fabrication of the fluidic device. While PDMS is easy to use and has high optical clarity, gas permeability, and biocompatibility, it can absorb small organic compounds, which might alter results in drug treatment.130 Additional research is needed to develop new materials for device microfabrication with quality/cost benefits.
Moreover, microfluidic chips need to be designed such that connections with microfluidic pumps can be easily implemented to introduce physiologically relevant fluid flows into the device channels, thereby reproducing the dynamic in vivo microenvironment. This aspect of microfluidic technology is the aim of “next-generation” chips which would include sensors for parameter measurements (e.g., flow, pressure, temperature, pH, oxygen, glucose, lactate, TEER, and electrical conduction) and integrated microscopic and microfluorimetric imaging capabilities to monitor the system overtime.98,124 Another relevant point to be considered pertains to the preparation of ECM-mimetic non-immunogenic biomaterials as an interface between neural and endothelial cells, providing transport, mechanical and structural properties similar to those of natural basement membranes. Additionally, a “universal medium” with optimal composition for all the cells present in the brain (endothelial cells, pericytes, astrocytes, neurons, and microglia) would be necessary, providing essential components for neural differentiation.98
Finally, one of the main challenges of microfluidic technologies lies in their technical robustness and reliability for in vitro testing. Although there have been few recent successes demonstrating that microfluidic models can mimic specific organ-level functions,98 the implementation of reliable testing tools for drug transport screening to fully replace animal model testing remains to be achieved. In vitro microfluidic models have the potential to contribute to a reduction in the use of animal models for experiments, accelerating research for the discovery of new effective drugs and/or drug carriers.
IX. ENHANCING DRUG TRANSPORT ACROSS THE BBB VIA NANOCARRIER-MEDIATED DRUG DELIVERY
In vivo animal studies and in vitro models have highlighted the BBB as the major obstacle in drug transport to the brain, indicating that the design of more efficient brain-delivery systems is key to improve therapeutic outcomes of patients affected by CNS pathologies. Indeed, only 3%–5% of compounds with positive in vitro and in vivo results on neurological disease models have reached clinical applications, mostly because of their inability to cross the BBB in vivo.131 For instance the humanized monoclonal antibody Solanezumab, designed for the treatment of Alzheimer's disease, failed to demonstrate clinical benefit in phase-3 trials, possibly due to the drug not actually reaching its target.132,133 Similarly, proteasome inhibitors which have demonstrated a potent anti-tumor effect on brain cancer models in vitro offered limited clinical benefits due to their poor affinity with the BBB.134 These examples suggest that treatment of CNS pathologies requires new delivery systems rather than new drugs.
Nano-size drug delivery systems (DDSs) based on inorganic or polymer nanoparticles (NPs) have been proposed for this purpose in virtue of their small size, tunable shape, and ease of surface modification that can be used to facilitate transport across the BBB via both, transcellular and paracellular pathways. Table II shows the main types of NPs used to deliver drugs to the brain tissue, their composition, average size, advantages, and limitations.
TABLE II.
Main NP types tested in BBB models, their average sizes, main advantages, and limitations. Reproduced with permission from Veszelka et al., Acta Biol. (Szeged) 59, 157–168 (2015). Copyright 2015 Authors, licensed under Creative Commons Attribution 3.0 License.72,82,83,87,135
| Type of NP | Composition | Size | Advantage | Limitations |
|---|---|---|---|---|
| Liposomes | Aqueous core surrounded by phospholipid bilayers | Small (<100 nm), large (>100 nm), and multi- (>500 nm) lamellar | Encapsulation of both hydrophilic and hydrophobic drugs | Poor control over release of the drug and low stability during storage |
| Solid lipid NPs | Hydrophobic core surrounded by triglycerides, fatty acids, and waxes | 10–500 nm | Low toxicity, high drug loading capacity, and controlled release | Encapsulation of only hydrophobic drugs |
| Polymeric NPs | Natural or synthetic degradable polymers | 10–1000 nm | Stability against enzymatic metabolism | Some have toxic hydrolysis by-products |
| Metal NPs | Gold, and titanium or iron oxides | 1–100 nm | Gold and titanium NPs: inert, ultra-small size, absorb, and scatter near infra-red light Iron NPs: can be paramagnetic |
Gold and titanium NPs: controversy on carcinogenicity Iron NPs: may induce oxidative stress |
| Quantum dots | Colloidal semi-conductor nano-scale crystal | 2–50 nm | Fluorescent targeting and imaging, long-term visualization, and extremely high stability to photobleaching | Cytotoxic in high concentrations |
While inorganic NPs offer better control of size and shape, their intrinsic toxicity and poor biodegradability have limited their application to imaging or theranostic purposes. On the other hand, polymeric NPs have been extensively investigated to deliver drugs to the CNS. Although size and shape control is more challenging, these platforms offer high versatility in terms of tunable polymer composition, drug encapsulation, and surface functionalization. Depending on the polymer of choice, polymeric NPs can encapsulate drugs of different molecular weights, physical properties and water solubility.58 Moreover, several surface modification strategies can be implemented to functionalize the particle surface with ligands that selectively interact with receptors over-expressed by BBB cells, resulting in enhanced CNS accumulation.
A. Surface-modified nanoparticles for targeted drug delivery to the CNS
NPs can be used to deliver drugs across the BBB in two main ways: (i) passive accumulation and (ii) active interaction with BBB cells.136 Passive targeting of the CNS is based on higher concentration gradients at the BBB that are achieved by enhancing the NP concentration in plasma. This implies that particles must circulate in the blood stream long enough to result in enhanced crossing of the BBB. As NPs are rapidly taken up by the Mononuclear Phagocyte System (MPS) and, consequently, cleared through the kidneys and the liver, MPS capture must be delayed and a “stealth” particle design has to be adopted. Typically, this is achieved by covering the particle surface with a flexible, hydrophilic layer of poly(ethylene glycol) (PEG) that is well known to delay clearance, extend circulation time and, consequently, enhance the chances of BBB passage.137 It has been demonstrated that particles with size ranging between 50 nm and 100 nm are passively taken up by BBB cells, resulting in favored transcellular transport. Indeed, virus-sized iron oxide nanoparticles (Ferumoxtran-10) have been successfully used to image CNS lesions in patients.138 On the other hand, Chertok et al. have shown that inorganic iron oxide NPs with a nominal size of about 100 nm could successfully accumulate into orthotropic 9L gliosarcoma-bearing rats only if an external magnetic guidance was used to force cranial accumulation of the particles. Without application of the external magnetic field, no contrast-enhancement (indicative of particle accumulation in the brain) was observed, suggesting that 100 nm may be the threshold size for passive BBB crossing, and that particles with larger sizes may require surface-modifications for active-transport.139
Active transport across the BBB involves receptor-mediated endocytosis or transcytosis as mentioned above. It requires surface-modification of NPs with ligands, antibodies, or small molecules that are typically a target for receptors over-expressed by BBB cells. For instance, Meng et al. have proposed chlorotoxin-conjugated iron oxide NPs (SPIOs) to actively target human and rat glioma models, as the glioma-specific chloride ion channel is sensitive to chlorotoxin.140 They showed that MRI contrast was strongly enhanced when conjugated platforms were used, as compared to the unfunctionalized SPIOs.141
The most common target used to enhance particle accumulation in the brain is the transferrin receptor (TfR), a transmembrane glycoprotein extensively expressed by endothelial cells in the BBB and highly over-expressed by brain cancer cells. Several NP platforms functionalized with transferrin (Tf), lactoferrin, TfR-specific antibodies (TfR-Ab) or peptides have been designed to target TfR. Hu et al. have designed PEG- poly-lactide (PLA) nanoparticles for brain targeting by using lactoferrin as a targeting ligand, coupled to the maleimide groups on PEG chains. After intra venous (i.v.) administration they showed a 3-fold increase in brain delivery of the fluorescent dye coumarin-6 with targeted nanoparticles as compared to their un-functionalized counterparts.142 Loureiro et al. designed TfR-Ab (OX26) conjugated poly(lactic-co-glycolic acid) (PLGA)-PEG NPs for brain delivery of iAβ5, a peptide for the treatment of Alzheimer's disease. Although an in vivo proof of concept was not provided, the authors showed significant cellular uptake in a porcine brain capillary endothelial BBB model, indicating that TfR-Ab has strong affinity for the BBB endothelial cells.143 Indeed, other studies highlighted that the use of TfR-Ab may result in too strong ligand-receptor interaction, causing the particles to remain trapped at the brain capillary endothelium without reaching the brain parenchyma.144,145 These considerations suggest careful selection of ligands as well as of the functionalization strategies to achieve effective BBB by passing.
Peptides able to selectively recognize BBB endothelial cells can also be used for active particle accumulation in the CNS. Dal Magro et al. used ApoE-functionalized solid lipid nanoparticles (SLN) for brain targeting after pulmonary administration. They showed significantly higher brain SLN accumulation for targeted nanoparticles delivered via the pulmonary route as compared to untargeted particles or to targeted particles delivered intra-venous (i.v.) or intra-peritoneal (i.p.).146 This work seems to suggest that the delivery route also plays a role in increasing particle accumulation in the brain. By reducing the circulation time of the NPs in the blood, this alternative delivery strategy aims at reducing particles uptake by the MPS, increasing the number of particles interacting with the BBB.147
Thus far, the majority of the available information on NP design including the tailoring of their surface composition has been derived from studies in animal models. This approach has some limitations, particularly regarding the transferability of the obtained results to the human case. Preliminary tests in in vitro models of the human BBB would allow for a more specific design of NPs, taking into account their applications in humans. On the other hand, testing in animal models still stands as a fundamental complementary step to test the NPs in the environment of a complex organism and get insights into immune response to NPs, NP toxicity, targeting, and accumulation in different tissues/organs, as well as to select the preferred delivery route for NPs.
B. Nanoparticle testing in in vitro BBB models
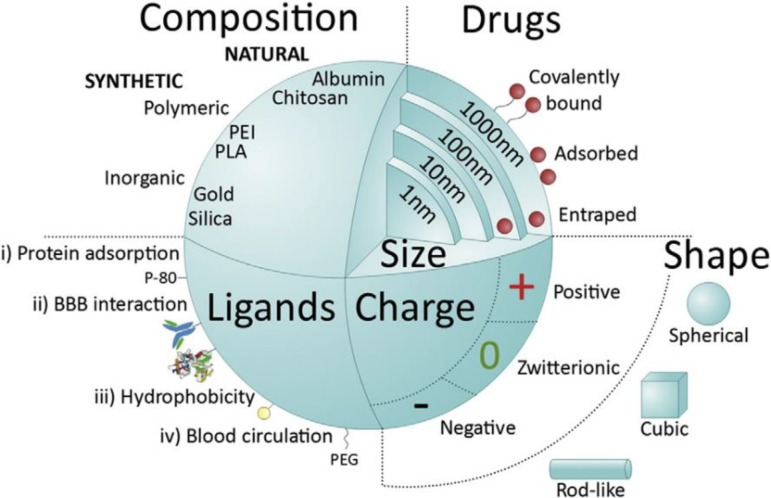
While in vitro BBB models have been used extensively for drug transport studies, drug-loaded NPs have only been recently integrated into these platforms, in an attempt to determine their efficacy and safety for use in humans. In this regard, microfluidic models allow for high-throughput screening of NP drugs to determine optimal chemical composition, size, and shape with respect to different applications (Fig. 11).58
FIG. 11.
Major NP features influencing systemic delivery across the BBB. Reproduced with permission from Saraiva et al., J. Controlled Release 235, 34–47 (2016). Copyright 2016 Elsevier.58
For instance, the surface composition of NPs affects their interaction with blood components, their duration of circulation in the vasculature, and their likelihood of barrier penetration to reach target cells/tissues. NP size is also a fundamental parameter, affecting cell uptake and intracellular localization of NPs,148 e.g., NPs with 50 nm size have been found to be optimal for effective internalization by the cells through receptor-mediated endocytosis.149,150 Additionally, in vitro models are also effective tools to understand the mechanisms by which NPs cross the BBB, in order to tailor NP properties for enhanced delivery to the brain. Hence, in accordance with the 3Rs principle, in vitro models play a key role in reducing the number of animal experiments needed to optimize NP design.
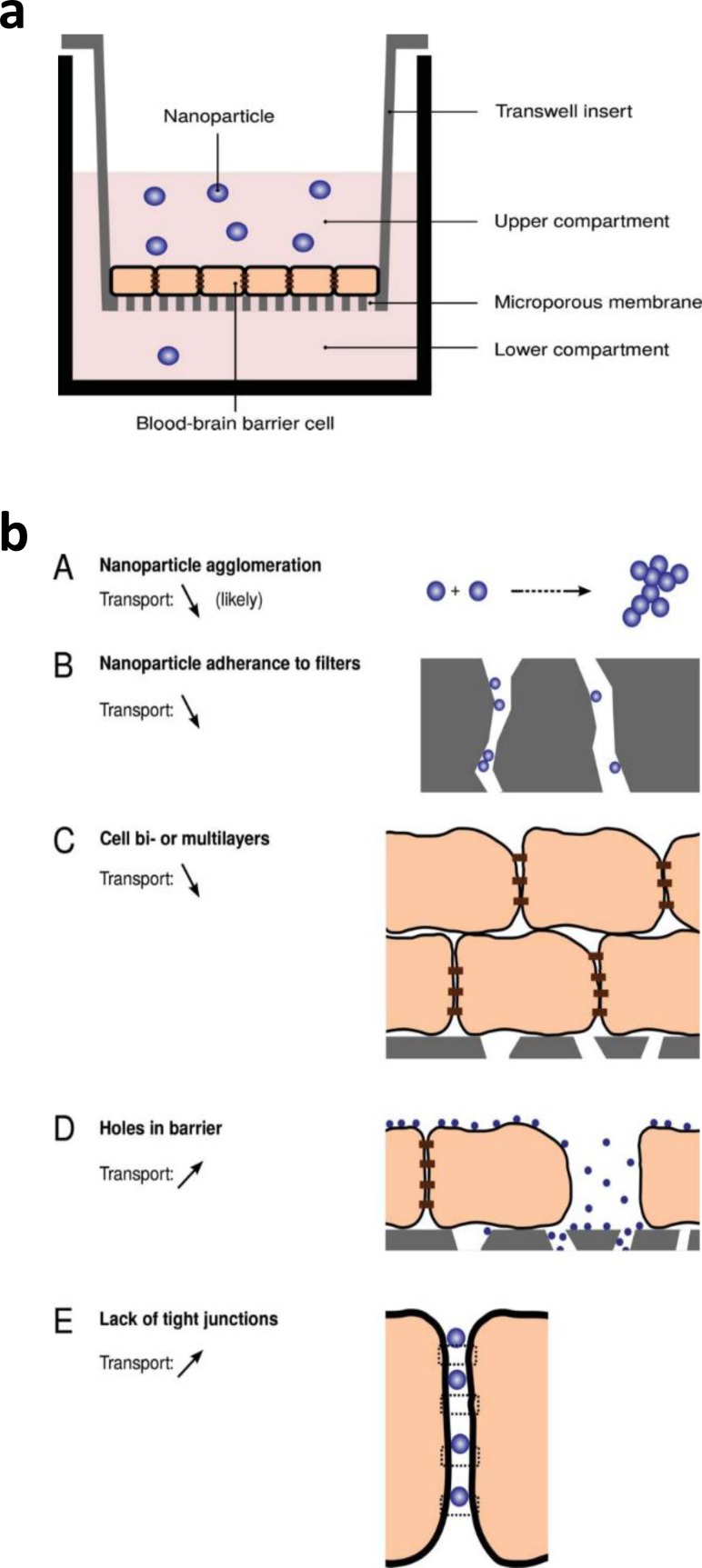
While intrinsically simple and well-established for the study of drug delivery, transwell BBB models do not always constitute an optimal platform for the study of NP delivery [Fig. 12(a)]. In fact, NPs may self-agglomerate or adhere to the transwell membrane pore walls, and imperfections in the BBB model (e.g., multi-layer structure, presence of holes, or lack of TJs) might render the results imprecise [Fig. 12(b)].150,151 Despite some of the issues surrounding transwell assays for NP delivery, these models have been widely used for NP testing, employing animal or human cells (Table III).
FIG. 12.
(a). Schematic of a BBB transwell model used for the testing of NP permeability. (b) Schematic representation of potential issues arising when using transwell BBB models for NP transport screening by Åberg, Tissue Barriers 4, e1143545 (2016). Copyright 2016 Authors, licensed under Creative Commons Attribution 3.0 License.151
TABLE III.
Relevant examples on the testing of NPs in BBB transwell models in the last decade (2008–2018). Adapted from Veszelka et al., Acta Biol. (Szeged) 59, 157–168 (2015). Copyright 2015 Authors, licensed under a Creative Commons Attribution 3.0 License.135 Abbreviations: BDNF, bone derived neurotrophic factor; BSA, bovine serum albumin; CRM197, a ligand of diphtheria toxin receptor; mAb, monoclonal antibody; MMA-SPM, methylmethacrylate–sulfopropylmethacrylate; MMP9, matrix metalloproteinase-9; N.D., no data; PBCA, poly(butylcyanoacrylate); PEG, polyethylene glycol; PLA, poly-lactide; PLGA, poly(lactic-co-glycolic acid); PVP, polyvinylpirrolidone; Qdots, quantum dots; RMP-7 (Cereport), bradykinin type II receptor agonist; MDCK, Madin–Darby canine kidney.
| NP type | Composition | Targeting ligand or coating | Cargo | NP size (nm) | EC type | Co-cultured cells | Result | References |
|---|---|---|---|---|---|---|---|---|
| Lipid NPs | Fluorescent magneto-liposomes | Transferrin | No | 130 | Primary human | Human astroglia | Increased NP permeability | Ding et al.152 |
| Solid lipid NPs | Solid lipid | Anti-insulin receptor mAb | Carmustine | 100-450 | Primary human | Human astroglia | Increased NP uptake and permeability | Kuo et al.153 |
| Metal NPs | Fe3O4 (magnetic) | No | BDNF | 60 | Primary human | Human astroglia | Magnetically guided NP permeability | Pilakka et al.154 |
| Metal NPs | Gold | Peptide targeting transferrin receptor | β-sheet breaker peptide | 15 | Primary bovine | Rat astrocytes | Increased NP permeability | Prades et al.155 |
| Metal NPs | Gold | PEG, transferrin, albumin | No | 5, 12, 25 | Human cerebral micro-vessel ECs | … | NP internalisation in lysosomes; rare transcytosis | Ye et al.156 |
| Polymer NPs | PLGA | Tween-20, BSA, Transferrin | Fluorescent dye | 63–90 | Primary bovine | Rat astroglia | Increased NP endocytosis | Chang et al.157 |
| Polymer NPs | PBCA | Ligand of diphtheria toxin receptor (CRM197) | Zidovudine | 87, 163, 195 | Primary human | Human astroglia | Increased NP uptake and permeability | Kuo et al.158 |
| Polymer NPs | MMA-SPM | Bradykinin type II receptor agonist (RMP-7) | Stavudine DelavirdineSaquinavir | 48, 13, 8 | Primary human | Human astroglia | Increased NP uptake and permeability | Kuo et al.159 |
| Polymer NPs | PEG-PLA | PVP coating | Curcumin | <100 | MDCK cells | … | Increased drug permeability | Cheng et al.160 |
| Polymer NPs | PLGA | … | Metoclopramid | N.D. | MDCK cells | … | Increased drug permeability | Nikandish et al.161 |
| Inorganic NPs | TiO2 | No | No | 25 | Primary rat | Rat astrocytes | Np uptake, transport and cellular toxicity. | Brun et al.162 |
| Inorganic NPs | TiO2 | No | No | 3–5 | Human cerebral micro-vessel ECs | … | NP internalization in lysosomes; rare transcytosis | Ye et al.156 |
| Inorganic NPs | Silica NPs | … | … | 50, 100, 200 | Human cerebral micro-vessel ECs | … | NP internalization in lysosomes; rare transcytosis | Ye et al.156 |
| Inorganic NPs | Silica NPs | No | No | 30, 100, 400 | Primary rat ECs | Rat astroglia and rat pericytes | Size dependent permeability | Hanada et al.163 |
| Inorganic NPs | Silica matrix magnetic core/green fluorescence | Prion or PEI (cationic) coating; surface hydroxyl groups (uncoated) | No | 500 | Human cerebral micro-vessel ECs | … | Internalization mechanism dependent on the surface composition | Georgieva et al.164 |
| Inorganic NPs | Mesoporous silica NPs | Bare or coated with PEG-PEI | No | 152–222 | RBE4 rat brain ECs and MDCK cells | … | Coating increased NP uptake | Baghirov et al.165 |
| QDots | QDots | No | siRNA for MMP9 | 15–20 | Primary human | Human astroglia | NP uptake and MMP9 gene silencing | Bonoiu et al.166 |
| QDots | QDots | Transferrin | No | Length: 26 width:6.5 | Primary human | Human astroglia | Concentration- and time-depending NP crossing | Xu et al.167 |
| QDots | QDots | Amino-, caboxy-, PEGylated Qdots | No | 30 | Primary rat ECs | Rat astroglia and rat pericytes | Amino-groups dependent permeability | Hanada et al.163 |
As an example, Hanada et al.163 developed a rapid, easy, and reproducible in vitro assay to screen the ability of NPs with different size and surface charge to cross a commercially available rat BBB model (RBT-24H, BBB Kit™, purchased from PharmaCo-Cell Co., Nagasaki, Japan) composed of primary rat brain endothelial cells, pericytes, and astrocytes. The testing of fluorescent silica NPs with different sizes (30, 100, 400 nm, and microparticles) and at 0.1–1.0 mg/ml concentration showed that permeability was significantly higher for NPs with 30 nm size (3.56 ± 1.62 × 10−6 cm/s). On the other hand, experiments using Qdots® NPs with optimal size (approximately 30 nm) and surface cationic, anionic, and neutral groups (amino-, carboxyl-, and PEGylated-Qdots, respectively) showed that amino-functionalised NPs more efficiently crossed the BBB model. This behaviour was attributed to the interaction of amino-functionalities with anionic phospholipids on the cell surface, leading to NP paracellular transport. However, the carboxyl-functionalised NPs mainly underwent endocytosis, slowing down their delivery to the basolateral compartment of the model. Finally, the study by Hanada et al.163 also evidenced that the use of serum-containing medium in in vitro tests may reduce permeability values, due to the interaction of proteins in the medium with the NP surface, possibly causing NP agglomeration.
Cheng et al.160 have analyzed the effectiveness of curcumin-loaded PVP-coated PEG-PLA NPs in crossing the BBB in a MDCK cell monolayer transwell model, followed by in vivo trials in Alzheimer's Disease Tg2576 Mice. The permeability of curcumin reached 1.8 × 10−6 cm/s from 15 to 45 min, then decreased and settled to a constant value from 75 min onward. The permeability of tetrahydrocurcumin (THC), a curcumin metabolite, was also measured showing a linear increase as a function of time. These findings indicated a transport mechanism based on transcytosis and/or endocytosis: curcumin-loaded NPs penetrated into the cells, the drug was released, partially metabolized, and then both curcumin and THC were delivered into the basal well. According to another hypothesis, curcumin was released from NPs in the apical medium, close to the cell membrane and then diffused into the cells. Regardless of the involved mechanism, curcumin encapsulation into NPs was found to improve its permeability to the BBB.168 Indeed, the measured permeability was in a range suggesting drug ability to penetrate the BBB in vivo.169
Although several in vitro studies in BBB transwell models have been followed by in vivo trials in animal models (e.g., Cheng et al.;160 Mc Carthy et al.170) correlations between in vitro and in vivo results are not available for drug-loaded nanocarriers. Additionally, dynamic microfluidic platforms and organ-on-chips models represent optimal tools for reliable testing of drugs and drug-loaded NPs, however there is no published data currently available on their use to assess the transport of NPs through the BBB. These observations underline that research in the field of biotransport of NPs in BBB models is still at the early phases and needs intense investigations in the next years. It is important to underline that in vitro experiments in BBB models using NPs should also evaluate cellular toxicity and the targeting ability of the NPs in an in vivo-like microenvironment.
X. CONCLUDING REMARKS
The BBB is characterized by unique features responsible for the maintenance of brain homeostasis. The presence of tight and adherens junctions regulates the transport of solutes across the barrier and molecular trafficking usually occurs through transcellular diffusion or active transport with carriers. A fundamental understanding of these transport mechanisms is needed for the development of drugs and solutes able to cross the BBB. While BBB transwell models continue to be widely used for the testing of drugs and drug-loaded NPs, microfluidic 3D BBB models are now regarded as the state-of-the-art in vivo-like platforms for reliable drug delivery testing.
Although the BBB is the main obstacle for therapeutics aimed at targeting neurological and brain degenerative diseases, as well as cancers, the field of BBB modeling has received less attention than that of brain disease treatment. Nevertheless, poor outcomes of many clinical trials for brain disease treatment should shift the focus back to the implementation of more physiologically relevant in vitro and in vivo BBB models, as well new strategies for drug release.171 Although in vitro platforms do not fully replicate the in vivo condition, microfluidic models are powerful and flexible tools to study transport across the BBB in normal and pathological states. Microfluidic platforms offer the possibility of reconstituting human organ-level physiological functions, clinically relevant disease phenotypes, and pharmacological responses that arise from structural and functional integration of multiple cell types.
The main advantage of microfluidic devices lies in their precise control of culture parameters allowing investigations that would be difficult, or even impossible, to be carried out in vivo. Unlike 2D culture systems, microfluidic models have the potential to serve as a new enabling platform to identify and validate the efficacy, toxicology, and safety of drugs toward potential targets, evaluate brain uptake, and assess permeability early in the drug discovery pipeline to increase success in clinical trials. This characteristic may allow more comprehensive and accurate predictions of complex drug responses in vivo. Fluid motions and cell culture substrates can also be controlled to reproduce various types of mechanical cues induced by physiological flow or pressure (for example, blood flow and interstitial flow) including shear stress, tension, and compression.
An integrative use of in vitro BBB models and in vivo techniques (together with advances in design of innovative nanocarriers and precise and personalized medicine) is likely to be of key importance in the development of CNS therapeutics with enhanced activity and improved BBB permeability. Given the complexities of organ function and regulatory requirements, it is unlikely that BBB microfluidic models will replace animal testing anytime soon to see a widespread use of this technology in basic and clinical research, however, it may be possible to progressively replace one animal-based assay at a time. Rigorous validation of this technology using not only animal data but, more importantly, the results of clinical trials is required to determine whether human in vitro models accurately represent human-relevant physiology and show predictive capability across broad drug classes and clinical outcomes.
ACKNOWLEDGMENTS
C.H. and R.D.K. acknowledge support from the National Cancer Institute (U01 CA202177). M.C. acknowledges support from Ermenegildo Zegna Founder's scholarship and the MIT-POLITO grant (BIOMODE-Compagnia di San Paolo) under the joint “Doctorate of Bioengineering and Medical-Surgical Sciences” of the University of Turin and Politecnico di Torino. C.M. acknowledges support from the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie Grant Agreement No 658665. M.C., V.C., and R.D.K. also acknowledge support from the NANOCAB project between MIT and POLITO.
Contributor Information
Valeria Chiono, Email: .
Roger D. Kamm, Email: .
References
- 1. Abbott N. J., Patabendige A. A. K., Dolman D. E. M., Yusof S. R., and Begley D. J., “Structure and function of the blood–brain barrier,” Neurobiol. Dis. 37, 13–25 (2010). 10.1016/j.nbd.2009.07.030 [DOI] [PubMed] [Google Scholar]
- 2. Barar J., Rafi M. A., Pourseif M. M., and Omidi Y., “Blood-brain barrier transport machineries and targeted therapy of brain diseases,” Bioimpacts 6, 225–248 (2016). 10.15171/bi.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cecchelli R. et al. , “Modelling of the blood-brain barrier in drug discovery and development,” Nat. Rev. Drug Discovery 6, 650–61 (2007). 10.1038/nrd2368 [DOI] [PubMed] [Google Scholar]
- 4. Abbott N. J., Rönnbäck L., and Hansson E., “Astrocyte-endothelial interactions at the blood-brain barrier,” Nat. Rev. Neurosci. 7, 41–53 (2006). 10.1038/nrn1824 [DOI] [PubMed] [Google Scholar]
- 5.Disorders, Brain Foundation.
- 6. Gaugler J., James B., Johnson T., Scholz K., and Weuve J., “2016 Alzheimer's disease facts and figures,” Alzheimers Dementia 12, 459–509 (2016). 10.1016/j.jalz.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 7. Rossi A. et al. , “Projection of the prevalence of Parkinson's disease in the coming decades: Revisited,” Mov. Disord. 33, 156–159 (2017). 10.1002/mds.27063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D'Andrea G., Palombi L., Minniti G., Pesce A., and Marchetti P., “Brain metastases: Surgical treatment and overall survival,” World Neurosurg. 97, 169–177 (2017). 10.1016/j.wneu.2016.09.054 [DOI] [PubMed] [Google Scholar]
- 9. Löscher W. and Potschka H., “Blood-brain barrier active efflux transporters: ATP-binding cassette gene family,” NeuroRX 2, 86–98 (2005). 10.1602/neurorx.2.1.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Helm M. W., van der Meer A. D., Eijkel J. C. T., van den Berg A., and Segerink L. I., “Microfluidic organ-on-chip technology for blood-brain barrier research,” Tissue Barriers 4, e1142493 (2016). 10.1080/21688370.2016.1142493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sohet F. and Daneman R., “Genetic mouse models to study blood-brain barrier development and function,” Fluids Barriers CNS 10, 1 (2013). 10.1186/2045-8118-10-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mak I. W. Y., Evaniew N., and Ghert M., “Lost in translation: Animal models and clinical trials in cancer treatment,” Am. J. Transl. Res. 6, 114–118 (2014). [PMC free article] [PubMed] [Google Scholar]
- 13. Chesselet M.-F. and Carmichael S. T., “Animal models of neurological disorders,” Neurotherapeutics 9, 241–244 (2012). 10.1007/s13311-012-0118-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pamies D., Hartung T., and Hogberg H. T., “Biological and medical applications of a brain-on-a-chip,” Exp. Biol. Med. 239, 1096–1107 (2014). 10.1177/1535370214537738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pardridge W. M., “Blood-brain barrier delivery,” Drug Discovery Today 12, 54–61 (2007). 10.1016/j.drudis.2006.10.013 [DOI] [PubMed] [Google Scholar]
- 16. Chen Y. and Liu L., “Modern methods for delivery of drugs across the blood–brain barrier,” Adv. Drug Delivery Rev. 64, 640–665 (2012). 10.1016/j.addr.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 17. Jo D. H., Kim J. H., Heo J.-I., J. H., Kim , and Cho C.-H., “Interaction between pericytes and endothelial cells leads to formation of tight junction in hyaloid vessels,” Mol. Cells 36, 465–471 (2013). 10.1007/s10059-013-0228-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alvarez J. I. et al. , “The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence,” Science 334, 1727–1731 (2011). 10.1126/science.1206936 [DOI] [PubMed] [Google Scholar]
- 19. Lien C. F. et al. , “Absence of glial α-dystrobrevin causes abnormalities of the blood-brain barrier and progressive brain edema,” J. Biol. Chem. 287, 41374–41385 (2012). 10.1074/jbc.M112.400044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alvarez J. I., Katayama T., and Prat A., “Glial influence on the blood brain barrier,” Glia 61, 1939–1958 (2013). 10.1002/glia.22575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luissint A.-C., Artus C., Glacial F., Ganeshamoorthy K., and Couraud P.-O., “Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation,” Fluids Barriers CNS 9, 23 (2012). 10.1186/2045-8118-9-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murray I. R. et al. , “Skeletal and cardiac muscle pericytes: Functions and therapeutic potential,” Pharmacol. Ther. 171, 65–74 (2017). 10.1016/j.pharmthera.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chintalgattu V. et al. , “Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity,” Sci. Transl. Med. 5, 187ra69 (2013). 10.1126/scitranslmed.3005066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park D. Y. et al. , “Plastic roles of pericytes in the blood–retinal barrier,” Nat. Commun. 8, 15296 (2017). 10.1038/ncomms15296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alimperti A. et al. , “Three-dimensional biomimetic vascular model reveals a RhoA, Rac1, and N-cadherin balance in mural cell–endothelial cell-regulated barrier function,” Proc. Natl. Acad. Sci. 114, 8758–8763 (2017). 10.1073/pnas.1618333114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cardoso F. L., Brites D., and Brito M. A., “Looking at the blood–brain barrier: Molecular anatomy and possible investigation approaches,” Brain Res. Rev. 64, 328–363 (2010). 10.1016/j.brainresrev.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 27. Balkovetz D. F., “Tight junction claudins and the kidney in sickness and in health,” Biochim. Biophys. Acta 1788, 858–863 (2009). 10.1016/j.bbamem.2008.07.004 [DOI] [PubMed] [Google Scholar]
- 28. Kirschner N. and Brandner J. M., “Barriers and more: Functions of tight junction proteins in the skin,” Ann. N. Y. Acad. Sci. 1257, 158–166 (2012). 10.1111/j.1749-6632.2012.06554.x [DOI] [PubMed] [Google Scholar]
- 29. Tokes A.-M., Schaff Z., Szasz A. M., and Kulka J., “The distribution of tight junctions and junctional proteins in the human body,” in ( Springer, Dordrecht, 2013), pp. 29–64. [Google Scholar]
- 30., edited by Najman A. ( InTech, 2012). [Google Scholar]
- 31. Bauer H.-C., Krizbai I. A., Bauer H., and Traweger A., “‘You Shall Not Pass’—Tight junctions of the blood brain barrier,” Front. Neurosci. 8, 392 (2014). 10.3389/fnins.2014.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bardet C. et al. , “Claudin loss-of-function disrupts tight junctions and impairs amelogenesis,” Front. Physiol. 8, 326 (2017). 10.3389/fphys.2017.00326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ebnet K., “Junctional adhesion molecules (JAMs): Cell adhesion receptors with pleiotropic functions in cell physiology and development,” Physiol. Rev. 97, 1529–1554 (2017). 10.1152/physrev.00004.2017 [DOI] [PubMed] [Google Scholar]
- 34. Campbell H. K., Maiers J. L., and DeMali K. A., “Interplay between tight junctions and adherens junctions,” Exp. Cell Res. 358, 39–44 (2017). 10.1016/j.yexcr.2017.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenberg G. A., “Cerebrospinal fluid: Formation, absorption, markers, and relationship to blood–brain barrier,” in , 2nd ed., edited by Caplan L. R. et al. ( Academic Press, 2017), Chap. 4, pp. 25–31. [Google Scholar]
- 36. Tietz A. and Engelhardt B., “Brain barriers: {{crosstalk}} between complex tight junctions and adherens junctions,” J. Cell Biol. 209, 493–506 (2015). 10.1083/jcb.201412147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dejana E., Tournier-Lasserve E., and Weinstein B. M., “The control of vascular integrity by endothelial cell junctions: Molecular basis and pathological implications,” Dev. Cell 16, 209–221 (2009). 10.1016/j.devcel.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 38. Luo Y. and Radice G. L., “N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis,” J. Cell Biol. 169, 29–34 (2005). 10.1083/jcb.200411127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Holzbaur E. L. F., Ligon L. A., Tokito M., and Karki S., “Dynein binds to β-catenin and may tether microtubules at adherens junctions,” Nat. Cell Biol. 3, 913 (2001). 10.1038/ncb1001-913 [DOI] [PubMed] [Google Scholar]
- 40. Fernández-Martín L. et al. , “Crosstalk between reticular adherens junctions and platelet endothelial cell adhesion molecule-1 regulates endothelial barrier function,” Arterioscler. Thromb. Vasc. Biol. 32, e90–e102 (2012). 10.1161/ATVBAHA.112.252080 [DOI] [PubMed] [Google Scholar]
- 41. Derada Troletti C., de Goede P., Kamermans A., and de Vries H. E., “Molecular alterations of the blood–brain barrier under inflammatory conditions: The role of endothelial to mesenchymal transition,” Biochim. Biophys. Acta 1862, 452–460 (2016). 10.1016/j.bbadis.2015.10.010 [DOI] [PubMed] [Google Scholar]
- 42. Rahman A., Tihanyi K., Choudhary I., and Vastag M., ( Bentham Science Publishers, 2011). [Google Scholar]
- 43. Karanth H. and Rayasa M., “Nanotechnology in Brain Targeting,” Int. J. Pharmaceutical Sci. Nanotech. 1(1), 9–24 (2018). [Google Scholar]
- 44. Banks W. A., “Characteristics of compounds that cross the blood-brain barrier,” BMC Neurol. 9, S3 (2009). 10.1186/1471-2377-9-S1-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arranz-Gibert P. et al. , “Lipid bilayer crossing—The gate of symmetry. water-soluble phenylproline-based blood-brain barrier shuttles,” J. Am. Chem. Soc. 137, 7357–7364 (2015). 10.1021/jacs.5b02050 [DOI] [PubMed] [Google Scholar]
- 46. Abbott N. J., “Blood–brain barrier structure and function and the challenges for CNS drug delivery,” J. Inherited Metab. Dis. 36, 437–449 (2013). 10.1007/s10545-013-9608-0 [DOI] [PubMed] [Google Scholar]
- 47. Fischer H., Gottschlich R., and Seelig A., “Blood-brain barrier permeation: Molecular parameters governing passive diffusion,” J. Membr. Biol. 165, 201–211 (1998). 10.1007/s002329900434 [DOI] [PubMed] [Google Scholar]
- 48. Lipinski C. A., Lombardo F., Dominy B. W., and Feeney P. J., “Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings,” Adv. Drug Delivery Rev. 64, 4–17 (2012). 10.1016/j.addr.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 49. Dahlin A. et al. , “Gene expression profiling of transporters in the solute carrier and ATP-binding cassette superfamilies in human eye substructures,” Mol. Pharm. 10, 650–663 (2013). 10.1021/mp300429e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu W., Dnyanmote A. V., and Nigam S. K., “Remote communication through solute carriers and ATP binding cassette drug transporter pathways: An update on the remote sensing and signaling hypothesis,” Mol. Pharmacol. 79, 795–805 (2011). 10.1124/mol.110.070607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nałęcz K. A., “Solute carriers in the blood–brain barier: Safety in abundance,” Neurochem. Res. 42, 795–809 (2017). 10.1007/s11064-016-2030-x [DOI] [PubMed] [Google Scholar]
- 52. Seelig A., “The role of size and charge for blood–brain barrier permeation of drugs and fatty acids,” J. Mol. Neurosci. 33, 32–41 (2007). 10.1007/s12031-007-0055-y [DOI] [PubMed] [Google Scholar]
- 53. Schinkel A. H., “P-Glycoprotein, a gatekeeper in the blood-brain barrier,” Adv. Drug Delivery Rev. 36, 179–194 (1999). 10.1016/S0169-409X(98)00085-4 [DOI] [PubMed] [Google Scholar]
- 54. Ni Z., Bikadi Z., Rosenberg M. F., and Mao Q., “Structure and function of the human breast cancer resistance protein (BCRP/ABCG2),” Curr. Drug Metab. 11, 603–617 (2010). 10.2174/138920010792927325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smith M. W. and Gumbleton M., “Endocytosis at the blood–brain barrier: From basic understanding to drug delivery strategies,” J. Drug Target. 14, 191–214 (2006). 10.1080/10611860600650086 [DOI] [PubMed] [Google Scholar]
- 56. Jones A. R. and Shusta E. V., “Blood-brain barrier transport of therapeutics via receptor-mediation,” Pharm. Res. 24, 1759–1771 (2007). 10.1007/s11095-007-9379-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hervé F., Ghinea N., and Scherrmann J.-M., “CNS delivery via adsorptive transcytosis,” AAPS J. 10, 455–472 (2008). 10.1208/s12248-008-9055-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saraiva C. et al. , “Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases,” J. Controlled Release 235, 34–47 (2016). 10.1016/j.jconrel.2016.05.044 [DOI] [PubMed] [Google Scholar]
- 59. Georgieva J. V., Hoekstra D., and Zuhorn I. S., “Smuggling drugs into the brain: An overview of ligands targeting transcytosis for drug delivery across the blood-brain barrier,” Pharmaceutics 6, 557–583 (2014). 10.3390/pharmaceutics6040557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wolburg H., Wolburg-Buchholz K., and Engelhardt B., “Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact,” Acta Neuropathol. 109, 181–190 (2005). 10.1007/s00401-004-0928-x [DOI] [PubMed] [Google Scholar]
- 61. Pieper C., Pieloch P., and Galla H. J., “Pericytes support neutrophil transmigration via interleukin-8 across a porcine co-culture model of the blood-brain barrier,” Brain Res. 1524, 1–11 (2013). 10.1016/j.brainres.2013.05.047 [DOI] [PubMed] [Google Scholar]
- 62. Konsman J. P., Drukarch B., and Van Dam A.-M., “(Peri)vascular production and action of pro-inflammatory cytokines in brain pathology,” Clin. Sci. 112, 1–25 (2007). 10.1042/CS20060043 [DOI] [PubMed] [Google Scholar]
- 63. Takeshita Y. and Ransohoff R. M., “Inflammatory cell trafficking across the blood-brain barrier (BBB): Chemokine regulation and in vitro models,” Immunol. Rev. 248, 228–239 (2012). 10.1111/j.1600-065X.2012.01127.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kienast Y. et al. , “Real-time imaging reveals the single steps of brain metastasis formation,” Nat. Med. 16, 116–122 (2010). 10.1038/nm.2072 [DOI] [PubMed] [Google Scholar]
- 65. Weidle U. H., Birzele F., Kollmorgen G., and Rüger R., “Dissection of the process of brain metastasis reveals targets and mechanisms for molecular-based intervention,” Cancer Genomics Proteomics 13, 245–258 (2016). [PubMed] [Google Scholar]
- 66. Fazakas C. et al. , “Transmigration of melanoma cells through the blood-brain barrier: Role of endothelial tight junctions and melanoma-released serine proteases,” PLoS One 6, e20758 (2011). 10.1371/journal.pone.0020758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rolland Y., Demeule M., Fenart L., and Béliveau R., “Inhibition of melanoma brain metastasis by targeting melanotransferrin at the cell surface,” Pigm. Cell Melanoma Res. 22, 86–98 (2009). 10.1111/j.1755-148X.2008.00525.x [DOI] [PubMed] [Google Scholar]
- 68. Boussommier-Calleja A., Li R., Chen M. B., Wong S. C., and Kamm R. D., “Microfluidics: A new tool for modeling cancer–immune interactions,” Trends Cancer 2, 6–19 (2016). 10.1016/j.trecan.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xu H. et al. , “A dynamic in vivo-like organotypic blood-brain barrier model to probe metastatic brain tumors,” Sci. Rep. 6, 36670 (2016). 10.1038/srep36670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lundquist S. et al. , “Prediction of drug transport through the blood-brain barrier in vivo: A comparison between two in vitro cell models,” Pharm. Res. 19, 976–981 (2002). 10.1023/A:1016462205267 [DOI] [PubMed] [Google Scholar]
- 71. Wolff A., Antfolk M., Brodin B., and Tenje M., “In vitro blood–brain barrier models—An overview of established models and new microfluidic approaches,” J. Pharm. Sci. 104, 2727–2746 (2015). 10.1002/jps.24329 [DOI] [PubMed] [Google Scholar]
- 72. Czupalla C. J., Liebner S., and Devraj K., “In vitro models of the blood–brain barrier,” in ( Humana Press, New York, NY, 2014), pp. 415–437. [DOI] [PubMed] [Google Scholar]
- 73. Helms H. C. et al. , “In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use,” J. Cereb. Blood Flow Metab. 36, 862 (2016). 10.1177/0271678X16630991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang Y. et al. , “In vitro model of the blood-brain barrier established by co-culture of primary cerebral microvascular endothelial and astrocyte cells,” Neural Regen. Res. 10, 2011–2017 (2015). 10.4103/1673-5374.172320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Siddharthan V., Kim Y. V., Liu S., and Kim K. S., “Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells,” Brain Res. 1147, 39–50 (2007). 10.1016/j.brainres.2007.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hatherell K., Couraud P. O., Romero I. A., Weksler B., and Pilkington G. J., “Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models,” J. Neurosci. Methods 199, 223–229 (2011). 10.1016/j.jneumeth.2011.05.012 [DOI] [PubMed] [Google Scholar]
- 77. Gibbons H. M. and Dragunow M., “Adult human brain cell culture for neuroscience research,” Int. J. Biochem. Cell Biol. 42, 844–856 (2010). 10.1016/j.biocel.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 78. Eigenmann D. E. et al. , “Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies,” Fluids Barriers CNS 10, 33 (2013). 10.1186/2045-8118-10-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kurokawa Y. K. et al. , “Human induced pluripotent stem cell-derived endothelial cells for three-dimensional microphysiological systems,” Tissue Eng. Part C 23, 474–484 (2017). 10.1089/ten.tec.2017.0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Calderon G. A. et al. , “Tubulogenesis of co-cultured human iPS-derived endothelial cells and human mesenchymal stem cells in fibrin and gelatin methacrylate gels,” Biomater. Sci. 5, 1652–1660 (2017). 10.1039/C7BM00223H [DOI] [PubMed] [Google Scholar]
- 81. Lippmann E. S. et al. , “Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells,” Nat. Biotechnol. 30, 783–791 (2012). 10.1038/nbt.2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lippmann E. S., Al-Ahmad A., Azarin S. M., Palecek S. P., and Shusta E. V., “A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources,” Sci. Rep. 4, 4160 (2014). 10.1038/srep04160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. He Y., Yao Y., Tsirka S. E., and Cao Y., “Cell-culture models of the blood–brain barrier,” Stroke J. Cereb. Circ. 45, 2514–2526 (2014). 10.1161/STROKEAHA.114.005427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wang Y. I., Abaci H. E., and Shuler M. L., “Microfluidic blood–brain barrier model provides in vivo-like barrier properties for drug permeability screening,” Biotechnol. Bioeng. 114, 184–194 (2017). 10.1002/bit.26045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Brown J. A. et al. , “Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor,” Biomicrofluidics 9, 054124 (2015). 10.1063/1.4934713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Adriani G., Ma D., Pavesi A., Kamm R. F., and Goh E. L. K., “A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood-brain barrier,” Lab Chip 17, 448–459 (2017). 10.1039/C6LC00638H [DOI] [PubMed] [Google Scholar]
- 87. Herland A. et al. , “Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood-brain barrier on a chip,” PLoS One 11, e0150360 (2016). 10.1371/journal.pone.0150360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bang S. et al. , “A low permeability microfluidic blood-brain barrier platform with direct contact between perfusable vascular network and astrocytes,” Sci. Rep. 7, 8083 (2017). 10.1038/s41598-017-07416-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cucullo L., Hossain M., Puvenna V., Marchi N., and Janigro D., “The role of shear stress in blood-brain barrier endothelial physiology,” BMC Neurosci. 12, 40 (2011). 10.1186/1471-2202-12-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Phan D. T. et al. , “Blood-brain barrier-on-a-chip: Microphysiological systems that capture the complexity of the blood-central nervous system interface,” Exp. Biol. Med. 242, 1669–1678 (2017). 10.1177/1535370217694100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kaya M. and Ahishali B., “Assessment of permeability in barrier type of endothelium in brain using tracers: Evans blue, sodium fluorescein, and horseradish peroxidase,” in ( Humana Press, 2011), pp. 369–382. [DOI] [PubMed] [Google Scholar]
- 92. Saunders N. R., Dziegielewska K. M., Møllgård K., and Habgood M. D., “Markers for blood-brain barrier integrity: How appropriate is evans blue in the twenty-first century and what are the alternatives?,” Front. Neurosci. 9, 385 (2015). 10.3389/fnins.2015.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hoffmann A. et al. , “High and low molecular weight fluorescein isothiocyanate (FITC)–dextrans to assess blood-brain barrier disruption: Technical considerations,” Transl. Stroke Res. 2, 106–111 (2011). 10.1007/s12975-010-0049-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Srinivasan B. et al. , “TEER measurement techniques for in vitro barrier model systems,” J. Lab. Autom. 20, 107–126 (2015). 10.1177/2211068214561025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Helm M. W. et al. , “Simple and stable transendothelial electrical resistance measurement in organs-on-chips,” in ( Chemical and Biological Micro Systems Society, 2015), pp. 771–773. [Google Scholar]
- 96. Griep L. M. et al. , “BBB on chip: Microfluidic platform to mechanically and biochemically modulate blood-brain barrier function,” Biomed. Microdevices 15, 145–150 (2013). 10.1007/s10544-012-9699-7 [DOI] [PubMed] [Google Scholar]
- 97. Shawahna R., Decleves X., and Scherrmann J.-M., “Hurdles with using in vitro models to predict human blood-brain barrier drug permeability: A special focus on transporters and metabolizing enzymes,” Curr. Drug Metab. 14, 120–136 (2013). 10.2174/138920013804545232 [DOI] [PubMed] [Google Scholar]
- 98. Bhatia S. N. and Ingber D. E., “Microfluidic organs-on-chips,” Nat. Biotechnol. 32, 760–772 (2014). 10.1038/nbt.2989 [DOI] [PubMed] [Google Scholar]
- 99. Shin Y. et al. , “Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels,” Nat. Protoc. 7, 1247–59 (2012). 10.1038/nprot.2012.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pan W., Stone K. P., Hsuchou H., Manda V. K., Zhang Y., and Kastin A. J., “Cytokine signaling modulates blood-brain barrier function,” Curr. Pharm. Des. 17, 3729–3740 (2011). 10.2174/138161211798220918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Macdonald J. A., Murugesan N., and Pachter J. S., “Endothelial cell heterogeneity of blood-brain barrier gene expression along the cerebral microvasculature,” J. Neurosci. Res. 88, 1457–1474 (2009). [DOI] [PubMed] [Google Scholar]
- 102. Kisler K., Nelson A. R., Montagne A., and Zlokovic B. V., “Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease,” Nat. Rev. Neurosci. 18, 419–434 (2017). 10.1038/nrn.2017.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pardridge W. M., “Drug transport across the blood-brain barrier,” J. Cereb. Blood Flow Metab. 32, 1959–1972 (2012). 10.1038/jcbfm.2012.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Miller D. S., Bauer B., and Hart A. M. S., “Modulation of P-glycoprotein at the blood-brain barrier: Opportunities to improve CNS pharmacotherapy,” Pharmacol. Rev. 60, 196–209 (2009). 10.1124/pr.107.07109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Probert L., “TNF and its receptors in the CNS: The essential, the desirable and the deleterious effects,” Neuroscience 302, 2–22 (2015). 10.1016/j.neuroscience.2015.06.038 [DOI] [PubMed] [Google Scholar]
- 106. Pan W., Hsuchou H., Tu H., and Kastin A. J., “Developmental changes of leptin receptors in cerebral microvessels: Unexpected relation to leptin transport,” Endocrinology 149, 877–885 (2008). 10.1210/en.2007-0893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wyss-Coray T., “Inflammation in Alzheimer disease: Driving force, bystander or beneficial response?,” Nat. Med. 12, 1191–1197 (2006). 10.1038/nm1474 [DOI] [PubMed] [Google Scholar]
- 108. Chaudhuri A., Duan F., Morsey B., Persidsky Y., and Kanmogne G. D., “HIV-1 activates proinflammatory and interferon-inducible genes in human brain microvascular endothelial cells: Putative mechanisms of blood-brain barrier dysfunction,” J. Cereb. Blood Flow Metab. 28, 697–711 (2008). 10.1038/sj.jcbfm.9600567 [DOI] [PubMed] [Google Scholar]
- 109. Cucullo L., Marchi N., Hossain M., and Janigro D., “A dynamic in vitro BBB model for the study of immune cell trafficking into the central nervous system,” J. Cereb. Blood Flow Metab. 31, 767–777 (2011). 10.1038/jcbfm.2010.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shusta E. V., “Blood-brain barrier genomics, proteomics, and new transporter discovery,” NeuroRx 2, 151–161 (2005). 10.1602/neurorx.2.1.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Poller B. et al. , “The human brain endothelial cell line hCMEC/D3 as a human blood-brain barrier model for drug transport studies,” J. Neurochem. 107, 1358–1368 (2008). 10.1111/j.1471-4159.2008.05730.x [DOI] [PubMed] [Google Scholar]
- 112. Urich E., Lazic S. E., Molnos J., Wells I., and Freskgård P. O., “Transcriptional profiling of human brain endothelial cells reveals key properties crucial for predictive in vitro blood-brain barrier models,” PLoS One 7, e38149 (2012). 10.1371/journal.pone.0038149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Appelt-Menzel A. et al. , “Establishment of a human blood-brain barrier co-culture model mimicking the neurovascular unit using induced pluri- and multipotent stem cells,” Stem Cell Rep. 8, 894–906 (2017). 10.1016/j.stemcr.2017.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Prabhakarpandian B. et al. , “SyM-BBB: A microfluidic blood brain barrier model,” Lab Chip 13, 1093–1101 (2013). 10.1039/c2lc41208j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Osaki T., Shin Y., Sivathanu V., Campisi M., and Kamm R. D., “In vitro microfluidic models for neurodegenerative disorders,” Adv. Healthcare Mater. 7, 1700489 (2017). 10.1002/adhm.201700489 [DOI] [PubMed] [Google Scholar]
- 116. Ohtsuki S. et al. , “Quantitative targeted absolute proteomic analysis of transporters, receptors and junction proteins for validation of human cerebral microvascular endothelial cell line hCMEC/D3 as a human blood-brain barrier model,” Mol. Pharm. 10(1), 289–296 (2012). 10.1021/mp3004308 [DOI] [PubMed] [Google Scholar]
- 117. Sano Y. et al. , “Establishment of a new conditionally immortalized human brain microvascular endothelial cell line retaining an in vivo blood-brain barrier function,” J. Cell. Physiol. 225, 519–528 (2010). 10.1002/jcp.22232 [DOI] [PubMed] [Google Scholar]
- 118. Franke H., Galla H. J., and Beuckmann C. T., “An improved low-permeability in vitro-model of the blood-brain barrier: Transport studies on retinoids, sucrose, haloperidol, caffeine and mannitol,” Brain Res. 818, 65–71 (1999). 10.1016/S0006-8993(98)01282-7 [DOI] [PubMed] [Google Scholar]
- 119. Zhang Y. et al. , “Porcine brain microvessel endothelial cells as an in vitro model to predict in vivo blood-brain barrier permeability,” Drug Metab. Dispos. 34, 1935–1943 (2006). 10.1124/dmd.105.006437 [DOI] [PubMed] [Google Scholar]
- 120. Canfield S. G. et al. , “An isogenic blood–brain barrier model comprising brain endothelial cells, astrocytes, and neurons derived from human induced pluripotent stem cells,” J. Neurochem. 140, 874–888 (2017). 10.1111/jnc.13923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Rist R. J. et al. , “F-actin cytoskeleton and sucrose permeability of immortalised rat brain microvascular endothelial cell monolayers: Effects of cyclic AMP and astrocytic factors,” Brain Res. 768, 10–18 (1997). 10.1016/S0006-8993(97)00586-6 [DOI] [PubMed] [Google Scholar]
- 122. Yusof S. R., Avdeef A., and Abbott N. J., “In vitro porcine blood-brain barrier model for permeability studies: PCEL-X software pKaFLUXmethod for aqueous boundary layer correction and detailed data analysis,” Eur. J. Pharm. Sci. 65, 98–111 (2014). 10.1016/j.ejps.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 123. Kim J. A. et al. , “Collagen-based brain microvasculature model in vitro using three-dimensional printed template,” Biomicrofluidics 9, 024115 (2015). 10.1063/1.4917508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Esch E. W., Bahinski A., and Huh D., “Organs-on-chips at the frontiers of drug discovery,” Nat. Rev. Drug Discovery 14, 248–260 (2015). 10.1038/nrd4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Booth R. and Kim H., “Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB),” Lab Chip 12, 1784 (2012). 10.1039/c2lc40094d [DOI] [PubMed] [Google Scholar]
- 126. Quadrato G., Brown J., and Arlotta P., “The promises and challenges of human brain organoids as models of neuropsychiatric disease,” Nat. Med. 22, 1220–1228 (2016). 10.1038/nm.4214 [DOI] [PubMed] [Google Scholar]
- 127. Cho C. F. et al. , “Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents,” Nat. Commun. 8, 15623 (2017). 10.1038/ncomms15623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Grskovic M., Javaherian A., Strulovici B., and Daley G. Q., “Induced pluripotent stem cells—Opportunities for disease modelling and drug discovery,” Nat. Rev. Drug Discovery 10, 915–929 (2011). 10.1038/nrd3577 [DOI] [PubMed] [Google Scholar]
- 129. Prantil-Baun R. et al. , “Physiologically-based pharmacokinetic and pharmacodynamic analysis enabled by microfluidically linked organs-on-chips,” Annu. Rev. Pharmacol. Toxicol. 58, 37–64 (2016). 10.1146/annurev-pharmtox-010716-104748 [DOI] [PubMed] [Google Scholar]
- 130. Domanskya K., Lesliea D. C., McKinneya J., Frasera J. P., Sliza J. D., Hamkins-Indika T., Hamiltona G. A., Bahinskia A., and Ingber D. E., “Clear castable polyurethane elastomer for fabrication of microfluidic devices,” Lab Chip 13, 3956–3964 (2013). 10.1039/c3lc50558h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Pardridge W. M., “Drug and gene delivery to the brain: The vascular route,” Neuron 36, 555–558 (2002). 10.1016/S0896-6273(02)01054-1 [DOI] [PubMed] [Google Scholar]
- 132. Honig L. S., Vellas B., Woodward M., Boada M., Bullock R., Borrie M., Hager K., Andreasen N., Scarpini E., Liu-Seifert H., Case M., Dean R. A., Hake A., Sundell K., Poole Hoffmann V., Carlson C., Khanna R., Mintun M., DeMattos R., Selzler K. J., and Siemers E., “Trial of Solanezumab for Mild Dementia Due to Alzheimer's Disease,” N Engl J Med 378, 321–330 (2018). 10.1056/NEJMoa1705971 [DOI] [PubMed] [Google Scholar]
- 133. Abbott A. and Dolgin E., “News in focus,” Nature 540, 15–16 (2016). 10.1038/nature.2016.21045 [DOI] [PubMed] [Google Scholar]
- 134. Huang W. J., Chen W. W., and Zhang X., “Proteasome inhibitors in glioblastoma,” Oncol. Lett. 13, 1058–1062 (2017). 10.3892/ol.2017.5585 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135. Veszelka S., Bocsik A., Walter F. R., Hantosi D., and Deli M. A., “Blood-brain barrier co-culture models to study nanoparticle penetration: Focus on co-culture systems,” Acta Biol. (Szeged) 59, 157–168 (2015). [Google Scholar]
- 136. Tosi G. et al. , “The ‘fate’ of polymeric and lipid nanoparticles for brain delivery and targeting: Strategies and mechanism of blood–brain barrier crossing and trafficking into the central nervous system,” J. Drug Delivery Sci. Technol. 32, 66–76 (2016). 10.1016/j.jddst.2015.07.007 [DOI] [Google Scholar]
- 137. Belletti D. et al. , “Exploiting the versatility of cholesterol in nanoparticles formulation,” Int. J. Pharm. 511, 331–340 (2016). 10.1016/j.ijpharm.2016.07.022 [DOI] [PubMed] [Google Scholar]
- 138. Manninger S. P. et al. , “An exploratory study of ferumoxtran-10 nanoparticles as a blood-brain barrier imaging agent targeting phagocytic cells in CNS inflammatory lesions,” Am. J. Neuroradiol. 26, 2290–2300 (2005). [PMC free article] [PubMed] [Google Scholar]
- 139. Chertok B. et al. , “Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors,” Biomaterials 29, 487–496 (2008). 10.1016/j.biomaterials.2007.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Meng X. et al. , “Specific targeting of gliomas with multifunctional superparamagnetic iron oxide nanoparticle optical and magnetic resonance imaging contrast agents,” Acta Pharmacol. Sin. 28, 2019–2026 (2007). 10.1111/j.1745-7254.2007.00661.x [DOI] [PubMed] [Google Scholar]
- 141. Bakhtiary Z. et al. , “Targeted superparamagnetic iron oxide nanoparticles for early detection of cancer: Possibilities and challenges,” Nanomed. Nanotechnol. Biol. Med. 12, 287–307 (2016). 10.1016/j.nano.2015.10.019 [DOI] [PubMed] [Google Scholar]
- 142. Hu K. et al. , “Lactoferrin conjugated PEG-PLGA nanoparticles for brain delivery: Preparation, characterization and efficacy in Parkinsons disease,” Int. J. Pharm. 415, 273–283 (2011). 10.1016/j.ijpharm.2011.05.062 [DOI] [PubMed] [Google Scholar]
- 143. Loureiro J. A. et al. , “Cellular uptake of PLGA nanoparticles targeted with anti-amyloid and anti-transferrin receptor antibodies for Alzheimer's disease treatment,” Colloids Surf. B 145, 8–13 (2016). 10.1016/j.colsurfb.2016.04.041 [DOI] [PubMed] [Google Scholar]
- 144. Moos T. and Morgan E. H., “Restricted transport of anti-transferrin receptor antibody (OX26) through the blood-brain barrier in the rat,” J. Neurochem. 79, 119–129 (2001). 10.1046/j.1471-4159.2001.00541.x [DOI] [PubMed] [Google Scholar]
- 145. Paris-robidas S., Emond V., Tremblay C., and Soulet D., “In vivo labeling of brain capillary endothelial cells after intravenous injection of monoclonal antibodies targeting the transferrin receptor,” Mol. Pharmacol. 80, 32–39 (2011). 10.1124/mol.111.071027 [DOI] [PubMed] [Google Scholar]
- 146. Dal Magro R. et al. , “ApoE-modified solid lipid nanoparticles: A feasible strategy to cross the blood-brain barrier,” J. Controlled Release 249, 103–110 (2017). 10.1016/j.jconrel.2017.01.039 [DOI] [PubMed] [Google Scholar]
- 147. Gartziandia O. et al. , “Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration,” Colloids Surf. B 134, 304–313 (2015). 10.1016/j.colsurfb.2015.06.054 [DOI] [PubMed] [Google Scholar]
- 148. Chithrani B. D., Ghazani A. A., and Chan W. C. W., “Determing the size and shape dependence of gold nanoparticles uptake into mammalian cells,” Nano Lett. 6, 662–668 (2006). 10.1021/nl052396o [DOI] [PubMed] [Google Scholar]
- 149. Lu F., Wu S. H., Hung Y., and Mou C. Y., “Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles,” Small 5, 1408–1413 (2009). 10.1002/smll.200900005 [DOI] [PubMed] [Google Scholar]
- 150. Jiang W., Kim B. Y. S., Rutka J. T., and Chan W. C. W., “Nanoparticle-mediated cellular response is size-dependent,” Nat. Nanotechnol. 3, 145–150 (2008). 10.1038/nnano.2008.30 [DOI] [PubMed] [Google Scholar]
- 151. Åberg C., “Quantitative analysis of nanoparticle transport through in vitro blood-brain barrier models,” Tissue Barriers 4, e1143545 (2016). 10.1080/21688370.2016.1143545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ding H. et al. , “Enhanced blood-brain barrier transm,” Nanotechnology 25(5), 055101 (2014). 10.1088/0957-4484/25/5/055101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kuo Y. C. and Shih-Huang C. Y., “Solid lipid nanoparticles carrying chemotherapeutic drug across the blood-brain barrier through insulin receptor-mediated pathway,” J. Drug Target. 21, 730–738 (2013). 10.3109/1061186X.2013.812094 [DOI] [PubMed] [Google Scholar]
- 154. Pilakka-Kanthikeel S., Atluri V. S. R., Sagar V., and Nair M., “Targeted brain derived neurotropic factors (BDNF) delivery across the blood-brain barrier for neuro-protection using magnetic nano carriers: An in-vitro study,” PLoS One 8, e62241 (2013). 10.1371/journal.pone.0062241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Prades R. et al. , “Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor,” Biomaterials 33, 7194–7205 (2012). 10.1016/j.biomaterials.2012.06.063 [DOI] [PubMed] [Google Scholar]
- 156. Ye D. et al. , “Nanoparticle accumulation and transcytosis in brain endothelial cell layers,” Nanoscale 5, 11153 (2013). 10.1039/c3nr02905k [DOI] [PubMed] [Google Scholar]
- 157. Chang J. et al. , “Characterization of endocytosis of transferrin-coated PLGA nanoparticles by the blood-brain barrier,” Int. J. Pharm. 379, 285–292 (2009). 10.1016/j.ijpharm.2009.04.035 [DOI] [PubMed] [Google Scholar]
- 158. Kuo Y. C. and Chung C. Y., “Transcytosis of CRM197-grafted polybutylcyanoacrylate nanoparticles for delivering zidovudine across human brain-microvascular endothelial cells,” Colloids Surf. B 91, 242–249 (2012). 10.1016/j.colsurfb.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 159. Kuo Y. C. and Lee C. L., “Methylmethacrylate-sulfopropylmethacrylate nanoparticles with surface RMP-7 for targeting delivery of antiretroviral drugs across the blood-brain barrier,” Colloids Surf. B 90, 75–82 (2012). 10.1016/j.colsurfb.2011.09.048 [DOI] [PubMed] [Google Scholar]
- 160. Cheng K. K. et al. , “Highly stabilized curcumin nanoparticles tested in an in vitro blood–brain barrier model and in alzheimer's disease Tg2576 mice,” AAPS J. 15, 324–336 (2013). 10.1208/s12248-012-9444-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Nikandish N., Hosseinzadeh L., Azandaryani A. H., and Derakhshandeh K., “The role of nanoparticle in brain permeability: An in-vitro BBB model,” Iran. J. Pharm. Res. 15, 403–413 (2016). [PMC free article] [PubMed] [Google Scholar]
- 162. Brun E., Carrière M., and Mabondzo A., “In vitro evidence of dysregulation of blood-brain barrier function after acute and repeated/long-term exposure to TiO2 nanoparticles,” Biomaterials 33, 886–896 (2012). 10.1016/j.biomaterials.2011.10.025 [DOI] [PubMed] [Google Scholar]
- 163. Hanada S. et al. , “Cell-based in vitro blood-brain barrier model can rapidly evaluate nanoparticles' brain permeability in association with particle size and surface modification,” Int. J. Mol. Sci. 15, 1812–1825 (2014). 10.3390/ijms15021812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Georgieva J. V. et al. , “Surface characteristics of nanoparticles determine their intracellular fate in and processing by human blood-brain barrier endothelial cells in vitro,” Mol. Ther. 19, 318–325 (2011). 10.1038/mt.2010.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Baghirov H. et al. , “Feasibility study of the permeability and uptake of mesoporous silica nanoparticles across the blood-brain barrier,” PLoS One 11, 1–22 (2016). 10.1371/journal.pone.0160705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Bonoiu A. et al. , “MMP-9 gene silencing by a quantum dot-siRNA nanoplex delivery to maintain the integrity of the blood brain barrier,” Brain Res. 1282, 142–155 (2009). 10.1016/j.brainres.2009.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Xu G. et al. , “Bioconjugated quantum rods as targeted probes for efficient transmigration across an in vitro blood-brain barrier,” Bioconjugate Chem. 19, 1179–1185 (2008). 10.1021/bc700477u [DOI] [PubMed] [Google Scholar]
- 168. Shen L. and Ji H. F., “Contribution of degradation products to the anticancer activity of curcumin,” Clin. Cancer Res. 15, 7108 (2009). 10.1158/1078-0432.CCR-09-1749 [DOI] [PubMed] [Google Scholar]
- 169. Wang Q. et al. , “Evaluation of the MDR-MDCK cell line as a permeability screen for the blood-brain barrier,” Int. J. Pharm. 288, 349–359 (2005). 10.1016/j.ijpharm.2004.10.007 [DOI] [PubMed] [Google Scholar]
- 170. Mc Carthy D. J., Malhotra M., O'Mahony A. M., Cryan J. F., and O'Driscoll C. m., “Nanoparticles and the blood-brain barrier: Advancing from in-vitro models towards therapeutic significance,” Pharm. Res. 32, 1161–1185 (2015). 10.1007/s11095-014-1545-6 [DOI] [PubMed] [Google Scholar]
- 171. Pangalos M. N., Schechter L. E., and Hurko O., “Drug development for CNS disorders: strategies for balancing risk and reducing attrition,” Nat. Rev. Drug Discovery 6, 521–32 (2007). 10.1038/nrd2094 [DOI] [PubMed] [Google Scholar]