Abstract
Aims
Data available on the fetal safety of trimethoprim–sulfamethoxazole (TMP‐SMX) exposure during pregnancy remains scarce and inconclusive. A previous study assessing the link between TMP‐SMX exposure during pregnancy and the risk of spontaneous abortion (SA) did not control for protopathic bias and indication bias.
Methods
We conducted a nested control study (n = 77 429 pregnancies including 7039 cases of SA and 70 390 controls) within the Quebec Pregnancy Cohort. For each case of SA, we selected 10 controls at the index date that were matched on gestational age and year of pregnancy. TMP‐SMX exposure was defined as either having filled at least one prescription between the first day of gestation (1DG) and the index date, or as having filled a prescription before pregnancy but with a duration overlapping the 1DG (102 pregnancies exposed to TMP‐SMX, including 25 cases of SA and 77 controls).
Results
Adjusting for potential confounders, TMP‐SMX exposure was associated with an increased risk of SA (AOR 2.94, 95% C 1.89–4.57, 25 exposed cases). Similar results were found after controlling for indication bias and protopathic bias.
Conclusion
Given that this drug is widely use in HIV patients to prevent opportunistic infections and malaria, there is an urgent need to identify potential data sources in Africa for analysis of early pregnancy exposure to TMP‐SMX.
Keywords: indication bias, pregnancy, protopathic bias, spontaneous abortion, trimethoprim–sulfamethoxazole
What is Already Known about this Subject
Data available on the fetal safety of TMP‐SMX exposure during pregnancy remains scarce and inconclusive.
To date, only one study evaluated the association between the use of TMP‐SMX during pregnancy and the risk of spontaneous abortion (SA) as a primary outcome, in Denmark.
Findings from research should be interpreted with caution given that indication bias and protopathic bias could not be ruled out.
What this Study Adds
TMP‐SMX exposure was associated with an increased risk of SA.
Similar results were found even after controlling for indication bias and protopathic bias.
Given that TMP‐SMX is also widely use to prevent opportunistic infections in HIV patients and malaria during pregnancy, there is an urgent need to replicate this finding in regions where these diseases are prevalent.
Introduction
Trimethoprim–sulfamethoxazole (TMP‐SMX) is a combination of antibiotics prescribed to treat a vast range of infections including urinary and lower respiratory tract infections 1. In HIV‐ infected patients, it has been shown to be effective at preventing opportunistic infections such as Pneumocystis pneumonia 1. Despite evidence regarding potential associations between folate deficiencies and neural tube defects and other congenital anomalies 2, guidelines related to the use of TMP‐SMX among pregnant HIV‐infected women remains controversial.
The World Health Organization (WHO) recommends the use of TMP‐SMX for prophylaxis against opportunistic infections at any stage of pregnancy in eligible women living with HIV 3.
Conversely, guidelines for the management of Pneumocystis pneumonia in women in their first trimester in the US recommends the use of TMP‐SMX for the treatment of this opportunistic infection because of its considerable benefit. However, given theoretical concerns about possible teratogenicity associated with first‐trimester TMP‐SMX exposure, it suggests that for prophylaxis, health care providers should consider using alternative regimens such as aerosolized pentamidine or oral atovaquone during this period 4.
Current evidence suggests that daily TMP‐SMX use in pregnancy may be as effective as intermittent preventive therapy with three doses of sulfadoxine‐pyrimethamine (SP‐IPTp), which is the cornerstone for malaria control in pregnancy. Indeed, several studies have shown that the two drugs have a similar risk of preterm birth, stillbirth, neonatal death and spontaneous abortions in infected HIV pregnant women 5, 6, 7. Another study has reported that daily TMP‐SMX use is associated with reduced malaria parasitaemia and anaemia compared with SP‐IPTp in infected HIV pregnant women 8. This reduction was even greater when TMP‐SMX was added to another effective antimalarial 8, 9. Recent data also suggest that this drug can be used for malaria prophylaxis in children and non‐pregnant HIV‐infected, and several trials are ongoing on malaria prevention during pregnancy 1.
In Canada, TMP‐SMX is among the first‐line treatment of uncomplicated urinary tract infection (UTI) which occurs usually in women of childbearing age 10.
Data available on the fetal safety of TMP‐SMX exposure during pregnancy remains scarce and inconclusive. To date, a median number of 39 women (interquartile range (IQR): 11–265 women, range: 5–7595 women) with evidence of in utero exposure to TMP‐SMX in the first trimester of pregnancy have been reported in nine studies in the literature 11, 12, 13, 14, 15, 16, 17, 18, 19. The main indication of use of TMP‐SMX was urinary tract infection (99.6%). All the studies were conducted in western countries and the majority were of poor quality 2.
Of the 10 studies reported in the literature, only one study evaluated the association between the use of TMP‐SMX during pregnancy (265 exposed pregnancies in the first trimester) and the risk of spontaneous abortion (SA) as primary outcome, in Denmark. Their findings suggested a two‐ fold increased risk of SA (adjusted hazard ratio, 2.04; 95% CI 1.43–2.91); however, a confounding by indication bias and protopathic bias could not be ruled out 13.
Therefore, we aimed to look at the link between TMP‐SMX exposure during pregnancy and the risk of SA controlling for indication bias and protopathic bias.
Methods
Setting
Within the Quebec pregnancy cohort (QPC) 20 we conducted a nested case–control study. The QPC is an ongoing population‐based cohort with prospective data collection on all pregnancies of women covered by the Quebec Public Prescription Drug Insurance, from January 1998 to December 2009, in the province of Quebec, Canada. Indeed, this cohort includes information on all prescription a pregnant woman covered by the Quebec Public Prescription Drug Insurance received during her pregnancy and one year before this period. The QPC is built by linking four administrative databases: RAMQ (medical and pharmaceutical data), Med‐Echo (hospitalizations), ISQ (births/deaths), and MELS (Ministry of Education data) using unique personal identifiers 20. For each pregnancy, information was obtained from province‐wide databases and linked using unique personal identifiers. The first day of the last menstrual period (first day of gestation: 1DG) was defined using data on gestational age, which was validated by patients charts and vultrasound measures.
Women who were 15–45 years old on the first day of gestation (1DG) and continuously covered by the RAMQ drug plan for at least 12 months before and during their pregnancies were included. We excluded pregnancies with exposure to a known teratogen and planned abortion 21, 22. Pregnancies with multiple exposure to antibiotics, or pregnancies exposed to antibiotics other than TMP‐SMX or penicillins, as well as pregnancies exposed to other anti‐infectives within the time window of interest, were also excluded.
Outcome
Pregnant women who were 15–45 years old on the first day of gestation (1DG) and continuously covered by the RAMQ drug plan for at least 12 months before and during their pregnancies were included in our cohort. The 1DG was the cohort entry. Within this cohort, we identified each case of SA defined as a pregnancy with a diagnosis or procedure related to SA before the 20th week of gestation (ICD‐9 codes 630–634 and ICD‐10 codes O01‐O03).
Using a nested case–control approach, we randomly selected control among pregnancies who did not have a spontaneous abortion at the index date (i.e., the calendar date of each SA). Therefore, we selected 10 controls for each case of SA at the index date (calendar date of SA) and matched them by gestational age and year of pregnancy. Given that gestational age is a known risk factor for SA, matching on this variable at the time of the event allows a control to have similar risk of having SA to a case with regard to the duration of gestation.
Given that a woman in early pregnancy can have an SA that can be mistaken with a monthly bleeding, we considered SA that occurred only between the 6th and 19th weeks of gestation to minimize a misclassification of the outcome.
TMP‐SMX exposure
We defined exposure to TMP‐SMX as either having filled at least one prescription between the 1DG and the index date, or as having filled a prescription for TMP‐SMX before pregnancy but with a duration that overlapped the first date of gestation. We hypothesized that a pregnant woman who received a prescription of TMP‐SMX between the 1DG and the index date (i.e., the calendar date of SA) would have taken at least one pill of this antibiotic. In addition, a study comparing the concordance between filling prescriptions in QPC and maternal reports showed that the positive predictive value (PPV) and negative predictive value (NPV) of antibiotics prescription in the QPC were high (PPV = 86.7%, 95% CI 69.5%–100% and NPV = 92.3%, 95% CI 86.4%–98.2%, respectively) 23.
We used two comparator groups: (1) an active treatment comparator group defined as pregnancies with exposure to penicillins (amoxicillin, phenoxy methyl penicillin, amoxicillin–potassium clavulanate, ivampicillin, ampicillin and cloxacillin sodium; and (2) a non‐exposure category defined as pregnancies with no exposure to antibiotics during the time window of interest.
Covariates
We assessed potential confounders for three periods: (1) on the 1DG: socio‐demographic variables; (2) in the year before and during pregnancy until index date using physician‐based diagnoses or filled prescriptions of related: maternal chronic co‐morbidities (depression, asthma, diabetes mellitus, chronic hypertension, thyroid disorder, epilepsy, endometriosis, and rheumatoid polyarthritis and systemic lupus erythematosus; maternal infections (urinary tract infection, respiratory tract infection, bacterial vaginosis and sexually transmitted infections); (3) in the year before pregnancy: healthcare utilization and history of planned and spontaneous abortion.
Statistical analysis
We calculated crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) using the conditional generalized estimation equation (GEE) to take into account the within‐subject correlation (correlation between risk sets). We also conducted several sensitivity analyses.
(1) To account for protopathic bias, we used TMP‐SMX exposure between the 1DG until 15 days before index date. (2) To control for indication bias (urinary tract infection is the main indication of use of TMP‐SMX in Quebec 24), we restricted our analysis to a cohort of pregnancies with UTI (defined as pregnancies with at least one diagnosis of UTI from the beginning of the pregnancy until index date). (3) We assumed that risk sets were independent and performed a conditional regression analysis. (4) Given that we adjusted for many covariates that might be highly correlated, we assessed the association between TMP‐SMX and an increased risk of SA adjusting only for well‐established risk factors that included maternal age 25, 26, 27, history of previous SA 27, 28 and endometriosis (a proxy of infertility) 27, 29, 30, 31, 32, 33. (5) To ensure that maternal chronic co‐morbidities were not mediators lying on the causal pathway between prescription of TMP‐SMX and SA, we conducted a sensitivity analysis where maternal comorbidities were assessed up to the beginning of pregnancy.
Results
We included 165 009 pregnancies that met the inclusion criteria in our study, 7039 (4.2%) of whom had a clinically detected SA. The mean gestational age at the index date was 14.3 ± 2.8 weeks (median: 14 weeks; interquartile range: 12–17 weeks; range: 6–19 weeks). Women whose pregnancies resulted in a SA were more likely to be older, to live alone and to have a diagnosis of infections and comorbidities (depression, asthma, epilepsy and hypertension) when compared to 70 390 matched controls; they were also more likely to use healthcare services during the year before pregnancy (see Table 1 for full description of the baseline characteristics of the study sample).
Table 1.
Characteristics of study population
| Characteristics | Cases (n = 7039) | Controls (n = 70390) | Unadjusted OR | P value* |
|---|---|---|---|---|
| Gestational age at index date (weeks); mean (SD) ** | ||||
| Socio‐demographics status ( n , %) a | 14.3 ± 2.8 | 14.3 ± 2.8 | 0.9904 | |
| Maternal age (years), mean (SD) ** | 28.9 ± 6.3 | 27.9 ± 5.6 | <0.001 | |
| <18 (ref.) | 104 (1.5) | 1035 (1.5) | 1.00 | |
| 18–34 | 5415 (76.9) | 59 612 (84.7) | 1.10 (0.90–1.34) | |
| >34 | 1520 (21.6) | 9743 (13.8) | 1.69 (1.59–1.80) | <0.001 |
| Urban residence (vs. rural residence) | 5816 (82.6) | 57 841 (82.1) | 1.03 (0.97–1.10) | 0.3431 |
| Recipient of social assistance (vs. RAMQ adherent) | 1765 (25.1) | 17 648 (25.1) | 1.00 (0.95–1.06) | 0.9958 |
| Education (≤12 years vs. >12 years) | 2933 (41.7) | 29 710 (42.2) | 0.98 (0.93–1.03) | 0.3818 |
| Living alone (vs. couple) | 1304 (18.5) | 10 592 (15.1) | 1.27 (1.20–1.36) | <0.001 |
| During year before until index date b ( n , %) a | ||||
| Comorbidities | ||||
| Depression c (yes vs. no) | 1328 (18.9) | 9702 (13.8) | 1.42 (1.34–1.52) | <0.001 |
| Asthma c (yes vs. no) | 987 (14.0) | 8335 (11.8) | 1.20 (1.12–1.29) | <0.001 |
| Diabetes mellitus c (yes vs. no) | 128 (1.8) | 1233 (1.8) | 1.04 (0.87–1.24) | 0.6844 |
| Hypertension c (yes vs. no) | 213 (3.0) | 1779 (2.5) | 1.20 (1.04–1.38) | 0.0118 |
| Thyroid disorders c (yes vs. no) | 278 (4.0) | 2581 (3.7) | 1.08 (0.95–1.22) | 0.2304 |
| Urinary tract infection d (yes vs. no) | 745 (10.6) | 6838 (9.7) | 1.10 (1.01–1.18) | 0.0193 |
| Respiratory tract infection d (yes vs. no) | 2325 (33.0) | 21 091 (30.0) | 1.15 (1.09–1.21) | <0.001 |
| Sexually transmitted diseases d (yes vs. no) | 259 (3.7) | 2563 (3.6) | 1.01 (0.89–1.15) | 0.8699 |
| Endometriosis d (yes vs. no) | 72 (1.0) | 441 (0.6) | 1.53 (1.19–1.96) | <0.001 |
| Bacterial vaginosis d (yes vs. no) | 578 (8.2) | 5424 (7.7) | 1.07 (0.98–1.17) | 0.1303 |
| Epilepsy c (yes vs. no) | 142 (2.0) | 881 (1.3) | 1.60 (1.34–1.91) | <0.001 |
| Uterine malformations d (yes vs. no) | 5 (<0.1) | 29 (<0.1) | 1.70 (0.67–4.27) | 0.2546 |
| In the year before the first day of gestation | ||||
| Health care utilization ( n , %) a | ||||
| Inpatient or emergency visit | 2530 (35.9) | 29 030 (32.6) | 1.15 (1.09–1.20) | <0.001 |
| No. of visits to physicians | ||||
| 0 (ref.) | 1900 (27.0) | 20 976 (29.8) | 1.00 | |
| 1–2 | 1404 (20.0) | 15 556 (22.1) | 0.99 (0.93–1.06) | |
| ≥3 | 3735 (53.0) | 33 858 (48.1) | 1.20 (1.15–1.26) | <0.001 |
| Medications | ||||
| Penicillins use (yes vs. no) | 1369 (19.5) | 12 824 (18.2) | 1.07 (1.02–1.14) | 0.0110 |
| *** TMP‐SMX use (yes vs. no) | 89 (1.3) | 763 (1.1) | 1.16 (0.94–1.44) | 0.1665 |
| Other drugs excluding anti‐infective | ||||
| 0 (ref.) | 2179 (31.0) | 24 084 (34.2) | 1.00 | |
| 1–2 | 1597 (22.7) | 16 134 (22.9) | 1.09 (1.02–1.16) | |
| ≥3 | 3263 (46.3) | 30 172 (42.9) | 1.18 (1.12–1.25) | <0.001 |
| Obstetrics complications | ||||
| History of prior SA **** (yes vs. no) | 140 (2.0) | 746 (1.1) | 1.85 (1.55–2.21) | <0.001 |
| History of prior abortion (yes vs. no) | 431 (6.1) | 3128 (4.4) | 1.38 (1.25–1.1) | <0.001 |
| During pregnancy ( n , %) a | ||||
| *** TMP‐SMX use e , f (yes vs. no) | 25 (0.4) | 77 (0.1) | 3.11 (2.01–4.81) | <0.0001 |
| Penicillins f , g (yes vs. no) | 427 (6.1) | 4777 (6.8) | 0.89 (0.81–0.99) | <0.0001 |
P‐value calculated to compared cases of SA and controls using Pearson χ2 test for categorical variables and a t‐test for continuous variables.
SD, standard deviation;
TMP‐SMX, trimethoprim–sulfamethoxazole;
SA, spontaneous abortion.
Unless otherwise stated.
Index date = date of spontaneous abortion for cases and corresponding date for matched controls.
Based on ICD‐9 or ICD‐10 codes or prescription filled for chronic diseases (hypertension, diabetes, asthma, depression, etc.).
Based on ICD‐9 or ICD‐10 codes for urinary tract infections, respiratory tract infections, bacterial vaginosis, endometriosis, sexually transmitted disease and uterine malformations.
Include pregnancies having filled at least one prescription for trimethoprim–sulfamethoxazole only between the start of pregnancy and the index date.
No exposure to antibiotics.
Include pregnancies having filled at least one prescription for penicillins only between the start of pregnancy and the index date.
TMP‐SMX exposure and risk of SA
After adjustment for potential confounders, we found that TMP‐SMX was associated with an increased risk of SA when compared to no exposure to antibiotics (AOR 2.94, 95% CI 1.89–4.57, 25 exposed cases) (Table 2 and Figure 1). TMP‐SMX exposure was also linked to an increased risk of SA when compared to penicillins (AOR 3.54, 95% CI 2.25–5.55, 25 exposed cases) (Table 2). Similar results were found with our main analysis when we restricted our cohort to pregnancy with a UTI diagnosis (AOR 9.19, 95% CI 4.31–19.56, 15 exposed cases) or when we looked at TMP‐SMX exposure from the 1DG until 15 days before the index date (AOR 2.99, 95% CI 1.86–4.80, 22 exposed cases) (Table 2). When we assumed an independence between risk sets, results obtained from a conditional regression analysis remain similar to those produced with a GEE analysis (AOR 2.97, 95% CI 1.88–4.71, 25 exposed cases). When adjusting only for well‐established risk factors for SA, our result was also similar to findings obtained from the main analysis (AOR 3.13, 95% CI 2.01–4.87, 25 exposed cases). When maternal chronic co‐morbidities were measured up to the beginning of pregnancy, our results remain consistent with findings obtained from the main analysis as well (AOR 2.91, 95% CI 1.87–4.53, 25 exposed cases).
Table 2.
TMP‐SMX exposure in pregnancy and risk of SA, odd ratio (95% CI)a
| No. of cases | Adjusted OR (95% CI) | |
|---|---|---|
| Analyses | ||
| No use of antibiotics | 6587 (93.5) | 1.00 |
| TMP‐SMX | 25 (0.4) | 2.94 (1.89–4.57) |
| Active comparator groups | ||
| Penicillins | 427 (6.1%) | 1.00 |
| TMP‐SMX | 25 (0.4%) | 3.54 (2.25–5.55) |
| Women with UTI b | ||
| Penicillins | 32 (17.4%) | 1.00 |
| TMP‐SMX | 15 (8.2%) | 9.19 (4.31–19.56) |
| Timing of TMP‐SMX exposure in pregnancy c | ||
| No use of antibiotics | 6664 (94.7%) | 1.00 |
| TMP‐SMX | 22 (0.3%) | 2.99 (1.86–4.80) |
CI, confidence interval; OR, odds ratio.
Adjusted for the following variables: maternal age on the 1DG, maternal marital status (living alone or cohabiting), receipt of social assistance during pregnancy, education level in years (≤12 or >12), and area of residence on the 1DG (urban or rural); maternal chronic co‐morbidities assessed using physician‐based diagnoses or filled prescriptions of related medications in the year before and during pregnancy until index date (chronic hypertension, depression, diabetes mellitus, asthma, epilepsy, uterine malformations, polyarthritis rheumatoid and systemic lupus erythematosus, endometriosis, thyroid disorders; Maternal infections assessed using physician‐based diagnoses in the year before and during pregnancy until index date (urinary tract infection, respiratory tract infection, bacterial vaginosis, and sexually transmitted diseases); Use of health services in the year before pregnancy; and history of planned and spontaneous abortion.
Pregnancies with at least one diagnosis codes of UTI from the beginning of pregnancy until the index date
Pregnancies exposed from the beginning of pregnancy until 15 days before index date.
Figure 1.
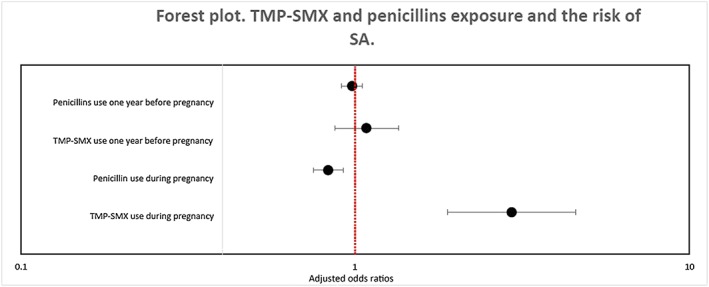
Trimethoprim–sulfamethoxazole and penicillin exposure and the risk of SA (comparator groups: no antibiotic use). Values greater than 1.0 indicate an increased risk of spontaneous abortion
Discussion
To our knowledge this is the first study to investigate the association between the use of TMP‐SMX exposure and the risk of SA after controlling for confounding by indication bias and protopathic bias. Our study showed that TMP‐SMX exposure was associated with a 2.94‐fold increased risk of SA when compared to no exposure to antibiotics and with a 3.54‐fold increased risk of SA when compared to penicillins. These results were consistent with a Danish study which showed a two‐fold increased risk of SA after TMP‐SMX exposure during the first trimester of pregnancy 13.
The Canadian Antimicrobial Resistance Surveillance System reported that resistance of E. coli to TMP‐SMX has increased from 9% in 2004 to 16% in 2014 in Canada 34. In Quebec, TMP‐SMX is the first line treatment of urinary tract infection in the general population along with β‐lactams (amoxicillin, cephalosporins), nitrofurantoin and fluoroquinolones 24. In the presence of asymptomatic bacteriuria, acute cystitis in pregnancy, the recommended antibiotics are amoxicillin, cephalexin, nitrofurantoin (after 36 weeks of gestation) and TMP‐SMX (second and third trimester of pregnancy) 35, 36, 37, 38. Given that we adjusted for several known proxies of resistance of E. coli to TMP‐SMX (prior use of TMP‐SMX as well as prior healthcare utilization) 39, 40, we are confident that our results could not be fully explained by indication bias. In addition, when we compared pregnant women exposed to TMP‐SMX to those exposed to penicillin (level of resistance of E. coli to penicillin varied between 10% and 32% from 2013 to 2014 in Quebec) 41, the results were consistent with the main analysis. Therefore, it is less likely that the resistance of E. coli to TMP‐SMX fully explains our results.
Our study also found in a cohort including pregnancies with a diagnosis of UTI during pregnancy, TMP‐SMX exposure was associated with a nine‐fold increased risk of SA when compared to penicillin exposure. Given that we adjusted for hospital‐based diagnosis of UTI as well, which is a proxy for symptomatic and severe UTI, we are confident that indication bias by severity would not fully explain our results.
We also found an almost three‐fold increased risk of SA when TMP‐SMX exposure was assessed from the 1DG until 15 days before the index date as compared to no exposure to antibiotics during the same period. When we adjusted only for well‐established risk factors for SA, our findings were similar to our main analysis.
Trimethoprim is one of the TMP‐SMX compounds that may support the biological plausibility of these findings, given its folate antagonist effect 42, 43. Trimethoprim crosses the placenta and may interfere with folate metabolism in trophoblast cells by its inhibition of dihydrofolate reductase (DHFR) that may result in SA due to the inhibition of DNA synthesis 43.
Though TMP‐SMX is among the first‐line treatments of UTI in the general population, its prevalence of use during pregnancy was low in our cohort. This is consistent with a previous study describing the trends in anti‐infective drug use during pregnancy in Québec that showed a decrease of TMP‐SMX use in favour of nitrofurantoin or amoxicillin during pregnancy. A potential explanation of the decrease of TMP‐SMX prescription during pregnancy in Québec was related to physicians' awareness of the potential risk of birth defects associated with the use of folic acid antagonists in early pregnancy. Therefore, physicians in Québec were more confident in prescribing nitrofurantoin or amoxicillin instead of TMP‐SMX during pregnancy. The strength of our study included accurate information on filled prescription, as well as prospectively and routinely collected data on physician‐based diagnoses or procedures related to SA, which limited the potential for detection bias. We were able to control for confounding by the main indication of use of TMP‐SMX by restricting our cohort in pregnancies with a diagnosis of UTI during pregnancy. To ensure that TMP‐SMX was prescribed before the occurrence of SA, we conducted a sensitivity analysis where we restricted our exposure to pregnant women who receive a prescription of TMP‐SMX between the 1DG and 15 days before the calendar date of SA (i.e., date of the diagnosis of SA) and the results were similar to our main analysis. Therefore, we are confident that a protopathic bias would not fully explain our finding.
To ensure that multicollinearity would not affect our results, we adjusted only for well‐established risk factors and our results also remain similar to findings obtained from our main analysis. Therefore, it is less likely that multicollinearity would influence the conclusions of our study. Also, when maternal chronic co‐morbidities were measured up to the beginning of pregnancy, our results remained consistent with findings obtained from the main analysis. Finally, using a nested case–control design allowed us to select controls from the same source population as the cases, which limited the potential for selection bias.
The main limitation of our study includes missing information in QPC on potentially important confounders such as smoking, folic acid and alcohol intake. However, by using active comparator group such as penicillins, we indirectly adjusted for lifestyles by design. Therefore, unmeasured confounding, if present, would not fully explain this finding.
Filled prescription might not have reflected actual intake. However, De Jong et al. 44 reported that 94% of all drugs dispensed to pregnant women are actually taken. In addition, filled prescription in the QPC has been validated against maternal reports with a high positive and negative predictive value for antibiotics exposure (PPV = 86.7% and NPV = 92.3%) 23, 45.
We included only clinically detected SA. Therefore, if the use of TMP‐SMX was associated with an increased risk of SA that was not clinically detected, our findings would underestimate the true risk. However, if no association was found between the use of TMP‐SMX and the risk of SA that was not clinically detected, any misclassification resulting from the non‐inclusion of undetected SA would be non‐differential and again our estimates should remain conservative.
Given the number of comparisons made in our study, we cannot rule out chance finding. The number of exposed cases to TMP‐SMX was small, which produced an estimate of the risk with a wide confidence interval. As such, we could not completely rule out a bias that might move the estimate away from the null when the number of the outcome is small 46; however, our results were consistent with the finding of the previous Danish study, so we are confident that a bias due to the small number of exposed SA would not fully explain our findings.
Finally, our cohort included women of lower socioeconomic status insured by the RAMQ for their medications. Although this will not affect the validity of our results, it might alter their generalizability 47.
TMP‐SMX exposure during early pregnancy was associated with an increased risk of SA even after controlling for protopathic bias and indication bias. Given that this drug is widely use in HIV patients to prevent opportunistic infections and malaria, there is an urgent need to identify potential data sources in Africa for analysis of early pregnancy exposure to TMP‐SMX.
Competing Interests
A.B. is a consultant for plaintiffs in litigations involving antidepressants and birth defects. F.T.M. and O.S. report no competing interests.
This work was supported by the Réseau Québécois de recherche sur l'usage des médicaments. F.T.M. is the recipient of the Sainte‐Justine Hospital Foundation and the Foundation of Stars scholarship from the Faculty of Pharmacy of the University of Montreal and is scholarship holder of the Canadian Network for Advanced Interdisciplinary Methods for comparative effectiveness research (CAN‐AIM). A.B. is recipient of a career award from the FRSQ and is on the endowment Research Chair of the Famille Louis‐Boivin on Medications, Pregnancy and Lactation at the Faculty of Pharmacy of the University of Montreal. The funding body had no involvement in the data collection or analysis, the preparation of the manuscript, or the decision to submit the paper for publication.
Contributors
All authors conceived and designed this study. Data were acquired by A.B. Statistical analyses were carried out by F.T.M. under the supervision of O.S. and A.B. and all authors interpreted the data. The manuscript was drafted by F.T.M. and all authors were involved in the critical revision and approval of the final manuscript.
Muanda, F. T. , Sheehy, O. , and Bérard, A. (2018) Use of trimethoprim–sulfamethoxazole during pregnancy and risk of spontaneous abortion: a nested case control study. Br J Clin Pharmacol, 84: 1198–1205. doi: 10.1111/bcp.13542.
References
- 1. Church JA, Fitzgerald F, Walker AS, Gibb DM, Prendergast AJ. The expanding role of co‐trimoxazole in developing countries. Lancet Infect Dis 2015; 15: 327–339. [DOI] [PubMed] [Google Scholar]
- 2. Ford N, Shubber Z, Jao J, Abrams EJ, Frigati L, Mofenson L. Safety of cotrimoxazole in pregnancy: a systematic review and meta‐analysis. J Acquir Immune Defic Syndr 2014; 66: 512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach 2006. Available at http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf (last accessed 23 March 2016). [PubMed]
- 4. Anonymous . Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV‐1‐infected adults and adolescents. Department of Health and Human Services, 2016. Available at https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf (last accessed 24 March 2016).
- 5. Manyando C, Njunju EM, Mwakazanga D, Chongwe G, Mkandawire R, Champo D, et al Safety of daily co‐trimoxazole in pregnancy in an area of changing malaria epidemiology: a phase 3b randomized controlled clinical trial. PloS one 2014; 9: e96017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dow A, Kayira D, Hudgens MG, Van Rie A, King CC, Ellington S, et al The effect of cotrimoxazole prophylactic treatment on malaria, birth outcomes, and postpartum CD4 count in HIV‐infected women. Infect Dis Obstet Gynecol 2013; 2013: 340702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klement E, Pitche P, Kendjo E, Singo A, D'Almeida S, Akouete F, et al Effectiveness of co‐trimoxazole to prevent Plasmodium falciparum malaria in HIV‐positive pregnant women in sub‐Saharan Africa: an open‐label, randomized controlled trial. Clin Infect Dis 2014; 58: 651–659. [DOI] [PubMed] [Google Scholar]
- 8. Kapito‐Tembo A, Meshnick SR, van Hensbroek MB, Phiri K, Fitzgerald M, Mwapasa V. Marked reduction in prevalence of malaria parasitemia and anemia in HIV‐infected pregnant women taking cotrimoxazole with or without sulfadoxine‐pyrimethamine intermittent preventive therapy during pregnancy in Malawi. J Infect Dis 2011; 203: 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gonzalez R, Desai M, Macete E, Ouma P, Kakolwa MA, Abdulla S, et al Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV‐infected women receiving cotrimoxazole prophylaxis: a multicenter randomized placebo‐controlled trial. PLoS Med 2014; 11: e1001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Institut national d'excellence en santé et en services sociaux (INESSS) . Infections urinaires chez l'adulte. Available at: https://www.inesss.qc.ca/fileadmin/doc/CDM/UsageOptimal/Guides-serieI/CdM-Antibio1-InfectionsUrinaires-Adultes-fr.pdf (accessed 24 March 2016).
- 11. Bailey RR, Bishop V, Peddie BA. Comparison of single dose with a 5‐day course of co‐trimoxazole for asymptomatic (covert) bacteriuria of pregnancy. Aust N Z J Obstet Gynaecol 1983; 23: 139–141. [DOI] [PubMed] [Google Scholar]
- 12. Angelakis E, Million M, D'Amato F, Rouli L, Richet H, Stein A, et al Q fever and pregnancy: disease, prevention, and strain specificity. Eur J Clin Microbiol Infect Dis 2013; 32: 361–368. [DOI] [PubMed] [Google Scholar]
- 13. Andersen JT, Petersen M, Jimenez‐Solem E, Broedbaek K, Andersen EW, Andersen NL, et al Trimethoprim use in early pregnancy and the risk of miscarriage: a register‐based nationwide cohort study. Epidemiol Infect 2013; 141: 1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yaris F, Kadioglu M, Kesim M, Unsal M, Yaris E, Ulku C, et al Urinary tract infections in unplanned pregnancies and fetal outcome. Eur J Contracept Reprod Health Care 2004; 9: 141–146. [DOI] [PubMed] [Google Scholar]
- 15. Matok I, Gorodischer R, Koren G, Landau D, Wiznitzer A, Levy A. Exposure to folic acid antagonists during the first trimester of pregnancy and the risk of major malformations. Br J Clin Pharmacol 2009; 68: 956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brumfitt W, Pursell R. Trimethoprim‐sulfamethoxazole in the treatment of bacteriuria in women. J Infect Dis 1973; 128 (Suppl): 657–665. [DOI] [PubMed] [Google Scholar]
- 17. Jungmann EM, Mercey D, DeRuiter A, Edwards S, Donoghue S, Booth T, et al Is first trimester exposure to the combination of antiretroviral therapy and folate antagonists a risk factor for congenital abnormalities? Sex Transm Infect 2001; 77: 441–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muanda FT, Sheehy O, Berard A. Use of antibiotics during pregnancy and the risk of major congenital malformations: a population based cohort study. Br J Clin Pharmacol 2017; 83: 2557–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hansen C, Andrade SE, Freiman H, Dublin S, Haffenreffer K, Cooper WO, et al Trimethoprim‐sulfonamide use during the first trimester of pregnancy and the risk of congenital anomalies. Pharmacoepidemiol Drug Saf 2016; 25: 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berard A, Sheehy O. The Quebec Pregnancy Cohort – prevalence of medication use during gestation and pregnancy outcomes. PloS One 2014; 9: e93870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kulaga S, Zargarzadeh AH, Berard A. Prescriptions filled during pregnancy for drugs with the potential of fetal harm. BJOG 2009; 116: 1788–1795. [DOI] [PubMed] [Google Scholar]
- 22. Koren G, Pastuszak A, Ito S. Drugs in pregnancy. N Engl J Med 1998; 338: 1128–1137. [DOI] [PubMed] [Google Scholar]
- 23. Zhao JP, Sheehy O, Gorgui J, Berard A. Can we rely on pharmacy claims databases to ascertain maternal use of medications during pregnancy? Birth Defects Res 2017; 109: 423–431. [DOI] [PubMed] [Google Scholar]
- 24. Guide pour remplir le Formulaire de demande d'inscription d'un médicament. Institut national d'excellence en santé et en services sociaux (INSS). Québec, February 2007. Available at https://www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Fiches_insc ription/Guide‐remplir‐formulaire.pdf (last accessed 29 December 2017).
- 25. Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ (Clinical Research ed) 2000; 320: 1708–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de la Rochebrochard E, Thonneau P. Paternal age and maternal age are risk factors for miscarriage; results of a multicentre European study. Hum Reprod 2002; 17: 1649–1656. [DOI] [PubMed] [Google Scholar]
- 27. Maconochie N, Doyle P, Prior S, Simmons R. Risk factors for first trimester miscarriage – results from a UK population‐based case‐control study. BJOG 2007; 114: 170–186. [DOI] [PubMed] [Google Scholar]
- 28. Regan L, Braude PR, Trembath PL. Influence of past reproductive performance on risk of spontaneous abortion. BMJ (Clinical Research ed) 1989; 299: 541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hakim RB, Gray RH, Zacur H. Infertility and early pregnancy loss. Am J Obstet Gynecol 1995; 172: 1510–1517. [DOI] [PubMed] [Google Scholar]
- 30. Gray RH, Wu LY. Subfertility and risk of spontaneous abortion. Am J Public Health 2000; 90: 1452–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Donnez J, Van Langendonckt A, Casanas‐Roux F, Van Gossum JP, Pirard C, Jadoul P, et al Current thinking on the pathogenesis of endometriosis. Gynecol Obstet Invest 2002; 54 (Suppl. 1): 52–58 discussion 59–62. [DOI] [PubMed] [Google Scholar]
- 32. Wei M, Cheng Y, Bu H, Zhao Y, Zhao W. Length of menstrual cycle and risk of endometriosis: a meta‐analysis of 11 case‐control studies. Medicine (Baltimore) 2016; 95: e2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zullo F, Spagnolo E, Saccone G, Acunzo M, Xodo S, Ceccaroni M, et al Endometriosis and obstetrics complications: a systematic review and meta‐analysis. Fertil Steril 2017; 108: 667–672 e5. [DOI] [PubMed] [Google Scholar]
- 34. Canadian antimicrobial resistance surveillance system – report 2016. Public Health Agency of Canada. September 2016. Available at https://www.canada.ca/content/dam/phacaspc/documents/services/publications/drugs-health-products/antibiotic-resistanceantibiotique/antibiotic-resistance-antibiotique-2016-eng.pdf (last accessed 29 December 2017).
- 35. Schnarr J, Smaill F. Asymptomatic bacteriuria and symptomatic urinary tract infections in pregnancy. Eur J Clin Invest 2008; 38 (Suppl. 2): 50–57. [DOI] [PubMed] [Google Scholar]
- 36. Dashe JS, Gilstrap LC 3rd. Antibiotic use in pregnancy. Obstet Gynecol Clin North Am 1997; 24: 617–629. [DOI] [PubMed] [Google Scholar]
- 37. Einarson A, Shuhaiber S, Koren G. Effects of antibacterials on the unborn child: what is known and how should this influence prescribing? Paediatr Drugs 2001; 3: 803–816. [DOI] [PubMed] [Google Scholar]
- 38. Sahm DF, Thornsberry C, Mayfield DC, Jones ME, Karlowsky JA. Multidrug‐resistant urinary tract isolates of Escherichia coli: prevalence and patient demographics in the United States in 2000. Antimicrob Agents Chemother 2001; 45: 1402–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hillier S, Roberts Z, Dunstan F, Butler C, Howard A, Palmer S. Prior antibiotics and risk of antibiotic‐resistant community‐acquired urinary tract infection: a case‐control study. J Antimicrob Chemother 2007; 60: 92–99. [DOI] [PubMed] [Google Scholar]
- 40. Hertz FB, Schonning K, Rasmussen SC, Littauer P, Knudsen JD, Lobner‐Olesen A, et al Epidemiological factors associated with ESBL‐ and non ESBL‐producing E. coli causing urinary tract infection in general practice. Infect Dis (Lond) 2016; 48: 241–245. [DOI] [PubMed] [Google Scholar]
- 41. Guide régional d'antibiothérapie et profil de sensibilité des bactéries courantes. Comité de gérance des antimicrobiens. Centre intégré de santé et des services de santé (CISSS ) de Chaudière‐Appalaches. Québec. November 2015. Available at http://www.cisssca.gouv.qc.ca/fileadmin/documents/Professionnels/Guide_de_pratique_clinique/GUI_DSP_Antibiotherapie_2016‐02‐11_FIN.pdf (last accessed 29 December 2017).
- 42. Schweitzer BI, Dicker AP, Bertino JR. Dihydrofolate reductase as a therapeutic target. FASEB J 1990; 4: 2441–2452. [DOI] [PubMed] [Google Scholar]
- 43. Baird DT. Mode of action of medical methods of abortion. J Am Med Womens Assoc (1972) 2000; 55: 121–126. [PubMed] [Google Scholar]
- 44. De Jong van den Berg LT, Feenstra N, Sorensen HT, Cornel MC. Improvement of drug exposure data in a registration of congenital anomalies. Pilot‐study: pharmacist and mother as sources for drug exposure data during pregnancy. EuroMAP Group. Europen Medicine and Pregnancy Group. Teratology 1999; 60: 33–36. [DOI] [PubMed] [Google Scholar]
- 45. Jobin‐Gervais K, Sheehy O, Berard A. Can we rely on pharmacy claims databases to ascertain maternal use of medications during pregnancy? Pharmacoepidemiol Drug Saf 2013; 22: 155. [DOI] [PubMed] [Google Scholar]
- 46. Greenland S. Bayesian perspectives for epidemiological research. II. Regression analysis. Int J Epidemiol 2007; 36: 195–202. [DOI] [PubMed] [Google Scholar]
- 47. Berard A, Lacasse A. Validity of perinatal pharmacoepidemiologic studies using data from the RAMQ administrative database. Can J Clin Pharmacol 2009; 16: e360–e369. [PubMed] [Google Scholar]


