Abstract
Background
Children with congenital adrenal hyperplasia (CAH) are exposed to fluctuating cortisol and androgen levels. The effects these hormonal states have on bone mineral density (BMD) and body composition are not well studied.
Objective
Compare BMD and body composition, including visceral adipose tissue (VAT) and Android:Gynoid (A:G) ratio, in children with CAH vs. healthy age-, sex-, and BMI-matched controls.
Methods
Forty-two cases with CAH (average age 12.3 years [SE 3]; 17 males) and 101 controls underwent a dual-energy X-ray absorptiometry scan. Total body BMD (TBMD) Z-scores were adjusted for height-for-age Z-scores (TBMDHAZ). Hydrocortisone dose (mg/m2/day) was averaged over the past year. Bone age Z-scores were used as a surrogate for long-term androgen exposure in cases. Statistical analyses comparing cases and controls accounted for matched groups using mixed linear models.
Results
Children with CAH had lower TBMD (0.81 vs. 1.27, p=0.003) and TBMDHAZ Z-scores (−0.51 vs. −0.01, p=0.001) than controls. In CAH cases, TBMD and TBMDHAZ Z-scores were positively correlated with bone age Z-scores (respectively, r=0.63, p<0.0001; r=0.51, p=0.001) but were not associated with HC dose. VAT and the A:G ratio did not differ significantly between children with CAH and controls and neither was associated with HC dose.VAT was not associated with bone age Z-score.
Conclusion
Lower BMD was observed in CAH cases compared with controls though no differences in body composition were identified. Among CAH cases, increased chronic androgen exposure, as measured by bone age Z-scores, was associated with higher BMD but was not associated with VAT.
Keywords: congenital adrenal hyperplasia, bone mineral density, body composition, visceral adipose tissue, glucocorticoids, hydrocortisone, androgen
INTRODUCTION
Congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency is characterized by impaired cortisol synthesis leading to excessive production of adrenal androgen, which through aromatization leads in turn to increased production of estrogen. Lifelong cortisol replacement is needed in the classic forms and in symptomatic non-classic CAH patients. In growing children, hydrocortisone (HC) is recommended at 10–15 mg/m2/day in three divided doses to avoid the adverse impact on growth from long-acting steroids.1 The challenge in treating CAH is to provide sufficient cortisol replacement to suppress overproduction of adrenal androgens (hyperandrogenemia) while avoiding the effects of cortisol excess (hypercortisolemia). Adding to the challenge is HC’s short median elimination half-life in CAH children, 58 min (range: 41–105 min), allowing most of the HC dose to be eliminated from the body within 4–5 hrs.2 Because of HC’s short half life, the recommended 3 times a day dosing schedule exposes children to alternating periods of hypo- and hypercortisolemia with resultant hyperandrogenemia throughout each day.
Evidence for the effects of increased adrenal sex steroids in CAH and glucocorticoid therapy on bone mineral density (BMD) are mixed, with some studies finding no difference in BMD between children with CAH and controls3–8, others finding lower BMD in children with CAH,9–15 and one study finding increased BMD.16 While androgens have an established positive effect on BMD through stimulation of osteoblastic activity17, glucocorticoids can have an opposite detrimental effect. Glucocorticoids reduce BMD through several mechanisms including decreasing osteoblastic activity, decreasing calcium absorption in the gastrointestinal tract, enhancing renal losses of calcium, and increasing bone resorption.18
The effects of adrenal sex steroids and glucocorticoids on measures of body composition, such as visceral adipose tissue (VAT) and the Android:Gynoid (A:G) ratio, have not been well studied in children with CAH. This area of investigation is important given that children and adults with CAH may have higher risk for cardiovascular disease compared to the general population.19 VAT is associated with increased cardiometabolic risk,20 while the A:G ratio is associated with elevated triglyceride levels and lower HDL levels and is a better predictor of cardiovascular risk than BMI.21 Although the A:G ratio can be calculated from dual-energy X-ray absorptiometry (DXA) measures22, data quantifying VAT in children with CAH are limited, and no studies have examined VAT using DXA. Estimation of abdominal VAT using DXA has been validated in adults23, and DXA has been shown to provide a reliable estimate in children as well22.
The aims of this study were to: 1) compare BMD in children with CAH to healthy controls; 2) compare VAT and the A:G ratio in children with CAH to healthy controls; and 3) determine the relationship between BMD and VAT in children with CAH and in healthy controls.
METHODS
Subjects
A retrospective cohort of 42 cases with CAH (all 21-hydroxylase deficiency; age range 7.6–17.7 years; 40% male; 19 with salt-wasting, 13 with simple-virilizing, and 10 with non-classic) were identified. Each case had molecular and biochemical testing to help confirm the diagnosis of CAH. Controls (43% male) were identified from a separate cross-sectional study evaluating cardiometabolic risk factors and vascular health in children ages 8–18 years and matched to cases based on age (±1 year), sex, and BMI Z-score (± 0.5 SD). On average, 2.4 controls (range 1–4) were matched to each case. The study was approved by the University of Minnesota Institutional Review Board.
Anthropometrics
Each case and control underwent height and weight measurements and a DXA scan as part of either clinical (cases) or research (controls) protocols. For controls, height and weight measurements were taken three times on the day of the DXA exam on a calibrated, wall-mounted stadiometer and averaged. For cases, height and weight measurements were obtained in a similar fashion from the closest clinic visit to the DXA exam (average 9 days from the DXA exam). Height and weight Z-scores were calculated using GenenCALC 3.0 Software. BMI Z-scores were generated for all cases and controls using standard growth charts from the Center for Disease Control (CDC)24. Tanner staging of puberty was assessed either at the closest clinic visit to the DXA exam or on the day of the DXA exam (controls). Breast development was assessed in both female cases and controls. For males, testicular volume was measured in cases only, with a volume of 4 cc or greater being considered pubertal. Pubic hair was assessed in male cases and controls and female cases.
DXA
DXA scans for both cases and controls were performed using GE Lunar iDXA system (iDXA, General Electric Medical Systems, Madison, WI, USA). Total body bone mineral density (TBMD) Z-scores were calculated using pediatric software, and TBMD Z-scores were adjusted for height-for-age Z-scores (TBMDHAZ) applying the method of Zemel, et al, 201025 to the present study's control subjects. Total body composition measures (percent total tissue fat, android fat mass, and gynoid fat mass) were calculated from DXA using enCore™ software (platform version 16.3, General Electric Medical Systems, Madison, WI, USA). Estimates of abdominal visceral adipose tissue (VAT) were obtained using methods previously described for children and adolescents.23 Android (abdominal) fat was measured using a region-of-interest automatically defined with a caudal limit placed at the top of the iliac crest and its height set to twenty percent of the distance from the top of the iliac crest to the base of the skull.26 The gynoid region (hip/gluteal) is located mid-pelvis to mid-thigh, with the upper limit set below the iliac crest a distance 1.5 times the height of the android region and the lower limit set a distance of 2 times the height of the android region.26 All scans were reviewed for accurate placement of the android box. By default, a VAT value of 2 grams or less was outside the precision of the machine and considered to be inaccurate, either because the value was below the threshold to detect VAT or the DXA machine could not accurately measure the VAT. Only one case and 5 controls had a VAT value of 2 grams or less; these values were removed from analyses.
Glucocorticoids
Most cases with CAH were being treated with HC (79%). There were 2 cases on prednisone and 3 cases on dexamethasone who had transitioned from HC after reaching their final adult heights. Five cases with non-classic CAH were not on treatment with glucocorticoids but still had evidence of hyperandrogenemia with advanced bone age and/or growth acceleration. Prednisone and dexamethasone doses were converted to equivalent HC doses in mg/m2/day using standard glucocorticoid equivalencies (20 mg of HC = 5 mg of prednisone = 0.4 mg of dexamethasone).27 For each case, the mean HC dose was calculated from treatment doses recorded at available clinic visits performed within 3, 6, and 12 months prior the DXA exam (mean number of clinic visits was 2.8).
Bone age
A bone age radiograph was performed only in cases with CAH, and bone age Z-scores were used as a surrogate measure of androgen exposure. The bone age radiograph closest in time to the DXA scan (median 3 months) was interpreted using the Greulich and Pyle method. Bone age Z-scores for cases were calculated using the following formula: (bone age of the case − mean bone age for the case’s chronological age)/(standard deviation of the bone age for the case’s chronological age)28. Bone age means and standard deviations for girls and boys were obtained from Greulich and Pyle.
Statistical Analysis
Preliminary analyses showed that the distribution of VAT Mass was skewed to the right (i.e., had a long upper tail) so it was more appropriate to the use the common log (log to base 10) of VAT Mass (log10 VAT Mass) as a dependent variable. Log10 VAT Mass was also used as a predictor in analyses of BMD measures.
All analyses that included both cases and controls used a mixed linear model. The random effect was a cluster consisting of a case and its 1 to 4 matched controls. Fixed effects were case versus control and other covariates of interest (e.g., VAT Mass) or adjusters. The analysis used the restricted likelihood method; adjusted averages are SAS’s least-squares means.
Analyses that included cases only used one-way ANOVA or multiple linear regression and the closely associated Pearson’s correlation (r).
All analyses were done using JMP (v. 12.0 Pro, SAS Institute Inc, Cary, NC).
RESULTS
Table 1 shows characteristics of CAH cases and controls. The groups did not differ significantly in ethnicity, height standard deviation, weight standard deviation, or Tanner Stage. The salt-wasting subtype accounted for 45% of cases, followed by simple-virilizing (31%), and non-classic (24%). The mean HC (or its equivalent) dose for the CAH cases receiving glucocorticoids was 11.3 mg/m2/day.
Table 1.
Characteristics of CAH Cases and Controls
| Characteristic | Cases | Controls | SE difference |
P-value |
|---|---|---|---|---|
| N | 42 | 101 | ||
| Sex, Male, n (%) | 17 (40%) | 43 (43%) | ||
| Age, mean (SE) | 12.34 (0.45) | 12.48 (0.44) | 0.08 | 0.09 |
| Ethnicity, n (%) | 0.71 | |||
| White Non-Hispanic | 36 (86%) | 81 (80%) | ||
| Other | 6 (14%) | 20 (20%) | ||
| Height SD, mean (SE) | 0.67 (0.18) | 0.38 (0.12) | 0.21 | 0.16 |
| Weight SD, mean (SE) | 1.07 (0.13) | 1.08 (0.12) | 0.09 | 0.91 |
| BMI Z-score, mean (SE) | 0.95 (0.13) | 1.02 (0.13) | 0.04 | 0.09 |
| Tanner stage, n (%) | 0.76 | |||
| Testicles (male) | ||||
| Tanner 1 | 10 (59%) | NA | ||
| Tanner 2–3 | 3 (18%) | NA | ||
| Tanner 4–5 | 4 (24%) | NA | ||
| Pubic hair (male) | ||||
| Tanner 1 | 4 (24%) | 13 (31%) | ||
| Tanner 2–3 | 7 (41%) | 13 (31%) | ||
| Tanner 4–5 | 6 (35%) | 16 (38%) | ||
| Breast (female) | ||||
| Tanner 1 | 7 (28%) | 10 (18%) | ||
| Tanner 2–3 | 9 (36%) | 23 (40%) | ||
| Tanner 4–5 | 9 (36%) | 24 (42%) | ||
| Pubic hair (female) | ||||
| Tanner 1 | 4 (17%) | NA | ||
| Tanner 2–3 | 8 (33%) | NA | ||
| Tanner 4–5 | 12 (50%) | NA | ||
| CAH subtype, n (%) | N/A | |||
| Salt-wasting | 19 (45%) | |||
| Simple-virilizing | 13 (31%) | |||
| Non-classic | 10 (24%) | |||
| Hydrocortisone, average dose, mg/m2/day | 11.3 (2.9) | N/A | ||
| Bone age Z-score | 1.34 (2.02) | NA |
BMI, body mass index; NA, not available; N/A, not applicable; CAH, congenital adrenal hyperplasia.
BMD
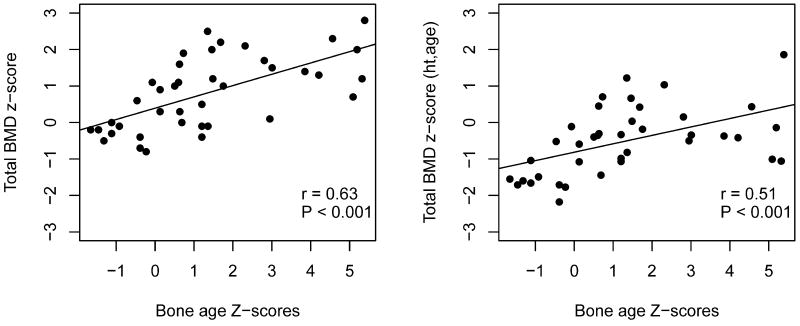
Cases had lower TBMD Z-scores compared to controls (0.81 vs. 1.27, p=0.003) (Table 2). This difference remained significant after adjusting for height-for-age Z-scores (i.e., Zemel-adjustment, −0.51 vs. −0.01, p=0.001). TBMD Z-scores were positively correlated with bone age Z-scores in cases with CAH (r=0.63, p<0.0001), even after adjusting for height-for-age Z-scores (r=0.51, p=0.001) (Figure 1). TBMD and TBMDHAZ Z-scores were not associated with average HC dose (p=0.68 and p=0.67 respectively), CAH subtype (p=0.22 and p=0.50 respectively), or age at diagnosis (p=0.18 and p=0.23 respectively).
Table 2.
CAH Cases and Controls compared according to BMD and Body Composition Measures
| BMD or Body Composition Measures |
Cases | Controls | Control − Case |
P-value |
|---|---|---|---|---|
| TBMD Z-scores | 0.81 (0.14) | 1.27 (0.11) | 0.47 (0.15) | 0.003* |
| TBMDHAZ Z-scores | −0.51 (0.14) | −0.01 (0.10) | 0.49 (0.15) | 0.001* |
| logVAT Mass | 2.00 (0.08) | 2.11 (0.07) | 0.11 (0.07) | 0.11 |
| A:G ratio | 0.405 (0.01) | 0.411 (0.01) | 0.006 | 0.33 |
| Percent Total Tissue Fat | 30.6 (1.5) | 32.4 (1.4) | 0.94 | 0.052 |
Written as adjusted average (standard error); CAH, congenital adrenal hyperplasia; BMD, bone mineral density; TBMD, total body bone mineral density; TBMDHAZ, total body bone mineral density Z-scores adjusted for height-for-age Z-scores; VAT, visceral adipose tissue; A:G, Android:Gynoid ratio
Indicates a significant value
Figure 1.
Correlation plots between total bone mineral density (TBMD) Z-scores and bone age Z-scores (left) and TMBD Z-scores adjusted for height-for-age Z-scores (TBMDHAZ) and bone age Z-scores (right) for CAH Cases
Body Composition
VAT did not differ significantly between cases and controls (adjusted average log10 VAT Mass 2.00 vs. 2.11, p=0.11) (Table 2). Adjusting VAT for height SD, age, and sex had negligible effects on this comparison; VAT still did not differ between cases and controls after adjustment (p=0.16). Among CAH cases, the association between VAT and bone age Z-scores trended in the negative direction (r=−0.26) but did not reach significance (p=0.11). VAT was not significantly associated with HC dose (p=0.98), CAH subtype (p=0.36), or age at diagnosis (p=0.15). Although cases with CAH had slightly lower percent total tissue fat (30.6% in cases vs. 32.4% in controls, p=0.052) and A:G ratios (0.405 in cases vs. 0.411 in controls, p=0.33) than controls, the differences did not reach significance. The A:G ratio was not associated with average HC dose (p=0.08), CAH subtype (p=0.29), or age at diagnosis (p=0.33).
BMD vs. VAT
In CAH cases, TBMD and TBMDHAZ Z-scores and log10 VAT were negatively correlated after adjusting for BMI Z-score (for TBMD, slope= −0.66, p=0.024; for TBMDHAZ, slope= −0.96, p=<0.001). The correlation between TBMDHAZ Z-scores and VAT remained upon further adjustment for bone age Z-score (for TBMD, p=0.30; for TBMDHAZ, p<0.001). VAT was not associated with TBMD or TBMDHAZ Z-scores in controls after adjustment for BMI Z-score (for TBMD, slope= −0.005, p=0.98; for TBMDHAZ, slope= −0.17, p=0.38).
DISCUSSION
We found that children with CAH had lower TBMD Z-scores compared to controls, with the difference being about the same with or without adjustment for height-for age. TBMD and TBMDHAZ Z-scores were positively correlated with bone age Z-scores but were not associated with HC dose, CAH subtype, or age at diagnosis. While studies in the literature have assessed BMD in children with CAH, comparison between the studies is difficult due to varying age ranges (and pubertal stage), dosage and type of glucocorticoid used; inconsistently reported subtypes; and variable measures of control assessment of androgen exposure. Therefore, it is not surprising that studies have reported BMD to be reduced9, 10, 12–15, increased16 and or no different3–8 in children with CAH.
Among studies that reported decreased BMD in CAH children compared to controls, two studies found that higher HC dose (or its equivalents) was associated with lower BMD Z-scores,12, 14 whereas Demirel et al15 and our study found no association. The rest of the studies did not examine this relationship.9, 10, 13 Most children in the two studies that found a negative relationship were on long-acting glucocorticoids. The finding of no relationship between total daily HC dose and decreased BMD suggests that other factors such as dose distribution, individual cortisol pharmacokinetics, glucocorticoid receptor sensitivity and type of glucocorticoid, all of which determine cortisol and androgen exposures over the course of the day, may play a role in the impact of glucocorticoids on BMD. For example, in our study, patients were treated with HC within the recommended range, but this did not prevent the presumed adverse effects of glucocorticoids on BMD as potentially some patients were more glucocorticoid sensitive or had decreased cortisol clearance and/or increased half-life, and therefore had higher biologically active glucocorticoid available at the tissue level.
Among studies that reported decreased BMD, the relationship between advanced bone age and BMD Z-scores was examined only in de Almeida Freire et al, who found, as we did, that in CAH cases advanced bone age was associated with higher BMD Z-scores.10 This finding is not surprising as higher bone age Z-scores indicate greater androgen exposure which can lead to an increase in BMD through androgen stimulation of osteoblastic activity.17 Aromatization of elevated adrenal androgen to estrogen may also have some protective effects on bone. Estrogen reduces the release of inflammatory cytokines such as IL-6, IL-1, and TNF-alpha from osteoblasts and decreases osteoclastic activity by suppressing RANK-L-induced osteoclast differentiation.29 In addition, estrogen can increase osteoprotegerin production30 and prevent glucocorticoid-induced apoptosis in osteoblasts.31
Several studies have found no difference in BMD Z-scores between children with CAH and controls.3–8, 11 In some of these studies, national reference data were used instead of age-, sex-, and BMI-matched controls;3, 4, 7, 11 BMD was not adjusted for height-for-age Z-score,4–8 which could lead to under- or overestimation of BMD Z-score25; the CAH subtype3, 4 or the type of glucocorticoid was not always specified4, 7; and in one study, only females with CAH were evaluated.8 Three of these studies found no association between HC dose and BMD Z-scores4, 8, 11, and the rest did not examine the relationship.3, 5–7
Only one study found increased BMD in children with CAH16, but that study differed from ours in that it included not only children with 21-hydroxylase deficiency but also 11β-hydroxylase deficiency; did not use age-, sex-, and BMI-matched controls; did not adjust BMD Z-scores for height-for-age Z-scores; did not examine measures of androgen control; did not specify the type of glucocorticoid used; and did not specify the pubertal status of the patients.
Our study was the first to use DXA to calculate VAT in children with CAH and found no difference in VAT between cases and controls. Only one previous study, by Kim et al, quantified VAT in children with CAH using a computed tomography scan and found increased VAT in 28 children with CAH.32 Several factors other than the measurement method may account for this difference. One of them is the dose of HC used, which was higher in the study by Kim et al., 19.5 (SD 5.4) mg/m2/day vs. 11.3 (SD 2.9) mg/m2/day in our study. In addition, CAH cases in Kim et al. were shorter (height Z-score: −0.96 vs. 0.67) and more overweight/obese (BMI Z-score: 1.31 vs 0.95), indicating higher glucocorticoid exposure. High doses of glucocorticoids increase VAT by promoting the differentiation of pre-adipocytes into central fat33 and by decreasing adrenal androgen production though suppression of the hypothalamic-pituitary adrenal axis. Androgen, mostly through aromatization to estrogen, increases lipolysis, helps prevent the differentiation of pre-adipocytes into adipocytes, and, in VAT specifically, inhibits lipoprotein lipase (which promotes fat accumulation).34 While we did not find a significant negative association between bone age Z-scores and VAT, the association trended in this direction, which may imply that increased adrenal sex steroids have a role in decreasing VAT in children with CAH.
We found a negative correlation between VAT and TBMD and TBMDHAZ Z-scores in the CAH cases, but did not find this association in controls. Adipose tissue secretes various inflammatory cytokines, such as TNF-a and IL-6, which enhance osteoclast differentiation and activation and inhibit osteoclast apoptosis.35 Altered hypothalamic-pituitary-adrenal axis and chronic fluctuations of cortisol and adrenal sex steroids may affect fat distribution and may increase secretion of inflammatory cytokines from VAT, leading to the inverse relationship between VAT and bone mineral density.35
The strengths of our study were the use of age-, sex-, and BMI-matched controls; adjustment of BMD Z-scores for height-for-age Z-scores given that CAH patients tend to be taller than their peers during childhood; having most of our subjects on HC; and use of bone age Z-scores to assess chronic androgen exposure rather than single measures of steroid concentrations which reflect control only at the time of measurement. Limitations of our study include its retrospective design as well as not having the testicular exam or bone age performed in controls. Cumulative glucocorticoid dosing since birth was not calculated as this information was not available in all patients. However, we examined the effect of age at diagnosis on BMD as an indirect measure of duration of glucocorticoid exposure and found no relationship between BMD and age at diagnosis. In conclusion, we found that CAH cases had lower TBMD than controls and that VAT and BMD were negatively associated in CAH cases. Bone age Z-scores and BMD were positively correlated, displaying the positive effects of androgen exposure in increasing BMD. Future longitudinal studies in children with CAH to examine the impact of chronic hormonal imbalances on BMD and VAT are needed, including duration of over- and under-cortisol exposure as well as androgen exposure over the course of the day.
Acknowledgments
not applicable
Financial disclosure: Funding for this project was provided in part by the National Heart, Lung, and Blood Institute/NIH (R01HL110957), the National Center for Advancing Translational Sciences/NIH (UL1TR000114), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)/NIH NORC Grant Number P30 DK050456, and the Clinical and Translational Science Award/NIH (8UL1TR000114-02). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Kelly receives research support (drug/placebo) from Astra Zeneca Pharmaceuticals and serves as a consultant for Novo Nordisk, Orexigen, and Vivus Pharmaceuticals but does not accept personal or professional income for these activities.
Abbreviations
- CAH
congenital adrenal hyperplasia
- BMD
bone mineral density
- VAT
visceral adipose tissue
- BA
bone age
- HC
hydrocortisone
- A:G
Android:Gynoid
Footnotes
Conflict of Interest Statement: The other authors have nothing to declare.
References
- 1.Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC, Endocrine S. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4133–4160. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarafoglou K, Zimmerman CL, Gonzalez-Bolanos MT, Willis BA, Brundage R. Interrelationships among cortisol, 17-hydroxyprogesterone, and androstenendione exposures in the management of children with congenital adrenal hyperplasia. J Investig Med. 2015;63:35–41. doi: 10.1097/JIM.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 3.Fleischman A, Ringelheim J, Feldman HA, Gordon CM. Bone mineral status in children with congenital adrenal hyperplasia. J Pediatr Endocrinol Metab. 2007;20:227–235. doi: 10.1515/jpem.2007.20.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkielstain GP, Kim MS, Sinaii N, Nishitani M, Van Ryzin C, Hill SC, Reynolds JC, Hanna RM, Merke DP. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97:4429–4438. doi: 10.1210/jc.2012-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gussinye M, Carrascosa A, Potau N, Enrubia M, Vicens-Calvet E, Ibanez L, Yeste D. Bone mineral density in prepubertal and in adolescent and young adult patients with the salt-wasting form of congenital adrenal hyperplasia. Pediatrics. 1997;100:671–674. doi: 10.1542/peds.100.4.671. [DOI] [PubMed] [Google Scholar]
- 6.Mora S, Saggion F, Russo G, Weber G, Bellini A, Prinster C, Chiumello G. Bone density in young patients with congenital adrenal hyperplasia. Bone. 1996;18:337–340. doi: 10.1016/8756-3282(96)00003-8. [DOI] [PubMed] [Google Scholar]
- 7.Girgis R, Winter JS. The effects of glucocorticoid replacement therapy on growth, bone mineral density, and bone turnover markers in children with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1997;82:3926–3929. doi: 10.1210/jcem.82.12.4320. [DOI] [PubMed] [Google Scholar]
- 8.Elnecave RH, Kopacek C, Rigatto M, Keller Brenner J, Sisson de Castro JA. Bone mineral density in girls with classical congenital adrenal hyperplasia due to CYP21 deficiency. J Pediatr Endocrinol Metab. 2008;21:1155–1162. doi: 10.1515/jpem.2008.21.12.1155. [DOI] [PubMed] [Google Scholar]
- 9.Cameron FJ, Kaymakci B, Byrt EA, Ebeling PR, Warne GL, Wark JD. Bone mineral density and body composition in congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1995;80:2238–2243. doi: 10.1210/jcem.80.7.7608286. [DOI] [PubMed] [Google Scholar]
- 10.de Almeida Freire PO, de Lemos-Marini SH, Maciel-Guerra AT, Morcillo AM, Matias Baptista MT, de Mello MP, Guerra G., Jr Classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency: a cross-sectional study of factors involved in bone mineral density. J Bone Miner Metab. 2003;21:396–401. doi: 10.1007/s00774-003-0434-6. [DOI] [PubMed] [Google Scholar]
- 11.Garcia Alves PA, Junior, Schueftan DL, de Mendonca LM, Farias ML, Beserra IC. Bone mineral density in children and adolescents with congenital adrenal hyperplasia. Int J Endocrinol. 2014;2014:806895. doi: 10.1155/2014/806895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metwalley KA, El-Saied AR. Bone mineral status in Egyptian children with classic congenital adrenal hyperplasia. A single-center study from Upper Egypt. Indian J Endocrinol Metab. 2014;18:700–704. doi: 10.4103/2230-8210.139236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paganini C, Radetti G, Livieri C, Braga V, Migliavacca D, Adami S. Height, bone mineral density and bone markers in congenital adrenal hyperplasia. Horm Res. 2000;54:164–168. doi: 10.1159/000053253. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann A, Sido PG, Schulze E, Al Khzouz C, Lazea C, Coldea C, Weber MM. Bone mineral density and bone turnover in Romanian children and young adults with classical 21-hydroxylase deficiency are influenced by glucocorticoid replacement therapy. Clin Endocrinol (Oxf) 2009;71:477–484. doi: 10.1111/j.1365-2265.2008.03518.x. [DOI] [PubMed] [Google Scholar]
- 15.Demirel F, Kara O, Tepe D, Esen I. Bone mineral density and vitamin D status in children and adolescents with congenital adrenal hyperplasia. Turk J Med Sci. 2014;44:109–114. doi: 10.3906/sag-1301-114. [DOI] [PubMed] [Google Scholar]
- 16.Lin-Su K, New MI. Effects of adrenal steroids on the bone metabolism of children with congenital adrenal hyperplasia. Ann N Y Acad Sci. 2007;1117:345–351. doi: 10.1196/annals.1402.040. [DOI] [PubMed] [Google Scholar]
- 17.Manolagas SC, O'Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol. 2013;9:699–712. doi: 10.1038/nrendo.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seibel MJ, Cooper MS, Zhou H. Glucocorticoid-induced osteoporosis: mechanisms, management, and future perspectives. Lancet Diabetes Endocrinol. 2013;1:59–70. doi: 10.1016/S2213-8587(13)70045-7. [DOI] [PubMed] [Google Scholar]
- 19.Mooij CF, Webb EA, Claahsen van der Grinten HL, Krone N. Cardiovascular health, growth and gonadal function in children and adolescents with congenital adrenal hyperplasia. Arch Dis Child. 2016 doi: 10.1136/archdischild-2016-311910. [DOI] [PubMed] [Google Scholar]
- 20.Direk K, Cecelja M, Astle W, Chowienczyk P, Spector TD, Falchi M, Andrew T. The relationship between DXA-based and anthropometric measures of visceral fat and morbidity in women. BMC Cardiovasc Disord. 2013;13:25. doi: 10.1186/1471-2261-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samsell L, Regier M, Walton C, Cottrell L. Importance of android/gynoid fat ratio in predicting metabolic and cardiovascular disease risk in normal weight as well as overweight and obese children. J Obes. 2014;2014:846578. doi: 10.1155/2014/846578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosch TA, Dengel DR, Kelly AS, Sinaiko AR, Moran A, Steinberger J. Visceral adipose tissue measured by DXA correlates with measurement by CT and is associated with cardiometabolic risk factors in children. Pediatr Obes. 2015;10:172–179. doi: 10.1111/ijpo.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, Ergun DL. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring) 2012;20:1313–1318. doi: 10.1038/oby.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 25.Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, Mahboubi S, Shepherd JA, Hangartner TN, Frederick MM, Winer KK, Kalkwarf HJ. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95:1265–1273. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stults-Kolehmainen MA, Stanforth PR, Bartholomew JB, Lu T, Abolt CJ, Sinha R. DXA estimates of fat in abdominal, trunk and hip regions varies by ethnicity in men. Nutr Diabetes. 2013;3:e64. doi: 10.1038/nutd.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller WL. Clinical review 54: Genetics, diagnosis, and management of 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1994;78:241–246. doi: 10.1210/jcem.78.2.8106606. [DOI] [PubMed] [Google Scholar]
- 28.Greulich WaSIP. Radiographic Atlas of Skeletal Development of the Hand and Wrist. Stanford University Press; Stanford, California: 1950. [Google Scholar]
- 29.Shevde NK, Bendixen AC, Dienger KM, Pike JW. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci U S A. 2000;97:7829–7834. doi: 10.1073/pnas.130200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Spelsberg TC, Riggs BL. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140:4367–4370. doi: 10.1210/endo.140.9.7131. [DOI] [PubMed] [Google Scholar]
- 31.Gohel A, McCarthy MB, Gronowicz G. Estrogen prevents glucocorticoid-induced apoptosis in osteoblasts in vivo and in vitro. Endocrinology. 1999;140:5339–5347. doi: 10.1210/endo.140.11.7135. [DOI] [PubMed] [Google Scholar]
- 32.Kim MS, Ryabets-Lienhard A, Dao-Tran A, Mittelman SD, Gilsanz V, Schrager SM, Geffner ME. Increased Abdominal Adiposity in Adolescents and Young Adults With Classical Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency. J Clin Endocrinol Metab. 2015;100:E1153–1159. doi: 10.1210/jc.2014-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasquali R, Vicennati V, Gambineri A, Pagotto U. Sex-dependent role of glucocorticoids and androgens in the pathophysiology of human obesity. Int J Obes (Lond) 2008;32:1764–1779. doi: 10.1038/ijo.2008.129. [DOI] [PubMed] [Google Scholar]
- 34.Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. 2004;5:197–216. doi: 10.1111/j.1467-789X.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 35.Russell M, Mendes N, Miller KK, Rosen CJ, Lee H, Klibanski A, Misra M. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab. 2010;95:1247–1255. doi: 10.1210/jc.2009-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]