Abstract
Timely follow-up for positive cancer screening results remains suboptimal and the evidence base to inform decisions on optimizing the timeliness of diagnostic testing is unclear. This systematic review evaluated published studies regarding time to follow-up after a positive screening for breast, cervical, colorectal, and lung cancers. The quality of available evidence was very-low or low across cancers with potential attenuated or reversed associations from confounding by indication in most studies. Overall, evidence suggested that the risk for poorer cancer outcomes rises with longer wait times that varies within and across cancer types, which supports performing diagnostic testing as soon as feasible after the positive result, but evidence for specific time targets is limited. Within these limitations, we provide our opinion on cancer-specific recommendations for times to follow-up and how existing guidelines relate to the current evidence. Thresholds set should consider patient worry, potential for loss to follow-up with prolonged wait times, and available resources. Research is needed to better guide the timeliness of diagnostic follow-up, including considerations for patient preferences and existing barriers, while addressing methodological weaknesses. Research is also needed to identify effective interventions for reducing wait times for diagnostic testing, particularly in underserved or low-resource settings.
MeSH key words: Neoplasm, early diagnosis, breast, cervix uteri, colon, lung, early detection of cancer, mass screening
Introduction
The Institute of Medicine (now National Academy of Medicine) identified improving the timeliness and patient-centeredness of care as important unmet health care priorities.1–3 Screening has been shown to reduce the risk of death from some cancers and is currently recommended at Grade A or B by the US Preventive Services Task Force (USPSTF) in eligible persons for breast, cervical, colorectal, and lung cancers,4–7 which enables full coverage of those services under the Affordable Care Act.8 To realize the benefits of screening, and depending on the type of test used, an individual with a positive screening result must undergo follow-up evaluation, including diagnostic testing, to guide clinical decision-making.4–7,9–18 However, there is considerable variation in the time to receipt of follow-up diagnostic testing among patients with positive screening results,19–23 and it is unclear how such differences influence cancer outcomes. The ideal timing of follow-up testing is unknown.
For several reasons, conventional wisdom has been to deliver diagnostic follow-up for a positive screening with minimal delay. First, cancer may progress over time from precancerous or early (more curable) tumors to advanced (less curable) cancer.24 Prompt diagnostic testing enables detection earlier in the course of carcinogenesis and may thus reduce mortality risk.25 Second, prompt diagnostic testing is part of patient-centered care,1–3 and may reduce worry from uncertainties about the procedure.26–31 Third, provider- or system-related delays in follow-up may increase the likelihood that diagnostic testing may not occur at all due to new logistical hurdles such as changes in patient contact information or insurance coverage.32 However, the assumption that quicker is always better is largely unproven in terms of patient outcomes. Rapid follow-up efforts may require more health care resources to implement and need to be weighed against the benefits and opportunity costs.33 Thus, evidence is needed to guide clinicians and health systems on the timeliness of diagnostic testing after a positive screening test.
This systematic review aimed to identify evidence from the published literature to inform recommendations for clinical practice and quality improvement on the timeliness of diagnostic testing for positive cancer screening results. We evaluated the following key question: what is the association between the time from the positive screening result and receipt of diagnostic testing on the risk of cancer progression and death for breast, cervical, colorectal, and lung cancers?
Methods
This review was conducted by a trans-disciplinary team of cancer-specific experts in the Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) consortium supported by the National Cancer Institute. PROSPR is comprised of research centers at diverse healthcare systems across the United States.34 Our review focused on breast, cervical, colorectal, and lung cancers because of existing recommendations and policies on screening,4–8 and thus the need for guidance on follow-up evaluation after a positive screen.
Conceptual Framework
Our review was guided by an analytical framework that was drawn from the conceptual model of the screening process developed by the PROSPR consortium.35 We focused on the steps between the receipt of a positive screening result and cancer diagnosis and death (Figure 1). Thus, our literature search sought to identify studies that addressed cancer diagnosis or cancer mortality as the outcome. We considered studies on intermediate outcomes such as precancerous lesions or tumor size as reasonable proxies where direct evidence was lacking.
Figure 1.
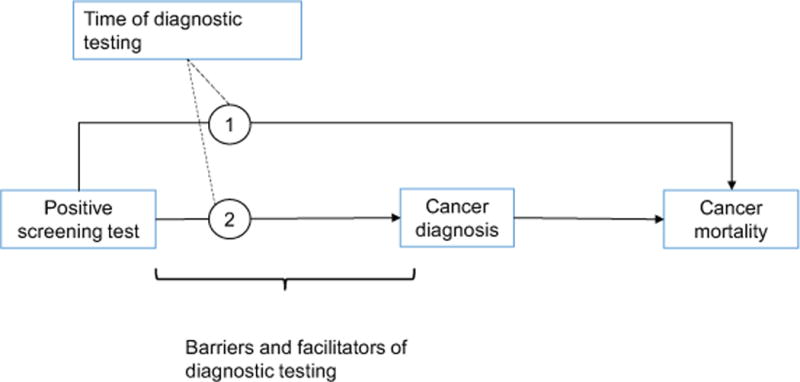
Analytic framework for the systematic review on time to diagnosis after positive screen. This model indicates how the timing of diagnostic testing can impact the pathways between (1) the positive screening test and cancer mortality and (2) the positive screening test and cancer diagnosis.
Selection criteria
To be included, articles had to be written in English; published in a peer-reviewed journal between January 1, 1998 and December 31, 2017 and indexed in MEDLINE; conducted in an average-risk population, except in lung cancer; used study designs that provided empirical evidence such as randomized controlled trials, cohort or case-control, systematic review, or meta-analysis; and evaluated our key question. The timeframe includes the date of some of the earliest screening guidelines for currently used strategies,36 and it seemed likely that relevant literature prior to 1998 would be included in more recent publications. We also included modeling studies but excluded publications that were unlikely to provide empirical evidence, such as commentaries, editorials, and opinion pieces (see Supporting Information 1 for specific inclusion and exclusion criteria).
We sought to restrict our review to studies on asymptomatic patients who are undergoing screening, which included studies in organized screening programs. However, we considered studies of wait time from symptomatic presentation to diagnosis or treatment to provide context, since the underlying biological processes may be similar in asymptomatic patients.
Data sources and search
We used a systematic search strategy for each cancer type that included general screening terms (e.g., diagnosis, screening, early detection of cancer), cancer-specific screening terms (e.g., mammogram, cytology, Papanicolaou (Pap) test, fecal immunochemical test (FIT), or low-dose computed tomography (LDCT)), terms capturing time to diagnostic testing (e.g., time, follow-up, compliance, completion, biopsy), general terms for positive results (e.g., abnormal, positive, suspicious), and cancer-specific terms for positive results (e.g., breast cancer, high-grade squamous intraepithelial lesion (HSIL), adenocarcinoma, or lung nodule). To enhance complete capture of published literature on our key question, the cancer-specific teams reviewed references in articles and existing guidelines and other relevant peer-reviewed literature identified in publications in their professional networks or collaborative work. The search criteria were then refined to improve results (see Supporting Information 2 for search terms). We performed four separate standardized searches in MEDLINE, one for each cancer type.
Data extraction
Articles retrieved through the search were screened by two trained reviewers for inclusion criteria using standardized procedures. Disagreements were resolved by two senior team members (KAR and NBG) with adjudication by a third (CAD) when necessary. Eligible unique articles were then reviewed by each cancer-specific expert group to generate evidence summaries (breast: KA and AMM; cervical: CMW and PEC; colorectal: CAD, DAC, EAH; and lung: CAD and AV). The quality of the evidence for each cancer was evaluated with the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group taxonomy.37 We used Cochrane tools for assessing risk of bias in cohort studies, which we rated using the ROBINS-I criteria as low, moderate, serious, or critical.38 We provide summaries under each cancer type, but the small numbers of eligible articles precluded a formal meta-analyses. In each study assessed, we also examined if confounding by indication was likely present.39,40
Definitions of confounding by indication in follow-up diagnostic testing
Confounding by indication occurs when the clinical reason for an intervention or treatment (the predictor) is related to the outcome under study. In the specific context of time of diagnostic testing for positive cancer screening results, clinicians may prioritize follow-up testing for more severe screening findings or for patients who report symptoms in addition to a positive screen. For example, patients with highly suspicious findings on LDCT may be followed-up more quickly; such patients are at higher risk of more advanced or fatal disease. This type of confounding would distort a positive association between time to diagnostic testing and cancer mortality such that estimates from analyses are attenuated, null, or even reversed to falsely suggest that a longer wait time is protective.
Definitions of a positive screening test result
We used consensus definitions to standardize the review across cancer types (Table 1). A positive screening was defined as the result or set of results from a screening test or tests in an eligible asymptomatic person that indicate the need for a confirmatory diagnostic test (such as imaging, visualization and/or biopsy). A diagnostic test was defined as a test used to determine the presence or absence of precancerous abnormality or invasive cancer following a positive screening result. An imaging or endoscopic test performed as follow-up for a positive screen was considered a diagnostic test even if biopsy was not performed. However, tests performed as part of multi-step screening processes or to triage an abnormal screening were not considered diagnostic testing: examples include repeat mammogram for an American College of Radiology Breast Imaging-Reporting and Data System (BI-RADS) score of 0, follow-up testing for a single positive human papillomavirus (HPV) DNA test with normal cytology, or surveillance for some lung nodules.
Table 1.
Summary of definitions of positive screening, choice of diagnostic testing, and test characteristics
| Cancer type | Positive screening test criteria | Diagnostic tests | Test performance characteristics* |
|---|---|---|---|
| BREAST | • Mammography with BI-RADS 4 or 5 | • Core needle biopsy • Fine-needle aspiration biopsy • Open biopsy |
• Sensitivity: 87%; specificity: 98%43 • Sensitivity: 74%; specificity: 96%43 • Sensitivity ≥98%44 |
| CERVICAL | • ** HPV+ & ASC-US+55 • **≥LSIL55 • HPV 16/18 in women ≥ 25y56 • Persistent (≥12 months) hrHPV+55 |
• Colposcopy-directed biopsy***
|
• Sensitivity: 60.6% (95% CI, 54.8% to 66.6%)65 • Sensitivity: 85.6% (95% CI, 80.3% to 90.2%)65 • Sensitivity: 95.6% (95% CI, 91.3% to 99.2%)65 |
| COLORECTAL | • Positive guaiac FOBT, FIT, or multitarget DNA • Polyp or lesion during sigmoidoscopy, CTC |
• Colonoscopy | • Sensitivity: 95%; specificity: 86%87 |
| LUNG | • LDCT indicating suspicious nodule or nodule that has increased in size since prior scan | • Imaging (PET scan) • Sputum cytology • Lung biopsy |
• Sensitivity: 97%; specificity: 78%104 • Sensitivity: 66%; specificity: 99%101 • Sensitivity: 90%; specificity: 97%101 |
Test characteristics calculated relative to the following gold standards: Breast – surgical biopsy; Cervical – presence of any HSIL found in at least one of four biopsies; Colorectal - tandem colonoscopy; Lung – modified gamma camera, semiquantitative and qualitative methods or semiquantitative methods only (imaging); histological confirmation or follow-up ≥ 1 year (sputum cytology and lung biopsy).
Management options may vary if the woman is pregnant or ages 21-24 years
Test characteristics vary by the number of biopsies taken
Abbreviations: +=positive; ASC-US=atypical squamous cells of undetermined significance; BI-RADS=Breast Imaging Reporting and Data System; LSIL=low-grade squamous intraepithelial lesion; ≥LSIL=LSIL or more severe cytologic abnormalities; HPV=Human papillomavirus; hr=high risk; CIN2+, cervical intraepithelial neoplasia grade 2 or more severe diagnoses; FIT=fecal immunochemical test; FOBT=fecal occult blood test; LDCT, low-dose computed tomography; NBCCEDP, National Breast and Cervical Cancer Early Detection Program; PET, positron emission tomography
Results
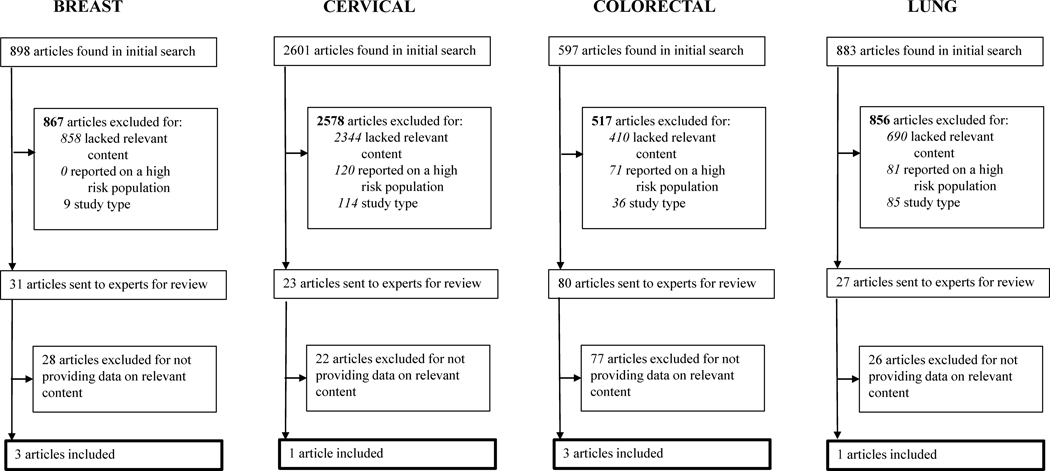
The results of the search are shown in Figure 2, and are presented along with evidence syntheses under each cancer type. Each section begins with an overview of screening processes followed by the evidence summary, contextual information, and recommendations offered by the PROSPR group.
Figure 2.
Study flow diagram for the selection of included studies in the systematic review
Breast cancer
Overview of Screening and Diagnostic Testing for Breast Cancer
Breast cancer is the most common cancer and the second leading cause of cancer death in US women, with more advanced disease and lower survival in black women.41 Breast cancer risk is related to age, family history, inheritable genetic susceptibility including BRCA1 and BRCA2 mutations, and other risk factors such as reproductive factors, breast density, obesity, alcohol intake, and exposure to hormones or radiation.41 Screening for breast cancer is recommended at Grade B for 50-74-year-old women by the USPSTF, but recommendations vary across public health and professional organizations’ guidelines on ages to start and stop screening.7,13,42
Mammography is the primary screening modality for breast cancer and has been recommended by various groups since the 1970’s.36 Findings on breast cancer screening are generally reported using the BI-RADS, with categories 4 or 5 recommended to receive a core needle or open surgical biopsy.9,10 Ultrasound and stereotactic-guided core needle biopsy has a sensitivity of >97% and specificity of 92-99% for cancer diagnosis with open biopsy as the standard (Table 1).9,43 Core needle biopsy without imaging guidance has lower sensitivity (91%) but similar specificity to open biopsy.44 Serious harms are relatively uncommon (<1%) and include bleeding, infection, vasovagal reactions, and procedure-related anxiety.26 The risk of harms is lower with core needle than with open biopsy.44
Quality metrics based on expert opinion for the time between a positive screening result and biopsy include the National Quality Measures for Breast Centers™ (NQMBC) program of the National Consortium of Breast Centers, Inc. and the Breast and Cervical Cancer Early Detection Program (BCCEDP), supported by the Centers for Disease Control and Prevention.45 These guidelines include both the time for resolution of an indeterminate (BI-RADS 0) and positive (BI-RADS 4/5) mammogram. BCCEDP sets a benchmark of 60 days or less from screening to diagnosis while the NQMBC™ ranks participating centers against each other and aims for each center to perform above the 25th percentile on 75% of measures. The National Accreditation Program for Breast Centers does not address time to diagnostic testing.46
Evidence Synopsis for Breast Cancer
The search yielded 898 unique articles for breast cancer and after initial screening, 31 articles were selected for review, and 3 were deemed acceptable by breast cancer team,47–49 including a multi-model microsimulation study on breast, cervical, and colorectal cancers by PROSPR investigators in collaboration with the CISNET (Cancer Intervention and Surveillance Modeling Network) group (see Supporting Information 3 for a list of final included articles).49
Olivotto et al. examined the association between the time interval from a positive screening mammogram to diagnostic testing and tumor characteristics among 4,465 women diagnosed with breast cancer in five Canadian organized breast cancer screening programs between 1990 and 1996 (Table 3).47 On average, women with mammograms that are more suspicious for cancer had diagnostic testing sooner than those who had less suspicious results (31 days for high suspicion vs. 47 days for others, p<0.0001). In regression models controlling for level of suspicion, compared with diagnostic testing at 29-84 days after the positive result, there was no statistically significant difference in risk for tumor size >10 mm or lymph node metastases for women with diagnostic testing at 85-140 days or 141-364 days after the positive screening exam. Those with a 365-728-day wait-time had a significantly increased odds of tumors >10 mm (odds ratio [OR]: 1.51, 95% confidence interval [CI] 1.05-2.16) and of lymph node metastases (OR: 2.16, CI 1.48-3.15). The associations were even greater for a 729-1092-day wait-time (OR: 2.11, CI 1.15-3.86 and 3.19, CI 1.84-5.55, respectively) (Figure 3).47
Table 3.
Summary of estimates from studies included in the review
| Study, year | Country | Study design | Sample size, n | Risk of bias | Days from positive screen to diagnosis* | Outcomes measured | Associations (Outcome, OR or RR with 95% confidence interval) |
|---|---|---|---|---|---|---|---|
| BREAST | |||||||
| Olivotto et. al.47, 2002 | Canada | Retrospective cohort | 4465 | Serious | Median: 41 | OR, Reference category: 29-84-day interval | |
| Tumor size >10mm | 0-28-day: 1.59 (1.36, 1.87) 85-140-day: 0.88 (0.66, 1.17) 141-364-day: 1.17 (0.88, 1.56) 365-728-day: 1.51 (1.05, 2.16) 729-1092-day: 2.11 (1.15, 3.86) |
||||||
| Lymph node involvement | 0-28-day: 1.37 (1.16, 1.62) 85-140-day: 0.98 (0.67, 1.42) 141-364-day: 1.19 (0.84, 1.69) 365-728-day: 2.16 (1.48, 3.15) 729-1092-day: 3.19 (1.84, 5.55) |
||||||
| Ganry et. al.48, 2004 | France | Retrospective cohort | 1984 | Serious | Mean (SD): High suspicion lesions – 59 (47) Low suspicion lesions – 78 (52) |
OR, Reference category: <30-day: | |
| Tumor size >10mm | 30-90-day: 1.37 (0.91, 1.63) 90-180-day: 1.32 (0.86, 1.96) >180-day: 1.77 (1.02, 2.85) |
||||||
| Lymph node involvement | 30-90-day: 0.99 (0.67, 1.42) 90-180-day: 1.41 (0.98, 1.98) >180-day: 2.01 (1.29, 3.03) |
||||||
| Rutter et. al.49, 2017 | United States | Microsimulation modeling | NA | n/a | 0 to 365 days (simulated) | RR/LYG, Reference category: 0-day | |
| Late-stage at diagnosis** | 90-day: RR=1.08 365-day: RR=1.26 |
||||||
| Life years gained (LYG) | 90-day: ↓ 17.3% | ||||||
| CERVICAL | |||||||
| Rutter et. al.49, 2017 | United States | Microsimulation modeling | NA | n/a | 0 to 365 days (simulated) | RR/LYG, Reference category: 0-day | |
| Late-stage at diagnosis** | 90 - day: RR=0.99 365-day: RR=0.98 |
||||||
| LYG | - ↓0.8% | ||||||
| COLORECTAL | |||||||
| Corley et. al.94, 2017 | United States | Retrospective cohort | 70,124 | Moderate | Median (IQR) (days): 37 (23-62) | OR, Reference category: 8-30-day | |
| CRC diagnosis | 31-60-day: 0.92 (0.83, 1.02) 61-90-day: 0.95 (0.82, 1.10) 91-180-day: 0.98 (0.82, 1.16) 181-270-day: 1.03 (0.99, 1.72) 271-365-day: 1.48 (1.05, 2.08) >365-day: 2.25 (1.89, 2.68) |
||||||
| Late-stage at diagnosis** | 31-60-day: 0.85 (0.69, 1.04) 61-90-day: 0.78 (0.58, 1.04) 91-180-day: 0.98 (0.71, 1.35) 181-270-day: 1.32 (0.80, 2.18) 271-365-day: 1.97 (1.14, 3.42) >365-day: 3.22 (2.44, 4.25) |
||||||
| Rutter et. al.49, 2017 | United States | Microsimulation modeling | NA | n/a | 0 to 365 days (simulated) | RR/LYG, Reference category: 0-day | |
| Late-stage at diagnosis** | 90-day: RR=1.03 365-day: RR=1.11-1.12 |
||||||
| LYG | 90-day: ↓2.0-2.7% | ||||||
| Meester et. al.95, 2016 | United States | Microsimulation modeling | 10 million (simulated) | n/a | 0 to 365 days (simulated) | Reference category: 0-14 days | |
| CRC incidence | ↑0.3% higher risk for every 30-day interval | ||||||
| CRC mortality | 90-day: ↑4% 365-day: ↑16% |
||||||
| LUNG | |||||||
| Sonavane et al.110, 2017 | United States | Retrospective cohort | 462 | Serious | Median (IQR): 132 (49-484) |
Lung cancer death | For each 180-day interval OR: 1.07 (0.90, 1.20) |
OR = Odds ratio; RR=relative risk or hazard ratios; LYG=life years gained
Late-stage = distant or regional, or stage II or IV at diagnosis
Also provided an estimate of 17.3% decrement in life years gained (LYG) with an interval of 90 days
Figure 3.
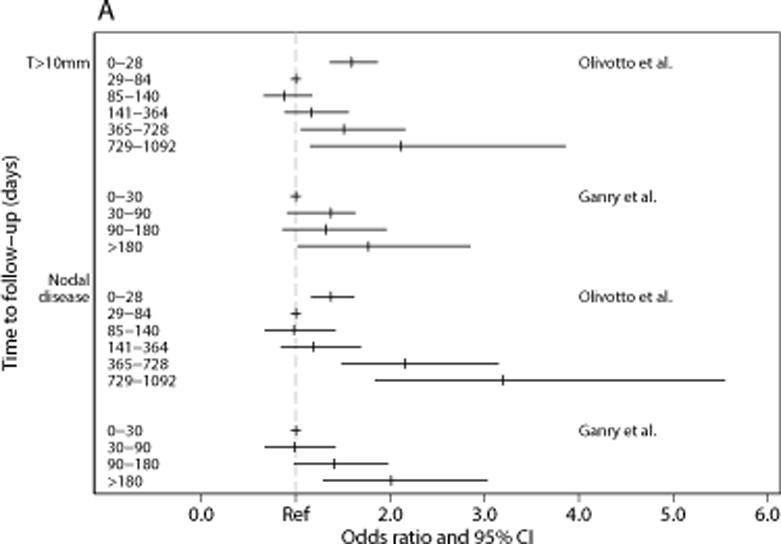
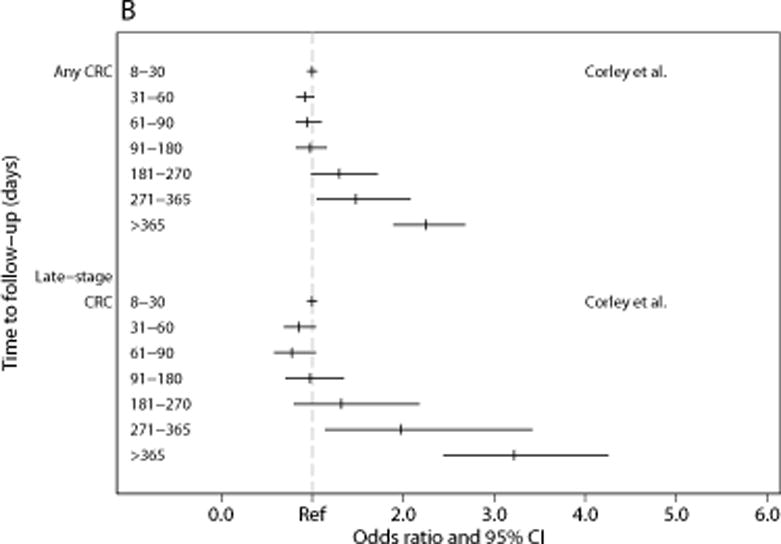
(a) and (b). Association between intervals for diagnostic testing and outcomes for (a) breast cancer and (b) colorectal cancer. Abbreviations: T=tumor size; CI=confidence interval
Ganry et al. analyzed 1,984 women who underwent screening in the Somme area in France and were found to have intermediate or high suspicion screens between 1996 and 2000; 159 of the women were diagnosed with breast cancer on follow up.48 They reported shorter time to diagnostic testing for high suspicion than for intermediate suspicion screening results. Compared with diagnostic testing within 30 days, women with >180-days interval to diagnostic testing had significantly increased risk for tumor size >10 mm (OR: 1.77, CI 1.02-2.85) and of lymph node metastases (OR: 2.01, CI 1.29-3.03) (Table 3). There was a statistically non-significant increased risk of larger tumor size and nodal metastases in 90-180 days.48
Finally, the PROSPR multi-model microsimulation study evaluated the effect of time to diagnostic testing after an abnormal mammogram. The study suggested no difference in the lifetime risk of breast cancer for wait-times of 90 days to 1 year, but the relative risk for late-stage disease was 1.08 at 90 days and 1.26 by 1 year. The modeling yielded a 17.3% decrement in estimated life years gained (LYG) when diagnostic testing occurred at 90 days.49
Contextual Evidence for Breast Cancer
Literature on wait-times in breast cancer diagnosis in women presenting with breast cancer-related symptoms provides indirect evidence for the key question. A 1999 systematic review of 87 studies found that women with wait-times of 3-6 months or longer had lower 5-year survival after diagnosis (OR for death: 1.47, CI 1.42-1.53 compared to <3 months).50 That review was updated with additional 26 articles that included studies examining the association between time to diagnostic testing from symptoms and stage at diagnosis and survival.51 Although the majority of these studies demonstrated an association between longer wait-times and poorer survival, several recent studies either did not find an association or found that patients with longer wait-times had better survival, likely due to confounding by indication.
Conclusion and Strength of Evidence for Breast Cancer Screening
Overall, there was limited evidence for a specific optimal time to diagnostic testing after a positive screening mammogram. One study48 suggested a non-significant increase in disease outcomes risk as early as 90 days after the positive screen date, while another did not observe worse outcomes until after 365-728 days. Thus, the current evidence is not sufficient to determine a precise timing for diagnostic testing, and the quality of evidence is low due to the small number of studies with inconsistent results, which used observational data and had evidence of confounding by indication.52 Therefore, the current published literature does not confirm or refute the 60-day threshold set by the BCCEDP. Given evidence of an increase in risk after 90 days, the 60-day goal set in CDC quality guidelines is a reasonable target for quality improvement after a positive breast cancer screening. Beyond existing practice standards, our conclusion reflects biological plausibility of disease progression with prolonged wait-times, the need for diagnostic testing to occur before discernible increases in risk, and consideration of potential patient anxiety and preferences.
Cervical cancer
Overview of Screening and Diagnostic Testing for Cervical Cancer
In the US, an estimated 12,820 women were diagnosed with cervical cancer and 4,210 women died of the disease in 2017.41 Persistent infection by oncogenic HPV is the primary etiologic factor in cervical cancer.41 HPV types 16 and 18 account for 70% of cases worldwide and an additional 10-12 HPV strains cause the remaining 30%. Cervical cancer is thus potentially preventable through vaccination against HPV. Current screening practices take advantage of the extended multi-step carcinogenic process of progression from incident to persistent HPV infection through cervical intraepithelial neoplasia (CIN) to invasive disease that occurs over an average of 20-25 years.53,54
Cervical cancer screening is recommended as Grade A by the USPSTF. Screening, historically with the Pap test, is credited with the large reductions in cervical cancer incidence and mortality over decades.5,15,36 Recent guidelines include recommendations for HPV-cytology co-testing every 5 years among women aged 30-64 years, with a shift towards HPV-only testing55,56 that is included in new USPSTF recommendations.
The definition of a positive screen depends on age, the presence of persistent high-risk HPV infection, and the severity and persistence of cytological abnormalities.14,15,18,55,57,58 A low-grade squamous intraepithelial lesion (LSIL) or more severe cytology, or HPV-positive atypical squamous cells of undetermined significance (ASC-US) is an indication for colposcopy in the US.18,59 Many other countries, especially those with organized screening programs, follow mild abnormalities such as LSIL (watchful waiting) until there is evidence of persistence. Increasing prevalence of HPV vaccination and identification of new biomarkers may change screening and diagnostic practices.60,61
Colposcopy is the primary diagnostic approach for a positive cervical cancer screen (Table 1) and includes endocervical sampling when endocervical canal extension is suspected, such as atypical glandular cells or adenocarcinoma in situ.55 Colposcopy performance is variable due to subjectivity in where and how many biopsies are taken and low-quality procedures may lead to false reassurance.62–64 Colposcopy sensitivity ranges from 60.6% for a single biopsy to 95.6% for three biopsies (Table 1).65 Current practice guidelines recommend no biopsies in low-risk women without visible acetowhite lesions; biopsy of all acetowhite lesions; and consideration for forgoing colposcopy in favor of treatment in women at very high risk for cervical precancer and cancer (such as at least two high-grade cytology, high-grade colposcopy finding, and/or positive HPV16/18).66,67
Harms from colposcopy are relatively few, but include bleeding, infection, and minor discomfort from prolonged speculum examination, application of acetic acid, and biopsy.68 Women may experience anxiety prior to, during, and after colposcopy.27 Colposcopy identifies CIN2, which may regress,69,70 but typically triggers excisional treatment,55 which can increase the risk of pre-term births by two-fold.71–73
Recommendations on time to diagnostic testing exist in a number of countries based on expert consensus.74 Metrics for time to diagnostic testing for a positive cervical screening in the US were implemented by BCCEDP.75 The interval set by the BCCEDP was originally ≤ 60 days but was subsequently changed in 2009 to ≤ 90 days based on expert opinion and pragmatic considerations of the ability of different health systems to achieve timely follow-up.76
Evidence Synopsis for Cervical Cancer
The final literature search yielded 2,601 unique articles for cervical cancer; 23 articles were selected for review after initial screening, and only the multi-model microsimulation study was deemed relevant.49 The PROSPR multi-model microsimulation study found that longer times to colposcopy led to lower lifetime benefit of screening with 1.4% fewer cancers prevented at about 90 days. There was an estimated decrement of 0.8% and 1.4% in life-years gained with 90- and 180-day intervals to colposcopy, respectively, compared to immediate follow-up (Table 3).49
Contextual Evidence for Cervical Cancer
As with other cancers, severity of abnormalities have been associated with follow-up of a positive Pap and poorer outcomes, thus women suspected to have a higher likelihood of severe disease may receive follow-up sooner.77 Minority, uninsured, and lower socioeconomic status (SES) women have lower probabilities of completing follow-up after a positive screening test result,52,78 and also have higher risk of disease.
Conclusion and Strength of Evidence for Cervical Cancer
The available evidence on the optimal time to diagnostic testing is of very low quality primarily due to the absence of published empirical studies. However, risk of cervical cancer is higher in underserved populations likely due to failure to screen or inadequate follow-up of abnormal cytology, and a modeling study suggests disease progression with longer wait-times.23,79 The current convention is to perform colposcopy within 30 days for suspected invasive disease, within 60 days for high-grade cytology, and within 90 days, but not exceeding 180 days, for women with lower-grade cytology (such as persistent HPV with ASC-US and LSIL).80–83 Because lesions with a low probability of invasive cancer may regress or progress only slowly over time, the urgency of follow-up after a positive cervical cancer screen appropriately depends on the severity of the abnormality, and the 90-day BCCEDP benchmark is a reasonable target for quality improvement, with shorter time intervals if there is suspected invasive disease or high-grade cytology.
Colorectal cancer
Overview of Screening and Diagnostic Testing for Colorectal Cancer
Colorectal cancer (CRC) ranks third in the number of new cancer cases reported annually and is the second leading cause of cancer death among US men and women combined.41 CRC risk is most strongly related to age and genetic susceptibility, including Lynch Syndrome, as well as lifestyle-related factors.41 CRC screening is recommended as Grade A by the USPSTF.4 Screening takes advantage of well-defined precancerous states and the slow progression to invasive disease. Screening has been shown to reduce the risk of death84–86 and is recommended by major national groups.4,11
Guidelines-recommended tests for screening average-risk individuals beginning at age 50 through 75 or 84, depending on prior screening history and life expectancy, include fecal immunochemical test (FIT), fecal DNA, computed tomography (CT) colonography, sigmoidoscopy, and optical colonoscopy. FIT and guaiac-based fecal occult blood tests (gFOBT) are the most widely used tests worldwide and are recommended either annually or biennially. CT colonography and flexible sigmoidoscopy are both less commonly used in the US. Colonoscopy, performed every 10 years, is the most commonly used screening test in the US and can both visualize and perform diagnostic evaluation of detected lesions at the time of screening. To realize the benefits of non-colonoscopy screening, a positive result requires diagnostic colonoscopy with biopsy or polypectomy, as appropriate. The sensitivity and specificity of colonoscopy is estimated to be 95% and 86%, respectively, but depend on the type and size of lesions (Table 1),87 and also vary across performing providers.88,89
Harms of colonoscopy are relatively uncommon and vary in severity from minor to major. Harms increase with age, comorbidity, and use of polypectomy and are mainly gastrointestinal (such as bleeding or perforation), and cardiovascular events (such as myocardial infarction or angina, arrhythmias, cardiac arrest, and syncope, hypotension or shock).30,90 There is also procedure-related fear.28,29
There are few published guidelines or quality metrics for completion of diagnostic testing after a positive CRC screen. A 2006 Canadian Association of Gastroenterology consensus panel recommended 60 days or less from positive screening to colonoscopy.91 The Veterans Health Administration’s (VHA) policy sets a 60-day target for completion of diagnostic colonoscopy from the date of the positive fecal test result;92 the evidence brief for that policy identified only one small study that we included in our contextual literature.93
Evidence Synopsis for Colorectal Cancer
The final literature search yielded 597 unique articles for CRC; after initial screening, 80 articles were selected for review, and 3 were deemed acceptable by the CRC team (See Supporting Information 3 for a list of final included articles),49,94,95 including two modeling studies.49,95
In the largest available study, Corley et al. examined the relationship between time to colonoscopy and risk of any CRC and late-stage CRC in a retrospective cohort of 70,124 patients who had a positive FIT within Kaiser Permanente Southern or Northern California’s organized screening programs between 2010-2014 (Table 3).94 Compared to patients who received colonoscopy 8-30 days after a positive FIT, the study found a trend towards increasing CRC risk after 180 days that became statistically significant at >270 days (OR: 1.48, CI 1.05-2.08) (see Figure 3). Similar times for significant increases in risk were observed for late-stage disease.94,96
Meester and colleagues95 used a well-known and validated microsimulation model that has been used to inform the USPSTF to simulate an average-risk cohort of 10 million 50-75-year-old U.S. adults undergoing annual FIT screening. They estimated the effect of time to diagnostic colonoscopy on disease progression and CRC mortality. The simulated CRC incidence without screening was 64.8 cases per 1,000 and mortality was 26.8 per 1,000. Compared to receiving diagnostic testing within 2 weeks of a positive FIT (incidence of 35.5 cases per 1,000), each additional month interval was estimated to increase both CRC incidence and mortality by 0.1 per 1,000 (a 0.3% and 1.4% monthly increase and 4% and 16% increase with a 12-month wait-time, respectively). The results were similar for fecal-DNA screening, but were twice as large for gFOBT.95 The PROSPR multi-model study used two CRC separate modeling strategies side-by-side. That study reported a RR of 1.01-1.02 for lifetime disease risk at 3 months (compared to immediate diagnosis) and 1.05 at 12 months, a RR of 1.03 for late-stage disease risk at 3 months and 1.11-1.12 at 12 months, and an estimated decrement of 2.0-2.7% in LYG with a 3-month interval to diagnostic testing.49
Contextual Evidence for Colorectal Cancer
In a small observational study (n=231), Gellad et al. examined the association between the timing of diagnostic testing following a positive gFOBT and risk of colorectal neoplasia.93 Median time to colonoscopy was 201 days; the study found that each additional 30-day wait-time for colonoscopy increased the odds of detecting any colorectal neoplasia (cancer or adenoma) (OR: 1.10, CI 1.02-1.19), but they did not evaluate specific time intervals and there was no statistically significant association when the analysis was restricted to advanced neoplasia (OR: 1.07, CI 0.98-1.18).93 A VHA study examined the time between referral for colonoscopy and CRC diagnosis between 2000-2005 in 269 veterans at a single center.97 The number of days between referral to CRC diagnosis was >90 days in 30% of patients. Shorter time to cancer diagnosis was associated with the presence of symptoms and abnormal laboratory findings. An interval of >40 days was correlated with lower mortality risk (hazard ratio: 0.61, CI 0.39-0.96) but became statistically non-significant after adjustment for tumor stage (hazard ratio: 0.75, CI 0.47-1.21). An analysis restricted to 100 patients referred as a result of positive screen (FOBT or sigmoidoscopy) did not find a statistically significant association, but the estimates were not reported.97
Conclusion and Strength of Evidence for Colorectal Cancer
Overall, there was limited evidence for a specific optimal time to diagnostic testing after a positive screening non-colonoscopy result for CRC. There is empirical evidence from a single study of increased risk for CRC incidence and higher stage in a dose-response fashion for wait-times of over 180 days. Modeling results support these findings, suggesting the potential for increased risk with each additional 1-month of wait time for diagnostic testing. The quality of evidence was low due to the small number of empirical observational studies, with some support from modeling studies.
The current published literature does not confirm or refute the 60-day threshold for follow-up for positive CRC screen set by the VHA and the Canadian panels.91,92 The current evidence is not sufficient on the precise timing for diagnostic testing. However, given evidence of an increase in risk after 180 days and modeling estimates suggesting a monotonic increase in risk over time, a 90-day goal is a reasonable target for quality improvement for positive CRC screening results, depending on the resources available, and colonoscopy capacity, but wait times of longer than 180 days should be avoided. This conclusion reflects biologically plausible disease progression with prolonged wait-times, the need to perform diagnostic before discernible increases in risk, the heterogeneity of disease detected by some screening tests (FIT and gFOBT are better at detecting cancers than precancerous lesions), existing practice standards, the available evidence, and consideration of the potential for patient anxiety and preferences.
Lung cancer
Overview of Screening and Diagnostic Testing for Lung Cancer
Lung cancer is the most common and most lethal malignancy in the US with disproportionately high mortality rates in some populations such as black men.41,98 Lung cancer is caused primarily by tobacco use and thus the high prevalence of comorbid tobacco-related conditions. Screening for lung cancer using LDCT is recommended by the USPSTF and other national groups based primarily on the National Lung Screening Trial (NLST) results.6,12,16,17 The USPSTF recommends annual screening as Grade B in those who are 55-80 years old, have ≥30 pack-years of smoking, are current smokers or quit in the past 15 years, and are willing and able to undergo surgery.6 Medicare limits coverage to ages 55-77 years and requires documented shared decision-making prior to screening and for clinical details to be provided to a registry, due partly to risks from high false-positivity rates.16 The American Academy of Family Physician does not formally endorse lung cancer screening and concluded that the evidence was insufficient.99
LDCT results are classified using the Lung CT Screening Reporting and Data System (Lung-RADS). Lesions classified as Lung-RADS 4B or 4X (and some 4A lesions) require a chest CT or Positron emission tomography–CT (PET-CT) and tissue confirmation using CT- or bronchoscopy-guided biopsy.100–102 The approach and procedures used for diagnostic confirmation (such as flexible bronchoscopy with transbronchial needle aspiration, endobronchial ultrasound guided-needle aspiration, transthoracic fine needle aspiration or biopsy, thoracoscopic pleural biopsy, mediastinoscopy, sputum cytology, or thoracentesis) depend on the clinical and radiological findings. There are several emerging technology refinements (such as navigational bronchoscopy) to aid diagnosis of peripheral lesions.103
The overall sensitivity and specificity of PET-CT is 96.8% and 77.8%, respectively (Table 1).104 The sensitivity and specificity of procedures for tissue sampling depend on the type, size, and location of the lesions; the sensitivity of flexible bronchoscopy is about 88% for central lesions, and the sensitivity of transthoracic needle biopsy is about 90%.101 Harms from diagnostic procedures vary across procedures used and are more common in older patients with comorbid conditions such as chronic obstructive pulmonary disease. Pneumothorax is the most common harm, which is reported in 5% of image-guided pleural biopsies and 15% of needle biopsies, but only about one-half require chest tube.105 Other harms include hemorrhage, hypoxemia, bradycardia, and death. There is also short-term procedure-related anxiety.31
Few published recommendations exist on benchmarks for timing of diagnostic testing after a positive lung cancer screen and there is high variability in the definition of wait intervals used in the current literature.106 The RAND Corporation recommended diagnostic confirmation within 60 days of presentation.107 The American College of Chest Physicians recommended “timely and efficient” delivery for patients with suspected or known lung cancer,108 and emphasize consideration for competing needs for resources to set local performance metrics.108 The UK recommends urgent referral (defined as ≤ 2 weeks) to a specialist for patients with suspected cancer on chest roentgenogram or CT.109
Evidence Synopsis for Lung Cancer
The final literature search yielded 883 unique articles for lung cancer; after initial screening, 27 articles were selected for further review, but only one was deemed relevant (see Supporting Information 3 for a list of final included articles).110 Sonavane et al. examined the association between time to diagnostic testing and tumor stage and survival over 7 years of follow-up in 462 NLST participants who had a positive baseline LDCT and were diagnosed with lung cancer within three years (Table 3). Many patients received diagnostic testing >12 months after the LDCT.110 Patients with less aggressive LDCT findings (such as nodules that are <10 mm, pure ground glass, or have smooth margins) took longer to receive diagnostic testing. There were fewer stage I/II cancers (55% versus 76%) or adenocarcinomas (53% versus 78%) in the lowest quartile of time to diagnostic testing than the highest quartile (p<0.005), and thus the lung cancer death rate was highest in the first quartile (44%, 25%, 30%, and 26%, for quartiles 1-4, respectively). There was no statistically significant association of time to diagnostic testing with lung cancer death (OR: 1.06, 95% CI 0.90-1.20, for each additional 180-day interval) after adjustment for tumor characteristics and LDCT findings.110
Contextual Evidence for Lung Cancer
In a Japanese study of 198 patients with lung cancer diagnosed through screening roentgenograms, the 5-year survival rate was lower in the group with a 1-year wait-time for follow-up (21%, n=45) than those who had follow-up sooner (51%, n=153) (hazard ratio [HR] 2.15, CI 1.20-3.84).111 Another Japanese study of 83 screening-detected cases found a difference in survival between those diagnosed with clinical staging interval <4 months after the screening result and those who took longer to be diagnosed (p=0.05).112
In a Canadian study, Byrne et al.113 used retrospective data to evaluate tumor size and stage in patients with biopsy-confirmed non-small cell lung cancer (NSCLC) during two periods of differing waiting times for diagnostic confirmation after positive imaging test (n=66 in 2009, 48 days; IQR: 29.0 – 84.0 versus n=85 in 2011, 81.0 days, IQR: 41.0-139.5). Tumor size and stage were higher in the group with longer median wait time (p<0.001). A small study (n=29) reported an increase of up to 373% in tumor size over a median 54-day period (range: 18-131) of work-up,114 consistent with the estimated doubling time of 20-300 days for NSCLC.115 In a US cohort study of 482 consecutive patients with NSCLC with a median of 16 (IQR: 6-43) days from imaging to diagnosis, Yorio et al. found no association between time to diagnostic testing and overall survival.116 Some low-quality studies have reported paradoxical findings of lower mortality risk with longer delay in receipt of care that likely reflects quicker follow-up for those with more severe LDCT results.
Conclusion and Strength of Evidence for Lung Cancer
Available evidence on the optimal time to diagnostic testing for lung cancer is of very low quality, primarily due to the paucity of studies assessing time to diagnosis on the key question in an LDCT screening population. However, indirect evidence is consistent with the notion that patients with suspected lung cancer detected at screening should undergo diagnostic work soon after the positive result, but the evidence is insufficient to provide a specific timeframe for diagnostic work-up to occur. The RAND Corporation recommended 60-day timeframe provides a reasonable target for quality improvement efforts.
In efforts to improve the quality of care for lung cancer screening, consistent with biological plausibility and patient-centered care, those with Lung-RADS 4B or 4X should undergo timely follow-up diagnostic evaluation with PET-CT and/or biopsy; work-up should be performed within 60 days of the positive result date to reduce the risk of disease progression. This recommendation does not apply to nodules for which surveillance is indicated.
Summary Discussion
This review evaluated the current evidence on the association between timing of diagnostic testing after a positive screening result and risk of progression of precancerous lesions or cancer and mortality for four cancers for which screening is recommended at Grade A or B by the USPSTF.4–7 We found limited direct evidence in the published literature on our key question, due mainly to a small number of studies and the presence of confounding by indication.39,40 Additional supporting evidence came from studies in non-screening populations that examined time from onset of symptoms to diagnostic testing or treatment or when screening was performed using tests that are not recommended in the US.111,112 The available evidence suggests an increased risk of cancer, later stage cancer, or mortality with increasing time between a positive cancer screening result and receipt of diagnostic testing; however, the patterns differ across cancers and specific thresholds remain unclear.
Gaps in the Evidence of Time to Diagnostic Testing
The primary gap in the evidence is the paucity of studies on the key question with sufficient power to examine effect of wait times on mortality from the specific cancers. Randomized clinical trials are infeasible for assessing the effect of delay in diagnostic testing after a positive screen because of ethical concerns. There are several challenges of observational studies, principally confounding by indication, as defined previously, from prioritizing follow-up of patients with high-suspicion screening results or concurrent symptoms. Consequently, observational studies may show a null or reverse association between time to diagnostic testing and cancer outcomes, especially during the early phases of follow-up, as reported in many studies.39,47,50,51,106,110 For instance, in one study of screening mammograms, the higher the level of suspicion for cancer the shorter interval to diagnostic testing (31 days for high suspicion vs. 47 days for other, p<0.0001); thus women with higher likelihood of cancer on mammograms were worked up more quickly.47 Myrdal et al. and Annakkaya et al. both found that longer delay from symptom to treatment initiation was correlated with better prognosis for NSCLC.117,118 Another study found that patients with longer wait times between referral and treatment had a higher proportion of earlier stage lung cancer.119
Studies of the time to diagnostic testing may be less susceptible to bias or confounding for screening results that are only reported qualitatively as positive or negative. Unlike cancer screening results that report lesion phenotype based on probability of cancer diagnosis (e.g., BI-RADS, Lung-RADS, or colonoscopy), results of fecal-based CRC screening tests are only reported qualitatively as positive or negative. A positive FIT simply signifies the presence of occult blood; studying outcomes in that population in large, closed systems with centralized organized screening programs that are able to track patients over time may be less subject to confounding, provided the timing of follow-up by patients or providers is not influenced by the presence of symptoms.94,96 Additionally, natural experiments, such as those occurring during roll out of screening programs or natural variations in practice patterns, may provide insights on the impact of time to diagnostic testing. Because of the limited evidence base, several existing recommendations on time to diagnostic testing are based on either historical patterns of care, consideration of patient-centered care, expert opinion, or policy mandates.
Well-calibrated natural history models can provide valuable insights on the effect of time to diagnostic testing, but findings from such studies depend on the specification of the model parameters and should be interpreted with caution. We did not have modeling estimates on lung cancer screening because it was not included in the first phase of PROSPR and we are unaware of any other microsimulation models that address our key question. Thus, additional modeling studies are needed to inform decisions on the effect of delay on the benefits and harms of lung cancer screening.
We included only published studies in our review, which is subject to publication bias. However, we found evidence for and against timely diagnostic testing, which argues against substantial publication bias.
Other considerations on the recommendations for timeliness of diagnostic testing
Despite the limitations of the current literature, strong biological plausibility and the available evidence support the notion that longer waiting times from a positive screen to diagnosis diminish the benefits of screening. Earlier time to diagnostic testing decreases the risk of progression of preneoplastic disease to cancer and from early-stage to late-stage cancers. Another consideration is the possibility that the longer the time period between positive screening and diagnostic evaluation, the higher the potential that patients may change contact information, insurance coverage, or providers, further complicating coordination of care and follow-up. Additionally, some patients may never receive follow-up after a positive test and only receive confirmatory diagnosis after symptoms appear. These directly influence the likelihood of successful cancer prevention or cure.25 There is also the potential for worry or anxiety about the uncertainty of a cancer diagnosis from a positive result.120,121
For those reasons, some systems offer same-day follow-up for positive mammograms. An analysis of 18,245 women in NQMBC™ found a median time to diagnostic testing of 6.5 days (interquartile range (IQR), 4.0-10.5 days) from a positive mammogram.122 The median time to diagnostic testing from positive mammogram have decreased by 2 days in BCCEDP between 1996-2000 and 2001-2005.123 Immediate follow-up may not be feasible for other cancer types because of greater resources needed for same-day evaluation and the need for preparation and transition to different settings to continue care. Some diagnostic procedures, such as colonoscopy, require special preparation, sedation, and additional personnel.
Improving Diagnostic Testing After Positive Screen
There is a strong national imperative to improve the timeliness of care.1–3 There is consistent evidence that the overall proportions of people receiving follow-up, and the times to follow-up testing after a positive screen, are generally suboptimal and vary across populations and health systems.19–23 Studies in the PROSPR consortium show that the probability of diagnostic colonoscopy after a positive fecal test plateaus at approximately 80% after 6 months in systems with highly organized screening, and at lower levels in systems with less organized follow-up.19 Less than 75% of women receive follow-up within variously defined time metrics after an abnormal cervical cancer screening.23
These suboptimal rates may reflect appropriate shared clinical decisions to forego further testing, or stem from social, cultural, financial, structural, or other barriers at individual, provider, or system levels. Such factors include low-SES, employment status, rurality, perceived discrimination or health-related stigma.23,52,78,124–130 Provider- and system-related problems with care delivery are crucial.3,124 Method of result notification (with telephone and letter notification associated with longer delays than in-person notification), as well as the use of navigators, rapid diagnostic units, and organized screening programs influence timeliness of follow-up.33,127,131 Procedures that reduce the number of interfaces involved (such as use of core needle biopsy rather than open biopsy for abnormal mammograms) may reduce the time to biopsy.132
Thus, overcoming barriers to timely follow-up likely requires multilevel interventions to mitigate multiple barriers,133 but evidence is limited regarding effective interventions.2,3 A recent systematic review of interventions to improve follow-up of positive FIT found only limited evidence that supported use of patient navigators, or giving providers reminders or performance data.134 Other studies also suggest a role for patient navigation as well as use of electronic health record (EHR) flags,124,135,136 counseling, and education.77 Improving EHR “cross-talk” across different health systems may also minimize losses to follow-up.
Conclusion
The effectiveness of cancer screening is predicated upon performing diagnostic testing to confirm the absence or presence of precancer or cancer, and the stage of disease when cancer is found. Failure to do so undermines the benefits of screening and may lead to poor cancer outcomes. Further, attention to timely follow-up is a critical part of routine clinical care.1–3 Current evidence shows that diagnostic testing is being prioritized based on severity of findings when available, but also suggests that longer wait times for diagnostic testing may be harmful. Overall, the evidence suggests that the risk for poorer cancer outcomes rises with longer wait times, which supports performing diagnostic testing as soon as feasible after the positive result. However, evidence for specific times for diagnostic testing is limited across cancers, and the available evidence does not confirm or refute existing guidelines that set targets for quality improvement. Local benchmarks may take available resources into account. More research is needed to provide evidence-based metrics for optimizing follow-up timing that best balances benefits and harms at both patient and system levels and patient preferences. It is crucial for future studies to address methodological weaknesses in current studies, principally, confounding by indication.39,137 Research is needed to guide interventions on reducing time to diagnosis for vulnerable and minority populations and patients with identified barriers to timely follow-up.
Supplementary Material
Table 2.
Evidence summary for the timing of follow-up diagnostic testing after a positive screening test
| Cancer type | studies, k | Study types | Quality of evidence | Summary | Existing recommended targets | Suggested targets, based on consensus opinion |
|---|---|---|---|---|---|---|
| Breast | 3 | Cohort Modeling |
Low | ↑ risk of tumor size >10mm or nodal involvement at 365 days or longer in one study47 and >180 days in another.48 Modeling suggest ↑ risk of late-stage and ↓ LYG by 90 days.49 |
60 days45 | Perform diagnostic testing within 60 days, but no later than 90 days |
| Cervical | 1 | Modeling | Very low | Modeling detected adverse outcomes after 90 days.49 | 90 days76 | Perform diagnostic testing within 90 days |
| Colorectal | 3 | Cohort Modeling |
Low | ↑ risk of cancer and late-stage disease at >180 days.94 Modeling found ↑ risk with each 30-day increment in wait time,95 and 2.0-2.7% decrement in LYG at 90 days.49 |
60 days91,92 | Perform diagnostic testing within 90 days, and not later than 180 days |
| Lung | 1 | Cohort | Very low | Reverse association in an NLST study.110 Contextual studies report ↑tumor size and stage with median wait time of 48 days vs. 81 days;113 and survival differences at a 4-month cut-point,112 but another found no significant relationship with overall survival over a median of 16 days.116 | 60 days107 Urgent referral109 |
Perform diagnostic testing within 60 days |
Abbreviations: LYG = Life years gained (estimated from modeling studies); NLST = the National Lung Screening Trial
Acknowledgments
We thank Jessica Chubak, PhD for helpful comments on the manuscript.
Grant Support: This study was supported in part by an award (number R01CA213645, U54CA163262, U54CA163261, U54CA 164336) from the National Cancer Institute of the National Institutes of Health. The views expressed here are those of the authors only and do not represent any official position of the National Cancer Institute or National Institutes of Health.
Footnotes
Disclaimer: No part of this study has been presented in any form and the authors have no conflicts of interest. Dr. Doubeni is a member of the US Preventive Services Task Force (USPSTF). This article does not necessarily represent the views and policies of the USPSTF.
Stacey A. Fedewa is employed by the American Cancer Society, which received a grant from Merck, Inc. for intramural research outside the submitted work; however, her salary is solely funded through American Cancer Society funds.
References
- 1.Kaplan GS. Health Care Scheduling and Access: A Report From the IOM. JAMA. 2015;314(14):1449–1450. doi: 10.1001/jama.2015.9431. [DOI] [PubMed] [Google Scholar]
- 2.IOM (Institute of Medicine) Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C: National Academy of Sciences; 2001. [Google Scholar]
- 3.IOM (Institute of Medicine) Transforming Health Care Scheduling and Access: Getting to Now. Washington, D.C: National Academy of Sciences; 2015. [PubMed] [Google Scholar]
- 4.U. S. Preventive Services Task Force. Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315(23):2564–2575. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 5.U. S. Preventive Services Task Force. Moyer VA. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891. W312. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 6.U. S. Preventive Services Task Force. Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 7.U. S. Preventive Services Task Force. Siu AL. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(4):279–296. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 8.Koh HK, Sebelius KG. Promoting prevention through the Affordable Care Act. N Engl J Med. 2010;363(14):1296–1299. doi: 10.1056/NEJMp1008560. [DOI] [PubMed] [Google Scholar]
- 9.Bruening W, Fontanarosa J, Tipton K, Treadwell JR, Launders J, Schoelles K. Systematic review: comparative effectiveness of core-needle and open surgical biopsy to diagnose breast lesions. Ann Intern Med. 2010;152(4):238–246. doi: 10.7326/0003-4819-152-1-201001050-00190. [DOI] [PubMed] [Google Scholar]
- 10.D’Orsi CJ, Sickles EA, Mendelson EB, et al. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 11.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 12.National Lung Screening Trial Research T. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oeffinger KC, Fontham ET, Etzioni R, et al. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update From the American Cancer Society. JAMA. 2015;314(15):1599–1614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saslow D, Runowicz CD, Solomon D, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52(6):342–362. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 15.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2017: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2017;67(2):100–121. doi: 10.3322/caac.21392. [DOI] [PubMed] [Google Scholar]
- 17.Wender R, Fontham ET, Barrera E, Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63(2):107–117. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ, Conference AS-SC 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287(16):2120–2129. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- 19.Chubak J, Garcia MP, Burnett-Hartman AN, et al. Time to Colonoscopy after Positive Fecal Blood Test in Four U.S. Health Care Systems. Cancer Epidemiol Biomarkers Prev. 2016;25(2):344–350. doi: 10.1158/1055-9965.EPI-15-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eggleston KS, Coker AL, Luchok KJ, Meyer TE. Adherence to recommendations for follow-up to abnormal Pap tests. Obstet Gynecol. 2007;109(6):1332–1341. doi: 10.1097/01.AOG.0000266396.25244.68. [DOI] [PubMed] [Google Scholar]
- 21.Laiyemo AO, Doubeni C, Pinsky PF, et al. Race and colorectal cancer disparities: health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010;102(8):538–546. doi: 10.1093/jnci/djq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy AM, Kim JJ, Beaber EF, et al. Follow-Up of Abnormal Breast and Colorectal Cancer Screening by Race/Ethnicity. Am J Prev Med. 2016;51(4):507–512. doi: 10.1016/j.amepre.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yabroff KR, Washington KS, Leader A, Neilson E, Mandelblatt J. Is the promise of cancer-screening programs being compromised? Quality of follow-up care after abnormal screening results. Med Care Res Rev. 2003;60(3):294–331. doi: 10.1177/1077558703254698. [DOI] [PubMed] [Google Scholar]
- 24.Michor F, Iwasa Y, Nowak MA. Dynamics of cancer progression. Nat Rev Cancer. 2004;4(3):197–205. doi: 10.1038/nrc1295. [DOI] [PubMed] [Google Scholar]
- 25.Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112(Suppl 1):S92–107. doi: 10.1038/bjc.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson HD, Pappas M, Cantor A, Griffin J, Daeges M, Humphrey L. Harms of Breast Cancer Screening: Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164(4):256–267. doi: 10.7326/M15-0970. [DOI] [PubMed] [Google Scholar]
- 27.Vesco KK, Whitlock EP, Eder M, et al. Screening for Cervical Cancer: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Rockville (MD): 2011. [PubMed] [Google Scholar]
- 28.Bynum SA, Davis JL, Green BL, Katz RV. Unwillingness to participate in colorectal cancer screening: examining fears, attitudes, and medical mistrust in an ethnically diverse sample of adults 50 years and older. Am J Health Promot. 2012;26(5):295–300. doi: 10.4278/ajhp.110113-QUAN-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green AR, Peters-Lewis A, Percac-Lima S, et al. Barriers to screening colonoscopy for low-income Latino and white patients in an urban community health center. J Gen Intern Med. 2008;23(6):834–840. doi: 10.1007/s11606-008-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin JS, Piper MA, Perdue LA, et al. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;315(23):2576–2594. doi: 10.1001/jama.2016.3332. [DOI] [PubMed] [Google Scholar]
- 31.Humphrey L, Deffebach M, Pappas M, et al. Screening for Lung Cancer: Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Rockville (MD): 2013. [PubMed] [Google Scholar]
- 32.Khakbazan Z, Taghipour A, Latifnejad Roudsari R, Mohammadi E. Help seeking behavior of women with self-discovered breast cancer symptoms: a meta-ethnographic synthesis of patient delay. PLoS One. 2014;9(12):e110262. doi: 10.1371/journal.pone.0110262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Racz JM, Holloway CM, Huang W, Hong NJ. Improving patient flow and timeliness in the diagnosis and management of breast abnormalities: the impact of a rapid diagnostic unit. Curr Oncol. 2016;23(3):e260–265. doi: 10.3747/co.23.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.https://healthcaredelivery.cancer.gov/prospr/.
- 35.Beaber EF, Kim JJ, Schapira MM, et al. Unifying screening processes within the PROSPR consortium: a conceptual model for breast, cervical, and colorectal cancer screening. J Natl Cancer Inst. 2015;107(6):djv120. doi: 10.1093/jnci/djv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Cancer Society. Chronological History of ACS Recommendations on Early Detection of Cancer. 2004. [Google Scholar]
- 37.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kyriacou DN, Lewis RJ. Confounding by Indication in Clinical Research. JAMA. 2016;316(17):1818–1819. doi: 10.1001/jama.2016.16435. [DOI] [PubMed] [Google Scholar]
- 40.Freemantle N, Marston L, Walters K, Wood J, Reynolds MR, Petersen I. Making inferences on treatment effects from real world data: propensity scores, confounding by indication, and other perils for the unwary in observational research. BMJ. 2013;347:f6409. doi: 10.1136/bmj.f6409. [DOI] [PubMed] [Google Scholar]
- 41.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 42.Expert Panel on Breast I. Mainiero MB, Moy L, et al. ACR Appropriateness Criteria(R) Breast Cancer Screening. J Am Coll Radiol. 2017;14(11S):S383–S390. doi: 10.1016/j.jacr.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 43.Wang M, He X, Chang Y, Sun G, Thabane L. A sensitivity and specificity comparison of fine needle aspiration cytology and core needle biopsy in evaluation of suspicious breast lesions: A systematic review and meta-analysis. Breast. 2017;31:157–166. doi: 10.1016/j.breast.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Dahabreh IJ, Wieland LS, Adam GP, Halladay C, Lau J, Trikalinos TA. Core Needle and Open Surgical Biopsy for Diagnosis of Breast Lesions: An Update to the 2009 Report. Rockville (MD): 2014. [PubMed] [Google Scholar]
- 45.Filella X, Molina R, Jo J, Mas E, Ballesta AM. Serum phosphohexose isomerase activities in patients with colorectal cancer. Tumour Biol. 1991;12(6):360–367. doi: 10.1159/000217737. [DOI] [PubMed] [Google Scholar]
- 46.Filella X, Molina R, Bedini JL, Jo J, Joseph J, Ballesta AM. Clinical usefulness of CEA as tumor marker in patients with colorectal cancer. J Nucl Med Allied Sci. 1990;34(4 Suppl):107–110. [PubMed] [Google Scholar]
- 47.Olivotto IA, Gomi A, Bancej C, et al. Influence of delay to diagnosis on prognostic indicators of screen-detected breast carcinoma. Cancer. 2002;94(8):2143–2150. doi: 10.1002/cncr.10453. [DOI] [PubMed] [Google Scholar]
- 48.Ganry O, Peng J, Dubreuil A. Influence of abnormal screens on delays and prognostic indicators of screen-detected breast carcinoma. J Med Screen. 2004;11(1):28–31. doi: 10.1177/096914130301100107. [DOI] [PubMed] [Google Scholar]
- 49.Rutter CM, Kim JJ, Meester RG, et al. Effect of Time to Diagnostic Testing for Breast, Cervical, and Colorectal Cancer Screening Abnormalities on Screening Efficacy: A Modeling Study. Cancer Epidemiology, Biomarkers & Prevention. 2017 Nov 17; doi: 10.1158/1055-9965.EPI-17-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353(9159):1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 51.Caplan L. Delay in breast cancer: implications for stage at diagnosis and survival. Front Public Health. 2014;2:87. doi: 10.3389/fpubh.2014.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Durham DD, Robinson WR, Lee SS, et al. Insurance-Based Differences in Time to Diagnostic Follow-up after Positive Screening Mammography. Cancer Epidemiol Biomarkers Prev. 2016;25(11):1474–1482. doi: 10.1158/1055-9965.EPI-16-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Selby JV, Friedman GD, Quesenberry CP, Jr, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326(10):653–657. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 54.Castle PE, Fetterman B, Akhtar I, et al. Age-appropriate use of human papillomavirus vaccines in the U.S. Gynecol Oncol. 2009;114(2):365–369. doi: 10.1016/j.ygyno.2009.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27. doi: 10.1097/LGT.0b013e318287d329. [DOI] [PubMed] [Google Scholar]
- 56.Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. J Low Genit Tract Dis. 2015;19(2):91–96. doi: 10.1097/LGT.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 57.Solomon D, Schiffman M, Tarone R, group AS Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001;93(4):293–299. doi: 10.1093/jnci/93.4.293. [DOI] [PubMed] [Google Scholar]
- 58.Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999;281(17):1605–1610. doi: 10.1001/jama.281.17.1605. [DOI] [PubMed] [Google Scholar]
- 59.Kurman RJ, Henson DE, Herbst AL, Noller KL, Schiffman MH. Interim guidelines for management of abnormal cervical cytology. The 1992 National Cancer Institute Workshop. JAMA. 1994;271(23):1866–1869. [PubMed] [Google Scholar]
- 60.Castle PE, Sideri M, Jeronimo J, Solomon D, Schiffman M. Risk assessment to guide the prevention of cervical cancer. J Low Genit Tract Dis. 2008;12(1):1–7. doi: 10.1097/lgt.0b013e31815ea58b. [DOI] [PubMed] [Google Scholar]
- 61.Schiffman M, Wentzensen N, Khan MJ, et al. Preparing for the Next Round of ASCCP-Sponsored Cervical Screening and Management Guidelines. J Low Genit Tract Dis. 2017;21(2):87–90. doi: 10.1097/LGT.0000000000000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jeronimo J, Schiffman M. Colposcopy at a crossroads. Am J Obstet Gynecol. 2006;195(2):349–353. doi: 10.1016/j.ajog.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 63.Gage JC, Hanson VW, Abbey K, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108(2):264–272. doi: 10.1097/01.AOG.0000220505.18525.85. [DOI] [PubMed] [Google Scholar]
- 64.Massad LS, Jeronimo J, Schiffman M, National Institutes of Health/American Society for C, Cervical Pathology Research G Interobserver agreement in the assessment of components of colposcopic grading. Obstet Gynecol. 2008;111(6):1279–1284. doi: 10.1097/AOG.0b013e31816baed1. [DOI] [PubMed] [Google Scholar]
- 65.Wentzensen N, Walker JL, Gold MA, et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol. 2015;33(1):83–89. doi: 10.1200/JCO.2014.55.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wentzensen N, Massad LS, Mayeaux EJ, Jr, et al. Evidence-Based Consensus Recommendations for Colposcopy Practice for Cervical Cancer Prevention in the United States. J Low Genit Tract Dis. 2017;21(4):216–222. doi: 10.1097/LGT.0000000000000322. [DOI] [PubMed] [Google Scholar]
- 67.Wentzensen N, Schiffman M, Silver MI, et al. ASCCP Colposcopy Standards: Risk-Based Colposcopy Practice. J Low Genit Tract Dis. 2017;21(4):230–234. doi: 10.1097/LGT.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 68.Khan MJ, Werner CL, Darragh TM, et al. ASCCP Colposcopy Standards: Role of Colposcopy, Benefits, Potential Harms, and Terminology for Colposcopic Practice. J Low Genit Tract Dis. 2017;21(4):223–229. doi: 10.1097/LGT.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 69.Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol. 2009;113(1):18–25. doi: 10.1097/AOG.0b013e31818f5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trimble CL, Piantadosi S, Gravitt P, et al. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLA phenotype. Clin Cancer Res. 2005;11(13):4717–4723. doi: 10.1158/1078-0432.CCR-04-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Castanon A, Landy R, Brocklehurst P, et al. Risk of preterm delivery with increasing depth of excision for cervical intraepithelial neoplasia in England: nested case-control study. BMJ. 2014;349:g6223. doi: 10.1136/bmj.g6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367(9509):489–498. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 73.Sasieni P, Castanon A, Landy R, et al. Risk of preterm birth following surgical treatment for cervical disease: executive summary of a recent symposium. BJOG. 2016;123(9):1426–1429. doi: 10.1111/1471-0528.13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mayeaux EJ, Jr, Novetsky AP, Chelmow D, et al. Systematic Review of International Colposcopy Quality Improvement Guidelines. J Low Genit Tract Dis. 2017;21(4):249–257. doi: 10.1097/LGT.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 75.Ryerson AB, Bernard VB, Majors A. National Breast and Cervical Cancer Early Detection Program: Summarizing the first 12 years of partnerships and progress against breast and cervical cancer. 2017 Accessed at: https://www.cdc.gov/cancer/nbccedp/pdf/national_report.pdf.
- 76.Centers for Disease Control and Prevention. NBCCEDP Monthly Newsletter. 2009. [Google Scholar]
- 77.Eggleston KS, Coker AL, Das IP, Cordray ST, Luchok KJ. Understanding barriers for adherence to follow-up care for abnormal pap tests. J Womens Health (Larchmt) 2007;16(3):311–330. doi: 10.1089/jwh.2006.0161. [DOI] [PubMed] [Google Scholar]
- 78.Tabnak F, Muller HG, Wang JL, Zhang W, Howell LP. Timeliness and follow-up patterns of cervical cancer detection in a cohort of medically underserved California women. Cancer Causes Control. 2010;21(3):411–420. doi: 10.1007/s10552-009-9473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Janerich DT, Hadjimichael O, Schwartz PE, et al. The screening histories of women with invasive cervical cancer, Connecticut. Am J Public Health. 1995;85(6):791–794. doi: 10.2105/ajph.85.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Canadian Partnership Against Cancer P-CCSN. Cervical Cancer Screening in Canada: Setting Targets for Program Performance. 2013 Nov 13; [Google Scholar]
- 81.Canadian Partnership Against Cancer. Cervical Cancer Screening in Canada: Monitoring and Evaluations of Quality Indicators. January 2011 - December 2013. [Google Scholar]
- 82.International Agency for Research on Cancer. Cancer Screening in the European Union: Report on the implementation of the Council Recommendation on cancer screening. 2017 [Google Scholar]
- 83.Mayeaux EJ, Jr, Novetsky AP, Chelmow D, et al. ASCCP Colposcopy Standards: Colposcopy Quality Improvement Recommendations for the United States. J Low Genit Tract Dis. 2017;21(4):242–248. doi: 10.1097/LGT.0000000000000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doubeni CA, Corley DA, Quinn VP, et al. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut. 2018;67(2):291–298. doi: 10.1136/gutjnl-2016-312712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Atkin W, Wooldrage K, Parkin DM, et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet. 2017;389(10076):1299–1311. doi: 10.1016/S0140-6736(17)30396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103(6):1541–1549. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 87.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies: Modeling Study for the US Preventive Services Task Force. JAMA. 2016;315(23):2595–2609. doi: 10.1001/jama.2016.6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Corley DA, Levin TR, Doubeni CA. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370(26):2541. doi: 10.1056/NEJMc1405329. [DOI] [PubMed] [Google Scholar]
- 89.Fedewa SA, Flanders WD, Ward KC, et al. Racial and Ethnic Disparities in Interval Colorectal Cancer Incidence: A Population-Based Cohort Study. Ann Intern Med. 2017;166(12):857–866. doi: 10.7326/M16-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150(12):849–857. W152. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 91.Paterson WG, Depew WT, Pare P, et al. Canadian consensus on medically acceptable wait times for digestive health care. Can J Gastroenterol. 2006;20(6):411–423. doi: 10.1155/2006/343686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peterson K, Carson S, Humphrey L, Helfand M. Patients with Positive Screening Fecal Occult Blood Tests: Evidence Brief on the Relationship Between Time Delay to Colonoscopy and Colorectal Cancer Outcomes. VA-ESP Project #09-199. 2013 [Google Scholar]
- 93.Gellad ZF, Almirall D, Provenzale D, Fisher DA. Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Dig Dis Sci. 2009;54(11):2497–2502. doi: 10.1007/s10620-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Corley DA, Jensen CD, Quinn VP, et al. Association Between Time to Colonoscopy After a Positive Fecal Test Result and Risk of Colorectal Cancer and Cancer Stage at Diagnosis. JAMA. 2017;317(16):1631–1641. doi: 10.1001/jama.2017.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meester RG, Zauber AG, Doubeni CA, et al. Consequences of Increasing Time to Colonoscopy Examination After Positive Result From Fecal Colorectal Cancer Screening Test. Clin Gastroenterol Hepatol. 2016;14(10):1445–1451 e1448. doi: 10.1016/j.cgh.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Doubeni CA, Corley DA, Levin TR. Time to Diagnostic Testing After a Positive Colorectal Cancer Screening Test. JAMA. 2017;318(5):483. doi: 10.1001/jama.2017.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wattacheril J, Kramer JR, Richardson P, et al. Lagtimes in diagnosis and treatment of colorectal cancer: determinants and association with cancer stage and survival. Aliment Pharmacol Ther. 2008;28(9):1166–1174. doi: 10.1111/j.1365-2036.2008.03826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.American Cancer Society. Cancer Facts & Figures 2018. Atlanta: American Cancer Society; 2018. [Google Scholar]
- 99.American Academy of Family Physicians. Clinical Preventive Service Recommendation: Lung Cancer. Patient Care. 2014 http://www.aafp.org/patient-care/clinical-recommendations/all/lung-cancer.html. Accessed December 3, 2017.
- 100.Roetzheim RG, Gonzalez EC, Ferrante JM, Pal N, Van Durme DJ, Krischer JP. Effects of health insurance and race on breast carcinoma treatments and outcomes. Cancer. 2000;89(11):2202–2213. doi: 10.1002/1097-0142(20001201)89:11<2202::aid-cncr8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 101.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e142S–e165S. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]
- 102.American College of Radiology. Lung CT Screening Reporting and Data System (Lung-RADS™) 2014 https://www.acr.org/Quality-Safety/Resources/LungRADS. Accessed Jan 8, 2017.
- 103.Belanger AR, Akulian JA. An update on the role of advanced diagnostic bronchoscopy in the evaluation and staging of lung cancer. Ther Adv Respir Dis. 2017;11(5):211–221. doi: 10.1177/1753465817695981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001;285(7):914–924. doi: 10.1001/jama.285.7.914. [DOI] [PubMed] [Google Scholar]
- 105.Benamore RE, Scott K, Richards CJ, Entwisle JJ. Image-guided pleural biopsy: diagnostic yield and complications. Clin Radiol. 2006;61(8):700–705. doi: 10.1016/j.crad.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 106.Jacobsen MM, Silverstein SC, Quinn M, et al. Timeliness of access to lung cancer diagnosis and treatment: A scoping literature review. Lung Cancer. 2017;112:156–164. doi: 10.1016/j.lungcan.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 107.Lung JR. Cancer. In: Asch S, Kerr E, Hamilton E, editors. Quality of Care for Oncologic Conditions and HIV: A Review of the Literature and Quality Indicators. RAND Corporation; 2000. pp. 133–172. [Google Scholar]
- 108.Ost DE, Jim Yeung SC, Tanoue LT, Gould MK. Clinical and organizational factors in the initial evaluation of patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e121S–e141S. doi: 10.1378/chest.12-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baldwin DR, White B, Schmidt-Hansen M, Champion AR, Melder AM, Guideline Development G Diagnosis and treatment of lung cancer: summary of updated NICE guidance. BMJ. 2011;342:d2110. doi: 10.1136/bmj.d2110. [DOI] [PubMed] [Google Scholar]
- 110.Sonavane SK, Pinsky P, Watts J, Jr, et al. The relationship of cancer characteristics and patient outcome with time to lung cancer diagnosis after an abnormal screening CT. Eur Radiol. 2017;27(12):5113–5118. doi: 10.1007/s00330-017-4886-9. [DOI] [PubMed] [Google Scholar]
- 111.Kashiwabara K, Koshi S, Itonaga K, Nakahara O, Tanaka M, Toyonaga M. Outcome in patients with lung cancer found on lung cancer mass screening roentgenograms, but who did not subsequently consult a doctor. Lung Cancer. 2003;40(1):67–72. doi: 10.1016/s0169-5002(02)00505-6. [DOI] [PubMed] [Google Scholar]
- 112.Kanashiki M, Satoh H, Ishikawa H, Yamashita YT, Ohtsuka M, Sekizawa K. Time from finding abnormality on mass-screening to final diagnosis of lung cancer. Oncol Rep. 2003;10(3):649–652. [PubMed] [Google Scholar]
- 113.Byrne SC, Barrett B, Bhatia R. The impact of diagnostic imaging wait times on the prognosis of lung cancer. Can Assoc Radiol J. 2015;66(1):53–57. doi: 10.1016/j.carj.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 114.O’Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol) 2000;12(3):141–144. doi: 10.1053/clon.2000.9139. [DOI] [PubMed] [Google Scholar]
- 115.Ost DE, Gould MK. Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med. 2012;185(4):363–372. doi: 10.1164/rccm.201104-0679CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yorio JT, Xie Y, Yan J, Gerber DE. Lung cancer diagnostic and treatment intervals in the United States: a health care disparity? J Thorac Oncol. 2009;4(11):1322–1330. doi: 10.1097/JTO.0b013e3181bbb130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Myrdal G, Lambe M, Hillerdal G, Lamberg K, Agustsson T, Stahle E. Effect of delays on prognosis in patients with non-small cell lung cancer. Thorax. 2004;59(1):45–49. [PMC free article] [PubMed] [Google Scholar]
- 118.Annakkaya AN, Arbak P, Balbay O, Bilgin C, Erbas M, Bulut I. Effect of symptom-to-treatment interval on prognosis in lung cancer. Tumori. 2007;93(1):61–67. doi: 10.1177/030089160709300111. [DOI] [PubMed] [Google Scholar]
- 119.Comber H, Cronin DP, Deady S, Lorcain PO, Riordan P. Delays in treatment in the cancer services: impact on cancer stage and survival. Ir Med J. 2005;98(8):238–239. [PubMed] [Google Scholar]
- 120.Haas J, Kaplan C, McMillan A, Esserman LJ. Does timely assessment affect the anxiety associated with an abnormal mammogram result? J Womens Health Gend Based Med. 2001;10(6):599–605. doi: 10.1089/15246090152543184. [DOI] [PubMed] [Google Scholar]
- 121.Keyzer-Dekker CM, van Esch L, de Vries J, et al. An abnormal screening mammogram causes more anxiety than a palpable lump in benign breast disease. Breast Cancer Res Treat. 2012;134(1):253–258. doi: 10.1007/s10549-012-2025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaufman CS, Shockney L, Rabinowitz B, et al. National Quality Measures for Breast Centers (NQMBC): a robust quality tool: breast center quality measures. Ann Surg Oncol. 2010;17(2):377–385. doi: 10.1245/s10434-009-0729-5. [DOI] [PubMed] [Google Scholar]
- 123.Richardson LC, Royalty J, Howe W, Helsel W, Kammerer W, Benard VB. Timeliness of breast cancer diagnosis and initiation of treatment in the National Breast and Cervical Cancer Early Detection Program, 1996-2005. Am J Public Health. 2010;100(9):1769–1776. doi: 10.2105/AJPH.2009.160184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tejeda S, Darnell JS, Cho YI, Stolley MR, Markossian TW, Calhoun EA. Patient barriers to follow-up care for breast and cervical cancer abnormalities. J Womens Health (Larchmt) 2013;22(6):507–517. doi: 10.1089/jwh.2012.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yabroff KR, Breen N, Vernon SW, Meissner HI, Freedman AN, Ballard-Barbash R. What factors are associated with diagnostic follow-up after abnormal mammograms? Findings from a U.S. National Survey. Cancer Epidemiol Biomarkers Prev. 2004;13(5):723–732. [PubMed] [Google Scholar]
- 126.Rojas C, Zhou MK, Khamis HJ, Amesse L. Analysis of patterns of patient compliance after an abnormal Pap smear result: the influence of demographic characteristics on patient compliance. J Low Genit Tract Dis. 2013;17(3):298–302. doi: 10.1097/LGT.0b013e31826b683e. [DOI] [PubMed] [Google Scholar]
- 127.Perez-Stable EJ, Afable-Munsuz A, Kaplan CP, et al. Factors influencing time to diagnosis after abnormal mammography in diverse women. J Womens Health (Larchmt) 2013;22(2):159–166. doi: 10.1089/jwh.2012.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carter-Harris L. Lung cancer stigma as a barrier to medical help-seeking behavior: Practice implications. J Am Assoc Nurse Pract. 2015;27(5):240–245. doi: 10.1002/2327-6924.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carter-Harris L, Hermann CP, Schreiber J, Weaver MT, Rawl SM. Lung cancer stigma predicts timing of medical help-seeking behavior. Oncol Nurs Forum. 2014;41(3):E203–210. doi: 10.1188/14.ONF.E203-E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dunn J, Garvey G, Valery PC, et al. Barriers to lung cancer care: health professionals’ perspectives. Support Care Cancer. 2017;25(2):497–504. doi: 10.1007/s00520-016-3428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chiarelli AM, Muradali D, Blackmore KM, et al. Evaluating wait times from screening to breast cancer diagnosis among women undergoing organised assessment vs usual care. Br J Cancer. 2017;116(10):1254–1263. doi: 10.1038/bjc.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Plotogea A, Chiarelli AM, Mirea L, et al. Clinical and prognostic factors associated with diagnostic wait times by breast cancer detection method. Springerplus. 2014;3:125. doi: 10.1186/2193-1801-3-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baron RC, Melillo S, Rimer BK, et al. Intervention to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers a systematic review of provider reminders. Am J Prev Med. 2010;38(1):110–117. doi: 10.1016/j.amepre.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 134.Selby K, Baumgartner C, Levin TR, et al. Interventions to Improve Follow-up of Positive Results on Fecal Blood Tests: A Systematic Review. Ann Intern Med. 2017;167(8):565–575. doi: 10.7326/M17-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Battaglia TA, Bak SM, Heeren T, et al. Boston Patient Navigation Research Program: the impact of navigation on time to diagnostic resolution after abnormal cancer screening. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1645–1654. doi: 10.1158/1055-9965.EPI-12-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Markossian TW, Darnell JS, Calhoun EA. Follow-up and timeliness after an abnormal cancer screening among underserved, urban women in a patient navigation program. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1691–1700. doi: 10.1158/1055-9965.EPI-12-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brookhart MA, Sturmer T, Glynn RJ, Rassen J, Schneeweiss S. Confounding control in healthcare database research: challenges and potential approaches. Med Care. 2010;48(6 Suppl):S114–120. doi: 10.1097/MLR.0b013e3181dbebe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.