Abstract
Objective
Post-infection HIV viral control and immune correlates analysis of the RV144 vaccine trial indicate a potentially critical role for Fc receptor-mediated antibody functions. However, the influence of functional antibodies in clade C infection is largely unknown.
Design
Plasma samples from 361 chronic subtype C-infected, antiretroviral therapy-naïve participants were tested for their HIV-specific isotype and subclass distributions, along with their Fc receptor-mediated functional potential.
Method
Total IgG, IgG subclasses and IgA binding to p24 clade B/C and gp120 consensus C proteins were assayed by multiplex. Antibody-dependent uptake of antigen-coated beads and Fc receptor-mediated NK cell degranulation were evaluated as surrogates for antibody-dependent cellular phagocytosis (ADCP) and antibody-dependent cellular cytotoxicity (ADCC) respectively.
Results
p24 IgG1 was the only subclass associated with viral control (p=0.01), with higher p24-specific ADCP and ADCC responses detected in individuals with high p24 IgG1. Although p24 IgG1 levels were enriched in subjects with elevated Gag-specific T cell responses, these levels remained an independent predictor of low viral loads (p=0.04) and high CD4 counts (p=0.004) after adjusting for Gag-specific T cell responses and for protective HLA class I alleles.
Conclusion
p24 IgG1 levels independently predict viral control in HIV-1 clade C infection. Whether these responses contribute to direct antiviral control via the recruited killing of infected cells via the innate immune system or simply mark a qualitatively superior immune response to HIV, is uncertain, but highlights the role of p24-specific antibodies in control of clade C HIV-1 infection.
Keywords: HIV-1 subtype C, chronic, ADCP, NK cell degranulation, antibody, p24, Gag
Background
In 2016 alone, there were an estimated 1.8 million new HIV-1 infections globally; a figure that highlights the relentless spread of this virus and the urgent need for an efficacious vaccine[1]. In 2009, the RV144 trial, a phase III HIV-1 human vaccine trial, was the first to show modest efficacy[2]. Immune correlates analysis identified binding antibodies to the viral envelope variable loops 1 and 2 as the primary correlates of reduced risk from infection[3]. However, in secondary analyses, Fc-mediated antibody-dependent cellular cytotoxicity (ADCC) preferentially mediated by IgG3 was also associated with reduced risk of infection in the absence of IgA[4, 5], suggesting a potentially critical role for non-neutralizing antibody Fc effector activities in protection from HIV-1 infection.
In addition to ADCC, antibodies mediate additional extra-neutralizing functions relevant in HIV-1 infection, such as antibody-mediated cellular phagocytosis (ADCP), through their capacity to interact with Fc receptors expressed on innate immune cells[6, 7]. Importantly, both the magnitude and breadth of the ADCC response has been linked to slow disease progression[8–12], and higher ADCC activity has been observed in rare individuals capable of controlling viral replication in the absence of antiretroviral therapy (ART)[13]. Furthermore, HIV-1 transmission from mothers to their breast-feeding infants is significantly reduced in women with high ADCC activity in breast milk[14]. In animal models, vaccine-induced ADCC and antibody-dependent cell-mediated viral inhibition responses have been associated with protection and reduced viral set point[15–20]. Moreover, prevention of simian-human immunodeficiency virus infection via vaccination has also been linked to enhanced ADCP activity[19, 20], which was also enriched in RV144 vaccinees[5, 21]. Together, these observations have renewed interest in dissecting the importance of Fc effector functions in natural infection, particularly against clades that contribute to the greatest HIV-1 burden globally.
The antibody response to HIV-1 proteins is largely dominated by IgG1 antibodies, with variable levels of other antibody subclasses (IgG2, IgG3 and IgG4)[22]. In natural infection, however, levels of gp120-specific antibodies do not correlate with markers of disease progression despite their potential antiviral activity[23–31]. In contrast, anti-p24 antibody levels have been shown to correlate with viral control in several studies, largely focused on subtype B cohorts (summarized by French et al.[32]). However, because p24 is not expressed on the cell surface, the direct contribution of p24-specific antibodies to antiviral control is unclear, but has also been proposed as a surrogate marker of an intact CD4 T cell response[33].
Because the humoral immune response may differ between clade B and C infected individuals[34], here we aimed to define the Fc receptor-mediated functional profiles of gp120- and p24-specific antibody responses in the context of HIV-1 subtype C infection. Similar to previous studies[32, 35], we found that levels of p24-specific but not gp120-specific IgG1 were associated with lower viral loads and higher CD4 counts. Furthermore, individuals with high p24 IgG1 levels had higher Fc-mediated responses that showed a trend towards viral control and higher CD4 counts. The association between levels of p24 IgG1 and viral loads and CD4 counts was maintained even after adjusting for the impact of Gag-specific CD4 and CD8 T cell responses and protective HLA class I alleles (HLA-I). These data indicate that p24-specific IgG1 may independently contribute to viral control and further studies are warranted to ascertain their direct or indirect roles in antiviral immunity.
Materials and Methods
Study Subjects
361 archived plasma samples from the Sinikithemba cohort[36], a study of HIV-1 subtype C–infected, ART-naïve individuals, established in Durban, South Africa were included in this study. All study participants provided written informed consent, and the study protocol was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal.
Customized IgG Subclass Binding Assay
To determine the relative levels of each HIV-1 specific isotype/subclass in plasma, we adopted a customized subclass binding assay described previously[37]. We used carboxylated microspheres (luminex) coupled to HIV-1 recombinant proteins of interest: p24 subtype B/C and gp120 consensus C (Immune Technology Corp).
Antibody-Dependent Cellular Phagocytosis (ADCP)
The ability of plasma antibodies to mediate ADCP was determined as previously described using gp120 consensus C and p24 subtype B/C protein (Immune Technology Corp)[38]. Antigen-bound fluorescent neutravidin beads (Invitrogen) were incubated with 1:100 dilution of plasma antibodies in the presence of THP-1 cells. HIV negative plasma and media alone were used as negative controls and pooled HIV positive plasma was used as a positive control. The cells were analyzed for bead uptake by flow cytometry and the phagocytic score representing iMFI values (integrated Mean Fluorescence Intensity=frequency x MFI)[39] was calculated. Flow cytometry gating strategy is shown in Supp Figure 1A.
While previous studies[38] have confirmed that gp120-specific ADCP activity is mediated by FcγR mechanisms, this has not been confirmed when using p24 protein as an antigen. Therefore as an additional control, 6 randomly selected HIV positive plasma samples were purified for IgG using Melon Gel (Thermo Fisher Scientific). THP-1 cells were pre-incubated with FcγRII (Abcam) or FcγRIII (Sigma) blocking antibodies for 30 min prior to assaying for p24-specific ADCP responses as described above (Supp Figure 1B).
Natural Killer (NK) Cell degranulation as a surrogate for ADCC
We used a modified ELISA-based protocol for detection of CD107a as a surrogate marker of NK cell–mediated cytolysis[40]. This assay has previously been shown to be associated with FcγRIIIa binding capacity of antigen-specific antibodies[41, 42]. A 96-well plate was coated overnight at 4°C with 150ng of recombinant protein per well,. 2% BSA blocked plates were used as antigen controls. The next day the plates were washed 6 times with PBS, 50μl of plasma (diluted 1:100) was added to each well, and incubated at 37°C for 2 hours. HIV negative plasma samples or media alone were used as negative controls, while HIVIG (pooled HIV Immunoglobulin G, NIH AIDS Reagents Program) was used as a positive control. The plates were washed and 5 × 104 NK cells enriched via negative selection from healthy blood donors (RosetteSep, Stemcell Technologies,) were added to each well in the presence of Brefeldin A (Biolegend), Golgi stop, and anti-CD107a-PE-Cy5 (BD Biosciences). The plate was incubated for 5 hours at 37°C and 5% CO2. Following incubation, cells were stained with anti-CD3-AF700, anti-CD56-PE-Cy7, anti-CD16-APC (BD), fixed with Perm A, permeabilized using Perm B (Invitrogen), and stained with anti-IFNγ-APC and anti-MIP1β-PE (BD). The cells were then fixed with 2% paraformaldehyde and analyzed by flow cytometry (gating strategy shown in Supp Figure 2).
Gag-Specific CD4+ and CD8+ T cell responses
Gag-specific IFN-γ CD4+ T cell responses were detected by intracellular cytokine staining (ICS) using freshly isolated PBMCs as previously described[43] staining for anti-human CD4 APC and anti-human IFNγ FITC. Gag-specific IFNγ CD8+ T cell were detected by IFNγ enzyme-linked immunosorbent spot (ELISpot) using overlapping peptides spanning Gag subtype C as previously described[44].
Statistical Analysis
Non-parametric Spearman’s rank tests were used to test for correlations, and a 2-tailed Mann Whitney test was used to evaluate unmatched groups. p values less than 0.05 were considered significant. To assess the effect of p24 IgG1 on viral load and CD4 count, multiple linear regression was used. IgG1p24 values were z-score normalized (standardized) to have a mean of zero and a standard deviation of one. We considered a variable to be a potential confounder of the effect of p24 IgG1 if it was shown to have a significant relationship with the outcome in univariate analysis or if its inclusion in the model resulted in a 10% or greater change in the estimated coefficients. A square root transformation was applied to CD4 count and a log transformation was applied to viral loads, which resulted in approximately normally distributed values. Analysis was performed using Graphpad Prism version 6 (Graphpad Software) and Stata Statistical Software: Release 13 (StataCorp LP).
Results
p24 IgG1 levels predict viral control in chronic HIV-1 subtype C infection
Previous studies, largely in clade B infected cohorts, have shown that p24- but not gp120-specific antibody levels are a prognostic marker of disease progression[25, 28–32]. Thus, to first define whether similar relationships were observable in clade C infection, a cross-sectional study including a cohort of 361 HIV-infected subjects (median viral load: 4.8 log10 HIV-RNA copies/ml, interquartile range (IQR) 3.9-5.3; median CD4 count: 339 cells/mm3, IQR 226-492) were profiled for gp120- or p24-specific antibodies. HIV-specific IgG to both proteins were detected in all individuals, however, neither gp120- nor p24-specific antibody levels correlated with either viral loads or CD4 counts (Fig 1A and C-left).
Figure 1. p24 IgG1 levels predict viral control in chronic HIV-1 subtype C infection.
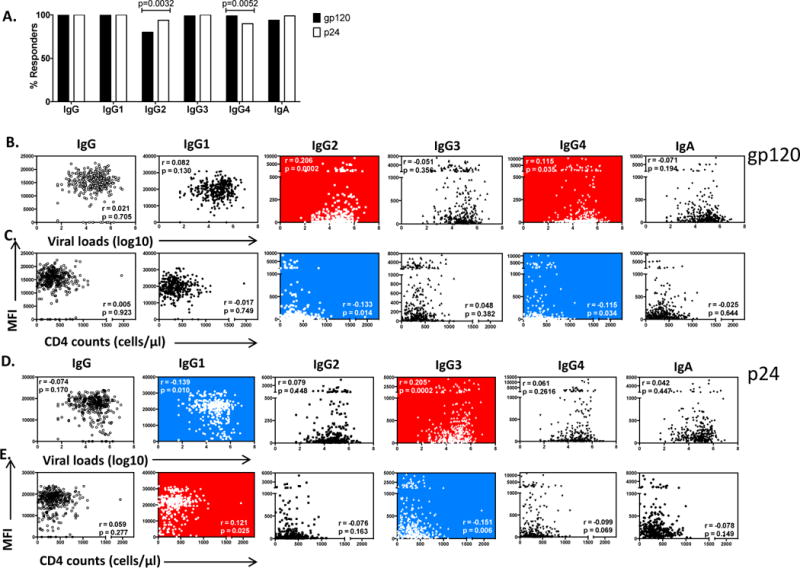
Using recombinant protein coupled to fluorescent beads and diluted plasma, a customized luminex assay was performed to determine the levels of HIV-1 gp120- and p24 specific antibodies. A). describes % of positive responders. A positive threshold was determined as 3× mean +2SD of HIV negatives. The relationship between the mean fluorescence intensity (MFI) of gp120 total IgG and isotype specific binding antibodies and viral loads or CD4 counts is depicted in panels B) and C) while p24 is shown in panel D) and E. Plots with red and blue background represent significant positive and negative correlations respectively while a white background denotes non-significant relationships. p-values were calculated by Spearman’s correlation.
Next we determined whether relative differences in gp120- or p24-specific Ig subclass specific responses correlated with measures of viral control. Similar to reports from other subtypes[22], the antibody responses directed at both gp120 and p24 were dominated by IgG1 responses. IgG3 directed to p24 was also observed in all individuals, and gp120-IgG3 responses were detected in most individuals (99%). Conversely, levels of other subclasses were lower and less frequently detected: p24- and gp120-specific IgA were detected in 94% and 99% of the cohort respectively Conversely, levels of other subclasses were lower and less frequently detected: gp120- and p24-specific IgA were detected in 94% and 99% of the cohort respectively, while gp120- and p24-specific IgG2 were detected in 80% and 94% of subjects respectively (p=0.0032), and gp120- and p24-specific IgG4 were detected in 99% and 90% respectively (p=0.0052) (Fig. 1).
To further probe the relationship between antigen-subclass specific responses and viral control, the relationships between individual antigen-specific subclasses and markers of disease progression were assessed. Higher gp120-specific IgG2 and IgG4 responses were associated with higher viral loads (p=0.0002, r=0.206 and p=0.035, r=0.115 respectively) (Fig. 1A) and were negatively associated with CD4 counts (p=0.014, r= −0.133 and p=0.034, r= −0.115 respectively) (Fig. 1B). Similarly, increasing levels of IgG3 responses targeting p24 were also associated with higher viral loads (p=0.0002, r=0.205) (Fig. 1C) and lower CD4 counts (p=0.006, r = −0.151) (Fig. 1D). In contrast, Gag p24 IgG1 levels were negatively correlated with viral loads (p=0.01, r= −0.139) (Fig. 1C) and positively correlated with CD4 counts (p=0.025, r=0.121) (Fig. 1D). These data suggest that higher levels of p24-IgG3 subclass selection profiles in chronic subtype C infection are associated with poor viral control, while p24-specific IgG1 responses in chronic subtype C infection may be a marker of viral control.
Elevated p24 IgG1 levels track with enhanced Fc effector activity
While antibodies directed against internal structural proteins of the virion have a limited capacity to mediate neutralization, these antibodies may contribute to viral control via non-neutralizing antiviral functions[45, 46]. Thus, we next profiled the ADCP and ADCC activity of these antibodies and found that p24-specific phagocytic activity was detectable in all individuals while only low-level p24-specific ADCC activity was observed, in a minority of subjects. Detectable p24-specific NK cell degranulation (CD107a) was observed in 34% of the tested subjects, and IFNγ and MIP1β secretion was detected in 47% and 16% of the subjects, respectively, with strong correlations observed between all three functions (p=0.004 to p<0.0001) (Fig 2A). We then determined the relationship between antibody levels and functional activity and found that p24 IgG1 was positively correlated with both ADCP phagoscore and CD107a expression (p<0.0001 and p=0.033 respectively, Fig 2B). Interestingly, there was a clear distribution of high and low IgG1 levels when considering ADCP phagoscore. Using the median value as a cutoff for p24 IgG1 levels, we found that individuals with high p24 IgG1 levels were able to mediate significantly higher ADCP and ADCC (CD107a) responses (p<0.0001 Fig. 2C-left and p=0.027 respectively; Fig. 2C-right). These data suggest that p24 antibodies may be effective at mediating Fc effector functions.
Figure 2. p24 IgG1 levels correlate with both ADCP phagoscore and CD107a expression levels.
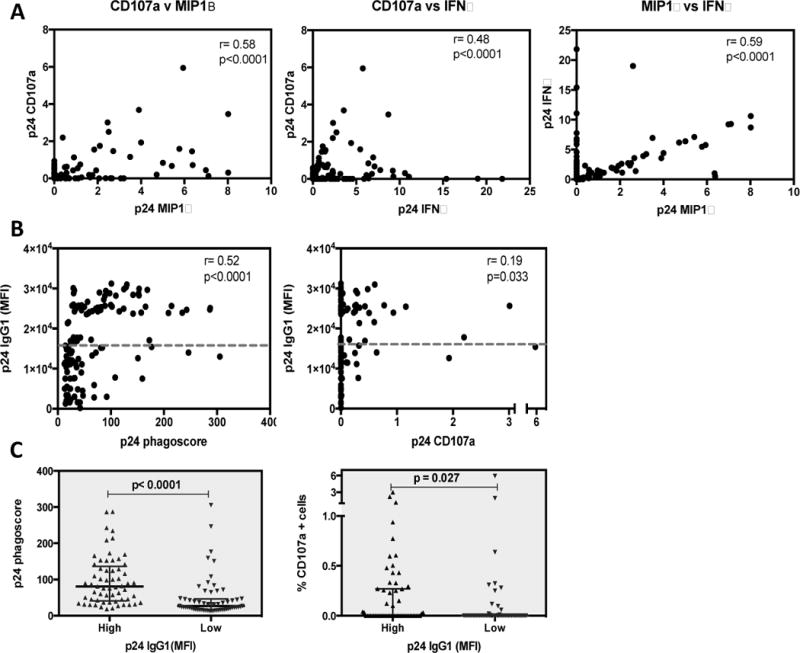
(A) Antibody mediated NK cell activation measured by expression of CD107a degranulation as a surrogate for ADCC, IFNγ cytokine secretion and MIP1β were assayed in the presence of p24 protein as a surrogate for ADCC activity. All measurements of NK activation were high correlated with each other. (B) Antibody dependent cellular phagocytosis (ADCP) of fluorescently labelled bead conjugated to p24 protein by monocyte cell line THP-1 was assessed. The relationship between the mean fluorescence intensity (MFI) of p24 specific IgG1 with ADCP phagoscore (left) and CD107a levels (right) are depicted. The grey dotted line on each graph represents the median p24 specific IgG1 levels. (C) Using the median, individuals were dichotomized into 2 groups with high and low p24 IgG1. The differences in magnitude of p24 ADCP (left) and ADCC (right) responses in the groups are shown respectively. Correlations between two variables were calculated by Spearman’s test. Difference between two groups were calculated by 2-tailed Mann-Whitney test.
Higher p24-specific Fc effector activity trends with markers of disease progression
Given that higher p24 IgG1 levels were associated with higher Fc effector activity, we next investigated the relationship between p24-specific Fc effector activity and markers of disease progression. While we did not observe a significant relationship between the expression of CD107a and viral load (p=0.562, r= −0.055) (Fig. 3B), a trend towards higher CD4 counts (p=0.065, r=0.172) was observed (Fig. 3D). In addition, the magnitude of p24-specific ADCP responses trended towards a negative correlation with viral load (p=0.086, r= −0.157; Fig. 3A) and a positive correlation with CD4 count (p=0.082, r=0.158; Fig. 3C), suggesting that p24-specific antibody functions are associated, albeit weakly, to viral control.
Fig. 3. Higher p24-specific Fc effector activity trends with markers of disease progression.
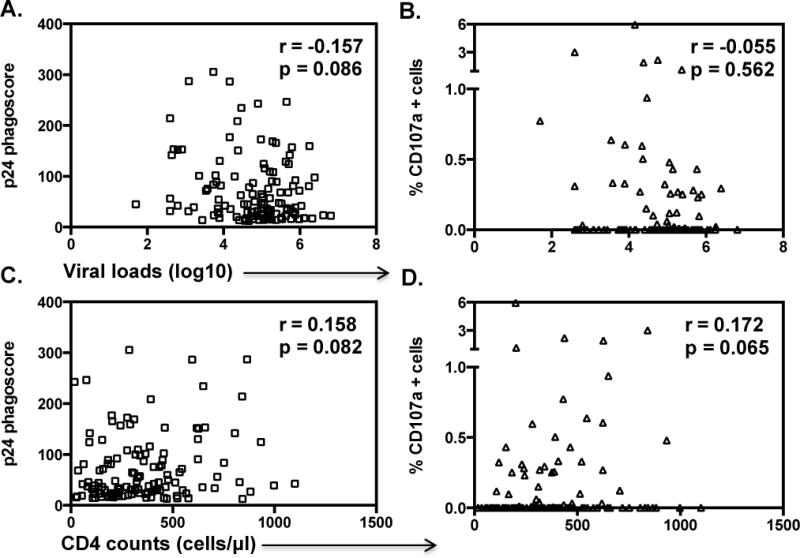
Diluted plasma was used to cross link and initiate phagocytosis of FITC fluorescent beads conjugated to p24 protein (ADCP) by THP-1 cells. Antibody mediated NK activation (detection of CD107a used as a surrogate for ADCC) was measured in the presence of p24 protein. The relationship between the magnitude of ADCP and ADCC responses and viral loads shown in panels A and B while panels C and D show the relationship with CD4 counts. p-values were calculated by Spearman’s correlation.
p24 IgG1 levels are not a simple surrogate of protective HLA class I antiviral activity
To next determine whether p24-specific antibodies may contribute to antiviral control, we examined the relationship between p24-specific IgG1 responses and traditional markers of HIV-1 control. Specifically, particular HLA class I alleles have been linked to slower disease progression, through the induction of superior CD8+ T cell activity in this cohort[36, 44, 47]. Of the 361 individuals, those with protective HLA class I alleles (HLA-B*57:01, HLA-B*58:01, HLA-B*81:00 and HLA-B*81:01) had significantly lower viral loads (p=0.002) and higher CD4 counts (p=0.003). Thus to define whether p24 IgG1 was independently associated with antiviral control individuals with protective HLA-I alleles were removed from the analysis (n=24). Interestingly, individuals with high p24 IgG1 levels maintained higher ADCP (p<0.0001) and ADCC (p=0.035), (Fig. 4A, B) and continued to exhibit lower viral loads (p=0.004) and higher CD4 counts (p=0.019) (Fig. 4C, D), indicating that the relationship between p24 specific IgG1 responses and enhanced viral control was not solely influenced by protective HLA-I alleles and therefore may represent an independent predictor of enhanced viral control.
Figure 4. Relationship between HLA alleles, CD4 and CD8 Gag responses and Gag p24 IgG1 levels.
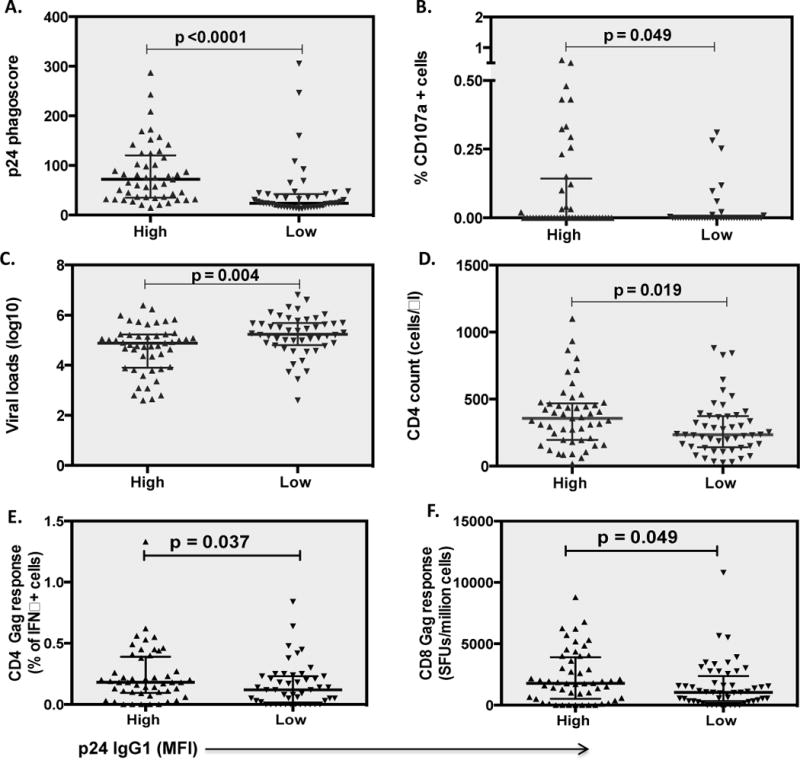
Patients with protective HLA alleles (HLA-B*57:01, HLA-B*58:01 and HLA-B* 81:00/01) were removed from this analysis and individuals with no protective alleles were dichotomized into groups of high and low p24 IgG1 levels. The panels show the difference in the magnitude of ADCP (A) and ADCC (B) responses, viral loads (C) and CD4 counts (D). Gag CD4 and CD8 activity was measured by ICS and ELISpot respectively and reported as % of cells positive for IFNγ or as number of spot forming units (SFUs/million PBMCs) respectively. Differences in CD4 and CD8 Gag responses in the dichotomized groups of individuals with high and low p24 IgG1 respectively are shown in panels A and B. p-values were calculated by 2-tailed Mann-Whitney test
p24 IgG1 levels independently mark protective HIV-1 control
Both CD4+ and CD8+ T cell responses have also been linked to protective immunity in this cohort[36, 43, 48]. Moreover, because enhanced helper CD4 T cell responses may promote more effective antibody responses in addition to viral control it is possible that elevated p24 antibodies may simply represent a marker of preserved T cell immunity rather than a direct antiviral mechanism of control. Thus, to parse out the relationship between T cell mediated antiviral control and p24-specific antibody responses, we next examined the inter-relationship between p24 IgG1 responses and both arms of the T cell response in these individuals. The frequency of Gag-specific CD4 T cells (by ICS) and magnitude of CD8 T cells (by ELISpot) was associated with lower viral loads, (p<0.0001 for both) and higher CD4 counts (p=0.0002 and 0.138) respectively in these individuals, as previously observed[36]. Moreover, when individuals were split into those with high or low p24 IgG1 levels, subjects with high p24 IgG1 levels had significantly higher Gag-specific CD4 (p=0.037) and CD8 (p=0.049) responses (Fig. 4E, F) suggesting that p24-specific humoral immune responses are induced in parallel to protective Gag T cell immunity.
However, to gain insights into whether p24-specific IgG1 immunity was simply a surrogate of a more effective T cell immune response or if it represented an independent marker of enhanced viral control, factors that were associated with viral control and elevated CD4 counts in univariate analyses including IgG1p24 levels, CD4/CD8-T cell Gag responses (dichotomized as having any or no response) and the presence of protective HLA-I alleles, were considered in a multivariate analysis. The CD4 T cell Gag response remained a significant predictor of viral load and CD4+ T cell counts (p=0.002 and p<0.001, respectively) (Table 1). Yet, most interestingly, even after adjusting for CD4 or CD8 Gag responsiveness and carriage of protective HLA-I alleles, IgG1 p24 antibody responses remained significantly associated with lower viral loads (p=0.030) and higher CD4 counts (p=0.005, Table 1). Hence, p24 IgG1 antibodies independently predict viral control and preservation of CD4 cells and likely complement T cell–mediated antiviral immunity in the control of viral replication.
Table 1.
Univariate and multivariate analysis of relationships with Viral Loads or CD4 counts
|
|
||||
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
|
|
||||
| Variable | Coefficient (SE) | p value | Coefficient (SE) | p value |
| Viral loada | ||||
|
| ||||
| p24 IgG1b | −0.14 (0.05) | 0.009 | −0.13 (0.06) | 0.030 |
| CD4 Gag | −0.46 (0.12) | <0.001 | −0.40 (0.13) | 0.002 |
| CD8 Gag | −0.42 (0.16) | 0.008 | −0.31 (0.16) | 0.052 |
| Protective HLA | −0.56 (0.13) | <0.001 | −0.37 (0.14) | 0.012 |
|
| ||||
| CD4 countc | ||||
|
| ||||
| p24 IgG1b | 0.87 (0.32) | 0.007 | 0.96 (0.34) | 0.005 |
| CD4 Gag | 3.71 (0.70) | <0.001 | 3.55 (0.72) | <0.001 |
| CD8 Gag | 1.95 (0.94) | 0.038 | 1.61 (0.89) | 0.072 |
| Protective HLA | 1.50 (0.81) | 0.066 | 1.29 (0.81) | 0.115 |
Log transformed
z-score normalized
square root transformed
Discussion
Despite the growing appreciation for the role of non-neutralizing antibody functions in both antiviral control[9–13] and protection from HIV-1 infection[4, 5, 14], little is known about the role of these functions in the context of HIV-1 subtype C viral control, which predominates the current global epidemic. Thus, here, we aimed to define the role of Fc-mediated antibody activities in chronic subtype C infection in a cross-sectional cohort of HIV-1-infected subjects at the heart of the South African epidemic, in Durban. Similar to previous studies of other viral subtypes[23–31], the levels of gp120-specific total IgG and other IgG subclasses did not correlate with viral control. Instead, as previously reported[32] p24-specific IgG1 responses were associated with antiviral control and slower progression to disease. Moreover, these antibodies have the capacity to mediate both cytotoxicity and clear infected cells via phagocytosis, providing two potential antiviral mechanisms by which these antibodies may contribute to antiviral control. Finally, while elevated CD4 and CD8 T cell responses were induced among individuals with the highest p24-specific IgG1 responses, multivariate modeling demonstrated that the p24-specific IgG1 levels independently predicted HIV-1 control, suggesting for the first time that p24-specific functional IgG1 antibodies may contribute directly to antiviral immunity in a manner complementary to known mechanisms of cellular immune control of HIV-1.
The selection of the poorly functional gp120 IgG2 and IgG4 was associated with poor viral control, likely by dampening antibody functionality due to their lower affinity for Fc-receptors, as has been previously reported[5]. However, interestingly, while IgG3, the most functional antibody subclass, has been linked to reduced risk of HIV-1 infection in RV144 vaccinees[4, 5], here, elevated gp120- or p24-specific IgG3 levels were not observed in subjects with markers of slower disease progression. Rather, elevated p24-specific IgG3 responses were associated with higher viral loads and lower CD4 T cell counts. As IgG3 is the first subclass in the immunoglobulin heavy locus, the selection of IgG3 antibodies is a signature of recently selected B cells, that have not rearranged to IgG1, IgG2, or IgG4[49]. Thus, these data suggest that p24 IgG3 antibody responses in chronic infection may represent a marker of a less mature B cell response, which lack provision of B cell cross switch recombination to other subclasses, while, in contrast, enhanced p24 IgG1 responses may contribute most effectively to post-infection antiviral immunity in the setting of Clade C HIV-1 infection.
While the association between p24-specific antibody levels and markers of disease control has been reported in several studies[32], the mechanism by which p24-specific IgG1 antibodies contribute to antiviral immunity remains unclear. One possibility includes a scenario where higher virus loads drive the formation of p24-containing immune complexes; although, this hypothesis has been refuted by a study done by Fenouillet et al. who showed that acid dissociation of immune complexes did not increase p24 levels [50]. Alternatively, p24-specific IgG1 antibodies may only be a marker of an intact immune response, where preserved p24-specific CD4+ helper T cell responses sustain elevated levels of p24-specific B cell responses p24-specific IgG1 but do not individually contribute to viral control[27, 51]. Our data, however, challenges this last hypothesis. Although we show that although p24-specific IgG1 levels are associated with higher CD4 T cell numbers (Fig. 1D) as well as elevated CD4+ T cell help (Fig. 4E), the ability to predict lower viral loads is sustained after controlling for CD4 T cell numbers and Gag-specific CD4 responses (Table 1). Thus, our data indicates that p24-specific IgG1 responses, beyond being only a marker of a more effective overall immune response, may also contribute independently to viral control.
Whether Gag is exposed on the virion surface remains a subject of debate. Although rare studies[52–54] have detected cell surface p24 on infected cells, these studies have only been conducted on cell lines, and surface expression was only observed at high, non-physiological rates of infection. However, it is possible that Gag may be exposed during delivery of HIV-1 Gag to the plasma membrane for virion assembly[55], particularly in the setting of early apoptosis when the inner-cell lipid layer flips out to expose phosphotidyl-serine, potentially exposing cytosolic anchored membrane proteins as well[56]. It is also plausible that in addition to native protein expression on the cell surface, processed Gag peptides may be recognized by antibodies in the groove of MHC molecules on the surface of infected cells to induce ADCP or ADCC, though this has yet to be demonstrated. Importantly, Fc effector responses to other non-envelope protein derived-peptides have been reported[12, 45, 46, 57–59]. Indeed Tjiam et. al. recently showed p24 IgG1 and IgG2 enhanced dendritic cell-mediated opsonophagocytic responses in individuals who controlled viremia[46] and these same opsonophagocytic responses were associated with early viral control, interestingly specifically mediated by p24 IgG1[45]. In our study, only p24-specific IgG1 levels were associated with higher ADCP and ADCC responses that trended towards viral control. Unlike Tjiam’s study, p24-specific IgG2 levels did not correlated with Fc function, potentially due to the different assays used, which measure different Fc functions and utilize different effector cells. Although in our study, these p24-specific Fc effector functions did not achieve significance with markers of disease progression (Fig 4, p-value range=0.062–0.086), antibodies are capable of mediating multiple other Fc-driven effector functions beyond ADCP and ADCC, including complement fixation, neutrophil activation, DC-activation as well as recruiting multiple other FcγR-expressing effector cells. Furthermore, all our assays were tested utilizing total plasma, which includes multiple different immunoglobulin Isotypes including IgA, IgM, IgE along with other serum proteins including complement which were not assessed. Future studies testing for alternative Fc effector functions using purified IgG and isolated p24-specific IgG1 could provide greater insights.
Additionally, antibodies have recently been shown to not only drive extra-cellular antiviral activity, but also to target pathogens within cells. In fact, the intracellular host restriction factor TRIM21 is an intracellular cytoplasmic Fc receptor that can drive rapid and profound autophagy. In this context, interactions between TRIM21 and cytosolic antibody-coated antigens triggers an innate immune intracellular signaling cascade that ultimately results in the induction of autophagy[60]. Thus, it is plausible that p24-specific antibodies able to gain access to the cytoplasm may drive the rapid elimination of infected cells. Taken together, these observations suggest that p24-specific IgG1 antibodies may contribute to antiviral control via a spectrum of diverse mechanisms, thereby driving viral control by unconventional Fc-driven means.
This study used a large cohort that has been well characterized for cellular responses associated with disease outcome, enabling a holistic understanding of the relationship between HIV-specific antibody responses and function with markers of slower disease progression. Importantly, the study demonstrated the independent predictive value of p24-specific IgG1 responses on markers of disease progression, highlighting a potential role for Gag-specific antibodies in antiviral control. However because this study used cross-sectional samples from a cohort of chronically infected individuals with unknown dates of infection, it remains unclear whether p24-specific antibodies actively contribute to reduced disease progression. Thus future longitudinal studies on acutely infected patients may shed light on the impact of p24-specific IgG1 antibodies on rates of disease progression.
In summary, these data confirm the importance of antibody isotype selection in HIV-1 clade C infection and show that p24-specific IgG1 and not gp120-specific antibodies are associated with markers of slower HIV-1 disease progression. Moreover, p24-specific antibodies have the capacity to mediate Fc effector functions and predict viral control independent of Gag-specific CD4 and CD8 T cell responses or the presence of protective HLA-I alleles. Determining both the mechanisms of antiviral activity of Gag-specific antibodies will provide us with a greater understanding of its potential as a target for an antibody-based vaccine, particularly in light of its more conserved nature.
Supplementary Material
A. The flow cytometry gating strategy for the ADCP assay is illustrated. THP-1 cells were acquired by FACS and selected for singlets. THP-1 cells were then assessed for uptake of p24 coated Beads (FITC). Representative histograms of Negative control sample (HIV negative plasma) and HIV positive control sample (Pooled HIV plasma) are illustrated. Phagocytic scores were calculated representing iMFI values (integrated MFI: frequency x MFI). For ease of presentation, reported phagocytic values are divided by 105. B. To confirm that p24-specific ADCP activity is mediated by Fcgamma Recpetor activation of effector cells, THP-1 effector cells were pre-incubated with FcgR2 (CD32) or Fcg3 (CD16) blocking antibody. Purified IgG was isolated from 6 randomly selected HIV-1 positive plasma samples using Melon Gel according to manufacturer’s recommendations. P24-specific ADCP activity was then assessed from the 6 HIV-1 purified IgG samples (Black circles) compared with 3 HIV negative samples (white squares). Differences between groups were calculated using Wilcoxon matched pairs signed rank test.
The flow cytometry gating strategy is described above. Enriched NK cells (via negative selection RosetteSep kit) were acquired and selected for singlets. NK cell population was confirmed and selected for as CD3 negative lymphocytes, that express CD56+/CD16+. Frequency of NK cells that express CD107a, IFNγ or MIP1β were determined. Responses from a representative negative control sample (HIV negative plasma) and HIV positive control sample (HIVIG-pooled HIV Immunoglobulin G) are shown.
Acknowledgments
We thank the study participants, the clinical and laboratory staff of the HIV Pathogenesis Programme. This work was supported by the National Institute of Health (R01 AI080289 and R01 A102660-01), the Bill and Melinda Gates Foundation CAVD (OPP1032817: Leveraging Antibody Effector Function), the Ragon Institute of MGH, MIT and Harvard, the South African Research Chairs Initiative, the Victor Daitz Foundation and an International Early Career Scientist Award from the Howard Hughes Medical Institute to T.N., National Health and Medical Research Council and the American Foundation for AIDS Research Mathilde Krim Fellowship to AWC.
Footnotes
This work was presented in part at the AIDS conference 7-10th October 2013 in Barcelona Spain. Title; “p24 specific Antibody Dependent Cellular Phagocytosis in Associated with Antiviral Control in Chronic HIV-1 Subtype C Infection.”
Author contributions:
AWC, JMM, PG, BW, TN, GA conceived the study, AWC, JMM, BN, AL, HR, YR conducted experiments. AWC, JMM, MG, TR conducted the analysis. AWC, JMM, TN, GA wrote the manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.http://www.unaids.org/en/resources/fact-sheet.
- 2.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. The New England journal of medicine. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 3.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. The New England journal of medicine. 2012;366(14):1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Science translational medicine. 2014;6(228):228ra239. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Science translational medicine. 2014;6(228):228ra238. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 6.Forthal D, Hope TJ, Alter G. New paradigms for functional HIV-specific nonneutralizing antibodies. Current opinion in HIV and AIDS. 2013;8(5):393–401. doi: 10.1097/COH.0b013e328363d486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung AW, Alter G. Dissecting the Antibody Constant Region Protective Immune Parameters in HIV Infection. Future Virol. 2014;9(4):397–414. [Google Scholar]
- 8.Chung AW, Navis M, Isitman G, Wren L, Silvers J, Amin J, et al. Activation of NK cells by ADCC antibodies and HIV disease progression. Journal of acquired immune deficiency syndromes. 2011;58(2):127–131. doi: 10.1097/QAI.0b013e31822c62b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J, Rinaldo C, et al. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. Journal of immunology. 1996;157(5):2168–2173. [PubMed] [Google Scholar]
- 10.Ahmad R, Sindhu ST, Toma E, Morisset R, Vincelette J, Menezes J, et al. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J Clin Immunol. 2001;21(3):227–233. doi: 10.1023/a:1011087132180. [DOI] [PubMed] [Google Scholar]
- 11.Forthal DN, Landucci G, Robinson WE., Jr Lymphokine-activated cytotoxicity in peripheral blood mononuclear cells of severely immunocompromised HIV-infected patients. Scandinavian journal of immunology. 1997;45(1):91–95. doi: 10.1046/j.1365-3083.1997.d01-367.x. [DOI] [PubMed] [Google Scholar]
- 12.Wren LH, Chung AW, Isitman G, Kelleher AD, Parsons MS, Amin J, et al. Specific antibody-dependent cellular cytotoxicity responses associated with slow progression of HIV infection. Immunology. 2013;138(2):116–123. doi: 10.1111/imm.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambotte O, Pollara J, Boufassa F, Moog C, Venet A, Haynes BF, et al. High Antibody-Dependent Cellular Cytotoxicity Responses Are Correlated with Strong CD8 T Cell Viral Suppressive Activity but Not with B57 Status in HIV-1 Elite Controllers. PloS one. 2013;8(9):e74855. doi: 10.1371/journal.pone.0074855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS pathogens. 2012;8(6):e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Roman VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R, et al. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. Journal of immunology. 2005;174(4):2185–2189. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- 16.Hidajat R, Xiao P, Zhou Q, Venzon D, Summers LE, Kalyanaraman VS, et al. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. Journal of virology. 2009;83(2):791–801. doi: 10.1128/JVI.01672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao P, Zhao J, Patterson LJ, Brocca-Cofano E, Venzon D, Kozlowski PA, et al. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. Journal of virology. 2010;84(14):7161–7173. doi: 10.1128/JVI.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482(7383):89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, McNally AG, et al. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell. 2013;155(3):531–539. doi: 10.1016/j.cell.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, et al. Protective efficacy of adenovirus-protein vaccines against SIV challenges in rhesus monkeys. Science. 2015;349(6245):320–324. doi: 10.1126/science.aab3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung AW, Kumar MP, Arnold KB, Yu WH, Schoen MK, Dunphy LJ, et al. Dissecting Polyclonal Vaccine-Induced Humoral Immunity against HIV Using Systems Serology. Cell. 2015;163(4):988–998. doi: 10.1016/j.cell.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomaras GD, Haynes BF. HIV-1-specific antibody responses during acute and chronic HIV-1 infection. Current opinion in HIV and AIDS. 2009;4(5):373–379. doi: 10.1097/COH.0b013e32832f00c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binley JM, Klasse PJ, Cao Y, Jones I, Markowitz M, Ho DD, et al. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. Journal of virology. 1997;71(4):2799–2809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrieu JM, Eme D, Venet A, Audroin C, Tourani JM, Stern M, et al. Serum HIV antigen and anti-P24-antibodies in 200 HIV seropositive patients: correlation with CD4 and CD8 lymphocyte subsets. Clinical and experimental immunology. 1988;73(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- 25.Hogervorst E, Jurriaans S, de Wolf F, van Wijk A, Wiersma A, Valk M, et al. Predictors for non- and slow progression in human immunodeficiency virus (HIV) type 1 infection: low viral RNA copy numbers in serum and maintenance of high HIV-1 p24-specific but not V3-specific antibody levels. The Journal of infectious diseases. 1995;171(4):811–821. doi: 10.1093/infdis/171.4.811. [DOI] [PubMed] [Google Scholar]
- 26.Sheppard HW, Ascher MS, McRae B, Anderson RE, Lang W, Allain JP. The initial immune response to HIV and immune system activation determine the outcome of HIV disease. Journal of acquired immune deficiency syndromes. 1991;4(7):704–712. [PubMed] [Google Scholar]
- 27.Teeuwsen VJ, Lange JM, Keet R, Schattenkerk JK, Debouck C, van den Akker R, et al. Low number of functionally active B lymphocytes in the peripheral blood of HIV-1-seropositive individuals with low p24-specific serum antibody titers. AIDS. 1991;5(8):971–979. doi: 10.1097/00002030-199108000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Zwart G, van der Hoek L, Valk M, Cornelissen MT, Baan E, Dekker J, et al. Antibody responses to HIV-1 envelope and gag epitopes in HIV-1 seroconverters with rapid versus slow disease progression. Virology. 1994;201(2):285–293. doi: 10.1006/viro.1994.1293. [DOI] [PubMed] [Google Scholar]
- 29.Turbica I, Simon F, Besnier JM, LeJeune B, Choutet P, Goudeau A, et al. Temporal development and prognostic value of antibody response to the major neutralizing epitopes of gp120 during HIV-1 infection. Journal of medical virology. 1997;52(3):309–315. [PubMed] [Google Scholar]
- 30.Farzadegan H, Henrard DR, Kleeberger CA, Schrager L, Kirby AJ, Saah AJ, et al. Virologic and serologic markers of rapid progression to AIDS after HIV-1 seroconversion. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1996;13(5):448–455. doi: 10.1097/00042560-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 31.Weber JN, Clapham PR, Weiss RA, Parker D, Roberts C, Duncan J, et al. Human immunodeficiency virus infection in two cohorts of homosexual men: neutralising sera and association of anti-gag antibody with prognosis. Lancet. 1987;1(8525):119–122. doi: 10.1016/s0140-6736(87)91964-7. [DOI] [PubMed] [Google Scholar]
- 32.French MA, Abdulai LN, Fernandez S. Isotype Diversification of IgG Antibodies to HIV Gag Proteins as a Therapeutic Vaccination Stragegy for HIV Infection. Vaccine. 2013;1:328–342. doi: 10.3390/vaccines1030328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee K, Klasse PJ, Sanders RW, Pereyra F, Michael E, Lu M, et al. IgG subclass profiles in infected HIV type 1 controllers and chronic progressors and in uninfected recipients of Env vaccines. AIDS research and human retroviruses. 2010;26(4):445–458. doi: 10.1089/aid.2009.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizzardini G, Piconi S, Ruzzante S, Fusi ML, Lukwiya M, Declich S, et al. Immunological activation markers in the serum of African and European HIV-seropositive and seronegative individuals. AIDS. 1996;10(13):1535–1542. doi: 10.1097/00002030-199611000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Banerjee K, Klasse PJ, Sanders RW, Pereyra F, Michael E, Lu M, et al. IgG subclass profiles in infected HIV type 1 controllers and chronic progressors and in uninfected recipients of Env vaccines. AIDS research and human retroviruses. 2010;26(4):445–458. doi: 10.1089/aid.2009.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright JK, Brumme ZL, Carlson JM, Heckerman D, Kadie CM, Brumme CJ, et al. Gag-protease-mediated replication capacity in HIV-1 subtype C chronic infection: associations with HLA type and clinical parameters. Journal of virology. 2010;84(20):10820–10831. doi: 10.1128/JVI.01084-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown EP, Licht AF, Dugast AS, Choi I, Bailey-Kellogg C, Alter G, et al. High-throughput, multiplexed IgG subclassing of antigen-specific antibodies from clinical samples. Journal of immunological methods. 2012;386(1–2):117–123. doi: 10.1016/j.jim.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ackerman ME, Moldt B, Wyatt RT, Dugast AS, McAndrew E, Tsoukas S, et al. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. Journal of immunological methods. 2011;366(1–2):8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nature medicine. 2007;13(7):843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 40.Jegaskanda S, Job ER, Kramski M, Laurie K, Isitman G, de Rose R, et al. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J Immunol. 2013;190(4):1837–1848. doi: 10.4049/jimmunol.1201574. [DOI] [PubMed] [Google Scholar]
- 41.Wines BD, Vanderven HA, Esparon SE, Kristensen AB, Kent SJ, Hogarth PM. Dimeric FcgammaR Ectodomains as Probes of the Fc Receptor Function of Anti-Influenza Virus IgG. Journal of immunology. 2016;197(4):1507–16. doi: 10.4049/jimmunol.1502551. [DOI] [PubMed] [Google Scholar]
- 42.Kristensen AB, Lay WN, Ana-Sosa-Batiz F, Vanderven HA, Madhavi V, Laurie KL, et al. Antibody Responses with Fc-Mediated Functions after Vaccination of HIV-Infected Subjects with Trivalent Influenza Vaccine. Journal of virology. 2016;90(12):5724–5734. doi: 10.1128/JVI.00285-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramduth D, Day CL, Thobakgale CF, Mkhwanazi NP, de Pierres C, Reddy S, et al. Immunodominant HIV-1 Cd4+ T cell epitopes in chronic untreated clade C HIV-1 infection. PloS one. 2009;4(4):e5013. doi: 10.1371/journal.pone.0005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nature medicine. 2007;13(1):46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 45.Tjiam MC, Sariputra L, Armitage JD, Taylor JP, Kelleher AD, Tan DB, et al. Control of early HIV-1 infection associates with plasmacytoid dendritic cell-reactive opsonophagocytic IgG antibodies to HIV-1 p24. AIDS. 2016;30(18):2757–2765. doi: 10.1097/QAD.0000000000001242. [DOI] [PubMed] [Google Scholar]
- 46.Tjiam MC, Taylor JP, Morshidi MA, Sariputra L, Burrows S, Martin JN, et al. Viremic HIV Controllers Exhibit High Plasmacytoid Dendritic Cell-Reactive Opsonophagocytic IgG Antibody Responses against HIV-1 p24 Associated with Greater Antibody Isotype Diversification. J Immunol. 2015;194(11):5320–8. doi: 10.4049/jimmunol.1402918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brumme Z, Wang B, Nair K, Brumme C, de Pierres C, Reddy S, et al. Impact of select immunologic and virologic biomarkers on CD4 cell count decrease in patients with chronic HIV-1 subtype C infection: results from Sinikithemba Cohort, Durban, South Africa. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;49(6):956–964. doi: 10.1086/605503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramduth D, Chetty P, Mngquandaniso NC, Nene N, Harlow JD, Honeyborne I, et al. Differential immunogenicity of HIV-1 clade C proteins in eliciting CD8+ and CD4+ cell responses. The Journal of infectious diseases. 2005;192(9):1588–1596. doi: 10.1086/496894. [DOI] [PubMed] [Google Scholar]
- 49.Murphy KTP, Walport M, et al. Janeway’s immunobology. (8th) 2012:868. [Google Scholar]
- 50.Fenouillet E, Blanes N, Coutellier A, Gluckman JC. Relationship between anti-p24 antibody levels and p24 antigenemia in HIV-infected patients. AIDS research and human retroviruses. 1993;9(12):1251–1255. doi: 10.1089/aid.1993.9.1251. [DOI] [PubMed] [Google Scholar]
- 51.Trkola A, Kuster H, Leemann C, Oxenius A, Fagard C, Furrer H, et al. Humoral immunity to HIV-1: kinetics of antibody responses in chronic infection reflects capacity of immune system to improve viral set point. Blood. 2004;104(6):1784–1792. doi: 10.1182/blood-2004-01-0251. [DOI] [PubMed] [Google Scholar]
- 52.Franke L, Grunow R, Meissner K, Porstmann T, von Baehr R. Inhibition of HIV-1 infection in vitro by murine monoclonal anti-p24 antibodies. Journal of medical virology. 1992;37(2):137–142. doi: 10.1002/jmv.1890370212. [DOI] [PubMed] [Google Scholar]
- 53.Grunow R, Franke L, Hinkula J, Wahren B, Fenyo EM, Jondal M, et al. Monoclonal antibodies to p24-core protein of HIV-1 mediate ADCC and inhibit virus spread in vitro. Clin Diagn Virol. 1995;3(3):221–231. doi: 10.1016/s0928-0197(94)00039-5. [DOI] [PubMed] [Google Scholar]
- 54.Nishino Y, Ohki K, Kimura T, Morikawa S, Mikami T, Ikuta K. Major core proteins, p24s, of human, simian, and feline immunodeficiency viruses are partly expressed on the surface of the virus-infected cells. Vaccine. 1992;10(10):677–683. doi: 10.1016/0264-410x(92)90089-3. [DOI] [PubMed] [Google Scholar]
- 55.Jouvenet N, Neil SJ, Bess C, Johnson MC, Virgen CA, Simon SM, et al. Plasma membrane is the site of productive HIV-1 particle assembly. PLoS biology. 2006;4(12):e435. doi: 10.1371/journal.pbio.0040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Badley AD, Pilon AA, Landay A, Lynch DH. Mechanisms of HIV-associated lymphocyte apoptosis. Blood. 2000;96(9):2951–2964. [PubMed] [Google Scholar]
- 57.Stratov I, Chung A, Kent SJ. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. Journal of virology. 2008;82(11):5450–5459. doi: 10.1128/JVI.01952-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Isitman G, Chung AW, Navis M, Kent SJ, Stratov I. Pol as a target for antibody dependent cellular cytotoxicity responses in HIV-1 infection. Virology. 2011;412(1):110–116. doi: 10.1016/j.virol.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madhavi V, Wines BD, Amin J, Emery S, Group ES. Lopez E, et al. HIV-1 Env- and Vpu-Specific Antibody-Dependent Cellular Cytotoxicity Responses Associated with Elite Control of HIV. Journal of virology. 2017;91(18) doi: 10.1128/JVI.00700-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McEwan WA, Tam JC, Watkinson RE, Bidgood SR, Mallery DL, James LC. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nature immunology. 2013;14(4):327–336. doi: 10.1038/ni.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. The flow cytometry gating strategy for the ADCP assay is illustrated. THP-1 cells were acquired by FACS and selected for singlets. THP-1 cells were then assessed for uptake of p24 coated Beads (FITC). Representative histograms of Negative control sample (HIV negative plasma) and HIV positive control sample (Pooled HIV plasma) are illustrated. Phagocytic scores were calculated representing iMFI values (integrated MFI: frequency x MFI). For ease of presentation, reported phagocytic values are divided by 105. B. To confirm that p24-specific ADCP activity is mediated by Fcgamma Recpetor activation of effector cells, THP-1 effector cells were pre-incubated with FcgR2 (CD32) or Fcg3 (CD16) blocking antibody. Purified IgG was isolated from 6 randomly selected HIV-1 positive plasma samples using Melon Gel according to manufacturer’s recommendations. P24-specific ADCP activity was then assessed from the 6 HIV-1 purified IgG samples (Black circles) compared with 3 HIV negative samples (white squares). Differences between groups were calculated using Wilcoxon matched pairs signed rank test.
The flow cytometry gating strategy is described above. Enriched NK cells (via negative selection RosetteSep kit) were acquired and selected for singlets. NK cell population was confirmed and selected for as CD3 negative lymphocytes, that express CD56+/CD16+. Frequency of NK cells that express CD107a, IFNγ or MIP1β were determined. Responses from a representative negative control sample (HIV negative plasma) and HIV positive control sample (HIVIG-pooled HIV Immunoglobulin G) are shown.


