Summary
Background
Despite reports of fungal infections in patients with inflammatory bowel disease (IBD), their clinical and microbiological characteristics remain unknown.
Objectives
The aim of this systematic review was to examine all available evidence regarding fungal infections in patients with IBD.
Methods
Systematic search of PubMed (through 27 May 2017) for studies providing data on clinical, microbiological, treatment and outcome data of fungal infections in patients with IBD. The primary study outcome was to record the most common fungal species in patients with IBD. Secondary outcomes were classified into 3 categories: (i) characteristics of fungal infections; (ii) data on IBD and (iii) treatment and outcomes of fungal infections in patients with IBD.
Results
Fourteen studies with data on 1524 patients were included in final analysis. The most common fungal infections in patients with IBD were caused by Candida species (903 infections); the most commonly reported site of Candida infection was the gastrointestinal tract. Available evidence shows that most fungal infections occur within 12 months of IBD treatment and within 6 months when anti-TNFa agents are used.
Conclusions
This systematic review thoroughly describes fungal infections in patients with IBD and provides important information for the early detection and management of these infections.
Keywords: Crohn’s, fungal infection, inflammatory bowel disease, opportunistic, systematic review, ulcerative colitis
1 | INTRODUC TION
Patients with inflammatory bowel disease (IBD) are at increased risk of infectious complications caused by bacteria, viruses, fungi and parasites. These pathogens may be common or opportunistic, ie microorganisms with limited pathogenic capacity under ordinary circumstances, but are able to cause serious disease under specific circumstances.1–3 Various factors have been associated with an increased risk of infection in these patients, including immunosuppressive and immunomodulatory therapy, severity of disease activity, comorbidity, narcotic analgesic treatment, surgery, malnutrition, leucopenia and older age.2–6 In particular, the evolution of treatment of IBD, that has introduced the use of anti-tumour necrosis factor a (anti-TNFa) agents, has led to an increased risk of infections in IBD.3,6
TNF-a inhibitors inhibit TNF-a, a central mediator in immunity with important roles in inflammatory cell migration and maturation, granuloma formation and production of inflammatory cytokines.7,8 TNF-a inhibition increases susceptibility to invasive fungal infection by defects in cellular immune response due to reduced interferon-γ production, decreased expression of pattern recognition receptors like Toll-like receptors and leucocyte apoptosis.9–12 Even though their mechanism of immunosuppression greatly varies, it involves alteration in intracellular signalling, inhibition of cytokine production and reduced leucocyte adhesion and migration.13–15 Lastly, glucocorticoids also increase the risk for fungal disease, through reduction in production of pro-inflammatory cytokines, neutrophil adhesion and chemotaxis, macrophage activation and other mechanisms.16,17
Fungal infections are a recognised cause of morbidity and poor outcomes in certain populations, such as in patients with haematologic and solid organ malignancy, and critically ill patients.18,19 Despite reports of fungal infections in patients with IBD, their clinical and microbiological characteristics have not been extensively described, and their significance in clinical practice and patient outcomes remain unknown. For this reason, the aim of this systematic review was to examine all available evidence in the literature regarding fungal infections in patients with IBD, focusing on the most common fungal causes of infection in IBD and their characteristics,
2 | ME THODS
2.1 | Data search
This review has adopted the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.20 Eligible studies were identified through search of Pubmed MEDLINE from inception with the following combination of text-words: (Inflammatory Bowel Disease*[tw] OR Crohn*[tw] OR Ulcerative Colitis[tw] OR colitis[tw] OR ileocolitis[tw] OR IBD[tw]) AND (fung*[tw] OR opportunistic[tw] OR infect*[tw]). Day of last search was 27th May 2017.
2.2 | Study selection
Studies that met the following criteria were included in analysis: (i) published in English; (ii) reported clinical data, laboratory data, infection treatment and/or outcomes and (iii) included patients with IBD who were diagnosed with a fungal infection. Studies with any of the following were excluded from analysis: case reports and case series with fewer than 4 patients (most of them were individual case reports); cases of fungal infections of the skin; experimental studies in animals; secondary research papers (eg reviews); editorials, perspectives and papers not reporting results of primary research; papers irrelevant to fungal infections in patients with IBD; papers not in English. Two investigators (PI, GS) independently reviewed the titles and abstracts of the citations for potentially relevant articles using Abstrackr21; the full-text publications of potentially relevant articles were retrieved and rescreened by the same 2 investigators. Disagreements were resolved by consensus with a third investigator (CT). Reference lists of included studies were searched for relevant articles.
2.3 | Outcomes of interest
The primary study outcome was to record the most common fungal species in patients with IBD. Secondary outcomes were classified into 3 categories: (i) characteristics of fungal infections; (ii) data on IBD and (iii) treatment and outcomes of fungal infections in patients with IBD.
2.4 | Data extraction and definitions
Data from each eligible study were independently extracted by 2 investigators (PI, GS). Extracted data included study type, year of publication and country; patient demographics (age, gender); IBD-related data (years of disease prior to infection, treatment of IBD, duration of treatment); infection-related data and microbiology (criteria or mode of diagnosis of fungal infection, predisposing factors for fungal infection, infection site, presence of complications, isolated fungal strains) and treatment (administered for the fungal infection) and outcomes (ie death). When applicable, invasive fungal infections were defined according to the revised European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group definitions.22 Recorded complications were any clinical deterioration or organ dysfunction that were considered by the authors to be related to the fungal infection. Relation of death to the index infection was reported according to the study authors. The quality of the evidence of the outcomes of included studies was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE).23
3 | RESULTS
3.1 | Literature search
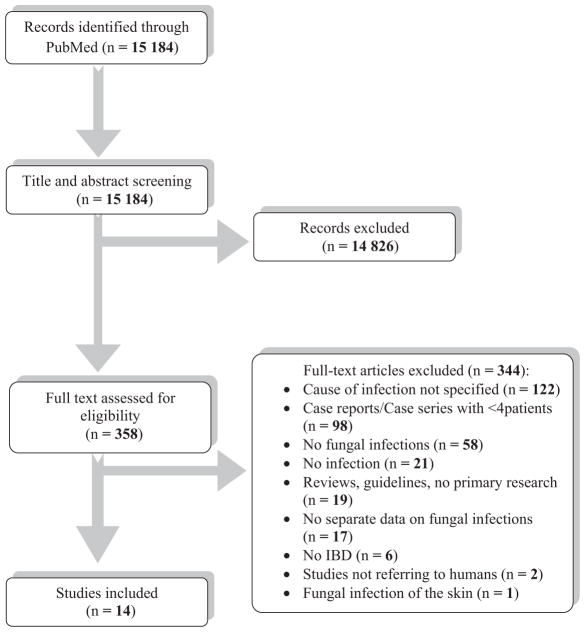
For this systematic review, we screened 15 184 non-duplicate citations from PubMed. After review of the titles and abstracts, 358 articles were retrieved for full-text review. We excluded 344 studies from our review: in 122 studies the microbiological cause of infection was not specified, 98 studies involved fewer than 4 cases, 58 studies had no fungal infections, 21 studies had no infections, 19 studies did not have primary research, 17 studies had no separate data on fungal infections, 6 studies did not have IBD patients, 2 studies did not involve humans and 1 study was on skin infections. No additional studies were found through hand-screening of the references of included studies. Finally, 14 studies4,5,24–35 met our inclusion criteria. Additional information was kindly provided by the corresponding authors of 3 studies.24,29,34 The review process is presented graphically in Figure 1.
FIGURE 1.
PRISMA flow diagram
3.2 | Study characteristics
The 14 included studies involved 1524 patients in total. Table 1 summarises the characteristics of included studies. Nine studies were conducted in North America,4,5,24–28,30,33 3 studies were performed in Asia29,31,32 and 2 studies were conducted in Europe.34,35 Eight studies involved both adult and paediatric patients,5,24,25,27,30–32,35 4 studies involved only adults4,28,29,33 and 2 studies involved exclusively paediatric patients.26,34 Age of patients ranged from 6 to 69.5 years. The majority of included studies were observational studies and case series; as a result the overall quality of the evidence that contributed to our systematic review was rated as low to very low.23
TABLE 1.
Study characteristics
| Study, year published, Country | Study description | Study age group | Number of patients (CD, UC, total) | Age range (y) | Gender male, n (%) |
|---|---|---|---|---|---|
| Lee et al, 2002, USA24a | Retrospective (Database surveillance) | Children & adults | 4 CD 0 UC 4 total |
11–42 (median 28.5) | 4 (100%) |
| Kaur et al, 2007, USA25 | Retrospective (Database surveillance) | Children & adults | 14 CD 2 UC 16 total |
NR | NR |
| Toruner et al, 2008, Ireland5a | Retrospective case-control | Children & adults | CD ND UC ND 29 total |
NR | NR |
| Dotson et al, 2011, USA26 | Retrospective case series | Children | 5 CD 0 UC 5 total |
13–21 (median 14) | 2 (40%) |
| Panaccione et al, 2011, USA27 | Prospective interventional single-arm trial | Adults | 4 CD 0 UC 4 total |
NR | NR |
| Lichtenstein et al, 2012, USA4 | Prospective observational registry | Adults | 5 CD 0 UC 5 total |
26.4–69.5 (mean 46.2) | NR |
| Deepak et al, 2013, USA28 | Retrospective (Database surveillance) | Adults | CD ND UC ND total 760 |
NR | NR |
| Naganuma et al, 2013, Japan29 | Prospective case-control | Adults | CD ND UC ND 6 total |
NR | NR |
| Seminerio et al, 2013, USA30 | Retrospective cohort | Children & adults | 21 CD 0 UC 21 total |
NR | NR |
| Park et al, 2014, South Korea31 | Retrospective cohort | Children & adults | 4 CD 0 UC 4 total |
NR | NR |
| Li et al, 2015, China32 | Retrospective cohort | Children & adults | 5 CD 0 UC 5 total |
NR | NR |
| McAuliffe et al, 2015, USA33 | Retrospective cohort | Adults | CD ND UC ND 656 total |
NR | NR |
| Penninck et al, 2016, France34a | Retrospective cohort | Children | 3 CD 1 UC 4 total |
6–17 (median 13) | 3 (75%) |
| Vögelin et al, 2016, Switzerland35a | Retrospective cohort | Children & adults | 3 CD 2 UC 5 total |
34–56 (mean 43.4) | 1 (20%) |
CD, Crohn’s disease; ND, not defined; NR, Not reported; UC, Ulcerative colitis.
More than 1 site of infection reported in some patients.
3.3 | Microbiology and characteristics of fungal infections
Clinical characteristics of included patients with IBD and fungal infections are presented in Table 2. All studies provided data on the microbiology of infections. Most fungal infections were caused by Candida species (903 infections, of which C. albicans were reported in 41, and C. tropicalis and C. glabrata in 1 case each, whereas the remaining were not reported),4,5,27–35 followed by Histoplasma capsulatum (140 infections),5,24,26,28,30,33 Pneumocystis jirovecii (19 infections),4,25,33 and Cryptococcus neoformans (1 infection).5 In 462 infections,28,30 fungal species were not reported. Table 3 classifies infection site per pathogen. According to the EORTC/MSG definitions which were applicable in 3 studies,5,24,26 34 cases were classified as proven mycosis and 4 cases were classified as probable; in the remaining cases, either clinical and/or mycological criteria were not applicable, or information was insufficient (Table 4).
TABLE 2.
Clinical characteristics and outcomes
| Study, year published | Total number of patients | Type of immunosuppressive treatment, n (%) | Criteria used for infection diagnosis | Site of fungal infections, n (%) | Microbiology of infections | Type of antifungal treatment, n (%) | Infection outcomes, n (%) |
|---|---|---|---|---|---|---|---|
| Lee et al, 200224 | 4 | Corticosteroids or DMARDs 4 (100%) Anti-TNFa (infliximab) 4 (100%) |
Tissue biopsy | Lower respiratory tract 4 (100%) Secondary bloodstream 3 (75%) Secondary upper respiratory 1 (sinusitis) (25%) Secondary liver 1 (25%) |
Histoplasma 4 (100%) | Amphotericin B 4 (100%) formulation NR Itraconazole (maintenance) 1 (25%) |
Clinical cure 4 (100%)a Mortality 0 (0%) |
| Kaur et al, 200725 | 16 | Corticosteroids NR DMARDs NR Anti-TNFa (infliximab) 16 (100%) |
NR | Lower respiratory tract 16 (100%) | Pneumocystis jiroveci 16 (100%) | NR | NR |
| Toruner et al, 20085 | 29b | Corticosteroids 11/14 (78.6%)b DMARDs 1/14 (7.1%)b Anti-TNFa (infliximab) 2/14 (14.3%)b |
Causative microorganism was identified (either by microbiologic or pathologic diagnosis), or there was a pathognomonic clinical picture | Oesophagus & blood & perianal 26 (89.7%) Lung 3 (10.3%) |
Candida species 26 (89.7%) Histoplasma capsulatum 2 (6.9%) Cryptococcus neoformans 1 (3.4%) |
NR | Mortality 0 (0%) |
| Dotson et al, 201126 | 5 | DMARDs 5 (100%) (6-MP 4[80%] MTX 1 [20%]) Anti-TNFa 5 (100%) (infliximab 4, adalimumab 1) |
Complement-fixation assay or culture from bronchoscopy specimen or positive Histoplasma antigen test (urine, blood) | Lower respiratory tract 5 (100%) | Histoplasma capsulatum 5 (100%) | Itraconazole 5 (100%) Amphotericin B (induction) formulation NR 2 (40%) |
Clinical cure 5 (100%) Mortality 0 (0%) |
| Panaccione et al, 201127 | 4 | Corticosteroids NR DMARDs NR Anti-TNFa (adalimumab) 4 (100%) |
NR | Oral 4 (100%) | Candida species 4 (100%) | NR | Mortality 0 (0%) |
| Lichtenstein et al, 20124 | 5 | Corticosteroids 2 (40%) DMARDs 2 (40%) Anti-TNFa (infliximab) 4 (80%) |
NR | Bloodstream 4 (80%) Lower respiratory tract 1 (20%) |
Candida 2 (40%)-unspecified Candida glabrata 1 (20%) Candida tropicalis 1 (20%) Pneumocystis jirovecii 1 (20%) |
NR | Mortality 1 (20%) |
| Deepak et al, 201328 | 760 | Corticosteroids 223 (29.3%) DMARDs 289 (38%) Anti-TNFa 719 (94.6%) |
NR | Respiratory tract 164 (21.6%) Gastrointestinal tract 13 (14.9%) Other ND 483 (63.5%) |
Candida species 197 (25.9%) Histoplasma capsulatum 113 (14.9%) Other ND 450 (59.2%) |
NR | Mortality 103 (13.6%) |
| Naganuma et al, 201329 | 6 | NR | NR | Oesophagus 6 (100%) | Candida albicans 6 (100%) | No antifungals 3 (50%) | Clinical cure 3 (50%)a |
| Seminerio et al, 201330 | 21 | Corticosteroids NR DMARDs NR Anti-TNFa 21 (100%) |
Candidiases diagnosed with culture. Other fungal infections NR | Oesophagus 4 (19%) Bloodstream 2 (9.5%) Wound ulcer 2 (9.5%) Lower respiratory tract 2 (9.5%) Other ND 10 (47.6%) |
Candida (unspecified) 8 (38.1%) Histoplasma capsulatum 3 (14.3%) Other ND 10 (47.6%) |
NR | NR |
| Park et al, 201431 | 4 | NR | Microrganism isolated from culture of abscess pus | Intra-abdominal abscess 4 (100%) | Candida species 4 (100%) | Drainage in 4 (100%) Antifungals NR |
NR |
| Li et al, 201532 | 5 | NR | Confirmation of abscess with imaging and positive culture of drained pus | Intra-abdominal abscesses 5 (100%) | Candida albicans 5 (100%) | Surgery or drainage in 5 (100%) Antifungals NR | NR |
| McAuliffe et al, 201533 | 656 | Corticosteroids NR DMARDs NR Anti-TNFa 60 (9.1%) |
NR | Respiratory tract 15 Other ND 641 |
Candida 641 (97.7%)c Histoplasma capsulatum 13 (2%) Pneumocystis jiroveci 2 (0.3%) |
NR | NR |
| Penninck et al, 201634 | 4 | NR | NR | Bloodstream 2 (50%) Digestive and surgical site infection 1 (25%) Digestive tract and anal 1 (25%) |
Candida albicans 4 (100%) | Liposomal Amphotericin B 2 (50%) Fluconazole 2 (50%) Drainage in 1 (25%) |
Clinical cure 4 (100%)a Mortality 0 (0%) |
| Vögelin et al, 201635 | 5 | Corticosteroids NR DMARDs 5 (100%) (AZA 4/5, MTX 2/5, tacrolimus 2/5, 6-MP 1/5) Anti-TNFa () 1 (20%) |
Oral, perianal or vaginal candidiasis was diagnosed by characteristic clinical appearance and response to antifungal therapy. In oesophageal candidiasis, endoscopic and histological confirmation | Oesophagus 2 (40%) Vaginal & perianal 1 (20%) Oral 1 (20%) Oesophagus & oral 1 (20%) |
Candida species 6d (100%) | NR | Clinical cure 5 (100%)a Mortality 0 (0%) |
6-MP, 6-mercaptopurine; AZA, azathioprine DMARDs, disease-modifying anti-rheumatic agents; MTX, methotrexate; ND, not defined; NR, not reported; TNF, tumour necrosis factor.
Defined as clinical resolution of the infection.
Data available only for 14/29 patients in this study.
Sites of Candida infection not specified.
Two Candida species were isolated in one infection.
TABLE 3.
Classification of infections per pathogen (n = 1063)
| Gastrointestinal tract | Respiratory tract | Bloodstream | Wound infection | Site not reported | |
|---|---|---|---|---|---|
| Candida spp. | 26 oesophagus, blood, perianala5 12 oesophagus27,28,33 9 intra-abdominal abscess29,30 4 oral251 both oesophagus & oral33 1 perianal-vaginal33 1 digestive-anal32 |
6 candidaemia or sepsisb4,28,32 | 2 wound ulceration (not otherwise specified)28 1 digestive and surgical wound infection32 |
840 infections4,26,31 | |
| Histoplasma capsulatum | 129 referred to as histoplasmosis26,28,31 11 lung5,22,24 |
||||
| Pneumocystis jirovecii | 19 lung4,23,31 | ||||
| Cryptococcus neoformans | 1 lung5 |
No further description provided.
2 cases were vascular line-associated.3
TABLE 4.
Application of the revised European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group definitions
| Study, year published | Total number of patients | Host factor | Clinical criteria | Mycological criteria | EORTC/MSG category | Comments |
|---|---|---|---|---|---|---|
| Lee et al, 200224 | 4 | R | R | R | Proven endemic mycosis | |
| Kaur et al, 200725 | 16 | R | NA | NA | NA | Pneumocystis pneumonia is not included in the criteria |
| Toruner et al, 20085 | 29 | R | R | R | Proven invasive fungal disease | |
| Dotson et al, 201126 | 5 | R | R | R | 4 probable/1 proven endemic mycoses | |
| Panaccione et al, 201127 | 4 | R | NR | NR | NA | Oral candidiasis is not mentioned in the criteria |
| Lichtenstein et al, 20124 | 5 | R | NR | NR | NA | |
| Deepak et al, 201328 | 760 | R | NR | NR | NA | |
| Naganuma et al, 201329 | 6 | R | NA | NA | NA | Oesophageal candidiasis is not mentioned in the criteria |
| Seminerio et al, 201330 | 21 | R | NR | NR | NA | Candidiasis diagnosed with culture. Other fungi NR |
| Park et al, 201431 | 4 | R | NA | NA | NA | Abscesses are not mentioned in the criteria (microorganism was isolated from abscess drainage) |
| Li et al, 201532 | 5 | R | NA | NA | NA | Abscesses are not mentioned in the criteria |
| McAuliffe et al, 201533 | 656 | R | NR | NR | NA | |
| Penninck et al, 201634 | 4 | R | NR | NR | NA | |
| Vögelin et al, 201635 | 5 | R | NA | NA | NA | Gastrointestinal candidiasis is not mentioned in the criteria |
NA, not applicable; NR, not reported; R, reported.
Risk factor analysis for opportunistic or fungal infections was performed in 8 studies,4,5,28,29,32–35 whereas 6 studies5,24,26,28,30,33 involving 1454 patients reported risk factors or predisposing conditions for fungal infections in IBD. The predominant condition associated with fungal infection in IBD was use of anti-TNFa agents, which was reported in all studies.5,24,26,28,30,33 In detail, predisposing conditions or risk factors included: use of anti-TNFa with or without additional immunomodulators or corticosteroids in 760 cases (including 197 cases of Candida infection and 113 cases of Histoplasmosis)28; use of anti-TNFa in 641 cases of Candida infection and 13 cases of histoplasmosis33; use of corticosteroids in 26 cases of Candida infection5; use of 6-mercaptopurine or azathioprine in addition to anti-TNFa agents in 5 cases of histoplasmosis26; use of prednisone, methotrexate and/or azathioprine in addition to anti-TNFa agents in 4 cases of histoplasmosis24; use of infliximab in 3 cases of histoplasmosis5; presence of central vascular line in 2 cases of candidaemia. 30 In addition, one study focused on patients with IBD and postoperative Candida infection34 and 2 studies included IBD patients with spontaneous intra-abdominal Candida abscess.31,32
Complications were present in 8 of 22 infections where data were provided.4,24,26,27,34 These included (more than one complications occurred in some patients): need for intensive care support (4 infections),24 sepsis (3 infections),4,34 disseminated intravascular coagulation (2 infections),24 surgical wound infection (2 infections)34 and abscess formation (1 infection).34
3.4 | Data on IBD
Sixty-eight patients suffered from Crohn’s disease and 5 patients suffered from ulcerative colitis; in 4 studies (1451 patients),5,28,29,33 the type of IBD was not defined. Duration of disease prior to infection, reported in 2 studies,26,34 was 6 months to 10 years (average, 33 months). In patients with available data, treatment for IBD included anti-TNFa agents in 837/1491 patients (infliximab in 52 patients, adalimumab in 5 patients, not defined in 780 patients),4,5,24–28,30,33,35 systematic corticosteroids (243/793 patients) 4,5,24,26,28,35 and disease-modifying anti-rheumatic drugs (DMARDs, 296/793 patients).4,5,24,26,28,35 Based on data from 6 studies,4,24–26,29,30 fungal infections occurred within 5 days–12 months of initiation of treatment for IBD; in 4 of these studies where infliximab was administered, fungal infections occurred within 5–180 days.4,24–26
3.5 | Treatment and outcomes
Data on treatment of fungal infections were available in 6 studies (24 patients).24,26,29,29,32,34 Antifungal treatment included amphotericin B (8 patients)24,26,34 and triazoles (8 patients),26,34 whereas in one study,29 no treatment was administered in 3 patients. Surgical intervention (either surgery or abscess drainage) was reported in 10 infections.31,32,34 Death related to the infection, according to the study authors, was reported in 104 out of 816 patients with available information on mortality.4,5,24,26–28,34,35 Of note, nearly all recorded deaths (103 patients) were recorded in one study.28
4 | DISCUSSION
The present systematic review provides important information on fungal infections in patients with IBD. Our findings show that Candida species are the predominant pathogens in fungal infections in patients with IBD, the most frequent sites of infection are the respiratory and gastrointestinal tract, and fungal infections occur early during treatment of IBD (especially with anti-TNFa agents). Despite study heterogeneity, our observations offer useful information for the management of patients with IBD, who are often characterised by comorbidity and considerable disability.1,36
Certain observations are worth noting on the microbiology of fungal infections. Although Candida was the predominant pathogen and candidaemia is considered the most frequent form of Candida infection,16 bloodstream infections were uncommon compared to other infection sites. Indeed, fungal infections most commonly affected the respiratory and gastrointestinal tract, whereas bloodstream infections represented fewer than 10% of infections in studies with available data. The fact that IBD lead to gastrointestinal mucosal injury, thus facilitating pathogen penetration, lends support to the higher frequency of gastrointestinal tract infections in these patients. It should be stated though, that in one study with a high number of Candida infections, the sites of Candida infections were not specified.33 Histoplasma capsulatum was the second most common fungus and was isolated exclusively in studies performed in the USA, where histoplasmosis is endemic. Therefore, local epidemiology remains an important consideration during management of infections in patients with IBD. Management of these infections in IBD patients is not different from other populations, whereas antifungal prophylaxis is currently recommended only for P. jirovecii in patients receiving certain combinations that include immunomodulators.3,37
A worthwhile remark is that no cases of invasive mould disease were included in this study. One reason is the fact that we excluded studies with few reported cases, consequently excluding numerous case reports. Another reasonable explanation is that the major risk factors for development of invasive aspergillosis are neutropenia and prolonged corticosteroid treatment, which are not the typical features of immunosupression used in patients with IBD.38 This is also shown in our study, where the majority of invasive fungal infections occurred within a short period of time after initiation of immunosupression. Another explanation could be associated with the pathophysiology of invasive fungal disease by Candida and moulds. Candida is thought to be able to invade the damaged epithelial barrier of the gut and cause invasive disease, which is quite reasonable in the case of IBD, especially with concurrent immunosuppression.16 On the other hand, invasive mould disease requires penetration of the alveolar epithelium, with no clear evidence suggesting a defective alveolar epithelium in patients with IBD.38
Currently available evidence does not allow for safe conclusions on infection severity and mortality. Although we observed considerable mortality rates (>10%), the majority of reported deaths were from one study,28 whereas exclusion of this study from analysis leaves a much lower reported death rate of 1 out of 56 patients with available outcome data. In addition, the burden of disease caused by fungal infections is difficult to define, as data on the presence or not of complications was available in a total of 22 patients from 5 studies. However, despite current lack of credible data on patient outcomes, it is known that invasive fungal infections carry significant morbidity and mortality and thus, early presumptive antifungal treatment can be of benefit in patients treated for IBD who develop a systematic infection.8,16
Fungal infections occurred within 12 months of treatment for IBD, indicating that immune-modifying treatment has an important association with these infections in IBD. In addition, the fact that most of these studies reported treatment with infliximab within 6 months of infection onset,4,24–26 raises the issue of early suspicion of fungal infection in patients with recent initiation of anti-TNFa agents. In line with our findings, a recent meta-analysis of randomised controlled trials showed a much higher relative risk for opportunistic infections in trials with short therapy duration (<8 weeks) compared to trials with longer therapy duration (>8 weeks).39 Furthermore, a previous review demonstrated that most fungal infections occurred a median of 55 days (IQR, 15–140 days) after initiation of treatment with infliximab.8 Concern over the risk of infection with the increasing use of biological therapies in IBD has led to several recent meta-analyses on this subject, which demonstrated an increased risk of infections and opportunistic infections in patients treated with biological agents.6,40 Corroborating with the above, we further found that prior or current use of anti-TNFa agents was a universal finding in all studies that performed risk factor analysis or explored predisposing factors for fungal infections in patients in IBD.5,24,26,28,30,33 There are certain limitations in our systematic review that should be acknowledged. First, significant heterogeneity between studies, especially as regards to design and outcomes, precludes concrete assumptions in several aspects, such as individual predisposing factors per site of fungal infection, infection severity, treatment and outcomes. Second, despite the notable number of included patients, a large proportion was derived from 2 studies, but only few data from these studies could be used in our review. Future studies should provide a more detailed description of risk factors, clinical characteristics, pathogen distribution and treatment of fungal infections in IBD. In addition, it is necessary to report duration of IBD treatment prior to infection, as well as details of treatment for IBD prior to infection (including type of treatment), to elucidate the association between specific agents and fungal infections.
In conclusion, the present systematic review outlines the clinical and microbiological profile of fungal infections in patients with IBD, with significant clinical implications. Published evidence shows that fungal pathogens, mainly Candida species, should be suspected in infections that occur early during treatment for IBD, especially when TNFa inhibitors are administered.
Footnotes
CONFLICT OF INTERESTS
All authors declare no conflict of interest.
ETHICAL APPROVAL
Not required.
References
- 1.Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 2.Viget N, Vernier-Massouille G, Salmon-Ceron D, Yazdanpanah Y, Colombel J-F. Opportunistic infections in patients with inflammatory bowel disease: prevention and diagnosis. Gut. 2007;57:549–558. doi: 10.1136/gut.2006.114660. [DOI] [PubMed] [Google Scholar]
- 3.Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–468. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT™ registry. Am J Gastroenterol. 2012;107:1409–1422. doi: 10.1038/ajg.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toruner M, Loftus EV, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–936. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Bonovas S, Fiorino G, Allocca M, et al. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1385–1397e10. doi: 10.1016/j.cgh.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 7.Sedger LM, McDermott MF. TNF and TNF-receptors: from mediators of cell death and inflammation to therapeutic giants – past, present and future. Cytokine Growth Factor Rev. 2014;25:453–472. doi: 10.1016/j.cytogfr.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Tsiodras S, Samonis G, Boumpas DT, Kontoyiannis DP. Fungal infections complicating tumor necrosis factor α blockade therapy. Mayo Clin Proc. 2008;83:181–194. [PubMed] [Google Scholar]
- 9.Van Der Meer JWM, Popa C, Netea MG. Side effects of anticytokine strategies. Neth J Med. 2005;63:78–80. [PubMed] [Google Scholar]
- 10.Tragiannidis A, Kyriakidis I, Zündorf I, Groll AH. Invasive fungal infections in pediatric patients treated with tumor necrosis alpha (TNF-α) inhibitors. Mycoses. 2017;60:222–229. doi: 10.1111/myc.12576. [DOI] [PubMed] [Google Scholar]
- 11.Netea MG, Van der Graaf CA, Vonk AG, Verschueren I, Van der Meer JW, Kullberg BJ. The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J Infect Dis. 2002;185:1483–1489. doi: 10.1086/340511. [DOI] [PubMed] [Google Scholar]
- 12.Bellocchio S, Montagnoli C, Bozza S, et al. The contribution of the toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol. 2004;172:3059–3069. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- 13.Konidari A, Matary WE. Use of thiopurines in inflammatory bowel disease: safety issues. World J Gastrointest Pharmacol Ther. 2014;5:63. doi: 10.4292/wjgpt.v5.i2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan ESL, Cronstein BN. Methotrexate—how does it really work? Nat Rev Rheumatol. 2010;6:175–178. doi: 10.1038/nrrheum.2010.5. [DOI] [PubMed] [Google Scholar]
- 15.Conaghan P, Lehmann T, Brooks P. Disease-modifying antirheumatic drugs. Curr Opin Rheumatol. 1997;9:183–190. doi: 10.1097/00002281-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. 2015;373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 17.Lionakis M, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362:1828–1838. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- 18.Chen SC, Sorrell TC, Chang CC, Paige EK, Bryant PA, Slavin MA. Consensus guidelines for the treatment of yeast infections in the haematology, oncology and intensive care setting, 2014: yeast treatment guidelines 2014. Intern Med J. 2014;44:1315–1332. doi: 10.1111/imj.12597. [DOI] [PubMed] [Google Scholar]
- 19.Fleming S, Yannakou CK, Haeusler GM, et al. Consensus guidelines for antifungal prophylaxis in haematological malignancy and haemopoietic stem cell transplantation, 2014: antifungal prophylaxis 2014. Intern Med J. 2014;44:1283–1297. doi: 10.1111/imj.12595. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 21.Wallace BC, Small K, Brodley CE, Lau J, Trikalinos TA. Deploying an interactive machine learning system in an evidence-based practice center: abstrackr. Proceedings of the 2nd ACM SIGHIT International Health Informatics Symposium IHI; New York, NY: ACM; 2012. pp. 819–824. [Google Scholar]
- 22.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J-H, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor α antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565–2570. doi: 10.1002/art.10583. [DOI] [PubMed] [Google Scholar]
- 25.Kaur N, Mahl TC. Pneumocystis jiroveci (carinii) pneumonia after infliximab therapy: a review of 84 cases. Dig Dis Sci. 2007;52:1481–1484. doi: 10.1007/s10620-006-9250-x. [DOI] [PubMed] [Google Scholar]
- 26.Dotson JL, Crandall W, Mousa H, et al. Presentation and outcome of histoplasmosis in pediatric inflammatory bowel disease patients treated with antitumor necrosis factor alpha therapy: a case series. Inflamm Bowel Dis. 2011;17:56–61. doi: 10.1002/ibd.21378. [DOI] [PubMed] [Google Scholar]
- 27.Panaccione R, Loftus EV, Binion D, et al. Efficacy and safety of adalimumab in Canadian patients with moderate to severe Crohn’s disease: results of the Adalimumab in Canadian SubjeCts with ModErate to Severe Crohn’s DiseaSe (ACCESS) trial. Can J Gastroenterol Hepatol. 2011;25:419–425. doi: 10.1155/2011/724813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deepak P, Stobaugh DJ, Ehrenpreis ED. Infectious complications of TNF-α inhibitor monotherapy versus combination therapy with immunomodulators in inflammatory bowel disease: analysis of the Food and Drug Administration Adverse Event Reporting System. J Gastrointestin Liver Dis. 2013;22:269–276. [PubMed] [Google Scholar]
- 29.Naganuma M, Kunisaki R, Yoshimura N, Takeuchi Y, Watanabe M. A prospective analysis of the incidence of and risk factors for opportunistic infections in patients with inflammatory bowel disease. J Gastroenterol. 2013;48:595–600. doi: 10.1007/s00535-012-0686-9. [DOI] [PubMed] [Google Scholar]
- 30.Seminerio JL, Loftus EV, Colombel J-F, Thapa P, Sandborn WJ. Infliximab for Crohn’s Disease: the first 500 patients followed up through 2009. Dig Dis Sci. 2013;58:797–806. doi: 10.1007/s10620-012-2405-z. [DOI] [PubMed] [Google Scholar]
- 31.Park S-K, Kim K-J, Lee S-O, et al. Ciprofloxacin usage and bacterial resistance patterns in Crohn’s disease patients with abscesses. J Clin Gastroenterol. 2014;48:703–707. doi: 10.1097/MCG.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 32.Li G, Ren J, Wu Q, et al. Bacteriology of spontaneous intra-abdominal abscess in patients with Crohn disease in china: risk of extended-spectrum beta-lactamase-producing bacteria. Surg Infect. 2015;16:461–465. doi: 10.1089/sur.2013.181. [DOI] [PubMed] [Google Scholar]
- 33.McAuliffe ME, Lanes S, Leach T, et al. Occurrence of adverse events among patients with inflammatory bowel disease in the HealthCore Integrated Research Database. Curr Med Res Opin. 2015;31:1655–1664. doi: 10.1185/03007995.2015.1065242. [DOI] [PubMed] [Google Scholar]
- 34.Penninck E, Fumery M, Armengol-Debeir L, et al. Postoperative complications in pediatric inflammatory bowel disease: a population-based study. Inflamm Bowel Dis. 2016;22:127–133. doi: 10.1097/MIB.0000000000000576. [DOI] [PubMed] [Google Scholar]
- 35.Vögelin M, Biedermann L, Frei P, et al. The impact of azathioprine-associated lymphopenia on the onset of opportunistic infections in patients with inflammatory bowel disease. PLoS ONE. 2016;11:e0155218. doi: 10.1371/journal.pone.0155218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumgart D, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 37.Ali T, Kaitha B, Mahmood S, Ftaisi A, Stone J, Bronze MS. Clinical use of anti-TNF therapy and increased risk of infections. Drug Healthc Patient Saf. 2013;5:79–99. doi: 10.2147/DHPS.S28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dagenais TRT, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive Aspergillosis. Clin Microbiol Rev. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ford AC, Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-α therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2013;108:1268–1276. doi: 10.1038/ajg.2013.138. [DOI] [PubMed] [Google Scholar]
- 40.Shah ED, Farida JP, Siegel CA, Chong K, Melmed GY. Risk for overall infection with anti-TNF and anti-integrin agents used in IBD: a systematic review and meta-analysis. Inflamm Bowel Dis. 2017;23:570–577. doi: 10.1097/MIB.0000000000001049. [DOI] [PubMed] [Google Scholar]