Abstract
Background:
Acute lymphoblastic leukemia (ALL) is one of the most common malignancies among children, characterized by mass production of leukemic blasts. Chemotherapy is the first step in routine treatment, although it may evoke considerable side effects. Matrine, an alkaloid extracted from a Chinese herb, Sophora alopecuroides flavescens Ait, may be protective. Several investigations have indicated pro-apoptotic and anti-proliferative effects in a diverse range of cancer cells.
Methods:
Matrine’s anti-cancer effects and associated mechanisms were assessed in human ALL B-lymphocytes, focusing on parameters of inflammatory change and apoptosis.
Results:
Treatment of ALL B-lymphocytes with matrine augmented ROS generation, and caused mitochondrial swelling and a decline in mitochondrial membrane potential. Significant up-regulation of the pro-apoptotic protein Bax and down-regulation of the anti-apoptotic Bcl-2 were also noted.
Conclusion:
Our results suggest that matrine may be a potential anticancer agent. However, additional studies are needed to clarify involved mechanisms.
Keywords: Matrine, ALL B-lymphocytes, mitochondria, Bcl-2/Bax, apoptosis
Introduction
Acute lymphoblastic leukemia (ALL) is a life-threatening malignancy in which lymphoid precursor cells are mainly involved. This disorder affects pediatric population with a high incidence between the ages of 2 to 5 years old (Schwab and Harrison, 2011; Roganovic, 2013).
The predominant intention of leukemia treatment is the completely eradicate injured cells. Chemotherapy is the first choice for treatment but almost all routine drugs have severe toxic side effects. Due to these unintentional adverse effects, many new compounds are introduced to substitute these drugs (Pui et al., 2004; Schwab and Harrison, 2011).
Sophora is a genus of approximately 45 species of small trees in the pea family Fabaceae. These species are native to southern Asia, Australasia, various Pacific islands and western South America (Jaktaji and Mohammadi, 2017). Different parts of Sophora plants, like flowers, stems, roots, and seeds have been used traditionally in Chinese medicines (Choudhary et al., 2000). Sophorea species contain a large amount of bioactive materials; the most phytochemically characterized compounds of this genus are flavonoids and alkaloids (e.g. quinolizidine alkaloids), particularly matrine and oxymatrine. The anti-tumor, anti-inflammatory, anti-viral and anti-pyretic activities of these compounds are considerably under investigation (Shi et al., 2014; Zheng et al., 2014). Recent researches demonstrated that matrine inhibit the growth of different kind of tumor cells by inducing apoptosis, so is capable of using in leukemia treatment(KÇÜKBOYACI, 2010).
Matrine, derived from Sophora flavescenes Ait, is a type of medicinal herbs that are used in traditional Chinese medicine. Its chemical formula is C15H24N2O, and its structural formula is shown in Figure 1. Matrine (C15H24N2O) is a unique tetracyclo-quinolizindine alkaloid extracted from Sophora flavescens. Matrine is a strong base. The molecule includes two nitrogen atoms; N1 belongs to the tertiary amine with trivalent bound on the heterocyclic ring and N16 in amide form which has not any basic characteristics. Because of its unique spatial conformation matrine can easily accept the proton; therefore, it has a high basicity.
Figure 1.
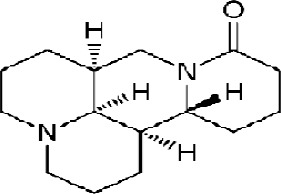
Chemical Structure of Matrine
Apoptosis is one of the important paths of cell destruction; hence the outcome of apoptosis malfunction is unbridled cell proliferation and resistance to cancer treatment. Mitochondria are the prominent organelles of the programmed cell death signaling (Wong, 2011). This process occurs via the certain changes in mitochondria such as mitochondrial membrane permeabilization (MMP) decline, migration of pro- and anti-apoptotic members such as Bax, Bak, and Bcl-2 to the outer surface of mitochondria (Kerr et al., 2013). Moreover, since mitochondria are the major source of intracellular ROS the redox state became an important issue in apoptosis management (Wlodkowic, 2011).
The aim of our investigation was to distinguish the role of matrine, on mitochondria-mediated apoptosis on B-ALL lymphocytes. Upstream parameters such as ROS generation, collapse of mitochondrial swelling, mitochondrial membrane potential (MMP), and also the expression of Bax and Bcl-2 was tested (Del et al., 2015).
Materials and methods
Chemicals
Trypan blue, 2′,7′-dichlorofuorescin diacetate (DCFH-DA), Rhodamine123, bovine serum albumin (BSA), N-(2-hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid) (HEPES) and matrine, RPMI1640 and FBS (Fetal Bovine serum) were purchased from Gibco, Life Technologies, Grand Island, NY. Ficoll-paque PLUS was obtained from Ge Healthcare Bio-Science Company.
Plant Extract
Sophora alopecuroides
flavescens seeds were collected from Shahrekord, Chahar Mahal va Bakhtiary Province, Southwest of Iran. The seeds were air dried at room temperature and ground into powder. Total alkaloid extraction were performed as previously described by Pourahmad Jaktaji and Mohammadi (2018).
Selection of healthy donors and patients with ALL
Ten ALL patients aged 2-9 years were enrolled in this study. ALL was diagnosed and confirmed according to the definition of the World Health Organization (WHO) classification by oncologist. Only patients with no previous treatments within the last six months were included in this study. All 10 patients have sampled prior any medication. This study was approved by the Shahid Beheshti University of Medical Science’s ethics committee, and all the patients signed an informed consent form.
Isolation of Mitochondria
Mitochondria were isolated from the ALL B-lymphocytes by mechanical lysis and multiple centrifugations. Briefly, B-lymphocytes were washed with cold PBS and centrifuged at 450×g. The pellet was re-suspended in cold isolation buffer. Non- lysed cells and nuclei were spun down by centrifugation at 1,000 × g for 10 minutes. The supernatant was further centrifuged at 20000 × g for 25 minutes. The pellet, designated as the mitochondrial fraction, was suspended in assay buffer. The accuracy of mitochondrial isolation was determined by succinate dehydrogenase measurement (Rotem, 2005; Rezaei et al., 2014).
Succinate Dehydrogenases Activity Assay Using MTT Test
The activity of succinate dehydrogenases was examined by estimation of MTT reduction (Berridge MV, 1993). The colorimetric assay was performed by ELISA reader (Tecan, Rainbow hermo, Austria).
In this case, we used an internationally accepted protocol using at least three different concentrations as IC50/2 (sub-toxic concentration), IC50 (toxicity threshold concentration) and 2×IC50 (very toxic concentration). In our research the IC50 determined for matrine on isolated B-ALL lymphocyte mitochondria was approximately 3.4 mg/ml and therefore we used 1.68 mg/ml as sub-toxic and 6.75 mg/ml as very toxic concentrations. To determine the IC50 of matrine which defined as a concentration which decreased the activity of mitochondrial succinate dehydrogenase down to 50% and considered as toxicity threshold in accelerated cytotoxicity mechanisms screening (ACMS) technique, we applied 6 different concentrations obtained from the literature review (shown in Figure 2) on isolated B-ALL lymphocyte mitochondria using MTT assay. In order to determine the IC50 value for our investigated compound, dose-response curves were plotted and the IC50 determination was based on a regression plot of 6 different concentrations (data and curves not shown).
Figure 2.
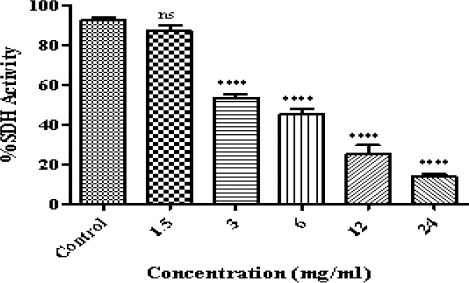
The Effect of Matrine on Succinate Dehydrogenase Activity ALL B-lymphocytes.The effect of matrine on succinate dehydrogenase activity in ALL mitochondria obtained from human B-lymphocytes were evaluated by MTT assay following 1 h of treatment. Values (mean ± S.D.) are from three independent experiments (n = 3). **** p < 0.0001.
Determination of Mitochondrial Swelling
Mitochondria suspensions were incubated in 96-well plates at 25 °C in swelling buffer supplemented with 1 mg/mL rotenone and 10 mmol/L succinate.
Various concentration of matrine was added after 10 minutes of pre-incubation. Mitochondrial swelling was measured spectrophotometrically within 60 minutes. Mitochondrial swelling results in a decrease in absorbance monitored at 540 nm (Rotem, 2005).
Determination of ROS formation
The mitochondrial ROS formation was performed using the fluorescent dye DCFH-DA. Briefly, isolated mitochondria were placed in respiration buffer. Following this step, DCFH-DA was added (final concentration, 10 μM) and then added different concentrations of matrine at 37 °C for an hour. Then, the fluorescence intensity was measured by fluorescence spectrophotometer at an excitation and emission wavelength 488 nm and 527 nm respectively (Kalyanaraman, 2012; Pourahmad et al., 2010).
Determination of MMP
Mitochondrial membrane potential assayed by a fluorescent dye, rhodamine 123. The mitochondrial fractions were incubated with 10 μM of rhodamine 123 in MMP assay buffer and then added various concentrations of matrine at 37 °C. The fluorescence intensity was monitored using fluorescence spectrophotometer at the excitation and emission wavelength of 490 nm and 535 nm, respectively (Hosseini, 2013; Talari et al., 2014).
Determination of Bax/Bcl-2 level: Quantitative real-time PCR
Bax and Bcl-2 mRNA levels were assessed by quantitative real-time PCR, using ACTB as a housekeeping gene for data normalization. A plot of Ct vs. log was used for quantification, using a standard curve obtained from serial dilutions of a reference cell cDNA. The thermal cycling conditions included a hot start step at 95 °C for 15 min followed by 40 cycles at 95 ºC for 15 s, 55 ºC for 30 s, and 72 ºC for 30 s (Cao, 2002).
Determination of caspase-3 activity
Caspase-3 activity was determined with the colorimetric assay in cell lysate of lymphocytes using Sigma’s caspase-3 assay kit (CASP-3-C) (Sakahira, 1998).
Determination of Cytochrome c Release
Following the pre-incubation of isolated mitochondria, tubes were centrifuged. The supernatant contained the cytochrome c released from the mitochondria (cytosolic fraction). The concentration of cytochrome c was determined using the Quantikine Human Cytochrome c Immunoassay kit.
Statistical analysis
Results are presented as mean ± SD. Assays were performed in triplicate and the mean was used for statistical analysis. Statistical significance was determined using the one-way ANOVA test, followed by the post-hoc Tukey test when appropriate. Statistical significance was set at P <0.05 and the parameters of mitochondrial dysfunction were analyzed by two way ANOVA and Bonferroni post-hoc test. In all graphs were expressed as mean ± SD and P< 0.001 was considered statistically significant.
Results
Succinate Dehydrogenase Activity
In this investigation, MTT assay was used to evaluate the impact of matrine on mitochondrial succinate dehydrogenase. Matrine can effectively inhibit succinate dehydrogenase activity in isolated ALL mitochondria (Figure 2).
ROS formation assay
Due to the fact that ROS have an important role in apoptosis, we tested if matrine could change the ROS level in ALL mitochondria. As shown in Figure 3, treatment with matrine at 1.68, 3.4, and 6.75 mg/ml for 60 minutes, significantly increased ROS generation (p < 0.0001).
Figure 3.
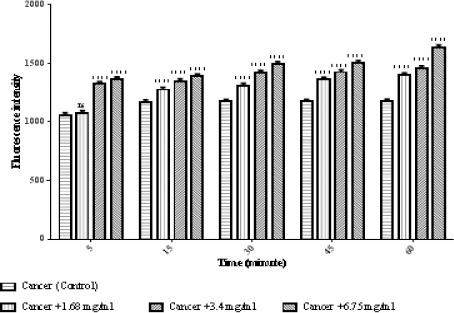
The Effect of Matrine on ROS Formation in ALL Mitochondria. Isolated mitochondria were obtained from ALL donors incubated with of matrine for 1 hour. Columns represent mean of DCF fluorescence intensity in ALL B-lymphocytes treated with matrine for 0–60 minutes. Values (mean ± S.D.) are from three independent experiments (n = 3). **** p < 0.0001.
MMP assay
We evaluated the impact of matrine on mitochondrial membrane potential. Treatment with different concentrations of matrine (1.68, 3.4, and 6.75 mg/ml for 60 minutes) significantly (p < 0.0001) reduced MMP mitochondria (Figure 4).
Figure 4.
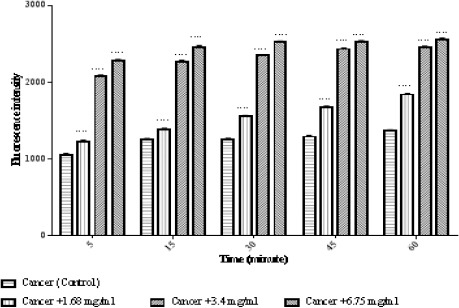
Effects of Matrine on MMP Decline in ALL Mitochondria. Columns represent mean of Rhodamine123 fluorescence intensity in ALL mitochondria treated with matrine for 5-60 minutes. Values (mean ± S.D.) are from three independent experiments (n = 3). **** p < 0.0001.
Mitochondrial swelling assay
Mitochondrial swelling followed up by detecting the absorbance of mitochondria through 60 minutes at 540 nm. As demonstrated in Figure 5, a great swelling in ALL mitochondria was observed following addition of different concentrations (1.68, 3.4, and 6.75 mg/ml) of matrine (Figure 5).
Figure 5.
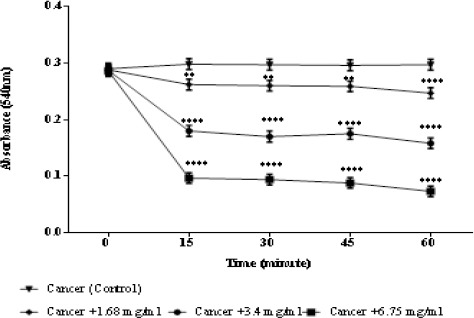
Effects of Matrine on Mitochondrial Swelling in ALL Mitochondria. Addition of matrine (1.68, 3.4, and 6.75 mg/ml) induces mitochondrial swelling in ALL B-lymphocyte mitochondria in a concentration depending manner. Values (mean ± S.D.) are from three independent experiments (n = 3).** p <0.01, **** p < 0.0001.
Determination of Bax/Bcl-2 level
As demonstrated in Figure 6 (A and B), the relative levels of Bax in ALL B-lymphocytes which treated by matrine were higher than untreated cancerous mitochondria. Moreover, the decrease in Bcl-2 was observed following treatment with the IC50 concentration of matrine.
Figure 6.
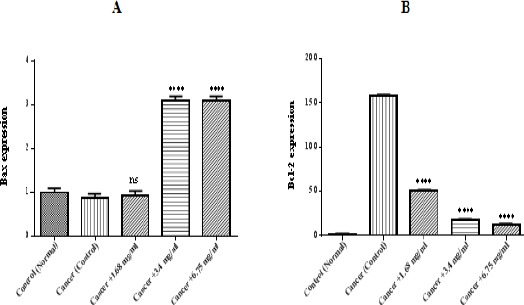
Determination of Bax/Bcl-2 Level. Columns represent relative mRNA expression of Bax (A) and BCL-2 (B) assessed by real-time quantitative RT-PCR. Values (mean ± S.D.) are from three independent experiments (n = 3), ** p <0.01.
Determination of Cytochrome c Release
As shown in Figure 7 matrine at IC50 (3.4 mg/ml) significantly (p < 0.01) caused a release of cytochrome c into incubation buffer. Our results revealed that matrine can cause mitochondrial permeability transition and cause cytochrome c expulsion from mitochondria. Moreover, cyclosporine-A, the MPT pore sealing agent, can significantly inhibit the release of cytochrome c.
Figure 7.
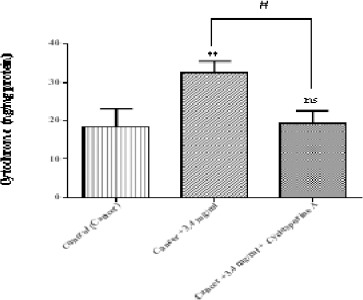
The Effect of Matrine on Cytochrome c Release in B-ALL Lymphocytes. Matrine (3.4 mg/ml) cause cytochrome c release in the ALL mitochondria. The amount of expelled cytochrome c from mitochondrial fraction into the suspension buffer was determined using human Cytochrome c ELISA kit as described in above. Values (mean ± S.D.) are from three independent experiments (n = 3). ** p < 0.01(control vs. matrine-treated cell), # p < 0.05 (matrine-treated cell vs. cyclosporine-A pretreated cells)
Determination of Caspase-3 Activity
As shown in Figure 8, matrine at IC50 (3.4 mg/ml) significantly (p < 0.01) enhanced the activity of caspae-3, the critical apoptosis mediator in ALL B-lymphocytes; however pretreatment by cyclosporine-A, the MPT pore sealing agent, can prevent caspase-3 activation.
Figure 8.
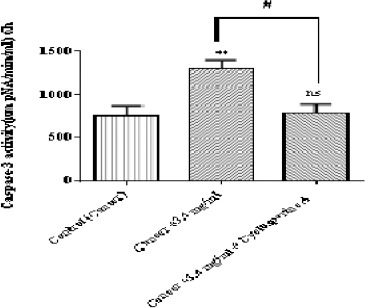
The Effect of Matrine on Caspase-3 Activity in B-ALL Lymphocytes. Caspase-3 activity was determined by Sigma-Aldrich kit. Columns represent caspse-3 activity (µM pNA/min/ml) in B-ALL lymphocytes treated with matrine for 6 h. Values (mean ± S.D.) are from three independent experiments (n = 3). ** p <0.01 (control vs. matrine-treated cell), # p < 0.05 (matrine-treated cell vs. cyclosporine-A pretreated cells)
Discussion
Natural compounds have been recently intended to use as an alternative medicine for many types of cancers including Leukemias. Since the majority of these compounds have considerable antitumor activities and besides most of them demonstrated low toxicities (Zhang, 2001; Stankovic, 2007). Matrine, an extraction from Sophora flavescens Ait, is one of these natural compounds. Recent studies have been demonstrated antitumor properties of matrine on breast carcinoma cell (Bcap-37), colon adenocarcinoma cell (HT-29), Glioma cell (C6), Hela cell, hepatocellular liver carcinoma cell (HepG2), erythromyeloblastoid leukemia cell (K-562), gastric adenocarcinoma cell (MKN45), leukemic monocyte lymphoma cell (U937), melanoma cell (A375) by induction of apoptosis which is regulated by different molecules (Gross et al., 1998; Liu, 2006; Qin, 2010; Sobolewski, 2010; Yixiang, 2010; Edlich et al., 2011). It has been identified that apoptosis process occurred through two leading routes, intrinsic and/or extrinsic pathways in which mitochondria play a crucial role (YI, 2000).
In this study, we inspected that matrine can trigger apoptosis. Our results showed that matrine is able to enhance ROS formation in mitochondria isolated from ALL B- lymphocytes which are followed by a decline of mitochondrial membrane potential. Our findings also revealed that matrine can induce mitochondrial swelling in time and concentration-dependent manner.
One of the most important pathogenic factors of any type of cancer is the imbalances in apoptotic/anti-apoptotic molecules. Bax is a define mitochondrial-dependent programmed cell death regulator. Bax generally located in the cytosol and during the onset of apoptosis translocates to the outer membrane of mitochondria. Following to the translocation of Bax, mitochondria lose their membrane potential (Δ ψm) and release the apoptogenic intramembrane protein, such as cytochrome c, into the cytosol (Edlich et al., 2011).
Our result showed that incubation of mitochondrial suspension with matrine at IC50 (3.4 mg/ml) significantly increased ROS formation. These reactive species may open MPT (mitochondrial permeability transition) pores by oxidation of thiol groups which consequently induced ΔΨm collapse, mitochondrial swelling and finally release of mitochondrial apoptogenic factors. It was also shown that the Bcl-2 and Bax genes expression in ALL mitochondria vastly changed which is probably due to ROS formation increase. Based on this fact, we determined expression levels of the pro- and anti-apoptotic Bax/Bcl-2 in ALL B-lymphocytes, the release of cytochrome-c and activation of caspase-3. Our results demonstrated that in contrast to Bcl-2, Bax was up-regulated after treatment of ALL mitochondria with matrine. We, therefore, concluded that matrine has a considerable role in initiating apoptosis on mitochondria isolated from ALL B-lymphocytes.
Results from our study demonstrated that matrine at IC50 (3.4 mg/ml) caused a release of the cytochrome c from mitochondria to cytosol which leads to activation of caspase-3 in human B-ALL lymphocytes.
In conclusion, the anticancer activity of matrine could associate with its apoptosis induction of ALL cells through mitochondrial pathway. This apoptotic process is presumably through the mitochondrial cascade, in which cytochrome c is released from mitochondria to cytosol and the released cytoplasmic cytochrome c, and then activates caspase-3. Moreover, cyclosporine-A, the MPT pore sealing agent, can significantly inhibit these phenomena.
Based on our findings, matrine may be a practical candidate as a chemotherapeutic agent against pediatric ALL.
References
- Berridge MV, Tan AS. Characterization of the cellular reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT):subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys. 1993;303:474–82. doi: 10.1006/abbi.1993.1311. [DOI] [PubMed] [Google Scholar]
- Cao Y, Prescott SM. Many actions of cyclooxygenase 2 in cellular dynamics and in cancer. J Cell Physiol. 2002;190:279–86. doi: 10.1002/jcp.10068. [DOI] [PubMed] [Google Scholar]
- Choudhary MI, Parvez K, Ahmed A, et al. Quinolizidine alkaloids from Sophora alopecuroides. J Nat product. 2000;63:190–2. [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, et al. Bcl-x L retrotranslocates Bax from the mitochondria into the cytosol. Cell. 2011;145:104–16. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A, Jockel J, Wei MC, et al. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–85. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini M-J, Shaki F, Ghazi-Khansari M, et al. Toxicity of vanadium on isolated rat liver mitochondria:a new mechanistic approach. Metallomics. 2013;5:152–66. doi: 10.1039/c2mt20198d. [DOI] [PubMed] [Google Scholar]
- Hsu Y-T, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-XL during apoptosis. Proc Natl Acad Sci U S A. 2018;94:3668–72. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman B, Darley-Usmar V, Davies KJ, et al. Measuring reactive oxygen and nitrogen species with fluorescent probes:challenges and limitations. Free Radic Biol Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–26. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kucukboyaci N, Adiguzel N, Ozkan S, et al. Alkaloid profiles and biological activities of different Sophora jaubertii extracts. Turk J Pharm Sci. 2010;7:1–8. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu X-S, Jiang J, Jiao X-Y, et al. Matrine-induced apoptosis in leukemia U937 cells:involvement of caspases activation and MAPK-independent pathways. Planta Med. 2006;72:501–6. doi: 10.1055/s-2006-931534. [DOI] [PubMed] [Google Scholar]
- Molday L, Cook NJ, Kaupp U, et al. The cGMP-gated cation channel of bovine rod photoreceptor cells is associated with a 240-kDa protein exhibiting immunochemical cross-reactivity with spectrin. J Biol Chem. 1990;265:18690–5. [PubMed] [Google Scholar]
- Moore VDG, Schlis KD, Sallan SE, et al. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood. 2008;111:2300–9. doi: 10.1182/blood-2007-06-098012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourahmad Jaktaji R, Mohammadi P. The effect of total alkaloid extract of local Sophora alopecuroides on MIC and intracellular accumulation of ciprofloxacin, and acrA expression in ciprofloxacin high resistance Escherichia coli clones. J Glob Antimicrob Resist. 2017;12:55–60. doi: 10.1016/j.jgar.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Pourahmad J, Eskandari MR, Shakibaei R, Kamalinejad M. A search for hepatoprotective activity of aqueous extract of Rhus coriaria L. against oxidative stress cytotoxicity. Food Chem Toxicol. 2010;48:854–8. doi: 10.1016/j.fct.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Pui C-H, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004a;350:1535–48. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- Pui C-h, Relling MV, Downing JR, et al. Magnetic fields and leukemia. N Engl J Med. 2004b;351:102. doi: 10.1056/NEJM200407013510120. [DOI] [PubMed] [Google Scholar]
- Qin X-G, Hua Z, Shuang W, et al. Effects of matrine on HepG2 cell proliferation and expression of tumor relevant proteins in vitro. Pharm Biol. 2010;48:275–81. doi: 10.3109/13880200903104101. [DOI] [PubMed] [Google Scholar]
- Rotem R, Heyfets A, Fingrut O, et al. Jasmonates:Novel anticancer agents acting directly and selectively on human cancer cell mitochondria. Cancer Res. 2005;65:1984–93. doi: 10.1158/0008-5472.CAN-04-3091. [DOI] [PubMed] [Google Scholar]
- Sakahira H EMaNS. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–9. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- Schwab C, Harrison CJ. Acute lymphoblastic leukaemia. Methods Mol Biol (Clifton, N.J.) 2011;730:99–117. doi: 10.1007/978-1-61779-074-4_8. [DOI] [PubMed] [Google Scholar]
- Shi Z, Chen J, Li C-y, et al. Antitumor effects of concanavalin A and Sophora flavescens lectin in vitro and in vivo. Acta Pharmacol Sin. 2014;35:248–56. doi: 10.1038/aps.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolewski C, Cerella C, Dicato M, et al. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. J Cell Biol. 2010;2010 doi: 10.1155/2010/215158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanković T, Marston E. Molecular mechanisms involved in chemoresistance in paediatric acute lymphoblastic leukaemia. Srp Arh Celok Lek. 2008;136:187–92. doi: 10.2298/sarh0804187s. [DOI] [PubMed] [Google Scholar]
- Talari M, Seydi E, Salimi A, et al. Dracocephalum:novel anticancer plant acting on liver cancer cell mitochondria. Biomed Res Int Article ID 892170. 2014 doi: 10.1155/2014/892170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodkowic D, Telford W, Skommer J, et al. Apoptosis and beyond:cytometry in studies of programmed cell death. Methods Cell Biol. 2011;103:55. doi: 10.1016/B978-0-12-385493-3.00004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RS. Apoptosis in cancer:from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao-Ming Y. Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell Res. 2000;10:161–7. doi: 10.1038/sj.cr.7290045. [DOI] [PubMed] [Google Scholar]
- Yixiang H, Shenghui Z, Jianbo W, et al. Matrine induces apoptosis of human multiple myeloma cells via activation of the mitochondrial pathway. Leuk Lymphoma. 2010;51:1337–46. doi: 10.3109/10428194.2010.488708. [DOI] [PubMed] [Google Scholar]
- Zhang L, Jiang J, Tam J, et al. Effects of matrine on proliferation and differentiation in K-562 cells. Leuk Res. 2001;25:793–800. doi: 10.1016/s0145-2126(00)00145-4. [DOI] [PubMed] [Google Scholar]
- Zheng K, Li C, Shan X, et al. Study on isolation of chemical constituents from Sophora Flavescens ait. And their anti-glioma effects. Afr J Tradit Complement Altern Med. 2014;11:156–60. doi: 10.4314/ajtcam.v11i1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]


