Abstract
Two of the primary cues used to localize the sources of sounds are interaural level differences (ILDs) and interaural time differences (ITDs). We conducted two experiments to explore how practice affects the human discrimination of values of ILDs and ongoing ITDs presented over headphones. We measured discrimination thresholds of 13 to 32 naive listeners in a variety of conditions during a pretest and again, 2 weeks later, during a posttest. Between those two tests, we trained a subset of listeners 1 h per day for 9 days on a single ILD or ITD condition. Listeners improved on both ILD and ITD discrimination. Improvement was initially rapid for both cue types and appeared to generalize broadly across conditions, indicating conceptual or procedural learning. A subsequent slower-improvement stage, which occurred solely for the ILD cue, only affected conditions with the trained stimulus frequency, suggesting that stimulus processing had fundamentally changed. These different learning patterns indicate that practice affects the attention to, or low-level encoding of, ILDs and ITDs at sites at which the two cue types are processed separately. Thus, these data reveal differences in the effect of practice on ILD and ITD discrimination, and provide insight into the encoding of these two cues to sound-source location in humans.
A listener who determines the position of a singing bird concealed among tree leaves, a jet passing overhead hidden by clouds, or a car approaching from behind, does so by using several auditory cues to the location of sound sources. Here we report the results of two investigations into how practice influences the ability of human listeners to discriminate small differences in each of two of these cues, interaural level differences (ILDs) and interaural time differences (ITDs).
In humans, the horizontal location, or azimuth, of sound sources is computed from differences in the information that arrives at the two ears. At frequencies above about 1.5 kHz, listeners determine sound azimuth primarily from sensitivity to differences in sound level at the two ears. These interaural level differences occur because the head forms a sound barrier between the two ears, so sounds are attenuated at the ear farthest from the source relative to the ear nearest to the source (1, 2). At frequencies below about 1.5 kHz, listeners determine sound azimuth primarily from sensitivity to differences in the arrival time of the sound at the two ears. These interaural time differences arise because there is distance between the two ears, so sounds reach the ear nearest to the sound source first and the other ear later (1, 2). For sound durations greater than about 150 ms, listeners are far more sensitive to differences at the two ears in the ongoing fine time structure of the sound than in the onset time of the sound (3, 4). These ongoing time differences are equivalent to interaural phase differences (IPDs) for tonal stimuli, but will be referred to as ITDs in this paper.
There have been many investigations into whether human listeners can learn to improve their ability to locate or discriminate the spatial position of sound sources. The goal of most of these experiments was to determine whether listeners could learn to adapt to altered sound-localization cues (5–14). These studies addressed the issue of whether listeners can learn to associate modified cue values with correct locations. Only a few investigators have examined the simpler question of whether listeners can improve their ability to locate or discriminate sound-source positions represented by normal, unaltered cues. These experiments reported how practice affected the ability of listeners to use binaural (15, 16) and monaural (17, 18) localization cues in a localization task, or to discriminate binaural ILD or ITD cues in precedence-effect (19, 20) or masking-level-difference (21, 22) tasks. However, to date, there has been no systematic investigation of, or comparison between, the patterns of learning and generalization of the discrimination of ILD and ITD cues in basic ILD- and ITD-discrimination tasks. We chose to examine these issues because of their potential to provide insight into the human encoding of ILDs and ITDs and the relative plasticity of these cues.
Our primary goals were to determine whether multihour training helps listeners learn to improve their ability to discriminate changes in ILDs or ITDs, and if so, to determine whether that learning generalizes to untrained conditions. Toward this end, we gave naive listeners 9 h of practice on ILD or ITD discrimination in one single stimulus condition, and then tested them on a variety of untrained conditions. Each condition had three characteristics: the type of interaural cue that was manipulated (ILD or ITD), the standard value of that cue, and the frequency of the tonal stimulus. We varied each of those characteristics independently to determine whether learning generalized from the trained to untrained versions of that specific characteristic. To independently control the ILD and ITD cues, we presented all of the stimuli over headphones. Under such conditions, the location of the sound image appears to be within the listener's head at a lateral position between the ears that is determined by the values of the ILD and ITD cues.
Materials and Methods
Task.
On each trial, we presented two digitally generated tones of the same frequency, one to each ear, in each of two visually marked observation periods. In one observation period, we presented the tones with a fixed ILD or ITD, referred to as the standard. In most conditions, the standard ILD was 0 dB and the standard ITD was 0 μs, so the sound image was located on or near the median plane. In the other observation period, we presented the tones with a comparison ILD or ITD equal to the standard ILD or ITD plus a variable ΔILD or ΔITD. The ΔILD or ΔITD always favored the right ear. The comparison ILD or ITD occurred randomly in either the first or the second observation period. The listener indicated which of the two observation periods contained the comparison by pressing a key on a computer keyboard. We indicated visually whether the response was correct or incorrect after every trial throughout the experiment. The comparison ILD or ITD varied adaptively across trials to determine the ΔILD or ΔITD needed to discriminate the comparison from the standard (see Procedure).
Organization of Experiments.
We collected data in two experiments referred to as the ILD-training and ITD-training experiments. Both experiments consisted of a pretest, a training phase, and a posttest. In the pre- and posttests of each experiment, we measured thresholds for ILD and ITD discrimination in every listener in five conditions (listed in Fig. 2; three conditions were common to both experiments). Each condition is denoted by the type of interaural cue manipulated, the standard value of that cue, and the frequency of the tones. For example, in the condition ILD 0 dB at 4 kHz, the ILD was manipulated, the ILD of the standard was 0 dB, and the tones were presented at 4 kHz.†
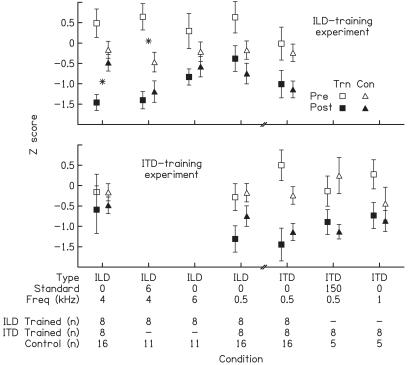
Figure 2.
Generalization to untrained conditions. Mean pretest (open symbols) and posttest (filled symbols) z scores for the five conditions tested in the ILD training (Top) and ITD training (Bottom) experiments. Results are plotted for the trained (squares) and control (triangles; same data plotted in both panels for conditions common to both experiments) listeners. Error bars represent ± 1 SEM across listeners. Asterisks indicate significant differences between the trained and control listeners on the pre- or posttest z scores. The number of listeners tested (n) is listed for each listener group and condition.
The training phase occurred between the pre- and posttests. It consisted of 1 h of practice per day for 9 or 10 days in either the ILD 0 dB at 4 kHz or the ITD 0 μs at 0.5 kHz conditions. Only a subset of randomly chosen listeners, referred to as trained listeners, participated in the training phase. The remaining listeners, referred to as control listeners, participated only in the pre- and posttests. The pre- and posttests were separated by an average of 14.4 days for both listener groups.
Stimuli.
When the variable was ILD, we presented the tone to the right ear at 70 dB sound pressure level (SPL) plus 0.5 times the total ILD, and the tone to the left ear at 70 dB SPL minus 0.5 times the total ILD. This technique helped to keep the perceived overall level of the sound image constant across different ILDs. In all ILD conditions, we presented the tones to both ears in the same starting phase (ITD = 0 μs), but chose the starting phase at random in each observation period.
When the ITD was varied, we turned the tones to both ears on and off together, but adjusted the starting phase of each tone such that the tone to the right ear led that to the left by an ongoing time difference equal to the total ITD. The tone presented to the right ear had a phase chosen at random on each observation period and the tone to the left ear had a starting phase determined by the ITD. In all ITD conditions, we presented the tones to both ears at 70 dB SPL (ILD = 0 dB).
In all conditions, the tones had a total duration of 300 ms, including 10-ms rise/fall ramps. There were 650 ms of silence between the tones in the two observation periods.
Procedure.
Within each 60-trial block, we adjusted the comparison ILD or ITD adaptively by decreasing its value after every three consecutive correct responses and increasing its value after each incorrect response. At its minimum, the comparison ILD or ITD was equal to the standard ILD or ITD. We noted the values at which the direction of change reversed from decreasing to increasing or increasing to decreasing, referred to as reversals. After discarding the first three reversals, we estimated the 79% correct point on the psychometric function by taking the average value of the remaining reversals (23). We based each estimate on the average value of a minimum of four reversals, excluding the first three reversals in each block. Throughout this paper, we express threshold as the ΔILD or ΔITD needed to achieve 79% correct discriminations.
For the ILD conditions, the step size was 0.5 dB until the third reversal and 0.25 dB thereafter. The ΔILD at the start of each block of trials was typically 6 dB in the pre- and posttests, and 2 dB above the threshold of the previous day in the training phase. For the ITD conditions, the step size was 0.2 log10-ITD units until the third reversal and 0.05 log10-ITD units thereafter (24). The starting ΔITD was always 0 μs, forcing the listener to guess on the first trial. We encouraged listeners to focus on the ILD or ITD cue by requiring them to mark the perceived lateral position of the standard sound on a schematic diagram of a head before beginning each condition, and to listen to sample trials in which, by their own description, the lateral position of the sound image changed.
We collected 5 blocks (300 trials) in each of the five conditions during the pre- and posttests. Listeners completed all of the blocks in one condition before proceeding to the next. We randomized the order of the conditions across listeners, but used the same order in both the pre- and posttests for any given listener. We collected 12 blocks (720 trials) on each day during the 9 to 10 days of the training phase. We combined the results of the control listeners across the two experiments in each of the three conditions common to both experiments.
Listeners.
Thirty-two normal-hearing volunteers (22 females, 10 males) between the ages of 18 and 44 years (mean 21.5 years) served as listeners. All were paid for their participation. None of the listeners had previous experience in any psychoacoustic task. There were 8 trained and 11 control listeners in the ILD-training experiment, and a separate group of 8 trained and 5 control listeners in the ITD-training experiment.
Results
Learning in the Trained Conditions.
Our first goal was to determine whether multihour training helps listeners to improve their ability to discriminate ILDs or ITDs. Both trained and control listeners learned on ILD as well as ITD discrimination, but multihour training affected only ILD discrimination. In the ILD-training experiment (Fig. 1 Upper Left), control listeners improved significantly between the pre- and posttest in the trained condition of ILD 0 dB at 4 kHz (t15 = 3.04, P = 0.008), but ILD-trained listeners improved more than controls. Mean thresholds did not differ significantly between groups on the pretest (t22 = 1.67, P = 0.109), and were at the high end of the range previously reported for naive or briefly trained listeners (25–27). However, thresholds were significantly lower for trained than control listeners on the posttest (t22 = −3.03, P = 0.006). Correspondingly, one-way ANOVAs with repeated measures revealed statistically significant changes in performance over sessions for trained listeners as a group, both with (F10,70 = 14.90, P < 0.0001) and without (F8,56 = 8.09, P < 0.0001) the pre- and posttest data.
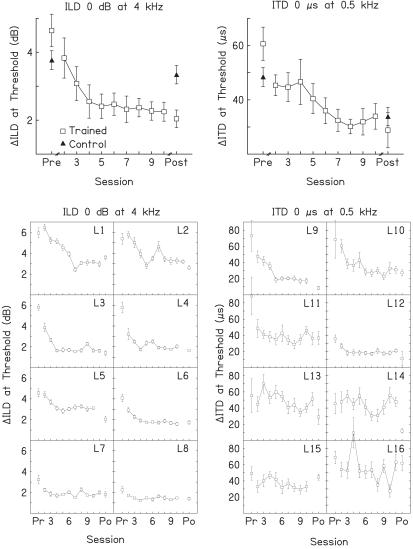
Figure 1.
Learning in the trained conditions. Pretest, posttest, and training-session thresholds for the discrimination of ILDs (Left) and ITDs (Right) in the trained conditions for trained (□) and control (▴) listeners. Shown are the group mean (Top) and individual (Bottom) thresholds. The error bars represent ± one SEM across listeners (Top) and within listeners (Bottom). Two 300-ms tones at the indicated frequencies (4 kHz or 0.5 kHz) were presented either with the indicated standard value of interaural difference (0 dB or 0 μs) or with a larger value that favored the right ear. Threshold is expressed as the difference in the values necessary to discriminate the larger from the standard values on 79% of trials.
Individually, a majority of the ILD-trained listeners showed learning during the training phase (Fig. 1 Lower Left). We judged that an individual listener had learned during the training phase if the results of that listener showed a significant change in threshold across training sessions according to a one-way ANOVA, and yielded a significant negative slope when fitted with a regression line (28). We set α at 0.05 for both tests. Six of the eight ILD-trained listeners (L1–L6) met these criteria.
In the ITD-training experiment both the trained (t7 = 4.33, P = 0.0034) and control (t15 = 3.32, P = 0.005) listeners improved significantly between the pre- and posttest in the trained ITD 0 μs at 0.5 kHz condition, but, in contrast to the results of the ILD-training experiment, the ITD-trained listeners learned no more than controls (Fig. 1 Upper Right). The pretest thresholds of both groups were within the range previously reported for naive or briefly trained listeners (25, 26). Here, the ITD thresholds did not differ significantly between the trained and control listeners on either the pretest (t22 = 1.85, P = 0.078) or the posttest (t22 = −0.76, P = 0.458). Note that the mean learning curve is misleading. Performance for trained listeners as a group changed significantly over sessions, as assessed both with (F10,70 = 6.05, P < 0.0001) and without (F8,56 = 3.65, P = 0.002) the pre- and posttest data. However, only two of the eight ITD-trained listeners showed significant learning during the training phase (Fig. 1 Lower Right; L9 and L10).
Generalization to Untrained Conditions.
Our second goal was to determine whether learning on trained ILD or ITD conditions generalizes to untrained conditions. To assess generalization, we placed the ILD and ITD results on the same measurement scale by converting the pre- and posttest thresholds to z scores. We calculated z scores for both the pre- and posttests for each listener in each condition relative to the mean and standard deviation of the pretest thresholds measured in all listeners, both trained and control. We then analyzed the z scores separately for each experiment by using a 2 × 2 × 5 ANOVA on group (trained vs. control), time (pre vs. post), and condition, with repeated measures on time. Because the number of control listeners often differed across conditions, we did not use repeated measures on condition in these analyses. We further analyzed significant interactions with t tests (29).
Both trained and control listeners improved on every ILD condition, but the ILD-trained listeners generalized their training-phase learning, if at all, only to an untrained standard cue value (6 dB) at the trained frequency (4 kHz) (Fig. 2 Upper). The ANOVA revealed a significant main effect for time (F1,100 = 154.24, P < 0.0001) and significant interactions between time and group (F1,100 = 27.87, P < 0.0001) and among time, group, and condition (F4,100 = 3.15, P = 0.018). Both ILD-trained and control listeners improved significantly between the pre- and posttests in every condition (paired t tests, every P value < 0.026). Most importantly, the pre- and posttest z scores differed significantly between the groups in only two cases: (i) the posttest z scores were smaller for the trained listeners in the trained condition of ILD 0 dB at 4 kHz (t22 = −3.03, P = 0.006), and (ii) the pretest z scores were larger for the trained listeners in the untrained condition of ILD 6 dB at 4 kHz (t17 = 2.82, P = 0.012). Thus, at the time of the posttest, the ability of the ILD-trained listeners differed from controls only in the trained condition, suggesting no generalization of their training-induced learning to other conditions. However, if pretest thresholds are taken into account, the overall magnitude of the improvement was also greater for the trained than the control listeners in the one untrained condition that used the trained frequency (ILD 6 dB at 4 kHz), suggesting generalization to an untrained standard value at the trained frequency. Note that, in that condition, the similarity in the posttest z scores between trained and control listeners occurred because 9 of the 10 lowest pretest thresholds across all listeners belonged to controls. Trained listeners had clearly lower posttest thresholds than those control listeners with similarly high pretest thresholds (for individual data, see Fig. 3, second row from top).
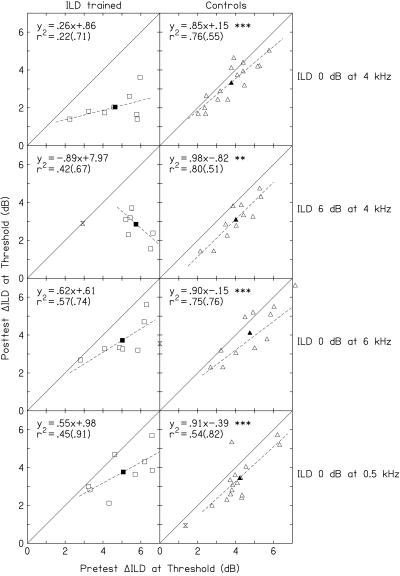
Figure 3.
Relationship between pretest thresholds and the amount of learning by individual listeners on the ILD conditions. Pretest (abscissa) and posttest (ordinate) thresholds for individual listeners (open symbols), and their group means (filled symbols), in the four ILD conditions. Results are shown for the ILD-trained (Left) and control (Right) listeners. We determined the linear regression of y on x for each data set (dashed lines), excluding values (hourglasses) for which the pretest threshold was either more than two standard deviations above or below the mean of all listeners, or was flagged by a Leverage test (30) during the fitting process. The regression-line slope and intercept, as well as the sample r2 and standard error of the estimate for the fit (in parentheses), are listed in each panel. Asterisks indicate slopes that were significantly different from zero.
Both trained and control listeners also improved on every ITD condition, but, in contrast to the ILD-trained listeners, the ITD-trained listeners did not learn more than controls on any condition (Fig. 2 Lower). Here the ANOVA revealed only a significant main effect for time (F1,88 = 60.62, P < 0.0001), and a significant interaction between time and condition (F4,88 = 3.48, P = 0.011). The three-way interaction was not significant. To further analyze the significant time-by-condition interaction, we combined the scores of the trained and control listeners within each condition. Listeners improved significantly between the pre- and posttests in every condition (paired t tests, every P < 0.011). The only difference between conditions was that the posttest z scores were smaller for the ITD 0 μs at 0.5 kHz than the ILD 0 dB at 4 kHz condition (t24 = 2.43, P = 0.019).
Relationship Between Pretest Thresholds and the Amount of Learning by Individual Listeners.
To examine the relationship between the pretest thresholds and the amount of learning in individual listeners, we determined the linear regression of the posttest (y) on the pretest (x) thresholds for each listener group and condition (Figs. 3 and 4). We excluded from all analyses values (hourglasses) for which the pretest threshold was either more than two standard deviations above or below the mean of all listeners, or was flagged by a Leverage test, which helps identify outliers in the independent variable space in regression analyses (30). Multihour training on the trained ILD condition primarily affected trained listeners whose pretest thresholds were high, but only in conditions in which trained listeners learned more than controls (Fig. 3 Left, top two rows). In contrast, short-term exposure to a variety of ILD conditions, such as that received by the control listeners, affected listeners uniformly in each condition, regardless of their pretest thresholds (Fig. 3 Right). The regression-line slopes were shallower for the ILD-trained listeners (ranging from 0.62 to −0.89) than for the control listeners (ranging from 0.85 to 0.98) in every ILD condition. However, tests of the homogeneity of regression indicated that the regression-line slopes differed significantly between trained and control listeners only in the trained ILD 0 dB at 4 kHz condition (Fig. 3, row 1; F1,20 = 7.46, P = 0.013) and the untrained ILD 6 dB at 4 kHz condition (Fig. 3, row 2; F1,14 = 10.89, P = 0.005).
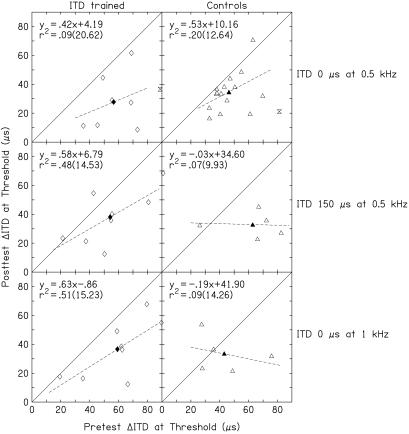
Figure 4.
Relationship between pretest thresholds and the amount of learning by individual listeners on the ITD conditions. Same as Fig. 3, but for the three ITD conditions.
In contrast, the influence of both multihour training on the trained ITD condition and short-term exposure to a variety of ITD conditions was only weakly related to the initial thresholds in those conditions (Fig. 4). Here, there were no significant differences in the regression-line slopes between trained and control listeners in any condition.
Retention.
Finally, we assessed the retention of learning 1 month after the end of training, in five of the eight ILD-trained listeners. Those listeners retained both their learning in the trained condition and their generalization pattern. A 2 × 5 ANOVA on posttest time (original vs. 1 month) and condition, performed on their z scores, revealed that neither main effect nor the interaction was statistically significant. We obtained no remeasured values from the listeners in the ITD-training experiment.
Discussion
Different Time Courses of Learning for ILD and ITD Discrimination.
The present data show that the time course of learning is longer for ILD than for ITD discrimination. The learning of ILD discrimination occurred in two stages. It began with a stage of rapid improvement, demonstrated by the learning of control listeners, and continued with a stage of slower improvement, demonstrated by the greater learning of trained than control listeners. To our knowledge, this is the first systematic examination of the learning of ILD discrimination. In contrast to ILD-discrimination learning, the learning of ITD discrimination generally occurred within the initial rapid-improvement stage, demonstrated by the equivalent learning of trained and control listeners in every ITD condition. The latter result is consistent with several previous reports that performance based on unaltered ITD cues does not change with long-term practice (20, 22), but differs from other reports that such performance does improve (19, 21).
Rapid-Improvement Stage.
Learning on interaural-cue discrimination during the rapid-improvement stage appears to generalize broadly across conditions and thus may reflect procedural learning. On average, control listeners learned uniformly during the rapid-improvement stage on all conditions within each cue type. We cannot determine the precise form of that learning from the present data, because we randomized the order of the pre- and posttest conditions across listeners. However, we now have data that suggest listeners learned rapidly on the first ILD- or ITD-discrimination condition and then immediately generalized their learning to subsequently tested conditions (J. A. Ortiz, B.A.W., M.B.F. and J. Pillai, unpublished data.). Such rapid learning with broad generalization has been termed conceptual (31) or procedural (32) learning, because it appears to represent the acquisition of the general procedural requirements of the task without regard to the specific condition. Procedural learning is unlikely to result from fundamental changes in stimulus processing of lateralization cues. Rather, when complete, procedural learning is more likely to provide an initial window into the limits of that processing by naive listeners. By this account, listeners showed rapid (procedural) learning on both ILD and ITD discrimination because both tasks shared the same basic requirements.
Slower-Improvement Stage.
Learning on interaural-cue discrimination during the slower-improvement stage, which occurred only for the ILD cue, was specific to the trained stimulus frequency, but generalized to an untrained standard ILD value. Others have noted that learning in tasks of sound lateralization and localization was specific to the spectrum of the trained sound (refs. 6, 18, and 21; for an exception see ref. 19). This frequency-specific learning parallels the location-specific learning of many visual and somatosensory tasks (e.g., refs. 33–35), because stimulus frequencies in the auditory system and stimulus locations in the visual and somatosensory systems are all encoded topographically on the receptor surfaces. Similarly, the generalization of learning to an untrained standard value is reminiscent of the generalization of improvements in visual stereoacuity to untrained visual depths at the trained location (36), because interaural and interocular cues are both encoded, by similar mechanisms (37), through the convergence of information from the two ears in the auditory brainstem or from the two eyes in the visual cortex.
Slow learning with limited generalization has been termed perceptual (31) or stimulus (32) learning, because it appears to represent fundamental changes in the processing of specific aspects of the trained stimulus. Here, these changes may have influenced either the distribution of attentional weight given to, or the low-level encoding of, stimuli with particular features. By this account, that listeners showed slower (stimulus) learning on ILD, but not on ITD discrimination, indicates that training can modify the processing of unaltered pure-tone ILDs, but not of ITDs, in adult listeners.
There are at least four potential explanations for why multihour training improved ILD but not ITD discrimination. One possibility is that it was easier to learn ILD than ITD discrimination, because the ILD conditions used an easier testing procedure. There is some evidence that easy, as opposed to difficult, testing paradigms facilitate perceptual learning (38). Here, at first glance, the testing procedure appears to have been easier for the ILD than the ITD conditions. In each adaptive track, listeners progressed from clearly audible to just detectable differences in the ILD conditions, but from inaudible to just detectable differences in the ITD conditions. However, we think these small differences in testing procedures are unlikely to account for the present results for three reasons. First, the adaptive tracks yielded estimates of 79% correct detections in both the ILD and ITD conditions. Thus, both condition types were equally difficult. Second, listeners appear to have performed similar numbers of trials near their threshold values in both the ILD and ITD conditions. The first adaptive-track reversal occurred, on average, on the 14th of 60 trials for the trained listeners in both the trained ILD and ITD conditions (based on 13 randomly selected tracks per condition). Third, all listeners heard clearly discriminable standard and comparison ILDs or ITDs during sample trials before beginning each adaptive track. Such experience is very similar to that which has induced the onset of learning in difficult testing paradigms (38).
A second possible explanation for why there were training-induced improvements on ILD but not ITD discrimination is that listeners in our ILD-training experiment focused on a monaural-intensity cue, whereas listeners in our ITD-training experiment focused on the intended interaural cue. Because we did not randomly vary the stimulus intensity within or across trials, listeners could have based their discrimination performance in the ILD conditions on a monaural-intensity cue rather than on the intended ILD cue (39). No such alternative cue was available in the ITD conditions. Therefore, the different learning patterns for ILD and ITD discrimination may have resulted because listeners were learning to perform markedly different (monaural versus interaural) tasks.
Although this explanation is theoretically possible, we also think it unlikely for three reasons. First, listeners reported using a change in the lateral position of the sound image to perform both the ILD- and ITD-discrimination tasks. Second, mean thresholds for ILD discrimination are higher than those for monaural intensity discrimination at 0.25 and 2 kHz (but not at 6 kHz), even when the overall stimulus level in the ILD tests is not randomized (40). Thus, listeners appear to use the ILD cue when it is present, even though their performance could be better if they used the available monaural-intensity cue. Third, the learning of intensity discrimination differs from that of ILD discrimination. We have preliminary data in which listeners trained for 6 h (n = 6) showed no more improvement than controls (n = 6) on intensity discrimination at 1 kHz (B.A.W. and D. A. Abrams, unpublished data.). In contrast, our ILD-trained listeners learned markedly more than controls on ILD discrimination at 4 kHz. Thus, it is unlikely that our ILD-trained listeners exclusively used a monaural intensity cue. Alternatively, the ILD-trained listeners may have initially used one cue, either the ILD or monaural-intensity cue, and switched to the opposite cue during training. In this scenario, ILD-discrimination learning would reflect a change in cue choice rather than in sensory discrimination. It seems unlikely that such learning would follow a smooth progression that was similar across all listeners, and generalize to an untrained location but not untrained frequencies, as we observed.
A third, more likely, reason why multihour training affected ILD but not ITD discrimination is that our adult listeners had to learn to attend to the ILD cue, because they were already highly practiced on, and thus readily attended to, the ITD cue. ITDs appear to govern the perceived location of sounds, as long as those sounds include frequencies below about 2 kHz (41–43). Given that many everyday sounds, such as speech, include low frequencies, our experimentally naive listeners may have had more experience with ITD-based, than ILD-based, localization. Because localization and lateralization abilities are related (e.g., ref. 44), our listeners were likely already more practiced at ITD than ILD discrimination in our lateralization task. Thus, the frequency-specific learning of the ILD cue may reflect the increasing ability of our listeners to attend selectively to that less commonly used cue at the trained stimulus frequency. This explanation is a variant of previous proposals that training improves the accuracy of the memory for, or increases the attention to, specific aspects of the trained stimulus (e.g., refs. 45 and 46; for reviews see refs. 47 and 48).
Finally, a fourth possible account for the demonstration of training-induced learning for ILD, but not ITD, discrimination is that our adult listeners had to modify the low-level stimulus encoding of the trained cue, and could do so for ILDs, but not for ITDs. The low-level encoding of ILDs and ITDs differs markedly (e.g., ref. 37). ILDs are initially encoded in the lateral superior olive primarily through excitatory inputs from the ipsilateral and inhibitory inputs from the contralateral side. In contrast, ITDs are initially encoded in the medial superior olive primarily through coincidence detectors that receive excitatory input from both the ipsilateral and contralateral sides. If training-induced learning reflects changes in low-level stimulus encoding (e.g., ref. 49), the presence of such learning for ILD, but not ITD, discrimination would result from the differences in their initial encoding. By this account, the encoding of ILDs, but not ITDs, is malleable, suggesting that the balancing of excitation and inhibition is more plastic than coincidence detection. Further, the specificity of ILD-discrimination learning to the frequency of the trained stimulus would indicate that the changes in the encoding of ILDs occurred in frequency-specific channels. It is worth noting that encoding changes probably do not occur at a level in the system in which sound location is determined by the combined information available from all cues, because changes there should affect the processing of ILDs and ITDs equally.
Conclusions
These data demonstrate differences in the relative plasticity of the processing of each of two interaural cues to sound-source location: interaural level differences (ILDs) and interaural time differences (ITDs). On our discrimination task, conceptual or procedural learning occurred for both cue types, but perceptual or stimulus learning occurred only for the ILD cue. Thus, whereas procedural learning may have tapped the same processing site for the ILD and ITD cues, stimulus learning appears to have differentially affected sites at which ILDs and ITDs are processed separately. Furthermore, the stimulus learning on ILD discrimination was specific to the frequency of the trained sound, but not to its standard lateral position. This generalization pattern indicates that the ILD-processing site modified by multihour training with a single standard sound is organized by frequency. That same site either is not organized by sound-source position or is organized by position but all positions are affected uniformly by training. These experiments on discrimination learning thus provide insight into the human encoding of cues to sound-source location.
Acknowledgments
We thank R. Theiss for her assistance with data collection, and Drs. A. Bradlow, R. Dye, and S. Zecker for their advice. D. Abrams, R. Badri, J. Mossbridge, J. Ortiz, W. Wright, Drs. W. Grantham, D. Moore, R. Withnell, and two anonymous reviewers provided helpful comments on earlier drafts of this paper. This work was supported by the National Institutes of Health/National Institute for Deafness and Other Communication Disorders (Grants R29-DC02997 and RO1 DC04453) and the McDonnell–Pew Program in Cognitive Neuroscience.
Abbreviations
- ILD
interaural level difference
- ITD
interaural time difference
- SPL
sound pressure level
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
For the nominal standard ITD of 150 μs, the ITD was indeed 150 μs for all five control listeners and for five of the eight trained listeners, but was 100 μs for the remaining three trained listeners (L9, L13, and L15).
References
- 1.Rayleigh L. Philosophical Magazine. 1907;13:214–232. [Google Scholar]
- 2.Fedderson W E, Sandel T T, Teas D C, Jeffress L A. J Acoust Soc Am. 1957;29:988–991. [Google Scholar]
- 3.Tobias J V, Schubert E D. J Acoust Soc Am. 1959;31:1595–1605. [Google Scholar]
- 4.Hafter E R, Dye R H, Gilkey R H. J Acoust Soc Am. 1979;65:471–477. doi: 10.1121/1.382346. [DOI] [PubMed] [Google Scholar]
- 5.Held R. Am J Psychol. 1955;68:526–548. [PubMed] [Google Scholar]
- 6.Feinstein S H. J Acoust Soc Am. 1973;53:393–399. doi: 10.1121/1.1913335. [DOI] [PubMed] [Google Scholar]
- 7.Stouffer J L. J Acoust Soc Am. 1975;57:1212–1213. doi: 10.1121/1.380547. [DOI] [PubMed] [Google Scholar]
- 8.Florentine M. J Am Audiol Soc. 1976;1:243–251. [PubMed] [Google Scholar]
- 9.Russell G. Percept Motor Skills. 1976;43:647–653. doi: 10.2466/pms.1976.42.2.647. [DOI] [PubMed] [Google Scholar]
- 10.Javer A R, Schwarz D W F. J Otolaryngol. 1995;24:111–117. [PubMed] [Google Scholar]
- 11.McPartland J L, Culling J F, Moore D R. Hear Res. 1997;113:165–172. doi: 10.1016/s0378-5955(97)00142-1. [DOI] [PubMed] [Google Scholar]
- 12.Hofman P M, Van Riswick J G A, Van Opstal A J. Nat Neurosci. 1998;1:417–421. doi: 10.1038/1633. [DOI] [PubMed] [Google Scholar]
- 13.Shinn-Cunningham B G, Durlach N I, Held R M. J Acoust Soc Am. 1998;103:3656–3666. doi: 10.1121/1.423088. [DOI] [PubMed] [Google Scholar]
- 14.Shinn-Cunningham B G, Durlach N I, Held R M. J Acoust Soc Am. 1998;103:3667–3676. doi: 10.1121/1.423107. [DOI] [PubMed] [Google Scholar]
- 15.Terhune J M. Scand Audiol. 1985;14:125–131. doi: 10.3109/01050398509045933. [DOI] [PubMed] [Google Scholar]
- 16.Recanzone G H, Makhamra S D D R, Guard D C. J Acoust Soc Am. 1998;103:1085–1097. doi: 10.1121/1.421222. [DOI] [PubMed] [Google Scholar]
- 17.Musicant A D, Butler R A. Percept Psychophys. 1980;28:236–240. doi: 10.3758/bf03204379. [DOI] [PubMed] [Google Scholar]
- 18.Butler R A. Percept Psychophys. 1987;41:1–7. doi: 10.3758/bf03208206. [DOI] [PubMed] [Google Scholar]
- 19.Saberi K, Perrott D R. J Acoust Soc Am. 1990;87:1732–1737. doi: 10.1121/1.399422. [DOI] [PubMed] [Google Scholar]
- 20.Litovsky R Y, Hawley M L, Fligor B J, Zurek P M. J Acoust Soc Am. 2000;108:2345–2352. doi: 10.1121/1.1312361. [DOI] [PubMed] [Google Scholar]
- 21.Hafter E R, Carrier S C. J Acoust Soc Am. 1970;47:1041–1047. doi: 10.1121/1.1912003. [DOI] [PubMed] [Google Scholar]
- 22.Trahiotis C, Bernstein L R, Buell T N, Spektor Z. J Acoust Soc Am. 1990;87:1359–1361. doi: 10.1121/1.399513. [DOI] [PubMed] [Google Scholar]
- 23.Levitt H. J Acoust Soc Am. 1971;49:467–477. [PubMed] [Google Scholar]
- 24.Saberi K. J Acoust Soc Am. 1995;98:1803–1806. doi: 10.1121/1.413379. [DOI] [PubMed] [Google Scholar]
- 25.Koehnke J, Culotta C P, Hawley M L, Colburn H S. Ear Hear. 1995;16:331–353. doi: 10.1097/00003446-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein L R, Trahiotis C, Hyde E L. J Acoust Soc Am. 1998;103:2069–2078. doi: 10.1121/1.421378. [DOI] [PubMed] [Google Scholar]
- 27.Hawley M L. Ph.D. dissertation. Boston: Boston Univ.; 2000. [Google Scholar]
- 28.Wright B A, Buonomano D V, Mahncke H W, Merzenich M M. J Neurosci. 1997;17:3956–3963. doi: 10.1523/JNEUROSCI.17-10-03956.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keppel G. Design and Analysis: A Researcher's Handbook. Englewood Cliffs, NJ: Prentice-Hall; 1982. [Google Scholar]
- 30.Velleman P F, Welsch R E. Am Stat. 1981;35:234–242. [Google Scholar]
- 31.Recanzone G H, Schreiner C E, Merzenich M M. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson K, Summerfield A Q. Ear Hear. 1996;17:51–65. doi: 10.1097/00003446-199617031-00006. [DOI] [PubMed] [Google Scholar]
- 33.Berardi N, Fiorentini A. J Physiol. 1987;384:633–647. doi: 10.1113/jphysiol.1987.sp016474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karni A, Sagi D. Nature (London) 1993;365:250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- 35.Schoups A A, Vogels R, Orban G. J Physiol. 1995;483:797–810. doi: 10.1113/jphysiol.1995.sp020623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sowden P, Davies I, Rose D, Kaye M. Perception. 1996;25:1043–1052. doi: 10.1068/p251043. [DOI] [PubMed] [Google Scholar]
- 37.Irvine D R F. In: The Mammalian Auditory Pathway: Neurophysiology. Popper A N, Fay R R, editors. New York: Springer; 1992. pp. 153–231. [Google Scholar]
- 38.Ahissar M, Hochstein S. Nature (London) 1997;387:401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- 39.Grantham D W. J Acoust Soc Am. 1984;75:1191–1194. doi: 10.1121/1.390769. [DOI] [PubMed] [Google Scholar]
- 40.Rowland R C, Tobias J V. J Speech Hear Res. 1967;10:745–756. doi: 10.1044/jshr.1004.745. [DOI] [PubMed] [Google Scholar]
- 41.Blauert J. Spatial Hearing: The Psychophysics of Human Sound Localization. Cambridge, MA: MIT Press; 1983. [Google Scholar]
- 42.Trahiotis C, Bernstein L R. J Acoust Soc Am. 1986;79:1950–1957. doi: 10.1121/1.393202. [DOI] [PubMed] [Google Scholar]
- 43.Wightman F L, Kistler D J. J Acoust Soc Am. 1992;91:1648–1661. doi: 10.1121/1.402445. [DOI] [PubMed] [Google Scholar]
- 44.Jeffress L A, Taylor R W. J Acoust Soc Am. 1961;33:482–483. [Google Scholar]
- 45.Ahissar M, Hochstein S. Proc Natl Acad Sci USA. 1993;90:5718–5722. doi: 10.1073/pnas.90.12.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mollon J D, Danilova M V. Spat Vis. 1996;10:51–58. doi: 10.1163/156856896x00051. [DOI] [PubMed] [Google Scholar]
- 47.Gilbert C D. Physiol Rev. 1998;78:467–485. doi: 10.1152/physrev.1998.78.2.467. [DOI] [PubMed] [Google Scholar]
- 48.Goldstone R L. Ann Rev Psychol. 1998;49:585–612. doi: 10.1146/annurev.psych.49.1.585. [DOI] [PubMed] [Google Scholar]
- 49.Karni A. Cog Brain Res. 1996;5:39–48. doi: 10.1016/s0926-6410(96)00039-0. [DOI] [PubMed] [Google Scholar]