Key Points
Thromboembolism is a frequent treatment-related complication in ALL patients aged 1 to 45 years, with a cumulative incidence of 7.9%.
Patients aged ≥10 years are at highest risk during asparaginase treatment, and may be candidates for preemptive antithrombotic prophylaxis.
Abstract
Thromboembolism frequently occurs during acute lymphoblastic leukemia (ALL) therapy. We prospectively registered thromboembolic events during the treatment of 1772 consecutive Nordic/Baltic patients with ALL aged 1 to 45 years who were treated according to the Nordic Society of Pediatric Hematology and Oncology ALL2008 protocol (July 2008-April 2017). The 2.5-year cumulative incidence of thromboembolism (N = 137) was 7.9% (95% confidence interval [CI], 6.6-9.1); it was higher in patients aged at least 10 years (P < .0001). Adjusted hazard ratios (HRas) were associated with greater age (range, 10.0-17.9 years: HRa, 4.9 [95% CI, 3.1-7.8; P < .0001]; 18.0-45.9 years: HRa, 6.06 [95% CI, 3.65-10.1; P < .0001]) and mediastinal mass at ALL diagnosis (HRa, 2.1; 95% CI, 1.0-4.3; P = .04). In a multiple absolute risk regression model addressing 3 thromboembolism risk factors, age at least 10 years had the largest absolute risk ratio (RRage, 4.7 [95% CI, 3.1-7.1]; RRenlarged lymph nodes, 2.0 [95% CI, 1.2-3.1]; RRmediastinal mass, 1.6 [95% CI, 1.0-2.6]). Patients aged 18.0 to 45.9 years had an increased hazard of pulmonary embolism (HRa, 11.6; 95% CI, 4.02-33.7; P < .0001), and patients aged 10.0 to 17.9 years had an increased hazard of cerebral sinus venous thrombosis (HRa, 3.3; 95% CI, 1.5-7.3; P = .003) compared with children younger than 10.0 years. Asparaginase was truncated in 38/128 patients with thromboembolism, whereas thromboembolism diagnosis was unassociated with increased hazard of relapse (P = .6). Five deaths were attributable to thromboembolism, and patients younger than 18.0 years with thromboembolism had increased hazard of dying compared with same-aged patients without thromboembolism (both P ≤ .01). In conclusion, patients aged at least 10 years could be candidates for preemptive antithrombotic prophylaxis. However, the predictive value of age 10 years or older, enlarged lymph nodes, and mediastinal mass remain to be validated in another cohort.
Visual Abstract
Introduction
Survival of children with Philadelphia-chromosome negative (Ph−) ALL is now higher than 90% with the best contemporary treatment, and the recent introduction of pediatric-inspired therapy has similarly improved cure rates among adults.1-9 This partly reflects increased use of asparaginase (ASP), and thromboembolism (TE) has become a frequent and serious treatment-related toxicity challenging protocol adherence and cure rates.10-15
The incidence of symptomatic thrombosis in childhood ALL is approximately 5%, as reported in larger studies,16,17 and has been shown to increase with age.13,15,17-19 When including asymptomatic cases, TE has been reported in as many as 37% to 73% of children with ALL.20,21 In adult ALL, the reported incidence ranges between 1.4% and 2.2% at the time of diagnosis22,23; rising to 4.5% to 41% during chemotherapy.3,8,13,17,24-28 In both children and adults, TE frequently coincides with ASP and corticosteroid administration.8,11,15,23,29 ASP has been associated with decreased levels of procoagulant factors (factor V [FV], FVII, FVIII, FIX, FX, and FXI), fibrinogen, plasminogen, protein C, protein S, and antithrombin III (AT), resulting in decreased thrombin inhibition combined with increased thrombin generation.12,18,28,30,31 In contrast, evidence regarding the association with corticosteroids is inconclusive, although corticosteroids may inhibit fibrinolysis by increasing levels of plasminogen activator inhibitor-1.12,32
Because data on TE risk in children and adults treated uniformly are lacking, the primary objectives of this study were to explore the cumulative incidence and clinical characteristics of TE in patients with Ph− ALL aged 1 to 45 years treated according to the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 protocol; the risk factors associated with TE; TE influence on subsequent ALL treatment, mortality, and relapse; and the efficacy and safety of antithrombotic therapy introduced after TE.
Patients and methods
Study population
Study patients aged 1 to 45 years were diagnosed from July 2008 to February 2016 with either B-cell precursor (BCP-) or T-cell (T-) ALL and treated according to the NOPHO ALL2008 protocol in Denmark, Estonia, Finland, Iceland, Lithuania, Norway, and Sweden. Of 1861 patients with ALL, the following were excluded: 21 patients with acute leukemia of ambiguous lineage, 54 with ALL predisposition syndromes (eg, Down syndrome or ataxia telangiectasia), and 1 not treated according to the ALL2008 protocol. One hundred fifty patients with ALL developed TE, of whom 13 were excluded because of missing imaging confirmation of TE (N = 1), superficial thrombophlebitis (N = 1), septic emboli (N = 1), central venous line (CVL) dysfunction registered as asymptomatic TE (N = 2), and missing data (N = 8). Thus, a total of 1772 patients with Ph− ALL, among whom were 137 registered TE cases, were included in the study. Twenty predefined toxicities, including TE, were prospectively included in the NOPHO registry through online mandatory toxicity registration at 3-month intervals, with a compliance rate of 98.9%.19,33 TE detailed data were subsequently retrieved by local clinicians or national NOPHO TE Working Group coordinators. Data on patient demographics, ALL characteristics, and treatment were retrospectively collected on 1 July 2017, from this NOPHO thrombosis database and the NOPHO ALL registry, respectively.
Protocol treatment and antithrombotic prophylaxis guidelines
The NOPHO ALL2008 protocol has been previously described.15,19,33,34 Treatment stratification involved assignment to 4 risk groups: standard risk (SR), intermediate risk (IR), high risk (HR), and high risk with hematopoietic stem cell transplantation (HR-SCT) in first complete remission (CR1) guided by tumor burden at diagnosis, immunophenotype, cytogenetics, and minimal residual disease (MRD) levels at treatment days 15, 29, and 79 (or after the second HR block). Of note, children were randomly assigned to receive pegylated ASP (PegASP), administered at 2-week (control group) vs 6-week (experimental group) intervals from treatment weeks 14 to 33 (ie, during delayed intensification [DI] and early maintenance-1). This randomization was closed March 1, 2016, after which all children received PegASP at 6-week intervals from week 14.35 A description of the NOPHO ALL2008 protocol treatment including dosage of corticosteroids and PegASP, together with an overview of the protocol (supplemental Figure 1, available on the Blood Web site), are presented in the supplemental Data.
No common recommendations for routine antithrombotic prophylaxis exist in the ALL2008 protocol. In the case of a symptomatic TE event during treatment, low molecular weight heparin (LMWH) twice daily for 1 to 3 months (but at minimum until PegASP therapy cessation) was recommended without PegASP truncation. However, local management of symptomatic CVL-related thrombosis in regard to removal or maintenance of CVL, and management of platelet count lower than 50 × 109/L in regard to transfusion of platelets or dosage lowering in antithrombotic therapy, were nonuniform across centers.36 Preemptive screening for inherited thrombophilia was only recommended in the case of family history of TE, including genetic predisposition (eg, AT, protein C, or protein S deficiency), as TE prophylaxis during high-risk periods could be relevant in such patients. In the case of a TE event, thrombophilia screening, including protein S, protein C, AT, fibrinogen, D-dimer, antiphospholipid antibodies, FV Leiden mutation G1691A, FII mutation G20210A, homocysteine, lipoprotein(a), and FVIII, was recommended.
The ALL2008 protocol was approved by the National Medicines Agencies and the relevant national or regional ethics committees in each participating country. Clinical Trial Registration: EudraCT 2008-003235-20 and 2011-000908-18 (Lithuania). All patients had given written informed consent, and the study was conducted in accordance with the Declaration of Helsinki (version 2008).
TE-related event definitions
Symptomatic/asymptomatic and venous/arterial TE cases identified through clinical evaluation and confirmed by imaging were included in this study and evaluated according to TE international Ponte di Legno consensus criteria.37 The date of TE diagnosis was established as the date of the first confirmatory imaging, or the death date in cases of TE diagnosed by autopsy. Asymptomatic TE cases included were detected by imaging because of other non-TE symptoms, and were treated with systemic anticoagulation, as required by definition. Cases of superficial thrombophlebitis, septic embolism, and CVL dysfunction resulting from thrombosis without other symptoms were excluded.
Bleeding events during antithrombotic therapy were categorized as minor or major bleeding. Major bleeding was defined as a nontraumatic fatal bleeding and/or symptomatic hemorrhage in a critical area or organ (intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome) and/or bleeding causing a decrease in hemoglobin level of at least 2 g/dL (1.24 mmol/L) within 1 day or leading to transfusion of 2 or more units of whole blood or red blood cells.38 Minor bleeding was defined as any nontraumatic, nonskin bleeding not fulfilling criteria for major bleeding. Information on bleeding was collected 3 months after the start of antithrombotic treatment.
Statistical analyses
Study patients were followed from the point of ALL diagnosis until the date of the first event (relapse, death including death during induction and in CR1, or second malignant neoplasm), SCT in CR1, loss to follow-up/abandonment of therapy, last follow-up in the registry, or 1 April 2017, whichever came first. Thus, patients in CR1 were followed for a minimum of 13 months postdiagnosis. Of 91 children (age, 1.0-17.9 years) with TE, 58 had previously been reported.15 The median follow-up time was estimated using the reversed Kaplan-Meier method. The cumulative incidences of first TE were estimated using the Aalen-Johansen estimator, considering relapse, death, and second malignant neoplasm as competing events, and the estimates were compared with Gray’s test. The TE incidence rate during different protocol treatment phases was calculated as the number of new TE cases during a specific period divided by the person-time-at-risk throughout the observation period. The incidence rate ratio of TE during vs before PegASP treatment was estimated by Poisson regression. The person-time before PegASP was calculated from ALL diagnosis until day 30 (time of first PegASP dose), the date of the first event, SCT, or loss to follow-up; whichever came first. The person-time on PegASP treatment was calculated from day 30 until truncation of PegASP, the last PegASP dose (both +4 weeks; the cutoff time of measurable PegASP acitivity,39 regardless of PegASP randomization), the date of the first event, SCT, or loss to follow-up, whichever came first. Time to first TE was analyzed in a Cox proportional hazards regression model including relevant selected clinical-, disease-, and treatment-related characteristics. As a secondary outcome, time to first TE was analyzed separately for each TE subtype, including sex, age group, and mediastinal mass. The body mass index (BMI) z-scores were based on age and sex.40 As a sensitivity analysis of the predefined age groups (1.0-9.9 years, 10.0-17.9 years, and 18.0-45.9 years), new age groups were identified as each containing approximately 25% of the TE events (1.0-5.9 years, 6.0-14.9 years, 15.0-20.9 years, and 21.0-45.9 years) and were included in the full model. Variable selection for a prediction model was done with the lasso method, using 10-fold cross-validation, including all baseline variables known at ALL diagnosis with age groups 1.0 to 9.9 years and 10.0 to 45.9 years. Absolute risk ratios of TE were estimated using an absolute risk regression model.41 To evaluate the predictive ability of risk factors, we calculated time-dependent inverse probability of censoring weighting estimates of sensitivity and specificity; the latter defining controls as a patient without TE as opposed to a patient free of any event. To investigate the association between TE and time to death, we used a Cox model with TE as a time-dependent variable. The model included an interaction between TE and age group and time to TE. Hence, the hazard ratios for TE vs non-TE can be reported only for a specific age group for a specific time to TE. When investigating the association between TE and time to relapse, the Cox model included only patients aged at least 18 years and the time-dependent TE variable, as all relapses among patients with TE occurred in the oldest age group. As sensitivity analyses, the models were stratified by treatment group day 29. The association between time to death and TE subtype was investigated by pairwise comparison in a simple Cox regression model with delayed entry at first TE diagnosis and adjusted for multiple testing, using Turkey’s method. All models involving antithrombotic prophylaxis, treatment group, and/or MRD day 29 were analyzed with delayed entry at day 29. In all Cox models, relevant interactions and the proportional hazards assumption were investigated. Two-sided P values < .05 were considered statistically significant. All statistical analyses were carried out using the statistical computing software R, version 3.2.0 (R Core Team).
Results
Patient and treatment characteristics
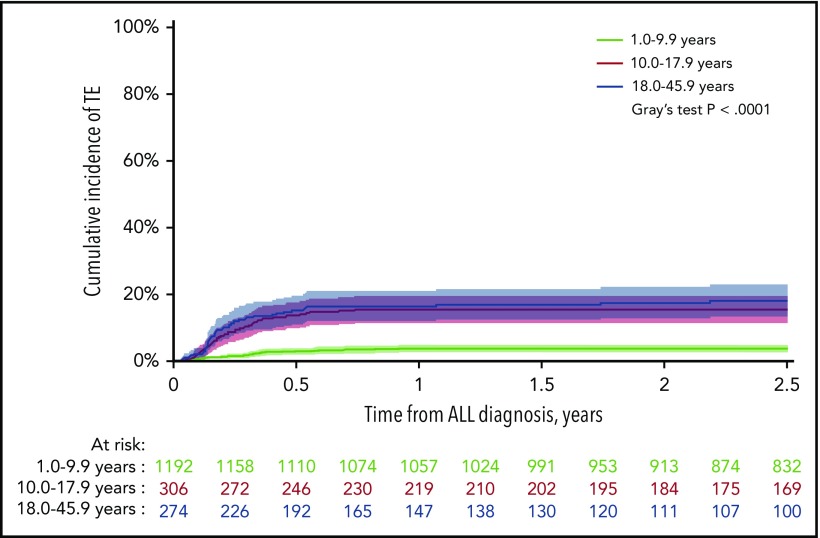
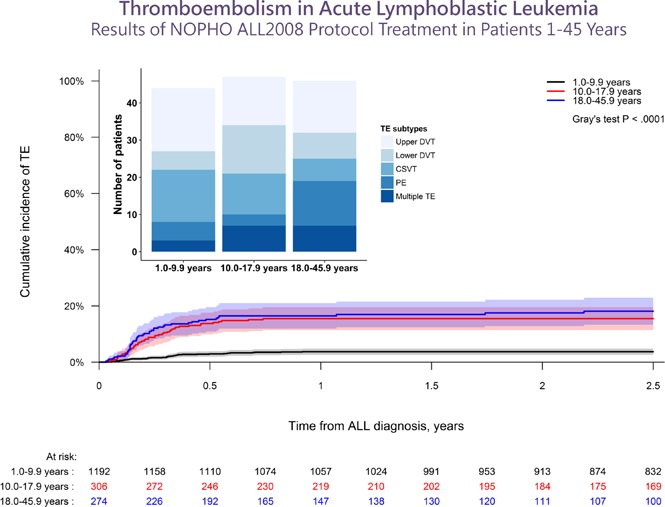
After a median follow-up of 4.3 years (interquartile range [IQR], 2.5-6.4 years), the 2.5-year cumulative incidence of first-time TE (137/1772) during therapy was 7.9% (95% CI, 6.6%-9.1%), of which 92% (N = 126) presented with symptomatic TE and a 2.5-year cumulative incidence of 7.2% (95% CI, 6.0%-8.4%). The 2.5-year cumulative incidence of any TE, as well as symptomatic TE, was significantly higher in patients aged at least 10 years, being 3.7%, 15.5%, and 18.1% in patients younger than 10.0 years, aged 10.0 to 17.9 years, and aged 18.0 years or older, respectively (P < .0001 for both; Figure 1; supplemental Figure 2). The clinical characteristics of the patients are summarized in Tables 1 and 2. Indwelling CVLs were present in 93% of supradiaphragmatic and 64% of asymptomatic TE events. Infection was reported in 42% of patients at the time of TE, in whom 32% showed positive blood cultures.
Figure 1.
The cumulative incidence of TE. The cumulative incidence of TE by age groups with 95% CIs and patients at risk. The 2.5-year cumulative incidences were: 3.7% for 1.0 to 9.9 years (95% CI, 2.6%-4.8%); 15.5% for 10.0 to 17.9 years (95% CI, 11.3%-19.4%); 18.1% for 18.0 to 45.9 years (95% CI, 13.2-22.8).
Table 1.
Clinical characteristics
| N (%) | 2.5-y cumulative incidence of TE, % | 95% CI | P | |
|---|---|---|---|---|
| All patients | 1772 | 7.87 | 6.60-9.13 | — |
| Age groups | ||||
| 1.0-9.9 y | 1192 (67) | 3.73 | 2.64-4.80 | <.0001 |
| 10.0-17.9 y | 306 (17) | 15.5 | 11.3-19.4 | |
| 18.0-45.9 y | 274 (16) | 18.1 | 13.2-22.8 | |
| Sex | ||||
| Female | 786 (44) | 6.41 | 4.68-8.12 | .05 |
| Male | 986 (56) | 9.06 | 7.22-10.9 | |
| Immunophenotype | ||||
| BCP | 1492 (84) | 6.81 | 5.51-8.09 | .0002 |
| T-cell | 280 (16) | 13.6 | 9.43-17.6 | |
| WBC | ||||
| <100 × 109/L | 1535 (87) | 7.95 | 6.57-9.31 | .7 |
| ≥100 × 109/L | 237 (13) | 7.35 | 3.92-10.7 | |
| Mediastinal mass | ||||
| No | 1611 (91) | 6.97 | 5.70-8.22 | <.0001 |
| Yes | 139 (8) | 17.3 | 10.8-23.4 | |
| Missing | 22 (1) | — | — | |
| Palpable splenomegaly | ||||
| No | 1475 (83) | 7.66 | 6.28-9.02 | .4 |
| Yes | 262 (15) | 9.30 | 5.69-12.8 | |
| Missing | 35 (2) | — | — | |
| Enlarged lymph nodes ≥3 cm | ||||
| No | 1611 (91) | 7.15 | 5.87-8.41 | <.0001 |
| Yes | 132 (7) | 16.8 | 10.1-22.9 | |
| Missing | 29 (2) | — | — | |
| CNS status* | ||||
| CNS1 | 1544 (87) | 7.99 | 6.62-9.35 | .6 |
| CNS2/CNS3 | 219 (12) | 6.88 | 3.46-10.2 | |
| Missing | 9 (1) | — | — | |
| BMI | ||||
| ≤−2SD | 86 (5) | 8.31 | 2.22-14.0 | .4 |
| >−2SD to <+2SD | 1552 (87) | 7.61 | 6.27-8.93 | |
| ≥+2SD | 119 (7) | 11.2 | 5.24-16.7 | |
| Missing | 15 (1) | — | ||
| Induction† | ||||
| Prednisolone | 1382 (78) | 7.05 | 5.68-8.41 | .02 |
| Dexamethasone | 390 (22) | 10.8 | 7.60-13.9 | |
| All patients (delayed entry at day 29) | 1737 | |||
| Antithrombotic prophylaxis | ||||
| No | 1690 (97) | 7.04 | 5.80-8.26 | .1 |
| Yes | 47 (3) | 13.1 | 2.75-22.3 | |
| MRD day 29 | ||||
| <0.001 | 1145 (66) | 6.77 | 5.30--8.21 | .2 |
| ≥0.001 to <0.05 | 452 (26) | 8.26 | 5.68-10.8 | |
| ≥0.05 | 81 (5) | 16.5 | 0.00-30.8 | |
| Missing | 59 (3) | — | — | |
| Treatment group day 29 | ||||
| SR | 780 (45) | 5.53 | 3.91-7.13 | .04 |
| IR | 653 (37) | 8.97 | 6.74--11.1 | |
| HR/HR-SCT | 302 (17) | 7.93 | 4.62-11.1 | |
| Missing | 2 (1) | — | — |
CNS, central nervous system; SD, standard deviation.
CNS1 (no blasts on cytospin and no other signs of CNS leukemia), CNS2 (> 0 and <5 cells/µL cerebrospinal fluid with blasts on cytospin and no other signs of CNS leukemia), and CNS3 (≥ 5 cells/µL cerebrospinal fluid with blasts on cytospin, cranial nerve palsy, intracranial “leukemic” mass on magnetic resonance imaging (MRI), eye involvement confirmed by MRI, or a biopsy to reflect ALL).
Induction treatment included prednisolone (BCP-ALL and white blood cell count [WBC] <100 × 109/L) or dexamethasone (T-ALL and/or WBC ≥ 100 × 109/L).
Table 2.
TE characteristics
| All TE | CSVT | PE | Supradiaphragmatic TE* | Infradiaphragmatic TE* | Multiple sites | |
|---|---|---|---|---|---|---|
| Types of TE, N (%) | 137 | 31 (23) | 20 (15) | 44 (32) | 25 (18) | 17 (12) |
| Venous/arterial/unknown origin | 126 (92)/2 (1)/9 (7) | 31 (100)/0/0 | 20 (100)/0/0 | 42 (95)/0/2 (5) | 16 (64)/2 (8)†/7 (28) | 17 (100)/0/0 |
| Median age (IQR), y | 15.6 (6.4-21.5) | 11.1 (5.2-16.8) | 22.4 (12.6-28.4) | 13.8 (5.4-19.3) | 16.0 (12.2-19.0) | 17.6 (12.9-31.1) |
| Median time to TE from ALL diagnosis (range), d | 80 (1-799) | 51 (16-799) | 66 (14-636) | 105 (21-391) | 70 (1-246) | 85 (37-198) |
| Diagnostic methods, N (%) | ||||||
| ULS | 50 (36) | 0 | 0 | 27 (61) | 20 (80) | 3 (18) |
| MRI and/or CT | 58 (42) | 30 (97) | 18 (90) | 4 (9) | 2 (8) | 4 (24) |
| TTE | 3 (2) | 0 | 0 | 3 (7) | 0 | 0 |
| Angiography | 2 (1) | 0 | 0 | 2 (5) | 0 | 0 |
| ULS + MRI and/or CT | 16 (12) | 0 | 0 | 4 (9) | 3 (12) | 9 (53) |
| ULS + MRI + angiography | 1 (1) | 0 | 0 | 1 (2) | 0 | 0 |
| ULS + chest X-ray + angiography | 1 (1) | 0 | 0 | 1 (2) | 0 | 0 |
| ULS + angiography | 1 (1) | 0 | 0 | 1 (2) | 0 | 0 |
| ULS + V/Q scan | 1 (1) | 0 | 0 | 0 | 0 | 1 (6) |
| Angiography + TTE | 1 (1) | 0 | 0 | 1 (2) | 0 | 0 |
| Autopsy | 3 (2) | 1 (3) | 2 (10) | 0 | 0 | 0 |
| Symptomatic/asymptomatic TE, N (%) | 126 (92)/11 (8) | 31 (100)/0 | 17 (85)/3 (15) | 37 (84)/7 (16) | 24 (96)/1 (4) | 17 (100)/0 |
| CVL-related‡ | 44 (32)/7 (5) | — | — | 34 (77)/7 (16) | 6 (24)/0 | 4 (24)/0 |
| Median time to TE after last CVL insertion (IQR), d | 61 (34-108) | — | — | 69 (40-109) | 60 (24-106) | 47 (28-125) |
| Infection, N (%) | 57 (42) | 6 (19) | 11 (55) | 17 (39) | 13 (52) | 10 (59) |
| Positive blood culture§ | 18 (13) | 1 (3) | 4 (20) | 5 (11) | 3 (12) | 5 (29) |
CSVT, cerebral sinus venous thrombosis; PE, pulmonary embolism; TTE, transthoracic echocardiography; ULS, ultrasonography; V/Q scan, ventilation/perfusion scan.
Supra- and infra-diaphragmatic TE are defined as deep venous thrombosis occurring at any site in the upper and lower venous system, respectively.
Both venous and arterial TE in 1 case
CVL-related is defined as a possible relation between the site of CVL and the development of TE. Information not available in 1 patient.
The causative agent was from the Candida genus (N=1), Pseudomonas genus (N=1), and unknown (N=16).
TE occurred during induction in 14 patients (0.10 cases per person-year), during SR/IR consolidation-1 in 47 patients (0.20 cases per person-year), during SR/IR DI-1/consolidation-2 in 28 patients (0.16 cases per person-year), during HR blocks in 21 patients (0.14 cases per person-year), during maintenance-1 in 24 SR/IR patients and in 1 HR patient (0.02 cases per person-year), and during maintenance-2 in 1 IR patient and in 1 HR patient (0.001 cases per person-year). PegASP activity was measurable in 82 patients and not measurable in 7 of 89 patients with TE during treatment with this information available. In addition, 72% of the TE cases (99/137) occurred within 4 weeks of the last PegASP administration. The incidence rate ratio of TE during vs before PegASP treatment was 1.3 (95% CI, 0.75-2.31; P = .4). After a median of 9 doses (IQR, 6-12 doses; range, 1-15 doses), PegASP treatment was truncated because of TE in 38 of 128 patients with TE with this information available, which included 17 patients with CSVT, 12 patients with supra-/infradiaphragmatic TE, 7 patients with multiple TE, and 2 patients with PE.
In a multiple Cox regression analysis with delayed entry at day 29 (N = 1594; 114 TE events), the adjusted hazard ratio (HRa) of TE was significantly increased in ages 10.0 to 17.9 years (HRa, 4.9; 95% CI, 3.1-7.8; P < .0001) and ages 18.0 to 45.9 years (HRa, 6.06; 95% CI, 3.65-10.1; P < .0001) compared with children younger than 10.0 years, and for mediastinal mass at ALL diagnosis (HRa, 2.1; 95% CI, 1.0-4.3; P = .04; Table 3). The abovementioned associations remained unchanged when including only patients with BCP-ALL (presence of mediastinal mass in N = 8; HRa, 4.2; 95% CI, 1.3-14.0; P = .02; remaining results not shown). When including the new 4 age groups in the Cox model, no difference in the estimates was observed. The adjusted TE-specific hazard was significantly increased in patients aged 6.0 to 14.9 years (HRa, 2.0; 95% CI, 1.2-3.5; P = .01), 15.0 to 20.9 years (HRa, 7.74; 95% CI, 4.52-13.2; P < .0001), and 21.0 to 45.9 years (HRa, 6.54; 95% CI, 3.69-11.6; P < .0001), using 1.0 to 5.9 years as reference. In neither of the Cox models did the estimates change markedly when including all 1772 patients by removing the delayed entry at day 29 and associated variables (antithrombotic prophylaxis, treatment group, and MRD day 29). From a TE-specific Cox model including baseline variables at ALL diagnosis (with age groups ≥10 and <10 years because of similar estimates for the oldest groups in Tables 1 and 3), we selected age, enlarged lymph nodes, and mediastinal mass for prediction based on the lasso method, using 10-fold cross-validation. In a multiple absolute risk regression model with 1723 patients (132 patients with TE) with no missing data, age had the largest absolute risk ratio (RRage, 4.7 [95% CI, 3.1-7.1]; RRenlarged lymph nodes, 2.0 [95% CI, 1.2-3.1]; RRmediastinal mass, 1.6 [95% CI, 1.0-2.6]). The predicted cumulative incidences by all 8 combinations of the 3 risk factors based on this model are shown in supplemental Figure 3. Investigating each selected variable separately, age group had the highest ability to identify patients at risk for TE within 2.5 years from ALL diagnosis (sensitivity, 66.6%), but the lowest ability to correctly identify patients who would not develop TE (specificity, 74.2%; enlarged lymph nodes: sensitivity, 15.8% and specificity, 93.2%; mediastinal mass: sensitivity, 18.1% and specificity, 93.4%). Of the 1723 patients, only 28 patients had all 3 risk factors: 128 patients had 2, and 663 patients harbored only 1 of these 3 risk factors.
Table 3.
Multiple analyses of TE with delayed entry at day 29 (N = 1594)
| N | TE-specific HRa | 95% CI | P | |
|---|---|---|---|---|
| Age groups | ||||
| 1.0-9.9 y | 1116 | 1.00 [ref.] | ||
| 10.0-17.9 y | 274 | 4.9 | 3.1-7.8 | <.0001 |
| 18.0-45.9 y | 204 | 6.1 | 3.7-10.1 | <.0001 |
| Sex | ||||
| Female | 715 | 1.00 [ref.] | ||
| Male | 879 | 1.3 | 0.9-2.0 | .1 |
| BMI | ||||
| >−2SD to <+2SD | 79 | 1.00 [ref.] | ||
| ≤−2SD | 1412 | 1.4 | 0.6-3.2 | .5 |
| ≥+2SD | 103 | 0.8 | 0.4-1.5 | .4 |
| WBC | ||||
| <100 × 109/L | 1396 | 1.00 [ref.] | ||
| ≥100 × 109/L | 198 | 1.0 | 0.5-2.1 | >.9 |
| Immunophenotype | ||||
| BCP | 1361 | 1.00 [ref.] | ||
| T-cell | 233 | 1.3 | 0.4-4.1 | .7 |
| CNS status | ||||
| CNS1 | 1397 | 1.00 [ref.] | ||
| CNS2/CNS3 | 197 | 0.9 | 0.4-1.6 | .6 |
| Mediastinal mass | ||||
| No | 1468 | 1.00 [ref.] | ||
| Yes | 126 | 2.1 | 1.0-4.3 | .04 |
| Enlarged lymph nodes ≥3 cm | ||||
| No | 1480 | 1.00 [ref.] | ||
| Yes | 114 | 1.3 | 0.7-2.4 | .4 |
| Palpable splenomegaly | ||||
| No | 1351 | 1.00 [ref.] | ||
| Yes | 243 | 1.3 | 0.8-2.0 | .3 |
| Induction | ||||
| Dexamethasone | 323 | 1.00 [ref.] | ||
| Prednisolone | 1271 | 1.4 | 0.4-4.4 | .6 |
| Antithrombotic prophylaxis | ||||
| No | 1555 | 1.00 [ref.] | ||
| Yes | 39 | 0.8 | 0.3-1.9 | .6 |
| MRD day 29 | ||||
| <0.001 | 1095 | 1.00 [ref.] | ||
| ≥0.001 to <0.05 | 425 | 1.1 | 0.6-2.0 | .7 |
| ≥0.05 | 74 | 2.3 | 0.7-7.0 | .2 |
| Treatment group day 29 | ||||
| SR | 741 | 1.00 [ref.] | ||
| IR | 611 | 0.9 | 0.5-1.6 | .8 |
| HR/HR-SCT | 242 | 0.5 | 0.2-1.4 | .2 |
ref., reference.
No significantly increased subtype TE-specific hazard (adjusted for age and sex) was associated with the presence of mediastinal mass at ALL diagnosis for first-time PE (20 events; P = .4), CSVT (30 events; P = .8), supradiaphragmatic TE (42 events; P = .05), infradiaphragmatic TE (25 events; P = .2), or TE at multiple sites (17 events; P = .2), respectively (supplemental Table 1). Adults aged 18.0 to 45.9 years had an increased hazard of PE compared with children younger than 10.0 years, and this accounted for 12 of 20 PE cases (adjusted PE-specific HRa, 11.6; 95% CI, 4.02-33.7; P < .0001). Adolescents aged 10.0 to 17.9 years had an increased hazard of CSVT compared with children younger than 10.0 years and accounted for 11 of 30 CSVT cases (adjusted CSVT-specific, HRa 3.3; 95% CI, 1.5-7.3; P = .003; supplemental Table 1). The same pattern was observed when using the new 4 age groups.
Laboratory findings
Measurement of D-dimer at TE diagnosis was available in 106 of 137 patients, of whom 28% (30/106) had values within the normal range (<0.5 mg/L), whereas the median of those remaining was 1.5 mg/L (IQR, 0.9-2.8 mg/L; range, 0.5-2000). Measurement of AT at TE diagnosis was available in 69 of 137 patients, of whom 41% (28/69) had values lower than 80% (lower normal limit), with a median of 60% (IQR, 44%-68%; range, 1.26%-79%). The thrombocyte count at TE diagnosis ranged from 6 to 762 ×109/L (median, 159 ×109/L; IQR, 105-296 ×109/L; unknown in N = 6). Nine percent had a thrombocyte count lower than 50 ×109/L at TE diagnosis (12/131), and none of them developed major bleeding complications. Inherited thrombophilia was investigated in 72 of 137 patients, of whom 11% (8/72) showed positive, with heterozygous FV Leiden mutation (N = 5), heterozygous FII mutation (N = 2), and both heterozygous FV Leiden mutation and FII mutation (N = 1).
Antithrombotic treatment: safety and efficacy
Seventeen percent of all study patients aged 17 years or older (48/289), who were followed at several adult hematology departments in Denmark, Norway, and Estonia, were treated with LMWH prophylaxis, and 13% of them developed first-time TE (6/48). None of the patients treated at the pediatric departments received antithrombotic prophylaxis. The antithrombotic therapy after TE consisted mainly of LMWH (in total, N = 130) as monotherapy (N = 111), preceded by tissue plasminogen activator/vitamin K antagonists/unfractionated heparin in 7 patients for a maximum of 4 days, combined with tissue plasminogen activator/vitamin K antagonists/direct oral anticoagulants/AT substitution in 9 patients, or followed by direct oral anticoagulants in 3 patients. The remaining patients received unfractionated heparin (N = 2) or no anticoagulation treatment (N = 5); the latter included TE diagnosed at autopsy (N = 3), removal of CVL (N = 1), and high risk of bleeding during treatment with extracorporal membrane oxygenation (thrombocyte count, 13 × 109/L) because of multiple PEs (N = 1). Major bleeding complications during antithrombotic therapy occurred in 3% (4/137) and included intraperitoneal hemorrhage during unfractionated heparin (N = 1), hemothorax during tissue plasminogen activator combined with LMWH (N = 1), intracranial hemorrhage during LMWH (N = 1), and unknown type of major bleeding during LMWH (N = 1). No death attributable to major bleeding was identified. Minor bleeding complications occurred in 3% (4/137) during LMWH treatment. A second TE event was seen in 5% of the patients with TE (7/137), with a median age of 12 years (IQR, 7-27 years; range, 2-45 years) and occurred 10 to 143 days after diagnosis of the first TE (median, 62 days; IQR, 20-91 days), despite ongoing LMWH treatment in 6 patients (86%). PegASP was truncated in 3 of these 7 patients after the first TE event.
Mortality and relapse
Death occurred in 67 (including 12 patients with TE) of 1772 patients and was directly attributable to TE in 5 of these cases; PE (respiratory compromise, N = 1), CSVT (cerebral herniation/intracerebral hemorrhage, N = 3), and infradiaphragmatic TE (total liver failure, N = 1). When analyzing time to death, the hazard of death was significantly increased in younger patients with TE compared with younger patients without TE (the size of the effect varied with onset of TE, here shown at 80 days after ALL diagnosis); ages 1.0 to 9.9 years (HRa, 10.1; 95% CI, 4.05-25.3; P < .0001) and 10.0 to 17.9 years (HRa, 4.51; 95% CI, 1.39-14.7; P = .01). No difference in hazard of death was seen when comparing patients aged at least 18 years with or without TE (HRa, 1.1; 95% CI, 0.2-5.0; P = .9). Among the patients with TE, no difference in the hazard of death was found when comparing patients diagnosed with PE, CSVT, supradiaphragmatic TE, infradiaphragmatic TE, and TE at multiple sites. Leukemic relapse in CR1 occurred in 134 of 1772 patients during the study period, including 7 TE patients (all ≥18 years), among whom PegASP was truncated in 2 patients. The hazard of relapse did not differ significantly between patients aged 18 years or older with or without TE (HRa, 0.8; 95% CI, 0.4-1.9; P = .6).
Stratifying the abovementioned models by treatment group on day 29 and adjusting for sex left the results unchanged.
Discussion
This is the first study to demonstrate a markedly increased TE risk among adolescents and adults with ALL treated according to the same protocol as children. The primary causes of this age-related TE risk remain uncertain, but may involve age-related decline in anticoagulant and fibrinolytic parameters42 that provides a prothrombotic state likely reflecting the changes in endogenous sex hormones. Sex hormones play a pivotal role in the modulation of insulin resistance and (pre-)metabolic syndrome, which increases in frequency during puberty43 and has been correlated with prothrombotic factors, impaired fibrinolytic capacity, and reduced antioxidative defense.44
The presence of a mediastinal mass (18% of the patients with TE) at ALL diagnosis was associated with a 2-fold increased hazard of TE. Although the presence of enlarged lymph nodes at ALL diagnosis (16% of the patients with TE) was not found to be significant in the multiple Cox model, in our absolute risk model, both the presence of mediastinal mass and enlarged lymph nodes significantly increased the TE risk, indicating an individual contribution to the TE risk. Nonetheless, these data support the fact that developing TE could be an effect of leukemia burden, including mechanical compression of vessels predisposing to venous TE, compatible with nearly all TE events being of venous origin (92%), in line with previous studies.11,12,15,23,26
Not surprisingly, the majority of TE events occurred during treatment phases containing PegASP, with the highest frequency found among SR/IR patients during consolidation phases. This contrasts with previous studies that report more than 90% of TE events occurring during induction,12,16,26,29 and potentially implicates ASP usage during induction therapy. However, we found no significant difference in incidence rate of TE during vs before PegASP treatment. Yet, we did not include the 2 doses of PegASP at weeks 99 to 101 in HR DI in the incidence rate ratio calculation, but this would not influence the results to any relevant degree. Moreover, importantly, lack of access to the PegASP randomization data (to be published later) posed a great limitation. The ALL2008 protocol calls for PegASP administration at the end of induction, when patients are tapering off corticosteroids, and concomitant administration of corticosteroids and PegASP is also part of SR/IR DI-1/consolidation-2 and maintenance-1. This has previously been reported to increase TE rates,16 as observed during consolidations phases in ALL2008 SR/IR patients. The use of dexamethasone instead of prednisolone during induction has been reported to significantly reduce TE onset.45 However, we were unable to confirm this in the multiple Cox analysis (induction dexamethasone versus prednisolone, P = .6).
TE occurrence altered the subsequently scheduled ALL treatment by truncating PegASP administration in 38 of 128 patients. Importantly, previous studies have linked PegASP discontinuation with inferior survival.46,47 However, we found no association between TE development and the hazard of relapse. In contrast, hazard of death was significantly increased (≥5-fold) in younger patients with TE compared with younger patients without TE.
Apart from older age, chemotherapy, and malignancy itself, other risk factors for venous TE include CVLs, infections, immobilization, oral contraceptives, and inherited thrombophilia traits.12,13,18,48 Indwelling CVLs were present in the majority of supradiaphragmatic TE events. Infection was reported in 42% of patients at the time of TE, in whom 32% showed positive blood cultures, which supports the existing evidence of infection-induced activation of coagulation.49,50 Inherited thrombophilia was only investigated in a limited number of patients with TE and was positive in 11%, which corresponds to the reported incidence among healthy individuals.51 However, as systematic screening of inherited thrombophilia was only performed in a subset of TE patients, the prevalence may be underestimated.
The main strengths of this study are the population-based design, including the use of the same protocol across a broad age range, combined with systematic, prospective toxicity registration with an almost 100% compliance rate, thus providing a unique platform for exploration of TE. The limitations include lack of data on TE-specific risk factors for patients without TE. In addition, laboratory analyses at the time of TE were performed at multiple centers across countries in a nonstandardized manner. Moreover, randomization of PegASP administration was nonuniformly managed between pediatric and adult departments. Not least, subgroup analyses of the different TE types were limited and resulted in wide CIs because of the small number of events in each subgroup, and as such, some of the results should be interpreted with caution. Finally, no TE screening was performed; hence, asymptomatic TE cases were detected by chance and may be underestimated.
In summary, we found that patients aged at least 10 years at ALL diagnosis constitute a group at high risk for TE development during chemotherapy including ASP, and patients younger than 18 years have an increased hazard of dying when developing TE. The predictive abilities of the TE risk factors age 10 years or older, presence of enlarged lymph nodes, and mediastinal mass remain to be validated in another cohort, and these patient subsets may then be candidates for future randomized trials of preemptive antithrombotic prophylaxis.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank all patients involved in the study and their colleagues at the ALL centers for reporting data to the NOPHO ALL registry and completing the TE registration forms.
This work was supported by research grants from the Research Foundation of Rigshospitalet (University of Copenhagen; C.U.R.), King Christian the 10th Foundation (C.U.R.), the Danish Acute Leukemia Group (C.U.R.), Krista and Viggo Petersen’s Foundation (C.U.R.), the Danish Childhood Cancer Foundation (K. Schmiegelow), and the Danish Cancer Society (K. Schmiegelow).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.U.R. designed the study, collected, analyzed, and interpreted data, and wrote and edited the manuscript; K. Schmiegelow served as principal investigator for NOPHO ALL2008, designed the study, interpreted data, and critically reviewed the manuscript; N.T. served as an adult investigator, designed the study, interpreted data, and critically reviewed the manuscript; R.T. served as a childhood investigator, designed the study, collected data, interpreted data, and critically reviewed the manuscript; K.G. interpreted data and critically reviewed the statistical analyses and manuscript; O.J.N. designed the study, collected data, interpreted data, and critically reviewed the manuscript; T.L.F. served as a childhood investigator, designed the study, collected data, supervised toxicity reporting, and edited the manuscript; H.V.H.M. served as investigator of MRD analyses, collected data, and edited the manuscript; B.K.A., U.T., E.R., K.B.J., P.H., Ó.G.J., S.S.T., and K. Saks served as childhood investigators, designed the study, collected data, and edited the manuscript; H.H., P.Q.-P., U.W.-K., L.G., and M.P. served as adult investigators, designed the study, collected data, and edited the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kjeld Schmiegelow, Department of Pediatrics and Adolescent Medicine, Rigshospitalet, University of Copenhagen, Blegdamsvej 9, 2100 Copenhagen, Denmark; e-mail: kjeld.schmiegelow@regionh.dk.
References
- 1.Redaelli A, Laskin BL, Stephens JM, Botteman MF, Pashos CL. A systematic literature review of the clinical and epidemiological burden of acute lymphoblastic leukaemia (ALL). Eur J Cancer Care (Engl). 2005;14(1):53-62. [DOI] [PubMed] [Google Scholar]
- 2.Hallböök H, Gustafsson G, Smedmyr B, Söderhäll S, Heyman M; Swedish Adult Acute Lymphocytic Leukemia Group; Swedish Childhood Leukemia Group . Treatment outcome in young adults and children >10 years of age with acute lymphoblastic leukemia in Sweden: a comparison between a pediatric protocol and an adult protocol. Cancer. 2006;107(7):1551-1561. [DOI] [PubMed] [Google Scholar]
- 3.Storring JM, Minden MD, Kao S, et al. . Treatment of adults with BCR-ABL negative acute lymphoblastic leukaemia with a modified paediatric regimen. Br J Haematol. 2009;146(1):76-85. [DOI] [PubMed] [Google Scholar]
- 4.Schmiegelow K, Forestier E, Hellebostad M, et al. ; Nordic Society of Paediatric Haematology and Oncology . Long-term results of NOPHO ALL-92 and ALL-2000 studies of childhood acute lymphoblastic leukemia. Leukemia. 2010;24(2):345-354. [DOI] [PubMed] [Google Scholar]
- 5.Toft N, Schmiegelow K, Klausen TW, Birgens H. Adult acute lymphoblastic leukaemia in Denmark. A national population-based retrospective study on acute lymphoblastic leukaemia in Denmark 1998-2008. Br J Haematol. 2012;157(1):97-104. [DOI] [PubMed] [Google Scholar]
- 6.Pui CH, Pei D, Campana D, et al. . A revised definition for cure of childhood acute lymphoblastic leukemia. Leukemia. 2014;28(12):2336-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pui CH, Yang JJ, Hunger SP, et al. . Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015;33(27):2938-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeAngelo DJ, Stevenson KE, Dahlberg SE, et al. . Long-term outcome of a pediatric-inspired regimen used for adults aged 18-50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2015;29(3):526-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toft N, Birgens H, Abrahamsson J, et al. . Results of NOPHO ALL2008 treatment for patients aged 1-45 years with acute lymphoblastic leukemia. Leukemia. 2018;32(3):606-615. [DOI] [PubMed] [Google Scholar]
- 10.Gugliotta L, Mazzucconi MG, Leone G, et al. ; The GIMEMA Group . Incidence of thrombotic complications in adult patients with acute lymphoblastic leukaemia receiving L-asparaginase during induction therapy: a retrospective study. Eur J Haematol. 1992;49(2):63-66. [DOI] [PubMed] [Google Scholar]
- 11.Athale UH, Chan AK. Thrombosis in children with acute lymphoblastic leukemia: part I. Epidemiology of thrombosis in children with acute lymphoblastic leukemia. Thromb Res. 2003;111(3):125-131. [DOI] [PubMed] [Google Scholar]
- 12.Payne JH, Vora AJ. Thrombosis and acute lymphoblastic leukaemia. Br J Haematol. 2007;138(4):430-445. [DOI] [PubMed] [Google Scholar]
- 13.Ku GH, White RH, Chew HK, Harvey DJ, Zhou H, Wun T. Venous thromboembolism in patients with acute leukemia: incidence, risk factors, and effect on survival. Blood. 2009;113(17):3911-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lund B, Åsberg A, Heyman M, et al. ; Nordic Society of Paediatric Haematology and Oncology . Risk factors for treatment related mortality in childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer. 2011;56(4):551-559. [DOI] [PubMed] [Google Scholar]
- 15.Tuckuviene R, Ranta S, Albertsen BK, et al. . Prospective study of thromboembolism in 1038 children with acute lymphoblastic leukemia: a Nordic Society of Pediatric Hematology and Oncology (NOPHO) study. J Thromb Haemost. 2016;14(3):485-494. [DOI] [PubMed] [Google Scholar]
- 16.Caruso V, Iacoviello L, Di Castelnuovo A, et al. . Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood. 2006;108(7):2216-2222. [DOI] [PubMed] [Google Scholar]
- 17.Grace RF, Dahlberg SE, Neuberg D, et al. . The frequency and management of asparaginase-related thrombosis in paediatric and adult patients with acute lymphoblastic leukaemia treated on Dana-Farber Cancer Institute consortium protocols. Br J Haematol. 2011;152(4):452-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Truelove E, Fielding AK, Hunt BJ. The coagulopathy and thrombotic risk associated with L-asparaginase treatment in adults with acute lymphoblastic leukaemia. Leukemia. 2013;27(3):553-559. [DOI] [PubMed] [Google Scholar]
- 19.Toft N, Birgens H, Abrahamsson J, et al. . Toxicity profile and treatment delays in NOPHO ALL2008-comparing adults and children with Philadelphia chromosome-negative acute lymphoblastic leukemia. Eur J Haematol. 2016;96(2):160-169. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell LG, Andrew M, Hanna K, et al. ; Prophylactic Antithrombin Replacement in Kids with Acute Lymphoblastic Leukemia Treated with Asparaginase Group (PARKAA) . A prospective cohort study determining the prevalence of thrombotic events in children with acute lymphoblastic leukemia and a central venous line who are treated with L-asparaginase: results of the Prophylactic Antithrombin Replacement in Kids with Acute Lymphoblastic Leukemia Treated with Asparaginase (PARKAA) Study. Cancer. 2003;97(2):508-516. [DOI] [PubMed] [Google Scholar]
- 21.Farinasso L, Bertorello N, Garbarini L, et al. . Risk factors of central venous lines-related thrombosis in children with acute lymphoblastic leukemia during induction therapy: a prospective study. Leukemia. 2007;21(3):552-556. [DOI] [PubMed] [Google Scholar]
- 22.Ziegler S, Sperr WR, Knöbl P, et al. . Symptomatic venous thromboembolism in acute leukemia. Incidence, risk factors, and impact on prognosis. Thromb Res. 2005;115(1-2):59-64. [DOI] [PubMed] [Google Scholar]
- 23.De Stefano V, Sorà F, Rossi E, et al. . The risk of thrombosis in patients with acute leukemia: occurrence of thrombosis at diagnosis and during treatment. J Thromb Haemost. 2005;3(9):1985-1992. [DOI] [PubMed] [Google Scholar]
- 24.Elliott MA, Wolf RC, Hook CC, et al. . Thromboembolism in adults with acute lymphoblastic leukemia during induction with L-asparaginase-containing multi-agent regimens: incidence, risk factors, and possible role of antithrombin. Leuk Lymphoma. 2004;45(8):1545-1549. [DOI] [PubMed] [Google Scholar]
- 25.Mohren M, Markmann I, Jentsch-Ullrich K, Koenigsmann M, Lutze G, Franke A. Increased risk of venous thromboembolism in patients with acute leukaemia. Br J Cancer. 2006;94(2):200-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caruso V, Iacoviello L, Di Castelnuovo A, Storti S, Donati MB. Venous thrombotic complications in adults undergoing induction treatment for acute lymphoblastic leukemia: results from a meta-analysis. J Thromb Haemost. 2007;5(3):621-623. [DOI] [PubMed] [Google Scholar]
- 27.Hunault-Berger M, Chevallier P, Delain M, et al. ; GOELAMS (Groupe Ouest-Est des Leucémies Aiguës et Maladies du Sang) . Changes in antithrombin and fibrinogen levels during induction chemotherapy with L-asparaginase in adult patients with acute lymphoblastic leukemia or lymphoblastic lymphoma. Use of supportive coagulation therapy and clinical outcome: the CAPELAL study. Haematologica. 2008;93(10):1488-1494. [DOI] [PubMed] [Google Scholar]
- 28.Grace RF, DeAngelo DJ, Stevenson KE, et al. . The use of prophylactic anticoagulation during induction and consolidation chemotherapy in adults with acute lymphoblastic leukemia. J Thromb Thrombolysis. 2018;45(2):306-314. [DOI] [PubMed] [Google Scholar]
- 29.Christ TN, Stock W, Knoebel RW. Incidence of asparaginase-related hepatotoxicity, pancreatitis, and thrombotic events in adults with acute lymphoblastic leukemia treated with a pediatric-inspired regimen [published online ahead of print 29 March 2017]. J Oncol Pharm Pract. doi:10.1177/1078155217701291. [DOI] [PubMed]
- 30.Leone G, Gugliotta L, Mazzucconi MG, et al. . Evidence of a hypercoagulable state in patients with acute lymphoblastic leukemia treated with low dose of E. coli L-asparaginase: a GIMEMA study. Thromb Haemost. 1993;69(1):12-15. [PubMed] [Google Scholar]
- 31.Mitchell L, Hoogendoorn H, Giles AR, Vegh P, Andrew M. Increased endogenous thrombin generation in children with acute lymphoblastic leukemia: risk of thrombotic complications in L’Asparaginase-induced antithrombin III deficiency. Blood. 1994;83(2):386-391. [PubMed] [Google Scholar]
- 32.van Zaane B, Nur E, Squizzato A, et al. . Systematic review on the effect of glucocorticoid use on procoagulant, anti-coagulant and fibrinolytic factors. J Thromb Haemost. 2010;8(11):2483-2493. [DOI] [PubMed] [Google Scholar]
- 33.Frandsen TL, Heyman M, Abrahamsson J, et al. . Complying with the European Clinical Trials directive while surviving the administrative pressure - an alternative approach to toxicity registration in a cancer trial. Eur J Cancer. 2014;50(2):251-259. [DOI] [PubMed] [Google Scholar]
- 34.Toft N, Birgens H, Abrahamsson J, et al. . Risk group assignment differs for children and adults 1-45 yr with acute lymphoblastic leukemia treated by the NOPHO ALL-2008 protocol. Eur J Haematol. 2013;90(5):404-412. [DOI] [PubMed] [Google Scholar]
- 35.Albertsen BK, Abrahamsson J, Lund B, et al. . Intermittent vs continuous asparaginase to reduce asparaginase-associated toxicities: a NOPHO ALL2008 randomized study. [abstract]. Blood. 2017;130(suppl 1). Abstract 1275. [DOI] [PubMed] [Google Scholar]
- 36.Biss TT, Payne JH, Hough RE, et al. . Strategies to prevent and manage thrombotic complications of acute lymphoblastic leukemia in children and young people vary between centers in the United Kingdom. J Pediatr Hematol Oncol. 2016;38(3):221-226. [DOI] [PubMed] [Google Scholar]
- 37.Schmiegelow K, Attarbaschi A, Barzilai S, et al. ; Ponte di Legno toxicity working group . Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. Lancet Oncol. 2016;17(6):e231-e239. [DOI] [PubMed] [Google Scholar]
- 38.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. [DOI] [PubMed] [Google Scholar]
- 39.Tram Henriksen L, Gottschalk Højfeldt S, Schmiegelow K, et al. ; Nordic Society of Pediatric Hematology and Oncology, NOPHO Group . Prolonged first-line PEG-asparaginase treatment in pediatric acute lymphoblastic leukemia in the NOPHO ALL2008 protocol-Pharmacokinetics and antibody formation. Pediatr Blood Cancer. 2017;64(12):e26686. [DOI] [PubMed] [Google Scholar]
- 40.Nysom K, Molgaard C, Hutchings B, Michaelsen KF. Body mass index of 0 to 45-y-old Danes: reference values and comparison with published European reference values. Int J Obes Relat Metab Disord. 2001;25(2):177-184. [DOI] [PubMed] [Google Scholar]
- 41.Gerds TA, Scheike TH, Andersen PK. Absolute risk regression for competing risks: interpretation, link functions, and prediction. Stat Med. 2012;31(29):3921-3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Appel IM, Hop WC, van Kessel-Bakvis C, Stigter R, Pieters R. L-Asparaginase and the effect of age on coagulation and fibrinolysis in childhood acute lymphoblastic leukemia. Thromb Haemost. 2008;100(2):330-337. [PubMed] [Google Scholar]
- 43.Agirbasli M, Agaoglu NB, Orak N, et al. . Sex hormones and metabolic syndrome in children and adolescents. Metabolism. 2009;58(9):1256-1262. [DOI] [PubMed] [Google Scholar]
- 44.Dimitrijevic-Sreckovic V, Colak E, Djordjevic P, et al. . Prothrombogenic factors and reduced antioxidative defense in children and adolescents with pre-metabolic and metabolic syndrome. Clin Chem Lab Med. 2007;45(9):1140-1144. [DOI] [PubMed] [Google Scholar]
- 45.Nowak-Göttl U, Ahlke E, Fleischhack G, et al. . Thromboembolic events in children with acute lymphoblastic leukemia (BFM protocols): prednisone versus dexamethasone administration. Blood. 2003;101(7):2529-2533. [DOI] [PubMed] [Google Scholar]
- 46.Silverman LB, Gelber RD, Dalton VK, et al. . Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97(5):1211-1218. [DOI] [PubMed] [Google Scholar]
- 47.Pullarkat V, Martinez D, Ji L, Douer D. The number of peg-asparaginase doses administered is a determinant of relapse risk in adult ALL treated with a pediatric-like regimen. Blood. 2013;122(21):3915. [Google Scholar]
- 48.Roininen S, Laine O, Kauppila M, et al. . A minor role of asparaginase in predisposing to cerebral venous thromboses in adult acute lymphoblastic leukemia patients. Cancer Med. 2017;6(6):1275-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hack CE. Tissue factor pathway of coagulation in sepsis. Crit Care Med. 2000;28(9 Suppl):S25-S30. [DOI] [PubMed] [Google Scholar]
- 50.Mateos MK, Trahair TN, Mayoh C, et al. . Clinical Predictors of Venous Thromboembolism during Therapy for Childhood Acute Lymphoblastic Leukemia [abstract]. Blood. 2016;128(22). Abstract 1182. [Google Scholar]
- 51.Zöller B, García de Frutos P, Hillarp A, Dahlbäck B. Thrombophilia as a multigenic disease. Haematologica. 1999;84(1):59-70. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.