Abstract
Arsenic trioxide (As2O3) is an environmental carcinogen and a putative endocrine disruptor. Resveratrol has been shown to reverse As2O3-induced oxidative damage. In immortalized but nontransformed estrogen receptor α-negative human breast cells (MCF10A), we observed that 25 μM resveratrol ameliorated As2O3-induced cytotoxicity. As2O3, in the presence or absence of 25 μM resveratrol, induced quinone reductase (NAD(P)H quinone dehydrogenase 1), via the induction of NFE2-related factor 2. As2O3 caused a repression of cytochrome P450 (CYP)1B1, but the addition of 25 μM resveratrol rescued the expression of cytochrome P450 1B1 and kept it at a constant level. Therefore, 25 μM resveratrol can modulate the effects of As2O3 on enzymes involved in estrogen metabolism.
Introduction
Arsenic is the 20th most abundant element in the earth’s crust, and trivalent arsenic trioxide (As2O3) contaminates groundwater in many places, leading to arsenic poisoning or arsenicosis.1 Arsenic and inorganic arsenic compounds have been classified as IARC Group 1 carcinogens,2 with lung, bladder, kidney, and liver as the predominant targets. At the same time, As2O3 (Trisenox or arsenic trioxide) is being used as a chemotherapy drug against acute promyelocytic leukemia (APL), where it selectively kills the leukemic cells but allows the proper development of regular blood cells.3 Interestingly, arsenic has long been implicated in endocrine disruption. Target genes for glucocorticoids, androgens, mineralocorticoids, and progestin receptors have also been reported to be regulated by inorganic arsenic in a biphasic dose-response fashion.4 To address such documented carcinogenicity and putative endocrine disruption, the U.S. Environmental Protection Agency has set the maximum contaminant level for As2O3 at 10 ppb.5
The principal pathways implicated in As2O3 toxicity result from reactive oxygen species, oxidative DNA damage, and induction of apoptosis. Resveratrol, a well-known dietary stilbene, has been shown to protect normal human bronchial epithelial cells from As2O3 toxicity by maintaining glutathione homeostasis.6 Cardiotoxicity, a major side effect of using As2O3 for APL, could be ameliorated in Wistar rats by resveratrol, via the maintenance of a balanced expression of the NFE2-related factor 2 (Nrf2)-heme oxygenase (HO) 1 pathway, and by promoting arsenic efflux from cells.7 Employing similar mechanisms, resveratrol has been reported to protect from As2O3-induced nephrotoxicity in male Wistar rats8 and from hepatotoxicity in Chinese Dragon-Li cats.9 Finally, research by at least one group has demonstrated the ability of inorganic arsenic to promote carcinogenesis via a nonestrogen receptor (ER)-mediated pathway: chronic exposure (18 weeks) to environmentally relevant 0.5 μM arsenite promoted cancer cell phenotypes in human prostate epithelial stem/progenitor cells (WPE-stem)10 and in their isogenic parental RWPE-1 cells (30 weeks).11 Chronic exposure (24 weeks) to low-level arsenite (500 nM) has also been reported to do the same thing in human breast epithelial cells (MCF10A) via the overexpression of aromatase.12 To the best of our knowledge, no studies have explored any protective role of resveratrol in As2O3-induced carcinogenesis via non-ER-mediated pathways.
Estrogens have been implicated in the development of a variety of cancers. Several types of evidence suggest the role of estrogen in tumor development in an ER knock-out transgenic mouse model of breast cancer,13 as well as in the transformation of ERα-negative breast epithelial cells (MCF10F).14 To explain such receptor-independent pathways for cancer initiation, it has been hypothesized that the metabolism of estrogens/androgens generates catechol quinones that can react with DNA, leading to the formation of apurinic sites and mutations, to initiate oncogenic transformation.15 In high-risk groups and cancer patients (women: breast, ovarian, and thyroid; men: prostate and non-Hodgkin lymphoma), an imbalance in estrogen metabolism stems from irregular expression patterns of one or more of the four key estrogen-metabolizing enzymes: cytochrome P450 19 or aromatase, cytochrome P450 1B1 (CYP1B1), catechol-O-methyltransferase, and quinone reductase (NAD(P)H quinone dehydrogenase 1 (NQO1)).15 Certain naturally occurring or synthetic compounds have the ability to alter the expression of some of these enzymes; for example, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) can induce CYP1B1, whereas resveratrol antagonizes this effect. At the same time, resveratrol can induce NQO1.15 Our laboratory has shown that preincubation with 25 μM resveratrol for 48 h significantly lowered the production of estrogen-DNA adduct formation in ERα-negative human breast epithelial MCF10F cells.16 Therefore, in this study, the effects of As2O3 on the expression levels of key estrogen-metabolizing enzymes (NQO1 and CYP1B1), and on the signaling molecule Nrf2, were studied in the presence or absence of 25 μM resveratrol in immortalized but nontransformed ERα-negative human breast epithelial cells (MCF10A).
Results and Discussion
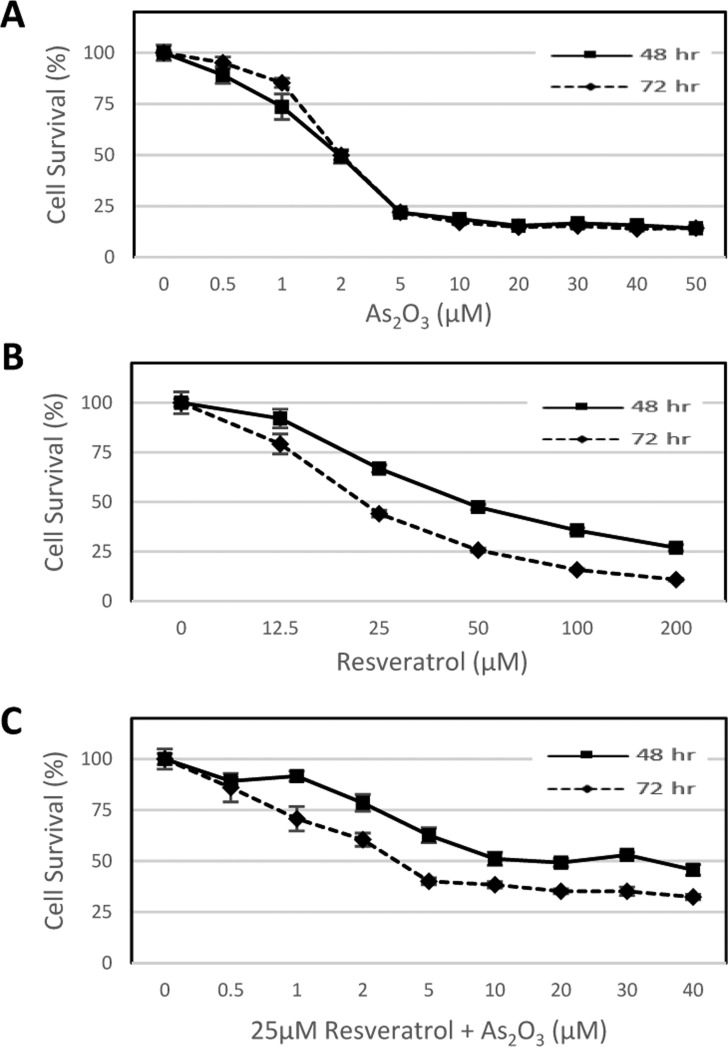
As2O3 exhibited cytotoxicity at both 48 and 72 h, with an IC50 at 1.45 μM for 48 h treatment (Figure 1A), which is an environmentally significant exposure. However, the presence of resveratrol, which also has some cytotoxicity (Figure 1B) at 25 μM, reduced this cytotoxicity by shifting the survival curves to the right. The IC50 for the combined 48 h As2O3 and resveratrol treatment was 28.69 μM. The rightward shift in the survival curve was lesser for 72 h treatment compared to that for 48 h treatment (Figure 1C). This indicates that the rescue effect from resveratrol is more pronounced at 48 h.
Figure 1.
Viability of MCF10A cells treated with As2O3 with and without resveratrol. (A) Arsenic trioxide (As2O3) alone. (B) Resveratrol alone. (C) Arsenic trioxide (As2O3) + 25 μM resveratrol. Data are presented as mean ± standard error of the mean cell survival (%) from six independent experiments (n = 6).
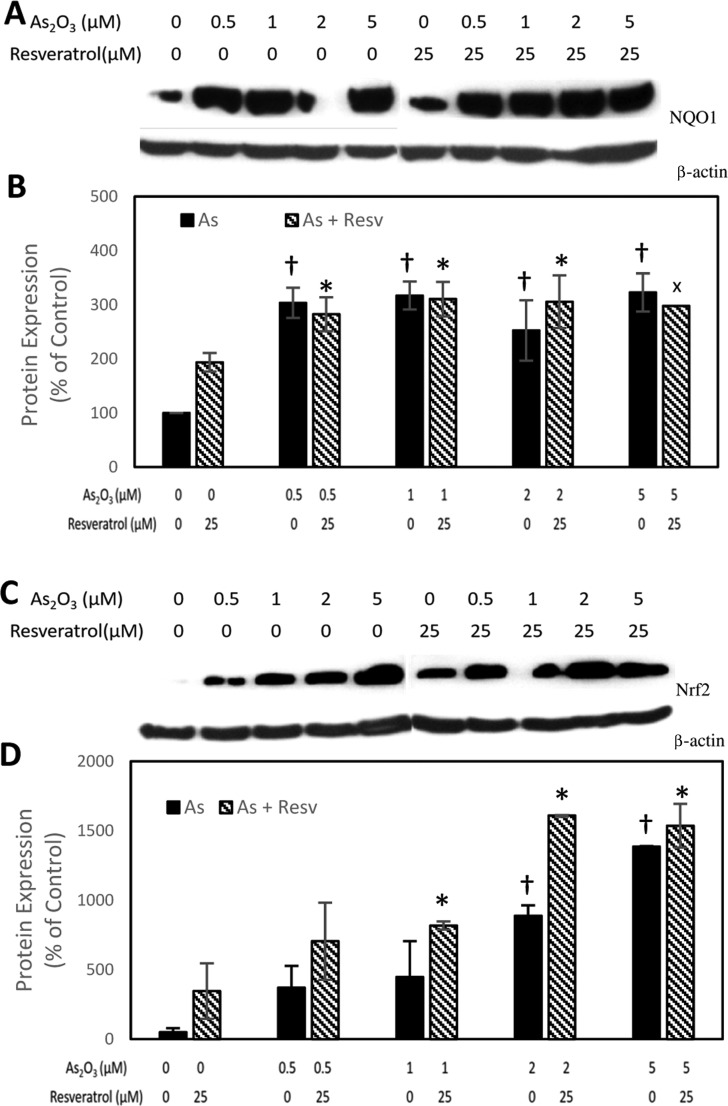
As2O3, with or without 25 μM resveratrol, induced the enzyme NQO1 in a dose-dependent manner, but no additive or synergistic effects were observed (Figure 2A,B). Resveratrol has been shown to act via the signaling molecule Nrf2, and NQO1 has been implicated as a downstream gene of Nrf2.17 This prompted us to study the effect of As2O3 on the expression level of Nrf2. As2O3, in both the presence and absence of 25 μM resveratrol, induced Nrf2 in a dose-dependent fashion, but again no additive or synergistic effects were observed (Figure 2C,D). Our finding is in accordance with a study in mouse hepatoma cell line hepa1c1c7, in which arsenic was shown to induce Nrf2 protein in a dose-dependent fashion, leading to a robust induction of NQO1.18 Furthermore, in MCF10A cells the extent of induction was much greater for Nrf2 compared to that for NQO1, but this may stem from the fact that resting cells have a very low-level basal expression of Nrf2 (t1/2 = 10–20 min).19 Similarly, disproportionate induction of NQO1 and Nrf2 by As2O3 has been reported in male Kunming mouse testis in both the presence and absence of antioxidants such as lutein.20
Figure 2.
Induction of NQO1 (A, B) and Nrf2 (C, D) by As2O3 with and without resveratrol. (A, C) Representative images. (B, D) Quantitative data. Data are presented as mean ± standard error of the mean protein expression (%) from three independent experiments (n = 3), X = mean of two experiments. A † indicates significant difference (p < 0.05) between As2O3 treatment groups and control, whereas an * indicates significant difference (p < 0.05) between As2O3 treatment groups and control in the resveratrol group.
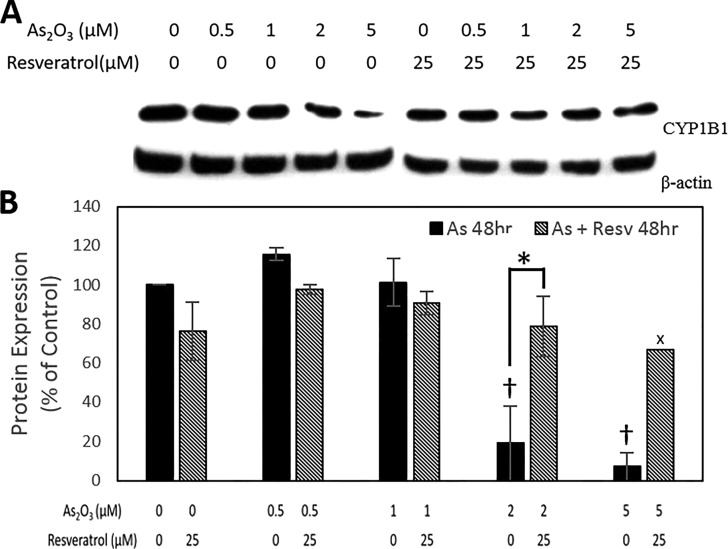
CYP1B1 is another key estrogen-metabolizing enzyme that is overexpressed in a variety of cancers, including breast cancer, and is subject to regulation through both hormonal and a putative aryl hydrocarbon receptor (AhR) pathways; the latter can be modulated by resveratrol.21 Previous studies on the male C57Bl/6 mouse heart have reported that 12.5 mg/kg As(III) induces CYP1B1 mRNA levels by 150%.22 We observed, however, that As2O3 treatment (0.5–5 μM) of human breast epithelial MCF10A cells caused a repression of CYP1B1 protein in a dose-dependent manner. Resveratrol was earlier shown to downregulate TCDD-induced CYP1B1 expression in MCF10F cells.23 Interestingly, the addition of 25 μM resveratrol rescued As2O3-suppressed CYP1B1 expression and kept it at a nearly constant level (Figure 3A,B). To confirm this interesting observation, the expression of CYP1B1 enzyme was further studied simultaneously in three different nontransformed cell lines derived from human breast epithelia, namely MCF10A, MCF10F, and MCF12F cells. In this study, the cells were also treated with a higher dose of 7.5 μM As2O3. The repressive effect of As2O3 on CYP1B1 expression was less pronounced in MCF10F cells, leaving little room for resveratrol to exert any rescue effect (Supporting Information, Figure S1B). Furthermore, such repressive effect of As2O3 on CYP1B1 expression was altogether absent in MCF12F cells (Supporting Information, Figure S1C). Collectively, these results suggest different degrees of modulatory effects of As2O3. One possibility is its differential cytotoxicity, as we have observed between MCF10A and MCF12F cells (IC50 value for MCF10A is 1.45 μM for 48 h treatment, whereas the same for MCF12F is 11.65 μM; Figure 1A vs Supporting Information Figure S2). As2O3 is not considered a ligand for AhR; therefore, the observed effects of As2O3 might be mediated through a hormonal pathway.
Figure 3.
Repression of CYP1B1 by As2O3 with and without resveratrol. (A) Representative image. (B) Quantitative data. Data are presented as mean ± standard error of the mean protein expression (%) from three independent experiments (n = 3), X = mean of two experiments. A † indicates significant difference (p < 0.05) between As2O3 treatment groups and control, whereas an * indicates significant difference (p < 0.05) between one particular As2O3 treatment with the inclusion or exclusion of resveratrol.
Conclusions
In the present study involving nontransformed breast epithelial MCF10A cells, 25 μM resveratrol was shown to ameliorate As2O3-induced cytotoxicity, with the beneficial effect being more prominent after 48 h treatment. As expected, both As2O3 and resveratrol induced the expression of the catechol quinone-quenching enzyme NQO1, possibly via the induction of the signaling protein Nrf2. To the best of our knowledge, however, this is the first time resveratrol has been shown to rescue As2O3-suppressed CYP1B1 expression back to the baseline levels. Collectively, these results suggest that 25 μM resveratrol has the ability to modulate the effects of As2O3 on the expression of estrogen-metabolizing enzymes in MCF10A cells. Whether such modulation leads to any potential benefit in estrogen metabolism and carcinogenesis needs to be addressed in future studies of the effects of As2O3 and resveratrol on estrogen metabolism.
Experimental Procedures
Cytotoxicity
Cells were seeded in a 96-well plate (seeding density: MCF10A 3000 cells per well, MCF12F 5000 cells per well) in estrogen- and phenol-red-indicator-free media and treated with 0.5–50 μM total concentration of As2O3 (purity > 99.5%; Sigma-Aldrich, St. Louis, MO), 12.5–200 μM total concentration of resveratrol (purity ≥ 98%; Cayman Chemical, Ann Arbor, MI), or 0.5–50 μM total concentration of As2O3 + 25 μM resveratrol for two time points (48 and 72 h). Finally, MTT (Calbiochem, San Diego, CA) was used to assess the cell viability.
Protein Expression Studies
Expression levels of the key estrogen-metabolizing enzymes NQO1 and CYP1B1 and of cell signaling protein Nrf2 were studied under different treatment regimens. Cells were seeded in a 6-well plate (seeding density: MCF10A and MCF10F 0.8 × 106 cells per well, MCF12F 1 × 106 cells per well) in estrogen- and phenol-red-indicator-free media and treated with 0.5–5 μM total concentration of As2O3 ± 25 μM resveratrol for 48 h. Following cell lysis with radioimmunoprecipitation assay buffer containing protease inhibitor cocktail (Roche Diagnostics, Basel, Switzerland), total protein was estimated by the Bradford assay, and 10–30 μg of total protein was resolved on a 15% polyacrylamide gel and transferred to poly(vinylidene difluoride) membranes. The membranes were probed with anti-NQO1, anti-Nrf2 (Abcam, Cambridge, MA), anti-CYP1B1 (LSBio, Seattle, WA), or anti-β-actin (Santa Cruz, Dallas, TX) primary antibodies overnight at 4 °C and secondary antibodies for 1 h at room temperature. Detection was performed with ECL Western Blotting Detection Reagents (GE Healthcare/Amersham, Little Chalfont, U.K.). Both the treatment and western blotting were performed three times to achieve statistical significance.
Statistical Analysis
To determine whether the differences observed were statistically significant, a 2-sample t-test was performed. α was set at 0.05 for all statistical tests, and data with p < 0.05 were considered to be significantly different.
Acknowledgments
We thank Drs. Rizwan Ahmed and Amar B. Singh for their assistance with cell counting and densitometry of Western Blots.
Glossary
ABBREVIATIONS
- As2O3
arsenic trioxide
- NQO1
NAD(P)H quinone dehydrogenase 1
- Nrf2
NFE2-related factor 2
- CYP1B1
cytochrome P450 1B1
- CYP19
cytochrome P450 19
- COMT
catechol-O-methyltransferase
- E2
estradiol
- 4-OHE2
4-hydroxyestradiol
- E2-3,4-Q
estradiol-3,4-quinone
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01727.
The expression of CYP1B1 by As2O3 with and without resveratrol in MCF10A, MCF10F, and MCF12F cells; the viability of MCF12F cells treated with As2O3 (PDF)
Author Contributions
The manuscript was written through contributions of all authors.
This project was supported by an internal grant of the University of Nebraska Medical Center, awarded and administered by the College of Public Health, UNMC, Omaha, NE. Core support at the Eppley Institute was provided by grant P30 CA36727 from the National Cancer Institute at the National Institutes of Health.
The authors declare no competing financial interest.
Supplementary Material
References
- Saha J. C.; Dikshit A. K.; Bandyopadhyay M.; Saha K. C. A review of arsenic poisoning and its effects on human health. Crit. Rev. Environ. Sci. Technol. 1999, 29, 281–313. 10.1080/10643389991259227. [DOI] [Google Scholar]
- Straif K.; Benbrahim-Tallaa L.; Baan R.; Grosse Y.; Secretan B.; El Ghissassi F.; Bouvard V.; Guha N.; Freeman C.; Galichet L.; Cogliano V. A review of human carcinogens—part C: metals, arsenic, dusts, and fibres. Lancet Oncol. 2009, 10, 453–454. 10.1016/S1470-2045(09)70134-2. [DOI] [PubMed] [Google Scholar]
- Soignet S. L.; Maslak P.; Wang Z. G.; Jhanwar S.; Calleja E.; Dardashti L. J.; Corso D.; DeBlasio A.; Gabrilove J.; Scheinberg D. A.; Pandolfi P. P. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N. Engl. J. Med. 1998, 339, 1341–1348. 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- Bodwell J. E.; Gosse J. A.; Nomikos A. P.; Hamilton J. W. Arsenic disruption of steroid receptor gene activation: complex dose – response effects are shared by several steroid receptors. Chem. Res. Toxicol. 2006, 19, 1619–1629. 10.1021/tx060122q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Environmental Protection Agency Office of Water (4607) The Technical Fact Sheet: Final Rule for Arsenic in Drinking Water; 815-F-00-016; EPA, 2001. [Google Scholar]
- Chen C.; Jiang X.; Lai Y.; Liu Y.; Zhang Z. Resveratrol protects against arsenic trioxide-induced oxidative damage through maintenance of glutathione homeostasis and inhibition of apoptotic progression. Environ. Mol. Mutagen. 2015, 56, 333–346. 10.1002/em.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.; Guo C.; Gao R.; Ge M.; Zhu Y.; Zhang Z. The protective role of resveratrol against arsenic trioxide-induced cardiotoxicity. J. Evidence-Based Complementary Altern. Med. 2013, 2013, 1–8. 10.1155/2013/407839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.; Liu Y.; Ge M.; Jing J.; Chen Y.; Jiang H.; Yu H.; Li N.; Zhang Z. Protective effect of resveratrol on arsenic trioxide-induced nephrotoxicity in rats. Nutr. Res. Pract. 2014, 8, 220–226. 10.4162/nrp.2014.8.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.; Gao L.; Cheng Y.; Jiang J.; Chen Y.; Jiang H.; Yu H.; Shan A.; Cheng B. Resveratrol, a natural antioxidant, has a protective effect on liver injury induced by inorganic arsenic exposure. BioMed Res. Int. 2014, 2014, 1–7. 10.1155/2014/617202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar E. J.; Diwan B. A.; Waalkes M. P. Arsenic exposure transforms human epithelial stem/progenitor cells into a cancer stem-like phenotype. Environ. Health Perspect. 2010, 118, 108. 10.1289/ehp.0901059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achanzar W. E.; Brambila E. M.; Diwan B. A.; Webber M. M.; Waalkes M. P. Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. J. Natl. Cancer Inst. 2002, 94, 1888–1891. 10.1093/jnci/94.24.1888. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Tokar E. J.; Waalkes M. P. Arsenic-induced cancer cell phenotype in human breast epithelia is estrogen receptor-independent but involves aromatase activation. Arch. Toxicol. 2014, 88, 263–274. 10.1007/s00204-013-1131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W.; Wang J. P.; Li Y.; Bocchinfuso W. P.; Korach K. S.; Devanesan P. D.; Rogan E.; Cavalieri E.; Santen R. J. Tamoxifen versus aromatase inhibitors for breast cancer prevention. Clin. Cancer Res. 2005, 11, 925s–930s. [PubMed] [Google Scholar]
- Russo J.; Lareef M. H.; Balogh G.; Guo S.; Russo I. H. Estrogen and its metabolites are carcinogenic agents in human breast epithelial cells. J. Steroid Biochem. Mol. Biol. 2003, 87, 1–25. 10.1016/S0960-0760(03)00390-X. [DOI] [PubMed] [Google Scholar]
- Cavalieri E.; Rogan E. The molecular etiology and prevention of estrogen-initiated cancers: Ockham’s Razor: Pluralitas non est ponenda sine necessitate. Plurality should not be posited without necessity. Mol. Aspects Med. 2014, 36, 1–55. 10.1016/j.mam.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahid M.; Gaikwad N. W.; Ali M. F.; Lu F.; Saeed M.; Yang L.; Rogan E. G.; Cavalieri E. L. Prevention of estrogen–DNA adduct formation in MCF-10F cells by resveratrol. Free Radical Biol. Med. 2008, 45, 136–145. 10.1016/j.freeradbiomed.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z.; Bagi Z.; Feher A.; Recchia F. A.; Sonntag W. E.; Pearson K.; De Cabo R.; Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. 2010, 299, H18–H24. 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X.; Chen M. G.; Lin G. X.; Ma Q. Arsenic induces NAD(P)H-quinone oxidoreductase I by disrupting the Nrf2· Keap1· Cul3 complex and recruiting Nrf2· Maf to the antioxidant response element enhancer. J. Biol. Chem. 2006, 281, 23620–23631. 10.1074/jbc.M604120200. [DOI] [PubMed] [Google Scholar]
- Itoh K.; Wakabayashi N.; Katoh Y.; Ishii T.; O’connor T.; Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells 2003, 8, 379–391. 10.1046/j.1365-2443.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- Li S. G.; Xu S. Z.; Niu Q.; Ding Y. S.; Pang L. J.; Ma R. L.; Jing M. X.; Wang K.; Ma X. M.; Feng G. L.; Liu J. M.; et al. Lutein alleviates arsenic-induced reproductive toxicity in male mice via Nrf2 signaling. Hum. Exp. Toxicol. 2016, 35, 491–500. 10.1177/0960327115595682. [DOI] [PubMed] [Google Scholar]
- Spink D. C.; Spink B. C.; Cao J. Q.; DePasquale J. A.; Pentecost B. T.; Fasco M. J.; Li Y.; Sutter T. R. Differential expression of CYP1A1 and CYP1B1 in human breast epithelial cells and breast tumor cells. Carcinogenesis 1998, 19, 291–298. 10.1093/carcin/19.2.291. [DOI] [PubMed] [Google Scholar]
- Anwar-Mohamed A.; El-Sherbeni A. A.; Kim S. H.; Althurwi H. N.; Zordoky B. N.; El-Kadi A. O. Acute arsenic toxicity alters cytochrome P450 and soluble epoxide hydrolase and their associated arachidonic acid metabolism in C57Bl/6 mouse heart. Xenobiotica 2012, 42, 1235–1247. 10.3109/00498254.2012.693971. [DOI] [PubMed] [Google Scholar]
- Lu F.; Zahid M.; Wang C.; Saeed M.; Cavalieri E. L.; Rogan E. G. Resveratrol prevents estrogen-DNA adduct formation and neoplastic transformation in MCF-10F cells. Cancer Prev. Res. 2008, 1, 135–145. 10.1158/1940-6207.CAPR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.