Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a naturally occurring cationic peptide with potent immunosuppressant and cytoprotective activities. We now show that full length PACAP38 and to a lesser extent, the truncated form PACAP27, and the closely related vasoactive intestinal peptide (VIP) and secretin had antimicrobial activity against the Gram-negative bacteria Escherichia coli in the radial diffusion assay. PACAP38 was more potent than either the bovine neutrophil antimicrobial peptide indolicidin or the synthetic antimicrobial peptide ARVA against E. coli. PACAP38 also had activity against the Gram-positive bacteria Staphylococcus aureus in the same assay with comparable potency to indolicidin and ARVA. In the more stringent broth dilution assay, PACAP38 had moderate sterilizing activity against E. coli, and potent sterilizing activity against the Gram-negative bacteria Pseudomonas aeruginosa. PACAP27, VIP and secretin were much less active than PACAP38 in this assay. PACAP38 also had some activity against the Gram-positive bacteria Bacillus cereus in the broth dilution assay. Many exopeptidase-resistant analogs of PACAP38, including both receptor agonists and antagonists, had antimicrobial activities equal to, or better than PACAP38, in both assays. PACAP38 made the membranes of E. coli permeable to SYTOX Green, suggesting a classical membrane lytic mechanism. These data suggest that analogs of PACPAP38 with a wide range of useful biological activities can be made by judicious substitutions in the sequence.
Keywords: Antibiotic Resistance, Antimicrobial Peptides, Innate Immunity, Pituitary Adenylate Cyclase-Activating Polypeptide, Vasoactive Intestinal Peptide
Introduction
Antimicrobial peptides (AMP) are an indispensable component of the innate immune system of multicellular animals and plants [1]. Small changes in the amino acid sequence of AMP can sometimes result in very large changes in activity against different microbial species. The clinical use of AMP has been proposed as a strategy to overcome the frequent resistance of many common microbes to conventional antibiotics [2]. The usefulness of AMP as drugs has usually been limited by residual toxicity, host cell inhibition [3], and their short half-life in circulation due to rapid proteolysis and filtration by the kidney. The few AMP that have been approved by the Food and Drug Administration (FDA) are only used for topical applications [2]. Therefore, there is a need for AMP analogs that have fewer impediments in order to effectively treat certain infections. AMP with other useful biological activities, especially potent immunosuppressive or cytoprotective activity, could have a wide range of medical applications [4,5].
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a member of the secretin/growth hormone-releasing hormone (GHRH)/vasoactive intestinal peptide (VIP) family, with potent anti-inflammatory and potent cytoprotective properties [6–13]. PACAP exists as two amidated peptides with 38 or 27 amino acids, differing in the C-terminus. PACAP27 is homologous to VIP and secretin. PACAP is most abundant in the brain, but there are significant levels in other organs, including the thymus, spleen, lymph nodes, and duodenal mucosa [10]. The usefulness of PACAP as a drug, however, is limited by its very short half-life in the circulation following systemic administration due, in part, to rapid proteolysis, especially rapid exopeptidase activity at the amino terminus by the enzyme DPP IV [14]. There is a need for analogs of PACAP38 with increased circulation half-life and altered receptor activity/selectivity, goals that we have approached with rational design of PACAP analogs [15].
PACAP38 has physicochemical properties that are similar to many natural and synthetic antimicrobial peptides (AMP). For example, PACAP38 carries a highly basic charge of +10 at neutral pH. It has a significant compliment of hydrophobic residues with a structurally amphipathic arrangement, both hallmarks of canonical AMP [16]. Furthermore, there is evidence that other peptide hormones also have antibacterial activity [17,18]. Thus, here we examined the antimicrobial activity of PACAP and some analogs. PACAP38 and many analogs have potent antimicrobial activity against a panel of Gram-positive and Gram-negative pathogens. Activity was comparable to that of known synthetic and natural AMPs. These observations, combined with the potent physiological effects of PACAP, make it an intriguing candidate for development into a treatment for antibiotic resistant infections.
Materials and Methods
Peptide Synthesis
All peptides were synthesized using FMOC chemistry, purified by HPLC and verified by MALDI mass spectrometry [19].
Proteolysis Assay
Each peptide for the proteolysis assay was incubated in phosphate buffer at a concentration of 50 µM peptide with DPP IV at a concentration of 0.5 mg/ml at 25°C. Proteolysis was monitored by high-performance liquid chromatography (HPLC) over the course of a 72-hour incubation. The amount of proteolysis at the specified time was calculated as the area under the curve of the peptide peak for the peptide incubated with DPP IV divided by the area under the curve of the peptide peak for the same peptide incubated without DPP IV.
Microbiological assays
Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (PA01, ATTC 47085) and Staphylococcus aureus (ATCC 25923) and Bacillus cereus were used in these assays. Broth dilution sterilization and radial diffusion assays were performed as described elsewhere [3]. The minimum inhibitory concentration was determined to be the lowest concentration at which a zone of inhibition can be observed (radial diffusion), or the lowest peptide concentration at which no bacterial proliferation was observed (broth dilution).
Red Blood Cell Hemolysis Assay
Fresh human O+ human red blood cells (RBC) were used in hemolysis assays. Lysis was measured by hemoglobin release as described in detail elsewhere [3,20].
Bacterial Membrane Disruption Assay
Peptides, at 20 µM were incubated with bacteria in the presence of 5 µM SYTOX Green. Entry of SYTOX Green was measured by fluorescence [20].
Statistical Analyses
All measurements of minimum sterilizing/inhibitory activity were performed as serial dilution experiments where the minimum effective concentration is noted. Experiments were repeated at least 3 time and the minimum concentrations were averaged. The results were expressed as the mean ± SD. Multiple comparisons were analyzed using a one-way ANOVA, with Tukey-Kramer post-hoc tests.
Results
Antimicrobial Activity
We performed two classical antibacterial assays for this work. The radial diffusion assay measures inhibition of bacterial growth in a zone around where an antibiotic has been applied to an agar gel. The minimum inhibitory concentration (MIC) in a radial diffusion experiment is the lowest concentration at which any inhibition is detectable. The broth dilution assay is a much more stringent, all-or-none sterilization assay. In the broth dilution assay only complete sterilization is noted as positive activity. The MIC for this assay is the lowest concentration that completely sterilizes bacteria in media.
All four of the naturally occurring peptides, PACAP38, PACAP27, VIP and secretin had antimicrobial activity against the Gram-negative bacteria E. coli in the radial diffusion assay, with PACAP38 having the highest potency, i.e. the lowest minimum inhibitory concentration (MIC) (Table 1). Similarly, PACAP38 has potent sterilizing activity against P. aeruginosa in the more stringent broth dilution assay. PACAP38 also had antimicrobial activity against the Gram-positive bacteria S. aureus in the radial diffusion (Table 1). PACAP27, VIP and secretin did not have any detectable effect against S. aureus in either assay (Table 1). El Karim et al. [17] have also reported that VIP had activity against E. coli, but not against S. aureus.
TABLE 1.
Antimicrobial activity of some members of the secretin/vasoactive intestinal peptide family
| Peptide | EC Radial Diffusion |
SA Radial Diffusion |
EC Broth |
PA Broth |
SA Broth |
BC Broth |
|---|---|---|---|---|---|---|
| PACAP38 | 0.76 | 3.54 | 28.3 | 1.2 | >30 | 23.3 |
| PACAP27 | 4.40** | N/A*** | >30 | 23.6 | >30 | >30 |
| VIP | 6.90*** | N/A*** | >30 | 30.0 | >30 | >30 |
| Secretin | 14.75*** | N/A*** | >30 | 30.0 | >30 | >30 |
| ARVA | 1.57 | 3.46 | 2.3 | 4.3 | 5.1 | 6.4 |
| indolicidin | 2.78 | 3.66 | 6.24 | >50 | 7.9 | 10.7 |
For comparison, we measured, in parallel, the activity the bovine neutrophil AMP indolicidin [21,22] and the potent broad spectrum synthetic AMP ARVA (Arg-Arg-Gly-Trp-Ala-Leu-Arg-Leu-Val-Leu-Ala-Tyr-NH2; [20,23]. These two peptides have activities that are very typical for the most active families of AMPs [3]. In the radial diffusion assays PACAP38 was more potent than either of these two control AMPs against E. coli and had very similar MIC against S. aureus. In the broth dilution assay, PACAP38 is much better than the controls against P. aeruginisa.
In table 2 we show the antimicrobial activities of PACAP and VIP analogs that have agonist activity [19,24]. In Table 3 we show data for analogs that have antagonist activity [25,26], and in Table 4 we show data for the subset of analogs that have pipecolic acid (piperidine-2-carboxylic acid) or isonepecotic acid (piperidine-4-carboxylic acid) in position 3 [19]. Most of the 16 full length analogs studied had good antimicrobial activity against E. coli in the radial diffusion assay, Similarly, all of the full length PACAP38 analogs, except for [Lys38-palmitoyl]PACAP38, had antimicrobial activity against the Gram-positive bacterium S. aureus in the radial diffusion assay.
TABLE 2.
Antimicrobial activity of some PACAP/VIP receptor agonists
| Peptide | EC Radial Diffusion |
SA Radial Diffusion |
EC Broth |
PA Broth |
SA Broth |
BC Broth |
|---|---|---|---|---|---|---|
| PACAP38 | 0.76 | 3.54 | 28.3 | 1.2 | >30 | 23.3 |
| [D-Ser2]PACAP38 | 0.41 | 1.30 | >30 | 6.1 | >30 | 13.6 |
| [Lys38-palmitoyl]PACAP38 | N/A*** | N/A*** | 9.1 | 6.1 | >30 | 4.9 |
| [Iaa1]PACAP38 | 2.11 | 24.75*** | >30 | 1.8 | >30 | 4.8 |
| [Iac1]PACAP38 | 0.95 | 1.89 | 16.6 | 2.7 | >30 | 20.4 |
| [Iac1,Nal6]PACAP38 | 1.93 | 7.04 | 16.3 | 0.8 | >30 | 5.9 |
| [Pro3]PACAP38 | 1.46 | 23.03*** | >30 | 1.8 | >30 | 8.9 |
| [Ala22]PACAP38 | 2.01 | 17.37*** | 2.15 | 0.52 | >30 | 4.84 |
| [Iaa1,D-Ser2]PACAP38 | 1.65 | 9.72 | >30 | 1.17 | >30 | >30 |
| [Iaa1,D-Ser2,Ala16,17,22,D-Lys38]PACAP38 | 1.26 | 7.47 | >30 | 1.17 | >30 | >30 |
| [Aib16,28,Ala17,Lys34,D-Lys38]PACAP38 | 0.72 | 4.62 | 7.3 | 1.2 | >30 | 13.3 |
| PACAP27 | 4.40** | N/A*** | >30 | 23.6 | >30 | >30 |
| VIP | 6.90*** | N/A*** | >30 | 30.0 | >30 | >30 |
| [Ala2,8,9,16,19,20,21,24,25]VIP | 12.28*** | N/A*** | >30 | 5.9 | >30 | >30 |
| ARVA (control AMP) | 1.57 | 3.46 | 2.3 | 4.3 | 5.1 | 6.4 |
TABLE 3.
Antimicrobial activity of some PACAP/VIP receptor antagonists
| Peptide | EC Radial Diffusion |
SA Radial Diffusion |
EC Broth |
PA Broth |
SA Broth |
BC Broth |
|---|---|---|---|---|---|---|
| PACAP38 | 0.76 | 3.54 | 28.3 | 1.2 | >30 | 23.3 |
| [Sar4]PACAP38 | 0.91 | 8.95 | 24.5 | 1.2 | >30 | 20.0 |
| PACAP(6–38) | 1.36 | 1.81 | >30 | 30.0 | >30 | >30 |
TABLE 4.
Antimicrobial activity of PACAP38 analogs
| Peptide | EC Radial Diffusion |
SA Radial Diffusion |
EC Broth |
PA Broth |
SA Broth |
BC Broth |
|---|---|---|---|---|---|---|
| PACAP38 | 0.76 | 3.54 | 28.3 | 1.2 | >30 | 23.3 |
| [Pip3]PACAP38 | 1.01 | 2.70 | >30 | 8.9 | >30 | 8.9 |
| [N-acetyl-His1,Pip3]PACAP38 | 1.03 | 1.45 | >30 | 20.0 | >30 | 7.3 |
| [Ini3,Aib16,28,Ala17,Lys34,D-Lys38]PACAP38 | 1.69 | 6.11 | 24.5 | 0.96 | >30 | >30 |
| [Pip3,Aib16,28,Ala17,Lys34,D-Lys38]PACAP38 | 1.13 | 5.52 | 2.15 | 0.52 | >30 | 3.95 |
| [Pip3,Aib16,28,Ala17,21,Lys34,D-Lys38]PACAP38 | 1.48 | 4.97 | 1.43 | 0.64 | >30 | 7.26 |
To better assess sequence-structure-activity relationships, we used ANOVA on each radial diffusion column to determine which of the variants studied had statistically significant differences from PACAP38 in the radial diffusion assays. In each Table, variants with activity that is significantly different from PACAP38 are marked with asterisks indicating the significance level. Unmarked variants were not significantly different (p > 0.05).
In the broth dilution assay, all of the full length analogs have good activity against P. aeruginosa, similar to the activity of PACAP38 (Table 2). Further, some had improved activity against other microbes in the broth dilution assay against which PACAP38 has poor activity. For example four of the full length analogs have MIC < 10 µM against E. coli in the broth dilution assay, and seven have MIC < 10 µM against B. cereus. Sterilization of S. aureus in the broth dilution assay is especially challenging for AMPs [3], as shown here by the observation that only ARVA had a measurable MIC against S. aureus in the broth dilution assay. Despite the lack of sterilization, PACAP38 and many of its analogs have inhibitory activity against S. aureus in radial diffusion.
Exopeptidase Resistance
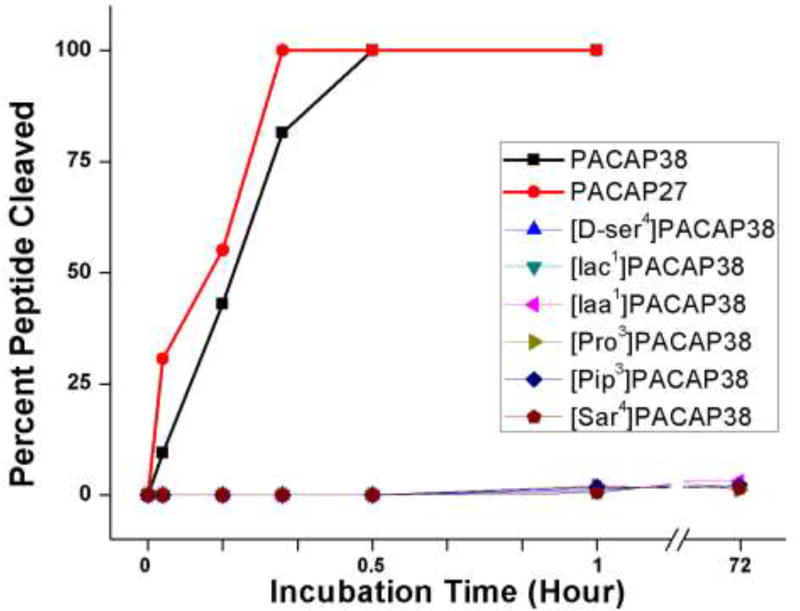
PACAP38 is thought to be rapidly cleaved and inactivated in vivo by the aminodipeptidase DPP IV [27,28]. PACAP analogs with non-natural amino acids near the N-terminus have been assumed to be resistant to DPP IV. Here we tested that assumption directly with purified DPP IV enzyme using PACAP38 analogs with non-natural amino acids in positions one, three or four. PACAP38 and PACAP27 were almost completely cleaved by DPP IV in less than 30 minutes (Fig. 1). On the other hand, there was no indication that any analog tested was cleaved at all by DPP IV even after 72 hours. The susceptibility of the analogs to DPP IV is negligible on any reasonable pharmacological or biological timescale.
Fig. 1.
The proteolysis of PACAP27, PACAP38 and PACAP38 analogs by dipeptidyl peptidase IV. Each peptide was incubated in phosphate buffer (pH 7.4) at a concentration of 50 µM with DPP IV at a concentration of 0.5 mg/ml at 22°C. Proteolysis was monitored by HPLC over the course of the 72-hour incubation. The lines for all of the PACAP analogs are overlapping. Iaa, imidazole-4-acetic acid; Iac, imidazole-4-acrylic acid; Pip, pipecolic acid; Sar, sarcosine.
Hemolysis
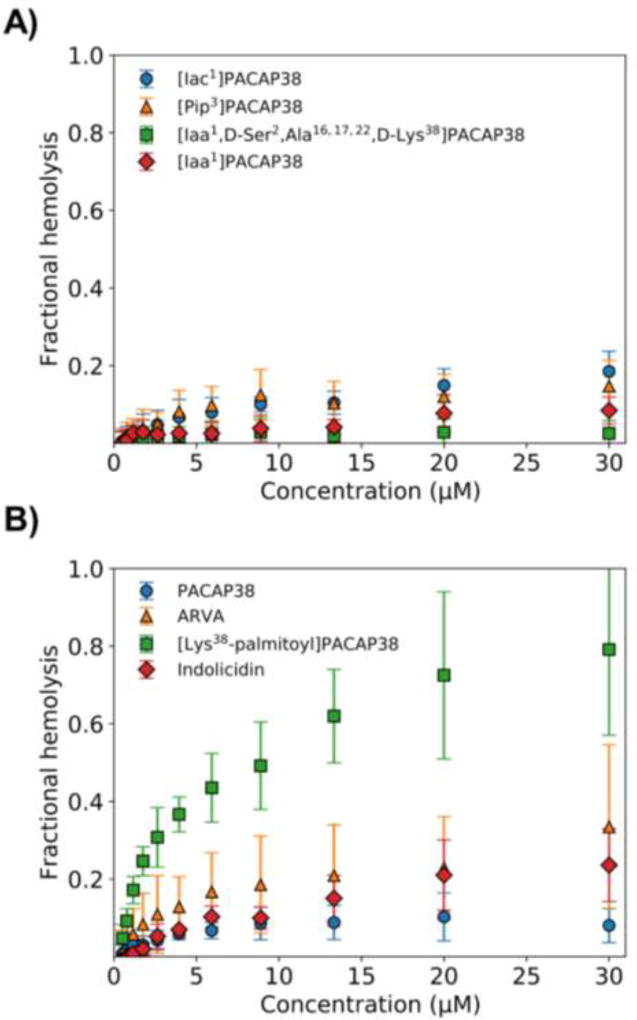
Hemolysis of red blood cells (RBC) has long been used as an index of toxicity of AMP against mammalian cells [29]. Here we measured the effect of PACAP38, some analogs, and control AMP, against human RBCs. PACAP38 was less toxic toward RBC than either the naturally occurring bovine neutrophil AMP indolicidin or the synthetic AMP ARVA (Fig. 2). Similarly, all four of the exopeptidase-resistant PACAP38 analogs tested (Fig. 1) were less toxic toward RBC than either indolicidin or ARVA (Fig. 2). The only exception was the analog of PACAP38 with a palmitate attached to the N-terminal lysine. This analog had dramatically increased toxicity toward RBC.
Fig. 2.
The lysis of human red blood cells by PACAP38 and PACAP38 analogs. Serial dilutions of each peptide were incubated with human RBC in PBS (pH 7.4) for one hour at 37°C and then the cells were spun down at 2000 rpm for five minutes. The optical density of the supernatant was measured at 410 nm to determine the amount of hemoglobin released. Iaa, imidazole-4-acetic acid; Iac, imidazole-4-acrylic acid; Pip, pipecolic acid.
Membrane Permeabilization
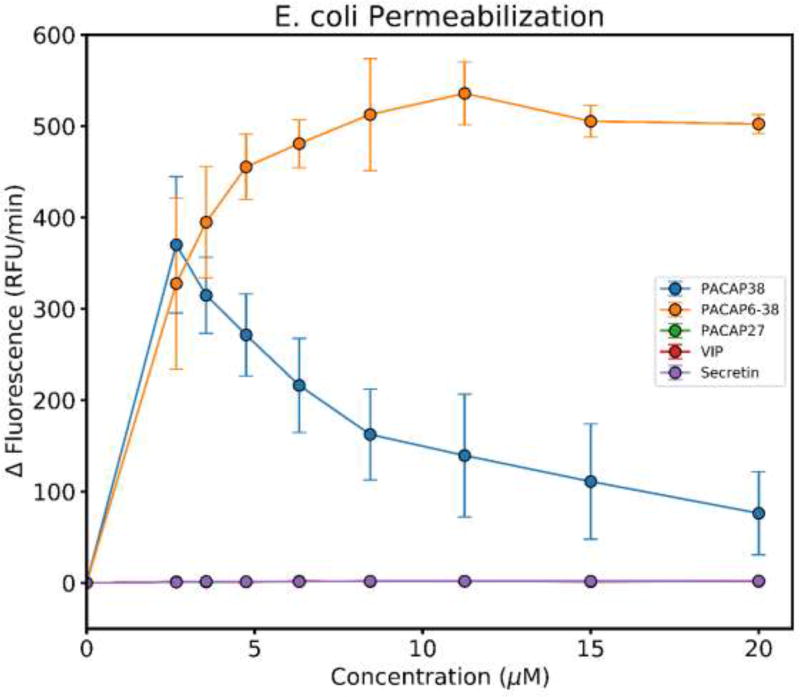
Many synthetic and natural AMP sterilize bacteria by permeabilization of the bacterial cytoplasmic membrane [1]. Therefore, we tested PACAP38 and some analogs for membrane permeabilizing activity. PACAP38 and PACAP(6–38) made the membranes of E. coli permeable to SYTOX Green at a low concentration, consistent with this classical AMP mechanism [20,23]. The decrease of SYTOX Green fluorescence rate at high PACAP38 concentration is likely due to competition for DNA binding sites between the cationic peptide and cationic SYTOX Green. Interestingly, the shorter analogs PACAP27, VIP and secretin did not enable any entry of SYTOX Green suggesting differences in the size of the membrane defects, or a different mechanism of action altogether at the concentrations tested (Fig. 3),
Fig. 3.
Disruption of bacterial cell membranes by PACAP38 and PACAP38 analogs. The fluorescence of the membrane impermeable, cationic DNA-binding dye, SYTOX Green was measured immediately after the addition of each peptide and then after 30 minutes. The change in fluorescence was plotted as ΔRFU/min for each concentration of each peptide. The decrease in the rate of SYTOX Green fluorescence increase at high PACAP concentrations is probably due to electrostatic competition between the cationic peptide and SYTOX Green for DNA binding sites. The rate of fluorescence change for PACAP27, VIP and secretin are all exactly zero, such that the points overlay.
Discussion
We show here that PACAP38 and many full length, exopeptidase-resistant analogs, both agonists and antagonists, have potent antibacterial activity that is mechanistically similar to the action of other natural and synthetic AMP on the bacterial membranes. At the same time they have low toxicity against human RBCs. The potency and species selectivity of the antimicrobial activity of PACAP analogs can be changed independently of the receptor-dependent biological activity profile. Therefore, we conclude that PACAP38 analogs with a wide range of useful biological activities can be made by judicious substitutions to the PACAP38 template.
Structure-Activity Relationships
The structural diversity of AMP are very large, but all structural classes share a positive net charge and some amphipathicity which enable them to bind to and permeabilize anionic bacterial membranes [1,16]. The observations that PACAP38 and PACAP(6–38) make bacterial membranes permeable to SYTOX Green indicates that classical membrane permeabilization is the basis for their bactericidal activity, and that of all full-length analogs. PACAP38 is, in fact, both cationic and amphipathic. The first ~20 residues comprise a sequence with a high propensity to fold into an amphipathic α-helix when bound to membranes. In solution, residues 7–23 are α-helical [30,31]. These N-terminal 20 residues have a net charge of +5. The next 6 residues are strictly hydrophobic and provide amphipathicity along the sequence. The C-terminal 12 residues of PACAP38 are less amphipathic, but are highly cationic and contribute an additional charge of +6. The fact that PACAP27 and PACAP(6–38) have lower antimicrobial activity than PACAP38 in most assays indicates that both the central amphipathicity and terminal charges are needed for full activity. Similarly, the weaker activity, and lack of membrane permeabilization, for secretin and VIP (which are homologous to PACAP27) supports this conclusion. The changes to the N-terminal amino acids of PACAP38 that enable exopeptidase resistance cause generally small changes in antimicrobial activity, often improving it (See Tables 2–4). This is consistent with the structure-function relationship just described because these changes neither reduce positive charge nor decrease amphipathicity significantly.
Comparative Localization of AMP and PACAP38
The idea that PACAP38 evolved to have anti-infective activity in addition to its other activities is supported by the observation that PACAP38 also killed the bloodstream (infective) form of the protozoan parasite Trypanosoma brucei [32]. PACAP(6–38) was also effective against the infective form of T. brucei. However, the procyclic (noninfective) form of T. brucei was resistant to PACAP38. Furthermore, PACAP38 has been found in tissues and fluids that are known to contain high concentrations of other AMP. For example, PACAP38 has been found in the epithelial surface of the nose, tongue, larynx, and trachea [33]. PACAP38 has also been found in human breast milk [34] and tears [35]. Whether PACAP38 interacts synergistically with any of the AMP in any of these tissues or fluids (e.g.,[36]) is currently unknown. PACAP and its analogs have an additional advantage over other AMP in that they are transported across the blood brain barrier [37].
Development of Clinical Applications
PACAP38 has been administered to healthy human volunteers by investigators in at least six academic laboratories in the European Union [38–45] and to a patient with multiple myeloma in the U.S. under a FDA-approved single-patient protocol [46] without any indication of serious side-effects. We expect that the PACAP38 analogs described above would have a similar high safety profiles and could be developed into therapeutics, including the example conditions described below.
Inflammation contributes significantly to the pathogenesis of many medical disorders. Immunosuppressive agents, such as corticosteroids, are among the most widely used drugs in modern medicine. However, the commonly used immunosuppressive agents also increase the risk of infection. PACAP38 and analogs with agonist activity have potent receptor-mediated immunosuppressive activity [6]. Unlike other immunosuppressive agents, PACAP38 and its analogs also have intrinsic broad-spectrum antibiotic activity which could offset some of the risk of infection caused by their receptor-mediated immunosuppressive activity and thus could possibly be developed into monotherapeutics and/or adjunctive therapeutics for a wide range of medical disorders [15]. Furthermore, PACAP38 and PACAP38 analogs with agonist activities have potent wound-healing properties [47,48], which may also act synergistically with antimicrobial activity.
The potent antimicrobial activity of PACAP38 agonists against P. aeruginosa in combination with its receptor-dependent effects could also be beneficial in the treatment of cystic fibrosis. P. aeruginosa is the most common pathogen in the lung of patients with cystic fibrosis and is the dominant cause of mortality [49]. PACAP has already been shown to have anti-inflammatory [8,50], antifibrotic [51], and bronchodilator [52] activities. Activation of the VPAC1 receptor by PACAP38 has been shown to stimulate the anion channel activity of the cystic fibrosis transmembrane conductance regulator (CFTR) in human epithelial cells [53] and to increase the insertion of functional ΔF508 CFTR protein into the apical membrane of human epithelial cells [54,55]. As a final example of the possibilities, we note that stimulation of mucus secretion from goblet cells via activation of VPAC2 receptors [56,57] may be dramatically reduced by replacing Tyr in position 22 of PACAP with Ala or Aib [19,58,59] and Table 2.
Highlights.
PACAP38 has broad spectrum antimicrobial activity
Full length agonist and antagonist variants retain antimicrobial activity
Receptor activation and antimicrobial activity can be independently manipulated
Antimicrobial activity results from bacterial membrane permeabilization
Acknowledgments
FUNDING SUPPORT
This work was supported in part by grants from the National Institutes of Health (Grants AI 119104 and GM 111824 to W.C.W.); and the Akira Arimura Foundation (to J.L.M.).
ABBREVIATIONS
- AMP
antimicrobial peptide
- ARVA
Arg-Arg-Gly-Trp-Ala-Leu-Arg-Leu-Val-Leu-Ala-Tyr-NH2
- CFTR
cystic fibrosis transmembrane conductance regulator
- CFU
colony-forming unit
- CNS
central nervous system
- DPP IV
dipeptidyl peptidase IV
- FDA
Food and Drug Administration
- GHRH
growth hormone-releasing hormone
- HPLC
high-performance liquid chromatography
- MRSA
methicillin-resistant Staphylococcus aureus
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PBS
phosphate-buffered saline
- RBC
red blood cell
- TSB
trypticase soy broth
- VIP
vasoactive intestinal peptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORSHIP CONTRIBUTIONS CGS and JH designed and performed experiments. DHC synthesized and purified the peptides. CGS, WCW and JM analyzed data. JM, WCW and CGS wrote the manuscript.
Values listed are minimum inhibitory concentration (MIC) in units of µM peptide. Each value is the mean of at least three independent measurements. Most standard deviations are 10–40% of the mean, with an average of ~30%. “>30” means that MIC is greater than 30 µM in the broth dilution assay. N/A means that no activity was observed in radial diffusion with 20 µM peptide. EC: E. coli, SA: S. aureus, PA: p. aeruginosa, BC: B. cereus. When compared to PACAP38 by ANOVA, the natural PACAP38 analogs have lower activity in radial diffusion (**, p<0.01, ***, p<0.001).
Values listed are minimum inhibitory concentration (MIC) in units of µM peptide. Each value is the mean of at least three independent measurements. Most standard deviations are 10–40% of the mean, with an average of ~30%. “>30” means that MIC is greater than 30 µM in the broth dilution assay. N/A means that no activity was observed in radial diffusion with 20 µM peptide. EC: E. coli, SA: S. aureus, PA: p. aeruginosa, BC: B. cereus. Aib, α-aminoisobutyric acid; Iaa, imidazole-4-acetic acid; Iac, imidazole-4-acrylic acid; Nal, naphthylalanine; When compared to PACAP38 by ANOVA, the analogs marked with * have significantly lower activity in radial diffusion (**, p<0.01, ***, p<0.001).
Values listed are minimum inhibitory concentration (MIC) in units of µM peptide. Each value is the mean of at least three independent measurements. Most standard deviations are 10–40% of the mean, with an average of ~30%. “>30” means that MIC is greater than 30 µM in the broth dilution assay. N/A means that no activity was observed in radial diffusion with 20 µM peptide. EC: E. coli, SA: S. aureus, PA: p. aeruginosa, BC: B. cereus. Sar, sarcosine. When compared to PACAP38 by ANOVA, none of these analogs had significantly different activity in radial diffusion against either organism.
Values listed are minimum inhibitory concentration (MIC) in units of µM peptide. Each value is the mean of at least three independent measurements. Most standard deviations are 10–40% of the mean, with an average of ~30%. “>30” means that MIC is greater than 30 µM in the broth dilution assay. N/A means that no activity was observed in radial diffusion with 20 µM peptide. EC: E. coli, SA: S. aureus, PA: p. aeruginosa, BC: B. cereus. Ini, isonipecotic acid; Pip, pipecolic acid. When compared to PACAP38 by ANOVA, none of these analogs had significantly different activity in radial diffusion against either organism.
DISCLOSURES
DHC and JLM are co-inventors on multiple World (PCT) patents about the properties and uses of PACAP and PACAP analogs. All of these patents have been assigned to the Administrators of the Tulane Educational Fund.
References
- 1.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 2.Baltzer SA, Brown MH. Antimicrobial peptides: promising alternatives to conventional antibiotics. J Mol Microbiol Biotechnol. 2011;20:228–235. doi: 10.1159/000331009. [DOI] [PubMed] [Google Scholar]
- 3.Starr CG, He J, Wimley WC. Host cell interactions are a significant barrier to the clinical utility of peptide antibiotics. ACS Chem Biol. 2016;11:3391–3399. doi: 10.1021/acschembio.6b00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandenburg K, Heinbockel L, Correa W, Lohner K. Peptides with dual mode of action: Killing bacteria and preventing endotoxin-induced sepsis. Biochim Biophys Acta. 2016;1858:971–979. doi: 10.1016/j.bbamem.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Hancock RE, Haney EF, Gill EE. The immunology of host defense peptides: beyond antimicrobial activity. Nat Rev Immunol. 2016;16:321–334. doi: 10.1038/nri.2016.29. [DOI] [PubMed] [Google Scholar]
- 6.Delgado M, Martinez C, Pozo D, Calvo JR, Leceta J, Ganea D, Gomariz RP. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activation polypeptide (PACAP) protect mice from lethal endotoxemia through the inhibition of TNF-α and IL-6. J Immunol. 1999;162:1200–1205. [PubMed] [Google Scholar]
- 7.Kong L-Y, Maderdrut JL, Jeohn G-H, Hong J-S. Reduction of lipopolysaccharide-induced neurotoxicity in mixed cortical neuron/glia cultures by femtomolar concentrations of pituitary adenylate cyclase-activating polypeptide. Neuroscience. 1999;91:493–500. doi: 10.1016/s0306-4522(98)00606-x. [DOI] [PubMed] [Google Scholar]
- 8.Ganea D, Delgado M. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) as modulators of both innate and adaptive immunity. Crit Rev Oral Biol Med. 2002;13:229–237. doi: 10.1177/154411130201300303. [DOI] [PubMed] [Google Scholar]
- 9.Arimura A, Li M, Batuman V. Potential protective action of pituitary adenylate cyclase-activating polypeptide (PACAP-38) on in vitro and in vivo models of myeloma kidney injury. Blood. 2006;107:661–668. doi: 10.1182/blood-2005-03-1186. [DOI] [PubMed] [Google Scholar]
- 10.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BCK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Maderdrut JL, Lertora JJL, Arimura A, Batuman V. Renoprotection by pituitary adenylate cyclase-activating polypeptide in multiple myeloma and other kidney diseases. Regul Pept. 2008;145:24–32. doi: 10.1016/j.regpep.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Khan A-M, Maderdrut JL, Li M, Toliver HL, Coy DH, Simon EE, Batuman V. Pituitary adenylate cyclase-activating polypeptide prevents contrast-induced nephropathy in a novel mouse model. Physiol Rep. 2013;1:e00163, 1–14. doi: 10.1002/phy2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waschek JA. VIP and PACAP: neuropeptide modulators of CNS inflammation, injury, and repair. Br J Pharmacol. 2013;169:512–523. doi: 10.1111/bph.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green BD, Irwin N, Flatt PR. Pituitary adenylate cyclase-activating peptide (PACAP): assessment of dipeptidyl peptidase IV degradation, insulin-releasing activity and antidiabetic potential. Peptides. 2006;27:1349–1358. doi: 10.1016/j.peptides.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Coy DH, Maderdrut JL. PCT/US2014/041597. New Analogs of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) and Methods for their Use. 2014:84.
- 16.Wimley WC. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chemical Biology. 2010;5:905–17. doi: 10.1021/cb1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Karim IA, Linden GJ, Orr DF, Lundy FT. Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J Neuroimmunol. 2008;200:11–16. doi: 10.1016/j.jneuroim.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Singh J, Joshi S, Mumtaz S, Maurya N, Ghosh I, Khanna S, Natarajan VT, Mukhopadhyay K. Enhanced Cationic Charge is a Key Factor in Promoting Staphylocidal Activity of α-Melanocyte Stimulating Hormone via Selective Lipid Affinity. Scientific Reports. 2016;6:31492. doi: 10.1038/srep31492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramos-Álvarez I, Mantey SA, Nakamura T, Nuche-Berenguer B, Moreno P, Moody TW, Maderdrut JL, Coy DH, Jensen RT. A structure-function study of PACAP using conformationally-restricted analogs: identification of PAC1 receptor-selective PACAP agonists. Peptides. 2015;66:26–42. doi: 10.1016/j.peptides.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rathinakumar R, Wimley WC. High-throughput discovery of broad-spectrum peptide antibiotics. FASEB J. 2010;24:3232–3238. doi: 10.1096/fj.10-157040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selsted ME, Novotny MJ, Morris WL WL, Tang YQ, Smith W, Cullor JS. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J Biol Chem. 1992;267:4292–4295. [PubMed] [Google Scholar]
- 22.Staubitz P, Peschel A, Nieuwenhuizen WF, Otto M, Götz F, Jung G, Jack RW. Structure-function relationships in the tryptophan-rich, antimicrobial peptide indolicidin. J Pept Sci. 2001;7:552–564. doi: 10.1002/psc.351. [DOI] [PubMed] [Google Scholar]
- 23.Rathinakumar R, Walkenhorst WF, Wimley WC. Broad-spectrum antimicrobial peptides by rational combinatorial design and high-throughput screening: The importance of interfacial activity. J Am Chem Soc. 2009;131:7609–7617. doi: 10.1021/ja8093247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Igarashi H, Ito T, Mantey SA, Pradhan TK, Hou W, Coy DH, Jensen RT. Development of simplified VIP analogs with receptor selectivity and stability for human VPAC receptors. J Pharmacol Exp Ther. 2005;315:370–381. doi: 10.1124/jpet.105.088823. [DOI] [PubMed] [Google Scholar]
- 25.Robberecht P, Gourlet P, De Neef P, Woussen-Colle MC, Vandermeers-Piret MC, Vandermeers A, Christophe J. Structural requirements for the occupancy of pituitary adenylate-cyclase-activating-peptide (PACAP) receptors and adenylate cyclase activation in human neuroblastoma NB-OK-1 cell membranes. Discovery of PACAP(6–38) as a potent antagonist. Eur J Biochem. 1992;207:239–246. doi: 10.1111/j.1432-1033.1992.tb17043.x. [DOI] [PubMed] [Google Scholar]
- 26.Bourgault S, Vaudry D, Ségalas-Milazzo I, Guilhaudis L, Couvineau A, Laburthe M, Vaudry H, Fournier A. Molecular and conformational determinants of pituitary adenylate cyclase-activating polypeptide (PACAP) for activation of the PAC1 receptor. J Med Chem. 2009;52:3308–3316. doi: 10.1021/jm900291j. [DOI] [PubMed] [Google Scholar]
- 27.Ahrén B, Hughes TE. Inhibition of dipeptidyl peptidase-4 augments insulin secretion in response to exogenously administered glucagon-like peptide-1, glucose-dependent insulinotropic polypeptide, pituitary adenylate cyclase-activating polypeptide, and gastrin-releasing peptide in mice. Endocrinology. 2005;146:2055–2059. doi: 10.1210/en.2004-1174. [DOI] [PubMed] [Google Scholar]
- 28.Zhu L, Tamvakopoulos C, Xie D, Dragovic J, Shen X, Fenyk-Melody JE, Schmidt K, Bagchi A, Griffin PR, Thornberry NA, Sinha-Roy R R. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: in vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1–38) J Biol Chem. 2003;278:22418–22423. doi: 10.1074/jbc.M212355200. [DOI] [PubMed] [Google Scholar]
- 29.Matsuzaki K, Sugishita K, Fujii N, Miyajima K. Molecular basis for membrane selectivity of an antimicrobial peptide, magainin 2. Biochemistry. 1995;34:3423–3429. doi: 10.1021/bi00010a034. [DOI] [PubMed] [Google Scholar]
- 30.Laburthe M, Couvineau A, Tan V. Class II G protein-coupled receptors for VIP and PACAP: structure, models of activation and pharmacology. Peptides. 2007;28:1631–1639. doi: 10.1016/j.peptides.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 31.Hollenstein K, de Graaf C, Bortolato A, Wang MW, Marshall FH, Stevens RC. Insights into the structure of class B GPCRs. Trends Pharmacol Sci. 2014;35:12–22. doi: 10.1016/j.tips.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Rey E, Chorny A, Delgado M. VIP: an agent with license to kill infective parasites. Ann N Y Acad Sci. 2006;1070:303–308. doi: 10.1196/annals.1317.032. [DOI] [PubMed] [Google Scholar]
- 33.Moller K, Zhang YZ, Håkanson R, Luts A, Sjölund B, Uddman R, Sundler F. Pituitary adenylate cyclase activating peptide is a sensory neuropeptide: immunocytochemical and immunochemical evidence. Neuroscience. 1993;57:725–732. doi: 10.1016/0306-4522(93)90018-b. [DOI] [PubMed] [Google Scholar]
- 34.Borzsei R, Mark L, Tamas A, Bagoly T, Bay C, Csanaky K, Banki E, Kiss P, Vaczy A, Horvath G, Nemeth J, Szauer F, Helyes Z, Reglodi D. Presence of pituitary adenylate cyclase activating polypeptide-38 in human plasma and milk. Eur J Endocrinol. 2009;160:561–565. doi: 10.1530/EJE-08-0911. [DOI] [PubMed] [Google Scholar]
- 35.Nakamachi T, Ohtaki H, Seki T, Yofu S, Kagami N, Hashimoto H, Shintani N, Baba A, Mark L, Lanekoff I, Kiss P, Farkas J, Reglodi D, Shioda S. PACAP suppresses dry eye signs by stimulating tear secretion. Nat Commun. 2016;7:12034. doi: 10.1038/ncomms12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zerweck J, Strandberg E, Bürck J, Reichert J, Wadhwani P, Kukharenko O, Ulrich AS. Homo- and heteromeric interaction strengths of the synergistic antimicrobial peptides PGLa and magainin 2 in membranes. Eur Biophys J. 2016;45:535–547. doi: 10.1007/s00249-016-1120-7. [DOI] [PubMed] [Google Scholar]
- 37.Banks WA, Kastin AJ, Komaki G, Arimura A. Passage of pituitary adenylate cyclase activating polypeptide1–27 and pituitary adenylate cyclase activating polypeptide1–38 across the blood-brain barrier. J Pharmacol Exp Ther. 1993;267:690–696. [PubMed] [Google Scholar]
- 38.Warren JB, Cockcroft JR, Larkin SW, Kajekar R, Macrae A, Ghatei MA, Bloom SR. Pituitary adenylate cyclase activating polypeptide is a potent vasodilator in humans. J Cardiovasc Pharmacol. 1992;20:83–87. [PubMed] [Google Scholar]
- 39.Hammond PJ, Talbot K, Chapman R, Ghatei MA, Bloom SR. Vasoactive intestinal peptide, but not pituitary adenylate cyclase-activating peptide, modulates the responsiveness of the gonadotroph to LHRH in man. J Endocrinol. 1993;137:529–532. doi: 10.1677/joe.0.1370529. [DOI] [PubMed] [Google Scholar]
- 40.Chiodera P, Volpi R, Capretti L, Coiro V. Effects of intravenously infused pituitary adenylate cyclase-activating polypeptide on arginine vasopressin and oxytocin secretion in man. Neuroreport. 1995;6:1490–1492. doi: 10.1097/00001756-199507310-00006. [DOI] [PubMed] [Google Scholar]
- 41.Chiodera P, Volpi R, Capretti L, Caffarri G, Magotti MG, Coiro V. Effects of intravenously infused pituitary adenylate cyclase-activating polypeptide on adenohypophyseal hormone secretion in normal men. Neuroendocrinology. 1996;64:242–246. doi: 10.1159/000127124. [DOI] [PubMed] [Google Scholar]
- 42.Filipsson K, Tornøe K, Holst J, Ahrén B. Pituitary adenylate cyclase-activating polypeptide stimulates insulin and glucagon secretion in humans. J Clin Endocrinol Metab. 1997;82:3093–3098. doi: 10.1210/jcem.82.9.4230. [DOI] [PubMed] [Google Scholar]
- 43.Birk S, Sitarz JT, Petersen KA, Oturai PS, Kruuse C, Fahrenkrug J, Olesen J. The effect of intravenous PACAP38 on cerebral hemodynamics in healthy volunteers. Regul Pept. 2007;140:185–191. doi: 10.1016/j.regpep.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Doberer D, Gschwandtner M, Mosgoeller W, Bieglmayer C, Heinzl H, Petkov V. Pulmonary and systemic effects of inhaled PACAP38 in healthy male subjects. Eur J Clin Invest. 2007;37:665–672. doi: 10.1111/j.1365-2362.2007.01832.x. [DOI] [PubMed] [Google Scholar]
- 45.Murck H, Steiger A, Frieboes RM, Antonijevic IA. Pituitary adenylate cyclase-activating peptide affects homeostatic sleep regulation in healthy young men. Am J Physiol. 2007;292:853–857. doi: 10.1152/ajpendo.00152.2006. [DOI] [PubMed] [Google Scholar]
- 46.Li M, Maderdrut JL, Lertora JJL, Batuman V. Intravenous infusion of pituitary adenylate cyclase-activating polypeptide (PACAP) in a patient with multiple myeloma and myeloma kidney: a case study. Peptides. 2007;28:1891–1895. doi: 10.1016/j.peptides.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Chéret J, Lebonvallet N, Buhé V, Carre JL, Misery L, Le Gall-Ianotto C. Influence of sensory neuropeptides on human cutaneous wound healing process. J Dermatol Sci. 2014;74:193–203. doi: 10.1016/j.jdermsci.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Ma Y, Zhao S, Wang X, Shen S, Ma M, Xu W, Hong A. A new recombinant PACAP-derived peptide efficiently promotes corneal wound repairing and lacrimal secretion. Invest Ophthalmol Vis Sci. 2015;56:4336–4349. doi: 10.1167/iovs.15-17088. [DOI] [PubMed] [Google Scholar]
- 49.Winstanley C, O'Brien S, Brockhurst MA. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol. 2016;24:327–337. doi: 10.1016/j.tim.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prasse A, Zissel G, Lützen N, Schupp J, Schmiedlin R, Gonzalez-Rey E, Rensing-Ehl A, Bacher G, Cavalli V, Bevec D, Delgado M, Müller-Quernheim J. Inhaled vasoactive intestinal peptide exerts immunoregulatory effects in sarcoidosis. Am J Respir Crit Care Med. 2010;182:540–548. doi: 10.1164/rccm.200909-1451OC. [DOI] [PubMed] [Google Scholar]
- 51.Sun W, Tadmori I, Yang L, Delgado M, Ganea D. Vasoactive intestinal peptide (VIP) inhibits TGF-α1 production in murine macrophages. J Neuroimmunol. 2000;107:88–99. doi: 10.1016/s0165-5728(00)00245-9. [DOI] [PubMed] [Google Scholar]
- 52.Lindén A, Cardell LO, Yoshihara S, Nadel JA. Bronchodilation by pituitary adenylate cyclase-activating peptide and related peptides. Eur Respir J. 1999;14:443–451. doi: 10.1034/j.1399-3003.1999.14b34.x. [DOI] [PubMed] [Google Scholar]
- 53.Dérand R, Montoni A, Bulteau-Pignoux L, Janet T, Moreau B, Muller JM, Becq F. Activation of VPAC1 receptors by VIP and PACAP-27 in human bronchial epithelial cells induces CFTR-dependent chloride secretion. Br J Pharmacol. 2004;141:698–708. doi: 10.1038/sj.bjp.0705597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rafferty S, Alcolado N, Norez C, Chappe F, Pelzer S, Becq F, Chappe V. Rescue of functional F508del cystic fibrosis transmembrane conductance regulator by vasoactive intestinal peptide in the human nasal epithelial cell line JME/CF15. J Pharmacol Exp Ther. 2009;331:2–13. doi: 10.1124/jpet.109.155341. [DOI] [PubMed] [Google Scholar]
- 55.Alcolado N, Conrad DJ, Rafferty S, Chappe FG, Chappe VM. VIP-dependent increase in F508del-CFTR membrane localization is mediated by PKCε. Am J Physiol Cell Physiol. 2011;301:C53–C65. doi: 10.1152/ajpcell.00568.2009. [DOI] [PubMed] [Google Scholar]
- 56.Ríos JD, Zoukhri D, Rawe IM, Hodges RR, Zieske JD, Dartt DA. Immunolocalization of muscarinic and VIP receptor subtypes and their role in stimulating goblet cell secretion. Invest Ophthalmol Vis Sci. 1999;40:1102–1111. [PubMed] [Google Scholar]
- 57.Kreda SM, Davis CW, Rose MC. CFTR, mucins, and mucus obstruction in cystic fibrosis. Cold Spring Harb Perspect Med. 2012;2:a009589. doi: 10.1101/cshperspect.a009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Igarashi H, Ito T, Hou W, Mantey SA, Pradhan TK, Ulrich CD, 2nd, Hocart SJ, Coy DH, Jensen RT. Elucidation of vasoactive intestinal peptide pharmacophore for VPAC1 receptors in human, rat, and guinea pig. J Pharmacol Exp Ther. 2002;30 m:37–50. doi: 10.1124/jpet.301.1.37. [DOI] [PubMed] [Google Scholar]
- 59.Igarashi H, Ito T, Pradhan TK, Mantey SA, Hou W, Coy DH, Jensen RT. Elucidation of the vasoactive intestinal peptide pharmacophore for VPAC2 receptors in human and rat and comparison to the pharmacophore for VPAC1 receptors. J Pharmacol Exp Ther. 2002;303:445–460. doi: 10.1124/jpet.102.038075. [DOI] [PubMed] [Google Scholar]