Abstract
Objective
Protein S (PS) is a plasma protein that directly inhibits the coagulation factor IXa (FIXa) in vitro. Because elevated FIXa is associated with increased risk of venous thromboembolism, it is important to establish how PS inhibits FIXa function in vivo. The goal of this work is to confirm direct binding of PS with FIXa in vivo, identify FIXa amino acid residues required for binding PS in vivo, and use an enzymatically active FIXa mutant that is unable to bind PS to measure the significance of PS-FIXa interaction in hemostasis.
Approach & Results
We demonstrate that PS inhibits FIXa in vivo by associating with the FIXa heparin-binding exosite. We used fluorescence tagging, immunohistochemistry, and protein-protein crosslinking to show in vivo interaction between FIXa and PS. Importantly, platelet co-localization required a direct interaction between the two proteins. FIXa and PS also co-immunoprecipitated from plasma, substantiating their interaction in a physiological milieu. PS binding to FIXa and PS inhibition of the intrinsic Xase complex required residues K132, K126 and R170 in the FIXa heparin-binding exosite. A double mutant, K132A/R170A, retained full activity but could not bind to PS. Crucially, Hemophilia B mice infused with FIXa K132A/R170A displayed an accelerated rate of fibrin clot formation compared with wild type FIXa.
Conclusions
Our findings establish PS as an important in vivo inhibitor of FIXa. Disruption of the interaction between PS and FIXa causes an increased rate of thrombus formation in mice. This newly discovered function of PS implies an unexploited target for antithrombotic therapeutics.
Keywords: Protein S, Thrombosis, Hemostasis, Platelets, Factor IXa
Introduction
To prevent undesirable thrombotic complications, the natural anticoagulants antithrombin, activated protein C (APC), tissue factor pathway inhibitor (TFPI), and Protein S (PS) regulate coagulant activity.1–6 Deficiencies in these anticoagulants are associated with increased risks of venous thromboembolism and higher rates of stroke and myocardial infarction.1, 7–10 Homozygous PS deficiency causes fatal neonatal purpura fulminans, a dramatic example of the critical role of PS in regulation of human blood coagulation.11, 12 Similarly, heterozygous PS deficiency is associated with increased familial risk of venous thromboembolism.13–15 Consistent with these clinical manifestations, gene ablation of PS in mice causes embryonic lethality related to consumptive coagulopathy and intracranial hemorrhage.16, 17
Despite the biological significance of PS, to now, there has not been definitive identification of the in vivo biochemical mechanism(s) for PS function. PS was originally identified as a cofactor for APC in the inactivation of factor Va (FVa).1 Subsequent studies demonstrated APC-independent anticoagulant activity for PS, notably as a cofactor for TFPI in the inhibition of factor Xa.9, 18 Additionally, FXa was directly inhibited by plasma PS that contained Zn2+.19 Likewise, direct binding of PS to FVIII resulted in decreased anticoagulant activity.20 These disparate PS mechanisms require re-examination in a physiological context because multimeric PS structures effectively inhibit phospholipid-dependent reactions at low phospholipid concentrations (i.e., < 50 µmole/liter), and therefore interfere during functional analysis of PS.21
Ex vivo modeling of blood coagulation demonstrates that factor X (FX) activation by the intrinsic Xase complex (FIXa-FVIIIa) is the rate-limiting step for thrombin generation. 22, 23 FIXa binds substrates and inhibitors poorly due to a partially occluded active site; incorporation of FIXa into the intrinsic Xase complex results in a dramatic enhancement in catalytic activity.24–26 The FIXa heparin-binding exosite (HBE) is key to regulation of protease activity by cofactor and inhibitors. Mutagenesis of this exosite modestly reduces FIXa-FVIIIa affinity by destabilizing the critical interaction with the FVIIIa A2 domain.27 The HBE is targeted by select glycosaminoglycans for antithrombin-independent inhibition of intrinsic Xase activity in serpin-deficient human plasmas28–31, and the HBE interacts directly with the heparin component of the antithrombin-heparin complex to accelerate FIXa inhibition.32, 33
We demonstrated previously that, in vitro, PS functions as an independent regulator of the intrinsic coagulation pathway; PS inhibits both free FIXa and the Xase complex, thereby directly inhibiting FX activation.34 The goals of the present study were to define the PS binding site(s) on FIXa and establish the contribution of PS-mediated FIXa inhibition to in vivo regulation of blood coagulation. Westmark et al.33 characterized recombinant FIX proteins possessing mutations in the HBE. Their results demonstrated a significant disparity between protease activity of purified proteins and plasma-based thrombin generation activity, suggesting involvement of an unidentified plasma inhibitor of FIXa. We show that the FIXa HBE is critical for PS binding and inhibition of FIXa in physiological environments, including the activated platelet surface, in human plasma and in murine thrombosis models. Particularly, we identified a FIXa HBE double mutant, K132A/R170A that did not bind PS yet retained full enzymatic activity. Critically, this FIXa mutant caused an increased rate of thrombus formation in Hemophilia B mice, a result that confirmed that PS maintains hemostasis and prevents thrombosis by inhibiting FIXa.
Materials and Methods
Materials
Reagents
Human PS, FX, FIXa, α-thrombin, and a Dansyl-Glu-Gly-Arg chloromethyl ketone active site blocked-FIXa (DEGR-FIXa) were purchased from Hematologic Technologies Inc. (Essex Junction, VT). DEGR forms of the mutant FIXa proteins were produced in-house according to Lollar and Fass.35 The FXa-specific substrate N-2-benzyloxycarbonyl-D-arginyl-L-arginine p-nitroanilide dihydrochloride (S-2765) was purchased from DiaPharma (West Chester, OH). 1,2-dioleoyl-3-sn-phosphatidylcholine and 1,2-dioleoyl-sn-glycero-3-phospho-L-serine (PS) were obtained from Avanti Polar Lipids (Alabaster, AL). All solvents were high-performance liquid chromatography grade.
A plasmid with a wild type human FIX gene was a generous gift from Valder R. Arruda (University of Pennsylvania). Gla-deleted FIXa was prepared by enzymatic digestion with chymotrypsin.36 Gla-EGF1-deleted FIXa was provided by Jim Huntington (Cambridge Research Institute, UK)37; its concentration was determined by titrating with antithrombin as described38. A sheep anti-human PS polyclonal antibody (PAHPS-S) and a sheep anti-human FIX polyclonal antibody, which binds both FIX and FIXa (PAHFIX-S), were purchased from Hematologic Technologies Inc. A rat anti-mouse PS monoclonal antibody (MAB4976) was purchased from R&D Systems (Minneapolis, MN).
Methods
Expression and Purification of Recombinant Proteins
HEK293 cells stably transfected with a plasmid encoding FIX R170L was employed for expression. 39 This recombinant FIX (rFIX) protein was purified from conditioned media using a FIX Select column.40 Uncarboxylated rFIX was generated by withholding vitamin K from the growth and expression media. Recombinant human FIX wild type, K126A, N129A, K132A, R150A, R165A, R170A, K126A/K132A, and K132A/R170A proteins (chymotrypsin numbering) were expressed in VKOR/HEK293 cells (stably transfected with vitamin K epoxide reductase) and purified to homogeneity from conditioned media as described.27, 33 FIX protein concentrations were determined by absorbance at 280 nm using an extinction coefficient (ε0.1%) of 1.32. rFIX proteins (1 µg) produced a single band on 10% SDS-PAGE gels stained with Gel-Code Blue as previously demonstrated.33 Purified rFIX was activated to rFIXa with human FXIa (150:1 substrate:enzyme molar ratio) at 37 °C for 1.5 h, followed by depletion of factor XIa by immunoabsorption with polyclonal anti-FXIa antisera cross-linked to Affi-gel beads in a spin column. FIXa active sites were quantitated by titration with antithrombin as described.41PS was purchased from Hematologic Technologies Inc., devoid of the zinc that affects PS activity in vivo.42
Binding of FIXa and PS with activated platelets
An ex vivo experiment with Alexa-Fluor-647-labeled FIXa and Alexa-Fluor-488-labeled PS was performed to detect binding of these two proteins to activated platelets. Washed platelets (5 × 108) were diluted 1:10 in Tyrode’s buffer containing 2 mM CaCl2 and activated for 15 minutes with FIXa or PS and 500 ng/mL convulxin and 500 µM Par 4 agonist. Annexin V staining was included as a positive control. Samples were immediately analyzed by flow cytometry (N = 4) as described.43
Co-Immunoprecipitation of FIXa and Protein S from human FIX-deficient plasma
Wild type or mutant FIXa (1 nM) was added to 5 mL of citrated FIX-deficient plasma that was thawed at 37 °C. The plasma was incubated on a shaker at 4 °C for 30 minutes and precleared with Poly-AG beads. Plasma was then centrifuged at 10,000 × g and 5 µg of FIXa antibody (PAHFIX-S) was added to the supernatant. FIXa was immunoprecipitated with a FIXa antibody (PAHFIX-S) and immunoblotted for PS with a sheep polyclonal anti-PS antibody (PAHPS-S).44
Direct binding of Protein S to DEGR-FIXa
To assess the interaction between PS and different mutant forms of FIXa, the steady state fluorescence of active site-labeled FIXa was measured in the presence of increasing concentrations of PS. FIXa was labeled with 1, 5-Dansyl-Glu-Gly-Arg (DEGR) to yield DEGR-FIXa.45 Protein S was titrated into a reaction containing 75 nM DEGR-FIXa and 50 µM phosphatidylserine/phosphatidylcholine vesicles in 20 mM Tris-HCl (pH 7.4), 5 mM Ca2+, and 150 mM NaCl. Fluorescence intensity was measured at 23°C with a Spex® FluoroLog-3 spectrofluorometer (Jobin Yvon Inc., Edison, NJ), using an excitation wavelength of 340 nm (bandpass 5 nm) and an emission wavelength of 540 nm (bandpass 5 nm). Average intensity was recorded over a 10 second period. The fluorescence intensity data were fit to a hyperbola (see Data Analysis section below) to determine the mutant FIXa Kds for binding to PS.
Competitive binding of mutant human FIXa to Protein S in the presence of DEGR-FIXa
To further verify the interaction between PS and the mutant forms of FIXa, a competitive binding assay utilizing fluorescence anisotropy was performed with DEGR-FIXa and untagged FIXa mutants. 25 nM DEGR-FIXa was incubated with 60 nM PS in 20 mM Tris-HCl (pH 7.4), 5 mM Ca2+, and 150 mM NaCl. As concentrations of the FIXa mutants were varied from 0–350 nM, anisotropy was measured at 23°C with a Spex® FluoroLog-3 spectrofluorometer (Jobin Yvon Inc., Edison, NJ), using an excitation wavelength of 340 nm (bandpass 8 nm) and an emission wavelength of 540 nm (bandpass 8 nm); anisotropic values were derived from the average of 10 readings. The data were fit to a sigmoidal curve and Ki was determined for each protein as described below in Data Analysis.
FIX activation by FXIa
5 µM FIX was incubated with 25 nM FXIa with and without 7.5 uM PS at 37°C in 20 mM Tris-HCl, 150 mM NaCl, and 5 mM CaCl2 at pH 7.4. Samples containing 3 ug total cleaved and uncleaved FIX were taken at 1, 5, 10, 15, 30, 45, 60, 90, and 120-minute time points and were quenched with 2.5 mM EDTA and 15 mg/mL SBTI on ice for 5 minutes. 6X reducing SDS-PAGE loading buffer was then added and the samples were immediately heated at 95 °C. The samples were separated on a 12% SDS-PAGE gel, and the cleaved bands were quantitated by densitometry on a GE Amersham Imager 600.
FX activation by FIXa-phospholipid
FIXa (20 nM wild type or mutant) was incubated for 5 min at 37°C with 200 µM (25:75) phosphatidylserine:phosphatidylcholine vesicles, 0.4 mM chromogenic substrate (S-2765), and 0–300 nM Protein S, in separate wells of a clear bottom microplate containing 50 mM Tris-HCl (pH 7.4), 5 mM Ca2+, 175 mM NaCl, and 0.6% polyethyleneglycol.46, 47 Reactions were initiated by addition of 150 nM FX, and the absorbance was recorded for 30 minutes at 405 nm.46, 48 For the immunoblotting analysis, FX activation by FIXa was determined by incubating 20 nM FIXa in assay buffer (20 mM Tris-HCl at pH 7.4, 150 mM NaCl, 5 mM CaCl2,) with 200 µM phosSer containing vesicles in the presence or absence of PS (300 nM) at 37 °C for 5 min, followed by addition of FX (150 nM). Aliquots were withdrawn at various times and reactions were terminated by addition of EDTA (to 10 mM final) and SDS (to 1%); the samples were resolved on 10% polyacrylamide-SDS gels, followed by immunoblotting with anti-FX antibody.
FX activation by the Xase complex
FIXa (0.5 nM wild type or mutant) was incubated for 10 min at 37 °C with 200 µM 25:75 phosphatidylserine:phosphatidylcholine vesicles, 0.4 mM chromogenic substrate (S-2765), and 0–250 nM Protein S, in separate wells of a clear bottom microplate containing the same buffer as the experiment with FIXa alone.34 Simultaneously, to produce FVIIIa, 50 nM of a B-domain-deleted FVIII (from Bayer) was incubated with 5 nM α-thrombin for 4 minutes at room temperature.34 This reaction was stopped by adding 40 nM hirudin and the mixture was left to stand for 1 min. During this time, 150 nM FX was added to the wells followed by 0.4 nM FVIIIa to initiate the reaction. The absorbance at 405 nm was recorded for 5 min.34, 49, 50 The rate of activation of FX was plotted against PS concentration and the data were fit by SigmaPlot to a hyperbolic decay equation to determine the Ki for each FIXa mutant.
Data Analysis
From each kinetic assay, the mean values (n=3–4) were taken from the data collected at various PS concentrations in binding or activity studies. These data were fit to a nonlinear regression by means of a hyperbolic, sigmoid, or exponential decay function. This method generated values that described the concentration at which either the reaction was 50% inhibited with respect to rate of FIXa activity (Xa generation assays), 50% of DEGR-FIXa was displaced from PS by untagged FIXa (competitive anisotropy), or 50% of FIXa was bound to PS (fluorescence binding), i.e., the values: Ki, IC50, and Kd, respectively. SigmaPlot was used to calculate the standard deviation and associated error for these values.
Statistics
All the experiments were performed three times and the data were presented as Mean ± SD. The figures 1a data were analyzed by one-way ANOVA followed by Student t test and the p values < 0.05 were considered statistically significant.
Figure 1. FIXa interacts with PS in vitro and ex vivo.
(A) Flow cytometry was used to quantify the binding of fluorescently labeled FIXa and PS to resting platelets (black bars) and convulxin/PAR-4 peptide-activated platelets (blue bars). Minimal interactions with Gla-containing proteins are seen on the resting platelets. In contrast, binding of both FIXa and PS is observed upon platelet activation, as reflected by increased mean fluorescent intensity (MFI). (B) A forward scatter of resting and activated platelets exposed to fluorescently tagged FIXa (top) and PS (bottom) demonstrated that platelet size decreases upon activation. These smaller, activated, “coated,” platelets showed an increase in fluorescence when binding to FIXa or PS. (C) Flow cytometry studies of resting and activated platelets were performed with fluorescently tagged FIXa (1 nmole/liter) and PS (140 nmole/liter) (labeled above each plot). A fluorescence shift into Q1 indicates FIXa binding to platelets, whereas a shift into Q4 suggests PS binding. Movement into Q2 indicates co-localization of the two proteins on the same platelet (see plot of ‘PS + FIXa’). All panels in (C) were derived from experiments that utilized activated platelets, except for the upper left panel, which describes resting platelets exposed to both FIXa and PS. (D) The flow cytometry studies in (C) were repeated on activated platelets with des-Gla FIXa in place of wild type FIXa. A shift to Q2 is observed in the Des-Gla FIXa + PS experiment, despite the fact that des-Gla FIXa alone is unable to bind platelets; this result demonstrated that PS was required to recruit des-Gla FIXa to the platelet surface. (E) Co-immunoprecipitation of FIXa with PS, and vice versa, revealed that FIXa interacts with PS in plasma. FIXa (1 nmole/liter) was added (lanes with +) or was not added (lanes with -) to FIX-deficient plasma. This plasma was immunoprecipitated with an IgG control antibody (both blots IgG lane) or with an anti-FIXa or anti-PS antibody (top blot and bottom blot, respectively), and immunoblotted for PS or FIXa (top blot and bottom blot, respectively). Initial plasma samples which were supplemented with FIXa are shown on both blots in the ‘Input’ lane. Molecular weights of bands (kDa) are shown to the right of the blots. The values in Figure 1A are expressed as mean ± STDEV (n=3 replicates). *, p<0.05 vs resting platelet (by t-test).
Modified aPTT utilizing PS-FIX double depleted Plasma
40 µL of PS-FIX double depleted plasma was added to a 96-well microplate. 5 µL of FIXa (~0.1nM, final), 5 µL of PS (150/300 nM, final), and 40 µL of Pacific Hemostasis™ KONTACT reagent were added; the solution was shaken and incubated at 37 °C for 5 minutes. The reaction was initiated with 40 µL of 25 mM CaCl2 and absorbance was measured at 405 nm. The clotting times were then converted to FIXa activity by measuring the clotting time of each parameter 51, FIXa and PS separately and together in PS-FIX double depleted plasma. The FIX-PS double immuno-depleted plasma was supplemented with various concentrations of WT FIXa, R150A and K132A/R170A mutant FIXa, in the absence or presence of 150 or 300 nM PS, and aPTT clotting time was determined as described above. A standard curve was generated by plotting clotting times versus log FIXa concentration and fitted to a linear plot. Using this standard curve, clotting times were converted to equivalent FIXa coagulant activity. Thus, clotting times determined in the presence and absence of PS were converted into relative FIXa coagulant activity. Relative FIX coagulant activity was determined from the ratio of FIXa + PS coagulant activity to FIXa alone coagulant activity and plotted as average ± STDEV (Figure S6B). Baseline clotting times were obtained by supplementation of 100 pM FIXa WT, R150A or K132A/R170A into FIX-PS double immuno-depleted plasma in the absence and presence of 150 and 300 nM PS.
Thrombin generation assay utilizing PS-FIX double depleted Plasma
40 µL of PS-FIX double depleted plasma was added to a 96-well microplate. 10 µL of FIXa (~0.8 nM, final), 10 µL of PS (0–250 nM, final), and 10 µL of Technothrombin® Thrombin Generation Assay (TGA) Reagent B (RB) were added, shaken, and incubated at 37°C for 5 minutes. The reaction was initiated with 50 µL of a mixture of 15 mM CaCl2 and z-Gly-Gly-Arg-AMC (0.5 mM final), and fluorescence was measured on a SpectraMax plate reader with emission and excitation wavelengths of 360 nm and 460 nm, respectively.
Murine electrolytic model of venous thrombosis
For in vivo investigation of the localization of FIX, FIXa, and PS in an active thrombus, a murine model of venous thrombosis was used 52. Adult, male wild-type C57Bl/6 mice were anesthetized with intraperitoneal pentobarbital (50 mg/kg body weight). Prior to thrombus induction, anti-Factor IX/IXa labeled with Alexa-Fluor-647 (Invitrogen) and human PS (Enzyme Research Laboratories, South Bend, IN) labeled with Alexa-Fluor-532 were co-injected into the jugular vein (approximately 10 ng of each) of the anesthetized mice. A 75-micron-diameter iron-containing wire was used to deliver a brief (30 seconds) electrolytic injury to the surface of the surgically exposed, un-dissected femoral vein, using an anodal voltage of 1.5 volts. The site was strobe-irradiated with green and red beam-expanded laser light (532 and 650 nm, respectively), with time-lapse image capture in a 2.4 X 3.2 mm field. A growing thrombus developed, with peak growth at 20–60 minutes. A low-light digital camera (DVC 1412) with filter wheel was used for image capture of fluorescence emissions over the course of 60 min.
Recombinant FIX (BeneFix, Pfizer) was injected intravenously into hemophilia B mice (C57Bl/6 mice, backcrossed 13X.) at a dose of 250 IU/kg, either unlabeled or labeled with the ultraviolet (UV) light-activated crosslinking molecule Sulfo-SANPAH (sulfosuccinimidyl 6-(4'-azido-2'-nitrophenylamino) hexanoate; Thermo Scientific, Cat No 22589). The infrarenal inferior vena cava was exposed after 5 minutes of recombinant FIX injection, and an electrolytic injury was applied to the outer surface of the vein at a point just proximal to the iliac bifurcation, delivering by direct contact 3 volts of anodal direct current for 6–8 minutes through a blunted 25-gauge steel cannula. Fifteen minutes after the induction, the exposed vessel and thrombus site were irradiated with a UV source (350–380 nm) continuously for 30 minutes to induce protein-protein crosslinking. The thrombus was then harvested from the inside of the vessel and frozen for protein analysis. Clots were dissolved in RIPA buffer, and equivalent protein concentrations from each sample, with or without SANPAH, were immunoblotted with FIX and PS antibodies. Fluorescence blotting was performed in a similar manner; however, either human FIX or a sulfo-SANPAH tagged human FIX, both pre-labeled with Alexa-Fluor-647, along with human PS pre-labeled with Alexa-Fluor-532, were injected into WT mice prior to clot formation.
FIXa Infusion-Induced Clotting in Hemophilia B Mice
For the locally infused active FIX proteins, a 30-gauge needle connected to a catheter and syringe was inserted into an ankle vein of an anesthetized FIX knockout mouse. The syringe was attached to a syringe injector, with slow infusion at a rate of 3 microliters per minute. An anti-fibrin antibody (59D8) isolated from ascites with specificity to fibrin and not fibrinogen 52 (permission for use kindly provided by Dr. Marschall Runge) labeled with Alexa-Fluor-647 and rhodamine 6G (to label platelets) was injected via the jugular vein prior to thrombus induction, for labeling and subsequent imaging of fibrin and platelets, respectively. An electrolytic injury was generated on the surface of the ipsilateral femoral vein just distal to the inguinal ligament, using the blunt end of a microsurgical needle touching the outer surface of the vein for 30 seconds with a 1.5-volt anodal voltage delivery, conferring a free radical injury within the vein as a nidus for thrombus development. The induction site was subsequently imaged with 532-nm and 650-nm laser excitation, capturing fluorescence emissions in time-lapse videos for 60 minutes, with subsequent off-line analysis and normalization of fluorescence intensities for inter-animal comparisons, as described.52 FIXa WT (Benefix FIXa) or R150A was infused upstream from the thrombus induction site via an ankle vein at a rate of 2 µg/h, or FIXa K132A/R170A at a rate of 1.3 µg/h; controls received infusion of physiologic saline. Infusions were started at the time of onset of the electrolytic injury and were maintained at a flow rate of 3 uL/minute for 60 minutes. Enzymatically equivalent doses of Benefix FIXa, FIXa R150A and K132A/R170A for infusion were determined by the rate of FX activation in an intrinsic Xase assay containing 0.1 nM FIXa, 1 nM FVIIIa, 150 nM FX and 200 µM of 25% phosphatidylserine vesicles (data not shown). The injury site was imaged for 60 minutes under fluorescence image capture with time-lapse acquisition 52.
Results
Interaction of FIXa and PS on platelets and in plasma
We used flow cytometry to evaluate the ability of FIXa and PS to bind to the surface of activated platelets. We incubated activated platelets with increasing concentrations of fluorescently labeled FIXa or PS and observed a steady increase in the mean fluorescence intensity which indicated individual binding of the proteins to platelets (Figure 1A, blue bars). Resting platelets demonstrated a negligible response (Figure 1A, black bars).53
A forward side-scatter (Figure 1B) showed that activation with convulxin/PAR-4 peptide resulted in a population of smaller (lower FSC-H) platelets binding both FIXa and PS. These smaller platelets are characteristic of “coated platelets," produced by exposure to multiple strong agonists.54, 55 A hallmark of coated platelets is surface exposure of phosphatidylserine, a negatively charged phospholipid that facilitates the assembly of the membrane bound enzyme complexes of coagulation. Furthermore, the PS- and FIX-positive platelets stained positive with annexin V (Figure 1A), which selectively binds membranes containing exposed phosphatidylserine. We also used annexin V and unlabeled PS to compete with fluorescently labeled PS in binding to activated platelets. Upon addition of different concentrations of either annexin V or unlabeled PS, we observed a gradual but marked decrease in fluorescently labeled PS binding to the platelets, indicating that PS binds to phosphatidylserine-positive platelets (Figure S1).
We continued to assess in more detail the co-localization of the proteins on platelets. No significant binding was detected with resting platelets (Figure 1C, top left), but co-localization of FIXa and PS was observed on activated platelets (Figure 1C, bottom right panel).53, 56 To assess whether co-localization occurred by interaction between the proteins, we employed a form of FIXa lacking the membrane-binding Gla domain (des-Gla FIXa). Labeled des-Gla FIXa did not bind to activated platelets in the absence of PS (Figure 1D, left panel). However, when activated platelets were incubated with both labeled des-Gla FIXa and PS, the proteins co-localized on the platelet surface, an effect suggesting that the platelet binding-competent PS pulled des-Gla FIXa down to the platelet surface (Figure 1D, right panel). The median fluorescence intensity for PS+FIXa was 4336, whereas the median fluorescence intensity for PS+des-Gla FIXa was 3997(Figure 1C and D). These results indicated that the Gla domain of FIXa is not required for binding to PS.
The ability of FIXa and PS to interact in human plasma was evaluated by co-immunoprecipitation. We added WT FIXa (1 nM) to FIX-deficient plasma and found that the FIXa co-immunoprecipitated with PS (Figure 1E, top blot lane 4); we did not detect PS in the immunoprecipitate from plasma that either lacked FIXa (Figure 1E, top lane 2) or that was incubated with a control IgG antibody (Figure 1E, top blot, lane 3). The failure to immunoprecipitate PS under the latter two conditions indicated that PS had not bound adventitiously to the immunoabsorbent. Finally, reverse co-immunoprecipitation of this plasma with anti-human PS antibody confirmed PS binding to FIXa in plasma (Figure 1E, bottom blot).
Interaction of FIXa and PS in mouse thrombi
Fluorescently labeled human anti-FIX antibody (red) and human PS (green) were injected into wild type mice, and an electrolytic injury was applied to the femoral vein to induce thrombi. The monoclonal anti FIX/FIXa antibody was checked to confirm that it does not affect FIXa activity. The labeled proteins co-localized over the entire area of a clot for the 20 minutes following injury (Figure 2A). The fluorescence intensities exhibited a correlated increase, suggesting that FIXa and PS co-localized during the process of thrombus formation.52 Labeled control antibody or albumin did not localize in the thrombus.52
Figure 2. FIX interacts with PS in vivo.
(A) Following the injection of Alexa-Fluor-647 labeled anti-FIXa (panel 1) and Alexa-Fluor-532 labeled human PS (panel 2) into wild type mice, a thrombus was induced by a brief electrolytic injury to the femoral vein; diffuse partial colocalization of the fluorescent molecules was observed in the merged image (3). A continuous correlated increase in fluorescence intensity was evident during the clotting process. (B) Femoral vein clots formed in hemophilia B mice, supplemented with FIX or FIX-SANPAH, were exposed to UV light to crosslink FIX-SANPAH to associated proteins. Immunoblots of clots from 1) FIX-injected mice and 2) FIX-SANPAH-injected mice were probed for FIX (top blot) and PS (bottom blot). Higher molecular weight forms (molecular weight in kD is shown to the left of the blots) are seen in both blots for FIX-SANPAH, but not for FIX, suggesting that FIX associated with PS. (C) A fluorescent blot of plasma from a hemophilia B mouse, injected with FIXSANPAH that was conjugated with Alexa-Fluor-647, and PS that was conjugated with Alexa- Fluor-532 is (1) probed for FIX and (2) probed for PS. (3) shows an overlay of lanes 1 and 2, exhibiting a band at ~140 kDa, in similar fashion to the blots from (B). Color has been added to highlight the overlap of bands, denoted in yellow. Scale Bar- 300 um.
In vivo association between FIXa and PS was further assessed by protein-protein crosslinking in hemophilia B mice. We injected recombinant FIX, covalently modified with the UV-inducible crosslinker SANPAH, after which we applied an electrolytic injury to the infrarenal vena cava, followed by UV irradiation and immunoblotting of resulting thrombi. Immunoblots demonstrated an intense FIX band at ~60 kDa and a higher molecular weight species at ~140 kDa. The higher molecular weight band represented crosslinked material because we observed only a single FIX band when we used unmodified FIX (Figure 2B, upper panel). The band at ~140 kDa represents a complex between FIXa and PS because PS binds to FIX ~5 fold weaker (as described below) than with FIXa, and it is probable that FIXa will form a complex with PS in the clot. Similarly, immunoblotting for PS following FIX-SANPAH injection revealed two bands, the free form of PS at ~80 kDa and a crosslinked form of PS at ~140 kDa. We did not observe the 140-kDa species in mice injected with unmodified FIX (Figure 2B, lower panel). The 140-kDa FIXa and PS bands were evaluated in UV-irradiated mouse plasma that contained fluorescently labeled, SANPAH-tagged FIXa and fluorescently labeled PS (Figure 2C). When immunoblotted for FIXa, these plasma samples showed a similar ~140 kDa band (Figure 2C, Left Lane), in addition to multiple, unidentified higher molecular weight species that we attribute to differences between the plasma and thrombus environments. We performed non SANPAH controls for FIXa and PS and the figure is provided as a supplemental figure (figure VIII).
Direct and competitive binding of FIXa mutants to PS
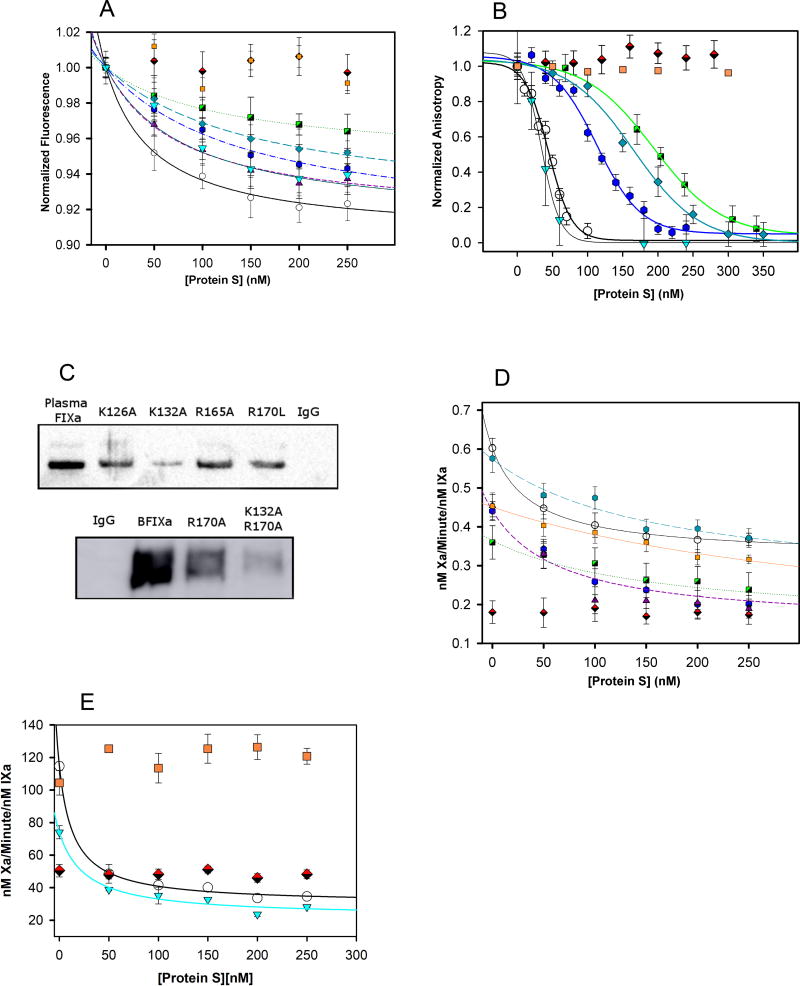
We previously demonstrated that titrating a DEGR-FIXa-PS complex with unfractionated heparin reduced the fluorescence anisotropy (Figure S2), suggesting that the binding sites for heparin and PS overlap within FIXa. Thus, we labeled with DEGR the active sites of previously characterized FIXa mutants having alanine substitutions in the HBE27, 33, 41 to evaluate the contribution of the HBE in PS binding. The interactions of these labeled FIXa mutants with PS were detected in a direct binding assay by changes in fluorescence intensity.48 We observed an exponential decay in fluorescence with addition of PS, suggesting a conformational change in DEGR-FIXa upon PS binding (Figure 3A). FIXa wild type (WT) exhibited a Kd ~ 51 ± 7 nmole/liter.34 Several FIXa proteins with mutations in the HBE demonstrated marked reductions in PS affinity (i.e., increased Kd), including FIXa K126A (118 ± 20 nmole/liter), K132A (148 ± 39 nmole/liter), and K132A/R170A and K126A/K132A (binding not detectable) (Table I). Additionally, the FIXa Padua mutant (R170L) bound PS with significantly reduced affinity (157 ± 33 nmole/liter).
Figure 3. FIXa Protease Domain Single-Site Mutant Kinetics.
(A) DEGR-FIXa Mutant Binding Fluorescence: 75 nmole/liter DEGR-FIXa was titrated with increasing concentrations of PS; fluorescence was measured and normalized. (B) DEGR-FIXa Fluorescence Anisotropy Binding Competition: 50 nmole/liter DEGR-FIXa was incubated with 80 nmole/liter PS, then titrated with unlabeled FIXa constructs at concentrations from 0–300 nmole/liter; the anisotropy was measured and normalized. (C) 1 nmole/liter of each FIXa construct was added to FIX- deficient plasma, immunoprecipitated with anti-FIX antibody, and immunoblotted for PS. The top blot was generated using X-ray film, whereas the bottom blot was scanned with an Amersham Imager 600. The particular Factor IXa construct that was supplemented into the FIX- deficient plasma is labeled above each blot. (D) Effect of PS on FXa generation by the FIXa- phospholipid complex: 20 nmole/liter FIXa proteins were incubated with 0.4 mM S-2765 chromogenic substrate and 0–250 nmole/liter PS at 37°C. The reactions were initiated by the addition of 150 nmole/liter FX to each well and absorbance was measured at 405 nm, (E) Effect of PS on FXa generation by the intrinsic Xase complex: performed as above in the presence of FIXa (0.5 nM) and FVIIIa (0.4 nM), complexed in the physiologically relevant Xase complex.
Error bars represent STDEV on all plots (n= 3). No significant differences (compared with FIXa WT) were observed in the inhibition kinetics of FIXa R150A or R165A by PS (data not shown). Legend: [Protein: Symbol]

Table 1.
FIXa Protease Mutant Construct Activity and Binding Kinetics
| FIXa Protease Mutant |
Kd of FIXa Binding by Protein S (nmole/liter) |
FIXa IC50 in Anisotropy Competition (nmole/liter) |
Ki of FIXa Activity Inhibition by Protein S (nmole/liter) |
|---|---|---|---|
| WT | 51 ± 12 | 63 ± 27 | 35 ± 26 |
| K126A | 118 ± 34 | 140 ± 24 | 100 ± 52 |
| N129A | 77 ± 64 | 92 ± 45 | 72 ± 31 |
| K132A | 148 ± 67 | 213 ± 79 | 132 ± 52 |
| R150A | 48 ± 14 | 43 ± 29 | 48 ± 33 |
| R165A | 54 ± 12 | 68 ± 36 | 33 ± 19 |
| R170L | 157 ± 57 | 163 ± 47 | 175 ± 90 |
| K132AR170A | ND | ND | 271± 119 |
| K126A K132A | ND | ND | <1% Activity |
Various kinetic parameters for PS binding to FIXa WT and mutants (protease variants) are shown. The table displays mean apparent Kd (direct binding of DEGR-FIXa with PS by fluorescence intensity), IC50 (competition of FIXa with DEGR-FIXa towards binding to PS by fluorescence anisotropy), or Ki (inhibition of FX activation by FIXa in presence of increasing concentration of PS by proteolysis of synthetic substrate S-2765) ± STDEV, (n=3 replicates of each) which were determined by fitting curves to data (average of three data sets) collected in the described fluorescent or activity assays. ND = Not Determinable, due to weak binding or low activity (<1% activity).
We used a fluorescence anisotropy-based binding assay to assess competition by mutant FIXa proteins in DEGR-FIXa binding to PS (Figure 3B). The IC50 values obtained from these competition assays followed the trend of binding affinities obtained from the direct binding assays (FIXa WT > K126A > R132A > R170L > K132A/R170A and K126A/K132A). PS binding to the R150A mutant, which demonstrates markedly reduced inhibition by antithrombin, was similar to its binding of wild type FIXa. Furthermore, the zymogen form of FIX showed an IC50 value ~ 200 nmole/liter (data not shown), indicating a significantly weaker interaction with PS compared with FIXa. In sum, the general agreement between the direct and competitive binding assays demonstrated that the FIXa HBE contributes to the interaction with PS.
We also measured the ability of FIXa mutants to co-immunoprecipitate PS from plasma (Figure 3C). The relative intensities of the PS immunoprecipitation bands inversely correlated with the Kd values determined in the fluorescence binding assays, with the faintest bands in the co-IPs corresponding to the highest dissociation constants (Figure 3C). Importantly, as demonstrated by immunoblotting, the various FIXa mutants showed negligible differences in antibody affinity (Figure S3). These data confirmed that the effects of the FIXa HBE mutations on PS binding affinity in vitro were retained in the plasma environment.
Effect of PS on FX activation by FIXa mutants
We assessed the effect of select mutations in the FIXa HBE on the ability of PS to inhibit FX activation by the FIXa-phospholipid and the intrinsic Xase (FIXa-FVIIIa) complexes. Titration of the FIXa-phospholipid complex with PS resulted in partial inhibition of FX activation (~45% maximal inhibition for wild type FIXa) with resistance to PS inhibition observed for multiple FIXa HBE mutants (Figure 3D). Furthermore, the relative Ki values for the FIXa mutants correlated with the dissociation constants (KD) observed in the PS binding studies (Table I). Similarly, titration of the intrinsic Xase complex with PS also resulted in ~65% inhibition of FX activation by WT FIXa, a result that agreed with our previous work34. Thus, PS binds not only to free FIXa, but it also binds as an allosteric inhibitor to the physiologically relevant Xase complex (Figure 3E). The mechanism of Xase inhibition is similar to the inhibition of the FIXa-phospholipid complex because the FIXa HBE mutants resistant to PS inhibition demonstrated a similar trend when incorporated into the Xase complex. Under these conditions, PS did not inhibit FX activation by either FIXa K132A/R170A or K126A/K132A, findings consistent with the loss of PS binding to these mutant proteases. Moreover, the double mutant K132A/R170A showed 1.5-fold (data not shown) higher activity than wild type FIXa in the absence of PS, but we did not detect a change in the activity of K132A/R170A after PS addition (Figure 3E). We did monitor the activation of FX by FIXa in the presence of phospholipid vesicles in SDS gel (see Methods) at different time intervals, and our data indicated that PS inhibits the formation of FXa (determined by the appearance of heavy chain) (Figure S5).
Effect of Gla and EGF1 domain deletions on FIXa binding to PS
We examined the ability of PS to bind FIXa lacking either the Gla domain (des-Gla)( amino acid numbering, 40–415), the Gla and EGF1 domains (des-Gla-EGF1)( amino acid numbering, 88–425), or γ-carboxylation. In both the direct and competitive binding assays, uncarboxylated FIXa demonstrated only slightly weaker binding (Kd 65 ± 15 nmole/liter), whereas des-Gla FIXa had a more pronounced reduction (Kd 124 ± 38 nmole/liter) in PS affinity (Table II, Figure 4A and B).57 Both des-Gla and uncarboxylated FIXa retained the ability to co-immunoprecipitate PS from plasma (Figure 4C). In contrast, the des-Gla-EGF1 mutant neither bound to PS (Figure 4A and B) nor co-immunoprecipitated PS from plasma (Figure 4C). Enzymatic analysis of FX activation ccould be obtained only for the uncarboxylated WT derivative in the Xase complex (Figure 4D), likely due to the important role of the N-terminal Gla domains in the productive assembly of membrane-bound coagulation complexes.58, 59 The binding and immunoprecipitation data suggested that while the Gla-domain was largely dispensable for the PS interaction, we could not exclude a contribution of the EGF-1 domain to FIXa-PS binding. A summary of the putative intermolecular interactions of FIXa with PS is displayed on a representative structure in Figure 5. Each residue or domain analyzed is displayed on a gradient from green to yellow to red (untested residues in blue), where red and green indicate, respectively, the most and least necessary residues in the FIXa-PS interaction.
Table 2.
FIXa Gla Deletion Construct Activity and Binding Kinetics
| FIXa Gla Construct |
Kd of FIXa Binding by Protein S (nmole/liter) |
FIXa IC50 in Anisotropy Competition (nmole/liter) |
Ki of FIXa Activity Inhibition by Protein S (nmole/liter) |
|---|---|---|---|
| Des-Gla | 124 ± 66 | 135 ± 21 | <1% (Active Xase Ki = 120 ± 93) |
| Des-Gla-EGF1 | ND | ND | <1% Activity |
| Uncarboxylated | 65 ± 26 | 77 ± 28 | <1% (Active Xase Kd = 46 ± 41) |
Various kinetic parameters for PS binding to FIXa domain variants are shown. The table displays mean apparent Kd (direct binding of DEGR-FIXa with PS by fluorescence intensity), IC50 (competition of FIXa with DEGR-FIXa towards binding to PS by fluorescence anisotropy), or Ki (inhibition of FX activation by FIXa in presence of increasing concentration of PS by proteolysis of synthetic substrate S-2765) ± STDEV (n=3 replicates), which were determined by fitting curves to data (average of three data sets) collected in the described fluorescent or activity assays. ND = Not Determinable, due to weak binding or low activity (<1% activity).
Figure 4. Identification of additional FIXa domains necessary for FIX-PS interaction.
(A) DEGR-FIXa Mutant Binding Fluorescence: 75 nmole/liter DEGR-FIXa and its deletions constructs were titrated with various concentrations of PS; fluorescence was measured and normalized. (B) DEGR-FIXa Fluorescence Anisotropy Binding Competition: 50 nmole/liter DEGR-FIXa was incubated with 80 nmole/liter PS, then titrated with unlabeled FIXa deletion constructs having concentrations from 0–300 nmole/liter and anisotropy was measured. (C) FIXa constructs (labeled above blot), at a final concentration of 1 nmole/liter, were added to FIX deficient plasma, immunoprecipitated with anti-FIX antibody, and immunoblotted for PS. (D) FXa Generation Assay by Xase Complex: 0.5 nmole/liter FIXa constructs were incubated with 0.4 mM S-2765 chromogenic substrate and 0–250 nmole/liter PS at 37°C. Reactions were initiated by the addition of 150 nmole/liter FX and 0.4 nmole/liter FVIIIa to each well; absorbance was measured at 405 nm. Error bars represent STDEV on all plots (n= 3). Legend: [Protein or Co-IP Control: Symbol (Co-IP Number)]

Figure 5. Structural Identification of Binding Site of PS on FIXa.
The porcine FIXa structure (PDBID:1PFX) is displayed with relevant amino acid residues designated by a color gradient from green to red: Green indicates residues not required for PS interaction, yellow indicates residues that contribute weakly to PS interaction, and red indicates residues required for strong interaction with PS. Domains are indicated in italics. Porcine and human FIX share 84% peptide sequence identity, and each of the relevant residues that were mutated in the protease domain are conserved between the porcine and human proteins.
Effect of PS on FIX activation by FXIa
To assess other consequences of the PS-FIXa and PS-FIX interactions, we examined the effect of PS on the rate of FXIa-mediated activation of FIX. We monitored activation of FIX by FXIa by SDS-PAGE analysis of samples over time with and without PS present. Densitometric quantitation of protein band volume indicated that there was no effect of PS on the rate of FIX activation, which showed >95% complete cleavage by 90 minutes without or with PS at a 1:200 molar ratio of protease to zymogen (Figure S4, supplement). These results suggested that the inhibitory effect of PS is primarily due to direct interaction with FIXa.
Modified aPTT and thrombin generation in FIX-PS double-immunodepleted plasma
The effect of PS on aPTT-based coagulant activity for FIXa WT, FIXa K132A/R170A, and R150A was examined in FIX/PS double-immunodepleted plasma (FIX-PS-ID plasma) supplemented with 0, 150, or 300 nmole/liter PS (described in Methods). We measured the coagulant activity of FIXa with no PS present relative to coagulant activity in the presence of 150 and 300 nM PS; the data are presented in Table III and Supplementary Figure 6A. At 300 nM PS, the activities of wild type and R150A FIXa decreased by ~90%, whereas the activity of K132A/R170A decreased by 58%. The resistance of FIXa K132A/R170A relative to WT and R150A with respect to prolongation of clotting time by PS was consistent with the reduced PS binding affinity and reduced inhibitor potency of PS for FIXa K132A/R170A (Table I).
Table 3.
Clotting activity relative to no PS of FIXa at Varied PS Concentrations in FIX-PS Double Immunodepleted Plasma
| FIXa Construct | 0 nmole/liter PS | 150 nmole/liter PS | 300 nmole/liter PS |
|---|---|---|---|
| WT FIXa | 1 ± 0.074 | 0.288 ± 0.006 | 0.121 ± 0.002 |
| R150A | 1 ± 0.008 | 0.310 ± 0.005 | 0.098 ± 0.004 |
| K132A R170A | 1 ± 0.065 | 0.677 ± 0.025 | 0.417 ± 0.019 |
The table shows relative FIXa coagulant activity with respect to no PS binding obtained from titration of FIX-PS double immunodepleted plasma with 100 pmole/liter FIXaWT, R150A, and K132AR170A in the absence and presence of PS. The values represent the relative coagulant activity of 100 pmole/liter FIXa WT, in the absence of protein S compared with activity after supplementation of 150 or 300 nM PS into the FIX-PS double depleted plasma. Results are reported as mean relative coagulation activity fraction ± STDEV (n = 3 replicates for each).
The relative resistance of FIXa K132A/R170A coagulant activity to inhibition by PS in an APTT-based assay mirrored the inability of FIXa K132A/R170A to bind PS. To further examine the physiological consequence of this impaired interaction, we evaluated the ability of FIXa WT, R150A and K132A/R170A to support plasma thrombin generation in the FIX-PS-ID plasma. Assays were initiated with limiting TF (0.4 pM) in the presence of 10 pmole/liter FIXa and increasing PS concentrations (Figure 6). Titration of PS into the plasma caused a decrease in peak thrombin for FIXa WT, FIXa R150A, and K132A/R170A (Figure 6A, 6B, and 6C). FIXa K132A/R170A exhibited only a 41% decrease in peak thrombin generation in the presence of 150 nmole/liter PS, compared to FIXa WT which demonstrated a 76% decrease, confirming the contribution of the FIXa HBE to the anticoagulant activity of PS. The decrease in peak thrombin for FIXa R150A at 150 nmole/liter PS was similar to FIXa WT (73%), suggesting that reduced FIXa inhibition by antithrombin does not significantly enhance thrombin generation under these conditions. The peak thrombin and endogenous thrombin potential (ETP) values for FIXa WT, K132A/R170A and R150A FIXa at 0, 150 nM, and 300 nM PS are presented in supplementary Tables S1 and S2, respectively.
Figure 6. Thrombin Generation in FIX-PS Double Immunodepleted Plasma.
Thrombin Generation assays were performed in FIX-PS-ID plasma supplemented with 10 pmole/liter WT FIXa (A) R150A FIXa (B) or K132A/R170A FIXa (C), along with concentrations of PS ranging from 0–250 nmole/liter (TGA were performed at least three times for each protein). Reactions were performed in the presence of a limiting concentration of tissue factor (TF) to selectively generate thrombin through the intrinsic pathway. The addition of PS resulted in reduced thrombin generation as demonstrated by peak thrombin values (D). FIXa K132A/R170A demonstrated a relatively smaller reduction in peak thrombin generation in the presence of PS compared to WT, likely due to its reduced ability to bind PS.Legend: (A–C): 0nmole/liter, 50nmole/liter, 100nmole/liter,150nmole/liter, 200nmole/liter, 250nmole/liter PS. (D) WT, R150A, K132A/R170A.
FIXa infusion-induced clotting in Hemophilia B mice
Because of its markedly reduced affinity for PS (Table I) and resistance to PS anticoagulant effects in plasma (Table III, Figure 6), we employed FIXa K132A/R170A to probe in vivo PS function in a murine thrombosis model. Hemophilia B mice were continuously infused with FIXa WT, R150A, or K132AR170A upstream of an electrolytic injury. FIXa WT (BeneFIXa) or R150A were infused at a rate of 2 µg/hr, and FIXa K132A/R170A was infused at 1.3 µg/hr because, in the FX activation assay, K132A/R170A had 1.5-fold higher activity compared with wild type and R150A FIXa. Importantly, FIXa K132A/R170A demonstrated increased fibrin accumulation relative to FIXa WT or R150A; this increase was seen at all time points beyond 10 minutes to the end of the experiment (60 min). The fibrin data analyzed with ANOVA were significant (p < 0.005). In post-hoc Fisher Least Significant Difference tests, the vehicle-infused thrombi had less fibrin accumulation than each of the other groups (p < 0.005); infusion of the double mutant resulted in significantly higher fibrin accumulation compared with infusion of the other two proteins (p < 0.05). The graphs (Figure 7B and C) are averages, with the colored error bars showing SEMs (unidirectional, to avoid too much noise; data points taken every 10 seconds in the graphs). The enhanced fibrin accumulation for the PS-resistant FIXa mutant indicates enhanced in vivo activity, consistent with its specific resistance to PS-dependent inhibition in the aPTT and thrombin generation assays. Thus, a protease with minimal affinity for PS, FIXa K132A/R170A, demonstrated enhanced in vivo coagulant activity following an electrolytic femoral vein injury in hemophilia B mice. Differences in platelet accumulation did not achieve significance (p = 0.292). These results compellingly indicate the existence of a physiologically significant interaction between PS and the HBE of FIXa.
Figure 7. FIXa Infusion-Induced Clot Formation in Hemophilia B Mice.
(A) Representative image capture of thrombus induction site during local, upstream infusion of vehicle, WT, R150A and K132A/R170A FIXa at 1, 15, 30, 45, and 60 minutes. Each video (see supplemental materials) shows a frame of 2.4 × 3.2 mm, with the femoral vein flowing from upper left to lower right and with the thrombus induction site, where the thrombus is most concentrated on the left. Platelets appear green and anti-fibrin antibody (showing fibrin accumulation) is in red. These data were quantitated for fluorescence intensity of fibrin (B) and platelet accumulation (C) over the 60-minute imaging run. Data were normalized for the amount of injected fluorophore and animal weight (surrogate of blood volume), from images captured every 10 seconds. Lines are averages with error bars showing STDEV (n=3/data set).
Discussion
Our objectives were to define the PS binding site(s) on FIXa and establish PS inhibition of FIXa as an authentic component of the regulation of blood coagulation. We employed FIXa proteins with amino acid substitutions and domain deletions to identify a region extensively overlapping the FIXa heparin-binding exosite (HBE) as critical for PS interaction. Direct and competition binding assays and co-immunoprecipitation from human plasma identified FIXa residues critical for interaction with PS, including K132 ≥ R170 > K126 (chymotrypsinogen numbering; Figure 3, Table I). Similarly, the resistance to PS inhibition of FIXa with these HBE mutations, either as free FIXa or in the intrinsic Xase complex, was proportional to the effects of the mutations on FIXa-PS affinity (Figure 3D). FX activation by FIXa, monitored in gel (Figure S5) indicated direct PS effects on FIXa rather than FXa in the Xase studies using purified components as FXa formation was clearly inhibited in the presence of PS (Figure S5). In contrast, the FIXa Gla domain was not required for PS interaction because deletion of the domain or a lack of γ-carboxylation had little or no effect on PS binding affinity and co-immunoprecipitation from plasma (Figures 1, 4 & Table 2). Deletion of both the Gla and EGF1 domains resulted in a complete lack of PS-FIXa binding and co-immunoprecipitation, suggesting that EGF1 contributes to this interaction (Figure 4). The EGF domains function as spacers to position the active site of FIX/FIXa and related coagulation proteases at a suitable distance from the biological membrane for interaction with activators, cofactors, and substrates60, 61. As the tertiary structure of the EGF2-protease domain protein is intact 37, these results suggest that FIXa and PS may have an extended protein-protein interaction on the membrane surface. Finally, the affinity of PS for FIXa (KD ~ 50 nmole/liter) is consistent with the expected physiological concentration of free PS (~150 nmole/liter) in human plasma and at least four-fold greater than the affinity for the FIX zymogen.
To establish that the inhibition of FIXa by PS is a bona fide component of the regulation of blood coagulation, we compared the activities of wild type FIXa and FIXa K132A/R170A in the presence of PS in multiple physiologic environments. FIXa K132A/R170A was the key to these studies because it had dramatically reduced PS binding but full enzymatic activity (Figures 3D, 3E, and 6 and Table III). We employed the active protease forms of the recombinant FIX proteins to exclude potential differences in zymogen activation. In plasma immunodepleted of both FIX and PS, FIXa K132A/R170A was relatively resistant to the anticoagulant effect of PS (compared with wild type FIXa) in both aPTT-based coagulant activity (Table III) and thrombin generation assays (Figure 6). In contrast, FIXa R150A behaved similarly to wild type protease, suggesting that FIXa inhibition by antithrombin does not substantially influence these endpoints. These results clearly demonstrated the contribution of the FIXa HBE to regulation of coagulation by PS in these physiological environments. Further, we confirmed the FIXa HBE-PS interaction in mice.52 In the electrolytic injury model, direct imaging of thrombi demonstrated co-localization of fluorescently labeled FIXa and PS (Figure 2A). In the infrarenal inferior vena cava injury model, immunoblot analysis of thrombi demonstrated crosslinking of SANPAH-FIXa with PS, a result that further substantiated the in vivo occurrence of this protein-protein interaction (Figures 2B and C). Finally, the ability of these proteases to initiate thrombus formation was assessed in hemophilia B mice. Following injury, FIXa K132A/R170A-injected mice produced significantly greater total fibrin accumulation compared with mice injected with FIXa WT and R150A (p=0.001; ANOVA and posthoc Fisher’s LSD test), whereas differences in platelet accumulation were insignificant (p=0.292)(Figure 7). These results confirm the in vivo relevance of interaction between PS and FIXa in regulation of coagulation. Our data from in vivo and in vitro experiments, in the presence of plasma PS concentration of 150 nM, further suggest that the natural PS plasma concentration exerts an anticoagulant function by directly inhibiting FIXa.
The FIXa HBE contributes to cofactor stability and allosteric communication within the intrinsic Xase complex, antithrombin-independent inhibition of intrinsic Xase activity and plasma thrombin generation by select glycosaminoglycans, and inhibition by antithrombin-heparin. 24–26, 28, 29, 31, 32, 62–65 Our findings establish that interaction of PS with the FIXa HBE is another important APC-independent regulatory mechanism for the propagation phase of coagulation. This critical regulatory role of the FIXa HBE may provide alternative approaches to therapeutic anticoagulation. Currently available treatments include small molecules that directly target the active site of FXa or thrombin (ex: rivaroxaban or dabigatran), or indirectly target coagulation protease active sites via activation of antithrombin (unfractionated and low molecular weight heparins).66, 67. While these anticoagulants are efficacious, they are all associated with a clinically significant risk of bleeding. As an alternative to targeting active sites, the functional exosites of coagulation proteases are an under-explored set of therapeutic targets. Here, we have shown that PS targets the heparin-binding exosite of FIXa. Thus, we suggest that our work has established the basis for exploring a novel, exosite-based therapeutic strategy for the management of thrombosis based on the FIXa-PS interaction.
Supplementary Material
Highlights.
Protein S contributes significantly to hemostasis by binding the protease domain of FIXa, thereby directly inhibiting FIXa.
The heparin-binding exosite of FIXa is critical for Protein S binding.
A double mutation in FIXa, R132A/R170A abolishes Protein S binding.
Disruption of the Protein S-FIXa interaction in mice causes an increased rate of thrombosis.
Acknowledgments
We thank Dr. Howard Fried (UNC, Chapel Hill) for editorial assistance and Dr. Dougald Monroe for help in analyzing the clotting data.
Source of Funding: This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health grant, 5R01HL118557-02 (R.M.) and a Special Project Award from the Bayer Hemophilia Awards Program (J.P.S.)
Non-standard Abbreviations and Acronyms
- PS
Protein S
- FIXa
Factor IXa
- FX
Factor X
- PhosSer
Phosphatidylserine
- APC
Activated Protein C
- TFPI
Tissue Factor Pathway Inhibitor
- FV
Factor V
- FVIIIa
factor VIIIa
- DEGR
Dansyl-Glu-Gly-Arg chloromethyl ketone
- HBE
Heparin-binding exosite
Footnotes
Disclosure: None
References
- 1.Arnljots B, Dahlback B. Protein-s as an in-vivo cofactor to activated protein-c in prevention of microarterial thrombosis in rabbits. Journal of Clinical Investigation. 1995;95:1987–1993. doi: 10.1172/JCI117883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bucciarelli P, Rosendaal FR, Tripodi A, Mannucci PM, De Stefano V, Palareti G, Finazzi G, Baudo F, Quintavalla R, Girte Risk of venous thromboembolism and clinical manifestations in carriers of antithrombin, protein c, protein s deficiency, or activated protein c resistance - a multicenter collaborative family study. Arteriosclerosis Thrombosis and Vascular Biology. 1999;19:1026–1033. doi: 10.1161/01.atv.19.4.1026. [DOI] [PubMed] [Google Scholar]
- 3.Dahm A, Van Hylckama Vlieg A, Bendz B, Rosendaal F, Bertina RM, Sandset PM. Low levels of tissue factor pathway inhibitor (tfpi) increase the risk of venous thrombosis. Blood. 2003;101:4387–4392. doi: 10.1182/blood-2002-10-3188. [DOI] [PubMed] [Google Scholar]
- 4.Kosch A, Kuwertz-Broking E, Heller C, Kurnik K, Schobess R, Nowak-Gottl U. Renal venous thrombosis in neonates: Prothrombotic risk factors and long-term follow-up. Blood. 2004;104:1356–1360. doi: 10.1182/blood-2004-01-0229. [DOI] [PubMed] [Google Scholar]
- 5.Rezende SM, Simmonds RE, Lane DA. Coagulation, inflammation, and apoptosis: Different roles for protein s and the protein s-c4b binding protein complex. Blood. 2004;103:1192–1201. doi: 10.1182/blood-2003-05-1551. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W, Swanson R, Izaguirre G, Xiong Y, Lau LF, Olson ST. The heparin-binding site of antithrombin is crucial for antiangiogenic activity. Blood. 2005;106:1621–1628. doi: 10.1182/blood-2005-02-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horowitz IN, Galvis AG, Gomperts ED. Arterial thrombosis and protein s deficiency. Journal of Pediatrics. 1992;121:934–937. doi: 10.1016/s0022-3476(05)80346-4. [DOI] [PubMed] [Google Scholar]
- 8.Simmonds RE, Ireland H, Lane DA, Zoller B, Garcia de Frutos P, Dahlback B. Clarification of the risk for venous thrombosis associated with hereditary protein s deficiency by investigation of a large kindred with a characterized gene defect. Ann Intern Med. 1998;128:8–14. doi: 10.7326/0003-4819-128-1-199801010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Hackeng TM, Sere KM, Tans G, Rosing J. Protein s stimulates inhibition of the tissue factor pathway by tissue factor pathway inhibitor. Proc Natl Acad Sci USA. 2006;103:3106–3111. doi: 10.1073/pnas.0504240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ten Kate MK, van der Meer J. Protein s deficiency: A clinical perspective. Haemophilia. 2008;14:1222–1228. doi: 10.1111/j.1365-2516.2008.01775.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosing J, Maurissen LFA, Tchaikovski SN, Tans G, Hackeng TM. Protein s is a cofactor for tissue factor pathway inhibitor. Thrombosis research. 2008;122:S60–S63. doi: 10.1016/S0049-3848(08)70021-5. [DOI] [PubMed] [Google Scholar]
- 12.Marlar RA, Neumann A. Neonatal purpura fulminans due to homozygous protein c or protein s deficiencies. Semin Thromb Hemost. 1990;16:299–309. doi: 10.1055/s-2007-1002683. [DOI] [PubMed] [Google Scholar]
- 13.Makris M, Leach M, Beauchamp NJ, Daly ME, Cooper PC, Hampton KK, Bayliss P, Peake IR, Miller GJ, Preston FE. Genetic analysis, phenotypic diagnosis, and risk of venous thrombosis in families with inherited deficiencies of protein s. Blood. 2000;95:1935–1941. [PubMed] [Google Scholar]
- 14.Comp PC, Nixon RR, Cooper MR, Esmon CT. Familial protein s deficiency is associated with recurrent thrombosis. J Clin Invest. 1984;74:2082–2088. doi: 10.1172/JCI111632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwarz HP, Fischer M, Hopmeier P, Batard MA, Griffin JH. Plasma protein s deficiency in familial thrombotic disease. Blood. 1984;64:1297–1300. [PubMed] [Google Scholar]
- 16.Saller F, Brisset AC, Tchaikovski SN, Azevedo M, Chrast R, Fernandez JA, Schapira M, Hackeng TM, Griffin JH, Angelillo-Scherrer A. Generation and phenotypic analysis of protein s-deficient mice. Blood. 2009;114:2307–2314. doi: 10.1182/blood-2009-03-209031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burstyn-Cohen T, Heeb MJ, Lemke G. Lack of protein s in mice causes embryonic lethal coagulopathy and vascular dysgenesis. J Clin Invest. 2009;119:2942–2953. doi: 10.1172/JCI39325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosing J, Maurissen LF, Tchaikovski SN, Tans G, Hackeng TM. Protein s is a cofactor for tissue factor pathway inhibitor. Thrombosis research. 2008;122(Suppl 1):S60–63. doi: 10.1016/S0049-3848(08)70021-5. [DOI] [PubMed] [Google Scholar]
- 19.Heeb MJ, Prashun D, Griffin JH, Bouma BN. Plasma protein s contains zinc essential for efficient activated protein c-independent anticoagulant activity and binding to factor xa, but not for efficient binding to tissue factor pathway inhibitor. FASEB J. 2009;23:2244–2253. doi: 10.1096/fj.08-123174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koppelman SJ, Hackeng TM, Sixma JJ, Bouma BN. Inhibition of the intrinsic-factor-x activating complex by protein-s - evidence for a specifric binding of protein-s to factor-viii. Blood. 1995;86:1062–1071. [PubMed] [Google Scholar]
- 21.Sere KM, Willems GM, Rosing J, Hackeng TM. Protein s multimers are generated in vitro and affect protein s structure-function analyses. Seminars in Hematology. 2006;43:S111–S120. doi: 10.1053/j.seminhematol.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 22.Rand MD, Lock JB, van't Veer C, Gaffney DP, Mann KG. Blood clotting in minimally altered whole blood. Blood. 1996;88:3432–3445. [PubMed] [Google Scholar]
- 23.Hoffman M, Monroe DM, Oliver JA, Roberts HR. Factors ixa and xa play distinct roles in tissue factor-dependent initiation of coagulation. Blood. 1995;86:1794–1801. [PubMed] [Google Scholar]
- 24.Hopfner KP, Lang A, Karcher A, Sichler K, Kopetzki E, Brandstetter H, Huber R, Bode W, Engh RA. Coagulation factor ixa: The relaxed conformation of tyr99 blocks substrate binding. Structure. 1999;7:989–996. doi: 10.1016/s0969-2126(99)80125-7. [DOI] [PubMed] [Google Scholar]
- 25.Brandstetter H, Bauer M, Huber R, Lollar P, Bode W. X-ray structure of clotting factor ixa: Active site and module structure related to xase activity and hemophilia b. Proc Natl Acad Sci USA. 1995;92:9796–9800. doi: 10.1073/pnas.92.21.9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zogg T, Brandstetter H. Activation mechanisms of coagulation factor ix. Biol Chem. 2009;390:391–400. doi: 10.1515/BC.2009.057. [DOI] [PubMed] [Google Scholar]
- 27.Yuan Q-P, Walke EN, Sheehan JP. The factor ixa heparin-binding exosite is a cofactor interactive site: Mechanism for antithrombin-independent inhibition of intrinsic tenase by heparin. Biochemistry. 2005;44:3615–3625. doi: 10.1021/bi047934a. [DOI] [PubMed] [Google Scholar]
- 28.Sheehan JP, Kobbervig CE, Kirkpatrick HM. Heparin inhibits the intrinsic tenase complex by interacting with an exosite on factor ixa. Biochemistry. 2003;42:11316–11325. doi: 10.1021/bi0342923. [DOI] [PubMed] [Google Scholar]
- 29.Sheehan JP, Walke EN. Depolymerized holothurian glycosaminoglycan and heparin inhibit the intrinsic tenase complex by a common antithrombin-independent mechanism. Blood. 2006;107:3876–3882. doi: 10.1182/blood-2005-07-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buyue Y, Misenheimer TM, Sheehan JP. Low molecular weight heparin inhibits plasma thrombin generation via direct targeting of factor ixa: Contribution of the serpin-independent mechanism. J Thromb Haemost. 2012;10:2086–2098. doi: 10.1111/j.1538-7836.2012.04892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buyue Y, Sheehan JP. Fucosylated chondroitin sulfate inhibits plasma thrombin generation via targeting of the factor ixa heparin-binding exosite. Blood. 2009;114:3092–3100. doi: 10.1182/blood-2009-02-203661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang LK, Manithody C, Rezaie AR. Localization of the heparin binding exosite of factor ixa. Journal of Biological Chemistry. 2002;277:50756–50760. doi: 10.1074/jbc.M208485200. [DOI] [PubMed] [Google Scholar]
- 33.Westmark PR, Tanratana P, Sheehan JP. Selective disruption of heparin and antithrombin-mediated regulation of human factor ix. J Thromb Haemost. 2015;13:1053–1063. doi: 10.1111/jth.12960. [DOI] [PubMed] [Google Scholar]
- 34.Chattopadhyay R, Sengupta T, Majumder R. Inhibition of intrinsic xase by protein s: A novel regulatory role of protein s independent of activated protein c. Arteriosclerosis Thrombosis and Vascular Biology. 2012;32:2387–2393. doi: 10.1161/ATVBAHA.112.250928. [DOI] [PubMed] [Google Scholar]
- 35.Lollar P, Fass DN. Inhibition of activated porcine factor ix by dansyl-glutamyl-glycyl-arginyl-chloromethylketone. Arch Biochem Biophys. 1984;233:438–446. doi: 10.1016/0003-9861(84)90465-x. [DOI] [PubMed] [Google Scholar]
- 36.Morita T, Kisiel W. Calcium binding to a human factor ixa derivative lacking gamma-carboxyglutamic acid: Evidence for two high-affinity sites that do not involve beta-hydroxyaspartic acid. Biochem Biophys Res Commun. 1985;130:841–847. doi: 10.1016/0006-291x(85)90493-0. [DOI] [PubMed] [Google Scholar]
- 37.Johnson DJ, Langdown J, Huntington JA. Molecular basis of factor ixa recognition by heparin-activated antithrombin revealed by a 1.7-a structure of the ternary complex. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:645–650. doi: 10.1073/pnas.0910144107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hopfner KP, Brandstetter H, Karcher A, Kopetzki E, Huber R, Engh RA, Bode W. Converting blood coagulation factor ixa into factor xa: Dramatic increase in amidolytic activity identifies important active site determinants. EMBO J. 1997;16:6626–6635. doi: 10.1093/emboj/16.22.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crudele JM, Finn JD, Siner JI, Martin NB, Niemeyer GP, Zhou S, Mingozzi F, Lothrop CD, Jr, Arruda VR. Aav liver expression of fix-padua prevents and eradicates fix inhibitor without increasing thrombogenicity in hemophilia b dogs and mice. Blood. 2015;125:1553–1561. doi: 10.1182/blood-2014-07-588194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pilli VS, Plautz WE, Monroe DM, Majumder R. A novel one-step purification of mouse factor ix. Thrombosis research. 2016;139:125–126. doi: 10.1016/j.thromres.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Misenheimer TM, Buyue Y, Sheehan JP. The heparin-binding exosite is critical to allosteric activation of factor ixa in the intrinsic tenase complex: The role of arginine 165 and factor x. Biochemistry. 2007;46:7886–7895. doi: 10.1021/bi7004703. [DOI] [PubMed] [Google Scholar]
- 42.Heeb MJ, Marzec U, Gruber A, Hanson SR. Antithrombotic activity of protein s infused without activated protein c in a baboon thrombosis model. Thromb Haemost. 2012;107:690–698. doi: 10.1160/TH11-10-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jonnalagadda D, Izu LT, Whiteheart SW. Platelet secretion is kinetically heterogeneous in an agonist-responsive manner. Blood. 2012;120:5209–5216. doi: 10.1182/blood-2012-07-445080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golemis E. Protein-protein interactions: A molecular cloning manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2002. [Google Scholar]
- 45.Krishnaswamy S. Prothrombinase complex assembly: Contributions of protein-protein and protein-membrane interactions toward complex formation. J Biol Chem. 1990;265:3708–3718. [PubMed] [Google Scholar]
- 46.Majumder R, Weinreb G, Lentz BR. Efficient thrombin generation requires molecular phosphatidylserine, not a membrane surface. Biochemistry. 2005;44:16998–17006. doi: 10.1021/bi051469f. [DOI] [PubMed] [Google Scholar]
- 47.Banerjee M, Majumder R, Weinreb G, Wang J, Lentz BR. Role of procoagulant lipids in human prothrombin activation: Soluble phosphatidylserine upregulates and directs factor x(a) to appropriate peptide bonds in prothrombin. Biochemistry. 2002;41:950–957. doi: 10.1021/bi0116902. [DOI] [PubMed] [Google Scholar]
- 48.Majumder R, Weinreb G, Zhai X, Lentz BR. Soluble phosphatidylserine triggers assembly in solution of a prothrombin-activating complex in the absence of a membrane surface. J Biol Chem. 2002;277:29765–29773. doi: 10.1074/jbc.M200893200. [DOI] [PubMed] [Google Scholar]
- 49.Larson PJ, Stanfield-Oakley SA, VanDusen WJ, Kasper CK, Smith KJ, Monroe DM, High KA. Structural integrity of the gamma-carboxyglutamic acid domain of human blood coagulation factor ixa is required for its binding to cofactor viiia. J Biol Chem. 1996;271:3869–3876. doi: 10.1074/jbc.271.7.3869. [DOI] [PubMed] [Google Scholar]
- 50.Camire RM, Bos MH. The molecular basis of factor v and viii procofactor activation. J Thromb Haemost. 2009;7:1951–1961. doi: 10.1111/j.1538-7836.2009.03622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pratt CW, Monroe DM. Microplate coagulation assays. BioTechniques. 1992;13:430–433. [PubMed] [Google Scholar]
- 52.Cooley BC. In vivo fluorescence imaging of large-vessel thrombosis in mice. Arterioscler Thromb Vasc Biol. 2011;31:1351–1356. doi: 10.1161/ATVBAHA.111.225334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah MD, Bergeron AL, Dong JF, Lopez JA. Flow cytometric measurement of microparticles: Pitfalls and protocol modifications. Platelets. 2008;19:365–372. doi: 10.1080/09537100802054107. [DOI] [PubMed] [Google Scholar]
- 54.Dale GL. Coated-platelets: An emerging component of the procoagulant response. J Thromb Haemost. 2005;3:2185–2192. doi: 10.1111/j.1538-7836.2005.01274.x. [DOI] [PubMed] [Google Scholar]
- 55.Jobe SM, Wilson KM, Leo L, Raimondi A, Molkentin JD, Lentz SR, Di Paola J. Critical role for the mitochondrial permeability transition pore and cyclophilin d in platelet activation and thrombosis. Blood. 2008;111:1257–1265. doi: 10.1182/blood-2007-05-092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stolla M, Stefanini L, Andre P, Ouellette TD, Reilly MP, McKenzie SE, Bergmeier W. Caldag-gefi deficiency protects mice in a novel model of fcgamma riia-mediated thrombosis and thrombocytopenia. Blood. 2011;118:1113–1120. doi: 10.1182/blood-2011-03-342352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freedman SJ, Blostein MD, Baleja JD, Jacobs M, Furie BC, Furie B. Identification of the phospholipid binding site in the vitamin k-dependent blood coagulation protein factor ix. J Biol Chem. 1996;271:16227–16236. doi: 10.1074/jbc.271.27.16227. [DOI] [PubMed] [Google Scholar]
- 58.Rawala-Sheikh R, Ahmad SS, Monroe DM, Roberts HR, Walsh PN. Role of gamma-carboxyglutamic acid residues in the binding of factor ixa to platelets and in factor-x activation. Blood. 1992;79:398–405. [PubMed] [Google Scholar]
- 59.Zhong D, Smith KJ, Birktoft JJ, Bajaj SP. First epidermal growth factor-like domain of human blood coagulation factor ix is required for its activation by factor viia/tissue factor but not by factor xia. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:3574–3578. doi: 10.1073/pnas.91.9.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Persson E, Madsen JJ, Olsen OH. The length of the linker between the epidermal growth factor-like domains in factor viia is critical for a productive interaction with tissue factor. Protein Sci. 2014;23:1717–1727. doi: 10.1002/pro.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Persson KE, Villoutreix BO, Thamlitz AM, Knobe KE, Stenflo J. The n-terminal epidermal growth factor-like domain of coagulation factor ix: Probing its functions in the activation of factor ix and factor x with a monoclonal antibody. J Biol Chem. 2002;277:35616–35624. doi: 10.1074/jbc.M205930200. [DOI] [PubMed] [Google Scholar]
- 62.Yuan QP, Walke EN, Sheehan JP. The factor ixa heparin-binding exosite is a cofactor interactive site: Mechanism for antithrombin-independent inhibition of intrinsic tenase by heparin. Biochemistry. 2005;44:3615–3625. doi: 10.1021/bi047934a. [DOI] [PubMed] [Google Scholar]
- 63.Buyue Y, Misenheimer TM, Sheehan JP. Low molecular weight heparin inhibits plasma thrombin generation via direct targeting of factor ixa: Contribution of the serpin-independent mechanism. Journal of Thrombosis and Haemostasis. 2012;10:2086–2098. doi: 10.1111/j.1538-7836.2012.04892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buyue Y, Whinna HC, Sheehan JP. The heparin-binding exosite of factor ixa is a critical regulator of plasma thrombin generation and venous thrombosis. Blood. 2008;112:3234–3241. doi: 10.1182/blood-2008-01-136820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Westmark PR, Tanratana P, Sheehan JP. Selective disruption of heparin and antithrombin-mediated regulation of human factor ix. Journal of Thrombosis and Haemostasis. 2015;13:1053–1063. doi: 10.1111/jth.12960. [DOI] [PubMed] [Google Scholar]
- 66.Graff J, Harder S. Anticoagulant therapy with the oral direct factor xa inhibitors rivaroxaban, apixaban and edoxaban and the thrombin inhibitor dabigatran etexilate in patients with hepatic impairment. Clin Pharmacokinet. 2013;52:243–254. doi: 10.1007/s40262-013-0034-0. [DOI] [PubMed] [Google Scholar]
- 67.Rimsans J, Sylvester KW, Connors JM. Direct thrombin inhibitor for lvad thrombosis: A closer look. Clin Appl Thromb Hemost. 2016 doi: 10.1177/1076029616672583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.