Abstract
Drug delivery systems (DDS) that allow spatially and temporally controlled release of drugs are of particular interest in the field of drug delivery. These systems create opportunities for individually tailored doses of drugs to be administered as well as reduce side effects by localizing the initial drug dose to the organ of interest. We present an electroresponsive DDS in the form of a bioresorbable nanocomposite film which operates at low voltages (<−2 V). The method is based on electrochemically generating local pH changes at an electrode surface to induce dissolution of a pH-sensitive polymer, which is used as the carrier material. We previously demonstrated this proof-of-concept using a poly(methyl methacrylate-co-methacrylic acid) (co-PMMA) copolymer commercially marketed as Eudragit S100 (EGT). However, as EGT is soluble at a pH above 7, experiments were performed in isotonic saline solutions (pH ~6.4). In this work, we have synthesized co-PMMA with a variety of monomer ratios to shift the solubility of the copolymer to higher pH values, and developed a polymer that can be used under physiologically relevant conditions. The generalizability of this system was demonstrated by showing controlled release of different drug molecules with varying parameters like size, hydrophobicity, and pKa. Fluorescein, a hydrophilic model compound, meloxicam, a hydrophobic anti-arthritic medication, curcumin, a small molecule with anti-cancer therapeutic potential, and insulin, a polypeptide hormone used in the treatment of hypoglycemia, could all be released on demand with minimal leakage. The drug loading achieved was ~32 wt% by weight of the co-polymer.
Graphical Abstract
Drug release into a buffered solution at physiological pH from a pH-sensitive, drug-loaded, carrier polymer via electrically induced localized pH changes.
Introduction
In present day medicine, finding innovative strategies for treating diseases is an important research pursuit to address the challenges of existing drug delivery systems (DDS). For example, novel controlled and localized DDSs can permit higher efficiency at lower doses as well as reduced side effects compared to conventional systemic oral or parenteral delivery routes. Such controlled DDSs are particularly promising for the treatment of chronic diseases like cancer, neurological disorders, chronic pain, and diabetes as they can increase patient adherence to medication, reduce side effects of drugs compared to systemic delivery, and offer the possibility of meeting the need for a responsive drug delivery with high controllability.1
Stimuli-responsive polymers have been used to develop controlled DDSs. Some common stimuli used to evoke drug release include light,2,3 pH change,4,5 ultrasound,6 temperature change,4 and electricity.5,7,8 The use of electric stimuli appears to offer major advantages over the other techniques. The instrumentation for electrical stimulation is well developed, of high precision, and could be easily miniaturized to build minimally-invasive implantable devices. Furthermore, electric stimuli can be tightly controlled by changing the voltage, current, and duration of the stimulus. The advent of “transient” electronics based on bio-resorbable materials makes electroresponsive DDSs more feasible and desirable.9 Depending on the positioning of the device, voltage could be generated using ether an external electro-conducting skin patch10 to trigger drug release near the skin or miniaturized implants that are controlled by ultrasound11 and radiofrequency12 to reach more deeply into the body. Electroresponsive materials may be broadly classified as (i) electroactive hydrogels, which use the effect of de-swelling or eroding induced by electric stimuli in order to release a loaded drug,8,13 (ii) conducting polymers, which release charged drugs by partial oxidation or reduction of the polymer backbone,14 or (iii) electroresponsive layer-by-layer (LbL) films that rely on the electrostatic properties of the drug molecules and an oppositely charged protective layer which is dissolved or destabilized by application of electric stiumuli.15–17 Typically, hydrogels require higher voltages (2–25 V) for drug release. Conducting polymers operate at 0.5 – 3 V; however, they are not biodegradable and are well-suited predominantly for charged drugs. LbL films are promising in terms of biocompatibility, but rely heavily on the electrostatic properties of the drug molecules. As a consequence, their interaction with the coating layer render them applicable only for charged drugs.8,18
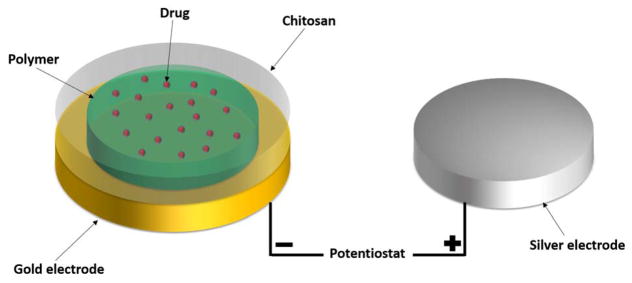
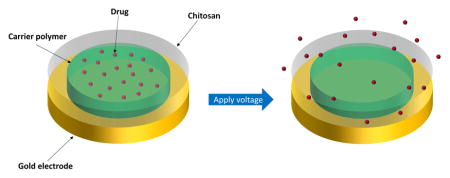
We present a different type of DDS. Our DDS is composed of two polymer layers dropcast onto a gold electrode creating a nanocomposite film (Figure 1). A poly(methyl methacrylate-co-methacrylic acid) (co-PMMA) copolymer is used as a drug-carrying layer and chitosan (CHT) is used as a protective layer. When voltage is applied to the gold electrode, a local pH change is induced at the electrode by reduction of water:
Fig. 1.
Schematic setup of our prepared electrodes.
The localized pH change leads to dissolution of the carrier-polymer, thereby releasing the entrapped drug. The protective chitosan layer is crucial as it prevents delamination of the co-PMMA from the electrode when voltage is applied. However, unlike an LbL film, chitosan does not play an active role in modulating drug release.
In our previous work, we demonstrated the potential of this DDS by performing proof-of-principle experiments in saline solution (pH ~6.4) using a co-PMMA commercially marketed as Eudragit S100 (EGT) which is soluble at a pH above 7.5 The major advantages of the system lie in the use of only FDA-approved materials and the low voltage (<−2 V) needed to control the system. However, this system is not physiologically relevant as the polymer will dissolve at the biological pH of 7.4 with concomitant drug release without any externally applied voltage.5,19–21
To overcome this limitation, we have synthesized copolymers that are based on the same two monomers as EGT: methacrylic acid (AA) and methyl methacrylate (MMA), but with different ratios. The ratio of AA:MMA in EGT is 1:2. The pH-sensitivity of these copolymers is based on the content of acidic or basic groups in each monomer, which are ionized by accepting or releasing protons dependent on the pH. The amount of hydrophobic and hydrophilic monomer influences the pH range over which the ionization takes place and therefore the copolymer is soluble.22 We hypothesized that by increasing the proportion of the hydrophobic methyl methacrylate (MMA) in the copolymer, we can increase the pH at which the carrier-polymer dissolves and thereby releases the drug. We have identified the monomer-ratio 1:5 to be optimal for releasing drugs at a physiological pH. We further show that the synthesized copolymer can be used for releasing drugs with significantly different properties such as size, hydrophobizity, and pKa into Ringer’s solution. Ringer’s solution is a carbonate buffer that is isotonic with blood and has a pH of 7.4 to mimic biological conditions. The drugs chosen for the experiments are used for the treatment of chronic diseases which would particularly benefit from high controllability of this system. Whereas fluorescein (FL) and its sodium salt (FL-Na) are used as drug surrogates, commonly used drugs like meloxicam (MX) as anti-arthritic medication or insulin (FITC-INS) for hyperglycemia were tested. Experiments were also run with curcumin (CM), a promising molecule with potential anti-cancer activity.
Materials
Eudragit S100 was received as a gift from Evonik Industries. Fluorescein free acid and disodium salt, sodium hydroxide, Falcon 96-well plates, chitosan (low molecular weight, molecular weight 50 000–190 000 Da), curcumin, Insulin-FITC labeled human (Lot # SLBG4503V), dimethyl sulfoxide (DMSO), methyl methacrylate (MMA), methacrylic acid (AA) and azobisisobutyronitrile (AIBN) were purchased from Sigma-Aldrich. Meloxicam was obtained from TCI America. DropSens-electrodes (DRP-220AT) were acquired from Metrohm and were used for all stimulation experiments.
Methods
All experiments and measurements were performed at room temperature in triplicate, unless otherwise stated.
Synthesis of poly(methyl methacrylate-co-methacrylic acid)
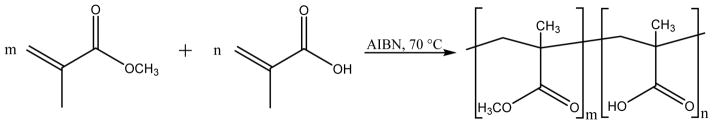
To synthesize the copolymers in different molar ratios of AA:MMA in a range from 1:3 to 1:9 a free radical polymerization method was used. (Table 1) The needed amounts of methyl methacrylate (MMA) and methacrylic acid (AA) were mixed in bulk in glass tubes to make a 3 mL reaction mixture. As initiator 0.1 g/mL azobisisobutyronitrile (AIBN) was added and the glass tubes were sealed. The reaction mixture was heated to 70 °C and kept at this temperature under constant stirring until full polymerization was achieved (~5 minutes). Afterwards the resulting copolymer was dissolved in acetone and poured into water. The solid product was filtered, washed with water, and dried under vacuum.
Table 1.
Used volumes for synthesis of different molar ratios and actual ratios of co-PMMA.
| Polymer | Volume/mL | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| P3 | P4 | P5 | P6 | P7 | P8 | P9 | |
| AA | 2.373 | 2.504 | 2.590 | 2.650 | 2.695 | 2.730 | 2.757 |
| MMA | 0.627 | 0.496 | 0.410 | 0.350 | 0.305 | 0.270 | 0.243 |
|
| |||||||
| Molar ratio (AA:MMA) | 1:3 | 1:4 | 1:5 | 1:6 | 1:7 | 1:8 | 1:9 |
Scanning electron microscopy (SEM) imaging
The electrodes were prepared as mentioned above without loading any drug and broken into two pieces along the center of the nanofilm. Each piece was mounted onto aluminum stubs and sputter-coated with Au/Pd. The images were taken with a Zeiss Sigma FESEM.
Molecular Weight and its distribution of co-PMMA
To determine the molecular weight and its distribution, 3 mg of each synthesized polymer were dissolved in 1 mL tetrahydrofuran (THF) and measured by gel permeation chromatography (GPC).
Preparation of drug-loaded polymer nanofilms
Disposable screen-printed gold working electrodes (WE) with silver pseudo-reference electrodes (Dropsens drp-220AT) were used for all experiments. All polymers were dissolved in DMSO to make a solution with a concentration of 20 μg/μL. The polymer solution was mixed in a 1:1 (9:1 for fluorescein) ratio with a drug solution with a concentration of 10 μg/μL in DMSO. 2 μL (1.41 μL for FL) of the resulting solution was deposited on the WE. The samples were then dried for 30 min in a 65 °C oven to ensure removal of the DMSO. 10 μL of a 0.01 % chitosan solution in 0.1 M HCL was dropcast onto the top of the sample and allowed to dry at room temperature.
Calibration curves for FL, Na-FL, MX, CM, and IN
For every drug, 1 μL of a 10 μg/μL (1 μg/μl for FL and Na-FL) solution in DMSO was added to a well filled with 200 μL of Ringer’s solution. After mixing, 100 μL of resultant solution were placed in another well filled with 100 μL of Ringer’s solution. By repeating this procedure a series of 7 half-dilutions were prepared in which a 8th well was left with only Ringer’s solution. Afterwards 100 μL of 0.1 N NaOH solution were added to every well. The absorbance of each well containing different concentrations of various drugs were measured by using a plate reader. For MX the absorbance was scanned in a range from 230 nm to 550 nm. For the measurement of the absorbance of the other drugs, filters at wavelengths of 492 nm and 562 nm (450 nm and 630 nm for CM) were used.
Timed release of FL after a single pulse
200 μL of Ringers solution was placed on the WE. The electrode was shorted such that the silver electrode acted as both the counter and reference electrode. A stimulus of −1.5 V was applied for 60 s. The samples were collected at 1, 3, 5, 7, and 15 min after the stimulus. Sampling consists of removing 100 μL of solution from the WE, placing it into a well in a 96-well plate and replacing with 100 μL of fresh Ringers solution. Each 100 μL sample in the 96-well plate was diluted with 100 μL 0.1 N NaOH and run on an Azure Biosystems microplate reader. The sample concentration was determined by comparison to the standard curve.
Pulsed release of FL, FL-Na, CM, MX, and FITC-Insulin
The pulsed release of different drugs was tested by using a constant voltage or current. For the voltage experiments 200 μL of Ringer’s solution were pipetted onto the electrode followed by repeatedly applied stimuli of −1.5 V for 20 s once every 6 min. The measurement ended after 10 pulses to make a one-hour scan. The solution was sampled 4 min after each pulse. As a control, an electrode was run in parallel without applying voltage. The experiments using constant current used stimuli of −300 μA for 20 sec once every 3 min and ended after 20 pulses to make a one-hour scan. The preparation of the samples and absorbance measurements followed the same scheme as already mentioned above.
Effect of voltage, time, and current on release of FL
To measure the effect of voltage and time on the release on FL the setup of the electrodes, preparation of the samples and measurement of the absorbance were repeated in the same way as already mentioned above. Different stimuli of −0.5 V, −0.8 V, −1 V, and −1.5 V were repeatedly applied for 20 s every 4 min. After 5 pulses the measurement was ended to make a 20-min scan. One electrode was run without any voltage applied as a control. The effect of current was determined by applying a single 20 s stimulus with different currents of −50 μA, −100 μA, −200 μA, and −300 μA.
Results and discussion
Synthesis and characterization of co-PMMA nanofilms
In our previous work, we introduced EGT (AA:MMA is 1:2) as a polymer that can release drugs into an isotonic saline solution (pH 6.4) with repeated electric stimuli.5 Ideally, a release of the drug should only appear when voltage is applied. However, as EGT is soluble above pH 7, it leaked significantly at the physiological pH of 7.4, and therefore, it is not practical for physiological use. To address this limitation, we have performed a systematic study by increasing the amount of methyl methacrylate (MMA) in the copolymer in order to increase the pH value at which the polymer would be soluble. (Table 1) Based on the work of Barba et al., we hypothesized that the increase in MMA in the copolymer would solve solubility issue at a physiological pH.22,23
Gel permeation chromatography (GPC) was used to determine the average molar weights of each co-PMMA. Every polymer composition showed a similar range of molecular weights (Mw~5–7·104 g mol−1 and Mn~1–3·104 g mol−1), which can be found in the supplementary information.
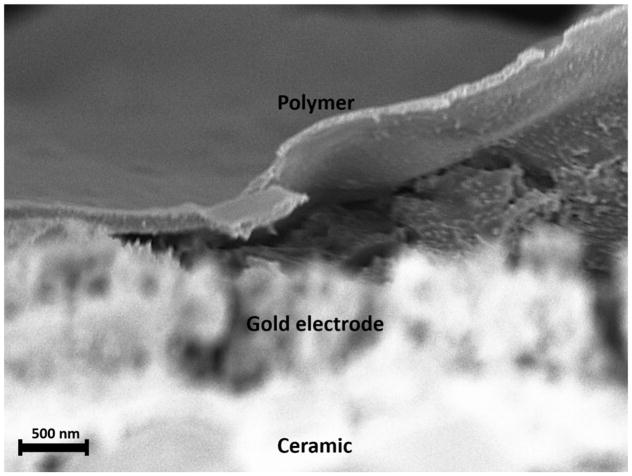
The size of the polymer films on the electrodes were determined by SEM imaging. The electrodes coated with nanofilms were broken in the middle and sputter-coated with Au/Pd. The image shows the polymer film on the electrode surface with a thickness of ~300 nm. (Figure 3)
Fig. 3.
SEM image taken of the surface of a prepared electrode. The polymer layer peeled off the electrode due to mechanical agitation during breaking the electrode. Average thickness of the polymer layer towards the center was ~300 nm.
Determination of optimal co-PMMA for drug release at physiological pH
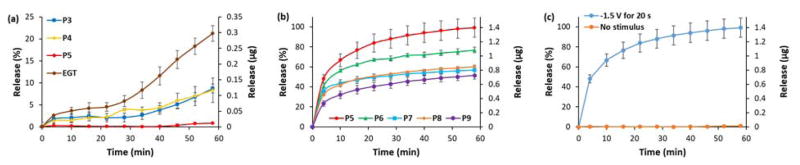
Our first goal was to identify a polymer that minimizes leakage at pH 7.4. To evaluate the influence of increasing the amount of MMA in the copolymer on drug leakage, we encapsulated fluorescein (FL) as a model compound into co-PMMA made with each monomer ratio (Table 1) and tested its passive release in Ringer’s solution over one hour. No electric stimulus was applied. FL was used as a drug surrogate for the ease of detection and quantification. Thereafter, each DDS was tested by applying a voltage of −1.5 V between the coated DDS electrode and a silver counter electrode for 20 seconds. This pulse was repeated every 5 min for a total of 10 electric stimuli. The total time of applied voltage was 3.17 min over 1 h.
As expected, a high amount of FL (21 % over 1 hour) leaked out of the EGT layer caused by its solubility at the pH of 7.4. Shown in Fig. 4a, the leakage of FL tends to decrease for higher ratios of MMA in the copolymer, up until the polymer composition with a ratio of 1:5 (AA:MMA) which stopped releasing the drug without an electric stimulus (<1 % over 1 h). The copolymers with higher ratios in terms of MMA showed similar behavior. The decreased drug leakage with an increasing amount of MMA in the copolymer matches the expected shift in solubility of the carrier polymer at a physiological pH at higher ratios.22,23
Fig. 4.
FL release from polymer-CHT films in Ringer’s solution with 10 pulses of −1.5 V for 20 s. (a) FL leakage from polymer films, (b) release of non-leaking polymer films and (c) behavior of P5. Full set of data for each co-PMMA ratio can be found in the supplemental information.
Every tested copolymer released a reasonable amount of drug when electrically stimulated. The polymer with a ratio of 1:5 could release up to 99 % of FL after 10 pulses of 20 s, whereas the percentage of released drug diminished with an increasing amount of MMA in the polymer – with co-PMMA 1:9 releasing the least amount of drug at 51 % under the same stimulation parameters. (Fig. 4b). This experiment also demonstrated that the localized pH change at the surface of the electrode is strong enough to overpower the buffer.
As proposed, we were able to shift the solubility of the drug-loaded layer by increasing the amount of MMA in the copolymer, which led to a decrease in leakage at a biological pH value. The side effect of the increasing ratio of MMA was the decrease of total drug release.
The optimal polymer for physiological use was deemed to be P5 for which the FL leakage was minimal, but stimulated FL release was maximal. P5 was probed further by testing the release of other drugs as well as the influence of time and voltage on the release. (Fig. 4c)
Pulsed release of FL
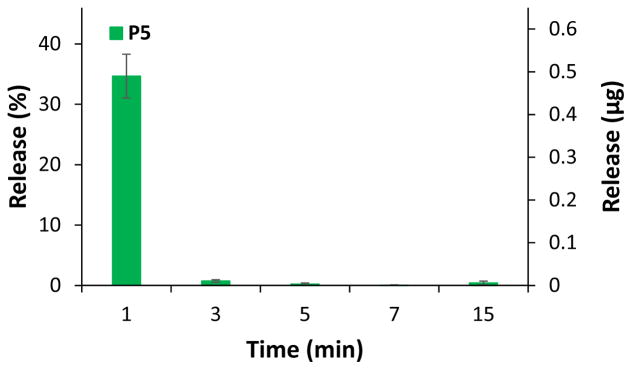
To make sure the polymer film would not continue to release drug molecules following electric stimulus, we studied the behavior of drug release after a single pulse. The tests were performed with P5 using FL as drug surrogate. In order to determine the timed release of the molecule in response to the electric stimulus in a buffer system close to human serum like Ringer’s solution, a 60 s long pulse with voltage of −1.5 V was applied. Samples were collected at fixed times (1 min, 3 min, 5 min, 7 min, and 15 min) after the stimulus.
Every polymer showed a similar release pattern, in which the major part of the drug was already released into the solution after 1 min. A very small amount of released FL could be found after 3 min whereas after a longer period of time no further release could be observed (Figure 5). The results show that the local pH change induced by an electric stimulus in a buffer solution is compensated fairly quickly. To confirm this hypothesis, the pH was measured before and after the pulse, showing no difference in value. The results further demonstrate that the layer of CHT does not pose as a significant barrier to diffusion of the FL released from the co-PMMA.
Fig. 5.
Timed release pattern of FL after a single electric stimulus of −1.5 V for 60 s for P5. Full set of data for each co-PMMA ratio can be found in the supplemental information.
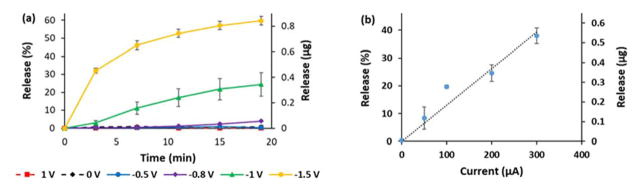
Effect of voltage, time, and current on FL release
To understand the effect of different voltages on the DDS, we tested the release of FL by P5 in Ringer’s solution using 5 repeated 20-s pulses every 4 min at different voltages (+1 V, 0 V, −0.5 V, −0.8 V, −1 V, and −1.5 V). The experiments with a fixed current were run as a single 20-s pulse at different values (−50 μA, −100 μA, −200 μA, and −300 μA).
As expected, no release of FL into Ringer’s solution could be observed at +1 V, 0 V, as well as −0.5 V. This is another confirmation for our proposed release mechanism, which is based on the reduction of water induced by applied negative voltages of at least −0.6 V vs. Ag/AgCl.25,26 At more negative voltages, the release of FL into the solution increased with a total release of 84 % of FL after 5 stimuli with −1.5 V. (Figure 6) This behavior is expected as the more negative electrical potentials create a larger local pH change at the electrode surface, which in turn releases more rapidly drug from the polymer film.
Fig. 6.
Release pattern of FL in Ringer’s solution for P5 at different (a) voltages and (b) currents.
Also, we were able to show a linear effect between the increase of current and the release of FL. The possibility of influencing the release of a drug surrogate like FL by changing the parameters voltage, time and current leads to a highly controllable and finely tunable DDS.
Pulsed release of FL-Na, CM, MX, and FITC-INS at physiological pH
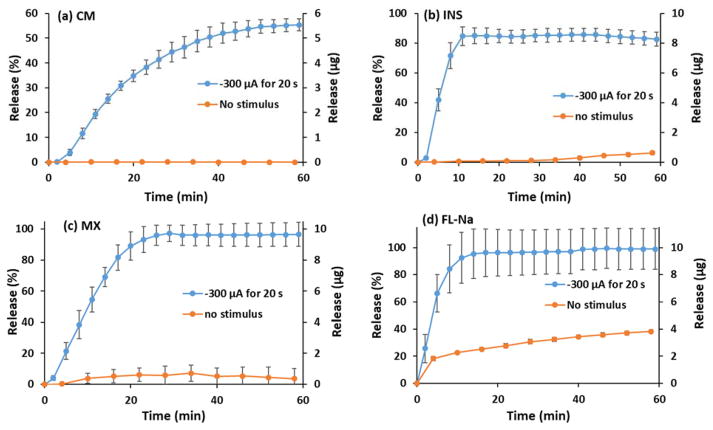
Most drug delivery systems are made for releasing a specific drug. In order to demonstrate the generality of our DDS, we repeated the pulsed-release experiment with FL-Na,27 as a model hydrophilic compound and drug surrogate, and three different drugs. Curcumin (CM)28 is a hydrophilic molecule that has a bright spectrum of pharmacological properties due to its inhibitory effects on metabolic enzymes and has potential use in various chronic diseases. Meloxicam (MX)29 is a nonsteroidal anti-inflammatory drug with hydrophobic properties, which is used as a fever reducer or for its analgesic behavior. Insulin is a hormone that regulates metabolism and is commonly used in the treatment of diabetes. For the ease of detection a FITC-labeled version of the peptide (FITC-INS)30,31 was used. Each drug was chosen because of their applications in the treatment of chronical diseases, in which a controlled delivery system can aid the delivery of these particular drugs and higher their efficiency. 20 pulses at −300 μA for 20 s each were applied every 3 min to P5 loaded with the different drugs. The same drugs were tested using P5 as carrier-polymer applying 10 pulses of −1.5 V for 20 s every 6 min. Control experiments were performed without the application of voltage or current to characterize leakage from these films. The results of the experiments with constant voltage can be found in the supplementary information.
Every DDS prepared with different drugs loaded on P5 (drug-loading 32 wt%) showed a controllable release pattern with each of the drugs. Figure 7 shows the release and leak of the different molecules. It appears that 99 % of the loaded FL-Na could be released by repeated electric stimuli. However, 38 % of FL-Na leaked out into the solution without an external stimulus. This behavior can be explained with the very high water solubility (500 mg mL−1) of this hydrophilic molecule.27 Contrary to this result, the MX and FITC-INS showed only leaks of 7 % and 6 %, respectively, from minor passive diffusion. This could be attributed to the hydrophobicity of MX (water solubility = 7.15 μg mL−1)29 and combination of hydrophobicity and size of FITC-INS (molecular weight = 5907.57 g mol−1).31 The amount of stimulated drug released is around 82 % for INS and 92 % for. CM did not show any leaking issues and up to 3 % of the incorporated drug could be released under the given stimulation conditions. Despite major differences in properties like size, molecular weight, pKa and hydrophobicity, every molecule could be controllably released into a solution at a physiological pH value. The results are promising considering no further optimization of parameters like the amount of drug-loading, the duration of electric stimuli or the molecular weight of the loaded polymer was performed. After the experiments were finished, a sodium hydroxide solution was put onto the electrodes to test, whether the non-released drug molecules were electrically degraded or remained encapsulated within the coffee ring at the edges of the polymer film.5 The results of absorbance measurements added up to 100 % of the incorporated drugs, which confirms that the remaining drug is stuck in the coffee rings on the electrodes and does not get degraded. Only CM showed a possible degradation of about 10 %.
Fig. 7.
Pulsed release and leakage with −300 μA for 20 s each of (a) CM, (b) INS, (c) MX, and (d) FL-Na with P5 as carrier-polymer. Because of the inhomogeneity of the polymer film, the release-current profile is not linear in this case. The results of the experiments with constant voltage can be found in the supplementary information.
Conclusions
We could enhance the properties of our developed electro-responsive drug delivery system by changing the ratio of the AA-MMA-copolymer on which the drugs are loaded. By increasing the amount of MMA in the polymer we were able to shift the solubility of the nanolayer to higher pH values in order to make it more stable under biological conditions. Furthermore, it could be shown that the release patterns in terms of the influence of applied voltage are in line with our previous studies. Various drugs could be controllably released into Ringer’s solution with minor to no passive diffusion from the layer despite their very different physicochemical properties. Notably, less water-soluble drugs show reduced or no passive release. Further studies on this DDS could lead to an optimization of different variables including controlling the stimulation parameter (e.g., duration of electric stimuli, magnitude of current, etc.) as well as altering the composition of the nanofilm (e.g., molecular mass of the polymer or amount of drug-loading). This DDS is a promising way to design on-demand drug delivery devices which, when coupled to bio-resorbable electronics, could controllably release drugs to fight different chronic illnesses, especially where repeated drug doses are needed.
Supplementary Material
Fig. 2.
Reaction of methyl methacrylate (MMA, m) and methacrylic acid (AA, n) to synthesize poly(methyl methacrylate-co-methacrylic acid) with different m : n ratios.
Acknowledgments
We are grateful to Devleena Samanta for helpful discussions and Evonik Industries for the gifted Eudragit S100. SEN thanks the German Academic Exchange Service (DAAD) for providing a research opportunity, and CFC thanks the Center for a Molecular Analysis and Design (CMAD) for a fellowship. This work was supported by NIH R01-grant 12409085.
Footnotes
Conflicts of interest
There are no conflicts to declare.
References
- 1.Wang Y, Kohane DS. Nat Rev Mater. 2017;2:17020. [Google Scholar]
- 2.Shanmugam V, Selvakumar S, Yeh CS. Chem Soc Rev. 2014;43:6254–6287. doi: 10.1039/c4cs00011k. [DOI] [PubMed] [Google Scholar]
- 3.McCoy CP, Rooney C, Edwards CR, Jones DS, Gorman SP. J Am Chem Soc. 2007;129:9572–9573. doi: 10.1021/ja073053q. [DOI] [PubMed] [Google Scholar]
- 4.Schmaljohann D. Adv Drug Delivery Rev. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Samanta D, Mehrotra R, Margulis K, Zare RN. Nanoscale. 2017;9:16429–16436. doi: 10.1039/c7nr06443h. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara KW. Adv Drug Delivery Rev. 2008;60:1097–1102. doi: 10.1016/j.addr.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Tavares AC, Gauthier MA. J Mater Chem B. 2016;4:3019–3030. doi: 10.1039/c6tb00049e. [DOI] [PubMed] [Google Scholar]
- 8.Murdan S. J Controlled Release. 2003;92:1–17. doi: 10.1016/s0168-3659(03)00303-1. [DOI] [PubMed] [Google Scholar]
- 9.Fu KK, Wang Z, Dai J, Carter M, Hu L. Chem Mater. 2016;28:3527–3539. [Google Scholar]
- 10.Kalia YN, Naik A, Garrison J, Guy RH. Adv Drug Delivery Rev. 2004;56:619–658. doi: 10.1016/j.addr.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Charthad J, Baltsavias S, Samanta D, Chang TC, Weber MJ, Hosseini-Nassab N, Zare RN, Arbabian A. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS; 2016; pp. 541–544. [DOI] [PubMed] [Google Scholar]
- 12.Hwang SW, Huang X, Seo JH, Song JK, Kim S, Hage-Ali S, Chung HJ, Tao H, Omenetto FG, Ma Z, Rogers JA. Adv Mater. 2013;25:3526–3531. doi: 10.1002/adma.201300920. [DOI] [PubMed] [Google Scholar]
- 13.Kwon IC, Bae YH, Kim SW. Nature. 1991;354:291–293. doi: 10.1038/354291a0. [DOI] [PubMed] [Google Scholar]
- 14.Svirskis D, Travas-Sejdic J, Rodgers A, Garg S. J Controlled Release. 2010;146:6–15. doi: 10.1016/j.jconrel.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Delcea M, Möhwald H, Skirtach AG. Adv Drug Delivery Rev. 2011;63:730–747. doi: 10.1016/j.addr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Boulmedais F, Tang CS, Keller B, Vörös J. Adv Funct Mater. 2006;16:63–70. [Google Scholar]
- 17.Schmidt DJ, Moskowitz JS, Hammond PT. Chem Mater. 2010;22:6416–6425. doi: 10.1021/cm102578j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guarino V, Zuppolini S, Borriello A, Ambrosio L. Polymers. 2016;8:185. doi: 10.3390/polym8050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigues S, Dionísio M, López CR, Grenha A. J Funct Biomater. 2012;3:615–641. doi: 10.3390/jfb3030615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. [accessed 30 January 2018];Asacol. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/019651s023lbl.pdf.
- 21.Thakral S, Thakral N, Majumdar DK. Expert Opin Drug Delivery. 2013;10:131–149. doi: 10.1517/17425247.2013.736962. [DOI] [PubMed] [Google Scholar]
- 22.Barba AA, Dalmoro A, De Santis F, Lamberti G. Polym Bull. 2009;62:679–688. [Google Scholar]
- 23.Yessine MA, Lafleur M, Petereit HU, Meier C, Leroux JC. Biophys Biochim Acta. 2003;1613:28–38. doi: 10.1016/s0005-2736(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 24.Giordanengo R, Viel S, Hidalgo M, Allard-Breton B, Thévand A, Charles L. Analytica Chimica Acta. 2009;654:49–58. doi: 10.1016/j.aca.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Lie A, Guex A, Vachicouras N, Hight AE, Brown MC, Lee DJ, Phanie S, Lacour P. J Mater Chem B. 2015;3:5021–5027. doi: 10.1039/c5tb00099h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Apollo NV, Maturana MI, Tong W, Nayagam DAX, Shivdasani MN, Foroughi J, Wallace GG, Prawer S, Ibbotson MR, Garrett DJ. Adv Funct Mater. 2015;25:3551–3559. [Google Scholar]
- 27. [accessed 30 January 2018];Fluorescein sodium – DrugBank. https://www.drugbank.ca/salts/DBSALT001432.
- 28. [accessed 30 January 2018];Curcumin – DrugBank. https://www.drugbank.ca/drugs/DB11672.
- 29. [accessed 30 January 2018];Meloxicam – DrugBank. https://www.drugbank.ca/drugs/DB00814.
- 30. [accessed 30 January 2018];Insulin Human – DrugBank. https://www.drugbank.ca/drugs/DB00030.
- 31. [accessed 30 January 2018];FITC-labeled Insulin – SigmaAldrich. https://www.sigmaaldrich.com/catalog/product/sigma/i3661.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.