Abstract
Aims
Recent studies have shown that in more than half of apparently unexplained sudden cardiac arrests (SCA), a specific aetiology can be unmasked by a careful evaluation. The characteristics and the extent to which such cases undergo a systematic thorough investigation in real-life practice are unknown.
Methods and results
Data were analysed from an ongoing study, collecting all cases of out-of-hospital cardiac arrest in Paris area. Investigations performed during the index hospitalization or planned after discharge were gathered to evaluate the completeness of assessment of unexplained SCA. Between 2011 and 2016, among the 18 622 out-of-hospital cardiac arrests, 717 survivors (at hospital discharge) fulfilled the definition of cardiac SCA. Of those, 88 (12.3%) remained unexplained after electrocardiogram, echocardiography, and coronary angiography. Cardiac magnetic resonance imaging yielded the diagnosis in 25 (3.5%) cases, other investigations accounted for 14 (2.4%) additional diagnoses, and 49 (6.8%) patients were labelled as idiopathic ventricular fibrillation (IVF) (48.7 ± 15 years, 69.4% male). Among those labelled IVF, only 8 (16.3%) cases benefited from a complete workup (including pharmacological testing). Younger patients [odds ratio (OR) 6.00, 95% confidence interval (CI) 1.80–22.26] and those admitted to university centres (OR 3.60, 95% CI 1.12–12.45) were more thoroughly investigated. Genetic testing and family screening were initiated in only 9 (18.4%) and 12 (24.5%) cases, respectively.
Conclusion
Our findings suggest that complete investigations are carried out in a very low proportion of unexplained SCA. Standardized, systematic approaches need to be implemented to ensure that opportunities for specific therapies and preventive strategies (including relatives) are not missed.
Keywords: Sudden death, Ventricular fibrillation, Workup, Prevention, Genetics, Family
Introduction
Sudden cardiac arrest (SCA), defined as a natural and unexpected collapse of presumed cardiac aetiology,1 is a major contributor to cardiovascular mortality (∼ 50%), with an estimated number of 300 000 cases a year in Europe.2,3
While SCA is principally related to coronary artery disease (80%),4 other causes include cardiomyopathies and electrical disorders, which are of particular importance due to their often dominant inherited (or familial) pattern and the potential for preventive measures among relatives.5–11 The diagnosis of idiopathic ventricular fibrillation (IVF) is reserved for cases where there is no evidence of underlying structural or electrical heart disease.12,13 However, this requires a thorough and comprehensive evaluation and should be a diagnosis of exclusion. Studies have suggested that a definitive diagnosis could be unmasked in more than half of apparently unexplained SCA, through a complete systematic medical assessment.14–16 This is crucial from several perspectives including prognostication, implementation of specific therapy when possible, appropriate lifestyle counselling, and also optimization of screening and prevention among relatives.5,8,10,11,17,18
However, clinical characteristics of unexplained cardiac arrests and the extent to which such a comprehensive cardiac assessment is carried out in the real-life setting has not been investigated so far at the community level. Hence, we aimed to describe characteristics of patients labelled as IVF, evaluate the completeness of investigations in these cases, and address factors associated with more exhaustive investigations through a large ongoing prospective registry of SCA in Paris Area.
Methods
Study setting
The methodology of this study is consistent with the STROBE checklist for observational studies.19 The Paris Sudden Death Expertise Center (Paris-SDEC) registry is an ongoing study and has been described previously.20–22 Briefly, it is a prospective population-based registry comprising Paris and suburbs, encompassing a residential population of approximately 6.7 million and covering 762 km2. Owing to a close collaboration with all the pre-hospital emergency medical services (EMS), every adult (≥18 years) case of out-of-hospital cardiac arrest occurring in the area of interest is systematically and prospectively enrolled in the Paris-SDEC registry since May 2011. In Paris, management of out-of-hospital cardiac arrest involves the firefighters and mobile emergency units, including at least one trained physician in emergency medicine. Patients in whom a return of spontaneous circulation is achieved are referred to a centre with an intensive care unit (ICU) and coronary intervention facilities. Patients aged less than 18 years and cardiac arrests occurring outside the geographical area of interest are excluded. Regular audits on the registry show that 99% of cardiac arrest cases admitted alive to the hospital are detected.20
The study is conducted in compliance with Good Clinical Practice, French Law, and the French data protection law. Data file of the Paris-SDEC registry was declared to and authorized by the French data protection committee (Commission Nationale de l'Informatique et des Libertés, CNIL).
Study population
Among all cases of out-of-hospital cardiac arrest collected in the Paris-SDEC registry, survivors of SCA of cardiac origin were analysed. According to definitions from recent guidelines,1 SCA was defined as an unexpected cardiac arrest without obvious extra-cardiac cause, occurring with a rapid witnessed collapse within 1 h after the onset of symptoms, or if unwitnessed, occurring within the 24 h after the last contact, in the absence of a prior terminal condition. Those likely due to other non-cardiac circumstances (such as trauma, drowning, hanging etc.) were excluded.
Data collection
Utstein23 templates for patient data collection were followed. General data included demographic characteristics and location of SCA (residential or public setting). Pre-hospital data recorded included SCA circumstances, preceding warning symptoms, presence of bystander, bystander cardiopulmonary resuscitation before EMS arrival, presence of shockable rhythm before advanced life support, and time intervals from collapse to basic life support and from basic life support to return of spontaneous circulation. Past medical history (cardiac disease, cardiovascular risk factors, comorbidities, treatment), final diagnosis and preventive measures implemented [implantable cardioverter defibrillator (ICD), family screening] were collected from both ICU and cardiology medical reports. The diagnostic process was undertaken by the local medical staff, but two investigators reviewed each medical report to ensure that appropriate diagnostic criteria were used, and provided final central adjudication. In cases of divergent opinion, a third expert was asked to arbitrate. The vital status and the neurological outcome at hospital discharge were assessed for all patients. Survival with favourable neurological prognosis was defined by a Cerebral Performance Category (CPC) score 1 or 2, with 1 representing full recovery or mild disability and 2, moderate disability but independent in activities of daily living.
Assessment of medical investigations
Medical investigations initiated and carried out among survivors were at the discretion of the managing medical team (real-world setting). Data regarding all investigations performed during the index hospitalization for SCA or planned subsequently after discharge were gathered to evaluate the completeness of initial assessment in cases labelled as IVF. The referent physician of each case was also contacted to collect all examinations performed and clinical events during the follow-up. In addition to 12-lead electrocardiogram (ECG), echocardiography and coronary angiography, cardiac magnetic resonance imaging (MRI) and provocative tests (ergonovine, ajmaline, and adrenaline) are those which have been demonstrated to have substantial yield in this clinical setting.14,15,24–34 Complete medical investigation was therefore defined as performance of cardiac MRI, ergonovine challenge, and pharmacological tests, besides the traditional tests. Diagnostic criteria were based on the latest international guidelines or major previous reports (see Supplementary material online, File S1).12,31,32,34,35 Data on performance of electrophysiological study,13,36 exercise testing,14 Holter-ECG,37 cardiac biopsy,38 signal averaged ECG,39 right ventricular angiography,40 Fourier phase analysis of gated blood pool single-photon emission computed tomography,41 myocardial voltage mapping,42 and genetic tests were also collected, as these tests can potentially reveal specific phenotypes in some cases of unexplained SCA.
We finally hypothesized that academic centres (with dedicated electrophysiology units), and specific patients’ characteristics, could be associated with more thorough investigations.
Statistical analysis
Continuous data were expressed as mean ± standard deviation. Categorical data were expressed as frequencies and percentages. Comparisons used the χ2 or Fisher’s exact test for categorical variables and Student’s t-test or Mann–Whitney–Wilcoxon test, when appropriate, for continuous variables. Univariate logistic regressions were used to assess the association of completeness of investigations with patient characteristics such as age and sex, as well as treating centre. Missing data on study variables were no more than 1%, except for EMS call before SCA occurrence (15.6%), collapse to basic life support (9.2%) and basic life support to return of spontaneous circulation (14.1%) delays. Results were considered statistically significant at P-value <0.05. All analyses were two-tailed. Statistical analysis was performed using R software, version 3.3.2 (R Project for Statistical Computing).
Results
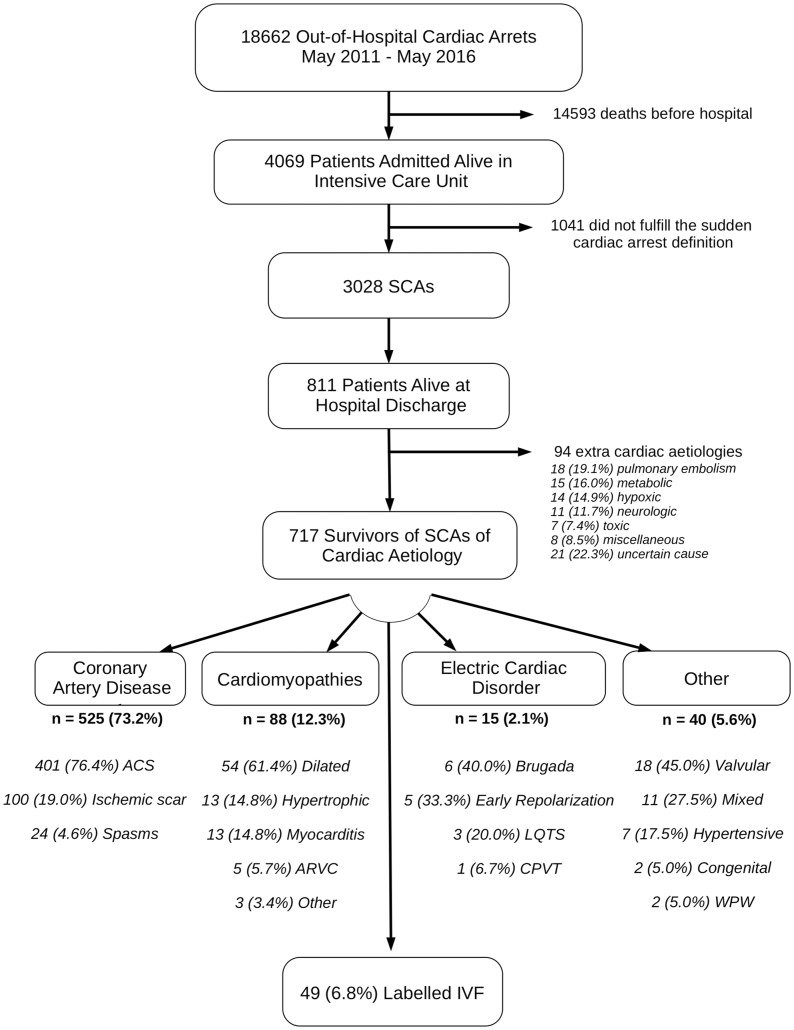
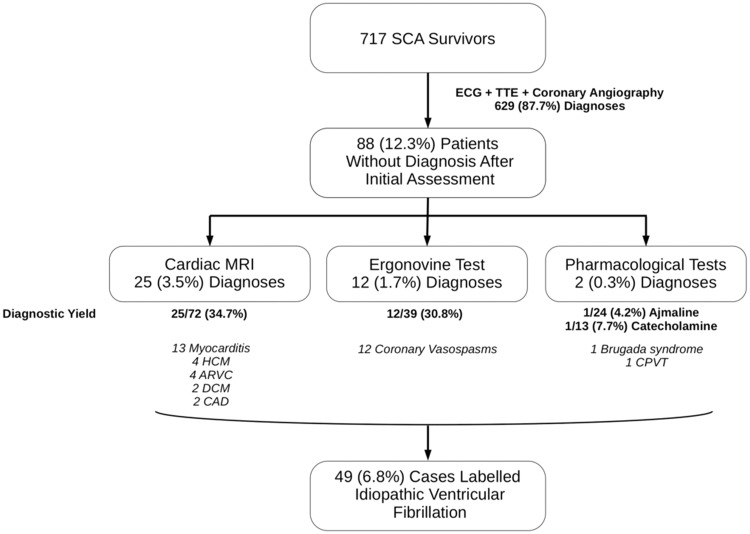
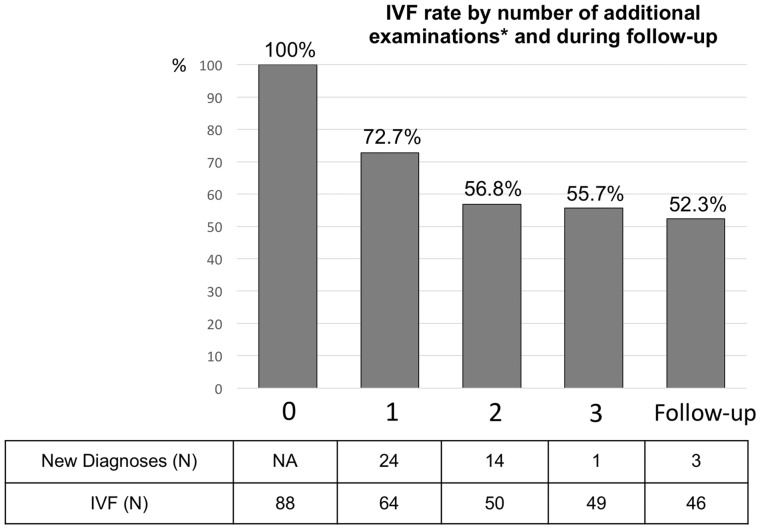
Between May 2011 and 2016, among the 18 622 out-of-hospital cardiac arrests, 717 survivors at hospital discharge from 48 different hospitals fulfilled the definition of cardiac SCA. Coronary artery disease was the main underlying aetiology (525 patients, 73.2%), followed by cardiomyopathies (88 patients, 12.3%) (Figure 1, flow chart). Among the 717 SCA survivors, ECG, coronary angiogram as well as echocardiography established the diagnosis in 629 (87.7%) cases. Cardiac MRI yielded the diagnosis in another 25 (3.5%) cases. Other investigations accounted for 14 (2.4%) additional diagnoses (Figure 2). Among the 88 patients without a diagnosis after the initial evaluation (ECG, echocardiogram, and coronary angiography), the proportion of unexplained SCA decreased from 100% to 55.7% after performance of subsequent examinations (cardiac MRI, ergonovine test, and pharmacological tests, Figure 3). In this group (unexplained SCA after initial evaluation), cardiac MRI revealed the diagnosis in 34.7%, ergonovine test in 30.8%, catecholamine test in 7.7%, and ajmaline challenge in 4.2% of investigated cases (diagnostic yield, Figure 2). Finally, a diagnosis of IVF was considered in 49 patients managed in 13 different hospitals, with no evidence of underlying structural or electrical heart disease discovered within the ambit of the performed investigations.
Figure 1.
Flow chart of the study. ACS, acute coronary syndrome; ARVC, arrhythmogenic right ventricular cardiomyopathy; CPVT, catecholaminergic polymorphic ventricular tachycardia; IVF, idiopathic ventricular fibrillation; LQTS, long QT syndrome; SCA, sudden cardiac arrest; WPW, Wolff–Parkinson–White syndrome.
Figure 2.
Yield of different aetiological examinations. ARVC, arrhythmogenic right ventricular cardiomyopathy; CAD, coronary artery disease; CPVT, catecholaminergic polymorphic ventricular tachycardia; DCM, dilated cardiomyopathy; ECG, electrocardiogram; HCM, hypertrophic cardiomyopathy; SCA, sudden cardiac arrest; TTE, transthoracic echocardiography.
Figure 3.
Unexplained sudden cardiac arrest (‘idiopathic ventricular fibrillation’) rate by number of additional examinations after the initial workup (electrocardiogram, echocardiography, and coronary angiography) and during follow-up. *Cardiac MRI, ergonovine challenge, and pharmacological tests (ajmaline or cathecolamine) were considered for the initial evaluation. The three additional diagnoses made during the follow-up were revealed by two ergonovine challenges (coronary vasospasms) and one exercise testing (long QT syndrome). IVF, idiopathic ventricular fibrillation.
Characteristics of IVF cases compared with other SCA are summarized in Table 1. Idiopathic ventricular fibrillation patients were younger (48.7 ± 14.8 vs. 57.8 ± 14.2 years, P < 0.001), had fewer traditional cardiovascular risk factors, and greater likelihood of family history of SCA (18.4% vs. 3.9%, P < 0.001). Emergency medical services were less frequently called prior to SCA in the IVF group (0% vs. 18.1%, P = 0.001), mostly related to a lower proportion of warning cardiac symptoms reported (defined as chest pain, dyspnoea, palpitations or faintness/syncope in the 4 weeks before SCA, 16.3% vs. 44.9%, P < 0.001). Average collapse to basic life support time was longer (5.2 ± 4.9 vs. 3.0 ± 4.1 min, P < 0.001) in IVF patients. The observed differences in sex distribution (69.4% vs. 80.8% of male, P = 0.080) and proportion of cases occurring during sport activity (16.7% vs. 9.6%, P = 0.132) were not statistically significant.
Table 1.
Characteristics of patients labelled idiopathic ventricular fibrillation compared with other sudden cardiac arrests
| IVFs (n = 49), n (%) | Other SCAs (n = 668), n (%) | P-value | |
|---|---|---|---|
| Age (years), mean ± SD | 48.7 ± 14.8 | 57.8 ± 14.2 | <0.001 |
| Male sex | 34 (69.4) | 540 (80.8) | 0.080 |
| Cardiovascular risk factors | |||
| Current/ex-smoking | 19/7 (38.8/14.3) | 292/121 (43.8/18.2) | 0.449 |
| Hypertension | 8 (16.3) | 241 (36.2) | 0.005 |
| Dyslipidaemia | 8 (16.3) | 204 (30.6) | 0.035 |
| Overweight (BMI >25 kg/m2) | 16 (32.7) | 274 (41.1) | 0.309 |
| Coronary heredity | 8 (16.3) | 80 (12.0) | 0.508 |
| Diabetes mellitus | 5 (10.2) | 86 (12.9) | 0.744 |
| Family history of SCA | 9 (18.4) | 26 (3.91) | <0.001 |
| Cardiac warning symptoms | 8 (16.3) | 300 (44.9) | <0.001 |
| Public location | 30 (61.2) | 398 (59.6) | 0.940 |
| EMS call prior to SCA onset | 0 (0) | 102 (18.1) | 0.001 |
| Bystander | 48 (98.0) | 645 (96.6) | 1.000 |
| Bystander CPR | 38 (77.6) | 527 (79.6) | 0.872 |
| Collapse to basic life support (min), mean ± SD | 5.2 ± 4.9 | 3.0 ± 4.1 | <0.001 |
| Basic life support to ROSC (min), mean ± SD | 19.8 ± 13.8 | 19.1 ± 18.4 | 0.758 |
| Sport-related SCA | 8 (16.7) | 64 (9.6) | 0.132 |
BMI, body mass index; CPR, cardiopulmonary resuscitation; EMS, emergency medical service; IVF, idiopathic ventricular fibrillation; ROSC, return of spontaneous circulation; SCA, sudden cardiac arrest; SD, standard deviation.
In the IVF group, investigations were more comprehensive, but inconsistently performed (Table 2). In addition to the almost universally performed ECG, echocardiogram, and coronary angiography (47 of 49 cases), the most frequent other examinations conducted were cardiac MRI in 40 (81.6%) patients, ajmaline challenge in 21 (42.9%), provocative testing with ergonovine in 19 (38.8%), and electrophysiological study in 12 (24.5%), most often with isoprenaline infusion during the study [10 (20.4%) patients]. Genetic testing was initiated in only 9 (18.4%) patients during the index hospital stay, while other investigations such as 24 h Holter-ECG (6, 12.2%), right ventricular angiography (5, 10.2%), exercise testing (4, 8.2%), signal averaged ECG (2, 4.1%), and cardiac scintigraphy (1, 2.0%) were performed in a very low proportion of cases. Cardiac biopsy, adrenaline challenge and voltage mapping were not performed. Only 8 (16.3%) patients benefited from all three examinations shown to have the highest diagnostic yield (cardiac MRI, ergonovine, and pharmacological testing, Figure 2). Younger patients [odds ratio (OR) 6.00, 95% confidence interval (CI) 1.80–22.26, P = 0.005 for patients <45 vs. >45 years] and those admitted to university centres with dedicated electrophysiology units as compared with non-academic hospitals (OR 3.60, 95% CI 1.12–12.45, P = 0.035) were more thoroughly investigated. There was no significant difference according to sex (P = 0.371). No complication was reported during the investigations, and there was no correlation between the length of hospital stay after ICU discharge and the number of investigations performed (average of 11.5 ± 5.3 days, r = 0.25, P = 0.087).
Table 2.
Medical investigations of cases labelled idiopathic ventricular fibrillation (performed during the index hospitalization following the sudden cardiac arrest or planned subsequently after discharge)
| IVFs (n = 49), n (%) | |
|---|---|
| Coronary angiography | 47 (95.9) |
| Cardiac MRI | 40 (81.6) |
| Provocative testing | |
| Ergonovine | 19 (38.8) |
| Ajmaline | 21 (42.9) |
| Isoprenaline | 10 (20.4) |
| Adenosine | 2 (4.1) |
| Adrenaline | 0 (0) |
| Electrophysiological study | 12 (24.5) |
| Genetic testing | 9 (18.4) |
| Holter-ECG | 6 (12.2) |
| Right ventricular angiography | 5 (10.2) |
| Exercise testing | 4 (8.2) |
| Signal averaged ECG | 2 (4.1) |
| Coronary CT | 1 (2.0) |
| Cardiac scintigraphy (for ARVC) | 1 (2.0) |
| Cardiac biopsy | 0 (0) |
ARVC, arrhythmogenic right ventricular cardiomyopathy; ECG, electrocardiogram; MRI, magnetic resonance imaging.
All 49 patients with a diagnosis of IVF had a favourable neurological outcome (CPC Score 1 or 2), and were implanted with an ICD for secondary prevention. Family screening was initiated during initial hospitalization in only 12 (24.5%) patients. The median follow-up was 48.7 months (range 11.7–71.2), with comprehensive data available in 46 (93.9%) patients. Aetiological investigations performed during follow-up after the initial evaluation were exercise testing in six patients, Holter-ECG in four, genetic testing in four, ergonovine challenge in three, and cardiac scintigraphy in two patients. These additional examinations eventually revealed two coronary vasospasms and one long QT syndrome (unmasked by exercise testing, Figure 3). Regarding genetic findings, one variant of unknown significance was found in the KCNQ1 gene in the patient with long QT syndrome diagnosed by exercise testing. During the follow-up, 10 (21.7%) IVF patients had a recurrence of ventricular arrhythmia (ventricular fibrillation or sustained ventricular tachycardia), after a median time of 281 days (range 64–1088 days), with an annual incidence rate of 5.7% (95% CI 2.8–10.3%). These patients also experienced a significant burden of inappropriate shocks [8 (17.4%) patients].
Discussion
To the best of our knowledge, this is the first study evaluating the completeness of medical assessment among survivors of unexplained SCA in the community. Our findings suggest that in a real-life setting, there is a significant lack of comprehensive cardiac investigation, leading to an overuse of the diagnosis of IVF. These findings call for the implementation of a standardized, systematic diagnostic approach in such cases.
Maximizing the ability to establish a diagnosis in ‘unexplained’ SCA has several implications. Firstly, it allows for provision of specific medical therapies, which might otherwise not be considered in a particular case. Although ICD implantation is almost universally done after SCA without acute or reversible cause, targeted pharmacological treatment can reduce ventricular arrhythmias and device therapies, as can implementation of essential lifestyle changes (avoiding high-risk drugs in long QT and Brugada syndromes, limiting excessive alcohol intake and immediate treatment of fever with antipyretic drugs in Brugada syndrome, and limiting or avoiding sports activities in exercise-related rhythm disorders).12,43 Secondly, because patients with SCA and no structural heart disease most of the time turn out to have inherited cardiac disorders, obtaining a diagnosis is crucial to perform targeted screening of family members, allowing early diagnosis and implementation of primary prevention for these patients, before the potential occurrence of a life-threatening event as first presentation.5,8,10,11,44,45
It has been tested and demonstrated that in the absence of a clear aetiology after ECG, echocardiography, and coronary angiography, a global workup strategy using a systematic algorithm including in particular pharmacological, exercise, and genetic testing, may allow the identification of cardiac abnormalities in more than half of apparently unexplained SCA.14–16 In our population, less than 20% of the cases labelled IVF received a comprehensive investigation. In particular, while cardiac MRI was performed in the majority of cases (80%), coronary vasospasm provocative testing and ajmaline tests were clearly underused. Also, the very low rate of exercise testing and Holter-ECG recording could have led to missed opportunities to diagnose catecholaminergic polymorphic ventricular tachycardia cases or unmask some long QT syndromes.46 Concerning adrenaline challenge and QT analysis, its predictive value and interpretation remains debated and often challenging, whereas it is much more useful and simple to perform both an exercise test and a 12-lead 24 h Holter recording to observe specific changes in repolarization during the recovery phase or night-time. These striking findings illustrate the gap between results reported by highly specialized electrophysiology teams working in tertiary centres, and our real-world approach involving numerous unselected hospitals. Moreover, our results emphasize that very few investigations are performed during follow-up subsequent to the initial evaluation during index hospitalization. The last consensus document defining the minimal requirements for the diagnosis of IVF was published more than two decades ago in 1997, and clearly needs updating in the light of recent advances in imaging, genetic and molecular testing.13 While recent European guidelines have underlined the crucial role of family screening, stating a clear diagnostic approach with ECG, echocardiography (and/or MRI), Holter-ECG, exercise testing, signal averaged ECG, and provocative test with ajmaline (when Brugada syndrome is suspected), the need for further thorough investigations in the index case is left to the treating specialist’s discretion. The value of genetic tests has also been emphasized in case of clinical suspicion of a specific phenotype with an inherited cardiac disorder.12 Although targeted genetic testing has been reported to find causative mutations in up to almost 50% of cases of apparently unexplained SCA,11 genetic screening for a large panel of genes in IVF patients is not currently recommended,12,43 due to its low yield in the absence of a specific clinical suspicion to guide testing, where multiple rare variants of unknown significance cannot formally be considered as causal in the absence of informative familial analyses. Nevertheless, being initiated in only 9 (18.4%) patients in the present study, there is a clear, present scope to enhance the role of genetic testing in the diagnostic armamentarium, particularly in the light of promising results from recent studies.46
Screening of first-degree relatives of an unexplained SCA is essential, and several studies have shown its clinical relevance. Krahn and colleagues.10 reported cardiac abnormalities in 17–30% (depending on levels of diagnostic strength) of a large population of 398 first-degree family members of unexplained SCA. In addition to enabling early, specific management in case of identification of a clear phenotype in relatives, family screening also has the potential to improve rates of aetiological diagnosis among index cases. As demonstrated by Jiménez-Jáimez et al.,15 when no cause of SCA is identified after conventional testing, family screening can sometimes establish the diagnosis retrospectively in the index case, because penetrance and expressivity among gene carriers in the same family is variable.
Our study highlights that a diagnosis of IVF is made in a significant minority of SCA cases (6.8%). Although its exact incidence is unknown and will probably decline with advancements in diagnostic testing and the discovery of new primary arrhythmia syndromes, similar rates from 5 to 10% have been reported in the literature.47,48 It represents, for example, a higher proportion of SCA than identified electrical primary disorders in our study [15 (2.1%) patients], although, as discussed above, inadequate medical investigations probably underestimate the true proportion of channelopathies. We also identified several clinical characteristics of cases labelled IVF. Patients were younger, with a distinct and lower cardiovascular risk factors profile, and a relatively good proportion of them had a family history of SCA (18.4%). Although circumstances of occurrence were similar to other SCAs, relatively fewer of them had specific cardiac symptoms during the weeks prior to the event, with resultant less pre-SCA EMS call and thus longer collapse to basic life support duration. These findings fit particularly with a primary electrical cardiac disorder, characterized by propensity toward ventricular fibrillation in an otherwise normal heart, and emphasize the challenge represented by the prediction of such cases, where SCA may be the inaugural event and alternative preventive strategies have to be sought.49–51
Study limitations
The present study has certain limitations. Firstly, our hospital data are extracted from medical records. While a central committee performed adjudication, this process was based on medical files provided by the different hospitals. Furthermore, we collected the performance of specialized key cardiac investigations, which are unlikely to be underreported in medical reports. We also contacted the referral physician of each IVF case to ensure that all examinations performed were collected. However, family screening, often implemented after completion of all clinical testing can be significantly delayed or initiated by other practitioners, and might be missed in some instances. Secondly, our data are based on a large population with numerous centres involved, but due to the limited number of cases as well as the relatively short study period, we were not able to adequately assess temporal trends in investigation of SCA over the years. Thirdly, disparities in the extent of SCA investigation may exist both regionally and in different Health systems and caution has to be exercised in generalizing these results. Finally, our main result focuses on investigations performed during hospitalization or planned at discharge, because our objective was to assess the comprehensiveness of the initial workup by the different teams in current practice before labelling SCA as IVF, but diagnoses could emerge remotely, especially in this population where a close follow-up has been shown to be essential.16,52,53 However, our data on follow-up emphasize that very few investigations are performed after the initial evaluation.
Conclusion
Our findings from a real-life setting emphasize the extent to which unexplained SCAs are insufficiently investigated and the diagnosis of IVF is probably overused, despite evidence supporting the yield of exhaustive medical investigations in this specific setting. Less than 20% of patients eventually benefit from a complete workup in this context, underlining the importance of referring these patients to expert centres. Guidelines have to be framed, promoting a standardized and systematic diagnostic strategy to improve the proportion of definite diagnosis in apparently unexplained SCA and allow optimized management and better preventive strategies, including among family members.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
Paris-SDEC Executive Committee is part of the ESCAPE-NET project, a €10 million funding from the European Commission (Horizon 2020 programme) to develop research on sudden cardiac death in Europe.
Funding
The Paris-SDEC activities are supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), Paris Descartes University, Assistance Publique - Hôpitaux de Paris, Fondation Coeur et Artères, Global Heart Watch, Fédération Française de Cardiologie, Société Française de Cardiologie, Fondation Recherche Medicale, as well as industrial partners (Medtronic, St Jude Medical, Boston Scientific, Liva Nova, and Biotronik).
Conflict of interest: none declared.
References
- 1. Fishman GI, Chugh SS, Dimarco JP, Albert CM, Anderson ME, Bonow RO, Buxton AE, Chen P-S, Estes M, Jouven X, Kwong R, Lathrop DA, Mascette AM, Nerbonne JM, O'Rourke B, Page RL, Roden DM, Rosenbaum DS, Sotoodehnia N, Trayanova NA, Zheng Z-J.. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation 2010;122:2335–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M.. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J 2016;37:3232–3245. [DOI] [PubMed] [Google Scholar]
- 3. Atlas Writing Group, Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M, Wilkins E, Wright L, Vos R, Bax J, Blum M, Pinto F, Vardas P.. European Society of Cardiology: cardiovascular disease statistics 2017. Eur Heart J 2018;39:508–579. [DOI] [PubMed] [Google Scholar]
- 4. Waldmann V, Bougouin W, Karam N, Albuisson J, Cariou A, Jouven X, Marijon E. Sudden cardiac death: a better understanting for a better prevention. Ann Cardiol Angeiol (Paris) 2017;66:230–238. [DOI] [PubMed] [Google Scholar]
- 5. Behr ER, Dalageorgou C, Christiansen M, Syrris P, Hughes S, Tome Esteban MT, Rowland E, Jeffery S, McKenna WJ.. Sudden arrhythmic death syndrome: familial evaluation identifies inheritable heart disease in the majority of families. Eur Heart J 2008;29:1670–1680. [DOI] [PubMed] [Google Scholar]
- 6. Winkel BG, Holst AG, Theilade J, Kristensen IB, Thomsen JL, Ottesen GL, Bundgaard H, Svendsen JH, Haunsø S, Tfelt-Hansen J.. Nationwide study of sudden cardiac death in persons aged 1-35 years. Eur Heart J 2011;32:983–990. [DOI] [PubMed] [Google Scholar]
- 7. Cerrone M, Priori SG.. Genetics of sudden death: focus on inherited channelopathies. Eur Heart J 2011;32:2109–2118. [DOI] [PubMed] [Google Scholar]
- 8. Semsarian C, Ingles J, Wilde AAM.. Sudden cardiac death in the young: the molecular autopsy and a practical approach to surviving relatives. Eur Heart J 2015;36:1290–1296. [DOI] [PubMed] [Google Scholar]
- 9. Brugada J, Brugada R.. Sudden death in structurally normal heart: we have learned a lot, but still a long way to go. Eur Heart J 2016;37:638–639. [DOI] [PubMed] [Google Scholar]
- 10. Steinberg C, Padfield GJ, Champagne J, Sanatani S, Angaran P, Andrade JG, Roberts JD, Healey JS, Chauhan VS, Birnie DH, Janzen M, Gerull B, Klein GJ, Leather R, Simpson CS, Seifer C, Talajic M, Gardner M, Krahn AD.. Cardiac abnormalities in first-degree relatives of unexplained cardiac arrest victims A report from the Cardiac Arrest Survivors with Preserved Ejection Fraction Registry. Circ Arrhythm Electrophysiol 2016;9:e004274. [DOI] [PubMed] [Google Scholar]
- 11. Mellor G, Laksman ZWM, Tadros R, Roberts JD, Gerull B, Simpson CS, Klein GJ, Champagne J, Talajic M, Gardner M, Steinberg C, Arbour L, Birnie DH, Angaran P, Leather R, Sanatani S, Chauhan VS, Seifer C, Healey JS, Krahn AD.. Genetic testing in the evaluation of unexplained cardiac arrest: from the CASPER (Cardiac Arrest Survivors with Preserved Ejection Fraction Registry). Circ Cardiovasc Genet 2017;10:e001686. [DOI] [PubMed] [Google Scholar]
- 12. Authors/Task Force Members, Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck K-H, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ.. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 13. Survivors of out-of-hospital cardiac arrest with apparently normal heart need for definition and standardized clinical evaluation. Circulation 1997;95:265–272. [DOI] [PubMed] [Google Scholar]
- 14. Krahn AD, Healey JS, Chauhan V, Birnie DH, Simpson CS, Champagne J, Gardner M, Sanatani S, Exner DV, Klein GJ, Yee R, Skanes AC, Gula LJ, Gollob MH.. Systematic assessment of patients with unexplained cardiac arrest: Cardiac Arrest Survivors with Preserved Ejection Fraction Registry (CASPER). Circulation 2009;120:278–285. [DOI] [PubMed] [Google Scholar]
- 15. Jiménez-Jáimez J, Peinado R, Grima EZ, Segura F, Moriña P, Sánchez Muñoz JJ, Mazuelos F, Cózar R, Gimeno JR, Heras RP, Monserrat L, Domingo D, Ortiz-Genga M, Fernández Pastor J, Álvarez M, Tercedor L.. Diagnostic approach to unexplained cardiac arrest (from the FIVI-Gen Study). Am J Cardiol 2015;116:894–899. [DOI] [PubMed] [Google Scholar]
- 16. Herman ARM, Cheung C, Gerull B, Simpson CS, Birnie DH, Klein GJ, Champagne J, Healey JS, Gibbs K, Talajic M, Gardner M, Bennett MT, Steinberg C, Janzen M, Gollob MH, Angaran P, Yee R, Leather R, Chakrabarti S, Sanatani S, Chauhan VS, Krahn AD.. Outcome of apparently unexplained cardiac arrest: results from investigation and follow-up of the prospective Cardiac Arrest Survivors with Preserved Ejection Fraction Registry. Circ Arrhythm Electrophysiol 2016;9:e004012. [DOI] [PubMed] [Google Scholar]
- 17. Wellens HJ, De Vreede J, Gorgels AP.. Sudden cardiac death. How to reduce the number of victims? Eur Heart J 1995;16:7–9. [DOI] [PubMed] [Google Scholar]
- 18. Wellens HJJ, Schwartz PJ, Lindemans FW, Buxton AE, Goldberger JJ, Hohnloser SH, Huikuri HV, Kääb S, La Rovere MT, Malik M, Myerburg RJ, Simoons ML, Swedberg K, Tijssen J, Voors AA, Wilde AA.. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J 2014;35:1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elm EV, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bougouin W, Lamhaut L, Marijon E, Jost D, Dumas F, Deye N, Beganton F, Empana J-P, Chazelle E, Cariou A, Jouven X.. Characteristics and prognosis of sudden cardiac death in Greater Paris: population-based approach from the Paris Sudden Death Expertise Center (Paris-SDEC). Intensive Care Med 2014;40:846–854. [DOI] [PubMed] [Google Scholar]
- 21. Maupain C, Bougouin W, Lamhaut L, Deye N, Diehl J-L, Geri G, Perier M-C, Beganton F, Marijon E, Jouven X, Cariou A, Dumas F.. The CAHP (Cardiac Arrest Hospital Prognosis) score: a tool for risk stratification after out-of-hospital cardiac arrest. Eur Heart J 2016;37:3222–3228. [DOI] [PubMed] [Google Scholar]
- 22. Marijon E, Bougouin W, Cariou A, Jost D, Carli P, Combes A, Leenhardt A, Jouven X. Sudden death expertise centre: a multi disciplinary approach for sudden death. Arch Cardiovasc Dis 2011;104:555–557. [DOI] [PubMed] [Google Scholar]
- 23. Perkins GD, Jacobs IG, Nadkarni VM, Berg RA, Bhanji F, Biarent D, Bossaert LL, Brett SJ, Chamberlain D, Caen AR, de Deakin CD, Finn JC, Gräsner J-T, Hazinski MF, Iwami T, Koster RW, Lim SH, Ma MH-M, McNally BF, Morley PT, Morrison LJ, Monsieurs KG, Montgomery W, Nichol G, Okada K, Ong MEH, Travers AH, Nolan JP; Utstein Collaborators. Cardiac Arrest and Cardiopulmonary Resuscitation Outcome Reports: update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: a Statement for Healthcare Professionals From a Task Force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia) ;and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation 2015;96:328–340. [DOI] [PubMed] [Google Scholar]
- 24. Baritussio A, Zorzi A, Dastidar AG, Susana A, Mattesi G, Rodrigues JCL, Biglino G, Scatteia A, Garate ED, Strange J, Cacciavillani L, Iliceto S, Nisbet A, Angelini GD, Corrado D, Marra MP, Bucciarelli-Ducci C.. Out of hospital cardiac arrest survivors with inconclusive coronary angiogram: impact of cardiovascular magnetic resonance on clinical management and decision-making. Resuscitation 2017;116:91–97. [DOI] [PubMed] [Google Scholar]
- 25. Hennig A, Salel M, Sacher F, Camaioni C, Sridi S, Denis A, Montaudon M, Laurent F, Jais P, Cochet H.. High-resolution three-dimensional late gadolinium-enhanced cardiac magnetic resonance imaging to identify the underlying substrate of ventricular arrhythmia. Europace; doi:10.1093/europace/eux278. Published online ahead of print 23 October 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Subbiah R, Gula LJ, Klein GJ, Skanes AC, White J, Yee R, Krahn AD.. Workup of the cardiac arrest survivor: for the symposium on sudden cardiac death for progress in cardiovascular diseases. Prog Cardiovasc Dis 2008;51:195–203. [DOI] [PubMed] [Google Scholar]
- 27. Meune C, Joly L-M, Chiche J-D, Charpentier J, Leenhardt A, Rozenberg A, Carli P, Sauval P, Weber S, Cracan A, Spaulding C.. Diagnosis and management of out-of-hospital cardiac arrest secondary to coronary artery spasm. Resuscitation 2003;58:145–152. [DOI] [PubMed] [Google Scholar]
- 28. Kobayashi N, Hata N, Shimura T, Yokoyama S, Shirakabe A, Shinada T, Tomita K, Murakami D, Takano M, Seino Y, Matsumoto H, Mashiko K, Mizuno K.. Characteristics of patients with cardiac arrest caused by coronary vasospasm. Circ J 2013;77:673–678. [DOI] [PubMed] [Google Scholar]
- 29. Rolf S, Bruns H-J, Wichter T, Kirchhof P, Ribbing M, Wasmer K, Paul M, Breithardt G, Haverkamp W, Eckardt L.. The ajmaline challenge in Brugada syndrome: diagnostic impact, safety, and recommended protocol. Eur Heart J 2003;24:1104–1112. [DOI] [PubMed] [Google Scholar]
- 30. Somani R, Krahn AD, Healey JS, Chauhan VS, Birnie DH, Champagne J, Sanatani S, Angaran P, Gow RM, Chakrabarti S, Gerull B, Yee R, Skanes AC, Gula LJ, Leong-Sit P, Klein GJ, Gollob MH, Talajic M, Gardner M, Simpson CS.. Procainamide infusion in the evaluation of unexplained cardiac arrest: from the Cardiac Arrest Survivors with Preserved Ejection Fraction Registry (CASPER). Heart Rhythm 2014;11:1047–1054. [DOI] [PubMed] [Google Scholar]
- 31. Krahn AD, Gollob M, Yee R, Gula LJ, Skanes AC, Walker BD, Klein GJ.. Diagnosis of unexplained cardiac arrest: role of adrenaline and procainamide infusion. Circulation 2005;112:2228–2234. [DOI] [PubMed] [Google Scholar]
- 32. Krahn AD, Healey JS, Chauhan VS, Birnie DH, Champagne J, Sanatani S, Ahmad K, Ballantyne E, Gerull B, Yee R, Skanes AC, Gula LJ, Leong-Sit P, Klein GJ, Gollob MH, Simpson CS, Talajic M, Gardner M.. Epinephrine infusion in the evaluation of unexplained cardiac arrest and familial sudden death: from the cardiac arrest survivors with preserved Ejection Fraction Registry. Circ Arrhythm Electrophysiol 2012;5:933–940. [DOI] [PubMed] [Google Scholar]
- 33. Shimizu W, Noda T, Takaki H, Nagaya N, Satomi K, Kurita T, Suyama K, Aihara N, Sunagawa K, Echigo S, Miyamoto Y, Yoshimasa Y, Nakamura K, Ohe T, Towbin JA, Priori SG, Kamakura S.. Diagnostic value of epinephrine test for genotyping LQT1, LQT2, and LQT3 forms of congenital long QT syndrome. Heart Rhythm 2004;1:276–283. [DOI] [PubMed] [Google Scholar]
- 34. Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, Shimizu W, Schulze-Bahr E, Tan H, Wilde A.. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 2005;111:659–670. [DOI] [PubMed] [Google Scholar]
- 35. Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Merz B, Noel C.. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J 2017;38:2565–2568. [DOI] [PubMed] [Google Scholar]
- 36. Klein GJ, Bashore TM, Sellers TD, Pritchett EL, Smith WM, Gallagher JJ.. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. N Engl J Med 1979;301:1080–1085. [DOI] [PubMed] [Google Scholar]
- 37. Leenhardt A, Glaser E, Burguera M, Nürnberg M, Maison-Blanche P, Coumel P.. Short-coupled variant of torsade de pointes. A new electrocardiographic entity in the spectrum of idiopathic ventricular tachyarrhythmias. Circulation 1994;89:206–215. [DOI] [PubMed] [Google Scholar]
- 38. Strain JE, Grose RM, Factor SM, Fisher JD.. Results of endomyocardial biopsy in patients with spontaneous ventricular tachycardia but without apparent structural heart disease. Circulation 1983;68:1171–1181. [DOI] [PubMed] [Google Scholar]
- 39. Leclercq JF, Coumel P.. Late potentials in arrhythmogenic right ventricular dysplasia. Prevalence, diagnostic and prognostic values. Eur Heart J 1993;14Suppl E:80–83. [DOI] [PubMed] [Google Scholar]
- 40. Daubert C, Mabo P, Druelles P, Foulgoc JL, De Place C, Paillard F.. Benefits and limits of selective right ventricular cineangiography in arrhythmogenic right ventricular dysplasia. Eur Heart J 1989;10:46–48. [DOI] [PubMed] [Google Scholar]
- 41. Casset-Senon D, Philippe L, Babuty D, Eder V, Fauchier L, Fauchier JP, Pottier JM, Cosnay P.. Diagnosis of arrhythmogenic right ventricular cardiomyopathy by Fourier analysis of gated blood pool single-photon emission tomography. Am J Cardiol 1998;82:1399–1404. [DOI] [PubMed] [Google Scholar]
- 42. Avella A, d'Amati G, Pappalardo A, Re F, Silenzi P, Laurenzi F, de Girolamo P, Pelargonio G, Russo A, Baratta P, Messina G, Zecchi P, Zachara E, Tondo C.. Diagnostic value of endomyocardial biopsy guided by electroanatomic voltage mapping in arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Cardiovasc Electrophysiol 2008;19:1127–1134. [DOI] [PubMed] [Google Scholar]
- 43. Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang C-E, Huikuri H, Kannankeril P, Krahn A, Leenhardt A, Moss A, Schwartz PJ, Shimizu W, Tomaselli G, Tracy C, Reviewers D, Ackerman M, Belhassen B, Estes NAM, Fatkin D, Kalman J, Kaufman E, Kirchhof P, Schulze-Bahr E, Wolpert C, Vohra J, Refaat M, Etheridge SP, Campbell RM, Martin ET, Quek SC; Heart Rhythm Society; European Heart Rhythm Association; Asia Pacific Heart Rhythm Society. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Europace 2013;15:1389–1406. [DOI] [PubMed] [Google Scholar]
- 44. Gimeno JR, Lacunza J, García-Alberola A, Cerdán MC, Oliva MJ, García-Molina E, López-Ruiz M, Castro F, González-Carrillo J, de la Morena G, Valdés M.. Penetrance and risk profile in inherited cardiac diseases studied in a dedicated screening clinic. Am J Cardiol 2009;104:406–410. [DOI] [PubMed] [Google Scholar]
- 45. Schwartz PJ. Cascades or waterfalls, the cataracts of genetic screening are being opened on clinical cardiology. J Am Coll Cardiol 2010;55:2577–2579. [DOI] [PubMed] [Google Scholar]
- 46. Leinonen JT, Crotti L, Djupsjöbacka A, Castelletti S, Junna N, Ghidoni A, Tuiskula AM, Spazzolini C, Dagradi F, Viitasalo M, Kontula K, Kotta M-C, Widén E, Swan H, Schwartz PJ.. The genetics underlying idiopathic ventricular fibrillation: a special role for catecholaminergic polymorphic ventricular tachycardia? Int J Cardiol 2018;250:139–145. [DOI] [PubMed] [Google Scholar]
- 47. Viskin S, Belhassen B.. Idiopathic ventricular fibrillation. Am Heart J 1990;120:661–671. [DOI] [PubMed] [Google Scholar]
- 48. Conte G, Caputo ML, Regoli F, Marcon S, Klersy C, Adjibodou B, Del Bufalo A, Moccetti T, Auricchio A.. True idiopathic ventricular fibrillation in out-of-hospital cardiac arrest survivors in the Swiss Canton Ticino: prevalence, clinical features, and long-term follow-up. Europace 2017;19:259–266. [DOI] [PubMed] [Google Scholar]
- 49. Adler A, Gollob MH.. Primary prevention of idiopathic ventricular fibrillation: not for the faint of heart. Heart Rhythm 2016;13:913–914. [DOI] [PubMed] [Google Scholar]
- 50. Karam N, Marijon E, Jouven X.. Opening a new front in the fight against sudden cardiac death: is it time for near-term prevention? Int J Cardiol 2017;237:10–12. [DOI] [PubMed] [Google Scholar]
- 51. Marijon E, Uy-Evanado A, Dumas F, Karam N, Reinier K, Teodorescu C, Narayanan K, Gunson K, Jui J, Jouven X, Chugh SS. Warning symptoms are associated with survival from sudden cardiac arrest. Ann Int Med 2016;164:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vittoria Matassini M, Krahn AD, Gardner M, Champagne J, Sanatani S, Birnie DH, Gollob MH, Chauhan V, Simpson CS, Hamilton RM, Talajic M, Ahmad K, Gerull B, Chakrabarti S, Healey JS.. Evolution of clinical diagnosis in patients presenting with unexplained cardiac arrest or syncope due to polymorphic ventricular tachycardia. Heart Rhythm 2014;11:274–281. [DOI] [PubMed] [Google Scholar]
- 53. Brugada J, Brugada P.. What to do in patients with no structural heart disease and sudden arrhythmic death? Am J Cardiol 1996;78:69–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.