Abstract
Parathyroid hormone (PTH) is produced and secreted by the parathyroid glands and has primary effects on kidney and bone. During the pathological growth of one or more parathyroid glands, the plasma level of PTH increases and causes primary hyperparathyroidism (PHPT). This disease is normally characterised by hyperparathyroid hypercalcaemia. In PHPT a continuously elevated PTH stimulates the kidney and bone causing a condition with high bone turnover, elevated plasma calcium and increased fracture risk. If bone resorption is not followed by a balanced formation of new bone, irreversible bone loss may occur in these patients. Medical treatment can help to minimise the loss of bone but the cure of PHPT is by parathyroidectomy. After operation, bone mineral density increases during the return to normal bone metabolism. Supplementation with calcium and vitamin D after operation may improve the normalisation to normal bone metabolism with a secondary reduction in fracture risk.
Keywords: Primary hyperparathyroidism, bone mineral density, parathyroidectomy, vitamin D, PTH, calcium, bone remodelling, fracture
Primary hyperparathyroidism (PHPT) is characterised by hypercalcaemia with a constantly elevated or inappropriately high plasma concentration of parathyroid hormone (PTH). This hormone, an 84 amino-acid peptide synthesised in the parathyroid glands, is the major regulator of calcium homeostasis and exerts its effects mainly on kidney and bone, where the PTH 1 receptor is expressed.1 This membrane-bound receptor is activated by the N-terminal end of PTH and causes intracellular activation through a G-protein coupled mechanism.1 The main stimulus for PTH secretion is low plasma calcium (Ca2+) levels. The concentration of Ca2+ is tightly regulated by complex feedback mechanisms where the calcium-sensing receptor (CaSR) is a main regulator. The sensitivity of the CaSR can graphically be explained by a sigmoidal curve, where small changes in Ca2+ can lead to significant changes in the subsequent PTH secretion. As a result of low Ca2+, PTH is secreted by the parathyroid cells. The PTH stimulation acts on kidney cells by increasing renal tubular reabsorption of calcium2 and the conversion of 25-hydroxy vitamin D (25OHD) to 1,25-dihydroxy vitamin D (1,25(OH)2D) by activation of the renal 1α-hydroxylase.3 Secondary, 1,25(OH)2D increases the calcium absorption from the intestine. In the bone, PTH controls Ca2+ release to the extracellular fluid4 with both a rapid release from a calcium pool as a buffer mechanism and the more slow mechanism with stimulation of increased bone turnover.4 The effect is an increase in plasma Ca2+. Also plasma phosphate is regulated by PTH but in the opposite direction. Hence, an increased plasma PTH leads to increased renal phosphate excretion5 with decreased plasma phosphate levels.
PTH is a major regulator of bone remodelling, the process by which the skeleton is being renewed constantly throughout life (see Figure 1).
Figure 1: The Role of Parathyroid Hormone (PTH) on the Osteoclasts through the PTH Receptor on Osteoblasts.
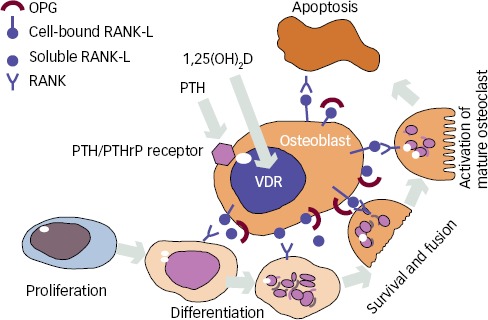
See text for further Information of receptor activator of nuclear factor-ĸβ (RANK), RANK ligand (RANK-L) and osteoprotegerin (OPG). 1,25(OH)2D = 1,25-dihydroxy vitamin D; PTHrP = parathyroid hormone (PTH)-related protein; VDR = vitamin D receptor.
The primer of bone remodelling is bone resorption by osteoclasts, which after a reversal phase changes to bone formation by osteoblasts.6 This process of bone remodelling is critical to maintain healthy bone. The regulation of the remodelling process goes primarily through the osteoblasts, which expresses receptors for both PTH and 1,25(OH)2D.7 It is believed that PTH mainly has an indirect stimulation on osteoclasts by binding to neighbouring osteoblasts.8 Elevated PTH levels induce an increased release of receptor activator of nuclear factor-κβ ligand (RANKL), which binds to its receptor (RANK) on osteoclast precursor cells leading to activation and formation of osteoclasts.9 In younger and otherwise healthy individuals, increased activation of osteoclasts is normally followed by a balanced formation of new bone under the process of bone remodelling10 (see Figure 2). However, this increased resorption of bone is seen in both trabecular and cortical bone in PHPT and causes a temporary, but reversible, bone loss if the remodelling cavities are refilled with new bone.
Figure 2: Coupled and Balanced Bone Remodelling.
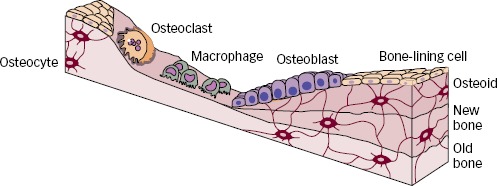
Presentation of coupled and balanced bone remodelling where any bone loss under resorption is reversible due to the balanced bone formation.
The PTH-induced increase in bone turnover is probably the main reason for reduced bone mineral density (BMD) in PHPT. However, if the remodelling process is not balanced, the increased bone resorption will lead to a catabolic state with a subsequent loss of bone. This mechanism may also explain part of the reduced BMD in PHPT. The distribution of balanced and unbalanced bone remodelling is not known.
Histomorphometry on iliac crest biopsies in PHPT has shown increased bone remodelling in PHPT primarily in the trabecular bone11 and to a lesser degree in the cortical bone where the metabolism is at a much lower level.12 Importantly, no disruption in the remodelling process was seen and the bone balance per remodelling cycle in the trabecular bone was not decreased in PHPT. Trabecular structure was not deteriorated. It was demonstrated that the trabecular lattice was in some way protected from the accelerated bone loss seen in normal women during menopause. The most pronounced effect on cortical bone was a 30 % increase of porosity, whereas the thickness of the cortical bone shell was not decreased in this study. Discrepant results have been reported from other authors with measures of cortical thinning in PHPT.13 Increased cortical porosity and cortical thinning may both contribute to the decreased BMD and increased fracture risk in PHPT patients.
In mild to moderate PHPT the general picture concerning BMD is reductions in BMD at all measured sites and reduced weight adjusted BMD compared with controls.14–16 The affection is usually found to be only modest in the spine (mainly trabecular bone) with a more severely affection of the long bones in the appendicular skeleton (mainly cortical bone).17 Accordingly, the low BMD in PHPT is considered to reflect a reversible bone loss due to a high turnover state and increased cortical porosity. However, an irreversible bone loss may occur if the high turnover state causes trabecular perforations or cortical thinning.
New emerging technologies, such as micro-computed tomography (μCT) and high-resolution peripheral quantitative CT (HRpQCT) have provided further insights into 3D microarchitecture of bone.18–21 In a study by Dempster et al. μCT of iliac crest biopsies from PHPT patients showed a well-preserved microarchitecture compared with postmenopausal controls.19 However, they used a very small sample size with only five postmenopausal female controls. With HRpQCT, cortical and trabecular bone can be examined noninvasively with high resolution. Two papers on PHPT patients and controls have described decreased total, cortical and trabecular volumetric BMD (vBMD) in the radius.18,20 The reduced vBMD in the radius seems to arise from decreases in cortical and trabecular thickness, but, contrary to histomorphometric studies, a decrease in trabecular number and increased trabecular spacing were also found suggesting an irreversible bone loss in both cortical and trabecular bone compartments.18,20
There are few studies that have evaluated fracture in PHPT.22–24 In a study from Vestergaard et al.22 the risk of fracture was almost doubled in PHPT patients compared with controls. The increased risk was observed at all skeletal sites in both the peripheral and the axial skeleton.
Changes in Bone Remodelling and Bone Mass after Surgery
Changes in bone turnover and bone structure have been evaluated by histomorphometry in a study by Steiniche et al.25 comparing iliac crest biopsies before and 3 years after parathyroidectomy (PTX). Reduced bone turnover, but no changes in trabecular structure, were observed.25 Activation frequency, bone formation rate and amount of osteoid surfaces all decreased following PTX. Furthermore, there was a small increase in relative cortical width and a decrease in cortical porosity.
Measurements of low BMD in PHPT with a T-score ≤2.5 at any site was an indication for PTX in the 2002 International Guidelines of asymptomatic PHPT and has not been changed.26,27 In most patients there will be an immediate BMD increase in the spine and hip following surgical cure of PHPT, while BMD of the forearm only improves at a slower rate.28,29 In a meta-analysis, the mean (95 % confidence interval [CI]) BMD increase 1 year after PTX was 4.2 % (2.6;5.9) in the spine and 3.5 % (1.4;5.5) in the femoral neck.29 Most of the change in BMD arises from a decrease in the PTH-induced bone turnover with secondary refilling of remodelling spaces.25 In previous studies by our group, most of the postoperative BMD increase was seen in the first 6 months of a 3-year study period after successful cure by PTX.30 However, during the following 2.5 years, BMD increased continually at the spine, hip and forearm (see Figure 3). We know that patients with young age and high preoperative plasma levels of PTH and ionised calcium (Ca2+) have the highest BMD increases postoperatively, whereas gender does not seem to affect the postoperative BMD increase.28 On the other hand, the increase in BMD is impaired in patients with reduced kidney function.28,31 In patients with renal failure the hyperparathyroidism is often secondary to kidney disease and these patients are not covered in the present paper.
Figure 3: Bone Turnover Markers and Bone Mineral Density over 3 Years following Surgical Cure by Parathyroidectomy.
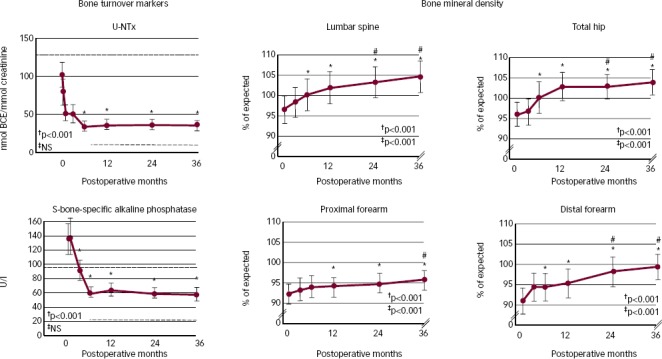
†0 to 3 years; ‡6 months to 3 years. *Values different from baseline by Dunn’s method; p<0.05. #Values different from 6 months by Dunn’s method; p<0.05. BCE = bone collagen equivalent; U-NTx = n-telopeptide, cross-linked, urine; y = years. Source: Reused with permission from Bone.30
Long-term data on patients with mild PHPT who have been followed without PTX have shown that BMD measurements were stable for up to 10 years.32 A significant BMD decrease was reported from 10–15 years after diagnosis but although the decrease in BMD seems to be minimal in untreated PHPT patients for many years, there is without doubt a stable BMD increase after PTX. In the same observational study, patients had significant BMD increases 1 year after PTX in the spine, femoral neck and forearm.32
In a study from Hansen et al.21 assessing effects of PTX by HRpQCT scans, trabecular vBMD and trabecular number increased following PTX whereas trabecular spacing decreased. In contrast to the changes in the forearm, HRpQCT scans of the distal tibia have not revealed significant microarchitectural abnormalities in PHPT patients18,20 and no major changes after PTX have been observed.21 Accordingly, it may be that weight-bearing (tibia) and non-weight-bearing (radius) bone is affected differentially in PHPT. Collectively, there is evidence of improved microarchitecture in both cortical and trabecular bone after PTX. The main increase in BMD following PTX seems to arise from a lowering of bone turnover causing refilling of the remodelling spaces with increased mineralisation.
The shift from a high to a low bone turnover state following PTX leads to increased deposit of calcium in the bone. Due to the increased calcium demands, a hungry bone syndrome may ensue following PTX. This is characterised by normocalcaemia (or hypocalcaemia) with elevated plasma PTH levels (secondary hyperparathyroidism). The syndrome is primarily seen in patients with severe PHPT (very high bone turnover) and vitamin D deficiency.33,34 In a few studies35,36 it has been found that combined supplementation with calcium and vitamin D have prevented secondary hyperparathyroidism after PTX better than calcium alone. This was recently confirmed in a randomised, controlled trial without differences between groups regarding the postoperative BMD increase.37 Up to 1 year after surgery the risk of fractures was increased in the study by Vestergaard et al.22 but thereafter the risk returns to the level of controls. In the same study, there was no difference in fracture-free survival between PHPT patients who were operated or not.22 However, we do not have evidence form randomised trials with fracture as endpoint and the fracture risk in asymptomatic PHPT without a BMD decrease may not be equally increased. To meet the increased risk of fracture in these patients, a treatment with anti-osteoporotic medication after PTX may be necessary if the postoperative BMD increase does not improve the fracture risk sufficiently.
Conclusion
Constantly elevated PTH levels in PHPT induce an increased bone turnover with a decreased BMD and an increased fracture risk. The affection varies in different compartments and at different skeletal sites. The decreased BMD is mainly due to a reversible bone loss and refilling of remodelling spaces following surgical cure increases BMD in both trabecular and cortical bone leading to a normalisation of fracture risk. The return to normal bone metabolism after operation may be improved with calcium and vitamin D supplementation.
References
- 1.Sneddon W. B., Magyar C. E., Willick G. E.. et al. Ligand-selective dissociation of activation and internalization of the parathyroid hormone (PTH) receptor: Conditional efficacy of PTH peptide fragments. Endocrinology. 2004;145:2815–23. doi: 10.1210/en.2003-1185. [DOI] [PubMed] [Google Scholar]
- 2.van Abel M., Hoenderop J. G., van der Kemp A. W.. et al. Coordinated control of renal ca(2+) transport proteins by parathyroid hormone. Kidney Int. 2005;68:1708–21. doi: 10.1111/j.1523-1755.2005.00587.x. [DOI] [PubMed] [Google Scholar]
- 3.Brenza H. L., Kimmel-Jehan C., Jehan F.. et al. Parathyroid hormone activation of the 25-hydroxyvitamin D3-1alpha-hydroxylase gene promoter. Proc Natl Acad Sci U S A. 1998;95:1387–91. doi: 10.1073/pnas.95.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parfitt A. M.. Misconceptions (3): Calcium leaves bone only by resorption and enters only by formation. Bone. 2003;33:259–63. doi: 10.1016/j.bone.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Bergwitz C., Juppner H.. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med. 2010;61:91–104. doi: 10.1146/annurev.med.051308.111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eriksen E. F.. Normal and pathological remodeling of human trabecular bone: Three dimensional reconstruction of the remodeling sequence in normals and in metabolic bone disease. Endocr Rev. 1986;7:379–408. doi: 10.1210/edrv-7-4-379. [DOI] [PubMed] [Google Scholar]
- 7.Norman A. W.. Minireview: Vitamin D receptor: New assignments for an already busy receptor. Endocrinology. 2006;147:5542–8. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 8.Wada T., Nakashima T., Hiroshi N.. et al. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Esbrit P., Alcaraz M. J.. Current perspectives on parathyroid hormone (PTH) and PTH-related protein (PTHrP) as bone anabolic therapies. Biochem Pharmacol. 2013;85:1417–23. doi: 10.1016/j.bcp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Andersen T. L., Abdelgawad M. E., Kristensen H. B.. et al. Understanding coupling between bone resorption and formation: Are reversal cells the missing link? Am J Pathol. 2013;183:235–46. doi: 10.1016/j.ajpath.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Christiansen P., Steiniche T., Vesterby A.. et al. Primary hyperparathyroidism: Iliac crest trabecular bone volume, structure, remodeling, and balance evaluated by histomorphometric methods. Bone. 1992;13:41–9. doi: 10.1016/8756-3282(92)90360-9. [DOI] [PubMed] [Google Scholar]
- 12.Christiansen P., Steiniche T., Brockstedt H.. et al. Primary hyperparathyroidism: Iliac crest cortical thickness, structure, and remodeling evaluated by histomorphometric methods. Bone. 1993;14:755–62. doi: 10.1016/8756-3282(93)90207-q. [DOI] [PubMed] [Google Scholar]
- 13.Parisien M., Silverberg S. J., Shane E.. et al. The histomorphometry of bone in primary hyperparathyroidism: Preservation of cancellous bone structure. J Clin Endocrinol Metab. 1990;70:930–38. doi: 10.1210/jcem-70-4-930. [DOI] [PubMed] [Google Scholar]
- 14.Mosekilde L.. Primary hyperparathyroidism and the skeleton. Clin Endocrinol (Oxf) 2008;69:1–19. doi: 10.1111/j.1365-2265.2007.03162.x. [DOI] [PubMed] [Google Scholar]
- 15.Silverberg S. J., Shane E., de la Cruz L.. et al. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res. 1989;4:283–91. doi: 10.1002/jbmr.5650040302. [DOI] [PubMed] [Google Scholar]
- 16.Grey A. B., Evans M. C., Stapleton J. P.. et al. Body weight and bone mineral density in postmenopausal women with primary hyperparathyroidism. Ann Intern Med. 1994;121:745–9. doi: 10.7326/0003-4819-121-10-199411150-00003. [DOI] [PubMed] [Google Scholar]
- 17.Christiansen P., Steiniche T., Brixen K.. et al. Primary hyperparathyroidism: Biochemical markers and bone mineral density at multiple skeletal sites in danish patients. Bone. 1997;21:93–9. doi: 10.1016/s8756-3282(97)00078-1. [DOI] [PubMed] [Google Scholar]
- 18.Stein E. M., Silva B. C., Boutroy S.. et al. Primary hyperparathyroidism is associated with abnormal cortical and trabecular microstructure and reduced bone stiffness in postmenopausal women. J Bone Miner Res. 2013;28:1029–40. doi: 10.1002/jbmr.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dempster D. W., Muller R., Zhou H.. et al. Preserved three-dimensional cancellous bone structure in mild primary hyperparathyroidism. Bone. 2007;41:19–24. doi: 10.1016/j.bone.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen S., Beck Jensen J. E., Rasmussen L.. et al. Effects on bone geometry, density, and microarchitecture in the distal radius but not the tibia in women with primary hyperparathyroidism: A case-control study using HR-pQCT. J Bone Miner Res. 2010;25:1941–7. doi: 10.1002/jbmr.98. [DOI] [PubMed] [Google Scholar]
- 21.Hansen S., Hauge E. M., Rasmussen L.. et al. Parathyroidectomy improves bone geometry and microarchitecture in female patients with primary hyperparathyroidism. A 1-year prospective controlled study using high resolution peripheral quantitative computed tomography. J Bone Miner Res. 2012;27:1150–58. doi: 10.1002/jbmr.1540. [DOI] [PubMed] [Google Scholar]
- 22.Vestergaard P., Mollerup C. L., Frokjaer V. G.. et al. Cohort study of risk of fracture before and after surgery for primary hyperparathyroidism. BMJ. 2000;321:598–602. doi: 10.1136/bmj.321.7261.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanderWalde L. H., Liu I. L., O’Connell T. X.. et al. The effect of parathyroidectomy on bone fracture risk in patients with primary hyperparathyroidism. Arch Surg. 2006;141:885–9. 889–91. doi: 10.1001/archsurg.141.9.885. discussion. [DOI] [PubMed] [Google Scholar]
- 24.Khosla S., Melton L. J.. 3rdWermers R. A.. et al. Primary hyperparathyroidism and the risk of fracture: A population-based study. J Bone Miner Res. 1999;14:1700–7. doi: 10.1359/jbmr.1999.14.10.1700. [DOI] [PubMed] [Google Scholar]
- 25.Steiniche T., Christiansen P., Vesterby A.. et al. Primary hyperparathyroidism: Bone structure, balance, and remodeling before and 3 years after surgical treatment. Bone. 2000;26:535–43. doi: 10.1016/S8756-3282(00)00260-X. [DOI] [PubMed] [Google Scholar]
- 26.Bilezikian J. P., Potts J. T. Jr., Fuleihan G.. et al. Summary statement from a workshop on asymptomatic primary hyperparathyroidism: A perspective for the 21st century. J Clin Endocrinol Metab. 2002;87:5353–61. doi: 10.1210/jc.2002-021370. [DOI] [PubMed] [Google Scholar]
- 27.Bilezikian J. P., Khan A. A., Potts J. T. Jr.. Third International Workshop on the Management of Asymptomatic Primary Hyperthyroidism Guidelines for the management of asymptomatic primary hyperparathyroidism: Summary statement from the third international workshop. J Clin Endocrinol Metab. 2009;94:335–9. doi: 10.1210/jc.2008-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolighed L., Vestergaard P., Heickendorff L.. et al. BMD improvements after operation for primary hyperparathyroidism. Langenbecks Arch Surg. 2013;398:113–20. doi: 10.1007/s00423-012-1026-5. [DOI] [PubMed] [Google Scholar]
- 29.Sankaran S., Gamble G., Bolland M.. et al. Skeletal effects of interventions in mild primary hyperparathyroidism: A metaanalysis. J Clin Endocrinol Metab. 2010;95:1653–62. doi: 10.1210/jc.2009-2384. [DOI] [PubMed] [Google Scholar]
- 30.Christiansen P., Steiniche T., Brixen K.. et al. Primary hyperparathyroidism: Effect of parathyroidectomy on regional bone mineral density in danish patients: A three-year follow-up study. Bone. 1999;25:589–95. doi: 10.1016/s8756-3282(99)00207-0. [DOI] [PubMed] [Google Scholar]
- 31.Sitges-Serra A., Garcia L., Prieto R.. et al. Effect of parathyroidectomy for primary hyperparathyroidism on bone mineral density in postmenopausal women. Br J Surg. 2010;97:1013–9. doi: 10.1002/bjs.7044. [DOI] [PubMed] [Google Scholar]
- 32.Rubin M. R., Bilezikian J. P., McMahon D. J.. et al. The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocrinol Metab. 2008;93:3462–70. doi: 10.1210/jc.2007-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biskobing D. M.. Significance of elevated parathyroid hormone after parathyroidectomy. Endocr Pract. 2010;16:112–7. doi: 10.4158/EP09122.RA. [DOI] [PubMed] [Google Scholar]
- 34.Witteveen J. E., van Thiel S., Romijn J. A.. et al. Hungry bone syndrome: Still a challenge in the post-operative management of primary hyperparathyroidism: A systematic review of the literature. Eur J Endocrinol. 2013;168:R45–53. doi: 10.1530/EJE-12-0528. [DOI] [PubMed] [Google Scholar]
- 35.Beyer T. D., Solorzano C. C., Prinz R. A.. et al. Oral vitamin D supplementation reduces the incidence of eucalcemic PTH elevation after surgery for primary hyperparathyroidism. Surgery. 2007;141:777–83. doi: 10.1016/j.surg.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 36.Carty S. E., Roberts M. M., Virji M. A.. et al. Elevated serum parathormone level after ‘concise parathyroidectomy’ for primary sporadic hyperparathyroidism. Surgery. 2002;132:1086–92. 1092–3. doi: 10.1067/msy.2002.128479. discussion. [DOI] [PubMed] [Google Scholar]
- 37.Norenstedt S., Pernow Y., Zedenius J.. et al. Vitamin D supplementation after parathyroidectomy – effect on bone mineral density – a randomized double blind study. J Bone Miner Res. 2014;29:960–7. doi: 10.1002/jbmr.2102. [DOI] [PubMed] [Google Scholar]


