Abstract
A dengue vaccine has been pursued for more than 50 years and, unlike other flaviviral vaccines such as that against yellow fever, progress has been slow. In this review, we describe progress toward the first licensed dengue vaccine Dengvaxia, which does not give complete protection against disease. The antibody response to the dengue virion is reviewed, highlighting immunodominant yet poorly neutralizing responses in the context of a highly dynamic structurally flexible dengue virus particle. Finally, we review recent evidence for cross-reactivity between antibody responses to Zika and dengue viruses, which may further complicate the development of broadly protective dengue virus vaccines.
Great Debates
What are the most interesting topics likely to come up over dinner or drinks with your colleagues? Or, more importantly, what are the topics that don't come up because they are a little too controversial? In Immune Memory and Vaccines: Great Debates, Editors Rafi Ahmed and Shane Crotty have put together a collection of articles on such questions, written by thought leaders in these fields, with the freedom to talk about the issues as they see fit. This short, innovative format aims to bring a fresh perspective by encouraging authors to be opinionated, focus on what is most interesting and current, and avoid restating introductory material covered in many other reviews.
The Editors posed 13 interesting questions critical for our understanding of vaccines and immune memory to a broad group of experts in the field. In each case, several different perspectives are provided. Note that while each author knew that there were additional scientists addressing the same question, they did not know who these authors were, which ensured the independence of the opinions and perspectives expressed in each article. Our hope is that readers enjoy these articles and that they trigger many more conversations on these important topics.
Dengue virus (DENV) is a flavivirus transmitted to man by Aedes mosquitos, principally Aedes aegypti. There are estimated to be ∼400 million infections annually of which around a quarter are symptomatic and 1%–5% of these can present with severe disease characterized by vascular leak and hemorrhage, termed dengue hemorrhagic fever. There are four distinct serotypes of dengue, which differ by 30%–35% amino acid identity; infection with one serotype leads to lifelong immunity to that serotype but not to infection with the other serotypes (Sabin 1952; Guzman et al. 2007). In many countries in the tropics and subtropics, all four serotypes frequently cocirculate or cyclically replace each other, meaning that multiple sequential infections are common or indeed the norm.
ENHANCED DISEASE ON SECONDARY INFECTION
One of the interesting immunological features of dengue is that the most severe symptoms occur more frequently following a secondary or sequential infection than occur following a primary infection, implying that some form of acquired immune response to the primary infection is driving more severe outcomes on subsequent reinfection.
One theory for more severe disease occurring on secondary infection is antibody-dependent enhancement (ADE), which was put forward by Halstead some 40 years ago (Halstead and O’Rourke 1977a,b). The ADE hypothesis proposes that during a secondary infection, antibodies formed to the primary infecting DENV, which differs substantially in sequence from the secondary infecting serotype, will not be of sufficient avidity or concentration to fully neutralize the secondary infecting dengue serotype (Screaton et al. 2015). Instead, they may partially coat and opsonize the virus for Fc-receptor-mediated uptake into myeloid cells, such as monocytes/macrophages, which are believed to be the principal site for virus replication, thus driving higher virus loads.
DENGUE VACCINES
The first successful flavivirus vaccine against yellow fever virus (YFV) was developed by Theiler in the 1930s and the same attenuated 17D strain is still in use today. Successful vaccines have also been produced against Japanese encephalitis virus (live attenuated or inactivated virus) and tick-borne encephalitis virus (inactivated virus).
There have been many different approaches to developing a vaccine against DENV over the last 50 years, and these vary from live attenuated and inactivated whole-virus vaccines to subunit, vaccine-like particles (VLPs), and DNA-based approaches. A central tenet to vaccine design to date is that individual DENV serotypes lead to type-specific protective immunity, meaning that vaccines are constructed as a tetravalent formulation, containing components from each of the four DENV serotypes.
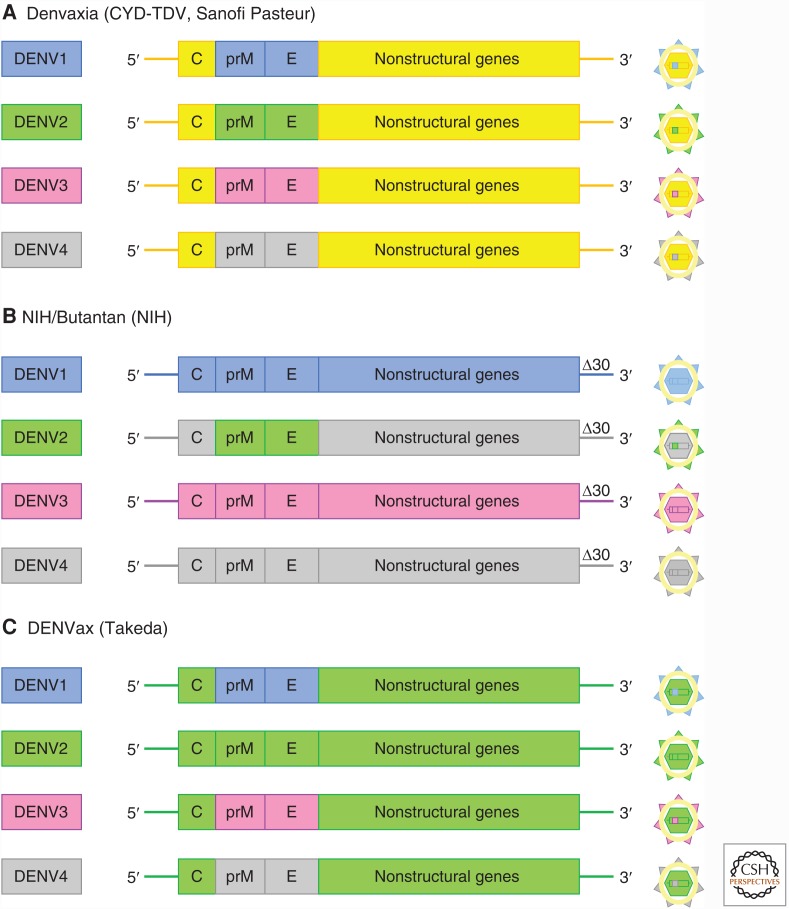
The most advanced DENV vaccines are live attenuated tetravalent formulations of which there are three leading candidates produced by Sanofi Pasteur, Takeda, and National Institutes of Health (NIH)/Butantan; the former has been licensed and the other two are entering phase III trials. All three vaccines contain precursor membrane (prM) protein and E-sequences from the four DENV serotypes grafted onto an attenuated backbone containing the nonstructural components. The Takeda and NIH vaccines use DENV as the backbone, whereas the Sanofi vaccine uses the YFV 17D as backbone (Fig. 1) (Screaton et al. 2015).
Figure 1.
Construction of three leading live attenuated dengue virus (DENV) vaccines. (A) The Sanofi Pasteur vaccine Denvaxia contains four chimeric live flaviviruses, each derived from the genome of the yellow fever virus 17D vaccine strain (shown in yellow) with the precursor membrane (prM) and envelope (E) gene segments replaced by the corresponding gene segments of each of the four DENV serotypes (DENV1 to DENV4). (B) The National Institutes of Health (NIH)/Butantan vaccine contains a mixture of four recombinant DENV genomes color coded to represent the origins of the component parts. The vaccine strains were attenuated by deleting 30 nucleotides (Δ30) from the 3′ untranslated region of the dengue viral genome. (C) The DENVax vaccine from Takeda contains a mixture of four recombinant DENV2 genomes. (From Screaton et al. 2015; reprinted, with permission.)
The Sanofi vaccine has undergone extensive phase IIb and III trials, which have shown an overall efficacy of 66%, with efficacy against DENV2 consistently lower than the other serotypes (Sabchareon et al. 2012; Capeding et al. 2014; Villar et al. 2015). The efficacy was somewhat below expectations based on early phase work in which the vaccine produced respectable in vitro neutralizing antibody titers and has prompted both a search for an explanation for this discrepancy and also better correlates of protective immunity. One suggestion for the less-than-expected performance of this vaccine was that it may not have produced a full T-cell response as only the structural antigens were from dengue, while the nonstructural antigens came from YFV. Further analysis of the trials also suggested that the vaccine-protected individuals who were previously dengue exposed but was less efficacious in vaccinees who were dengue-naïve at enrollment (Capeding et al. 2014; Villar et al. 2015).
Follow-up of the Sanofi vaccine trials substantiated the vaccine efficacy, but there was also a safety signal in the younger age groups included in the trial (Hadinegoro et al. 2015). Specifically, 3 years postvaccination, there were more hospitalizations in those aged <9 years in the vaccine group than in the control group. One explanation for this is that, as the <9-year age group is likely to be enriched for dengue-naïve subjects, the vaccine may be priming but not protecting these naïve individuals and leading to ADE on natural dengue infection.
The Sanofi vaccine, Dengvaxia, has been licensed for use in a number of countries and the World Health Organization (WHO) Strategic Advisory Group of Experts on immunization has recommended its use in those >9 years in areas of high dengue transmission with >70% dengue seropositivity and estimated that deployment of the vaccine in such areas will reduce hospitalization by 10%–30% over a 30-year time frame (see who.int/immunization/sage/meetings/2016/april/SAGE_April_2016_Meeting_Web_summary.pdf) (Screaton et al. 2015).
WHY HAVE DENGUE VACCINES PROVED PROBLEMATIC TO PRODUCE?
The challenge for dengue vaccines is formidable and part of this challenge relates directly to the fact that there are four related serotypes:
The vaccine will need to be effective against four similar but distinct serotypes.
The risk of ADE mandates that a successful vaccine will produce protection against all four serotypes, otherwise the vaccine may prime individuals for more severe disease on secondary infection.
Tetravalent formulations need to produce balanced immunity between the four serotypes when coadministered.
Original antigenic sin may complicate vaccine strategies relying on multiple boosts and, in endemic countries, a vaccine will ideally be given to both dengue-naïve and previously dengue-exposed vaccinees.
In addition to these challenges, there may be additional features unique to DENV, which are described below.
INCOMPLETE prM CLEAVAGE
Two transmembrane-anchored structural proteins are found in the glycoprotein shell of dengue virions, prM, and envelope protein (E) (Mukhopadhyay et al. 2005; Li et al. 2008; Yu et al. 2008; Screaton et al. 2015). Virions bud into the endoplasmic reticulum (ER) initially as immature forms in which 180 copies of E and 180 copies of prM are arranged into trimers (3prM:3E), giving the virion a spiky appearance. In the acidic environment of the Golgi, the virion undergoes a conformational change whereby it reassorts from prM/E trimers to prM/E dimers. Also in the Golgi, prM is cleaved by furin protease and, following cleavage, the pr peptide remains associated with the virion and disassociates on release of the virus particle from the infected cell. In dengue, prM cleavage is frequently incomplete, leading to the production of a spectrum of viral particles containing differing prM:E ratios, varying from the spiky, fully immature particle in which prM remains associated with E, to the mature virus particle, in which prM is fully cleaved and 180 copies of the E protein are arranged into 90 head-to-tail dimers to form smooth virus particles (see Fig. 3a in Mackenzie et al. 2004 and Fig. 3c in Kuhn et al. 2002). Between the fully immature and fully mature particles, a spectrum of partially mature forms exist, which have been shown by cryoelectron microscopy (cryo-EM) to contain varying amounts of smooth (E-dimer) and spiky (prM/E trimer) surfaces (Junjhon et al. 2008).
To add to this complexity, there are cell-type-specific differences in the degree of prM cleavage; insect cells produce high-prM virus particles, whereas primary human dendritic cells (DCs) produce relatively low prM virus particles (Dejnirattisai et al. 2010). Analysis of the memory B-cell response following DENV infection shows a high proportion of cells that produce antibodies reacting to prM (Dejnirattisai et al. 2010). These antibodies are potent inducers of ADE but show poor neutralization, which plateaus at ∼50%. The reason for this plateau is that fully mature DENV particles contain no prM and are therefore not a target for anti-prM antibodies. Low prM particles do not contain enough prM antigen to allow neutralization, but can still be opsonized and promote ADE; therefore, only a fraction of prM-containing particles can be neutralized. We believe that, ideally, prM responses should be minimized or avoided in DENV vaccines; however, prM is an obligatory component of all live attenuated or inactivated virus vaccines currently under investigation.
STRUCTURAL FLEXIBILITY OF THE DENGUE VIRION
Further complexity is added by the structural flexibility of DENV. The dengue virion can adopt a variety of different conformations with differing thermodynamic stabilities in a process termed breathing. This can affect the accessibility of some antigenic sites that may be occluded in some conformations but exposed in others and explains why binding of some antibodies may be enhanced by prolonged incubation or by increased temperature (Dowd et al. 2014). A “bumpy” conformation of DENV2 has been described in which the virus particle is expanded and the interaction of the 90 E-dimers is changed relative to the standard mature virus particle, which may disrupt some quaternary epitopes formed between opposing dimers (Fibriansah et al. 2013; Zhang et al. 2013). It is interesting in this regard that the Zika virus (ZIKV) particles seem to be relatively more rigid than DENV particles (Goo et al. 2016; Kostyuchenko et al. 2016).
THE IMMUNODOMINANT FUSION LOOP EPITOPE
Many antibodies have been made to dengue starting with mouse monoclonals and, more recently, several hundred human monoclonal antibodies have been produced by different investigators. Antibodies binding the fusion loop epitope (FLE) are a major immunodominant component of the response to DENV (Smith et al. 2013; Dejnirattisai et al. 2015). During virus infection, acidification in an endosomal compartment triggers conformational reorganization of the E-protein from dimers to trimers exposing the fusion peptide, which drives apposition of viral and host-cell membranes, allowing membrane fusion and escape of the viral RNA into the host-cell cytoplasm (Screaton et al. 2015).
In DENVs, access to the FLE is not restricted to virions at endosomal pH but also found at neutral pH. This is likely attributed to two of the processes described above. The presence of prM in partially mature DENV particles increases accessibility of the FLE (Cherrier et al. 2009; Dejnirattisai et al. 2015). Anti-FLE monoclonal antibodies (mAbs) can fully neutralize high-prM DENV produced in insect cells. However, low-prM DENV produced in primary human DCs, which represents virus produced in the infected host following the initial mosquito inoculation, cannot be fully neutralized by anti-FLE mAbs, typically plateauing at ∼80%, yet anti-FLE mAbs can potently induce ADE (Dejnirattisai et al. 2015).
In addition to the presence of prM, structural flexibility or breathing of the DENV E-dimer may also allow access to the FLE. Interestingly, the FLE is highly conserved between DENVs and ZIKV and anti-FLE produced from DENV-infected patients can bind with high affinity to monomeric ZIKV E-protein (Dejnirattisai et al. 2016; Stettler et al. 2016). However, these anti-FLE mAbs fail to neutralize ZIKV infection but promote ADE, which is consistent with the concept that ZIKV is more rigid than the DENVs, thereby limiting access to the FLE (Barba-Spaeth et al. 2016; Dejnirattisai et al. 2016; Kostyuchenko et al. 2016).
We believe that the immunodominance of the FLE in DENV infection may be related to the incomplete cleavage of prM and to structural flexibility of the virus. Because anti-FLE antibodies poorly neutralize low-prM-containing viruses. The ideal DENV vaccine would thus aim to minimize responses to the FLE.
CONFORMATIONAL QUATERNARY EPITOPES
Analysis of panels of human anti-DENV mAbs has identified a number that are potently neutralizing with 50% neutralization titer (NT50) values into the low picomolar range (Screaton et al. 2015). The most potent antibodies react to conformational epitopes on the E-protein that are only found when E is displayed on virus particles, but not on recombinant monomeric-E (Screaton et al. 2015). A number of such epitopes have now been structurally characterized, most of which are serotype-specific; mAb-1F4 (DENV1) binds E-monomers only when in the intact virion, mAb-HM14c10 (DENV1) binds to two opposing E-dimers, mAb-5J7 (DENV3) binds three adjacent monomers, and mAb-2D22 (DENV2) binds two monomers in the E-dimer (Teoh et al. 2012; Fibriansah et al. 2014, 2015a,b; Screaton et al. 2015).
We have recently reported a new epitope for conformational quaternary antibodies, the E-dimer epitope (EDE), of which two categories EDE1 and EDE2 are distinguished by the lack of sensitivity or sensitivity to removal of glycan at position N153 in E, respectively (Dejnirattisai et al. 2015). A number of such antibodies were isolated from dengue-infected patients and their epitopes mapped by X-ray crystallography and cryo-EM (Dejnirattisai et al. 2015; Rouvinski et al. 2015). The antibodies bind across the interface of two head-to-tail E-monomers making up the E-dimer (Fig. 2). They occupy a site where prM binds to E as it passes through the Golgi, which is highly conserved between all DENV serotypes; hence, many of the EDE mAbs are broadly neutralizing of all four serotypes. The EDE-mAbs are potently neutralizing in the low picomolar range and, unlike the anti-FLE antibodies described above, they potently neutralize high- and low-prM-content viruses produced in insect and DC, respectively.
Figure 2.
Anti-E-dimer epitope (EDE) monoclonal antibodies (mAbs) cross-react between dengue virus (DENV) and Zika virus (ZIKV). (A,B) Binding of EDE mAb-A11 to the DENV E-dimer. (From Rouvinski et al. 2015; reprinted, with permission, from Nature Publishing Group.) (C) Conservation of amino acid sequence and footprint of the EDE between DENV and ZIKV. (From Barba-Spaeth et al. 2016; reprinted, with permission, from Nature Publishing Group © 2016.)
In summary, antibodies to conformational epitopes seem to be the most potent neutralizers of DENV. The mAbs themselves are potential prophylactics or therapeutics and elicitation of broadly neutralizing antibodies to the EDE should be prioritized in future vaccine approaches.
DENGUE AND ZIKA INTERACTIONS
ZIKV was first isolated in 1947 and, until recently, has been relatively understudied because infection was frequently asymptomatic, caused relatively mild disease, and cases were largely sporadic with no epidemic activity (Musso and Gubler 2016). This has dramatically changed with large-scale outbreaks spreading eastward across the Pacific reaching Brazil in 2014, leading to an explosive epidemic spreading across South America associated with Guillain-Barré syndrome (estimated risk 0.24%) and a large increase in cases of microcephaly in children born to mothers infected during pregnancy, particularly the first trimester (estimated risk, 1%–22%) (Brasil et al. 2016; Cauchemez et al. 2016). The WHO declared Zika a global health emergency in February 2016 and there is now a concerted effort to develop a ZIKV vaccine.
ZIKV is a flavivirus most closely related to the DENV serocomplex (41%–46% amino acid difference in the envelope protein) and like DENV is also transmitted by the A. aegypti mosquito (Musso and Gubler 2016). In South America, there has been geographical spread of DENV, meaning that in recent ZIKV-affected areas DENV seropositivity is frequently 80% or more (Brathwaite Dick et al. 2012; Castanha et al. 2013). The difficulty in distinguishing previous DENV or ZIKV infection serologically suggests that there is substantial cross reactivity in the antibody responses to the two viruses (Lanciotti et al. 2008).
This cross-reaction leads to the possibility that anti-DENV responses may be either protective against ZIKV infection or, by promoting ADE, may actually increase ZIKV replication. Several reports have now explored this possibility (Castanha et al. 2016; Dejnirattisai et al. 2016; Priyamvada et al. 2016; Stettler et al. 2016), showing that serum from dengue-immune donors strongly binds to ZIKV by enzyme-linked immunosorbent assay (ELISA). Although most of these sera are non-neutralizing of ZIKV, some anti-dengue serum samples showed quite respectable neutralization of ZIKV (50% activity in focus reduction neutralization test [FRNT50] <1 in 200) (Dejnirattisai et al. 2016; Priyamvada et al. 2016). However, although most anti-DENV serum samples fail to neutralize ZIKV, they potently promote ADE of ZIKV in vitro and ADE induced by an anti-dengue serum has also been demonstrated in a murine model (Bardina et al. 2017).
Analysis of panels of monoclonal antibodies made from DENV- or ZIKV-infected donors have now been reported (Dejnirattisai et al. 2010, 2015; Dai et al. 2016; Priyamvada et al. 2016; Sapparapu et al. 2016; Stettler et al. 2016; Swanstrom et al. 2016; Wang et al. 2016; Robbiani et al. 2017). These mAbs show substantial cross-reaction between DENV and ZIKV, suggesting that ZIKV could be considered as a fifth member of the DENV serocomplex. Interestingly, antibodies generated from DENV-infected donors, which are directed to the FLE and which can neutralize DENV bind strongly to recombinant ZIKV envelope protein but show poor neutralization of ZIKV, yet still potently promote ADE of ZIKV infection (Barba-Spaeth et al. 2016; Dejnirattisai et al. 2016; Paul et al. 2016). This may be the result of differences in breathing of the DENV and ZIKV virions as described above (Kostyuchenko et al. 2016).
Although most anti-dengue mAbs show poor neutralization of ZIKV anti-EDE mAbs, particularly the EDE1 subclass show potent neutralization of ZIKV in the low picomolar range (Dejnirattisai et al. 2016). Crystal structures of EDE1 antibodies binding to the ZIKV envelope dimer reveal the strong conservation of this epitope between ZIKV and DENV (Barba-Spaeth et al. 2016).
The cross-reaction between serological responses to ZIKV and DENV may have implications for the pathogenesis of ZIKV infection by driving increased virus replication by ADE of ZIKV in previously DENV-exposed individuals, and there is also the possibility the ADE may directly drive transplacental spread of ZIKV leading to fetal brain infection and microcephaly. The close serological cross-reactivity between DENV and ZIKV needs to be borne in mind in future vaccine development. It is possible that dengue vaccination may prime individuals for ADE of future ZIKV infection and conversely that ZIKV vaccination may lead to ADE of future DENV infection. In addition, it is likely that, in the future, DENV and ZIKV vaccines will need to be deployed in populations that have already been naturally exposed to one or other viruses, and original antigenic sin may well shape the subsequent response to the vaccine in a way that could differ substantially from vaccination of DENV/ZIKV-naïve individuals.
SUMMARY
Following >50 years of work, the first dengue vaccine has been licensed for use primarily in previously DENV-exposed individuals in areas of high endemicity. Two further tetravalent live attenuated vaccines from Takeda and NIH/Butantan, which differ from Dengvaxia in terms of the vector backbones, are reaching phase III and the results of these trials are awaited. If these two products fail to advance greatly on Dengvaxia, new approaches will be required to control the spread of DENV infection. We believe that anti-prM and anti-FLE responses to DENV are not desirable and that their immunodominance may indeed be an immune-evasion strategy used by DENV. One possible new avenue is to produce a subunit immunogen consisting of stabilized E-dimers; this would both remove the need for prM and also restrict breathing of the E-dimer, reducing accessibility and immunogenicity of the FLE. Furthermore, because the EDE response is broadly neutralizing between the DENVs and extends to ZIKV, a universal pan-DENV or DENV/ZIKV immunogen may be possible.
Footnotes
Editors: Shane Crotty and Rafi Ahmed
Additional Perspectives on Immune Memory and Vaccines: Great Debates available at www.cshperspectives.org
REFERENCES
- Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Lorière E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, et al. 2016. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 536: 48–53. [DOI] [PubMed] [Google Scholar]
- Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere JJ, Brown JA, Nachbagauer R, Foster GA, Krysztof D, Tortorella D, et al. 2017. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 356: 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P, Pereira JP Jr, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, et al. 2016. Zika virus infection in pregnant women in Rio de Janeiro—Preliminary report. N Engl J Med 375: 2321–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brathwaite Dick O, San Martín JL, Montoya RH, del Diego J, Zambrano B, Dayan GH. 2012. The history of dengue outbreaks in the Americas. Am J Trop Med Hyg 87: 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, et al. 2014. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 384: 1358–1365. [DOI] [PubMed] [Google Scholar]
- Castanha PM, Cordeiro MT, Martelli CM, Souza WV, Marques ET Jr, Braga C. 2013. Force of infection of dengue serotypes in a population-based study in the northeast of Brazil. Epidemiol Infect 141: 1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanha PM, Nascimento EJ, Cynthia B, Cordeiro MT, de Carvalho OV, de Mendonça LR, Azevedo EA, França RF, Rafael D, Marques ET Jr. 2016. Dengue virus (DENV)-specific antibodies enhance Brazilian Zika virus (ZIKV) infection. J Infect Dis. 215: 781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, Salje H, Van Kerkhove MD, Abadie V, Garel C, et al. 2016. Association between Zika virus and microcephaly in French Polynesia, 2013–15: A retrospective study. Lancet 387: 2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier MV, Kaufmann B, Nybakken GE, Lok SM, Warren JT, Chen BR, Nelson CA, Kostyuchenko VA, Holdaway HA, Chipman PR, et al. 2009. Structural basis for the preferential recognition of immature flaviviruses by a fusion-loop antibody. EMBO J 28: 3269–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Song J, Lu X, Deng YQ, Musyoki AM, Cheng H, Zhang Y, Yuan Y, Song H, Haywood J, et al. 2016. Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe 19: 696–704. [DOI] [PubMed] [Google Scholar]
- Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, et al. 2010. Cross-reacting antibodies enhance dengue virus infection in humans. Science 328: 745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W, Wongwiwat W, Supasa S, Zhang X, Dai X, Rouvinski A, Jumnainsong A, Edwards C, Quyen NT, Duangchinda T, et al. 2015. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol 16: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, et al. 2016. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with Zika virus. Nat Immunol 17: 1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd KA, Mukherjee S, Kuhn RJ, Pierson TC. 2014. Combined effects of the structural heterogeneity and dynamics of flaviviruses on antibody recognition. J Virol 88: 11726–11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibriansah G, Ng TS, Kostyuchenko VA, Lee J, Lee S, Wang J, Lok SM. 2013. Structural changes in dengue virus when exposed to a temperature of 37°C. J Virol 87: 7585–7592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibriansah G, Tan JL, Smith SA, de Alwis AR, Ng TS, Kostyuchenko VA, Ibarra KD, Wang J, Harris E, de Silva A, et al. 2014. A potent anti-dengue human antibody preferentially recognizes the conformation of E protein monomers assembled on the virus surface. EMBO Mol Med 6: 358–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibriansah G, Ibarra KD, Ng TS, Smith SA, Tan JL, Lim XN, Ooi JS, Kostyuchenko VA, Wang J, de Silva AM, et al. 2015a. Cryo-EM structure of an antibody that neutralizes dengue virus type 2 by locking E protein dimers. Science 349: 88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibriansah G, Tan JL, Smith SA, de Alwis R, Ng TS, Kostyuchenko VA, Jadi RS, Kukkaro P, de Silva AM, Crowe JE, et al. 2015b. A highly potent human antibody neutralizes dengue virus serotype 3 by binding across three surface proteins. Nat Commun 6: 6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goo L, Dowd KA, Smith AR, Pelc RS, DeMaso CR, Pierson TC. 2016. Zika virus is not uniquely stable at physiological temperatures compared to other flaviviruses. mBio 7: e01396–e01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman MG, Alvarez M, Rodriguez-Roche R, Bernardo L, Montes T, Vazquez S, Morier L, Alvarez A, Gould EA, Kouri G, Halstead SB, et al. 2007. Neutralizing antibodies after infection with dengue 1 virus. Emerg Infect Dis 13: 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadinegoro SR, Arredondo-García JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, et al. 2015. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 373: 1195–1206. [DOI] [PubMed] [Google Scholar]
- Halstead SB, O’Rourke EJ. 1977a. Antibody-enhanced dengue virus infection in primate leukocytes. Nature 265: 739–741. [DOI] [PubMed] [Google Scholar]
- Halstead SB, O’Rourke EJ. 1977b. Dengue viruses and mononuclear phagocytes. I: Infection enhancement by non-neutralizing antibody. J Exp Med 146: 201–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junjhon J, Lausumpao M, Supasa S, Noisakran S, Songjaeng A, Saraithong P, Chaichoun K, Utaipat U, Keelapang P, Kanjanahaluethai A, et al. 2008. Differential modulation of prM cleavage, extracellular particle distribution, and virus infectivity by conserved residues at nonfurin consensus positions of the dengue virus pr-M junction. J Virol 82: 10776–10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuchenko VA, Lim EX, Zhang S, Fibriansah G, Ng TS, Ooi JS, Shi J, Lok SM. 2016. Structure of the thermally stable Zika virus. Nature 533: 425–428. [DOI] [PubMed] [Google Scholar]
- Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, et al. 2002. Structure of dengue virus: Implications for flavivirus organization, maturation, and fusion. Cell 108: 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14: 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Lok SM, Yu IM, Zhang Y, Kuhn RJ, Chen J, Rossmann MG. 2008. The flavivirus precursor membrane-envelope protein complex: Structure and maturation. Science 319: 1830–1834. [DOI] [PubMed] [Google Scholar]
- Mackenzie JS, Gubler DJ, Petersen LR. 2004. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 10: S98–S109. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Kuhn RJ, Rossmann MG. 2005. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol 3: 13–22. [DOI] [PubMed] [Google Scholar]
- Musso D, Gubler DJ. 2016. Zika virus. Clin Microbiol Rev 29: 487–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul LM, Carlin ER, Jenkins MM, Tan AL, Barcellona CM, Nicholson CO, Michael SF, Isern S. 2016. Dengue virus antibodies enhance Zika virus infection. Clin Transl Immunology 5: e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, Pattanapanyasat K, Chokephaibulkit K, Mulligan MJ, Wilson PC, et al. 2016. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci 113: 7852–7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani DF, Bozzacco L, Keeffe JR, Khouri R, Olsen PC, Gazumyan A, Schaefer-Babajew D, Avila-Rios S, Nogueira L, Patel R, et al. 2017. Recurrent potent human neutralizing antibodies to Zika virus in Brazil and Mexico. Cell 169: 597–609.e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvinski A, Guardado-Calvo P, Barba-Spaeth G, Duquerroy S, Vaney MC, Kikuti CM, Navarro Sanchez ME, Dejnirattisai W, Wongwiwat W, Haouz A, et al. 2015. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature 520: 109–113. [DOI] [PubMed] [Google Scholar]
- Sabin AB. 1952. Research on dengue during World War II. Am J Trop Med Hyg 1: 30–50. [DOI] [PubMed] [Google Scholar]
- Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, et al. 2012. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: A randomised, controlled phase 2b trial. Lancet 380: 1559–1567. [DOI] [PubMed] [Google Scholar]
- Sapparapu G, Fernandez E, Kose N, Bin Cao, Fox JM, Bombardi RG, Zhao H, Nelson CA, Bryan AL, Barnes T, et al. 2016. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature 540: 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screaton G, Mongkolsapaya J, Yacoub S, Roberts C. 2015. New insights into the immunopathology and control of dengue virus infection. Nat Rev Immunol 15: 745–759. [DOI] [PubMed] [Google Scholar]
- Smith SA, de Alwis AR, Kose N, Harris E, Ibarra KD, Kahle KM, Pfaff JM, Xiang X, Doranz BJ, de Silva AM, et al. 2013. The potent and broadly neutralizing human dengue virus-specific monoclonal antibody 1C19 reveals a unique cross-reactive epitope on the bc loop of domain II of the envelope protein. mBio 4: e00873–00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, et al. 2016. Specificity, cross-reactivity and function of antibodies elicited by Zika virus infection. Science 353: 823–826. [DOI] [PubMed] [Google Scholar]
- Swanstrom JA, Plante JA, Plante KS, Young EF, McGowan E, Gallichotte EN, Widman DG, Heise MT, de Silva AM, Baric RS. 2016. Dengue virus envelope dimer epitope monoclonal antibodies isolated from dengue patients are protective against Zika virus. mBio 7: e01123-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh EP, Kukkaro P, Teo EW, Lim AP, Tan TT, Yip A, Schul W, Aung M, Kostyuchenko VA, Leo YS, et al. 2012. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci Transl Med 4: 139ra183. [DOI] [PubMed] [Google Scholar]
- Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramírez JO, Carrasquilla G, et al. 2015. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 372: 113–123. [DOI] [PubMed] [Google Scholar]
- Wang Q, Yang H, Liu X, Dai L, Ma T, Qi J, Wong G, Peng R, Liu S, Li J, et al. 2016. Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus. Sci Transl Med 8: 369ra179. [DOI] [PubMed] [Google Scholar]
- Yu IM, Zhang W, Holdaway HA, Li L, Kostyuchenko VA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J. 2008. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science 319: 1834–1837. [DOI] [PubMed] [Google Scholar]
- Zhang X, Sheng J, Plevka P, Kuhn RJ, Diamond MS, Rossmann MG. 2013. Dengue structure differs at the temperatures of its human and mosquito hosts. Proc Natl Acad Sci 110: 6795–6799. [DOI] [PMC free article] [PubMed] [Google Scholar]