Abstract
Membrane integrity is essential in maintaining sperm viability, signaling, and motility, which are essential for fertilization. Sperm are highly susceptible to oxidative stress, as they are rich in sensitive polyunsaturated fatty acids (PUFA), and are unable to synthesize and repair many essential membrane constituents. Because of this, sperm cellular membranes are important targets of this process. Membrane Lipid Replacement (MLR) with glycerophospholipid mixtures (GPL) has been shown to ameliorate oxidative stress in cells, restore their cellular membranes, and prevent loss of function. Therefore, we tested the effects of MLR on sperm by tracking and monitoring GPL incorporation into their membrane systems and studying their effects on sperm motility and viability under different experimental conditions. Incubation of sperm with mixtures of exogenous, unoxidized GPL results in their incorporation into sperm membranes, as shown by the use of fluorescent dyes attached to GPL. The percent overall (total) sperm motility was increased from 52±2.5% to 68±1.34% after adding GPL to the incubation media, and overall sperm motility was recovered from 7±2% after H2O2 treatment to 58±2.5%)(n = 8, p<0.01) by the incorporation of GPL into sperm membranes. When sperm were exposed to H2O2, the mitochondrial inner membrane potential (MIMP), monitored using the MIMP tracker dye JC-1 in flow cytometry, diminished, whereas the addition of GPL prevented the decrease in MIMP. Confocal microscopy with Rhodamine-123 and JC-1 confirmed the mitochondrial localization of the dyes. We conclude that incubation of human sperm with glycerolphospholipids into the membranes of sperm improves sperm viability, motility, and resistance to oxidizing agents like H2O2. This suggests that human sperm might be useful to test innovative new treatments like MLR, since such treatments could improve fertility when it is adversely affected by increased oxidative stress.
Introduction
Sperm are highly specialized and must traverse a changing environment to fertilize oocytes. From the total number of sperm found initially in an ejaculate (200–600 million), only 200–300 will come close to eggs, and from those, only one sperm will eventually fertilize an egg [1]. Sperm are surrounded by a unique plasma membrane, and inside there are a number of other membranes that separate various sperm organelles, including mitochondria. There is increasing evidence that the membranes of sperm (SM) are more than just an inert barrier system [2]. Lipids in the SM, especially the glycerolphospholipids (GPL), are compositionally disparate in different membrane regions and undergo changes during sperm maturation where they also function as an energy storage system. In sperm, GPL and sphingomyelin are characterized by the presence of long-chain and very-long-chain polyunsaturated fatty acids (PUFA) (indeed, almost 30% of the fatty acids are PUFA) [3–7] [8–11].
During normal sperm maturation, the continuity between the plasma membrane and intracellular organelle membranes change [12, 13], and there are several modifications in membrane lipid compositions that influence membrane-membrane interactions, fluidity and mobility of lipids within the SM [13, 14]. These changes in lipid compositions in SM are essential for membrane fusion and are required for the bending and fusion of the membrane domains in the plasma membrane and organelles, while recruiting proteins from various membranes and the cytosol. The SM also exert appropriate control over signal processing mediators, such as calcium ions (Ca2+) [15]. Lipids from different SM are subject to exchange and renewal by various mechanisms, such as endocytosis, exocytosis, contacts between different membranes and non-membrane lipid vesicles and by non-vesicular lipid carrier trafficking [15, 16]. For example, the compositions of lipids in the SM have been shown to be susceptible to compositional changes by the incorporation/fusion of nanoliposomes [17].
As in other cells, the SM are particularly sensitive to oxidative stress, especially from Reactive Oxygen and Nitrogen Species (ROS/RNS). The production of ROS/RNS in sperm has been known since the 1940s and was found to be important in sperm function and viability [18–21]. In addition, lipid peroxidation was found to affect SM integrity, leading to the loss of cytosolic components and finally cell death [22]. The high concentrations of PUFA compared to saturated lipids in SM makes them highly susceptible to lipid peroxidation. Indeed, reductions in sperm motility have been associated with lipid peroxidation of SM [2, 3, 23–32], and the loss of sperm motility with time has been used as an indirect estimation of oxidative stress and rate of lipid peroxidation [33]. Reductions in sperm motility caused by SM lipid peroxidation were also reported to decrease fertility [8, 34–36]. The natural defense mechanisms of sperm against lipid peroxidation are mostly superoxide dismutase (SOD) and the glutathione peroxidase/reductase system (GPX/GRD). Both are essential, but the SOD defense system seems to be more variable between human sperm samples [3, 37]. The GPX/GRD system appears to be limited by the glucose-6-phosphate dehydrogenase-catalyzed rate of production of NADPH. In addition, seminal plasma can also act as a natural scavenger against lipid peroxidation [3]. These natural defense systems can be overloaded in pathological conditions, leading to deficiencies in male reproduction [23, 37–42].
Oxidative stress and the generation of ROS/RNS also have an impact on sperm mitochondrial function. Oxidative stress and excess ROS/RNS increase the activity of mitochondrial proteins, such as BCL-2, which stimulate the release of mitochondrial proapoptotic factors into the cytosol, activating caspases and harming mitochondrial membrane integrity with loss of the mitochondrial inner membrane chemical/electrical potential (MIMP) [43–45]. The over-production of ROS/RNS also reduces the ATP levels in sperm [46]. There is a direct relationship between loss of MIMP and sperm viability, because the loss of MIMP leads to the reduction of ATP production during cell respiration, leading eventually to cell death [45, 46]. Thus when mitochondria are affected by oxidative stress, there is a loss in MIMP, and this is followed by alterations in many sperm functions, such as motility, viability and the ability to undertake fertilization [47].
The SM is especially damaged during cryopreservation procedures, altering the viability of sperm recovered from frozen samples [48]. That is the reason why most studies on membrane replacement have been limited to its possible use in cryopreservation. Previous studies attempting to restore membrane function in sperm have been done with phospholipids in cryopreserved ram sperm [49], or omega-3 PUFA to cryopreserve turkey sperm [50]. A similar approach has been recently tested for cryopreserved-human sperm using phosphatidylserine present in a freezing supplement (Trolox) [51]. Finally, there are reports of freezing supplements that have used phosphatidylcholine from soybean to increase the cryotolerance of human sperm [52]. Although these studies were relatively successful, they did not explore the use of membrane lipid replacement (MLR) at the molecular level, nor they did not use GPL mixtures or mixtures mimicking most of the SM components, and they did not test oxidative stress damage to sperm incubated at 37°C.
We have used dietary MLR to replace oxidized mitochondrial membrane lipids to improve MIMP and restore function [53, 54]. MLR utilizes mixtures of cell membrane GPL, plus fructooligosaccharides for protection against oxidative, acid and enzymatic damage, in order to safely replace damaged, oxidized, membrane GPL through diet. In preliminary studies MLR with GPL have been shown to improve human sperm motility and viability [55]. Here, we examined the ability of sub-μm-sized micelles prepared from GPL to modify human SM. We also tested human sperm to see if GPL could protect from the loss of motility and viability due to the effects of oxidative stress. Finally, we also examined if the incubation of human sperm with sub-μm-sized micelles prepared from GPL could prevent the loss of MIMP produced by oxidizing agents like hydrogen peroxide (H2O2). Our results suggest that human sperm can be used to test innovative new treatments like MLR in order to improve male fertility when it is adversely affected by increased oxidative stress.
Material and methods
Ethics
The procedure to obtain human sperm was done following ethics procedures approved by the National Ethics Committee at the School of Medicine, Universidad de la República (Res# 070153-001013-14). The donors comprised a random group of 24 adult males with normozoospermia and an average age of 32±1 years. An Informed Consent document was approved by the Ethics Committee and signed by all participants.
Experimental procedures
Obtaining fresh human sperm samples, incubation procedure and preparation of the sub-μm-sized micelles
Human sperm were obtained by ejaculation from young males of reproductive age, (range, 27–39 years). Once obtained, human semen samples were submitted to liquefaction for 30 min, followed by a standard swim-up protocol in accordance with standard procedures, to enrich the preparation of healthy sperm [56]. We excluded sperm samples that yield a viable sperm low count (less than 1 x 106 sperm/ml) and/or more than 60% non-motile sperm cells per field after a one h incubation. After processing the semen samples with a sperm swim-up procedure, the samples were centrifuged at 300 × g for 5–10 min and finally resuspended in HAM-F10 medium (GIBCO, Thermofisher cat N° 11550), at an approximate concentration of 20–30 × 106 sperm/ml. To obtain the desired concentrations we measured the diluted initial sperm count from the semen with a Neubauer hematocytometer chamber or a Cell-VU chamber with a grid of standard dimernsions. We counted the sperm in five volumes of the 0.1 mm3 (or μl) central counting square. Then we multiplied that result by 10000 to know the concentration per ml, and finally we multiplied that for the dilution factor to know the sperm concentration in the undiluted sample. The original undiluted sample was then diluted to obtain approximate concentrations of 20–30 × 106 sperm/ml. Sperm selected by the swim-up procedure were placed in a sterile incubator at 37°C and 5% CO2, with constant mixing a shaker inside the incubator. The selected sperm preparations were incubated under different conditions for average time of 2–3 h. Sperm had to be incubated for up to 2h to reach a steady state due to the kinetics of incorporation of GPL into bilayers, nano-micelles and other vesicles that mimic membrane composition. An almost full substitution of the lipids followed hyperbolic Michaelis-Menten-like kinetics [57]. The incubation procedure offered several advantages as it allowed; (a) an exact time of exposure of the SM to the sub-μm-sized micelles; (b) an adequate monitoring of the viable sperm at different times of incubation; (c) an improvement of the impact of the motile sperm with the sub-μm-micelles; and finally (d) testing in parallel of different experimental conditions, such as incubation with oxidizing agents. To promote oxidative stress incubation was performed with 300 μM H2O2 added to HAM-F10, in accordance with previous studies [58, 59]. To evaluate the antioxidant properties of GPL mixtures, sub-μm-sized micelles were added to sperm incubated in HAM-F10 with 300 μM H2O2 using a shaker, following the procedure described above. To avoid interference from sub-μm-sized micelles in the measurements of sperm motility all samples were centrifugated after the incubations at 300 x g for 5 min, and the sperm to be tested were taken predominantly from the middle of the centrifugation vial. Centrifugation at low speed does not alter the characteristics of sperm samples [60, 61].
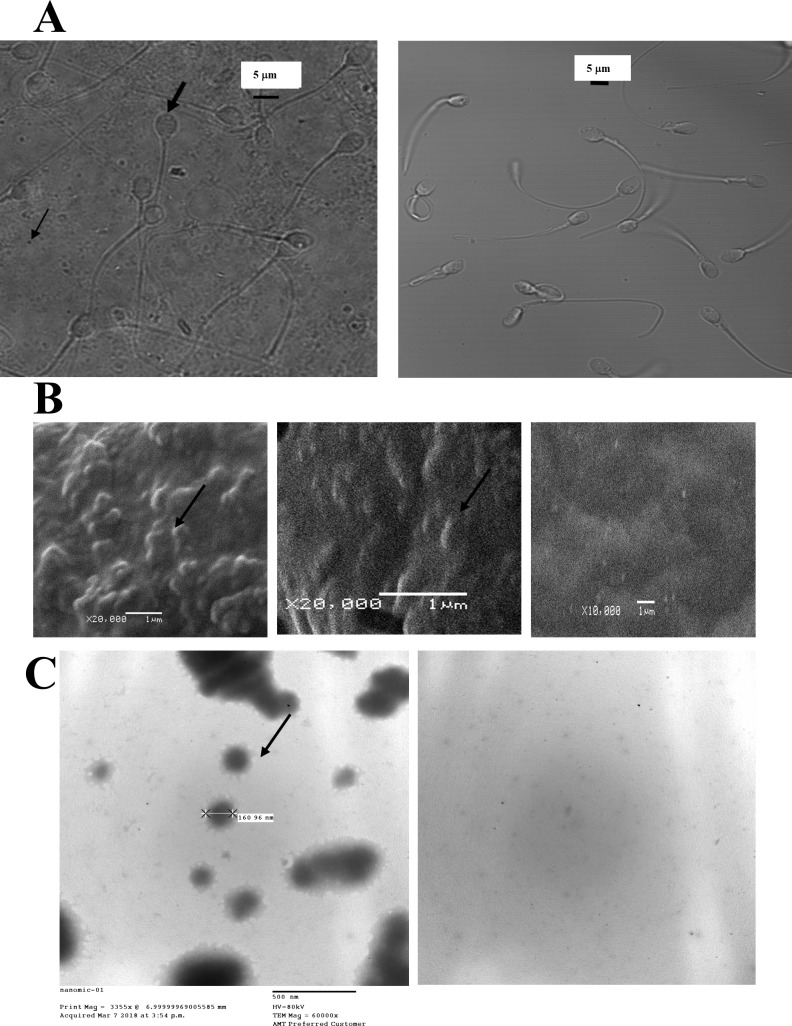
GPL in physiological solutions spontaneously form bilayers or micelles, and if ultrasonicated at low concentrations, tend to form smaller micelles in the diameter range of nm or sub-μm. We used freshly prepared mixtures of GPL and fatty acids of precise composition that mimic the GPL composition of mitochondrial membranes (NTFactor Lipids®, Nutritional Therapeutics, Inc. of Hauppuage, NY, USA). This composition of GPL is known (see [62]) and has proved to be successful for in vivo MLR in several human diseases and conditions in various reports [53, 63–66]. The advantage of using a mixture like this with precise proportions of GPL and unsaturated fatty acids is that it closely mimics the compositions of biological membranes. When used in the incubation procedures, the GPL were added to the incubation media with less than 0.1% ethanol to enhance solubility. Control incubation medium was HAM-F10. The addition of ethanol <0.1% did not cause significant variations in data (P = 0.95, n = 8). GPL micelles were prepared at concentrations up to 3% GPL mixtures in the incubation media (typically, 0.1–1% was used). The GPL mixture was ultrasonicated at 20 KHz for 15–25 min, using a probe sonicator plus a 50W Virtis virsonic 475 device (Virtis/SP Industries, Gardiner, NY, 12525, USA), similar to that reported for the constitution of nanocapsules [67–71]. The resulting product was purified as sub-μm-sized micelles with a CL-4B sepharose chromatography size exclusion column, or alternatively using a sterile 0.2 μm Millipore filter similar to filling patch clamp pipettes [72, 73]. Applying this procedure, we obtained sub-μm-sized micelles that mixed well with the media and that were small enough to be incorporated into the SM. Fig 1A (right), shows human sperm isolated after an incubation period of 6 h, without the addition of a GPL mixture. The background is clear and homogeneous in this image. Fig 1A (left), shows ultrasonicated 3% GPL mixtures after a 6-h incubation with human sperm without shaking to enhance the observation of the sub-μm-sized micelles. The background shows a mesh of precipitated sub-μm-sized micelles, some of them isolated and some fusing with themselves. Following Abbe's law, the average size of the micelles we obtained was in the resolution limit of a light microscope (~210 nm for 100x objective lense with NA 1.45 and visible light wavelength). To obtain a better resolution of the sub-μm-sized micelles, we observed the micelles with a Scanning Electron Microscope (Jeol JSM-5900LV, Tokyo, Japan) and with a transmission electron microscope (Jeol JEM-1010 Tokyo, Japan). To observe the micelles with the scanning electron microscope the micelles were dried and placed into a low-pressure vacuum and sputter coated with gold using a coating device (Denton Vacuum, Desk II, NJ, USA). Fig 1B shows the results obtained on a control surface (right) and on a surface with precipitated sub-μm-sized GPL micelles. Precipitated micelles can be seen isolated (middle) or parts of thin layered films (left). Sub-μm-sized micelles rarely exceeded 1 μm in diameter and were usually at the limit of optical resolution (200–250 nm). The composition of the atomic elements in the micelles in physiological saline solutions (after spraying with gold) was obtained by X-ray Fluorescence (see the blue dots in S1A Fig). The same analysis was also done as a control for the graphite surface sprayed with gold (see S1B Fig). Nitrogen, Oxygen, Sodium, Potassium, and Chloride could only be detected in the dots corresponding to the micelles and not in the control. These results were repeated and observed in a transmission electron microscope. Negative staining of the micelles was done using phosphotungstic acid [74]. The average size of the micelles measured with the transmission electron microscope was consistent with the measurements in the light and scanning electron microscopes. A micelle measuring 160 nm is identified in Fig 1C. They are usually denser in the middle, and a star-shaped outer layer is often observed surrounding them. Small micelles can join into larger micelles as a consequence of the dehydration process necessary to observe them in an electron microscope. The background image without micelles is shown to the right.
Fig 1. Aggregation of glycerophospholipids into sub-μm-sized micelles.
(A) Images of sperm and GPL micelles in a confocal microscope with visible light. Precipitated ultra-sonicated sub-μm-sized GPL micelles (thin arrow) with human sperm (thick arrow) after a 6-h incubation with GPL (left), or human sperm after a similar incubation in media without GPL (right). (B) Precipitated GPL micelles seen in a Scanning Electron Microscope. The left image shows the micelles as protruding images from a thin-layered film (thin arrow). In other regions, isolated micelles can also be seen (middle image, thin arrow). The control image without GPL micelles is shown to the right. (C) GPL micelles observed in a transmission electron microscope with negative staining. To the left single GPL micelles (thin arrow) can be seen, and some micelles colliding and forming larger micelles can be observed. A background image without GPL micelles is shown to the right.
Preparation of fluorescent sub-μm-sized GPL micelles
To observe the direct incorporation of GPL into SM, we covalently attached the dye Rhodamine 123 to the carboxyl and phosphate residues in the GPL mixture (especially phosphatidylserine, phospatidic acid and fatty acids components, though GPL like phosphatidylcoline can react as well) [75, 76]. The cross-linking conjugation reaction was elicited with water-soluble carbodiimide 1-etil-3-(3-dimetilaminopropil)carbodiimide) (EDC, Thermofisher, USA), under mildly acidic conditions (pH 4–6) [75]. The stability of the active ester was improved using N-hydroxysulfoxuccinimide (sulfo-NHS, Thermo Fisher, USA). All of the conjugation reactions were achieved with EDC carbodiimide, according to previous publications [75, 77]. The amount and isolation of the bioconjugate Rhodamine 123 crosslinked to the GPL mixture was evaluated by the procedure by Nakajima et al. 1995 [78]. The GPL mixture with crosslinked Rhodamine 123 remained fluorescent, and it was then filtered with a 0.2 μm filter. To observe the fluorescence of the conjugates SM were incubated with the cross-linked GPL-Rhodamine 123 and then washed using centrifugation and resuspension. This was used solely to test the incorporation of the sub-μm-sized micelles, prepared using the GPL mixture, into the SM, and it was not used for the functional assays described in this paper.
Computer Assisted Sperm Analysis
Computer Assisted Sperm Analysis (CASA) was used to test the motion characteristics of sperm immediately after the incubation procedure was complete. This was performed with a Microptic Sperm Class Analyzer software (SCA) (Microptic, Barcelona, Spain). A volume of 5–15 μl of sperm was used for the incubation procedure to be tested (concentration 20-40x106 sperm/ml measuring). Sperm were loaded into a Cell-VU sperm counting chamber (Millenium Sciences Inc., NY, NY) and placed on slide warmers at 37°C. Sperm samples in the chambers were observed with a Nikon Eclipse E200-LED microscope using a 10X phase objective (Nikon Corporation, Tokyo, Japan), with a Basler ACA 780-75GC camera (Basler AG, Ahrensburg, Germany) connected to a computer with the SCA automated software. The acquisition rate to obtain the videos and idealize the trajectories of sperm in the samples was set to 25 frames/sec. For each Cell-VU chamber loaded, the automatic count for the motility parameters used to evaluate a sperm sample to be tested were set according to WHO standards [79], and the instrument measured a minimum of 8–10 different randomly selected microscopic fields (200–500 sperm/field). The values analyzed were total motility (TM,%), progressive motility (PM,%), velocity according to the smoothed path (VAP, μm/s), velocity according to the straight path (VSL, μm/s), velocity according to the actual path (VCL, μm/s), amplitude of lateral head displacement (ALH, μm), head beat-cross frequency (BCF, Hz), straightness (STR, %) and linearity index (LIN, %). We focused our reports on the most significant variables for our studies, such as overall or total motility, TM, VSL, VCL and VAP for fast, slow and non-progressive sperm with slow, medium or fast velocities. The procedure was repeated three times for each experimental condition during incubation (control, 0.1–1% GPL, 300 μM H2O2, 300 μM H2O2 plus 0.1–1% GPL). This type of analysis was performed for samples from 8 different males to get an estimation of the relative variation of dispersion among the different experiments and samples. The variation coefficients obtained varied from 7 to 28% according to the different WHO velocity motility parameters examined and individuals assayed, and this was consistent with several reports [80–82]. Excel spreadsheets were automatically obtained for each sample data collection and also for the total numbers of samples in each different experimental condition.
Monitoring the MIMP by flow cytometry using the JC-1 dye
The remaining sperm suspension was used for fluorescence measurements to evaluate the MIMP of the samples using the dye JC-1. In functional mitochondria, a strongly negative MIMP favors the accumulation of the cationic JC-1 probe as an aggregate inside the organelle, yielding red fluorescence. In unhealthy mitochondria, MIMP is less negative and the accumulation of JC-1 into the mitochondria is reduced, favoring its accumulation as a monomer that elicits green fluorescence. A 1000x stock solution of 5,5',6,6' -tetra-chloro-1,1',3,3'-tetraethylben-imidazolyl-carbocyanine iodide (JC-1, Sigma, USA), was prepared at 5 mg/mL in dimethylsulfoxide (DMSO, D8779, Sigma, USA). The JC-1 stock solution was divided into aliquots and stored at -20°C. One ml of the sperm suspension was incubated with JC-1 prepared and diluted 1000 times from the stock solution, yielding a final concentration of 5 μg/ul. Sperm were loaded with the dye under different experimental conditions in the dark in an incubator for 30–40 min at 37°C with 5% CO2. After loading, sperm were centrifuged at low speed, and then they were resuspended in various solutions under the different experimental conditions. In each sample, we confirmed staining with JC-1 by removing and observing a small sample on a slide using an epifluorescence microscope, exciting the dye with an Argon laser at 488 nm. The remaining volume of each of the samples was analyzed using a FACSCalibur Flow Cytometer (Becton Dickinson, Mountain View, CA, USA) with the CELLQuest software (Becton Dickinson). We determined the forward scatter (FSC) and side scatter (SSC) of the sperm samples. The FSC and SSC regions corresponding to the JC-1 stained sperm were determined for the acquisition of normal sperm [83]. Samples in the flow cytometer were analyzed to obtain at least 10,000 events for each sample. The samples were excited at 488 nm and emitted light was collected with emission filters at 530 nm (green fluorescence: FL1, dye monomers) and at 585 nm (red fluorescence: FL2, dye aggregates) [84, 85]. The photomultiplier was set for logarithmic scale. The ratio of red/green fluorescence is linearly related to the mitochondrial membrane potential [85]. The larger the red-to-green fluorescent ratio, the more negative the average MIMP, which translates to more active and healthier (functional) mitochondria [84, 85]. Dead/live sperm ratios were determined using 10 μg/ml propidium iodide (PI) from a stock solution of 1mg/ml in water (Sigma Aldrich, USA). PI is a membrane impermeant dye that is generally excluded from viable cells. It binds to double-stranded DNA by intercalating between base pairs. It is excited at 488 nm and emits at a maximum wavelength of 617 nm [86]. A negative control of sperm unstained with the JC-1 dye was also routinely obtained. The results for each experimental condition were analyzed with FlowJo software (FlowJo LLC, Oregon, USA) and displayed either as a dot plot or histograms of events at the green or red wavelength. The GPL mixture used for these experiments was prepared as described above, without covalently attached Rhodamine 123.
Confocal imaging of live human sperm
The samples of sperm incubated under different experimental conditions were loaded with JC-1 or Rhodamine 123 dyes (Sigma-Aldrich, USA) to evaluate MIMP [87, 88]. The fluorescence intensity of Rhodamine 123 excited at 488 nm and with an emission at 530 nm is linearly related to the MIMP [87]. The stronger the fluorescent signal at this wavelength, the more negative is the MIMP. We also used JC-1 as a dye to evaluate MIMP as the red/green fluorescence ratio [88]. The loading procedure for both dyes was the same, and it was performed as described for the flow cytometry experiments. After loading, washing, and resuspension of the sperm, they were immobilized for live cell imaging. To immobilize live sperm, the samples were placed for 15 min on thin coverslips covered with poly-L-Lysine (Sigma Aldrich, USA) in semi-sterile conditions in an incubator at 37°C and 5% CO2, following the procedure described by Wennemuth et al., 1998 [89]. Coverslips with the samples from each experimental condition were placed in a 35 mm microincubator chamber and held at 37°C for observation under a Leica SP5 confocal microscope (Leica GmbH, Germany). The dye Rhodamine 123 was excited with an Argon Laser at 488 nm, and the emitted light was collected at 530 nm. JC-1 was excited at 488 nm, and the emitted light was collected at both 530 nm (green fluorescence) and at 585 nm (red fluorescence). Images were obtained with either 40X, 63X or 100X oil immersion objectives lenses and were acquired in xyt scanning mode at 512x512 pixels. To avoid out-of-focus imaging and collection of light from several planes in the size range of a sperm, the pinhole was usually set at 1.5–2.5 Airy Units. Image processing was done using the Leica LAS AF or LAS X suites (Leica GmbH, Germany) and Image J. The GPL mixture used for these experiments was prepared as described above.
Analysis of the results, statistics and figure preparation
The data obtained from applying the methods previously described to each of the eight samples of human sperm under the different experimental conditions was analyzed for statistical analysis using Sigmaplot 11 (Systat Software Inc. USA). Average values and standard error of the mean were obtained for each condition and paired or independent Mann-Whitney Rank t-tests were applied when comparing two samples, in order to know if the changes that occurred with the treatment was greater than would be expected by chance (significance was determined at least with p<0.05). If the comparison was made for more than two treatments within the samples, the statistical analyses to test the significance of the differences of the mean for multiple experimental conditions were performed following the Tukey One-Way ANOVA test, using either Sigmaplot 11 or the SPSS software (IBM, USA). Dose-Response curves were fitted by a Hill model plus an initial constant (Do), R = Do + A*Dn /(IC50n + Dn), where R is the response, D is the dose and the parameters are maximum variation, A; half maximal dose, IC50 and hill number, n. To get the best Hill parameters that fit the observed values, non-linear regression by non-linear least squares, between the data and the model, was performed. Flow cytometry dot plots and cytograms, were obtained using the CELLQuest (Becton Dickinson) and the FlowJo software (FlowJo, LLC, USA). For image analysis, raw images were imported with Image J (NIH, USA) or the LAS-AF Suite (Leica, Germany) and fluorescence intensity along lines were obtained.
Results
Ultrasonication of glycerolphospholipids creates sub-μm-sized micelles that can incorporate GPL into sperm membranes
When sonicated GPL were mixed with human sperm, the sperm heads increased in size, suggesting the incorporation of the GPL mixture into the sperm plasma membrane. When the average sperm head area in a control solution was compared to sperm head areas in an ultrasonicated 1% GPL solution, the average area of the sperm heads measured by phase contrast during CASA experiments was significantly greater. The size of the sperm heads increased from an average of 16 μm to 19 μm (p<0.05, independent t-test, n = 8). This result held for static or mobile sperm (at all velocities). The GPL were likely incorporated into the plasma membrane and other sperm membranes, but this was not as obvious as with the sperm heads. In Fig 2 the mean areas of the sperm heads versus the concentration of the GPL mixture are shown as normalized histograms (Fig 2A). The size of the sperm head area was dependent on the concentration of GPL (Fig 2B). Increasing the GPL concentrations enlarged the sperm head area, demonstrating that these variables are related.
Fig 2. Increase in size of the sperm heads after incubation with sub-μm-sized GPL micelles.
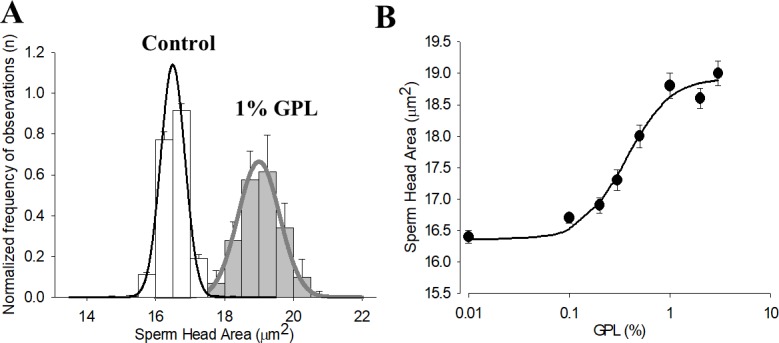
(A) Histogram and normal distribution of sperm head size after incubation in control and in 1% GPL mixture. (B) Sperm Head Area and GPL mixture concentration.
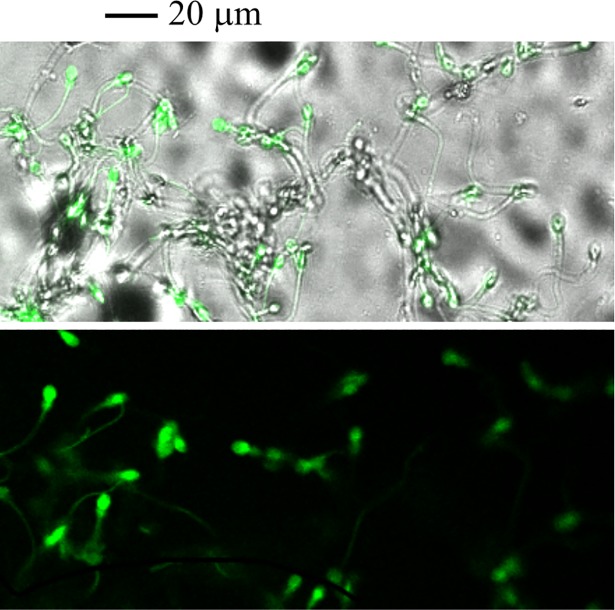
Sperm with incorporated, labeled, ultrasonicated GPL are shown in Fig 3. Sperm were incubated with a cross-linked GPL-Rhodamine 123 and then washed using centrifugation and resuspension. High laser intensities at 488 nm had to be used to get light emission from sub-μm-sized micelles. These fluorescent bioconjugates were used solely to observe the incorporation of the GPL into sperm membranes. Most of the fluorescence is seen in the sperm middle piece and in the sub-equatorial region of the sperm head. This suggests that labeled GPL in the mixture can incorporate into these regions of the sperm, at least at the level of the plasma membrane. It has been shown that the mitochondrial membranes and the plasma membrane in the mitochondrial sheet around the mid-piece are interrelated and possibly linked, at least transiently [90].
Fig 3. Incorporation of GPL from sub-μm-sized micelles into sperm membranes can be observed with fluorescent GPL crosslinked with Rhodamine 123.
Human sperm were incubated with sub-μm-sized micelles made of GPL crosslinked with Rhodamine 123. Images of stained sperm are shown merged with trans-illumination (upper) or in fluorescence mode alone (lower). Note that more than one-half of the sperm are strongly fluorescent.
Since the lipid composition of the sperm plasma membrane is a major determinant of sperm motility [91], we expected to observe changes in sperm motility with the incorporation of exogenous GPL. To test this hypothesis and get functional data on the incorporation of GPL from the sub-μm-sized micelles into sperm membranes, we measured the motility of human sperm with Computer Assisted Sperm Analysis (CASA) after incubation of various concentrations of sub-μm-sized micelles made with different concentrations of GPL. The results of overall motility and fastest motility versus the concentration of GPL are shown in Fig 4. With increasing concentrations of GPL, both types of motility were increased up to a maximum of 10%. The solid lines represent the best fit of the Hill equation to the average values of overall and fastest motility observed at each GPL concentration. At concentrations above 1% of the GPL mixture there was a tendency to observe a reduction in motility because of the size of GPL micelles interfering with sperm displacement, and possibly because the sperm heads became too heavy to sustain motility. If ultrasonication was not done to the GPL, the micelles also interfered with the normal kinetic movements of the sperm. When sperm were separated from the lipid micelles by low-speed centrifugation, the enhanced motility of sperm cells due to the GPL mixtures was more obvious. After incubation and centrifugation at 300–500 x g for 10 min at 37°C, the increase in sperm motility can be seen in the GPL-incubated samples, especially at concentrations of GPL above 0.5%, supporting the findings reported in Figs 1 and 2. The data indicate that ultrasonicated GPL transfer their lipids to the SM, modifying their composition and function. Next, we evaluated if MLR with the GPL could reduce the damage to SM produced by exposure to oxidizing agents.
Fig 4. Dose response curves of overall and fastest sperm motility versus concentration of GPL.
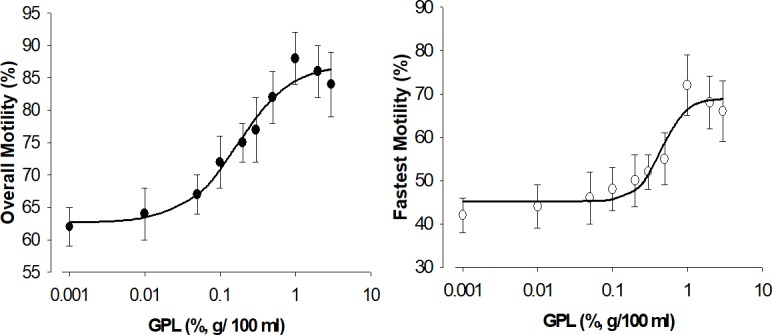
Both types of velocities are increased by incubation of sperm with sub-μm-sized micelles prepared from GPL. The solid line represents the best fit of a Hill equation. The effect is more pronounced (IC50 of approximately 0.5%) for the fastest sperm (right) compared with 0.15% for the overall motile sperm (left).
Incubation of human sperm with an ultrasonicated GPL mixture corrects reduced motility produced after incubation with hydrogen peroxide
Oxidative stress is a major determinant of membrane lipid peroxidation and sperm motility [40–42]. We expect reduced damage from oxidative stress by lipid peroxidation when sperm are incubated with unoxidized GPL like those present in our GPL mixture. We tested this hypothesis by measuring sperm motility under four different experimental incubation conditions (control, GPL, H2O2, GPL plus H2O2).
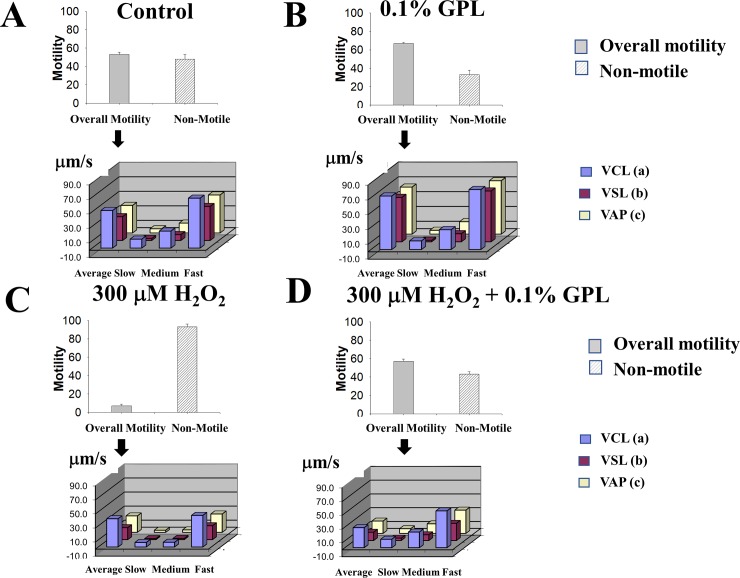
In Fig 5, the average motility percentage and the distribution bar charts of sperm velocities in μm/s are presented for the WHO velocity variables obtained by CASA under various experimental conditions. In Fig 5A, the control sperm that are motile were found to be approximately 52±2.5% of total sperm, after an incubation period of 3 h in HAM F10 medium (n = 8). We analyzed the distribution of velocities for the motile fraction of sperm. Motile sperm, according to the WHO, can be fast, medium or slow progressive [79]. The plot of velocity versus the type of motility for the three sperm velocities (following WHO standards), automatically reported by CASA analysis, are shown for a control incubation. Each velocity is indicated next to the bars (VCL, VLS, and VAP, shown as blue, purple and yellow), and it contains the average velocity for the motility and its distribution as fast, medium or slow velocities (Fig 5A). The same analysis was performed for sperm incubated for 3–4 h in sonicated 0.1% GPL (Fig 5B). When ultra-sonicated 0.1% GPL were present during incubation, the overall motility fraction increased from 52±2.5 to 68±1.34%, and the non-motile sperm decreased from 48±5 to 32±5% (n = 8 experiments, p<0.001). In addition, there was also a statistically significant increase of the fastest motility produced by incubating sperm in sonicated 0.1% GPL (68% compared to 52%) (n = 8 experiments, p<0.001). The results indicate that incubation in 0.1% GPL is non-toxic for the sperm, as monitored by their motility. Moreover, this increase could be beneficial, as it increased significantly their fastest motility for all velocities examined (VAP, VCL and VLS). Sperm motility is one of the best indicators of semen quality [92]. We promoted oxidative stress by addition of 300 μM H2O2 for 3 h to the incubation medium and found that the motility monitored by CASA [79, 93] was significantly reduced (Fig 5C). Overall motility (grey) was reduced to 7±2% and immotile sperm (dashed white) increased to 93±2% of the sperm (n = 8,p<0.0001). All of the velocities of all types were dramatically reduced in agreement with previous reports [59, 94]. The average sperm velocities diminished dramatically to about 6 μm/s (n = 8, p<0.01), except for the fast velocities (30 μm/s). However, co-incubating the sperm with 300 μM H2O2 containing additionally 0.1% ultra-sonicated GPL, increased the overall motility from 7% to 58% and decreased the non-motile sperm from 93% to 42% (n = 8 experiments, p<0.01) (Fig 5D). Though all the velocities were increased for each type of motile sperm, the differences were more pronounced for slow and medium velocities (increases from 6 μm/s to 10 and 20 μm/s), whereas the fast velocities increased from 30 μm/s to 42 μm/s (see Fig 5D). These results suggest that ultrasonicated GPL can prevent the damage to sperm and their motility by agents that promote oxidative stress, such as H2O2. Thus, exposure to 0.1% GPL ameliorates the reduction of motility observed by exposure to H2O2, suggesting that the GPL mixture it is an effective agent to protect sperm against oxidative stress (one of the main causes of infertility).
Fig 5. Analysis of motility of mature human sperm from adult males incubated under different experimental conditions (control, GPL, H2O2, GPL plus H2O2).
For each experimental condition (listed on the top of each graph set) the percent overall motility (grey bar) and immotile sperm (dashed white bar) are shown. Below these plots are shown the curvilinear velocity (VCL), the straight line velocity (VSL) and the average path velocity (VAP) in μm/s, for each type of sperm motile pattern (average, slow, medium and fast motility)(n = 8 experiments). (A) control (B) 0.1% GPL (C) 300 μM H2O2 (D) 300 μM H2O2 plus 0.1% GPL.
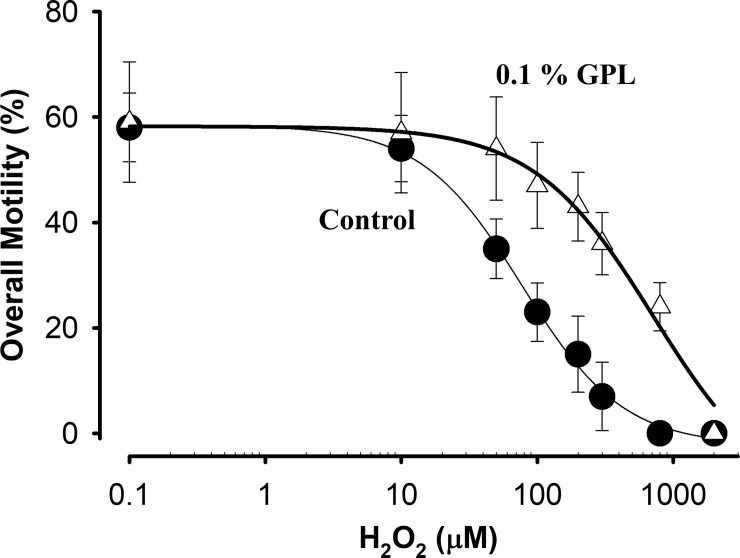
We next examined the protective effect of ultra-sonicated 0.1% GPL on sperm motility when incubated together with different H2O2 concentrations. Dose-Response curves of sperm motility versus increasing H2O2 concentrations in control and in the presence of 0.1% GPL are shown in Fig 6. Overall motility was analyzed after an incubation period of 3 h. The effects of H2O2 concentrations on overall motility for the control (filled circles, without GPL) and for the incubation in 0.1% GPL (white triangles) are shown. The solid line represents the best fit of a Hill equation. The IC50 for overall motility changes from 80±15 to 700±20 μM when 0.1% GPL are added. The protective effect of GPL was observed at all concentrations of H2O2 tested. The results suggest a protective role of GPL against oxidative stress affecting sperm motility when they are exposed to different concentrations of H2O2.
Fig 6. Dose response curves of overall motility versus hydrogen peroxide concentration.
The data correspond to the average motility obtained after incubation in the presence of varying concentrations of H2O2 (solid circles) or in the presence of varying concentrations of H2O2 with 0.1% GPL added (open triangles) (n = 8 experiments). The solid lines represent the best fit of a Hill equation to the data.
Incubation of human sperm with a GPL mixture reduces the loss of mitochondrial membrane potential promoted by incubation with hydrogen peroxide
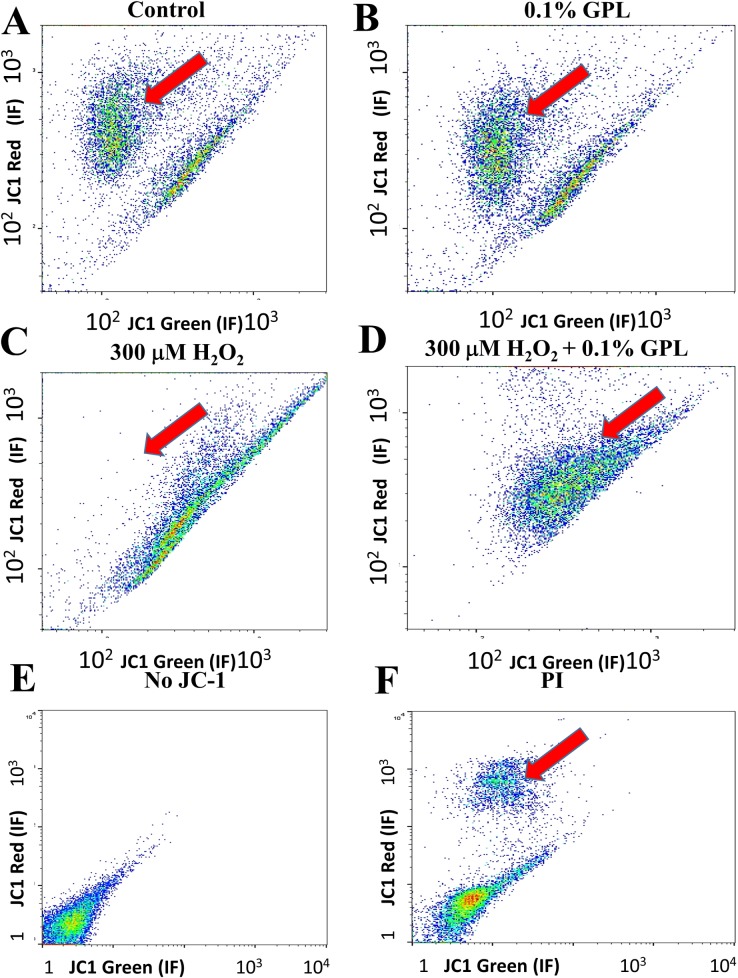
Since the viability and motility of sperm are related to the health of their mitochondria [95, 96], and because we have observed incorporation of GPL in regions of sperm where mitochondria are abundant, we sought to determine if these effects are correlated with mitochondrial function. Moreover, most of the ROS/RNS damage in sperm is linked to mitochondrial dysfunction. To examine if GPL can restore mitochondrial function in a population of sperm we performed flow cytometry of sperm loaded with JC-1, a fluorescent dye reporter of MIMP. Maintenance of MIMP is directly related to mitochondrial function [97]. Since JC-1 fluorescent ratio at 535/595 nm is directly related to MIMP, the red-to-green dye ratio of JC-1 was examined in a mixture of sperm incubated under different conditions: various control samples, 0.1% GPL, 300 μM H2O2, 300 μM H2O2 plus 0.1% GPL and exposure to the toxic agent propidium iodide (PI). JC-1 is a ratiometric dye, and the greater the ratio of red versus green intensities for the cells, the healthier they are; thus as the ratio red/green fluorescence increases, the more negative is the MIMP, and the more high-energy molecules produced. Fig 7 shows comparisons in the log-log scale of individual sperm red-to-green fluorescence for each experimental condition. The data are represented from blue, green, yellow to red dots indicating lower to higher densities/probabilities of the results obtained with sperm for each pair of fluorescence intensities for red and green emission. Higher ratios of red versus green emission indicate healthier spermatozoids, because the distribution reports collectively a more negative MIMP. Two types of data point clouds can be distinguished. One type of data cloud can be found on a diagonal following an almost direct proportional ratio of red-to-green fluorescence with a slope of approximately 1. These sperm, are likely to be damaged or at least not in a healthy condition (Fig 7A). The red line separates another cloud of data points with a higher red/green fluorescent ratio, indicating functional maintenance of the MIMP. These are mostly healthy sperm (see large red arrows). In Fig 7B the same experiment was performed with sperm were incubated with 0.1% GPL. Since the results are similar to the control in Fig 7A, GPL must be non-toxic to the sperm and may even increase slightly their viability. This is apparent because the cloud of healthy spermatozoids in the GPL-treated sample has the same or larger area than that observed in the control, suggesting that GPL treatment can improve the control viability. When sperm were incubated with a toxic oxidizing agent (300 μM H2O2), the cloud of healthy sperm almost disappears, and only the diagonal cloud corresponding to non-healthy sperm remains (Fig 7C). The addition of 0.1% GPL to the incubation along with 300 μM H2O2 promotes the appearance of healthy sperm (red arrow, Fig 7D). This plot is clearly different from that observed after incubation with 300 μM H2O2, suggesting a protective role of GPL against the loss of MIMP promoted by H2O2. The plot in Fig 7E corresponds to the experimental control without JC-1 and indicates that the low levels of fluorescence shown at the left-bottom of each panel represent nonspecific emission. To evaluate sperm cell viability and membrane integrity an intercalating fluorescent agent (PI) was applied during the incubation with the JC-1 dye. PI is also toxic to cells, promoting cell death [98]. Although most of the sperm died during the incubation, yielding a pattern similar to JC-1-unstained sperm, some sperm survived corresponding to the position of the cloud shown in Fig 7A and 7D (red arrow, Fig 7F).
Fig 7. Dot plots from flow cytometry of sperm loaded with JC-1, a mitochondrial reporter, under different experimental conditions.
(A) Results obtained after control incubation of sperm. (B) Incubation of sperm with 0.1% GPL. (C) Incubation of sperm with 300 μM H2O2. (D) Incubation of sperm with 300 μM H2O2 with the addition of 0.1% GPL. (E) Control obtained with unstained sperm (without JC-1). (F) Cells stained with JC-1 and exposed to the toxic molecule PI.
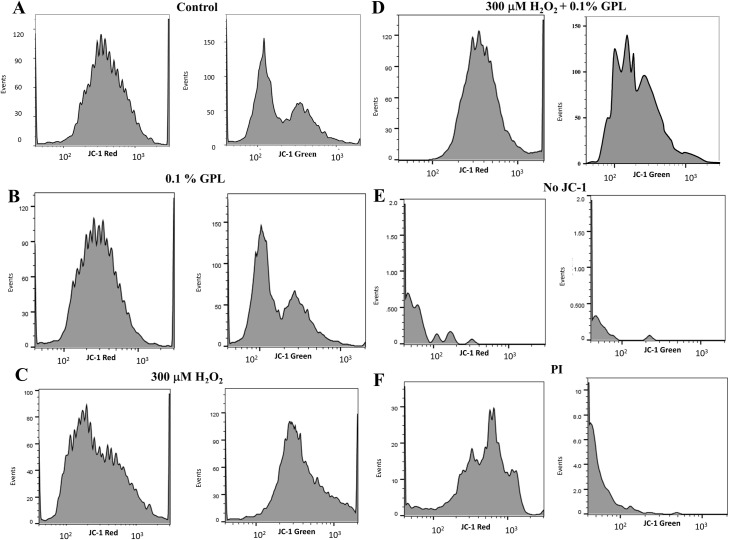
Flow cytometry fluorescence histograms using JC-1 plot the number of events for red (left panels) and green (right panels) fluorescent intensities under different experimental conditions (Fig 8). The area under each curve represents the number of sperm found at different fluorescence intensities (semi-log plot). Fig 8A shows the control histograms. There is only one red fluorescence peak at an intensity of 400 arbitrary units (Fig 8A left panel). The range of events is distributed at fluorescence intensities between 100 and 2000 (log scale). There were two peaks in the green fluorescence histogram, one at 100 and another at 400 arbitrary units (Fig 8A right panel). The red-to-green ratio for the lower peak was approximately 4, suggesting that the highest peak at a lower green intensity corresponds to healthy sperm. When comparing the distribution of red versus green fluorescence in both plots, the green fluorescence peak that corresponds to the red fluorescence peak was lower than the other green fluorescence peak, and therefore this lower peak represents unhealthy sperm with lower MIMP. In Fig 8B the same results were obtained after incubation of the sperm with 0.1% GPL (red fluorescence peak ~ 350 arbitrary units, same values for green). This result indicates that the GPL does not promote damage to the sperm. In Fig 8C the sperm were incubated with 300 μM H2O2. The red fluorescent intensity was shifted towards lower values with a peak at about 130 arbitrary units, and the green fluorescent intensity became one peak centered at about 300 arbitrary units of fluorescence intensity. These results indicated that most of the sperm have a dramatically lower ratio of red/green fluorescence after treatment with H2O2, suggesting a dramatic loss of MIMP and an increase in unhealthy sperm. Fig 8D shows the effect of adding 0.1% GPL to the sperm in the presence of 300 μM H2O2. The peak for red fluorescence was shifted towards 250 fluorescence intensity units, whereas the histogram for green fluorescence revealed a bimodal plot similar to that observed in Fig 8A and 8B. The peak at a lower intensity was positioned at about 120 arbitrary units, whereas the peak at a higher intensity was positioned at about 300 arbitrary units. The reappearance of two peaks of green fluorescence suggested a protective effect of the GPL from the oxidative stress produced by 300 μM H2O2. In Fig 8E the control histograms for red and green fluorescence without the addition of JC-1 are shown. Thus, the fluorescence measured in Fig 7 and Fig 8A–8D were due to JC-1 and were not artifacts. In Fig 8F, the histograms are shown after incubation with PI. PI molecules can enter sperm that have lost membrane integrity and are also toxic to sperm. With PI treatment the red fluorescence peak was observed at approximately 700 arbitrary units, corresponding to surviving viable sperm. The number of events in the vertical axis in Fig 8F are quite low in comparison with Fig 8A–8D, and the green fluorescence is also low, mostly at intensities below 100, indicating the presence of non-viable sperm after exposure to PI. This control indicates that the results in Fig 8A–8D reflect measurements with viable sperm.
Fig 8. Histograms of red and green fluorescence intensity of sperm stained with JC-1.
(A) Histograms of fluorescence of sperm red (left panels) and green (right panels) fluorescence intensities obtained after the control incubation (semi-log scale). (B) Fluorescence histograms of sperm obtained after incubation with 0.1% GPL. (C) Fluorescence histograms of sperm obtained after incubation in 300 μM H2O2. (D) Fluorescence histograms of sperm after exposure to 300 μM H2O2 in the presence of 0.1% GPL. (E) Fluorescence histograms of sperm cells without JC-1. (F) Fluorescence histograms of sperm in the presence of propidium iodide (PI).
In summary, the incubation of human sperm with a GPL mixture reduced the loss of MIMP after incubation with hydrogen peroxide. Similar incubations were performed using Rhodamine 123 and JC-1, and the sperm were examined under a confocal microscope. The results confirmed the cytofluorographic studies.
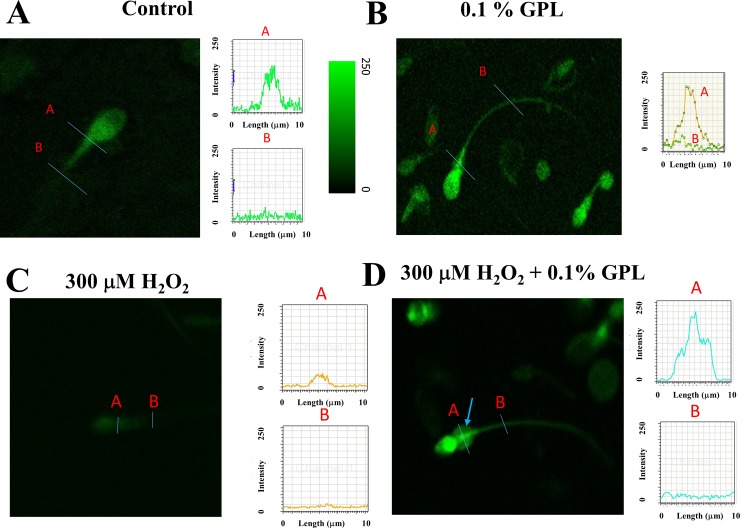
Flow cytometry has the advantage of examining thousands of cells under different experimental conditions, but it cannot evaluate the origin of the fluorescent signal. To better understand this, as well as the improvement of viability over time in sperm incubated with GPL we examined sperm under a confocal microscope after loading with dyes that can evaluate MIMP. In Fig 9, a typical sperm loaded with the Rhodamine 123 dye under different conditions for 3 h (control, 0.1% GPL, 300 μM H2O2 and 0.1% GPL plus 300 μM H2O2) is shown. Though in each panel we show just one sperm cell stained with the dye Rhodamine 123, we performed the analysis under each condition for 150 sperm randomly selected from different fields for each sperm sample. After incubation in control media, segments A and B of the sperm were rich in or devoid of mitochondria, respectively (Fig 9A). The fluorescence intensity for those sperm segments A and B are shown to the right with the same scale for the ordinate axis. The higher the fluorescent intensity observed in segment A, the healthier the mitochondria. The pseudo-color intensity scale is also shown. The peak of fluorescence intensity in segment A is between 150 and 200. Similar results were observed in 59±5% of the total number of randomly selected sperm analyzed under this experimental condition (n = 8 samples). Fig 9B shows a confocal image of sperm after incubation with 0.1% GPL and stained with Rhodamine 123. The neck of a sperm (segment A) has an intense fluorescence intensity (peak A, to the right), compared with other regions of the sperm (such as segment B shown in peak B, to the right). The fluorescence intensity in the dye-intense regions can reach values of approximately 200 arbitrary units (see graph to the right) [99–101]. Similar results were observed in 64±7% of the total number of randomly selected sperm analyzed under this experimental condition (n = 8 samples). Another interesting point is that when sperm were incubated with GPL, a cytoplasmic droplet appeared in many sperm cells. It has been suggested that such cytoplasmic droplets are indicative of stronger resistance to oxidative stress and other damaging events [102–104]. Sperm membrane channels and signaling processes localized in the sperm head are essential for the acrosomal reaction and successful fertilization [104, 105]. In Fig 9C the results obtained after incubation of sperm in 300 μM H2O2 are shown. Most of the sperm loaded with the Rhodamine 123 dye showed low fluorescence intensity for segments in the sperm neck (segment A). The maximum values observed were around 50 fluorescence arbitrary units. This reduction implies that exposure to 300 μM H2O2 results in oxidative stress to mitochondria. The loss of staining suggests that exposure to 300 μM H2O2 results in unhealthy sperm due to the loss of mitochondrial function. Similar results were observed in 84±8% of the total number of randomly selected sperm analyzed under this experimental condition (n = 8 samples). Co-incubation of sperm with GPL plus 300 μM H2O2 restores the fluorescence values of segment A to 200 arbitrary units. The peak of fluorescence for segment A is in a similar range to those found in Fig 9A and 9B, suggesting again a protective role of GPL for the mitochondrial oxidative stress produced by H2O2. Most of the sperm have cytoplasmic droplets (see light blue arrows). It has been hypothesized that the cytoplasmic droplet appears as a response to environmental stress [102–104]. Similar results were observed in 54±3% of the total number of randomly selected sperm analyzed under this experimental condition (n = 8 samples). The results under confocal microscopy obtained with Rhodamine 123, under the same conditions as described in Figs 7 and 8, are consistent with the results described previously. This suggests that GPL incorporate into the sperm membranes and partition into the mitochondria, possibly removing and substituting GPL for peroxidized lipids.
Fig 9. Confocal images of human sperm stained with Rhodamine 123 under different experimental conditions.
Segment A has been inserted in a sperm sector enriched in mitochondria, whereas segment B has been placed in a sector devoid of mitochondria. Fluorescence intensity plots from segments A and B are shown to the right. (A) A typical spermatozoon stained with Rhodamine 123 under control conditions. (B) A sperm stained with Rhodamine 123 in medium containing 0.1% GPL. (C) A typical spermatozoon stained with Rhodamine 123 in medium containing 300 μM H2O2. (D) A sperm stained with Rhodamine 123 in 300 μM H2O2 with 0.1% GPL.
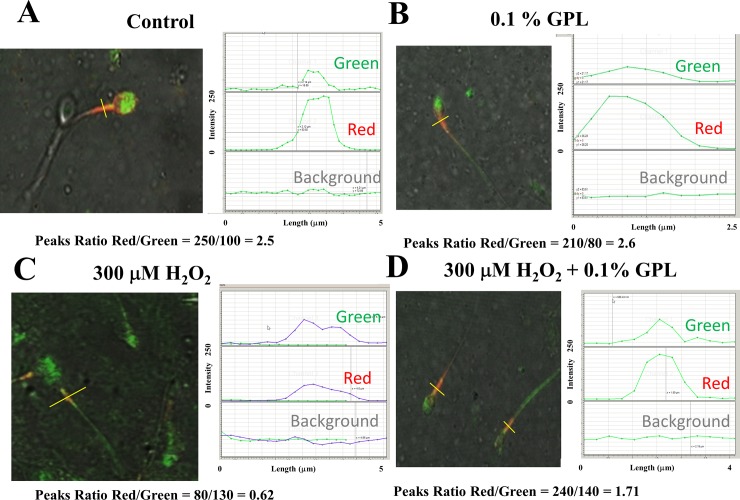
To better assess the MIMP and fluorescence in different sperm segments, such as the neck/midpiece, we have used JC-1 instead of Rhodamine 123, along with acquiring images at low magnification to evaluate multiple sperm at the same time (Fig 10). This approach was applied to approximately 150 randomly selected sperm in different fields for each of the samples. A typical image of the stained sperm for each experimental condition is shown in each panel. Using JC-1, the red/green ratio of the peak signal at the midpiece was calculated to obtain an estimation of MIMP and thus the health of sperm (yellow lines). The plots to the right labeled as Green, Red, and Background, represent the levels of fluorescence intensity along the yellow segment through the spermatozoon, with the same scale for the ordinate axis. The red/green ratio was almost 2.5±0.1 for the control, indicating that mitochondria stained with JC-1 show negative MIMPs (<-100 mV) (n = 16, p<0.01) (Fig 10A) [99]. Similar results were observed in 55±3% of the total number of randomly selected sperm analyzed under this experimental condition (n = 16 samples). This same type of experiment was performed in the presence of 0.1% GPL, yielding a ratio of 2.6±0.15 (n = 14, p<0.01)(Fig 10B). The ratio red/green fluorescence was similar but showed a slight improvement to that seen in Fig 10A. Similar results were observed in 60±8% of the total number of randomly selected sperm analyzed under this experimental condition (n = 14 samples).The incubation in 300 μM H2O2 shows mitochondrial oxidative stress and loss of the MIMPs to less than -50 mV (Fig 10C). The ratio red/green falls to approximately 0.62±0.12 (n = 10, p<0.01). Similar results were observed in 89±5% of the total number of randomly selected sperm analyzed under this experimental condition (n = 8 samples).Fig 10D shows that the addition of 0.1% GPL to the incubation with 300 μM H2O2, restored, at least partially, the red/green ratio to 1.7±0.18 (n = 16, p<0.01). Similar results were observed in 56±4% of the total number of randomly selected sperm analyzed under this experimental condition (n = 16 samples).This result is consistent with a protective effect of GPL against mitochondrial oxidative stress [64–66]. Again, these experiments agree with the notion that the GPL have a protective effect against mitochondrial oxidative stress promoted by H2O2.
Fig 10. Confocal images of human sperm stained with JC-1 under different experimental conditions.
(A) Live imaging of sperm incubated in control medium. (B) The same experiment as in A, but in the presence of 0.1% GPL. (C) Sperm incubated in 300 μM H2O2. (D) Sperm co-incubated with 300 μM H2O2 and 0.1% GPL.
Discussion
Membrane Lipid Replacement (MLR) has been used as a novel therapeutic approach to substitute damaged lipids in biological membranes [53, 62]. It has been shown to be useful to ameliorate the symptom severity in many chronic diseases, such as fibromyalgia, chronic fatigue syndrome, metabolic diseases, among others [53, 106–108]. It has been hypothesized that fresh, undamaged GPL can substitute for damaged GPL in biological membranes. Damage to GPL in biological membranes is an inevitable process that occurs naturally during cell aging or may be increased in pathological conditions [109–111]. Mitochondrial membranes are especially susceptible and relevant targets in these processes, as they are one of the main sources of ROS, which in turn is one of the leading causes of membrane lipid peroxidation [109]. It has been suggested by indirect evidence that MLR can replace damaged lipids, not only in the plasma membrane but also in mitochondrial membranes [54, 107, 108, 112]. However, there remains a lack of experimental evidence showing the incorporation of GPL into biological membranes (plasma and intracellular membranes). One goal of this report was to prepare GPL in a suitable way to create sub-μm-sized micelles that can be used to collect direct optical and functional experimental evidence of the incorporation of GPL into biological membranes through MLR. Once we established that, another aim using sperm as model cells was to test if MLR with GPL can diminish the functional damage produced by oxidative stress and reduce the impact of oxidizing agents on mitochondrial membranes. Our results suggest that incubation of sperm with sub-μm-sized micelles prepared with a GPL mixture can result in the incorporation of GPL into the SM. In addition, incubation with this mixture reduced the damage of oxidizing agents and increased the motility of sperm, while preventing the loss of MIMP produced by these agents.
Physical evidence of GPL incorporation into sperm membranes
We created sub-μm-sized GPL micelles through ultrasonication and subsequent purification through size exclusion chromatography as described in methods and observed in Fig 1. We chose spermatozoa as model cells to directly test MLR and the incorporation of fresh GPL into their membranes. Sperm GPL are abundant with PUFAs that alter sperm function, and sperm can be monitored when their membranes are oxidized. In addition, they are individual cells that are easy to evaluate in terms of their viability and function, and they are motile, scanning all particles at the surface where they move. Sperm motility is also critically dependent on the constitution of SM lipids and on the health of their mitochondria [27, 31, 47, 91, 113, 114]. Thus the incorporation of GPL into sperm membranes was considered much easier to follow than other cell types where features of the cell morphology are not so distinct. The incubation of sperm with different concentrations of sub-μm-sized GPL micelles resulted in increases in the area of the head (see Fig 2). Previous MLR studies have shown that it is likely that MLR GPL are distributed into all cellular membranes, but the estimation of the incorporation of GPL from the sub-μm-sized micelles into SM was only detectable on the basis of membrane surface area in the head of the sperm. As the first SM to contact sub-μm-sized micelles are generally the plasma membrane of the head, it is likely that sub-μm-sized micelles deposit more GPL there as well.
The energy needed for lipid incorporation into membranes may also change with the degree of curvature of the membrane [115]. Thus, MLR GPL incorporation into SM may be heterogeneous in different membranes and even in the same membrane in different regions. To directly observe the incorporation of GPL from the sub-μm-sized micelles into SM we prepared a crosslinked GPL mixture with Rhodamine 123 using EDC water soluble ceramides [75, 78]. This procedure has been used before with liposomes to report lipid incorporation into mitochondrial membranes [72]. After these procedures, the GPL-linked Rhodamine 123 was still fluorescent and formed sub-μm-sized micelles as before (see Fig 3). Our experiments suggested that GPL from sub-μm-sized micelles incorporated into the SM. We next gathered functional evidence of GPL incorporation into SM by monitoring functional changes, such as changes in sperm motility.
Functional evidence of GPL incorporation into sperm membranes
Sperm plasma membranes have restricted spaces to free lateral diffusion [116], and there are differences in lipid compositions and fluidities of the plasma membranes in sperm in the head and other regions [8, 117]. However, there is also evidence suggesting that most of the membranes in sperm and other cells that show structural asymmetries are in contact with intracellular membranes and exchange lipids through direct contact and transfer by non-vesicular transport or other pathways [2, 16, 19, 118, 119]. Thus, the incorporation of GPL into the plasma membrane will likely impact intracellular membranes in sperm organelles, such as the residual nuclear envelope (RNE) and mitochondria. These organelles play important roles in the motility of sperm [113, 114, 120–125]. Damage to membrane lipids in these organelles and in the plasma membrane has been reported to diminish sperm motility [8, 55, 91, 126]. Hence, we determined if there were observable changes in basal conditions after incubating sperm with sub-μm-sized micelles containing GPL. Fig 4 examines the result of such an experiment, where incorporating GPL from sub-μm-sized micelles at different concentrations caused different effects. We observed a dual effect on sperm motility by increasing the concentration of GPL. At high GPL concentrations (>3%), the size of micelles started to interfere with the measurements of the motility of sperm, leading to confusing results. Even though we separated micelles from sperm in all our measurements, a small residual fraction remained. This small fraction became significant at high GPL concentrations, especially at concentrations >3%, interfering with the estimations of sperm motility. At lower concentrations, the size of the micelles did not interfere with the CASA measurements. It was at these concentrations that we observed significant increases in sperm motility with the incubation of GPL in sub-μm-sized micelles, especially when oxidative stress was promoted in the sperm by the addition of H2O2.
The richest SM areas in phospholipid contents are usually near the post-acrosomal regions around the sperm neck and the RNE [127]. The RNE is a calcium storage organelle, and it has been implicated as an important source for calcium release in sperm [128]. This is especially interesting because of the possible continuity in SM and the involvement of the RNE in the process of signaling within sperm [12, 128]. The RNE is also enriched in sterols and phosphoinositides [129]. These are important signaling molecules that are involved in calcium homeostasis, and they are also critically involved in many essential sperm functions, such as motility, capacitation, acrosome reaction and fertilization [128, 130–132]. Phosphoinositides constitute 25% of the GPL mixture. This is important because it suggests that MLR can affect or contribute to calcium homeostasis. Moreover, there are several plasma membrane ion channels implicated in the regulation of calcium homeostasis modulated by the surrounding lipid environment [133–136]. For example, CatSper channels are critical for many sperm functions, and they are dramatically modulated by progesterone, which in turn is critically dependent on the membrane lipid constitution for its appropriate action [136–140]. These results suggest several possibilities related to the mechanism of action of MLR, as it suggests that MLR can indirectly affect the functioning of several membrane proteins like ion channels and also effect intracellular calcium homeostasis.
Protective role of GPL against the loss of function in motility degraded by oxidative stress
One of the main mechanisms of damage to membrane lipids leading to cell dysfunction is lipid peroxidation [141]. PUFA are especially sensitive to lipid peroxidation [142]. Lipid peroxidation results in several mechanisms that can result in cell dysfunction and death, including the loss of fluidity and organization of the membranes and increased non-specific water permeability of the membrane bilayer [143].
Compared to other membranes, SM are particularly enriched in PUFA [6–9]. Because of this, SM are particularly susceptible to oxidative stress [4, 23]. In human sperm, there are multiple causes of oxidative stress [144], and such stress has been implicated as a major determinant of male infertility [40, 41, 145–148]. Considering this and the results discussed above, we decided to promote oxidative stress in sperm by incubating them with H2O2 and testing to see if the incubation with GPL sub-μm-sized micelles can reduce ROS damage [58, 149]. Our functional test was to measure the motility of human sperm under four different incubation conditions (control, with GPL sub-μm-sized micelles, with H2O2 and with H2O2 and plus GPL sub-μm-sized micelles) (Fig 5) and to obtain a dose-response curve of sperm motility against concentration of H2O2 with or without GPL sub-μm-sized GPL micelles (Fig 6). The fact that motility during co-incubation of H2O2 plus GPL sub-μm-sized GPL micelles, can restore sperm motility to values close to those obtained for the control, suggests that MLR with GPL can prevent the oxidative stress promoted by H2O2 in the SM. Furthermore, the protection against oxidative stress by H2O2 occurred at all doses. It has been shown that one of the targets and mechanism of damage of H2O2 exposure in human sperm are phospholipids [24, 26]. Sub-μm-sized GPL micelles made from fresh GPL mixtures are resistant to peroxidation [62]. These results are consistent with the findings in Figs 1–4, suggesting that lipids from sub-μm-sized GPL micelles incorporate into SM and protect these membranes. Moreover, they also suggest that MLR with GPL might be useful for treating those cases of infertility where oxidative stress is increased. As healthy mitochondria are a key to healthy fertile sperm [150], we next tested if the incubation with GPL sub-μm-sized GPL micelles could influence sperm mitochondrial function.
Protective role of GPL against the loss of MIMP promoted by oxidative stress
Once the added sub-μm-sized GPL micelles contact sperm plasma membranes, they can transfer their constituents directly to SM. However, transfer to internal membranes may occur via different mechanisms, such as non-vesicular lipid exchange and membrane-membrane contact [5, 18, 118]. Alternatively, GPL incorporated into the SM may activate metabolic or signaling pathways (through ion channels, membrane receptors or second messengers) that could have indirect effects on internal membranes. Liposomes that fuse with the plasma membrane have been used to show translocation of lipids between the mitochondria and the plasma membrane [72]. The endoplasmic reticulum, with functional continuity to the plasma membrane, is a major provider of mitochondrial lipids [151, 152]. Mitochondria and glycolysis are the main sources of energy production needed for sperm motility. Mitochondria are also one of the main sources of ROS involved in sperm oxidative stress [96, 144, 153]. Excessive ROS production in the mitochondria impacts sperm on many cell levels, among them are damage to lipids in the plasma membrane and mitochondrial membranes, resulting in loss of MIMP [154, 155]. An important cellular target of oxidative stress is cardiolipin, a major constituent of the mitochondrial inner membrane [156]. A key precursor of cardiolipin is phosphatidylglycerol, a component of our GPL mixture [62]. Providing precursors to critical mitochondrial molecules may be an important element in MLR enhancement in sperm function. There is indirect evidence that MLR with GPL can be a useful supplement in many chronic diseases, possibly by replacing damaged lipids in mitochondria [53, 54, 64, 66, 107]. We then decided to test if MLR with GPL incorporated into SM has a functional continuity that can impact internal membranes, either through lipid transfer or through the activation of pathways by the SM.
With this in mind, we took advantage of the easy access to the membrane systems in human sperm as a cell model and asked whether the loss of MIMP promoted by oxidative stress could be prevented by incubation with our mixture of GPL. Our first approach was to measure MIMP in a large population of sperm using flow cytometry of sperm loaded with the dye JC-1 that reports MIMP. The results presented in Figs 7 and 8 suggest that the GPL mixtures can prevent the loss of MIMP produced by H2O2, and it further suggests that the GPL reaches the mitochondria, replacing the damaged lipids with undamaged ones, restoring MIMP and mitochondrial function. We also tested this hypothesis in Figs 9 and 10, observing with a confocal microscope staining in mitochondria after incubating with dyes that report MIMP (Rhodamine 123 and JC-1).
We also found frequently cytoplasmic droplets in sperm incubated with GPL sub-μm-sized GPL micelles. In addition to suggesting that this could be evidence of GPL incorporation into the SM, it also suggests that GPL incorporation into internal membranes makes sperm more resistant to stress conditions. It has been suggested that the droplets are indicative of sperm oxidative resistance [102–104]. The same type of experiments using Rhodamine 123 were performed with the JC-1 dye with similar results indicating the type of dye used in the experiment did not affect the results. The ratio between incubation of sperm in control and the GPL mixture was similar, or even slightly better for the incubation with the GPL mixture. However, the ratio dropped to 0.62 when H2O2 was added, but the addition of the GPL mixture to the incubation with H2O2 restored the normal ratios. These results are consistent with the sperm motility and flow cytometry data and support the notion that MLR with GPL was incorporated into the mitochondrial membranes and prevented the loss of MIMP.
Conclusions
Sub-μm-sized GPL micelles, like liposomes, can fuse with biological membranes and deliver phospholipids that can replace damaged membrane phospholipids in order to restore membrane function. Once in the plasma membrane, these replacement phospholipids can partition or be transferred to other membranes in a cell and restore function. Since this approach does not interfere with other treatment strategies, it can also be used to supplement other pharmacological approaches without the problem of interference or counter-indications that can occur with the use of different drugs.
This type of replacement approach is particularly useful for the treatment of excess oxidative stress. Oxidative stress is related to mitochondrial activity, and we have shown here that MLR with GPL can restore the mitochondrial function when it is damaged by oxidative stress.
Our results have shown that: (a) we can substitute the damaged GPL with a mixture of exogenous GPL in a combination that resembles the plasma membrane composition (b) we also show that this approach is valuable under basal conditions, but also when there is an increment of lipid peroxidation promoted by H2O2; and (c) we demonstrate that the plasma membrane and organelles in the sperm have functional continuity, because our treatment can modify mitochondrial membranes and change mitochondrial membrane potential.
Finally, our results suggest that MLR could be a relevant approach for the treatment of infertility in males when it is linked to increased oxidative stress.
Supporting information
(A) Precipitated nano-micelles and spots picked for X-Ray fluorescence analysis (up) and number of counts versus energy plot (down). (B) Same as in (A) for a control surface.
(TIF)
Acknowledgments
GF wants to acknowledge the support from CSIC UdelaR, p944, p146, p91, Fortalecimiento; PEDECIBA and SNI ANII, Uruguay and a grant from Allergy Research Group, Alameda, California. Dr. Alberto Darszon and Dr. Claudia Treviño, are acknowledged by GF for their support with sperm techniques. Personnel from Fertilab is acknowledged for getting human sperm samples. Personnel from the Facultad de Medicina is acknowledged by their support in getting reactives and equipment. We are grateful to Alejandro Marquez, Magela Rodao and Gabriela Casanova for their support in the electron-microscope studies.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors declare that the Robert Settineri and Jose Montes are members of the following commercial companies: Sierra Productions Research, LLC and Fertilab respectively. Neither company participated in the study design, data collection and analysis, decision to publish, and only provided financial support in the form of authors' salaries and/or research materials. Robert Settineri participated in this paper in its writing, editing and reviewing of the manuscript. Jose Montes participated in this study by performing the swim-up procedures of the human sperm samples and also helped performing research and validating the results obtained in the lab. The funders provided support in the form of salaries for authors [CSIC and Universidad de la República, Uruguay for Gonzalo Ferreira, Carlos Costa, Veronica Bassaizteguy, Romina Cardozo and Marcelo Santos, IMM and Allergy Research Group for Garth L. Nicolson. Each funding organization did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are stated in the ‘author contributions’ section. In addition, CSIC is a national agency from the Universidad de la Republica, Montevideo, Uruguay, that provides grants for funding research in Uruguay, helping research groups to get the materials and equipment used during their research, as they did in this study (p944, p146, p91 to GF). Its purpose is mostly to improve human knowledge. Universidad de la República also paid the salaries of many of the authors of this paper. The Institute for Molecular Medicine is a non-profit organization directed by Dr. Prof. Emeritus Garth L. Nicolson dedicated to biomedical research. The Allergy Research Group is an organization dedicated to producing and marketing hypoallergenic nutritional supplements and has supported this research by donating equipment and purifying and making the lipid mixture used throughout many of the experiments in this paper in collaboration with Dr. Garth L. Nicolson and Dr. Gonzalo Ferreira.
References
- 1.Plant TM, Zeleznik AJ. Knobil and Neill's Physiology of Reproduction: Elsevier Science; 2014. [Google Scholar]
- 2.Tapia J, Macias‐Garcia B, Miro‐Moran A, Ortega‐Ferrusola C, Salido G, Peña F, et al. The membrane of the mammalian spermatozoa: much more than an inert envelope. Reproduction in Domestic Animals. 2012;47(s3):65–75. [DOI] [PubMed] [Google Scholar]
- 3.Lenzi A, Picardo M, Gandini L, Dondero F. Lipids of the sperm plasma membrane: from polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Human reproduction update. 1996;2(3):246–56. Epub 1996/05/01. . [DOI] [PubMed] [Google Scholar]
- 4.Lenzi A, Gandini L, Picardo M, Tramer F, Sandri G, Panfili E . Lipoperoxidation damage of spermatozoa polyunsaturated fatty acids (PUFA): scavenger mechanisms and possible scavenger therapies. Frontiers in bioscience: a journal and virtual library. 2000;5:E1–E15. . [DOI] [PubMed] [Google Scholar]
- 5.Lenzi A, Gandini L, Maresca V, Rago R, Sgro P, Dondero F, et al. Fatty acid composition of spermatozoa and immature germ cells. Molecular human reproduction. 2000;6(3):226–31. . [DOI] [PubMed] [Google Scholar]
- 6.Zanetti SR, Monclus Mde L, Rensetti DE, Fornes MW, Aveldano MI. Differential involvement of rat sperm choline glycerophospholipids and sphingomyelin in capacitation and the acrosomal reaction. Biochimie. 2010;92(12):1886–94. doi: 10.1016/j.biochi.2010.08.015 . [DOI] [PubMed] [Google Scholar]
- 7.Oresti GM, Penalva DA, Luquez JM, Antollini SS, Aveldano MI. Lipid Biochemical and Biophysical Changes in Rat Spermatozoa During Isolation and Functional Activation In Vitro. Biology of reproduction. 2015;93(6):140 doi: 10.1095/biolreprod.115.131201 . [DOI] [PubMed] [Google Scholar]
- 8.Zalata AA, Christophe AB, Depuydt CE, Schoonjans F, Comhaire FH. The fatty acid composition of phospholipids of spermatozoa from infertile patients. Molecular human reproduction. 1998;4(2):111–8. Epub 1998/05/23. . [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Soto JC, Domingo JC, Cordobilla B, Nicolas M, Fernandez L, Albero P, et al. Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Systems biology in reproductive medicine. 2016;62(6):387–95. Epub 2016/10/30. doi: 10.1080/19396368.2016.1246623 . [DOI] [PubMed] [Google Scholar]
- 10.Lin DS, Connor WE, Wolf DP, Neuringer M, Hachey DL. Unique lipids of primate spermatozoa: desmosterol and docosahexaenoic acid. Journal of lipid research. 1993;34(3):491–9. Epub 1993/03/01. . [PubMed] [Google Scholar]
- 11.Hartree EF, Mann T. Plasmalogen in ram semen, and its role in sperm metabolism. Biochemical Journal. 1959;71(3):423–34. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carothers ZB. Studies of spermatogenesis in the Hepaticae. 3. Continuity between plasma membrane and nuclear envelope in androgonial cells of Blasia. The Journal of cell biology. 1972;52(2):273–82. Epub 1972/02/01. ; PubMed Central PMCID: PMCPMC2108634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terasaki M, Jaffe LA, Hunnicutt GR, Hammer JA, 3rd. Structural change of the endoplasmic reticulum during fertilization: evidence for loss of membrane continuity using the green fluorescent protein. Developmental biology. 1996;179(2):320–8. Epub 1996/11/01. . [DOI] [PubMed] [Google Scholar]
- 14.Gadella BM, Luna C. Cell biology and functional dynamics of the mammalian sperm surface. Theriogenology. 2014;81(1):74–84. doi: 10.1016/j.theriogenology.2013.09.005 . [DOI] [PubMed] [Google Scholar]
- 15.Cohen R, Mukai C, Travis AJ. Lipid Regulation of Acrosome Exocytosis. Advances in anatomy, embryology, and cell biology. 2016;220:107–27. Epub 2016/05/20. doi: 10.1007/978-3-319-30567-7_6 . [DOI] [PubMed] [Google Scholar]
- 16.Prinz WA. Lipid trafficking sans vesicles: where, why, how? Cell. 2010;143(6):870–4. doi: 10.1016/j.cell.2010.11.031 ; PubMed Central PMCID: PMC3004474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasimanickam VR, Buhr MM. Fusion of Boar Sperm with Nanoliposomes Prepared from Synthetic Phospholipids. Reproduction in domestic animals = Zuchthygiene. 2016;51(4):461–6. Epub 2016/05/25. doi: 10.1111/rda.12702 . [DOI] [PubMed] [Google Scholar]
- 18.Jones R, Mann T. Lipid peroxidation in spermatozoa. Proceedings of the Royal Society of London Series B, Biological sciences. 1973;184(1074):103–7. Epub 1973/08/31. . [DOI] [PubMed] [Google Scholar]
- 19.Jones R, Mann T. Lipid peroxides in spermatozoa; formation, role of plasmalogen, and physiological significance. Proceedings of the Royal Society of London B: Biological Sciences. 1976;193(1113):317–33. [DOI] [PubMed] [Google Scholar]
- 20.MacLeod J. THE RÔLE OF OXYGEN IN THE METABOLISM AND MOTILITY OF HUMAN SPERMATOZOA. American Journal of Physiology—Legacy Content. 1943;138(3):512–8. [Google Scholar]
- 21.Tosic J, Walton A. Formation of hydrogen peroxide by spermatozoa and its inhibitory effect of respiration. Nature. 1946;158:485 Epub 1946/10/05. . [DOI] [PubMed] [Google Scholar]
- 22.Surai PF, Brillard JP, Speake BK, Blesbois E, Seigneurin F, Sparks NH. Phospholipid fatty acid composition, vitamin E content and susceptibility to lipid peroxidation of duck spermatozoa. Theriogenology. 2000;53(5):1025–39. Epub 2000/05/08. doi: 10.1016/S0093-691X(00)00249-1 . [DOI] [PubMed] [Google Scholar]
- 23.Aitken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biology of reproduction. 1989;41(1):183–97. . [DOI] [PubMed] [Google Scholar]
- 24.Bell M, Sikka S, Rajasekaran M, Hellstrom W. Time course of hydrogen peroxide induced changes in the lipid peroxidation of human sperm membranes. Advances in contraceptive delivery systems: CDS. 1992;8(1–2):144–50. Epub 1992/01/01. . [PubMed] [Google Scholar]
- 25.Hong CY, Lee MF, Lai LJ, Wang CP. Effect of lipid peroxidation on beating frequency of human sperm tail. Andrologia. 1994;26(2):61–5. . [DOI] [PubMed] [Google Scholar]
- 26.Suleiman SA, Ali ME, Zaki ZM, el-Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: protective role of vitamin E. Journal of andrology. 1996;17(5):530–7. . [PubMed] [Google Scholar]
- 27.Chen CS, Chao HT, Pan RL, Wei YH. Hydroxyl radical-induced decline in motility and increase in lipid peroxidation and DNA modification in human sperm. Biochemistry and molecular biology international. 1997;43(2):291–303. . [DOI] [PubMed] [Google Scholar]
- 28.Verma A, Kanwar KC. Human sperm motility and lipid peroxidation in different ascorbic acid concentrations: an in vitro analysis. Andrologia. 1998;30(6):325–9. . [DOI] [PubMed] [Google Scholar]
- 29.Engel S, Schreiner T, Petzoldt R. Lipid peroxidation in human spermatozoa and maintenance of progressive sperm motility. Andrologia. 1999;31(1):17–22. . [PubMed] [Google Scholar]
- 30.Zabludovsky N, Eltes F, Geva E, Berkovitz E, Amit A, Barak Y, et al. Relationship between human sperm lipid peroxidation, comprehensive quality parameters and IVF outcome. Andrologia. 1999;31(2):91–8. . [DOI] [PubMed] [Google Scholar]
- 31.Khosrowbeygi A, Zarghami N. Fatty acid composition of human spermatozoa and seminal plasma levels of oxidative stress biomarkers in subfertile males. Prostaglandins, leukotrienes, and essential fatty acids. 2007;77(2):117–21. doi: 10.1016/j.plefa.2007.08.003 . [DOI] [PubMed] [Google Scholar]
- 32.Ben Abdallah F, Fetoui H, Zribi N, Fakfakh F, Ammar-Keskes L. Antioxidant supplementations in vitro improve rat sperm parameters and enhance antioxidant enzyme activities against dimethoate-induced sperm damages. Andrologia. 2012;44 Suppl 1:272–9. Epub 2011/08/03. doi: 10.1111/j.1439-0272.2011.01177.x . [DOI] [PubMed] [Google Scholar]
- 33.Storey BT. Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Molecular human reproduction. 1997;3(3):203–13. Epub 1997/03/01. . [DOI] [PubMed] [Google Scholar]
- 34.Tavilani H, Doosti M, Nourmohammadi I, Mahjub H, Vaisiraygani A, Salimi S, et al. Lipid composition of spermatozoa in normozoospermic and asthenozoospermic males. Prostaglandins, leukotrienes, and essential fatty acids. 2007;77(1):45–50. Epub 2007/08/21. doi: 10.1016/j.plefa.2007.07.001 . [DOI] [PubMed] [Google Scholar]
- 35.Tavilani H, Doosti M, Abdi K, Vaisiraygani A, Joshaghani HR. Decreased polyunsaturated and increased saturated fatty acid concentration in spermatozoa from asthenozoospermic males as compared with normozoospermic males. Andrologia. 2006;38(5):173–8. Epub 2006/09/12. doi: 10.1111/j.1439-0272.2006.00735.x . [DOI] [PubMed] [Google Scholar]
- 36.Kodama H, Kuribayashi Y, Gagnon C. Effect of sperm lipid peroxidation on fertilization. Journal of andrology. 1996;17(2):151–7. . [PubMed] [Google Scholar]
- 37.Aitken RJ. Free radicals, lipid peroxidation and sperm function. Reproduction, fertility, and development. 1995;7(4):659–68. Epub 1995/01/01. . [DOI] [PubMed] [Google Scholar]
- 38.Wade MA, Jones RC, Murdoch RN, Aitken RJ. Motility activation and second messenger signalling in spermatozoa from rat cauda epididymidis. Reproduction. 2003;125(2):175–83. Epub 2003/02/13. . [DOI] [PubMed] [Google Scholar]
- 39.Aitken RJ, Jones KT, Robertson SA. Reactive oxygen species and sperm function—in sickness and in health. Journal of andrology. 2012;33(6):1096–106. Epub 2012/08/11. doi: 10.2164/jandrol.112.016535 . [DOI] [PubMed] [Google Scholar]
- 40.Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN. Oxidative stress and male reproductive health. Asian journal of andrology. 2014;16(1):31–8. Epub 2013/12/27. doi: 10.4103/1008-682X.122203 ; PubMed Central PMCID: PMCPMC3901879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aitken RJ, Gibb Z, Baker MA, Drevet J, Gharagozloo P. Causes and consequences of oxidative stress in spermatozoa. Reproduction, Fertility and Development. 2016;28(2):1–10. [DOI] [PubMed] [Google Scholar]
- 42.Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Molecular reproduction and development. 2017. Epub 2017/07/28. doi: 10.1002/mrd.22871 . [DOI] [PubMed] [Google Scholar]
- 43.Satoh T, Enokido Y, Aoshima H, Uchiyama Y, Hatanaka H. Changes in mitochondrial membrane potential during oxidative stress-induced apoptosis in PC12 cells. Journal of neuroscience research. 1997;50(3):413–20. Epub 1997/11/19. doi: 10.1002/(SICI)1097-4547(19971101)50:3<413::AID-JNR7>3.0.CO;2-L . [DOI] [PubMed] [Google Scholar]
- 44.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends in cell biology. 2008;18(4):157–64. doi: 10.1016/j.tcb.2008.01.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vander Heiden MG, Chandel NS, Li XX, Schumacker PT, Colombini M, Thompson CB. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(9):4666–71. doi: 10.1073/pnas.090082297 ; PubMed Central PMCID: PMC18290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uribe P, Treulen F, Boguen R, Sanchez R, Villegas JV. Nitrosative stress by peroxynitrite impairs ATP production in human spermatozoa. Andrologia. 2017;49(3). Epub 2016/05/03. doi: 10.1111/and.12615 . [DOI] [PubMed] [Google Scholar]
- 47.Losano J, Angrimani D, Dalmazzo A, Rui BR, Brito MM, Mendes CM, et al. Effect of mitochondrial uncoupling and glycolysis inhibition on ram sperm functionality. Reproduction in domestic animals = Zuchthygiene. 2017;52(2):289–97. Epub 2017/01/07. doi: 10.1111/rda.12901 . [DOI] [PubMed] [Google Scholar]
- 48.Kumaresan A, Kadirvel G, Bujarbaruah KM, Bardoloi RK, Das A, Kumar S, et al. Preservation of boar semen at 18 degrees C induces lipid peroxidation and apoptosis like changes in spermatozoa. Animal reproduction science. 2009;110(1–2):162–71. Epub 2008/02/26. doi: 10.1016/j.anireprosci.2008.01.006 . [DOI] [PubMed] [Google Scholar]
- 49.Quinn P, Chow P, White I. Evidence that phospholipid protects ram spermatozoa from cold shock at a plasma membrane site. Journal of reproduction and fertility. 1980;60(2):403–7. [DOI] [PubMed] [Google Scholar]
- 50.Zaniboni L, Cerolini S. Liquid storage of turkey semen: changes in quality parameters, lipid composition and susceptibility to induced in vitro peroxidation in control, n-3 fatty acids and alpha-tocopherol rich spermatozoa. Animal reproduction science. 2009;112(1–2):51–65. Epub 2008/05/20. doi: 10.1016/j.anireprosci.2008.04.002 . [DOI] [PubMed] [Google Scholar]
- 51.Nekoonam S, Nashtaei MS, Naji M, Zangi BM, Amidi F. Effect of Trolox on sperm quality in normozospermia and oligozospermia during cryopreservation. Cryobiology. 2016;72(2):106–11. Epub 2016/03/06. doi: 10.1016/j.cryobiol.2016.02.008 . [DOI] [PubMed] [Google Scholar]
- 52.Vireque AA, Tata A, Silva OF, LoTurco EG, Azzolini A, Ferreira CR, et al. Effects of n-6 and n-3 polyunsaturated acid-rich soybean phosphatidylcholine on membrane lipid profile and cryotolerance of human sperm. Fertility and sterility. 2016;106(2):273–83 e6. Epub 2016/04/24. doi: 10.1016/j.fertnstert.2016.03.044 . [DOI] [PubMed] [Google Scholar]
- 53.Nicolson GL, Rosenblatt S, Ferreira de Mattos G, Settineri R, Breeding PC, Ellithorpe RR, et al. Clinical Uses of Membrane Lipid Replacement Supplements in Restoring Membrane Function and Reducing Fatigue in Chronic Diseases and Cancer. Discoveries. 2016;4(1):e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicolson GL, Ellithorpe R. Lipid replacement and antioxidant nutritional therapy for restoring mitochondrial function and reducing fatigue in chronic fatigue syndrome and other fatiguing illnesses. Journal of Chronic Fatigue Syndrome. 2006;13(1):57–68. [Google Scholar]
- 55.Costa TG C., Ferreira G., Nicolson G.L. Lipid replacement with a membrane glycerolphospholipid formulation: enhancement of spermatoozoa motility and viability. Functional and Medical Foods for Chronic Diseases: Bioactive Compounds and Biomarkers 2015;18:202–6. [Google Scholar]
- 56.Ragni G, De Lauretis L, D'Ambrogio G, Pellegrini S, Maggioni P, Vegetti W, et al. Semen preparation by standard swim-up versus swim-up with test yolk buffer incubation in intrauterine insemination: a randomized study. Human reproduction (Oxford, England). 1998;13(7):1859–63. Epub 1998/09/18. . [DOI] [PubMed] [Google Scholar]
- 57.Abreu MS, Moreno MJ, Vaz WL. Kinetics and thermodynamics of association of a phospholipid derivative with lipid bilayers in liquid-disordered and liquid-ordered phases. Biophysical journal. 2004;87(1):353–65. doi: 10.1529/biophysj.104.040576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Misro MM, Choudhury L, Upreti K, Gautam D, Chaki SP, Mahajan AS, et al. Use of hydrogen peroxide to assess the sperm susceptibility to oxidative stress in subjects presenting a normal semen profile. International journal of andrology. 2004;27(2):82–7. Epub 2004/05/20. doi: 10.1046/j.0105-6263.2003.00451.x . [DOI] [PubMed] [Google Scholar]
- 59.du Plessis SS, McAllister DA, Luu A, Savia J, Agarwal A, Lampiao F. Effects of H(2)O(2) exposure on human sperm motility parameters, reactive oxygen species levels and nitric oxide levels. Andrologia. 2010;42(3):206–10. Epub 2010/05/27. doi: 10.1111/j.1439-0272.2009.00980.x . [DOI] [PubMed] [Google Scholar]
- 60.Malvezzi H, Sharma R, Agarwal A, Abuzenadah AM, Abu-Elmagd M. Sperm quality after density gradient centrifugation with three commercially available media: a controlled trial. Reproductive Biology and Endocrinology: RB&E. 2014;12 doi: 10.1186/1477-7827-12-121 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamanaka M, Tomita K, Hashimoto S, Matsumoto H, Satoh M, Kato H, et al. Combination of density gradient centrifugation and swim-up methods effectively decreases morphologically abnormal sperms. The Journal of Reproduction and Development. 2016;62(6):599–606. doi: 10.1262/jrd.2016-112 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicolson GL. Membrane Lipid Replacement: clinical studies using a natural medicine approach to restoring membrane function and improving health. International Journal of Clinical Medicine. 2016;7(02):133. [Google Scholar]
- 63.Nicolson GL, Ash ME. Membrane Lipid Replacement for chronic illnesses, aging and cancer using oral glycerolphospholipid formulations with fructooligosaccharides to restore phospholipid function in cellular membranes, organelles, cells and tissues. Biochimica et biophysica acta. 2017;1859(9 Pt B):1704–24. Epub 2017/04/23. doi: 10.1016/j.bbamem.2017.04.013 . [DOI] [PubMed] [Google Scholar]
- 64.Nicolson GL, Ash ME. Lipid Replacement Therapy: a natural medicine approach to replacing damaged lipids in cellular membranes and organelles and restoring function. Biochimica et biophysica acta. 2014;1838(6):1657–79. doi: 10.1016/j.bbamem.2013.11.010 . [DOI] [PubMed] [Google Scholar]
- 65.Nicolson GL. Lipid replacement therapy: a nutraceutical approach for reducing cancer-associated fatigue and the adverse effects of cancer therapy while restoring mitochondrial function. Cancer metastasis reviews. 2010;29(3):543–52. doi: 10.1007/s10555-010-9245-0 . [DOI] [PubMed] [Google Scholar]
- 66.Nicolson GL. Mitochondrial dysfunction and chronic disease: treatment with natural supplements. Alternative therapies in health and medicine. 2014;20 Suppl 1:18–25. Epub 2014/01/30. . [PubMed] [Google Scholar]
- 67.Liu TY, Liu KH, Liu DM, Chen SY, Chen IW. Temperature‐Sensitive Nanocapsules for Controlled Drug Release Caused by Magnetically Triggered Structural Disruption. Advanced Functional Materials. 2009;19(4):616–23. [Google Scholar]
- 68.Li J, Wang X, Zhang T, Wang C, Huang Z, Luo X, et al. A review on phospholipids and their main applications in drug delivery systems. Asian journal of pharmaceutical sciences. 2015;10(2):81–98. [Google Scholar]
- 69.Zhao J, Pispas S, Zhang G. Effect of Sonication on Polymeric Aggregates Formed by Poly (ethylene oxide)‐Based Amphiphilic Block Copolymers. Macromolecular Chemistry and Physics. 2009;210(12):1026–32. [Google Scholar]
- 70.Zhang T, Wang H, Ye Y, Zhang X, Wu B. Micellar emulsions composed of mPEG-PCL/MCT as novel nanocarriers for systemic delivery of genistein: a comparative study with micelles. International journal of nanomedicine. 2015;10:6175 doi: 10.2147/IJN.S91348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang L, Tonggu L. Membrane protein reconstitution for functional and structural studies. Science China Life sciences. 2015;58(1):66–74. doi: 10.1007/s11427-014-4769-0 . [DOI] [PubMed] [Google Scholar]
- 72.Biswas S, Dodwadkar NS, Sawant RR, Koshkaryev A, Torchilin VP. Surface modification of liposomes with rhodamine-123-conjugated polymer results in enhanced mitochondrial targeting. Journal of drug targeting. 2011;19(7):552–61. doi: 10.3109/1061186X.2010.536983 ; PubMed Central PMCID: PMC3492939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ogden D, STANFIELD P, editors. Patch clamp techniques for single channel and whole-cell recording Microelectrode techniques: the Plymouth workshop handbook; 1994. [Google Scholar]
- 74.Franken LE, Boekema EJ, Stuart MC. Transmission electron microscopy as a tool for the characterization of soft materials: application and interpretation. Advanced Science. 2017;4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hermanson GT. Bioconjugate Techniques: Elsevier Science; 2013. [Google Scholar]
- 76.Rao KS, Rani SU, Charyulu DK, Kumar KN, Lee BK, Lee HY, et al. A novel route for immobilization of oligonucleotides onto modified silica nanoparticles. Analytica chimica acta. 2006;576(2):177–83. Epub 2007/08/29. doi: 10.1016/j.aca.2006.06.019 . [DOI] [PubMed] [Google Scholar]
- 77.Iwasawa T, Wash P, Gibson C, Rebek J. Reaction of an Introverted Carboxylic Acid with Carbodiimide. Tetrahedron. 2007;63(28):6506–11. doi: 10.1016/j.tet.2007.03.075 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakajima N, Ikada Y. Mechanism of amide formation by carbodiimide for bioconjugation in aqueous media. Bioconjugate chemistry. 1995;6(1):123–30. . [DOI] [PubMed] [Google Scholar]
- 79.Organization WH. WHO laboratory manual for the examination and processing of human semen. 2010. [PubMed]
- 80.Graves JE, Higdon HL 3rd, Boone WR, Blackhurst DW. Developing techniques for determining sperm morphology in today's andrology laboratory. Journal of assisted reproduction and genetics. 2005;22(5):219–25. Epub 2005/07/29. doi: 10.1007/s10815-005-4925-3 ; PubMed Central PMCID: PMCPMC3455498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yeung CH, Cooper TG, Nieschlag E. A technique for standardization and quality control of subjective sperm motility assessments in semen analysis. Fertility and sterility. 1997;67(6):1156–8. Epub 1997/06/01. . [DOI] [PubMed] [Google Scholar]
- 82.Rajashri M, Reddy KRC, Kumari GA, Kumari NN. COEFFICIENT OF VARIATION ASSESSMENT FOR SEMINAL TRAITS EVALUATED BY COMPUTER ASSISTED SEMEN ANALYSIS (CASA). 2016.
- 83.Kasai T, Ogawa K, Mizuno K, Nagai S, Uchida Y, Ohta S, et al. Relationship between sperm mitochondrial membrane potential, sperm motility, and fertility potential. Asian journal of andrology. 2002;4(2):97–103. Epub 2002/06/27. . [PubMed] [Google Scholar]
- 84.Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(9):3671–5. ; PubMed Central PMCID: PMC51514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reers M, Smiley ST, Mottola-Hartshorn C, Chen A, Lin M, Chen LB. [29] Mitochondrial membrane potential monitored by JC-1 dye. Methods in enzymology. 1995;260:406–17. [DOI] [PubMed] [Google Scholar]
- 86.Garner DL, Johnson LA. Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biology of reproduction. 1995;53(2):276–84. . [DOI] [PubMed] [Google Scholar]
- 87.Baracca A, Sgarbi G, Solaini G, Lenaz G. Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F0 during ATP synthesis. Biochimica et Biophysica Acta (BBA)—Bioenergetics. 2003;1606(1):137–46. https://doi.org/10.1016/S0005-2728(03)00110-5. [DOI] [PubMed] [Google Scholar]
- 88.Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess ΔΨ changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Letters. 1997;411(1):77–82. https://doi.org/10.1016/S0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- 89.Wennemuth G, Eisoldt S, Bode HP, Renneberg H, Schiemann PJ, Aumuller G. Measurement of calcium influx in surface-fixed single sperm cells: efficiency of different immobilization methods. Andrologia. 1998;30(3):141–6. . [DOI] [PubMed] [Google Scholar]
- 90.Barroso G, Taylor S, Morshedi M, Manzur F, Gavino F, Oehninger S. Mitochondrial membrane potential integrity and plasma membrane translocation of phosphatidylserine as early apoptotic markers: a comparison of two different sperm subpopulations. Fertility and sterility. 2006;85(1):149–54. doi: 10.1016/j.fertnstert.2005.06.046 . [DOI] [PubMed] [Google Scholar]
- 91.Lucio CF, Brito MM, Angrimani D, Belaz K, Morais D, Zampieri D, et al. Lipid composition of the canine sperm plasma membrane as markers of sperm motility. Reproduction in domestic animals = Zuchthygiene. 2017;52 Suppl 2:208–13. Epub 2016/11/04. doi: 10.1111/rda.12860 . [DOI] [PubMed] [Google Scholar]
- 92.Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, et al. Sperm morphology, motility, and concentration in fertile and infertile men. The New England journal of medicine. 2001;345(19):1388–93. 10.1056/NEJMoa003005. 11794171. doi: 10.1056/NEJMoa003005 [DOI] [PubMed] [Google Scholar]
- 93.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Human reproduction update. 2010;16(3):231–45. doi: 10.1093/humupd/dmp048 . [DOI] [PubMed] [Google Scholar]
- 94.Chaki SP, Misro MM. Assessment of human sperm function after hydrogen peroxide exposure. development of a vaginal contraceptive. Contraception. 2002;66(3):187–92. Epub 2002/10/18. . [DOI] [PubMed] [Google Scholar]
- 95.Uribe P, Boguen R, Treulen F, Sanchez R, Villegas JV. Peroxynitrite-mediated nitrosative stress decreases motility and mitochondrial membrane potential in human spermatozoa. Molecular human reproduction. 2015;21(3):237–43. Epub 2014/11/27. doi: 10.1093/molehr/gau107 . [DOI] [PubMed] [Google Scholar]
- 96.Amaral S, ST R, Baptista M, Sousa MI, Silva A, Escada-Rebelo S, et al. Mitochondrial Functionality and Chemical Compound Action on Sperm Function. Current medicinal chemistry. 2016;23(31):3575–606. Epub 2016/04/26. . [DOI] [PubMed] [Google Scholar]
- 97.Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. BioTechniques. 2011;50(2):98–115. doi: 10.2144/000113610 ; PubMed Central PMCID: PMC3115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao H, Oczos J, Janowski P, Trembecka D, Dobrucki J, Darzynkiewicz Z, et al. Rationale for the real-time and dynamic cell death assays using propidium iodide. Cytometry Part A: the journal of the International Society for Analytical Cytology. 2010;77(4):399–405. doi: 10.1002/cyto.a.20867 ; PubMed Central PMCID: PMC3646415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Biasi S, Gibellini L, Cossarizza A. Uncompensated Polychromatic Analysis of Mitochondrial Membrane Potential Using JC-1 and Multilaser Excitation. Current protocols in cytometry. 2015;72:7 32 1–11. doi: 10.1002/0471142956.cy0732s72 . [DOI] [PubMed] [Google Scholar]
- 100.Chen LB. Mitochondrial membrane potential in living cells. Annual review of cell biology. 1988;4:155–81. doi: 10.1146/annurev.cb.04.110188.001103 . [DOI] [PubMed] [Google Scholar]
- 101.Evenson DP, Darzynkiewicz Z, Melamed MR. Simultaneous measurement by flow cytometry of sperm cell viability and mitochondrial membrane potential related to cell motility. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 1982;30(3):279–80. doi: 10.1177/30.3.6174566 . [DOI] [PubMed] [Google Scholar]
- 102.Cooper TG, Yeung CH, Fetic S, Sobhani A, Nieschlag E. Cytoplasmic droplets are normal structures of human sperm but are not well preserved by routine procedures for assessing sperm morphology. Human reproduction (Oxford, England). 2004;19(10):2283–8. doi: 10.1093/humrep/deh410 . [DOI] [PubMed] [Google Scholar]
- 103.Cooper TG. Cytoplasmic droplets: the good, the bad or just confusing? Human reproduction (Oxford, England). 2005;20(1):9–11. doi: 10.1093/humrep/deh555 . [DOI] [PubMed] [Google Scholar]
- 104.Xu H, Yuan SQ, Zheng ZH, Yan W. The cytoplasmic droplet may be indicative of sperm motility and normal spermiogenesis. Asian journal of andrology. 2013;15(6):799–805. doi: 10.1038/aja.2013.69 ; PubMed Central PMCID: PMC3854046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Orta G, Ferreira G, José O, Treviño CL, Beltrán C, Darszon A. Human spermatozoa possess a calcium‐dependent chloride channel that may participate in the acrosomal reaction. The Journal of physiology. 2012;590(11):2659–75. doi: 10.1113/jphysiol.2011.224485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nicolson GL. Lipid replacement/antioxidant therapy as an adjunct supplement to reduce the adverse effects of cancer therapy and restore mitochondrial function. Pathology oncology research: POR. 2005;11(3):139–44. . [DOI] [PubMed] [Google Scholar]
- 107.Nicolson GL, Conklin KA. Reversing mitochondrial dysfunction, fatigue and the adverse effects of chemotherapy of metastatic disease by molecular replacement therapy. Clinical & experimental metastasis. 2008;25(2):161–9. Epub 2007/12/07. doi: 10.1007/s10585-007-9129-z . [DOI] [PubMed] [Google Scholar]
- 108.Nicolson GL. Metabolic syndrome and mitochondrial function: molecular replacement and antioxidant supplements to prevent membrane peroxidation and restore mitochondrial function. Journal of cellular biochemistry. 2007;100(6):1352–69. doi: 10.1002/jcb.21247 . [DOI] [PubMed] [Google Scholar]
- 109.Ademowo OS, Dias HKI, Burton DGA, Griffiths HR. Lipid (per) oxidation in mitochondria: an emerging target in the ageing process? Biogerontology. 2017;18(6):859–79. Epub 2017/05/26. doi: 10.1007/s10522-017-9710-z ; PubMed Central PMCID: PMCPMC5684309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Agmon E, Stockwell BR. Lipid homeostasis and regulated cell death. Current opinion in chemical biology. 2017;39:83–9. Epub 2017/06/24. doi: 10.1016/j.cbpa.2017.06.002 ; PubMed Central PMCID: PMCPMC5581689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morris G, Walker AJ, Berk M, Maes M, Puri BK. Cell Death Pathways: a Novel Therapeutic Approach for Neuroscientists. Molecular neurobiology. 2017. Epub 2017/10/21. doi: 10.1007/s12035-017-0793-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Agadjanyan M, Vasilevko V, Ghochikyan A, Berns P, Kesslak P, Settineri RA, et al. Nutritional supplement (NT Factor™) restores mitochondrial function and reduces moderately severe fatigue in aged subjects. Journal of Chronic Fatigue Syndrome. 2003;11(3):23–36. [Google Scholar]
- 113.Volpe S, Leoci R, Aiudi G, Lacalandra GM. Relationship between motility and mitochondrial functional status in canine spermatozoa. Reproduction in domestic animals = Zuchthygiene. 2009;44 Suppl 2:275–8. Epub 2009/09/17. doi: 10.1111/j.1439-0531.2009.01457.x . [DOI] [PubMed] [Google Scholar]
- 114.Piomboni P, Focarelli R, Stendardi A, Ferramosca A, Zara V. The role of mitochondria in energy production for human sperm motility. International journal of andrology. 2012;35(2):109–24. Epub 2011/09/29. doi: 10.1111/j.1365-2605.2011.01218.x . [DOI] [PubMed] [Google Scholar]
- 115.Helfrich P, Jakobsson E. Calculation of deformation energies and conformations in lipid membranes containing gramicidin channels. Biophysical journal. 1990;57(5):1075–84. doi: 10.1016/S0006-3495(90)82625-4 ; PubMed Central PMCID: PMC1280812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nicolson GL, Yanagimachi R. Mobility and the restriction of mobility of plasma membrane lectin-binding components. Science. 1974;184(4143):1294–6. . [DOI] [PubMed] [Google Scholar]
- 117.Oresti GM, Luquez JM, Furland NE, Aveldano MI. Uneven distribution of ceramides, sphingomyelins and glycerophospholipids between heads and tails of rat spermatozoa. Lipids. 2011;46(12):1081–90. Epub 2011/08/09. doi: 10.1007/s11745-011-3601-x . [DOI] [PubMed] [Google Scholar]
- 118.Suzuki-Toyota F, Ito C, Maekawa M, Toyama Y, Toshimori K. Adhesion between plasma membrane and mitochondria with linking filaments in relation to migration of cytoplasmic droplet during epididymal maturation in guinea pig spermatozoa. Cell and tissue research. 2010;341(3):429–40. Epub 2010/08/03. doi: 10.1007/s00441-010-1012-6 . [DOI] [PubMed] [Google Scholar]
- 119.Wolfe CA, James PS, Mackie AR, Ladha S, Jones R. Regionalized lipid diffusion in the plasma membrane of mammalian spermatozoa. Biology of reproduction. 1998;59(6):1506–14. Epub 1998/11/26. . [DOI] [PubMed] [Google Scholar]
- 120.Turner RM. Moving to the beat: a review of mammalian sperm motility regulation. Reproduction, fertility, and development. 2006;18(1–2):25–38. Epub 2006/02/16. . [DOI] [PubMed] [Google Scholar]
- 121.Paoli D, Gallo M, Rizzo F, Baldi E, Francavilla S, Lenzi A, et al. Mitochondrial membrane potential profile and its correlation with increasing sperm motility. Fertility and sterility. 2011;95(7):2315–9. Epub 2011/04/22. doi: 10.1016/j.fertnstert.2011.03.059 . [DOI] [PubMed] [Google Scholar]
- 122.Agnihotri SK, Agrawal AK, Hakim BA, Vishwakarma AL, Narender T, Sachan R, et al. Mitochondrial membrane potential (MMP) regulates sperm motility. In vitro cellular & developmental biology Animal. 2016;52(9):953–60. Epub 2016/10/22. doi: 10.1007/s11626-016-0061-x . [DOI] [PubMed] [Google Scholar]
- 123.Suarez SS. Control of hyperactivation in sperm. Human reproduction update. 2008;14(6):647–57. doi: 10.1093/humupd/dmn029 . [DOI] [PubMed] [Google Scholar]
- 124.Ho HC, Suarez SS. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca(2+) store is involved in regulating sperm hyperactivated motility. Biology of reproduction. 2001;65(5):1606–15. Epub 2001/10/24. . [DOI] [PubMed] [Google Scholar]
- 125.Chang H, Suarez SS. Two distinct Ca(2+) signaling pathways modulate sperm flagellar beating patterns in mice. Biology of reproduction. 2011;85(2):296–305. Epub 2011/03/11. doi: 10.1095/biolreprod.110.089789 ; PubMed Central PMCID: PMC3142258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhu L, Inaba K. Lipid rafts function in Ca2+ signaling responsible for activation of sperm motility and chemotaxis in the ascidian Ciona intestinalis. Molecular reproduction and development. 2011;78(12):920–9. Epub 2011/09/03. doi: 10.1002/mrd.21382 . [DOI] [PubMed] [Google Scholar]
- 127.Kan FW, Lin Y. Topographical distribution of phospholipids in boar sperm plasma and intracellular membranes as revealed by freeze-fracture cytochemistry. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 1996;44(7):687–701. Epub 1996/07/01. . [DOI] [PubMed] [Google Scholar]
- 128.Ho HC, Suarez SS. Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biology of reproduction. 2003;68(5):1590–6. Epub 2003/02/28. doi: 10.1095/biolreprod.102.011320 . [DOI] [PubMed] [Google Scholar]
- 129.Byrne RD, Barona TM, Garnier M, Koster G, Katan M, Poccia DL, et al. Nuclear envelope assembly is promoted by phosphoinositide-specific phospholipase C with selective recruitment of phosphatidylinositol-enriched membranes. Biochemical Journal. 2005;387(Pt 2):393–400. Epub 2004/11/24. doi: 10.1042/BJ20040947 ; PubMed Central PMCID: PMCPMC1134967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Breitbart H. Intracellular calcium regulation in sperm capacitation and acrosomal reaction. Molecular and cellular endocrinology. 2002;187(1–2):139–44. Epub 2002/05/04. . [DOI] [PubMed] [Google Scholar]
- 131.Costello S, Michelangeli F, Nash K, Lefievre L, Morris J, Machado-Oliveira G, et al. Ca2+-stores in sperm: their identities and functions. Reproduction. 2009;138(3):425–37. Epub 2009/06/23. doi: 10.1530/REP-09-0134 ; PubMed Central PMCID: PMCPMC3552241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Publicover SJ, Giojalas LC, Teves ME, de Oliveira GS, Garcia AA, Barratt CL, et al. Ca2+ signalling in the control of motility and guidance in mammalian sperm. Frontiers in bioscience: a journal and virtual library. 2008;13:5623–37. Epub 2008/05/30. . [DOI] [PubMed] [Google Scholar]
- 133.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nature reviews Neuroscience. 2006;7(12):921–31. Epub 2006/11/23. doi: 10.1038/nrn1992 . [DOI] [PubMed] [Google Scholar]
- 134.Reyes N, Salkoff L, Brum G, Pizarro G, Ferreira G, editors. Functional coupling of L-type Ca2+ channels and calcium activated potassium channels (BK) in tsA 201 cells. Biophysical journal; 2001: BIOPHYSICAL SOCIETY 9650 ROCKVILLE PIKE, BETHESDA, MD 20814–3998 USA. [Google Scholar]
- 135.Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel beta-subunits. News in physiological sciences: an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society. 2002;17:156–61. Epub 2002/07/24. . [DOI] [PubMed] [Google Scholar]
- 136.Lishko PV, Kirichok Y, Ren D, Navarro B, Chung JJ, Clapham DE. The control of male fertility by spermatozoan ion channels. Annual review of physiology. 2012;74:453–75. Epub 2011/10/25. doi: 10.1146/annurev-physiol-020911-153258 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chung JJ, Miki K, Kim D, Shim SH, Shi HF, Hwang JY, et al. CatSperzeta regulates the structural continuity of sperm Ca2+ signaling domains and is required for normal fertility. eLife. 2017;6 doi: 10.7554/eLife.23082 ; PubMed Central PMCID: PMC5362262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011;471(7338):387–91. Epub 2011/03/18. doi: 10.1038/nature09767 . [DOI] [PubMed] [Google Scholar]
- 139.Carlson JC, Gruber MY, Thompson JE. A study of the interaction between progesterone and membrane lipids. Endocrinology. 1983;113(1):190–4. Epub 1983/07/01. doi: 10.1210/endo-113-1-190 . [DOI] [PubMed] [Google Scholar]
- 140.Witte TS, Schafer-Somi S. Involvement of cholesterol, calcium and progesterone in the induction of capacitation and acrosome reaction of mammalian spermatozoa. Animal reproduction science. 2007;102(3–4):181–93. Epub 2007/09/18. doi: 10.1016/j.anireprosci.2007.07.007 . [DOI] [PubMed] [Google Scholar]
- 141.Mylonas C, Kouretas D. Lipid peroxidation and tissue damage. In vivo (Athens, Greece). 1999;13(3):295–309. Epub 1999/08/25. . [PubMed] [Google Scholar]
- 142.Brodnitz MH. Autoxidation of saturated fatty acids. A review. Journal of Agricultural and Food Chemistry. 1968;16(6):994–9. [Google Scholar]
- 143.Wong-Ekkabut J, Xu Z, Triampo W, Tang IM, Tieleman DP, Monticelli L. Effect of lipid peroxidation on the properties of lipid bilayers: a molecular dynamics study. Biophysical journal. 2007;93(12):4225–36. doi: 10.1529/biophysj.107.112565 ; PubMed Central PMCID: PMC2098729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sabeti P, Pourmasumi S, Rahiminia T, Akyash F, Talebi AR. Etiologies of sperm oxidative stress. International journal of reproductive biomedicine (Yazd, Iran). 2016;14(4):231–40. Epub 2016/06/29. ; PubMed Central PMCID: PMCPMC4918773. [PMC free article] [PubMed] [Google Scholar]
- 145.Venkatesh S, Deecaraman M, Kumar R, Shamsi MB, Dada R. Role of reactive oxygen species in the pathogenesis of mitochondrial DNA (mtDNA) mutations in male infertility. The Indian journal of medical research. 2009;129(2):127–37. Epub 2009/03/19. . [PubMed] [Google Scholar]
- 146.Sikka SC. Relative impact of oxidative stress on male reproductive function. Current medicinal chemistry. 2001;8(7):851–62. Epub 2001/05/29. . [DOI] [PubMed] [Google Scholar]
- 147.Ko EY, Sabanegh ES Jr., Agarwal A. Male infertility testing: reactive oxygen species and antioxidant capacity. Fertility and sterility. 2014;102(6):1518–27. Epub 2014/12/03. doi: 10.1016/j.fertnstert.2014.10.020 . [DOI] [PubMed] [Google Scholar]
- 148.Chen SJ, Allam JP, Duan YG, Haidl G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Archives of gynecology and obstetrics. 2013;288(1):191–9. Epub 2013/04/02. doi: 10.1007/s00404-013-2801-4 . [DOI] [PubMed] [Google Scholar]
- 149.Guthrie HD, Welch GR. Effects of reactive oxygen species on sperm function. Theriogenology. 2012;78(8):1700–8. Epub 2012/06/19. doi: 10.1016/j.theriogenology.2012.05.002 . [DOI] [PubMed] [Google Scholar]
- 150.Amaral A, Lourenco B, Marques M, Ramalho-Santos J. Mitochondria functionality and sperm quality. Reproduction. 2013;146(5):R163–74. Epub 2013/08/01. doi: 10.1530/REP-13-0178 . [DOI] [PubMed] [Google Scholar]
- 151.Scharwey M, Tatsuta T, Langer T. Mitochondrial lipid transport at a glance. Journal of cell science. 2013;126(Pt 23):5317–23. Epub 2013/11/06. doi: 10.1242/jcs.134130 . [DOI] [PubMed] [Google Scholar]
- 152.Flis VV, Daum G. Lipid Transport between the Endoplasmic Reticulum and Mitochondria. Cold Spring Harbor Perspectives in Biology. 2013;5(6). doi: 10.1101/cshperspect.a013235 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Plaza Davila M, Martin Munoz P, Tapia JA, Ortega Ferrusola C, Balao da Silva CC, Pena FJ. Inhibition of Mitochondrial Complex I Leads to Decreased Motility and Membrane Integrity Related to Increased Hydrogen Peroxide and Reduced ATP Production, while the Inhibition of Glycolysis Has Less Impact on Sperm Motility. PloS one. 2015;10(9):e0138777 Epub 2015/09/26. doi: 10.1371/journal.pone.0138777 ; PubMed Central PMCID: PMCPMC4583303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wong YT, Ruan R, Tay FE. Relationship between levels of oxidative DNA damage, lipid peroxidation and mitochondrial membrane potential in young and old F344 rats. Free radical research. 2006;40(4):393–402. Epub 2006/03/07. doi: 10.1080/10715760600556074 . [DOI] [PubMed] [Google Scholar]
- 155.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiological reviews. 2014;94(3):909–50. doi: 10.1152/physrev.00026.2013 ; PubMed Central PMCID: PMC4101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schlame M, Ren M, Xu Y, Greenberg ML, Haller I. Molecular symmetry in mitochondrial cardiolipins. Chemistry and physics of lipids. 2005;138(1–2):38–49. Epub 2005/10/18. doi: 10.1016/j.chemphyslip.2005.08.002 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Precipitated nano-micelles and spots picked for X-Ray fluorescence analysis (up) and number of counts versus energy plot (down). (B) Same as in (A) for a control surface.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.