Abstract
This study sought to evaluate the muscle metaboreflex in heart failure patients with reduced ejection fraction (HFrEF), with an emphasis on the interaction between cardiac and peripheral vascular hemodynamics across multiple levels of metaboreceptor activation. In 23 HFrEF patients (63 ± 2 yrs) and 15 healthy controls (64 ± 3 yrs), we examined changes in mean arterial pressure (MAP), cardiac output (CO), systemic vascular conductance (SVC), effective arterial elastance (Ea), stroke work (SW), and forearm deoxyhemoglobin concentration during metaboreceptor activation elicited by post-exercise circulatory occlusion (PECO) following three levels of static-intermittent handgrip exercise (15, 30, and 45% maximal voluntary contraction (MVC)). Across workloads, the metaboreflex-induced increase in deoxyhemoglobin and MAP were similar between groups. However, in controls, the pressor response was driven by changes in CO (Δ495 ± 155, Δ564 ± 156, Δ666 ± 217 ml/min), while this change was accomplished by intensity-dependent reductions in SVC in patients with HFrEF (Δ−4.9 ± 1.5, Δ−9.1 ± 1.9, Δ−12.7 ± 1.8 ml/min/mmHg). This differential response contributed to the exaggerated increases in Ea in HFrEF compared to controls, coupled with a blunted response in SW in the HFrEF patients. Together, these findings indicate a preserved role of the metaboreflex-induced pressor response in HFrEF, but suggest that this response is governed by changes in the peripheral circulation. The net effect of this response appears to be maladaptive, as it places a substantial hemodynamic load on the left ventricle that may exacerbate left ventricular systolic dysfunction and contribute to exercise intolerance in this patient population.
Keywords: Heart Failure, Metaboreflex, Exercise, Arterial Blood Pressure
Introduction
Heart failure with reduced ejection fraction (HFrEF) is a clinical syndrome which is commonly linked to exercise intolerance (Wilson et al., 1983; Sullivan & Hawthorne, 1995). While there are many contributing factors to exercise intolerance in this patient population, maladaptation of skeletal muscle has been increasingly recognized, with specific interest focused on the muscle metaboreflex. Activation of this reflex pathway is mediated by metabolically-sensitive group IV afferent fibers (metaboreceptors) originating in skeletal muscle, which increase efferent sympathetic nervous system activity in an effort to augment perfusion of the exercising skeletal muscle through increases in arterial blood pressure (ABP) (O'Leary & Augustyniak, 1998; Crisafulli et al., 2007; Amann et al., 2011). Whether the metaboreflex is altered in HFrEF patients remains an ongoing topic of debate (Middlekauff & Sinoway, 2007; Piepoli & Coats, 2007), with evidence for both similar (Sterns et al., 1991; Carrington et al., 2001; Notarius et al., 2001; Kon et al., 2004) and exaggerated (Piepoli et al., 1996; Shoemaker et al., 1998; Silber et al., 1998; Piepoli et al., 1999; Piepoli & Coats, 2007) reflex increases in mean arterial blood pressure (MAP) during metaboreflex activation. These disparate findings suggest that significant uncertainty remains regarding disease-related changes in the muscle metaboreflex in HFrEF patients, as well as the contribution of this reflex to the cardiovascular response to exercise.
Beyond the simple determination of the pressor response, further insight into the importance of metaboreceptor activation in patients with HFrEF may be gained by considering the relative contribution of changes in cardiac (i.e. cardiac output, CO) and peripheral vascular (i.e. systemic vascular conductance, SVC) hemodynamics to the increase in MAP in these patients. Interestingly, in an animal model of systolic heart failure (HF), the contribution of these factors to the overall metaboreflex-induced pressor response have been documented to be solely due to a reduction in SVC across exercise intensities (Hammond et al., 2000). This is vastly different than the response observed in healthy animals, where the metaboreflex-induced increase in MAP is predominantly due to an increase in CO at low to moderate exercise intensities, with a shift towards a reliance on SVC to increase MAP only during high intensity efforts, when the ability to increase CO was compromised (Augustyniak et al., 2001). In humans, only one study to date has examined cardiac and peripheral vascular contributions to the metaboreflex in HFrEF. In this study, Crisafulli et al. (2007) reported a metaboreflex-induced increase in MAP which was predominantly driven by an increase in CO in healthy individuals and by a reduction in SVC in patients with HFrEF, suggesting a greater role of the peripheral vasculature in governing the pressor response. However, this study only included one level of metaboreceptor activation, leaving uncertainty regarding the differential nature of the response that has been demonstrated in an animal model of systolic HF (Hammond et al., 2000).
Whether the metaboreflex-mediated increase in MAP is achieved by cardiac or peripheral vascular mechanisms may be of functional significance in HFrEF patients, as this reflex may further stress cardiac muscle through a substantial increase in afterload. Indeed, SVC, a measure of systemic vascular tone, represents the non-pulsatile component of arterial afterload (Yin & Avolio, 1987; Kass & Kelly, 1992), and considering that patients with HFrEF are known to be afterload-sensitive (Asanoi et al., 1989; Kameyama et al., 1991; Schwartzenberg et al., 2012a), these patients can experience severe impairments in left ventricular systolic function when arterial afterload is increased (Kameyama et al., 1991). Thus, while the metaboreflex response is typically viewed as an effective way to increase perfusion of the exercising muscle by increasing perfusion pressure in healthy individuals, this reflex may, in fact, exacerbate existing left ventricular systolic dysfunction in HFrEF patients if metaboreceptor activation results in a marked reduction in SVC.
Thus, this study aimed to use post-exercise circulatory occlusion (PECO) following static-intermittent handgrip exercise across a range of exercise intensities to comprehensively investigate the interaction between cardiac and peripheral vascular responses to metaboreceptor activation in HFrEF. We hypothesized that, compared to controls,: 1) HFrEF patients would exhibit similar increases in MAP across all levels of metaboreceptor activation, 2) HFrEF patients would exhibit a greater dependence on reductions in SVC than increases in CO to achieve the metaboreflex-induced pressor response, and 3) HFrEF patients would exhibit a greater increase in effective arterial elastance (Ea) and an attenuated increase in functional left ventricular systolic work in response to metaboreceptor activation.
Methods
Ethical Approval
Protocol approval and written informed consent were obtained according to University of Utah and Salt Lake City Veterans Affairs Medical Center Institutional Review Board requirements (IRB #40212, approved 06/16/2010), in compliance with clause 35 of the Declaration of Helsinki.
Subjects
23 NYHA class II-III HFrEF patients (22 males, 1 female) and 15 healthy controls (14 males, 1 female) of similar age were recruited either by word of mouth or in the HF clinics at the University of Utah Health Sciences Center and the Salt Lake City VA Medical Center. All subjects were nonsmokers, and controls were not taking any prescription medication and were free of overt cardiovascular disease, as indicated by a health history questionnaire. All studies were performed in a thermoneutral environment, with subjects reporting to the laboratory fasted, and not having performed any exercise within 24 hours of the study.
Handgrip Exercise and Metaboreceptor Activation
Subjects were instrumented with a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands) on the non-exercising arm, a 3-lead ECG (Biopac, Goleta, CA, U.S.A.) to measure heart rate, and a pneumatic blood pressure cuff distal to the antecubital fossa on the exercising arm to isolate the metaboreflex following exercise. Subjects remained in the supine position for the duration of the protocol. After ∼20 minutes of rest, baseline measurements were taken over the course of 1 minute. Each subject's maximal voluntary contraction (MVC) was then established by taking the highest value recorded of three maximal contractions using a handgrip dynamometer (TSD121C, Biopac Systems, Goleta, CA). Static-intermittent handgrip exercise was performed at three intensities based on each subject's MVC (15, 30, and 45% of MVC). The subjects squeezed the dynamometer to the sound of a metronome (1 Hz) and real-time force output was displayed on a computer monitor so that subjects could evaluate their effort and make corrections when necessary. Each bout of handgrip exercise lasted 3 minutes, and was followed by 2 minutes of PECO to isolate the metaboreflex, with measurements taken during the final minute. Forearm ischemia was achieved through the inflation of the pneumatic blood pressure cuff on the exercising arm to suprasystolic pressures (>250 mmHg) 5 seconds prior to cessation of exercise. A 5 minute recovery period was given after each period of metaboreceptor activation to allow cardiovascular variables to return to resting values. If cardiovascular variables did not return to resting values after 5 minutes, additional rest was given.
Measurements
Hemodynamic Variables
Stroke volume (SV), heart rate (HR), CO, and ABP were determined non-invasively (Finapres Medical Systems BV, Amsterdam, The Netherlands). SV was calculated using the Modelflow method which includes age, sex, height, and weight in its algorithm (Beatscope version 1.1; Finapres Medical Systems BV, Amsterdam, The Netherlands) (Bogert & van Lieshout, 2005), and has been documented to accurately track CO during a variety of experimental protocols including exercise (Sugawara et al., 2003; van Lieshout et al., 2003; de Vaal et al., 2005; de Wilde et al., 2009). Pulse pressure (PP), a measure of pulsatile arterial afterload, the non-resistive oscillatory component of arterial afterload (Kelly et al., 1992; Chemla et al., 1998) was calculated as: PP (mmHg) = systolic arterial pressure (SAP) – diastolic arterial pressure (DAP). Total arterial compliance (TAC), an index of pulsatile arterial afterload which takes into account the effect of SV (Chemla et al., 1998; Reil et al., 2013) was calculated as: Total arterial compliance (ml/mmHg) = SV/PP. MAP was calculated as: MAP (mmHg) = DAP + (PP*0.33). End systolic arterial pressure (Pes) was calculated as (Kelly et al., 1992): Pes = 0.9*SAP. CO was calculated as: CO (L/min) = SV*HR. SVC, a measure of systemic vascular tone and the non-pulsatile (mean resistive) component of arterial afterload (Kass & Kelly, 1992) was calculated as: SVC (ml/min/mmHg) = CO/MAP. Systemic vascular resistance (SVR) was calculated as: SVR (mmHg/L/min) = MAP/CO. Ea, an index of total arterial afterload (both pulsatile and non-pulsatile arterial afterload) (Kelly et al., 1992; Reil et al., 2013) was calculated as: Ea (mmHg/ml) = Pes/SV. Stroke work (SW), a measure of functional left ventricular systolic work (Sunagawa et al., 1985; Kass & Kelly, 1992) was calculated as: SW (mmHg*ml) = Pes*SV. Rate pressure product (RPP), an index of myocardial oxygen consumption (Kitamura et al., 1972) was calculated as: RPP (AU) = SAP*HR.
Near Infrared Spectroscopy
To determine muscle microvascular deoxyhemoglobin (DeLorey et al., 2003) during exercise and metaboreceptor activation, in a subset of subjects (HFrEF = 13; control = 9), near infrared spectroscopy (NIRS) measurements were made in the brachioradialis and the flexor carpi radialis muscles. A frequency-domain multi-distance NIRS system was utilized (Oxiplex TS, ISS, Champaign, IL) that allows the absolute quantification of deoxyhemoglobin concentrations, expressed in μM (Hueber et al., 2001). Prior to use, the probe was calibrated using a block with known absorption characteristics to calculate the absorption and scattering coefficients. Prior to placement, the skin covering the brachioradialis and the flexor carpi radialis was cleaned and double-sided adhesive tape was used to seat the diode, which was covered and further secured with coban (3M, St. Paul, MN). The data were acquired at 0.5 Hz, and 1 minute averages were calculated during the last minute of each exercise bout and during the final minute of PECO.
Data Analysis
Statistics were performed using commercially available software (SigmaStat 3.10; Systat Software, Point Richmond, CA). For both the exercise and metaboreceptor activation portion of the protocol, a 2×4 repeated measures ANOVA (α < 0.05) (group: 2 levels; controls vs. HFrEF) (workload or metaboreflex activation: 4 levels; rest, 15, 30, and 45% of MVC) was utilized to determine the exercise and metaboreflex-induced alterations in hemodynamic measurements. The Holm-Sidak method was used for alpha adjustment and post hoc analysis.
Results
Subject characteristics
Baseline characteristics of the control subjects and HFrEF patients are displayed in Table 1. Disease-specific characteristics and medications of patients with HFrEF are presented in Table 2. Handgrip MVC force was similar between controls (27 ± 6 kg) and patients with HFrEF (25 ± 7 kg).
Table 1. Subject characteristics.
| Controls (n = 15) | HFrEF (n = 23) | |
|---|---|---|
| Age, yrs | 64 ± 11 | 63 ± 10 |
| Height, cm | 176 ± 7 | 175 ± 5 |
| Weight, kg | 80 ± 15 | 85 ± 16 |
| Body mass index, kg/m2 | 26 ± 5 | 28 ± 4 |
| Maximum voluntary contraction, kg | 27 ± 5 | 25 ± 5 |
| Glucose, mg/dl | 85 ± 17 | 99 ± 18 |
| Total cholesterol, mg/dl | 192 ± 35 | 155 ± 40 |
| Triglycerides, mg/dl | 143 ± 66 | 131 ± 56 |
| HDL, mg/dl | 49 ± 11 | 39 ± 10* |
| LDL, mg/dl | 124 ± 29 | 96 ± 27 |
HFrEF, heart failure with reduced ejection fraction; HDL, high density lipoprotein; LDL, low density lipoprotein. Data are expressed as mean ± SD.
Significant difference from control, P <0.05.
Table 2. Disease - specific characteristics and medications.
| HFrEF (n =23) | |
|---|---|
| Disease-specific characteristics | |
| Left ventricular ejection fraction, % (mean ±SEM) | 22 ± 12 |
| Diagnosis (ischemic) | 14 / 23 |
| Diagnosis (non-ischemic) | 9 / 23 |
| NYHA class II | 16 / 23 |
| NYHA class III | 7 / 23 |
| Diabetic | 4 / 23 |
| Medications | |
| β-Blocker | 23 / 23 |
| ACE inhibitor | 17 / 23 |
| Angiotensin receptor inhibitor | 4 / 23 |
| Statin | 18 / 23 |
| Diuretic | 18 / 23 |
| Aldosterone inhibitor | 4 / 23 |
| Calcium channel inhibitor | 1 / 23 |
| Digoxin | 4 / 23 |
| Anticoagulant | 13 / 23 |
| Antiarrhythmic | 1 / 23 |
| Erythropoiesis - stimulating agent | 1 / 23 |
HFrEF, heart failure with reduced ejection fraction; NYHA, New York Heart Association; ACE, angiotensin-converting enzyme.
Rest and exercise hemodynamics
Cardiac and peripheral vascular hemodynamics for both groups are presented in Table 3. At rest, there were no significant differences in deoxyhemoglobin, MAP, CO, or SVC in HFrEF patients compared to controls. Exercise elicited similar intensity-dependent increases in deoxyhemoglobin and MAP between groups. The changes in MAP were dictated by increases in CO across workloads in control subjects, and reductions in SVC in HFrEF patients. This was complemented by substantially attenuated increases in SAP and exaggerated increases in DAP in HFrEF compared to control subjects. These blood pressure differences resulted in a lower PP across exercise intensities in HFrEF compared to controls. However, when factoring in the differences in SV on PP, as expressed by TAC, there were no significant differences in TAC between groups at any workload. Ea was significantly increased across all workloads in both groups, however, the increases were significantly greater in HFrEF patients compared to controls at the two highest workloads. SW increased significantly across all workloads in the control subjects, with no significant difference from rest demonstrated by the HFrEF patients. In contrast, RPP was similar across all workloads in both groups.
Table 3. Cardiac and peripheral vascular hemodynamics at rest and during acute exercise.
| Workload (%MVC) | Rest | 15% | 30% | 45% |
|---|---|---|---|---|
| Controls | ||||
| Mean arterial pressure, mmHg | 83 ± 7 | 92 ± 9† | 96 ± 9† | 103 ± 7† |
| Systolic arterial pressure, mmHg | 119 ± 11 | 140 ± 15† | 143 ± 19† | 154 ± 12 |
| Diastolic arterial pressure, mmHg | 66 ± 6 | 68 ± 8 | 73 ± 6† | 77 ± 6† |
| Pulse pressure, mmHg | 53 ± 7 | 71 ± 14† | 70 ± 16† | 77 ± 10† |
| Heart rate, beats/min | 57 ± 6 | 64 ± 7† | 65 ± 9† | 68 ± 9† |
| Stroke volume, ml/beat | 109 ± 19 | 110 ± 18 | 111 ± 17 | 110 ± 16 |
| Cardiac output, L/min | 6.3 ± 1.2 | 7.0 ± 1.4† | 7.2 ± 1.4† | 7.4 ± 1.4† |
| Systemic vascular conductance, ml/min/mmHg | 75 ± 14 | 77 ± 18 | 75 ± 14 | 72 ± 14 |
| Systemic vascular resistance, mmHg/L/min | 14 ± 2 | 14 ± 3 | 14 ± 2 | 14 ± 2 |
| Total arterial compliance, ml/mmHg | 2.2 ± 0.6 | 1.6 ± 0.5† | 1.7 ± 0.8† | 1.5 ± 0.3† |
| Effective arterial elastance, mmHg/ml | 1.0 ± 0.2 | 1.2 ± 0.2† | 1.2 ± 0.2† | 1.3 ± 0.2† |
| Stroke work, mmHg*ml | 11,700 ± 2,293 | 13,920 ± 3,149† | 14,357 ± 3,243† | 15,308 ± 2,645† |
| Rate pressure product, AU | 6,821 ± 983 | 8,924 ± 1,311† | 9,354 ± 1,987† | 10,417 ± 1,706† |
| Deoxyhemoglobin, μM (n=9) | 27 ± 7 | 34 ± 8† | 37 ± 8† | 39 ± 8† |
| HFrEF | ||||
| Mean arterial pressure, mmHg | 84 ± 15 | 93 ± 16† | 95 ± 17† | 102 ± 16† |
| Systolic arterial pressure, mmHg | 117 ± 20 | 128 ± 23† | 131 ± 24† | 141 ± 25*† |
| Diastolic arterial pressure, mmHg | 67 ± 14 | 75 ± 14† | 77 ± 16† | 83 ± 13† |
| Pulse pressure, mmHg | 50 ± 6 | 53 ± 15* | 53 ± 17* | 58 ± 18*† |
| Heart rate, beats/min | 67 ± 9* | 69 ± 12† | 71 ± 11† | 73 ± 14† |
| Stroke volume, ml/beat | 83 ± 17* | 81 ± 17* | 79 ± 20* | 76 ± 20* |
| Cardiac output, L/min | 5.4 ± 1.3 | 5.5 ± 1.2* | 5.6 ± 1.6* | 5.4 ± 1.3* |
| Systemic vascular conductance, ml/min/mmHg | 68 ± 19 | 62 ± 16*† | 61 ± 21*† | 55 ± 15*† |
| Systemic vascular resistance, mmHg/L/min | 17 ± 1 | 18 ± 1† | 20 ± 3*† | 21 ± 1*† |
| Total arterial compliance, ml/mmHg | 1.8 ± 0.6 | 1.6 ± 0.3 | 1.6 ± 0.7 | 1.4 ± 0.6† |
| Effective arterial elastance, mmHg/ml | 1.4 ± 0.6 | 1.6 ± 0.7† | 1.6 ± 1.0*† | 1.8 ± 0.9*† |
| Stroke work, mmHg*ml | 8,654 ± 2,223 | 9,253 ± 2,250* | 9,192 ± 529* | 9,562 ± 3,097* |
| Rate pressure product, AU | 7,677 ± 1,488 | 8,902 ± 2,158† | 9,215 ± 2,184† | 10,322 ± 2,774† |
| Deoxyhemoglobin, μM (n=13) | 28 ± 4 | 32 ± 4† | 35 ± 5† | 36 ± 4† |
MVC, maximum voluntary contraction; HFrEF, heart failure with reduced ejection fraction. Data are expressed as mean ± SD.
Significant difference from control, P<0.05;
Significant difference from rest, P<0.05.
Metaboreflex-induced changes in hemodynamics
Cardiac and peripheral vascular hemodynamics during metaboreceptor activation via PECO are presented in Table 4. Metaboreceptor activation provoked similar increases in tissue deoxyhemoglobin and MAP (Figure 1, top panel) across increasing levels of activation between groups. However, the metaboreflex-induced increases in MAP were due exclusively to increases in CO in controls (Figure 1, middle panel), and reductions in SVC in the patients with HFrEF (Figure 1, bottom panel). Similar to exercise, HFrEF patients exhibited a blunted increase in SAP across increasing levels of metaboreceptor activation and exaggerated increases in DAP compared to controls (Table 4). This led to a significantly attenuated increase PP in the patients with HFrEF, who only established an increase in PP at the highest level of metaboreceptor activation. However, when factoring in the significantly greater increases in SV induced by the metaboreflex exhibited by the control subjects compared to HFrEF patients (expressed as TAC), no difference was evident between groups at any level of metaboreceptor activation (Table 4). Metaboreceptor activation provoked minimal increases in Ea in controls, who only exhibited a significant increase at the highest level (Figure 2). In contrast, HFrEF patients had significant increases in Ea across all levels of metaboreceptor activation and were significantly different from controls at the highest two levels (Figure 2). SW was significantly increased by metaboreceptor activation at every level in the control group, and only at the highest activation level in HFrEF patients, but was significantly lower across all levels of metaboreceptor activation in HFrEF compared to controls (Figure 3, top panel). These differences in SW between groups were due to significantly blunted changes in Pes (Figure 3, middle panel) and SV (Figure 3, bottom panel) induced by metaboreceptor activation in HFrEF patients compared to the control group. Across all levels of metaboreceptor activation, RPP was similar between groups.
Table 4. Cardiac and peripheral vascular hemodynamics at rest and during metaboreceptor activation.
| Metaboreceptor activation | Rest | 15% | 30% | 45% |
|---|---|---|---|---|
| Controls | ||||
| Mean arterial pressure, mmHg | 83 ± 7 | 90 ± 11† | 93 ± 3† | 100 ± 2† |
| Systolic arterial pressure, mmHg | 119 ± 11 | 132 ± 18† | 139 ± 5† | 150 ± 5† |
| Systolic arterial pressure, ΔmmHg | - | 13 ± 10† | 19 ± 3† | 34 ± 4† |
| Diastolic arterial pressure, mmHg | 66 ± 6 | 69 ± 8† | 70 ± 2† | 74 ± 1† |
| Diastolic arterial pressure, ΔmmHg | - | 4 ± 5† | 4 ± 1† | 8 ± 1† |
| Pulse pressure, mmHg | 53 ± 10 | 62 ± 13† | 68 ± 14† | 75 ± 19† |
| Pulse Pressure, ΔmmHg | - | 9 ± 5† | 16 ± 3† | 23 ± 4† |
| Heart rate, beats/min | 57 ± 6 | 58 ± 8 | 59 ± 2 | 58 ± 2 |
| Stroke volume, ml/beat | 109 ± 19 | 118 ± 19† | 118 ± 5† | 118 ± 5† |
| Cardiac output, L/min | 6.3 ± 1.2 | 6.8 ± 1.2† | 6.9 ± 0.3† | 6.8 ± 0.3† |
| Systemic vascular conductance, ml/min/mmHg | 75 ± 14 | 76 ± 13 | 75 ± 3 | 68 ± 3† |
| Systemic vascular resistance, mmHg/L/min | 14 ± 3 | 14 ± 2 | 14 ± 1 | 15 ± 1 |
| Total arterial compliance, ml/mmHg | 2.2 ± 0.6 | 2.0 ± 0.5 | 1.8 ± 0.7† | 1.7 ± 0.6† |
| Total arterial compliance, Δml/mmHg | - | -0.2 ± 0.5 | -0.3 ± 0.8† | -0.3 ± 0.9† |
| Effective arterial elastance, mmHg/ml | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.2 ± 0.2† |
| Stroke work, mmHg*ml | 11,700 ± 2,293 | 13,921 ± 2691† | 14,799 ± 3,469† | 15,941 ± 3,904† |
| Rate pressure product, AU | 6,821 ± 983 | 7,659 ± 1,653† | 8,217 ± 1,843† | 8,680 ± 1,260† |
| Deoxyhemoglobin, μM (n=9) | 27 ± 5 | 48 ± 9† | 50 ± 9† | 51 ± 10† |
| HFrEF | ||||
| Mean arterial pressure, mmHg | 84 ± 15 | 90 ± 16† | 94 ± 3† | 100 ± 4† |
| Systolic arterial pressure, mmHg | 117 ± 21 | 125 ± 24† | 129 ± 5† | 140 ± 6† |
| Systolic arterial pressure, ΔmmHg | - | 8 ± 8† | 12 ± 2*† | 23 ± 3*† |
| Diastolic arterial pressure, mmHg | 67 ± 14 | 73 ± 15† | 77 ± 3† | 81 ± 3† |
| Diastolic arterial pressure, ΔmmHg | - | 7 ± 4† | 9 ± 1*† | 13 ± 1*† |
| Pulse pressure, mmHg | 50 ± 14 | 52 ± 17 | 53 ± 16* | 59 ± 19*† |
| Pulse pressure, ΔmmHg | - | 3 ± 4* | 3 ± 6* | 10 ± 8*† |
| Heart rate, beats/min | 67 ± 9* | 68 ± 11* | 70 ± 11* | 68 ± 2* |
| Stroke volume, ml/beat | 83 ± 17* | 81 ± 18* | 77 ± 4* | 80 ± 4* |
| Cardiac output, L/min | 5.4 ± 1.3 | 5.5 ± 1.2* | 5.3 ± 0.3* | 5.4 ± 0.3* |
| Systemic vascular conductance, ml/min/mmHg | 68 ± 19 | 63 ± 18*† | 58 ± 4*† | 55 ± 4*† |
| Systemic vascular resistance, mmHg/L/min | 17 ± 10 | 18 ± 9† | 20 ± 2*† | 21 ± 3*† |
| Total arterial compliance, ml/mmHg | 1.8 ± 0.6 | 1.7 ± 0.6 | 1.6 ± 0.6 | 1.5 ± 0.6† |
| Total arterial compliance, Δml/mmHg | - | -0.1 ± 0.3 | -0.2 ± 0.4 | -0.5 ± 0.8† |
| Effective arterial elastance, mmHg/ml | 1.4 ± 0.6 | 1.6 ± 0.7*† | 1.7 ± 0.8*† | 1.7 ± 0.8*† |
| Stroke work, mmHg*ml | 8,654 ± 2,223 | 9,021 ± 2,266* | 8,896 ± 2,541* | 9,982 ± 3,043*† |
| Rate pressure product, AU | 7,677 ± 1,489 | 8,572 ± 1,131† | 8,990 ± 2,044† | 9,445 ± 2,286† |
| Deoxyhemoglobin, μM (n=13) | 28 ± 5 | 47 ± 8† | 50 ± 8† | 53 ± 7† |
MVC, maximum voluntary contraction; HFrEF, heart failure with reduced ejection fraction. Data are expressed as mean ± SD.
Significant difference from control, P<0.05;
Significant difference from rest, P<0.05.
Figure 1.
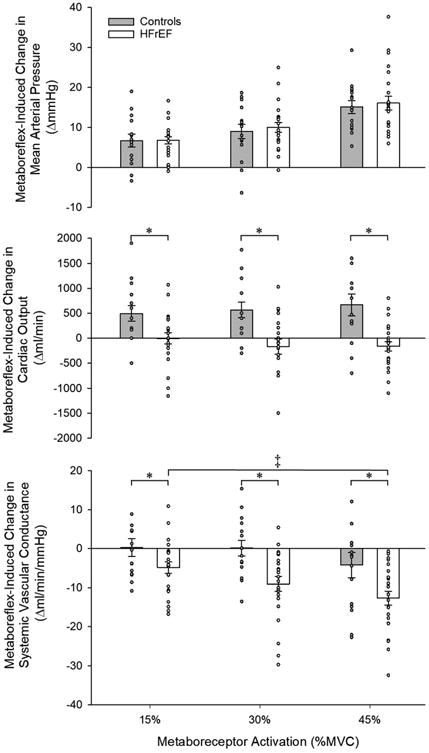
Metaboreflex-induced changes in mean arterial pressure (top), cardiac output (middle), and systemic vascular conductance (bottom), in control subjects and heart failure patients with reduced ejection fraction (HFrEF). * Significant difference from control, P<0.05; ‡ Significant difference from 15% MVC, P<0.05.
Figure 2.
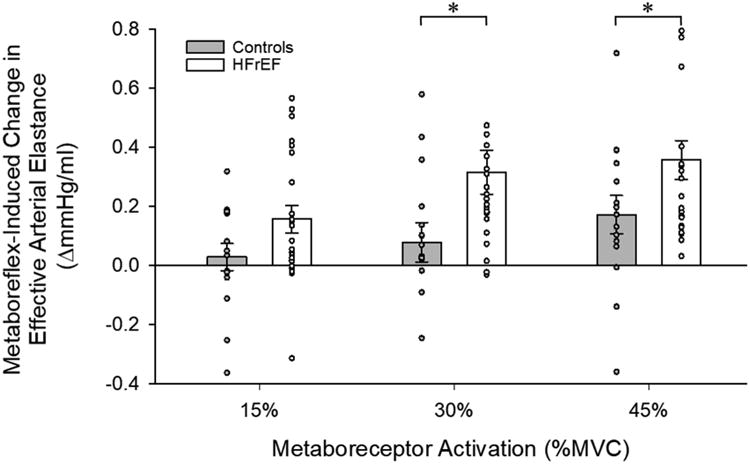
Metaboreflex-induced changes in effective arterial elastance in control subjects and heart failure patients with reduced ejection fraction (HFrEF). * Significant difference from control, P<0.05.
Figure 3.
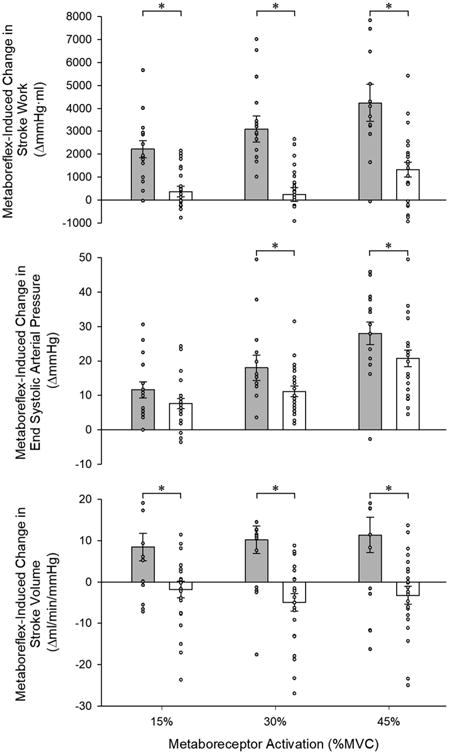
Metaboreflex-induced changes in stroke work (top), end systolic pressure (middle), and stroke volume (bottom) in control subjects and heart failure patients with reduced ejection fraction (HFrEF). * Significant difference from control, P<0.05.
Discussion
This study sought to comprehensively examine the muscle metaboreflex in HFrEF patients and healthy control subjects of a similar age, with an emphasis on investigating the cardiac and peripheral vascular hemodynamic contributions to the metaboreflex-induced pressor response. Across multiple levels of metaboreceptor activation, the increase in MAP was similar between groups, providing new evidence refuting a disease-related exaggeration of the muscle metaboreflex-induced pressor response in HFrEF. However, a disease-specific, discrete pattern of cardiac and peripheral vascular hemodynamic changes was observed between groups. In control subjects, the pressor response induced by metaboreceptor activation was driven by an increase in CO, with no significant change in SVC. In contrast, progressively greater reductions in SVC contributed to the pressor response in patients with HFrEF, while CO remained unchanged. The functional consequence of relying upon changes in SVC to govern the pressor response during metaboreflex activation in the patients with HFrEF was evident through marked increases in total arterial afterload, which appears to have provoked a reduction in myocardial efficiency during metaboreceptor activation. Together, these findings indicate a preserved role of the muscle metaboreflex-induced pressor response in HFrEF. However, the shift to an increase in peripheral vasoconstriction to drive this response in patients with HFrEF appears to represent a maladaptive process which places a substantial hemodynamic load on the left ventricle, potentially exacerbating the underlying impairment in left ventricular systolic function and thereby contributing to the exercise intolerance present in this patient population.
Metaboreflex contribution to the exercise-induced changes in mean arterial pressure
It is well established that patients with HFrEF suffer from a nearly insurmountable intolerance to physical exertion (Wilson et al., 1983; Sullivan & Hawthorne, 1995), which may be due, at least in part, to maladaptations in skeletal muscle. Indeed, Drs. Coats and Piepoli (Coats et al., 1994; Piepoli et al., 1999) have hypothesized that abnormalities in sensory reflex activity in skeletal muscle may contribute to the exercise limitations in HFrEF, the so-called “muscle hypothesis” of heart failure. Located within the skeletal muscle are two distinct sensory afferent fiber types; group III afferent fibers, which are predominately mechanically sensitive (mechanoreceptors) and group IV afferent fibers (metaboreceptors) which are principally sensitive to metabolites produced during exercise (Kaufman & Hayes, 2002). Collectively, these reflex pathways serve to increase sympathetic nervous system activity, which ultimately increase perfusion pressure (O'Leary & Augustyniak, 1998; Crisafulli et al., 2007; Amann et al., 2011). In HFrEF patients, some aspect of this reflex response appears to be dysfunctional.
While it is difficult to completely isolate these respective reflex pathways, PECO has become a widely adopted experimental approach whereby metabolic byproducts produced during exercise are trapped distal to the point of occlusion, activating group IV afferent fibers with minimal input from the group III fibers (Alam & Smirk, 1937). Interestingly, despite extensive use of this technique over the past 80 years in both healthy humans and patient populations, the exact role of metaboreceptor activation in the cardiovascular response to exercise in HFrEF remains a topic of ongoing debate. Indeed, using microneurography for direct assessment of muscle sympathetic nerve activity (MSNA), evidence can be found for blunted (Sterns et al., 1991), similar (Middlekauff et al., 2004), and increased (Notarius et al., 2001) activity during PECO in HFrEF patients compared to healthy individuals. Likewise, evidence exists for both similar (Sterns et al., 1991; Carrington et al., 2001; Notarius et al., 2001; Kon et al., 2004) and exaggerated (Piepoli et al., 1996; Shoemaker et al., 1998; Silber et al., 1998; Piepoli et al., 1999; Piepoli & Coats, 2007) reflex increases in MAP during metaboreflex activation in HFrEF patients, indicating that significant uncertainty remains as to whether the metaboreflex-induced pressor response is altered in this patient group.
In the present study, we employed the PECO technique following three different handgrip exercise intensities in an effort to comprehensively evaluate the muscle metaboreflex in HFrEF patients compared to healthy control subjects of a similar age. As illustrated in Figure 1 (top panel), we observed a metaboreflex-induced pressor response that was almost identical between groups across all levels of metaboreceptor activation. These results are in disagreement with some of the earliest work on this topic (Shoemaker et al., 1998; Piepoli et al., 1999), and may be explained by differences in experimental protocols, including differing handgrip exercise paradigms and methods of activating the muscle metaboreflex (PECO vs. limb positive pressure). To our knowledge, this is the first study to perform PECO following multiple intensities of static-intermittent handgrip exercise, providing a comprehensive and systematic assessment of the pressor response across multiple levels of metaboreceptor activation. The present findings thus confirm and extend observations from previous work (Sterns et al., 1991; Carrington et al., 2001; Notarius et al., 2001; Kon et al., 2004), providing new evidence refuting a disease-related exaggeration of the pressor response induced by the muscle metaboreflex in patients with HFrEF.
Cardiac and peripheral vascular hemodynamic contributions to metaboreflex-induced changes in MAP
While a large number of studies have focused on elucidating the strength of the metaboreflex-induced pressor response in HFrEF, limited work has been undertaken to examine the variables contributing to this rise in MAP. In a healthy animal model, Augustyniak et al. (Augustyniak et al., 2001) documented that the rise in MAP triggered by metaboreceptor activation was achieved via two distinct, but complimentary, mechanisms. Specifically, during mild and moderate exercise intensities, the increase in MAP was due solely to increases in CO, with a shift towards a reliance on SVC to increase MAP only during high intensity exercise, when the ability to increase CO was compromised. In contrast to this somewhat dichotomous response, work from the same group reported that the metaboreflex-induced increases in MAP in an animal model of systolic HF were primarily due to reductions in SVC (Hammond et al., 2000), indicating that the pressor response was achieved almost exclusively via sympathetic vasoconstriction of the peripheral vasculature. Based on these findings, the authors concluded that the inability of the metaboreflex to increase CO in this animal model of HF is likely detrimental, as only a single mechanism appears to be available for this reflex pathway to increase perfusion pressure, and ultimately, blood flow to exercising skeletal muscle.
The present study builds upon the previous findings to human HF, documenting metaboreflex-induced increases in MAP in patients with HFrEF (Figure 1, top panel) that were primarily accomplished through reductions in SVC (Figure 1, bottom panel), with virtually no changes in CO (Figure 1, middle panel). This was in marked contrast to the response observed in healthy control subjects, where increases in CO played a dominant role in increasing MAP during metaboreceptor activation (Figure 1, middle panel). To our knowledge, only one other study in humans has examined the roles of CO and SVC in increasing MAP during metaboreceptor activation in patients with HFrEF. Crisafulli et al. (2007) reported a metaboreflex-induced increase in MAP which was predominantly driven by an increase in CO in healthy individuals and by a reduction in SVC in patients with HFrEF, suggesting a greater role of the peripheral vasculature in governing the metaboreflex-induced pressor response in the patient group. However, this previous study only investigated the hemodynamic alterations induced by one level of metaboreceptor activation (30% MVC), compared to the three levels of exercise in the present study. The importance of examining multiple levels of metaboreceptor activation should not be underestimated. Indeed, as outlined above, the relative contribution of CO and SVC to the pressor response evoked by metaboreceptor activation has been shown to differ according to exercise intensity in an animal model (Augustyniak et al., 2001), and it was thus anticipated that a similar, intensity-dependent response would be observed in the present study. This was indeed the case, and thus the current study may be viewed as providing a comprehensive investigation into the role of CO and SVC in the metaboreflex-induced pressor response in systolic HF in humans by identifying an intensity-dependent reduction in SVC during metaboreflex activation in HFrEF, thus indicating a proportionally greater role of SVC in increasing MAP in this patient group.
Arterial afterload and left ventricular systolic function
The manner by which metaboreceptor activation elicits an increase in MAP may be particularly significant when considering the relationship between the left ventricle and the peripheral vasculature in HFrEF patients. Indeed, SVC represents the non-pulsatile component of arterial afterload (Yin & Avolio, 1987; Kass & Kelly, 1992) and it is well-established that patients with HFrEF are afterload-sensitive (Asanoi et al., 1989; Kameyama et al., 1991; Schwartzenberg et al., 2012a). Thus, these patients face certain impairment in left ventricular systolic function if arterial afterload is increased (Kameyama et al., 1991). In the present study, at all levels of metaboreceptor activation, HFrEF patients exhibited an exaggerated increase in Ea, an index of total arterial afterload, compared to control subjects who only exhibited a significant augmentation in Ea at the highest level of metaboreceptor activation (Figure 2). As there were no differences between groups in the reduction in TAC (Table 4), a measure of the pulsatile component of arterial afterload, SVC is likely the primary contributor to the exaggerated increase in total arterial afterload induced by metaboreceptor activation exhibited in HFrEF. These findings thus confirm and extend former work (2007), providing additional evidence for an augmented arterial afterload induced by the metaboreflex-driven changes in SVC in HFrEF.
This metaboreflex-induced increase in arterial afterload in HFrEF appears to have deleterious cardiac effects. Indeed, metaboreceptor activation provoked much smaller increases in SW (a measure of functional left ventricular systolic work) in patients with HFrEF compared to healthy control subjects (Figure 3, top panel), an impairment that is likely related to the exaggerated increase in arterial afterload in this cohort. This reduction in SW is particularly relevant when viewed in the context of the metabolic cost, as determined by RPP, which was similar between groups across all levels of metaboreceptor activation (Table 4). Taken together, the SW and RPP responses point to a reduction in myocardial efficiency in HFrEF, as less left ventricular systolic work was performed for a similar metabolic cost in the patient group. These cardiac indices therefore suggest that the metaboreflex-induced reductions in SVC and the associated increases in arterial afterload come at a steep cost to HFrEF patients, and may actually limit functional left ventricular systolic work and reserve capacity by placing a substantial hemodynamic load on the failing heart. Further studies with direct measurements of left ventricular hemodynamics are warranted to explore this interesting possibility.
Experimental Considerations
The present study is not without limitations. We acknowledge that the arterial afterload calculations used in the current study are typically based on aortic pressure measurements (Kelly et al., 1992), and this variable was calculated from measurements obtained non-invasively via finger photoplethysmography in the present study. Though peripheral ABP measurements may not always reflect central pressures due to wave amplification descending the arterial tree (Williams et al., 2006; Safar et al., 2009), central ABP measurements may also be augmented due to reflected pressure waveforms (Safar et al., 2009), limiting the discrepancy between central and peripheral pressure measurements (Kroeker & Wood, 1955; Kelly et al., 1992; Nussbacher et al., 1999). While effective arterial elastance has been used as an index of total arterial afterload in many studies with diverse clinical populations (Kussmaul et al., 1993; Borlaug & Kass, 2008; Borlaug et al., 2009; Schwartzenberg et al., 2012b; Eleid et al., 2013), we recognize that this index has not directly been validated in HFrEF patients. Recent evidence suggests that the cardiovascular response to metaboreflex activation is also abnormal in heart failure patients with a preserved ejection fraction (HFpEF) (Roberto et al., 2017), findings that are relevant to the current study given that many patients with systolic dysfunction also suffer from some level of diastolic dysfunction, ranging from abnormal relaxation to restrictive filling (Naqvi, 2003; Lang et al., 2015). However, patients in the present study did not demonstrate any echocardiographic evidence of diastolic dysfunction as defined by current guidelines (Nagueh et al., 2016), suggesting the reported findings are specific to the HFrEF phenotype. We enrolled HFrEF patients on optimized pharmacotherapy, and no medications were withheld on experimental days. We therefore cannot exclude the possibility that existing drug therapy may have affected our measurements, particularly cardiac responses, though it is noteworthy that this represents the “real world” in which optimally medicated patients live. Finally, we acknowledge that use of the Modelflow method for estimation of stroke volume may not provide the same level of precision as that provided by more invasive techniques, though it is noteworthy that good agreement in tracking CO changes has recently been documented with these two methodologies in heart disease patients (de Wilde et al., 2007).
Conclusions
This study has identified a preserved role of the metaboreflex-induced pressor response in HFrEF patients, and provides evidence that the rise in MAP is governed almost entirely by the peripheral circulation in this patient population. The net effect of this response appears to be maladaptive, as it places a substantial hemodynamic load on the heart, exacerbates the underlying impairment of systolic function, and likely contributes to exercise intolerance in this patient group.
Acknowledgments
Funding. Funded in part by grants from the National Institutes of Health (HL118313) and the U.S. Department of Veterans Affairs (RX001311, RX001697).
New Findings: The central question of this study is whether HFrEF patients exhibit a greater dependence on cardiac or peripheral vascular hemodynamics across multiple levels of muscle metaboreflex activation provoked by post-exercise circulatory occlusion. The main findings of this study is that the metaboreflex-induced pressor response in HFrEF patients is governed almost entirely by the peripheral circulation, which places a substantial hemodynamic load on the failing heart. This maladaptive response exacerbates the disease-related impairment of systolic function that is a hallmark feature of HFrEF, and may therefore contribute to exercise intolerance in this patient group.
Abbreviations
- ABP
Arterial Blood Pressure
- CO
Cardiac Output
- DAP
Diastolic Arterial Pressure
- Ea
Effective Arterial Elastance
- HF
Heart Failure
- HFrEF
Heart Failure with Reduced Ejection Fraction
- HR
Heart Rate
- MAP
Mean Arterial Pressure
- MVC
Maximal Voluntary Contraction
- NIRS
Near-Infrared Spectroscopy
- PECO
Post-Exercise Circulatory Occlusion
- Pes
End-Systolic Arterial Pressure
- PP
Pulse Pressure
- RPP
Rate Pressure Product
- SAP
Systolic Arterial Pressure
- SVC
Systemic Vascular Conductance
- SVR
Systemic Vascular Resistance
- SW
Stroke Work
Footnotes
Author Contributions. All data collection took place at the Utah Vascular Research Laboratory located at the Veterans Affairs Salt Lake City Geriatric, Research, Education, and Clinical Center. ZBOK, JFL, AB, MAHW, JNN, JS, RSR, and DWW contributed to conception or design of the work; ZBOK, JFL, AB, MAHW, RSR, and DWW contributed to acquisition, analysis, or interpretation of data for the work; ZBOK, JFL, AB, MAHW, JNN, JS, RSR, and DWW contributed to drafting the work or revising it critically for important intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
References
- Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol. 2011;589:3855–3866. doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanoi H, Sasayama S, Kameyama T. Ventriculoarterial coupling in normal and failing heart in humans. Circ Res. 1989;65:483–493. doi: 10.1161/01.res.65.2.483. [DOI] [PubMed] [Google Scholar]
- Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O'Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol. 2001;280:H1645–1652. doi: 10.1152/ajpheart.2001.280.4.H1645. [DOI] [PubMed] [Google Scholar]
- Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol. 2005;90:437–446. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Fail Clin. 2008;4:23–36. doi: 10.1016/j.hfc.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington CA, Fisher WJ, Davies MK, White MJ. Muscle afferent and central command contributions to the cardiovascular response to isometric exercise of postural muscle in patients with mild chronic heart failure. Clin Sci (Lond) 2001;100:643–651. [PubMed] [Google Scholar]
- Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P, Lecarpentier Y. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol. 1998;274:H500–505. doi: 10.1152/ajpheart.1998.274.2.H500. [DOI] [PubMed] [Google Scholar]
- Coats AJ, Clark AL, Piepoli M, Volterrani M, Poole-Wilson PA. Symptoms and quality of life in heart failure: the muscle hypothesis. Br Heart J. 1994;72:S36–39. doi: 10.1136/hrt.72.2_suppl.s36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol. 2007;292:H2988–2996. doi: 10.1152/ajpheart.00008.2007. [DOI] [PubMed] [Google Scholar]
- de Vaal JB, de Wilde RB, van den Berg PC, Schreuder JJ, Jansen JR. Less invasive determination of cardiac output from the arterial pressure by aortic diameter-calibrated pulse contour. Br J Anaesth. 2005;95:326–331. doi: 10.1093/bja/aei189. [DOI] [PubMed] [Google Scholar]
- de Wilde RB, Geerts BF, Cui J, van den Berg PC, Jansen JR. Performance of three minimally invasive cardiac output monitoring systems. Anaesthesia. 2009;64:762–769. doi: 10.1111/j.1365-2044.2009.05934.x. [DOI] [PubMed] [Google Scholar]
- de Wilde RB, Schreuder JJ, van den Berg PC, Jansen JR. An evaluation of cardiac output by five arterial pulse contour techniques during cardiac surgery. Anaesthesia. 2007;62:760–768. doi: 10.1111/j.1365-2044.2007.05135.x. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Kowalchuk JM, Paterson DH. Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol. 2003;95:113–120. doi: 10.1152/japplphysiol.00956.2002. [DOI] [PubMed] [Google Scholar]
- Eleid MF, Nishimura RA, Sorajja P, Borlaug BA. Systemic hypertension in low-gradient severe aortic stenosis with preserved ejection fraction. Circulation. 2013;128:1349–1353. doi: 10.1161/CIRCULATIONAHA.113.003071. [DOI] [PubMed] [Google Scholar]
- Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O'Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol. 2000;278:H818–828. doi: 10.1152/ajpheart.2000.278.3.H818. [DOI] [PubMed] [Google Scholar]
- Hueber DM, Franceschini MA, Ma HY, Zhang Q, Ballesteros JR, Fantini S, Wallace D, Ntziachristos V, Chance B. Non-invasive and quantitative near-infrared haemoglobin spectrometry in the piglet brain during hypoxic stress, using a frequency-domain multidistance instrument. Phys Med Biol. 2001;46:41–62. doi: 10.1088/0031-9155/46/1/304. [DOI] [PubMed] [Google Scholar]
- Kameyama T, Asanoi H, Ishizaka S, Sasayama S. Ventricular load optimization by unloading therapy in patients with heart failure. Journal of the American College of Cardiology. 1991;17:199–207. doi: 10.1016/0735-1097(91)90728-r. [DOI] [PubMed] [Google Scholar]
- Kass DA, Kelly RP. Ventriculo-arterial coupling: concepts, assumptions, and applications. Ann Biomed Eng. 1992;20:41–62. doi: 10.1007/BF02368505. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res. 2002;12:429–439. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]
- Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Jorgensen CR, Gobel FL, Taylor HL, Wang Y. Hemodynamic correlates of myocardial oxygen consumption during upright exercise. J Appl Physiol. 1972;32:516–522. doi: 10.1152/jappl.1972.32.4.516. [DOI] [PubMed] [Google Scholar]
- Kon H, Nakamura M, Arakawa N, Hiramori K. Muscle metaboreflex is blunted with reduced vascular resistance response of nonexercised limb in patients with chronic heart failure. J Card Fail. 2004;10:503–510. doi: 10.1016/j.cardfail.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Kroeker EJ, Wood EH. Comparison of simultaneously recorded central and peripheral arterial pressure pulses during rest, exercise and tilted position in man. Circ Res. 1955;3:623–632. doi: 10.1161/01.res.3.6.623. [DOI] [PubMed] [Google Scholar]
- Kussmaul WG, 3rd, Altschuler JA, Matthai WH, Laskey WK. Right ventricular-vascular interaction in congestive heart failure. Importance of low-frequency impedance. Circulation. 1993;88:1010–1015. doi: 10.1161/01.cir.88.3.1010. [DOI] [PubMed] [Google Scholar]
- Lang RM, Goldstein SA, Kronzon I, Khandheria B, Mor-Avi V American Society of Echocardiography. ASE's comprehensive echocardiography. Elsevier; Philadelphia, PA: 2015. [Google Scholar]
- Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi J, Patel J. Muscle mechanoreceptor sensitivity in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1937–1943. doi: 10.1152/ajpheart.00330.2004. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Sinoway LI. Increased mechanoreceptor stimulation explains the exaggerated exercise pressor reflex seen in heart failure. J Appl Physiol (1985) 2007;102:492–494. doi: 10.1152/japplphysiol.00994.2006. discussion 496. [DOI] [PubMed] [Google Scholar]
- Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Naqvi TZ. Diastolic function assessment incorporating new techniques in Doppler echocardiography. Rev Cardiovasc Med. 2003;4:81–99. [PubMed] [Google Scholar]
- Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol. 2001;280:H969–976. doi: 10.1152/ajpheart.2001.280.3.H969. [DOI] [PubMed] [Google Scholar]
- Nussbacher A, Gerstenblith G, O'Connor FC, Becker LC, Kass DA, Schulman SP, Fleg JL, Lakatta EG. Hemodynamic effects of unloading the old heart. Am J Physiol. 1999;277:H1863–1871. doi: 10.1152/ajpheart.1999.277.5.H1863. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Augustyniak RA. Muscle metaboreflex increases ventricular performance in conscious dogs. Am J Physiol. 1998;275:H220–224. doi: 10.1152/ajpheart.1998.275.1.H220. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation. 1996;93:940–952. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Ponikowski P, Clark AL, Banasiak W, Capucci A, Coats AJ. A neural link to explain the “muscle hypothesis” of exercise intolerance in chronic heart failure. Am Heart J. 1999;137:1050–1056. doi: 10.1016/s0002-8703(99)70361-3. [DOI] [PubMed] [Google Scholar]
- Piepoli MF, Coats AJ. Increased metaboreceptor stimulation explains the exaggerated exercise pressor reflex seen in heart failure. J Appl Physiol (1985) 2007;102:494–496. doi: 10.1152/japplphysiol.00994a.2006. discussion 496-497. [DOI] [PubMed] [Google Scholar]
- Reil JC, Tardif JC, Ford I, Lloyd SM, O'Meara E, Komajda M, Borer JS, Tavazzi L, Swedberg K, Bohm M. Selective heart rate reduction with ivabradine unloads the left ventricle in heart failure patients. Journal of the American College of Cardiology. 2013;62:1977–1985. doi: 10.1016/j.jacc.2013.07.027. [DOI] [PubMed] [Google Scholar]
- Roberto S, Mulliri G, Milia R, Solinas R, Pinna V, Sainas G, Piepoli MF, Crisafulli A. Hemodynamic response to muscle reflex is abnormal in patients with heart failure with preserved ejection fraction. J Appl Physiol (1985) 2017;122:376–385. doi: 10.1152/japplphysiol.00645.2016. [DOI] [PubMed] [Google Scholar]
- Safar ME, Protogerou AD, Blacher J. Statins, central blood pressure, and blood pressure amplification. Circulation. 2009;119:9–12. doi: 10.1161/CIRCULATIONAHA.108.824532. [DOI] [PubMed] [Google Scholar]
- Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. Journal of the American College of Cardiology. 2012a;59:442–451. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
- Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012b;59:442–451. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Kunselman AR, Silber DH, Sinoway LI. Maintained exercise pressor response in heart failure. J Appl Physiol (1985) 1998;85:1793–1799. doi: 10.1152/jappl.1998.85.5.1793. [DOI] [PubMed] [Google Scholar]
- Silber DH, Sutliff G, Yang QX, Smith MB, Sinoway LI, Leuenberger UA. Altered mechanisms of sympathetic activation during rhythmic forearm exercise in heart failure. J Appl Physiol. 1998;84:1551–1559. doi: 10.1152/jappl.1998.84.5.1551. [DOI] [PubMed] [Google Scholar]
- Sterns DA, Ettinger SM, Gray KS, Whisler SK, Mosher TJ, Smith MB, Sinoway LI. Skeletal muscle metaboreceptor exercise responses are attenuated in heart failure. Circulation. 1991;84:2034–2039. doi: 10.1161/01.cir.84.5.2034. [DOI] [PubMed] [Google Scholar]
- Sugawara J, Tanabe T, Miyachi M, Yamamoto K, Takahashi K, Iemitsu M, Otsuki T, Homma S, Maeda S, Ajisaka R, Matsuda M. Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol Scand. 2003;179:361–366. doi: 10.1046/j.0001-6772.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Hawthorne MH. Exercise intolerance in patients with chronic heart failure. Prog Cardiovasc Dis. 1995;38:1–22. doi: 10.1016/s0033-0620(05)80011-8. [DOI] [PubMed] [Google Scholar]
- Sunagawa K, Maughan WL, Sagawa K. Optimal arterial resistance for the maximal stroke work studied in isolated canine left ventricle. Circ Res. 1985;56:586–595. doi: 10.1161/01.res.56.4.586. [DOI] [PubMed] [Google Scholar]
- van Lieshout JJ, Toska K, van Lieshout EJ, Eriksen M, Walloe L, Wesseling KH. Beat-to-beat noninvasive stroke volume from arterial pressure and Doppler ultrasound. Eur J Appl Physiol. 2003;90:131–137. doi: 10.1007/s00421-003-0901-8. [DOI] [PubMed] [Google Scholar]
- Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Martin JL, Ferraro N, Weber KT. Effect of hydralazine on perfusion and metabolism in the leg during upright bicycle exercise in patients with heart failure. Circulation. 1983;68:425–432. doi: 10.1161/01.cir.68.2.425. [DOI] [PubMed] [Google Scholar]
- Yin FCP, Avolio AP. Ventricular/vascular coupling: clinical, physiological, and engineering aspects. Springer-Verlag; New York: 1987. [Google Scholar]


