Abstract
Background and Aims
High rates of loss to follow-up represent a significant challenge to clinical trials of pharmacological treatments for methamphetamine (MA) use disorder. We aimed to estimate and test the relationship between achieving and maintaining abstinence in the initial weeks of study participation and subsequent retention in such trials, hypothesizing that participants able to achieve early abstinence would be less likely to drop out.
Design
Data from four randomized controlled trials (RCTs) of pharmacological treatments for MA use disorder were pooled and analyzed using a random-effects approach.
Setting
All trials were conducted in the greater Los Angeles, CA, USA area.
Participants
A total of 440 participants were included; trials were conducted between 2004 and 2014.
Measurements
Participants’ ability to achieve a brief period of initial abstinence was measured as the number of MA-negative urine screens completed in the first 2 weeks of the trials. Outcomes were the likelihood of dropout, i.e. missing two consecutive weeks of scheduled urine drug screens, and the number of days participants were retained in the trials.
Findings
Study participants achieved an average of three (of six possible) negative urine screens during the first 2 weeks of the trials, 51% dropped out and the average number of days retained was 60 (of 90 maximum). Each additional negative urine screen achieved during the first 2 weeks of the study reduced multiplicatively the odds of dropout by 41% [odds ratio (OR) = 0.59, 95% confidence interval (CI) = 0.53, 0.66]. Abstinence was also a significant predictor of retention time; the hazard ratio for non-completion was 0.75 per additional negative urine screen (95% CI = 0.71, 0.80).
Conclusions
Participants in randomized controlled trials of pharmacological treatments for methamphetamine use disorder who are able to achieve a brief period of early abstinence are retained longer in the trials and are less likely to drop out overall.
Keywords: Abstinence, clinical trials, dropout, methamphetamine, pharmacotherapy, retention
INTRODUCTION
Globally, there are an estimated 35.7 million people who use amphetamines and related stimulants recreationally [1]. The physical, psychological, interpersonal and economic harms associated with methamphetamine (MA) use disorder are well documented, and represent significant public health, financial, legal and social challenges [2–4]. There is currently no approved pharmacological substitute or treatment for MA dependence and withdrawal [5,6], highlighting the need to identify additional candidates and continue to conduct clinical trials.
Loss to follow-up rates that are commonly as high as 50% represent a significant challenge in clinical trials for MA use disorder [7]. High rates of dropout can increase study duration and costs and severely harm statistical power and precision [8,9], increasing the likelihood of making Type II errors in hypothesis testing (failing to detect a truly non-null treatment effect). Additionally, loss to follow-up can create selection bias [10] and problems with generalizability, and results may be biased if the ‘missing data’ assumptions of statistical analyses are not met [11–13]. Many trials of potential pharmacological treatments for MA use disorder utilize a form of single imputation where missing urine MA screens are assumed to be MA-positive, which may bias estimates of treatment effects [12].
Several reports have shown a relationship between early treatment response and positive outcomes in clinical trials of substance use treatments [14–18]. Early abstinence is associated with better retention in out-patient behavioral and pharmacological treatment programs for opiate use [19–21]. However, characteristics and motivations for seeking treatment may differ substantially between patients entering treatment programs and those enrolling in clinical trials. Similarly, differences probably exist between individuals who primarily use opiates and those who use MA in their proclivity to remain enrolled in studies.
The aims of this study were to: (1) estimate and test the relationship between achievement of a brief period of abstinence early in study participation and subsequent retention in clinical trials of pharmacological treatments for MA dependence, (2) test whether this relationship was modified by medication treatment, contingency management (CM) or baseline MA use and (3) estimate and test the ‘main effects’ of treatment, CM and baseline MA use on retention. We hypothesized that participants able to achieve a brief period of early abstinence would be more likely to be retained in their respective trials.
METHODS
To address the study aims, we conducted a secondary analysis of pooled data from four completed clinical trials of pharmacotherapies for MA dependence: one trial of sertraline [22], one of modafinil [23] and two of bupropion ([24,25]; referred to hereafter as the bupro1 and bupro2 trials). This study was not a formal meta-analysis of all potentially relevant trials; these four trials were included because they are all completed with major findings published, they share many key design elements and their data could be incorporated readily into a pooled analysis. Ethical approval was approved prior to initiation of all trials, and all participants provided written informed consent before participation.
Study designs
The four trials shared common design elements; all compared active pharmacotherapy conditions versus placebo during a 12-week active medication treatment period. During the trials, all participants provided thrice-weekly urine drug screens. Drug screens were scheduled regularly, and not conducted randomly. The primary outcomes for all trials were treatment effectiveness score, defined as the number of negative urine drug screens achieved during the active medication phase, and end-of-treatment abstinence, defined as having no MA-positive urine screens during the last 2 weeks of treatment and not more than two screens missing.
There were also some differences between the trial protocols. Notably, while the bupro1 and modafinil trials included CM for all participants throughout the active treatment period, the sertraline trial randomized participants to CM or no CM, and the bupro2 trial did not include CM at all. All CM interventions provided food vouchers or gift cards in increasing amounts for consecutive MA-free samples. Missing or MA-positive samples were not awarded vouchers, and the value of the next MA-free urine sample was reduced to the starting amount (see [22–24] for additional details). The sertraline trial also had slightly modified inclusion criteria compared to the others. All subjects in all trials met the DSM-IV criteria for MA dependence; however, in the bupro1, bupro2 and modafinil trials, subjects were also required to provide a MA-positive urine screen prior to enrollment. The urine test was not required in the sertraline trial, resulting in a sample with less frequent MA use at baseline.
Measures
In this analysis, we aimed to evaluate the impact of achieving a brief period of initial abstinence, defined as the number of MA-negative urine screens completed (of six possible) during the first 2 weeks of the active treatment period on the likelihood of retention in the trials. We defined and analyzed retention using two different methods. First, a participant was considered to have dropped out if he or she missed 2 weeks of urine screens in a row, i.e. six consecutive screens, at any point in the study after the first 2 weeks. Defining dropout in this way is consistent with the trial protocols, which all specified that an individual’s participation was terminated if 2 consecutive weeks of study visits were missed. Secondly, we analyzed retention time by computing the number of days retained for each participant using a method that could be standardized across trials. The first urine drug screen was considered to have happened on day zero, and participants were credited with 7 days for each full week they were retained and 2 days for each screen completed during the final week of their participation. Thus, for example, if a participant failed to return after the second screen during the fourth week of the trial, that participant’s retention time was calculated as (3 completed weeks × 7) + (2 additional screens × 2) = 25 days. Using this methodology, the last scheduled screen during the active medication phase of each study (i.e. week 12, screen 3) occurred at day 90, which represents the maximum retention time; participants with less retention time were coded as lost. This variable approximates closely the actual number of days retained in the trials, with slight discrepancies due to different participant start times with respect to weekends, holidays or other periods when drug screens were not scheduled. Other variables included in the analyses were medication treatment (an indicator for active medication group versus placebo), CM (an indicator for CM received versus no CM received), age, sex and baseline MA use, measured as the self-reported number of days used in the month prior to enrollment.
Statistical analyses
Prior to analysis, predictors, outcomes and covariates were described and compared between studies using χ2 tests and analysis of variance (ANOVA). We also analyzed initial abstinence as a function of age, gender, baseline MA use, medication and CM treatments using negative binomial regression, including study as a random effect. Retention time was graphically described using Kaplan–Meier methods.
For the primary analyses, we modeled dropout with a mixed-effects logistic regression model and number of days retained using a mixed-effects Cox proportional hazards model. The primary predictor of interest was initial abstinence, as potentially moderated by medication treatment group, CM or baseline MA use. Covariates were age and sex. Moderation hypotheses were tested by examining P-values for interaction parameters between initial abstinence and the moderating variables. In order to account for heterogeneity between studies in these pooled analyses, study was included as a random effect in logistic regression and Cox models. Additionally, because the value of the initial abstinence variable changed during the time-period where subjects were at risk of dropping out (and indeed, many subjects were not retained for even the first 2 weeks), it was treated as a time-varying covariate in the Cox model.
As secondary analyses, we examined the effects of medication treatment, CM and baseline MAuse on dropout and days retained while excluding initial abstinence from the analyses. All reported test statistics represent single-parameter, one degree of freedom tests. All analyses were conducted in R version 3.2.5 [26] using the gmodels [27], survival [28], lsmeans [29], ggplot2 [30] and lme4 [31] packages.
RESULTS
Seven participants from the sertraline trial were missing covariate data (age, sex or baseline MA use) and were excluded from analyses; all other participants from the trials were included. The final analytical sample size was 440: 212 (48%) from the sertraline trial, 71 from the modafinil trial (16%), 73 from the bupro1 trial (17%) and 84 from the bupro2 trial (19%). Each trial allocated participants randomly to treatment or control in a roughly 1 : 1 ratio, thus the analytical sample included 225 active medication participants (51%) and 215 placebo participants (49%). Fifty-seven per cent of participants (n = 251) received CM.
Participants in the study averaged 35 ± 9.3 years of age and 67% were male (n = 294). Participants self-reported using MA an average of 7.9 ± 9.1 days in the month preceding baseline assessment. In total, 51% (n = 226) of participants dropped out of their trials, and the average number of days retained in the trials was 60.3 ± 30.8. Forty-nine participants (11%) dropped out during the first 2 weeks of study participation. Descriptive statistics for demographic and treatment related variables, in total and stratified by study and treatment group, are presented in Table 1.
Table 1.
Demographic and treatment-related variables by study and treatment group.
| Total (n = 440), mean (SD)/n (%) | Sertraline (n = 212) | Bupropion 1 (n = 73) | Bupropion 2 (n = 84) | Modafinil (n = 71) | Active medication treatment (n = 225) | Placebo (n = 215) | |
|---|---|---|---|---|---|---|---|
| Age | 35.0 (9.3) | 32.7 (7.2)*** | 34.6 (10.3) | 38.4 (10.1) | 38.4 (10.6) | 35.3 (9.4) | 34.7 (9.1) |
| Male sex | 294 (67%) | 129 (61%)** | 47 (64%) | 68 (81%) | 50 (70%) | 148 (66%) | 146 (68%) |
| Baseline MA use (no. of days in past month) | 7.9 (9.1) | 2.1 (2.9)*** | 15.7 (10.5) | 10.1 (6.4) | 14.5 (10.8) | 7.8 (9.0) | 8.0 (9.2) |
| Active medication treatment condition | 225 (51%) | 112 (53%) | 36 (49%) | 43 (51%) | 34 (48%) | – | – |
| Contingency management condition | 251 (57%) | 107 (50%)a | 73 (100%) | 0 | 71 (100%) | 128 (57%) | 123 (57%) |
| Number of MA-negative urine screens during first two weeks of study | 3.0 (2.3) | 3.3 (2.4) | 2.5 (2.4) | 2.9 (2.2) | 3.0 (2.2) | 2.9 (2.3) | 3.2 (2.4) |
| Missed 2 consecutive weeks of drug screens | 226 (51%) | 100 (47%) | 43 (59%) | 42 (50%) | 41 (58%) | 127 (56%) | 99 (46%) |
| Number of days until last screen | 60.3 (30.8) | 61.6 (31.7) | 57.8 (29.3) | 61.3 (30.1) | 57.8 (30.9) | 58.6 (30.2) | 62.0 (31.5) |
P < 0.01,
P < 0.001.
No between-groups tests were performed for this variable because differences were by design. MA = methamphetamine; SD = standard deviation.
On average, participants achieved 3.0 ± 2.3 MA-negative urine screens during the first 2 weeks of the trials. Receiving CM treatment was associated with achieving more negative urine screens [incidence rate ratio (IRR) = 1.25, 95% confidence interval (CI) = 1.07, 1.46)], while increased baseline MA use (IRR = 0.96, 95% CI = 0.95, 0.97) and female gender (IRR = 0.79, 95% CI = 0.67, 0.97) were associated with fewer.
Dropout
A logistic regression analysis was conducted predicting dropout as a function of initial abstinence and medication treatment group, baseline MA use and CM, controlling for age and sex. Initial abstinence impacted the likelihood of dropout significantly, such that each additional negative urine screen during the first 2 weeks of the study reduced the odds of dropout multiplicatively by 41% [odds ratio (OR) = 0.59, 95% CI = 0.53, 0.66]. Interactions between abstinence and treatment (χ2 = 0.60, P = 0.439), abstinence and CM (χ2 = 0.13, P = 0.717) and abstinence and baseline MA use (χ2 = 3.04, P = 0.081) were not significant. Thus, we did not find evidence that the association between abstinence and dropout was modified by treatment, CM or baseline MA use. Table 2 presents model parameter estimates, P-values and confidence intervals, and Fig. 1 presents the adjusted probability of dropout as a function of initial abstinence.
Table 2.
Fixed-effect parameter estimates from multivariable logistic regression analysis of dropout and Cox proportional hazards analysis of number of days retained in the study.
| Parameter | Dropout | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Estimate | SE | Wald p | OR | Lower CL | Upper CL | |
| Intercept | 2.29 | 0.54 | ||||
| Baseline MA use | −0.01 | 0.01 | 0.494 | 0.99 | 0.96 | 1.02 |
| Age | −0.01 | 0.01 | 0.245 | 0.99 | 0.96 | 1.01 |
| Female sexa | 0.02 | 0.25 | 0.936 | 1.02 | 0.63 | 1.66 |
| Placebo treatmentb | −0.37 | 0.22 | 0.096 | 0.69 | 0.44 | 1.07 |
| Contingency managementc | 0.29 | .24 | 0.222 | 1.33 | 0.84 | 2.12 |
| Initial abstinence (1-unit increase)d | −0.52 | 0.07 | <0.001 | 0.59 | 0.53 | 0.66 |
| Parameter | Days retained in study | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Estimate | SE | Wald p | HR | Lower CL | Upper CL | |
| Baseline MA use | −0.005 | 0.007 | 0.450 | 0.99 | 0.98 | 1.01 |
| Age | −0.02 | 0.01 | 0.016 | 0.98 | 0.97 | 0.99 |
| Female sexa | 0.09 | 0.12 | 0.450 | 1.10 | 0.86 | 1.40 |
| Placebo treatmentb | −0.08 | 0.12 | 0.430 | 0.93 | 0.73 | 1.16 |
| Contingency managementc | 0.07 | 0.12 | 0.560 | 1.08 | 0.84 | 1.37 |
| Initial abstinence (1-unit increase)d | −0.29 | 0.03 | <0.001 | 0.75 | 0.71 | 0.80 |
Ref = male sex.
Ref = active medication treatment.
Ref = no contingency management.
Initial abstinence is defined as the number of MA-negative urine screens achieved during the first 2 weeks of the trials. MA = methamphetamine; SE = standard error; CL = confidence limit; HR = hazard ratio; OR = odds ratio.
Figure 1.
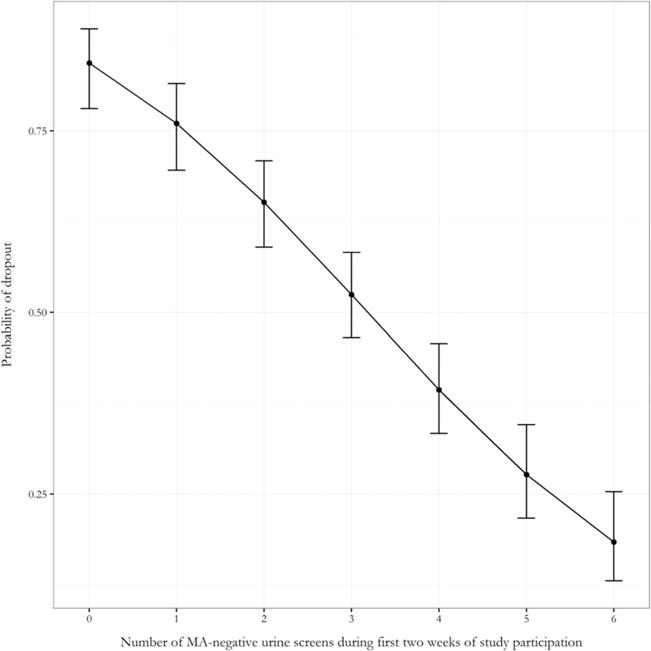
Adjusted probability of dropout (2 vided during the first 2 weeks of the study
Excluding initial abstinence from the model, increased baseline MA use was associated with greater likelihood of dropout (OR =1.04 per each additional day MA was used in the month preceding baseline, 95% CI = 1.02, 1.06) and placebo group participants were less likely to drop out than those in the active medication group (OR = 0.64, 95% CI = 0.43, 0.94). There was no evidence that CM was associated with dropout (χ2 = 0.38, P = 0.539).
Days retained in the trials
In order to describe and visualize retention time, a Kaplan–Meier analysis was performed, stratified by length of initial abstinence period achieved, and the resulting survival curves are presented in Fig. 2. As can be seen in the figure, additional negative urine screens achieved in the first 2 weeks of treatment were associated with longer retention time. The primary analysis of retention time was a Cox proportional hazards model including initial abstinence as a time-varying covariate (see Table 2 for model results). Initial abstinence was associated significantly with retention time; the hazard ratio for dropping out was 0.75 per one additional negative urine screen during the first 2 weeks of treatment, 95% CI = 0.71, 0.80. There was no evidence that abstinence interacted with treatment (χ2 = 0.81, P = 0.368) or CM (χ2 = 0.92, P = 0.336); however, initial abstinence interacted with baseline MA use to predict retention time ((χ2 = 6.30, P = 0.012). The results are shown graphically in Fig. 3; as can be seen in the figure, increased baseline MA use attenuated the relationship between initial abstinence and retention, such that the HR for dropout per 1-unit increase in abstinence approached null as baseline MA use increased. Age was also associated with retention time, such that older participants were less likely to drop out [hazard ratio (HR) = 0.92 per 5-year increase in age, 95% CI = 0.87, 0.98].
Figure 2.
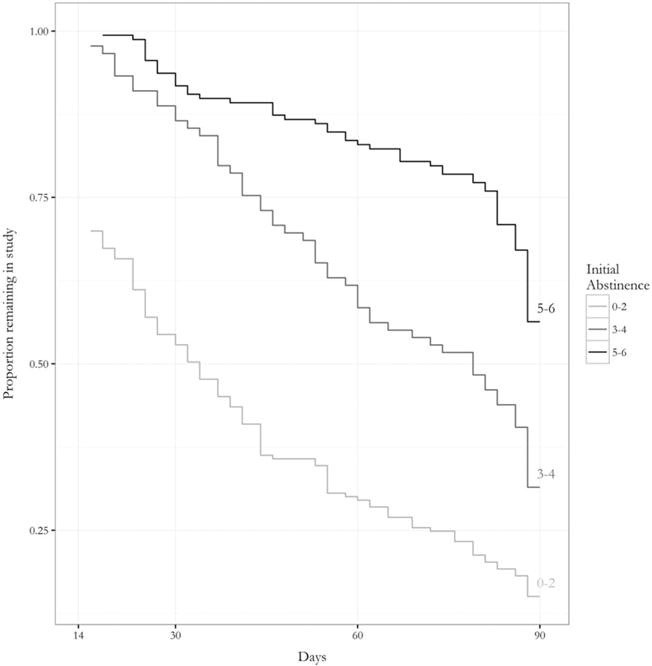
Proportion remaining in the study after week 2 as a function of the number of negative urine screens provided during the first 2 weeks of the study (initial abstinence)
Figure 3.
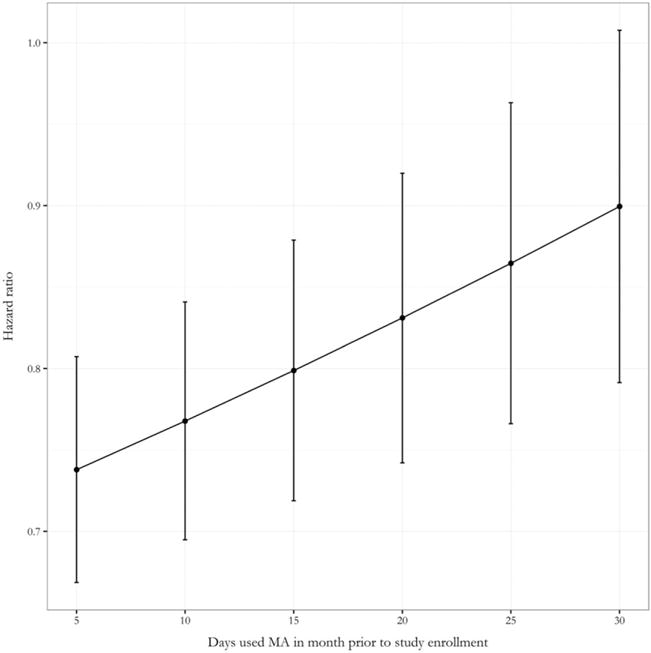
Hazard ratio for dropout per additional MA-negative urine screen during the first 2 weeks of study participation as a function of the number of days MA was used in the month preceding study entry
In a model excluding initial abstinence, baseline MA use was associated with decreased retention time, HR = 1.03, 95% CI = 1.01, 1.04, but there was no evidence that treatment (χ2 = 2.67, P = 0.102) or CM (χ2 = 1.62, P = 0.203) impacted retention time.
DISCUSSION
This pooled analysis of four randomized controlled trials of pharmacological therapies for MA dependence showed a strong impact of early abstinence on retention observed across all trials. When dropout (defined as 2 consecutive weeks of missed urine drug screenings after the first 2 weeks of the study) was analyzed, each additional negative screen during the first 2 weeks of treatment reduced the odds of subsequent dropout multiplicatively by approximately 40%. Similarly, when days retained in the study were analyzed, each additional negative screen during the first 2 weeks of treatment was associated with a relative decrease in the likelihood of non-completion of approximately 25%. The findings of this study suggest that investigators may be able to identify study participants at high risk of dropout by their failure to achieve abstinence during the initial weeks of the trial, providing an opportunity to intervene to increase retention.
Broadly, there are two plausible explanations for these observations, with different implications for intervention. Achievement of a brief period of initial abstinence could be a causal determinant of retention in MA users, either directly or mediated by a phenomenon such as delay discounting, which is common among individuals with MA use disorder [32,33]. Many addicted patients also exhibit the inability to delay gratification, and those able to achieve a brief period of initial abstinence may believe that they are receiving an effective treatment and be more likely to continue participation, whereas those unable to achieve abstinence may lose faith and drop out. If early abstinence is related causally to retention, intervening directly to increase the likelihood of abstinence could increase retention, e.g. a form of compulsory abstinence during the early treatment period, such as an in-patient detoxification phase, may be effective. Alternatively, investigators could focus intensively on relapse prevention during the initial treatment period or employ an adaptive design that initiates additional retention strategies for individuals who demonstrate poor levels of abstinence during the initial part of the trial. An immediately and highly effective medication might also result in high rates of retention among the treated. Conversely, it may be that achievement of initial abstinence has no causal effect on retention and the observed association is the result of a shared common cause, such as readiness to quit MA. In these and other clinical trials where participants enroll for a variety of reasons (i.e. some may not be treatment-seeking), subjects’ levels of readiness or motivation to quit using MA could impact both early abstinence and retention. Early interventions to increase readiness and motivation may thus increase retention.
We did not find that effects differed between participants in the active medication and placebo groups, nor that treatment group predicted initial abstinence independently. None of the study medications were very effective at reducing MA use; as mentioned previously, a highly effective medication may increase both retention and treatment success. Although previous research has shown that contingency management may impact retention positively in substance use treatment [34,35], we failed to find a ‘main effect’ of CM on retention in our analyses. However, we found that CM impacted initial abstinence positively, such that participants receiving CM were more likely to achieve approximately one additional negative urine screen during the first 2 weeks of the trial. Although we cannot rule out confounding of this relationship (CM was randomized in only one trial), our findings suggest that CM may have an indirect positive impact on retention by increasing the likelihood of achieving a period of early abstinence.
Our study also found that higher levels of MA use prior to enrollment increased the likelihood of dropout, decreased the likelihood of achieving initial abstinence and attenuated the initial abstinence–retention relationship. This supports previous findings that heavy MA users are the most challenging to retain in treatment [36] and suggests that even a successful intervention to increase early abstinence may be less effective in retaining those with more severe levels of addiction. Many studies have shown that severity of addiction is associated with worse treatment outcomes among MA users [6,37,38], perhaps reflecting underlying neurobiological deficits or cognitive dysfunction [39–41]. Those with the most severe levels of MA use disorder may also be the ones unable to respond both to study medications and the motivational and incentive interventions to increase retention.
As a secondary data analysis, this study was limited by its inability to assess other factors found to be associated with retention in substance abuse treatment, for example, race/ethnicity [42] or comorbid mental disorders [43,44]. The available data do not allow us to identify whether the effect of abstinence on retention is causal, as we do not have data on all potential confounders (e.g. motivation to quit MA). Thus, we emphasize that these findings represent predictions or associations, not causal relationships. Additionally, our primary predictor, initial abstinence, is confounded with attendance at the study clinic during the first 2 weeks, as participants needed to attend in order to provide a negative urine screen. However, inverting the analysis and examining the number of positive urine screens shows significantly increased risk of dropout with additional positive screens. This suggests that abstinence, and not just attendance, is a key component in retention; simply attending the clinic during the first 2 weeks of treatment is not enough to predict long-term retention. We were also unable to account for differences in the reason for dropout (e.g. loss to follow-up versus voluntary withdrawal), although the vast majority of participants were simply lost to follow-up. In addition, all four trials included in this study had strict inclusion criteria. Results may not be generalizable to treatment settings outside similar clinical trials, although the purpose of this study was to examine retention in such trials.
Although a great deal of effort is dedicated to retaining participants, dropout remains a major challenge in clinical trials involving substance users. However, previous studies have shown that high rates of retention are possible [45–47]. This study found that retention was related strongly to participants’ ability to achieve and maintain early abstinence and moderated by baseline MA use, which may strengthen researchers’ ability to identify those at the greatest risk of dropout and intervene before they are lost. Findings also suggest that MA trials may benefit from more sophisticated study designs; for example, those where the targeted sample size is adjusted based on indicators of trial progress, such as rates of early treatment abstinence. Improving retention rates in clinical trials will undoubtedly aid in the detection of a successful treatment for MA use disorder.
Acknowledgments
K.H. declares that he has received research funding from Pfizer, Medicinova and Alkermes. S.S. declares that he has received supplies for clinical trials from Pfeizer and Medicinova.
Footnotes
Declaration of interests
R.C. and B.Q. declare that they have no conflicts of interest.
References
- 1.United Nations Office on Drugs and Crime. World Drug Report. 2016 Available at: https://www.unodc.org/doc/wdr2016/WDR_2016_Chapter_1.pdf (accessed 6 February, 2017)(Archived at http://www.webcitation.org/6o4sTko92)
- 2.Darke S, Kaye S, McKetin R, Duflou J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev. 2008;27:253–62. doi: 10.1080/09595230801923702. [DOI] [PubMed] [Google Scholar]
- 3.Degenhardt L, Mathers B, Guarinieri M, Panda S, Phillips B, Strathdee SA, et al. Meth/amphetamine use and associated HIV: implications for global policy and public health. Int J Drug Policy. 2010;21:347–58. doi: 10.1016/j.drugpo.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe-Galloway S, Ryan S, Hansen K, Hullsiek B, Muli V, Malone AC. Effects of methamphetamine abuse beyond individual users. J Psychoact Drugs. 2009;41:241–8. doi: 10.1080/02791072.2009.10400534. [DOI] [PubMed] [Google Scholar]
- 5.Courtney KE, Ray LA. Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend. 2014;143:11–21. doi: 10.1016/j.drugalcdep.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brensilver M, Heinzerling KG, Shoptaw S. Pharmacotherapy of amphetamine-type stimulant dependence: an update. Drug Alcohol Rev. 2013;32:449–60. doi: 10.1111/dar.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Mana C, Castells X, Torrens M, Capella D, Farre M. Efficacy of psychostimulant drugs for amphetamine abuse or dependence. Cochrane Database Syst Rev. 2013;(9) doi: 10.1002/14651858.CD009695.pub2. Art. No.: CD009695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Easterbrook PJ, Matthews DR. Fate of research studies. J R Soc Med. 1992;85:71–6. doi: 10.1177/014107689208500206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman LM, Furberg C, DeMets DL, Reboussin DM, Granger CB. Fundamentals of Clinical Trials. New York: Springer; 1998. [Google Scholar]
- 10.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–25. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 11.Sordo L, Bravo MJ, Barrio G, Indave BI, Degenhardt L, Pastor-Barriuso R. Potential bias due to outcome-related loss to follow-up in cohort studies on incidence of drug injection: systematic review and meta-analysis. Addiction. 2015;110:1247–57. doi: 10.1111/add.12940. [DOI] [PubMed] [Google Scholar]
- 12.McPherson S, Barbosa-Leiker C, Burns GL, Howell D, Roll J. Missing data in substance abuse treatment research: current methods and modern approaches. Exp Clin Psychopharmacol. 2012;20:243–50. doi: 10.1037/a0027146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McPherson S, Barbosa-Leiker C, Mamey MR, McDonell M, Enders CK, Roll J. A ‘missing not at random’ (MNAR) and ‘missing at random’ (MAR) growth model comparison with a buprenorphine/naloxone clinical trial. Addiction. 2015;110:51–8. doi: 10.1111/add.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morral AR, Belding MA, Iguchi MY. Identifying methadone maintenance clients at risk for poor treatment response: pretreatment and early progress indicators. Drug Alcohol Depend. 1999;55:25–33. doi: 10.1016/s0376-8716(98)00176-8. [DOI] [PubMed] [Google Scholar]
- 15.Plebani JG, Kampman KM, Lynch KG. Early abstinence in cocaine pharmacotherapy trials predicts successful treatment outcomes. J Subst Abuse Treat. 2009;37:313–7. doi: 10.1016/j.jsat.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDermott KA, Griffin ML, Connery HS, Hilario EY, Fiellin DA, Fitzmaurice GM, et al. Initial response as a predictor of 12-week buprenorphine-naloxone treatment response in a prescription opioid-dependent population. J Clin Psychiatry. 2015;76:189–94. doi: 10.4088/JCP.14m09096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kampman KM, Volpicelli JR, Mulvaney F, Rukstalis M, Alterman AI, Pettinati H, et al. Cocaine withdrawal severity and urine toxicology results from treatment entry predict outcome in medication trials for cocaine dependence. Addict Behav. 2002;27:251–60. doi: 10.1016/s0306-4603(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 18.Brensilver M, Heinzerling KG, Swanson AN, Shoptaw SJ. Placebo-group responders in methamphetamine pharmaco-therapy trials: the role of immediate establishment of abstinence. Exp Clin Psychopharmacol. 2012;20:430–5. doi: 10.1037/a0029210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcovitz DE, McHugh RK, Volpe J, Votaw V, Connery HS. Predictors of early dropout in outpatient buprenorphine/naloxone treatment. Am J Addict. 2016;25:472–7. doi: 10.1111/ajad.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein MD, Cioe P, Friedmann PD. Buprenorphine retention in primary care. J Gen Intern Med. 2005;20:1038–41. doi: 10.1111/j.1525-1497.2005.0228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warden D, Subramaniam GA, Carmody T, Woody GE, Minhajuddin A, Poole SA, et al. Predictors of attrition with buprenorphine/naloxone treatment in opioid dependent youth. Addict Behav. 2012;37:1046–53. doi: 10.1016/j.addbeh.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoptaw S, Huber A, Peck J, Yang X, Liu J, Jeff D, et al. Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85:12–8. doi: 10.1016/j.drugalcdep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Heinzerling KG, Swanson AN, Kim S, Cederblom L, Moe A, Ling W, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109:20–9. doi: 10.1016/j.drugalcdep.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96:222–32. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinzerling KG, Swanson AN, Hall TM, Yi Y, Wu Y, Shoptaw SJ. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014;109:1878–86. doi: 10.1111/add.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Core Team R. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 27.Warnes G, Bolker B, Lumley T, Johnson R. gmodels: Various R Programming Tools for Model Fitting. (R package version 2.16.2). [Google Scholar]
- 28.Therneau T. A Package for Survival Analysis in S, version 2.38. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 29.Lenth RV. Least-squares means: the R Package lsmeans. J Stat Softw. 2016;69:1–33. [Google Scholar]
- 30.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer Science and Business Media; 2009. [Google Scholar]
- 31.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 32.Stevens L, Verdejo-Garcia A, Roeyers H, Goudriaan AE, Vanderplasschen W. Delay discounting, treatment motivation and treatment retention among substance-dependent individuals attending an in inpatient detoxification program. J Subst Abuse Treat. 2015;49:58–64. doi: 10.1016/j.jsat.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berl) 2006;188:162–70. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- 34.Secades-Villa R, Garcia-Rodriguez O, Fernandez-Hermida JR. Contingency management for substance use disorders in Spain: Implications for research and practice. Prev Med. 2015;80:82–8. doi: 10.1016/j.ypmed.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a National Drug Abuse Treatment Clinical Trials Network Study. Arch Gen Psychiatry. 2005;62:1148–56. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- 36.Brecht ML, Greenwell L, Anglin MD. Methamphetamine treatment: trends and predictors of retention and completion in a large state treatment system (1992–2002) J Subst Abuse Treat. 2005;29:295–306. doi: 10.1016/j.jsat.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Dean AC, London ED, Sugar CA, Kitchen CM, Swanson AN, Heinzerling KG, et al. Predicting adherence to treatment for methamphetamine dependence from neuro-psychological and drug use variables. Drug Alcohol Depend. 2009;105:48–55. doi: 10.1016/j.drugalcdep.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elkashef A, Kahn R, Yu E, Iturriaga E, Li SH, Anderson A, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107:1297–306. doi: 10.1111/j.1360-0443.2011.03771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreno-Lopez L, Albein-Urios N, Martinez-Gonzalez JM, Soriano-Mas C, Verdejo-Garcia A. Prefrontal gray matter and motivation for treatment in cocaine-dependent individuals with and without personality disorders. Front Psychol. 2014;5:52. doi: 10.3389/fpsyt.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dean AC, Kohno M, Morales AM, Ghahremani DG, London ED. Denial in methamphetamine users: associations with cognition and functional connectivity in brain. Drug Alcohol Depend. 2015;151:84–91. doi: 10.1016/j.drugalcdep.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moeller SJ, Fleming SM, Gan G, Zilverstand A, Malaker P, d’Oleire Uquillas F, et al. Metacognitive impairment in active cocaine use disorder is associated with individual differences in brain structure. Eur Neuropsychopharmacol. 2016;26:653–62. doi: 10.1016/j.euroneuro.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mennis J, Stahler GJ. Racial and ethnic disparities in outpatient substance use disorder treatment episode completion for different substances. J Subst Abuse Treat. 2016;63:25–33. doi: 10.1016/j.jsat.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Elmquist J, Shorey RC, Anderson SE, Stuart GL. The relationship between generalized anxiety symptoms and treatment dropout among women in residential treatment for substance use disorders. Subst Use Misuse. 2016;51:835–9. doi: 10.3109/10826084.2016.1155612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samuel DB, LaPaglia DM, Maccarelli LM, Moore BA, Ball SA. Personality disorders and retention in a therapeutic community for substance dependence. Am J Addict. 2011;20:555–62. doi: 10.1111/j.1521-0391.2011.00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cottler LB, Compton WM, Ben-Abdallah A, Horne M, Claverie D. Achieving a 96.6 percent follow-up rate in a longitudinal study of drug abusers. Drug Alcohol Depend. 1996;41:209–17. doi: 10.1016/0376-8716(96)01254-9. [DOI] [PubMed] [Google Scholar]
- 46.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 47.Zweben A, Fucito LM, O’Malley SS. Effective strategies for maintaining research participation in clinical trials. Drug Inf J. 2009;43:459–67. doi: 10.1177/009286150904300411. [DOI] [PMC free article] [PubMed] [Google Scholar]


