Abstract
Significance: Redox imbalance may lead to overproduction of reactive oxygen and nitrogen species (ROS/RNS) and subsequent oxidative tissue damage, which is a critical event in the course of neurodegenerative diseases. It is still not fully elucidated, however, whether oxidative stress is the primary trigger or a consequence in the process of neurodegeneration.
Recent Advances: Increasing evidence suggests that oxidative stress is involved in the propagation of neuronal injury and consequent inflammatory response, which in concert promote development of pathological alterations characteristic of most common neurodegenerative diseases.
Critical Issues: Accumulating recent evidence also suggests that there is an important interplay between the lipid endocannabinoid system [ECS; comprising the main cannabinoid 1 and 2 receptors (CB1 and CB2), endocannabinoids, and their synthetic and metabolizing enzymes] and various key inflammatory and redox-dependent processes.
Future Directions: Targeting the ECS to modulate redox state-dependent cell death and to decrease consequent or preceding inflammatory response holds therapeutic potential in a multitude of oxidative stress-related acute or chronic neurodegenerative disorders from stroke and traumatic brain injury to Alzheimer's and Parkinson's diseases and multiple sclerosis, just to name a few, which will be discussed in this overview. Antioxid. Redox Signal. 29, 75–108.
Keywords: : endocannabinoid, neuroprotection, oxidative/nitrative stress, neurodegeneration, free radicals, inflammation
Introduction
Even though the therapeutic properties of the plant Cannabis sativa (marijuana) have been known since ancient times, Δ9-tetrahydrocannabinol (THC), the most abundant psychoactive constituent of hemp, was discovered only in the mid-60s (212). Since this important discovery, our knowledge on cannabinoids has greatly expanded by the identification of G protein-coupled cannabinoid 1 and 2 receptors (CB1 and CB2 receptor, respectively) (69, 204, 229) and by the elucidation of their intracellular signaling pathways (139, 321). Intriguingly, several small lipid mediators have been identified acting as endogenous cannabinoid receptor ligands, including arachidonoyl ethanolamide (or anandamide [AEA]) (70) and 2-arachidonoyl glycerol (2-AG) (211, 320). The synthesis and breakdown of these endogenous mediators have been extensively studied. During the past two decades, endocannabinoids in vitro have also been reported to act on other targets, including nuclear peroxisome proliferator-activated receptors (PPARs) (37), transient receptor potential vanilloid type 1 (TRPV1) (365) channel, and G protein-coupled receptor 55 (GPR55) and G protein-coupled receptor 119 (GPR119) [reviewed by Refs. (107, 270)].
Moreover, novel pathways of endocannabinoid metabolism have been discovered and different lipid mediators [e.g., virodhamine (275), noladin ether (88), endocannabinoid-derived prostanoids, and peptide endocannabinoids] with endocannabinoid-like activity have been identified (172, 173, 220). In parallel with these important discoveries, it also became clear that modulation of the endocannabinoid system (ECS) holds great therapeutic potential in multiple neurodegenerative (and other) diseases [for review, see Refs. (187, 197, 249)]. Furthermore, various constituents of marijuana (e.g., cannabidiol) (116) turned out to have important biological activities with translational potential for the treatment of human diseases (149, 187).
Dysregulation of endocannabinoid production and/or receptor expression have been reported under different pathophysiological conditions, including nociception (2, 290), emotional disorders (80, 114), energy imbalance (59, 81, 305), neurodegenerative diseases (5, 85, 87), cancer (337), diabetes and metabolic disorders (81, 247, 322), and cardiovascular disease (226, 280, 314) among others.
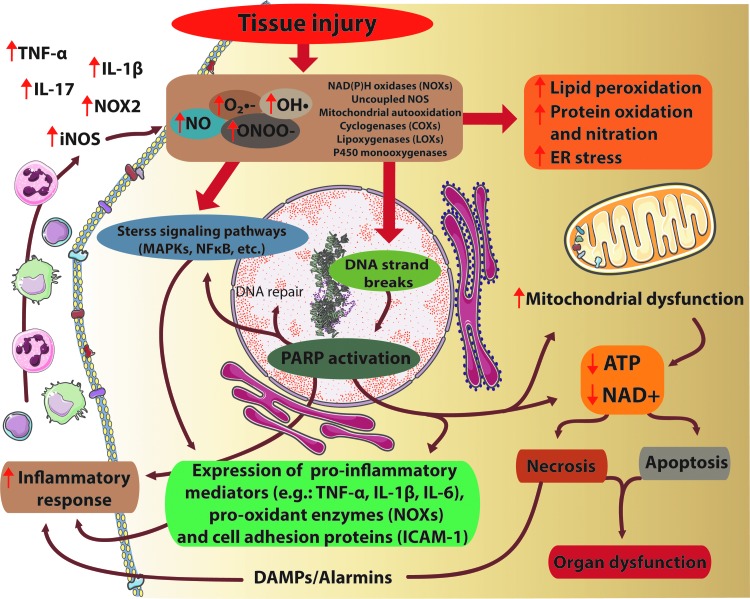
Accumulating evidence implicates enhanced oxidative/nitrative stress in the propagation of neuronal injury and consequent inflammatory response, which in concert promote development of pathological alterations and cell demise, characteristic of most common neurodegenerative diseases (the common mechanisms of redox-dependent processes involved in tissue injury are illustrated in Fig. 1).
FIG. 1.
Interplay of oxidative/nitrative stress, inflammation, and cell death pathways in tissue injury. Tissue injury ultimately results in excessive O2•−, OH• production by NADPH oxidases (NOXs), uncoupled nitric oxide synthase (NOS) enzymes, mitochondrial autooxidation, lipoxigenases, or by the P450 monooxygenase system. Furthermore, in the presence of NO, the reactive free radical peroxynitrite (ONOO−) can form as well. All these agents cause massive cell damage, lipid peroxidation, mitochondrial damage, endoplasmic reticulum (ER) stress, impaired calcium handling, and nitration of tyrosine residue of different proteins. Tyrosine nitration disturbs the structure of proteins, alterations in the catalytic activity of enzymes, and impairs cell signaling pathways. Moreover, ONOO− -induced DNA damage and the subsequent overactivation of the NAD+-consuming poly(APD-ribose) polymerase-1 (PARP-1) enzyme compromise energy homeostasis. These detrimental effects of excessive formation of reactive oxygen/nitrogen species (ROS/RNS) ultimately lead to energy failure, neurotoxicity and neuronal death. On the other hand, ROS can enhance the formation of proinflammatory and pro-oxidant proteins by stimulation of MAPK or NFκB pathways, resulting in enhanced expression of proinflammatory mediators, facilitating the inflammatory response, leukocyte recruitment that contributes to an enhanced NO production by inducible NOS (iNOS), and further aggravation of ROS production. MAPK, mitogen-activated protein kinase; NAD+, nicotinamide adenine dinucleotide; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; NO, nitric oxide; O2•−, superoxide; OH•, hydroxyl radical. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Numerous studies also suggest an important interplay between the endocannabinoid lipid signaling system, plant-derived cannabinoids such as cannabidiol, and various redox-dependent processes (17, 138, 155, 227, 251, 336).
In this review, we focus on the role of the ECS and its therapeutic modulation in different oxidative stress-related neurodegenerative diseases.
Cannabinoid Signaling
CB1 receptor
The first endogenous cannabinoid receptor (CB1) has been originally identified in rat brain samples by Devane et al. (69). CB1 receptors are abundantly expressed in different areas of the brain, including cerebral cortex, hippocampus, caudate-putamen, substantia nigra pars reticulata, globus pallidus, entopeduncular nucleus, and cerebellum, as well as in the spinal cord (143).
The distribution of CB1 receptor in the central and peripheral nervous system reflects its pivotal functions: CB1 receptors are involved in modulation nociception, memory, anxiety, stress, depression, or addiction [reviewed by Ref. (222)]. At the cellular level, CB1 receptors are expressed by both central and peripheral neurons and also by non-neuronal cells such as astrocytes, microglial cells, cerebrovascular smooth muscle, and endothelial cells (25, 90, 101, 139, 281). CB1 receptors, located at central and peripheral nerve terminals, are able to modulate (inhibit) the release of excitatory and/or inhibitory neurotransmitters when activated (139, 161). On the other hand, CB1 receptors found on the postsynaptic neuron are able to control the activity of certain ligand-driven ion channels (e.g., N-methyl-d-aspartate [NMDA] receptors), thereby providing on-demand protection against acute excitotoxicity (202). Interestingly, CB1 receptors have been documented to present in the mitochondria of neurons and might be involved in modulation of neuronal energy homeostasis, revealing a new mechanism of action of CB1 receptor signaling in the brain (23, 125, 196). Moreover, activation of CB1 receptors in cat cerebrovascular smooth muscle cells has been shown to decrease the opening of L-type calcium channels (101), whereas in endothelial cells, it stimulates Ca2+ influx (108), suggesting that endocannabinoids might play an essential role in the regulation of cerebral arterial tone and reactivity in a CB1 receptor-dependent manner (25). Similarly, astrocytes expressing CB1 receptors might be involved in the regulation of local blood flow by producing nitric oxide (NO) and they also have the ability to regulate energy supply of neurons by increasing the rate of glucose oxidation and ketone body utilization [reviewed by Ref. (316)]. Low levels of functional CB1 receptors have been also described in the adipose tissue (59, 81), in liver (199), kidney (111, 155, 228), skeletal muscle and cardiovascular system (16, 177, 189, 226, 252), and certain immune cells, such as macrophages (77, 319).
CB1 receptors belong to the heterotrimeric G protein-coupled receptor superfamily, characterized by having seven transmembrane domains. Activation of CB1 receptors may lead to subsequent inhibition of adenylate cyclase, initiating depletion of intracellular cyclic adenosine monophosphate (cAMP) that results in reduction of protein kinase A (PKA) activity. On the other hand, it has been also reported that CB1 receptor may stimulate adenylate cyclase, thereby being able to increase intracellular cAMP levels (71).
CB1-driven modulation of PKA activity has impact on the activity of mitogen-activated protein kinase (MAPK) cascade that can influence cell fate, depending on the actual circumstances [reviewed by Ref. (36)]. Furthermore, CB1 receptors are also able to control ion channels in the neuronal cell membrane: upon G protein-coupled activation, cannabinoids may positively influence inwardly rectifying K+ currents in a βγ-subunit-mediated manner, resulting in an elevated resting potential in neurons (113, 127). Moreover, neuronal calcium channels can be inhibited by cannabinoids in the same manner (113). Since the expression of CB1 in neurons is largely restricted to presynaptic terminals, the effects of cannabinoids on these ion channels suggest a pivotal role in modulating presynaptic functions and consequent neurotransmitter release (161).
CB2 receptor
The second main CB2 has been initially identified in immune and hematopoietic cells (94, 229). The abundant CB2 expression in immune cells suggests a unique immunomodulatory role of cannabinoids (195). Besides classic immune tissues (thymus, bone marrow, spleen), other peripheral tissues also show CB2 receptor expression [including the liver (158), pancreatic beta cells (156), bone (246), myocardium (18, 67, 219), and vasculature (280)]. Although the presence of CB2 receptors in microglial cells has been previously confirmed, their existence in neurons remains controversial (201, 253, 338, 342). Intriguingly, CB2 expression in microglia appears to be upregulated in certain oxidative stress-related neuropathologies, including Alzheimer's disease, multiple sclerosis (MS), or Parkinson's disease (9, 57, 254, 357); however, little is known about how free radicals are able to induce CB2 receptor expression.
The neuroprotective effect of CB2 agonists is associated with suppression of microglia activation, leading to inhibition of neurotoxic factor release and attenuation of neuronal cell damage (78, 167). Perivascular microglial cells show high CB2 expression, suggesting a potential role of CB2 in the regulation of cerebral blood flow and in preserving the integrity of the blood–brain barrier under neuroinflammatory conditions [reviewed by Ref. (25)].
Activation of CB2 ultimately results in cAMP decline, leading to reduced PKA activity with a consequent decrease in the inhibitory phosphorylation of MAPK cascade (36). By triggering the MAPK pathway, CB2-mediated cannabinoid signaling enables the influence of cell survival, proliferation, or stress response (139). In contrast to CB1 receptors, CB2 is not known to be able to modulate ion channel activity.
Increasing body of evidence suggests that endocannabinoids may serve as ligands for other receptors, including TRPV1 (365), PPARs (37) GPR55 (154), and GPR119 (248); however, the distinct role of these receptors in endocannabinoid signaling needs to be confirmed, particularly in vivo [reviewed by Refs. (22, 107)].
Emerging recent evidence also indicates that both CB1 and CB2 ligands may exhibit biased signaling [e.g., certain ligands for the same receptor could affect distinct signaling pathways (181, 200, 311)]. Better understanding of these important differences may have very important implications for the development of future cannabinoid-based ligands for therapeutic use.
Endocannabinoid Metabolism
AEA, as the first described endocannabinoid, was identified in porcine brain in 1992 (70). The second key endocannabinoid, 2-AG, was discovered in canine intestines in the mid-90s (211, 320). AEA and 2-AG are the most abundant endocannabinoids that can be found in the central nervous system (CNS) as well as in all peripheral tissues.
Although endocannabinoids are thought to be produced on demand in response to certain stimuli, there is some evidence for their intracellular trafficking, storage, and even degradation in adiposomes (133, 160, 244) emphasizing a complex underlying mechanism of endocannabinoid signaling. 2-AG has been suggested to be the main endogenous agonist of CB2 receptors, whereas AEA has higher affinity to CB1 receptors (249). Moreover, it is known that 2-AG appears in higher concentrations in tissues than AEA (321). Although the biosynthesis and metabolism of AEA and 2-AG have been well established, it is still unclear how these endocannabinoids are transported across the cell membrane. Increasing body of evidence suggests that AEA and 2-AG can be taken up by cells via facilitated diffusion in a protein transporter‐mediated manner (132, 208, 242, 352).
Although pharmacological modulators have been designed to influence endocannabinoid uptake (Table 1), proteins that facilitate and control their transport have not been identified yet. Further studies are needed to clarify the underlying molecular mechanism of endocannabinoid transport across the cell membrane and to elucidate the role of other intracellular binding proteins involved in endocannabinoid trafficking [reviewed by Ref. (242)].
Table 1.
Examples of Synthetic or Natural Compounds Modulating Endocannabinoid Metabolism and Signaling
| Name of compounds | Effect on eCB metabolism or CB receptors | Ref. |
|---|---|---|
| Marijuana-derived compounds | ||
| Δ9-THC | Mixed cannabinoid receptor agonists | (253, 270) |
| Cannabidiol | Depending on assay/condition antagonist of CB1/CB2 receptor agonists in CB1- and CB2-expressing cells or tissues | (179, 209, 269–271) |
| Cannabigerol | (46, 130, 270) | |
| THCV | Depending on assay/condition CB2 agonist, CB1 inverse agonist | (269) |
| Endocannabinoids | ||
| Anandamide | Agonists with higher selectivity for CB1 | (253, 270) |
| 2-AG | Agonists with higher selectivity for CB2 | (253, 270) |
| Synthetic compounds | ||
| HU-210 | Mixed CB1/2 agonists | (269, 270) |
| WIN 55,212-2 | (270) | |
| CP 55940 | (269, 270) | |
| ACEA | Agonists with higher selectivity for CB1 | (253, 268, 270) |
| Methanandamide | Agonists with higher selectivity for CB1, FAAH-resistant compound | (268, 270) |
| JWH-015 | Agonists with higher selectivity for CB2 | (253, 268, 270) |
| JWH-133 | (253, 268, 270) | |
| HU-910 | (137) | |
| HU-308 | (253, 268, 270) | |
| AM1241 | (253, 270) | |
| O-1966 | (253, 345) | |
| O-3853 | (362) | |
| LEI-101 | (225) | |
| SR141716A (Rimonabant) | CB1 inverse agonist/antagonist | (253, 270) |
| AM251 | (268, 270) | |
| Taranabant | (90, 270) | |
| SR144528 | CB2 inverse agonists/antagonists | (253, 268, 270) |
| AM630 | (253, 268, 270) | |
| JTE-907 | (253, 268, 270) | |
| PF-3845 | FAAH inhibitors | (32, 323) |
| URB597 | (128, 231) | |
| URB937 | (55) | |
| BIA 10-2474 | (326) | |
| JNJ-42165279 | (162) | |
| JZL-184 | MAGL inhibitors | (272, 359) |
| JZL-195 (dual FAAH, MAGL inhibitor) | (32, 162) | |
| KML29 | (144) | |
| JZP 361 | (262) | |
| URB602 | (56) | |
| AM404 | Inhibitors of the putative endocannabinoid membrane transporters | (221, 242) |
| The farinosone-C analog BSL-34 | (39, 242) | |
| OMDM-2 | (52, 242) | |
| UCM707 | (52, 242) | |
| VDM11 | (66, 242) | |
| KT-109 | DAGL inhibitors | (140, 141) |
| LEI-105 | (12) | |
2-AG, 2-arachidonoyl glycerol; ACEA, arachidonoyl-2-chloroethylamide; eCB, endocannabinoids; CB1, cannabinoid 1 receptor; CB2, cannabinoid 2 receptor; DAGL, diacylglycerol lipase; FAAH, fatty acid amide hydrolase; MAGL, monoacylglycerol lipase; THC, Δ9-tetrahydrocannabinol; THCV, Δ9-tetrahydrocannabivarin.
AEA can be produced by neurons in vitro when stimulated with membrane-depolarizing agents (73). 2-AG can also be released by neurons in a Ca2+-driven manner (317). Virtually all other cell types have been documented to be a source of endocannabinoids in the brain, including astrocytes and microglia, as well as cells of the cerebral vasculature (25, 341, 346). Recent studies also highlight that tissue injury promotes the release of endocannabinoids and eicosanoids (44, 243, 249). Furthermore, in the last few decades, a series of other endogenous small lipid mediators have been identified, evoking cannabinoid-like effects, including the polyunsaturated fatty acid derivatives dihomo-γ-linolenoyl ethanolamide and docosatetraenoyl ethanolamide (121); 2-AG ether (also known as noladin ether) (120); O-arachidonoyl ethanolamine (or virodhamine) (275); and N-arachidonoyl dopamine (NADA) (340). However, these endocannabinoid-like substances are synthesized in very low amounts and are found to be unstable molecules in vivo, with uncertain physiological role.
Additionally, it has been recently documented that the α-hemoglobin-derived peptide hemopressin (Hpα) is able to bind to the CB1 receptor in vitro and exerts CB1 receptor inverse agonistic effects (126). Hpα reduced food intake in rodents in a CB1 receptor-mediated manner (76). This finding suggests that not only polyunsaturated fatty acid derivatives but even certain proteins are able to induce cannabinoid-like effects (20, 109).
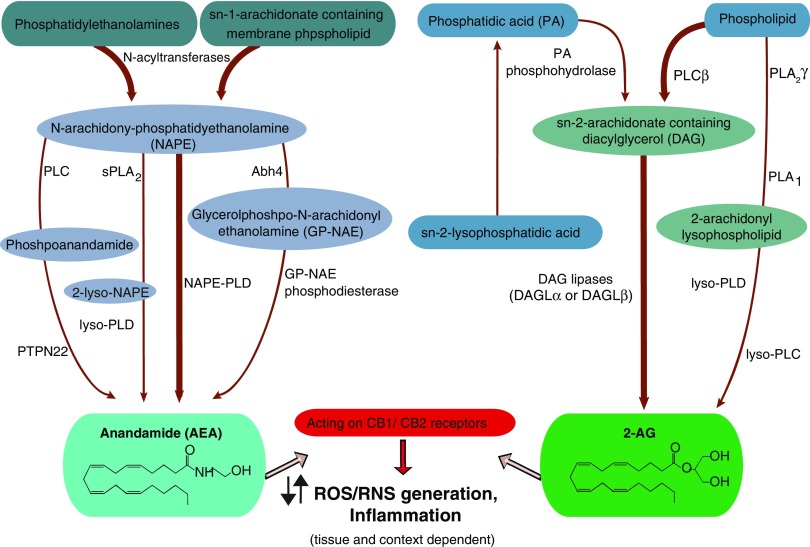
The complex metabolic root of AEA and 2-AG has been investigated in detail [for review, see Refs. (3, 198)]. The biosynthesis of AEA is mainly related to the hydrolysis of its membrane-bound precursor N-acylphosphatidylethanolamine (NAPE) by a phospholipase D (NAPE-PLD) (Fig. 2). This enzyme is able to synthetize a wide range of endogenous ethanolamines.
FIG. 2.
Synthesis of the major endocannabinoids, AEA and 2-AG. The synthesis of AEA starts with the hydrolysis of the precursor N-acylphosphatidylethanolamine (NAPE) by a phospholipase D (NAPE-PLD). AEA can be formed from NAPE in a phospholipase C (PLC)-mediated manner as well, yielding phosphoanandamide, which is then dephosphorylated by the tyrosine phosphatase PTPN22. Moreover, the sequential deacylation of NAPE by α/β-hydrolase 4 (Abhd4) results in the formation of glycerophospho-arachidonoyl ethanolamide. The subsequent cleavage of glycerophosphate residue can also result in the formation of AEA. For the synthesis of 2-AG, the major precursor is the diacylglycerol (DAG), then DAG is converted to 2-AG by diacylglycerol lipase (DAGLα or DAGLβ) enzymes. On the other hand, 2-AG may be formed from other arachidonate-containing DAGs mediated by the calcium-independent phospholipase A2γ (PLA2γ), thereby yielding a 2-arachidonoyl lysolipid that can be further converted to 2-AG by lysophospholipase D (lyso-PLD). Stimulating CB1/CB2 receptors, these endocannabinoids act oppositely in different tissues and might contribute to the formation of ROS/RNS. The most common biosynthetic pathways are indicated by thick arrows. 2-AG, 2-arachidonoyl glycerol; AEA, anandamide; CB1, cannabinoid 1 receptor; CB2, cannabinoid 2 receptor; PTPN22, protein tyrosine phosphatase, nonreceptor type 22. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
There are also other parallel pathways for the generation of AEA such as the phospholipase C (PLC)-mediated hydrolysis of NAPE yielding phosphoanandamide, which is then dephosphorylated by the tyrosine phosphatase protein tyrosine phosphatase, nonreceptor type 22 (PTPN22), and the inositol 5′ phosphatase SHIP1. Bacterial endotoxin-induced synthesis of anandamide in macrophages is mediated in this manner (190). Moreover, the sequential deacylation of NAPE by α/β-hydrolase 4 (Abhd4) results in the formation of glycerophospho-arachidonoyl ethanolamide. The subsequent cleavage of glycerophosphate residue can also contribute to the formation of anandamide (Fig. 2) (308).
The degradation of AEA is mediated by the membrane-linked fatty acid amide hydrolase (FAAH), yielding arachidonic acid and glycerol (Fig. 2). Additionally, increasing number of evidences emphasize that AEA may be metabolized by other enzymes, including cyclooxygenase-2 (COX-2), lipoxygenases, and members of the microsomal cytochrome P450 system [for review, see Refs. (4, 207, 310, 351, 353)]. Consistently, the resulting AEA derivatives are capable of promoting endocannabinoid-like effects (117, 168, 171, 306, 310, 313, 330).
For the biosynthesis of 2-AG, the major precursor is diacylglycerol (DAG), one of the main products of PLC (261); DAG is then further converted to 2-AG by diacylglycerol lipase (DAGL) enzyme (Fig. 2) (30). This enzyme has two different isoforms (DAGLα and DAGLβ) with a distinct tissue distribution pattern (72). 2-AG may also be formed independently from DAGLs: arachidonate containing DAGs can be liberated from the cell membrane by calcium-independent phospholipase A2γ (PLA2γ), thereby yielding a 2-arachidonoyl lysolipid that can be further converted to 2-AG by lysophospholipase D (lyso-PLD) (152).
The serine hydrolase monoacylglycerol lipase (MAGL) is the key enzyme in the conversion of 2-AG to arachidonic acid and glycerol (74), although other enzymes are also thought to be involved in degradation of 2-AG such as the α/β hydrolase domains 6 and 12 (Abhd6 and Abhd12, respectively, see Fig. 3) (238). Similar to AEA, 2-AG may be a substrate for COX-2, for lipoxygenases and for microsomal cytochrome P450 enzymes, suggesting a significant interplay between endocannabinoid and eicosanoid signaling (142, 171–173, 220) (Fig. 3).
FIG. 3.
Catabolism of AEA and 2-AG. The degradation of AEA is driven by the membrane-linked fatty acid amide hydrolase (FAAH) yielding arachidonic acid and glycerol. Then, arachidonic acid can be converted to prostaglandins, leukotrienes, and hydroxyeicosatetraenoic acids (HETEs) or epoxyeicosatrienoic acids (EETs) by COXs, LOXs, and by the microsomal cytochrome P450 system, respectively. On the other hand, AEA might be metabolized by cyclooxygenase-2 (COX-2), lipoxygenases (LOXs), or the members of the CYP 450 as well, yielding different AEA derivatives such as prostaglandin ethanolamine esters, hydroxyeicosatetraenoic ethanolamines (HETE-EAs), or epoxyeicosatrienoic ethanolamines (EET-EAs), respectively. Monoacylglycerol lipase (MAGL) is the key enzyme in the conversion of 2-AG to arachidonic acid and glycerol, although other enzymes are thought to be involved in the degradation of 2-AG such as the α/β hydrolase domains 6 and 12 (Abhd6 and Abhd12, respectively). Similarly to AEA, 2-AG may serve as a substrate for COX-2, for LOXs, and for microsomal cytochrome P450 enzymes (CYP 450) as well, yielding prostaglandin glycerol esters, hydroxyeicosatetraenoic glycerol (HETE-Gs), or epoxyeicosatrienoic glycerol (EET-Gs) derivatives, respectively. Thus, the degradation of endocannabinoids indirectly contributes to the formation of proinflammatory mediators. The most common catabolic pathways are highlighted by thick arrows. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Implication of Endocannabinoid Signaling in Reactive Oxygen Species Formation
Intracellular source of free radicals
On the one hand, the detrimental effect of the excess of reactive oxygen and nitrogen species (ROS/RNS) is well recognized in various types of diseases/pathologies, including but not limited to atherosclerosis, certain neurodegenerative diseases (e.g., Alzheimer's disease), diabetes and its complications, aging, various types of cancers, ischemia/reperfusion (I/R) injury, and inflammatory diseases [see review Refs. (50, 61, 148, 163, 230, 250, 283, 336)].
On the other hand, recent evidence highlights the distinct role of free radicals in different physiological processes such as cell growth (332), proliferation (40), and modulation of the activity of different enzymes and ion channels (15). Accordingly, the role of NO under physiological conditions has been widely studied. NO is physiologically produced by many cells of the brain, including neurons, endothelial cells, or glial cells (astrocytes, oligodendrocytes, and microglia) in a Ca2+-calmodulin-dependent manner. Under normal conditions, neuronal and endothelial nitric oxide synthase (nNOS/NOS1 and eNOS/NOS3) and endothelial cells (eNOS or NOS3) produce a remarkable amount of NO in the nanomolar range, which is essential for maintaining cerebral blood flow. NO is also involved in the regulation of neurotransmission, synaptic plasticity, and modulation of neuroendocrine functions since it can act as a second messenger activating soluble guanylyl cyclase, hence triggering the cyclic guanosine monophosphate-protein kinase G signaling axis (112, 218, 250).
Under various pathophysiological conditions associated with neuroinflammation, large amount of NO is produced in the brain due to the elevated expression of inducible nitric oxide synthase (iNOS/NOS2) in glial cells, infiltrating phagocytes, and vascular cells [reviewed by Ref. (250)]. This event plays a crucial role in the escalation of oxidative–nitrative stress and has deleterious effects on cell viability (Fig. 1).
Elevated NO level can contribute to the generation of peroxynitrite (ONOO−), a powerful oxidant and nitrating species. NO by interacting with superoxide (O2•−) generated by various sources (Fig. 1) leads to the rapid diffusion-limited formation of ONOO− (206, 218). The excessive formation of ONOO− promotes lipid peroxidation, mitochondrial damage, and depletion of glutathione, leading to impaired antioxidant capacity (Fig. 1). Tyrosine nitration by ONOO− disrupts the structure and function of various key proteins with the consequent formation of neoepitopes, alterations in the catalytic activity of enzymes, destruction of cytoskeleton, and impairment of cell signal pathways. ONOO−-induced oxidative DNA damage and the subsequent overactivation of the nicotinamide adenine dinucleotide (NAD+)-consuming nuclear enzyme poly(APD-ribose) polymerase-1 (PARP-1) compromise cellular energy homeostasis (Fig. 1). These detrimental effects of ROS/RNS-mediated cytotoxicity ultimately lead to energy failure, mitochondrial dysfunction, neurotoxicity, and neuronal death (145, 249, 297, 327).
In addition to impaired mitochondria, NADPH oxidase (NOX) enzymes can also be significant sources of ROS during tissue injury, similar to xanthine oxidoreductase, uncoupled eNOS, cytochrome P450 monooxygenases, and lipoxygenases (Fig. 3) [reviewed by Refs. (206, 292)]. A wide range of substances, including the inflammatory mediator arachidonic acid or oxidized low-density lipoprotein particles, can contribute to the association of NOX subunits leading to the consequent generation of O2•− (324). Moreover, superoxide may serve as a precursor for other free radicals, such as hydroxyl radical (OH•) (Fig. 1). Furthermore, OH• can be formed from hydrogen peroxide (H2O2) in an Fe2+-catalyzed nonenzymatic reaction (Fig. 1). Hydroxyl radical is a highly reactive compound compared with O2•−, exhibiting a higher reaction rate with proteins, DNA, or membrane lipids (mainly unsaturated fatty acids), thus it contributes to the generation of more free radicals and propagation of oxidative stress (206, 245, 250).
Interplay between ROS and endocannabinoid signaling
Oxidative stress is implicated in different neurodegenerative complications through the initiation of diverse signaling and transcriptional pathways [reviewed by Refs. (178, 335)]; however, little is known on how expression of enzymes is involved in endocannabinoid synthesis/degradation and cannabinoid receptors modulated/regulated by ROS. Based on a recent report, 2-AG biosynthesis may depend on the presence of NOX-derived oxyradicals (205). The authors have revealed that upon NOX activation, the elevated level of free radicals activated DAGLβ and resulted in an augmented level of 2-AG in macrophages. Nox2-overexpressing COS7 cells synthesized larger amounts of 2-AG when compared with controls (205). In the presence of either NOX or DAGLβ inhibitors in DAGL β-overexpressing COS7 cells, 2-AG levels were reduced (205), indicating a unique interplay between oxyradical production and endocannabinoid synthesis.
Increasing evidence also underscores the significance of endocannabinoid signaling in large number of pathological conditions characterized by enhanced ROS production [see review Refs. (249, 251)]. Activation of vascular CB1 receptors on endothelium and or smooth muscle cells by excessive levels of endocannabinoids during tissue injury may promote activation of p38 and JNK–MAPKs and increase ROS generation, thereby facilitating activation of cell death pathways (281) (Figs. 4 and 5). It has also been shown that AEA enhances prostaglandin E2 (PGE2) and 8-iso-prostaglandin F2α (8-iso-PGF2α) level, similar to free radical formation via enhancing COX-2 activity in lipopolysaccharide (LPS)-challenged astrocytes and microglial cells (237). Recent findings also highlight the ROS-generating effect of angiotensin II in endothelial and vascular smooth muscle cells (VSMCs) (240, 241, 325). A major consequence of angiotensin II receptor type 1 (AT1 receptor) stimulation by angiotensin II is the activation of NOX (240, 241). CB1 receptor activation has been shown to be more pronounced following AT1 receptor stimulation (329), suggesting a costimulatory effect of these two signaling pathways in ROS generation (Fig. 5).
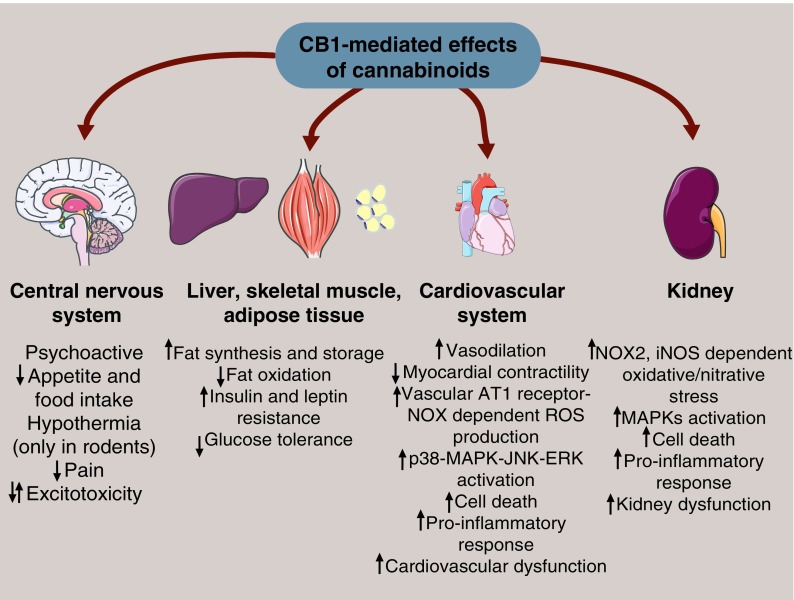
FIG. 4.
CB1-mediated effects of cannabinoids in different tissues. Cannabinoids provoke multiple responses in tissues via stimulating CB1 receptor signaling. Triggering CB1 receptors in the central nervous system has psychoactive features and elicits appetite loss; hypothermia (observed only in rodents) decreases pain sensation and has multiple effects on excitotoxicity according to the cell-specific localization of CB1 receptor. Furthermore, in other organs involved in the regulation of lipid metabolism and energy homeostasis, cannabinoids trigger fat accumulation and decrease fatty acid oxidation, increase insulin and leptin resistance, and decrease glucose tolerance in a CB1 receptor-mediated manner. Moreover, the effect of CB1 receptor activation in the cardiovascular system has detrimental effects on myocardial contractility, decreases blood pressure by the initiation of vasodilation, impairs endothelial function leading to the formation and release of proinflammatory mediators, and facilitates vascular ROS/RNS production in an MAPK-JNK-ERK-mediated manner leading to cell death. Similarly, CB1 signaling in the kidney also leads to renal dysfunction by triggering NOX2 and iNOS expression that results in excessive oxidative/nitrative stress. Enhanced cell death can occur by MAPK activation leading to proinflammatory response. ERK, extracellular signal-regulated kinases; JNK, c-Jun N-terminal kinase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
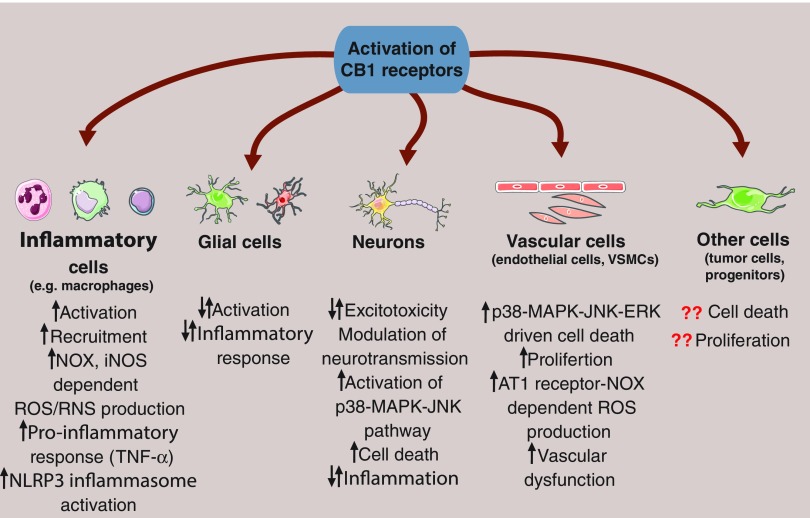
FIG. 5.
Effects of the activation of CB1 receptor at cellular level. Activation of CB1 receptor signaling in presynaptic neurons can modulate neurotransmission by silencing the release of transmitters. Hence, CB1 receptors expressed in glutamatergic neurons can reduce glutamate-induced excitotoxicity, thereby attenuating cell death. In addition, CB1 receptor activation in GABAergic neurons might lead to a depressed inhibitory activity and concomitant overactivation of excitatory circuits. Moreover, CB1 stimulation might lead to p38-MAPK-JNK-dependent cell death and proinflammatory responses. In glial cells, cannabinoids can activate and facilitate inflammatory response, upregulate iNOS, and trigger ROS production. In contrast, attenuated activation and decreased ROS production of glial cells have been reported as well via the CB1-dependent stimulation of the cAMP-PKA axis. Furthermore, CB1 receptor signaling is able to promote the activation of inflammatory cells (e.g., neutrophils and monocytes/macrophages), stimulate their recruitment, initiate ROS/RNS formation in an NOX and iNOS-dependent manner, provoke inflammasome activation, and proinflammatory response. In addition, CB1 receptor signaling promotes p38-MAPK-mediated cell death in the vasculature, enhances proliferation of vascular smooth muscle cells (VSMCs), and facilitates angiotensin II receptor type 1 (AT1)-mediated ROS formation via triggering NOX expression and activity in endothelial cells resulting in vascular dysfunction. However, the role of CB1 receptor activation in other cell types (e.g., neuronal progenitors or tumor cells) is still unclear. Question marks indicate that the presence of CB1 receptor in certain cell types are still questionable. cAMP, cyclic adenosine monophosphate; NLRP3, NACHT, LRR, and PYD domain-containing protein 3 (also known as cryopyrin); PKA, protein kinase A. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Tiyerili et al. have shown that the CB1 receptor inverse agonist/antagonist rimonabant reduces angiotensin II-mediated NOX activity in vivo and in vitro (325). Furthermore, rimonabant decreased AT1 receptor expression in the aortic wall and cultured VSMCs (325).
Several studies also demonstrated that CB1 receptor activation promotes ROS generation and inflammatory response in macrophages, involving p38MAPK activation [reviewed in Ref. (315)].
In contrast, the neuroprotective role of CB1 receptor has been documented in a mouse model of Parkinson's disease (54). This neuroprotection by nonselective CB1 agonists (WIN55,212-2 and HU210) was accompanied by increased survival of nigrostriatal dopaminergic neurons in the striatum, suppression of NOX and ROS production, and reduced expression of proinflammatory cytokines from activated microglia. Similarly, it has been also reported that AEA protected hippocampal neurons from oxidative injury by decreasing intracellular ROS and lowering the expression of NOX2 in a CB1 receptor-mediated manner. All these effects were abolished by simultaneous administration of CB1 antagonist AM251 or by CB1-siRNA (153). Because of this great diversity of results, further studies are required to uncover the contribution of CB1 receptor signaling to ROS formation, which is apparently context and cell-type dependent and may be a secondary/indirect consequence of various processes (Figs. 4 and 5).
Activation of CB2 attenuates vascular inflammation by decreasing endothelial expression of adhesion molecules (e.g., intercellular adhesion molecule 1 [ICAM-1] and vascular cell adhesion molecule 1 [VCAM-1]) and transendothelial migration of leukocytes. It also decreases pathological microglial activation, thereby indirectly attenuating oxidative/nitrative stress and subsequent cell death (253, 280, 314).
An enhanced CB2 expression was demonstrated in cerebral vessels of human brains from subjects with HIV-1 encephalitis (108, 282). In a rodent model of LPS-induced encephalitis, leukocyte-endothelial adhesion was also evaluated on the surface of cortical vessels, which was significantly inhibited by CB2 receptor agonists. CB2 activation reduced the expression of adhesion molecules required for leukocyte recruitment and upregulated tight junction protein expression, thereby improving blood–brain barrier function (267, 282, 289).
CB2 receptor stimulation in CNS was also shown to reduce iNOS expression and NO production in activated microglial cells via inhibition of extracellular signal-regulated kinase (ERK)-1/2 phosphorylation in CNS inflammation (214) (Figs. 6 and 7). The nonselective cannabinoid receptor agonist WIN 55,212-2 inhibited HIV-1 envelope glycoprotein gp120-induced interleukin (IL)-1β production and synapse loss that was reversed by CB2 receptor antagonist (164). These results suggest that CB2 agonists may have therapeutic potential in HIV-1-associated neurocognitive disorder commonly presented in patients with AIDS.
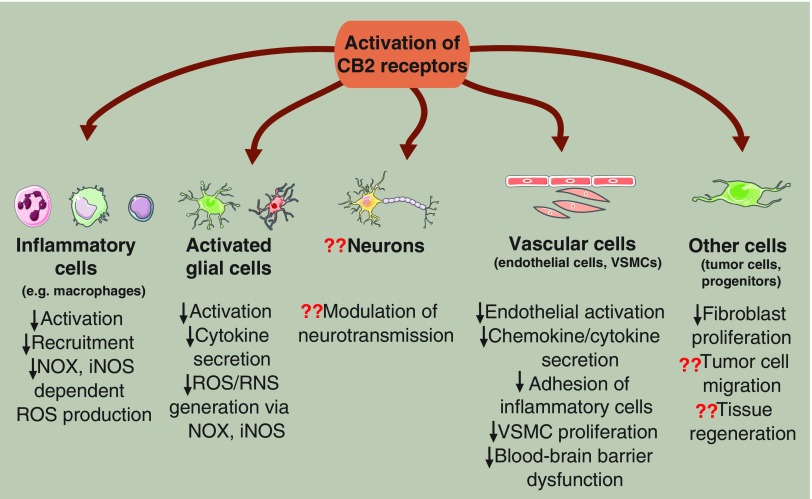
FIG. 6.
Effects of the activation of CB2 receptor at cellular level. Expression of CB2 receptor and its role in the modulation of neurotransmission are still a matter of debate; however, it is well known that CB2 receptor messenger ribonucleic acid (mRNA) levels rise during the activation of microglial and/or astroglial cells. Moreover, stimulation of CB2 receptor signaling is able to attenuate glial activation in neuroinflammation. Furthermore, CB2 activation can reduce the release of cytokines/chemokines (TNF-α, IL-1β) originated from glial cells and diminish the formation of ROS/RNS produced by NOX and iNOS. In addition, CB2 receptor stimulation attenuates leukocyte recruitment and mitigates inflammation and ROS/RNS production in inflammatory cells as well. Moreover, in the vasculature, CB2 receptor activation can attenuate endothelial activation, cytokine/chemokine release, inflammatory cell adhesion and transmigration, VSMC proliferation, and blood–brain barrier dysfunction. In other cell types, it has been reported that fibroblast proliferation can be decreased in a CB2 receptor-mediated manner; however, its role in tissue regeneration or in tumor cell formation and migration is still questionable. Question marks show that the involvement of CB2 receptor in certain processes is still a matter of debate. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
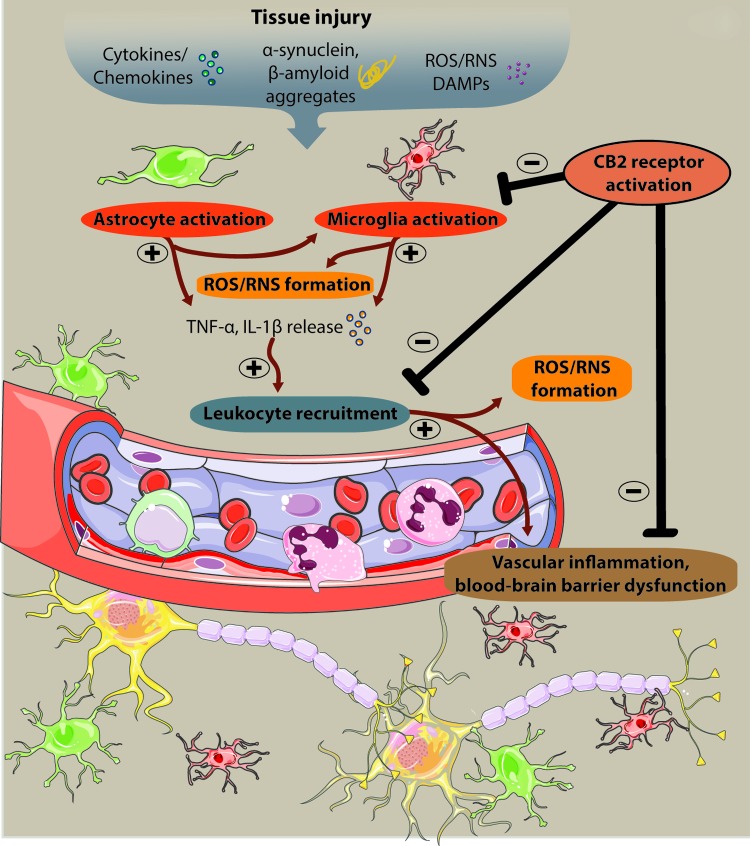
FIG. 7.
The role of cannabinoid signaling in oxidative stress-related neurodegenerative diseases. The involvement of innate immune system in the development and progression of different neuroinflammatory diseases is well described. Activation of microglial and/or astroglial cells is a key event during neuroinflammation and is triggered by numerous factors such as proinflammatory cytokines/chemokines, α-synuclein, β-amyloid aggregates, elevated levels of ROS/RNS, or by damage-associated molecular patterns (DAMPs) derived from necrotic cells. Following glial activation, soluble factors (TNF-α, IL-1β) trigger leukocyte recruitment and contribute to the aggravation of inflammation, production of ROS/RNS, and neuronal death. Hence, drugs targeting CB2 receptors have multiple actions on ROS-mediated neuroinflammation since it can decrease ROS/RNS production in activated glial cells, attenuate vascular inflammation, improve blood–brain barrier function, and prevent leukocyte recruitment, thereby attenuating neuronal cell death. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
All the abovementioned findings emphasize the potent anti-inflammatory effect of CB2 signaling in immune cells, which is associated with attenuated ROS production and reduced neuronal cell death (Figs. 6 and 7).
Notably, the nonpsychoactive marijuana-derived cannabinoid, cannabidiol (CBD), has been shown to exert potent neuroprotective effects against neuroinflammation. CBD was shown to prevent H2O2-induced oxidative damage in neuronal cultures and was more protective against glutamate-induced neurotoxicity than either ascorbate or alpha-tocopherol, emphasizing its potent antioxidant and anti-inflammatory effects (116).
Similarly, Mecha et al. have reported that CBD protected oligodendrocyte progenitor cells challenged by LPS/interferon (IFN)-γ (210). CBD effectively decreased ROS production, caspase-3 activation, and attenuated endoplasmic reticulum stress-induced apoptosis via mechanisms that do not depend on CB1, CB2, TRPV1, or PPARγ signaling (210), suggesting a unique cannabinoid receptor-independent neuroprotective mechanism most likely related to the direct antioxidant and anti-inflammatory effects of this compound.
Role of Endocannabinoids in the Pathophysiology of Oxidative Stress-Related Neurodegenerative Disorders
Stroke
Acute ischemic stroke is one of the leading causes of death worldwide and a prominent cause of hospitalization with acquired disability. Ischemic stroke results from the reduction of cerebral blood flow due to a transient or permanent thrombotic occlusion of a major cerebral artery. A complex cascade of molecular events is activated during cerebral ischemia that eventually leads to neuronal cell death (Fig. 1).
The immediate consequence of ischemia is a significant depletion of tissue adenosine triphosphate (ATP) levels that is associated with dissipation of ionic gradients across the neuronal cell membrane normally maintained by Na+/K+-ATPases. The dysregulation of normal ionic homeostasis ultimately leads to rapid depolarization of neurons and glia, followed by activation of voltage-dependent Ca2+ channels and Ca2+ overload (188). These events induce the release and accumulation of excitatory neurotransmitters (mainly glutamate) in the synaptic cleft, triggering the activation of two distinct ionotropic receptors, the NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. NMDA receptors exacerbate Ca2+ overload allowing inward Ca2+ transport, whereas AMPA receptor activation facilitates intracellular Na+ accumulation and a consequent brain edema (188). The pathological alterations elicited by the actions of glutamate on these receptors are also known as excitotoxicity.
The massive increase in intracellular Ca2+ contributes to the activation of various enzymes, including phospholipases, cyclooxygenases, NOS, and proteolytic enzymes. Moreover, it also stimulates the release of free radicals, which triggers lipid peroxidation, DNA injury, and mitochondrial dysfunction that can eventually jeopardize cellular function and survival (188, 288).
Intracellular calcium overload and oxidative stress promote the activation of different transcription factors, including nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), hypoxia-inducible factor 1 (HIF-1), and signal transducer and activator of transcription 3 (STAT3). Thus, they trigger the production of inflammatory cytokines (tumor necrosis factor alpha [TNF-α], IL-1β), increased expression of iNOS and COX-2, adhesion molecules (ICAM-1, selectins), and the recruitment of activated leukocytes culminating in local tissue inflammation (75).
It has been widely accepted that endocannabinoids can be produced in response to certain stimuli, for example, overactivation of the glutamatergic NMDA and AMPA receptors (119, 159, 161). Recent findings have revealed that cerebral ischemia is associated with the increase of tissue N-acetylethanolamine and endocannabinoid levels; however, the course of presented endocannabinoid mediators highly depends on the model and the type of brain insult (Tables 2 and 3) (82, 98, 118, 232, 236, 257).
Table 2.
In Vitro Evidence of the Interplay of Endocannabinoids and Oxidative/Nitrative Stress
| Model | Changes in eCB level | Changes in cannabinoid receptor expression | Changes in oxidative stress markers following eCB targeted treatment | Ref. |
|---|---|---|---|---|
| Cultured mouse cortical neurons | Not measured | Not measured | CB1/CB2 activation by WIN 55,212-2 or by AEA reduced neuronal cell death and attenuated oxidative stress induced by Fe2+. Protection was abolished by the administration of rimonabant. | (165) |
| PC12 pheochromocytoma cell line | Not measured | Not measured | 6-OHDA-induced cell death was attenuated by AEA. | (217) |
| Rat glioma C6.9 cell line and primary rat cortical astrocytes | Not measured | Not measured | CBD treatment induced apoptosis in glioma cells via stimulation of ceramide synthesis; however, It did not affect viability of primary astrocytes. Long-term CBD incubation prevented H2O2-induced cell loss and abrogated sensitization to oxidative stress in serum-deprived astrocytes. | (45) |
| C6 glioma cell line | Not measured | Not measured | THC dose-dependently increased cell damage and death in the presence of agents generating ROS in a CB1-mediated manner. | (110) |
| Murine microglial BV-2 cell line or primary rat microglia culture | Not measured | High expression of CB2 in both BV-2 cells and primary microglia cells | Both CB1 and CB2 agonists attenuated LPS-induced iNOS expression, NO production and ROS generation. | (287) |
| Microglial BV-2 cell line | Not measured | Not measured | CBD and THC repressed the expression of several proinflammatory genes (CCL2, CCL7, CXCL14, CCL6, and CCL9). Interestingly, the authors found and increased ROS formation in BV-2 cells induced by CBD via the upregulation of genes involved in redox homeostasis. | (157) |
| Microglial BV-2 cell line | Not measured | Not measured | CBD and THC decreased the production and release of IL-1β, IL-6, and IFN-β following LPS challenge. CBD, but not THC, reduced the activity of the NF-κB pathway and activated STAT3 signaling. | (175) |
| Primary mouse microglia culture | Not measured | Not measured | NADA prevented ROS formation and PGE2 generation, whereas AEA exerted the opposite effects in LPS-primed primary microglial cells. | (237) |
| Human primary mDC and pDC | High expression of FAAH in pDC cells derived from MS patients. | High expression of CB2 in mDCs and pDCs originated from MS subjects. | mDCs produced high level of IL-6 and IL-12, whereas pDCs accounted for lower levels of IFN-γ in MS subjects compared with healthy controls. AEA inhibited cytokine production of dendritic cells as well as their ability to generate Th1 and Th17 lineages. | (53) |
6-OHDA, 6-hydroxydopamine; AEA, anandamide; CBD, cannabidiol; CCL, C-C motif chemokine; H2O2, hydrogen peroxide; IFN, interferon; IL, interleukin; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; mDC, myeloid dendritic cell; MS, multiple sclerosis; NADA, N-arachidonoyl dopamine; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; NO, nitric oxide; pDC, plasmacytoid dendritic cell; PGE2, prostaglandin E2; ROS, reactive oxygen species; STAT3, signal transducer and activator of transcription 3; Th1, T helper 1; Th17, T helper 17.
Table 3.
Implication of Targeting the Endocannabinoid System in Oxidative Stress-Related Neurodegenerative Diseases
| Model | Changes in eCB level | Changes in cannabinoid receptor expression | Drug used | Observation | Changes in oxidative stress markers following eCB targeted treatment | Ref. |
|---|---|---|---|---|---|---|
| Mouse model of permanent focal cerebral ischemia | Not measured | Not measured | Not used | Increased infarct volume was found in CB1−/− mice compared with wild-type mice. | Not measured | (259) |
| Rat model of cerebral ischemia | Not measured | Not measured | CB1 activation by HU-210 | HU-210 decreased motor disability and infarct volume that were abolished by the administration of rimonabant. | Not measured | (185) |
| Significantly elevated brain AEA level. 2-AG was slightly increased | Not measured | CB1/CB2 activation by WIN 55,212-2 CB1 blockade by either rimonabant or LY320105 | Rimonabant or LY320105 reduced cerebral infarct volume and neuronal cell death, whereas the administration of WIN 55,212-2 was ineffective. | Not measured | (232) | |
| Not measured | CB1 receptor expression was unchanged in cultured cortical neurons following hypoxia | CB1/CB2 activation by WIN 55,212-2 | WIN 55,212-2 treatment reduced cerebral infarct volume and neuronal cell death. Improved neurological function was also noted. | Not measured | (233) | |
| Mouse model of cerebral (I/R) injury | Not measured | Not measured | CB2 activation by O-1966 | The treatment improved neurologic function and reduced brain edema induced by transient mid cerebral artery occlusion. | O-1966 reduced inflammatory responses and leukocyte recruitment. | (356) |
| Rat and porcine model of brain hypoxia | Not measured | Not measured | CBD | CBD treatment improved neuronal cell survival in newborn rats exposed to short-term hypoxia | Reduced expression of inflammatory mediators (TNF-α), improved antioxidant capacity. | (263, 264) |
| Mouse model of TBI | Elevated brain 2-AG following CHI | Not measured | Exogenous 2-AG or rimonabant | 2-AG exerted neuroprotection by inhibiting the early inflammatory response, improved blood–brain barrier function. Moreover, administration of 2-AG improved neurological performance and decreased edema that was completely abolished in CB1−/− mice. | 2-AG inhibited TNF-α, IL-1β, and IL-6 expression and improved brain antioxidant capacity after CHI. | (255, 256) |
| Not measured | Not measured | CB2 receptor stimulation with 0–1966 and JWH-133 | CB2 stimulation reduced blood–brain barrier permeability and suppressed microglial activation and monocyte recruitment. | CB2 receptor agonist decreased TNF-α protein level, attenuated vascular ICAM-1 expression, and decreased iNOS expression. | (6, 79) | |
| Not measured | Not measured | MAGL inhibitor JZL-184 | Inhibition of 2-AG metabolism promoted neurologic recovery: spatial learning and memory were improved following TBI | JZL-184 treatment reduced the production of proinflammatory cytokines, astroglial activation. | (359) | |
| Drug treatment increased brain AEA level | Not measured | The selective FAAH inhibitor PF3845 | PF3845 attenuated TBI-induced anxiety, reduced deficits in hippocampus-dependent memory performance and in fine motor coordination via activation of CB1 and CB2 receptors | PF3845 upregulated the expression of the antiapoptotic Bcl-2 and Hsp70/72 proteins, decreased caspase-3 activity, and suppressed COX-2 and iNOS expression | (323) | |
| Mouse model of experimental MS | Not measured | Not measured | Dexanabinol | Dexanabinol significantly reduced maximal EAE score when given once at the onset of disease. | Dexanabinol reduced inflammatory response in the brain and spinal cord in ALS mice. | (1) |
| Not measured | Not measured | CB1−/− mice | Neurofilament and myelin basic protein levels decreased over the course of experimental ALS. In addition, concomitant neuronal/axonal loss and demyelination were observed. | CB1−/− mice displayed increased Caspase-3 activation | (150) | |
| Not measured | Not measured | CBD | Administration of CBD at the onset of EEA reduced axonal loss, infiltration of T cells, and microglial activation. | CBD treatment was accompanied instead reduced microglial activation and T cell recruitment in the spinal cord. | (174) | |
| Mouse model of experimental ALS | Increased spinal 2-AG and AEA level | Not measured | Not used | Progressive neuronal damage increased endocannabinoid production in ALS mice. | Not measured | (347) |
| AβPP/PS1 mice and wild-type littermate | Not measured | Not measured | CB2 receptor activation by JWH-133. | JWH-133 induced cognitive improvement in AβPP/PS1 mice. | JWH-133 reduced microglial IL-1β, IL-6, IL-10, and TNF-α production increased 4-HNE content and enhanced the expression of SOD1 and SOD2 around senile plaques. | (10) |
| Mouse model of Alzheimer's disease induced by β-amyloid fragment injection | Increased hippocampal 2-AG, but not AEA, level | Enhanced CB2, but not CB1, expression in the treated hemisphere | The endocannabinoid reuptake inhibitor VDM11 | β-Amyloid fragment injection significantly increased hippocampal cell loss. VDM11 improved memory retention. | VDM11 treatment decreased iNOS, COX-2, and Caspase-3 expression following β-amyloid fragment injection. | (333) |
| Postmortem human brain tissues from patients with Alzheimer's disease | Enhanced FAAH expression in glial cells associated with senile plaque | Enhanced CB2 expression in glial cells associated with senile plaque | Not used | FAAH and CB2 receptors have an overexpressed feature in glial cells linked to the inflammatory process that accompanied with Alzheimer's disease. | Not measured | (24) |
| Rodent model of experimental Parkinson's disease induced by 6-OHDA | Not measured | CB1 receptor expression was unchanged in lesioned brain regions. CB2 expression was not measured | THC/CBD treatment | Chronic administration of either THC or CBD significantly decreased neurodegeneration produced by a unilateral injection of 6-OHDA into the medial forebrain bundle. | Not measured | (184) |
| Not measured | Not measured | ACEA; WIN 55,212-2; HU-308; AM-404; or CBD treatment | Daily administration of the CB1 agonist ACEA or WIN 55,212-2 did not reverse neuronal damage, whereas HU-308 produced a slight recovery. AM-404 produced a marked recovery of 6-OHDA-induced depletion. | SOD expression was increased by CBD in the affected region compared with vehicle control. | (97) | |
| Mouse model of experimental Parkinson's disease induced by MPTP | Not measured | Not measured | The nonselective cannabinoid receptor agonist WIN 55,212-2 | WIN 55,212-2 exerted neuroprotection against MPTP-induced loss of nigrostriatal dopaminergic neurons This protection was independent from CB1 receptor activation. | Not measured | (276) |
| Not measured | Not measured | WIN 55,212-2 and HU-210 treatment | WIN 55,212-2 and HU-210 attenuated nigrostriatal neuronal cell loss. | Decreased NOX activation, reduced IL-1β and TNF-α expression levels were observed mediated via CB1 receptor. | (54) | |
| Mouse model of experimental Huntington's disease induced by malonate injection | Not measured | Increased expression of CB2 following injection of malonate | ACEA; HU-308 or CBD treatment | HU-308 and CBD treatment attenuated malonate-induced cell loss likely through a mechanism involving glial cells. ACEA treatment failed to improve cell death. | Activation of CB2 receptors significantly reduced the levels of TNF-α. | (295) |
4-HNE, 4-hydroxynonenal; ACEA, arachidonoyl-2-chloroethylamide; ALS, amyotrophic lateral sclerosis; Bcl-2, B cell lymphoma 2 protein; CHI, closed head injury; COX-2, cyclooxygenase-2; EAE, experimental autoimmune encephalomyelitis; Hsp70/72, heat shock protein 70/72; I/R, ischemia/reperfusion (injury); MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NOX, NADPH oxidase; TBI, traumatic brain injury; TNF-α, tumor necrosis factor alpha.
Although initial studies described an endocannabinoid-driven neuroprotection against cerebral ischemia, the exact role of endocannabinoids in focal ischemic insult is still controversial. Shen and his coworkers have reported cannabinoid receptor-mediated silencing of excitatory neuronal activity in cultured rat hippocampal neurons (302). The nonselective cannabinoid receptor agonist WIN 55,212-2 reduced neuronal cell death in a CB1-dependent manner via attenuated presynaptic glutamate release, thereby decreasing excitotoxicity and cell death (115, 161, 165, 302). WIN 55,212-2 was also able to reduce infarct volumes in vivo when administered before the induction of both global and focal cerebral ischemia (233).
Interestingly, activation of the recently discovered mitochondrial CB1 receptor by using arachidonoyl-2-chloroethylamide (ACEA) has been shown to exert neuroprotection against ischemia/reperfusion injury via decreasing oxidative stress, lactate dehydrogenase release, and caspase-3 activation in vitro, as well as by ameliorating neurological scores in vivo. ACEA-induced neuroprotection was completely abolished by the selective cell-permeant CB1 receptor antagonist AM251, but only partially diminished by the selective cell-impermeant CB1 receptor antagonist hemopressin (196), suggesting that mitochondrial CB1 expression plays a unique role in cannabinoid-driven neuroprotection and might directly regulate mitochondrial ROS formation under this pathological condition.
Furthermore, it has been also documented that CB1 activation by using another agonist (HU-210) conferred neuroprotection in a permanent focal middle cerebral ischemia model (185). Infarct volumes and motor disability were significantly reduced in animals treated with HU-210. The neuroprotective effect of HU-210 was partially reversed by coadministration of rimonabant. However, it was completely abolished by maintaining the core temperature of treated animals to the levels observed in controls, suggesting that it was mediated by CB1-dependent hypothermia. Even though cannabinoids are able to lower blood pressure, reduction of cerebral blood flow was not observed in the border zone by the CB1 receptor agonist HU-210 following permanent middle cerebral artery occlusion (185).
Consistently, it has been also documented that CB1−/− mice had increased mortality, increased infarct volume, and severe neurological defects following permanent focal cerebral ischemia. The excitation-induced neurotoxicity was also more severe in CB1-deficient mice compared with their wild-type littermates (259).
In contrast, evidence supporting the detrimental effect of CB1 signaling following transient middle cerebral artery occlusion has been shown by Muthian et al. (232). The authors have revealed that the detrimental effect of CB1 receptor activation was mediated by the acutely elevated brain AEA levels following the insult. They also showed that administration of CB1 receptor inverse agonist/antagonist rimonabant before middle cerebral ischemia exhibited a significant reduction in infarct volume and a remarkable improvement in neurological function compared with the vehicle-treated group. In line with this finding, others have also shown the neuroprotective effect of CB1 receptor blockade in focal cerebral ischemia models emphasizing different pathways in the protection of rimonabant against cerebral ischemia (265, 361). The plausible explanation for the opposing effects of CB1 inhibition versus studying CB1 knockouts is still unclear (could be, in part, explained by poor temperature control and central hypothermic effects of CB1 agonists in rodents); however, further studies are required for the clarification of actual status of CB1-related endocannabinoid signaling in cerebral ischemia.
Concerning the effect of CB2-mediated signaling, it has been documented in an elegant study performed by Zhang and his coworkers that activation of CB2 receptors by using CB2 agonist, O-1966, reduced inflammatory responses (leukocyte adhesion and invasion) and improved neurologic function following insult in cerebral ischemia/reperfusion injury (Figs. 6 and 7). Moreover, CB2 receptor knockout mice showed larger cerebral infarct volumes and worse neurological function compared with wild-type mice (360).
In another study, the coadministration of rimonabant and O-1966 exerted greater protection against cerebral ischemia/reperfusion injury compared with CB2 receptor activation alone, although the neurological scores were rather similar in these groups (361). Consequently, CB2 receptor activation exerted neuroprotective effects through the attenuation of cerebral microcirculatory dysfunction and activation of microglial cells, with consequent proinflammatory response and oxidative/nitrative stress. Thus, CB2 receptor-driven signaling can indirectly attenuate ROS production and oxidative/nitrative (Figs. 6 and 7) stress following cerebral ischemia/reperfusion injury (249, 267, 280, 282, 289).
The neuroprotective effect of CBD has been evaluated in focal cerebral ischemia models as well. Hayakawa et al. have reported that the neuroprotective effect of THC is manifested mainly via the CB1 receptor and consequent temperature-dependent mechanisms, whereas the protective effects of CBD are independent of CB1 and of hypothermia (122). Moreover, CBD mitigated impaired cerebral microcirculation after reperfusion, inhibited myeloperoxidase (MPO) activity in neutrophils, (124), and inhibited high-mobility group box1 protein (HMGB1) expression and release from monocyte/macrophages, as well as glial activation induced by cerebral ischemia (123), suggesting a direct anti-inflammatory and an indirect antioxidant property of this plant-derived cannabinoid.
Traumatic brain injury
Traumatic brain injury (TBI) is one of the leading causes of morbidity and mortality among young individuals with limited therapeutic options (21, 134). The acute brain damage presented after primary injury as the consequence of an external mechanical force results in contusion, laceration, and diffuse injuries. Secondary injury immediately presented after primary damage is accompanied with the activation of a complex cascade of molecular and cellular immune responses leading to neuroinflammation, excitotoxicity, oxidative/nitrative stress, and impaired calcium homeostasis, mitochondrial dysfunction, neuronal injury, and death. Moreover, post-traumatic changes in cognitive and neurological function might be presented as well [see review Refs. (58, 213, 309, 355)].
Inflammation has been shown to be a key player in TBI (355). Proinflammatory markers, including IL-1β, IL-6, and TNF-α, are released from activated astrocytes and/or microglial cells that engage leukocyte recruitment and exacerbate brain injury (Fig. 6). It has been also revealed that the extent of neuroinflammatory response in TBI closely correlates with the outcome (304, 349, 355). Recent findings also highlight that the repetitive head injury in athletes or in war veterans is associated with the development of brain atrophy, β- amyloid plaque formation, tau phosphorylation, and TDP-43 pathologies. All these events can contribute to the presence of clinical syndromes of cognitive impairment (35, 309).
Cannabinoids have been proposed to be neuroprotective against TBI. Previously, studies demonstrated that both AEA and 2-AG levels are increased in the brain following closed head injury (CHI) (304). Administration of exogenous 2-AG was able to attenuate CHI-induced neuropathology that was partially abolished by the administration of rimonabant. Moreover, 2-AG moderately improved neurological performance and attenuated edema formation when administered to CB1−/− mice subjected to CHI, suggesting that 2-AG-driven neuroprotection is not solely mediated via CB1 receptors (255). Exogenous 2-AG also decreased blood–brain barrier permeability and suppressed the acute expression of IL-1β, IL-6, and TNF-α and augmented the levels of endogenous antioxidants (256), indicating a unique role of 2-AG in TBI as an endogenous signaling mediator maintaining brain homeostasis against harmful insults (257, 358).
Along with these findings, it has been recently described that CB1 receptor expression decreases in the affected brain area after TBI and negatively correlates with edema formation and behavioral impairments (191). In contrast, an acute mitochondrial CB1 receptor upregulation has been documented after TBI proposing a dual role of mitochondrial CB1 signaling. First, it might aggravate metabolic defects and energy failure by inhibition of the mitochondrial cAMP-PKA signaling axis. Second, CB1 activation mitigates energy insufficiency by acting on the ATPase complex and attenuates neuronal apoptosis via activation of the mitochondrial protein kinase B (AKT) pathway (350).
Regarding CB2 receptor signaling, it should be noted that an increased CB2 receptor expression has been found after TBI that was associated with high neurological deficit, whereas no correlation with edema was noted (191). It is apparently due to activation of microglial cells that upregulate CB2 upon stimulation. Accordingly, activation of CB2 receptor signaling by using agonists 0–1966 and JWH-133 was shown to be neuroprotective in TBI by attenuating neuroinflammation via decreasing the mRNA level of iNOS in macrophage/microglia and reducing TNF-α level in the injured cortex, whereas pharmacological blockade or genetic deletion of the CB2 receptor worsened inflammation (6, 79). These findings support earlier observations that modulation of CB2 signaling decreases macrophage infiltration and microglial activation that might be an important target of further preclinical investigations (Figs. 6 and 7).
Along with the beneficial effect of CB2 receptor activation upon TBI, inhibition of the major 2-AG-metabolizing enzyme MAGL by using the inhibitor JZL-184 exerted neuroprotection via increasing brain 2-AG level and reducing the formation of proinflammatory prostaglandins in a repetitive mild CHI model (359). Additionally, TDP-43 aggregation and tau phosphorylation were also attenuated by MAGL inactivation.
Inhibition of FAAH by using the selective inhibitor PF-3845 has been shown to exert neuroprotection via increasing endogenous AEA level in mouse model of TBI (323). The authors revealed that chronic treatment with PF-3845 following TBI can reverse impairments in fine motor movement, memory, and anxiety-like behavior. In addition, it reduced neurodegeneration in the dentate gyrus and upregulated the expression of both B cell lymphoma 2 protein (Bcl-2) and heat shock protein 70/72 (Hsp70/72) in cortex and hippocampus, thereby improving cell survival in these areas. PF-3845 also suppressed the formation of amyloid precursor and the expression of iNOS and COX-2 (323).
With regard to human data, there are only few studies exploring the effect of cannabinoids (particularly CBD or THC) on neurological indices in patients with TBI. Nevertheless, according to recently published data assessing the effect of THC consumption on outcomes in TBI, a positive correlation has been observed between THC consumption and decreased mortality in adult patients with TBI (239).
In contrast, it has been also concluded by others that cannabis use might act as a neurological stressor and represent a risk factor following TBI in late adolescent and young individuals (278).
Taken together, neuroinflammation plays an important role in the propagation of neuronal damage following TBI. Therefore, reduction of proinflammatory processes, including microglial activation, blood–brain barrier dysfunction, leukocyte recruitment, and attenuation of oxidative/nitrative stress, might be an important therapeutic aim. Targeting CB2 signaling offers an alternative approach to improve these outcomes. In contrast, the exact role of CB1 receptor signaling in TBI needs to be further elucidated by utilizing rigorous temperature control.
Multiple sclerosis
Multiple sclerosis (MS) is an immune-mediated inflammatory disease causing randomly formed focal damage in the brain, affecting both the gray and white matter of the CNS. Increasing number of evidences highlight the role of the innate immune system in development and progression of MS [reviewed by Ref. (96)]. During the progression of MS, IFN-γ-secreting T helper 1 (Th1) cells and IL-17-secreting Th17 cells infiltrate the affected region of CNS, initiating microglial activation and local immune response that eventually lead to neuronal myelin sheath loss, impaired conductive properties, and axonal loss (62). Even though drugs targeting the immune system are capable of attenuating the progression of this disease in certain cases, there is no curative treatment for MS. Inflammatory plaques in MS patients show increased immunoreactivity for iNOS and nitrotyrosine (38, 131) associated with high levels of nitrate and nitrite in urine and serum (104, 105). The nitration of tyrosine residues in proteins of the blood–brain barrier and consequent dysfunction allow the further influx of inflammatory cells (64, 135).
Cannabinoids have a unique anti-inflammatory potential in the development and progression of MS (Tables 1 and 2). In the chronic phase of the disease, which is characterized by a profound neurodegeneration and hind limb spasticity in animal models, elevated AEA, palmitoylethanolamide (PEA), and 2-AG levels have been reported in response to neuronal damage (13, 14). Interestingly, spasticity was rapidly increased after administration of rimonabant, suggesting that alleviation of hind limb spasticity is CB1 dependent and can be a part of an endogenous compensatory mechanism mediated by the elevated level of endocannabinoids (53, 277). Accordingly, exogenous administration of AEA and 2-AG and N-acylethanolamine PEA has been shown to ameliorate the development of this disease in a mouse model of experimental MS (also known as experimental autoimmune encephalomyelitis [EAE]) (14, 53).
The rate of neurodegeneration in MS was decreased by the administration of CB1 receptor agonists (60). Consistently with this, CB1 receptor-deficient mice with EAE displayed more rapid neurodegeneration. These studies suggested that the neuroprotective role of CB1 signaling may be manifested via the inhibition of glutamate-induced excitotoxicity and oxidative stress (203, 277), which are important contributors to the course of neurodegeneration in MS (274).
Recent studies also showed that the CB2 receptor agonist, JWH-133, ameliorated tremor and spasticity in EAE mice (13). Similarly, treatment with another CB2 agonist, HU-308, attenuated the development of EAE in mice (301). In the latter study, CB2 receptor activation promoted autophagy in the spinal cord of EAE mice and inhibited the expression and activation of NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) inflammasome in BV2 microglia cell line in vitro (301). Consistently with these observations, CB2−/− mice displayed greater vulnerability to neurofilament degeneration, inflammation, apoptosis, and axonal damage in EAE mice similarly to CB1-deficient mice (150, 254, 294).
Additionally, a protective effect of the MAGL inhibitor, JZ-L184, was documented in cultured oligodendrocytes as well as in EAE mice. JZL-184 suppressed cell death by mild activation of AMPA receptors in oligodendrocytes in vitro and preserved the integrity of myelin sheath and suppressed microglial activation in vivo (26). This effect was imitated by the administration of 2-AG and by AEA in a concentration-dependent manner. Interestingly, inhibition of the AEA-metabolizing enzyme FAAH with URB597 was ineffective in attenuating oligodendrocyte cell death. The authors concluded that oligodendrocyte protection from excitotoxicity of MAGL blockade results in the reduction of AMPA-induced cytosolic calcium overload, mitochondrial membrane depolarization, and production of ROS (26). It should be also noted that chronic MAGL inhibition is associated with downregulation/desensitization of brain CB1 receptors (298), thus the role of MAGL in the context of MS needs to be further elucidated.
As for the nonpsychoactive natural cannabinoids, it has been shown that CBD ameliorated clinical signs of EAE that was accompanied by diminished axonal damage and inflammation, microglial activation, and T cell recruitment (174). However, a recent finding indicates that coadministration of PEA with CBD was not as effective in EAE compared with each drug administered alone, indicating that there is an antagonistic interaction between CBD and PEA in protection against EAE (279). THC has also been shown to diminish the progression of MS in EAE by attenuating the inflammatory process, which appears to be CB1 dependent (93). Similarly, the beneficial effect of the nonpsychotropic synthetic cannabinoid dexanabinol (HU-211) was also evaluated on EAE (1). The authors reported a reduction in the inflammatory response in the brain and spinal cord in EAE mouse treated with HU-211.
The abovementioned results encouraged clinicians to test the feasibility of THC/CBD in reduction of the rate of neurological damage and refractory spasticity both in acute and chronic phases of MS (89, 192, 300). However, the results of these clinical trials were inconsistent, and clearly more investigations are required with greater sample size to evaluate whether cannabinoids can lower the progression of this disease.
Collectively, diminution of inflammation during the course of MS is an important therapeutic approach. Drugs inhibiting TNF-α release, reducing edema, free radical production, and improving blood–brain barrier integrity might be useful therapeutically to slow down the rate of disease progression in MS.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS, also known as Lou Gehrig's disease) is a progressive neurodegenerative disorder that primarily affects motor neurons in the spinal cord, brainstem, and cortex, leading to muscle weakness, paralysis of respiratory muscles, and eventually death. The majority of ALS is thought to be sporadic; however, about 10% of all cases have familial origin. The major histologic sign of ALS is the formation of inclusion bodies in certain brain regions, which comprise apoptotic neurons and the surrounding activated astrocytes.
As for the genetic background of ALS, two major factors have been identified playing a pivotal role in the development of this disease: an inherited autosomal mutation of the superoxide dismutase-1 (SOD-1)-encoding gene leading to impaired neuronal antioxidant capacity (291). Additionally, an expansion of a hexanucleotide sequence in the noncoding region of the C9ORF72 gene has been shown to be associated with ALS (286). Notably, other genes might be involved in the progress of this form of neurodegeneration (170). Numerous mechanisms have been implicated in the pathogenesis of ALS, including neurofilament accumulation, dysregulation of axonal transport, glutamate-mediated excitotoxicity, neuroinflammation and consequent microglial activation, and oxidative stress (29, 284, 318, 363) (Figs. 6 and 7). However, neither of these mechanisms has been identified to be the major cause of ALS.
Elevated levels of AEA and 2-AG have been documented in transgenic G93A-SOD1 mice that can recapitulate several features of ALS. Witting and her coworkers suggested that the increase in endocannabinoid levels in G93A-SOD1 mice might be a part of an endogenous defense mechanism, which is mediated via the AEA-CB1 receptor signaling axis (347). Similarly, Bilsland et al. have reported that genetic ablation of FAAH in these G93A-SOD1 mice and the consequent increase of AEA level significantly lowered the progression of ALS (28, 29). Moreover, WIN 55,212-2 treatment of G93A-SOD1 mice reduced the clinical signs of the disease; however, either FAAH ablation or treatment with the nonselective cannabinoid receptor agonist WIN 55,212-2 had no effect on life span of G93A-SOD1 mice compared with controls (28). These results suggest a neuroprotective role of cannabinoids in ALS that is not exclusively mediated via CB1 receptor and may involve CB2 receptor signaling.
Rossi and his coworkers have demonstrated an altered sensitivity of cannabinoid CB1 receptors controlling both glutamate and GABA transmission in the striatum of G93A-SOD1 mice, suggesting that adaptation of the ECS might be involved in the pathophysiology of ALS (293).
The protective effect of specific CB2 receptor agonists has been evaluated in ALS as well. Notably, CB2 receptors existing primarily in the periphery are dramatically upregulated in the affected areas, likely due to the activation of microglial cells (303, 328, 347). It has been recently reported that daily administration of CB2 agonist AM-1241 to G93A-SOD1 mice at the onset of clinical signs can increase the survival upon disease onset and improve the outcome (303), suggesting that CB2 agonists might attenuate local inflammation, motor neuron degeneration, and preserve motor function.
Moreno-Martet et al. have conducted a study with a Sativex®-like combination of phytocannabinoids (THC and CBD-enriched plant extract) in G93A-SOD1 mice (224). Even though the treatment preserved motor neurons in the spinal cord of SOD-1 mutant mice, it failed to preserve the integrity of neuromuscular junctions, achieving only poor neurological recovery. On the other hand, a marked upregulation of CB2 receptors was demonstrated in G93A-SOD1 mice; however, other genes involved in endocannabinoid metabolism and signaling were unchanged. Importantly, there were no changes in animal survival (224).
In spite of the increasing number of preclinical studies, there are only relatively few clinical trials evaluating the effect of THC/CBD in humans with ALS (7, 343). People with ALS have reported that cannabis consumption can alleviate some of their symptoms, including pain, muscle spasms, depression, and excessive production of saliva (7). However, there are no convincing clinical data so far investigating the benefits of plant-derived cannabinoids in ALS.
Taken together, ROS produced in motor neurons in response to excitotoxic activation can induce an oxidative disruption of glutamate transport in surrounding astrocytes, leading to the expansion of excitotoxic stress that can trigger the progression of ALS (284, 285). CB1 receptor stimulation was shown to block TNF-α-induced upregulation of AMPA glutamate receptors and exerted protection from excitotoxic death in hippocampal neuron cultures (364). Mitochondrial abnormalities have been found to play a critical role in the development of ALS, amplifying ROS production (169, 348). Therefore, it is conceivable that drugs targeting CB1 receptor signaling might limit the extent of oxidative damage in motor neurons associated with glutamate receptor hyperstimulation; however, the direct link between ROS-induced neuronal death and endocannabinoids needs to be elucidated in this disease. On the other hand, targeting CB2 signaling by selective receptor agonist may indirectly reduce oxidative/nitrative stress in ALS by attenuating microglial activation, inflammatory response, and subsequent oxidative stress.
Parkinson's disease
Oxidative/nitrative stress has been shown to increase in neurons with age due to their postmitotic character, which may also result in neuronal damage, dysfunction, and cell death. Accordingly, an ROS-dependent impairment of neuronal function can be observed in Parkinson's disease as the most common form of synucleinopathy (103).
Postmortem analysis of human brain samples with Parkinson's disease showed an augmented ROS production in the affected regions. Peroxynitrite-mediated nitration of certain tyrosine residues in the neuronal presynaptic protein α-synuclein has been shown to alter its folding, resulting in protein dysfunction, impaired protein degradation, and subsequent formation of Lewy bodies. Peroxynitrite further contributes to the progression of Parkinson's disease through nitration of tyrosine hydroxylase, the rate-limiting enzyme in the biosynthesis of dopamine, causing progressive nigrostriatal dopaminergic neuron loss resulting in motor disturbances and cognitive impairment (31, 103, 176, 312). Moreover, certain tyrosine residues determine the substrate specificity of monoamino oxidase B (MAO B). Thus, nitration of these tyrosine residues also leads to altered dopamine metabolism (102).
Furthermore, peroxynitrite is known to be involved in reduction of the intracellular glutathione level in substantia nigra by inactivating glutathione reductase (27). As early events in parkinsonism, dysfunctional glutathione reductase and the impaired function of mitochondrial complex I exacerbate oxidative stress, leading to neuronal cell death (235).
Since oxidative/nitrative stress is a hallmark of parkinsonism, the possible role of cannabinoids has been investigated in this pathophysiological condition as well (Tables 2 and 3). The effect of targeted activation of CB1 receptors in parkinsonism remains controversial in the literature. García-Arencibia et al. have reported that daily administration of selective CB1 receptor agonist, ACEA, or the nonselective cannabinoid receptor agonist, WIN 55,212-2, failed to reverse 6-hydroxydopamine-induced dopamine depletion in the affected region (97). In contrast, another study has concluded that activation of CB1 receptor-mediated signaling contributed to enhanced survival of nigrostriatal dopaminergic neurons in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced model of Parkinson's disease (54). This protective effect was accompanied by suppression of NOX enzymes, decreased ROS production, and reduced expression and release of proinflammatory cytokines originating from activated microglia. Nevertheless, activation of CB1 receptor might have disadvantages in parkinsonism since the hypokinetic effect of CB1 activation might worsen the symptoms of Parkinson's disease such as bradykinesia. In contrast, inhibition of CB1-mediated signaling attenuated Parkinson's disease-associated akinesia (85).
Interestingly, the specific FAAH inhibitor, URB597, has been found to be protective in probenecid and MPTP-induced experimental parkinsonism (47). URB597 prevented MPTP-induced motor impairment, but it failed to preserve dopamine levels in the substantia nigra or regulate glial cell activation. The symptomatic relief of URB597 was also studied in haloperidol-induced catalepsy assays, whereas the anticataleptic effect of URB597 was abolished by either pharmacological blockade or genetic ablation of CB1 and CB2 receptors, suggesting a pivotal role of FAAH inhibition and concomitant AEA increase in the motor symptoms of Parkinson's disease. However, the role of CB2 receptor in symptomatic relief should be uncovered.
Similarly, it was shown that the nonselective cannabinoid receptor agonist WIN 55,212-2 protected against nigrostriatal neuron loss in mouse model of MPTP-induced neurotoxicity and neuroinflammation. The neuroprotective effect of WIN 55,212-2 was associated with elevated dopamine and 3,4-dihydroxyphenylacetic acid levels in the substantia nigra pars compacta and dorsal striatum. In addition, the authors have documented significant microglial activation and concomitant upregulation of CB2 receptors in the ventral midbrain. On the other hand, treatment with the CB2 receptor agonist JWH015 attenuated MPTP-induced microglial activation, whereas genetic ablation of CB2 receptors aggravated neuronal death (276).
Neuroprotective effect of the MAGL inhibitor JZL-184 was also assessed in the dopaminergic-like SH-SY5Y cell line exposed to 1-methyl-4-phenylpyridinium iodide (MPP+) toxicity (11). The protective effect of JZL-184 on cell survival was abolished by the CB2 receptor antagonist AM630 and it was mimicked by the CB2 receptor agonist JWH133. Additionally, rimonabant, a CB1 receptor inverse agonist/antagonist, did not affect JZL-184-induced cell survival. Thus, MAGL inhibition provides further evidence for the beneficial effects of CB2 signaling in an in vitro model of Parkinson's disease.
Studies investigating the effect of plant-derived CBD revealed that CBD is able to attenuate mitochondrial dysfunction and oxidative damage and upregulate the mRNA level of enzymes in endogenous defenses against oxidative stress in experimental models of Parkinson's disease (97, 99, 184).
To date, there are no clinical data addressing the neuroprotective effects of cannabinoids and assessing the effects of THC and/or CBD in patients with Parkinson's disease. Clinical studies mainly focus on the relief of specific symptoms, including bradykinesia, tremor, and levodopa-induced dyskinesia (91, 92, 215); however, preclinical findings support the concept that an effective therapy against Parkinson's disease should aim to improve antioxidant capacity. Control of inflammatory response by CB2 receptor activation and inter-related oxidative/nitrative stress might be beneficial, and the blockade of CB1 receptor signaling could reduce motor inhibition. However, the exact role of CB1 receptor signaling in parkinsonism needs to be better understood.
Alzheimer's disease
Alzheimer's disease is characterized by progressive neurodegeneration due to the formation of β-amyloid plaques, neurofibrillary tangles, gliosis, and neuroinflammatory response (Fig. 6). All these effects lead to loss of cognitive function (344). Interestingly, oxidative stress has been shown to be an early event in the progression of neurodegeneration associated with Alzheimer's disease (41, 136, 216, 223). Increased expression of nNOS has been documented in astrocytes surrounding β-amyloid plaques in human subjects (307). An elevated iNOS and eNOS expression in neurofibrillary tangles has also been reported by other groups, suggesting that enhanced production of NO by reactive astrocytes and formation of peroxynitrite play a pivotal role in the pathogenesis of Alzheimer's disease (65, 193, 194). Protein nitration is increased in the cortex, neocortex, or in hippocampus in human subjects suffering from Alzheimer's disease (43, 129), coupled with decreased antioxidant capacity and reduced superoxide dismutase, catalase, and glutathione peroxidase activity in the affected brain regions (258, 354).
The relationship between Alzheimer's disease and the cannabinoid system has been extensively investigated (Tables 1 and 2). Several studies revealed that β-amyloid-induced progressive hippocampal neurodegeneration is associated with increased 2-AG, but not AEA, production in vivo (5, 87, 333). Controversially, augmented FAAH activity has been confirmed in astrocytes associated with senile plaques that lead to increased generation of arachidonic acid, aggravating inflammatory stimuli (24, 63).
Increased CB1 expression has been shown at earlier stages of Alzheimer's disease in hippocampal regions and internal layers of frontal cortex, which was decreased at the advanced stage of disease.
Elevation of 2-AG and modulation of CB2 receptor signaling either by using specific CB2 agonists (151, 170) or by inhibiting 2-AG-degrading enzyme MAGL might result in reduced microglial activation, decreased inflammatory response, attenuated oxidative stress, and attenuated β-amyloid plaque accumulation in the affected brain areas (49, 272). Notably, MAGL inhibition has been reported to prevent neuroinflammation, decrease neurodegeneration and maintain the integrity of hippocampal synaptic structure and function, and improve long-term synaptic plasticity, thereby supporting spatial learning and attenuating memory impairments in animals with Alzheimer's disease (49) (Figs. 6 and 7).
The nonpsychoactive phytocannabinoid, CBD, has been demonstrated to be neuroprotective in vitro and in vivo in Alzheimer's disease models (146). Neuronal cells pretreated with CBD showed diminished iNOS expression and reduced hyperphosphorylation of tau protein following Aβ treatment (83). Moreover, ROS levels, lipid peroxidation products, and caspase-3 activation were also attenuated by CBD (147). Similarly, mice treated with CBD before Aβ exposure also displayed an impaired iNOS and IL-1β protein expression and reduced NO and IL-1 β release (84).
Moreover, CBD dose-dependently decreased glial fibrillary acidic protein (GFAP) expression, suggesting attenuated microglial activation and neuroinflammation. However, the protection of CBD in neuronal cells cannot be fully attributed to its antioxidant properties; further clarification of its pharmacology is required to explain the mechanism of action (299).
In spite of the numerous promising results with cannabinoids in preclinical models of Alzheimer's disease, suitable clinical studies are still lacking in patients suffering from Alzheimer's disease. Clinical studies conducted so far have focused on the symptom-relieving effects of cannabinoids (dronabinol, CBD); however, they did not investigate the effect of cannabinoids on the progression of this devastating disease (339). Since oxidative/nitrative stress and NO production by active astrocytes and microglial cells in neurofibrillary tangles are the hallmarks of Alzheimer's disease even at early phases, therapies targeting ROS production might hold great therapeutic potential in slowing progression of the disease.
Huntington's disease
Huntington's disease is a dominantly inherited, progressive, autosomal neuropsychiatric disorder. The prevalence of Huntington's disease is lower than the other abovementioned neurodegenerative complications. It is characterized by a progressive neurodegeneration in the basal ganglia and certain cortical regions ultimately leading to the presence of involuntary movements, dystonia, psychiatric symptoms, and dementia (8, 100). The massive neuronal damage and cell loss are caused by an expansion of a trinucleotide (CAG) repeat encoding glutamine in the gene of huntingtin. This polyglutamine extension of huntingtin leads to conformational changes and abnormal protein–protein interactions.
Moreover, mutant huntingtin can bind to certain transcription factors resulting in a decreased expression of specific genes that might be crucial in neuronal survival (100). Substantial evidence also suggests an important role of oxidative stress and excessive production of NO in the development and progression of Huntington's disease (42). Increased iNOS expression, enhanced ONOO− generation, and elevated 3-nitrotyrosine (3-NT) formation have been shown in neuronal, glial, and vascular cells of diseased human brains as well as in animal models of Huntington's disease (48, 95, 112, 266). To date, there is no curative treatment for Huntington's disease, and only palliative care is available to reduce motor neuronal disturbances, chorea, and dystonia.
A decreased expression of CB1 receptors has been documented in the subset of neurons of the lateral striatum, cortex, and hippocampus before the clinical onset of Huntington's disease, suggesting involvement of the cannabinoid system in the development of this neurodegenerative disorder (19, 68, 106, 180, 334). Furthermore, mutant huntingtin has been suggested to play a unique role in the depletion of CB1 receptors by silencing CB1 transcription at the level of gene expression (182). Moreover, loss of CB1 function has been shown to contribute to cognitive, behavioral, and motor deficits in animal models of Huntington's disease (33, 34). Accordingly, targeting CB1 receptors located on cortical glutamatergic neurons that project to the striatum attenuated neuronal loss rather than the CB1-mediated silencing of GABAergic striatal neurons underlining the importance of different subsets of CB1-expressing neurons in the pathology of Huntington's disease (51).
In addition, it has been recently shown that compounds acting selectively on CB2 receptors are able to attenuate the progression of Huntington's disease by ameliorating inflammation and microglial activation in striatum following malonate-induced damage (295). The authors also documented that activation of CB2 receptors significantly reduced the levels of proinflammatory TNF-α, which was elevated in the malonate-induced lesion. In addition, mice deficient in CB2 receptor were more sensitive to malonate-induced striatal cell loss compared with wild-type animals (295). Accordingly, in another animal model of Huntington's disease, induction of striatal excitotoxicity by quinolinic acid administration exacerbated brain edema, microglial activation, proinflammatory mediator release, and neuron degeneration. All these effects were more severe in CB2 receptor-deficient mice and were attenuated by administration of the selective CB2 receptor agonist HU-308 to wild-type mice (295).
The nonspecific DAGL inhibitor O-3841 and the specific MAGL inhibitor JZL-184 were also tested in a malonate-induced striatal damage model (331). Surprisingly, the inhibition of 2-AG biosynthesis by using the nonspecific DAGL inhibitor O-3841 was neuroprotective following striatal malonate treatment due to the attenuation of overproduction of the neuroinflammatory prostaglandin E2 glyceryl ester (PGE2-G), formed by COX-2-mediated oxygenation of 2-AG. Accordingly, MAGL inhibition by JZL-184 or the administration of PGE2-G exacerbated the malonate-induced striatal damage (331).
The neuroprotective effect of CBD has been also evaluated in animal models of Huntington's disease. CBD exerted neuroprotection when striatal atrophy was induced by the mitochondrial toxin 3-nitropropionic acid (3-NP) (296). 3-NP-induced neuronal loss mimics mitochondrial defects with the subsequent generation of ROS in patients suffering from Huntington's disease. This protection was independent from cannabinoid receptor activation and presumably caused by the antioxidant nature of CBD. The neuroprotective potential of THC has been also evaluated in rats with striatal degeneration induced by malonate (183). Administration of THC increased malonate-induced striatal cell loss compared with vehicle. Surprisingly, rimonabant also enhanced malonate-induced neuronal damage in a greater extent, suggesting complex overlapping mechanism in the pathology of Huntington's disease (183).
The effects of cannabinoids have been also studied in patients with Huntington's disease, although these clinical trials evaluated only the alleviation of specific symptoms, such as chorea and behavioral disturbances [reviewed by Ref. (86)], and were not conclusive. Clearly, further studies are required to assess the role of interaction of cannabinoids and ROS production in the disease progression.
Conclusions
Endocannabinoid production may be triggered during tissue injury by oxidative stress and inflammation. In turn, endocannabinoids may also control oxidative stress by directly or indirectly modulating CB1 and CB2 signaling or CB receptor-independent processes (e.g., COX-2-dependent eicosanoid pathways). Consequently, this may lead to either tissue-protective or damaging effects depending on the injury and cell/tissue type and/or context. Better understanding of these complex interactions and tissue-specific differences may lead to better cannabinoid-based therapies in the future.
For the moment, it appears that targeting CB2 signaling with selective agonists in various neuroinflammatory/neurodegenerative diseases may represent a promising therapeutic avenue to attenuate inflammation and inter-related oxidative/nitrative stress if the impending target validation studies and studies with novel ligands are successful. Certain cannabis-based natural constituents, such as cannabidiol, may also have therapeutic potential to treat certain human diseases associated with oxidative stress and inflammation. Tables 1–3 summarize the most commonly used cannabinoid ligands (Table 1), evidence on the interplay of endocannabinoids and oxidative stress (Table 2), and implication of targeting the ECS in oxidative stress-related neurodegenerative diseases (Table 3).
Abbreviations Used
- 2-AG
2-arachidonoyl glycerol
- 3-NP
3-nitropropionic acid
- 3-NT
3-nitrotyrosine
- 4-HNE
4-hydroxynonenal
- 6-OHDA
6-hydroxydopamine
- 8-iso-PGF2α
8-iso-prostaglandin F2α
- Abhd12
α/β-hydrolase 12
- Abhd4
α/β-hydrolase 4
- Abhd6
α/β-hydrolase 6
- ACEA
arachidonoyl-2-chloroethylamide
- AEA
anandamide
- AKT
protein kinase B
- ALS
amyotrophic lateral sclerosis
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AT1 receptor
angiotensin II receptor type 1
- ATP
adenosine triphosphate
- Bcl-2
B cell lymphoma 2 protein
- cAMP
cyclic adenosine monophosphate
- CB1
cannabinoid 1 receptor
- CB2
cannabinoid 2 receptor
- CBD
cannabidiol
- CCLs
C-C motif chemokines
- cGMP
cyclic guanosine monophosphate
- CHI
closed head injury
- CNS
central nervous system
- COXs
cyclooxygenases (also known as prostaglandin-endoperoxide synthases)
- COX-2
cyclooxygenase-2
- CXCLs
C-X-C motif chemokines
- CYP 450
microsomal cytochrome P450 enzymes
- DAG
diacylglycerol
- DAGLα
diacylglycerol lipase α
- DAGLβ
diacylglycerol lipase β
- DAMPs
damage-associated molecular patterns
- DNA
deoxyribonucleic acid
- EAE
experimental autoimmune encephalomyelitis
- ECS
endocannabinoid system
- EET
epoxyeicosatrienoic acids
- EET-EAs
epoxyeicosatrienoic ethanolamines
- EET-Gs
epoxyeicosatrienoic glycerols
- eNOS (NOS3)
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinases
- FAAH
fatty acid amide hydrolase
- GFAP
glial fibrillary acidic protein
- GPR119
G protein-coupled receptor 119
- GPR55
G protein-coupled receptor 55
- H2O2
hydrogen peroxide
- HETE-EAs
hydroxyeicosatetraenoic ethanolamines
- HETE-Gs
hydroxyeicosatetraenoic glycerols
- HETEs
hydroxyeicosatetraenoic acids
- HIF-1
hypoxia-inducible factor 1
- Hsp70/72
heat shock protein 70/72
- ICAM-1
intercellular adhesion molecule 1
- IFN-γ
interferon-γ
- IL-1β
interleukin 1β
- IL-6
interleukin 6
- IL-10
interleukin 10
- IL-17
interleukin 17
- iNOS (NOS2)
inducible nitric oxide synthase
- JNKs
c-Jun N-terminal kinases
- LPS
lipopolysaccharide
- lyso-PLD
lysophospholipase D
- MAGL
monoacylglycerol lipase
- MAO B
monoamino oxidase B
- MAPK
mitogen-activated protein kinase
- MPO
myeloperoxidase
- MPP+
1-methyl-4-phenylpyridinium iodide
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- mRNA
messenger ribonucleic acid
- MS
multiple sclerosis
- NAD+
nicotinamide adenine dinucleotide
- NADA
N-arachidonoyl dopamine
- NADPH
nicotinamide adenine dinucleotide phosphate (reduced form)
- NAPE
N-acylphosphatidylethanolamine
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NLRP3
NACHT, LRR, and PYD domain-containing protein 3 (also known as cryopyrin)
- NMDA receptor
N-methyl-D-aspartate receptor
- nNOS (NOS1)
neuronal nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
- NOXs
NADPH oxidases
- O2•−
superoxide
- OH•
hydroxyl radical
- ONOO−
peroxynitrite
- PARP-1
poly(APD-ribose) polymerase-1
- PEA
palmitoylethanolamide
- PGE2
prostaglandin E2
- PGE2-G
prostaglandin E2 glyceryl ester
- PKA
protein kinase A
- PLA2γ
calcium-independent phospholipase A2γ
- PLC
phospholipase C
- PLD
phospholipase D
- PPARs
peroxisome proliferator-activated receptors
- PTPN22
protein tyrosine phosphatase, nonreceptor type 22
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD-1
superoxide dismutase-1
- STAT3
signal transducer and activator of transcription 3
- TBI
traumatic brain injury
- Th1 cells
T helper 1 cells
- Th17 cells
T helper 17 (IL-17-secreting) cells
- THC
Δ9-tetrahydrocannabinol
- THCV
Δ9-Tetrahydrocannabivarin
- TNF-α
tumor necrosis factor alpha
- TRPV1
transient receptor potential vanilloid type 1
- VCAM-1
vascular cell adhesion molecule 1
- VSMCs
vascular smooth muscle cells
Acknowledgments
Authors are indebted to Dr. George Kunos, the Scientific Director of NIAAA, for his support and for critical reading of the manuscript. The authors are grateful to Servier Medical Art for providing professionally designed high-quality images that were used for creating the figures of this review. Servier Medical Art presentations are available on www.servier.com
References
- 1.Achiron A, Miron S, Lavie V, Margalit R, and Biegon A. Dexanabinol (HU-211) effect on experimental autoimmune encephalomyelitis: implications for the treatment of acute relapses of multiple sclerosis. J Neuroimmunol 102: 26–31, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, and Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci 10: 870–879, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander SPH. and Kendall DA. The complications of promiscuity: endocannabinoid action and metabolism. Br J Pharmacol 152: 602–623, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alhouayek M. and Muccioli GG. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol Sci 35: 284–292, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Altamura C, Ventriglia M, Martini MG, Montesano D, Errante Y, Piscitelli F, Scrascia F, Quattrocchi C, Palazzo P, Seccia S, Vernieri F, and Di Marzo V. Elevation of plasma 2-arachidonoylglycerol levels in Alzheimer's disease patients as a potential protective mechanism against neurodegenerative decline. J Alzheimers Dis 46: 497–506, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Amenta PS, Jallo JI, Tuma RF, Hooper DC, and Elliott MB. Cannabinoid receptor type-2 stimulation, blockade, and deletion alter the vascular inflammatory responses to traumatic brain injury. J Neuroinflammation 11: 191, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amtmann D, Weydt P, Johnson KL, Jensen MP, and Carter GT. Survey of cannabis use in patients with amyotrophic lateral sclerosis. Am J Hosp Palliat Care 21: 95–104, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Anderson KE. Huntington's disease and related disorders. Psychiatr Clin North Am 28: 275–290, x, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Aso E. and Ferrer I. CB2 cannabinoid receptor as potential target against Alzheimer's disease. Front Neurosci 10: 243, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aso E, Juves S, Maldonado R, and Ferrer I. CB2 cannabinoid receptor agonist ameliorates Alzheimer-like phenotype in AbetaPP/PS1 mice. J Alzheimers Dis 35: 847–858, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Aymerich MS, Rojo-Bustamante E, Molina C, Celorrio M, Sanchez-Arias JA, and Franco R. Neuroprotective effect of JZL184 in MPP(+)-treated SH-SY5Y cells through CB2 receptors. Mol Neurobiol 53: 2312–2319, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Baggelaar MP, Chameau PJ, Kantae V, Hummel J, Hsu KL, Janssen F, van der Wel T, Soethoudt M, Deng H, den Dulk H, Allara M, Florea BI, Di Marzo V, Wadman WJ, Kruse CG, Overkleeft HS, Hankemeier T, Werkman TR, Cravatt BF, and van der Stelt M. Highly selective, reversible inhibitor identified by comparative chemoproteomics modulates diacylglycerol lipase activity in neurons. J Am Chem Soc 137: 8851–8857, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, and Layward L. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature 404: 84–87, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Makriyannis A, Khanolkar A, Layward L, Fezza F, Bisogno T, and Di Marzo V. Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J 15: 300–302, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Bartosz G. Reactive oxygen species: destroyers or messengers? Biochem Pharmacol 77: 1303–1315, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Bátkai S, Mukhopadhyay P, Harvey-White J, Kechrid R, Pacher P, and Kunos G. Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in vivo in cirrhotic rats. Am J Physiol Heart Circ Physiol 293: H1689–H1695, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bátkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, Hong F, Harvey-White J, Jafri A, Haskó G, Huffman JW, Gao B, Kunos G, and Pacher P. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J 21: 1788–1800, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bátkai S. and Pacher P. Endocannabinoids and cardiac contractile function: pathophysiological implications. Pharmacol Res 60: 99–106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battista N, Bari M, Tarditi A, Mariotti C, Bachoud-Levi AC, Zuccato C, Finazzi-Agro A, Genitrini S, Peschanski M, Di Donato S, Cattaneo E, and Maccarrone M. Severe deficiency of the fatty acid amide hydrolase (FAAH) activity segregates with the Huntington's disease mutation in peripheral lymphocytes. Neurobiol Dis 27: 108–116, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Bauer M, Chicca A, Tamborrini M, Eisen D, Lerner R, Lutz B, Poetz O, Pluschke G, and Gertsch J. Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors. J Biol Chem 287: 36944–36967, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayir H, Kochanek PM, and Clark RS. Traumatic brain injury in infants and children: mechanisms of secondary damage and treatment in the intensive care unit. Crit Care Clin 19: 529–549, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Begg M, Pacher P, Bátkai S, Osei-Hyiaman D, Offertáler L, Mo FM, Liu J, and Kunos G. Evidence for novel cannabinoid receptors. Pharmacol Ther 106: 133–145, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Benard G, Massa F, Puente N, Lourenco J, Bellocchio L, Soria-Gomez E, Matias I, Delamarre A, Metna-Laurent M, Cannich A, Hebert-Chatelain E, Mulle C, Ortega-Gutierrez S, Martin-Fontecha M, Klugmann M, Guggenhuber S, Lutz B, Gertsch J, Chaouloff F, Lopez-Rodriguez ML, Grandes P, Rossignol R, and Marsicano G. Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nat Neurosci 15: 558–564, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Benito C, Núñez E, Tolón RM, Carrier EJ, Rábano A, Hillard CJ, and Romero J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J Neurosci 23: 11136–11141, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benyo Z, Ruisanchez E, Leszl-Ishiguro M, Sandor P, and Pacher P. Endocannabinoids in cerebrovascular regulation. Am J Physiol Heart Circ Physiol 310: H785–H801, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernal-Chico A, Canedo M, Manterola A, Victoria Sanchez-Gomez M, Perez-Samartin A, Rodriguez-Puertas R, Matute C, and Mato S. Blockade of monoacylglycerol lipase inhibits oligodendrocyte excitotoxicity and prevents demyelination in vivo. Glia 63: 163–176, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Bharath S. and Andersen JK. Glutathione depletion in a midbrain-derived immortalized dopaminergic cell line results in limited tyrosine nitration of mitochondrial complex I subunits: implications for Parkinson's disease. Antioxid Redox Signal 7: 900–910, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Bilsland LG, Dick JR, Pryce G, Petrosino S, Di Marzo V, Baker D, and Greensmith L. Increasing cannabinoid levels by pharmacological and genetic manipulation delay disease progression in SOD1 mice. FASEB J 20: 1003–1005, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Bilsland LG, Nirmalananthan N, Yip J, Greensmith L, and Duchen MR. Expression of mutant SOD1 in astrocytes induces functional deficits in motoneuron mitochondria. J Neurochem 107: 1271–1283, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams E-J, Gangadharan U, Hobbs C, Marzo VD, and Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol 163: 463–468, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanchard-Fillion B, Souza JM, Friel T, Jiang GC, Vrana K, Sharov V, Barron L, Schoneich C, Quijano C, Alvarez B, Radi R, Przedborski S, Fernando GS, Horwitz J, and Ischiropoulos H. Nitration and inactivation of tyrosine hydroxylase by peroxynitrite. J Biol Chem 276: 46017–46023, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Blankman JL. and Cravatt BF. Chemical probes of endocannabinoid metabolism. Pharmacol Rev 65: 849–871, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blazquez C, Chiarlone A, Bellocchio L, Resel E, Pruunsild P, Garcia-Rincon D, Sendtner M, Timmusk T, Lutz B, Galve-Roperh I, and Guzman M. The CB(1) cannabinoid receptor signals striatal neuroprotection via a PI3K/Akt/mTORC1/BDNF pathway. Cell Death Differ 22: 1618–1629, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blazquez C, Chiarlone A, Sagredo O, Aguado T, Pazos MR, Resel E, Palazuelos J, Julien B, Salazar M, Borner C, Benito C, Carrasco C, Diez-Zaera M, Paoletti P, Diaz-Hernandez M, Ruiz C, Sendtner M, Lucas JJ, de Yebenes JG, Marsicano G, Monory K, Lutz B, Romero J, Alberch J, Gines S, Kraus J, Fernandez-Ruiz J, Galve-Roperh I, and Guzman M. Loss of striatal type 1 cannabinoid receptors is a key pathogenic factor in Huntington's disease. Brain 134: 119–136, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Blennow K, Hardy J, and Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron 76: 886–899, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Bosier B, Muccioli GG, Hermans E, and Lambert DM. Functionally selective cannabinoid receptor signalling: therapeutic implications and opportunities. Biochem Pharmacol 80: 1–12, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, and Casellas P. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur J Pharmacol 517: 174–181, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Broholm H, Andersen B, Wanscher B, Frederiksen JL, Rubin I, Pakkenberg B, Larsson HB, and Lauritzen M. Nitric oxide synthase expression and enzymatic activity in multiple sclerosis. Acta Neurol Scand 109: 261–269, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Burch P, Chicca A, Gertsch J, and Gademann K. Functionally optimized neuritogenic farinosone C analogs: SAR-study and investigations on their mode of action. ACS Med Chem Lett 5: 172–177, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med 18: 775–794, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Butterfield DA. and Boyd-Kimball D. Amyloid beta-peptide(1–42) contributes to the oxidative stress and neurodegeneration found in Alzheimer disease brain. Brain Pathol 14: 426–432, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butterfield DA, Howard BJ, and LaFontaine MA. Brain oxidative stress in animal models of accelerated aging and the age-related neurodegenerative disorders, Alzheimer's disease and Huntington's disease. Curr Med Chem 8: 815–828, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Butterfield DA. and Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med 32: 1050–1060, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Cao Z, Mulvihill MM, Mukhopadhyay P, Xu H, Erdélyi K, Hao E, Holovac E, Haskó G, Cravatt BF, Nomura DK, and Pacher P. Monoacylglycerol lipase controls endocannabinoid and eicosanoid signaling and hepatic injury in mice. Gastroenterology 144: 808–817.e15, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carracedo A, Geelen MJ, Diez M, Hanada K, Guzman M, and Velasco G. Ceramide sensitizes astrocytes to oxidative stress: protective role of cannabinoids. Biochem J 380: 435–440, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cascio MG, Gauson LA, Stevenson LA, Ross RA, and Pertwee RG. Evidence that the plant cannabinoid cannabigerol is a highly potent alpha2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br J Pharmacol 159: 129–141, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Celorrio M, Fernandez-Suarez D, Rojo-Bustamante E, Echeverry-Alzate V, Ramirez MJ, Hillard CJ, Lopez-Moreno JA, Maldonado R, Oyarzabal J, Franco R, and Aymerich MS. Fatty acid amide hydrolase inhibition for the symptomatic relief of Parkinson's disease. Brain Behav Immun 57: 94–105, 2016 [DOI] [PubMed] [Google Scholar]
- 48.Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, and Friedlander RM. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 6: 797–801, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Chen R, Zhang J, Wu Y, Wang D, Feng G, Tang YP, Teng Z, and Chen C. Monoacylglycerol lipase is a therapeutic target for Alzheimer's disease. Cell Rep 2: 1329–1339, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y-R. and Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res 114: 524–537, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiarlone A, Bellocchio L, Blazquez C, Resel E, Soria-Gomez E, Cannich A, Ferrero JJ, Sagredo O, Benito C, Romero J, Sanchez-Prieto J, Lutz B, Fernandez-Ruiz J, Galve-Roperh I, and Guzman M. A restricted population of CB1 cannabinoid receptors with neuroprotective activity. Proc Natl Acad Sci U S A 111: 8257–8262, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chicca A, Marazzi J, Nicolussi S, and Gertsch J. Evidence for bidirectional endocannabinoid transport across cell membranes. J Biol Chem 287: 34660–34682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiurchiu V, Cencioni MT, Bisicchia E, De Bardi M, Gasperini C, Borsellino G, Centonze D, Battistini L, and Maccarrone M. Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Ann Neurol 73: 626–636, 2013 [DOI] [PubMed] [Google Scholar]
- 54.Chung YC, Bok E, Huh SH, Park J-Y, Yoon S-H, Kim SR, Kim Y-S, Maeng S, Park SH, and Jin BK. Cannabinoid receptor type 1 protects nigrostriatal dopaminergic neurons against MPTP neurotoxicity by inhibiting microglial activation. J Immunol 187: 6508–6517, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, Tontini A, Sanchini S, Sciolino NR, Spradley JM, Hohmann AG, Calignano A, Mor M, Tarzia G, and Piomelli D. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci 13: 1265–1270, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Comelli F, Giagnoni G, Bettoni I, Colleoni M, and Costa B. The inhibition of monoacylglycerol lipase by URB602 showed an anti-inflammatory and anti-nociceptive effect in a murine model of acute inflammation. Br J Pharmacol 152: 787–794, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Concannon RM, Okine BN, Finn DP, and Dowd E. Upregulation of the cannabinoid CB2 receptor in environmental and viral inflammation-driven rat models of Parkinson's disease. Exp Neurol 283: 204–212, 2016 [DOI] [PubMed] [Google Scholar]
- 58.Corps KN, Roth TL, and McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol 72: 355–362, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cota D, Marsicano G, Tschöp M, Grübler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thöne-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst ACE, Pasquali R, Lutz B, Stalla GK, and Pagotto U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 112: 423–431, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Croxford JL, Pryce G, Jackson SJ, Ledent C, Giovannoni G, Pertwee RG, Yamamura T, and Baker D. Cannabinoid-mediated neuroprotection, not immunosuppression, may be more relevant to multiple sclerosis. J Neuroimmunol 193: 120–129, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Csiszar A, Podlutsky A, Wolin MS, Losonczy G, Pacher P, and Ungvari Z. Oxidative stress and accelerated vascular aging: implications for cigarette smoking. Front Biosci (Landmark Ed) 14: 3128–3144, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, and Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421: 744–748, 2003 [DOI] [PubMed] [Google Scholar]
- 63.D'Addario C, Di Francesco A, Arosio B, Gussago C, Dell'Osso B, Bari M, Galimberti D, Scarpini E, Altamura AC, Mari D, and Maccarrone M. Epigenetic regulation of fatty acid amide hydrolase in Alzheimer disease. PLoS One 7: e39186, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Groote MA, Ochsner UA, Shiloh MU, Nathan C, McCord JM, Dinauer MC, Libby SJ, Vazquez-Torres A, Xu Y, and Fang FC. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc Natl Acad Sci U S A 94: 13997–14001, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de la Monte SM, Sohn YK, Etienne D, Kraft J, and Wands JR. Role of aberrant nitric oxide synthase-3 expression in cerebrovascular degeneration and vascular-mediated injury in Alzheimer's disease. Ann N Y Acad Sci 903: 61–71, 2000 [DOI] [PubMed] [Google Scholar]
- 66.De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, and Di Marzo V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett 483: 52–56, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Defer N, Wan J, Souktani R, Escoubet B, Perier M, Caramelle P, Manin S, Deveaux V, Bourin MC, Zimmer A, Lotersztajn S, Pecker F, and Pavoine C. The cannabinoid receptor type 2 promotes cardiac myocyte and fibroblast survival and protects against ischemia/reperfusion-induced cardiomyopathy. FASEB J 23: 2120–2130, 2009 [DOI] [PubMed] [Google Scholar]
- 68.Denovan-Wright EM, and Robertson HA. Cannabinoid receptor messenger RNA levels decrease in a subset of neurons of the lateral striatum, cortex and hippocampus of transgenic Huntington's disease mice. Neuroscience 98: 705–713, 2000 [DOI] [PubMed] [Google Scholar]
- 69.Devane WA, Dysarz FA, Johnson MR, Melvin LS, and Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34: 605–613, 1988 [PubMed] [Google Scholar]
- 70.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, and Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258: 1946–1949, 1992 [DOI] [PubMed] [Google Scholar]
- 71.Di Marzo V. CB1 receptor antagonism: biological basis for metabolic effects. Drug Discov Today 13: 1026–1041, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res 60: 77–84, 2009 [DOI] [PubMed] [Google Scholar]
- 73.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, and Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372: 686–691, 1994 [DOI] [PubMed] [Google Scholar]
- 74.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, and Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A 99: 10819–10824, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dirnagl U, Iadecola C, and Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22: 391–397, 1999 [DOI] [PubMed] [Google Scholar]
- 76.Dodd GT, Mancini G, Lutz B, and Luckman SM. The peptide hemopressin acts through CB1 cannabinoid receptors to reduce food intake in rats and mice. J Neurosci 30: 7369–7376, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dol-Gleizes F, Paumelle R, Visentin V, Marés A-M, Desitter P, Hennuyer N, Gilde A, Staels B, Schaeffer P, and Bono F. Rimonabant, a selective cannabinoid CB1 receptor antagonist, inhibits atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 29: 12–18, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, Hoertnagl H, Raine CS, Schneider-Stock R, Nitsch R, and Ullrich O. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron 49: 67–79, 2006 [DOI] [PubMed] [Google Scholar]
- 79.Elliott MB, Tuma RF, Amenta PS, Barbe MF, and Jallo JI. Acute effects of a selective cannabinoid-2 receptor agonist on neuroinflammation in a model of traumatic brain injury. J Neurotrauma 28: 973–981, 2011 [DOI] [PubMed] [Google Scholar]
- 80.Emrich HM, Leweke FM, and Schneider U. Towards a cannabinoid hypothesis of schizophrenia: cognitive impairments due to dysregulation of the endogenous cannabinoid system. Pharmacol Biochem Behav 56: 803–807, 1997 [DOI] [PubMed] [Google Scholar]
- 81.Engeli S, Böhnke J, Feldpausch M, Gorzelniak K, Janke J, Bátkai S, Pacher P, Harvey-White J, Luft FC, Sharma AM, and Jordan J. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 54: 2838–2843, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.England TJ, Hind WH, Rasid NA, and O'Sullivan SE. Cannabinoids in experimental stroke: a systematic review and meta-analysis. J Cereb Blood Flow Metab 35: 348–358, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Esposito G, De Filippis D, Carnuccio R, Izzo AA, and Iuvone T. The marijuana component cannabidiol inhibits beta-amyloid-induced tau protein hyperphosphorylation through Wnt/beta-catenin pathway rescue in PC12 cells. J Mol Med (Berl) 84: 253–258, 2006 [DOI] [PubMed] [Google Scholar]
- 84.Esposito G, Scuderi C, Savani C, Steardo L, Jr., De Filippis D, Cottone P, Iuvone T, Cuomo V, and Steardo L. Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br J Pharmacol 151: 1272–1279, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fernández-Ruiz J. The endocannabinoid system as a target for the treatment of motor dysfunction. Br J Pharmacol 156: 1029–1040, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fernandez-Ruiz J, Moro MA, and Martinez-Orgado J. Cannabinoids in neurodegenerative disorders and stroke/brain trauma: from preclinical models to clinical applications. Neurotherapeutics 12: 793–806, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fernández-Ruiz J, Romero J, and Ramos JA. Endocannabinoids and neurodegenerative disorders: Parkinson's disease, Huntington's chorea, Alzheimer's disease, and others. Handbf Exp Pharmacol 231: 233–259, 2015 [DOI] [PubMed] [Google Scholar]
- 88.Fezza F, Bisogno T, Minassi A, Appendino G, Mechoulam R, and Di Marzo V. Noladin ether, a putative novel endocannabinoid: inactivation mechanisms and a sensitive method for its quantification in rat tissues. FEBS Lett 513: 294–298, 2002 [DOI] [PubMed] [Google Scholar]
- 89.Flachenecker P, Henze T, and Zettl UK. Nabiximols (THC/CBD oromucosal spray, Sativex(R)) in clinical practice—results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity. Eur Neurol 71: 271–279, 2014 [DOI] [PubMed] [Google Scholar]
- 90.Fong TM, Guan XM, Marsh DJ, Shen CP, Stribling DS, Rosko KM, Lao J, Yu H, Feng Y, Xiao JC, Van der Ploeg LH, Goulet MT, Hagmann WK, Lin LS, Lanza TJ, Jr., Jewell JP, Liu P, Shah SK, Qi H, Tong X, Wang J, Xu SS, Francis B, Strack AM, MacIntyre DE, and Shearman LP. Antiobesity efficacy of a novel cannabinoid-1 receptor inverse agonist, N-[(1S,2S)-3-(4-chlorophenyl)-2-(3-cyanophenyl)-1-methylpropyl]-2-methyl-2-[[5-(t rifluoromethyl)pyridin-2-yl]oxy]propanamide (MK-0364), in rodents. J Pharmacol Exp Ther 321: 1013–1022, 2007 [DOI] [PubMed] [Google Scholar]
- 91.Fox SH, Lang AE, and Brotchie JM. Translation of nondopaminergic treatments for levodopa-induced dyskinesia from MPTP-lesioned nonhuman primates to phase IIa clinical studies: keys to success and roads to failure. Mov Disord 21: 1578–1594, 2006 [DOI] [PubMed] [Google Scholar]
- 92.Frankel JP, Hughes A, Lees AJ, and Stern GM. Marijuana for parkinsonian tremor. J Neurol Neurosurg Psychiatry 53: 436, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fujiwara M. and Egashira N. New perspectives in the studies on endocannabinoid and cannabis: abnormal behaviors associate with CB1 cannabinoid receptor and development of therapeutic application. J Pharmacol Sci 96: 362–366, 2004 [DOI] [PubMed] [Google Scholar]
- 94.Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, Bouaboula M, Shire D.LE Fur G, and Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem 232: 54–61, 1995 [DOI] [PubMed] [Google Scholar]
- 95.Galpern WR, Matthews RT, Beal MF, and Isacson O. NGF attenuates 3-nitrotyrosine formation in a 3-NP model of Huntington's disease. Neuroreport 7: 2639–2642, 1996 [DOI] [PubMed] [Google Scholar]
- 96.Gandhi R, Laroni A, and Weiner HL. Role of the innate immune system in the pathogenesis of multiple sclerosis. J Neuroimmunol 221: 7–14, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.García-Arencibia M, González S, de Lago E, Ramos JA, Mechoulam R, and Fernández-Ruiz J. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson's disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res 1134: 162–170, 2007 [DOI] [PubMed] [Google Scholar]
- 98.Garcia-Ovejero D, Arevalo-Martin A, Petrosino S, Docagne F, Hagen C, Bisogno T, Watanabe M, Guaza C, Di Marzo V, and Molina-Holgado E. The endocannabinoid system is modulated in response to spinal cord injury in rats. Neurobiol Dis 33: 57–71, 2009 [DOI] [PubMed] [Google Scholar]
- 99.García C, Palomo-Garo C, García-Arencibia M, Ramos J, Pertwee R, and Fernández-Ruiz J. Symptom-relieving and neuroprotective effects of the phytocannabinoid Δ9-THCV in animal models of Parkinson's disease. Br J Pharmacol 163: 1495–1506, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gardian G. and Vecsei L. Huntington's disease: pathomechanism and therapeutic perspectives. J Neural Transm (Vienna) 111: 1485–1494, 2004 [DOI] [PubMed] [Google Scholar]
- 101.Gebremedhin D, Lange AR, Campbell WB, Hillard CJ, and Harder DR. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am J Physiol 276: H2085–H2093, 1999 [DOI] [PubMed] [Google Scholar]
- 102.Geha RM, Rebrin I, Chen K, and Shih JC. Substrate and inhibitor specificities for human monoamine oxidase A and B are influenced by a single amino acid. J Biol Chem 276: 9877–9882, 2001 [DOI] [PubMed] [Google Scholar]
- 103.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, and Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290: 985–989, 2000 [DOI] [PubMed] [Google Scholar]
- 104.Giovannoni G, Heales SJ, Silver NC, O'Riordan J, Miller RF, Land JM, Clark JB, and Thompson EJ. Raised serum nitrate and nitrite levels in patients with multiple sclerosis. J Neurol Sci 145: 77–81, 1997 [DOI] [PubMed] [Google Scholar]
- 105.Giovannoni G, Silver NC, O'Riordan J, Miller RF, Heales SJ, Land JM, Elliot M, Feldmann M, Miller DH, and Thompson EJ. Increased urinary nitric oxide metabolites in patients with multiple sclerosis correlates with early and relapsing disease. Mult Scler 5: 335–341, 1999 [DOI] [PubMed] [Google Scholar]
- 106.Glass M, Dragunow M, and Faull RL. The pattern of neurodegeneration in Huntington's disease: a comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington's disease. Neuroscience 97: 505–519, 2000 [DOI] [PubMed] [Google Scholar]
- 107.Godlewski G, Offertáler L, Wagner JA, and Kunos G. Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Other Lipid Mediat 89: 105–111, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Golech SA, McCarron RM, Chen Y, Bembry J, Lenz F, Mechoulam R, Shohami E, and Spatz M. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res Mol Brain Res 132: 87–92, 2004 [DOI] [PubMed] [Google Scholar]
- 109.Gomes I, Grushko JS, Golebiewska U, Hoogendoorn S, Gupta A, Heimann AS, Ferro ES, Scarlata S, Fricker LD, and Devi LA. Novel endogenous peptide agonists of cannabinoid receptors. FASEB J 23: 3020–3029, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Goncharov I, Weiner L, and Vogel Z. Delta9-tetrahydrocannabinol increases C6 glioma cell death produced by oxidative stress. Neuroscience 134: 567–574, 2005 [DOI] [PubMed] [Google Scholar]
- 111.Gruden G, Barutta F, Kunos G, and Pacher P. Role of the endocannabinoid system in diabetes and diabetic complications. Br J Pharmacol 173: 1116–1127, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guix FX, Uribesalgo I, Coma M, and Munoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol 76: 126–152, 2005 [DOI] [PubMed] [Google Scholar]
- 113.Guo J. and Ikeda SR. Endocannabinoids modulate N-Type calcium channels and G-protein-coupled inwardly rectifying potassium channels via CB1 cannabinoid receptors heterologously expressed in mammalian neurons. Mol Pharmacol 65: 665–674, 2004 [DOI] [PubMed] [Google Scholar]
- 114.Haller J, Varga B, Ledent C, Barna I, and Freund TF. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur J Neurosci 19: 1906–1912, 2004 [DOI] [PubMed] [Google Scholar]
- 115.Hampson AJ, Bornheim LM, Scanziani M, Yost CS, Gray AT, Hansen BM, Leonoudakis DJ, and Bickler PE. Dual effects of anandamide on NMDA receptor-mediated responses and neurotransmission. J Neurochem 70: 671–676, 1998 [DOI] [PubMed] [Google Scholar]
- 116.Hampson AJ, Grimaldi M, Axelrod J, and Wink D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A 95: 8268–8273, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hampson AJ, Hill WAG, Zan-Phillips M, Makriyannis A, Leung E, Eglen RM, and Bornheim LM. Anandamide hydroxylation by brain lipoxygenase:metabolite structures and potencies at the cannabinoid receptor. Biochim Biophys Acta 1259: 173–179, 1995 [DOI] [PubMed] [Google Scholar]
- 118.Hansen HH, Schmid PC, Bittigau P, Lastres-Becker I, Berrendero F, Manzanares J, Ikonomidou C, Schmid HH, Fernandez-Ruiz JJ, and Hansen HS. Anandamide, but not 2-arachidonoylglycerol, accumulates during in vivo neurodegeneration. J Neurochem 78: 1415–1427, 2001 [DOI] [PubMed] [Google Scholar]
- 119.Hansen HS, Lauritzen L, Strand AM, Moesgaard B, and Frandsen A. Glutamate stimulates the formation of N-acylphosphatidylethanolamine and N-acylethanolamine in cortical neurons in culture. Biochim Biophys Acta 1258: 303–308, 1995 [DOI] [PubMed] [Google Scholar]
- 120.Hanuš L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, and Mechoulam R. 2-Arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci U S A 98: 3662–3665, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hanus L, Gopher A, Almog S, and Mechoulam R. Two new unsaturated fatty acid ethanolamides in brain that bind to the cannabinoid receptor. J Med Chem 36: 3032–3034, 1993 [DOI] [PubMed] [Google Scholar]
- 122.Hayakawa K, Mishima K, Abe K, Hasebe N, Takamatsu F, Yasuda H, Ikeda T, Inui K, Egashira N, Iwasaki K, and Fujiwara M. Cannabidiol prevents infarction via the non-CB1 cannabinoid receptor mechanism. Neuroreport 15: 2381–2385, 2004 [DOI] [PubMed] [Google Scholar]
- 123.Hayakawa K, Mishima K, Irie K, Hazekawa M, Mishima S, Fujioka M, Orito K, Egashira N, Katsurabayashi S, Takasaki K, Iwasaki K, and Fujiwara M. Cannabidiol prevents a post-ischemic injury progressively induced by cerebral ischemia via a high-mobility group box1-inhibiting mechanism. Neuropharmacology 55: 1280–1286, 2008 [DOI] [PubMed] [Google Scholar]
- 124.Hayakawa K, Mishima K, Nozako M, Hazekawa M, Irie K, Fujioka M, Orito K, Abe K, Hasebe N, Egashira N, Iwasaki K, and Fujiwara M. Delayed treatment with cannabidiol has a cerebroprotective action via a cannabinoid receptor-independent myeloperoxidase-inhibiting mechanism. J Neurochem 102: 1488–1496, 2007 [DOI] [PubMed] [Google Scholar]
- 125.Hebert-Chatelain E, Reguero L, Puente N, Lutz B, Chaouloff F, Rossignol R, Piazza PV, Benard G, Grandes P, and Marsicano G. Cannabinoid control of brain bioenergetics: exploring the subcellular localization of the CB1 receptor. Mol Metab 3: 495–504, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Heimann AS, Gomes I, Dale CS, Pagano RL, Gupta A, de Souza LL, Luchessi AD, Castro LM, Giorgi R, Rioli V, Ferro ES, and Devi LA. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci U S A 104: 20588–20593, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Henry DJ. and Chavkin C. Activation of inwardly rectifying potassium channels (GIRK1) by co-expressed rat brain cannabinoid receptors in Xenopus oocytes. Neurosci Lett 186: 91–94, 1995 [DOI] [PubMed] [Google Scholar]
- 128.Henry RJ, Kerr DM, Finn DP, and Roche M. FAAH-mediated modulation of TLR3-induced neuroinflammation in the rat hippocampus. J Neuroimmunol 276: 126–134, 2014 [DOI] [PubMed] [Google Scholar]
- 129.Hensley K, Maidt ML, Yu Z, Sang H, Markesbery WR, and Floyd RA. Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer brain indicates region-specific accumulation. J Neurosci 18: 8126–8132, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hill AJ, Williams CM, Whalley BJ, and Stephens GJ. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol Ther 133: 79–97, 2012 [DOI] [PubMed] [Google Scholar]
- 131.Hill KE, Zollinger LV, Watt HE, Carlson NG, and Rose JW. Inducible nitric oxide synthase in chronic active multiple sclerosis plaques: distribution, cellular expression and association with myelin damage. J Neuroimmunol 151: 171–179, 2004 [DOI] [PubMed] [Google Scholar]
- 132.Hillard CJ, Edgemond WS, Jarrahian A, and Campbell WB. Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. J Neurochem 69: 631–638, 1997 [DOI] [PubMed] [Google Scholar]
- 133.Hillard CJ. and Jarrahian A. Cellular accumulation of anandamide: consensus and controversy. Br J Pharmacol 140: 802–808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Holm L, Cassidy JD, Carroll LJ, and Borg J. Neurotrauma task force on mild traumatic brain injury of the WHOCC. Summary of the WHO collaborating centre for neurotrauma task force on mild traumatic brain injury. J Rehabil Med 37: 137–141, 2005 [DOI] [PubMed] [Google Scholar]
- 135.Hooper DC, Spitsin S, Kean RB, Champion JM, Dickson GM, Chaudhry I, and Koprowski H. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci U S A 95: 675–680, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Horiguchi T, Uryu K, Giasson BI, Ischiropoulos H, LightFoot R, Bellmann C, Richter-Landsberg C, Lee VM-Y, and Trojanowski JQ. Nitration of tau protein is linked to neurodegeneration in tauopathies. Am J Pathol 163: 1021–1031, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Horvath B, Magid L, Mukhopadhyay P, Batkai S, Rajesh M, Park O, Tanchian G, Gao RY, Goodfellow CE, Glass M, Mechoulam R, and Pacher P. A new cannabinoid CB2 receptor agonist HU-910 attenuates oxidative stress, inflammation and cell death associated with hepatic ischaemia/reperfusion injury. Br J Pharmacol 165: 2462–2478, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Horváth B, Mukhopadhyay P, Haskó G, and Pacher P. The endocannabinoid system and plant-derived cannabinoids in diabetes and diabetic complications. Am J Pathol 180: 432–442, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol 168: 53–79, 2005 [DOI] [PubMed] [Google Scholar]
- 140.Hsu KL, Tsuboi K, Adibekian A, Pugh H, Masuda K, and Cravatt BF. DAGLbeta inhibition perturbs a lipid network involved in macrophage inflammatory responses. Nat Chem Biol 8: 999–1007, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hsu KL, Tsuboi K, Whitby LR, Speers AE, Pugh H, Inloes J, and Cravatt BF. Development and optimization of piperidyl-1,2,3-triazole ureas as selective chemical probes of endocannabinoid biosynthesis. J Med Chem 56: 8257–8269, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hu SS-J, Bradshaw HB, Chen JS-C, Tan B, and Walker JM. Prostaglandin E2 glycerol ester, an endogenous COX-2 metabolite of 2-arachidonoylglycerol, induces hyperalgesia and modulates NFκB activity. Br J Pharmacol 153: 1538–1549, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hu SS-J. and Mackie K. Distribution of the endocannabinoid system in the central nervous system. Handb Exp Pharmacol 231: 59–93, 2015 [DOI] [PubMed] [Google Scholar]
- 144.Ignatowska-Jankowska BM, Ghosh S, Crowe MS, Kinsey SG, Niphakis MJ, Abdullah RA, Tao Q, ST ON, Walentiny DM, Wiley JL, Cravatt BF, and Lichtman AH. In vivo characterization of the highly selective monoacylglycerol lipase inhibitor KML29: antinociceptive activity without cannabimimetic side effects. Br J Pharmacol 171: 1392–1407, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ischiropoulos H. and Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest 111: 163–169, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Iuvone T, Esposito G, De Filippis D, Scuderi C, and Steardo L. Cannabidiol: a promising drug for neurodegenerative disorders? CNS Neurosci Ther 15: 65–75, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Iuvone T, Esposito G, Esposito R, Santamaria R, Di Rosa M, and Izzo AA. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J Neurochem 89: 134–141, 2004 [DOI] [PubMed] [Google Scholar]
- 148.Ivanova DG. and Yankova TM. The free radical theory of aging in search of a strategy for increasing life span. Folia Med 55: 33–41, 2013 [DOI] [PubMed] [Google Scholar]
- 149.Izzo AA, Borrelli F, Capasso R, Di Marzo V, and Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci 30: 515–527, 2009 [DOI] [PubMed] [Google Scholar]
- 150.Jackson SJ, Pryce G, Diemel LT, Cuzner ML, and Baker D. Cannabinoid-receptor 1 null mice are susceptible to neurofilament damage and caspase 3 activation. Neuroscience 134: 261–268, 2005 [DOI] [PubMed] [Google Scholar]
- 151.Jayant S, Sharma BM, Bansal R, and Sharma B. Pharmacological benefits of selective modulation of cannabinoid receptor type 2 (CB2) in experimental Alzheimer's disease. Pharmacol Biochem Behav 140: 39–50, 2016 [DOI] [PubMed] [Google Scholar]
- 152.Jenkins CM, Cedars A, and Gross RW. Eicosanoid signalling pathways in the heart. Cardiovasc Res 82: 240–249, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jia J, Ma L, Wu M, Zhang L, Zhang X, Zhai Q, Jiang T, Wang Q, and Xiong L. Anandamide protects HT22 cells exposed to hydrogen peroxide by inhibiting CB1 receptor-mediated type 2 NADPH oxidase. Oxid Med Cell Longev 2014: 893516, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, Riddick M, Dowell S, Staton PC, Green P, Shabon U, Bao W, Aiyar N, Yue T-L, Brown AJ, Morrison AD, and Douglas SA. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br J Pharmacol 152: 825–831, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jourdan T, Szanda G, Rosenberg AZ, Tam J, Earley BJ, Godlewski G, Cinar R, Liu Z, Liu J, Ju C, Pacher P, and Kunos G. Overactive cannabinoid 1 receptor in podocytes drives type 2 diabetic nephropathy. Proc Natl Acad Sci U S A 111: E5420–E5428, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Juan-Picó P, Fuentes E, Javier Bermúdez-Silva F, Javier Díaz-Molina F, Ripoll C, Rodríguez de Fonseca F, and Nadal A. Cannabinoid receptors regulate Ca2+ signals and insulin secretion in pancreatic β-cell. Cell Calcium 39: 155–162, 2006 [DOI] [PubMed] [Google Scholar]
- 157.Juknat A, Pietr M, Kozela E, Rimmerman N, Levy R, Coppola G, Geschwind D, and Vogel Z. Differential transcriptional profiles mediated by exposure to the cannabinoids cannabidiol and Delta9-tetrahydrocannabinol in BV-2 microglial cells. Br J Pharmacol 165: 2512–2528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Julien B, Grenard P, Teixeira-Clerc F, Van Nhieu JT, Li L, Karsak M, Zimmer A, Mallat A, and Lotersztajn S. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 128: 742–755, 2005 [DOI] [PubMed] [Google Scholar]
- 159.Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, Mackie K, and Piomelli D. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol 68: 1196–1202, 2005 [DOI] [PubMed] [Google Scholar]
- 160.Kaczocha M, Glaser ST, Chae J, Brown DA, and Deutsch DG. Lipid droplets are novel sites of N-acylethanolamine inactivation by fatty acid amide hydrolase-2. J Biol Chem 285: 2796–2806, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Katona I. and Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med 14: 923–930, 2008 [DOI] [PubMed] [Google Scholar]
- 162.Keith JM, Jones WM, Tichenor M, Liu J, Seierstad M, Palmer JA, Webb M, Karbarz M, Scott BP, Wilson SJ, Luo L, Wennerholm ML, Chang L, Rizzolio M, Rynberg R, Chaplan SR, and Breitenbucher JG. Preclinical characterization of the FAAH inhibitor JNJ-42165279. ACS Med Chem Lett 6: 1204–1208, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim GH, Kim JE, Rhie SJ, and Yoon S. The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol 24: 325–340, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim HJ, Shin AH, and Thayer SA. Activation of cannabinoid type 2 receptors inhibits HIV-1 envelope glycoprotein gp120-induced synapse loss. Mol Pharmacol 80: 357–366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim SH, Won SJ, Mao XO, Jin K, and Greenberg DA. Involvement of protein kinase A in cannabinoid receptor-mediated protection from oxidative neuronal injury. J Pharmacol Exp Ther 313: 88–94, 2005 [DOI] [PubMed] [Google Scholar]
- 166. This reference has been deleted.
- 167.Klegeris A, Bissonnette CJ, and McGeer PL. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br J Pharmacol 139: 775–786, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Koda N, Tsutsui Y, Niwa H, Ito S, Woodward DF, and Watanabe K. Synthesis of prostaglandin F ethanolamide by prostaglandin F synthase and identification of Bimatoprost as a potent inhibitor of the enzyme: new enzyme assay method using LC/ESI/MS. Arch Biochem Biophys 424: 128–136, 2004 [DOI] [PubMed] [Google Scholar]
- 169.Kong J. and Xu Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. J Neurosci 18: 3241–3250, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Koppel J, Vingtdeux V, Marambaud P, d'Abramo C, Jimenez H, Stauber M, Friedman R, and Davies P. CB2 receptor deficiency increases amyloid pathology and alters tau processing in a transgenic mouse model of Alzheimer's disease. Mol Med 20: 29–36, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kozak KR, Crews BC, Morrow JD, Wang L-H, Ma YH, Weinander R, Jakobsson P-J, and Marnett LJ. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem 277: 44877–44885, 2002 [DOI] [PubMed] [Google Scholar]
- 172.Kozak KR, Gupta RA, Moody JS, Ji C, Boeglin WE, DuBois RN, Brash AR, and Marnett LJ. 15-Lipoxygenase metabolism of 2-arachidonylglycerol generation of a peroxisome proliferator-activated receptor α agonist. J Biol Chem 277: 23278–23286, 2002 [DOI] [PubMed] [Google Scholar]
- 173.Kozak KR, Rowlinson SW, and Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem 275: 33744–33749, 2000 [DOI] [PubMed] [Google Scholar]
- 174.Kozela E, Lev N, Kaushansky N, Eilam R, Rimmerman N, Levy R, Ben-Nun A, Juknat A, and Vogel Z. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br J Pharmacol 163: 1507–1519, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kozela E, Pietr M, Juknat A, Rimmerman N, Levy R, and Vogel Z. Cannabinoids Delta(9)-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-kappaB and interferon-beta/STAT proinflammatory pathways in BV-2 microglial cells. J Biol Chem 285: 1616–1626, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kuhn DM, Sadidi M, Liu X, Kreipke C, Geddes T, Borges C, and Watson JT. Peroxynitrite-induced nitration of tyrosine hydroxylase: identification of tyrosines 423, 428, and 432 as sites of modification by matrix-assisted laser desorption ionization time-of-flight mass spectrometry and tyrosine-scanning mutagenesis. J Biol Chem 277: 14336–14342, 2002 [DOI] [PubMed] [Google Scholar]
- 177.Kunos G, Járai Z, Bátkai S, Goparaju SK, Ishac EJN, Liu J, Wang L, and Wagner JA. Endocannabinoids as cardiovascular modulators. Chem Phys Lipids 108: 159–168, 2000 [DOI] [PubMed] [Google Scholar]
- 178.Lambeth JD. and Neish AS. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu Rev Pathol 9: 119–145, 2014 [DOI] [PubMed] [Google Scholar]
- 179.Laprairie RB, Bagher AM, Kelly ME, and Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol 172: 4790–4805, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Laprairie RB, Bagher AM, Kelly ME, and Denovan-Wright EM. Biased type 1 cannabinoid receptor signaling influences neuronal viability in a cell culture model of Huntington disease. Mol Pharmacol 89: 364–375, 2016 [DOI] [PubMed] [Google Scholar]
- 181.Laprairie RB, Bagher AM, Kelly ME, Dupre DJ, and Denovan-Wright EM. Type 1 cannabinoid receptor ligands display functional selectivity in a cell culture model of striatal medium spiny projection neurons. J Biol Chem 289: 24845–24862, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Laprairie RB, Kelly ME, and Denovan-Wright EM. Cannabinoids increase type 1 cannabinoid receptor expression in a cell culture model of striatal neurons: implications for Huntington's disease. Neuropharmacology 72: 47–57, 2013 [DOI] [PubMed] [Google Scholar]
- 183.Lastres-Becker I, Bizat N, Boyer F, Hantraye P, Brouillet E, and Fernandez-Ruiz J. Effects of cannabinoids in the rat model of Huntington's disease generated by an intrastriatal injection of malonate. Neuroreport 14: 813–816, 2003 [DOI] [PubMed] [Google Scholar]
- 184.Lastres-Becker I, Molina-Holgado F, Ramos JA, Mechoulam R, and Fernández-Ruiz J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson's disease. Neurobiol Dis 19: 96–107, 2005 [DOI] [PubMed] [Google Scholar]
- 185.Leker RR, Gai N, Mechoulam R, and Ovadia H. Drug-induced hypothermia reduces ischemic damage: effects of the cannabinoid HU-210. Stroke; a J Cereb Circ 34: 2000–2006, 2003 [DOI] [PubMed] [Google Scholar]
- 186. This reference has been deleted.
- 187.Ligresti A, De Petrocellis L, and Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol Rev 96: 1593–1659, 2016 [DOI] [PubMed] [Google Scholar]
- 188.Lipton P. Ischemic cell death in brain neurons. Physiol Rev 79: 1431–1568, 1999 [DOI] [PubMed] [Google Scholar]
- 189.Liu J, Gao B, Mirshahi F, Sanyal AJ, Khanolkar AD, Makriyannis A, and Kunos G. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem J 346: 835–840, 2000 [PMC free article] [PubMed] [Google Scholar]
- 190.Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim H-Y, and Kunos G. A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A 103: 13345–13350, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lopez-Rodriguez AB, Acaz-Fonseca E, Viveros MP, and Garcia-Segura LM. Changes in cannabinoid receptors, aquaporin 4 and vimentin expression after traumatic brain injury in adolescent male mice. Association with edema and neurological deficit. PLoS One 10: e0128782, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lorente Fernandez L, Monte Boquet E, Perez-Miralles F, Gil Gomez I, Escutia Roig M, Bosca Blasco I, Poveda Andres JL, and Casanova-Estruch B. Clinical experiences with cannabinoids in spasticity management in multiple sclerosis. Neurologia 29: 257–260, 2014 [DOI] [PubMed] [Google Scholar]
- 193.Luth HJ, Holzer M, Gartner U, Staufenbiel M, and Arendt T. Expression of endothelial and inducible NOS-isoforms is increased in Alzheimer's disease, in APP23 transgenic mice and after experimental brain lesion in rat: evidence for an induction by amyloid pathology. Brain Res 913: 57–67, 2001 [DOI] [PubMed] [Google Scholar]
- 194.Luth HJ, Munch G, and Arendt T. Aberrant expression of NOS isoforms in Alzheimer's disease is structurally related to nitrotyrosine formation. Brain Res 953: 135–143, 2002 [DOI] [PubMed] [Google Scholar]
- 195.Lynn AB. and Herkenham M. Localization of cannabinoid receptors and nonsaturable high-density cannabinoid binding sites in peripheral tissues of the rat: implications for receptor-mediated immune modulation by cannabinoids. J Pharmacol Exp Ther 268: 1612–1623, 1994 [PubMed] [Google Scholar]
- 196.Ma L, Jia J, Niu W, Jiang T, Zhai Q, Yang L, Bai F, Wang Q, and Xiong L. Mitochondrial CB1 receptor is involved in ACEA-induced protective effects on neurons and mitochondrial functions. Sci Rep 5: 12440, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Maccarrone M, Bab I, Biro T, Cabral GA, Dey SK, Di Marzo V, Konje JC, Kunos G, Mechoulam R, Pacher P, Sharkey KA, and Zimmer A. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci 36: 277–296, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Maccarrone M, Guzman M, Mackie K, Doherty P, and Harkany T. Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nat Rev Neurosci 15: 786–801, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mallat A. and Lotersztajn S. Endocannabinoids and liver disease. I. Endocannabinoids and their receptors in the liver. Ame J Physiol Gastrointest Liver Physiol 294: G9–G12, 2008 [DOI] [PubMed] [Google Scholar]
- 200.Mallipeddi S, Janero DR, Zvonok N, and Makriyannis A. Functional selectivity at G-protein coupled receptors: advancing cannabinoid receptors as drug targets. Biochem Pharmacol 128: 1–11, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, and Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem 95: 437–445, 2005 [DOI] [PubMed] [Google Scholar]
- 202.Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, and Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302: 84–88, 2003 [DOI] [PubMed] [Google Scholar]
- 203.Marsicano G, Moosmann B, Hermann H, Lutz B, and Behl C. Neuroprotective properties of cannabinoids against oxidative stress: role of the cannabinoid receptor CB1. J Neurochem 80: 448–456, 2002 [DOI] [PubMed] [Google Scholar]
- 204.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, and Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346: 561–564, 1990 [DOI] [PubMed] [Google Scholar]
- 205.Matthews AT, Lee JH, Borazjani A, Mangum LC, Hou X, and Ross MK. Oxyradical stress increases the biosynthesis of 2-arachidonoylglycerol: involvement of NADPH oxidase. Am J Physiol Cell Physiol 311: C960–C974, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Matthews AT. and Ross MK. Oxyradical stress, endocannabinoids, and atherosclerosis. Toxics 3: 481–498, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.McDougle DR, Kambalyal A, Meling DD, and Das A. Endocannabinoids anandamide and 2-arachidonoylglycerol are substrates for human CYP2J2 epoxygenase. J Pharmacol Exp Ther 351: 616–627, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.McFarland MJ, Porter AC, Rakhshan FR, Rawat DS, Gibbs RA, and Barker EL. A role for caveolae/lipid rafts in the uptake and recycling of the endogenous cannabinoid anandamide. J Biol Chem 279: 41991–41997, 2004 [DOI] [PubMed] [Google Scholar]
- 209.McPartland JM, Duncan M, Di Marzo V, and Pertwee RG. Are cannabidiol and Delta(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol 172: 737–753, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mecha M, Torrao AS, Mestre L, Carrillo-Salinas FJ, Mechoulam R, and Guaza C. Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Dis 3: e331, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, and Compton DR. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50: 83–90, 1995 [DOI] [PubMed] [Google Scholar]
- 212.Mechoulam R. and Gaoni Y. Hashish. IV. The isolation and structure of cannabinolic cannabidiolic and cannabigerolic acids. Tetrahedron 21: 1223–1229, 1965 [DOI] [PubMed] [Google Scholar]
- 213.Mechoulam R, Panikashvili D, and Shohami E. Cannabinoids and brain injury: therapeutic implications. Trends Mol Med 8: 58–61, 2002 [DOI] [PubMed] [Google Scholar]
- 214.Merighi S, Gessi S, Varani K, Simioni C, Fazzi D, Mirandola P, and Borea PA. Cannabinoid CB(2) receptors modulate ERK-1/2 kinase signalling and NO release in microglial cells stimulated with bacterial lipopolysaccharide. Br J Pharmacol 165: 1773–1788, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 215.Mesnage V, Houeto JL, Bonnet AM, Clavier I, Arnulf I, Cattelin F, Le Fur G, Damier P, Welter ML, and Agid Y. Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson disease. Clin Neuropharmacol 27: 108–110, 2004 [DOI] [PubMed] [Google Scholar]
- 216.Mhatre M, Floyd RA, and Hensley K. Oxidative stress and neuroinflammation in Alzheimer's disease and amyotrophic lateral sclerosis: common links and potential therapeutic targets. J Alzheimers Dis 6: 147–157, 2004 [DOI] [PubMed] [Google Scholar]
- 217.Mnich K, Finn DP, Dowd E, and Gorman AM. Inhibition by anandamide of 6-hydroxydopamine-induced cell death in PC12 cells. Int J Cell Biol 2010: 818497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Moncada S. and Bolanos JP. Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem 97: 1676–1689, 2006 [DOI] [PubMed] [Google Scholar]
- 219.Montecucco F, Lenglet S, Braunersreuther V, Burger F, Pelli G, Bertolotto M, Mach F, and Steffens S. CB(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J Mol Cell Cardiol 46: 612–620, 2009 [DOI] [PubMed] [Google Scholar]
- 220.Moody JS, Kozak KR, Ji C, and Marnett LJ. Selective oxygenation of the endocannabinoid 2-arachidonylglycerol by leukocyte-type 12-lipoxygenase. Biochemistry 40: 861–866, 2001 [DOI] [PubMed] [Google Scholar]
- 221.Moore SA, Nomikos GG, Dickason-Chesterfield AK, Schober DA, Schaus JM, Ying BP, Xu YC, Phebus L, Simmons RM, Li D, Iyengar S, and Felder CC. Identification of a high-affinity binding site involved in the transport of endocannabinoids. Proc Natl Acad Sci U S A 102: 17852–17857, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Moreira FA. and Lutz B. The endocannabinoid system: emotion, learning and addiction. Addict Biol 13: 196–212, 2008 [DOI] [PubMed] [Google Scholar]
- 223.Moreira PI, Siedlak SL, Aliev G, Zhu X, Cash AD, Smith MA, and Perry G. Oxidative stress mechanisms and potential therapeutics in Alzheimer disease. J Neural Transm (Vienna) 112: 921–932, 2005 [DOI] [PubMed] [Google Scholar]
- 224.Moreno-Martet M, Espejo-Porras F, Fernandez-Ruiz J, and de Lago E. Changes in endocannabinoid receptors and enzymes in the spinal cord of SOD1(G93A) transgenic mice and evaluation of a Sativex((R)) -like combination of phytocannabinoids: interest for future therapies in amyotrophic lateral sclerosis. CNS Neurosci Ther 20: 809–815, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mukhopadhyay P, Baggelaar M, Erdelyi K, Cao Z, Cinar R, Fezza F, Ignatowska-Janlowska B, Wilkerson J, van Gils N, Hansen T, Ruben M, Soethoudt M, Heitman L, Kunos G, Maccarrone M, Lichtman A, Pacher P, and Van der Stelt M. The novel, orally available and peripherally restricted selective cannabinoid CB2 receptor agonist LEI-101 prevents cisplatin-induced nephrotoxicity. Br J Pharmacol 173: 446–458, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mukhopadhyay P, Bátkai S, Rajesh M, Czifra N, Harvey-White J, Haskó G, Zsengeller Z, Gerard NP, Liaudet L, Kunos G, and Pacher P. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol 50: 528–536, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mukhopadhyay P, Horváth B, Rajesh M, Matsumoto S, Saito K, Bátkai S, Patel V, Tanchian G, Gao RY, Cravatt BF, Haskó G, and Pacher P. Fatty acid amide hydrolase is a key regulator of the endocannabinoid-induced myocardial tissue injury. Free Radic Biol Med 50: 179–195, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mukhopadhyay P, Pan H, Rajesh M, Bátkai S, Patel V, Harvey-White J, Mukhopadhyay B, Haskó G, Gao B, Mackie K, and Pacher P. CB1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. Br J Pharmacol 160: 657–668, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Munro S, Thomas KL, and Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365: 61–65, 1993 [DOI] [PubMed] [Google Scholar]
- 230.Murakami S, Noguchi T, Takeda K, and Ichijo H. Stress signaling in cancer. Cancer Sci 98: 1521–1527, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Murphy N, Cowley TR, Blau CW, Dempsey CN, Noonan J, Gowran A, Tanveer R, Olango WM, Finn DP, Campbell VA, and Lynch MA. The fatty acid amide hydrolase inhibitor URB597 exerts anti-inflammatory effects in hippocampus of aged rats and restores an age-related deficit in long-term potentiation. J Neuroinflammation 9: 79, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Muthian S, Rademacher DJ, Roelke CT, Gross GJ, and Hillard CJ. Anandamide content is increased and CB1 cannabinoid receptor blockade is protective during transient, focal cerebral ischemia. Neuroscience 129: 743–750, 2004 [DOI] [PubMed] [Google Scholar]
- 233.Nagayama T, Sinor AD, Simon RP, Chen J, Graham SH, Jin K, and Greenberg DA. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J Neurosci 19: 2987–2995, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. This reference has been deleted.
- 235.Naoi M. and Maruyama W. Future of neuroprotection in Parkinson's disease. Parkinsonism Relat Disord 8: 139–145, 2001 [DOI] [PubMed] [Google Scholar]
- 236.Natarajan V, Schmid PC, and Schmid HH. N-acylethanolamine phospholipid metabolism in normal and ischemic rat brain. Biochim Biophys Acta 878: 32–41, 1986 [DOI] [PubMed] [Google Scholar]
- 237.Navarrete CM, Fiebich BL, de Vinuesa AG, Hess S, de Oliveira AC, Candelario-Jalil E, Caballero FJ, Calzado MA, and Munoz E. Opposite effects of anandamide and N-arachidonoyl dopamine in the regulation of prostaglandin E and 8-iso-PGF formation in primary glial cells. J Neurochem 109: 452–464, 2009 [DOI] [PubMed] [Google Scholar]
- 238.Navia-Paldanius D, Savinainen JR, and Laitinen JT. Biochemical and pharmacological characterization of human alpha/beta-hydrolase domain containing 6 (ABHD6) and 12 (ABHD12). J Lipid Res 53: 2413–2424, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Nguyen BM, Kim D, Bricker S, Bongard F, Neville A, Putnam B, Smith J, and Plurad D. Effect of marijuana use on outcomes in traumatic brain injury. Am Surg 80: 979–983, 2014 [PubMed] [Google Scholar]
- 240.Nickenig G. and Harrison DG. The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: part I: oxidative stress and atherogenesis. Circulation 105: 393–396, 2002 [DOI] [PubMed] [Google Scholar]
- 241.Nickenig G. and Harrison DG. The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: Part II: AT(1) receptor regulation. Circulation 105: 530–536, 2002 [DOI] [PubMed] [Google Scholar]
- 242.Nicolussi S. and Gertsch J. Endocannabinoid transport revisited. Vitam Horm 98: 441–485, 2015 [DOI] [PubMed] [Google Scholar]
- 243.Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Hahn YK, Lichtman AH, Conti B, and Cravatt BF. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 334: 809–813, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Oddi S, Fezza F, Pasquariello N, De Simone C, Rapino C, Dainese E, Finazzi-Agro A, and Maccarrone M. Evidence for the intracellular accumulation of anandamide in adiposomes. Cell Mol Life Sci 65: 840–850, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Oddi S, Latini L, Viscomi MT, Bisicchia E, Molinari M, and Maccarrone M. Distinct regulation of nNOS and iNOS by CB2 receptor in remote delayed neurodegeneration. J Mol Med (Berl) 90: 371–387, 2012 [DOI] [PubMed] [Google Scholar]
- 246.Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, Tam J, Attar-Namdar M, Kram V, Shohami E, Mechoulam R, Zimmer A, and Bab I. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci U S A 103: 696–701, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Bátkai S, Harvey-White J, Mackie K, Offertáler L, Wang L, and Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 115: 1298–1305, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Overton HA, Babbs AJ, Doel SM, Fyfe MCT, Gardner LS, Griffin G, Jackson HC, Procter MJ, Rasamison CM, Tang-Christensen M, Widdowson PS, Williams GM, and Reynet C. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab 3: 167–175, 2006 [DOI] [PubMed] [Google Scholar]
- 249.Pacher P, Bátkai S, and Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58: 389–462, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Pacher P, Beckman JS, and Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pacher P. and Haskó G. Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning. Br J Pharmacol 153: 252–262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Pacher P. and Kunos G. Modulating the endocannabinoid system in human health and disease—successes and failures. FEBS J 280: 1918–1943, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pacher P. and Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog Lipid Res 50: 193–211, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Palazuelos J, Davoust N, Julien B, Hatterer E, Aguado T, Mechoulam R, Benito C, Romero J, Silva A, Guzman M, Nataf S, and Galve-Roperh I. The CB(2) cannabinoid receptor controls myeloid progenitor trafficking: involvement in the pathogenesis of an animal model of multiple sclerosis. J Biol Chem 283: 13320–13329, 2008 [DOI] [PubMed] [Google Scholar]
- 255.Panikashvili D, Mechoulam R, Beni SM, Alexandrovich A, and Shohami E. CB1 cannabinoid receptors are involved in neuroprotection via NF-kappa B inhibition. J Cereb Blood Flow Metab 25: 477–484, 2005 [DOI] [PubMed] [Google Scholar]
- 256.Panikashvili D, Shein NA, Mechoulam R, Trembovler V, Kohen R, Alexandrovich A, and Shohami E. The endocannabinoid 2-AG protects the blood-brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol Dis 22: 257–264, 2006 [DOI] [PubMed] [Google Scholar]
- 257.Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, and Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature 413: 527–531, 2001 [DOI] [PubMed] [Google Scholar]
- 258.Pappolla MA, Omar RA, Kim KS, and Robakis NK. Immunohistochemical evidence of oxidative [corrected] stress in Alzheimer's disease. Am J Pathol 140: 621–628, 1992 [PMC free article] [PubMed] [Google Scholar]
- 259.Parmentier-Batteur S, Jin K, Mao XO, Xie L, and Greenberg DA. Increased severity of stroke in CB1 cannabinoid receptor knock-out mice. J Neurosci 22: 9771–9775, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. This reference has been deleted.
- 261.Parrish JC. and Nichols DE. Serotonin 5-HT2A receptor activation induces 2-arachidonoylglycerol release through a phospholipase c-dependent mechanism. J Neurochem 99: 1164–1175, 2006 [DOI] [PubMed] [Google Scholar]
- 262.Patel JZ, Ahenkorah S, Vaara M, Staszewski M, Adams Y, Laitinen T, Navia-Paldanius D, Parkkari T, Savinainen JR, Walczynski K, Laitinen JT, and Nevalainen TJ. Loratadine analogues as MAGL inhibitors. Bioorg Med Chem Lett 25: 1436–1442, 2015 [DOI] [PubMed] [Google Scholar]
- 263.Pazos MR, Cinquina V, Gomez A, Layunta R, Santos M, Fernandez-Ruiz J, and Martinez-Orgado J. Cannabidiol administration after hypoxia-ischemia to newborn rats reduces long-term brain injury and restores neurobehavioral function. Neuropharmacology 63: 776–783, 2012 [DOI] [PubMed] [Google Scholar]
- 264.Pazos MR, Mohammed N, Lafuente H, Santos M, Martinez-Pinilla E, Moreno E, Valdizan E, Romero J, Pazos A, Franco R, Hillard CJ, Alvarez FJ, and Martinez-Orgado J. Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: role of 5HT(1A) and CB2 receptors. Neuropharmacology 71: 282–291, 2013 [DOI] [PubMed] [Google Scholar]
- 265.Pegorini S, Zani A, Braida D, Guerini-Rocco C, and Sala M. Vanilloid VR1 receptor is involved in rimonabant-induced neuroprotection. Br J Pharmacol 147: 552–559, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Perez-De La Cruz V, Gonzalez-Cortes C, Galvan-Arzate S, Medina-Campos ON, Perez-Severiano F, Ali SF, Pedraza-Chaverri J, and Santamaria A. Excitotoxic brain damage involves early peroxynitrite formation in a model of Huntington's disease in rats: protective role of iron porphyrinate 5,10,15,20-tetrakis (4-sulfonatophenyl)porphyrinate iron (III). Neuroscience 135: 463–474, 2005 [DOI] [PubMed] [Google Scholar]
- 267.Persidsky Y, Fan S, Dykstra H, Reichenbach NL, Rom S, and Ramirez SH. Activation of cannabinoid type two receptors (CB2) diminish inflammatory responses in macrophages and brain endothelium. J Neuroimmune Pharmacol 10: 302–308, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol 1–51, 2005 [DOI] [PubMed] [Google Scholar]
- 269.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol 153: 199–215, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, and Ross RA. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol Rev 62: 588–631, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Pertwee RG, Ross RA, Craib SJ, and Thomas A. (−)-Cannabidiol antagonizes cannabinoid receptor agonists and noradrenaline in the mouse vas deferens. Eur J Pharmacol 456: 99–106, 2002 [DOI] [PubMed] [Google Scholar]
- 272.Pihlaja R, Takkinen J, Eskola O, Vasara J, Lopez-Picon FR, Haaparanta-Solin M, and Rinne JO. Monoacylglycerol lipase inhibitor JZL184 reduces neuroinflammatory response in APdE9 mice and in adult mouse glial cells. J Neuroinflammation 12: 81, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. This reference has been deleted.
- 274.Pitt D, Werner P, and Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med 6: 67–70, 2000 [DOI] [PubMed] [Google Scholar]
- 275.Porter AC, Sauer J-M, Knierman MD, Becker GW, Berna MJ, Bao J, Nomikos GG, Carter P, Bymaster FP, Leese AB, and Felder CC. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther 301: 1020–1024, 2002 [DOI] [PubMed] [Google Scholar]
- 276.Price DA, Martinez AA, Seillier A, Koek W, Acosta Y, Fernandez E, Strong R, Lutz B, Marsicano G, Roberts JL, and Giuffrida A. WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Eur J Neurosci 29: 2177–2186, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Pryce G, Ahmed Z, Hankey DJ, Jackson SJ, Croxford JL, Pocock JM, Ledent C, Petzold A, Thompson AJ, Giovannoni G, Cuzner ML, and Baker D. Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain 126: 2191–2202, 2003 [DOI] [PubMed] [Google Scholar]
- 278.Rabner J, Gottlieb S, Lazdowsky L, and LeBel A. Psychosis following traumatic brain injury and cannabis use in late adolescence. Am J Addict 25: 91–93, 2016 [DOI] [PubMed] [Google Scholar]
- 279.Rahimi A, Faizi M, Talebi F, Noorbakhsh F, Kahrizi F, and Naderi N. Interaction between the protective effects of cannabidiol and palmitoylethanolamide in experimental model of multiple sclerosis in C57BL/6 mice. Neuroscience 290: 279–287, 2015 [DOI] [PubMed] [Google Scholar]
- 280.Rajesh M, Mukhopadhyay P, Bátkai S, Haskó G, Liaudet L, Huffman JW, Csiszar A, Ungvari Z, Mackie K, Chatterjee S, and Pacher P. CB2-receptor stimulation attenuates TNF-α-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am J Physiol Heart Circ Physiol 293: H2210–H2218, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Rajesh M, Mukhopadhyay P, Hasko G, Liaudet L, Mackie K, and Pacher P. Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. Br J Pharmacol 160: 688–700, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ramirez SH, Hasko J, Skuba A, Fan S, Dykstra H, McCormick R, Reichenbach N, Krizbai I, Mahadevan A, Zhang M, Tuma R, Son YJ, and Persidsky Y. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J Neurosci 32: 4004–4016, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Rani V, Deep G, Singh RK, Palle K, and Yadav UCS. Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sci 148: 183–193, 2016 [DOI] [PubMed] [Google Scholar]
- 284.Rao SD. and Weiss JH. Excitotoxic and oxidative cross-talk between motor neurons and glia in ALS pathogenesis. Trends Neurosci 27: 17–23, 2004 [DOI] [PubMed] [Google Scholar]
- 285.Rao SD, Yin HZ, and Weiss JH. Disruption of glial glutamate transport by reactive oxygen species produced in motor neurons. J Neurosci 23: 2627–2633, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Holtta-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chio A, Restagno G, Borghero G, Sabatelli M, Consortium I, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, and Traynor BJ. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72: 257–268, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ribeiro R, Wen J, Li S, and Zhang Y. Involvement of ERK1/2, cPLA2 and NF-kappaB in microglia suppression by cannabinoid receptor agonists and antagonists. Prostaglandins Other Lipid Mediat 100–101: 1–14, 2013 [DOI] [PubMed] [Google Scholar]
- 288.Rodrigo J, Fernandez AP, Serrano J, Peinado MA, and Martinez A. The role of free radicals in cerebral hypoxia and ischemia. Free Radic Biol Med 39: 26–50, 2005 [DOI] [PubMed] [Google Scholar]
- 289.Rom S, Zuluaga-Ramirez V, Dykstra H, Reichenbach NL, Pacher P, and Persidsky Y. Selective activation of cannabinoid receptor 2 in leukocytes suppresses their engagement of the brain endothelium and protects the blood-brain barrier. Am J Pathol 183: 1548–1558, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Romero TRL, Resende LC, Guzzo LS, and Duarte IDG. CB1 and CB2 cannabinoid receptor agonists induce peripheral antinociception by activation of the endogenous noradrenergic system. Anesth Analg 116: 463–472, 2013 [DOI] [PubMed] [Google Scholar]
- 291.Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 364: 362, 1993 [DOI] [PubMed] [Google Scholar]
- 292.Ross MK, Matthews AT, and Mangum LC. Chemical atherogenesis: role of endogenous and exogenous poisons in disease development. Toxics 2: 17–34, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Rossi S, De Chiara V, Musella A, Cozzolino M, Bernardi G, Maccarrone M, Mercuri NB, Carri MT, and Centonze D. Abnormal sensitivity of cannabinoid CB1 receptors in the striatum of mice with experimental amyotrophic lateral sclerosis. Amyotroph Lateral Scler 11: 83–90, 2010 [DOI] [PubMed] [Google Scholar]
- 294.Rossi S, Furlan R, De Chiara V, Muzio L, Musella A, Motta C, Studer V, Cavasinni F, Bernardi G, Martino G, Cravatt BF, Lutz B, Maccarrone M, and Centonze D. Cannabinoid CB1 receptors regulate neuronal TNF-alpha effects in experimental autoimmune encephalomyelitis. Brain Behav Immun 25: 1242–1248, 2011 [DOI] [PubMed] [Google Scholar]
- 295.Sagredo O, Gonzalez S, Aroyo I, Pazos MR, Benito C, Lastres-Becker I, Romero JP, Tolon RM, Mechoulam R, Brouillet E, Romero J, and Fernandez-Ruiz J. Cannabinoid CB2 receptor agonists protect the striatum against malonate toxicity: relevance for Huntington's disease. Glia 57: 1154–1167, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Sagredo O, Ramos JA, Decio A, Mechoulam R, and Fernandez-Ruiz J. Cannabidiol reduced the striatal atrophy caused 3-nitropropionic acid in vivo by mechanisms independent of the activation of cannabinoid, vanilloid TRPV1 and adenosine A2A receptors. Eur J Neurosci 26: 843–851, 2007 [DOI] [PubMed] [Google Scholar]
- 297.Sarchielli P, Galli F, Floridi A, Floridi A, and Gallai V. Relevance of protein nitration in brain injury: a key pathophysiological mechanism in neurodegenerative, autoimmune, or inflammatory CNS diseases and stroke. Amino Acids 25: 427–436, 2003 [DOI] [PubMed] [Google Scholar]
- 298.Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, Thomas EA, Selley DE, Sim-Selley LJ, Liu QS, Lichtman AH, and Cravatt BF. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci 13: 1113–1119, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Scuderi C, Filippis DD, Iuvone T, Blasio A, Steardo A, and Esposito G. Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders. Phytother Res 23: 597–602, 2009 [DOI] [PubMed] [Google Scholar]
- 300.Serpell MG, Notcutt W, and Collin C. Sativex long-term use: an open-label trial in patients with spasticity due to multiple sclerosis. J Neurol 260: 285–295, 2013 [DOI] [PubMed] [Google Scholar]
- 301.Shao BZ, Wei W, Ke P, Xu ZQ, Zhou JX, and Liu C. Activating cannabinoid receptor 2 alleviates pathogenesis of experimental autoimmune encephalomyelitis via activation of autophagy and inhibiting NLRP3 inflammasome. CNS Neurosci Ther 20: 1021–1028, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Shen M, Piser TM, Seybold VS, and Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci 16: 4322–4334, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Shoemaker JL, Seely KA, Reed RL, Crow JP, and Prather PL. The CB2 cannabinoid agonist AM-1241 prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis when initiated at symptom onset. J Neurochem 101: 87–98, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Shohami E, Cohen-Yeshurun A, Magid L, Algali M, and Mechoulam R. Endocannabinoids and traumatic brain injury. Br J Pharmacol 163: 1402–1410, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Silvestri C. and Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab 17: 475–490, 2013 [DOI] [PubMed] [Google Scholar]
- 306.Silvestri C, Martella A, Poloso NJ, Piscitelli F, Capasso R, Izzo A, Woodward DF, and Di Marzo V. Anandamide-derived prostamide F2α negatively regulates adipogenesis. J Biol Chem 288: 23307–23321, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Simic G, Lucassen PJ, Krsnik Z, Kruslin B, Kostovic I, and Winblad B. Bogdanovi. nNOS expression in reactive astrocytes correlates with increased cell death related DNA damage in the hippocampus and entorhinal cortex in Alzheimer's disease. Exp Neurol 165: 12–26, 2000 [DOI] [PubMed] [Google Scholar]
- 308.Simon GM. and Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for α/β-Hydrolase 4 in this pathway. J Biol Chem 281: 26465–26472, 2006 [DOI] [PubMed] [Google Scholar]
- 309.Smith DH, Johnson VE, and Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 9: 211–221, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Snider NT, Nast JA, Tesmer LA, and Hollenberg PF. A cytochrome P450-derived epoxygenated metabolite of anandamide is a potent cannabinoid receptor 2-selective agonist. Mol Pharmacol 75: 965–972, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Soethoudt M, Grether U, Fingerle J, Grim TW, Fezza F, de Petrocellis L, Ullmer C, Rothenhausler B, Perret C, van Gils N, Finlay D, MacDonald C, Chicca A, Gens MD, Stuart J, de Vries H, Mastrangelo N, Xia L, Alachouzos G, Baggelaar MP, Martella A, Mock ED, Deng H, Heitman LH, Connor M, Di Marzo V, Gertsch J, Lichtman AH, Maccarrone M, Pacher P, Glass M, and van der Stelt M. Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat Commun 8: 13958, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Souza JM, Giasson BI, Chen Q, Lee VM, and Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha -synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J Biol Chem 275: 18344–18349, 2000 [DOI] [PubMed] [Google Scholar]
- 313.Sridar C, Snider NT, and Hollenberg PF. Anandamide oxidation by Wild-Type and polymorphically expressed CYP2B6 and CYP2D6. Drug Metab Dispos 39: 782–788, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Steffens S. and Pacher P. Targeting cannabinoid receptor CB2 in cardiovascular disorders: promises and controversies. Br J Pharmacol 167: 313–323, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Steffens S. and Pacher P. The activated endocannabinoid system in atherosclerosis: driving force or protective mechanism? Curr Drug Targets 16: 334–341, 2015 [DOI] [PubMed] [Google Scholar]
- 316.Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 58: 1017–1030, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Stella N, Schweitzer P, and Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature 388: 773–778, 1997 [DOI] [PubMed] [Google Scholar]
- 318.Strom AL, Shi P, Zhang F, Gal J, Kilty R, Hayward LJ, and Zhu H. Interaction of amyotrophic lateral sclerosis (ALS)-related mutant copper-zinc superoxide dismutase with the dynein-dynactin complex contributes to inclusion formation. J Biol Chem 283: 22795–22805, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Sugamura K, Sugiyama S, Nozaki T, Matsuzawa Y, Izumiya Y, Miyata K, Nakayama M, Kaikita K, Obata T, Takeya M, and Ogawa H. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation 119: 28–36, 2009 [DOI] [PubMed] [Google Scholar]
- 320.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, and Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215: 89–97, 1995 [DOI] [PubMed] [Google Scholar]
- 321.Sugiura T. and Waku K. Cannabinoid receptors and their endogenous ligands. J Biochem 132: 7–12, 2002 [DOI] [PubMed] [Google Scholar]
- 322.Tam J, Vemuri VK, Liu J, Bátkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, Makriyannis A, and Kunos G. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest 120: 2953–2966, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Tchantchou F, Tucker LB, Fu AH, Bluett RJ, McCabe JT, Patel S, and Zhang Y. The fatty acid amide hydrolase inhibitor PF-3845 promotes neuronal survival, attenuates inflammation and improves functional recovery in mice with traumatic brain injury. Neuropharmacology 85: 427–439, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Tithof PK, Olivero J, Ruehle K, and Ganey PE. Activation of neutrophil calcium-dependent and -independent phospholipases A2 by organochlorine compounds. Toxicol Sci 53: 40–47, 2000 [DOI] [PubMed] [Google Scholar]
- 325.Tiyerili V, Zimmer S, Jung S, Wassmann K, Naehle CP, Lutjohann D, Zimmer A, Nickenig G, and Wassmann S. CB1 receptor inhibition leads to decreased vascular AT1 receptor expression, inhibition of oxidative stress and improved endothelial function. Basic Res Cardiol 105: 465–477, 2010 [DOI] [PubMed] [Google Scholar]
- 326.Tong J, Mizrahi R, Houle S, Kish SJ, Boileau I, Nobrega J, Rusjan PM, and Wilson AA. Inhibition of fatty acid amide hydrolase by BIA 10-2474 in rat brain. J Cereb Blood Flow Metab 37: 3636–3639, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Torreilles F, Salman-Tabcheh S, Guerin M, and Torreilles J. Neurodegenerative disorders: the role of peroxynitrite. Brain Res Brain Res Rev 30: 153–163, 1999 [DOI] [PubMed] [Google Scholar]
- 328.Tu PH, Gurney ME, Julien JP, Lee VM, and Trojanowski JQ. Oxidative stress, mutant SOD1, and neurofilament pathology in transgenic mouse models of human motor neuron disease. Lab Invest 76: 441–456, 1997 [PubMed] [Google Scholar]
- 329.Turu G, Simon A, Gyombolai P, Szidonya L, Bagdy G, Lenkei Z, and Hunyady L. The role of diacylglycerol lipase in constitutive and angiotensin AT1 receptor-stimulated cannabinoid CB1 receptor activity. J Biol Chem 282: 7753–7757, 2007 [DOI] [PubMed] [Google Scholar]
- 330.Ueda N, Yamamoto K, Yamamoto S, Tokunaga T, Shirakawa E, Shinkai H, Ogawa M, Sato T, Kudo I, Inoue K, Takizawa H, Nagano T, Hirobe M, Matsuki N, and Saito H. Lipoxygenase-catalyzed oxygenation of arachidonylethanolamide, a cannabinoid receptor agonist. Biochim Biophys Acta 1254: 127–134, 1995 [DOI] [PubMed] [Google Scholar]
- 331.Valdeolivas S, Pazos MR, Bisogno T, Piscitelli F, Iannotti FA, Allara M, Sagredo O, Di Marzo V, and Fernandez-Ruiz J. The inhibition of 2-arachidonoyl-glycerol (2-AG) biosynthesis, rather than enhancing striatal damage, protects striatal neurons from malonate-induced death: a potential role of cyclooxygenase-2-dependent metabolism of 2-AG. Cell Death Dis 4: e862, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, and Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84, 2007 [DOI] [PubMed] [Google Scholar]
- 333.van der Stelt M, Mazzola C, Esposito G, Matias I, Petrosino S, De Filippis D, Micale V, Steardo L, Drago F, Iuvone T, and Di Marzo V. Endocannabinoids and beta-amyloid-induced neurotoxicity in vivo: effect of pharmacological elevation of endocannabinoid levels. Cell Mol Life Sci 63: 1410–1424, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Van Laere K, Casteels C, Dhollander I, Goffin K, Grachev I, Bormans G, and Vandenberghe W. Widespread decrease of type 1 cannabinoid receptor availability in Huntington disease in vivo. J Nucl Med 51: 1413–1417, 2010 [DOI] [PubMed] [Google Scholar]
- 335.van Noort JM. Stress proteins in CNS inflammation. J Pathol 214: 267–275, 2008 [DOI] [PubMed] [Google Scholar]
- 336.Varga ZV, Giricz Z, Liaudet L, Haskó G, Ferdinandy P, and Pacher P. Interplay of oxidative, nitrosative/nitrative stress, inflammation, cell death and autophagy in diabetic cardiomyopathy. Biochim Biophys Acta 1852: 232–242, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Velasco G, Sánchez C, and Guzmán M. Endocannabinoids and Cancer. Handb Exp Pharmacol 231: 449–472, 2015 [DOI] [PubMed] [Google Scholar]
- 338.Viscomi MT, Oddi S, Latini L, Pasquariello N, Florenzano F, Bernardi G, Molinari M, and Maccarrone M. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J Neurosci 29: 4564–4570, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Volicer L, Stelly M, Morris J, McLaughlin J, and Volicer BJ. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer's disease. Int J Geriatr Psychiatry 12: 913–919, 1997 [PubMed] [Google Scholar]
- 340.Walker JM, Krey JF, Chu CJ, and Huang SM. Endocannabinoids and related fatty acid derivatives in pain modulation. Chem Phys Lipids 121: 159–172, 2002 [DOI] [PubMed] [Google Scholar]
- 341.Walter L, Franklin A, Witting A, Moller T, and Stella N. Astrocytes in culture produce anandamide and other acylethanolamides. J Biol Chem 277: 20869–20876, 2002 [DOI] [PubMed] [Google Scholar]
- 342.Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, and Stella N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci 23: 1398–1405, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Weber M, Goldman B, and Truniger S. Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trial. J Neurol Neurosurg Psychiatry 81: 1135–1140, 2010 [DOI] [PubMed] [Google Scholar]
- 344.Weksler ME, Gouras G, Relkin NR, and Szabo P. The immune system, amyloid-beta peptide, and Alzheimer's disease. Immunol Rev 205: 244–256, 2005 [DOI] [PubMed] [Google Scholar]
- 345.Wiley JL, Beletskaya ID, Ng EW, Dai Z, Crocker PJ, Mahadevan A, Razdan RK, and Martin BR. Resorcinol derivatives: a novel template for the development of cannabinoid CB(1)/CB(2) and CB(2)-selective agonists. J Pharmacol Exp Ther 301: 679–689, 2002 [DOI] [PubMed] [Google Scholar]
- 346.Witting A, Walter L, Wacker J, Moller T, and Stella N. P2X7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc Natl Acad Sci U S A 101: 3214–3219, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Witting A, Weydt P, Hong S, Kliot M, Moller T, and Stella N. Endocannabinoids accumulate in spinal cord of SOD1 G93A transgenic mice. J Neurochem 89: 1555–1557, 2004 [DOI] [PubMed] [Google Scholar]
- 348.Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, and Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 14: 1105–1116, 1995 [DOI] [PubMed] [Google Scholar]
- 349.Woodcock T. and Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol 4: 18, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Xu Z, Lv XA, Dai Q, Ge YQ, and Xu J. Acute upregulation of neuronal mitochondrial type-1 cannabinoid receptor and it's role in metabolic defects and neuronal apoptosis after TBI. Mol Brain 9: 75, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Yates ML. and Barker EL. Inactivation and biotransformation of the endogenous cannabinoids anandamide and 2-arachidonoylglycerol. Mol Pharmacol 76: 11–17, 2009 [DOI] [PubMed] [Google Scholar]
- 352.Yates ML. and Barker EL. Organized trafficking of anandamide and related lipids. Vitam Horm 81: 25–53, 2009 [DOI] [PubMed] [Google Scholar]
- 353.Zelasko S, Arnold WR, and Das A. Endocannabinoid metabolism by cytochrome P450 monooxygenases. Prostaglandins Other Lipid Mediat 116–117: 112–123, 2015 [DOI] [PubMed] [Google Scholar]
- 354.Zemlan FP, Thienhaus OJ, and Bosmann HB. Superoxide dismutase activity in Alzheimer's disease: possible mechanism for paired helical filament formation. Brain Res 476: 160–162, 1989 [DOI] [PubMed] [Google Scholar]
- 355.Zetterberg H, Smith DH, and Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol 9: 201–210, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Zhang F, Challapalli SC, and Smith PJW. Cannabinoid CB(1) receptor activation stimulates neurite outgrowth and inhibits capsaicin-induced Ca(2+) influx in an in vitro model of diabetic neuropathy. Neuropharmacology 57: 88–96, 2009 [DOI] [PubMed] [Google Scholar]
- 357.Zhang H, Hilton DA, Hanemann CO, and Zajicek J. Cannabinoid receptor and N-acyl phosphatidylethanolamine phospholipase D—evidence for altered expression in multiple sclerosis. Brain Pathol 21: 544–557, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Zhang J. and Chen C. Endocannabinoid 2-arachidonoylglycerol protects neurons by limiting COX-2 elevation. J Biol Chem 283: 22601–22611, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Zhang J, Teng Z, Song Y, Hu M, and Chen C. Inhibition of monoacylglycerol lipase prevents chronic traumatic encephalopathy-like neuropathology in a mouse model of repetitive mild closed head injury. J Cereb Blood Flow Metab 35: 443–453, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Zhang M, Adler MW, Abood ME, Ganea D, Jallo J, and Tuma RF. CB2 receptor activation attenuates microcirculatory dysfunction during cerebral ischemic/reperfusion injury. Microvasc Res 78: 86–94, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Zhang M, Martin BR, Adler MW, Razdan RJ, Kong W, Ganea D, and Tuma RF. Modulation of cannabinoid receptor activation as a neuroprotective strategy for EAE and stroke. J Neuroimmune Pharmacol 4: 249–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Zhang M, Martin BR, Adler MW, Razdan RK, Jallo JI, and Tuma RF. Cannabinoid CB(2) receptor activation decreases cerebral infarction in a mouse focal ischemia/reperfusion model. J Cereb Blood Flow Metab 27: 1387–1396, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Zhao P, Ignacio S, Beattie EC, and Abood ME. Altered presymptomatic AMPA and cannabinoid receptor trafficking in motor neurons of ALS model mice: implications for excitotoxicity. Eur J Neurosci 27: 572–579, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Zhao P, Leonoudakis D, Abood ME, and Beattie EC. Cannabinoid receptor activation reduces TNFalpha-induced surface localization of AMPAR-type glutamate receptors and excitotoxicity. Neuropharmacology 58: 551–558, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Zygmunt PM, Petersson J, Andersson DA, Chuang H-h, Sørgård M, Di Marzo V, Julius D, and Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400: 452–457, 1999 [DOI] [PubMed] [Google Scholar]