Abstract
Purpose
Mutations in Fanconi anemia (FA) genes are common in sporadic squamous cell carcinoma of the head and neck (HNSCC) and we have previously demonstrated that FA pathway depletion in HNSCC cell lines stimulates invasion. The goal of our studies was to use a systems approach in order to define FA pathway-dependent lipid metabolism, and to extract lipid-based signatures and effectors of invasion in FA-deficient cells.
Experimental Design
We subjected FA-isogenic HNSCC keratinocyte cell lines to untargeted and targeted lipidomics analyses to discover novel biomarkers and candidate therapeutic targets in FA-deficient cells. Cellular invasion assays were carried out in the presence and absence of N-butyldeoxynojirimycin (NB-DNJ), a biosynthetic inhibitor of the newly identified class of gangliosides, to investigate the requirement of ganglioside upregulation in FA-deficient HNSCC cells.
Results
The most notable element of the lipid profiling results was a consistent elevation of glycosphingolipids, and particularly the accumulation of gangliosides. Conversely, repression of this same class of lipids was observed upon genetic correction of FA patient derived HNSCC cells. Functional studies demonstrate that ganglioside upregulation is required for HNSCC cell invasion driven by FA pathway loss. The motility of non-transformed keratinocytes in response to FA loss displayed a similar dependence, thus supporting early and late roles for the FA pathway in controlling keratinocyte invasion through lipid regulation.
Conclusions
Elevation of glycosphingolipids including the ganglioside GM3 in response to FA loss stimulates invasive characteristics of immortalized and transformed keratinocytes. An inhibitor of glycosphingolipid biosynthesis NB-DNJ attenuates invasive characteristics of FA-deficient HNSCC cells.
Introduction
The Fanconi anemia pathway comprises 22 FA and many more FA-associated genes and their respective protein products (1–3). This pathway is essential for the repair of DNA interstrand crosslinks (4–7) with additional nuclear functions (8) in the suppression of non-homologous end joining (9,10), stabilization of DNA replication forks (11), and cell cycle progression (8,12). Loss of function of any of the 22 FA genes in the germline causes a pediatric disorder FA that is characterized by cellular and organismal sensitivity to inter-strand crosslinks (ICLs) (13), bone marrow failure with selection for acute myeloid leukemia, and the development of HNSCCs early in life with exceedingly poor outcomes (14–16). The FA pathway has been intensely studied for its molecular activities in genome repair (17–21). In response to crosslinks or stalled replication forks, the protein products of 8 FA genes, including FANCA, assemble near the lesion, forming the “FA core complex.” This complex triggers monoubiquitination and dimerization of 2 central pathway components FANCD2 and FANCI. The activated dimer then orchestrates recruitment of downstream endonucleases and specialized repair proteins – including the breast cancer susceptibility proteins BRCA1/FANCS, BRCA2/FANCD1, and BRIP/FANCJ (22). Whereas mechanistic aspects of FA complex formation and function have been well documented, it remains unclear why FA pathway loss causes aggressive tumor phenotypes specifically in squamous epithelium of the head and neck, anogenital tract and skin (16).
In the general population, HNSCC is the sixth most common human cancer type worldwide, and arises from epidermal keratinocytes (23–25). This cancer type alone accounts for 650,000 new cases diagnosed annually, and approximately 350,000 associated deaths (26,27). HNSCC includes tumors of the hypopharynx, oropharynx, nasopharynx and oral cavity, and is primarily caused by smoking, alcohol consumption and infection with high risk human papillomavirus type 16. Approximately 50% of patients first present with locally advanced or metastatic disease, associated with high morbidity and mortality. New biomarkers and therapeutic targets for improved detection and new treatments of invasive disease are urgently needed. We have recently associated the loss of FA gene function in HNSCCs that harbor a functional pathway with the acquisition of invasive characteristics (28). Furthermore, a significant proportion of sporadic HNSCCs harbor transcriptional repression and nonsynonymous mutations in FA and FA-related genes (28–30). In order to test the biological effects of FA pathway loss in this context, we published FA knockdown models of HNSCC using lentiviral shRNA vectors (28,29). FA gene loss stimulated cellular conversion of HNSCC cells from an epithelial to mesenchymal morphology, as well enhanced cell motility and invasion in transwell assays. These phenotypes were dependent on DNA-PK/Rac1 signaling and were reversed by shRNA-resistant FA gene expression. Similar data were obtained across a panel of HPV+ and HPV− cell lines, and in response to the knockdown of FANCA, FANCD2 and FANCJ. Taken together, FA gene loss stimulates HNSCC cell invasion, a major cause of cancer-related mortality in humans. The observed invasion in response to FA loss was linked to the formation of unique plasma membrane projections (28), and lipid dysregulation in the cellular plasma membrane is key to cancer cell invasion (31). Thus, the goal of our studies was to use a systems approach in order to define FA pathway-dependent lipid metabolism, and to extract lipid-based signatures and effectors of aggressive disease in FA-deficient cells.
Materials and Methods
Cell culture
Near diploid immortalized keratinocytes that form skin (NIKS), VU1365 cells, HPV-negative UM-SCC1 and HPV16 positive UM-SCC47 cell lines, were maintained as previously described (28,32–34). Irradiated J2-3T3 mouse fibroblasts were used as feeder cells for NIKS. Lentiviral FANCD2sh and FANCJsh gene knockdown was performed in NIKS, NOKS, UM-SCC1 and UM-SCC47 as a model of sporadic FA pathway loss. In each case, control cells were transduced in parallel with a non-targeting control vector (NTsh) as previously described (34). The VU1365 cell line was originally derived from an FA patient and was genetically corrected for the mutant FANCA gene using retroviral transduction, or was control transduced (33). J2-3T3, UM-SCC1, UM-SCC47 cells, and NIKS were initially obtained from the Lambert laboratory, and VU1365 cells were obtained from the de Winter laboratory (VU University Medical Cancer, Amsterdam, The Netherlands). NOKS were obtained from the Khan laboratory (University of Pittsburgh Medical Center, Pittsburgh, PA, USA). These cell lines were published in collaboration with the laboratories from which they were received. NOKS and NIKS are routinely tested for differentiation capacity in organotypic epithelial rafts. Routine testing for FA gene expression was performed in all isogenic cell pairs. No other testing was performed. The number of passages between thawing and use in the described experiments was below 12.
Metabolite standards and drugs
Ganglioside standards from ovine brain (GM1), bovine brain (GD1) and bovine milk (GM3, GD3) were purchased from Avanti Polar Lipids (Alabaster, AL) and Sigma-Aldrich (St Louis, MO). All solvents were of mass spectrometry grade and deionized water was obtained from a Milli-Q water system (Millipore, Milford, MA). The ganglioside biosynthesis inhibitor N-Butyldeoxynojirimycin (NB-DNJ), also referred as Miglustat and Zavesca®, was purchased from Sigma-Aldrich (St. Louis, MO).
Sample preparation and untargeted metabolomics analysis
Cells were grown and then plated at equal cell numbers. Cells were then harvested, counted, and subjected to two consecutive washes in PBS, followed by centrifugation cycles. Cell pellets were extracted by chloroform:methanol:water (1:2:0.7, v/v/v) twice to obtain lipid metabolites. The extracts were dried under liquid nitrogen and reconstituted in mobile phase solution with normalization by cell number before injection. Untargeted metabolomics analysis was performed on a Xevo G2-S quadrupole time-of-flight (Q-TOF) mass spectrometer interfaced with ACQUITY ultra-high performance liquid chromatography (UPLC) system (Waters, Milford, MA). An Acquity CSH C18 UPLC column (2.1 × 100 mm, 1.7 μm) was used in separation. Detailed UPLC-ESI-MSe methods are described in the Supplementary Methods. Deconvolution, peak alignment, and preliminary normalization were conducted on raw metabolomics data with Progenesis QI™. Each compound ion feature, i.e. deconvoluted peak in the mass chromatogram, is annotated by elution time with m/z ratio. Raw data were normalized by total compound ion intensity and with a global scalar derived from the logarithm ratio of each sample to the reference. Accurate molecular mass (m/z) was used to search against the Human Metabolome Database (HMDB) for putative compound identification. Acquired metabolite ions were also searched against an in-house lipid database combined with retention time. Putative marker metabolites were confirmed by targeted UPLC-MS/MS methods using authenticated standards.
Targeted metabolite analysis
Separation and quantification of known metabolites were performed on a UPLC coupled with Premier triple-quadruple mass spectrometry (Waters, Milford, MA). An Acquity UPLC CSH C18 column (2.1×100 mm, 1.7μm, Waters, Milford, MA) was applied and the details are described in the Supplementary Methods section. Multiple Reaction Monitoring (MRM) analysis based on a theoretical expansion approach was performed for quantification of gangliosides GM3, GM1, GD3 and GD1. The precursors of ganglioside GM3, i.e. glucosylceramide and lactosylceramide were measured with a similar method but in positive ion mode. The precursor and product ion pairs for MRM monitoring were either selected by MS/MS spectra of standards, or calculated theoretically when standards for a given ceramide length were not available (Supplementary Table 1). The composition of individual species in the standards was determined by the ratio of standard infusion ion intensity or provided by the supplier. The liquid chromatography system LC-20A (Shimadzu, Kyoto, Japan) coupled with 5500 QTrap mass spectrometry (ABI-SCIEX, Toronto, Canada) using multiple reaction monitoring (MRM) was used for specific quantification of the ganglioside GD3. A Kinetex Phenyl-Hexyl (50×4.6mm, 2.6μm, 100A) HPLC column (Phenomenex, Torres, CA) was used for injection of 10 μL sample solution. Additional details are described in the Supplementary Methods.
Invasion assays
BioCoat Matrigel transwell invasion assays were performed as per manufacturer’s instructions (BD Biosciences, San Jose, CA). Cells were starved 24h before experiment and plated into the top chamber in serum-free media, and allowed to invade through Matrigel towards serum-containing media in the bottom chamber. For UM-SCC1 and NOKs cells, 1.5 × 105 cells were plated, while for UM-SCC47 cells, 2.5 ×105 cells were plated. Invasion was allowed to proceed for 23 hours prior to fixation in methanol and staining with Giemsa. Total numbers of invaded cells were quantified for each transwell using ImageJ.
Organotypic epithelial raft culture
Three dimensional organotypic rafts were generated as previously described (32). Briefly, a total of 1×106 cells were plated on a collagen matrix containing fibroblasts. After 5 days, keratinocytes were lifted to the liquid-air phase which proliferate and differentiate into stratified epithelium. Rafts were harvested and fixed in 4% paraformaldehyde at 14 days after lifting, and embedded in paraffin. Sections were stained with hematoxylin and eosin.
Statistical analysis
For untargeted lipidomics analysis, discovery of lipid features significantly altered between different genotypes (i.e. NTsh vs FANCD2sh, NTsh vs FANCJsh) was conducted with univariate analysis. Statistical significance was determined using both fold changes and p-values. Student’s t-test and Mann-Whitney-Wilcoxon test were applied on the the lipidomics data. The high dimensional feature space of untargeted data considers the multiple hypothesis testing problem. Bonferroni adjusted p-value < 0.05 and false discovery rate (FDR) correction were used to control family-wise error rate. For targeted lipidomics analysis, statistical significance was determined using a two-tailed Student t-test. All statistical analyses were conducted with the R language and environment for statistical computing.
Results
Global lipidomics profiling of FA deficient immortalized keratinocytes reveals upregulation of the primitive ganglioside GM3
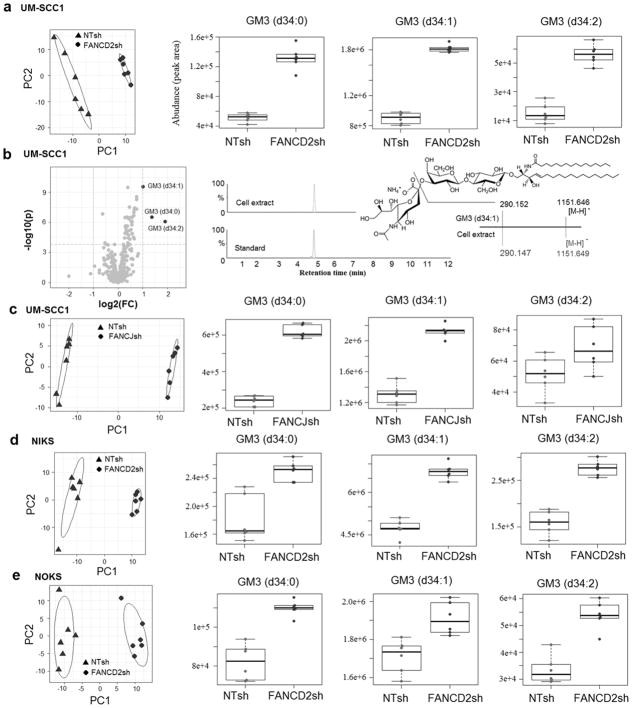
To identify lipids that are differentially regulated in FA-deficient versus FA-proficient cell lines (Fig. 1), we used an untargeted mass spectrometry (MS) based lipidomics approach. Specifically, UM-SCC1 cells were knocked down for FANCD2 of FANCJ, and normal foreskin keratinocytes (NIKS), as well as normal oral keratinocytes (NOKS) were knocked down for FANCD2, and knockdown validation was demonstrated by western blot analysis (28,32). All knockdown cells were compared to their respective nontargeted (NTsh) control cells. Over 300 lipid species were detectable by untargeted MS, and quantified in both positive and negative ion modes. Lipidomic profiles visualized by principal component analysis (PCA) revealed shared differences for the FANCD2sh and FANCJsh, compared to their respective NTsh cell lipidomes. For UM-SCC1 cells, 3 gangliosides of 312 lipid species were significantly regulated by FA status. We observed elevated levels of the ganglioside GM3 (d34:0), GM3 (d34:1), and GM3 (d34:2) in FANCD2sh cells compared to the NTsh control cells (Fig. 1a). The identity of gangliosides was first validated with authentic standards by comparing retention times and mass spectral fragmentation patterns, in addition to accurate mass measurements. In negative ionization, gangliosides with sialic acid (SA) in the glycan chain show a signature fragment ion, i.e. sialic acid at m/z 290. Observing the same pattern of SA ion with parent ion and identical retention time, we confirmed the identity of GM3 (d34:0), GM3 (d34:1) and GM3 (d34:2) (Fig. 1b, Supplementary Fig. 1a–1b). These ganglioside GM3 species were also significantly increased in FANCJsh UM-SCC1 cells compared to their NTsh counterparts (Fig. 1c). In NIKS cells, 26 of 312 lipids showed significant differences in FA deficient versus FA proficient cells (Supplementary Fig. 2a), and GM3 (d34:0), GM3 (d34:1), and GM3 (d34:2) were again significantly increased (Fig. 1d). A similar pattern was also observed in NOKS cells (Fig. 1e). Given the broad and consistent upregulation of GM3 in FA-deficient immortalized and transformed keratinocytes by untargeted metabolomics, we next carried out a targeted approach to quantify precursors and products of GM3, and to extend the analyses to FA patient derived HNSCC cells.
Figure 1. Global lipidomics profiling of FANCD2 and FANCJ knockdown UM-SCC1 cells (a–c), and FANCD2 knockdown NIKS (d) and NOKS (e).
Principal component analysis (PCA) of lipids of UM-SCC1 cells (312 lipids identified, n=6 per cell population) with NTsh and FANCD2sh knockdown, UM-SCC1 cells (231 lipids identified, n=6 per cell population) with NTsh and FANCJsh knockdown, NIKS cells (312 lipids identified, n=6 per cell population) with NTsh and FANCD2sh knockdown, NOKS cells (253 lipids, n=6 per cell population) with NTsh and FANCD2sh knockdown.
Volcano plot indicated several ganglioside GM3 species are significantly altered in the FANCD2sh knockdown UM-SCC1 cells. The p-value was calculated with 2-tailed student’s t test, thresholds for significance: Bonferroni adjusted p-value < 0.05, fold change > 2.
Identification of ganglioside GM3 (d34:1) by UPLC-MSe. The putative biomarkers ganglioside GM3 by database search was confirmed by an authentic standard with retention time, fragmentation ions, and standard addition with extracted ion chromatogram (EIC). Mass chromatogram was EIC using the molecular ion [M-H]−. Mass spectrum is for illustration showing only extracted ions of interest and relative intensity.
Box-and-whisker plots of identified ganglioside metabolites, p-values shown in the figure were not adjusted by Bonferroni correction. The p-value and fold change for identified gangliosides, i.e. gangliosides GM3 (d34:0), GM3 (d34:1), and GM3 (d34:2), are 3.0×10−7 and 2.6, 2.7×10−10 and 2.0, 8.5×10−7 and 3.8 in NTsh vs FANCD2sh UM-SCC1 cells (a); 1.3×10−9 and 2.6, 9.7×10−8 and 1.6, and 0.04 and 1.3 in NTsh vs FANCJsh UM-SCC1 cells (c); 8.0×10−4 and 1.4, 2.4×10−7 and 1.4, 2.2×10−6 and 1.7 in NTsh vs FANCD2sh NIKS cells (d); 4.7×10−5 and 1.3, 2.5×10−3 and 1.1, 4.9×10−5 and 1.6 in NTsh vs FANCD2sh NOKS cells (e).
Targeted analysis of members of the ganglioside biosynthesis pathway
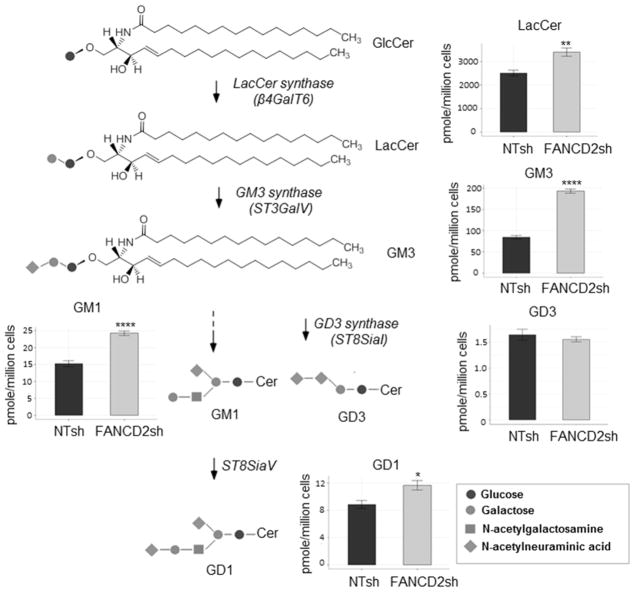
Although gangliosides only comprise a minor proportion of total cellular lipids in extra-neural tissues, their nature and composition is characteristic and related to the function of the specific cell type. In mammalian cells, Ganglioside biosynthesis is catalyzed by glycosyltransferases in the lumen of the Golgi apparatus, followed by ganglioside transport to the plasma membrane via exocytotic membrane flow (35). Successive lipid modifications occur en route via a number of key enzymes (Fig. 2). First, galactosyltransferase produces lactosylceramide from glucosylceramide by the transfer of a galactose residue from UDP. Second, GM3 synthase adds a sialic acid molecule to lactosylceramide for the production of the parent ganglioside GM3. More complex gangliosides are subsequently generated from GM3. For instance, GD3 arises from the linkage of an additional sialic acid moiety, while GM1a arises from the linkage of galactosamine and galactose to the glycan chain. The ganglioside GD1a is synthesized from GM1a by the addition of a sialic acid moiety. We found that total concentrations of lactosylceramide, GM3, and GM1 were significantly elevated in HPV-negative UM-SCC1 after FANCD2 knockdown (Fig. 2), NIKS, NOKS and HPV-positive UM-SCC47 cells (Supplementary Fig. 3a–c). Interestingly, while GD3 production was not changed in response to FA loss in UM-SCC1 cells, it was higher in NIKS, NOKS and UM-SCC47 cells. Overall these results demonstrate that FANCD2 knockdown stimulates the ubiquitous accumulation of some gangliosides, and that of others in a cell type specific fashion.
Figure 2. Ganglioside GM3 pathway is perturbed by FANCD2 knockdown.
UPLC-MS/MS analysis of UM-SCC1 cell (n=6 per cell population) with NTsh and FANCD2sh knockdown calculated with pmol/million cells in sum of all detected individual species of that lipid class were overlaid onto a stylized ganglioside pathway
FANCD2 loss of function regulates specific ganglioside species
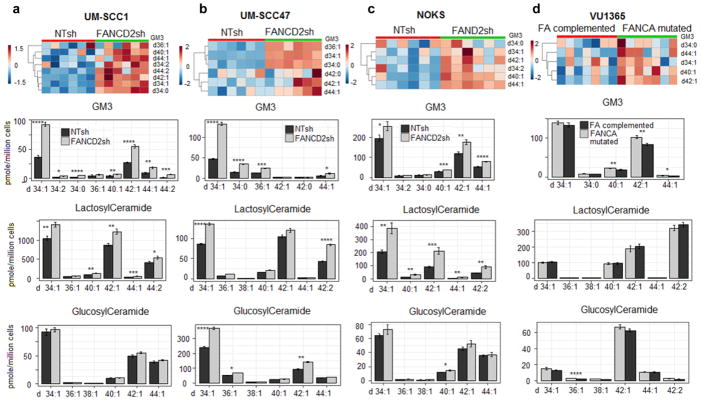
Within the sphingolipid family, each member consists of multiple species characterized by different acyl chain lengths and/or saturated versus unsaturated acyl chains. Both can confer unique chemical and biological functionalities (36,37). For instance, long and/or saturated acyl chains tend to result in diminished lipid fluidity and greater motion constraint when compared to their shorter and/or unsaturated counterparts (38). In the targeted UPLC-MS/MS assay to quantify the FA-dependent regulation of ganglioside precursors and products in FANCD2sh versus NTsh cells, 8 GM3 species with various acyl chain lengths were increased in FA-deficient compared to FA-proficient UM-SCC1 samples, and 6 GM3 species were increased in the respective UM-SCC47 samples (Fig. 3a–b). The heatmap displays relative levels of GM3 species in FANCD2sh versus NTsh treated UM-SCC1 and UM-SCC47 cells. These lipids including ganglioside GM3(d34:1), GM3(d34:0), and/or GM3(d34:2), were similarly increased in FANCD2sh versus NTsh treated NIKS and NOKS cells (Fig. 3c, Supplementary Fig. 2). In the HNSCC and normal cells, the gangliosides GM3 d34:1 and d42:1 tended to be the majority components accompanied by small amounts of d36:1, d38:1, d44:1 and other carbohydrate chain lengths. Finally, FANCA patient derived HNSCC VU1365 cells were tested for ganglioside regulation as a function of FA status. The cells were previously published, and had either been stably transduced with empty vector and were thus FANCA deficient, or had been transduced with a FANCA expression vector and were thus FANCA-competent (33). In line with the above FA knockdown data, gangliosides GM3 d40:1, d42:1 and d44:1 were detectable in the FANCA-deficient cells, and were distinctly repressed in FANCA-corrected cells (Fig. 3d). Precursors and downstream products of Ganglioside GM3 including glucosylceramides, lactosylceramides, ganglioside GM1 and GD3 with different acyl chain lengths were measured and perturbation of the glycosphingolipids were observed (Fig. 3 and Supplementary Fig. 4). Taken together, these data demonstrate that ganglioside biosynthesis is controlled by a functional FA pathway.
Figure 3. Targeted analysis of lipids of ganglioside GM3 metabolic pathway in NTsh and FANCD2 deficient SCC cells, UM-SCC1 (n=6 per cell population) (a), UM-SCC47 (n=6 per cell population) (b), NOKS cells (n=6 per cell population) (c), and FANCA mutated and complementation VU1365 cells (n=6 per cell population) (d).
Heatmap of targeted UPLC-MS/MS analysis of ganglioside GM3 species in NTsh and FANCD2 knockdown UM-SCC1, UM-SCC47, NOKS cells, and FANCA mutated and complementation VU1365 cells, the estimated false discovery rates (q value) for calling all p-values < 0.05 significant were 0.013 for UM-SCC1, 0.023 for UM-SCC47, 0.074 for NOKS, and 0.072 for VU1365 cells, i.e. under 10% false discovery rate.
Targeted quantitative analysis of glucosylceramides, lactosylceramides, and ganglioside GM3, in NTsh and FANCD2 knockdown UM-SCC1 and UM-SCC47 cells; NOKS and FANCA mutated and complementation VU1365 cells. * p-value < 0.05, **p-value < 0.01, *** p-value < 0.001, ****p-value < 0.0001 (two-tailed student’s t test)
Upregulated ganglioside biosynthesis stimulates invasion that is dependent upon FA loss
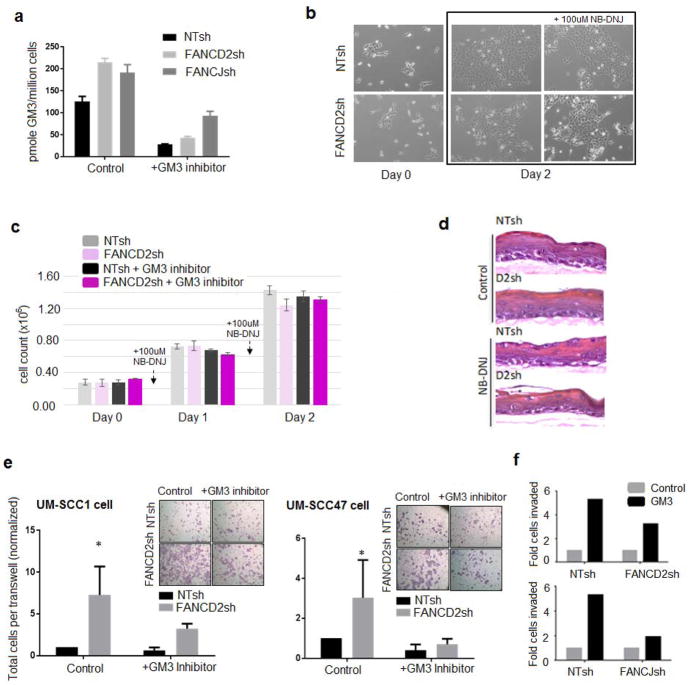
We have published previously that FA loss of function in HNSCC cells stimulates invasive characteristics (28). In order to determine whether ganglioside biosynthesis is required, we took advantage of a clinically relevant iminosugar NB-DNJ (miglustat/Zavesca) (39). NB-DNJ inhibits the enzyme glucosylceramide synthase which catalyzes the first step in the synthesis of glycosphingolipids, and is used for the treatment of Gaucher’s disease. To ensure that this dose sufficiently blocked ganglioside biosynthesis, cells were harvested for MS-based quantification of total GM3 levels after 48 hours. As predicted, levels of GM3 were dramatically repressed in the presence of NB-DNJ (Fig. 4a, Supplementary Fig. 5). This was observed regardless of FA status, in cells transduced with non-targeting vector and in cells knocked down for FANCD2 or FANCJ, respectively. UM-SCC1 cells harboring NTsh and FANCD2sh were plated at equal cell numbers, exposed to NB-DNJ, or not, for 48 hours, then photographed to show cellular morphology (Fig. 4b). Cells were harvested to quantify viable cell counts (Fig. 4c). Neither cellular morphology, nor viability, was affected by NB-DNJ at a dose of 100uM. NIKS and UM-SCC47 cells were also tested and similar results were observed (Supplementary Fig. 6a–b). Next, we determined whether NB-DNJ affected the growth and differentiation of normal human keratinocytes by culturing NIKS that were either control transduced or FANCD2 depleted in organotypic epithelial rafts as published (32). NB-DNJ did not affect raft morphology or thickness in these experiments (Fig. 4d), regardless of FA status. Our recent data demonstrated that FA loss of function in HNSCC cells is sufficient to stimulate tumor cell motility and invasion (28). To measure the functional requirement for ganglioside accumulation in the invasive capacity of FA-deficient cells, FANCD2- and FANCJ-depleted versus control UM-SCC1 and UM-SCC47 cells were exposed to NB-DNJ (or vehicle only), and subjected to transwell assays (Fig. 4e). FANCD2 and FANCJ loss stimulated invasion in both cell lines as previously published, and this phenotype was partly reversed by NB-DNJ. Thus, a block in ganglioside biosynthesis attenuates the invasive capacity of transformed HPV-negative and –positive HNSCC cells. In order to determine whether ganglioside accumulation was sufficient to stimulate UM-SCC1 cell invasion, 50uM of GM3 was added to the media of NTsh, FANCD2sh, or FANCJsh UM-SCC1 cells, followed by measurements of tumor cell invasion (Fig. 4f). GM3 stimulated cellular invasion, most notably in the NTsh control cells. The observed increase invasion in control cells was approximately 5-fold above baseline levels, whereas the increase in FANCD2sh and FANCJsh knockdown cells was 2- and 3-fold, respectively. Taken together, these data demonstrate that ganglioside accumulation in FA-deficient keratinocytes promotes FA-deficient HNSCC invasion. Since NB-DNJ/miglustat is already in clinical use for substrate reduction therapy in patients with Gaucher syndrome, this drug can now be explored for its ability to prevent or halt the progression of FA-deficient HNSCC.
Figure 4. Ganglioside GM3 upregulation contributes to invasion downstream from FA loss and GM3 inhibition suppresses FA-dependent invasion.
a) GM3 repression was measured by liquid chromatography-mass spectrometry using GM3 inhibitor (i.e. NB-DNJ) on intransduced UM-SCC1 cells (n=6 per cell population) with NTsh, FANCD2sh, and FANCJsh knockdown. b) NTsh and FANCD2sh UM-SCC1 cells were plated at equal cell numbers, and GM3 inhibitor (NB-DNJ) was added following cell attachment, and replenished in fresh media after 24 hrs. c) Cells were stained with trypan blue and viable cells counted (n=3 per cell population). d) Organotypic epithelial rafts derived from either control transduced or FANCD2 depleted UM-SCC1 cells. e) NTsh-, FANCD2sh transduced UM-SCC1 and UM-SCC47 cells, were cultured for 24 hours in 100uM NB-DNJ, followed by transwell invasion assays in the continued presence of the drug. The asterisk denotes the statistically significant difference of invasion (p-value < 0.05) between FANCD2sh transduced cells cultured without and with NB-DNJ (n=3 biological replicates for each cell population). f) 50uM of GM3 was added to the media of NTsh, FANCD2sh and FANCJsh UM-SCC1 cells for 24 hours, followed by transwell invasion assays in the continued presence of the GM3. Except for d) and f), all experiments are representative data from >2 biological repeats.
Discussion
Advanced HNSCC represents a devastating diagnosis in the general population, with early onset and significantly worse outcomes in individuals with FA. Beyond a presumed role for FA loss in tumor initiation, our recent data have linked the loss of this important DNA repair pathway to cytoskeletal reorganization, adhesion defects and invasion acquisition (29). These epithelial-mesenchymal transition-like phenotypes were shown to require inappropriate activation of the NHEJ-associated DNA damage sensor kinase DNA-PK and downstream stimulation of the Rac1 GTPase activity. They also require, as demonstrated here via lipidomic analyses, an abnormal accumulation of glycosphingolipids. Inhibition of ganglioside biosynthesis with a repurposed, FDA-approved drug attenuated HNSCC invasion cells, and the addition of the simple ganglioside GM3 stimulated invasion. This indicates gangliosides are sufficient and required for invasion downstream from FA loss, and points to lipid-based avenues for the prevention and treatment of advanced FA-deficient HNSCC.
Gangliosides are acidic glycosphingolipids harboring one or more sialic acid residues. These are key components of cellular membranes, located at the outer leaflet, in nearly all vertebrates. These lipids are often enriched in membrane microdomains which form lipid rafts and interact with phospholipids, cholesterol, and transmembrane proteins (40). Gangliosides are involved in cell adhesion (41), proliferation (42), migration (43), viral infection (44), as well as in regulation of signal transduction pathways (44–46). We found consistent upregulation of the monosialodihexosyl ganglioside GM3 and several derivative gangliosides in FA-deficient normal immortalized and transformed keratinocytes when compared with their isogenic, FA-proficient counterparts. The extent of suppression of GM3 by genetic correction of VU1365 cells was less pronounced than the extent of GM3 upregulation in FA knockdown cells. Whether this is due to a lesser effect of FANCA versus FANCD2 mutations, the individual’s adaptation to lifelong FA deficiency, or cell line variabilities which cannot be ruled out here remains unknown. Relevant mechanisms which stimulate ganglioside biosynthesis stimulation downstream from FA loss are unknown, but strictly dependent on the FA pathway as accumulation is reversible by FA gene complementation. FA proteins have been shown to act in transcriptional regulation (47) and a recent study also revealed that FA mesenchymal stromal cells (MSCs) overproduce a group of glycerophospholipids and the elevated levels of these lipids impair the hematopoietic supporting function of MSCs (48). Our RNA sequencing data and immunoblot analyses of ganglioside GM3 synthase (ST3GAL5) do not support a role for transcriptional regulation or protein accumulation (data not shown). Enzyme activity is the likely target, and thus identification of the responsible enzymes will require future isotope labeling studies of ganglioside biosynthesis. Whether ganglioside regulation depends upon, or is independent from, DNA-PK/Rac1 signaling is currently unknown.
Our results establish new links between a key DNA repair pathway and lipid metabolism in head and neck cancer cells, and demonstrate a consistent requirement of ganglioside accumulation for the aggressive invasion of FA pathway-deficient cells. Similar effects were observed in HPV positive or negative SCC cells and in normal keratinocytes. This is an important finding since gangliosides can have variable, even opposing effects, on the motility of distinct tumor types and cell lines (1,42,43). Furthermore, even within a given line, motility stimulation versus repression depends upon the types of signaling pathways that are engaged. For instance, in Hepa 1-6 hepatocellular carcinoma cells, GM3 inhibited EGF/EGFR-dependent motility but stimulated HGF/HGFR-dependent motility (49). Such transmembrane receptor specific mechanisms might be envisioned for the observed ganglioside-dependent invasion of FA-deficient HNSCC. Alternatively, since gangliosides are essential components of lipid rafts, and since lipid rafts nucleate a multitude of signal transduction complexes, ganglioside accumulation may de-regulate global signal transduction indirectly, via lipid raft structure and function (50). Finally, it is possible that ganglioside upregulation following FA loss may stimulate advanced tumor phenotypes directly via control of plasma membrane structure and fluidity. Interestingly, specific glycosphingolipids including the ganglioside GM3 are known to promote epithelial-to-mesenchymal transition (EMT) of human epithelial cell lines (51,52), and FA knockdown in UM-SCC1 cells was sufficient for plasma membrane abnormalities and EMT-like phenotypes (28). Dissecting direct from indirect effects of ganglioside-dependent invasion in HNSCC cells will be important for selective inhibition of downstream effectors towards therapy in FA-deficient settings.
Lipidomics is a rapidly growing area of research with major potential to identify clinically relevant biomarkers and drug targets for improved therapeutic outcomes, as was shown for atherosclerosis, diabetes, and Alzheimer’s disease (53,54). Using MS-based lipidomics studies, we demonstrate that FA DNA repair loss is associated with a distict lipid signature for potential diagnostic implications, and promotes advanced tumor phenotypes via lipid production that can be therapeutically targeted with minimal toxicity. NB-DNJ (Miglustat/Zavesca®), an FDA-approved imino sugar-based drug for the oral treatment of Gaucher disease and inhibitor of glycosphingolipid biosynthesis, diminished levels of the primitive ganglioside GM3 and specifically reduced the aggressive invasion of FA-deficient cancer cells. NB-DNJ has been investigated as a treatment for infantile and juvenile patients with ganglioside related disorders (55) and implicated in cancer treatment (56,57). Repurposing NB-DNJ or other glycosphingolipid synthesis inhibitors provides a potential new avenue to prevent or treat HNSCC in FA and in sporadic tumors that are FA-deficient. Interestingly, there may be clinical overlap between FA and Gaucher disease, including anemia, short stature, and cancer susceptibility, although squamous cell carcinoma is not common in the Gaucher population (58). Relevant mechanistic determinants that link FA with GD phenotypes remain to be defined.
Supplementary Material
Translational Relevance.
Tumor invasion in patients with squamous cell carcinoma of the head and neck (HNSCC) is a major cause of morbidity and mortality. Discovery of biomarkers and effectors of HNSCC progression remains imperative. Mutations in Fanconi anemia (FA) genes are common in sporadic SCCs. The FA DNA repair pathway suppresses advanced HNSCC phenotypes, and loss of this pathway may be common in HNSCC. Furthermore, germline loss of function mutations in FA genes causes FA, a genome instability syndrome where patients harbor extreme susceptibility to aggressive HNSCC. Here, we demonstrate that elevation of glycosphingolipids including the ganglioside GM3 in response to FA loss stimulates invasive characteristics of immortalized and transformed keratinocytes. An inhibitor of glycosphingolipid biosynthesis NB-DNJ attenuated invasive characteristics of FA-deficient HNSCC cells. This highlights new avenues for the diagnosis and treatment of advanced HNSCC through the repurposing of FDA-approved glycosphingolipid modulators.
Acknowledgments
This study was supported by RO1 CA102357 and by a grant from Fanconi Anemia Research Fund to S.I. Wells. The authors would like to acknowledge acknowledge the expert support and technical assistance of Dr. Junfang Zhao.
Footnotes
Disclosure of Potential Conflicts of Interest
The following authors have intellectual property related to this work: S.I. Wells, K.D.R. Setchell, X. Zhao, W. Zhang, L.E. Romick-Rosendale.
Authors’ Contributions
Conception and design: S.I. Wells, K.D.R. Setchell, X. Zhao, M.G. Brusadelli
Development of methodology: X. Zhao, M.G. Brusadelli, K.D.R. Setchell, S.I. Wells
Acquisition of data (provided cell lines, provided facilities, etc.): X. Zhao, M.G. Brusadelli
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): X. Zhao, M.G. Brusadelli, S.I. Wells, K.D.R. Setchell
Writing, review, and/or revision of the manuscript: X. Zhao, M.G. Brusadelli, S. Sauter, M. Butsch Kovacic, W. Zhang, L.E. Romick-Rosendale, P.F. Lambert, K.D.R. Setchell, S.I. Wells
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): X. Zhao, P.F. Lambert, M.G. Brusadelli, W. Zhang
Study supervision: K.D.R. Setchell, S.I. Wells
References
- 1.Bogliolo M, Surralles J. Fanconi anemia: a model disease for studies on human genetics and advanced therapeutics. Curr Opin Genet Dev. 2015;33:32–40. doi: 10.1016/j.gde.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Gueiderikh A, Rosselli F, Neto JBC. A never-ending story: the steadily growing family of the FA and FA-like genes. Genetics and molecular biology. 2017;40(2):398–407. doi: 10.1590/1678-4685-GMB-2016-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nepal M, Che R, Zhang J, Ma C, Fei P. Fanconi Anemia Signaling and Cancer. Trends in cancer. 2017;3(12):840–56. doi: 10.1016/j.trecan.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deans AJ, West SC. DNA interstrand crosslink repair and cancer. Nat Rev Cancer. 2011;11(7):467–80. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kottemann MC, Smogorzewska A. Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature. 2013;493(7432):356–63. doi: 10.1038/nature11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Martinez D, Liang CC, Cohn MA. Cellular response to DNA interstrand crosslinks: the Fanconi anemia pathway. Cell Mol Life Sci. 2016;73(16):3097–114. doi: 10.1007/s00018-016-2218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walden H, Deans AJ. The Fanconi anemia DNA repair pathway: structural and functional insights into a complex disorder. Annu Rev Biophys. 2014;43:257–78. doi: 10.1146/annurev-biophys-051013-022737. [DOI] [PubMed] [Google Scholar]
- 8.Ceccaldi R, Sarangi P, D’Andrea AD. The Fanconi anaemia pathway: new players and new functions. Nature reviews Molecular cell biology. 2016;17(6):337–49. doi: 10.1038/nrm.2016.48. [DOI] [PubMed] [Google Scholar]
- 9.Adamo A, Collis SJ, Adelman CA, Silva N, Horejsi Z, Ward JD, et al. Preventing nonhomologous end joining suppresses DNA repair defects of Fanconi anemia. Molecular cell. 2010;39(1):25–35. doi: 10.1016/j.molcel.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Pace P, Mosedale G, Hodskinson MR, Rosado IV, Sivasubramaniam M, Patel KJ. Ku70 corrupts DNA repair in the absence of the Fanconi anemia pathway. Science. 2010;329(5988):219–23. doi: 10.1126/science.1192277. [DOI] [PubMed] [Google Scholar]
- 11.Schlacher K, Wu H, Jasin M. A distinct replication fork protection pathway connects Fanconi anemia tumor suppressors to RAD51-BRCA1/2. Cancer Cell. 2012;22(1):106–16. doi: 10.1016/j.ccr.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nalepa G, Clapp DW. Fanconi anemia and the cell cycle: new perspectives on aneuploidy. F1000Prime Rep. 2014;6:23. doi: 10.12703/P6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kee Y, D’Andrea AD. Molecular pathogenesis and clinical management of Fanconi anemia. The Journal of clinical investigation. 2012;122(11):3799–806. doi: 10.1172/JCI58321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutler DI, Patel KR, Auerbach AD, Kennedy J, Lach FP, Sanborn E, et al. Natural history and management of Fanconi anemia patients with head and neck cancer: A 10 year follow-up. Laryngoscope. 2015 doi: 10.1002/lary.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR) Blood. 2003;101(4):1249–56. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- 16.Velleuer E, Dietrich R. Fanconi anemia: young patients at high risk for squamous cell carcinoma. Molecular and cellular pediatrics. 2014;1(1):9. doi: 10.1186/s40348-014-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alpi AF, Pace PE, Babu MM, Patel KJ. Mechanistic insight into site-restricted monoubiquitination of FANCD2 by Ube2t, FANCL, and FANCI. Molecular cell. 2008;32(6):767–77. doi: 10.1016/j.molcel.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Machida YJ, Machida Y, Chen Y, Gurtan AM, Kupfer GM, D’Andrea AD, et al. UBE2T is the E2 in the Fanconi anemia pathway and undergoes negative autoregulation. Molecular cell. 2006;23(4):589–96. doi: 10.1016/j.molcel.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Meetei AR, de Winter JP, Medhurst AL, Wallisch M, Waisfisz Q, van de Vrugt HJ, et al. A novel ubiquitin ligase is deficient in Fanconi anemia. Nature genetics. 2003;35(2):165–70. doi: 10.1038/ng1241. [DOI] [PubMed] [Google Scholar]
- 20.Smogorzewska A, Matsuoka S, Vinciguerra P, McDonald ER, 3rd, Hurov KE, Luo J, et al. Identification of the FANCI protein, a monoubiquitinated FANCD2 paralog required for DNA repair. Cell. 2007;129(2):289–301. doi: 10.1016/j.cell.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taniguchi T, Garcia-Higuera I, Andreassen PR, Gregory RC, Grompe M, D’Andrea AD. S-phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51. Blood. 2002;100(7):2414–20. doi: 10.1182/blood-2002-01-0278. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Walter JC. Mechanism and regulation of incisions during DNA interstrand cross-link repair. DNA repair. 2014;19:135–42. doi: 10.1016/j.dnarep.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillison ML. Current topics in the epidemiology of oral cavity and oropharyngeal cancers. Head & neck. 2007;29(8):779–92. doi: 10.1002/hed.20573. [DOI] [PubMed] [Google Scholar]
- 24.Lacko M, Braakhuis BJ, Sturgis EM, Boedeker CC, Suarez C, Rinaldo A, et al. Genetic susceptibility to head and neck squamous cell carcinoma. International journal of radiation oncology, biology, physics. 2014;89(1):38–48. doi: 10.1016/j.ijrobp.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 25.Parkin DM, Ferlay J, Curado MP, Bray F, Edwards B, Shin HR, et al. Fifty years of cancer incidence: CI5 I-IX. International journal of cancer Journal international du cancer. 2010;127(12):2918–27. doi: 10.1002/ijc.25517. [DOI] [PubMed] [Google Scholar]
- 26.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371(9625):1695–709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G, Da M, Zhang W, Wu H, Ye J, Chen J, et al. Alteration of serum lipid profile and its prognostic value in head and neck squamous cell carcinoma. Journal of oral pathology & medicine: official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2016;45(3):167–72. doi: 10.1111/jop.12344. [DOI] [PubMed] [Google Scholar]
- 28.Romick-Rosendale LE, Hoskins EE, Privette Vinnedge LM, Foglesong GD, Brusadelli MG, Potter SS, et al. Defects in the Fanconi Anemia Pathway in Head and Neck Cancer Cells Stimulate Tumor Cell Invasion through DNA-PK and Rac1 Signaling. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22(8):2062–73. doi: 10.1158/1078-0432.CCR-15-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romick-Rosendale LE, Lui VW, Grandis JR, Wells SI. The Fanconi anemia pathway: repairing the link between DNA damage and squamous cell carcinoma. Mutation research. 2013;743–744:78–88. doi: 10.1016/j.mrfmmm.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wreesmann VB, Estilo C, Eisele DW, Singh B, Wang SJ. Downregulation of Fanconi anemia genes in sporadic head and neck squamous cell carcinoma. ORL; journal for oto-rhino-laryngology and its related specialties. 2007;69(4):218–25. doi: 10.1159/000101542. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi H, Oikawa T. Membrane lipids in invadopodia and podosomes: key structures for cancer invasion and metastasis. Oncotarget. 2010;1(5):320–8. doi: 10.18632/oncotarget.100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoskins EE, Morreale RJ, Werner SP, Higginbotham JM, Laimins LA, Lambert PF, et al. The fanconi anemia pathway limits human papillomavirus replication. Journal of virology. 2012;86(15):8131–8. doi: 10.1128/JVI.00408-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lombardi AJ, Hoskins EE, Foglesong GD, Wikenheiser-Brokamp KA, Wiesmuller L, Hanenberg H, et al. Acquisition of Relative Interstrand Crosslinker Resistance and PARP Inhibitor Sensitivity in Fanconi Anemia Head and Neck Cancers. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21(8):1962–72. doi: 10.1158/1078-0432.CCR-14-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yablonska S, Hoskins EE, Wells SI, Khan SA. Identification of miRNAs dysregulated in human foreskin keratinocytes (HFKs) expressing the human papillomavirus (HPV) Type 16 E6 and E7 oncoproteins. MicroRNA. 2013;2(1):2–13. doi: 10.2174/2211536611302010002. [DOI] [PubMed] [Google Scholar]
- 35.Kolter T. Ganglioside biochemistry. ISRN biochemistry. 2012;2012:506160. doi: 10.5402/2012/506160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koybasi S, Senkal CE, Sundararaj K, Spassieva S, Bielawski J, Osta W, et al. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. The Journal of biological chemistry. 2004;279(43):44311–9. doi: 10.1074/jbc.M406920200. [DOI] [PubMed] [Google Scholar]
- 37.Senkal CE, Ponnusamy S, Rossi MJ, Bialewski J, Sinha D, Jiang JC, et al. Role of human longevity assurance gene 1 and C18-ceramide in chemotherapy-induced cell death in human head and neck squamous cell carcinomas. Molecular cancer therapeutics. 2007;6(2):712–22. doi: 10.1158/1535-7163.MCT-06-0558. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton KS, Briere K, Jarrell HC, Grant CW. Acyl chain length effects related to glycosphingolipid crypticity in phospholipid membranes: probed by 2H-NMR. Biochimica et biophysica acta. 1994;1190(2):367–75. doi: 10.1016/0005-2736(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 39.Stirnemann J, Belmatoug N, Camou F, Serratrice C, Froissart R, Caillaud C, et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int J Mol Sci. 2017;18(2) doi: 10.3390/ijms18020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groux-Degroote S, Guerardel Y, Delannoy P. Gangliosides: Structures, Biosynthesis, Analysis, and Roles in Cancer. Chembiochem: a European journal of chemical biology. 2017;18(13):1146–54. doi: 10.1002/cbic.201600705. [DOI] [PubMed] [Google Scholar]
- 41.Iwabuchi K, Yamamura S, Prinetti A, Handa K, Hakomori S. GM3-enriched microdomain involved in cell adhesion and signal transduction through carbohydrate-carbohydrate interaction in mouse melanoma B16 cells. The Journal of biological chemistry. 1998;273(15):9130–8. doi: 10.1074/jbc.273.15.9130. [DOI] [PubMed] [Google Scholar]
- 42.Wang XQ, Sun P, Go L, Koti V, Fliman M, Paller AS. Ganglioside GM3 promotes carcinoma cell proliferation via urokinase plasminogen activator-induced extracellular signal-regulated kinase-independent p70S6 kinase signaling. The Journal of investigative dermatology. 2006;126(12):2687–96. doi: 10.1038/sj.jid.5700469. [DOI] [PubMed] [Google Scholar]
- 43.Hashiramoto A, Mizukami H, Yamashita T. Ganglioside GM3 promotes cell migration by regulating MAPK and c-Fos/AP-1. Oncogene. 2006;25(28):3948–55. doi: 10.1038/sj.onc.1209416. [DOI] [PubMed] [Google Scholar]
- 44.You J, O’Hara SD, Velupillai P, Castle S, Levery S, Garcea RL, et al. Ganglioside and Non-ganglioside Mediated Host Responses to the Mouse Polyomavirus. PLoS pathogens. 2015;11(10):e1005175. doi: 10.1371/journal.ppat.1005175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merrill AH., Jr Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem Rev. 2011;111(10):6387–422. doi: 10.1021/cr2002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buch MH, Liaci AM, O’Hara SD, Garcea RL, Neu U, Stehle T. Structural and Functional Analysis of Murine Polyomavirus Capsid Proteins Establish the Determinants of Ligand Recognition and Pathogenicity. PLoS Pathog. 2015;11(10):e1005104. doi: 10.1371/journal.ppat.1005104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huard CC, Tremblay CS, Magron A, Levesque G, Carreau M. The Fanconi anemia pathway has a dual function in Dickkopf-1 transcriptional repression. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(6):2152–7. doi: 10.1073/pnas.1314226111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amarachintha S, Sertorio M, Wilson A, Li X, Pang Q. Fanconi Anemia Mesenchymal Stromal Cells-Derived Glycerophospholipids Skew Hematopoietic Stem Cell Differentiation Through Toll-Like Receptor Signaling. Stem cells. 2015;33(11):3382–96. doi: 10.1002/stem.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Huang X, Wang C, Li Y, Luan M, Ma K. Ganglioside GM3 exerts opposite effects on motility via epidermal growth factor receptor and hepatocyte growth factor receptor-mediated migration signaling. Molecular medicine reports. 2015;11(4):2959–66. doi: 10.3892/mmr.2014.3087. [DOI] [PubMed] [Google Scholar]
- 50.Cantu L, Corti M, Brocca P, Del Favero E. Structural aspects of ganglioside-containing membranes. Biochimica et biophysica acta. 2009;1788(1):202–8. doi: 10.1016/j.bbamem.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Kim SJ, Chung TW, Choi HJ, Kwak CH, Song KH, Suh SJ, et al. Ganglioside GM3 participates in the TGF-beta1-induced epithelial-mesenchymal transition of human lens epithelial cells. The Biochemical journal. 2013;449(1):241–51. doi: 10.1042/BJ20120189. [DOI] [PubMed] [Google Scholar]
- 52.Guan F, Handa K, Hakomori SI. Specific glycosphingolipids mediate epithelial-to-mesenchymal transition of human and mouse epithelial cell lines. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(18):7461–6. doi: 10.1073/pnas.0902368106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huynh K, Martins RN, Meikle PJ. Lipidomic Profiles in Diabetes and Dementia. Journal of Alzheimer’s disease: JAD. 2017;59(2):433–44. doi: 10.3233/JAD-161215. [DOI] [PubMed] [Google Scholar]
- 54.Kolovou G, Kolovou V, Mavrogeni S. Lipidomics in vascular health: current perspectives. Vascular health and risk management. 2015;11:333–42. doi: 10.2147/VHRM.S54874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maegawa GH, van Giersbergen PL, Yang S, Banwell B, Morgan CP, Dingemanse J, et al. Pharmacokinetics, safety and tolerability of miglustat in the treatment of pediatric patients with GM2 gangliosidosis. Molecular genetics and metabolism. 2009;97(4):284–91. doi: 10.1016/j.ymgme.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 56.Guerrera M, Ladisch S. N-butyldeoxynojirimycin inhibits murine melanoma cell ganglioside metabolism and delays tumor onset. Cancer letters. 2003;201(1):31–40. doi: 10.1016/s0304-3835(03)00459-2. [DOI] [PubMed] [Google Scholar]
- 57.Ranes MK, El-Abbadi M, Manfredi MG, Mukherjee P, Platt FM, Seyfried TN. N -butyldeoxynojirimycin reduces growth and ganglioside content of experimental mouse brain tumours. British journal of cancer. 2001;84(8):1107–14. doi: 10.1054/bjoc.2000.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mistry PK, Taddei T, vom Dahl S, Rosenbloom BE. Gaucher disease and malignancy: a model for cancer pathogenesis in an inborn error of metabolism. Critical reviews in oncogenesis. 2013;18(3):235–46. doi: 10.1615/critrevoncog.2013006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.