Abstract
Although prostate cancer (PCa) is clinically manageable during several stages of progression, survival is severely compromised once cells invade and metastasize to distant organs. Comprehending the pathobiology of invasion is required for developing efficacious targeted therapies against metastasis. Based on bioinformatics data, we predicted an association of melanoma differentiation associated gene-9 (syntenin, or syndecan binding protein (SDCBP)) in PCa progression. Using tissue samples from various Gleason stage PCa patients with adjacent normal tissue, a series of normal prostate and PCa cell lines (with differing tumorigenic/metastatic properties), mda-9/syntenin manipulated variants (including loss-of-function and gain-of-function cell lines), and CRISPR/Cas9 stable MDA-9/syntenin knockout cells, we now confirm the relevance of and dependence on MDA-9/syntenin in PCa invasion. MDA-9/syntenin physically interacted with insulin-like growth factor-1 receptor (IGF-1R) following treatment with insulin-like growth factor binding protein-2 (IGFBP-2), regulating downstream signaling processes that enabled STAT3 phosphorylation. This activation enhanced expression of MMP-2 and MMP-9, two established enzymes that positively regulate invasion. Additionally, MDA-9/syntenin-mediated upregulation of pro-angiogenic factors including IGFBP-2, IL-6, IL-8, and VEGF-A also facilitated migration of PCa cells. Collectively, our results draw attention to MDA-9/syntenin as a positive regulator of PCa metastasis, and the potential application of targeting this molecule to inhibit invasion and metastasis in PCa and potentially other cancers.
Keywords: MDA-9/Syntenin, prostate cancer, invasion, IGF-1R, STAT3
Introduction
As for most solid malignancies, mortality from prostate cancer (PCa), the most commonly diagnosed non-cutaneous cancer, results from widespread metastases (1). In this context, even treatment of localized PCa is fraught with significant morbidity, however, once metastasis occurs, there are few available treatment options for patients that have failed to respond to androgen deprivation therapies. Patients diagnosed in this advanced stage usually die within 12 to 18 months (1), emphasizing the need for better definition of the signaling cascades that regulate metastasis, which embodies three main processes: invasion, intravasation and extravasation. Invasion initiates with loss of cell-cell adherence of metastatic competent cells, which become motile and breakdown the extracellular matrix allowing them to migrate and enter the circulation (2). Multiple intrinsic transcriptional and epigenetic molecular programs regulate epithelial-to-mesenchymal transition (EMT). This process results in loss of intracellular adhesion and epithelial polarization, cytoskeleton rearrangement facilitating mobility, and release of extracellular proteases including matrix metalloproteinases (MMPs) that degrade the extracellular matrix promoting tumor spread. Secretion of active MMPs release matrix bound growth factors and cytokines further enabling invasion (3).
Defining genes that control invasion has potential to identify new target(s) for intervening in metastasis, which would have profound therapeutic implications. To achieve this objective, we employed subtraction hybridization (4). Melanoma differentiation associated gene-9 (mda-9), also known as syntenin, (mda-9/syntenin) was initially identified by subtraction hybridization as a novel gene displaying biphasic expression during terminal differentiation of human melanoma cells (5,6). mda-9/syntenin, a PDZ-domain protein, overexpressed in many types of human cancers, based on bioinformatics analyses http://cancergenome.nih.gov/, may function in tumor progression (7–9). Although both bioinformatics analyses and our observations (published and unpublished) indicate a positive correlation of mda-9/syntenin expression with aggressive phenotypes in multiple cancers, the precise roles of mda-9/syntenin in many of these neoplasms have not been ascertained. Suppression subtractive hybridization between a poorly invasive/non-metastatic and an invasive/metastatic breast cancer cell line identified mda-9/syntenin overexpression in metastatic cells (10). Overexpression of mda-9/syntenin is also evident in metastatic melanoma (11), breast (10), gastric (10) and bladder (12) cancer cells in comparison to their primary or poorly metastatic counterparts. Recently, we confirmed a role of this protein in glioma invasion (13). Notably, forced expression of mda-9/syntenin increased migration of non-metastatic cancer cells, which correlated with a more polarized distribution of F-actin and increased pseudopodia formation (11,14). Additionally, immunohistochemical analyses revealed a statistically significant continued increase in MDA-9/Syntenin expression from acquired melanocytic nevi to primary melanoma without or with progression to metastatic melanoma. These accumulated data strongly support the hypothesis that mda-9/syntenin functions as a positive regulator of melanoma metastasis (11,15–17) as well as aggressiveness in breast and gastric cancers, and might contribute to invasion of multiple additional cancers.
The insulin-like growth factor (IGF) signaling axis plays a pivotal role in prostate cancer progression, confirmed both in preclinical and clinical studies (18). This signaling axis consists of extracellular ligands (IGF-1 and IGF-2 with their binding partners, e.g., IGFBP-1 to -6) and receptors (IGF-1R and IGF-2R for IGF-1 and IGF-2, respectively). In 1998, Chan et al. first reported a positive correlation between IGF-1 levels and PCa risk (19), which was validated further in transgenic animals overexpressing IGF-1 in prostate epithelium that led to spontaneous neoplasia in the mouse prostate (20,21). Upon stimulation by specific ligands, IGF-1R is activated through auto phosphorylation resulting in phosphorylation of its’ downstream targets insulin receptor substrate-1 (IRS-1) and Shc adaptor proteins, which play decisive roles in cellular proliferation/differentiation/cellular homeostasis through PI3K/AKT or Ras/Raf/Mek pathways (22). Additionally, activation of IGF-1R also associates with cellular invasiveness and metastatic phenotypes through activation of IRS-1 that directly influences the beta catenin pathway (23), or by regulating secretion of matrix metalloproteinases (24). As a receptor kinase, IGF-1R influences other growth factors or receptors such as VEGF and EGFR (Reviewed by Tao et al.(25)) and regulates angiogenesis and proliferation. Accordingly, IGF-1R activation or its’ signaling cascade in PCa is fundamental, emphasizing clinical relevance and rationalizing the development of targeted therapeutics as reflected by the accelerated number of ongoing clinical trials employing inhibitors of the IFG-1R axis (reviewed by Heidegger et al.(22)).
The present study explores the relevance of MDA-9/Syntenin expression in PCa pathogenesis. We decipher a novel mechanistic signaling pathway for MDA-9/Syntenin in regulating PCa invasion. IGF-1R, through a physical interaction with MDA-9/Syntenin is central in this cascade. Overall, our findings show that MDA-9/Syntenin represents a novel molecular target for inhibiting invasion and metastasis, an ultimate cause of PCa-associated death.
Materials and Methods
Human cell lines
In the present study, the following cell lines were used, P69, RWPE-1, DU-145, PC-3, M12, M2182, ARCaP, ARCaP-E, and ARCaP-M (26). P69 (SV40 T antigen immortal normal human prostate epithelial cells), M2182 (progressed P69 cells displaying a tumorigenic phenotype) and M12 (progressed P69 cells displaying a tumorigenic and metastatic phenotype) were provided by Dr. Joy Ware, VCU School of Medicine, Richmond, VA. P69, M2182 and M12, provide a representative panel of human prostate cells (from normal immortal, tumorigenic, metastatic) and are described and cultured as indicated in our previous publication (26). Other cells, except ARCaP with its epithelial and metastatic variants, ARCaP-E and ARCaP-M, respectively, were obtained from ATCC (Manassas, VA) and maintained in culture as per ATCC recommendations. ARCaP, ARCaP-E and ARCaP-M cells were from Novicure Biotechnology (Birmingham, AL) and maintained in media recommended by the provider. HPV-immortal human prostate epithelial cells, RWPE-1, were from ATCC. All cell lines were routinely checked for mycoplasma contamination using commercial kits. The majority of experiments used RWPE-1, DU-145 and ARCaP-M cells. All of these cell lines were purchased recently (within the last 3 years) and were strictly maintained as recommended by the manufacturer.
Reagents and antibodies
Specific antibodies (MDA-9/Syntenin, Phospho-IGF-1R (Tyr-1135), IGF-1R, Phospho-Src (Tyr-416), Src, Phospho-FAK (Tyr-397), FAK, Phospho-STAT3 (Tyr-705), MMP-2, MMP-9, IGFBP-2, STAT3, Syntenin-2, β-actin and EF-1α) and reagents (IGFBP-2 and ELISA kits (IGFBP-2)) used in the present study are described in Supplementary Materials and Methods.
Tissue microarray
Two different PCa tissue microarrays, purchased from Novus Biologicals (Littleton, CO)) and USBiomax (Rockville, MD) were stained with MDA-9/Syntenin or IGFBP-2 antibody from Sigma-Aldrich (St. Louis). All clinical samples were received from a commercial source without any personal identifications, with the exception of sex (all are male), age and cancer stage. Images were captured by Vectra Polaris Quantitative Pathology Imaging System in VCU Cancer Mouse Models Developing Shared Resource Core. Staining intensity was quantified with inbuilt software supplied by PerkinElmer, Inc. (Houston, TX).
Real Time PCR
For qPCR, total RNA were extracted using miRNeasy kits (Qiagen, Valencia, CA) and cDNA was prepared as described (15). Quantitative qPCR was performed using an ABI ViiA7 fast real-time PCR system and Taqman gene expression assays according to the manufacturer’s protocol (Applied Biosystems, Foster City, CA).
Constructs and stable cell clones
Vectors and stable cell clones used in this study, including mda-9 and shmda-9 (15), and pRc.CMV.Stat3Y705F vectors and Pool 1 and Pool 2 mda-9/syntenin knockout, and sgRNA control clones are described in Supplementary Materials and Methods.
In vivo experiments
All in vivo experiments were performed using IACUC approved protocols. Metastasis assays using tail vein injection were performed by injecting 1 × 106 ARCaP-Luc cells, either infected with Ad.5/3-shcon or Ad.5/3-shmda-9, and animals were monitored for lung fluorescence by bioluminescence (BLI) at 48 days and survival was monitored over 90 days. Details can be found in Supplemental Materials and Methods.
Co-immunoprecipitation
Co-Immunoprecipitation was performed as described previously (15,17) using a kit from Pierce (Pierce Biotechnology, Rockford, IL).
Invasion assays
Boyden chamber assays tested the invasive properties of cancer cells (15,17). Briefly, cells were infected with control shRNA or mda-9/syntenin shRNA expressing Ad.5/3 and plated on the Matrigel-coated upper chamber. After 18 hours, invasive cells in the lower chamber were photographed and analyzed. All data presented, mean ± S.D.
Statistical analysis
All data represent mean ± S.D. from three independent experiments. Statistical analysis was performed using either Student t test (Microsoft excel), Pearson Correlation (GraphPad prism software). P < 0.05 was considered significant.
Results
MDA-9/Syntenin expression is elevated in PCa
Computational analysis identified mda-9/syntenin mRNA overexpression in PCa compared to normal prostate (8). Immunohistochemistry (IHC) of MDA-9/Syntenin protein expression in two tissue microarray (TMA) slides containing 8 adjacent tumor tissue sections and 64 human prostate tumor samples (from individual patients) at different disease stage (Stage II (n = 17) and Stage III (n = 47)) supported the genomic profiling data (Fig. 1A). MDA-9/Syntenin expression was significantly upregulated in Stage II and Stage III compared with adjacent normal tissue. Due to the low number of cases, samples from “Stage I (n = 3)”, “metastatic stage (n = 2)”, “hyperplasia (n = 3)”, “small acinar carcinoma (n = 1)” and “carcinoid (n = 1)” were not included in the final analysis. In the two cases from metastatic stage higher expression of MDA-9/Syntenin was observed, however, the staining scores were not significantly different from Stage III adenocarcinoma. Next, prostate tumor sections from a cohort of Hi-myc mice, a well-studied spontaneous PCa model which develops PIN by 13-weeks and invasive adenocarcinoma by 26-weeks of age, were analyzed for MDA-9/Syntenin expression (27). In 2-month old and older Hi-myc mice, MDA-9/Syntenin expression was elevated in prostate adenocarcinomas (Fig. 1B).
Figure 1. MDA-9/Syntenin is upregulated in advanced PCa.
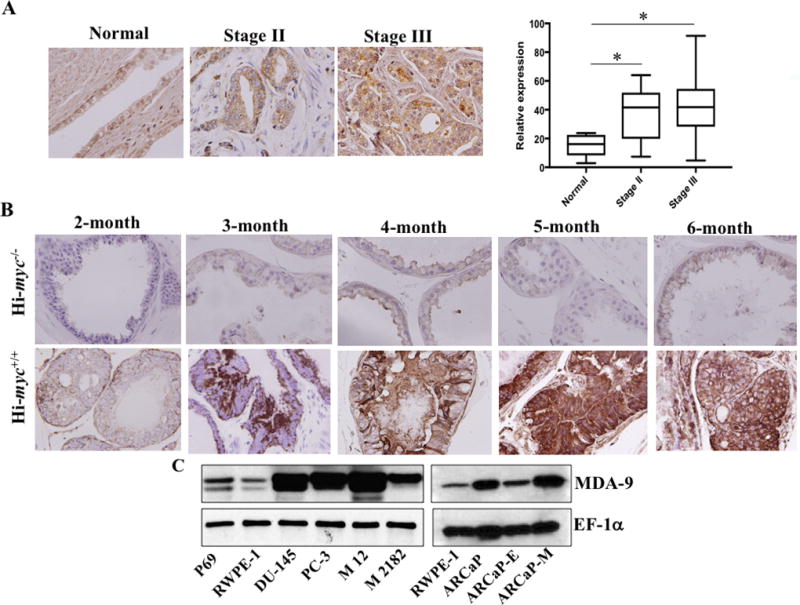
A) Representative photomicrographs of MDA-9/Syntenin expression in various stages of prostate adenocarcinoma. Staining intensity was measured using imaging software and the average value with S.D. for Stage II and III disease is presented. B) Prostates from Hi-Myc positive or negative male mice at different ages (n = 3) were collected, paraffin embedded and sectioned. Representative photomicrographs of MDA-9/Syntenin immunostained slides. C) Expression of MDA-9/Syntenin in P69, RWPE-1 and different human PCa cells.
mda-9/syntenin was elevated (Fig. 1C; Supplementary Fig. S1) in different human PCa cell lines in comparison with RWPE-1, a normal immortalized prostate epithelial cell line. Expression was significantly up-regulated in M12, a metastatic variant of P69 (non-tumorigenic prostate epithelial cells), suggesting an association of MDA-9/Syntenin with metastasis, which was supported further by in vitro invasion assays. MDA-9/Syntenin was also overexpressed in M2182 cells, which are tumorigenic but non-metastatic P69 variant cells, suggesting that in addition to metastasis, MDA-9/Syntenin might also contribute to tumorigenic phenotypes. In addition, higher expression of MDA-9/Syntenin was evident in mesenchymal ARCaP-M (metastatic variant) cells in comparison with epithelial variant ARCaP-E cells (Fig. 1C, right panel; Supplementary Fig. S1), providing additional confirmation of the potential relevance of MDA-9/Syntenin in PCa progression.
MDA-9/Syntenin expression regulates invasiveness of PCa cells
Invasion is an early event in metastasis (28). A role of MDA-9/Syntenin in invasion has been established in multiple cancer lineages (9). To investigate the potential role of this gene in PCa invasion, we overexpressed mda-9/syntenin in normal immortal RWPE-1 cells, using an adenovirus expressing mda-9/syntenin (Ad.5/3-mda-9) (15,17). Adenovirus expressing empty vector was used as control. Overexpression of mda-9/syntenin (Supplementary Fig. S2A) caused a robust up regulation of invasion ability (~6-fold) in these minimally invasive cells (Fig. 2A). Additionally, we silenced MDA-9/Syntenin expression in three aggressive human PCa cell lines, PC-3, DU-145, and ARCaP-M, using an mda-9/syntenin targeted shRNA carrying adenovirus (Ad.5/3-shmda-9) (15,17). A significant reduction (~30 to ~50%) in invasion was observed, which correlated with the down regulation of MDA-9/Syntenin expression (Fig. 2B; Supplementary Fig. S2B). Both PDZ domains affect MDA-9/Syntenin-mediated invasion, since ectopic expression of mutant constructs (Fig. 2C, Left panel: ΔPDZ1 and ΔPDZ2 (16,29)) containing only the PDZ1 (ΔPDZ2) or PDZ2 (ΔPDZ1) domain decreased invasion as compared with full length mda-9/syntenin (WT) (Fig. 2C, right panel). Deletion of the PDZ1 domain had greater impact on invasion than deleting the PDZ2 domain in PCa cells (Students t test, p=0.0002). A stable luciferase expressing ARCaP-M-shmda-9 cell line was engineered and introduced into athymic nude mice intravenously via tail vein injection. Mice were imaged to monitor lung metastasis progression. Tumor burden was reduced in the lungs of animals that received mda-9/syntenin manipulated (suppressed, Fig. 2D. panel i) ARCaP-M cells (Fig. 2D, panel ii). Mice receiving control cells had larger lungs (Fig. 2D, panel iii) and poorer survival in comparison with the mda-9/syntenin manipulated (suppressed) group (Fig. 2D, panel iv).
Figure 2. MDA-9/Syntenin regulates PCa progression.
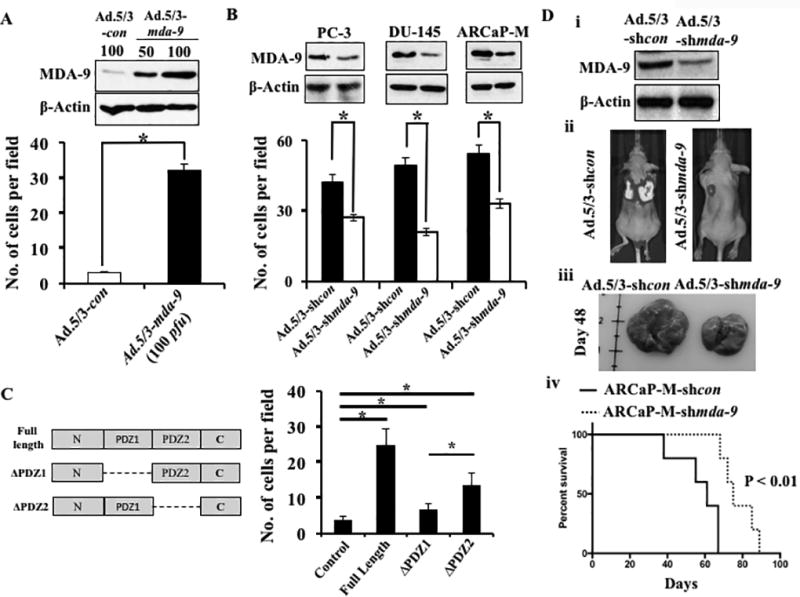
A) RWPE-1 cells infected with various adenoviruses at the indicated M.O.I. After 48 hours, infected cells were subjected to Matrigel invasion assays as described in Materials and methods. Expression of MDA-9/Syntenin was confirmed by Western blotting. B) MDA-9/Syntenin levels and invasiveness of different PCa cells after infection with Ad.5/3-shcon or Ad.5/3-shmda-9. C) Left panel: Schematic diagram of mutant mda-9/syntenin constructs, ΔPDZ1 and ΔPDZ2. Right panel: RWPE-1 cells transfected with full length and mutants of mda-9/syntenin and 48 hours after transfection invasion was evaluated. Number of invaded cells presented as a bar graph. Average with S.D. from three independent experiments. “*” represents statistical significance (p<0.05) between indicated groups. D) Downregulation of MDA-9/Syntenin was confirmed in Ad.shmda-9 infected cells (i). Luciferase expressing ARCaP-M cells carrying shcon or shmda-9 were inoculated I.V. into athymic nude mice by tail vein. Representative photographs of BLI (ii) and gross morphology of lungs (iii) 48 days after inoculation. Kaplan-Meier survival graph using Graph Pad software (iv).
MDA-9/Syntenin regulates PCa invasion through STAT3 activation
Many tumor-derived cell lines as well as human tumors, including PCa, express a constitutively active STAT3 protein (30–32). Consistent with previous observations, STAT3 activity (measured by phosphorylated (Tyr705) STAT3) was significantly higher in three aggressive PCa cells and showed a positive correlation with MDA-9/Syntenin levels (Fig. 3A vs. Fig. 1C for MDA-9/Syntenin). Next, we assessed the expression of phospho-STAT3 by Western blotting in mda-9/syntenin over-expressing RWPE-1 cells or in different PCa cells where mda-9/syntenin was silenced. As seen in Fig. 3B, forced expression of mda-9/syntenin upregulated STAT3 activation in RWPE-1 and ARCaP-E cells (Supplementary Fig. S3). Conversely, downregulation of mda-9/syntenin in different PCa cells (DU-145, ARCaP and ARCaP-M) negatively regulated STAT3 activation supporting a correlation between MDA-9/Syntenin expression and STAT3 activity (Supplementary Fig. S3). To provide additional confirmatory evidence, an additional experiment was conducted using STAT3 reporter constructs from SA Bioscience (Valencia, CA), which encode a firefly luciferase reporter gene under the control of a minimal CMV promoter and tandem repeats of the SIE transcriptional response element. These constructs can monitor both increases and decreases in the transcriptional activity of STAT3-containing dimers, and hence the activity of the STAT3 signaling pathway. Evidence for mda-9/syntenin-mediated STAT3 activation was verified by co-transfection of a STAT3 reporter with mda-9/syntenin or shmda-9 in RWPE-1 and DU-145 cells, respectively (Fig. 3C). Finally, the anti-invasive activity of mda-9/syntenin silencing was rescued by overexpressing a constitutively active STAT3 in transiently mda-9/syntenin knockdown PCa cells (Fig. 3D). Blocking secretion, using Brefeldin A, significantly reduced STAT3 activity in DU-145 and ARCaP-M cells indicating a role of secretion in activation (Fig. 4A). To further characterize the secretory molecules involved in MDA-9/Syntenin-mediated STAT3 regulation, we evaluated the role of IGFBP-2, a downstream secretory target of MDA-9/Syntenin (15), which was expressed at elevated levels in aggressive PCa cells (33,34). A TMA slide (Novus Biologicals) was immunostained for IGFBP-2 and compared with MDA-9/Syntenin stained sections. In 46 samples, including both normal (n = 8) and adenocarcinomas (8 and 30 cases from Stage II and Stage III, respectively), both Stage II and III cases showed significantly higher staining intensity compared with adjacent normal tissue (Fig. 4B). The values for MDA-9/Syntenin and IGFBP-2 from individual cases were analyzed for Pearson correlation using statistical software (Prism). A positive correlation (r2 = 0.4813, p<0.0001) between these two proteins was observed when comparing the 46 samples (Fig. 4C) or only Stage II (n = 8, r2 = 0.8451, p<0.0083) and Stage III (n = 30, r2 = 0.5965, p<0.0005) samples. Additionally, when comparing ARCaP series for both mda-9/syntenin and IGFBP-2 mRNA expression, maximum fold changes (relative to RWPE-1) for both genes was evident in ARCaP followed by ARCaP-M and ARCaP-E (Supplementary Fig. S4A). Similar patterns were also apparent in DU-145 and PC-3ML cells (metastatic PCa cells isolated from a bone metastatic lesion) (Supplementary Fig. S4A). Finally, using a loss-of-function experiment, we confirmed that mda-9/syntenin expression directly regulates igfbp-2 at both mRNA and protein level (Supplementary Fig. S4B and S4C). In contrast, manipulation of MDA-9/syntenin did not alter the expression of Syntenin-2 (Supplementary Fig. S4C and S4D). Downregulation of secretory IGFBP-2 was evident by ELISA in the media collected from mda-9/syntenin manipulated cell lines (Supplementary Fig. S4E). Co-transfection studies with various combinations of genetic manipulation defined the effects of IGFBP-2 on STAT3 activation and the potential role of MDA-9/Syntenin in this pathway. RWPE-1 cells, which have very low levels of MDA-9/Syntenin expression, displayed higher STAT3 activity following elevated mda-9/syntenin expression. This activity was significantly suppressed in the presence of shigfbp-2, indicating the importance of IGFBP-2 (Fig. 4D; Supplementary Fig. S5A) in MDA-9/Syntenin-mediated STAT3 activation. Similarly, transiently silencing mda-9/syntenin impacted STAT3 activation in DU-145 and ARCaP-M cells and the effect was more robust when both genes (mda-9/syntenin and igfbp-2) were knocked down) (Fig. 4E; Supplementary Fig. S5B). Loss-of-function studies in ARCaP-M and DU-145 cells confirmed that exogenous hIGFBP-2 (recombinant protein)-mediated STAT3 activation depended on endogenous MDA-9/Syntenin expression levels (the effects of hIGFBP-2 was not evident in mda-9/syntenin-knockdown cells, Fig. 4F; Supplementary Fig. S5C).
Figure 3. MDA-9/Syntenin regulates STAT3 activity in PCa cells.
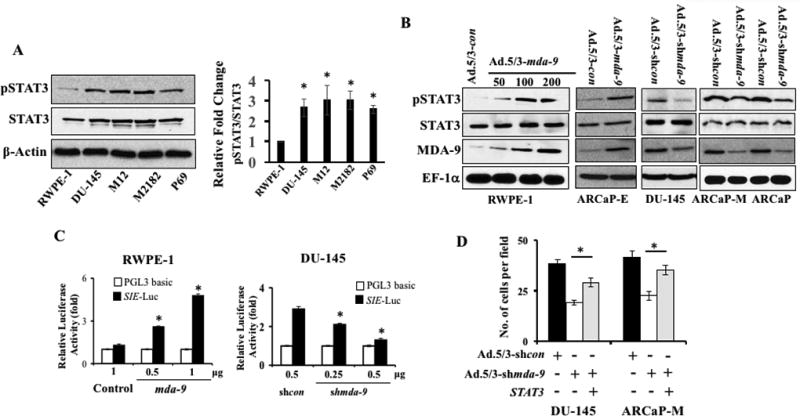
A) Western blotting analysis of phospho-STAT3 (Tyr705) and total STAT3 (left panel). Band intensity was quantified and relative-fold change in different cancer cells vs. RWPE-1 is presented (right panel). Average with S.D. from three independent experiments. “*” represents statistical significance (p<0.05) between indicated groups. B) Cells were either infected with adenoviruses for 48 hours and reseeded on fibronectin-coated plates. Total cell lysates prepared 1 hour after plating and Western blotting using indicated antibodies. C) Cells were co-transfected with a reporter gene and empty vector, mda-9/syntenin or mda-9/syntenin shRNA and after 48 hours, luciferase activity was measured. Data presented as fold-change vs. the control group (empty vector). D) Cells were co-transfected with different expression plasmids and 48 hours later, cells were trypsinized and invasion assayed. Cells counted using bright field microscopy.
Figure 4. MDA-9/Syntenin plays a decisive role in IGFBP-2-induced STAT3 activation.
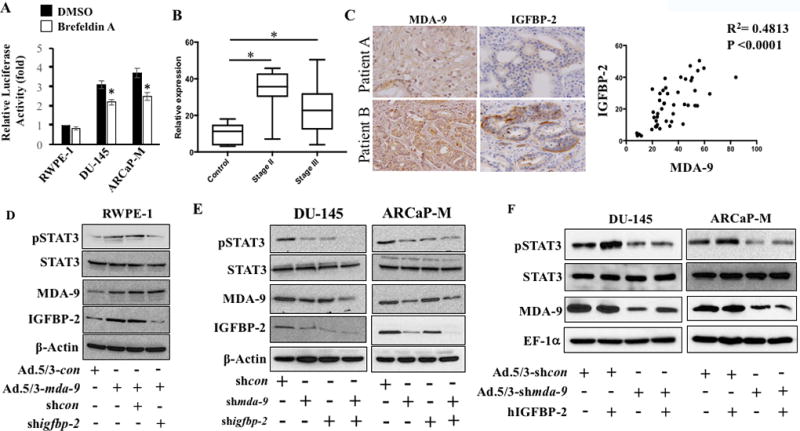
A) Cells transfected with a STAT3 reporter gene and after 36 hours were treated with Brefeldin A (5 µg/ml) for 30 min. Media was removed and cells were cultured an additional 3 hours in serum-free media and luciferase activity measured. B) Immunostaining for IGFBP-2 was performed in tissue microarray and staining intensity was quantified with Polaris Image Analysis software. The average value with S.D. from different stages is plotted. “*” represents statistical significance between groups. C) The expression of MDA-9/Syntenin and IGFBP-2 correlates in sections from the same patient (upper patient A (low expression) and patient B (high expression) bottom). Pearson correlation determined using 46 samples and presented with R2 and p value. D) RWPE-1 cells expressing MDA-9/Syntenin in presence of control shRNA or igfbp-2 shRNA for 48 hours. Cells were trypsinized and reseeded onto fibronectin-coated plates. Samples collected after 1 hour and Western blotting performed. E) Endogenous MDA-9/Syntenin and IGFBP-2 expression in PCa cells treated with shmda-9 and shigfbp-2 either alone or in combination. Cells were reseeded on fibronectin-coated plates for 1 hour. Western blotting with indicated antibodies. F) PCa cells infected with Ad.5/3-shcon or Ad.5/3-shmda-9 (100 pfu/cell) for 48 hours. Cells re-seeded on fibronectin-coated plates and treated with recombinant hIGFBP-2 (100 ng/ml) for 1 hour. Western blotting for indicated proteins.
MDA-9/Syntenin physically interacts with IGF-1R and activates STAT3
IGF-1R is a tyrosine kinase that phosphorylates STAT3 (35). IGF-1R, which belongs to the IGF receptor family, is a transmembrane receptor tyrosine kinase (RTK). In response to insulin-like growth factor 1 ligand binding, IGF-1R is activated via auto phosphorylation (Tyr 980), leading to activation of various signaling cascades including STAT3. Because IGF-1R can also potently activate the STAT3 pathway, one could envision a model in which IGFBP-2 (through binding with IGF-1) might activate STAT3 in an IGF-1R-dependent manner. To explore this possibility, we treated RWPE-1 with recombinant IGFBP-2 (hIGFBP-2) in the absence of serum and Western blotting was performed to determine auto phosphorylation, representing the activation state of IGF-1R. The presence of hIGFBP-2 in the absence of exogenous IGF-1 (not ruling out the possibility of endogenous- or cell-derived IGF-1 in the media) activated IGF-1R, which only occurred in the presence of MDA-9/Syntenin (Fig. 5A; Supplementary Fig. S6A). Enhanced STAT3 activation was also observed in these samples. These findings support the importance of MDA-9/Syntenin in IGF-1R-mediated STAT3 activation. Further experiments revealed that MDA-9 physically interacts and colocalizes with IGF-1R (Fig. 5B and C), which might play an essential role in transmitting activation signals to STAT3. To obtain more insight into potential binding site(s) and the consequences of this interaction, we overexpressed different deletion mutants (ΔPDZ1 and ΔPDZ2) of mda-9/syntenin in RWPE-1 cells and examined IGF-1R activation (Fig. 5D; Supplementary Fig. S6B). Expression of a PDZ1-deleted fragment (ΔPDZ1) failed to activate IGF-1R (following hIGFBP-2 treatment) indicating that the potential binding site of IGF-1R resides in the PDZ1 domain of MDA-9/Syntenin. STAT3 activity also correlated with IGF-1R activation further confirming that MDA-9/Syntenin-mediated STAT3 activation is a downstream consequence of MDA-9/IGF-1R interactions. Two PCa cells, with genetically modified mda-9/syntenin expression (using Ad.5/3-shmda-9), were exposed to exogenous hIGFBP-2 to investigate IGF-1R activation. As anticipated, lack of mda-9/syntenin expression robustly impacted IGFBP-2 activity (Fig. 5E; Supplementary Fig. S6C).
Figure 5. IGF-1R and MDA-9/Syntenin physically interact and regulate STAT3 activity.
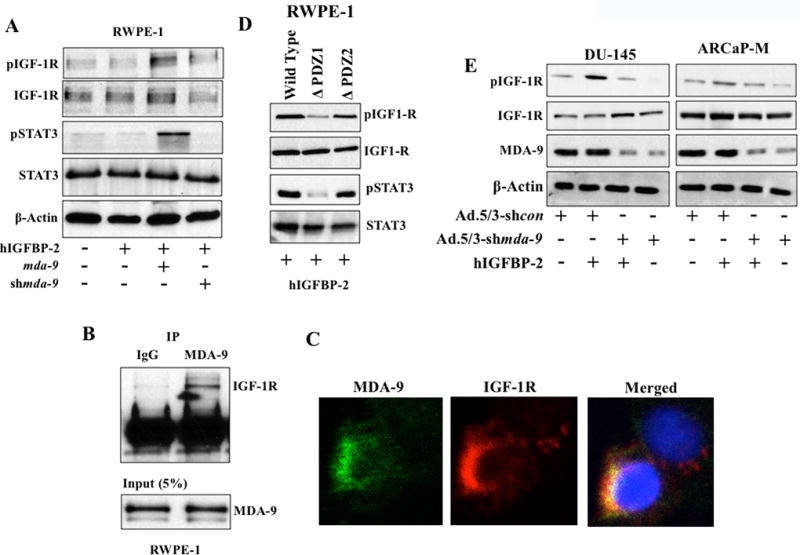
A) RWPE-1 cells treated with hIGFBP-2 under different conditions and phospho-IGF-1R expression determined by Western blotting. B) 200 µg of total protein from ARCaP-M cells incubated with MDA-9/Syntenin overnight for immunoprecipitation and Western blotting performed with anti-IGF-1R antibody. C) Immunofluorescence assay to determine co-localization of proteins. Confocal microscopy images of MDA-9/Syntenin and IGF-1R as “separate” or “merged” images. D) RWPE-1 cells transfected with wild type or mutant mda-9 vectors. 48 hours later, cells replated on fibronectin-coated plates for 30 mins and cell lysates analyzed using the indicated antibodies. E) PCa cell lines infected with Ad.5/3-shcon or Ad.5/3-shmda-9 for 48 hours. Cells reseeded on fibronectin-coated plates and treated with recombinant hIGFBP-2 (100 ng/ml) for 1 hour. Western blotting analysis to determine auto-phosphorylation.
Based on prior studies with MDA-9/Syntenin (11,15,16) and current experimental evidences we hypothesized that MDA-9/Syntenin and IGF-1R physically interact, enhancing stability and activating STAT3 through phosphorylation at tyrosine 705. Phospho-STAT3 forms a dimer and translocates into the nucleus to induce various genes that actively participate in PCa progression. To test this hypothesis, we determined the expression of MMP-2 and MMP-9, the most common, invasion-related downstream targets of STAT3 in mda-9/syntenin-knockdown cell variants (transient or stable). Knocking down mda-9/syntenin downregulated basal expression of MMP-2 and MMP-9 (Fig. 6A; Supplementary Fig. S7A and S7B). Exogenous stimulation (using recombinant hIGFBP-2) upregulated both proteins in wild type cells, however, this did not occur in mda-9/syntenin downregulated cells. This data and that provided in Fig. 4F (hIGFBP-2-mediated STAT3 activation) demonstrate that endogenous MDA-9/Syntenin expression is critical for IGFBP-2-mediated MMP-2 and MMP-9 up regulation through STAT3 activation. mda-9/syntenin-mediated downregulation of MMP-2 and MMP-9 was rescued when over expressing a constitutively active STAT3 (Fig. 6B and C; Supplementary Fig. S8A and S8B). To confirm these findings, we developed two stable mda-9/syntenin knockout variant ARCaP-M cells using the CRISPR/Cas9 approach, one with minimal (Pool 1) and the other with a complete knockout (Pool-2) (Fig. 7A) of expression. These knockout pools showed a significant downregulation of igfbp-2 (Fig. 7B), MMP-2 (Fig. 7C) and MMP-9 (Fig. 7C) mRNA. In addition, qPCR data from the complete knockout MDA-9/Syntenin cells (Pool 2) demonstrated a significant downregulation of STAT3 downstream targets, e.g., Interleukin-6 (IL-6), IL-8 (IL-8), and VEGF-A mRNA, further supporting the critical role of MDA-9/Syntenin in regulating STAT3 activity in PCa (Fig. 7D). These clones significantly lose their invasive potential in vitro in Boyden chamber assays and this phenotype is rescued significantly by a constitutive active STAT3 (Fig. 7E). A hypothetical model for MDA-9/Syntenin in regulating PCa progression via IGF-1R/STAT3 regulation is shown in Fig. 7F.
Figure 6. MMP-2 and MMP-9 are key regulators of MDA-9/Syntenin-mediated PCa invasion.
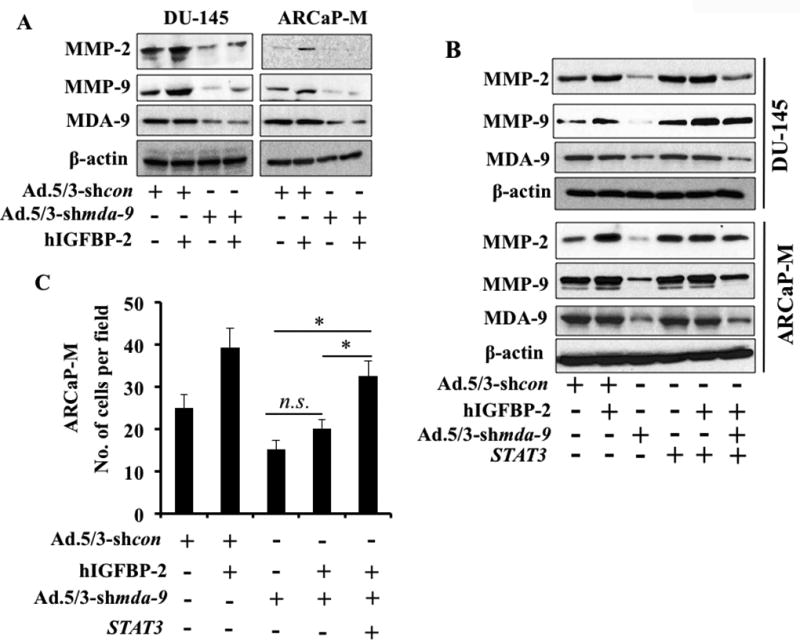
A) PCa cell lines infected with Ad.5/3-shcon or Ad.5/3-shmda-9 for 48 hours at 100 pfu/cell. Cells reseeded on fibronectin-coated plates and treated with recombinant hIGFBP-2 (100 ng/ml) for 1 hour. Western blotting to determine MMP-2 and MMP-9 expression. B) Cells were co-transfected/infected with different adenovirus/plasmids, followed by 48 hours incubation for transgene expression. Western blotting performed using anti-MMP-2 and anti-MMP-9. C) Cells were co-transfected with different expression plasmids. 48 hours later, cells were trypsinized and invasion determined. Average with S.D. from three independent experiments. “*” represents statistical significance (p<0.05) between indicated groups.
Figure 7. Knockout of MDA-9/Syntenin in PCa inhibits invasion.
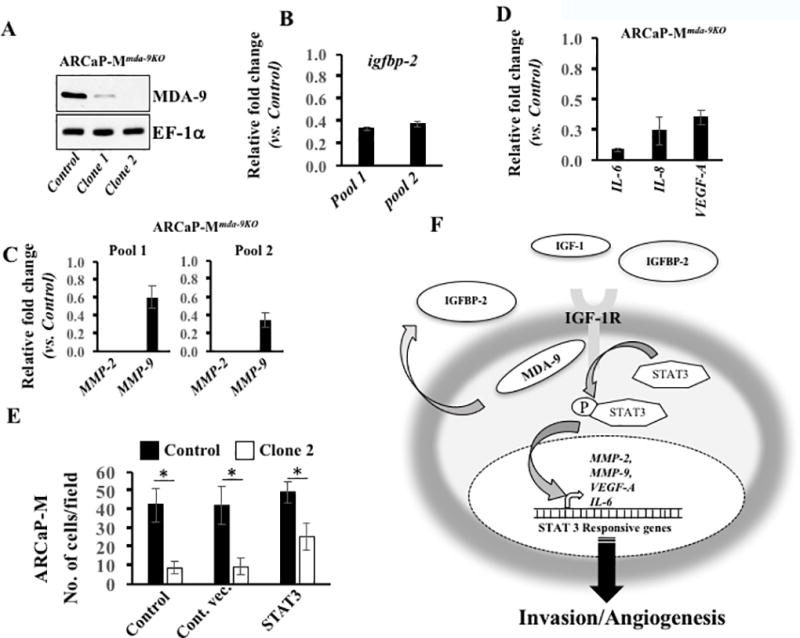
A) MDA-9/Syntenin expression in two clones from CRISPR/Cas9-mda-9/syntenin transfected cells. B & C) Expression of igfbp-2 (B) or MMP-2 and MMP-9 mRNA (C) determined in Pool-1 and Pool-2, presented as fold-change vs. control CRISPR/Cas9 transfected cells. D) Expression of indicated mRNA determined in Pool-2. Data presented as fold-change vs. control. E) Cells co-transfected with different expression plasmids. 48 hours later, cells trypsinized and invasion determined. Cells counted using bright field microscopy. Average with S.D. from three independent experiments. “*” represents statistical significance (p<0.05) between indicated groups. F) Hypothetical model of MDA-9/Syntenin-mediated PCa progression.
Discussion
Using computational-based analyses to interrogate online public data bases, confirmation was provided for the potential clinical significance of mda-9/syntenin with cancer prognosis (8). The bioinformatics data established and supported previous pre-clinical observations indicating a correlation of MDA-9/Syntenin with GBM (13) and melanoma (11) pathogenesis in clinical settings. Scrutiny of the public data bases also predicted a relationship between MDA-9/Syntenin expression and PCa prognosis. Moreover, it has been suggested that MDA-9/Syntenin is present in secreted exosomes (36), where its function requires clarification. Our present study confirms a direct relationship between expression of mda-9/syntenin and PCa progression and provides direct molecular mechanistic insights into precisely how this gene regulates PCa invasion (Fig. 7).
MDA-9/Syntenin is an adaptor scaffold protein that elicits its diverse functions by physically interacting with subsets of unique proteins in different regions of the cell (7,9). In melanoma, MDA-9/Syntenin physically interacts with Src (16) activating the transcription factor NFκB resulting in upregulation of invasion/angiogenesis associated genes (e.g., MMP-9, IGFBP-2). EGFR and MDA-9/Syntenin interaction was observed in urothelial cancer (12) and in radiation-treated GBM (37) cells. Menezes et al. demonstrated a physical interaction of MDA-9/Syntenin with TGFβ that induced EMT in breast cancer cells (29). Interestingly, MDA-9/Syntenin was also reported as a partner of the cytoplasmic domain of TGFβ receptor in the context of breast cancer (38). In head and neck cancer, small proline-rich protein 1B interacts with the PDZ domain of MDA-9/Syntenin regulating angiogenesis by upregulating VEGF-R (39). Additionally, a number of interacting partners and the consequences of these interactions have been documented in non-cancerous conditions. MDA-9/Syntenin interacts with the cytoplasmic domain of CD4 in T-helper cells allowing successful HIV entry (40). MDA-9/Syntenin and ALIX interactions are essential for exosome biogenesis (41). T-cell migration towards a chemoattractant is regulated by an association of MDA-9/Syntenin and myosin phosphatase Rho interacting protein (42). Thus, the diversity of interactions and the corresponding signaling changes in different cancerous or non-cancerous disorders suggests that specific interactions might be context dependent and influenced by both intrinsic (e.g., affinity, availability) and extrinsic (ECM, growth factors) factors. Defining the precise role of MDA-9/Syntenin in diverse pathological conditions is of value in clarifying its complete repertoire of functions. Our present study identified IGF-1R as a new MDA-9/Syntenin binding partner. Ligensa et al. identified a type I PDZ domain binding site ((S/T)XV) at the C-terminal of IGF-1R that could serve as the docking site for MDA-9/Syntenin (43). Further studies are necessary to define the critical genetic site(s) involved in defining IGF-1R-MDA-9/Syntenin interactions and what additional partner proteins may facilitate or antagonize these interactions.
Following IGF-1R binding to MDA-9/Syntenin STAT3 is activated. STAT3 is one of the downstream targets of IGF-1R. For example, Zong et al demonstrated activation of STAT3 by phosphorylation at tyrosine 705 position by IGF-1R upon IGF-1 stimulation (35). In pancreatic cancer, an IGF-1R-targeted small molecule inhibitor NVP-AEW541 elicited a direct effect on cell proliferation and abrogated cellular migration through inhibition of STAT3 activation (44). NT157, another IGF-1R targeting molecule, similarly caused significant down regulation of IGF-1R in a pre-clinical model of metastatic melanoma progression (45). A role of IGF-1R-mediated STAT3 activation (31) also occurs in PCa and its clinical relevance has been described (32). In summary, these studies and our previous collaborative study demonstrating that IL-6 activates STAT3 through IGF-1R in PCa (31) establishes a link between IGF-1R and STAT3 activation. These observations raise intriguing questions as to how STAT3 is recruited by IGF-1R. One study demonstrated that RACK1, an adaptor protein, served as a mediator and facilitated the recruitment of STAT3 with IGF-1R for phosphorylation (46). Theoretically, being an adaptor protein MDA-9/Syntenin may enable the assembly of a large complex containing both STAT3 and IGF-1R proteins, which requires experimental confirmation. Apart from inflammation, active STAT3 transcriptionally regulates various genes involved in survival, proliferation, invasion and angiogenesis (47). Since MDA-9/Syntenin did not show any direct impact on cell survival or proliferation in PCa cells based on in vitro experiments, we predict that MDA-9/Syntenin-mediated up regulation of STAT3 activity may be more relevant in regulating cell invasion rather than cell survival, which is experimentally supported in our present study.
Expression of IGFBP-2 is upregulated in PCa patients. Recently, a meta-analysis using 17 prospective and two cross-sectional data, which included more than ten thousand patients and healthy volunteers, demonstrated a positive correlation of IGFBP-2 with PCa risk. Along with IGFBP-2, this study also revealed that levels of IGF-1, IGF-2, IGFBP-3 positively correlated with disease progression (48). IGFBP-2 modulates IGF-1 function through IGF-1R. However, IGFBP-2 may also function in an IGF-1-independent manner via association with cytoplasmic or nuclear binding partners. Using cell fractionation approaches, IGFBP-2 was shown to translocate into the nucleus through importin-α, via a classical nuclear import mechanism, and activated VEGF-A transcription in multiple types of cancer including PCa (49). IGFBP-2 mediates EGFR-dependent STAT3 activation in glioblastoma cells. Although the activation of IGF-1R was not explored, IGFBP-2 and EGFR co-localized and accumulated in the nucleus, transactivated STAT3 and initiated the transcription of several STAT3-regulated genes including c-Myc, Bcl-xL, Cyclin D (50). Our experimental evidences suggest a potential role of IGFBP-2 in activation of STAT3 through IGF-1R in PCa, where MDA-9/Syntenin plays a decisive role.
Support is now provided for a signaling cascade in which MDA-9/Syntenin physically interacts with IGF-1R upon IGFBP-2 stimulation, resulting in STAT3 phosphorylation, thereby facilitating the expression of invasion/angiogenesis-related genes particularly MMP-2, MMP-9 and VEGF-A (Fig. 7). MDA-9/Syntenin-mediated MMP-9 and VEGF-A expression occurs in multiple cancers (11,12). The present study, firmly establishes MDA-9/Syntenin mechanistic functions in PCa pathogenesis, principally through interactions with partner protein(s) that are influenced by IGFBP-2, a protein upregulated and found in PCa patients’ body fluids (48). Although, both PDZ domains are important for MDA-9/Syntenin function (16), recent studies confirm the importance of the PDZ1 domain in forming relevant interactions independent of the PDZ2 domain. As discussed, the PDZ1 domain of MDA-9/Syntenin is the interacting domain with TGFβ1 (29), TGFβ1 receptor (38), small proline rich protein B (39) and now IGF-1R. We demonstrate that disrupting the MDA-9/Syntenin/IGF-1R/STAT3 loop profoundly affects PCa pathogenesis suggesting that targeting MDA-9/Syntenin could provide a strategy for inhibiting PCA progression and metastasis. We recently developed a novel MDA-9/Syntenin PDZ1-targeted small molecule pharmacological agent (PDZ1i) using a combination of fragment-based drug discovery techniques guided by in silico docking and NMR-based design (37). This small molecule was efficacious in interrupting the interaction of MDA-9/Syntenin with EGFR and inhibited downstream signaling involved in the invasion gain in radiation-surviving GBM cells. PDZ1i also displayed a strong synergistic effect when used in combination with radiation in terms of survival in animals containing a primary human GBM (37). Further studies, not in the scope of this paper, are clearly warranted to define the impact of PDZ1i on MDA-9/IGF-1R interactions and downstream signaling events in PCa progression and metastasis.
Supplementary Material
Significance.
This study provides new mechanistic insight into the pro-invasive role of MDA-9/Syntenin-1 in prostate cancer and has potential for therapeutic application to prevent prostate cancer metastasis.
Acknowledgments
The present research was supported in part by funding from NIH grant P50 CA058236 (to P.B. Fisher and Martin G. Pomper) and NCI Cancer Center Support Grant to VCU Massey Cancer Center P30 CA016059 (to P.B. Fisher and D. Sarker), the National Foundation for Cancer Research (NFCR) (to P.B. Fisher), the Human and Molecular Genetics Enhancement Fund (to S.K. Das and L. Emdad), VCU Massey Cancer Center (MCC) developmental funds (to P.B. Fisher) and VCU Institute of Molecular Medicine (VIMM) developmental funds (to P.B. Fisher, S.K. Das and L. Emdad). Services and products in support of the research project were also provided by the VCU Massey Cancer Center Cancer Mouse Model Shared Resource, supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059 (to P.B. Fisher and D. Sarkar). P.B. Fisher holds the Thelma Newmeyer Corman Chair in Cancer Research in the MCC. D. Sarkar is the Harrison Foundation Distinguished Professor in Cancer Research in the MCC.
Footnotes
Competing Interests
P.B. Fisher is a co-founder and has ownership interest in CTS, Inc. Virginia Commonwealth University, Johns Hopkins University and Columbia University have ownership interest in CTS. P.B. Fisher is also a co-founder and has ownership in InVaMet Therapeutics, Inc. The remaining authors declare no competing financial interests.
References
- 1.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–44. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 2.Sarkar D, Fisher PB. In: Cancer metastasis: biologic basis and therapeutics. Welch DR, Lyden DC, Psaila C, editors. New York: Cambridge University Press; 2011. p. 9. [Google Scholar]
- 3.Wan LL, Pantel K, Kang YB. Tumor metastasis: moving new biological insights into the clinic. Nat Med. 2013;19:1450–64. doi: 10.1038/nm.3391. [DOI] [PubMed] [Google Scholar]
- 4.Jiang H, Fisher PB. Use of a sensitive and efficient subtraction hybridization protocol for the identification of genes differentially regulated during the induction of differentiation in human melanoma cells. Mol Cell Different. 1993;1(3):285–99. [Google Scholar]
- 5.Lin JJ, Jiang HP, Fisher PB. Characterization of a novel melanoma differentiation-associated gene, mda-9, that is down-regulated during terminal cell differentiation. Molecular and Cellular Differentiation. 1996;4:317–33. [Google Scholar]
- 6.Lin JJ, Jiang H, Fisher PB. Melanoma differentiation associated gene-9, mda-9, is a human gamma interferon responsive gene. Gene. 1998;207:105–10. doi: 10.1016/s0378-1119(97)00562-3. [DOI] [PubMed] [Google Scholar]
- 7.Das SK, Bhutia SK, Kegelman TP, Peachy L, Oyesanya RA, Dasgupta S, et al. MDA-9/syntenin: a positive gatekeeper of melanoma metastasis. Front Biosci. 2012;17:1–15. doi: 10.2741/3911. [DOI] [PubMed] [Google Scholar]
- 8.Bacolod MD, Das SK, Sokhi UK, Bradley S, Fenstermacher DA, Pellecchia M, et al. Examination of Epigenetic and other Molecular Factors Associated with mda-9/Syntenin Dysregulation in Cancer Through Integrated Analyses of Public Genomic Datasets. Adv Cancer Res. 2015;127:49–121. doi: 10.1016/bs.acr.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kegelman TP, Das SK, Emdad L, Hu B, Menezes ME, Bhoopathi P, et al. Targeting tumor invasion: the roles of MDA-9/Syntenin. Expert Opin Ther Targets. 2015;19:97–112. doi: 10.1517/14728222.2014.959495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koo TH, Lee JJ, Kim EM, Kim KW, Kim HD, Lee JH. Syntenin is overexpressed and promotes cell migration in metastatic human breast and gastric cancer cell lines. Oncogene. 2002;21:4080–8. doi: 10.1038/sj.onc.1205514. [DOI] [PubMed] [Google Scholar]
- 11.Boukerche H, Su ZZ, Emdad L, Baril P, Balme B, Thomas L, et al. mda-9/Syntenin: a positive regulator of melanoma metastasis. Cancer Res. 2005;65:10901–11. doi: 10.1158/0008-5472.CAN-05-1614. [DOI] [PubMed] [Google Scholar]
- 12.Dasgupta S, Menezes ME, Das SK, Emdad L, Janjic A, Bhatia S, et al. Novel role of MDA-9/syntenin in regulating urothelial cell proliferation by modulating EGFR signaling. Clin Cancer Res. 2013;19:4621–33. doi: 10.1158/1078-0432.CCR-13-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kegelman TP, Das SK, Hu B, Bacolod MD, Fuller CE, Menezes ME, et al. MDA-9/syntenin is a key regulator of glioma pathogenesis. Journal of Neuro-oncology. Neuro Oncol. 2014;16:50–61. doi: 10.1093/neuonc/not157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grootjans JJ, Zimmermann P, Reekmans G, Smets A, Degeest G, Durr J, et al. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13683–8. doi: 10.1073/pnas.94.25.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das SK, Bhutia SK, Azab B, Kegelman TP, Peachy L, Santhekadur PK, et al. MDA-9/Syntenin and IGFBP-2 Promote Angiogenesis in Human Melanoma. Cancer Res. 2013;73:844–54. doi: 10.1158/0008-5472.CAN-12-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boukerche H, Su ZZ, Prevot C, Sarkar D, Fisher PB. mda-9/Syntenin promotes metastasis in human melanoma cells by activating c-Src. Proc Natl Acad Sci U S A. 2008;105:15914–9. doi: 10.1073/pnas.0808171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das SK, Bhutia SK, Sokhi UK, Azab B, Su ZZ, Boukerche H, et al. Raf kinase inhibitor RKIP inhibits MDA-9/syntenin-mediated metastasis in melanoma. Cancer Res. 2012;72(23):6217–26. doi: 10.1158/0008-5472.CAN-12-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocrine reviews. 2000;21:215–44. doi: 10.1210/edrv.21.3.0399. [DOI] [PubMed] [Google Scholar]
- 19.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–6. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan-Lefko PJ, Sutherland BW, Evangelou AI, Hadsell DL, Barrios RJ, Foster BA, et al. Enforced epithelial expression of IGF-1 causes hyperplastic prostate growth while negative selection is requisite for spontaneous metastogenesis. Oncogene. 2008;27:2868–76. doi: 10.1038/sj.onc.1210943. [DOI] [PubMed] [Google Scholar]
- 21.DiGiovanni J, Kiguchi K, Frijhoff A, Wilker E, Bol DK, Beltran L, et al. Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proc Natl Acad Sci U S A. 2000;97:3455–60. doi: 10.1073/pnas.97.7.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heidegger I, Massoner P, Sampson N, Klocker H. The insulin-like growth factor (IGF) axis as an anticancer target in prostate cancer. Cancer Letters. 2015;367:113–21. doi: 10.1016/j.canlet.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Playford MP, Bicknell D, Bodmer WF, Macaulay VM. Insulin-like growth factor 1 regulates the location, stability, and transcriptional activity of beta-catenin. Proc Natl Acad Sci U S A. 2000;97:12103–8. doi: 10.1073/pnas.210394297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang D, Samani AA, Brodt P. The role of the IGF-I receptor in the regulation of matrix metalloproteinases, tumor invasion and metastasis. Horm Metab Res. 2003;35:802–8. doi: 10.1055/s-2004-814143. [DOI] [PubMed] [Google Scholar]
- 25.Tao Y, Pinzi V, Bourhis J, Deutsch E. Mechanisms of disease: signaling of the insulin-like growth factor 1 receptor pathway--therapeutic perspectives in cancer. Nat Clin Pract Oncol. 2007;4:591–602. doi: 10.1038/ncponc0934. [DOI] [PubMed] [Google Scholar]
- 26.Dash R, Richards JE, Su ZZ, Bhutia SK, Azab B, Rahmani M, et al. Mechanism by which Mcl-1 regulates cancer-specific apoptosis triggered by mda-7/IL-24, an IL-10-related cytokine. Cancer Res. 2010;70:5034–45. doi: 10.1158/0008-5472.CAN-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–38. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 28.Martin LY Tracey A, Sanders Andrew J, Lane Jane, Jiang Wen G. Cancer Invasion and Metastasis: Molecular and Cellular Perspective. Metastatic Cancer: Clinical and Biological Perspectives edited by Rahul Jandial [Google Scholar]
- 29.Menezes ME, Shen XN, Das SK, Emdad L, Sarkar D, Fisher PB. MDA-9/Syntenin (SDCBP) modulates small GTPases RhoA and Cdc42 via transforming growth factor beta1 to enhance epithelial-mesenchymal transition in breast cancer. Oncotarget. 2016;7(49):80175–89. doi: 10.18632/oncotarget.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, et al. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23:3550–60. doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- 31.Rojas A, Liu G, Coleman I, Nelson PS, Zhang M, Dash R, et al. IL-6 promotes prostate tumorigenesis and progression through autocrine cross-activation of IGF-IR. Oncogene. 2011;30:2345–55. doi: 10.1038/onc.2010.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tam L, McGlynn LM, Traynor P, Mukherjee R, Bartlett JM, Edwards J. Expression levels of the JAK/STAT pathway in the transition from hormone-sensitive to hormone-refractory prostate cancer. Br J Cancer. 2007;97:378–83. doi: 10.1038/sj.bjc.6603871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura G, Kasuya J, Giannini S, Honda Y, Mohan S, Kawachi M, et al. Insulin-like growth factor (IGF) system components in human prostatic cancer cell-lines: LNCaP, DU145, and PC-3 cells. Int J Urol. 1996;3:39–46. doi: 10.1111/j.1442-2042.1996.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 34.Ho PJ, Baxter RC. Insulin-like growth factor-binding protein-2 in patients with prostate carcinoma and benign prostatic hyperplasia. Clin Endocrinol (Oxf) 1997;46:333–42. [PubMed] [Google Scholar]
- 35.Zong CS, Chan J, Levy DE, Horvath C, Sadowski HB, Wang LH. Mechanism of STAT3 activation by insulin-like growth factor I receptor. J Biol Chem. 2000;275:15099–105. doi: 10.1074/jbc.M000089200. [DOI] [PubMed] [Google Scholar]
- 36.Friand V, David G, Zimmermann P. Syntenin and syndecan in the biogenesis of exosomes. Biol Cell. 2015;107:331–41. doi: 10.1111/boc.201500010. [DOI] [PubMed] [Google Scholar]
- 37.Kegelman TP, Wu B, Das SK, Talukdar S, Beckta JM, Hu B, et al. Inhibition of radiation-induced glioblastoma invasion by genetic and pharmacological targeting of MDA-9/Syntenin. Proc Natl Acad Sci U S A. 2017;114:370–5. doi: 10.1073/pnas.1616100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwangbo C, Tae N, Lee S, Kim O, Park OK, Kim J, et al. Syntenin regulates TGF-beta1-induced Smad activation and the epithelial-to-mesenchymal transition by inhibiting caveolin-mediated TGF-beta type I receptor internalization. Oncogene. 2015;35(3):389–401. doi: 10.1038/onc.2015.100. [DOI] [PubMed] [Google Scholar]
- 39.Oyesanya RA, Bhatia S, Menezes ME, Dumur CI, Singh KP, Bae S, et al. MDA-9/Syntenin regulates differentiation and angiogenesis programs in head and neck squamous cell carcinoma. Oncoscience. 2014;1:725–37. doi: 10.18632/oncoscience.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordon-Alonso M, Rocha-Perugini V, Alvarez S, Moreno-Gonzalo O, Ursa A, Lopez-Martin S, et al. The PDZ-adaptor protein syntenin-1 regulates HIV-1 entry. Mol Biol Cell. 2012;23:2253–63. doi: 10.1091/mbc.E11-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–85. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 42.Sala-Valdes M, Gordon-Alonso M, Tejera E, Ibanez A, Cabrero JR, Ursa A, et al. Association of syntenin-1 with M-RIP polarizes Rac-1 activation during chemotaxis and immune interactions. J Cell Sci. 2012;125:1235–46. doi: 10.1242/jcs.094912. [DOI] [PubMed] [Google Scholar]
- 43.Ligensa T, Krauss S, Demuth D, Schumacher R, Camonis J, Jaques G, et al. A PDZ Domain Protein Interacts with the C-terminal Tail of the Insulin-like Growth Factor-1 Receptor but Not with the Insulin Receptor. J Biol Chem. 2001;276(36):33419–27. doi: 10.1074/jbc.M104509200. [DOI] [PubMed] [Google Scholar]
- 44.Moser C, Schachtschneider P, Lang SA, Gaumann A, Mori A, Zimmermann J, et al. Inhibition of insulin-like growth factor-I receptor (IGF-IR) using NVP-AEW541, a small molecule kinase inhibitor, reduces orthotopic pancreatic cancer growth and angiogenesis. Eur J Cancer. 2008;44:1577–86. doi: 10.1016/j.ejca.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Flashner-Abramson E, Klein S, Mullin G, Shoshan E, Song R, Shir A, et al. Targeting melanoma with NT157 by blocking Stat3 and IGF1R signaling. Oncogene. 2016;35:2675–80. doi: 10.1038/onc.2015.229. [DOI] [PubMed] [Google Scholar]
- 46.Zhang WZ, Zong CS, Hermanto U, Lopez-Bergami P, Ronai Z, Wang LH. RACK1 recruits STAT3 specifically to insulin and insulin-like growth factor 1 receptors for activation, which is important for regulating anchorage-independent growth. Mol Cell Biol. 2006;26:413–24. doi: 10.1128/MCB.26.2.413-424.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jarnicki A, Putoczki T, Ernst M. Stat3: linking inflammation to epithelial cancer - more than a "gut" feeling? Cell Div. 2010;5 doi: 10.1186/1747-1028-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Travis RC, Appleby PN, Martin RM, Holly JMP, Albanes D, Black A, et al. A Meta-analysis of Individual Participant Data Reveals an Association between Circulating Levels of IGF-I and Prostate Cancer Risk. Cancer Research. 2016;76:2288–300. doi: 10.1158/0008-5472.CAN-15-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Azar WJ, Zivkovic S, Werther GA, Russo VC. IGFBP-2 nuclear translocation is mediated by a functional NLS sequence and is essential for its pro-tumorigenic actions in cancer cells. Oncogene. 2014;33:578–88. doi: 10.1038/onc.2012.630. [DOI] [PubMed] [Google Scholar]
- 50.Chua CY, Liu Y, Granberg KJ, Hu L, Haapasalo H, Annala MJ, et al. IGFBP2 potentiates nuclear EGFR-STAT3 signaling. Oncogene. 2016;35:738–47. doi: 10.1038/onc.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


