Abstract
The gold-standard treatment for childhood amblyopia remains patching or penalizing the fellow eye, resulting in an average of about a one line (0.1 logMAR) improvement in visual acuity following ≈ 120 hours of patching (Stewart et al., 2004, 2007) in children 3 to 8 years old. However, compliance with patching and other treatment options is often poor. In contrast, fast-paced action video games can be highly engaging, and have been shown to yield broad-based improvements in vision and attention in adult amblyopia. Here, we pilot tested a custom-made action video game to treat children with amblyopia. Twenty-one (n=21) children (mean age 9.95 ± 3.14 [se]) with unilateral amblyopia (n=12 anisometropic and n=9 strabismic) completed 20 hours of game play either monocularly, with the fellow eye patched (n=11), or dichoptically, with reduced contrast to the fellow eye (n=10). Participants were assessed for visual acuity (VA), stereo acuity and reading speed at baseline, and following 10 and 20 hours of play. Additional exploratory analyses examined improvements after 6–10 weeks of completion of training (follow-up). Following 20 hours of training, VA improved on average by 0.14 logMAR (≈ 38%) for the dichoptic group and by 0.06 logMAR (≈ 15%) for the monocular group. Similarly, stereoacuity improved by 0.07 log arcsec (≈ 17%) following dichoptic training, and by 0.06 log arcsec (≈ 15%) following monocular training. Across both treatment groups, 7 of the 12 individuals with anisometropic amblyopia showed improvement in stereoacuity, while only 1 of the 9 strabismic individuals improved. Most improvements were largely retained at follow-up. Our feasibility study suggests that the action videogame approach may be used as an effective adjunct treatment for amblyopia in children, achieving results similar to those of the gold-standard treatment in shorter duration.
1. Introduction
While the consequences of abnormal visual development have been known for several centuries, millions of children go undiagnosed and therefore untreated every year. Current reports put the prevalence of amblyopia at about 2.4% of the population, affecting approximately 15 million children worldwide (Wu & Hunter, 2006). As a result, these patients face the possibility of permanent monocular vision loss and a greater likelihood of complete impairment if vision to the good eye is disturbed through injury or disease (Williams and Harrad, 2003). Amblyopia can also negatively impact one’s quality of life, resulting in reduced reading and fine motor skills, and may even negatively affect an individual’s self-image (Choong et al., 2004; Chua & Mitchell, 2004; Horwood et al., 2005; O’Connor et al., 2010b; O’Connor et al., 2009; Packwood et al., 1999; Rahi et al., 2006; Webber et al., 2008a,b).
Amblyopia is accompanied by widespread processing deficits in a range of visual functions that cannot be solely explained by abnormalities in primary visual cortex (see Kiorpes, 2006; Levi 2006, 2013 for reviews). Despite this, the standard treatment for amblyopia, refractive correction and occlusion (‘patching’) or penalization of the fellow (non-amblyopic) eye, focuses on improving visual acuity. While it is now clear that occlusion therapy can be effective, it also has some significant limitations. For one thing, patching is slow. For example, Stewart et al. (2007) report that it takes approximately 170 hours of patching for two lines of improvement in VA for a 4-year-old, and 236 hours for a similar effect in a 6-year-old. This jumps to over 400 hours for children older than 7 years of age (Fronius, Cirina, Ackermann, Kohnen & Diehl, 2014). Moreover, covering one eye is conspicuous, and requires the child to accept reduced visual perception while the fellow eye is covered. For these reasons, compliance can be very challenging. Further, the visual function of many children does not improve to normal levels. In fact, a substantial proportion of amblyopic children fail to achieve normal acuity even after extended periods of treatment (Birch and Stager, 2006; Birch et al., 2004; Repka et al., 2003; Repka et al., 2004; Repka et al., 2005; Rutstein et al., 2010; Stewart et al., 2004b; Wallace et al., 2006; Woodruff et al., 1994a). Even when vision is fully normalized, as many as 25% of patients experience a recurrence within the first year of treatment (PEDIG, 2004).
For these reasons, over the last two decades, there have been a number of attempts to develop more efficient treatments for childhood amblyopia, using perceptual learning and videogame techniques (see Levi & Li, 2009; Levi, 2012; Birch, 2013; Hess & Thompson, 2015; Levi, Knill & Bavelier, 2015 for reviews), either monocularly (with the amblyopic eye; AE) or dichoptically (with different information presented to the two eyes in order to reduce suppression and/or enhance fusion). A summary of the main studies testing such treatments in children is provided in Table 1.
Table 1.
Previous studies
| Approach | Study | N | Age (years) | Task | Duration (hours) | LogMAR gain | Stereo gain | Suppression change |
|---|---|---|---|---|---|---|---|---|
| Monocular PL & VG | ||||||||
| Li et al. (2007) | 2 | 9 &12 | Position discrimination in noise | ≈ 100 | 0.3 | 2/2 | ||
| Polat et al. (2009) | 5 | 7–8 | Gabor detection | ≈ 40 | 0.21 | 1/5 | Yes | |
| Liu et al. (2011) | 8–17 | Grating acuity | 40 – 60 | |||||
| - patched | 10 | 0.07 | 3/9 | |||||
| - not patched | 13 | 0.11 | 9/11 | |||||
| Kelly (2016) | 14 | 4.6–9.5 | patching | 28 | 0.07 | NS | Yes | |
| Holmes (2016) | 195 | 5–12.9 | patching | 224 | 0.14 | NS | ||
| Current study | 11 | 7–17 | VGP + Gabor | 20 | 0.06 | 5/11 | ||
| Mean | 0.14 | |||||||
| 95% CI | 0.07 | |||||||
| Dichoptic PL & VG | ||||||||
| Anti-suppression | Knox et al. (2012) | 14 | 5–14 | Motion coherence tetris; Balloon; Pong & Labyrinth | 5 | 0.09 | 7/14 | NS |
| Anti-suppression** | Li et al. (2014) | 4–12 | ||||||
| - binocular group | 45 | AE high contrast; FE low | 16 – 32 | 0.08 | NS | NS | ||
| - sham group | 24 | AE low contrast; FE high | 16 | NS | NS | NS | ||
| Anti-suppression** | Birch (2015) | 3–6.9 | ||||||
| - binocular group | 45 | AE high contrast; FE low | at least 16 | 0.09 | NS | NS | ||
| - sham group | 5 | AE low contrast; FE high | at least 16 | 0.02 | NS | NS | ||
| Anti-suppression | Kelly (2016) | 14 | 4.6–9.5 | binocular iPad adventure game | 10 | 0.15 | NS | Yes |
| Anti-suppression | Holmes (2016) | 190 | 5–12.9 | binocular iPad Tetris game | 112 | 0.11 | NS | |
| Anti-suppression | Li (2015) | 8 | 4–10 | dichoptic movies | 9.4 | 0.2 | NS | NS |
| Anti-suppression | Webber (2016) | 18 | 7–12 | Dichoptic iPod | 11.7 | 0.09 | 9/16 | Yes |
| iBIT | Cleary (2009) | 12 | 6–11 | Video AE; Frame NAE | 3.5 | 0.18 | Transient | |
| iBIT | Waddingham (2006) | 6 | 5.4–7.8 | Video AE; Frame NAE | 4.4 | 0.27 | - | |
| iBIT | Herbison (2013) | 4–8 | Video AE; Frame NAE | |||||
| iBIT | Herbison (2015) | 4–8 | 3 | |||||
| - Arm 1 | 24 | Video AE; BG OU | 0.1 | NS | ||||
| - Arm 2 | 26 | Action VG AE; BG OU | 0.06 | NS | ||||
| - Arm 3 | 25 | BG and FG OU | 0.03 | NS | ||||
| BBV | BBV | BBV | BBV | BBV | BBV | BBV | BBV | BBV |
| BBV | Bossi et al. (2017) | 22 | 3–11 | movie viewing | 75 | 0.27 | 9/22 | NS and NC |
| BBV+AE GP | Current study | 10 | 7 – 17 | dichoptic action VGP + Gabor | 20 | 0.14 | 3/10 | Yes but NC |
| Mean | 0.14 | |||||||
| Total | 680 | 95% CI | 0.04 | |||||
These appear to be the same study.
NS = Not significant
NC = not correlated
An important limitation on clinical adoption of these methods for treating amblyopia in children is compliance. Laboratory-based perceptual learning is generally repetitive and tedious. As a result, several groups have recently moved toward either gamified versions of perceptual learning tasks or full-fledged video games that exploit the appeal of video games developed for entertainment. However, gamified perceptual learning tasks may not have the same level of appeal and engagement as commercial action videogames. Unlike lab-based gamified perceptual learning, the video game industry is a multi-billion-dollar segment of the entertainment media, and designers face intense competition to create rich, immersive and engaging environments. The result is a more compelling experience that is more enjoyable and overcomes much of the tediousness experienced in perceptual learning regimes. Importantly, it is now well established that in normal adults, action videogames enhance various aspects of visual perception, above and beyond other video game genres such as social simulation games or Tetris (see for example, Green, Li and Bavelier, 2010).
While action video games were initially defined as first and third person shooter video games as developed by the video game industry, we (and others) now consider action video games as those that combine a number of features or game mechanics that facilitate brain plasticity and learning. Among these mechanics, are the need to execute actions under time constraints, a high load on divided attention, the appropriate switch between focused and divided attention as task demands vary, the requirement to plan at many different time scales from milliseconds to hours, and the use of variable value and time reward schedules, to cite a few (Green, Li and Bavelier, 2010). Thus, video games do not have to have violent content in order to be considered as action games.
Commercial action videogames are compelling and highly engaging. These games often include targets and enemies that move into and across the visual field. To succeed, players must be able to both distribute their attention widely and focus to the most relevant areas of the screen, and make spatial decisions under time pressure by aligning a cross hair or viewing scope to the target of interest. Once a decision has been made, the player receives immediate feedback in the form of points or negative consequences. Like perceptual learning, the level of game difficulty also increases as the players improve.
Action video games also trigger arousal and provide nuanced feedback on performance, which may be critical for efficient learning (Bavelier, Green, Pouget, & Schrater, 2012). Most importantly, however, action video games have a variety of salient content over the entire screen, leading to behavioral enhancements that are broader than the retinotopic and task specific changes that are often observed in PL (but see Xiao et al., 2008; Zhang et al., 2014). Playing action videogames results in significant improvements in a broad range of visual functions, from low-level to high level in normal adults (Green & Bavelier, 2007; Li et al., 2010; Li, Polat, Makous & Bavelier, 2009).
In contrast to neurotypical adults, adults with amblyopia, show improvements in vision after playing commercial vide games - either action (Medal of Honor) or non-action (Sim City) videogames (Li, Ngo, Nguyen & Levi, 2011; Li, Ngo & Levi, 2015) monocularly, with the fellow eye patched. For example, Li et al (2011, 2015) showed that playing videogames monocularly with the AE resulted in a broad range of improvements (visual acuity, stereoacuity, positional acuity, and spatial and temporal attention) in adults with amblyopia. However, an important principle of learning is that task difficulty should be adapted to the learner’s capacity. From this point of view, commercially available action video games designed by the industry for experienced gamers with normal vision may not be ideal, but should be modified to include easier levels adapted to the specific challenge of playing with degraded vision. Scaffolding the learning experience for the patient is a key design principle that should not be overlooked.
A number of recent studies have used dichoptic games, aimed at improving stereovision by reducing suppression and/or enhancing fusion for both adults (Hess & Thomson, 2015; Vedamurthy et al., 2015) and children (Kelly et al., 2016). For example, in a recent study, Kelly et al. (2016) had children play DigRush - a game in which children manipulate miners and their surroundings to dig for gold, while avoiding obstacles. However, to date there have not been studies using action video games (either monocular or dichoptic) with amblyopic children.
The aim of the current study was to test the feasibility and initial efficacy of using a customized action video game with a population of amblyopic children (age 7–17). While several groups have recently conducted studies with similar goals using both non-action games and movie viewing (see Table 1), they all cite motivation and compliance as challenging factors that may be limiting their results. Importantly, we compared the dichoptic videogame to an identical videogame played monocularly, with the fellow eye patched. Unlike the “sham” treatment where the content to the two eyes is reversed (i.e., high contrast to the fellow eye and low contrast to the weak eye, ensuring that the AE will be suppressed during play – e.g. Birch et al., 2015; Li et al., 2014), this control condition incorporates the traditional gold standard treatment. Our ‘patching-while-playing’ control should help provide further insight into whether dichoptic action videogame play yields greater improvement than monocular action videogame play. Previous studies in children have been equivocal, with some reporting greater improvement with dichoptic training (e.g. Kelly et. Al., 2016) and others reporting little or no advantage to the effects of dichoptic training (Tetris) over patching (e.g. Holmes et al., 2016).
2. Methods
2.1 Study Participants and Ethics Statement
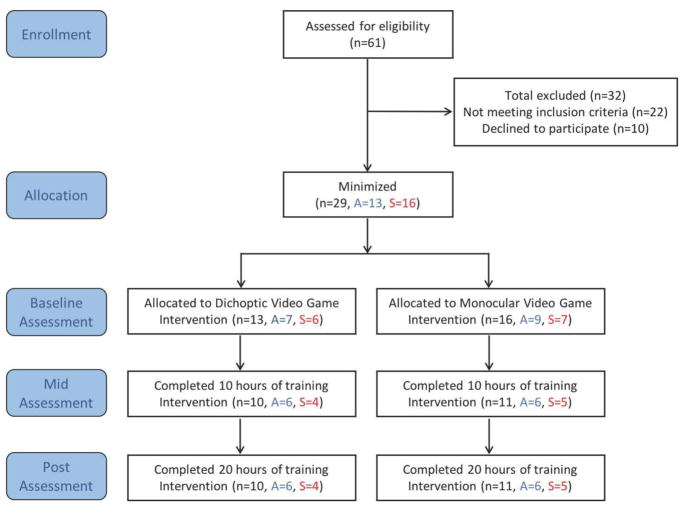
The study took place in research laboratories, at University of Rochester, NY and University of California, Berkeley, CA. The Institutional Human Subjects Review Boards at both institutions approved the study protocol. The study was conducted according to the tenets of the Declaration of Helsinki and informed consent was obtained from each participant and their parent/guardian. Twenty-one (N=21) children1 (mean age: 9.95 ± 3.14 [se], range 7–17 years) with unilateral amblyopia completed 20 hours of video game training and their data were analyzed. Participants were recruited through the eye clinic at both sites as well as through print advertisements at both locations. Three experienced optometrists, one at Rochester and two at Berkeley, provided complete eye exams for all participants prior to enrolling. Figure 1 shows the study design with numbers of participants screened, qualified and dropped out.
Figure 1.
Study design. Total number of participants included in each portion of the study (n), further divided into anisometropic amblyopes (A) and strabismic amblyopes (S).
The inclusion criteria were: (1) age 7–17 years old; (2) anisometropic amblyopia, strabismic amblyopia, or mixed (i.e., anisometropic and strabismic); (3) interocular visual acuity (VA) difference of at least 0.2 LogMAR; and (4) no history of eye surgery except those to correct strabismus. Exclusion criteria included: (1) non-concomitant or large angle constant strabismus (>30 prism diopters); and (2) any ocular pathological conditions (e.g., macular abnormalities) or nystagmus. All of our participants had normal or near normal VA in the fellow eye (FE - 20/12−1 - 20/25). The retinal health of all participants was assessed as normal, and they all had clear ocular media as assessed by ophthalmoscopy. Cover tests were used to assess ocular alignment at both distance and near.
Subject Classification
Study participants were categorized as either anisometropic (‘aniso’) or strabismic (‘strab’) amblyopes. Anisometropia was defined as ≥ 0.50D difference in spherical equivalent refraction or ≥ 1.5D difference in astigmatism in any meridian, between the two eyes (Wallace et al., 2011). Amblyopic subjects with anisometropia and an absence of manifest ocular deviation were classified as anisometropic amblyopes. Those with an ocular deviation (strabismus), as indicated by the cover test, were classified as strabismic amblyopes, irrespective of their refractive state, meaning that participants with both strabismus and anisometropia were defined as ‘strabismic’. Clinical details of study participants who completed the study are provided in Table S1. Several of the subjects had been recently treated (as indicated by the + in Table S1), and discussed in Section 3.34.
Correction of Refractive Error
Participants were instructed to wear their most recent optical prescription at all times, but were given trial frames with their refractive correction for training in the lab if they arrived without spectacles or contact lenses. Two children required an updated prescription. They were provided with full optical correction and were monitored for 6 to 8 weeks. They then returned to the lab for a new baseline assessment before starting the study.
2.2 Study Design Overview
The complete experimental design is detailed in Figure 1. Following consent and screening, eligible participants were assigned to one of two intervention groups: (1) dichoptic game group (n=13): playing the custom-made dichoptic videogame using a mirror stereoscope and balanced input (see description below); (2) monocular game group (n=16): playing the same game with the FE view turned off and that eye occluded with a black eye patch. Groups were assigned via minimization procedure (Taves, 1974; see Green, Strobach & Schubert, 2014 for a discussion), i.e. the first several participants were randomly assigned to a treatment and later participants were assigned to reduce the imbalance between the groups. This was particularly important to approximately balance the amblyopic subtypes (anisometropic and strabismic) for each training method.
Before starting the 20-hour intervention, participants completed a test battery (described in 2.4) to assess vision and related functions (‘baseline assessments’). Participants repeated the battery after the completion of 10 hours (‘mid-assessment’), and 20 hours (‘post-assessment’). Additionally, 12 of the participants who completed the study returned for one last assessment following a no-contact period of at least six-week (‘follow-up assessment’). Although this follow-up assessment was initially planned in our design, it became rapidly clear it would lead to a too great attrition rate and thus participants were kept in the study even if they made it clear they could not comply with a follow-up visit. This led to a self-selected subgroup at follow-up; for this reason, their data will be discussed separately from the main study.
2.3 Study Interventions
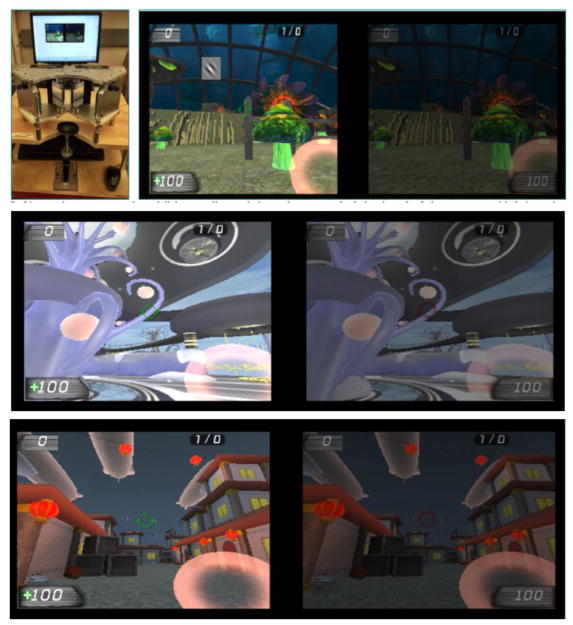
Participants from both groups were required to complete a total of 20 hours of experimental treatment, in sessions lasting approximately 1 hour, 1–3 times/week. Participants played a child-friendly action video game developed using the Unreal Tournament engine (see Figure 2 and Gambacorta et al., 2014). The general gaming principles were similar to those used in our adult videogame version (Bayliss et al., 2012, 2013; Vedamurthy et al., 2015 a & b). However, in the child-friendly version of the action game, we removed the violent elements of the original Unreal Tournament action game (Epic Games, 2004) while maintaining the motivating nature of a commercial game, as well as the heavy attentional load and relatively fast pacing of action video games.
Figure 2.
Screenshots from the custom-made child-friendly action video game. Top Left: Children in the dichoptic game play group aligned the game with a mirror stereoscope. Top right: Dichoptic display showing the image sent to the AE on the left and that sent to the NAE on the right, in the Magical Garden game world; during set-up, children adjusted the mirrors and contrast level of the NAE image, such that both images of the stereoscope were visible. Bottom Panels: The lower two screenshots show the two other game worlds, Amblyopia World (top) and Chinatown (bottom); Various games were included in order to keep the children engaged for as many as 20 hours.
Easier training levels were included so that young children with little gaming experience could master the skills required to play the game. The initial training levels included basic environmental cues and boundaries to keep the children focused while learning to move, pick up objects, and orient their pointer tools. Children were instructed to move throughout the scene, collect health points and tag one or more robot opponents. To tag the opponents, the fixation scope was aligned onto the center of the robot and the mouse was clicked to activate the pointer tool. This was operationally similar to a first-person-shooter game, but instead of guns, the pointer tools included a juice machine, bubble wand, and flower button.
Once basic proficiency with the game was achieved, the children played the tag game in one of three main worlds, each with a variety of objects and scenes, meaning players were exposed to many colors and spatial frequencies. Each world had a different theme, including the Magical Garden with imaginative plants, Amblyopia World with bridges and elevators to access the multiple stories, and Chinatown, with hidden alleys and takeout food boxes. To maintain engagement over the course of the entire study, additional robot opponents were added, and the difficulty level of the game was modulated based on the child’s individual progress, causing the robots to vary not only in number but also in speed, moving faster across the screen as the child progressed in the game.
A perceptual learning task was seamlessly integrated within the game, with a rotated Gabor patch that randomly appeared every few seconds in the view of the AE only (Vedamurthy et al., 2015a & b). Participants were required to respond to one orientation by tagging the target, and to the other orientation by either ignoring the patch or pressing the letter ‘E’. An incorrect response transformed the Gabor patch into a particularly powerful game enemy. The spatial frequency of the Gabor patch was adapted to maintain participant’s performance at 79% correct (Levitt, 1971). The Gabor patch task enabled us to monitor the AE’s resolution limit, while simultaneously serving as a suppression check (particularly important under dichoptic mode, see below), ensuring that the AE was actively engaged during game play.
Training took place in the lab, under supervision of research assistants. While logistically more challenging to the patients and their families, this design offers several key advantages. First, we can ensure that all patients receive the same training dosage with the proper optical correction. Second, we can have participants use a mirror-stereoscope to view the game content dichoptically, which is important for proper binocular alignment in some of the patients. For example, several of the strabismic amblyopes in the current study initially experienced difficulty fusing the dichoptic content. By starting with a very low contrast image in the fellow eye and adjusting the mirrors of the stereoscope until bifoveal alignment was achieved, patients learned to fuse the two images with practice. These patients became more proficient with maintaining fusion as the training progressed. While several previous studies used dichoptic content, it was presented with anaglyph or shutter glasses, which do not allow the same control over binocular alignment.
For all participants, the videogame was displayed on a gamma corrected monitor (Mitsubishi Diamond Pro 2070 SB), with resolution 1024 x 768 pixels and refresh rate of 60 Hz.
2.3.1 Dichoptic Game Mode
In the dichoptic game mode, the game was presented via a split screen view, allowing independent control of the images presented to the left and right eyes, and in particular their respective luminance and contrast. The split images of the game were viewed with a custom designed stereoscope at a distance of 68 cm. These dichoptic viewing conditions were designed to reduce suppression and promote fusion, while challenging the AE with an embedded psychophysical resolution task. Alpha blending (see Vedamurthy et al., 2015a & b for details) was used to balance the perceived image strength of the NAE with that of the AE eye at the start of each play session, in an effort to reduce suppression and facilitate fusion.
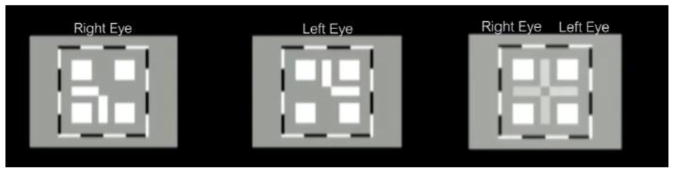
Each session began with both horizontal and vertical alignment of the dichoptic nonius lines by adjusting the mirrors of the stereoscope (Figure 3). The image to one eye was the bottom and left side of the cross, while the image to the other eye was the top and right side of the cross. With proper alignment, the image was a cross with a square cutout of the center, surrounded by four additional squares and a high contrast border that was visible in both eyes. Older children performed the alignment themselves. When necessary (for young children), the experimenter adjusted the mirrors. Children were shown key cards on how the cross should appear for each eye and both eyes together, and they were asked to draw the image as observed via the stereoscope. Confirmation of alignment was obtained after this iterative approach to ensure the nonius lines were aligned. After launching the game, the experimenter checked in again with the child to ensure that both parts of the fixation scope were visible. The experimenter also monitored the performance of the perceptual learning task to confirm that the child was not suppressing the AE.
Figure 3.
Dichoptic alignment: Fusion was achieved by aligning dichoptic horizontal and vertical lines to make a cross. A high contrast border and additional squares presented to both eyes provided context to aid in this process.
We note that there are important differences between our method of dichoptic presentation and those used by others. Our action video game presented the same image to each eye (except for Gabor patches and part of the fixation scope) with reduced luminance/contrast in the NAE, in an attempt to promote binocular fusion. Other dichoptic video game studies have presented different game elements to each eye so that binocular combination is required to play the game (see Hess et al., 2014a for a review). Both approaches have been reported to reduce binocular suppression as well as to improve VA and stereopsis (Hess & Thompson, 2015; Vedamurthy et al., 2015a).
2.3.2 Monocular Game Mode
Participants in the monocular game group played the custom videogame described above, but with the NAE display turned off, and a patch over this eye. Other features of the game, such as the perceptual learning task presented to the AE, were identical to the dichoptic group. Training parameters, such as game difficulty and duration of sessions were also kept the same in both groups.
2.4 Visual Function Assessments
Participants were required to wear their best optical correction (if any) for all visual assessments. Assessments included three main measures: visual acuity (VA), stereoacuity, and reading speed. Assessments were administered at baseline and following 10 and 20 hours of training. Follow-up assessments were conducted in 12 of the participants 6–10 weeks post training.
2.4.1 Main visual function assessments
Visual Acuity (VA)
Clinical VA at distance was measured using either Bailey-Lovie logMAR letter charts (UCB site), or using the high-contrast ETDRS format chart with Sloan optotypes (catalog No. 2104; Precision Vision, La Salle, Illinois; U of R site). Monocular acuities for both the AE and NAE, as well as binocular acuity were all measured with the same conditions.
Stereoacuity
Stereopsis was measured using the Randot Stereotest (Stereo Optical Co., Inc.; See description in Simons, 1981). Analyses were performed on the logarithm (base 10) of the stereoacuity values. Participants who ‘failed’ the stereo test were assigned a value of 800 arcsec (similar to Vedamurthy et al., 2015a and Wallace et al., 2011). Additional analyses were performed only for patients who had measurable stereoacuity initially, with similar results, i.e. there were no patients that went from no measureable stereoacuity to some consistently measurable stereoacuity.
Reading Speed
Amblyopic adults read slowly with their amblyopic eye (Levi, Song & Pelli, 2007), and children with amblyopia have reading impairments, even when using both eyes (Kelly et al., 2015). Therefore we evaluated reading speed for reading out-loud using the standardized MN Read Acuity Chart (Legge et al., 1989). This chart includes 16 lines, with a full sentence on each line, and each successive line is reduced in letter size by 0.1 Log units. The words chosen for the sentences are ones that commonly occur in second or third grade reading material. All but one child in the study could comfortably read the sentences with supra-threshold print size. This child was removed from the reading analysis. The test was run for each eye separately and then binocularly. One of two charts, each with the same parameters, was chosen for each viewing condition.
The time it takes to read each line, and the number of errors on that line were used to assess reading metrics. Basic reading speed was calculated in words per minute (WPM) after accounting for reading errors. We then calculated a difference reading speed score for each participant. This was derived by first calculating the reading speed difference (post minus pre) for each print size value, and averaging across the number of print sizes read by that participant. This difference between WPM was used for data analysis.
Missing data
Out of the 21 participants, one strabismic patient in the dichoptic group (S7) had missing data for the MN read sessions at 10 and 20h post-tests. One anisometrope patient did not have a 20hr time-point data for all 3 assessments. Another participant was missing stereo data for the 10hr time-point. Four other participants had missing MN read data at the 10hr time-point and 2 additional participants had missing MN read data at the 20hr time-point, due to data not being recorded correctly. We detail below at each step how missing data were treated.
2.4.2 Exploratory visual function measures
In-game Suppression (Inter Ocular Ratio – IOR)
For subjects in the dichoptic group, each session began with careful alignment of the stereoscope and reducing the luminance and contrast level of the NAE’s image (by adjusting the alpha value) relative to the AE’s image to perceptually equalize the input to the two eyes. The Inter-Ocular Ratio (IOR - the ratio of fellow, NAE to AE luminance/contrast) provides a convenient index for suppression (Ding & Levi, 2014; Vedamurthy et al., 2015b), with higher ratios indicating less suppression. IOR of 0 indicates complete suppression while IOR of 1 indicates no suppression. We averaged the IOR values in two-hour bins, and report the running average IOR.
2.5 Data Analysis
We report here two complementary analyses. In all cases, in order to assess any differences in the effects of training between the two treatment groups (monocular and dichoptic) and the two subject populations (anisometropic or strabismic), our analyses focused on performance differences over time. Since the three tests (visual acuity, stereo test and MN read) were on different scales, we first converted them to Z scores based on the values of each of the tests at baseline. Additionally, since for VA and stereo acuity lower values indicate better performance while for the MN read test higher values indicate better performance, we converted the z-scores for VA and stereo by multiplying them by -1.
Our first analysis was a repeated-measures multivariate analysis of variance (MANOVA). Given the small sample size and the presence of missing cells both at 10h and 20h evaluations, we selected the analysis with the least missing data, and focused on contrasting baseline and 20 hours performance. The dependent variables were the three main tests of visual acuity (VA), stereo acuity and MN read. The MANOVA was therefore run with the within-subject factors of time (2 levels: baseline and 20hours) and test (3 tests: VA, stereo and MN read) and the between-subject factors of treatment type (monocular vs dichoptic) and amblyopia type (anisometropic vs. strabismic / aniso-strab). We used the Huynh-Feldt correction for the model.
For this analysis, we handled missing data at the 20hr time point by replacing it with the 10hr time point data when possible (VA, stereo and MN read data for participant A9, MN read data for participant A10) and with the group average at 20hr when the 10hr data did not exist (MN read data for participant A8). One participant (S7) did not have any MN read data (at both 10 and 20hr time points) and was therefore excluded from analyses.
We then tested our hypotheses via growth modeling, which is known to be more robust in the face of missing data points, given our focus of performance changes over time. The growth models were accomplished through multilevel modeling (e.g., Singer & Willett, 2003), which estimates individual rates of change over time by generating individual intercepts and slopes for each subject. Missing data is tolerated in growth models assuming data is missing at random, and is based in Full Information Maximum Likelihood (FIML), a “gold standard” in treating missing data, which has been shown to be superior to complete case analysis (i.e., listwise or pairwise deletion; Enders, 2010). Individual rates of change were extracted for each participant for each of the three variables of interest. These estimated rates of change were then converted to z-scores, and then we tested whether groups observed different rates of change over time using a Multivariate Analysis of Variance (MANOVA). This analysis uses the rate of change over time for each of the dependent variables rather than a within-subjects factor of time. This allowed us to retain all participants with at least 2 complete data time points, using the z scores for the 3 relevant tests (VA, stereoacuity, MN read). The only participant (S7), who did not have two complete data time points (S7 missed MN read data at both 10 and 20hr time points), was excluded from the growth model analyses.
3. Results
We report descriptive statistics (Sections 3.2 and correlations) for all 21 participants who completed 20 hours of training. MANOVA and growth model statistics are reported for only those participants (N = 20) who had data for all three primary outcomes - visual acuity, stereo vision and reading speed. One strabismic patient in the dichoptic group (S7 – see Table 1) was not included in these analyses as he lacked reading speed data. A separate analysis of the interocular ratio (IOR) included data of 9 participants who completed 10 hours. IOR data for 2 observers were inadvertently not recorded.
3.1 Compliance
Drop-out rate was about 31% for the monocular group, and 23% for the dichoptic group. The main reason invoked was the substantial time commitment required for training in the lab. We note that the two groups were similar in age (9.7 ± 2.2 [se] years and 10.2 ± 4.0 [se] in the monocular and dichoptic groups, respectively), and in distribution of amblyopia type (≈ 45 % strabismic and 55% anisometropic in each group), but differed slightly, although not significantly (p = 0.35), in their baseline VA (0.50 ± 0.16 [se] vs. 0.58 ± 0.28 [se] logMAR in the monocular and dichoptic game groups, respectively).
3.2 Descriptive Results
In the section below we provide a description of changes seen in all 21 participants for visual acuity, stereo acuity, and reading speed. Of note, we report ‘raw’, non-transformed data in this section, with missing values being treated as such with no imputation. Thus, means extracted at the 10h and 20h time point are not necessarily matched in terms of patients they include (see Figure 4).
Figure 4.
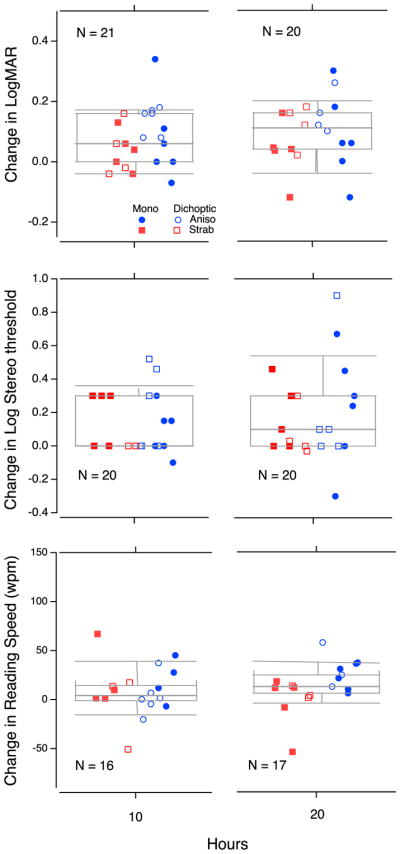
Box plots showing change in performance for visual acuity (top panels), stereoacuity (middle panels) and MN read (bottom panels). In each boxplot, the center horizontal bar is the median, the box shows the semi-interquartile range, and the whiskers the 9th and 91st percentile. Change scores are shown following 10 hours (left plots) and 20 hours (right plots) of training, relative to baseline. Changes are shown for monocular (filled symbols) and dichoptic (open symbols) training participants. Color- and shape-coding denotes amblyopia type: aniso (blue, circle) or strab (red, square). The number of participants contributing to each measurement is reported at the bottom of each plot. Note that the horizontal positions of the data points have been jittered to avoid overlap.
3.2.1 Changes in LogMAR Visual Acuity
The mean change in visual acuity (LogMAR) across all participants (n = 21) was 0.08 ± 0.02 [se] logMAR units following 10 hours of training, and 0.095 ± 0.02 [se] logMAR units (the equivalent of one line on a letter chart or ≈ 26%) from baseline to 20 hours (Figure 4 - top panels).
The dichoptic training group (n=10) improved by 0.1 ± 0.02 [se] logMAR units after 10 hours and by 0.14 ± 0.02 l[se] ogMAR units after 20 hours (when compared to their baseline data), whereas the monocular group (n=11) improved on average by 0.06 ± 0.03[se] logMAR units following both 10 and 20 hours of training.
Regardless of treatment group, individuals with anisometropia (‘aniso’) improved by 0.1 ± 0.03 [se] and by 0.11 ± 0.03 [se] logMAR units by 10 and 20 hours of training, respectively. This indicates that most change was achieved following 10 hours of training, and little change was seen with additional training. In contrast, individuals with strabismus (‘strab’) improved only by 0.04 ± 0.02 [se] logMAR units following 10 hours of training (from 0.62 ± 0.07 to 0.58 ± 0.07 [se] logMAR) and by a total of 0.07 ± 0.03 [se] logMAR units following 20 hours of training, indicating slow and consistent gains across each training period.
3.2.2 Changes in Stereoacuity
Two of the 11 subjects in the monocular training group (18.2%) and 3 of the 10 participants in the dichoptic training group (30%) failed the Randot stereo test at the baseline visit (we label them as ‘stereo blind’). None recovered stereopsis. Of those with initially measurable stereo (n=16), 8 subjects showed improved stereo acuity; however, overall the mean change across all study participants (n = 21) was 0.08 ± 0.05 [se] log arcsec (≈ 20%) following 10 hours of training, and 0.07 ± 0.07 [se] log arcsec (≈ 17%) from baseline to 20 hours.
Participants in the dichoptic group improved, on average, by 0.16 ± 0.07 [se] and by 0.07 ± 0.11 [se] log arcsec (45 and 17%) following 10 and 20 hours of training, respectively. (Note that because of missing data the 10h and 20h group differed in the participants they included. The 10h group included one anisometrope with no data at 20h and the 20h group including one strabismic patient with no data at 10h). The monocular training group improved only by 0.02 ± 0.06 [se] and by 0.06 ± 0.1[se] log arcsec (≈5 and 15%) following 10 and 20 hours of training.
Across both treatment groups, 7 of the 12 individuals with anisometropic amblyopia showed improvement in stereoacuity, while only 1 of the 9 strabismic individuals improved. The average improvement for the anisometropic group was 0.15 ± 0.06 [se] log arcsec (41%) following 10 hours and 0.2 ± 0.1 [se] log arcsec (58%) following 20 hours of training, thus showing continued improvement with increased training. In contrast, strabismic amblyopes showed no improvement at either 10 hours (0.002 ± 0.07 [se] log arcsec) or at 20 hours (worse by 0.1 ± 0.06 [se] log arcsec). Log-transformed stereoacuity data are plotted in Figure 4 (middle panel).
3.2.3 Changes in Reading Speed
We examined changes in reading speed (words read per minute, WPM) using the MN Read chart-based test. Overall, children were able to read slightly more words per minute in their AE following training. The mean change across all study participants was 7.5 ± 6.5[se] WPM following 10 hours of training (n=16), and 11.7 ± 5.9 [se] WPM following 20 hours of training (n=17; Figure 4, bottom panels).
The dichoptic training group’s reading speed was reduced following 10 hours (n=8), by 4.6 ± 7.3 WPM, but did improve, by 18.8 ± 8.4 WPM after 20 hours of training (n=6). The monocular group improved on average by 19.6 ± 8.9 [se] WPM following 10 hours (n=8), but only by 10.8 ± 7.7 [se] WPM following 20 hours of training (n=11).
Regardless of treatment group, anisometropic amblyopes improved by 6.8 ± 6.5 WPM following 10 hours (n=9) and by 26.1 ± 5.4 [se] WPM following 20 hours of training (n=9). In contrast, strabismic amblyopes improved by 8.4 ± 13 [se] WPM following 10 hours (n=7), and did not improve at all following 20 hours of training (change of -0.45 ± 8.2 [se] WPM; n=8).
3.3 Statistical Analyses
3.3.1 Omnibus MANOVA Results
We next examined the statistical robustness of the numerical trends described above using a MANOVA. The omnibus MANOVA (n=20) yielded a significant effect of time (F(1,16)=16.79, p<.001, partial η 2=.51), but no other significant main effects (test: F(2,32)=1.3, p=.28, partial η2=.076; group: F(1,16)=.07, p=.79, partial η 2=.005; amblyopia type: F(1,16)=2.68, p=.12, partial η2=.14). Additionally, there were significant interactions between time and amblyopia type (F(1,16)=4.94, p=.041, partial η2=.24), and between test and amblyopia type (F(2,32)=3.2, p=.048, partial η2=.17). All other interactions, including time by treatment group, were non-significant.
These results indicate that both modes of playing the action game (monocular and dichoptic play modes) yielded statistically similar changes across all three tests, but that these changes over time were different for the two patient populations, anisometropic and strabismic amblyopes. The interaction between test and amblyopia type implies that the two patient populations differed in their test scores. A closer look at the data shows that this was particularly true for stereoacuity. Results of the Omnibus MANOVA on the z-scores are provided in Supplementary materials (Figure S1).
3.3.2 Growth model results
To take into account all data points in our analysis (baseline, 10 hours, and 20 hours), we conducted a growth model analysis, yielding a single ‘change’ score for each participant for each measure. We then converted the change scores into z-scores, and ran a MANOVA analysis with the 3 tests (VA, stereo, MN read). In terms of overall growth, we found significant change over time for visual acuity (b = −.049, se = .012, |t| = 4.16, p = .001, 95% CI = −.074, −.024) and a trend for significant growth for MN read (b = 6.397, se = 3.044, |t| = 2.10, p = .051, 95% CI = −.022, 12.815) but not for stereoacuity (b = −.042, se = .037, |t| = 1.13, p = .270, 95% CI = −.120, .036).
The normalized z-score changes for the growth model are presented in Supplementary Materials (Table S2). In the subsequent MANOVA, the only additional significant effect was found for amblyopia type, for stereoacuity (F(1,16)=9.6, p=.007), Indicating larger stereoacuity changes over time in the anisometropic than in the strabismic patients. All other effects, including group, were non-significant.
3.3.3 Change between outcome measures not correlated
Although we found little difference between our two groups, both groups improved over time. It is thus of interest to ask whether a participant who improved in VA may be more likely than someone who showed no VA improvement to display gains in stereo or reading speed. Interestingly, none of the dependent measures were correlated: VA/Stereo: R2=0.19, p=0.43; VA/WPM: R2=0.29, p=0.2; Stereo/WPM: R2=0.29, p=0.2.
3.4 Exploratory Analyses
3.4.1 In-game Interocular Suppression (IOR)
Interocular suppression (IOR) was reduced during dichoptic game play. Ten children completed 20 hours of dichoptic game play: 4 strabismic and 6 anisometropic amblyopes. IOR data for one anisometropic observer (A13) was not recorded at all, and for another, (A9), was inadvertently not recorded for the last 10 hours. For the 9 children with some IOR data, IOR increased steadily and significantly - by, on average about a factor of 2.4, about twice that of adult amblyopes over the same 20 hours of training (Vedamurthy et al., 2015b). The symbols in Figure 5 (top panel) show the running average IOR (blue circles for anisometropic and red diamonds for strabismic). The dot-dashed line shows comparable data from adults (from Vedamurthy et al., 2015b). However, as reported for adults, there was no significant relationship between decreased suppression as measured by increased IOR and improved visual acuity (Figure 5, middle panel) or stereo acuity (Figure 5, bottom). Furthermore, similar to the data of adults, it is clear that the two participants with the greatest improvement in stereoacuity show little or no change in IOR.
Figure 5. In-game IOR Data. Top.
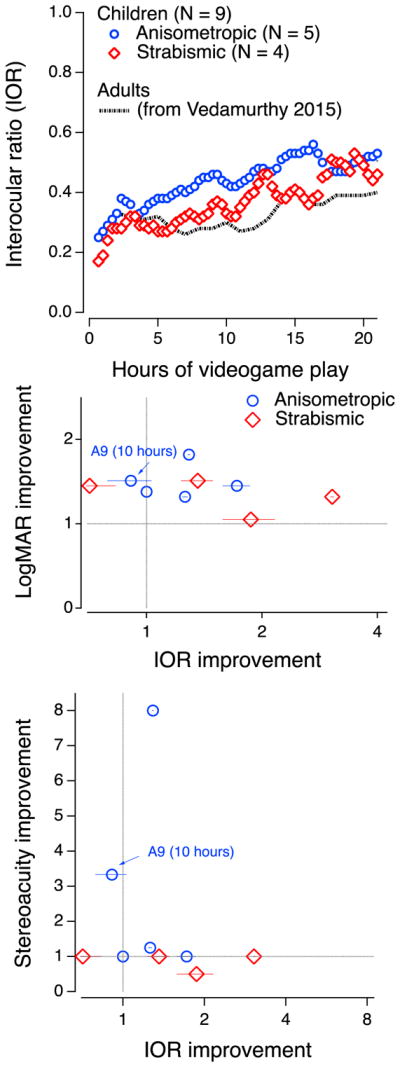
IOR as a function of hours of videogame play for anisometric (N=5 out of 6) and strabismic (N = 4) patients separately as well as for comparable data from our recent study in adults (dotted line - Vedamurthy et al., 2015). Middle: Improvement in VA as a function of changes in IOR from 0 to 20 hours of game play (except for A9). Bottom: improvement in stereo acuity as a function of changes in IOR from 0 to 20 hours of game play. Blue – data from anisometric participants. Red – data from strabismic participants. The data of observer A9 is highlighted in the two lower panels because IOR was only recorded for the first 10 hours).
3.4.2 Follow-up Assessments
Study participants were asked to return to follow-up assessments 6–10 weeks following completion of training. However, only 12 of the 20 participants returned (6 for each of the monocular and dichoptic training group). We therefore conducted an exploratory MANOVA with effects of time (baseline and follow up) and test (VA, and stereo only, since MN read did not have enough data points) and between-subject effects of group and amblyopia type. Follow-up data is summarized in Supplementary Figure S3.
We found that VA and stereo improvements were maintained at follow-up (significant effect of time: F(1,8)=68.4, p<.0005, partial η 2=.895), and that those improvements did not differ between the training groups (no significant effects of group or time X group interaction). However, improvements at follow-up were overall larger for VA than for stereo acuity, indicated by a significant effect of test (F(1,8)=34.2, p<.0005, partial η 2=.81) and a significant interaction of time X test (F(1,8) = 39.4, p<.0005, partial η 2=.83). Specifically, for VA, when only the children that returned for a follow-up were included (n=12) the 0.11 [se] 0.04 logMAR gain seen from baseline to post-20hr was numerically maintained at follow-up. For stereo acuity, the mean change from baseline to follow-up was 0.044 ± 0.10[se] log units.
3.3.4 Participant Factors
Given the mixed results of previous studies, we examined whether participant factors such as the child’s age and baseline level of visual acuity could be related to improvement on visual assessments. We found no relationship between age and VA improvement (R2=0.01, p=0.62), nor between baseline VA in the AE and VA improvement (R2=0.01, p=0.70). We also found no correlation between both participant factors (age and baseline VA) and stereoacuity change (age/stereo change: R2=0.04, p=0.39 and baseline VA/stereo change: R2=0.20, p=0.09). Similarly, there was no relationship between improvement in reading metrics and age (WPM: R2=0.02, p=0.55; CPS: R2=0.12, p=0.17) or baseline VA (WPM: R2=0.01, p=0.67; CPS: R2=0.01, p=0.70).
In contrast, treatment history was significantly correlated with the degree of improvement. Participants were classified as previously patched in the previous 6 months (n=6), not patched (n=12), or unknown treatment history (n=3). Those with unknown history were excluded from this analysis. The recently-patched group was slightly younger (mean age 8.33 ± 0.61 [se] years old) than the non-patched group (11.66 ± 1.06 [se] years old, t=1.80, p=0.05). However, both groups had similar starting VAs in their AE (0.53 ± 0.17 [se] logMAR in the recently-patched group, 0.57 ± 0.22 [se] logMAR in the non-patched group, t=0.41, p=0.34). While the recently-patched group had little to no improvement in VA (0.02 ± 0.05 [se] logMAR on average), the non-patched group had significantly more improvement (0.12 ± 0.03 [se] logMAR, t=1.97, p=0.03). Differences in stereoacuity (0.04 ± 0.09 [se] log units vs. 0.16 ± 0.11 [se] log units for the recently-patched and non-patched groups, respectively), and reading (WPM: 15.08 ± 19.49 [se] words vs. 16.50 ± 6.98 [se] words) were not significant.
While not all children demonstrated a significant improvement in VA, we looked to see if there was improvement in at least 2 of the 3 visual functions assessed. Our criteria for this was, an improvement of: 0.1 log unit or more in VA and stereo, or an increase of 20 wpm in reading speed. Eight of the twenty-one children achieved at least two of these criteria by the end of training. Seven were anisometropic (4/6 for monocular training vs. 3/6 for dichoptic training); however, only one strabismic child in the dichoptic group met at least 2 of the improvement criteria, and none of the strabismic children in the monocular group did so.
4. Discussion
The goal of this study was to evaluate a custom-made action video game for treatment of children with amblyopia, and to determine whether dichoptic game playing is more effective than playing the game monocularly with the amblyopic eye while the fellow eye is patched. Another goal was differences in outcomes between the two subgroups of amblyopia, anisometric and strabismic patients.
Our dichoptic approach, presenting a weak but visible stimulus to the dominant eye, receives some physiological support from a recent study of dichoptic masking in amblyopic monkeys (Shooner et al., 2017). Specifically, it provides evidence that the plasticity required to restore normal binocular function “need not include a weakening of amblyopic-eye suppression, but rather a strengthening of the amblyopic eye’s suppressive influence over the dominant eye” (Shooner et al., 2017, p. 16). Thus, presenting a weak but visible stimulus to the dominant eye may provide the requisite target signal for modulation of the dominant eye by the amblyopic eye, while sidestepping the reciprocal suppression that would otherwise reduce the amblyopic eye’s signals in the cortex.
We found that both groups benefitted from video game training, whether played dichoptically or monocularly with the fellow eye patched, and that these gains were largely maintained following a no-contact period of 6–10 weeks. Although VA, stereo acuity and reading speed improved slightly more for the dichoptic training group, these differences did not reach statistical significance, potentially given our small sample. Furthermore, these improvements were independent of one another, with no covariation among any of these 3 measures.
Amblyopia type had a significant effect on gains made following training, with anisometropic amblyopes showing greater gains on all measures compared with strabismic amblyopes. Most notable differences were seen for stereo acuity, where anisometropic individuals improved while the majority of strabismic amblyopes did not. In the sections below we discuss these results in light of similar studies in both adults and children with amblyopia.
4.1 Relationship to previous studies in children
Our main findings were that following 20 hours of training, VA improved on average by 0.14 logMAR (≈ 38%) for the dichoptic group and only by 0.06 logMAR (≈ 15%) for the monocular group. Improvements in stereoacuity were similar across training groups, with average improvement of 0.07 log arcsec (≈ 17%) following dichoptic training, and of 0.06 log arcsec (≈ 15%) following monocular training. Across both treatment groups, 7 of the 12 individuals with anisometropic amblyopia showed improvement in stereoacuity, while only 1 of the 9 strabismic individuals improved.
4.1.1 Gains in Visual Acuity
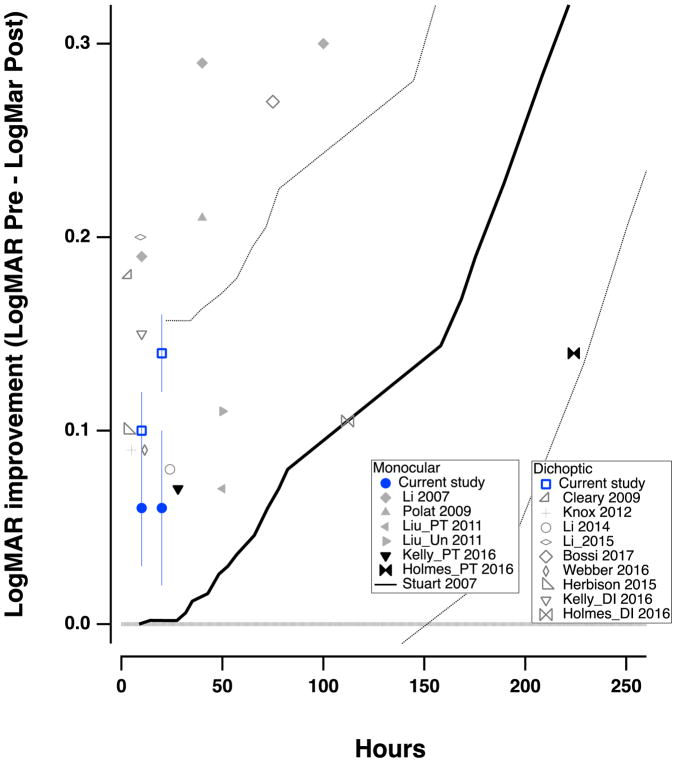
A review of the extant studies of videogame play and PL in childhood amblyopia suggests about sixteen studies with almost 700 patients. A summary of these data is presented in Figure 6 (see also Table 1). On average, despite wide variations in training paradigms, training duration and participants age, both monocular and dichoptic training yielded an average improvement of about 0.15 LogMAR (1.5 lines on an eye chart) in VA, with a range of benefit of ≈ 0.08 to 0.3 logMAR (see Table 1). Our results, showing improvement of 0.14 and 0.06 logMAR for the dichoptic and monocular groups, respectively, are consistent with those of previous studies.
Figure 6.
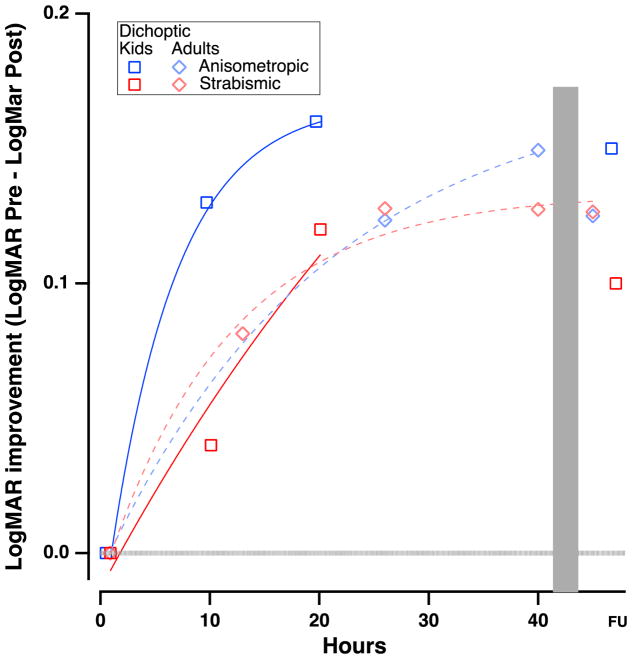
VA improvement data (pre-post) from previous studies examining treatments in children with amblyopia, as a function of hours of treatment. Solid gray symbols represent monocular perceptual learning or video game treatment. Black symbols show patching treatment; open symbols indicate dichoptic/binocular treatment. The blue symbols are from the current study. The lines in Figure 6 show the time course of monitored occlusion (solid line) and the 95% confidence intervals (dotted lines - from Stewart et al., 2007).
There are several potential accounts for this range of results. First, one major factor in our study was treatment history, where children that had recently undergone occlusion therapy were much less likely to show improvements in visual function. This has also been noted in several other children’s studies. For example, Holmes et al. (2016) report an improvement of 0.12 logMAR for all of the children that played a dichoptic game in their study; however, when including only children that had not recently patched, the mean improvement more than doubled to 0.25 logMAR. Similarly, Liu et al. (2011) reported a substantial difference in the response to perceptual learning in untreated children (see Liu_Un 2011 in Figure 6) compared to those who had been previously patched (see Liu_PT 2011 in Figure 6). This was also the case in the present study, with the recently patched children showing improvements of only 0.02 logMAR, but the non-patched ones showing an average improvement of 0.12 logMAR.
Two very recent studies should also be considered. First, the BRAVO RCT (Gao et al., 2018), which included both adults (up to 55 years of age, N = 49) and children (7 – 18 years of age, N = 58), many of whom had prior treatment. Patients were randomized into two groups. The active group played a dichoptic Ipod touch falling blocks video game with game elements split between the two eyes, and a contrast offset between the two eyes, for 1 hour per day for 16 weeks. The placebo group played the same game with identical images to the two eyes. Compliance was generally poor, especially in the younger age groups. VA improved by 0.06 LogMAR in the active group and by 0.07 LogMAR in the placebo group. Both groups showed small and similar reductions in suppression and improvements in stereopsis. Interestingly, the authors report no significant influence of age.
A second recent study is a PEDIG RCT (Manh et al., 2017), which included older children (13 – < 17 years of age) and hence is not included in Fig. 6 or Table 1. Patients were randomized into two groups: a ‘binocular’ group, which received the same treatment as in the BRAVO RCT above, and a ‘patching’ group, which wore a patch (prescribed for 2 hours/day) for 16 weeks. The mean VA improved by ≈.07 LogMAR in the binocular group and by ≈0.13 LogMAR in the patching group. However, compliance here too was poor, with only 13% of participants in the binocular group completing more than 75% of the prescribed treatment.
Second, results may have been affected by compliance with treatment, which varied widely among different studies, from near 100% for DIGRUSH, an action-adventure videogame developed specifically to be played dichoptically (Kelly et al., 2016) to 22% for a Tetris style game (i.e., only 22% of the children achieved greater than 75% of the prescribed play time - Holmes et al., 2016). Several groups send participants home with an iPad and anaglyphic glasses, or have the children complete the training on a computer at home (see Table 1). This design results in a wide variation in compliance, both within and between studies, causing large differences in dosage, despite approximately the same time period between assessments. Studies that report better compliance tend to have better outcomes (Li et al., 2007; Bossi et al., 2017; see full list in Table 1). In the current study, compliance was ensured by having the subjects play the game or watch movies under supervision. However, the difficulty of coming to the lab several times per week led to attrition. Thus, 21 of 29 subjects (72%) completed 20 hours of training, with number of weeks to completion varying widely between participants (from 3 to 20 weeks).
Importantly, our study, and many of the others illustrated in Table 1 and Fig. 6 suggest that the most important benefit of perceptual learning and videogame play in children is that 1 to 2 lines of improvement can be achieved in 10 to 20 hours of play, in contrast to more than 100 hours of occlusion (Stewart et al., 2007 - solid black line in Fig. 6).
Finally, since most of the experimental treatments have been tested over short durations (some only for a few hours, and none over 100 hours), it is not clear whether the maximum improvement possible is more limited than that obtained with prolonged patching.
4.1.2 Dichoptic vs. Monocular Training
As previously mentioned, it is important to understand whether dichoptic training provides an additional benefit beyond monocular training, as it is logistically more challenging. Also, the development of diplopia is an added concern with dichoptic training, although few if any cases have been reported. The present study allowed us to investigate the direct effect of dichoptic training since our two groups played the same game, but one monocularly and the other dichoptically. The dichoptic group showed larger improvements in VA; however, because our sample size was small, the difference is not statistically significant. This was also true for the main three outcome measures used in the study, that is not only VA, but also stereoacuity and reading speed. Interestingly 6/12 anisometropic subjects showed at least a 2-step improvement in stereoacuity and a final stereoacuity of better than 140arc sec (Levi, Knill & Bavelier, 2015) – 4/6 in the monocular group and 2/6 in the dichoptic group. In contrast, none of the nine strabismic patients met this criterion.
It is also evident in Fig. 6 that the range of improvement in children with amblyopia is similar for monocular PL, monocular videogame training and dichoptic training. Indeed, the studies showing the largest improvements are those of Li et al. (2007) who had children perform extensive PL monocularly and Bossi et al (2017) who had children watching dichoptic movies. Note that the subjects in both studies were previously untreated.
4.2 Action Video Game Training in Children vs. Adults
The adult study most similar to the current study is that of Vedamurthy et al. (2015a). In this study, adults with amblyopia either played a dichoptic action video game, or watched action movies while patching their NAE for 40 hours. They found that visual acuity (VA) improved on average by ≈ 0.14 logMAR (≈ 28%) in the action video game group, and 0.07 logMAR in the action movies group. Interestingly, patients with anisometropic amblyopia in the movies group showed similar VA improvements to those of the video game group, while subjects with strabismic amblyopia improved only following game play. Stereoacuity and reading speed, and contrast sensitivity improved more for the video game group participants compared with the movies group participants.
Figure 7 compares the VA data of the two studies. As can be seen in the figure, the children in the dichoptic group in the current study had VA improvements numerically similar to those of the adults who played the dichoptic action game in the Vedamurthy et al. study (2015a,b). Interestingly, the anisometropic children appear to improve slightly faster than the anisometropic adults (blue squares vs. blue diamonds in Figure 7) and faster than both the strabismic adults and children (red symbols in Figure 7).
Figure 7.
Comparison of children and adult gains following action video game play. Gains in visual acuity (pre-post) as a function of hours of training for current study in children (square symbols) and for our previous study in adults (diamond symbols; Vedamurthy et al., 2015)
4.3 Training effects in anisometropic vs. strabismic amblyopes
Vedamurthy et al. (2015a) reported very different outcomes for their adult anisometropic and strabismic groups. Their anisometropic group improved in both the control condition (watching action television shows while wearing an eye patch), and the experimental condition (playing the dichoptic action video game), while the strabismic group only improved in the experimental condition.
The results of our current study suggest tantalizing differences between anisometropic and strabismic children. The results of our current study suggest tantalizing differences between anisometropic and strabismic children. Indeed, there were significant interactions between time and amblyopia type, as described in Section 3.21. Accordingly, anisometropic amblyopes showed greater improvements following 20 hours in each of the 3 measures than did strabismic amblyopes. In addition, the growth model indicated larger stereoacuity changes over time in the anisometropic than in the strabismic patients. The most notable differences were seen for stereo acuity, where anisometropic individuals seemed to improve while the majority of strabismic amblyopes did not as documented in previous works (for a review of this point, see Levi, Knill & Bavelier, 2015).
4.4 Feasibility and other limitations of the study
Due to the challenging nature of visiting the lab 2 to 3 times a week, we had some drop-out in both training groups, for an overall drop-out rate of 28%. More work is needed to simplify the experimental treatment so that the training is portable and engaging. Several groups have looked into this. For example, Hess’s group developed a dichoptic Tetris game that can be played on an iPad with anaglyph glasses at home (Li et al., 2014, 2015; Birch et al., 2015), and the Nottingham group (Hussain, Astle, Webb & McGraw, 2014), developed a game that can be played on a computer at home with a patch over the NAE. Although certainly a great improvement, there remain a number of weaknesses with these designs. In both of these cases, compliance can still be an issue as the games can be played without the glasses, or without the patch. As Stewart et al. (2004) found with their occlusion-monitoring device, self or parental reports of wear time frequently do not match up with actual usage. Although our design was challenging in that it required children to travel to the lab after school or on weekends, we were able to directly monitor game play to make sure the children were following the rules and difficulty levels could be adjusted to maintain engagement.
As noted previously, children are more challenging to motivate in training studies, even when games rather than PL regimes are used. As such, several studies have noted more variability in results. Interestingly, there is also a great deal of variability in the response of children with amblyopia to patching, even when compliance is taken into account (Holmes, Lazar, Melia et al., 2011). Therefore, although several of our assessments did not show significant differences between training types, or patient group, it does not rule out the possibility that these differences may exist. We recognize our sample size remains quite small in the face of such potential variability.
4.5 Conclusions
The emphasis of our study was on the feasibility of using action video games for children with amblyopia, with the ultimate goal of determining whether the intervention was feasible and whether the dichoptic approach may have some added benefits compared to monocular training. While both forms of active video-game training (monocular and dichoptic) resulted in rapid improvements in visual acuity, our study indicates little advantage to a dichoptic approach, and calls for caution in running large RCTs contrasting two active video games one played dichoptically and the other not. This conclusion is very much in line with a recent RCT including 115 older children, adolescents and adults which also found no advantage of a dichoptic videogame over a binocular one (Gao et al. 2018).
Our study adds to the growing body of work showing that using active treatments (PL and video games) to treat amblyopia can be as effective (if not more) as traditional occlusion therapy. While we have made progress in understanding how video games can be used as a treatment for patients with amblyopia in the 7 years since Li et al. (2011) first reported on this topic, there are still many important questions that remain unanswered. In particular, we need to understand how videogame play affects oculomotor control. Eye movements skills are important for proper development of spatial attention and learning activities such as reading. Children with amblyopia have reading impairments, even when using both eyes (Kelly et al., 2015), thus understanding how to improve these functions is important to the clinical outcome of these patients. Binocular fixation stability and bifoveal alignment and fusion may be key to unlocking a holistic treatment for this developmental condition. Additionally, incorporating stereo cues, as was done in Vedamurthy et al. (2016) may lead to greater overall improvement, as this provides an additional cue to aid in sustained binocular fusion. Finally, easier set-ups, that include an assortment of engaging action video games in a portable unit, and the ability of researchers and clinicians to track the data regarding a patient’s compliance and progression, will be essential to future iterations of this work.
Supplementary Material
Highlights.
Playing a custom child friendly action videogame resulted in improved visual acuity
Improvement of 1.4 lines after 20 hours compared to more than 100 hours of patching
The action videogame approach may be an effective adjunct treatment for amblyopia
Acknowledgments
Supported by a grant R01EY020976 from the National Eye Institute, Bethesda, Md to DL and DB. In addition, we would like to thank our research assistants Sam Huang, Olga Pikul, Nuhamin Petros, Ivy Liu, Ashley-May Masa, Jaclyn Wittmer, Janet Lee, Hans Baertsch, Jessica Ho, Jessie Wong, Sheena Song, Lexi Lambeck, and Sean Noah for their help with data collection. We would also like to thank our optometry students, Jill Lobingier, and Kayee So for their help with performing visual screenings and assessments, as well as our study coordinators, Zakia Young, Ellen Ong, Rachel Spencer, that helped with scheduling. Finally, this work could not have happened without the support of Drs. Gearinger and DePaolis who referred patients for our study.
Footnotes
Both the NIH and our IRBs define children as individuals under 18 years of age.
Author contributions
CG and MN share co-first authorship. Study design and conceptualization: DB & DL; Video Game Development and Design: primarily JB with contributions from DB, IV, and DL; Video Game play test during game development: IV with contributions from DB & DL & MN; Piloting and fine-tuning of vision experiments: primarily IV with contributions of SH, MN and CG; Running the Study: IV, MN, and CG; Data analysis: MN, CG and JJ; Writing: primarily CG, DL and MN with all authors contributing. Figures: DL.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Gambacorta C, Huang S, Vedamurthy I, Nahum M, Bayliss J, Bavelier D, Levi D. Action Video Games as a Treatment of Amblyopia in Children: A Pilot Study of a novel, child-friendly action game. Poster presented at the Vision Sciences Society; Florida. May, 2014. [Google Scholar]
- Bayliss J, Vedamurthy I, Nahum M, Levi D, Bavelier D. Lazy Eye Shooter: Making a Game Therapy for Visual Recovery in Adult Amblyopia. Abstract accepted for presentation at the Human-Computer Interaction (HCI) international 2013 meeting; Las Vegas, Nevada, USA. 2013. [Google Scholar]
- Vedamurthy I, Nahum M, Bayliss J, Bavelier D, Levi D. Using a Custom-Made Version of the Unreal Tournament Video Game to Improve Vision in Individuals with Amblyopia. Poster presented at the Entertainment Software and Cognitive Neurotherapeutics Society meeting; 2013; Los Angeles, CA, USA. Mar, 2013. [Google Scholar]
- Bayliss JD, Vedamurthy I, Bavelier D, Nahum M, Levi DM. Lazy eye ehooter: a novel game therapy for visual recovery in adult amblyopia. IEEE International Games Innovation Conference Proceedings.2012. [Google Scholar]
- Bavelier D, Green CS, Pouget A, Schrater P. Brain plasticity through the life span: learning to learn and action video games. Annu Rev Neurosci. 2012;35:391–416. doi: 10.1146/annurev-neuro-060909-152832. [DOI] [PubMed] [Google Scholar]
- Birch EE. Amblyopia and binocular vision. Prog Retin Eye Res. 2013;33:67–84. doi: 10.1016/j.preteyeres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Li SL, Jost RM, Morale SE, De La Cruz A, Stager D, Jr, Dao L, Stager DR., Sr Binocular iPad treatment for amblyopia in preschool children. J AAPOS. 2015;19(1):6–11. doi: 10.1016/j.jaapos.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Stager DR., Sr Long-term motor and sensory outcomes after early surgery for infantile esotropia. J AAPOS. 2006;10(5):409–413. doi: 10.1016/j.jaapos.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Birch EE, Stager DR, Sr, Berry P, Leffler J. Stereopsis and long-term stability of alignment in esotropia. J AAPOS. 2004;8(2):146–150. doi: 10.1016/j.jaapos.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Bossi M, Tailor VK, Anderson EJ, Bex PJ, Greenwood JA, Dahlmann-Noor A, Dakin SC. Binocular Therapy for Childhood Amblyopia Improves Vision Without Breaking Interocular Suppression. Invest Ophthalmol Vis Sci. 2017;58(7):3031–3043. doi: 10.1167/iovs.16-20913. [DOI] [PubMed] [Google Scholar]
- Choong YF, Lukman H, Martin S, Laws DE. Childhood amblyopia treatment: psychosocial implications for patients and primary carers. Eye (Lond) 2004;18(4):369–375. doi: 10.1038/sj.eye.6700647. [DOI] [PubMed] [Google Scholar]
- Chua B, Mitchell P. Consequences of amblyopia on education, occupation, and long term vision loss. Br J Ophthalmol. 2004;88(9):1119–1121. doi: 10.1136/bjo.2004.041863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung ST, Kumar G, Li RW, Levi DM. Characteristics of fixational eye movements in amblyopia: Limitations on fixation stability and acuity? Vision Res. 2015;114:87–99. doi: 10.1016/j.visres.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M, Moody AD, Buchanan A, Stewart H, Dutton GN. Assessment of a computer-based treatment for older amblyopes: the Glasgow Pilot Study. Eye (Lond) 2009;23(1):124–131. doi: 10.1038/sj.eye.6702977. [DOI] [PubMed] [Google Scholar]
- Ding J, Levi DM. Rebalancing binocular vision in amblyopia. Ophthalmic Physiol Opt. 2014;34(2):199–213. doi: 10.1111/opo.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK. Applied Missing Data Analysis. Guilford Press; 2010. [Google Scholar]
- Fronius M, Cirina L, Ackermann H, Kohnen T, Diehl CM. Efficiency of electronically monitored amblyopia treatment between 5 and 16 years of age: new insight into declining susceptibility of the visual system. Vision Res. 2014;103:11–19. doi: 10.1016/j.visres.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Gambacorta C, Huang S, Vegamurthy I, Nahum M, Bayliss J, Bavelier D, Levi D. Action Video Games as a Treatment of Amblyopia in Children: A Pilot Study of a novel, child-friendly action game. Vision Sciences Society Annual Meeting Abstract. Journal of Vision. 2014 Aug;14:665. doi: 10.1167/14.10.665. [DOI] [Google Scholar]
- Gao TY, Guo CX, Babu RJ, Black JM, Bobier WR, Chakraborty A, Dai S, Hess RF, Jenkins M, Jiang Y, Kearns LS, Kowal L, Lam CSY, Pang PCK, Parag V, Pieri R, Raveendren RN, South J, Staffieri SE, Wadham A, Walker N, Thompson B, Team BS. Effectiveness of a Binocular Video Game vs Placebo Video Game for Improving Visual Functions in Older Children, Teenagers, and Adults With Amblyopia: A Randomized Clinical Trial. JAMA Ophthalmol. 2018 doi: 10.1001/jamaophthalmol.2017.6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CS, Bavelier D. Action-video-game experience alters the spatial resolution of vision. Psychol Sci. 2007;18(1):88–94. doi: 10.1111/j.1467-9280.2007.01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CS, Bavelier D. Learning, attentional control, and action video games. Curr Biol. 2012;22(6):R197–206. doi: 10.1016/j.cub.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CS, Strobach T, Schubert T. On methodological standards in training and transfer experiments. Psychol Res. 2014;78(6):756–772. doi: 10.1007/s00426-013-0535-3. [DOI] [PubMed] [Google Scholar]
- Green CS, Li R, Bavelier D. Perceptual learning during action video games. TopicS. Special issue on perceptual learning. 2010;2(2):202–216. doi: 10.1111/j.1756-8765.2009.01054.x. [DOI] [PubMed] [Google Scholar]
- Herbison N, MacKeith D, Vivian A, Purdy J, Fakis A, Ash IM, Cobb SV, Eastgate RM, Haworth SM, Gregson RM, Foss AJ. Randomised controlled trial of video clips and interactive games to improve vision in children with amblyopia using the I-BiT system. Br J Ophthalmol. 2016;100(11):1511–1516. doi: 10.1136/bjophthalmol-2015-307798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison N, Cobb S, Gregson R, Ash I, Eastgate R, Purdy J, Hepburn T, MacKeith D, Foss A group IBs. Interactive binocular treatment (I-BiT) for amblyopia: results of a pilot study of 3D shutter glasses system. Eye (Lond) 2013;27(9):1077–1083. doi: 10.1038/eye.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RF, Thompson B. Amblyopia and the binocular approach to its therapy. Vision Res. 2015;114:4–16. doi: 10.1016/j.visres.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Holmes JM, Lazar EL, Melia BM, Astle WF, Dagi LR, Donahue SP, Frazier MG, Hertle RW, Repka MX, Quinn GE, Weise KK Pediatric Eye Disease Investigator G. Effect of age on response to amblyopia treatment in children. Arch Ophthalmol. 2011;129(11):1451–1457. doi: 10.1001/archophthalmol.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JM, Manh VM, Lazar EL, Beck RW, Birch EE, Kraker RT, Crouch ER, Erzurum SA, Khuddus N, Summers AI, Wallace DK Pediatric Eye Disease Investigator G. Effect of a Binocular iPad Game vs Part-time Patching in Children Aged 5 to 12 Years With Amblyopia: A Randomized Clinical Trial. JAMA Ophthalmol. 2016;134(12):1391–1400. doi: 10.1001/jamaophthalmol.2016.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood J, Waylen A, Herrick D, Williams C, Wolke D. Common visual defects and peer victimization in children. Invest Ophthalmol Vis Sci. 2005;46(4):1177–1181. doi: 10.1167/iovs.04-0597. [DOI] [PubMed] [Google Scholar]
- Hussain Z, Astle AT, Webb BS, McGraw PV. The challenges of developing a contrast-based video game for treatment of amblyopia. Front Psychol. 2014;5:1210. doi: 10.3389/fpsyg.2014.01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Jost RM, Dao L, Beauchamp CL, Leffler JN, Birch EE. Binocular iPad Game vs Patching for Treatment of Amblyopia in Children: A Randomized Clinical Trial. JAMA Ophthalmol. 2016;134(12):1402–1408. doi: 10.1001/jamaophthalmol.2016.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Jost RM, De La Cruz A, Birch EE. Amblyopic children read more slowly than controls under natural, binocular reading conditions. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2015;19(6):515–520. doi: 10.1016/j.jaapos.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L. Visual processing in amblyopia: animal studies. Strabismus. 2006;14(1):3–10. doi: 10.1080/09273970500536193. [DOI] [PubMed] [Google Scholar]
- Knox PJ, Simmers AJ, Gray LS, Cleary M. An exploratory study: prolonged periods of binocular stimulation can provide an effective treatment for childhood amblyopia. Invest Ophthalmol Vis Sci. 2012;53(2):817–824. doi: 10.1167/iovs.11-8219. [DOI] [PubMed] [Google Scholar]
- Legge GE, Ross JA, Luebker A, LaMay JM. Psychophysics of reading. VIII. The Minnesota Low-Vision Reading Test. Optom Vis Sci. 1989;66(12):843–853. doi: 10.1097/00006324-198912000-00008. [DOI] [PubMed] [Google Scholar]
- Levi DM. Visual processing in amblyopia: human studies. Strabismus. 2006;14(1):11–19. doi: 10.1080/09273970500536243. [DOI] [PubMed] [Google Scholar]
- Levi DM. Linking assumptions in amblyopia. Vis Neurosci. 2013;30(5–6):277–287. doi: 10.1017/S0952523813000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Li RW. Perceptual learning as a potential treatment for amblyopia: a mini-review. Vision Res. 2009;49(21):2535–2549. doi: 10.1016/j.visres.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM. Prentice award lecture 2011: removing the brakes on plasticity in the amblyopic brain. Optom Vis Sci. 2012;89(6):827–838. doi: 10.1097/OPX.0b013e318257a187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Knill DC, Bavelier D. Stereopsis and amblyopia: A mini-review. Vision Res. 2015;114:17–30. doi: 10.1016/j.visres.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Song S, Pelli DG. Amblyopic reading is crowded. J Vis. 2007;7(2):21 21–17. doi: 10.1167/7.2.21. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. Journal of the Acoustic Society of America. 1971;49:467–477. [PubMed] [Google Scholar]
- Li RW, Ngo C, Nguyen J, Levi DM. Video-game play induces plasticity in the visual system of adults with amblyopia. PLoS Biol. 2011;9(8):e1001135. doi: 10.1371/journal.pbio.1001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RW, Ngo CV, Levi DM. Relieving the attentional blink in the amblyopic brain with video games. Sci Rep. 2015;5:8483. doi: 10.1038/srep08483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RW, Provost A, Levi DM. Extended perceptual learning results in substantial recovery of positional acuity and visual acuity in juvenile amblyopia. Invest Ophthalmol Vis Sci. 2007;48(11):5046–5051. doi: 10.1167/iovs.07-0324. [DOI] [PubMed] [Google Scholar]
- Li R, Polat U, Scalzo F, Bavelier D. Reducing backward masking through action video game training. Journal of Vision. 2010;10(14) doi: 10.1167/10.14.33. pii: 33. [DOI] [PubMed] [Google Scholar]
- Li R, Polat U, Makous W, Bavelier D. Enhancing the contrast sensitivity function through action video game training. Nat Neurosci. 2009;12(5):549–551. doi: 10.1038/nn.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SL, Reynaud A, Hess RF, Wang YZ, Jost RM, Morale SE, De La Cruz A, Dao L, Stager D, Jr, Birch EE. Dichoptic movie viewing treats childhood amblyopia. J AAPOS. 2015;19(5):401–405. doi: 10.1016/j.jaapos.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SL, Jost RM, Morale SE, De La Cruz A, Dao L, Stager D, Jr, Birch EE. Binocular iPad treatment of amblyopia for lasting improvement of visual acuity. JAMA Ophthalmol. 2015;133(4):479–480. doi: 10.1001/jamaophthalmol.2014.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SL, Jost RM, Morale SE, Stager DR, Dao L, Stager D, Birch EE. A binocular iPad treatment for amblyopic children. Eye (Lond) 2014;28(10):1246–1253. doi: 10.1038/eye.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Zhang T, Jia YL, Wang NL, Yu C. The therapeutic impact of perceptual learning on juvenile amblyopia with or without previous patching treatment. Invest Ophthalmol Vis Sci. 2011;52(3):1531–1538. doi: 10.1167/iovs.10-6355. [DOI] [PubMed] [Google Scholar]
- Manh VM, Holmes JM, Lazar EL, Kraker RT, Wallace DK, Kulp MT, Galvin JA, Shah BK, Davis PL Pediatric Eye Disease Investigator G. A Randomized Trial of a Binocular iPad Game Versus Part-Time Patching in Children Aged 13 To 16 Years With Amblyopia. Am J Ophthalmol. 2017 doi: 10.1016/j.ajo.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor AR, Birch EE, Anderson S, Draper H. Relationship between binocular vision, visual acuity, and fine motor skills. Optom Vis Sci. 2010;87(12):942–947. doi: 10.1097/OPX.0b013e3181fd132e. [DOI] [PubMed] [Google Scholar]
- O'Connor AR, Birch EE, Spencer R. Factors affecting development of motor skills in extremely low birth weight children. Strabismus. 2009;17(1):20–23. doi: 10.1080/09273970802679006. [DOI] [PubMed] [Google Scholar]
- Packwood EA, Cruz OA, Rychwalski PJ, Keech RV. The psychosocial effects of amblyopia study. J AAPOS. 1999;3(1):15–17. doi: 10.1016/s1091-8531(99)70089-3. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group. A comparison of atropine and patching treatments for moderate amblyopia by patient age, cause of amblyopia, depth of amblyopia, and other factors. Ophthalmology. 2003;110(8):1632–1637. doi: 10.1016/S0161-6420(03)00500-1. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group. Risk of amblyopia recurrence after cessation of treatment. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2004;8(5):420–428. doi: 10.1016/S1091853104001612. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group. A randomized trial of atropine versus patching for treatment of moderate amblyopia: Follow-up at 10 years of age. Archives of ophthalmology. 2008;126(8):1039. doi: 10.1001/archopht.126.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG, Robson JG. The design of a new letter chart for measuring contrast sensitivity. Clinical Vision Sciences. 1988;2:187–199. [Google Scholar]
- Polat U, Ma-Naim T, Spierer A. Treatment of children with amblyopia by perceptual learning. Vision Res. 2009;49(21):2599–2603. doi: 10.1016/j.visres.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Rahi JS, Cumberland PM, Peckham CS. Does amblyopia affect educational, health, and social outcomes? Findings from 1958 British birth cohort. BMJ. 2006;332(7545):820–825. doi: 10.1136/bmj.38751.597963.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka MX, Beck RW, Holmes JM, Birch EE, Chandler DL, Cotter SA, Hertle RW, Kraker RT, Moke PS, Quinn GE, Scheiman MM Pediatric Eye Disease Investigator G. A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch Ophthalmol. 2003;121(5):603–611. doi: 10.1001/archopht.121.5.603. [DOI] [PubMed] [Google Scholar]
- Repka MX, Cotter SA, Beck RW, Kraker RT, Birch EE, Everett DF, Hertle RW, Holmes JM, Quinn GE, Sala NA, Scheiman MM, Stager DR, Sr, Wallace DK Pediatric Eye Disease Investigator G. A randomized trial of atropine regimens for treatment of moderate amblyopia in children. Ophthalmology. 2004;111(11):2076–2085. doi: 10.1016/j.ophtha.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Repka MX, Holmes JM, Melia BM, Beck RW, Gearinger MD, Tamkins SM, Wheeler DT Pediatric Eye Disease Investigator G. The effect of amblyopia therapy on ocular alignment. J AAPOS. 2005;9(6):542–545. doi: 10.1016/j.jaapos.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutstein RP, Quinn GE, Lazar EL, Beck RW, Bonsall DJ, Cotter SA, Crouch ER, Holmes JM, Hoover DL, Leske DA, Lorenzana IJ, Repka MX, Suh DW. A randomized trial comparing Bangerter filters and patching for the treatment of moderate amblyopia in children. Ophthalmology. 2010;117(5):998–1004 e1006. doi: 10.1016/j.ophtha.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh AG, Otero-Millan J, Kumar P, Ghasia FF. Abnormal Fixational Eye Movements in Amblyopia. PLoS One. 2016;11(3):e0149953. doi: 10.1371/journal.pone.0149953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shooner C, Hallum LE, Kumbhani RD, Garcia-Marin V, Kelly JG, Majaj NJ, Movshon JA, Kiorpes L. Asymmetric dichoptic masking in visual cortex of amblyopic macaque monkeys. J Neurosci. 2017 doi: 10.1523/JNEUROSCI.1760-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford university press; 2003. [Google Scholar]
- Stewart CE, Moseley MJ, Stephens DA, Fielder AR. Treatment dose-response in amblyopia therapy: the Monitored Occlusion Treatment of Amblyopia Study (MOTAS) Invest Ophthalmol Vis Sci. 2004;45(9):3048–3054. doi: 10.1167/iovs.04-0250. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Stephens DA, Fielder AR, Moseley MJ, Cooperative R. Objectively monitored patching regimens for treatment of amblyopia: randomised trial. BMJ. 2007;335(7622):707. doi: 10.1136/bmj.39301.460150.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15(5):443–453. doi: 10.1002/cpt1974155443. [DOI] [PubMed] [Google Scholar]
- Vedamurthy I, Knill DC, Huang SJ, Yung A, Ding J, Kwon OS, Bavelier D, Levi DM. Recovering stereo vision by squashing virtual bugs in a virtual reality environment. Philos Trans R Soc Lond B Biol Sci. 2016;371(1697) doi: 10.1098/rstb.2015.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedamurthy I, Nahum M, Huang SJ, Zheng F, Bayliss J, Bavelier D, Levi DM. A dichoptic custom-made action video game as a treatment for adult amblyopia. Vision Res. 2015a;114:173–187. doi: 10.1016/j.visres.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedamurthy I, Nahum M, Bavelier D, Levi DM. Mechanisms of recovery of visual function in adult amblyopia through a tailored action video game. Sci Rep. 2015b;5:8482. doi: 10.1038/srep08482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddingham PE, Butler TK, Cobb SV, Moody AD, Comaish IF, Haworth SM, Gregson RM, Ash IM, Brown SM, Eastgate RM, Griffiths GD. Preliminary results from the use of the novel Interactive binocular treatment (I-BiT) system, in the treatment of strabismic and anisometropic amblyopia. Eye (Lond) 2006;20(3):375–378. doi: 10.1038/sj.eye.6701883. [DOI] [PubMed] [Google Scholar]
- Wallace DK, Edwards AR, Cotter SA, Beck RW, Arnold RW, Astle WF, Barnhardt CN, Birch EE, Donahue SP, Everett DF, Felius J, Holmes JM, Kraker RT, Melia M, Repka MX, Sala NA, Silbert DI, Weise KK Pediatric Eye Disease Investigator G. A randomized trial to evaluate 2 hours of daily patching for strabismic and anisometropic amblyopia in children. Ophthalmology. 2006;113(6):904–912. doi: 10.1016/j.ophtha.2006.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DK, Lazar EL, Melia M, Birch EE, Holmes JM, Hopkins KB, Kraker RT, Kulp MT, Pang Y, Repka MX, Tamkins SM, Weise KK Pediatric Eye Disease Investigator G. Stereoacuity in children with anisometropic amblyopia. J AAPOS. 2011;15(5):455–461. doi: 10.1016/j.jaapos.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber AL, Wood JM, Thompson B. Fine Motor Skills of Children With Amblyopia Improve Following Binocular Treatment. Invest Ophthalmol Vis Sci. 2016;57(11):4713–4720. doi: 10.1167/iovs.16-19797. [DOI] [PubMed] [Google Scholar]
- Webber AL, Wood JM, Gole GA, Brown B. Effect of amblyopia on self-esteem in children. Optom Vis Sci. 2008a;85(11):1074–1081. doi: 10.1097/OPX.0b013e31818b9911. [DOI] [PubMed] [Google Scholar]
- Webber AL, Wood JM, Gole GA, Brown B. The effect of amblyopia on fine motor skills in children. Invest Ophthalmol Vis Sci. 2008b;49(2):594–603. doi: 10.1167/iovs.07-0869. [DOI] [PubMed] [Google Scholar]
- Williams C, Harrad R. Amblyopia: contemporary clinical issues. Strabismus. 2006;14(1):43–50. doi: 10.1080/09273970500538090. [DOI] [PubMed] [Google Scholar]
- Woodruff G, Hiscox F, Thompson JR, Smith LK. Factors affecting the outcome of children treated for amblyopia. Eye (Lond) 1994;8(Pt 6):627–631. doi: 10.1038/eye.1994.157. [DOI] [PubMed] [Google Scholar]
- Wu C, Hunter DG. Amblyopia: diagnostic and therapeutic options. Am J Ophthalmol. 2006;141(1):175–184. doi: 10.1016/j.ajo.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Xiao LQ, Zhang JY, Wang R, Klein SA, Levi DM, Yu C. Complete transfer of perceptual learning across retinal locations enabled by double training. Curr Biol. 2008;18(24):1922–1926. doi: 10.1016/j.cub.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Cong LJ, Klein SA, Levi DM, Yu C. Perceptual learning improves adult amblyopic vision through rule-based cognitive compensation. Invest Ophthalmol Vis Sci. 2014;55(4):2020–2030. doi: 10.1167/iovs.13-13739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.