Abstract
Aims and Objectives:
The aim of the study was to estimate and compare aspartate aminotransferases (AST) and alanine aminotransferases (ALT) levels in saliva and serum of insulin-dependent diabetes mellitus (IDDM) and normal children, and the objective was to evaluate the significance of these enzymes in assessing the salivary gland injury in IDDM children.
Materials and Methods:
The study group comprised of thirty children clinically and biochemically diagnosed for IDDM and thirty healthy children of similar age in the control group. Saliva and serum samples were collected and enzyme assay was performed by optimized ultraviolet test method (according to International Federation of Clinical Chemistry). The values obtained were subjected to statistical analysis using Mann–Whitney U-test for comparison of the variables and Karl–Pearson's coefficient test for correlation. The SPSS 16.0, (SPSS Inc., Chicago, USA) was used for statistical analysis.
Results:
Higher values of AST (mean = saliva 91.60 IU/L) and ALT (mean = saliva 94.93 IU/L) were found in the saliva than in serum (AST serum = 15.83 IU/L) (ALT serum = 20.80 IU/L) among the patients and the differences were statistically highly significant (P = 0.001). A similar comparison in the control group did not show statistical significant difference (saliva – AST mean = 23.50 IU/L, ALT mean = 21.95 IU/L and serum – AST mean = 12.60 IU/L, ALT mean = 13.25 IU/L). On correlation between patient and normal group, greater values of AST and ALT were observed in saliva of patients and the difference was statistically highly significant ([AST-mean = patients 91.166 IU/L and controls = 23.500 IU/L] [ALT-mean = patients 94.933 IU/L and controls 21.950 IU/L]). The serum values of patients and controls did not show statistical difference. Although higher values of salivary ALT and AST were observed in patients with the disease for 0–5-year clinical duration (ALT mean = 100.21 IU/L and AST mean = 95.39 IU/L) than those with the disease for 6–10-year duration (ALT mean = 77.57 IU/L and AST mean = 77.28 IU/L), values were statistically nonsignificant.
Conclusion:
Elevated salivary AST and ALT levels in IDDM patients suggest the injury to salivary glands and could aid as a salivary marker for the involvement of salivary glands in IDDM.
Keywords: Alanine aminotransferase, aspartate aminotransferase, insulin-dependent diabetes mellitus, saliva, serum
INTRODUCTION
Diabetes is the most common metabolic disease of endocrine origin affecting a large number of people in the world.[1] It is a chronic multisystem disease of carbohydrate, fat, and protein metabolism caused due to a lack of insulin production because of autoimmune destruction of beta cells of pancreas. Prevalence of diabetes has increased or remained same in every country and it is believed that only a few Western European countries have a chance of meeting the target to halt the rise in diabetes by 2025. Type 1 diabetes, even though is rarer than type 2, accounts for about 5% and 15% of all diabetes mellitus (DM). Various complications may develop in diabetics endangering their health, survival, and high cost of care and medical expenditure.[2] Alongside the metabolic abnormalities, the common long-term complications involve several organs such as the eyes, kidneys, nerves, and blood vessels.[1,3]
As far as involvement of salivary glands in DM, the ultrastructural and immunocytochemical studies reveal degenerative changes in salivary glands caused by T- and B-lymphocytes very similar to that in Sjogren's syndrome. It is possible that salivary glands and pancreas share a common antigen and hence both are susceptible to autoimmune attack in insulin-dependent diabetes mellitus (IDDM) patients.[4] Studies on animal models reveal a common underlying pathophysiology that the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) blockade or TRAIL deficiency causes glutamic acid dehydrogenase (GAD) 65-specific immune responses in the pancreatic islets and salivary glands in DM mice.[4,5,6,7]
The oral manifestations in diabetes include xerostomia, dental calculi, dental caries, burning mouth syndrome, candidiasis, increased gingival and plaque index, periodontal disease, delayed and abnormal wound healing, fulminating gingival abscesses and granular subgingival proliferations, altered taste, mucormycosis, aspergillosis, geographic tongue, fissured tongue, and oral lichen planus. Both qualitative and quantitative properties of saliva have found to be altered in diabetics such as reduced salivary flow rate, low calcium but increased potassium and sodium levels, increased salivary antioxidants, and IgA levels. As oral health is directly or indirectly associated with salivary changes, many of the oral manifestations in diabetics are due to defective or altered salivary gland status.[8]
TRANSAMINASES (ASPARTATE AMINOTRANSFERASES AND ALANINE AMINOTRANSFERASES) AS DISEASE MARKERS
Transaminases or aminotransferases are a group of enzymes that catalyze the interconversion of amino acids and oxalo acids by transfer of amino groups. They are present in skeletal muscle, kidney, brain, pancreas, lungs, leukocytes, and erythrocytes but the heart and liver tissues contain them in relatively high concentrations, and hence damage to these organs leads to transaminase leakage into the blood.[9,10] The aspartate aminotransferases (AST) and alanine aminotransferases (ALT) assessment has been a routine test primarily in liver diseases such as cirrhosis, viral hepatitis, alcoholic hepatitis and others such as myocardial infarction, appendicitis, polymyositis, and dermatomyositis.[9,10,11,12,13]
As postulated by some scientists, an immunological damage could occur in salivary gland; a pathomechanism similar to that in diabetes in the beta cells of islets of Langerhans.[5,6,7] With the background of AST and ALT leakage from the damages tissues, few researchers have worked on these enzymes as markers in saliva of diabetic individuals. In one such primary study, Musumeci et al. found that the salivary glutamate oxaloacetate transaminase, glutamate pyruvate transaminase, and lactate dehydrogenase (LDH) being elevated in both IDDM and non-IDDM (NIDDM) patients.[14] Malicka et al. also observed high activity of salivary AST, ALT, and LDH in Type 1 and Type 2 diabetes patients and interestingly threefold increase in salivary AST and ALT levels in Type 1 compared to Type 2 diabetics.[15]
With a steady increase in the incidence of diabetes worldwide, this metabolic diseases present as a huge burden on the economy of the countries at large and pose as a life-threatening disease with multiple complications as far as diabetics are concerned. Hence, the present study was undertaken to primarily analyze the salivary AST and ALT levels in IDDM children in comparison with normal healthy children which could possibly serve as a marker in assessing the injury to salivary glands.
MATERIALS AND METHODS
This simple random controlled research study was performed after getting the ethical clearance from the local Review Board (TIDSHRC/EC 40/A/2016). The participants in the study were included only after obtaining informed consent from the parents of the children and the specialty hospital authority. The study spanned for a period of 9 months from July 2016 to April 2017.
For calculation of sample size, the assumptions taken into account are minimum 80% power, 5% significance level (significant at 95% confidence level), and the relative risk of failure for experimental subjects is 0.12, it is estimated that at least 25 experimental subjects are required in each group. For the safer side of the normality of data, a sample size of thirty subjects was considered in each group. The Type 1 probability error associated with the test is 0.05.
Necessary sample size (n) = (Z2 1− α/2 σ2)/d2
α = 5% (i.e., confidence level = 95%)
d = 5% (precision required)
σ = standard deviation
Probability error = Type 1
INCLUSION CRITERIA
The study group consisted of thirty patients who were clinically and biochemically diagnosed with IDDM according to the WHO criteria[16] reporting to specialty hospitals and thirty healthy normal individuals formed the control group visiting the outpatient department with no systemic diseases or taking any medications at least 3 months before the study.
EXCLUSION CRITERIA
Patients were excluded if they were on medications other than for IIDM, those with any other systemic disease apart from IDDM, oral soft-tissue lesions, and patients with tobacco-related habits if any.
METHOD OF COLLECTION OF SALIVA AND BLOOD SAMPLES
Before collecting the saliva, the subjects were instructed to rinse the mouth with normal water. They were made to sit with their head in bent position with open mouth, and 5 ml of saliva was collected into a sterile container. Clear saliva was centrifuged at 1500 rpm for 15 min and the supernatant was collected and used for the estimation of AST and ALT. Two milliliters of venous blood was collected from antecubital vein in a plain bottle for serum AST and ALT estimation. The collected samples were then sent for biochemical analysis.
METHODS FOR ESTIMATION OF ASPARTATE AMINOTRANSFERASES AND ALANINE AMINOTRANSFERASES
The salivary and serum AST and ALT levels were estimated using optimized ultraviolet-test (340 nm) according to International Federation of Clinical Chemistry and Laboratory Medicine.[17,18]
STATISTICAL ANALYSIS
The data obtained were subjected to statistical analysis using Mann–Whitney U-test for comparison of the variables and Karl–Pearson's coefficient test for correlation.
The Statistical Package for the Social Sciences version 16.0, (SPSS Inc., Chicago, USA) was used for statistical analysis.
RESULTS
Higher values of AST were found in the saliva than in serum among the patients (mean – saliva = 91.60 IU/L and serum = 15.83 IU/L) and the difference was statistically highly significant (P = 0.001). A similar comparison in the control group did not show statistical significant difference (mean – saliva = 23.50 IU/L and serum = 12.60 IU/L) [Table 1].
Table 1.
Comparison of aspartate aminotransferase and alanine aminotransferase levels in saliva and serum of insulin-dependent diabetes mellitus patients and normal individuals
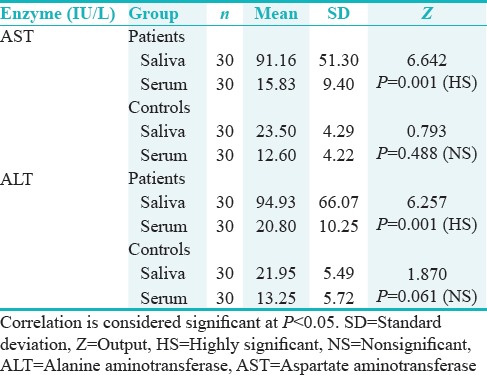
Similarly, increased levels of ALT were seen in the saliva than in serum within the patients group (mean – saliva = 94.93 IU/L and serum = 20.80 IU/L) and were statistically highly significant (P = 0.001), and in the control group, no significant difference was noted for a similar comparison (mean – saliva = 21.95 IU/L and serum = 13.25 IU/L) [Table 1].
On correlation between patient and normal group, greater values of AST and ALT were observed in saliva of patients and the difference was statistically highly significant ([mean AST – patients = 91.166 IU/L and controls = 23.500 IU/L] [mean ALT – patients = 94.933 IU/L and controls = 21.950 IU/L]) [Table 2 and Figure 1].
Table 2.
Comparison of the patient and normal group in relation to salivary aspartate aminotransferase and alanine aminotransferase levels
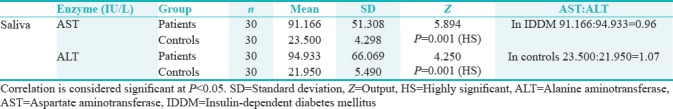
Figure 1.
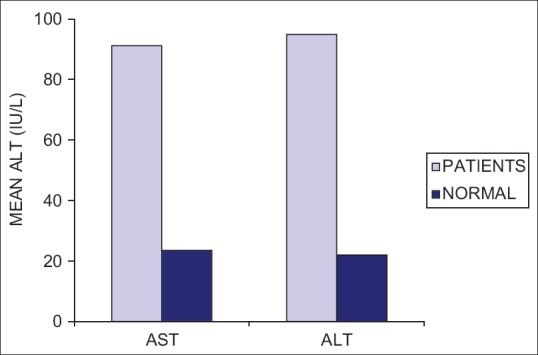
Comparison of AST and ALT levels in saliva of the patients and normal individuals. AST = Aspartate aminotransferases, ALT = Alanine aminotransferases, IU/L = International units per litre
The patients were grouped into two depending on the clinical duration of the disease as 0–5 and 6–10 years. Even though statistically nonsignificant, higher values of salivary ALT and AST were observed in patients with the disease for 0–5-year clinical duration than those with the disease for 6–10-year duration [Table 3 and Figure 2].
Table 3.
Comparison of aspartate aminotransferase and alanine aminotransferase levels in the saliva and serum of patients with insulin-dependent diabetes mellitus for 0.5- and 6-10-year clinical duration
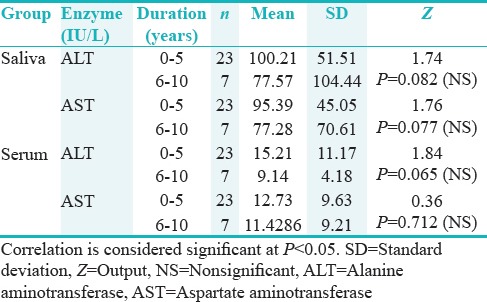
Figure 2.
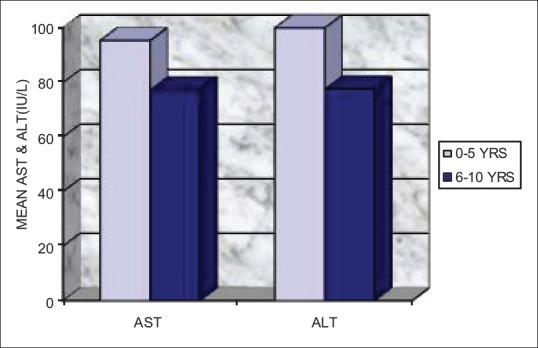
Comparison of AST and ALT levels in saliva of IDDM children with 0–5 and 6–10 years clinical duration of IDDM. AST = Aspartate aminotransferases, ALT = Alanine aminotransferases, IU/L = International units per liter, IDDM = Insulin-dependent diabetes mellitus
DISCUSSION
The primary outcome observed in the study was the increased levels of salivary AST and ALT enzymes in the IDDM children in comparison with the control group. Approximately a fourfold increase in salivary AST (mean = 91.16 IU/L) and ALT (mean = 94.93 IU/L) in the IDDM group was noted in comparison with the control group (AST mean = 23.50 IU/L and ALT mean 21.95 IU/L). The serum values remained almost same in both the patient and the control group (Patients – AST mean = 15.83 IU/L and ALT mean = 20.80 IU/L; controls – AST mean = 12.60 IU/L and ALT mean = 13.25 IU/L). Hence, a highly significant difference (P < 0.001) was noted between the salivary and serum levels of AST and ALT only in the IDDM group.
The secondary outcome observed was the difference in the salivary levels of AST and ALT in IDDM children with duration of the disease. Those with 0–5 years of disease duration had slightly high values of salivary AST (mean = 95.39 IU/L) and ALT (mean = 100.21 IU/L) than in those with 6–10 years of duration (mean, AST = 77.28 IU/L and ALT = 77.57 IU/L). Compared to the normal group, the IDDM children with 0–5 years of disease duration had fourfold increase in enzyme values and a threefold higher values were noted in the IDDM children with 6–10 years of disease duration possibly because of greater degree of injury to the salivary gland at the early stages of disease.
STRENGTH OF THE STUDY
The study is based on a noninvasive methodology of estimation of AST and ALT, reflecting the status of the salivary glands in IDDM patients
The concept of the study is supported by the earlier researchers through histological, ultrastructural, and immunological studies,[4,5,6,7] hence bear a strong correlation.
LIMITATIONS OF THE STUDY
The concept of the study is supported by the earlier researchers through histological, ultrastructural, and immunological studies.
The outcomes of the present study could be compared with those of few other studies available from the literature. Musumeci et al. observed that the salivary concentrations of AST in IDDM patients were similar to those found in the NIDDM but higher than those observed in normal subjects. ALT was higher in NIDDM than IDDM, which in turn was higher than in normal subjects. The salivary and serum values of AST and ALT were not correlated.[14] In the present study, the salivary AST and ALT in IDDM children were fourfold high compared to the control group. Here, the serum values were assessed but were within normal levels.
Malicka et al. ffound a twofold increase in the salivary AST and ALT levels in Type 1 DM patients in comparison with Type 2 DM and three times when compared to normal healthy individuals.[15] The results were similar to that obtained in the present study with almost four times higher values in IDDM patients compared to the control group.
Upon comparison with the clinical duration of the disease, we also found that the salivary AST and ALT levels were indirectly proportional to the clinical duration. At least onefold increase in values of salivary AST and ALT were seen in IDDM children with 0–5 years of clinical duration than those with 6–10 years. This finding was in parallel with those by Cinquini et al., where higher values were detected in the diabetic subgroup diagnosed for <4-year clinical duration with negative correlation between the enzymatic activities and duration of IDDM.[19] This finding is in correlation with the conditions associated with elevated transaminase enzymes in the early phases of the damage to the organs, as evidenced in acute myocardial infarction and acute hepatitis.[20,21] Hence, it is empirical from our results that immune-mediated cell damage could be present in the salivary glands of recently diagnosed diabetics and as opinioned by other researchers.[18]
As per estimation, presently, over 20 million diabetes patients to projected numbers around 57 million by the year 2025, diabetes is a disease of chief concern across the globe.[2,3] Along with many systemic and local complications, oral complications cause a major distress to the patients with DM. The involvement of salivary glands secondary to diabetes is one such complication[7,18] with manifestations such as xerostomia being the most common and others such as altered salivary organic and inorganic components with increased lather, total sugars, glucose, urea, total proteins, potassium, calcium, leptin, and nerve growth factor.[8,13,22]
Studies relating to the salivary transaminases published in diabetes are few in numbers. Musumeci et al. suggested the injury in salivary gland tissue through autoimmune mechanism similar to that found in the pancreatic cells of DM patients.[14] The concept was further strengthened through a study by Cinquini et al., revealing lymphocytic infiltration in the salivary glands of Type 1 diabetic children.[19] Backing this histological finding is the study by Markopoulos et al., who showed increased levels of GAD antibodies in serum and saliva of diabetic children (Type 1 DM) against the beta cells of islets of Langerhans of the pancreas and the salivary gland duct cells.[7] With these possible pathomechanisms, could there be injury occurring in the salivary gland tissue in diabetes and hence higher levels of salivary transaminase enzymes noted as in present and few previous research studies. Cinquini et al. opinioned that the determination of cytosolic enzymes in saliva might be useful for monitoring the diabetic involvement of salivary glands.[19]
The assessment of ratio between AST and ALT was done in IDDM children and normal controls [Table 2], and the same was compared to those in other diseases. The AST: ALT ratio in saliva of IDDM children (91.166:94.933 = 0.96) was slightly <1.0, with a marginal increase in ALT than AST (23.500:21.950 = 1.07), and the ratio was approximately 1.0 in the saliva of normal individuals. The studies pertaining to salivary AST and ALT ratio are neither reported in diabetics nor in other diseases. Hence, salivary AST and ALT ratio of the IDDM group in the present study were compared with serum AST and ALT ratios in other disease conditions reported in the literature. In acute and chronic viral hepatitis, both serum ALT and AST are elevated but usually ALT higher than AST with AST/ALT ratio <1.0.[15] A similar observation is found in the present study too. In alcoholic hepatitis, usually AST is high resulting in AST/ALT ratio of 1.5 or more.[23,24] Increased levels of ALT with a AST: ALT ratio of <0.8 are usually noted in nonalcoholic fatty liver disease, whereas >1.5 values are noted in alcoholic fatty liver disease.[25] This is in contrary to the salivary AST: ALT ratio in the present study where AST values were marginally elevated than ALT. Altered AST/ALT ratio is also noted in several other conditions such as rhabdomyolysis, particularly in polymyositis where ALT levels could be more than AST.[12] At large, tissue injuries cause elevation of transaminases but vary between type of tissues and the nature and duration of injury.[9,10,11,12,14,19,24,25]
With few limitations, the results from our study reveal increased activity salivary AST and ALT in IDDM patients, suggesting a possible leakage from the injured salivary gland tissues. Further researches in this regard at different stages of IDDM are required to enhance the knowledge of salivary transaminases in diabetic individuals. Even though, the serum AST and ALT were measured in our study, did not reveal significantly different values in patients and controls. This could be controversial point and need evaluation through further studies. Meanwhile, similar studies in adults with IDDM background with different clinical parameters seem appealing for researchers with similar interest.
CONCLUSION
Elevated levels of salivary AST and ALT enzymes in IDDM children could possibly be due to tissue damage occurring in the salivary glands secondary to autoimmune pathomechanism in islets of Langerhans’ cells in IDDM, which further could be responsible for the reduced functioning of these glands along with associated various oral complications. It can be concluded that salivary AST and ALT could be used as markers to assess the damage occurring in salivary glands.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
There are no conflicts of interest.
REFERENCES
- 1.American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40:S11–24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 2.Krug EG. Trends in diabetes: Sounding the alarm. Lancet. 2016;387:1485–6. doi: 10.1016/S0140-6736(16)30163-5. [DOI] [PubMed] [Google Scholar]
- 3.Britton R. Diabetes mellitus pathophysiology and complications. In: Colledge NR, Walker BR, Ralston SH, editors. Davidson's Principles and Practice of Medicine. 21st ed. UK: Elsevier; 2010. pp. 799–834. [Google Scholar]
- 4.Miyagawa J, Hanafusa T, Miyazaki A, Yamada K, Fujino-Kurihara H, Nakajima H, et al. Ultrastructural and immunocytochemical aspects of lymphocytic submandibulitis in the non-obese diabetic (NOD) mouse. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51:215–25. doi: 10.1007/BF02899031. [DOI] [PubMed] [Google Scholar]
- 5.Noorchashm H, Noorchashm N, Kern J, Rostami SY, Barker CF, Naji A, et al. B-cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice. Diabetes. 1997;46:941–6. doi: 10.2337/diab.46.6.941. [DOI] [PubMed] [Google Scholar]
- 6.Mi QS, Ly D, Lamhamedi-Cherradi SE, Salojin KV, Zhou L, Grattan M, et al. Blockade of tumor necrosis factor-related apoptosis-inducing ligand exacerbates type 1 diabetes in NOD mice. Diabetes. 2003;52:1967–75. doi: 10.2337/diabetes.52.8.1967. [DOI] [PubMed] [Google Scholar]
- 7.Markopoulos AK, Belazi MA, Drakoulakos D. Glutamic acid decarboxylase autoantibodies in saliva of children with type 1 diabetes. Diabetes Res Clin Pract. 1997;38:169–72. doi: 10.1016/s0168-8227(97)00078-8. [DOI] [PubMed] [Google Scholar]
- 8.Mauri-Obradors E, Estrugo-Devesa A, Jané-Salas E, Viñas M, López-López J. Oral manifestations of diabetes mellitus. A systematic review. Med Oral Patol Oral Cir Bucal. 2017;22:e586–94. doi: 10.4317/medoral.21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodwell VW, Kennelly PJ. Enzymes. In: Murray RK, Granner DK, Mayes PA, Rodwell VW, editors. Harper's Biochemistry. 26th ed. Connecticut: Appleton and Lange; 2003. pp. 49–72. [Google Scholar]
- 10.Giannini EG, Testa R, Savarino V. Liver enzyme alteration: A guide for clinicians. CMAJ. 2005;172:367–79. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toson EA, Shiha GE, Abdelgaleel AE. Fibrogenic/angiogenic linker for non-invasive assessment of hepatic fibrosis staging in chronic hepatitis C among Egyptian patients. Ann Hepatol. 2017;16:862–73. doi: 10.5604/01.3001.0010.5276. [DOI] [PubMed] [Google Scholar]
- 12.Mathur T, Shi Q, Niu J, Huang X, Pu C. Do muscle enzyme changes forecast liver injury in polymyositis/dermatomyositis patients treated with methylprednisolone and methotrexate? Ann Clin Lab Sci. 2016;46:266–9. [PubMed] [Google Scholar]
- 13.López-Pintor RM, Casañas E, González-Serrano J, Serrano J, Ramírez L, de Arriba L, et al. Xerostomia, hyposalivation, and salivary flow in diabetes patients. J Diabetes Res. 2016;2016:4372852. doi: 10.1155/2016/4372852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musumeci V, Cherubini P, Zuppi C, Zappacosta B, Ghirlanda G, Di Salvo S, et al. Aminotransferases and lactate dehydrogenase in saliva of diabetic patients. J Oral Pathol Med. 1993;22:73–6. doi: 10.1111/j.1600-0714.1993.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 15.Malicka B, Skoskiewicz-Malinowska K, Kaczmarek U. Salivary lactate dehydrogenase and aminotransferases in diabetic patients. Medicine (Baltimore) 2016;95:e5211. doi: 10.1097/MD.0000000000005211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paschou SA, Papadopoulou-Marketou N, Chrousos GP, Kanaka-Gantenbein C. On type 1 diabetes mellitus pathogenesis. Endocr Connect. 2018;7:R38–R46. doi: 10.1530/EC-17-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas L, editor. Clinical Laboratory Diagnostics. 1st ed. Frankfurt: TH-Books Verlagsgesellschaft; 1998. Alanine aminotransferase (ALT), aspartate aminotransferase (AST) pp. 55–65. [Google Scholar]
- 18.Moss DW, Henderson AR. Clinical enzymology. In: Burtis CA, Ashwood ER, editors. Tietz Textbook of Clinical Chemistry. 3rd ed. Philadelphia: W.B Saunders Company; 1999. pp. 617–721. [Google Scholar]
- 19.Cinquini I, Calisti L, Fierabracci V, Marrapese E, Egéa JC, Masiello P, et al. Enzymatic markers of salivary cell injury in saliva of type 1 diabetic children. Clin Oral Investig. 2002;6:21–3. doi: 10.1007/s00784-002-0154-7. [DOI] [PubMed] [Google Scholar]
- 20.Baars T, Neumann U, Jinawy M, Hendricks S, Sowa JP, Kälsch J, et al. In acute myocardial infarction liver parameters are associated with stenosis diameter. Medicine (Baltimore) 2016;95:e2807. doi: 10.1097/MD.0000000000002807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC. Public Policy Committee of the American Association for the Study of Liver Disease. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–70. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 22.Lamey PJ, Tvarijonaviciute A, Castillo C, Ceron JJ, Martinez-Subiela S, Tecles F, et al. Leptin and NGF in saliva of patients with diabetes mellitus type 2: A pilot study. J Oral Pathol Med. 2017;46:853–5. doi: 10.1111/jop.12587. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell MC, Friedman LS, McClain CJ. Medical Management of Severe Alcoholic Hepatitis; Expert Review from the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol. 2017;15:5–12. doi: 10.1016/j.cgh.2016.08.047. Doi.10.1016/j.cgh.2016.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Botros M, Sikaris KA. The de ritis ratio: The test of time. Clin Biochem Rev. 2013;34:117–30. [PMC free article] [PubMed] [Google Scholar]
- 25.Petta S, Wong VW, Cammà C, Hiriart JB, Wong GL, Vergniol J, et al. Serial combination of non-invasive tools improves the diagnostic accuracy of severe liver fibrosis in patients with NAFLD. Aliment Pharmacol Ther. 2017;46:617–27. doi: 10.1111/apt.14219. [DOI] [PubMed] [Google Scholar]


