Abstract
BACKGROUND:
Ziziphus jujuba belongs to family Rhamnaceae widely distributed in subtropical and tropical countries. It is used traditionally for several pharmacological purposes including anti-inflammation, antidiarrhoeal and antibacterial, as well as tonic and sometimes as hypnotic (sedative).
AIM:
To determine the in vivo antidiarrhoeal, antibacterial and anti-inflammatory activities of Z. jujuba fruit ethanolic extract.
METHOD:
The fruit was macerated and extracted by 95% (v/v) ethanol. The antidiarrhoeal activity was evaluated using castor oil and Escherichia coli induced diarrhoea mouse model. The antidiarrhoeal and antibacterial activity was investigated at graded doses (400-1200 mg/kg). The anti-inflammatory effects were tested using the carrageenan-induced paw oedema in female Wistar rats. Rat’s treatment groups received tragacanth, 100 mg/kg diclofenac sodium, 800 mg/kg, 1200 mg/kg or 1600 mg/kg of an ethanolic extract of Z. jujuba (EEZJ). All treatment groups were fed with the compounds one hour before carrageenan injection at of rat’s paw. Also, the EEZJ was further analysed by HPLC-PDA system for identification of the presence of betulinic acid and quercetin.
RESULTS:
EEZJ different doses did not show inhibitory activity against castor oil induced diarrhoea except for the higher (1200 mg/kg) dose. However, the frequency of defecation of stools and watery stool were reduced significantly when compared to control group (P ≤ 0.05 and P ≤ 0.01 respectively), resulted in overall 67% inhibition of diarrhoea. Our anti-inflammatory results demonstrated that EEZJ was able to inhibit the carrageenan-induced paw oedema in rats to a significant degree (p ≤ 0.05) and the paw volume and thickness of both left and right paw were affected compared to the negative control group.
CONCLUSION:
EEZJ possesses antidiarrhoeal and antibacterial activity in a dose depending manner and may provide a pharmacological basis for its clinical use in diarrheal diseases. The activity may partially be due to the presence of betulinic acid and quercetin.
Keywords: Ziziphus jujuba, Antidiarrhoeal, anti-inflammatory, Carrageenan-induced paw oedema, Betulinic acid, Quercetin
Introduction
Ziziphus jujuba Mill. (family: Rhamnaceae), is a thorny tree of medium height, whose fruit resembles buckthorn or olive fruit and had been widely consumed as both fruit and remedy for a long time all around the world. It has been listed among the first most valuable fruits in ancient Chinese medicine books. Moreover, in the Chinese herbal medicine, it is considered as one of the superior herbal medicines and thought to possess the effect of prolonging lifespan by purifying and nourishing blood, treating insomnia and help in digestion. Nowadays, Z. jujuba fruit is believed to be one of the nutritious foods as it contains plenty of nutrients which consists of amino acids, carbohydrates, minerals and vitamins [1] [2].
Regarding the geographical distribution, Z. jujuba is widely found in the tropical and subtropical regions of Asia and America as well as in the Mediterranean regions [3]. It is well known in the Arabian Peninsula since ancient time. However, it is believed to be originated in the Algerian town of Annaba, due to which it has been named Annab. Furthermore, the mature fruit of Z. jujuba is red to purplish black, resembling small dates. Therefore in China are known as Chinese red date or Chinese jujuba. The dried pulp of Z. jujuba is a source of essential unsaturated fatty acids. The main fatty acids in the jujube are oleic, linoleic (omega-6), palmitic, and palmitoleic acids. Jujube fruits contain various types of amino acids and proteins. The protein and free asparagines content are influenced and accumulated potentially during the ripening and harvesting stage [4].
Dietary fibre and fructose contents of the jujube fruit play a role in the regulation of blood sugar levels by slowing digestion [5]. The major sugars found in the jujube fruit are glucose, fructose, sucrose, rhamnose and sorbitol. The fruit is also abundant in vitamin C, which is one of the water-soluble antioxidants [6]. The postharvest sorting process is important for increasing the economic benefits and dietary values of the jujube fruit, especially vitamin C content protection during storage and marketing [7]. Moreover, the jujube is enriched, nevertheless to a lesser extent, with other vitamins including thiamin, riboflavin, niacin, vitamin B6, and vitamin A. Jujube fruit is also considered a good source of minerals such as magnesium, phosphorus, potassium, sodium, and zinc [6]. Various studies have shown that the jujube fruit contains many bioactive compounds, including triterpenic acids, flavonoids, cerebrosides, phenolic acids, α-tocopherol, β-carotene, and polysaccharides. Each constituent of the jujube presents some health benefits, thus making it a healthy food choice [8]. The total phenolic compounds in jujube fruit which is accounted for the antioxidant activities are higher compared to other common fruits, such as cherry, apple, persimmon, or red grape [9]. Flavonoids, phenolic acids, tannins, stilbenes, and lignans are derivatives of phenolic compounds [10] [11] [12].
The Indian jujuba, Ziziphus mauritiana Lam. and Z. jujuba Mill. Are the two main domesticated jujubes. The pantropical genus Ziziphus Mill. Includes approximately 170 species with a few species occurring in temperate regions. Recent studies on the biological activities of this fruit have supported the health benefits of jujube as both food and medicinal herb. Different parts of Z. jujuba are used traditionally for curing many kinds of illness including diabetes, diarrhoea, liver complaints, urinary disorders, obesity, skin infections, respiratory infections, anaemia, insomnia, cancer, and also for blood purification and modification of the gastrointestinal tract [ [6] 13] [14]. Z. jujuba is used traditionally as a tonic and sometimes as hypnotic-sedative. Additionally, there are studies that had been carried out to test its anxiolytic, anticancer, antimicrobial, anti-inflammatory, anti-allergy, cognitive, anti-nephritic, antioxidant and wound healing properties [8] [15].
The anxiolytic effect in vivo had been reported in a polyherbal substance which consists of seed extract of Z. jujuba [16]. The possibility of improving cytotoxic activity was suggested to be due to the presence of coumaroyl moiety at the carbon-3 position of the lupanetype triterpene, extracted from Z. jujuba [17]. Selective toxicity against cultured human melanoma cells is also performed by the triterpenes acid and betulinic acid extracted from Z. jujuba and Ziziphus mauritiana [18]. Notable inhibitory activity performed by ethanolic extract of Z. jujuba root on fungi Candida albicans, C. tropicalis, Aspergillus flavus and A. niger showed convincing antifungal activity [19]. Additionally, extract of root bark of Z. jujuba exhibited antibacterial activity against 20 bacteria [20].
A traditional Chinese prescription, Huangqin Tang with fruit content from Z. jujuba had shown remarkable anti-inflammatory and antispasmodic effect [21]. Additionally, Ziziphus mauritiana leaf extracts were also found to possess significant anti-inflammatory activity against carrageenan induced rat paw oedema [22]. The anti-allergic activity of the aqueous extracts of leaves of Z. jujuba was studied by measuring its inhibitory effect on hyaluronidase or bovine testes activation in vitro. Z. jujuba was shown to have strong antiallergic and anti-anaphylactic activity [23] [24]. A component of Z. jujuba extract, oleamide poses possible potential against Alzheimer’s disease as it could be a useful chemopreventative agent [25] [26]. Methanolic extract of Z. jujuba was found to show activation effect on choline acetyltransferase in vitro as high as 34.1%.
Moreover, Z. jujuba also exhibits possibly anti-nephritic effect by increasing renal blood flow and thus reducing inflammation of kidney [27]. Two reports studied 70 antioxidant Korean medicinal plants listed the in vitro antioxidant effect of Z. jujuba [28] [29]. The wounding healing effect had been reported on the extract of Z. jujuba root [30]. Furthermore, a rat model which uses an ointment formula at a dose 0.5% and 1% on topical application proved the wound healing activity of the extract of Z. jujuba root [31]. The main aim of the current studies was to further explore the anti-inflammatory and anti-diarrhoeal activities of ethanolic extract of Ziziphus jujube fruit.
Materials and Methods
The 1 ml and 10 ml disposable syringes, amber bottles, oral gavage and 27G needle, were purchased from Terumo Tokyo–Japan. Digital Plethysmometer (model 7140, Ugo Basile, Italy), weighing balance (A & D, Tokyo, Japan), freezer (Action International, Kuala Lumpur, Malaysia), refrigerator (Sharp Malaysia), cell culture incubator (CL-170B-8, ESCO, Singapore), rotary evaporator (Butchi Rotavapour model R-114, Büchi Labortechnik AG, Flawil Switzerland). The plastic cages, water bottles, white cloth, tissue paper, masks, gloves, vacuum pump and vortex, were from Autovortex SA6, Stuart Scientific, UK. HPLC machine (Waters 2695; WATERS CORPORATION, Milford, MA 01757 USA), Ultra-purified water machine (ELGA Labwater Purification System, High Wycombe HP14 3BY, UK).
The dried granules of fruits (2744.58 g) were extracted by cold maceration for 72 hours with 95% of ethanol. The solutions were filtered using vacuum filtration. The filtrates were then concentrated in a rotary evaporator to eliminate ethanol and yielded semi-solid extract. Immersion, filtration and rotary evaporation process were repeated for three times. The extract of Z. jujuba was preserved at 10°C until further use [24].
Different concentration of EEZJ, loperamide, and diclofenac sodium salt were freshly prepared in tragacanth compound powder suspension prior administration. Carrageenan powder was added into normal saline to create 1% w/v carrageenan solution at 4°C and stirred immediately. Any remaining lumps were dispersed by using vortex. The solution was warmed to 50°C with stirring. After that, the solution was incubated at 40°C for less than 24 hours before use [32].
Sample (EEZJ) and standards (betulinic acid and quercetin) were weighed and transferred into three 2 ml vials, respectively. After dissolved in 100% methanol, solutions were filtered through 0.45 µm membrane filters before HPLC analysis, Guo et al. [33].
The bacterial inoculum suspension of E. coli (ATCC 25927) was prepared by direct transfer of bacteria from stock culture into nutrient broth contained in the universal bottle. Few loops of stock culture were transferred into the sterile nutrient broth and incubated for 24 hours at 37°C prior administration. E. coli suspension was then compared with McFarland standards to obtain the desired turbidity of bacterial suspension. The minimum effective dose obtained from this test will determine the doses of choice in the in vivo antidiarrhoeal experiment and in vivo antibacterial experiment [33].
Following Sahoo et al. work [34] the preliminary in vivo assay using mice, six mice were separated randomly into two groups of three animals each. The turbidity of E. coli inoculum suspensions was adjusted to McFarland Standard No. 1 (3.0x108 CFU /ml), No. 2 (6.0x108 CFU/ml) prior administration to mice. Group 1 received 1 ml of E. coli inoculum suspension of McFarland Standard No.1 while group 2 received 1 ml of E. coli inoculum suspension of McFarland Standard No.2. Following treatment of E. coli suspension, mice were placed and observed for subsequence eight hours in the separated beaker which consists of white filter paper. The filter paper was changed hourly. Several parameters such as the weight of stools, the frequency of total stools and watery stools were taken for measuring purpose. After eight hours of observation, mice were sacrificed, and the intestines were removed. Any abnormalities such as ulceration, perforation and redness were observed and recorded. The minimum concentration of E. coli that induced abnormalities in intestines of the mice was used in the antibacterial activity experiment.
Base on experiment used by Ayalate and his colleagues [36], thirty mice with a weight of 20-35 g were separated into six groups of five animals each. All five groups of animals were fed orally with 1 ml of E. coli suspension using gavage needle with turbidity similar to McFarland Standard No. 1 three hours prior administration of suspension. Group 1 received 0.2 ml of tragacanth 2% orally and served as the control group while group 2 received antibiotic (Amoxicillin 260 mg/kg) and served as positive control. Group 3, 4 and 5 received 400 mg/kg, 800 mg/kg and 1200 mg/kg of EEZJ, respectively. Following treatment with drug and extraction suspension, mice were separated and placed individually into a different beaker containing a white filter paper and observed for the subsequent four hours. The white filter papers were changed hourly.
RA was calculated according to the following formula:
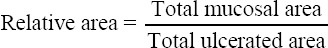
Meanwhile, approximately 2000 mg of stools of each mouse were collected and suspended into 5 ml of sterile phosphate-buffered saline (PBS) to produce suspension A, which underwent vigorous shaking by a vortex mixer for at least 15 minutes. Then, 100 µl of the suspension A was diluted into 10 ml of sterile PBS to form suspension B. Afterwards, 100 µL of the suspension B was spread on to the MacConkey agar plates and incubated at 37°C for 24 hours. Later the growth of bacterial colony was observed and counted by using colony counter.
A preliminary study was carried out to determine the dose of carrageenan to induce paw oedema in rats. Moreover, the effective doses of plant extract that exert an observable anti-diarrhoeal effect on castor oil induced diarrhoea, as well as anti-inflammatory effects on carrageenan-induced paw oedema were determined in the current study.
Following Wang et al. method [33], 30 mice were selected and divided randomly into six groups of five animals each. The weight of all mice was in the range of 20-35 g. Group 1 and group 2 were administered with 0.2 ml of 2% tragacanth and 4.2 mg/kg loperamide HCl respectively. Group 3, 4 and 5 received 400 mg/kg, 800 mg/kg and 1200 mg/kg of EEZJ, respectively. Mice acute diarrhoea was induced with 0.2 ml of castor oil one hour after administration of oral suspension. After the supply of castor oil, animals were placed in the separated beakers which consist of white filter paper. The filter papers were changed every hour for subsequence four hours. Mice were under direct observation for the onset of diarrhoea, weight of stools, and frequency of total stools and watery stools during the four hours. The proportion of the weight of watery stools was calculated based the formula that developed by Wang et al., [33].
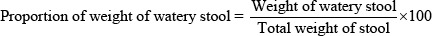
The percentage of inhibition of defaecation and diarrhoea were calculated according to the formula that developed by Lumpu et al., [37].
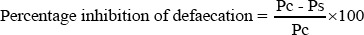
Where Pc is the average number of defecation of control group while Ps is the average number of defaecation of the test group.
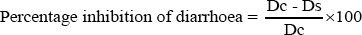
Where Dc is the average number of diarrhoea of control group, while Ds is the average number of diarrhoea of test group.
Acute inflammation was induced by subplantar injection of 0.1 ml carrageenan solution (1% w/v in normal saline) in the hind paw of each rat. Before injection, the rat paw was held still and steady to ensure the injection site was cleared and observable. Different groups of rats were administered either solution of Z. jujuba ethanolic extract (800 mg/kg, 1200 mg/kg or 1600 mg/kg), diclofenac sodium (100 mg/kg) or negative control solution (2% tragacanth) orally, one hour before carrageenan injection. The paw volume and thickness were measured by plethysmometer and digital calliper respectively immediately before carrageenan injection (0 h) and the consecutive six hours with the one-hour interval between two readings [38].
Paw volume was measured by dipping the rat paw into the tube until marked level, and the pedal switch was pressed to get the data value after the figure stabilised. The rat feet were kept still and steady during measurement process to improve accuracy and consistency. Every time after data value was taken, the meter was zeroed before next measurement. The thickness of the paw was taken at the marked line to ensure the consistency and accuracy of the data value by using a digital calliper.
The extent of inflammation was expressed as percent (%) oedema and calculated as below [39]:
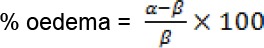
Where α and β are the paw volume/ thickness after carrageenan injection and paw volume/ thickness before carrageenan injection respectively. The anti-inflammatory effects were deduced from the extent of inflammation. The greater the extent of inflammation, the less anti-inflammatory effects exerted by that treatment group.
HPLC analysis was performed on a Waters 2695 Alliance HPLC system (Waters Corporation, United States of America), equipped with a quaternary pump solvent management system, an auto-sampler, and an on-line degasser. The separation was carried out with an XBridge™ C18 column (4.6 mm x 250 mm) while raw data were detected by Waters 2998 PDA and processed with EmpowerTM Software. The column temperature was 25◦C.
The mobile phase for betulinic acid HPLC qualification was composed of A (acetonitrile) and B (0.1 % acetic acid) at the flow rate of 1.0 ml/min. The elution concentration of mobile phase were: 0-5 min (A: 20%; B: 80%), 6-25 min (A: 70%; B: 30%), 26-45 min (A: 90%; B: 10%) and detected at the wavelength of 205nm. Re-equilibration duration was 15 minutes between individual runs [40].
HPLC analysis for qualification of quercetin was carried out by gradient elution beginning with a mobile phase A (acetonitrile) and B (0.2% of acetic acid) at the flow rate of 0.5 ml/min. The elution concentration of mobile phase were: 0-12 min (A: 30%; B: 70%), 12-13 min (A: 33%; B: 67%), 13-31 min (A: 48%; B: 52%), 31-35 min (A: 63%; B: 37%), 35-80 min (A: 100%; B: 0%) and detected at the wavelength of 205nm [41]. Re-equilibration duration was 15 minutes between individual runs.
The chromatographic peaks from EEZJ were identified by comparing the retention time to the reference standard compounds which underwent the same condition of elution. Each sample and reference standard were analysed for three times for the precision of the analysis.
Data obtained were analysed statistically using GraphPad PRISM. All data are presented as means a ± standard error of the mean (S.E.M). Data were evaluated by one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. The level of statistical significance was set at P ≤ 0.001 (***) or P ≤ 0.01 (**) or P ≤ 0.05 (*) when compared to control group.
Result
The yield of a crude ethanolic extract of Z. jujuba was 1620.68 g, from 2744.58 g. The yield percentage was 59.05%. 90% of the extract was subjected to in vivo test while another 10% was subjected to qualification analysis.
Two parameters were employed in this test, which is the number of colonies growing in the petri dish and the ulcer index. Positive control greatly reduces the number of colonies growing on petri dish; nevertheless, the results were not sufficient to be statistically significant. Test groups postulate a decreasing trend in reducing the number of colonies in a dose depending manner. However, the results are not statistically significant.
However, Table 1 shows that the EEZJ inhibited the growth of bacteria in vivo in a dose depending manner, although there were no ulcers observed in our experiment. Orally administered E. coli demonstrated some redness like inflammation and ulcerative presentation at the intestinal tissue during post-mortem session.
Table 1.
Antibacterial activity
| Compound | Dosage (mg/kg) | Number of Colonies | Ulcer Index |
|---|---|---|---|
| Control | - | 162.83 ± 49.57 | 0.82 ± 0.02 |
| Amoxicillin | 260 | 60.67 ± 18.11ns | 0.80 ± 0.00ns |
| EEZJ | 400 | 140.83 ± 48.14ns | 0.82 ± 0.04ns |
| EEZJ | 800 | 122.83 ± 52.64ns | 0.77 ± 0.05ns |
| EEZJ | 1200 | 117.00 ± 38.26ns | 0.88 ± 0.02ns |
(means ± S.E.M; n = 6); ns P ≥ 0.05 compared to control (One-way ANOVA followed by Dunnett’s Test); *P ≤ 0.05 compared to control (One-way ANOVA followed by Dunnett’s Test)’ **P ≤ 0.01 compared to control (One-way ANOVA followed by Dunnett’s Test).
Based on Table 2, the extractions produced no significant results in parameters such as the proportion of watery stools, and the onset of diarrhoea when compared statistically to the control group. However, there is a trend of reducing the proportion of the weight of watery stool in mice treated with different concentration of EEZJ. Not only that, but mice treated with EEZJ also delayed the onset of diarrhoea compared to control group. However, the results obtained were not statistically significant. Among three concentrations of EEZJ, only EEZJ 1200 mg/kg produced a significant result (P ≤ 0.01) in reducing a number of watery stools. This significant level was comparable with positive control as it also produced a significant result (P ≤ 0.01). Furthermore, EEZJ 1200 mg/kg was the only test group that produced a significant result (P ≤ 0.05) when compared to control group in reducing a total number of the stool. From Table 3, it shows increasing inhibition of both defaecation and diarrhoea frequency with increasing concentration of EEZJ. Positive control produced the similar significance level when compared to negative control (P ≤ 0.05) regarding reducing a total number of stool. Loperamide was the only group that showed the significant result in all parameters when compared to control group.
Table 2.
Preliminary antidiarrhoeal test
| Compound | Dosage (mg/kg) | The proportion of Weight of Watery Stool (%) | The onset of Diarrhoea (hour) | Total Number of Defecation | Number of Watery Stool |
|---|---|---|---|---|---|
| Control | - | 92.73 ± 6.70 | 1.00 ± 0.00 | 12.67 ± 1.20 | 11.67 ± 1.76 |
| EEZJ | 100 | 96.19 ± 3.62ns | 1.33 ± 0.33ns | 12.67 ± 1.76ns | 11.67 ± 2.33ns |
| EEZJ | 400 | 75.47 ± 8.81ns | 2.67 ± 0.33** | 13.67 ± 1.76ns | 9.00 ± 1.00ns |
(means ± S.E.M; n = 3); ns P ≥ 0.05 compared to control (One-way ANOVA followed by Dunnett’s Test); * P ≤ 0.05 compared to control (One-way ANOVA followed by Dunnett’s Test);
P ≤ 0.01 compared to control (One-way ANOVA followed by Dunnett’s Test).
Table 3.
In vivo antidiarrhoeal activity
| Compound | Dosage (mg/kg) | The proportion of Weight of Watery Stool (%) | The onset of Diarrhoea (hour) | Total Number of Stools | Number of Watery Stool | Inhibition of Defecation (%) | Inhibition of Diarrhoea (%) |
|---|---|---|---|---|---|---|---|
| Control | - | 83.07 ± 5.98 | 1.83 ± 0.31 | 16.33 ± 1.50 | 13.17 ± 2.06 | 0.0 | 0.0 |
| Loperamide | 4.2 | 42.49 ± 14.57* | 3.67 ± 0.56* | 6.83 ± 2.75* | 3.83 ± 1.72** | 58.16 | 70.89 |
| EEZJ | 400 | 77.34 ± 3.70ns | 2.33 ± 0.21ns | 11.17 ± 1.90ns | 9.00 ± 0.97ns | 31.63 | 31.66 |
| EEZJ | 800 | 73.00 ± 3.90ns | 2.33 ± 0.33ns | 10.50 ± 1.38ns | 8.67 ± 1.01ns | 35.70 | 34.17 |
| EEZJ | 1200 | 66.23 ± 12.06ns | 2.83 ± 0.48ns | 8.50 ± 1.82* | 4.33 ± 1.45** | 47.95 | 67.09 |
(means ± S.E.M; n = 6); ns P ≥ 0.05 compared to control (One-way ANOVA followed by Dunnett’s Test);
P ≤ 0.05 compared to control (One-way ANOVA followed by Dunnett’s Test);
P ≤ 0.01 compared to control (One-way ANOVA followed by Dunnett’s Test.
As shown in Table 4 and Table 5, anti-inflammatory activity of Z. jujuba is significant when compared to negative control group (2% tragacanth) all the time. The experimental findings from the carrageenan-induced rat paw oedema model showed that the Z. jujuba ethanolic extract reduced the paw volume significantly (P≤0.001) from 1 h to 6 h. Inhibition of paw oedema formation was shown in three doses of Z. jujuba ethanolic extract. However, the anti-inflammatory effect exerted by Z. jujuba ethanolic extract was less potent than that found with positive control drugs. Furthermore, the onset of anti-inflammatory action of Z. jujuba ethanolic extract was found to be comparable with that of control positive drug.
Table 4.
Percentage of right paw oedema (%) calculated from the change in paw volume after treatment
| Group | Treatment | Dose (mg/kg) | Percentage of right paw oedema (%) after treatment (Mean±SEM) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | |||
| 1 | EEZJ | 800 | 7.60 ± 1.12*** | 14.00 ± 1.58*** | 20.80 ± 1.72*** | 25.40 ± 1.69*** | 30.20 ± 2.08*** | 36.00 ± 2.68*** |
| 2 | EEZJ | 1200 | 8.00 ± 1.27*** | 13.40 ± 1.20*** | 18.40 ± 1.50*** | 24.00 ± 1.79*** | 30.00 ± 2.43*** | 32.80 ± 3.41*** |
| 3 | EEZJ | 1600 | 6.00 ± 0.45*** | 16.00 ± 1.76*** | 21.40 ± 1.33*** | 26.80 ± 1.78*** | 30.40 ± 1.86*** | 35.00 ± 2.24*** |
| 4 | Diclofenac Sodium | 100 | 3.20 ± 1.07*** | 8.00 ± 1.30*** | 13.60 ± 2.34*** | 18.00 ± 2.67*** | 21.20 ± 2.56*** | 23.20 ± 2.35*** |
| 5. | Control | 100 | 28.00 ± 4.72 | 45.80 ± 5.00 | 68.00 ± 5.06 | 89.20 ± 6.59 | 105.20 ± 10.10 | 114.00 ± 10.07 |
(means ± S.E.M; n = 5); ns P ≥ 0.05 compared to control (One-way ANOVA followed by Dunnett’s Test);* P ≤ 0.05 compared to control (One-way ANOVA followed by Dunnett’s Test);**P ≤ 0.01 compared to control (One-way ANOVA followed by Dunnett’s Test);
P ≤ 0.001 compared to control (One-way ANOVA followed by Dunnett’s Test).
Table 5.
Percentage of left paw oedema (%) calculated from the change in paw volume after treatment
| Group | Treatment | Dose (mg/kg) | Percentage of left paw oedema (%) after treatment (Mean ± SEM) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | |||
| 1 | EEZJ | 800 | 6.80 ± 1.16*** | 13.40 ± 2.54*** | 19.80 ± 3.09*** | 25.80 ± 3.80*** | 31.00 ± 4.38*** | 37.00 ± 4.45*** |
| 2 | EEZJ | 1200 | 6.00 ± 0.63*** | 12.60 ± 1.75*** | 17.40 ± 2.11*** | 24.40 ± 4.13*** | 29.40 ± 4.68*** | 32.20 ± 4.12*** |
| 3 | EEZJ | 1600 | 5.80 ± 0.37*** | 12.80 ± 1.36*** | 17.40 ± 1.17*** | 25.00 ± 1.82*** | 29.00 ± 1.92*** | 34.20 ± 2.48*** |
| 4 | Diclofenac Sodium | 100 | 2.20 ± 0.37*** | 6.40 ± 0.81*** | 10.20 ± 2.60*** | 14.00 ± 2.59*** | 17.60 ± 2.46*** | 19.80 ± 2.29*** |
| 5. | Control | 100 | 23.60 ± 4.78 | 41.80 ± 4.60 | 60.00 ± 4.37 | 78.00 ± 6.72 | 93.20 ± 6.58 | 102.60 ± 6.81 |
Means ± S.E.M; n = 5); ns P ≥ 0.05 compared to control (One-way ANOVA followed by Dunnett’s Test); * P ≤ 0.05 compared to control (One-way ANOVA followed by Dunnett’s Test); **P ≤ 0.01 compared to control (One-way ANOVA followed by Dunnett’s Test);
P ≤ 0.001 compared to control (One-way ANOVA followed by Dunnett’s Test).
According to Table 4 and 5, both three doses of Z. jujuba ethanolic extract successfully controlled the percentage of paw oedema under 40%. 1200 mg/kg Z. jujuba ethanolic extract treatment group has a higher percentage of paw oedema inhibition when compared to that of 800 mg/kg and 1600 mg/kg Z. jujuba ethanolic extract treatment groups. 800 mg/kg Z. jujuba ethanolic extract treatment group has weakest anti-inflammatory effect at the corresponding time point.
Apart from that, all three doses of Z. jujuba ethanolic extract showed a similar trend of oedema development. Additionally, the Z. jujuba ethanolic extract produced the dose-dependent significant anti-inflammatory activity as the lowest dose, 800 mg/kg showed least anti-inflammatory activity when compared to other two with the higher dose.
According to Table 6 and 7, the three doses of Z. jujuba ethanolic extract successfully controlled the thickness of paw that due to oedema when compared with that of the negative control group. The 1200 mg/kg Z. jujuba ethanolic extract treatment group has a higher percentage of paw oedema inhibition when compared to the 800 mg/kg and 1600 mg/kg Z. jujuba ethanolic extract treatment groups. However, all the three doses of Z. jujuba ethanolic extract exhibited lower anti-inflammatory activity than the positive control group.
Table 6.
Percentage of right paw oedema (%) calculated from the change in paw thickness after treatment
| Group | Treatment | Dose (mg/kg) | Percentage of right paw oedema (%) after treatment (Mean ± SEM) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | |||
| 1 | EEZJ | 800 | 14.00 ± 4.93ns | 19.33 ± 4.41ns | 21.67 ± 3.38** | 25.33 ± 2.91* | 20.33 ± 9.33** | 26.67 ± 7.13** |
| 2 | EEZJ | 1200 | 6.33 ± 1.45* | 14.33 ± 2.33* | 20.67 ± 1.76** | 22.67 ± 3.48** | 23.00 ± 5.20* | 21.00 ± 6.81*** |
| 3 | EEZJ | 1600 | 10.33 ± 0.88ns | 20.00 ± 1.53ns | 25.33 ± 0.88* | 24.67 ± 6.57* | 26.33 ± 5.78* | 24.67 ± 1.20** |
| 4 | Diclofenac Sodium | 100 | 3.00 ± 0.58** | 8.00 ± 1.00** | 10.00 ± 1.53*** | 8.66 ± 3.28*** | 10.00 ± 4.50** | 13.33 ± 3.18*** |
| 5. | Control | 100 | 19.33 ± 3.53 | 31.00 ± 4.73 | 39.00 ± 4.36 | 46.00 ± 2.52 | 56.00 ± 4.51 | 62.33 ± 3.71 |
Means ± S.E.M; n = 5); ns P ≥ 0.05 compared to control (One-way ANOVA followed by Dunnett’s Test);
P ≤ 0.05 compared to control (One-way ANOVA followed by Dunnett’s Test);
P ≤ 0.01 compared to control (One-way ANOVA followed by Dunnett’s Test);
P ≤ 0.001 compared to control (One-way ANOVA followed by Dunnett’s Test).
Table 7.
Percentage of left paw oedema (%) calculated from the change in paw thickness after treatment
| Group | Treatment | Dose (mg/kg) | Percentage of left paw oedema (%) after treatment (Mean ±SEM) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | |||
| 1 | EEZJ | 800 | 12.67 ± 3.18** | 22.00 ± 7.55ns | 28.67 ± 6.33ns | 27.33 ± 5.55** | 30.33 ± 6.94** | 30.67 ± 4.41*** |
| 2 | EEZJ | 1200 | 7.00 ± 0.58*** | 15.00 ± 1.53* | 19.33 ± 0.88** | 19.00 ± 1.16*** | 22.67 ± 1.67*** | 19.67 ± 1.76*** |
| 3 | EEZJ | 1600 | 10.33 ± 0.33** | 19.67 ± 2.73ns | 25.00 ± 3.00* | 31.33 ± 1.20* | 28.00 ± 4.36** | 27.33 ± 3.53*** |
| 4 | Diclofenac Sodium | 100 | 3.00 ± 0.58*** | 6.00 ± 1.00** | 8.33 ± 0.67*** | 8.33 ± 1.33*** | 6.33 ± 1.33*** | 12.00 ± 7.69*** |
| 5. | Control | 100 | 22.33 ± 2.03 | 31.33 ± 1.67 | 40.33 ± 2.33 | 46.67 ± 2.67 | 53.33 ± 1.20 | 56.00 ± 2.08 |
Means ± S.E.M; n = 5); ns P = 0.05 compared to control (One-way ANOVA followed by Dunnett's Test);
P = 0.05 compared to control (One-way ANOVA followed by Dunnett's Test);
P = 0.01 compared to control (One-way ANOVA followed by Dunnett's Test);
P = 0.001 compared to control (One-way ANOVA followed by Dunnett's Test).
The HPLC chromatograms of the reference standards and EEZJ are shown in the Fig. 1A, 1B, 1C and 1D, respectively. UV absorption, retention time and identification results of peaks are presented in Table 8.
Figure 1.
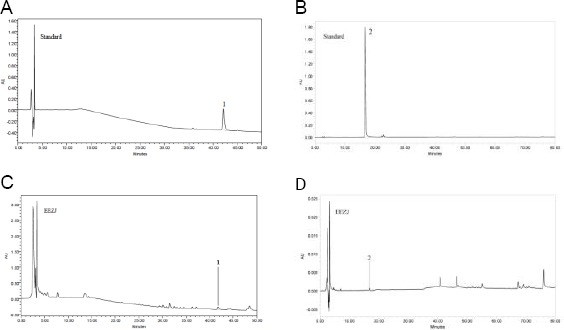
A) Chromatographic profile of standard; 1 = Betulinic acid; B) Chromatographic profile of standard; 2 = Quercetin; C) Chromatographic profile of ethanolic extract of Ziziphus jujube; 1 = Betulinic acid; D) Chromatographic profile of ethanolic extract of Ziziphus jujube; 2 = Quercetin
Table 8.
Method employed, retention time, and ultraviolet absorption of betulinic acid, quercetin and ethanolic extract of Ziziphus jujube
| Compound | Method | Constituent | Retention Time (min) | UV Absorption (nm) |
|---|---|---|---|---|
| Standard 1 | Zhang et al. 2008 | Betulinic Acid | 42.169 | 205 |
| EEZJ | Zhang et al. 2008 | Betulinic Acid | 42.169 | 205 |
| Standard 2 | Guo et al. 2011 | Quercetin | 16.821 | 205 |
| EEZJ | Guo et al. 2011 | Quercetin | 16.821 | 205 |
Peak 1 represented the present of betulinic acid at the retention time of 42.169 minutes from reference compound. EEZJ showed a peak at the same retention time. Hence, EEZJ consists of betulinic acid as the peak was comparable to reference compound (Fig. 1).
Peak 2 appeared at 16.821 minutes in the reference compound and represented as quercetin. EEZJ showed a peak at the same retention time which indicates the presence of quercetin in the extract tested (Fig. 1).
Discussion
People in different communities commonly use plant or plant derivatives to cure illnesses without scientific evidence. The current studies aimed to evaluate anti-diarrhoeal as well as anti-inflammatory activity potential of Z. jujuba fruit. Castor oil was given to mice to imitate the diarrhoea symptom while carrageenan was given to rats to induce inflammation at the paw of the rats.
Means ± S.E.M; n = 5); ns P ≥ 0.05 compared to control (One-way ANOVA followed by Dunnett’s Test); * P ≤ 0.05 compared to control (One-way ANOVA followed by Dunnett’s Test); ** P ≤ 0.01 compared to control (One-way ANOVA followed by Dunnett’s Test); *** P ≤ 0.001 compared to control (One-way ANOVA followed by Dunnett’s Test)
The minimum effective dose of EEZJ that determined in the preliminary test was brought into the experimental test. Moreover, two higher concentration of EEZJ (800 mg/kg and 1200 mg/kg) were added into the test to evaluate the effectiveness in gradual increasing dose.
Loperamide (loperamide hydrochloride) served as a positive control group in the experiment as it acts directly at the opioid receptors on the circular and longitudinal intestinal mucosa. Similar to other µ-receptor agonists, loperamide inhibits peristalsis of the intestine and prolongs the transit time of the digested content. Loperamide also stimulates the reabsorption process and alters the transport of water and electrolytes. Thus, it reduces the faecal volume and decreases the fluid and electrolyte loss. Loperamide not only used in acute diarrhoea patient but also successfully applied in chronic diarrhoea patient for several years without evidence of tolerance [42].
Based on the results obtained and presented in Table 3, mice treated with different concentrations of EEZJ produced a reduced number of watery dropping as well as a total number of faeces.
This result is similar to the studies done by Oh and Ryu [43], where they proposed that Korean oriental medicine which containing Z. jujuba fruit, can reduce the number of faeces of mice in five hours of observation. A similar observation was reported by Hu et al. [44] where a Chinese traditional medicine (Wei-Chang-An-Wan extract) containing Z. jujuba fruit was able to reduce the total number of faeces significantly and the number of watery dropping.
Stool weight depends on many factors such as water composition, number of bacteria and fibre content in the stool. In addition to this, total stool weight is highly correlated with the frequency of defaecation [45]. In our experiment, EEZJ did not only reduce the number of stool but also induced mice to produce a lesser weight of watery stool. Muller et al. [46] in their work concluded that the gastrointestinal transit time would affect the weight of stool where longer gastrointestinal transit time will produce lighter stool weight. Which was also tested by Rao and Lakshimi [44], where they also displayed the aqueous extract of Z. jujuba leaves to increase the gastrointestinal transit time. Hence, it may indicate that Z. jujuba fruit in our experiment may reduce stool weight by increasing the gastrointestinal transit time.
EEZJ did not only reduce stool weight and some faeces, but it also delayed the onset of first watery dropping compared to control group. Z. jujuba can reduce the intestinal motility, and this has been described by Rao and Lakshimi [47]. Hu et al. [44] demonstrated that Z. jujuba containing traditional medicine able to attenuate the gastrointestinal tract motility in a dose depending manner. Based on the same studies, Z. jujuba containing traditional medicine also decreased the spontaneous contraction of isolated jejunum in animals. In our study, the result showed that EEZJ could reduce the number of faeces as well as the weight of faeces in a dose depending manner. Moreover, it significantly attenuated the onset of first watery dropping from the mice. With these characteristics, EEZJ can be considered as possessing antidiarrhoeal activity against castor oil-induced diarrhoea.
Inflammation process causes an alteration in human body’s physiological responses and ultimately resulting in pain, heat, redness, swelling and loss of function. Carrageenan-induced paw oedema is an example of acute inflammation which results in swelling. It had been widely used as an experimental animal model to discover and evaluation of anti-inflammatory potential [48].
Carrageenan is a complex group of polysaccharides made up of repeating galactose-related monomers. The inflammatory response is usually quantified by an increase in paw size which is observed for around five hours after carrageenan injection [32]. Inhibition of carrageenan-induced inflammation has been shown to be highly predictive of anti-inflammatory activity in human inflammatory disease and doses of nonsteroidal anti-inflammatory drugs (NSAIDs) in this model correlate well with effective dose in patients [49]. Using antagonists of various mediators of inflammation showed that the inflammatory response to carrageenan consisted of three phases [50]. The primary phase is mediated by both histamine and 5-hydroxytryptamine. The second phase starts after one hour and persists for six hours after carrageenan injection. This phase is kinin-mediated.
The final phase is attributed to the local production of prostaglandins, which is derived from arachidonic acid by the action of cyclooxygenase (COX) enzymes. Inhibition of these enzymes is the basis of action of the NSAIDs of major clinical importance in the treatment of pain and inflammation [51]. Cyclooxygenase 2 (COX-2) is a pro-inflammatory enzyme which is in charge of making prostaglandins (PG), and it is also the site of action targeted by non-steroidal anti-inflammatory drugs such as diclofenac and COX-2 inhibitors. However, COX-2 is normally produced within the second hour after carrageenan is induced to cause inflammation. This means PG is not involved in oedema formation in the very first hour but instead contribute to oedema within second and the subsequent hours.
According to Di Lorenzo et al. [52], carrageenan injection evokes neutrophil infiltration immediately after injection and persists for six hours. O2-, OH and H2O2 which are derived from neutrophils, are suggested to cause a sustained increase in permeability through damaging the endothelial cells [53]. Thus, carrageenan-induced paw oedema has been an important tool in the development of NSAIDs. A role for neutrophil-derived reactive oxygen species, nitric oxide, and peroxynitrite in carrageenan-induced inflammation has also been identified, and some specific inhibitors have been identified which have potential clinical use.
Diclofenac sodium is superior to the other clinically established non-steroidal anti-inflammatory agents in carrageenan-induced paw oedema [54]. It exerts their effect by blocking prostaglandin synthetase system. In the present study, diclofenac sodium significantly expressed its anti-inflammatory activity in Table 4-7. The result of the present study showed and solidified that diclofenac sodium has significant anti-inflammatory activity.
According to the anti-inflammatory experiments that were carried out, Z. jujuba ethanolic extract successfully controlled the inflammation of the paw. At 1200 mg/kg dose of Z. jujuba ethanolic extract displayed the highest paw oedema inhibition. The mechanisms of action of Z. jujuba ethanolic extract may be similar to that diclofenac sodium whereby the anti-inflammatory effect observed might be due to the inhibition of expression and activity of COX-2.
Based on the chemical analysis done, it was found that Z. jujuba fruit contains both betulinic acid and quercetin in it. Earlier studies showed that most of the medical plants could counter dysentery and diarrhoeal incident. Most of the medical plants contain alkaloids, saponins, flavonoids, sterols and triterpenoid [55] and they are responsible for the mechanism of antidiarrhoeal activity. Betulinic acid belongs to the pentacyclic triterpenoid group [56], whereas quercetin belongs to flavonoids group [13]. From the earlier statement, we understand that both triterpenoid and flavonoids capable of possessing antidiarrhoeal activity. This is further support by Ezekwesili et al. [57] studies, where he described that quercetin relaxes the intestinal smooth muscle and inhibits bowel contraction which probably due to inhibition of intracellular release of calcium ions from the sarcoplasmic reticulum. Other than that, Begum et al. [58] claimed that triterpenoid also had been shown to possess antispasmodic activity in a dose depending manner.
From previous studies, betulinic acid is considered to have a potent anti-inflammatory activity where betulinic acid can reduce the production of TNF-α as well as nitric oxide in mice. Additionally, betulinic acid also found to promote the concentration of IL-10 upon LPS stimulation [59][60], and quercetin was able to regulate down the inflammatory response of in vitro bone marrow-derived macrophages. They further proof that quercetin to inhibit the release of cytokine and induce the nitric oxide synthase via inhibition of NF-κB pathway without modification of c-Jun N-terminal kinase activity.
Besides that, different kinds of methods were tried during the chemical analysis for better resolution and separation of target peak. All of it was run at the maximum wavelength of 205 nm as the study compound from Z. jujuba has wavelength maximum at 205 nm. According to Guo et al. [33], suggested acetonitrile to be used as a mobile phase because acetonitrile has low wavelength maximum compared to methanol where acetonitrile avoids blank interference during the analysis. Therefore, low peaks of EEZJ can be observed due to less blank interference. Guo et al. group reported the mobile phase with a buffered acid to produce a better separation of peaks. This is particularly in case of betulinic acid analysis as buffer acid improved the peak shape and eliminated the tailing of the target peak [61].
Acknowledgements
We would like to acknowledge financial support for this work from the Deanship of Scientific Research (DSR), University of Tabuk, Tabuk, Saudi Arabia (KSA), (under grant no: S-1437-0263). We would like to extend our appreciation and special thanks to the Faculty of Pharmacy, University Kebangsaan, Malaysia for their contribution in funding this work through student Projects programme.
Footnotes
Funding: This research was financially supported by the Deanship of Scientific Research (DSR), University of Tabuk, Tabuk, Saudi Arabia (KSA), (under grant no: S-1437-0263)
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Li J, Ding S, Ding X. Optimization of the ultrasonically assisted extraction of polysaccharides from Zizyphus jujuba cv. insixiaozao. J Food Eng. 2007;80:176–83. https://doi.org/10.1016/j.jfoodeng.2006.05.006. [Google Scholar]
- 2.Chen J, Liu X, Li Z, Qi A, Yao P, Zhou Z, Dong TTX, Tsim KWK. A Review of Dietary Ziziphus jujuba Fruit (Jujube):Developing Health Food Supplements for Brain Protection. Evid Based Complement Alternat Med. 2017;2017:3019568. doi: 10.1155/2017/3019568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plastina P, Bonofiglio D, Vizza D, Fazio A, Rovito D, Giordano C, et al. Identification of bioactive constituents of Ziziphus jujube fruit extracts exerting antiproliferative and apoptotic effects in human breast cancer cells. J Ethnopharmacol. 2012;140:325–32. doi: 10.1016/j.jep.2012.01.022. https://doi.org/10.1016/j.jep.2012.01.022 PMid:22301448. [DOI] [PubMed] [Google Scholar]
- 4.Choi SH, Ahn JB, Kim HJ, Im N. K, Kozukue N, Levin CE, Friedman M. Changes in free amino acid, protein, and flavonoid content in jujube (Ziziphus jujube) fruit during eight stages of growth and antioxidative and cancer cell inhibitory effects by extracts. J Agric Food Chem. 2012;60(41):10245–55. doi: 10.1021/jf302848u. https://doi.org/10.1021/jf302848u PMid:23046062. [DOI] [PubMed] [Google Scholar]
- 5.Gusakova SD, Sagdullaev SS, Aripov KN, Basher KH, Kurkcuoglu M, Demirci B. Isomers of plamitoleic acid in lipids and volatile substances from the fruits of Ziziphus jujuba. Chem Nat Compd. 1999;35:401–3. https://doi.org/10.1007/BF02282503. [Google Scholar]
- 6.Li J, Fan L, Ding S, Ding X. Nutritional composition of five cultivars of Chinese jujube. Food Chem. 2007;103:454–60. https://doi.org/10.1016/j.foodchem.2006.08.016. [Google Scholar]
- 7.Barrett DM, Lloyd B. Advanced preservation methods and nutrient retention in fruits and vegetables. J Sci Food Agric. 2012;92:7–22. doi: 10.1002/jsfa.4718. https://doi.org/10.1002/jsfa.4718 PMid:22102258. [DOI] [PubMed] [Google Scholar]
- 8.Gao Q.H, Wu CS, Wang M. The jujube (Ziziphus jujuba Mill.) fruit:a review of current knowledge of fruit composition and health benefits. J Agric Food Chem. 2013;61(14):3351–63. doi: 10.1021/jf4007032. https://doi.org/10.1021/jf4007032 PMid:23480594. [DOI] [PubMed] [Google Scholar]
- 9.Carlsen MH, Halvorsen BL, Holte K, Bøhn SK, Dragland S, Sampson L, et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. 2010;9:3. doi: 10.1186/1475-2891-9-3. https://doi.org/10.1186/1475-2891-9-3 PMid:20096093 PMCid:PMC2841576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R. Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanita. 2007;43:348–61. PMid:18209268. [PubMed] [Google Scholar]
- 11.Chang SC, Hsu BY, Chen BH. Structural characterization of polysaccharides from Zizyphus jujuba and evaluation of antioxidant activity. Int J Biol Macromol. 2010;1-47(4):445–53. doi: 10.1016/j.ijbiomac.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Choi S-H, Ahn J-B, Kozukue N, Levin CE, Friedman M. Distribution of free amino acids, flavonoids, total phenolics, and antioxidative activities of jujube (Ziziphus jujuba) fruits and seeds harvested from plants grown in Korea. J Agric Food Chem. 2011;22(59(12)):6594–604. doi: 10.1021/jf200371r. [DOI] [PubMed] [Google Scholar]
- 13.Pawlowska AM, Camangi F, Bader A, Braca A. Flavonoids of zizyphus jujuba l. And zizyphus spina-christi (L.) Wild (rhamnaceae) fruits. Food Chemistry. 2009;112:858–862. https://doi.org/10.1016/j.foodchem.2008.06.053. [Google Scholar]
- 14.Ghaly IS, Said A, Abdel-Wahhab MA. Zizyphus jujuba and Origanum majorana extracts protect against hydroquinone-induced clastogenicity. Environ Toxicol Pharmacol. 2008;25:10–9. doi: 10.1016/j.etap.2007.07.002. https://doi.org/10.1016/j.etap.2007.07.002 PMid:21783830. [DOI] [PubMed] [Google Scholar]
- 15.Mehreen A, Waheed M, Liaqat I, Arshad N. Phytochemical, Antimicrobial, and Toxicological Evaluation of Traditional Herbs Used to Treat Sore Throat. Biomed Res Int. 2016;2016:8503426. doi: 10.1155/2016/8503426. https://doi.org/10.1155/2016/8503426 PMid:27429983 PMCid:PMC4939213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin YC, Hsieh MT, Chen CF, Cheng HY, Peng WH. Anxiolytic effect of Ting-Chih-Wan in mouse behavior models of anxiety. Am J Chin Med. 2003;31:27–30. doi: 10.1142/S0192415X03000709. https://doi.org/10.1142/S0192415X03000709 PMid:12723754. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Min B, Lee C, Kim K, Kho Y. Cytotoxic triterpenoids from the fruits of Zizyphus jujuba. Planta Med. 2003;69:18–21. doi: 10.1055/s-2003-45155. [DOI] [PubMed] [Google Scholar]
- 18.Kim DSHL, Pezzuto JM, Pisha E. Synthesis of betulinic acid derivatives with activity against human melanoma. Bioorg Med Chem Lett. 1998;8:1707–1712. doi: 10.1016/s0960-894x(98)00295-9. https://doi.org/10.1016/S0960-894X(98)00295-9. [DOI] [PubMed] [Google Scholar]
- 19.Sarfaraz A, Ansari SH, Porchezhian E. Antifungal activity of alcoholic extracts of Ziziphus vulgaris and Acacia concinna. Hamdard Medicus. 2002;14-15:42–45. [Google Scholar]
- 20.Elmahi M, Essassi EM, Hamamouchi M, Hamamouchi J. Study on the antimicrobial and antibilharzia activity of Ziziphus vulgaris. Fitoterapia. 1997;68:34–36. [Google Scholar]
- 21.Huang LYW, Cai B, Li D, Liu J, Liu M. A preliminary study on the pharmacology of the compound prescription huangqin tang and its component drugs. Zhongguo Zhong Yao Za Zhi. 1990;5:115–128. [PubMed] [Google Scholar]
- 22.Shiv K, Ganachari MS, Banappa Nagoor VS. Antiinflammatory activity of Ziziphus jujuba Lam. leaves extract in rats. Journal of Natural Remedies. 2004;4:183–185. [Google Scholar]
- 23.Su BNCM, Farnsworth NR, Fong HH, Pezzuto JM, Kinghorn AD. Activity guided fractionation of the seeds of Ziziphus jujuba using a cyclooxygenase2 inhibitory assay. Planta Med. 2002;68:1125–1128. doi: 10.1055/s-2002-36354. https://doi.org/10.1055/s-2002-36354 PMid:12494342. [DOI] [PubMed] [Google Scholar]
- 24.Naik SR, Bhagat S, Shah PD, Tare AA, Ingawale D, Wadekar RR. Evaluation Of Anti-Allergic And Anti-Anaphylactic Activity Of Ethanolic Extract Of Zizyphus Jujuba Fruits In Rodents. Revista Brasileira de Farmacognosia (Brazillian Journal of Pharmacognosy) 2013;23:811–818. https://doi.org/10.1590/S0102-695X2013000500014. [Google Scholar]
- 25.Heo HJ, Park YJ, Suh YM, Choi SJ, Kim MJ, Cho HY, Chang YJ, Hong B, Kim HK, Kim E, Kim CJ, Kim BG, Shin DH. Effects of oleamide on choline acetyltransferase and cognitive activities. Biosci Biotechnol Biochem. 2003;67(6):23–27. doi: 10.1271/bbb.67.1284. https://doi.org/10.1271/bbb.67.1284 PMid:12843655. [DOI] [PubMed] [Google Scholar]
- 26.Zare-Zardini H, Tolueinia Hashemi B, Ebrahimi A, Fesahat L. F. Antioxidant and cholinesterase inhibitory activity of a new peptide from Ziziphus jujuba fruits. Am J Alzheimers Dis Other Demen. 2013;28(7):702–9. doi: 10.1177/1533317513500839. https://doi.org/10.1177/1533317513500839 PMid:24005854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HY, Han SW. Ziziphus jujuba and Codonopsis pilosula stimulate nitric oxide release in cultured endothelial cells and kidney tissues. Asia Pacific Journal of Pharmacology. 1996;11:26–29. [Google Scholar]
- 28.Na M, An R, Lee S, Hong N, Yoo J, Lee C, Kim J, Bae K. Screening of crude drugs for antioxidative activity. Korean Journal of Pharmacognosy. 2001;32:108–115. [Google Scholar]
- 29.Seong HK, Seong WC, Sang KY, Angho SY, Hyun SK, Myung HC. Comparison of antioxidant activities of seventy herbs that have been used in Korean traditional medicine 2008. Nutr Res Pract. 2008;2(3):143–151. doi: 10.4162/nrp.2008.2.3.143. https://doi.org/10.4162/nrp.2008.2.3.143 PMid:20126599 PMCid:PMC2814189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ansari SH, Bhatt D, Masihuddin M, Khan MU. Herbal Drugs. New Delhi: Jay Pee Publication; 2006. The wound healing and herbal drugs; pp. 460–468. [Google Scholar]
- 31.Chopda MZ, Mahajan RT. The wound healing plants of Jalgaon District, Maharashtra State, India. Ethanobotanical leaflets. 2009;13:1–32. [Google Scholar]
- 32.Morris CJ. Carrageenan-induced paw edema in the rat and mouse. Dlm. Winyard PG & Willoughby DA. Inflammation Protocols, hlm. 115-121. United States of America:Human Press. 2003. https://doi.org/10.1385/1-59259-374-7:115 . [DOI] [PubMed]
- 33.Guo S, Duan J, Tang Y, Su S, Shang E, Ni S, Qian D. High-Performance Liquid Chromatography –Two Wavelength Detection Of Triterpenoid Acids From The Fruits Of Ziziphus Jujuba Containing Various Cultivars In Different Regions And Classification Using Chemometric Analysis. J Pharm Biomed Anal. 2009;49:1296–1302. doi: 10.1016/j.jpba.2009.03.006. https://doi.org/10.1016/j.jpba.2009.03.006 PMid:19359121. [DOI] [PubMed] [Google Scholar]
- 34.Sahoo HB, Sagar R, Kumar A, Bhaiji A, Bhattamishra SK. Antidiarrhoeal investigation of Apium leptophyllum (Pers.) by modulation of Na+K+ATPase, nitrous oxide and intestinal transit in rats. Biomed J. 2016;39(6):376–381. doi: 10.1016/j.bj.2016.11.003. https://doi.org/10.1016/j.bj.2016.11.003 PMid:28043416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu M, Zhang N, Zhang L, Xiong M, Yang Y, Gu X, Shen X, Ding H. Response of a clinical Escherichia coli strain to repeated cefquinome exposure in a piglet tissue-cage model. BMC Vet Res. 2015;11:169. doi: 10.1186/s12917-015-0486-6. https://doi.org/10.1186/s12917-015-0486-6 PMid:26209108 PMCid:PMC4514946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aylate A, Agize M, Ekero D, Kiros A, Ayledo G, Gendiche K. In-Vitro and In-Vivo Antibacterial Activities of Croton macrostachyus Methanol Extract against E. coli and S. aureus. Adv Anim Vet Sci. 2017;5(3):107–114. [Google Scholar]
- 37.Lumpu SN, Lutete GT, Tona G, Kabangu OK, Kanyanga RC, Apers S, Pieters L, Vlietinck AJ. Assessment Of The Antidiarrhoeal Properties Of The Aqueous Extract, The 80% Methanol Extract And Its Soluble Fractions Of The Leaves Of Alstonia Congensis Engl. (Apocynaceae) In Wistar Rats. J Ethnopharmacol. 2012;142:620–626. doi: 10.1016/j.jep.2012.04.038. https://doi.org/10.1016/j.jep.2012.04.038 PMid:22609154. [DOI] [PubMed] [Google Scholar]
- 38.Winter CA, Risley EA, Nuss CW. Carrageenan-induced edema in hind paw of the rats as an assay for anti-inflammatory drugs. Proceedings of the Society of Experimental Biology and Medicine. 1962;111:544–547. doi: 10.3181/00379727-111-27849. https://doi.org/10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 39.Yam MF, Lim V, Ibrahim MS, Omar ZA, Lee FA, Noersal R, Muthana FA, Amirin S, Mohd Z.A. HPLC and anti-inflammatory studies of the flavonoid rich chloroform extract fraction of Orthosiphon Stamineus leaves. Molecules. 2010;15:4452–4466. doi: 10.3390/molecules15064452. https://doi.org/10.3390/molecules15064452 PMid:20657453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang M, Zhang Y, Xie J. Simultaneous Determination Of Jujuboside A, B And Betulinic Acid In Semen Ziziphi Spinosae By High Performance Liquid Chromatography-Evaporative Light Scattering Detection. J Pharm Biomed Anal. 2008;48:1467–1470. doi: 10.1016/j.jpba.2008.09.022. https://doi.org/10.1016/j.jpba.2008.09.022 PMid:18977107. [DOI] [PubMed] [Google Scholar]
- 41.Guo S, Duan J, Tang Y, Qian Y, Zhao J, Qian D, Su S, Shang E. Simultaneous Qualitative And Quantitative Analysis Of Triterpenic Acids, Saponins And flavonoids In The Leaves Of Two Ziziphus Species By HPLC–PDA–MS/ELSD. J Pharm Biomed Anal. 2011;56:264–270. doi: 10.1016/j.jpba.2011.05.025. https://doi.org/10.1016/j.jpba.2011.05.025 PMid:21665400. [DOI] [PubMed] [Google Scholar]
- 42.Regnard C, Twycross R, Mihalyo M, Wilcock A. Loperamide. J Pain Symptom Manage. 2011;42(2):319–32. doi: 10.1016/j.jpainsymman.2011.06.001. https://doi.org/10.1016/j.jpainsymman.2011.06.001 PMid:21703817. [DOI] [PubMed] [Google Scholar]
- 43.Oh S, Ryu B. Experimental Studies On The Antidiarrheal Effects Of Anjang-San. The Journal of Korean Oriental Medicine. 2011;32(6):54–66. [Google Scholar]
- 44.Rao GHJ, Lakshimi P. Anti Diarrhoeal Activity of Ziziphus jujuba Leaf Extract in Rats. International Journal of Pharma and Bio Sciences. 2012;13(1):532–538. [Google Scholar]
- 45.Seyed MKH, Seyed DH. Determination Of The Mean Daily Stool Weight, Frequency Of Defecation And Bowel Transit Time :Assessment Of 1000 Healthy Subjects in central Iran. Archieves of Iranian Medicine. 2000;3(4) [Google Scholar]
- 46.Muller-Lissner S.A. Effect If Wheat Bran On Weight Of Stool And Gastrointestinal Transit Time A Meta-Analysis. Br Med J (Clin Res Ed) 1988;296:615–617. doi: 10.1136/bmj.296.6622.615. https://doi.org/10.1136/bmj.296.6622.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu J, Gao WY, Ling NS, Liu CX. Antidiarrhoeal And Intestinal Modulatory Activities Of Wei-Chang-An-Wan Extract. J Ethnopharmacol. 2009;125:450–455. doi: 10.1016/j.jep.2009.07.027. https://doi.org/10.1016/j.jep.2009.07.027 PMid:19646520. [DOI] [PubMed] [Google Scholar]
- 48.Ouedraogo N, Tibiri A, Sawadogo R.W, Lompo M, Hay A.E, Koudou J, Dijoux Mg, Guissou I.P. Antioxidant antiinflammatory and analgesic activities of aqueous extract from stem bark of Pterocarpus erinaceus Poir. Fabaceae J Med Plant Res. 2011;5(10):2047–2053. [Google Scholar]
- 49.Otterness IG, Wiseman E.H, Gans D. A comparison of the carrageenan edema test and the ultraviolet light-induced erythema test as predictors of the clinical dose in rheumatoid arthritis. Agents Actions. 1979;9:177–183. doi: 10.1007/BF02024731. https://doi.org/10.1007/BF02024731 PMid:474303. [DOI] [PubMed] [Google Scholar]
- 50.Di Rosa M, Giroud JP, Willoughby DA. Studies of the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971;104:15–29. doi: 10.1002/path.1711040103. https://doi.org/10.1002/path.1711040103 PMid:4398139. [DOI] [PubMed] [Google Scholar]
- 51.Vane JR, Botting RM. New insights into the mode of action of anti-inflammatory drugs. Inflamm Res. 1995;44:1–10. doi: 10.1007/BF01630479. https://doi.org/10.1007/BF01630479 PMid:7664022. [DOI] [PubMed] [Google Scholar]
- 52.Di Lorenzo A, Fernandez-Hernando C, Clrino G, Sessa WC. Akt1 is critical for acute inflammation nd histamine-mediated vascular leakage. Proc Natl Acad Sci U S A. 2009;106(34):14552–14557. doi: 10.1073/pnas.0904073106. https://doi.org/10.1073/pnas.0904073106 PMid:19622728 PMCid:PMC2732859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chandrasoma P, Taylor CR. The acute inflammatory response. Concise Pathology. 3e. New York, NY, New York: McGraw-Hill Publishing Co; 1997. PMCid:PMC1191073. [Google Scholar]
- 54.Menasse R, Hedwall PR, Kraetz J, Pericin C, Riesterer L, Sallmann A, Ziel R, Jaques R. Pharmacological properties of diclofenac sodium and its metabolites. Scand J Rheumatology. 1978;22:5–16. doi: 10.3109/03009747809097211. https://doi.org/10.3109/03009747809097211. [DOI] [PubMed] [Google Scholar]
- 55.Otshudi AL, Vercruysse A, Foriers A. Contribution To The Ethnobotanical, Phytochemical And Pharmacological Studies Of Traditionally Used Medicinal Plants In The Treatment Of Dysentery And Diarrhoea In Lomela Area, Democratic Republic Of Congo (DRC) J Ethnopharmacol. 2000;71:411–423. doi: 10.1016/s0378-8741(00)00167-7. https://doi.org/10.1016/S0378-8741(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 56.Park SY, Kim HJ, Kim K.R, Lee SK, Lee CK, Park KK, Chung WY. Betulinic Acid, A Bioactive Pentacyclic Triterpenoid, Inhibits Skeletal-Related Events Induced By Breast Cancer Bone Metastases And Treatment. Toxicol Appl Pharmacol. 2014;275(2):152–62. doi: 10.1016/j.taap.2014.01.009. https://doi.org/10.1016/j.taap.2014.01.009 PMid:24463094. [DOI] [PubMed] [Google Scholar]
- 57.Ezekwesili JO, Nkemdilim UU, Okeke CU. Mechanism Of Antidiarrhoeal Effect Of Ethanolic Extract Of Psidium Guajava Leaves. Biokemistri. 2010;22(2):85–90. [Google Scholar]
- 58.Begum S, Hassan S.I, Siddiqui B.S, Shasheen F, Ghayur M.N, Gilani A.H. Triterpenoids From The Leaves of Psidium Guajava. Phytochemistry. 2002;61:399–403. doi: 10.1016/s0031-9422(02)00190-5. https://doi.org/10.1016/S0031-9422(02)00190-5. [DOI] [PubMed] [Google Scholar]
- 59.Jose Fernando OC, Jose Maria B-F, Gabriela Lemos A. M, Elisalva Teixera G, Cassio Santana M, Ricardo-dos-Santos Lain Carlos PC, Mileno Botelho PS. Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. Int Immunopharmacol. 2014;23:469–474. doi: 10.1016/j.intimp.2014.09.021. https://doi.org/10.1016/j.intimp.2014.09.021 PMid:25281393. [DOI] [PubMed] [Google Scholar]
- 60.Monica C, Desirre C, Saleta S, Isabel B, Jordi X, Julio G, Antonio Z. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-κB pathway. Int Immunopharmacol. 2005;35:584–592. doi: 10.1002/eji.200425778. [DOI] [PubMed] [Google Scholar]
- 61.Guo S, Duan J, Tang Y, Qian Y, Zhao J, Qian D, Su S, Shang E. Characterization Of Triterpenic Acids In Fruits Of Ziziphus Species By HPLC-ELSD-MS. J Agric Food Chem. 2010;58:6285–6289. doi: 10.1021/jf101022p. https://doi.org/10.1021/jf101022p PMid:20426471. [DOI] [PubMed] [Google Scholar]


