Key Clinical Message
Spinal lymphoma is a rare manifestation of a common canine hematopoietic neoplasia. Description of treatment, outcome, and MRI features are scarce. The latter can be heterogeneous, stressing the importance of lesion excision and analysis. Clinicians should also be aware of hypereosinophilia as accompanying paraneoplastic syndrome and its potential prognostic implications.
Keywords: Canine, hematopoietic neoplasm, hypereosinophilic syndrome, vertebral column
Case History
A 3‐year‐old female neutered Weimaraner was presented as an emergency because of a two‐day history of neck pain rapidly progressing to nonambulatory tetraparesis. An existing eosinophilia had been noted 2 days prior to referral (4.77 × 109/L; reference range, 0–1.25 × 109/L). Symptomatic treatment, with nonsteroidal anti‐inflammatories, resulted in little improvement.
Physical examination was within normal limits. On neurological examination, the dog was nonambulatory tetraparetic, with very mild movement in both pelvic limbs, but no movement in either thoracic limb. The segmental spinal reflexes were normal in the pelvic limbs; however, the flexor withdrawal reflexes were difficult to elicit in the thoracic limbs due to increased muscle tone. Moderate hyperesthesia was noted upon palpation and ventroflexion of the cervical spine. No other neurological abnormalities were detected. Neurological examination localized a lesion to the C1–C5 spinal cord segments; however, a more caudal (C6–C8) cervical segment localization, impairing the function of the musculocutaneous nerve and increasing the extensor tone due to upper motor neuron inhibition release of the radial nerve, could not be excluded. Differential diagnoses included intervertebral disk herniation, inflammation (immune‐mediated or infectious), and neoplasia.
The dog was anesthetized for MRI evaluation of the cervical vertebral column. A high field (1.5T) magnet (Magnetom Essenza, Siemens, Camberley, UK) was used to obtain T2‐weighted sequences in sagittal and transverse planes, T1‐weighted sequences in sagittal and transverse planes before and after intravenous administration of gadopentetate dimeglumine (Magnevist, Bayer Schering Pharma AG, Berlin, Germany) (0.1 mmol/kg), T2* images in transverse plane, short tau inversion‐recovery (STIR) sequences in sagittal plane, and 3D constructive interference steady state (CISS) sequences in dorsal plane. Magnetic resonance imaging demonstrated a dorsal, extradural T2‐weighted, T2*, STIR, and CISS homogeneously hyperintense mass at the level of C3 vertebra (Fig. 1A). The lesion seemed to extend dorsally to the left multifidus cervicis muscle through C3‐4 interarcuate space, showing identical MRI signal characteristics (Fig. 1A–C). On T1‐weighted precontrast images, the extradural and paraspinal lesion was slightly hyperintense to the spinal cord and epaxial musculature, and strong homogenous contrast enhancement was observed on postcontrast sequences (Fig. 1B and C). Severe spinal cord compression and T2‐weighted and STIR intramedullary hyperintensity, most likely consistent with edema, gliosis, and/or Wallerian degeneration, were also noted.
Figure 1.
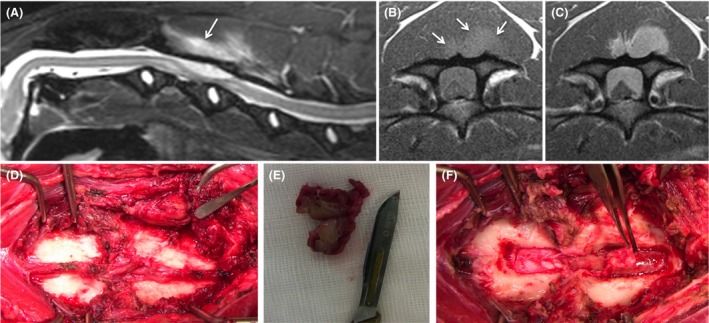
(A) Midsagittal STIR image of the cervical spine demonstrating a hyperintense well‐defined, dorsal extradural mass lesion at the level of C3, extending slightly caudal to C3‐4, and severely compressing the spinal cord which shows intramedullary hyperintensity consistent with edema, gliosis, and/or Wallerian degeneration. Note also a lesion of identical signal characteristics dorsal to C3 lamina (arrow). (B) Precontrast transverse T1‐weighted image at the level of caudal C3 showing the extradural and paraspinal mass (arrows) are slightly hyperintense to the spinal cord and epaxial musculature. (C) On the postcontrast transverse T1‐weighted image at the same level of (B), strong and homogenous contrast enhancement is observed in the mass tissue at both levels. (D) Intraoperative image of the exposed dorsal lamina of C3 (right) and C4 (left). The dorsal spinous processes have been removed. Note the well‐demarcated mass in the ventral belly of the left multifidus cervicis muscle (pointed by periosteal elevator). (E) Resected paraspinal mass incised to show its pale green discoloration. (F) C3–C4 dorsal laminae have been removed and elevation of a fibrous tissue layer connected to the paraspinal mass through the interarcuate space reveals a pale green gelatinous extradural mass extending cranially over the length of C3.
Cerebrospinal fluid (CSF) was collected from the cerebellomedullary cistern and L5‐6 under general anesthesia. Analysis of the cisternal sample revealed mild pleocytosis (17 nucleated cells/μL; reference range, <5 nucleated cells/μL) with normal protein concentration. Results of cytological examination indicated 57% of cells were neutrophils and 38% eosinophils (reference range for neutrophils and eosinophils in CSF, <2%) 1. Analysis of the lumbar sample demonstrated moderate eosinophilic pleocytosis (80 nucleated cells/μL, 68% eosinophils) and increased protein concentration (60 mg/dL; reference range, <45 mg/dL). Based on the MRI and CSF findings, inflammatory infectious or neoplastic disease was suspected.
The patient recovered well from the anesthetic and in view of normal arterial blood gas analysis, decompressive surgery was planned for the following day. Symptomatic treatment with antibiotics (enrofloxacin [Baytril 25 mg/mL, Bayer, Reading, Berkshire, UK] 4.5 mg/kg IV q 12 h and clindamycin [Antirobe capsules, 25 mg, 300 mg, Zoetis, Surrey, UK] 10.5 mg/kg PO q 12 h), analgesia (buprenorphine [Vetergesic multidose, 0.3 mg/mL, Ceva Animal Health Ltd, Bucks, UK] 0.015 mg/kg IV q 6 h and gabapentin [Gabapentin 300 mg capsules, ALP Swift Services Ltd, Malta] 9 mg/kg PO q 8 h), and anti‐inflammatories (dexamethasone [Dexafort suspension, 2 mg/mL, MSD Animal Health, Buckinghamshire, UK] 0.15 mg/kg IV q 24 h) was initiated. However, clinical signs progressed to tetraplegia and severe hypoventilation (PaCO2 57 mmHg; reference range, 32–49 mmHg), prompting intermittent positive pressure ventilation. Emergency dorsal laminectomy at C3–C4 was performed for spinal cord decompression and biopsy sampling. A firm, well‐demarcated, pale green colored mass was noted on the ventral belly of the left multifidus cervicis muscle (Fig. 1D and E). This mass was connected to the joint capsule/ligamentum flavum at C3–C4 interarcuate space. Removal of the dorsal laminae of C3–C4 revealed continuity with a fibrous tissue layer overlying an extradural mass of pale green gelatinous content (Fig. 1F). Debulking was continued and the dura and spinal cord appeared normal following decompression. The dog ventilated normally following recovery from anesthesia. Samples of the muscle and extradural mass were fixed in neutral‐buffered 10% formalin and submitted for histopathological analysis. Negative serological test results for Toxoplasma gondii and Neospora caninum were obtained at this stage.
Histopathological analysis of the paraspinal and extradural mass tissue found a poorly differentiated neoplastic large round cell population (nuclei greater than two red blood cell diameter), admixed with a high number of eosinophils (Fig. 2A). Immunohistochemistry showed the vast majority of neoplastic cells exhibited a moderate to strong membranous and intracytoplasmic staining for CD3 (Fig. 2B), consistent with a lymphosarcoma of T‐cell origin, likely a peripheral T‐cell lymphoma not otherwise specified (PTCL‐NOS) based on the World Health Organisation classification system 2. Further diagnostics were carried out, including abdominal ultrasound and thoracic computed tomography. Computed tomography of the thorax showed a bilobed soft tissue attenuating mass in the left lung lobe. Cytology was suspicious for lymphoid neoplasia, with a high number of lymphoblasts. On the abdominal ultrasound scan there was no marked lymphadenopathy; however, the splenic architecture was found to be heterogeneous. Hepatic and splenic fine needle aspirates were obtained, and cytological analysis showed no aberrant cells, although a high proportion of eosinophils were noted, likely a reflection of the high circulating population. As a result of the imaging and cytology findings, a diagnosis of Stage Vb T‐cell lymphoma, affecting the cervical spine and lungs, with circulating eosinophilia (Fig. 2C) and eosinophilic infiltrate of liver and spleen, was made. Based on the histopathological diagnosis, chemotherapy was initiated eight‐day postsurgery, with an intravenous bolus of vincristine (Vincristine Sulfate 1 mg/mL, Hospira UK Limited, Hurley, UK) (0.5 mg/m2), as a single dose, and oral prednisolone (Prednidale 5 mg tablets, Dechra, Staffordshire, UK) (40 mg/m2 q 24 h). Hematology samples taken prior to chemotherapy documented the presence of hypereosinophilia (Table 1). The dog's neurological status gradually improved, she was comfortable and marginally ambulatory at discharge 10 days post‐admission.
Figure 2.
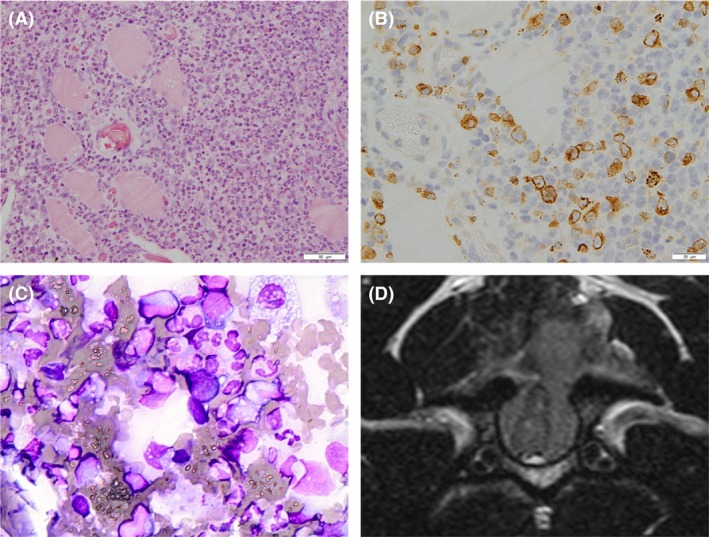
(A) Histopathological examination of the paraspinal mass tissue. Moderate numbers of neoplastic large lymphocytes admixed with high numbers of eosinophils are present, infiltrating between striated myocytes which often show degenerative changes (clear cytoplasmic vacuoles and loss of cross striations). Hematoxylin and eosin stain; scale bar: 50 μm. (B) Immunohistochemical examination. There is intense membranous and cytoplasmic immunolabeling of neoplastic T lymphocytes for CD3. Neoplastic lymphocytes have large nuclei (two red cells in diameter) with pale vesicular chromatin, sometimes with several nucleoli and scant to moderate amounts of pale amphophilic cytoplasm. There is moderate anisocytosis and anisokaryosis. Scale bar: 20 μm. (C) Peripheral blood smear cytology showing leucocytosis with predominant eosinophilia. Cells exhibit multilobed nuclei with an abundance of eosinophilic granules within the cytoplasm. Giemsa stain, original magnification X40. (D) Follow‐up transverse T2‐weighted MR image confirming a partially circumferential regrowth of the surgically resected mass at the level of C3, severely compressing the spinal cord, and extending dorsally to the paraspinal compartment through the laminectomy defect. As at initial diagnosis, the mass lesion was hyperintense to the spinal cord and epaxial musculature. Note also the multiple hyperintense intramedullary foci within the distorted spinal cord consistent with areas of Wallerian degeneration as confirmed on histological examination.
Table 1.
Hematology parameters during the course of disease and treatment
| Presurgery | Chemotherapy 1 | Chemotherapy 2 | Recurrence of clinical signs | Reference range | |
|---|---|---|---|---|---|
| RBC(×1012/L) | 5.24 | 5.66 | 6.13 | 5.36 | 5.5–8.5 |
| WBC(×109/L) | 10.84 | 12.89 | 17.87 | 22.82 | 6.0–12.0 |
| HCT (%) | 35.6 | 37.3 | 41.2 | 33.7 | 37–55 |
| Neutrophils (×109/L) | 4.661 | 4.769 | 7.863 | 8 | 3.0–11.8 |
| Lymphocytes (×109/L) | 0.867 | 2.062 | 1.251 | 1.37 | 1.0–4.8 |
| Monocytes (×109/L) | 0.434 | 0.387 | 0.179 | 0.25 | 0.15–1.35 |
| Eosinophils (×109/L) | 4.77 | 5.672 | 8.22 | 13.06 | 0.1–1.25 |
| Basophils (×109/L) | 0 | 0 | 0.179 | 0.14 | Rare |
| PLT (×109/L) | – | 206 | 359 | 344 | 200–500 |
Values in bold depict the progressive eosinophilia during the course of disease.
She was presented 1 week later, to continue chemotherapy. A routine hematology sample was taken prior to chemotherapy administration. The circulating eosinophilia had increased in comparison with previous samples (Table 1); however, clinical signs had continued to improve. On neurological reevaluation, the dog was ambulatory tetraparetic with marked proprioceptive deficits in all limbs and intact spinal reflexes. She received a second dose of chemotherapy, oral cyclophosphamide (Cyclophosphamide, 50 mg tablets, Baxter Oncology, Halle, Germany) (250 mg/m2), and continued oral prednisolone (Prednidale 5 mg tablets, Dechra) at a reduced dose, in line with the chemotherapy protocol (30 mg/m2 q 24 h).
Two days after receiving her second dose of chemotherapy, the dog was presented again as an emergency, due to recurrence of tetraplegia, hypoventilation, and marked neck pain. Hematology documented a further increase in the circulating eosinophilia (Table 1) and repeat MRI confirmed a circumferential regrowth of the surgically resected mass at the level of C3, compressing the spinal cord more severely than at diagnosis (Fig. 2D). At this point, 18 days after the initial presentation, the owner elected euthanasia due to disease progression.
Postmortem examination revealed a paraspinal, extradural mass partially attached to the meninges of the dorsolateral spinal cord within C3, infiltrating the epaxial muscles dorsal to the laminectomy site. Grossly, the mass was of a pale green color, possibly due to the presence of large numbers of eosinophils. There were also multiple nodules noted within the lung parenchyma. There were no other abnormalities noted on gross postmortem and no visible lymphadenopathy reported. Histopathology confirmed that the mass, while attached and infiltrating the dura mater with occasional neoplastic lymphocytes, was not infiltrating the spinal cord or leptomeninges. The cord, however, showed significant Wallerian‐like degeneration secondary to compression, and there were eosinophil aggregates surrounding the nerve roots and infiltrating the meninges. Histopathological examination of the extradural/paraspinal mass and lung parenchyma nodules was consistent with a neoplastic round cell population with a similar appearance to that described above, with a high number of eosinophils observed throughout the examined sections.
Discussion
Lymphoma is the most common canine hematopoietic neoplasm, accounting for 7–24% of all canine malignancies 3, 4. Lymphoma usually occurs in lymphoid organs, however, can arise from any tissue in the body 3, 4. Although multicentric lymphoma is the most common form, with 84% of canine lymphomas presenting with peripheral and possibly visceral lymphadenopathy, extranodal sites including the vertebral column and paraspinal tissues, can be affected 3. Lymphoma affecting the spinal cord is much less frequently reported in dogs than cats 5, 6.
Spinal tumors can be classified according to their anatomical location relative to the dura and the spinal cord as extradural, intradural/extramedullary, or intramedullary 5. Extradural spinal tumors occur most commonly in dogs, accounting for over 50% of diagnoses 5, 7. In spinal lymphoma, while intradural or intramedullary infiltration is common in cats, this presentation is rare in dogs 6, 7. Thus, in dogs, lymphoma affecting the spinal cord most commonly manifests as a primary or metastatic extradural tumor. In many instances, there can be osseous involvement and, less frequently, extension to the paraspinal tissues 7, 8, 9, 10, 11, 12, 13. Involvement of more than one spinal compartment (paraspinal tissues, vertebrae, and vertebral canal) has typically been observed in canine spinal lymphoma 9, 11, 12. However, evidence of tumor tissue within the vertebral canal, resulting in spinal cord compression, with extension to the paraspinal tissues through the intervertebral foramina and no evidence of bone involvement, has only been reported in three dogs 10, 12, 13. A similar pattern (the presence of an epidural [extradural] mass with foraminal extension but without vertebral involvement) has been reported in people 14. The dog described herein represents another case of involvement of the paraspinal and epidural compartments without invasion of the vertebrae, with the difference that, in this case, tumor tissue extended dorsally to the epaxial musculature through the interarcuate space and the ligamentum flavum.
Reports of MRI findings in canine spinal lymphoma are scarce but, in most cases, lesions were hypo‐ to isointense on T1‐weighted, iso‐ to hyperintense on T2‐weighted, and hyperintense on STIR images with moderate to strong contrast enhancement 10, 11, 12. However, there is variability in the MRI signal characteristics in the cases reported in the literature and in three dogs, the lesions were hyperintense on precontrast T1‐weighted images 8, 13. Likewise, the extradural and paraspinal lesions identified on MRI in this case were hyperintense on precontrast T1‐weighted images. This finding supports the previously reported heterogeneity of MRI features in canine spinal lymphoma and emphasizes the importance of biopsy sampling for definitive diagnosis.
Paraneoplastic syndromes are commonly found in lymphoma patients, with hypercalcemia, hyperglobulinemia, and hematological disturbances (e.g., anemia, immune‐mediated hemolytic anemia, and immune‐mediated thrombocytopenia) being the most frequently seen 3, 4, 15. Hypereosinophilia (defined as circulating eosinophil counts greater than 5 × 109/L) is a common hematological abnormality seen secondary to a variety of etiologies in the cat and dog, most commonly related to allergic, parasitic, or respiratory disease. However, it is rarely reported as a paraneoplastic syndrome in veterinary medicine 16. In the literature, hypereosinophilia has been noted across a range of canine neoplasms, including two cases of B‐cell (multicentric and splenic) and one T‐cell (intestinal) lymphoma 15, 17, 18. An association with feline T‐cell lymphoma (two intestinal and two other forms) and equine intestinal lymphoma has also been reported 19, 20, 21, 22. In addition, there are single historical reports of hypereosinophilia in cases of feline transitional cell carcinoma, canine mammary carcinoma, and canine oral fibrosarcoma 17, 23, 24.
In the human literature, hypereosinophilia is also recognized as an unusual paraneoplastic condition, more commonly seen in T‐cell lymphoproliferative disorders and found in approximately 15% of patients with Hodgkin's lymphoma 25, 26. The etiology of paraneoplastic hypereosinophilia is poorly understood; however, it has been associated with the production of lymphokines by neoplastic T lymphocytes. These interleukins, particularly IL‐3, IL‐5, and granulocyte–macrophage colony‐stimulating factor, inhibit eosinophil apoptosis, resulting in eosinophilia 15, 16, 26, 27. Hypereosinophilia itself is often of little clinical importance to the patient and treatment of the underlying neoplasm should result in resolution of the paraneoplastic condition 15. However, the degree of eosinophilia is potentially related to tumor burden, with higher tumor burden correlating with excessive interleukin production 27. As such, eosinophilia at the time of diagnosis can indicate high disease burden and, in primary cutaneous T‐cell lymphoma, it has been confirmed to be a poor prognostic indicator associated to disease progression and disease‐specific death 27. Additionally, recurrence of hypereosinophilia after successful cancer treatment can be seen as an early sign of relapse.
In human cases, hypereosinophilia can be present as a single hematological abnormality or part of a generalized leucocytosis and can infiltrate the tumor tissues, as well as being present peripherally, within the blood. In this case, the hypereosinophilia was generalized; seen in the spinal and pulmonary tumor tissue, peripherally in the circulation, in the liver and spleen, and also within the CSF. Interestingly, the eosinophil levels found within the CSF varied according to sampling location. It is likely that the lumbar sample exhibited a higher percentage of eosinophils due to its location, caudal to the tumor site, and the anatomical flow of CSF in a caudal direction. The definition of eosinophilia within the CSF is varied; however, greater than 5% eosinophils is generally considered nonspecific inflammation, while counts greater than 10% of the total nucleated cell population are required to diagnose eosinophilic meningitis in human medicine 28. In a previous study in dogs, CSF was considered eosinophilic if there was pleocytosis with an eosinophil percentage greater than 20 28. In this case, the eosinophilia in both cisternal and lumbar samples was greater than 20%, with the lumbar sample eosinophils accounting for 68% of the total nucleated cells.
There is little information available on therapeutic options and outcomes for canine spinal lymphoma. Cytoreductive surgery and chemotherapy, alone or combined, or in combination with radiotherapy have been reported 5, 7, 11. Regardless of therapeutic option, outcomes were generally poor.
Only one previous case of confirmed extradural T‐cell lymphoma has been reported to receive treatment consisting of cytoreductive surgery 9. However, in that case, chemotherapy was declined and, following an initial period of improvement, the dog was euthanized due to tumor recurrence confirmed on myelography 6 weeks postoperatively. Little is known about the appropriate therapeutic approach and prognosis for spinal T‐cell lymphoma in dogs, due to the paucity of the published literature. In human medicine, extradural lymphoma presenting with spinal cord compression is a rare occurrence and is typically treated with decompressive surgery, followed by local radiotherapy 29, 30, 31. Adjuvant chemotherapy, using a variety of protocols, has also been described, with studies supporting the use of systemic chemotherapy in addition to local treatment for optimal survival times 29, 30, 31. This multimodal approach has been shown to be effective in veterinary medicine also, with two reported cases receiving postoperative radiotherapy and chemotherapy, resulting in favorable survival times 7.
The initial clinical improvement in this case may have been due to the surgical decompression of the spinal cord and the anti‐inflammatory action of prednisolone rather than tumor response to chemotherapy. Tumor cells may have shown inherent resistance to the chemotherapy agents or rapidly acquired resistance, and the failure of the hypereosinophilia to improve would be consistent with a lack of response to chemotherapy.
Conclusion
To our knowledge, this is the first report of treatment by means of surgery and chemotherapy of a confirmed extradural T‐cell lymphoma with paraspinal extension and its outcome in a dog. Additionally, it documents an associated progressing hypereosinophilic paraneoplastic syndrome. This unusual finding of paraneoplastic hypereosinophilia was observed within the CSF as well as blood, and eosinophilic infiltrates were confirmed in the liver and spleen and admixed among neoplastic T lymphocytes. The etiology of hypereosinophilia is poorly understood; however, it is a recognized, although unusual, paraneoplastic condition associated with lymphoma in several species and it might be an indicator of prognosis. Further recognition of these tumors and associated syndromes may improve the adequacy of treatment and provide additional information on prognosis.
Authorship
KAM: collected information and was the writer of the manuscript. RJL: contributed to the evaluation and management of the patient, provided the magnetic resonance and intraoperative images, produced the figures and their descriptions, and contributed to the writing of the manuscript. JM: contributed to the evaluation and management of the patient and to the writing of the manuscript. KL: contributed to the evaluation and management of the patient and reviewed the manuscript. CM: assessed the histopathology, provided the neuropathology images, and reviewed the manuscript.
Conflict of Interest
This work was not supported by any grant and none of the authors of this paper have conflict of interest that could influence the content of the paper.
Clinical Case Reports 2018; 6(6): 999–1005
References
- 1. Wood, A. , Garosi L., and Platt S.. Cerebrospinal fluid analysis Pp. 121–136. Small Animal Neurological Emergencies. Manson Publishing; 2012. [Google Scholar]
- 2. Valli, V. E. , San Myint M., Barthel A., Bienzle D., Caswell J., Colbatzky F., et al. 2011. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet. Pathol. 48:198–211. [DOI] [PubMed] [Google Scholar]
- 3. Young, K. , Vail D., and Pinkerton M.. Canine Lymphoma and Lymphoid Leukaemias Pp. 608–638. Withrow and MacEwen's Small Animal Clinical Oncology, 5th Edition 2013. [Google Scholar]
- 4. Fournel‐Fleury, C. , Ponce F., Felman P., Blavier A., Bonnefont C., Chabanne L., et al. 2002. Canine T‐cell lymphomas: a morphological, immunological, and clinical study of 46 new cases. Vet. Pathol. 39:92–109. [DOI] [PubMed] [Google Scholar]
- 5. Bagley, R. S. 2010. Spinal neoplasms in small animals. Vet. Clin. North Am. Small Anim. Pract. 40:915–927. [DOI] [PubMed] [Google Scholar]
- 6. Marioni‐Henry, K. , Van Winkle T. J., Smith S. H., and Vite C. H.. 2008. Tumors affecting the spinal cord of cats: 85 cases (1980‐2005). J. Am. Vet. Med. Assoc. 232:237–243. [DOI] [PubMed] [Google Scholar]
- 7. Levy, M. S. , Kapatkin A. S., Patnaik A. K., Mauldin G. N., and Mauldin G. E.. 1997. Spinal tumours in 37 dogs: clinical outcome and longterm survival (1987‐1994). J. Am. Anim. Hosp. Assoc. 33:307–312. [DOI] [PubMed] [Google Scholar]
- 8. Kippenes, H. , Gavin P. R., Bagley R. S., Silver G. M., Tucker R. L., and Sande R. D.. 1999. Magnetic resonance imaging features of tumors of the spine and spinal cord in dogs. Vet. Radiol. Ultrasound. 40:627–633. [DOI] [PubMed] [Google Scholar]
- 9. Lamagna, B. , Lamagna F., Meomartino L., Paciello O., and Fatone G.. 2006. Polyostotic lymphoma with vertebral involvement and spinal extradural compression in a dog. J. Am. Anim. Hosp. Assoc. 42:71–76. [DOI] [PubMed] [Google Scholar]
- 10. Ortega, M. , and Castillo‐Alcala F.. 2010. Hind‐limb paresis in a dog with paralumbar solitary T‐cell lymphoma. Can. Vet. J. 51:480–484. [PMC free article] [PubMed] [Google Scholar]
- 11. Kornder, J. , Platt S. R., Eagleson J., Kent M., and Holmes S. P.. 2016. Imaging diagnosis—vertebral polyostotic lymphoma in a geriatric dog. Vet. Radiol. Ultrasound. 57:E42–E45. [DOI] [PubMed] [Google Scholar]
- 12. Allett, B. , and Hecht S.. 2016. Magnetic resonance imaging findings in the spine of six dogs diagnosed with lymphoma. Vet. Radiol. Ultrasound. 57:154–161. [DOI] [PubMed] [Google Scholar]
- 13. Veraa, S. , Dijkman R., Meij B. P., and Voorhout G.. 2010. Comaprative imaging of spinal extradural lymphoma in a Bordeaux dog. Can. Vet. J. 51:519–521. [PMC free article] [PubMed] [Google Scholar]
- 14. Laredo, J. , El Quessar A., Bossard P., and Vuillemin‐Bodaghi V.. 2001. Vertebral tumors and pseudotumors. Radiol. Clin. North Am. 39:137–163. [DOI] [PubMed] [Google Scholar]
- 15. Bergman, P. Paraneoplastic Syndromes Pp. 83–97. Withrow and MacEwen's Small Animal Clinical Oncology, 5th Edition Elsevier, 2013. [Google Scholar]
- 16. Lilliehöök, I. , and Tvedten H.. 2003. Investigation of hypereosinophilia and potential treatments. Vet. Clin. North Am. Small Anim. Pract. 33:1359–1378. [DOI] [PubMed] [Google Scholar]
- 17. Marchetti, V. , Benetti C., Citi S., and Taccini V.. 2005. Paraneoplastic hypereosinophilia in a dog with intestinal T‐cell lymphoma. Am. Soc. Vet. Clin. Pathol. 34:259–263. [DOI] [PubMed] [Google Scholar]
- 18. Tomiyasu, H. , Fujino Y., Ugai J., Goto‐Koshino Y., Ide T., Takahashi M., et al. 2010. Eosinophilia and eosinophilic infiltration into splenic B‐cell high‐grade lymphoma in a dog. J. Vet. Med. Sci. 72:1367–1370. [DOI] [PubMed] [Google Scholar]
- 19. Barrs, V. R. , Beatty J. A., McCandlish I. A., and Kipar A.. 2002. Hypereosinophilic paraneoplastic syndrome in a cat with intestinal T cell lymphosarcoma. J. Small Anim. Pract. 43:401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takeuchi, Y. , Takahashi M., Tsuboi M., Fujino Y., Uchida K., Ohno K., et al. 2012. Intestinal T‐cell lymphoma with severe hypereosinophilic syndrome in a cat. J. Vet. Med. Sci. 74:1057–1062. [DOI] [PubMed] [Google Scholar]
- 21. Cave, T. A. , Gault E. A., and Argyle D. J.. 2004. Feline epitheliotrophic T‐cell lymphoma with paraneoplastic eosinophilia ‐ immunochemotherapy with vinblastine and human recombinant interferon alpha(2b). Vet. Comp. Oncol. 2:91–97. [DOI] [PubMed] [Google Scholar]
- 22. Duckett, W. M. , and Matthews H. K.. 1997. Hypereosinophilia in a horse with intestinal lymphosarcoma. Can. Vet. J. 38:719–720. [PMC free article] [PubMed] [Google Scholar]
- 23. Sellon, R. K. , Rottman J. B., Jordan H. L., Wells M. R., Simpson R. M., Nelson P., et al. 1992. Hypereosinophilia associated with transitional cell carcinoma in a cat. J. Am. Vet. Med. Assoc. 201:591–592. [PubMed] [Google Scholar]
- 24. Losco, P. E. 1986. Local and peripheral eosinophilia in a dog with anaplastic mammary carcinoma. Vet. Pathol. 23:536–538. [DOI] [PubMed] [Google Scholar]
- 25. Cyriac, S. , Sagar T. G., Rajendranath R., and Rathnam K.. 2008. Hypereosinophilia in hodgkin lymphoma. Indian J. Haematol. Blood Transfus. 24:67–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murata, K. , Yamada Y., Kamihira S., Atogami S., Tsukasaki K., Momita S., et al. 1992. Frequency of eosinophilia in adult T‐cell leukemia/lymphoma. Cancer 69:966–971. [DOI] [PubMed] [Google Scholar]
- 27. Tancrède‐Bohin, E. , Ionescu M. A., de La Salmonière P., Dupuy A., Rivet J., Rybojad M., et al. 2004. Prognostic value of blood eosinophilia in primary cutaneous T‐cell lymphomas. Arch. Dermatol. 140:1057–1061. [DOI] [PubMed] [Google Scholar]
- 28. Windsor, R. C. , Sturges B. K., Vernau K. M., and Vernau W.. 2009. Cerebrospinal fluid eosinophilia in dogs. J. Vet. Intern. Med. 23:275–281. [DOI] [PubMed] [Google Scholar]
- 29. Eeles, R. A. , O'Brien P., Horwich A., and Brada M.. 1991. Non‐Hodgkin's lymphoma presenting with extradural spinal cord compression: functional outcome and survival. Br. J. Cancer 63:126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wallington, M. , Mendis S., Premawardhana U., Sanders P., and Shahsavar‐Haghighi K.. 1997. Local control and survival in spinal cord compression from lymphoma and myeloma. Radiother. Oncol. 42:43–47. [DOI] [PubMed] [Google Scholar]
- 31. Rathmell, A. J. , Gospodarowicz M. K., Sutcliffe S. B., and Clark R. M.. 1992. Localized extradural lymphoma: survival, relapse pattern and functional outcome. Radiother. Oncol. 24:14–20. [DOI] [PubMed] [Google Scholar]


