Abstract
Dietary phosphorus restriction is recommended to help control hyperphosphatemia in hemodialysis (HD) patients, but many high-phosphorus foods are important sources of protein. In this review, we examine whether restricting dietary phosphorus compromises protein status in HD patients. Although dietary phosphorus and protein are highly correlated, phosphorus intakes can range up to 600 mg/day for a given energy and protein intake level. Further, the collinearity of phosphorus and protein may be biased because the phosphorus burden of food depends on: (1) the presence of phosphate additives; (2) food preparation method; and (3) bioavailability of phosphorus; which are often unaccounted for in nutrition assessments. Ultimately, we argue that clinically relevant reductions in phosphorus intake can be made without limiting protein intake by avoiding phosphate additives in processed foods, using wet cooking methods such as boiling, and if needed, substituting high-phosphorus foods for nutritionally-equivalent foods that are lower in bioavailable phosphorus.
Keywords: Protein, Phosphorus, Energy, Diet, Hemodialysis
Introduction
Dietary phosphorus restriction is recommended in patients with chronic kidney disease (CKD) to help manage hyperphosphatemia1. However, these recommendations have been met with concerns, often relating to protein-energy malnutrition2. These concerns are particularly relevant to patients treated with hemodialysis (HD) because of protein losses in dialysate and greater protein catabolism from metabolic stress3.
The Phosphorus-Protein Dilemma
Numerous studies have noted the collinearity of dietary phosphorus and protein, and have developed linear regression equations and lines describing their relationship in CKD patients2,4–7 (Figure 1, Table 1). Based on the published equations or lines, the estimated amount of phosphorus in a diet containing 84 g/d of protein (1.2 g/kg/d3 for a 70-kg person) is approximately 1,050–1,400 mg/d (Table 1). Given the high estimated phosphorus-to-protein ratio of the diet and the strong correlation between dietary phosphorus and protein (R2 = 0.58–0.84, Table 1), many experts have expressed concerns that phosphorus restriction may put patients at-risk of inadequate protein intake with resultant protein-energy wasting2. Importantly, these regression equations may understate the problem because nutrient databases underestimate the phosphorus content of foods by approximately 25–30%8.
Figure 1.
Linear regression equation and line describing the relationship between dietary protein intake (x-axis) and dietary phosphorus intake (y-axis) in patients with chronic kidney disease (n=104)
The regression line (solid middle line) is described by the regression equation, y = 14.13(x) + 127.91, where y = phosphorus intake in mg, and x = protein intake in g. The dashed outermost lines describe the 95% confidence interval, and the two lines closed to the regression line describe the prediction interval.
Reproduced with permission from Boaz & Smetana, J Am Diet Assoc 1996;96:1268–1270.
Table 1.
Summary of estimated phosphorus intakes for an 84-gram protein diet based on published regression equations and lines linking protein to phosphorus intakes
| Study | Equation | R2 | Details | Phosphorus* |
|---|---|---|---|---|
| Boaz & Smetana, 199617 | P = [128 + (14 × Pro)] | 0.84 | FFQ, n=104 | 1,304 mg |
| Ruffino et al., 199815 | P = [42.4 + (15 × Pro)] | 0.79 | 5-day food record, n=60 | 1,304 mg |
| Kalantar-Zadeh et al., 201018 | P = [78 + (11.8 × Pro)] | 0.83 | 3-day food record, n=107 | 1,069 mg |
| Noori et al., 201019 | n/a | 0.58 | FFQ, n=224 | 1,400 mg† |
| Cupisti & D’Alessandro, 201120 | P = [102 + (12.9 × Pro)] | n/a | 3-day food record, n=260 | 1,187 mg |
| BalanceWise study | P = [100 + (11.5 × Pro)] | 0.73 | 3-day 24-hr recall, n=137 | 1,066 mg |
FFQ, food frequency questionnaire, n/a, not available, P, phosphorus, Pro, protein
Estimated amount of phosphorus provided in diet containing 84 g of protein (amount recommended for a 70-kg person on hemodialysis (1.2 g/kg/d)3
Value based on visual estimate of regression line because regression equation not provided in study
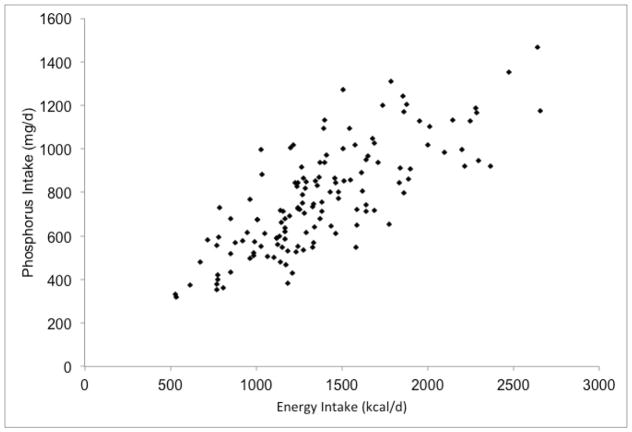
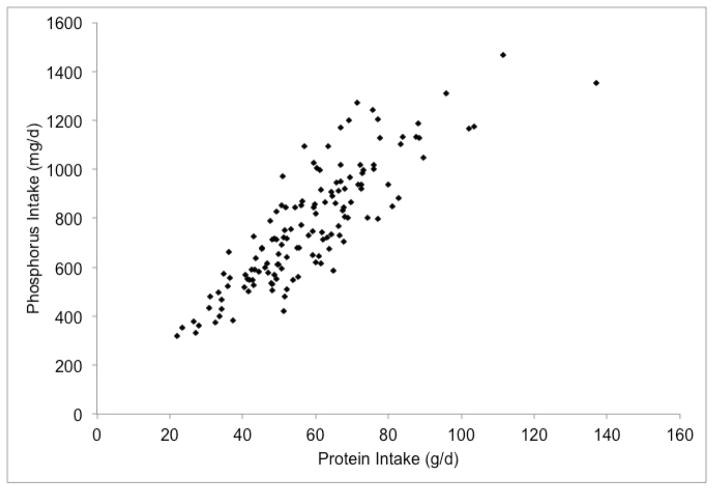
We have explored the phosphorus-protein relationship in the BalanceWise study, a behavioral intervention in CKD patients on thrice weekly HD9. At baseline, BalanceWise participants completed three, unscheduled 24-hour dietary recalls in a two-week period, including two weekdays (one dialysis, one non-dialysis) and a non-dialysis weekend day. The dietary recalls were analyzed using the Nutrition Data System for Research® (NDSR) software10. To improve estimates, we restricted our sample to participants with all three days of dietary collection (n=140/190) who reported consuming at least 500 kcal/d (n=137/140). As expected, there was a strong correlation between reported phosphorus and protein intakes (R2 = 0.73, Figure 2b), and the estimated amount of phosphorus for 84 g of protein greatly exceeded the 800 mg phosphorus target (Table 1).
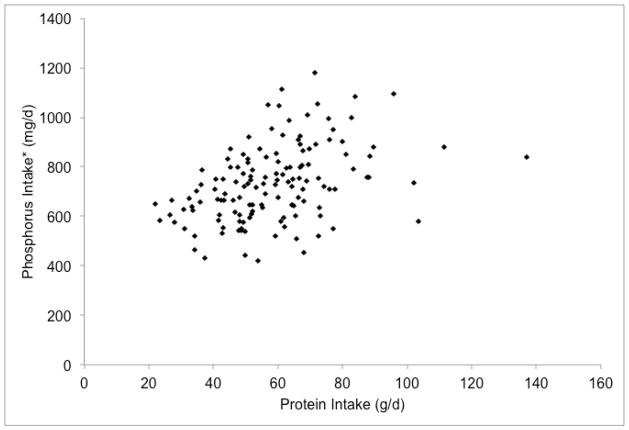
Figure 2.
Associations of reported dietary phosphorus intake with energy and protein intakes of hemodialysis patients in the BalanceWise study (n=137)
a. Energy x Phosphorus, R2=0.62; b. Phosphorus x Protein, R2=0.73; c. Adjusted Phosphorus* x Protein, R2=0.17
*Reported phosphorus intakes were adjusted for energy intake using the residual method with addition of median intakes to normalize values27.
Issues with the Dietary Phosphorus-Protein Dilemma
Although many good sources of dietary protein are also high in phosphorus, the phosphorus-to-protein ratios of high-protein foods vary considerably, from <5 mg/g (e.g., egg whites) to >30 mg/g (e.g., milk)11. Indeed, one common criticism of published phosphorus-protein regression equations is that phosphorus intakes vary considerably (~700 mg/d) for a given protein intake12–13. We evaluated the range of reported phosphorus intakes for given energy (500–999, 1000–1499, 1500–1999 or ≥2000 kcal/d) and protein intake levels (<15, 15–19.9 or ≥20 % of kcal) in the BalanceWise study. As shown in Figure 3, we found that the highest phosphorus intake was generally about two times greater than the lowest phosphorus intake for a given energy and protein subgroup, and varied more than 600 mg/d for some subgroups.
Figure 3.
Ranges in reported dietary phosphorus intake of hemodialysis patients in relation to energy and protein intake in the BalanceWise study (n=137)
Sample size of energy/protein subgroups are presented in parentheses of the energy intake group legend according to protein intake group as (Low/Mid/High). For example, five participants reported dietary intakes of 500–999 kcal/d with protein intakes of <15% kcal, and their phosphorus intake range was 354–571 mg/d.
Perhaps of greater concern than the measured variance in the phosphorus-protein relationship is the unmeasured sources of variance, including confounding and measurement error. Although the correlation of dietary phosphorus and protein appears strong, this relationship is substantially confounded by energy intake – patients who eat more tend to have higher intakes of all nutrients. When adjusting for energy intake using the residual method14, the proportion of phosphorus intake explained by protein intake went from 73% (Figure 2b) to 17% (Figure 2c). Similarly, when protein intake was added as an independent variable to the linear regression equation of energy and phosphorus, the proportion of phosphorus intake that was explained by the model increased modestly from 62% to 77%. The relative importance of energy and protein intake in determining dietary phosphorus intakes can also be clearly seen in Figure 3. Whereas the reported dietary phosphorus intake range increased across the energy intake levels (shown by different colors) for a given protein intake (shown by columns), only a small increase in the phosphorus intake was seen across the different protein intake levels for a given energy intake.
In addition to confounding, as pointed out by Sherman (2007)13, the range of dietary phosphorus intake for a given protein intake is greater than observed due to unaccounted for phosphorus from food additives in processed foods. Phosphorus additives are frequently used in both high-protein and low-protein processed foods11, but they are difficult to capture by dietary assessment and are often missing from nutrient databases. This omission results in measurement error for phosphorus intake that is likely random with regards to protein intake. Adding to this, cooking method (also unmeasured in many dietary assessments) greatly influences the phosphorus content of food. In particular, discarding the water when using wet cooking methods, such as boiling, can reduce the phosphorus content of beef and poultry by more than half, while retaining most of the protein15. Lastly, there is the issue of phosphorus bioavailability, which recently has drawn heightened attention. The bioavailability of dietary phosphorus varies widely from <10% for phytates to almost 100% for inorganic phosphorus, and differs by source (plant ≪ animal ≪ food additives), an important variable that is often not factored into nutrient analyses or dietary recommendations (e.g., consume <800 mg/d)16–17.
The overall measurement error of phosphorus intake estimates was recently assessed using urinary phosphorus as the reference18. In this study, although phosphorus intake assessed by weighed food record was moderately correlated with urinary phosphorus (Spearman’s rho = 0.53), visual assessment of the scatterplot indicates an even distribution of phosphorus ranging ~1000 mg across all urinary phosphorus levels (Figure 4).
Figure 4.

Association of dietary phosphorus intake (x-axis) with urinary phosphorus concentration (y-axis) in healthy volunteers (n=32)
UC = urine collection, DR = dietary record, rs = Spearman’s rho
Reproduced with permission from Morimoto et al, J Clin Biochem Nutr 2014;55(1):62–66.
Restricting Dietary Phosphorus Intake
Despite the apparent relationship between dietary phosphorus and protein, reductions in dietary phosphorus can be achieved in many HD patients without compromising protein status. For example, patients can limit or avoid processed foods prepared with phosphorus additives11. Importantly, similar phosphorus additive-free items are often available19–21. This approach is estimated to remove up to 600–700 mg of highly absorbed phosphorus per day20–21, and may significantly reduce serum phosphorus in HD patients with hyperphosphatemia22. Unfortunately, although most phosphorus additives can be readily identified using the product’s ingredients list, their presence may be difficult to discern for foods consumed outside the home or in certain processed items (e.g., enhanced meats)23. Further, choosing foods prepared without phosphorus additives may increase food costs by ~$2.00/day20. While cost may not be a deterrent for some patients, roughly one-third of HD patients in the BalanceWise study reported that their income did not meet their needs (32%), and/or that they had difficulty affording a healthy diet in the previous two months (39%)24.
Another potential target for reducing dietary phosphorus is to increase the proportion of meals that are prepared by the patients themselves. Through self-preparation, patients attain greater control over their dietary intake. This choice should decrease the intake of phosphorus additives25, and provides the opportunity to leech phosphorus from foods using wet cooking methods15. As an added benefit, this approach limits commercially-prepared foods, which tend to be high in sodium21,23,26, and wet cooking methods also help remove potassium from foods27. Unfortunately, preparing meals from scratch is not the cultural norm in many regions facing high incidence rates of CKD, and may be difficult to sustain, particularly given that most HD patients report being too tired to cook (59%)24.
Lastly, substituting foods that are high in phosphorus for similar foods that are lower in phosphorus may help reduce dietary phosphorus without limiting protein. Foods from the dairy food group are known to be very high in phosphorus, and therefore are usually among the targets for dietary phosphorus restriction. However, dairy products tend to be easily prepared and consumed, and are major sources of high biological value protein in the diet. Choosing dairy products that are lower in phosphorus (e.g., cottage cheese, low-phosphorus milk28), higher in protein (e.g., Greek yogurt), or plant-based alternatives that contain less bioavailable phosphorus (e.g., certain soy beverages) may be helpful. Although other substitutions (e.g., egg whites instead of whole eggs) may also be beneficial29, such tradeoffs should be pursued cautiously. Substitutions require that patients (and clinicians) consider the bioavailability of phosphorus as well as the other nutrients present in foods to avoid inadvertently compromising nutrition status (e.g., replacing milk with rice milk or almond milk, which are very low in protein and may not be fortified with vitamins A and D, and vitamin B12). In particular, protein-rich plant foods that are high in phytates (e.g., nuts, seeds, beans, peas, lentils) may be suitable substitutes for animal-based protein foods despite having higher phosphorus-to-protein ratios11 due to their relatively low phosphorus bioavailability16–17. Indeed, when vegetarian and meat diets containing equal amounts of phosphorus and protein were compared in patients with stage 3–4 CKD, serum phosphorus and fibroblast growth factor-23 concentrations were lower after consuming the vegetarian diet.30 Importantly, too many substitutions may become overwhelming, as many HD patients report difficulty keeping track of nutrients (56%), uncertainty about foods they should be eating (26%), and problems with finding the dialysis diet too complicated (49%)24
Practical Applications
In this article, we have presented findings suggesting that dietary phosphorus restriction can be achieved in HD patients without compromising protein status (Table 2). Eliminating phosphorus additives, preparing food at home using wet cooking methods, and substituting high-phosphorus foods are approaches that may help to lower dietary phosphorus, often without lowering protein intake. None of these approaches is without its challenges, and ultimately, the best approach to reducing dietary phosphorus will vary from patient to patient, and should be based on a thorough nutrition assessment. Renal dietitians are uniquely qualified in this regard, and should counsel patients on how to reduce their dietary phosphorus without compromising the nutritional quality of their diet. It may not be possible to achieve the recommended intake of 800 mg of phosphorus per day while consuming a diet that is both balanced and varied; however, this target may be misleading because it does not account for bioavailability of dietary phosphorus. Given the considerable measurement error, research is desperately needed to improve estimates of dietary phosphorus intake. In the meantime, the associations of dietary phosphorus with kidney disease health outcomes should be interpreted cautiously.
Table 2.
Recommendations for restricting dietary phosphorus in hemodialysis patients
| Recommendation | Potential benefit | Important considerations |
|---|---|---|
| 1. Choose commercial food items prepared without phosphorus-containing food additives | ||
| 2. Prepare foods at home, using wet cooking methods such as boiling (discard water) |
|
|
| 3. Substitute commonly eaten high-phosphorus foods with nutritionally equivalent foods that are lower in bioavailable phosphorus (use sparingly) |
|
|
Acknowledgments
Support: The work of this paper was supported by the following grants: NIH/NINR/R01-NR010135, NIH/NINR/NIDDK/NHLBI/NIA-K24-NR012226, NIH/NIA/R01-AG027017, NIH/NIA/P30-AG024827, NIH/NIA/K07-AG033174.
The authors thank the management and staff of the participating dialysis units from DaVita HealthCare Partners Inc., Dialysis Clinic Inc., and Fresenius Medical Care North America; research study dietitians Beth Hall, BA, RD, LDN, and Susan Stark, MS, RD, CSR, LDN, for conducting the interventions; Deborah Klinvex, BA, for conducting the dietary recall interviews, D. Scott Obrosky, MS, for developing the data tracking system, and Tienna Luster for data management.
Footnotes
Financial Disclosure Declaration: NIH played no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication. The authors have no financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guidelines for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int. 2009;76(Suppl 113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 2.Rufino M, de Bonis E, Martin M, Rebollo S, Martin B, Miquel R, Cobo M, Hernandez D, Torres A, Lorenzo V. Is it possible to control hyperphosphatemia with diet, without inducing protein malnutrition? Nephrol Dial Transplant. 1998;13(Suppl 3):65–67. doi: 10.1093/ndt/13.suppl_3.65. [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation (NKF), Kidney Disease Outcomes Quality Initiative (K/DOQI) Clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2000;35(Suppl 2):S1–S140. [Google Scholar]
- 4.Boaz M, Smetana S. Regression equation predicts dietary phosphorus intake from estimate of dietary protein intake. J Am Diet Assoc. 1996;96(12):1268–1270. doi: 10.1016/S0002-8223(96)00331-8. [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Gutekunst L, Mehrotra R, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(3):519–530. doi: 10.2215/CJN.06080809. [DOI] [PubMed] [Google Scholar]
- 6.Noori N, Kalantar-Zadeh K, Kovesdy CP, Bross R, Benner D, Kopple JD. Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis. Clin J Am Soc Nephrol. 2010;5(4):683–692. doi: 10.2215/CJN.08601209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cupisti A, D’Alessandro C. The impact of known and unknown dietary components to phosphorus intake. G Ital Nephrol. 2011;28(3):278–288. [PubMed] [Google Scholar]
- 8.Calvo MS, Moshfegh AJ, Tucker KL. Assessing the health impact of phosphorus in the food supply: Issues and considerations. Adv Nutr. 2014;5:104–113. doi: 10.3945/an.113.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sevick MA, Piraino BM, St-Jules DE, et al. A randomized trial to evaluate a technology-supported behavioral intervention to reduce dietary sodium in hemodialysis: Primary outcomes of the BalanceWise study. 2015 Jun; doi: 10.1053/j.jrn.2015.11.006. Submitted to J Ren Nutr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sievert YA, Schakel SF, Buzzard IM. Maintenance of a nutrient database for clinical trials. Control Clin Trials. 1989;10:416–425. doi: 10.1016/0197-2456(89)90006-8. [DOI] [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, Gutekunst L, Mehrotra R, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:519–530. doi: 10.2215/CJN.06080809. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A, Agarwal R. More on predicting dietary phosphorus intake. J Am Diet Assoc. 1997;97(6):583–584. doi: 10.1016/S0002-8223(97)00150-8. [DOI] [PubMed] [Google Scholar]
- 13.Sherman RA. Dietary phosphate restriction and protein intake in dialysis patients: A misdirected focus. Semin Dial. 2007;20(1):16–18. doi: 10.1111/j.1525-139X.2007.00204.x. [DOI] [PubMed] [Google Scholar]
- 14.Willett W. Nutritional Epidemiology. 3. New York: Oxford University Press; 2013. [Google Scholar]
- 15.Cupisti A, Comar F, Benini O, Lupetti S, D’Alessandro C, Barsotti G, Gianfaldoni D. Effect of boiling on dietary phosphorus and nitrogen intake. J Ren Nutr. 2006;16(1):36–40. doi: 10.1053/j.jrn.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Karp H, Ekholm P, Kemi V, Hirvonen T, Lamberg-Allardt C. Differences among total and in vitro digestible phosphorus content of meat and milk products. J Ren Nutr. 2012a;22(3):344–349. doi: 10.1053/j.jrn.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Karp H, Ekholm P, Kemi V, et al. Differenceds among total and in vitro digestible phosphorus content of plant foods and beverages. J Ren Nutr. 2012b;22(4):416–422. doi: 10.1053/j.jrn.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Morimoto Y, Sakuma M, Ohta H, et al. Estimate of dietary phosphorus intake using 24-h urine collection. J Clin Biochem Nutr. 2014;55(1):62–66. doi: 10.3164/jcbn.14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benini O, D’Alessandro C, Gianfaldoni D, Cupisti A. Extra-phosphorus load from food additives in commonly eaten foods: A real and insidious danger for renal patients. J Ren Nutr. 2011;21(4):303–308. doi: 10.1053/j.jrn.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Leon JB, Sullivan CM, Sehgal AR. The prevalence of phosphorus containing food additives in top selling foods in grocery stores. J Ren Nutr. 2013;23(4):265–270. doi: 10.1053/j.jrn.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrigan A, Klinger A, Choquette SS, et al. Contribution of food additives to sodium and phosphorus content of diets rich in processed foods. J Ren Nutr. 2014;24(1):13–19. doi: 10.1053/j.jrn.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan C, Sayre SS, Leon JB, et al. Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: A randomized controlled trial. JAMA. 2009;301(6):629–635. doi: 10.1001/jama.2009.96. [DOI] [PubMed] [Google Scholar]
- 23.Karalis M, Murphy-Gutekunst L. Enhanced foods: Hidden phosphorus and sodium in foods commonly eaten. J Ren Nutr. 2006;16(1):79–81. doi: 10.1053/j.jrn.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 24.St-Jules DS, Woolf K, Pompeii M, Sevick MA. Exploring problems in following the hemodialysis diet, and their relation to energy and nutrient intakes: The BalanceWise study. J Ren Nutr. 2015 Nov; doi: 10.1053/j.jrn.2015.10.002. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarathy S, Sullivan C, Leon JB, Sehgal AR. Fast food, phosphorus-containing additives, and the renal diet. J Ren Nutr. 2008;18(5):466–470. doi: 10.1053/j.jrn.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Arquette C. Are there fast-food choices for end-stage renal disease patients? A look at phosphorus and potassium content in common fast foods. J Ren Nutr. 2014;24(3):e19–e21. [Google Scholar]
- 27.Bethke PC, Jansky SH. The effect of boiling and leaching on the content of potassium and other minerals in potatoes. J Food Sci. 2008;73(5):H80–H85. doi: 10.1111/j.1750-3841.2008.00782.x. [DOI] [PubMed] [Google Scholar]
- 28.Oliverio S, Atcher L. Regular use of low-phosphorus milk significantly improves dietary satisfaction of patients without changing their serum phosphorus. Dial Transplant. 2006;35(4):1–3. [Google Scholar]
- 29.Taylor LM, Kalantar-Zadeh K, Markewich T, et al. Dietary egg whites for phosphorus control in maintenance haemodialysis patients: A pilot study. J Ren Care. 2011;37(1):16–24. doi: 10.1111/j.1755-6686.2011.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:257–264. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]