Abstract
Hemodialysis patients are often advised to limit their intake of high-potassium foods to help manage hyperkalemia. However, the benefits of this practice are entirely theoretical and not supported by rigorous randomized controlled trials. The hypothesis that potassium restriction is useful is based on the assumption that different sources of dietary potassium are therapeutically equivalent. In fact, animal and plant sources of potassium may differ in their potential to contribute to hyperkalemia. In this commentary, we summarize the historical research basis for limiting high-potassium foods. Ultimately, we conclude that this approach is not evidence-based and may actually present harm to patients. However, given the uncertainty arising from the paucity of conclusive data, we agree that until the appropriate intervention studies are conducted, practitioners should continue to advise restriction of high-potassium foods.
Keywords: Acid-base Equilibrium, Diet, Fruit, Vegetables, Potassium, Hemodialysis, End Stage Renal Disease
Introduction
Hyperkalemia is a life-threatening complication of end-stage renal disease (ESRD)1, and accounts for about one-quarter of emergent dialysis treatments2. Serum potassium concentration is a key determinant of the resting cell membrane potential of neurons and muscle fibers. Consequently, hyperkalemia is associated with a variety of neuromuscular complications including abdominal cramping, weakness, paresthesia, and most concerningly, cardiac arrhythmias that can result in cardiac arrest. Under normal conditions, the kidneys excrete the majority of excess dietary potassium (~80–90%) to help maintain potassium balance; however, this process becomes compromised as glomerular filtration declines.
To prevent and manage hyperkalemia, ESRD patients treated with intermittent hemodialysis (HD) are advised to follow a low-potassium diet (2,000–3,000 mg/d), which involves avoiding high-potassium, plant-based foods (>200 mg/portion), including nuts, seeds, beans, peas, lentils and many commonly consumed fruits and vegetables (e.g., tomatoes, potatoes, bananas)3. Although this approach seems prudent, numerous factors may modify the effect of dietary potassium on serum potassium concentrations (SK).
In this commentary, we evaluate the recommendation that HD patients should avoid high-potassium foods, considering: (1) observational studies of dietary potassium intake in relation to SK; (2) experimental studies on potassium kinetics in ESRD; and (3) nutritional characteristics of plant-based potassium. We will not attempt to review all of the many variables, including dialysis modalities and prescription, and medications, which have profound effects on SK values.
Dietary potassium and its relation to serum potassium
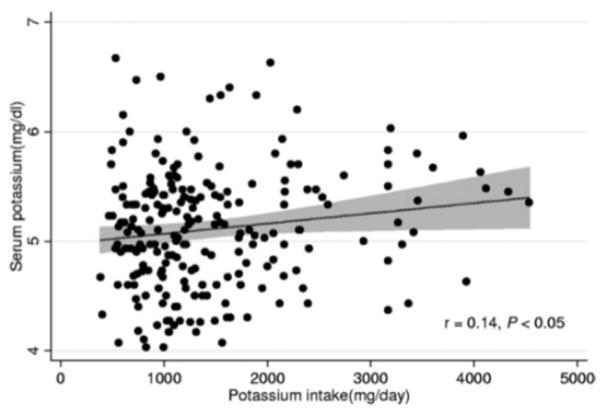
The assumption that dietary potassium intake is an important determinant of SK in HD patients is fundamental to the recommendation to avoid high-potassium foods. However, although potassium salts have been shown to result in post-prandial SK excursions in patients with chronic kidney disease (CKD, discussed below), dietary potassium intake appears to be weakly (if at all) associated with pre-dialysis SK in HD patients. In a secondary analysis of 224 HD patients in the Nutritional and Inflammatory Evaluation in Dialysis (NIED) study, Noori et al. found that reported dietary potassium explained only about 2% of the variance in quarterly mean pre-dialysis SK (r=0.14, p <0.05, Figure 1)4. The regression line describing this relationship indicates that, as reported dietary potassium intake went from a low of 500 mg/d to a high of 4,500 mg/d (a 9-fold difference), SK was only about 0.4 mEq/L higher (Figure 1).
Figure 1.
Associations of reported dietary potassium intake with serum potassium concentration in hemodialysis patients from the Nutritional and Inflammatory Evaluation in Dialysis (NIED) study (n=224)
Regression line (solid line) and 95% confidence interval (shaded area) are shown for the linear regression analysis.
Reproduced with permission from Noori et al., Am J Kidney Dis 2010;56(2):338–347.
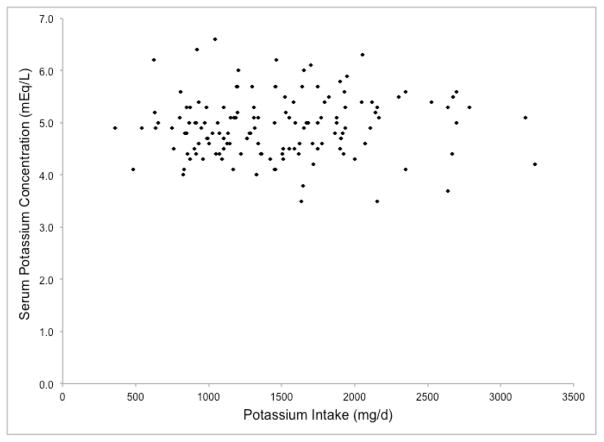
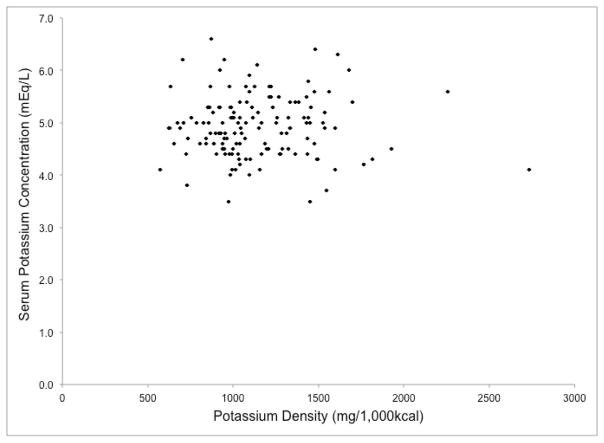
To confirm these findings, we investigated the associations of mean reported potassium intake (mg/d) and potassium density (mg/1,000 kcal) with pre-dialysis SK among 140 HD patients in the BalanceWise Study who completed three, 24-hour dietary recalls (1 dialysis weekday, 1 non-dialysis weekday, 1 non-dialysis weekend day)5. The scatterplots of these variables are shown in Figures 2a and 2b. No significant correlations were found between SK, and either absolute reported potassium intake (r=0.06, p=0.50) or potassium density (r=−0.003, p=0.97) (unpublished data). These associations remained non-significant after adjusting for age, sex, race, and body mass (p >0.05, data not shown).
Figure 2.
Associations of reported dietary potassium intake with serum potassium in hemodialysis patients from the BalanceWise Study (n=140)
a. Potassium Intake; r=0.06, p=0.50
b. Potassium Density; r=−0.003, p=0.97
Although high pre-dialysis SK is used clinically to assess hyperkalemia risk and is associated with worst survival in hemodialysis patients6, lack of a correlation between reported dietary potassium intake and pre-dialysis SK is not, in itself, evidence that high-potassium foods do not affect hyperkalemia risk in HD patients. Dietary potassium intake is measured with error, and SK reflects a complex interaction of numerous intrinsic factors, including nervous/endocrine signals (e.g., epinephrine, aldosterone, insulin), intracellular/extracellular chemical concentrations (e.g., osmolality, H+), circadian rhythms, and organ system functionality, which are influenced by environmental exposures such as diet and medications. It is possible that the association of dietary potassium intake with pre-dialysis SK is too weak to overcome these sources of measurement error, or that dietary potassium intake is correlated with SK when measured in other metabolic states (e.g., post-prandial, fasting). However, the lack of a discernable relationship between these variables in the BalanceWise study (Figures 2a and 2b) contradicts the belief that the amount of potassium consumed influences pre-dialysis SK in HD patients.
Distribution and excretion of potassium in kidney disease
Kidney disease has been recognized as a condition of impaired potassium tolerance for 100 years. In 1915, Smillie published findings from a series of functional tests performed in five patients with chronic nephritis. Patients ingested either 5-g or 10-g of potassium chloride (provides 2.6-g or 5.2-g potassium), and one of the patients given the 10-g dose later exhibited symptoms of weakness, collapse, abdominal distress, chest pain, vomiting, and cyanosis, which were attributed to potassium poisoning7. Despite some concerns, potassium salts continued to be regularly used as diuretics in patients with renal insufficiency to manage edema (standard dose of potassium nitrate provided approximately 4.8 grams of potassium per day)8.
In the 1940s, potassium balance studies by Winkler et al. and Keith & Osterberg demonstrated impaired renal clearance of potassium and higher SK in patients with renal insufficiency after ingesting 2-g to 5-g of potassium9–10. It was then concluded that caution should be exercised when using potassium-based diuretics in patients that were anuric9 or uremic (blood urea ≥100 mg/dL)10. In both studies, the increases in SK were highly variable, and less than predicted based on the dose and renal clearance of potassium.
It is now apparent that a portion of ingested potassium is temporarily distributed within a secondary (intracellular) compartment, thereby buffering its effect on SK. This extrarenal buffering of potassium was well demonstrated in a series of kinetics analyses conducted in one oliguric and four anuric HD patients11. In this study, 63–92% of intravenous potassium (0.3 mEq/kg/hr × 3 hours) exited the extracellular fluid, and the observed changes in SK were consistent with a two-compartment model with bidirectional flux between compartments.
Several factors are known to influence intracellular/extracellular shifts of potassium, including acid-base balance. In the kinetic series above, when the same dose of potassium was infused in an alkaline solution, significantly more potassium was dispersed into the secondary compartment11. Moreover, hydrogen ion concentrations and SK were found to be inversely correlated in HD patients (r=−0.66)12, and higher bicarbonate dialysate solutions resulted in more rapid SK decreases, despite removing less potassium13. Prolonged infusions of sodium bicarbonate in hyperkalemic HD patients were shown to decrease SK from 6.04 mmol/L to 5.30 mmol/L (p<0.01), although half of this decrease was attributed to extracellular volume expansion14. The biological mechanisms linking acidosis and hyperkalemia are incompletely understood, but appear to involve a complex interaction of numerous ion transporters (e.g., Na+-H+ exchanger, Na+/K+-ATPase), which help to maintain blood pH balance by indirectly leading to an exchange of H+ for K+ between intracellular and extracellular compartment15.
Insulin is another key determinant of potassium distribution in the body. Although insulin is generally recognized for its role in macronutrient metabolism, it also helps regulate potassium distribution and balance; potassium triggers and mediates insulin release16–18, and insulin, in turn, shifts potassium into cells by stimulating Na+/K+-ATPase activity19–20. Because dietary macronutrients, in particular glucose, also stimulate insulin release, they can help shift potassium intracelluarly21–22. The rise in SK following potassium ingestion is greatly attenuated if glucose is provided along with it23–24, although studies providing potassium and glucose in kidney disease patients and normal controls have produced conflicting results. Gonick et al. found no difference in peak SK between patients with glomerular or tubular kidney diseases or normal controls despite differences in urinary potassium excretion following potassium load of 0.75 mEq/kg (~2,050 mg potassium/70-kg person) in 8-oz of orange juice23. Other studies were conducted in a fasted state using lower doses of potassium (0.25 mmol/kg24–25 and 0.5 mmol/kg26), and found significantly higher peak SK in HD patients compared to controls. Importantly, fasting is known to increase SK in ESRD. In one of these studies24, the difference in the maximal change in SK between HD patients and controls was greatly attenuated after ingestion of carbohydrates (+0.41 mmol/L → +0.20 mmol/L). In the other study26, total CO2 (HCO3) concentrations were measured and found to be low (avg. 15±1.5 mEq/L) indicating metabolic acidosis, which was moderately, albeit non-significantly, correlated with peak increases in SK in HD patients (r=−0.53), and may have contributed to the observed differences between groups.
Although intracellular shifts of potassium help to prevent hyperkalemia in HD patients, excess dietary potassium must eventually be removed from the body. When the kidneys are unable to excrete the dietary potassium load (i.e. oliguria/anuria), the bowel becomes especially important for maintaining potassium balance. In the 1960s, Hayes et al. conducted a series of potassium balance studies demonstrating that potassium excretion in stool was three times higher in HD patients (avg. 37%) compared to normal controls (avg. 12%), reaching almost 80% of dietary potassium (up to 3,000 mg/d) for some HD patients. Importantly, fecal potassium content was directly proportional to dietary potassium intake and stool weight27. The increase in bowel potassium excretion in CKD was later shown to be primarily the result of potassium secretion into the bowel, rather than reduced dietary potassium absorption in the small intestine, an adaptation that may be due to greater high-conductance potassium channels on the apical surface of colonic epithelial cells28–29. Given the relatively high prevalence of constipation in HD patients (~53%)30, infrequent bowel movements may be an important determinant of hyperkalemia in HD patients.
Nutritional characteristics of plant foods
Many plants are naturally high in potassium, which make them an obvious target for dietary potassium restriction. However, in the aforementioned study by Noori et al.4, which reported a weak correlation between potassium intake and SK concentrations in HD patients, the top five sources of potassium were beef, chicken, Mexican food, hamburgers and legumes. It is possible that patients were already restricting high-potassium plant foods, or underreported their consumption (social desirability bias), but muscle-based animal products are naturally high in potassium, and may be enhanced with potassium-based food additives that can greatly increase potassium content31. The contribution of potassium additives to dietary potassium is largely unknown and unaccounted for in conventional nutrition assessments, but one analysis found that enhanced boneless loin strip steak contained 930 mg/100 g, nearly three times more than a similar, unenhanced product (311 mg/100 g)31. Meats are often absent from high-potassium foods lists3, despite containing more potassium than the recommended cut off (>200 mg/portion), and nearly as much or more potassium than many of the fruits and vegetables listed, including tomatoes (213 mg), bananas (211 mg), kiwi (215 mg), oranges (237 mg) and baked potatoes (471 mg) (from USDA National Nutrient Database for Standard Reference32 using portions provided by National Kidney Foundation)3.
Although potassium from different foods is chemically equivalent, other nutrients in food influence potassium distribution and excretion, as well as the relationship between potassium intake and health outcomes. Unlike meats, the metabolism of which leads to net acid production, and which are low in carbohydrates and contain no fiber, plant foods (especially fruits and vegetables) tend to yield net base production, and are high in both carbohydrates and fiber. Although the pH of oranges, for example, is acidic, the net result of orange juice ingestion is urinary alkalinization33. Therefore, compared to high potassium meats, potassium-rich plant foods may promote distribution of a greater proportion of dietary potassium intracellularly (alkaline and insulin-stimulating), and excretion of potassium in stool by increasing fecal bulk (dietary fiber). Moreover, the dietary fiber and phytonutrients (e.g., carotenoids, flavonoids, etc.) in plants may provide additional health benefits. There are no studies demonstrating differences in SK resulting from potassium ingested from animal versus plant products in HD patients.
Recent data have suggested that alkalinizing fruits and vegetables may have a beneficial effect of reducing the progression of CKD. A study conducted in stage 4 CKD patients found that increasing fruit and vegetable consumption for one year reduced metabolic acidosis and progressive kidney injury without increasing SK34. Unfortunately, due to concern regarding hyperkalemia, this study specifically selected non-diabetic patients with acidemia who had SK ≤4.6 mEq/L and did not require potassium-sparing diuretics.
Discussion
The practice of restricting potassium-rich foods appears to have begun in the mid-1960s, when the purpose of the ESRD diet was “to lower the production of protein catabolites, and to prevent wastage of body proteins”35. With this intent, Giordano36 and Giovannetti & Maggiore35 developed very low-protein diets, which were then modified for different cultures to treat severely uremic patients37–40. The Giordano diet included synthetic pudding made of sugar, starch, margarine or vegetable oil, amino acids, colored flavor, and rum or anise consumed over 3–4 meals, as well as lettuce (100 g) and apple (150 g) at the second and third meals36. The Giovannetti and Maggiore diet included eggs and some low-nitrogen fruits and vegetables, but the majority of energy came from unsalted butter and lard, vegetable oils, sugar honey, and maize and wheat starch35.
Despite the relatively low potassium content of these diets (~2,000 mg/d), patients were prone to developing hyperkalemia37–40. The etiology of hyperkalemia was unknown, but thought to involve acidosis and reduced urinary clearance of potassium37,40. Regardless of the cause, treatment included potassium restriction to about 1,000 mg/d as a prudent, albeit unproven measure37,39–40. Since that time, advances in HD and clinical practice, as well as changes in our food system have dramatically transformed the HD diet from the traditional low-protein Giordano-Giovannetti diet, and yet restriction of high-potassium foods have remained a component of the usual dietary prescription3, which many HD patients appear to follow41–43. Indeed, HD patients consistently report mean dietary potassium intakes well below the proposed upper limit of 3,000 mg/d3 and less than non-CKD matched controls41–43 with corresponding lower intakes of fruits and vegetables43 and other plant-derived compounds (e.g., dietary fiber, vitamin C, carotenoids)41–43. Of note, very few (<2%) of U.S. adults actually consume the Adequate Intake for potassium (4,700 mg/d), regardless of CKD status44.
Both then and now, the targeting of high-potassium foods is based on the assumption that all dietary potassium is therapeutically equivalent. Dietary potassium is not alone in being regarded this way. HD patients with hyperphosphatemia are advised to avoid many high-phosphorus foods that may contribute minimally to hyperphosphatemia due to their relatively low phosphorus bioavailability45–46. For example, sesame seeds, which are often eliminated because of their high phosphorus content (667 mg/100 g), actually have a relatively low digestible fraction of phosphorus (42 mg/100 g)47. Recently, a dietary intervention study demonstrated the food-specific effects of phosphorus in patients with chronic kidney disease48, and similar studies should be conducted with regard to potassium.
Unfortunately, health effects of dietary interventions are difficult to predict. The low-potassium diet changes more than the intake of high-potassium foods because improvisational cooking through substitutions can often be difficult (e.g. replacing tomatoes in spaghetti or chili), and the vigilance required to follow the many components of the renal diet (low-salt, low-phosphorus, low-potassium, high-protein), may overwhelm patients causing them to either give up, or to adopt a very simple diet from which they derive little pleasure or nutrition. When asked about factors that make adherence to the HD diet difficult (rated ≥3 on a 5-point Likert scale), many BalanceWise participants reported that the diet is bland and tasteless (59/140) and too complicated (49/139), and that it is hard to keep track of nutrient intakes (64/140)49. In addition to potentially impairing nutrition status and quality of life50, advising HD patients to limit or avoid many plant-based foods, especially fruits and vegetables, may contribute to adverse metabolic states (e.g., oxidative stress, inflammation, metabolic acidosis, dyslipidemias) and conditions (e.g., constipation, hypertension) that negatively impact the health of HD patients. Ultimately, we can only speculate as to the net health effects of liberalizing the HD diet to allow potassium-rich plant foods, and any such change should only be conducted in a controlled setting with close monitoring of other nutrition and health indices (e.g., protein status).
Implication for practice
In this commentary, we have examined the recommendations that HD patients should avoid high-potassium foods to manage hyperkalemia. Although up to half of severe hyperkalemia episodes in HD patients (>6.0 mmol/L) are attributed to the consumption of high-potassium foods51, the evidence linking high dietary potassium intake to hyperkalemia in HD patients is virtually non-existent. When determining the cause of hyperkalemia, it is important to consider non-dietary factors such as prolonged fasting, hyperosmolality, metabolic acidosis, tissue breakdown, constipation, and medications52. In the absence of empirical evidence, it is of course prudent to continue to recommend low-potassium diets to HD patients with hyperkalemia; however, the practice of advising patients to eliminate so many plant foods from the diet may be harmful, and must be evaluated.
Acknowledgments
The authors thank the management and staff of the participating dialysis units from DaVita HealthCare Partners Inc., Dialysis Clinic Inc., and Fresenius Medical Care North America; research study staff Linda J. Hough, MPH for managing the project; Beth Hall, BA, RD, LDN, and Susan Stark, MS, RD, CSR, LDN, for conducting the interventions; Deborah Klinvex, BA, for conducting the dietary recall interviews, D. Scott Obrosky, MS, for developing the data tracking system, and Tienna Luster for data management.
Support and Financial Disclosure Declaration. The work of this paper was supported by the following National Institutes of Health grants: NINR/R01-NR010135 & NINR/NIDDK/NHLBI/NIA- K24-NR012226. NIH played no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Footnotes
The authors have no financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Einhorn LM, Zhan M, Hsu YD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156–1162. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacchetti A, Stuccio N, Panebianco P, Torres M. ED hemodialysis for treatment of renal failure emergencies. Am J Emerg Med. 1999;17:305–307. doi: 10.1016/s0735-6757(99)90131-6. [DOI] [PubMed] [Google Scholar]
- 3.Potassium and your CKD diet. National Kidney Foundation (NKF) Web site; [Accessed August 10, 2015]. http://www.lib.jmu.edu/citation/amaguide.pdf Published 2015. [Google Scholar]
- 4.Noori N, Kalantar-Zadeh K, Kovesdy CP, et al. Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis. 2010;56(2):338–347. doi: 10.1053/j.ajkd.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sevick MA, Piraino BM, St-Jules DE, et al. No difference in sodium intake observed in a randomized trial with technology-supported behavioral interventions in adults undergoing maintenance hemodialysis in the United States: Primary outcome of the BalanceWise Study. J Ren Nutr. 2015 doi: 10.1053/j.jrn.2015.11.006. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovesdy CP, Regidor DL, Mehrotra R, Jing J, McAllister CJ, Greenland S, Kopple JD, Kalantar-Zadeh K. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:999–1007. doi: 10.2215/CJN.04451206. [DOI] [PubMed] [Google Scholar]
- 7.Smillie WG. Potassium poisoning in nephritis. Arch Int Med. 1915;16(2):330–339. [Google Scholar]
- 8.Keith NM, Binger MW. Diuretic action of potassium salts. J Am Med Assoc. 1935;105(2):1584–1591. [Google Scholar]
- 9.Winkler AW, Hoff FE, Smith PK. The toxicity of orally administered potassium salts in renal insufficiency. J Clin Invest. 1941;20(2):119–126. doi: 10.1172/JCI101204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keith NM, Osterberg AE. The tolerance for potassium in severe renal insufficiency: A study of ten cases. J Clin Invest. 1947;26(4):773–783. [PubMed] [Google Scholar]
- 11.Sterns RH, Feig PU, Pring M, Guzzo J, Singer I. Disposition of intravenous potassium in anuric man: A kinetic analysis. Kidney Int. 1979;15:651–660. doi: 10.1038/ki.1979.85. [DOI] [PubMed] [Google Scholar]
- 12.Morgan AG, Burkinshaw L, Robinson PJA, Rosen SM. Potassium balance and acid- base changes in patients undergoing regular haemodialysis therapy. Br Med J. 1970;1:779–783. doi: 10.1136/bmj.1.5699.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heguilen RM, Sciurano C, Bellusci AD, et al. The faster potassium-lowering effect of high dialysate bicarbonate concentrations in chronic haemodialysis patients. Nephrol Dial Transplant. 2005;20:591–597. doi: 10.1093/ndt/gfh661. [DOI] [PubMed] [Google Scholar]
- 14.Blumberg A, Weidmann P, Ferrari P. Effect of prolonged bicarbonate administration on plasma potassium in terminal renal failure. Kidney Int. 1992;41:369–374. doi: 10.1038/ki.1992.51. [DOI] [PubMed] [Google Scholar]
- 15.Aronson PS, Giebisch G. Effects of pH on potassium: New explanations for old observations. J Am Soc Nephrol. 2011;22:1981–1989. doi: 10.1681/ASN.2011040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiatt N, Davidson MB, Bonorris G. The effect of potassium chloride infusion on insulin secretion in vivo. Horm Metab Res. 1972;4(2):64–68. doi: 10.1055/s-0028-1094101. [DOI] [PubMed] [Google Scholar]
- 17.Gomez M, Curry DL. Potassium stimulation of insulin release by the perfused rat pancreas. Endocrinology. 1973;92(4):1126–1134. doi: 10.1210/endo-92-4-1126. [DOI] [PubMed] [Google Scholar]
- 18.Rowe JW, Tobin JD, Rosa RM, Andres R. Effect of experimental potassium deficiency on glucose and insulin metabolism. Metabolism. 1980;29(6):498–502. doi: 10.1016/0026-0495(80)90074-8. [DOI] [PubMed] [Google Scholar]
- 19.Alvestrand A, Wahren J, Smith D, DeFronzo RA. Insulin-mediated potassium uptake is normal in uremic and healthy subjects. Am J Physiol. 1984;246:E174–E180. doi: 10.1152/ajpendo.1984.246.2.E174. [DOI] [PubMed] [Google Scholar]
- 20.Al-Khalili L, Kotova O, Tsuchida H, et al. ERK1/2 mediates insulin stimulation of Na,K-ATPase by phosphorylation of the α-subunit in human skeletal muscle cells. J Biol Chem. 2004;279(24):25211–25218. doi: 10.1074/jbc.M402152200. [DOI] [PubMed] [Google Scholar]
- 21.Goto S, Sebata K, Watanabe H, et al. Effect of oral glucose administration on serum potassium concentration in hemodialysis patients. Am J Kidney Dis. 2005;46:697–705. doi: 10.1053/j.ajkd.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Chothia MY, Halperin ML, Rensburg MA, Hassan MS, Davids MR. Bolus administration of intravenous glucose in the treatment of hyperkalemia: A randomized controlled trial. Nephron Physiol. 2014;126(1):1–8. doi: 10.1159/000358836. [DOI] [PubMed] [Google Scholar]
- 23.Gonick HC, Kleeman CR, Rubini ME, Maxwell MH. Functional impairment in chronic renal disease III. Studies of potassium excretion. Am J Med Sci. 1971;261(1):281–290. doi: 10.1097/00000441-197105000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Allon M, Dansby L, Shanklin N. Glucose modulation of the disposal of an acute potassium load in patients with end-stage renal disease. Am J Med. 1993;94:475–482. doi: 10.1016/0002-9343(93)90081-Y. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez J, Oster JR, Perez GO. Impaired extrarenal disposal of an acute oral potassium load in patients with endstage renal disease on chronic hemodialysis. Mineral Electrolyte Metab. 1986;12:125–129. [PubMed] [Google Scholar]
- 26.Alvo M, Krsulovic P, Fernandez V, Espinoza AM, Escobar M, Marusic ET. Effect of a simultaneous potassium and carbohydrate load on extrarenal K hemeostasis in end-stage renal failure. Nephron. 1989;53:133–137. doi: 10.1159/000185725. [DOI] [PubMed] [Google Scholar]
- 27.Hayes CP, Jr, McLeod ME, Robinson RR. An extrarenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physicians. 1967;80:207–216. [PubMed] [Google Scholar]
- 28.Martin RS, Panese S, Virginillo M, et al. Increased secretion of potassium in the rectum of humans with chronic renal failure. Am K Kidney Dis. 1986;8(2):105–110. doi: 10.1016/s0272-6386(86)80120-2. [DOI] [PubMed] [Google Scholar]
- 29.Mathialahan T, Maclennan KA, Sandle LN, Verbeke C, Sandle GI. Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol. 2005;206:46–51. doi: 10.1002/path.1750. [DOI] [PubMed] [Google Scholar]
- 30.Murtagh FEM, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: A systematic review. Adv Chronic Kidney Dis. 2007;14(1):82–99. doi: 10.1053/j.ackd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Sherman RA, Mehta O. Phosphorus and potassium content of enhanced meat and poultry products: Implications for patients who receive dialysis. Clin J Am Soc Nephrol. 2009;4:1370–1373. doi: 10.2215/CJN.02830409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National nutrient database for standard reference 27. United States Department of Agriculture (USDA) Web site; [Accessed August 10, 2015]. www.ndb.nal.usda.gov/ndb/foods Published May 2015. [Google Scholar]
- 33.Odvina CV. Comparative value of orange juice versus lemonade in reducing stone-forming risk. Clin J Am Soc Nephrol. 2006;1(6):1269–1274. doi: 10.2215/CJN.00800306. [DOI] [PubMed] [Google Scholar]
- 34.Goraya N, Simoni J, Jo C, Wesson DE. A comparison of treatment metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol. 2013;8:371–381. doi: 10.2215/CJN.02430312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giovannetti S, Maggiore Q. A low-nitrogen diet with proteins of high biological value for severe chronic uraemia. Lancet. 1964;1(7341):1000–10003. doi: 10.1016/s0140-6736(64)91919-1. [DOI] [PubMed] [Google Scholar]
- 36.Giordano C. Use of exogenous and endogenous urea for protein synthesis in normal and uremic subjects. J Lab Clin Med. 1963;62:231–246. [PubMed] [Google Scholar]
- 37.Shaw AB, Bazzard FJ, Booth EM, Nilwarangkur S, Berlyne GM. The treatment of chronic renal failure by a modified Giovannetti diet. Q J Med. 1965;34:237–253. [PubMed] [Google Scholar]
- 38.Berlyne GM, Shaw AB. Giordano-Giovannetti diet in terminal renal failure. Lancet. 1965;286(7401):7–9. [Google Scholar]
- 39.Berlyne GM, Shaw AB, Nilwarangkur S. Dietary treatment of chronic renal failure: Experiences with a modified Giovannetti diet. Nephron. 1965;2:129–147. doi: 10.1159/000179399. [DOI] [PubMed] [Google Scholar]
- 40.Franklin SS, Gordon A, Kleeman CR, Maxwell MH. Use of a balanced low-protein diet in chronic renal failure. J Am Med Assoc. 1967;202(6):477–484. [PubMed] [Google Scholar]
- 41.Kalantar-Zadeh K, Kopple JD, Deepak S, Block D, Block G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr. 2002;12(1):17–31. doi: 10.1053/jren.2002.29598. [DOI] [PubMed] [Google Scholar]
- 42.Cupisti A, D’Alessandro C, Valeri A, et al. Food intake and nutrition status in stable hemodialysis patients. Ren Fail. 2010;32(1):47–54. doi: 10.3109/08860220903391234. [DOI] [PubMed] [Google Scholar]
- 43.Therrien M, Byham-Gray L, Denmark R, Beto J. Comparison of dietary intake among women on maintenance dialysis to a Women’s Health Initiative cohort: Results from the NKF-CRN Second National Research Question Collaborative Study. J Ren Nutr. 2014;24(2):72–80. doi: 10.1053/j.jrn.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Cogswell ME, Zhang Z, Carriquiry AL, et al. Sodium and potassium intakes among U.S. adults: NHANES 2003–2008. Am J Clin Nutr. 2012;96:647–657. doi: 10.3945/ajcn.112.034413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams C, Ronco C, Kotanko P. Whole grains in the renal diet – Is it time to reevaluate their role? Blood Purif. 2013;36:210–214. doi: 10.1159/000356683. [DOI] [PubMed] [Google Scholar]
- 46.Gallant KMH. Studying dietary phosphorus intake: The challenge of when a gram is not a gram. Am J Clin Nutr. 2015;102:237–238. doi: 10.3945/ajcn.115.116889. [DOI] [PubMed] [Google Scholar]
- 47.Karp H, Ekholm P, Kemi V, et al. Differenceds among total and in vitro digestible phosphorus content of plant foods and beverages. J Ren Nutr. 2012;22(4):416–422. doi: 10.1053/j.jrn.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:257–264. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.St-Jules E, Woolf K, Pompeii M, Sevick MA. An exploration of problems in following the hemodialysis diet, and their relation to energy and nutrient intakes: The BalanceWise Study. J Ren Nutr. 2015 doi: 10.1053/j.jrn.2015.10.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalantar-Zadeh K, Tortorici AR, Chen JLT, et al. Dietary restrictions in dialysis patients: Is there anything left to eat? Semin Dial. 2015;28(2):159–168. doi: 10.1111/sdi.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tzamaloukas AH, Avasthi PS. Temporal provide of serum potassium concentration in nondiabetic and diabetic outpatients on chronic dialysis. Am J Nephrol. 1987;7:101–109. doi: 10.1159/000167443. [DOI] [PubMed] [Google Scholar]
- 52.Pani A, Floris M, Rosner MH, Ronco C. Hyperkalemia in hemodialysis patients. Semin Dial. 2014;27(6):571–576. doi: 10.1111/sdi.12272. [DOI] [PubMed] [Google Scholar]