Abstract
Diabetic kidney disease (DKD) is the leading cause of end-stage kidney disease (ESKD) in the United States. Multiple risk factors contribute to DKD development, yet few interventions target more than a single DKD risk factor at a time. This manuscript describes the study protocol, recruitment, and baseline participant characteristics for the Simultaneous Risk Factor Control Using Telehealth to slOw Progression of Diabetic Kidney Disease (STOP-DKD) study. The STOP-DKD study is a randomized controlled trial designed to evaluate the effectiveness of a multifactorial behavioral and medication management intervention to mitigate kidney function decline at 3 years compared to usual care. The intervention consists of up to 36 monthly educational modules delivered via telephone by a study pharmacist, home blood pressure monitoring, and medication management recommendations delivered electronically to primary care physicians. Patients seen at seven primary care clinics in North Carolina, with diabetes and (1) uncontrolled hypertension and (2) evidence of kidney dysfunction (albuminuria or reduced estimated glomerular filtration rate [eGFR]) were eligible to participate. Study recruitment completed in December 2014. Of the 281 participants randomized, mean age at baseline was 61.9; 52% were male, 56% were Black, and most were high school graduates (89%). Baseline co-morbidity was high- mean blood pressure was 134/76 mmHg, mean body mass index was 35.7 kg/m2, mean eGFR was 80.7 ml/min/1.73m2, and mean glycated hemoglobin was 8.0%. Experiences of recruiting and implementing a comprehensive DKD program to individuals at high risk seen in the primary care setting are provided.
Keywords: randomized controlled trial, diabetes, chronic kidney disease, hypertension, telehealth, pharmacist, behavioral intervention
1. Introduction
Type 2 diabetic kidney disease (DKD) is the leading cause of end-stage kidney disease (ESKD) in the United States (US), accounting for over $25 billion in medical expenditures each year.1 The prevalence of DKD is increasing in direct proportion to diabetes prevalence; DKD is estimated to affect 3.3% of the general US population,2 and approximately 26-40% of those with diabetes.3,4 Several key risk factors contribute to the development of kidney disease among patients with diabetes, including poorly controlled comorbidities such as hypertension.5 Approximately 60% of individuals with DKD have uncontrolled blood pressure that increases the risk of ESKD, cardiovascular events, and death.6,7 The results of multiple randomized trials demonstrate that reducing blood pressure slows the decline of glomerular filtration rate (GFR).8, 9 Among those with uncontrolled hypertension, greater reductions in blood pressure result in even greater preservation of GFR. Therefore, efforts to slow or reverse DKD progression must improve control of the proximal determinants that lead to progression such as hypertension and related health behaviors.10
There is a growing consensus that the optimal management of DKD requires a comprehensive, multifactorial approach focusing on early risk factor control.11,12 Management includes the use of medications to control blood pressure, glucose, and lipid profiles, along with antiplatelet agents and lifestyle modifications including smoking cessation, healthy diet, exercise, and weight reduction. Although control of individual risk factors may be effective, multifactorial interventions have been evaluated with a variety of study designs, interventions, and intended targets (e.g., health system, providers, patients). 13,14,15,16–23 While addressing single risk factors or even a few together may be modestly effective,24 targeting multiple traditional risk factors concomitantly is more effective at reducing progression of kidney disease, ESKD, and death. 6,13,19,20,22,25–34,
Despite favorable effects of multifactorial interventions for DKD, inference is limited by their small sample sizes,20 lack of renal outcomes,35 and/or non-randomized study designs.36 Importantly, all of these interventions have required frequent face-to-face visits that limit the ability to translate them to populations more widely. In addition, most interventions have been conducted within demographically homogenous patient populations, limiting generalizability to the increasingly diverse DKD population and dissemination throughout the heterogeneous US health care system.
To address these gaps, we describe the development of a randomized controlled trial in adults with DKD that uses a multifactorial telehealth intervention targeting key risk factors in the progression of DKD. The primary specific aim is to test the hypothesis that patients with DKD and uncontrolled hypertension who receive a multifactorial telehealth intervention will have less progression defined as a smaller decrease in kidney function after 3 years when compared to an education control group. Secondary aims test the hypothesis that the intervention will result in greater improvement in blood pressure, blood glucose, and urinary albumin excretion relative to the control group.
2. Materials and Methods
2.1. Study overview
The Simultaneous Risk Factor Control Using Telehealth to slOw Progression of Diabetic Kidney Disease (STOP-DKD) study is a randomized controlled trial of adults with diabetic kidney disease (DKD) and poorly controlled hypertension randomized to receive a tailored multifactorial, clinical pharmacist-administered telehealth intervention or to an educational control group. All study procedures and protocols were approved by the Duke University Institutional Review Board.
2.2. Study population
Eligible patients were identified through primary care providers (PCPs) at 5 outpatient primary care clinics within the Duke University Health System (DUHS), two of which are Federally Qualified Health Centers. Duke Primary Care is comprised of Internal Medicine and Family Medicine clinics serving Durham County, NC and the surrounding areas. Eligible patients were identified through screening of the electronic health record, and introduced to the study through their primary care provider (PCP).
2.3. Eligibility criteria
Potentially eligible patients were ascertained through the Duke Enterprise Data Unified Content Explorer Research Portal (DEDUCE).37 DEDUCE is a web-based tool that queries electronic health data from clinical and billing systems within the Duke University Health System (DUHS). Patients identified through the DEDUCE data pull were further screened to confirm eligibility. Electronic screening took place monthly for patients who, during the preceding 3 years, met the following eligibility criteria: 1) adult (age ≥18 and ≤75 years); 2) regular use of the DUHS (≥2 primary care visits in 3 prior years); 3) diagnosis of type 2 diabetes (ICD-9 codes 250.×0, 250. ×2); 4) at least 2 serum creatinine values available in the 3 prior years, separated by at least 3 months; 5) eGFR greater than 45 ml/min/1.73m2 but less than 90 ml/min/1.73m2 on most recent creatinine, estimated by calculating an eGFR using the 4-variable Modification of Diet in Renal Disease Study [MDRD] equation; 6) evidence of diabetic nephropathy (either: i. presence of macroalbuminuria; ii. history of microalbuminuria prior to angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) therapy; iii. previous documentation of diabetic retinopathy or laser therapy38; iv. if only microalbuminuria and no iii, then urinalysis without hematuria, and no other renal etiologies [i.e., glomerulonephritis, polycystic kidney disease, membranous nephropathy, renal artery stenosis]); 7) poorly controlled hypertension (1year mean clinic systolic blood pressure [SBP]≥140 mmHg and/or diastolic blood pressure [DBP] ≥90 mmHg, and 8) prescribed hypertension and diabetes medications.
2.4. Expanded Inclusion Criteria
Use of the inclusion criteria noted above proved more challenging than anticipated and resulted in delayed enrollment. Clinic recruitment was expanded to include 2 more clinics for a total of 7 primary care clinics. Despite this expansion, variability in BP, eGFR, and albuminuria was found to be problematic for inclusion. Consequently, study inclusion criteria were expanded to include individuals with two blood pressure values of SBP ≥ 140 and/or DBP ≥ 90 over 1 year, regardless of 1 year average. A third expansion of inclusion criteria removed the upper eGFR threshold of 90 ml/min/1.73m2. We chose to compromise on kidney function criteria more so than BP, since hypertension remains a key driver of progressive kidney disease, even among those with milder DKD.
2.5. Exclusion criteria
Patients without telephone access; not proficient in English; residents of a long-term care facility or home health care patient; with impaired hearing, speech, or vision; participating in another clinical trial; planning to leave the area within 3 years; with pancreatic insufficiency or diabetes secondary to pancreatitis; who currently abuse alcohol (>14 alcoholic beverages weekly); with a diagnosis of non-diabetic kidney disease; with an active malignancy; or with a life-threatening illness and probable death within 4 years were excluded.
2.6. Recruitment of participants
Invitation letters signed by their PCP were mailed to patients requesting their participation in the study. Patients were given an option to opt-out of further communication with study staff. Those who did not opt-out within 10 days were subsequently contacted by study staff and screened for exclusion criteria.
2.7. Randomization
Eligible patients were randomized to one of the 2 study groups. Patient randomization was stratified by eGFR and race, where eGFR was dichotomized as 45-60 ml/min/1.73m2 versus > 60; race was dichotomized as Black versus non-Black. In order to assure allocation concealment, project statisticians performed the entire study randomization before enrollment using blocked randomization to ensure rolling balance between groups over the recruitment period. Randomization assignments by strata were loaded into the study tracking database and participant group assignment automatically generated at the baseline visit.
2.8. Treatment Arms
2.8.1 Education control group
Patients randomized to the education control group received primary care and management of DKD at the discretion of their provider, after being informed of the patient’s enrollment into the study. At baseline, the educational control group received material describing optimal management strategies for kidney disease developed by the National Kidney Disease Education Project (NKDEP).39
2.8.2 Multi-factorial Intervention
2.8.2.1 Theoretical Framework
The multi-factorial pharmacist intervention design was informed using the chronic care model (CCM)40 and health decision model (HDM)41. The CCM model was used to focus on linking informed, active patients with proactive provider teams. The CCM model acknowledges that a substantial portion of chronic care occurs outside of formal healthcare settings and highlights six core elements for the provision of optimal care of patients with chronic disease. The six core elements are addressed in the intervention in the following ways: community resources (e.g., connecting participants to resources for accessing low cost medications and exercise programs); the healthcare system (e.g. promotion of patient-provider communication and patient engagement); patient self-management (e.g., focus on self-care, behavior, and motivation); decision support (e.g., pharmacists using software to assist in guideline concordance medication treatment; delivery system redesign (e.g., using telephone-based pharmacists); and clinical information systems (e.g., using the electronic health record to inform providers of interim home blood pressure values and recommended medication changes).
The intervention is led by clinical pharmacists trained in motivational interviewing, and performed in close communication with participants’ PCPs, facilitating management of complex medication regimens. The revised HDM was used to identify behavioral factors to target. The HDM incorporates health beliefs and modifying factors that may explain poor disease control related to treatment adherence by examining factors that hinder or promote health behaviors. The intervention draws on this model by tailoring feedback relevant to each patient rather than reliance on a generic intervention protocol. Drawing on stages of change,42 and the revised.
HDM,41 the intervention addresses how to 1) set healthy goals and gain self-efficacy, 2) implement healthy behavior and monitor performance, and 3) maintain the behaviors and associated risk factor control over time in patients with DKD and uncontrolled hypertension.
2.8.2.2. Intervention Group
The intervention consists of medication management and behavioral educational modules developed from evidence-based DKD treatment recommendations. Each module is delivered via telephone by a clinical pharmacist monthly for three years. The clinical pharmacists utilize motivational interviewing techniques to help patients explore and resolve ambivalence regarding readiness for change in each component. To ensure the tailored information is standardized, the pharmacists use an intervention software application with predetermined scripts and patient-specific, tailored algorithms (Appendix 1). All patients in the intervention group receive a home BP monitor and are requested to use BP monitors to provide at least 3 values for at least one week prior to each monthly medication management call session. Patients are similarly asked to provide at least three blood sugar values for at least one week prior to each monthly session.
2.8.2.3. Intervention Components
Intervention components include medication management and behavioral-educational modules (Table 1). Key risk factors for the progression of DKD are addressed monthly via telephone over a 36-month time interval, including evaluation of medications, side effects, communication skills, health behaviors, health knowledge, and diabetes self-management. The activation frequency of each module (multiple modules make up an encounter) can vary; some modules may be activated at every encounter when the intervention is activated (medication adherence/update, side effects, and pill refill), while others are activated every 6 months (disease knowledge/risk perception, patient-physician communication, and health behaviors [smoking, activity/exercise, diet]). Key risk factors for the progression of DKD are addressed during the 36-month time interval. When needed, treatment recommendations were made based on evidence-based protocols (Appendix 1) and communicated to the participant’s PCP via the electronic health record. Verbal information is reinforced with written and visual material delivered to the patient in print.
Table 1.
STOP-DKD Intervention Components
| Focus | Intervention Component |
|---|---|
| Hypertension | Medication intensification focusing on achieving a 1-week average home blood pressure of <135/85mmHg. |
| Diabetes | Medication management focusing on achieving target hemoglobin A1C <8.0. |
| Albuminuria | Blood pressure control and use of ACE inhibitors and angiotensin receptor blockers (if tolerated) are prioritized as methods to reduce micro- and macro-albuminuria. |
| Hyperlipidemia |
|
| Smoking |
|
| Aspirin |
|
| Medication Management |
|
| Health Behaviors |
|
2.9. Data Collection, Follow-Up and Outcomes
All participants are assessed using an in-person study visit at the Duke University Center for Living Campus in Durham, NC at baseline, 12 months, 24 months, and 36 months (Figure 1). Anthropometric, biospecimen, and survey data are collected in detail at each visit and are outlined in Table 2.
Figure 1.
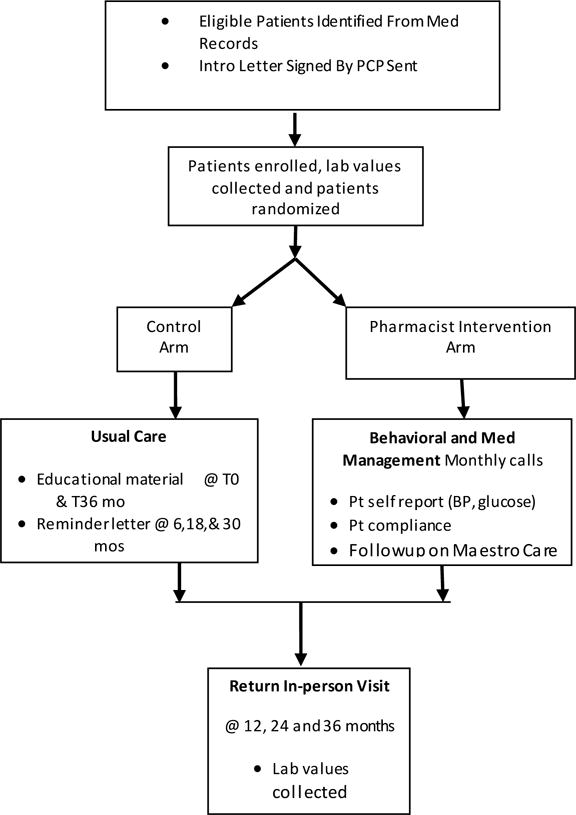
Flow of STOP-DKD Study Activities
Table 2.
Data collected at STOP DKD baseline and follow-up visits
| Study Assessments | ||||
|---|---|---|---|---|
| Baseline | 12 months | 24 months | 36 months | |
| Primary Outcome | ||||
| Change in Estimated Glomerular Filtration Rate (eGFR) | X | X | X | X |
| Secondary Outcome | ||||
| Change in blood pressure | X | X | X | X |
| Social and Demographic | ||||
| Gender*, age, marital status, work status, household income, and insurance | X | X | X | X |
| Social Support | X | X | X | X |
| Health Literacy (Rapid Estimate of Adult Literacy in Medicine) | X | X | ||
| Health Numeracy | X | X | ||
| Quality of Life (EuroQoL, EQ-5D | X | X | X | X |
| Medication Treatment Barriers (Catz et al. 2000) | X | X | X | X |
| Biomedical | ||||
| Height and weight | X | X | X | X |
| Blood pressure* | X | X | X | X |
| Ambulatory Blood Pressure Monitoring (optional) | X | |||
| Ankle Brachial Index (ABI) | X | X | ||
| Eye Acuity and Retinopathy Imaging | X | |||
| Serum Creatinine | X | X | X | X |
| Cy statin C | X | X | X | X |
| Urine Albumin | X | X | X | X |
| Hemoglobin A1c | X | X | X | X |
| Serum Glucose | X | X | X | X |
| Lipid Panel | X | X | X | X |
| Biorepository specimen collection (optional) | X | X | X | X |
| Psychological | ||||
| Depression (Patient Health Questionnaire-2) | X | X | X | X |
| Self-efficacy | X | X | X | X |
| Self-management | X | X | X | X |
| Chaos | X | X | X | X |
| Knowledge and Behaviors | ||||
| Sleep (Pittsburgh Sleep Quality Index) | X | X | X | X |
| Diet (Behavioral Risk Factor Surveillance System) | X | X | X | X |
| Functional Capacity (Duke Activity Status Index) | X | X | X | X |
| Kidney Disease Knowledge (National Health and Nutrition Examination Survey-CDC) | X | X | X | X |
| Diabetes Knowledge (National Health and Nutrition Examination Survey-CDC) | X | X | X | X |
| Cholesterol Knowledge (National Health and Nutrition Examination Survey-CDC) | X | X | X | X |
| Tobacco and Alcohol Use | X | X | X | X |
| Information Technology Usage (Pew Technology Use) | X | X | X | X |
assessed only at baseline
The primary outcome of interest is change in estimated glomerular filtration rate (eGFR) at three years; secondary outcomes include changes in blood pressure, blood glucose, hemoglobin A1C, and urinary albumin excretion. The primary hypothesis is a mean eGFR difference of −5ml/min/1.73m2 at 36 months between intervention and control groups. Other outcomes of interest include assessment of 1) psychological barriers to optimal DKD care, including depression,43 household chaos,44 perceived self-efficacy,45 and perceived diabetes self-management, 2) DKD and risk factor awareness and knowledge, 3) behaviors such as physical activity,46 diet,47 sleep,48 tobacco and alcohol use, 4) sociodemographic barriers to optimal DKD care, including health literacy and numeracy,49 quality of life,50 social support and barriers to medication treatment. Additional biomedical assessments include ankle brachial index, retinopathy screening, and biorepository specimen collection, and we later added an ambulatory blood pressure monitoring option.
2.10. Statistical Considerations
2.10.1. Data analysis
Assigned study group (intervention versus educational control) is the main independent variable, using an intent-to-treat analytic framework. The primary analysis is a comparison of the mean change in in eGFR from baseline to 36 months between the two study groups, using a two-sided 0.05 level t-test (“difference of differences” test). As a supplement to our primary analysis, a longitudinal linear mixed model will be carried out using all measurements of eGFR over three years to compare trajectories of the primary and secondary continuous outcome across study arms.51 In secondary analysis, race will be added to the models above to determine if changes in eGFR and/or the shapes of the trajectories over time differ by the two study race groups. Exploratory analyses will examine the impact on alternate measures of kidney function over time (e.g. change in slope of eGFR), as well as expand the longitudinal models to determine if trajectories over time are further explained by other baseline characteristics.
2.10.2. Sample size, power, and randomization
The study is designed to detect a −5 ml/min/1.73m2 difference in the mean change in eGFR from baseline to 3 years between intervention and control groups. The power calculations for the primary outcome assume a baseline eGFR standard deviation of 14 mmHg, a within-person correlation of 0.7, and a 10% drop-out rate. Under these assumptions, the target sample size of 300 (150 in each arm) has 95.6% power, and the actual sample size of 280 would have 85% power using a two-sided 0.05 level t-test.
A stratified randomization with blocking was used with a total of four strata, defined by two stratification variables – eGFR (eGFR ≤ 60.00 ml/min/1.73m2 vs. eGFR > 60.00 ml/min/1.73m2) and race (Black/non-Black). The stratification was used to generate reasonable balance between the intervention and control groups on two stratification factors, while the blocking was used to ensure rolling balance as the study progressed. The block size and order was known only to the statisticians generating the randomization scheme. The scheme was loaded into the tracking database and assignment automatically generated at the randomization visit.
2.11. Data & Safety Monitoring
2.11.1. Data & Safety Monitoring Board (DSMB)
A DSMB has been established to monitor data and oversee participant safety. The DSMB, comprised of three independent clinical researchers with related expertise, monitors participant recruitment and retention, adverse events, data quality, and outcome data. The DSMB meets biannually via teleconferencing to review protocols, procedures, and concerns related to research integrity and safety.
2.11.2 Adverse Events (AEs)
All AEs, either serious or non-serious, regardless of relationship to the study (i.e. related or not) or to the study intervention, are recorded in the study’s AE log. At each study visit, study personnel review the participant’s electronic health record and screen for emergency room visits, hospital admissions (planned or unplanned) and urgent care visits that occurred since the patient’s last annual follow-up visit. These AE’s are documented and detailed in the study’s AE log. In addition, participants are asked at each study visit about inter-current hospitalizations, emergency room visits, or urgent care visits since their last study visit. If a reported event occurred outside of the Duke Health System, study personnel obtain details surrounding the event using a signed patient medical release form. AE collection occurs throughout the study duration, and all AE’s are reviewed by study investigators monthly, who confirm the AE, note the seriousness of the AE, and determine its relation to the study on the AE log. The study’s AE log is reported to the DSMB for biannual review.
2.12. Resource Considerations
In order to understand the potential economic impact of the STOP-DKD intervention, we will perform an economic evaluation estimating total direct and indirect costs incurred over 3 years, and the long-term cost-effectiveness of the intervention based on projected estimates of lifetime costs and quality-adjusted life-years. Data to be collected for use in the economic evaluation includes: (1) all-cause medical resource use; (2) patient time; (3) health utilities; and (4) resources required to provide the telehealth and education interventions. This information will be ascertained via patient self-report and supplemented with information available from patients’ medical records. In order to evaluate the combined impact on long-term health benefits, we will utilize a well-validated epidemiological tool known as the Archimedes model.52,53
3. Results
3.1. Baseline demographic and clinical characteristics of participants
Participant recruitment details are shown in Figure 2. Enrollment concluded in December 2015. A total of 281 patients were randomized to treatment arms; 143 randomized to control and 138 randomized to receipt of the intervention. Baseline characteristics by treatment arm are presented in Table 3. Mean participant age at at baseline was 61.9, and 56% of the cohort is Black. The majority of participants have completed high school and have annual household incomes of <$60,000. Self-report of diabetes and hypertension diagnoses was generally high (93.2% and 91.1%, respectively), but self-reported CKD was low at 10.9%. Median BMI was 36.7 kg/m2, mean baseline blood pressure was 134/76 mmHg, and median eGFR was 80.7 ml/min/1.73m2. Approximately, one quarter of the sample reported literacy challenges, and approximately one-third of participants reported medication non-adherence.
Figure 2.
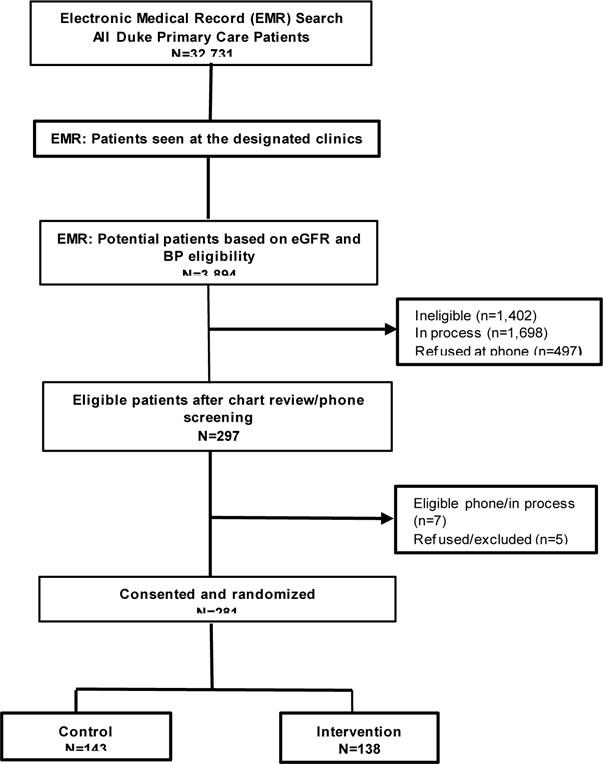
STOP-DKD participant recruitment and follow-up
Table 3.
Baseline demographics and clinical characteristics of participants
| n | Overall 281 |
Control 143 |
Intervention 138 |
|---|---|---|---|
|
| |||
| age in years, mean (SD) | 61.89 (8.83) | 61.33 (9.36) | 62.47 (8.25) |
|
| |||
| Sex | |||
| Female | 136 (48.4) | 75 (52.45) | 61 (44.2) |
| Male | 145 (51.6) | 68 (47.55) | 77 (55.8) |
|
| |||
| Race | |||
| Non-Hispanic white | 116 (41.28) | 60 (41.96) | 56 (40.58) |
| Non-Hispanic black | 156 (55.52) | 79 (55.24) | 77 (55.8) |
| Asian | 2 (0.71) | 0 (0) | 2 (1.45) |
| American Indian/Alaska Native | 1 (0.36) | 1 (0.7) | 0 (0) |
| Other/Hispanic | 6 (2.14) | 3 (2.1) | 3 (2.17) |
|
| |||
| Education | |||
| 1st to 8th grade | 4 (1.42) | 2 (1.4) | 2 (1.45) |
| 9 th to 11th grade | 24 (8.54) | 14 (9.79) | 10 (7.25) |
| 12th grade or GED | 62 (22.06) | 34 (23.78) | 28 (20.29) |
| Associates degree (AA or AS) | 22 (7.83) | 11 (7.69) | 11 (7.97) |
| College 1 to 3 years | 72 (25.62) | 34 (23.78) | 38 (27.54) |
| College graduate | 95 (33.81) | 46 (32.17) | 49 (35.51) |
| Missing | 2 (0.71) | 2 (1.4) | 0 (0) |
|
| |||
| Household income | |||
| Less than $15,000 | 33 (11.74) | 15 (10.49) | 18 (13.04) |
| $15,000-$29,999 | 49 (17.44) | 27 (18.88) | 22 (15.94) |
| $30,000-$59,999 | 77 (27.4) | 37 (25.87) | 40 (28.99) |
| $60,000-$89,999 | 59 (21) | 32 (22.38) | 27 (19.57) |
| $90,000 or more | 53 (18.86) | 25 (17.48) | 28 (20.29) |
| Don’t know | 6 (2.14) | 4 (2.8) | 2 (1.45) |
| Refused | 2 (0.71) | 1 (0.7) | 1 (0.72) |
| Missing | 2 (0.71) | 2 (1.4) | 0 (0) |
|
| |||
| Employment | |||
| Employed for wages, full-time | 80 (28.47) | 40 (27.97) | 40 (28.99) |
| Employed for wages, part-time | 10 (3.56) | 6 (4.2) | 4 (2.9) |
| Self-employed | 12 (4.27) | 6 (4.2) | 6 (4.35) |
| Not employ ed for wages | 7 (2.49) | 5 (3.5) | 2 (1.45) |
| Retired, not working | 97 (34.52) | 46 (32.17) | 51 (36.96) |
| Retired, working part-time or more | 26 (9.25) | 15 (10.49) | 11 (7.97) |
| Unable to work or disabled | 47 (16.73) | 23 (16.08) | 24 (17.39) |
| No response/missing | 2 (0.71) | 2 (1.4) | 0 (0) |
|
| |||
| Tobacco use in the past 12 months | |||
| No | 233 (82.92) | 120 (83.92) | 113 (81.88) |
| Yes | 44 (15.66) | 21 (14.69) | 23 (16.67) |
| Missing | 4 (1.42) | 2 (1.4) | 2 (1.45) |
|
| |||
| Self-reported medical history | |||
| Diabetes | |||
| No | 3 (1.07) | 2 (1.4) | 1 (0.72) |
| Borderline or pre-diabetes | 12 (4.27) | 6 (4.2) | 6 (4.35) |
| Yes | 262 (93.24) | 131 (91.61) | 131 (94.93) |
| Missing | 4 (1.42) | 4 (2.8) | 0 (0) |
| Hypertension | |||
| No | 22 (7.83) | 13 (9.09) | 9 (6.52) |
| Yes | 256 (91.1) | 127 (88.81) | 129 (93.48) |
| Don’t know | 1 (0.36) | 1 (0.7) | 0 (0) |
| Missing | 2 (0.71) | 2 (1.4) | 0 (0) |
| Cardiovascular disease* | |||
| No | 237 (84.34) | 118 (82.52) | 119 (86.23) |
| Yes | 44 (15.66) | 25 (17.48) | 19 (13.77) |
| Chronic kidney disease** | |||
| No | 137 (87.82) | 68 (83.95) | 69 (92.0) |
| Yes | 17 (10.9) | 11 (13.5) | 6 (8.0) |
| Don’t know | 1 (0.64) | 1 (1.23) | 0 (0) |
| Missing | 1 (0.64) | 1 (1.23) | 0 (0) |
| High cholesterol | |||
| No | 84 (29.89) | 42 (29.37) | 42 (30.43) |
| Yes | 187 (66.55) | 92 (64.34) | 95 (68.84) |
| Don’t know | 6 (2.14) | 6 (4.2) | 0 (0) |
| Missing | 4 (1.42) | 3 (2.1) | 1 (0.72) |
|
| |||
| Blood preesure (BP) variables, mmHg, mean (SD) | |||
| Systolic BP (n=279) | 134.33 (19.46) | 133.55 (22.34) | 135.14 (16.05) |
| Diastolic BP (n=279) | 76.31 (13.53) | 75.98 (14.95) | 76.65 (11.96) |
| Mean Arterial Pressure (n=279) | 71.33 (12.38) | 71.07 (12.07) | 71.6 (12.74) |
| BP ≥ 140/90 | |||
| No | 176 (62.63) | 93 (65.03) | 83 (60.14) |
| Yes | 103 (36.65) | 48 (33.57) | 55 (39.86) |
| Missing | 2 (0.71) | 2 (1.4) | 0 (0) |
|
| |||
| BMI, kg/m2 (n=279), mean (SD) | 35.66 (7.87) | 36.15 (8.45) | 35.16 (7.23) |
|
| |||
| Hemoglobin A1c, % (n=280), mean (SD) | 7.97 (1.8) | 7.98 (1.84) | 7.96 (1.77) |
|
| |||
| eGFR, ml/min/1.73m2, mean (SD) | 80.7 (21.69) | 80.09 (23.11) | 81.33 (20.17) |
| eGFR categories | |||
| 0 – 44 | 15 (5.34) | 9 (6.29) | 6 (4.35) |
| 45 – 59 | 35 (12.46) | 20 (13.99) | 15 (10.87) |
| 60-89 | 133 (47.33) | 68 (47.55) | 65 (47.1) |
| ≥90 | 98 (34.88) | 46 (32.17) | 52 (37.68) |
| Urinary ACR, mg/g (n=271), median [IQR] | 30 [10-125] | 30 [11-120] | 30 [9-131] |
| Urinary ACR categories | |||
| 0 – 29 | 136 (48.4) | 69 (48.25) | 67 (48.55) |
| 30 – 299 | 100 (35.59) | 52 (36.36) | 48 (34.78) |
| ≥ 300 | 35 (12.46) | 16 (11.19) | 19 (13.77) |
| Missing | 10 (3.56) | 6 (4.2) | 4 (2.9) |
|
| |||
| REALM score (n=273) | 60.68 (8.97) | 60.9 (8.29) | 60.47 (9.63) |
| REALM categories | |||
| ≤ 60 (limited health literacy) | 75 (26.69) | 38 (26.57) | 37 (26.81) |
| > 60 (normal health literacy) | 198 (70.46) | 99 (69.23) | 99 (71.74) |
| Missing | 8 (2.85) | 6 (4.2) | 2 (1.45) |
|
| |||
| Medication adherence score | 1.71 (1.62) | 1.82 (1.73) | 1.59 (1.5) |
| Medication adherence | |||
| ≤ 2 (adherent) | 182 (64.77) | 87 (60.84) | 95 (68.84) |
| > 2 (non-adherent) | 97 (34.52) | 54 (37.76) | 43 (31.16) |
| Missing | 2 (0.71) | 2 (1.4) | 0 (0) |
Not self-report; ascertained from the electronic medical record
Among the 156 participants with chronic kidney disease (eGFR <60mL/min/1.73m2) or urinary ACR ≥ 30mg/g
Abbreviations: BP= blood pressure; BMI=body mass index; eGFR= estimated glomerular filtration rate; ACR= albumin-to-creatinine ratio; REALM= Rapid Estimate of Adult Literacy in Medicine
3.2 Relationship with Primary Care Providers
The STOP-DKD Study was designed to promote patient self-management and engagement in health by encouraging patients to actively discuss ongoing health issues with their primary care providers. Study pharmacists also served as communication liaisons by providing treatment recommendations and raising provider awareness of both safety and clinical issues that were ascertained through study activities through communication with providers in the electronic medical record.
To further promote the concept of a team-based approach to patient care, STOP-DKD study team members (principal investigators, pharmacists, study coordinators) visited each of seven primary care providers following completion of recruitment activities to provide them with general and tailored updates of study participant recruitment and retention. Feedback from providers regarding communication strategies (e.g., need for more or less detail in digital messaging) were incorporated by study pharmacists for each individual provider request. Broad recommendations from a diverse sample of providers also allowed refinement of communication techniques (e.g. placing the phrase “STOP-DKD Study” in the electronic medical record message subject line) into study activities.
4. Discussion
The STOP-DKD study is an ongoing randomized clinical trial testing the effectiveness of a multifactorial treatment approach that simultaneously targets risk factors for DKD progression. Prior multifactorial interventions across a variety of study designs, interventions, and intended targets (e.g. health system, providers, patients)24,54–59 have been effective in improving intermediate outcomes including control of BP and metabolic abnormalities, but few have included a diverse sample of high-risk participants with comprehensive longitudinal follow-up as seen in STOP-DKD. Although the study is ongoing, our experiences with intervention development and implementation, recruitment and enrollment, and participant and provider engagement represent opportunities to evaluate the impact of practical and common study challenges on the intended outcomes in pragmatic research.
While the resultant STOP-DKD study population is comprised of a diverse, high-risk primary care population, recruitment difficulties and inclusion criteria expansion resulted in a study cohort with higher degrees of blood pressure control and preserved kidney function than initially intended. How this alteration in study sample characteristics will impact the effectiveness of the intervention remains to be determined. Examination of study engagement and treatment adherence by degree of risk factor control warrants further review, and will indisputably inform the development and personalization of future interventions in high-risk populations with varying degrees of informational and medication management needs.
The STOP-DKD study was designed with the objective of enhancing patient engagement by monthly telephone modules delivered via the same study pharmacist to promote continuity and build trust. Use of a standard voice telephone was explicitly chosen to eliminate any potential barriers to engagement by varying levels of participant digital readiness. Further, the pharmacists function as champions of evidenced-based DKD treatment strategies by communicating with treating primary care providers with minimal workflow interruptions. While not yet formally evaluated, patient engagement with phone calls appears to vary across several dimensions. Many participants spend more than the allotted time speaking with study pharmacists, while others are quick to complete intervention calls. Few, however, do not complete the calls at all. Examination of factors related to engagement and its association in outcomes will need to consider any bias by the two study pharmacists who conducted the intervention. Although modules are scripted, standardized, and undergo fidelity assessments, different approaches to delivering the scripted intervention material may lead to different levels of engagement and connection that influence patient health behaviors and self-management.
The STOP-DKD study has several strengths that should be highlighted. Implementation of a complex intervention in a real-world setting across several different clinics was facilitated by the use of telehealth. There is growing interest in such approaches to remotely monitor and manage patients with more advanced kidney disease.60–62 Despite growing clinical uptake of telehealth platforms, few studies have rigorously evaluated the impact of such telehealth interventions on (1) long-term (rather than intermediate) outcomes, or (2) the economic and resource considerations associated with the implementation of such telehealth strategies. Further, the STOP-DKD study population, over half of which is Black or male, provides a unique opportunity to exam the impact of a telehealth intervention in these high-risk groups. Finally, the inclusion of an assortment of behavioral and social support surveys and measures may help to identify subgroups who are more or less responsive to the intervention, and identify potential barriers or facilitators to behavioral health approaches.
5. Conclusion
Our study is the first randomized controlled trial to test the effectiveness of a multi-factorial telehealth intervention to improve outcomes in DKD. The racial diversity of our participants, the concomitant attention to both behavioral influences and medication management, and the efficient and sustainable approach to care delivery, allows us to evaluate the impact of such an intervention in the early course of DKD, when the long-term impact is likely to be the greatest.
Acknowledgments
All authors were supported by funding from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (1R01DK93938.) CJD is supported by grant 1K23DK099385 MJC is supported by a Career Development Award (CDA 13-261) from VA Health Services Research & Development. HBB is funded by a Senior Career Scientist Award VA HSR&D 08-027 and a grant from NIDDK (R34 DK102166). UDP was also supported by R34DK102166 and P30DK096493 prior to joining Gilead Sciences in 2016. CAD is partially supported by UL1TR001117 from the National Center for Advancing Translational Sciences. The authors would like to thank Ashley Cabacungan for her assistance in manuscript preparation.
Abbreviations
- ABI
ankle-brachial index
- ACE inhibitors
angiotensin converting enzyme inhibitors
- BMI
body mass index
- BP
blood pressure
- CCM
chronic care model
- CKD
chronic kidney disease
- DBP
diastolic blood pressure
- DEDUCE
Duke Enterprise Data Unified Content Explorer Research Portal
- DKD
diabetic kidney disease
- DUHS
Duke University Health System
- eGFR
estimated glomerular filtration rate
- HDM
health decision model
- LDL-C
low-density lipoprotein-C
- MDRD
Modification of Diet in Renal Disease Study
- PCP
primary care provider
- REALM
Rapid Estimate of Adult Literacy in Medicine
- SBP
systolic blood pressure
Appendix 1. STOP-DKD Treatment Protocols
STOP-DKD Cholesterol Treatment Protocol
STOP-DKD Smoking cessation Treatment Protocol
STOP-DKD Hypertension Treatment Protocol
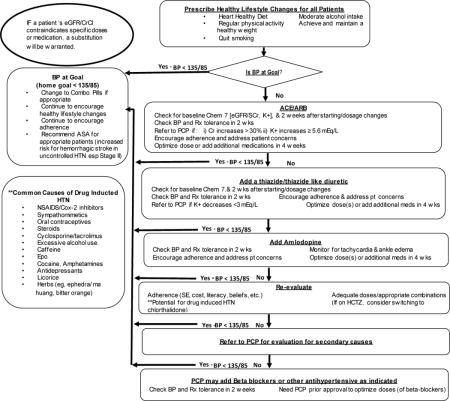
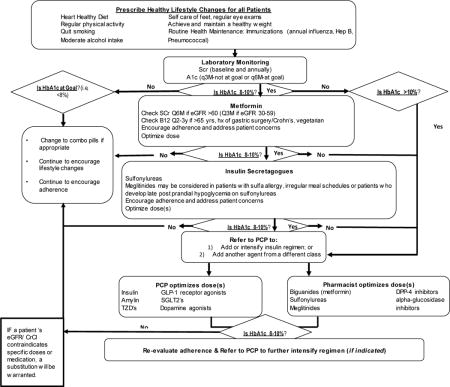
STOP-DKD Diabetes Treatment Protocol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: NCT01829256
References
- 1.Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. 2016 (Accessed 2017, at www.usrds.org/adr)
- 2.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–9. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afkarian M, Zelnick LR, Hall YN, et al. Clinical Manifestations of Kidney Disease Among US Adults With Diabetes, 1988-2014. JAMA. 2016;316:602–10. doi: 10.1001/jama.2016.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress and Possibilities. Clinical journal of the American Society of Nephrology : CJASN. 2017 doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988-2012. JAMA. 2015;314:1021–9. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 6.Plantinga LC, Miller ER, 3rd, Stevens LA, et al. Blood pressure control among persons without and with chronic kidney disease: US trends and risk factors 1999-2006. Hypertension. 2009;54:47–56. doi: 10.1161/HYPERTENSIONAHA.109.129841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palsson R, Patel UD. Cardiovascular complications of diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21:273–80. doi: 10.1053/j.ackd.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–50. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 9.Cheung AK, Rahman M, Reboussin DM, et al. Effects of Intensive BP Control in CKD. J Am Soc Nephrol. 2017;28:2812–23. doi: 10.1681/ASN.2017020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes care. 2014;37:2864–83. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaede P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353:617–22. doi: 10.1016/S0140-6736(98)07368-1. [DOI] [PubMed] [Google Scholar]
- 12.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–91. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 13.Pagels AA, Hylander B, Alvarsson M. A multi-dimensional support programme for patients with diabetic kidney disease J Ren Care. 2015;41:187–94. doi: 10.1111/jorc.12114. [DOI] [PubMed] [Google Scholar]
- 14.Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood) 2013;32:207–14. doi: 10.1377/hlthaff.2012.1061. [DOI] [PubMed] [Google Scholar]
- 15.Saheb Kashaf M, McGill ET, Berger ZD. Shared decision-making and outcomes in type 2 diabetes: A systematic review and meta-analysis. Patient Educ Couns. 2017 doi: 10.1016/j.pec.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes–2011. Diabetes care. 2011;34(Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–30. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 18.Bakris GL. Slowing nephropathy progression: focus on proteinuria reduction. Clin J Am Soc Nephrol. 2008;3(Suppl 1):S3–10. doi: 10.2215/CJN.03250807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zinellu A, Sotgia S, Mangoni AA, et al. Effects of Ramipril and Telmisartan on Plasma Concentrations of Low Molecular Weight and Protein Thiols and Carotid Intima Media Thickness in Patients with Chronic Kidney Disease. Dis Markers. 2016;2016:1821596. doi: 10.1155/2016/1821596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams A, Manias E, Walker R, Gorelik A. A multifactorial intervention to improve blood pressure control in co-existing diabetes and kidney disease: a feasibility randomized controlled trial. J Adv Nurs. 2012;68:2515–25. doi: 10.1111/j.1365-2648.2012.05950.x. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi R, Tamura K, Wakui H, et al. Effect of single-pill irbesartan/amlodipine combination-based therapy on clinic and home blood pressure profiles in Hypertension with chronic kidney diseases. Clin Exp Hypertens. 2016;38:744–50. doi: 10.1080/10641963.2016.1200063. [DOI] [PubMed] [Google Scholar]
- 22.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–92. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura H, Mizuno K, Ohashi Y, Yoshida T, Hirao K, Uchida Y. Pravastatin and cardiovascular risk in moderate chronic kidney disease. Atherosclerosis. 2009;206:512–7. doi: 10.1016/j.atherosclerosis.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 24.Farsaei S, Sabzghabaee AM, Zargarzadeh AH, Amini M. Effect of pharmacist-led patient education on glycemic control of type 2 diabetics: a randomized controlled trial. J Res Med Sci. 2011;16:43–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Meuleman Y, Hoekstra T, Dekker FW, et al. Sodium Restriction in Patients With CKD: A Randomized Controlled Trial of Self-management Support. Am J Kidney Dis. 2017;69:576–86. doi: 10.1053/j.ajkd.2016.08.042. [DOI] [PubMed] [Google Scholar]
- 26.Yamagata K, Makino H, Iseki K, et al. Effect of Behavior Modification on Outcome in Early- to Moderate-Stage Chronic Kidney Disease: A Cluster-Randomized Trial. PLoS One. 2016;11:e0151422. doi: 10.1371/journal.pone.0151422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayliss EA, Bhardwaja B, Ross C, Beck A, Lanese DM. Multidisciplinary team care may slow the rate of decline in renal function. Clin J Am Soc Nephrol. 2011;6:704–10. doi: 10.2215/CJN.06610810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai AY, Ishikawa H, Kiuchi T, Mooppil N, Griva K. Communicative and critical health literacy, and self-management behaviors in end-stage renal disease patients with diabetes on hemodialysis. Patient Educ Couns. 2013;91:221–7. doi: 10.1016/j.pec.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 29.Docherty NG, Canney AL, le Roux CW. Weight loss interventions and progression of diabetic kidney disease. Curr Diab Rep. 2015;15:55. doi: 10.1007/s11892-015-0625-2. [DOI] [PubMed] [Google Scholar]
- 30.Chan JC, So WY, Yeung CY, et al. Effects of structured versus usual care on renal endpoint in type 2 diabetes: the SURE study: a randomized multicenter translational study. Diabetes care. 2009;32:977–82. doi: 10.2337/dc08-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooney D, Moon H, Liu Y, et al. A pharmacist based intervention to improve the care of patients with CKD: a pragmatic, randomized, controlled trial. BMC Nephrol. 2015;16:56. doi: 10.1186/s12882-015-0052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tirosh A, Golan R, Harman-Boehm I, et al. Renal function following three distinct weight loss dietary strategies during 2 years of a randomized controlled trial. Diabetes care. 2013;36:2225–32. doi: 10.2337/dc12-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 34.Tonelli M, Moye L, Sacks FM, Kiberd B, Curhan G. Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Ann Intern Med. 2003;138:98–104. doi: 10.7326/0003-4819-138-2-200301210-00010. [DOI] [PubMed] [Google Scholar]
- 35.Sixt S, Beer S, Bluher M, et al. Long- but not short-term multifactorial intervention with focus on exercise training improves coronary endothelial dysfunction in diabetes mellitus type 2 and coronary artery disease. Ann Intern Med J. 2010;31:112–9. doi: 10.1093/eurheartj/ehp398. [DOI] [PubMed] [Google Scholar]
- 36.Hanai K, Babazono T, Uchigata Y. Effects of statins on the kidneys in patients with type 2 diabetes. Clin Exp Nephrol. 2017;21:633–42. doi: 10.1007/s10157-016-1329-x. [DOI] [PubMed] [Google Scholar]
- 37.Horvath MM, Winfield S, Evans S, Slopek S, Shang H, Ferranti J. The DEDUCE Guided Query tool: providing simplified access to clinical data for research and quality improvement. Journal of biomedical informatics. 2011;44:266–76. doi: 10.1016/j.jbi.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He F, Xia X, Wu XF, Yu XQ, Huang FX. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: a meta-analysis. Diabetologia. 2013;56:457–66. doi: 10.1007/s00125-012-2796-6. [DOI] [PubMed] [Google Scholar]
- 39.National Kidney Disease Education Program. (Accessed 2013 at https://www.niddk.nih.gov/health-information/communication-programs/nkdep.)
- 40.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA. 2002;288:1909–14. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 41.Bosworth HB, Oddone EZ. A model of psychosocial and cultural antecedents of blood pressure control. J Natl Med Assoc. 2002;94:236–48. [PMC free article] [PubMed] [Google Scholar]
- 42.Prochaska JOVW. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 43.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–92. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 44.Wong MD, Sarkisian CA, Davis C, Kinsler J, Cunningham WE. The association between life chaos, health care use, and health status among HIV-infected persons. J Gen Intern Med. 2007;22:1286–91. doi: 10.1007/s11606-007-0265-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polchert MJ. Cross cultural exploration of the perceived health competence scale Open Journal of Nursing. 2015;5:632–41. [Google Scholar]
- 46.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989;64:651–4. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 47.Diet History Questionnaire, Version 2.0.2010(n d) (Accessed 2014, at https://epi.grants.cancer.gov/dhq2/webquest/)
- 48.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 49.Davis TC, Crouch MA, Long SW, et al. Rapid assessment of literacy levels of adult primary care patients. Fam Med. 1991;23:433–5. [PubMed] [Google Scholar]
- 50.EuroQol Group. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 51.Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64:821–35. doi: 10.1053/j.ajkd.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 52.Eddy DM, Schlessinger L. Validation of the archimedes diabetes model. Diabetes care. 2003;26:3102–10. doi: 10.2337/diacare.26.11.3102. [DOI] [PubMed] [Google Scholar]
- 53.Eddy DM, Schlessinger L. Archimedes: a trial-validated model of diabetes. Diabetes care. 2003;26:3093–101. doi: 10.2337/diacare.26.11.3093. [DOI] [PubMed] [Google Scholar]
- 54.Norris SL, Nichols PJ, Caspersen CJ, et al. The effectiveness of disease and case management for people with diabetes A systematic review. Am J Prev Med. 2002;22:15–38. doi: 10.1016/s0749-3797(02)00423-3. [DOI] [PubMed] [Google Scholar]
- 55.Renders CM, Valk GD, Griffin SJ, Wagner EH, Eijk Van JT, Assendelft WJ. Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review. Diabetes care. 2001;24:1821–33. doi: 10.2337/diacare.24.10.1821. [DOI] [PubMed] [Google Scholar]
- 56.Sanchez I. Implementation of a diabetes self-management education program in primary care for adults using shared medical appointments. Diabetes Educ. 2011;37:381–91. doi: 10.1177/0145721711401667. [DOI] [PubMed] [Google Scholar]
- 57.Tricco AC, Ivers NM, Grimshaw JM, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet. 2012;379:2252–61. doi: 10.1016/S0140-6736(12)60480-2. [DOI] [PubMed] [Google Scholar]
- 58.Bowman BT, Kleiner A, Bolton WK. Comanagement of diabetic kidney disease by the primary care provider and nephrologist. Med Clin North Am. 2013;97:157–73. doi: 10.1016/j.mcna.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Helou N, Dwyer A, Shaha M, Zanchi A. Multidisciplinary management of diabetic kidney disease: a systematic review and meta-analysis. JBI Database System Rev Implement Rep. 2016;14:169–207. doi: 10.11124/JBISRIR-2016-003011. [DOI] [PubMed] [Google Scholar]
- 60.Frilling S. Medicare Telehealth Services and Nephrology: Policies for Eligibility and Payment. Adv Chronic Kidney Dis. 2017;24:46–50. doi: 10.1053/j.ackd.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 61.Krishna VN, Managadi K, Smith M, Wallace E. Telehealth in the Delivery of Home Dialysis Care: Catching up With Technology. Adv Chronic Kidney Dis. 2017;24:12–6. doi: 10.1053/j.ackd.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 62.Diamantidis CJ. A Fundamental Theorem of Telehealth. Adv Chronic Kidney Dis. 2017;24:4–5. doi: 10.1053/j.ackd.2016.11.001. [DOI] [PubMed] [Google Scholar]


