Abstract
Thermal ablation of large hepatic cavernous hemangiomas may lead to intravascular hemolysis, hemoglobinuria, and even acute renal failure. This study aimed to identify the risk factors associated with hemoglobinuria after ultrasonography-guided percutaneous microwave ablation for large hepatic cavernous hemangiomas. In our study, 11 related risk factors were analyzed using univariate and multivariate binary logistic regression model and Receiver operating characteristic curves to determine the contribution to hemoglobinuria after microwave ablation for 49 patients with 51 hepatic cavernous hemangiomas. By multivariate analysis, the ablation time (p = 0.021; Odds Ratio, 1.005), and the number of antenna insertions (p = 0.036; Odds Ratio, 3.568) were the independent risk factors associated with hemoglobinuria. The cutoff value for ablation time and the number of antenna insertions in predicting the presence of hemoglobinuria was 1185s (sensitivity, 75%; specificity, 69%) and 4.5 (sensitivity, 55%; specificity, 83%), respectively. Less than 5 of antenna insertions and less than 20 mins of ablation time may therefore be recommended in patients with microwave ablation of large hepatic cavernous hemangiomas, in order to reduce the occurrence of hemoglobinuria. This is the first report about the risk factors analysis associated with hemoglobinuria after thermal ablation for large hepatic cavernous hemangiomas.
Keywords: microwave ablation, hepatic cavernous hemangioma, ultrasound guidance, hemoglobinuria, risk factors
INTRODUCTION
Hepatic cavernous hemangiomas (HCHs) are the most common benign neoplasms in liver, with the prevalence in the general population as many as 20% in autopsy studies [1]. Hemangiomas are referred to as “giant” if greater than 4 cm in diameter [2]. Although most hemangiomas are asymptomatic and can be managed safely with observation alone, larger lesions may produce a variety of symptoms and signs, including abdominal pain, dyspepsia, jaundice, thrombocytopenia and even spontaneous rupture [3, 4]. The primary treatment is surgical resection, transarterial embolization, radiation therapy, and the use of a vascular endothelial growth factor (VEGF) inhibitor have also been reported [5–8]. Thermal ablation, such as radiofrequency ablation (RFA) and microwave ablation (MWA), was safe, well-tolerated, and effective in markedly shrinking large HCHs and improving symptoms in most patients [9–12]. Hemoglobinuria after thermal ablation of HCHs was a common side effect and and should be taken seriously. Hemoglobinuria is a type of abnormal urine (deep-yellow-colored or wine-colored) with hemoglobin (Hb), which is often found in intravascular hemolysis leading to cell-free Hb released into circulatory system [13]. Because hemangiomas are sinusoids and the main component inside is the blood, thermal ablation by inserting the ablation needle into the tumor can lead to massive red cell destruction, intravascular hemolysis, and even acute kidney injury. Van Tilborg AA et al. reported two patients with very large symptomatic HCHs who developed acute kidney injury (AKI) shortly after bipolar RF ablation, caused by massive heat-induced intravascular hemolysis [14]. Determining risk factors associated with hemoglobinuria after thermal ablation for large hepatic cavernous hemangiomas is beneficial for reducing the risk of ablation. To our knowledge, there has been no report about the risk factors analysis associated with hemoglobinuria after thermal ablation for large hepatic cavernous hemangiomas. This study aimed to identify the risk factors associated with hemoglobinuria after ultrasonography-guided percutaneous microwave ablation for large hepatic cavernous hemangiomas.
RESULTS
Ablation efficacy
All patients were performed MWA successfully. Technical effectiveness rate was 100% with a mean ablation time of 1251.2 ± 535.1 (range 480–2730) seconds. Within 3 days after ablation, 44 lesions were necrosis completely, and 5 lesions was more than 90% necrosis due to the peripheral of the tumor adjacent to dangerous location. The mean tumor volume shrinkage rate was 53.05 ± 23.81% within 3 days after ablation. (Figure 1) Out of 20 patients who had hemoglobinuria after microwave ablation, the color of urine and urine routine test recovered normally gradually after basification of urine and hydration treatment in 19 cases. One case developed acute kidney injury (AKI) shortly after MWA, caused by massive heat-induced intravascular hemolysis. Lab results showed AKI (creatinine 227 micromol/l and urea 13.8 mmol/l in the second day after MWA, creatinine 353 micromol/l and urea 15.1 mmol/l 3 days later after MWA). Because of progressive dyspnea and ongoing anuria haemodialysis through a femoral catheter was started. After 12 hemodialysis, 32 days later, the renal function gradually recovered and dialysis was stopped and the patient was discharged from the hospital 34 days after the procedure.
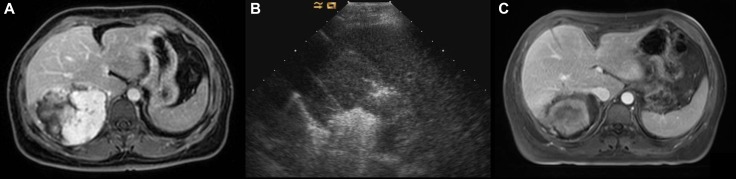
Figure 1. Images in a 39-year-old-woman who underwent MWA for hepatic cavernous hemangioma 10.4 cm in diameter.
(A) Preoperative contrast-enhanced MRI showed the hemangioma was hyperenhanced in portal vein phase in right lobe. (B) US image showed the two antennae were placed into the tumor and ablated simultaneously. (C) Postoperative contrast-enhanced MRI showed complete necrosis of the tumor and and the maximum diameter was reduced to 7.8 cm 3 days after MWA.
The preoperative characteristics and postoperative findings of the patients are shown in Table 1. Tumor maximum diameter was significantly larger among the patients with hemoglobinuria than among those with non-hemoglobinuria after MWA (p = 0.007). Ablation time was significantly longer among the patients with hemoglobinuria than among those with non-hemoglobinuria after MWA (p = 0.004). The number of antenna insertions was significantly more among the patients with hemoglobinuria than among those with non-hemoglobinuria after MWA (p = 0.005). Patients with hemoglobinuria use more energy than non hemoglobinuria patients after MWA (p = 0.001).There were no significant differences in liver function and renal function between the two groups before MWA. There were significant differences in ALT and AST level and renal function between the two groups in the second day after MWA (p < 0.05).
Table 1. Comparison of clinical parameters between hemoglobinuria group and non-hemoglobinuria group patients after microwave ablation for large hepatic cavernous hemangiomas.
| Variable | hemoglobinuria (n = 20) | Non-hemoglobinuria (n = 29) | P value |
|---|---|---|---|
| Age (y) | 43.85 ± 7.31 | 42.75 ± 8.89 | 0.653 |
| Gender (women/men) | 15/5 | 21/8 | 0.840 |
| Max diameter (cm) | 8.26 ± 1.92 | 6.89 ± 1.48 | 0.007 |
| Ablation time (s) | 1506.00 ± 440.86 | 1075.51 ± 529.82 | 0.004 |
| Tumor volume (ml) | 156.24 ± 80.89 | 119.62 ± 71.45 | 0.101 |
| No. of antenna insertion | 4.80 ± 1.32 | 3.72 ± 1.22 | 0.005 |
| Energy (J) | 154650 ± 59004 | 92151 ± 36367 | 0.001 |
| ALT-pre (U/L) | 18.55 ± 11.71 | 16.65 ± 12.02 | 0.591 |
| AST-pre (U/L) | 17.10 ± 6.54 | 15.75 ± 4.53 | 0.408 |
| STB-pre (U/L) | 13.41 ± 4.56 | 11.75 ± 3.97 | 0.195 |
| ALP-pre (U/L) | 60.77 ± 14.26 | 58.20 ± 11.10 | 0.495 |
| Cre-pre (μmol/L) | 67.81 ± 12.42 | 63.60 ± 11.56 | 0.239 |
| BUN-pre (mmol/L) | 4.70 ± 1.25 | 4.36 ± 1.13 | 0.329 |
| ALT-post (U/L) | 216.42 ± 189.54 | 100.22 ± 61.70 | 0.003 |
| AST-post (U/L) | 313.71 ± 281.30 | 145.73 ± 116.43 | 0.006 |
| STB-post (U/L) | 41.07 ± 31.86 | 27.37 ± 16.31 | 0.054 |
| ALP-post (U/L) | 56.52 ± 12.99 | 57.45 ± 12.45 | 0.823 |
| Cre-post (μmol/L) | 90.08 ± 47.12 | 59.87 ± 17.04 | 0.004 |
| BUN-post (mmol/L) | 6.95 ± 6.04 | 3.82 ± 1.59 | 0.013 |
| Tumor reduction rate (%) | 52.14 ± 25.35 | 53.97 ± 22.97 | 0.832 |
Abbreviations; ALT: alanine aminotransferase; AST: aspartate aminotransferase; STB: total bilirubin; ALP: alkaline phosphatase; Cre: creatinine; BUN: blood urea nitrogen; *-pre: index of preablation; *-post: index of postablation.
Risk factors of hemoglobinuria
By univariate analysis, tumor maximum diameter (p = 0.017), ablation time (p = 0.01), and the number of antenna insertions (p = 0.01) were statistically significant risk factors after MWA for HCHs. By multivariate analysis, the ablation time (p = 0.021; Odds Ratio, 1.005), and the number of antenna insertions (p = 0.036; Odds Ratio, 3.568) were the independent risk factors associated with hemoglobinuria after MWA for HCHs. (Table 2) When analyzed with use of ROC curves, ablation time (AUC, 0.759; 95% CI: 0.626, 0.892; P = 0.002) and the number of antenna insertions (AUC, 0.722; 95% CI: 0.573, 0.870; P = 0.009) were also significant factors for predicting hemoglobinuria. The cutoff value for ablation time in predicting the presence of hemoglobinuria was 1185s (sensitivity, 75%; specificity, 69%). The cutoff value for the number of antenna insertions in predicting the presence of hemoglobinuria was 4.5 (sensitivity, 55%; specificity, 83%) (Table 3).
Table 2. Univariate and multivariate analysis of clinical parameters related to hemoglobinuria after microwave ablation for large hepatic cavernous hemangiomas.
| Factor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| P Value | Odds Ratio | P Value | Odds Ratio | |
| Age | 0.645 | 1.017 | 0.872 | 0.987 |
| Gender | 0.840 | 1.143 | 0.093 | 27.720 |
| Max diameter | 0.017 | 1.668 | 0.860 | 1.104 |
| Tumor volume | 0.108 | 1.007 | 0.424 | 1.011 |
| ALT level | 0.584 | 1.014 | 0.155 | 1.171 |
| AST level | 0.408 | 1.048 | 0.906 | 0.970 |
| STB level | 0.197 | 1.100 | 0.320 | 1.196 |
| BUN level | 0.323 | 1.286 | 0.498 | 1.505 |
| Cre level | 0.236 | 1.031 | 0.126 | 1.126 |
| Ablation time | 0.010 | 1.002 | 0.021 | 1.005 |
| NO. of antenna insertion | 0.010 | 1.971 | 0.036 | 3.568 |
Abbreviations; ALT: alanine aminotransferase; AST: aspartate aminotransferase; STB: total bilirubin; ALP: alkaline phosphatase; Cre: creatinine; BUN: blood urea nitrogen.
Table 3. Sensitivity, specificity, and cutoff values for risk factors in predicting the presence of hemoglobinuria after microwave ablation for large hepatic cavernous hemangiomas.
| Factors | Cutoff value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Ablation time | 1185s | 75 | 69 |
| No. of antenna insertions | 4.5 | 55 | 83 |
DISCUSSION
For most patients with HCHs, open or laparoscopic surgical resection has been considered the preferred choice of treatment [17]. However, it is important to keep in mind that many of the trade-offs encountered during the treatment of cancer are not applicable to HCHs due to their benign natural history. Thus, a highly effective but morbid treatment would be a poor choice for most patients. Percutaneous thermal ablation, such as RFA and MWA, was safe, well-tolerated, and effective treatment method for most patients with HCHs [9–12]. However, due to the characteristics of the hemangioma itself, thermal ablation for hemangiomas may lead to a mass of red blood cells undergoing budding and fragmentation, which presumably resulted in a massive heat-induced intravascular hemolysis. Massive hemolysis can lead to various degrees of hemoglobinuria, hemolytic jaundice, anemia, and renal damage [11]. Determining risk factors associated with hemoglobinuria after thermal ablation for large HCHs is beneficial for reducing the risk of ablation, while there have been no report about it.
In our study, tumor maximum diameter, ablation time and the number of antenna insertions were statistically significant risk factors by univariate analysis. By multivariate analysis, we discovered that long ablation time and more antenna insertions during MWA can lead to hemoglobinuria with statistically significant cutoff values of 1185s (nearly 20 mins) and 4.5, respectively. Therefore, when treating large HCHs with MWA, the number of antenna insertions and the ablation time should be reduced to the absolute minimum, in order to prevent hemoglobinuria. Van Tilborg AA et al. reported two patients with very large symptomatic HCHs who occurred hemoglobinuria and developed acute kidney injury shortly after bipolar RF ablation, and thought it was related with the size of the ablation zone and length of the procedure [14]. The goal of MWA for hemangioma and other benign lesions is tumor conformal necrosis or most part necrosis, so the ablation zone is basically the same as the tumor volume, but tumor volume were not statistically significant risk factors by univariate analysis, while the maximum tumor diameter is statistically significant only in univariate analysis, not in multivariate analysis in our study. During thermal ablation for HCHs, hemoglobin is released upon erythrocyte destruction and is filtered by the glomerulus into the urinary space. In the urinary space, hemoglobin is degraded and releases hemepigments which are toxic to the kidney. Hemepigments can cause tubular injury by (1) tubular obstruction, (2) damage due to direct proximal tubular cell injury, and (3) vasoconstriction, resulting in reduced blood flow in the outer medulla [18]. So, proper treatments including adequate hydration to decrease the Hb concentration in circulation system, alkalization of urine to make Hb crystal dissolution and prevent tubular obstruction and even acute kidney injury.
This study had some limitations. First, hemoglobinuria was qualitatively determined by urine routine without quantitative analysis. Second, the present study was a retrospective analysis which was performed in a single institution. The results must be confirmed in another cohort or in a prospective multicenter-study.
MATERIALS AND METHODS
Patients
From January 2011 to December 2016, 49 patients (36 females, 13 male; average age 43.20 ± 8.22 years) with 51 giant hepatic hemangiomas (mean maximum diameter 7.45 ± 1.78 cm, range 4.1–12.6 cm) treated with image-guided percutaneous MWA were reviewed in this study. Among them, 20 patients had hemoglobinuria confirmed by urine routine after MWA. The patients were divided into two groups: hemoglobinuria group and non-hemoglobinuria group based on whether hemoglobinuria occurred after MWA.
Inclusion criteria for performing MWA were (1) definite diagnosis of a giant cavernous hemangioma > 4 cm based on the typical enhancement pattern on contrast-enhanced multiphase computed tomography (CT) or magnetic resonance imaging (MRI); (2) clinical symptoms typically caused by the giant hemangioma present for at least 1 year, including abdominal pain, nausea, vomiting, abdominal fullness. The diagnoses of HCHs were proven pathologically in all patients using US-guided core needle biopsy followed by ablation. This clinical application was approved by our institutional human research review committee. Written informed consent was obtained from all patients.
Microwave equipment and ablation technique
All treatments were performed in our institution and were carried out under US guidance with the patients under unconscious intravenous anesthesia (Propofol, 6–12 mg/kg/h; Ketamine, 1–2 mg/kg) in the operating room. The 2450 MHz or 915 MHz MW system was used. The 2450 MHz MW system (KY-2000, Kangyou Medical, China) consists of three independent MW generators, three flexible coaxial cables and three water-pumping machines, which can drive three 15-gauge cooled-shaft antennae (1.1 cm antenna tip) simultaneously. The 915MHz MW system (KY-2001, Kangyou Medical, China) consists of two independent MW generators, two flexible coaxial cables and two water-pumping machines, which can drive two 15-gauge cooled-shaft antennae (2.2 cm antenna tip) simultaneously. The two MW system generators are capable of producing 1–100 W of power output. All therapy was performed by two experienced radiologists according to the preoperative planning. Hydrodissection technique and thermal monitoring technique were applied for hemangiomas abutting vital structures to avoid thermal damage. The detailed procedures of ultrasound-guided MWA in patients with liver tumors were described in our previous publications [15, 16].
Evaluation methods
The patients’ blood tests (hepatic and renal function) and urine routine were taken before and after MWA. In the urine routine, dry chemistry analysis was used to detect Hb and flow cytometry was used to detect the red blood cells (RBCs). Hemoglobinuria was determined with the results of Hb positive and RBCs negative [13]. The wine-colored hemoglobinuria can be visually observed while deep-yellow-colored hemoglobinuria only can be detected by urine routine. Once abnormal results were discovered after MWA, hepatic or renal functions were tested every other day, and urine routine would be tested every time the patient urinated until all the results were normal. The data of tumor volume, ablation energy and ablation time in this study were all from one-session MWA. The tumor volume was calculated by the equation V = π*a*b*c/6, where a, b and c were three dimensions of the tumor measured by contrast-enhanced ultrasound. The number of antenna insertions was defined as the total number of antenna placements for each patient during ablation.
Statistical analysis
Data analysis was performed using SPSS17.0 for windows (SPSS Inc, Chicago, IL, USA) and the continuous data were expressed as means ± standard deviations (SD). Data were analyzed between the hemoglobinuria group and non-hemoglobinuria group by using the Student t test for unpaired data and the x2 test and Fisher exact test as appropriate. 11 related risk factors, including gender, age, tumor maximum diameter, tumor volume, ablation time, the number of antenna insertions, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (STB), blood urea nitrogen (BUN) and creatinine (Crea) were analyzed using univariate and multivariate binary logistic regression model method. Receiver operating characteristic (ROC) curves were constructed, and the area under the ROC curve (AUC) was calculated by using the trapezoidal rule. Optimal cutoff values for risk factors were selected to maximize sensitivity, specificity. All of the statistical tests were two-sided, and significance was set at p < 0.05.
CONCLUSIONS
In conclusion, the ablation time and No. of antenna insertion were the independent risk factors associated with hemoglobinuria when MWA for large HCHs. Less than 5 of antenna insertions and less than 20 mins of ablation time may therefore be recommended in patients with MWA of large HCHs, in order to reduce the occurrence of hemoglobinuria.
Abbreviations
- HCHs
hepatic cavernous hemangiomas
- MWA
microwave ablation
- ROC
receiver operating characteristic
- VEGF
vascular endothelial growth factor
- RFA
radiofrequency ablation
- AKI
acute kidney injury
Footnotes
Author contributions
The authors alone are responsible for the content and writing of the paper.
CONFLICTS OF INTEREST
The authors report no conflicts of interest.
FUNDING
This study was supported by the National Scientific Foundation Committee of China (grant numbers 81201167) and Beijing Nova Program (xx2013108).
REFERENCES
- 1.Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology. 1990;11:787–97. doi: 10.1002/hep.1840110512. [DOI] [PubMed] [Google Scholar]
- 2.Schnelldorfer T, Ware AL, Smoot R, Schleck CD, Harmsen WS, Nagorney DM. Management of giant hemangioma of the liver: resection versus observation. J Am Coll Surg. 2010;211:724–730. doi: 10.1016/j.jamcollsurg.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Corigliano N, Mercantini P, Amodio PM, Balducci G, Caterino S, Ramacciato G, Ziparo V. Hemoperitoneum from a spontaneous rupture of a giant hemangioma of the liver: report of a case. Surg Today. 2003;33:459–463. doi: 10.1007/s10595-002-2514-z. [DOI] [PubMed] [Google Scholar]
- 4.Watzke HH, Linkesch W, Hay U. Giant hemangioma of the liver (Kasabach-Merritt syndrome): successful suppression of intravascular coagulation permitting surgical removal. J Clin Gastroenterol. 1989;11:347–350. doi: 10.1097/00004836-198906000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Singh RK, Kapoor S, Sahni P, Chattopadhyay TK. Giant haemangioma of the liver: is enucleation better than resection? Ann R Coll Surg Engl. 2007;89:490–3. doi: 10.1308/003588407X202038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giavroglou C, Economou H, Ioannidis I. Arterial embolization of giant hepatic hemangiomas. Cardiovasc Intervent Radiol. 2003;26:92–6. doi: 10.1007/s00270-002-2648-8. [DOI] [PubMed] [Google Scholar]
- 7.Gaspar L, Mascarenhas F, da Costa MS, Dias JS, Afonso JG, Silvestre ME. Radiation therapy in the unresectable cavernous hemangioma of the liver. Radiother Oncol. 1993;29:45–50. doi: 10.1016/0167-8140(93)90172-5. [DOI] [PubMed] [Google Scholar]
- 8.Mahajan D, Miller C, Hirose K, McCullough A, Yerian L. Incidental reduction in the size of liver hemangioma following use of VEGF inhibitor bevacizumab. J Hepatol. 2008;49:867–80. doi: 10.1016/j.jhep.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 9.Ziemlewicz TJ, Wells SA, Lubner MA, Musat AI, Hinshaw JL, Cohn AR, Lee FT., Jr Microwave ablation of giant hepatic cavernous hemangiomas. Cardiovasc Intervent Radiol. 2014;37:1299–305. doi: 10.1007/s00270-014-0934-x. [DOI] [PubMed] [Google Scholar]
- 10.Park SY, Tak WY, Jung MK, Jeon SW, Cho CM, Kweon YO, Kim KC. Symptomatic-enlarging hepatic hemangiomas are effectively treated by percutaneous ultrasonography-guided radiofrequency ablation. J Hepatol. 2011;54:559–565. doi: 10.1016/j.jhep.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Gao J, Ke S, Ding XM, Zhou YM, Qian XJ, Sun WB. Radiofrequency ablation for large hepatic hemangiomas: Initial experience and lessons. Surgery. 2013;153:78–85. doi: 10.1016/j.surg.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Sharpe EE, 3rd, Dodd GD., 3rd Percutaneous radiofrequency ablation of symptomatic giant hepatic cavernous hemangiomas: report of two cases and review of literature. J Vasc Interv Radiol. 2012;23:971–975. doi: 10.1016/j.jvir.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Veerreddy P. Hemoglobinuria misidentified as hematuria: review of discolored urine and paroxysmal nocturnal hemoglobinuria. Clin Med Insights Blood Disord. 2013;6:7–17. doi: 10.4137/CMBD.S11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Tilborg AA, Dresselaars HF, Scheffer HJ, Nielsen K, Sietses C, van den Tol PM, Meijerink MR. RF Ablation of Giant Hemangiomas Inducing Acute Renal Failure: A Report of Two Cases. Cardiovasc Intervent Radiol. 2016;39:1644–1648. doi: 10.1007/s00270-016-1415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu F, Yu X, Cheng Z, Han Z, Sun Y, Liang P, Zhou F. Comparison of ultrasonography-guided percutaneous microwave ablation for subcapsular and nonsubcapsular hepatocellular carcinoma. Eur J Radiol. 2017;91:93–98. doi: 10.1016/j.ejrad.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Yu J, Yu XL, Han ZY, Cheng ZG, Liu FY, Zhai HY, Mu MJ, Liu YM, Liang P. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut. 2017;66:1172–1173. doi: 10.1136/gutjnl-2016-312629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miura JT, Amini A, Schmocker R, Nichols S, Sukato D, Winslow ER, Spolverato G, Ejaz A, Squires MH, Kooby DA, Maithel SK, Li A, Wu MC, et al. Surgical management of hepatic hemangiomas: a multi-institutional experience. HPB (Oxford) 2014;16:924–928. doi: 10.1111/hpb.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heyman SN, Rosen S, Fuchs S, Epstein FH, Brezis M. Myoglobinuric acute renal failure in the rat: a role for medullary hypoperfusion, hypoxia, and tubular obstruction. J Am Soc Nephrol. 1996;7:1066. doi: 10.1681/ASN.V771066. [DOI] [PubMed] [Google Scholar]