Abstract
In humans, hormonal regulation is crucial for the preparation of uterine environment leading to either successful implantation or menstrual cycle. Estrogen is a pivotal female steroid hormone that regulates the uterine dynamics along with progesterone in the estrous and menstrual cycles in humans. Estrogen signals act via nuclear estrogen receptor or membrane-bound receptor. The membrane-bound estrogen receptor plays a crucial role in the rapid response of estrogen in the uterine epithelium. Recently, RASD1 has received attention as a novel signal transducer of estrogen in various systems including female reproductive organs. In this review, we discuss the regulation of estrogen and RASD1 signaling in the uterus and also provide insights into RAS as a novel signaling molecule in repeated implantation failure.
Keywords: Estrogen, Estrogen receptor, RAS signaling, Repeated implantation failure, Uterus
INTRODUCTION
Uterus is an important reproductive organ in mammals, and is responsive to female hormones. The organ comprises three layers: endometrium, myometrium, and perimetrium. Endometrium is the innermost layer of the uterus, and contains luminal and glandular epithelium along with stromal region. The endometrium changes throughout the menstrual cycle in response to ovarian hormones, estrogen and progesterone. Estrogen is one of the crucial female steroid hormones and plays a key role in regulating physiological and cellular functions of various tissues including reproductive organs (1, 2). Estrogen orchestrates the cycle of uterine epithelium with another steroid hormone, progesterone, for successful uterine implantation after puberty. In this review, we will investigate the relationship between estrogen and the recently reported RAS signal as well as the implications for patient with repeated implantation failure.
ESTROGEN SIGNALING
Estrogen production from the ovary during the estrous or menstrual cycle
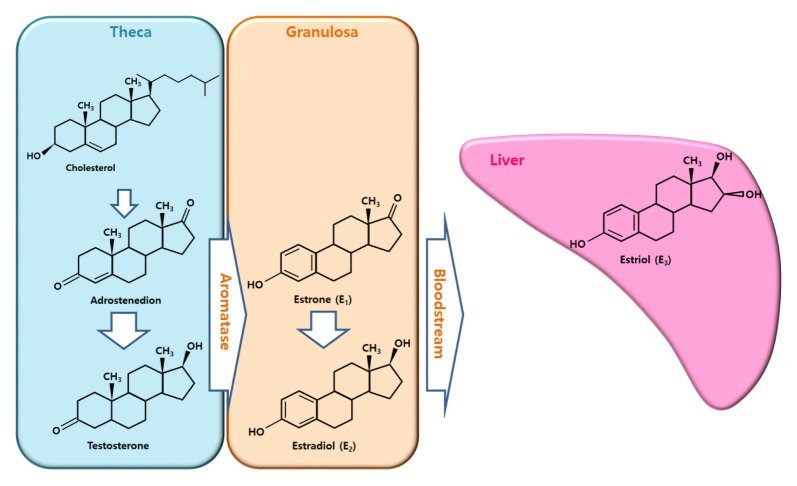
Estrogen exists in three forms such as estrone, estradiol, and estriol (1, 2). Estrogen is produced in various tissues including the ovary, placenta, fat, and the liver in females (3, 4). It is the primary source of estrogen production. Two different somatic cells known as theca and granulosa cells surround the oocyte in the ovarian follicles (3–5). Estrogen biosynthesis depends on P450 aromatase enzyme activity, which catalyzes the hydroxylation of estrogen precursors, adrostenedione or testosterone (Fig. 1). Androstenedione and testosterone are produced in theca cells from cholesterol via steroidogenesis, and diffuse into granulosa cells, which contain aromatases leading to estrogen synthesis during reproductive life. Estrogen production in the ovary changes periodically along with the estrous or menstrual cycle under the control of a gonadotropin, follicle-stimulating hormone. which is synthesized and secreted by the anterior pituitary (6).
Fig. 1.
Estrogen production. Estradiol is synthesized in the ovary based on the two-cell theory. In theca cells, androstenedione or testosterone is generated from cholesterol. In granulosa cells, estrone or estradiol is produced from androstenedione or testosterone by aromatase, which is a cell-specific enzyme. Estradiol in the blood is converted into estriol in the liver or placenta (modified from Hiller (1, 3)).
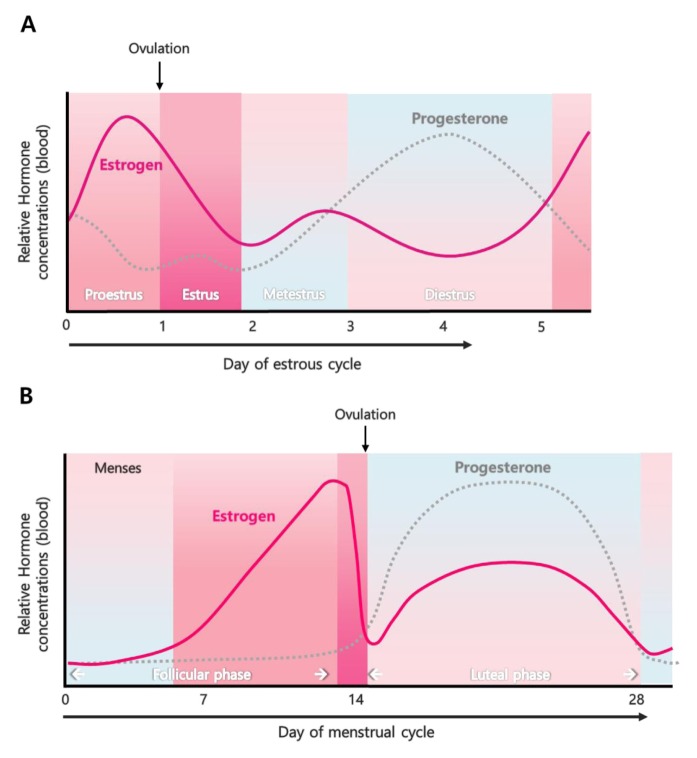
The estrogen produced in the ovary is secreted into bloodstream, bound to carrier proteins such as sex-hormone-binding globulin or albumin, and then delivered to target tissues (1, 7). Uterus is one of the main targets of estrogen. Uterus is an important secondary sex organ of the reproductive system responsive to female hormones in most mammals. The uterus consists of three layers, endometrium, myometrium and perimetrium. The growth and degeneration of uterine epithelium are dynamically and cyclically altered by estrogen and another steroid hormone, progesterone in rodents and humans (8). The dynamic regulation of uterine epithelium is precisely regulated by the endocrine system for preparation of uterine receptivity for successful implantation of embryo during early pregnancy (8). The estrous cycle in mice is divided into 4 stages, proestrus, estrous, metestrus, and diestrus, with four to five days (9). In rodents, the estrogen levels peak at proestrus stage followed by ovulation (10). The cycle in humans is called menstrual cycle which is approximately 28 days long. The uterine cycle during the menstrual cycle is divided into proliferative and secretory phases (11). Estrogen level is gradually increased in the follicular phase and peaks before the ovulation leading to thickening of the uterine endometrium (11). Human and mouse cycles vary, although the estrogen concentrations peak before the ovulation (Fig. 2).
Fig. 2.
Estrogen level during estrous cycle and menstrual cycle. (A) The estrous cycle is divided into four stages in mice: proestrus, estrus, metestrus, and diestrus. (B) The menstrual cycle is divided into two phases in humans: follicular phase and luteal phase (modified from Wang and Dey (11)).
Estrogen receptor signaling in the uterus
Estrogen levels are dynamically altered according to the cycle, and are closely related to changes in the uterine endometrium. Cunha et al. reported that estrogen induces cell proliferation and maintains secretory function in uterine epithelium (12). The role of estrogen in the uterine changes has been investigated in ovariectomized mice and knockout animal models. Estrogen induced epithelial cell proliferation in ovariectomized mice within 24 h of administration (13). Estrogen binds via two types of receptor: estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) (2). Estrogen receptors occur widely throughout the body including the uterus (14, 15) suggesting that ER signaling is important in fertility. However, the biological functions of the receptor are different. ERα knockout mice are infertile (16), whereas ERβ-deficient mice are fertile (17). ERα plays a crucial role in uterine development and function. The uterus in ERα knockout mice is hypoplastic (16). ERα signals and induces cellular proliferation and differentiation of uterine epithelium in response to estrogen (18) suggesting that estrogen regulates uterine physiology via its own receptor. Estrogen receptor mediates estrogen signaling via two signal transduction pathways (19), one of which is mediated via nuclear estrogen receptor. In the absence of estrogen, the receptor is present in cytoplasm. However, estrogen diffuses across the phospholipid membrane and binds with the cytoplasmic receptor. Estrogen binding to the receptor triggers translocation of ER into the nucleus and acts as a transcriptional factor via subsequent binding to specific DNA sequences found in the regulatory regions of estrogen-responsive target genes, called the estrogen response elements (ERE). This ERα nuclear receptor-mediated signaling is known as genomic actions.
A majority of ERα occurs in the cytoplasm or the nucleus, whereas a small portion of the receptor is located in the membrane (19). Compared with the nuclear receptor, the membrane-bound ERα signaling pathway triggers rapid transcription of estrogen to control the gene expression of target tissues resulting in rapid changes in the cellular events of hormone signal. The membrane-bound ERα is known to be associated with other complexes such as G proteins (20), tyrosine kinases (21, 22), caveolin-1/-2 (23), p130Cas (24), and striatin (25) suggesting that ERα-mediated signaling is complex resulting in an integrated cellular response with target cell specificity. These non-genomic signals are transmitted to the nucleus via various intracellular signaling pathways such as the mitogen-activated protein kinase (MAPK/ERK) and phosphoinositide 3-kinase (Pl3K/AKT) pathways (19, 26).
RAS SIGNALING
RAS superfamily
RAS proteins are encoded by Ras sarcoma (Ras) oncogenes and belong to a class of small GDP/GTP-binding guanine triphosphatases (GTPase) (27). The RAS superfamily consists of over 150 members including three major RAS isoforms: HRAS, NRAS, and KRAS (27), which play a key role in cellular processes such as proliferation, migration, adhesion, and differentiation (28). Abnormal signaling of RAS occurs in human diseases including cancer (29). RAS proteins mediate intracellular signal transduction of cellular responses by switching between active GTP-binding and inactive GDP-binding forms. The level of active and inactive RAS is regulated by guanine nucleotide exchange-factors (GEFs) and GTPase-activating proteins (GAPs) via interaction with other molecules and membrane-bound receptors (30, 31). GEFs catalyze the release of GDP and promote GTP binding to activate RAS, whereas GAPs stimulate intrinsic GTPase activity of RAS leading to GTP hydrolysis and inactivation of RAS (32). The relationship between RAS activation and ER-mediated signaling was investigated (33). in 2004. First, the p85-associated PI3-kinase, which is associated with ER, is activated via phosphorylation of a protein kinase C (PKC). The PKC induces phosphorylation and recruitment of RAS to the ER/Src/PI3K complex followed by RAS stimulation resulting in activation of RAF via the Src/Shc-SOS pathway in MCF-7 cells (33). The activation of RAS signals occurs via a cascade of mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathways. The activated RAS leads to activation of a serine/threonine-protein kinase, RAF (34), which in turn activates a serine/tyrosine/threonine kinase, MAPK/ERK kinase (MEK) (35) resulting in phosphorylation of MAPK/ERK.
RASD1 and RASD2
The RASD subfamily comprises two members, RASD1 and RASD2 (36, 37). RASD1 is a dexamethasone-induced Ras-related protein 1 (called dexras1) (38), whereas RASD2 is a Ras homolog enriched in striatum (called Rhes) (36). RASD1 was originally found in a mouse pituitary-derived cell line, AtT-20 (38). RASD2 was discovered in the striatum of rat brain (36). RASD1 and RASD2 contain longer C-termini compared with other members, although their C-termini vary (36). RASD1 contains four highly conserved GTP-binding and hydrolysis pockets, an effector loop, and a C-terminal CAAX box site for isoprenylation (39). RASD1 plays crucial roles in cellular signal transduction, growth, and proliferation via binding to receptor or interacting partners (40). RASD1 forms a heterodimer with G proteins, G1α and Gβγ. RASD1 suppresses cAMP-PKR-CREB pathway via interaction with G1α (41), and inhibits dopamine receptor-mediated sensitization through Gβγ interaction (42). In addition, RASD1 increases nitric oxide (NO) signal by interacting with neuronal nitric oxide synthase (nNOS) and CAPON in nervous system (43, 44). Cell growth is suppressed by RASD1 overexpression in several cells including NIH-3T3 fibroblast cells, MCF-7 breast cancer cell line and A549 lung adenocarcinoma cell line (45).
Regulation of RASD1 by estrogen
The expression of RASD1 was regulated by dexamehtasone treatment in AtT-20 cells (38). However, recent studies suggest that RASD1 is regulated by estrogen in several tissues including pituitary, uterus, and oviduct (37, 38, 46–48). In fact, RASD1 mRNA was exclusively detected in the brain and female reproductive organs (37, 46). Estrogen is known to regulate cell cycle in several organs including the uterus, mammary gland and pituitary (49). Kim et al. reported that the expression of RASD1 was dynamically regulated in the uterus during the estrous cycle (37). RASD1 was localized in the uterine epithelium at the proestrus stage, which is consistent with estrogen peaks in the blood (Fig. 2). Administration of estrogen significantly induced RASD1 expression in the uterine epithelium of ovariectomized mice and 3-week-old prepubertal mice (37). In addition, Jeong et al. reported that RASD1 expression was rapidly regulated by estrogen in regenerating oviduct after molting in hens (46). Wang et al. showed that RASD1 is essential for estrogen-responsive immediate early gene expression in rat pituitary cells (48). In fact, the uterine epithelium is rapidly and dynamically altered by estradiol and progesterone during the estrous cycle for preparation of pregnancy. Therefore, RASD1 might play a role in rapid signal transduction during uterine dynamics in response to estrogen.
RASD1 expression in the uterus of patients with repeated implantation failure
Interactions between blastocyst and uterus are a prerequisite for successful embryo implantation and the role of uterine endometrium plays a key role. Kim et al. reported a recent study suggesting that patients with repeated implantation failure (RIF) show aberrant gene expression in the endometrium (50) following dysregulation of several signaling pathways as well as genes. Interestingly, RASD1 expression was significantly reduced in RIF (Fig. 3) (50). In fact, the level of estrogen of RIF patients was lower than in control group (Fig. 3) suggesting that low estrogen level during menstrual cycle was related to failure of implantation.
Fig. 3.
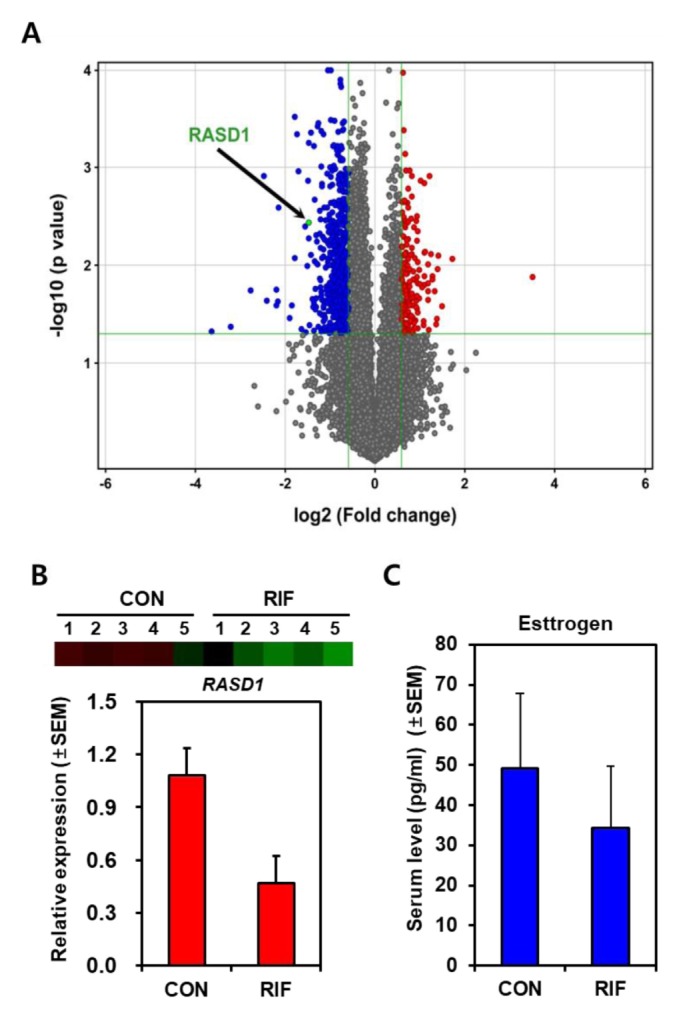
RASD1 expression in the uterine endometrium of RIF patients. (A) Gene profile analysis of RIF and the relative RASD1 location in volcano plot. (B) Semi-quantitative RT-PCR analysis was performed using total RNA extracted from uterine endometrial tissues of RIF patients (n = 5) and control women (n = 5). (C) Relative serum levels (pg/ml) of estrogen in RIF patients (n = 15) and control women (n = 7). Data modified from Choi (50).
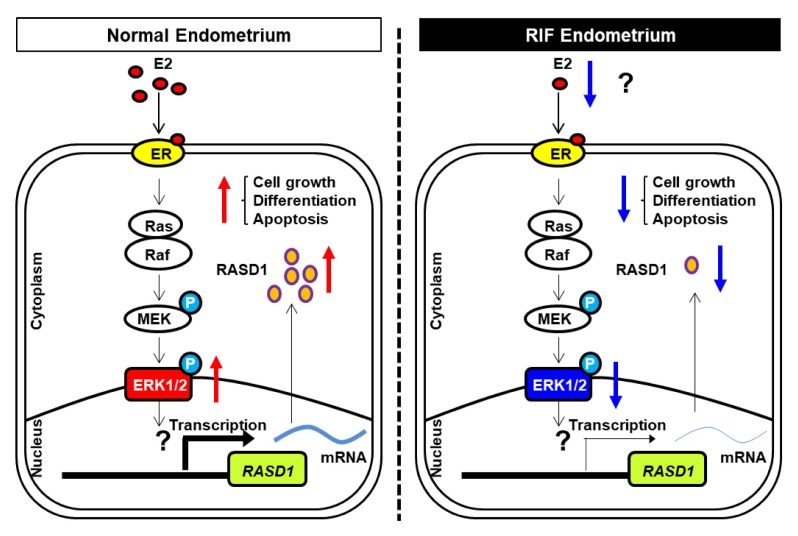
In normal conditions, RASD1 acts a signaling factor in uterine modulation leading to successful implantation, such as cellular growth and differentiation. By contrast, RIF patients show reduced estrogen levels leading to diminished RASD1 levels in the uterine endometrium, resulting in abnormal implantation via abnormal uterine regulation (Fig. 4).
Fig. 4.
Estrogen signaling pathway regulates RASD1 expression in uterine epithelium of control and RIF patients. In normal uterine endometrium, estrogen signals are transduced via the non-genomic action of estrogen receptor, which mediates the RAS/MEK/ERK pathway to regulate RASD1 expression for successful implantation. In RIF endometrium, decreased estrogen expression result in a reduction in the levels of RASD1 factors.
CONCLUSION
Uterus is the most dynamic organ, undergoing organ-specific proliferation and morphological changes, which are regulated by ovarian hormones including estrogen. The uterine epithelium is rapidly changed during the reproductive cycle. The signaling pathway requires a second messenger, such as RASD1, associated with estrogen receptor to orchestrate the uterine dynamics. The relationship between RASD1 and estrogen receptor-mediated signal transduction warrants further studies for a better understanding and insight into RIF clinically.
ACKNOWLEDGEMENTS
This was supported by a grant from the Ministry of Science, ICT & Future Planning (2015M2A2A7A03044831 & 2017M3 A9C6061361).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346:340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 2.Yasar P, Ayaz G, User SD, Gupur G, Muyan M. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod Med Biol. 2017;16:4–20. doi: 10.1002/rmb2.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited. Mol Cell Endocrinol. 1994;100:51–54. doi: 10.1016/0303-7207(94)90278-X. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman S. Are the differences between estradiol and other estrogens, naturally occurring or synthetic, merely semantical? J Clin Endocrinol Metab. 1996;81:850–851. doi: 10.1210/jcem.81.2.8636315. [DOI] [PubMed] [Google Scholar]
- 5.Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45:S116–124. doi: 10.1067/mjd.2001.117432. [DOI] [PubMed] [Google Scholar]
- 6.Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- 7.Anderson DC. Sex-hormone-binding globulin. Clin Endocrinol (Oxf) 1974;3:69–96. doi: 10.1111/j.1365-2265.1974.tb03298.x. [DOI] [PubMed] [Google Scholar]
- 8.Groothuis PG, Dassen HH, Romano A, Punyadeera C. Estrogen and the endometrium: lessons learned from gene expression profiling in rodents and human. Hum Reprod Update. 2007;13:405–417. doi: 10.1093/humupd/dmm009. [DOI] [PubMed] [Google Scholar]
- 9.Champlin AK, Dorr DL, Gates AH. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol Reprod. 1973;8:491–494. doi: 10.1093/biolreprod/8.4.491. [DOI] [PubMed] [Google Scholar]
- 10.Bingel AS. Timing of LH release and ovulation in 4-and 5-day cyclic mice. J Reprod Fertil. 1974;40:315–320. doi: 10.1530/jrf.0.0400315. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 12.Cunha GR, Lung B. The importance of stroma in morphogenesis and functional activity of urogenital epithelium. In Vitro. 1979;15:50–71. doi: 10.1007/BF02627079. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal AK, Durani S, Setty BS. Dose dependent modulation of receptor dynamics and uterine growth in immature rat by estradiol: importance of an additional nuclear binding at 24 hr for long-term (72 hr) uterine growth. Endokrinologie. 1982;79:235–241. [PubMed] [Google Scholar]
- 14.Bondesson M, Hao R, Lin CY, Williams C, Gustafsson JA. Estrogen receptor signaling during vertebrate development. Biochim Biophys Acta. 20151849:142–151. doi: 10.1016/j.bbagrm.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weihua Z, Saji S, Makinen S, et al. Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc Natl Acad Sci U S A. 2000;97:5936–5941. doi: 10.1073/pnas.97.11.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 19.Cheskis BJ, Greger JG, Nagpal S, Freedman LP. Signaling by estrogens. J Cell Physiol. 2007;213:610–617. doi: 10.1002/jcp.21253. [DOI] [PubMed] [Google Scholar]
- 20.Kumar P, Wu Q, Chambliss KL, et al. Direct interactions with G alpha i and G betagamma mediate nongenomic signaling by estrogen receptor alpha. Mol Endocrinol. 2007;21:1370–1380. doi: 10.1210/me.2006-0360. [DOI] [PubMed] [Google Scholar]
- 21.Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci U S A. 2004;101:2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song RX, McPherson RA, Adam L, et al. Linkage of rapid estrogen action to MAPK activation by ERalpha-Shc association and Shc pathway activation. Mol Endocrinol. 2002;16:116–127. doi: 10.1210/mend.16.1.0748. [DOI] [PubMed] [Google Scholar]
- 23.Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol. 2002;16:100–115. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- 24.Cabodi S, Moro L, Baj G, et al. p130Cas interacts with estrogen receptor alpha and modulates non-genomic estrogen signaling in breast cancer cells. J Cell Sci. 2004;117:1603–1611. doi: 10.1242/jcs.01025. [DOI] [PubMed] [Google Scholar]
- 25.Lu Q, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH. Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor alpha. Proc Natl Acad Sci U S A. 2004;101:17126–17131. doi: 10.1073/pnas.0407492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato S, Endoh H, Masuhiro Y, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 27.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 28.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 29.Simanshu DK, Nissley DV, McCormick F. RAS Proteins and Their Regulators in Human Disease. Cell. 2017;170:17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93:269–309. doi: 10.1152/physrev.00003.2012. [DOI] [PubMed] [Google Scholar]
- 32.McCormick F. Going for the GAP. Curr Biol. 1998;8:R673–674. doi: 10.1016/S0960-9822(98)70431-2. [DOI] [PubMed] [Google Scholar]
- 33.Castoria G, Migliaccio A, Di Domenico M, et al. Role of atypical protein kinase C in estradiol-triggered G1/S progression of MCF-7 cells. Mol Cell Biol. 2004;24:7643–7653. doi: 10.1128/MCB.24.17.7643-7653.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avruch J, Khokhlatchev A, Kyriakis JM, et al. Ras activation of the Raf kinase: tyrosine kinase recruitment of the MAP kinase cascade. Recent Prog Horm Res. 2001;56:127–155. doi: 10.1210/rp.56.1.127. [DOI] [PubMed] [Google Scholar]
- 35.Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:782–789. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falk JD, Vargiu P, Foye PE, et al. Rhes: A striatal-specific Ras homolog related to Dexras1. J Neurosci Res. 1999;57:782–788. doi: 10.1002/(SICI)1097-4547(19990915)57:6<782::AID-JNR3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Kim HR, Cho KS, Kim E, et al. Rapid expression of RASD1 is regulated by estrogen receptor-dependent intracellular signaling pathway in the mouse uterus. Mol Cell Endocrinol. 2017;446:32–39. doi: 10.1016/j.mce.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Kemppainen RJ, Behrend EN. Dexamethasone rapidly induces a novel ras superfamily member-related gene in AtT-20 cells. J Biol Chem. 1998;273:3129–3131. doi: 10.1074/jbc.273.6.3129. [DOI] [PubMed] [Google Scholar]
- 39.Graham TE, Key TA, Kilpatrick K, Dorin RI. Dexras1/AGS-1, a steroid hormone-induced guanosine triphosphate-binding protein, inhibits 3′,5′-cyclic adenosine monophosphate-stimulated secretion in AtT-20 corticotroph cells. Endocrinology. 2001;142:2631–2640. doi: 10.1210/endo.142.6.8209. [DOI] [PubMed] [Google Scholar]
- 40.McCormick F. Ras-related proteins in signal transduction and growth control. Mol Reprod Dev. 1995;42:500–506. doi: 10.1002/mrd.1080420419. [DOI] [PubMed] [Google Scholar]
- 41.Graham TE, Qiao Z, Dorin RI. Dexras1 inhibits adenylyl cyclase. Biochem Biophys Res Commun. 2004;316:307–312. doi: 10.1016/j.bbrc.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen CH, Watts VJ. Dexras1 blocks receptor-mediated heterologous sensitization of adenylyl cyclase 1. Biochem Biophys Res Commun. 2005;332:913–920. doi: 10.1016/j.bbrc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 43.Fang M, Jaffrey SR, Sawa A, Ye K, Luo X, Snyder SH. Dexras1: a G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron. 2000;28:183–193. doi: 10.1016/S0896-6273(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 44.Shen A, Chen M, Niu S, et al. Changes in mRNA for CAPON and Dexras1 in adult rat following sciatic nerve transection. J Chem Neuroanat. 2008;35:85–93. doi: 10.1016/j.jchemneu.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Vaidyanathan G, Cismowski MJ, Wang G, Vincent TS, Brown KD, Lanier SM. The Ras-related protein AGS1/RASD1 suppresses cell growth. Oncogene. 2004;23:5858–5863. doi: 10.1038/sj.onc.1207774. [DOI] [PubMed] [Google Scholar]
- 46.Jeong W, Bae H, Lim W, Bazer FW, Song G. RAS-related protein 1: an estrogen-responsive gene involved in development and molting-mediated regeneration of the female reproductive tract in chickens. Animal. 2017:1–8. doi: 10.1017/S1751731117003226. [DOI] [PubMed] [Google Scholar]
- 47.Lee Y, Kim KH, Yoon H, et al. RASD1 knockdown results in failure of oocyte maturation. Cell Physiol Biochem. 2016;40:1289–1302. doi: 10.1159/000453182. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Mitsui T, Ishida M, Izawa M, Arita J. Rasd1 is an estrogen-responsive immediate early gene and modulates expression of late genes in rat anterior pituitary cells. Endocr J. 2017;64:1063–1071. doi: 10.1507/endocrj.EJ17-0148. [DOI] [PubMed] [Google Scholar]
- 49.Foster JS, Henley DC, Ahamed S, Wimalasena J. Estrogens and cell-cycle regulation in breast cancer. Trends Endocrinol Metab. 2001;12:320–327. doi: 10.1016/S1043-2760(01)00436-2. [DOI] [PubMed] [Google Scholar]
- 50.Choi Y, Kim HR, Lim EJ, et al. Integrative analyses of uterine Transcriptome and microRNAome reveal compromised LIF-STAT3 signaling and progesterone response in the endometrium of patients with recurrent/repeated implantation failure (RIF) PLoS One. 2016;11:e0157696. doi: 10.1371/journal.pone.0157696. [DOI] [PMC free article] [PubMed] [Google Scholar]