Abstract
Background
Ulcerative colitis (UC) only involves the colonic mucosa. Yet, nearly 50% of UC patients who undergo total proctocolectomy with ileal pouch anal anastomosis (IPAA) develop UC-like inflammation of the ileal pouch (pouchitis). In contrast, patients with familial adenomatous polyposis (FAP) with IPAA develop pouchitis far less frequently. We hypothesized that pathogenic events associated with the development of UC are recapitulated by colonic-metaplastic transcriptomic reprogramming of the UC pouch.
Methods
We prospectively sampled pouch and pre-pouch ileum mucosal biopsies in UC patients with IPAA 4, 8, and 12 months after their pouch was in continuity. Mucosal samples were also obtained from FAP patients. Transcriptional profiles of the UC and FAP pouch and pre-pouch ileum were investigated via RNA sequencing and compared to data from a previously published microarray study.
Results
Unlike FAP patients, UC subjects exhibited a large set of differentially expressed genes (DEGs) between the pouch and pre-pouch ileum as early as 4 months after pouch functionalization. Functional pathway analysis of DEGs in the UC pouch revealed an enhanced state of immune/inflammatory response and extra cellular matrix remodeling. Moreover, >70% of DEGs mapped to published IBD microarray datasets displayed directional changes consistent with active UC, but not Crohn’s disease.
Conclusions
The UC pouch, well before histologic inflammation, already displays a systems-level gain of colon-associated and loss of ileum-associated genes. UC patients exhibit a unique transcriptomic response to ileal pouch creation that can be observed well before disease and may in part explain their susceptibility to the development of pouchitis.
Keywords: pouchitis, ulcerative colitis, familial adenomatous polyposis, RNA sequencing, ileal pouch
Introduction
The causes of human inflammatory bowel diseases (IBD) are still unknown although most believe they arise from an unfortunate convergence of genetic, environmental, and microbial factors - each necessary, but not sufficient to cause disease. Despite recent advances in medical therapy, approximately 30% of ulcerative colitis (UC) patients become medically refractory and undergo total proctocolectomy.1 Creation of an ileal pouch anal anastomosis (IPAA) from disease-free ileum has become the procedure of choice in these patients to preserve continence and intestinal continuity. Although UC does not affect the ileum prior to surgery, as many as 50% of patients develop an UC-like inflammatory condition ("pouchitis") in the pouch within two years after surgery.2–4 In contrast, non-IBD patients with the pre-cancerous disorder familial adenomatous polyposis (FAP) who undergo the same procedure develop pouchitis at a far lower rate (and their pouchitis is clinically different).5,6 This observation suggests that the underlying disease predisposition and the mechanisms of pathogenesis that lead to UC in the colon may be replicated within the ileal pouch after the fecal stream is re-established. Because risk and onset of IBD cannot be predicted, most studies are performed after diagnosis when many confounding variables (inflammation, medications, surgery, diet) obscure the distinction between causal and consequential events. The human pouchitis model, however, is not encumbered with many of the challenges that have limited IBD research. UC patients with IPAA provide a known, susceptible, and more homogenous population that can be easily sampled and followed prospectively from “time zero” before disease has occurred and therefore act as their own controls.
However, many studies of the UC pouch to date have been cross sectional, and unable to determine whether gene expression changes noted in the ileal pouch are transient or more lasting and related to underlying mechanisms of disease. Studies of the host have suggested that there may be partial colonic metaplasia that occurs in the ileal pouch.7–9 Villus shortening and crypt hyperplasia have been described, though histologic changes appear to be patchy and inconsistent. Some have argued that these changes may be secondary to inflammation.10 Increased sulphomucin production has also been described in the ulcerative colitis pouch, which is more characteristic of the colon, while FAP pouches retain a more sialomucin predominant mucus layer.11
Few studies have examined the gene expression changes that occur in the UC pouch and FAP pouch that may precede any histologic changes. Ben-Shachar et al examined gene expression as measured by DNA microarray in the UC normal terminal ileum, healthy pouches, chronic pouchitis, and Crohn’s like disease of the pouch.12 They found that patients with chronic pouchitis and Crohn’s disease like disease of the pouch had far more gene expression changes, and changes of higher magnitude than the normal pouch, as compared to UC ileum. However, this study was not focused on examining which host events precede the onset of inflammation, but rather which changes correlate with inflammation. Gene arrays by Paziewska et al have demonstrated that there are significant gene expression differences between the UC and FAP pouch, suggesting that there is something unique about the UC pouch that makes it more prone to pouchitis.13 Kabakchiev et al described down-regulation of some transporters and up-regulation of inflammatory pathways in the UC ileal pouch as compared to the pre-pouch ileum that correlates with outcome of no pouchitis vs pouchitis or Crohn’s like inflammation.14 Thus, several studies have noted that the UC pouch is unique as compared to the FAP pouch, and that the tissue in the ileal pouch undergoes re-programming that distinguishes it from the ileal tissue immediately above it.
In this study, we examine a homogenous, healthy group of UC patients from 4 months after their pouch is in continuity. Patients were followed longitudinally to act as their own controls at 8 months, and 12 months post pouch creation. Thus, this is the first pouch cohort to be followed longitudinally to understand which pouch gene expression changes occur early and persist over time. Pouch gene expression was examined by unbiased, highly sensitive RNA sequencing. We found that the UC pouch transcriptional profile is consistent with colonic metaplasia, changes in the extracellular matrix, enhanced immune activation, and suppressed xenobiotic metabolism and P450 signaling, i.e. changes that may explain why UC only involves the colon and why there is a propensity to develop pouchitis in the UC ileal pouch mucosa. These changes promote disease susceptibility, but are not sufficient by themselves to cause pouchitis. Additional environmental and/or microbial triggers are likely needed for the clinical onset of disease.
Materials and Methods
Patient cohort and sample collection
Patients were recruited for the prospective SHARE study at University of Chicago Medical Center and Mayo Clinic Rochester. Ulcerative colitis patients who underwent total proctocolectomy with IPAA and were over the age of 18 were eligible for the study. Participants underwent pouchoscopy at 4 months, 8 months, and 12 months after ileostomy takedown. At each visit, patient data including current medications, smoking status, and clinical symptoms were recorded. FAP patients who underwent total proctocolectomy with IPAA were recruited at the Mayo Clinic Rochester and underwent a one-time pouchoscopy. None of the FAP patient included in this study were genetically related. The Institutional Review Boards of University of Chicago and Mayo Clinic approved this study. All study subjects provided informed consent. Total RNA was isolated using the AllPrep DNA/RNA Mini Kit (QIAGEN, Cat No. 8020), RNA quality and integrity were confirmed by Nanodrop (A260/A280 ratios between 1.7 and 2.2) and Bio-Analyzer mini-gel assay.
RNA-Seq library preparation and sequencing
RNA quality was evaluated using the Bioanalyzer (Agilent, Santa Clara, CA). All RNA samples displayed RNA Integrity Number (RIN) >7. RNA-seq including cDNA library preparation was processed at the Genomics Core Facility of University of Chicago (https://fgf.uchicago.edu/). Total RNA in the amount of 100–500µg per sample was depleted of ribosomal RNA using the Ribo-Zero kit (Epicentre, Madison, WI). The directional (first strand) cDNA libraries were prepared following the guide of TruSeq Stranded Total RNA Sample Preparation kit. RNA was fragmented at 94°C for 6 min, followed by the first strand cDNA generation. Deoxy-UTP was incorporated in second strand synthesis in order to effectively quench the second strand during PCR amplification. After adenylation of the 3' end and ligation of adapters, fragments were selected and enriched with 10 cycles of PCR amplification. Clusters were generated by bridge amplification within paired-end flow cells using Illumina TruSeq PE Cluster Kit v3-cBot-HS according to manufacturer’s instructions (Illumina, San Diego, CA). The clusters on flow cells were then sequenced on the Illumina HiSeq2500 using TruSeq SBS kit v3. A total of 437 Gbase data were generated for cDNA libraries prepared from 72 samples using high output mode of 75bp paired-end (PE) reads (59 libraries) or 50bp single-end (SE) reads (16 libraries). Three libraries were sequenced twice with both 50bp SE reads and 75bp PE reads. Around 94.4% sequences passed quality checked (> Q30), yielding 2.7 billion passing filter (PF) clusters in total or ~40M PF clusters per sample.
RNA-seq reads processing and normalization
Raw data were processed with splice junction mapper Tophat.15 Briefly, reads in fastq format were aligned to the UCSC human genome hg19 using Tophat version 2.0.10 incorporated with the Bowtie version 2.1.0.16 Up to 3 mismatched nucleotides were allowed for the alignment of each read. Transcriptome assembly was performed using the R/Bioconductor package "Rsubread".17 The aligned .bam files were mapped to the reference transcriptome (UCSC known annotation).
The abundance of transcripts was summarized into RPKM (Reads Per Kilobase of transcript per Million mapped reads). Genes with a value of RPKM >1 in at least one sample were included for downstream analysis using the R/CRAN package 'limma'.18 The "voom" normalization implemented in "limma" package was applied to transform count data into log2-counts per million (logCPM) with associated precision weights.19 Between-sample normalization with the "quantile" method implemented in 'limma' was used to reduce noise associated with the clinical samples. The R/Bioconductor package "sva” was used to remove batch effects associated with the two library preparations in this study.19 Specifically, the "ComBat" algorithm implemented in "sva" was adopted to adjust for batch effects using an empirical Bayes framework.20 Correction of the batch effect was controlled using the 3 libraries that were sequenced twice using both 50bp SE and 75bp PE reads mode. The raw reads and processed data were deposited in NCBI Gene Expression Omnibus (GEO) database with accession number GSE81266, and Sequence Read Archive (SRA) database with accession number SRP074739.
Differential expression profiling
Unsupervised sample classification was performed using multi-dimensional scaling (MDS) plots. MDS, based on the Euclidean distance between each pair of samples, is an intrinsic function implemented in the R/Bioconductor package 'limma'.18 Differentially expressed genes (DEGs) were identified using the empirical Bayes (eBayes) moderated t-test implemented in the R/Bioconductor package "limma".21 P-values were adjusted for multiple comparisons using the Benjamini-Hochberg method.22 Differences between pouch and pre-pouch gene expression were also estimated independently of sample size using Cohen’s effect size, which was calculated from Cohen’s d = (Mean1-Mean2)/SD.pooled, where SD.pooled = sqr[ ((SD1)2 + (SD2)2)/2 ].23 The cross-platform comparison of effect size between RNA-seq and Morgan’s microarray data was performed by meta-analysis using R/Bioconductor package “GeneMeta”. Percentage of significant genes was determined by z score of average effect size followed by column-wise permutation within each dataset to control FDR.57
Quantitative Reverse Transcription Polymerase Chain Reaction
(RT qPCR): RNA was reverse transcribed by using anchored-oligo(dT) and random hexamer primers. Following reverse transcription, qPCR was performed on triplicate cDNA in LightCycler capillary according to the manufacturer’s protocol (Roche Applied Science). The expression of each candidate gene was calculated relative to GAPDH. Primer sequences are included below (forward, reverse ward): CEACAM7 FW 5'-CTTCAATCC GGTGGAGAACAA-3', RW 5'-CGCTGAGTAGAACGAGGGTC-3'; NXPE4 FW 5'-TGACATCCACAATCCCCAGTG-3', RW 5'-AGCCAAACTACAG GAGACAGG-3'; MUC4 FW 5'-ACAACAGCGACACTAGAGGG-3', RW 5'-ATCATCTGCGTGAG GGTGTC-3'; MUC1 FW 5'-AGTGCTTACAGTTGTTACGGGT-3', RW 5'-TCTCAGTAGAGCTGG GCACT-3'; GAPDH FW 5'-AATGGGCAGCCGTTAGGAAA-3', RW 5'-GCGCCCAATA CGACCA AATC-3'.
Canonical pathway and Gene Ontology analysis
Significant canonical pathways or gene interaction networks were analyzed by Ingenuity Pathway Analysis (IPA) software (Ingenuity Systems, Redwood City, CA) using one-tailed (referring to the overrepresented pathways) Fisher’s exact test with the criterion of Benjamini-Hochberg adjusted p-value <0.01. Significant biological processes of Gene Ontology were identified using R/Bioconductor package 'GOSim' with criterion of Benjamini-Yekutieli adjusted p<0.005.24
Sample classification and gene clustering
Sample and gene clustering based on a priori selected genes was performed using dChip software with the following parameters: distance matrice="1-correlation", linkage method="centroid".25 Self-organizing map (SOM) extracts the N most prominent patterns (where N is the number of nodes in the geometry) and arranges them so that similar patterns occur as neighbors in the SOM.26 We used the R package "SOM" to generate a 2-dimensional SOM with means and standard deviation of the centralized genes at different intestinal locations. The normalized RNA-seq data were further standardized before SOM clustering, so that each row has a mean of 0 and variance of 1.
Results
Demographic and clinical characteristics
Seventeen patients with UC and four patients with FAP were recruited at the University of Chicago and the Mayo Clinic Rochester. All patients underwent a total proctocolectomy with ileal pouch anal anastomosis (IPAA) as a standard of care. UC patients underwent a pouchoscopy for biopsy of the pre-pouch ileum and pouch at 4 months, 8 months, and 12 months after ileostomy closure (Supplemental Figure S1). None of these patients had pouchitis, as defined by the pouch disease activity index, during the time of their biopsies. For FAP patient samples, a single pouchoscopy with biopsies of the pouch and pre-pouch ileum was performed; these patients were 2–8 years removed from ileostomy closure. Demographic and clinical characteristics are as shown in Table 1. Pouch tissues were formalin-fixed, stained with hematoxylin and eosin, and assessed for histologic evidence of inflammation as well as colonic metaplasia by a blinded gastrointestinal pathologist. None of the biopsies showed significant metaplasia, characterized by villus shortening and crypt elongation. Clinical data analysis revealed that the proportion of pouchitis development was significantly higher in female than male UC patients in our small cohort (chi-sqr p=0.0133).
Table 1.
Demographics of study subjects and samples
| Clinical parameters | Patients n=21 |
Samples n=72 |
|---|---|---|
| Sex (Male /female) | 13/8 | |
| ---UC-H (UC remained healthy) | 9/2 | |
| ---UC-D (UC developing pouchitis) | 2/4 | |
| ---FAP (familial adenomatous polyposis ) | 2/2 | |
|
| ||
| Race (Caucasian/Other) | 19/2 | 62/10 |
|
| ||
| Mean age (UC-H / UC-D) | 35 / 33.8 | |
|
| ||
| Follow-up months mean (SD) | 13.5 (6.8) | |
|
| ||
| Ulcerative colitis (UC-H / UC-D) | 17 (11/6) | 64 (39/25) |
| ---pre-pouch ileum (UC-H / UC-D) | 16 (10/6) | 31 (19/12) |
| ---Pouch (UC-H / UC-D) | 17 (11/6) | 33 (20/13) |
| Months to ileostomy closure (UC): 4/8/12 | 17/11/16 | 29/20/15 |
| ---UC-H (UC remained healthy) | 11/7/13 | 17/13/9 |
| ---UC-D (UC developing pouchitis) | 6/4/3 | 12/7/7 |
|
| ||
| Familial adenomatous polyposis (FAP) | 4 | 8 |
Note: All biopsies of UC pouch and prepouch ileum were collected within 12 months after IPAA surgery.
The UC pouch and pre-pouch ileum have distinct gene expression profiles
To investigate how the transcriptome of the ileal mucosa responds to the creation of an ileal pouch, we performed deep sequencing of RNA (RNA-seq) isolated from pouch and pre-pouch ileum biopsies in UC and FAP patients. The pre-pouch ileum is the ileal segment immediately proximal to the pouch, that, even when UC pouchitis develops, is not involved by inflammation, thus serving as an internal control. We first performed unsupervised sample classification of UC pouch and pre-pouch ileum using multi-dimensional scaling (MDS) analysis. MDS highlighted the differences between the UC pouch and pre-pouch ileum at 4mo, 8mo or 12mo based on their global transcriptomic profiles (Figure 1A–C, respectively), despite the fact that the pouch is created from the same ileal tissue from which pre-pouch samples were obtained. We further combined all UC pouch and pre-pouch samples at the three time points for MDS, which also demonstrated very distinct differences between the UC pouch and pre-pouch regions (Figure 1D).
Figure 1. UC pouch and pre-pouch ileum have distinct gene expression profiles.
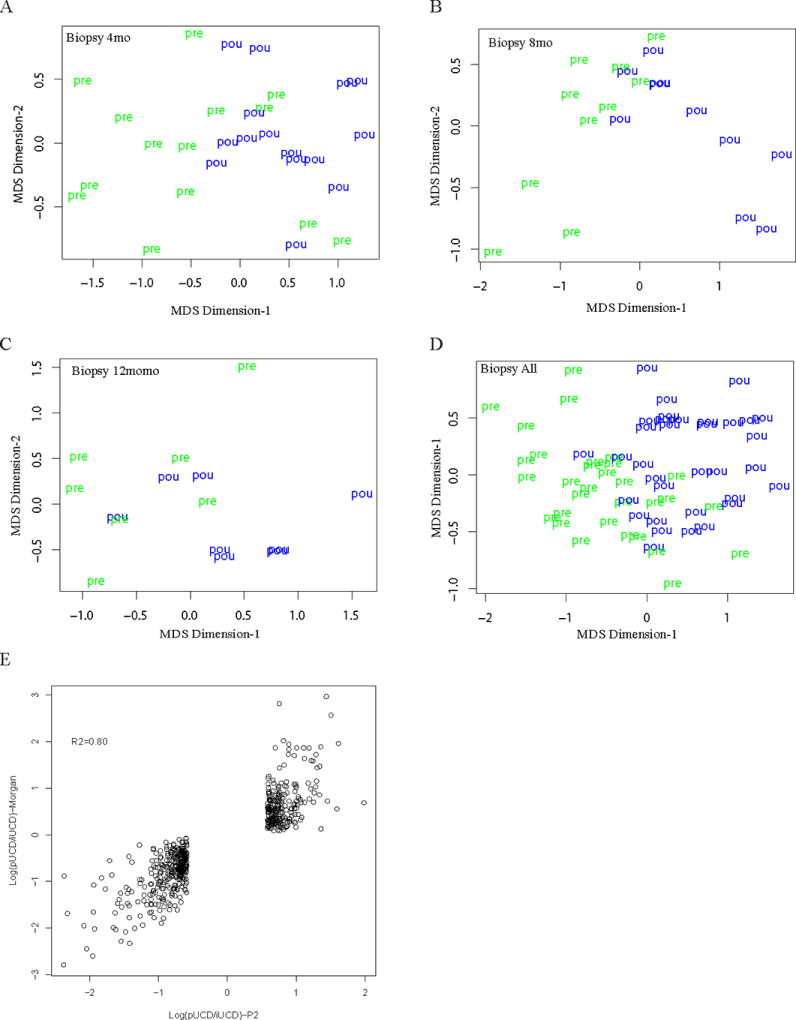
Multidimensional scaling (MDS) analysis was performed using RNA-seq data from pouch ("pou") and pre-pouch ileum ("pre") biopsies in UC patients at 4mo (A), 8mo (B), or 12mo (C) after ileostomy closure, or all time points combined (D). The MDS plots are shown with samples identified by location. (E). Concordance of gene expression changes in the UC pouch over short (<12mo) and long (>12mo) time scales. Each circle on the scatter plot represents a transcript whose expression significantly changes in the UC pouch after ileostomy closure, with distance along the x- and y-axes reflecting the log2-transformed magnitude of expression ratio between pouch and pre-pouch ileum. Values along the x-axis were computed from RNA-seq data in the present study, with criteria of fold-change > 1.5. and adjusted p<0.1. Values along the y-axis were calculated from published microarray data of pouchitis patients with biopsies collected >1 year after ileostomy closure (12).
We then identified differentially expressed genes (DEGs) between all pouch and pre-pouch ileum samples using the empirical Bayes-moderated t-test implemented in R/Bioconductor package "limma". Among our UC patient samples, we found 6521 DEGs between the pouch and pre-pouch ileum, including 3105 up- and 3416 down-regulated genes with criterion of adjusted p<0.1. In this set, 746 DEGs displayed at least a 1.5-fold change, including 378 up-regulated and 368 down-regulated genes in the pouch. In contrast to the UC pouch, none of the genes in the FAP pouch displayed significant changes when compared to the pre-pouch ileum with the same criterion of adjusted p<0.1 (Supplemental Figure S1). Given the rare nature of FAP, we had fewer FAP pouch-derived samples compared to UC pouch-derived samples. Therefore, we computed the Cohen’s effect size to assess the scale of differential gene expression between pouch and pre-pouch independent of sample size. This analysis indicated that the effect size of the pouch on ileal gene expression is overall greater in UC patients than FAP patients (Supplemental Figure S2A). This finding underscores the unique molecular characteristics of the UC pouch.
We compared our RNA-seq data to the previously published microarray data of pouch and pre-pouch ileum gene expression from 265 patients, including 233 with UC and 32 with FAP 27. This cross-sectional study compared expression profiles from intestinal biopsies taken >1 year after IPAA and included patients with or without pouchitis. Of the 746 DEGs from our project, 566 genes mapped to the microarray data from Morgan et al.27 For these 566 overlapping genes, the log2-transformed fold changes of the pouch to pre-pouch ileum were highly correlated between our dataset and the cross-sectional dataset of Morgan et al. 27 (Figure 1E). These findings indicate that the early transcriptomic alterations in our UC pouch cohort are maintained throughout the natural history of the ileal pouch, even after pouchitis develops.
Given the low number of FAP patients in our study cohort, we performed a meta-analysis including data from our RNA sequencing of UC pre-pouch ileum and pouch samples with the larger cohort of FAP samples (28 pre-pouch and 18 pouch samples) analyzed via microarray by Morgan et al. Meta-analysis was performed using the R/Bioconductor package "GeneMeta". Similar to our RNA-seq FAP samples shown in Figure S2A, this larger cohort of FAP patients also demonstrated a smaller pouch effect size than did our UC cohort, when compared by Z-score or percentage of differentially expressed genes (Supplemental Figure S2B & S2C). Moreover, only 5/46 colonic target markers identified in our UC pouch displayed differential expression (adjusted p<0.05) between FAP pouch and pre-pouch ileum in this FAP data, thus indicating a different transcriptomic pattern between the FAP and UC pouch in comparison to the corresponding pre-pouch ileal tissue.
The 378 up-regulated and 368 down-regulated genes in our UC pouch were uploaded into the Ingenuity Pathway Analysis (IPA) software to identify significantly enriched canonical pathways (Figure 2, and Supplemental Tables S2 and S3). Pathway analysis revealed transcriptional activation of the immune/inflammatory response and tissue remodeling pathways (Figure 2A), along with repression of lipid and xenobiotic metabolism (Figure 2B).
Figure 2. Canonical pathways associated with up- or down-regulated genes in the UC pouch.
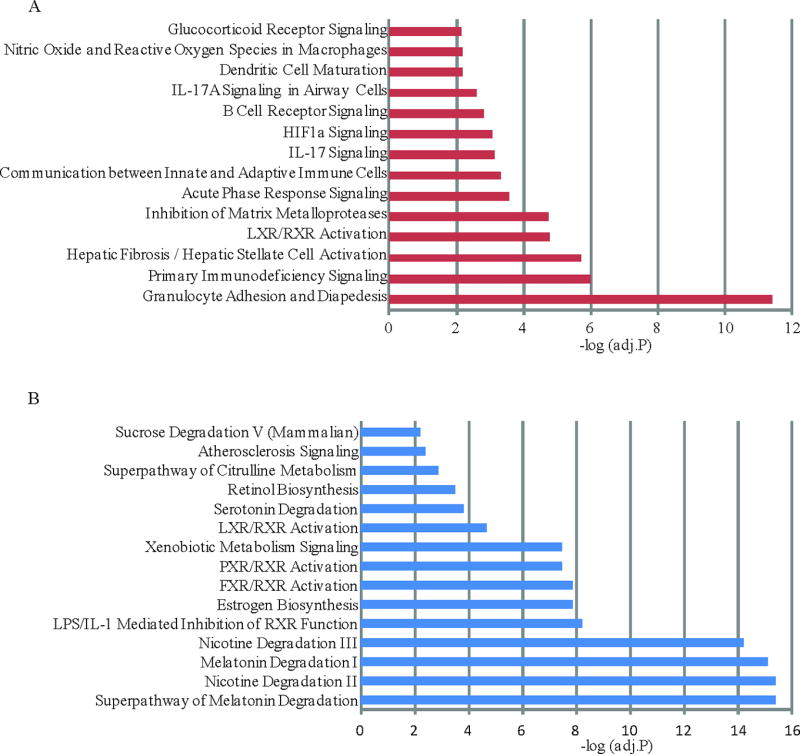
Differentially expressed genes (fold change >1.5) in pouch vs pre-pouch ileum were submitted to Ingenuity Pathway Analysis software to identify significant pathways using one-tailed Fisher’s exact test. The criterion of significance is Benjamini-Hochberg adjusted p<0.01, i.e. -log(adjusted P) > 2.0. (A) Pathways associated with up-regulated genes in pouch. (B) Pathways associated with down-regulated genes in pouch. The genes in these pathways can be found in Supplemental Table S2 or S3, respectively.
Altered barrier function may contribute to the pathogenesis of pouchitis and ulcerative colitis.28 We found that of the 138 genes in the KEGG tight junction pathway, 65 were differentially expressed between pouch and pre-pouch ileum in our UC cohort. Within this set of 65 genes, 40 genes were also differentially expressed between the inflamed pouch and uninvolved pre-pouch ileum mucosa in microarray analysis 27; importantly, 39 of these 40 genes displayed similarity in the direction and magnitude of expression change, whether analyzed by RNA-seq or microarray 27. (Supplemental Figure S3). In contrast, none of the tight junction genes demonstrated significant differences in expression between the pouch and pre-pouch in FAP patients in our cohort, in agreement with previous findings.27 Taken together, these data strongly suggest that changes in the expression of tight junction genes are a common feature of the ileal pouch in UC patients. These data support the idea that intestinal barrier dysfunction contributes to IBD risk.28
The UC pouch develops a colon-like transcriptome
We next examined whether the alterations in the pouch transcriptome in UC patients represented a shift to a more “colon-like” gene expression profile. DEGs between pouch and pre-pouch identified by RNA-seq (this study) or microarray 27 were compared against DEGs (fold change>2 and adjusted p<0.05) in microarray expression data for healthy human colon and ileum.29 A majority of the genes up-regulated in the UC pouch were also expressed at significantly higher levels in colon than in ileum, whereas the opposite tended to be true of down-regulated pouch genes (Figure 3A–C). This correlation held true regardless of disease state (pouchitis or no pouchitis), or method of transcriptome assay (microarray vs. RNA-seq). Taken together, these data confirm that the ileal pouch develops molecular features characteristic of colonic mucosa very early after IPAA, in the absence of visible histological changes. We hypothesize from our data that specific genes and pathway responses unique to colonic tissue render UC subjects susceptible to disease restricted to the colon. Interestingly, we found a slightly higher correlation of pouch gene expression to colonic gene expression in UC patients that went on to develop pouchitis (R2=0.8) (Figure 3B) than in those that remained healthy (R2=0.62) (Figure 3A). Thus, the degree to which the ileal pouch transcriptome resembles the colonic transcriptome may be an indicator of pouchitis risk, although the mechanisms underlying this shift are not understood.
Figure 3. The UC pouch transcriptome acquires colon-like features.
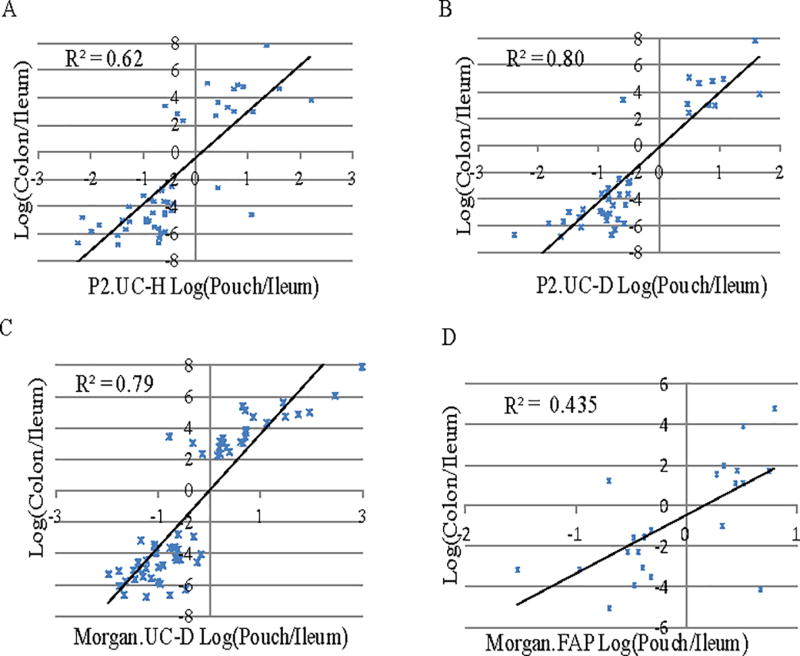
A–C, Concordance of transcript ratios between pouch/pre-pouch ileum with normal colon/ileum. Expression data for healthy human colon and ileum transcriptomes were retrieved from Comelli et al. (29). Each dot represents a single gene. (A) Correlation plot of log2-transformed (colon/ileum) expression ratio with (pouch/pre-pouch ileum) ratio in UC patients who did not develop pouchitis (UC-H). (B) Correlation plot of log2-transformed (colon/ileum) expression ratio with (pouch/pre-pouch ileum) ratio in UC patients who went on to develop pouchitis (UC-D). (C) Correlation plot of log2-transformed (colon/ileum) expression ratio with (pouch/pre-pouch ileum) ratio in pouchitis patients with active disease >1 year after ileostomy closure from re-analysis of previously published microarray dataset of Morgan, et al. (12). (D) Correlation plot of log2-transformed (colon/ileum) expression ratio with (pouch/pre-pouch ileum) ratio in FAP patients from re-analysis of previously published microarray dataset of Morgan, et al. (12). Coefficients of determination are shown in each graph.
Based on the correlation data in Figures 3A–B, we identified 46 genes that could serve as early-stage markers in the UC pouch for gain of colonic function (Quadrant 1 of Figure 3A–B, Table 2A) or loss of ileal function (Quadrant 3 of Figure 3A–B, Table 2B). All of the colonic and ileal markers identified in our cohort of UC patients in the first 12 months of their pouch were also found to have concordant expression changes amongst the patients with older pouches and active pouchitis in the study of Morgan et al. 27 (Figure 3C). In contrast, only 19 genes demonstrated consistent direction of changes in Morgan’s FAP pouch cohort and Comelli’s healthy colon cohort compared to ileum, and the correlation of alterations was much lower (Figure 3D, R2=0.435). We further validated four colonic biomarkers by RT qPCR on paired pouch and pre-pouch samples derived from an independent cohort of 13 UC patients. Consistent with the RNA-seq data, CEACAM7, NXPE4, MUC4 and MUC1 displayed significant up-regulation in the pouch as compared to the pre-pouch in the independent UC validation cohort (Figure 4).
Table 2.
| a. Colonic biomarkers: | ||||
|---|---|---|---|---|
| Symbols | logFC# | adj.P.Val# | logFC* | adj.P.Val* |
| CEACAM7 | 7.93 | 0.001 | 1.34 | 0.005 |
| FAM55D | 5.17 | 0.001 | 0.21 | 0.095 |
| CA2 | 5.04 | 0.043 | 0.79 | 0.007 |
| MUC4 | 4.91 | 0.011 | 0.90 | 0.005 |
| CD24 | 4.77 | 0.001 | 1.59 | 0.000 |
| IL1R2 | 4.75 | 0.006 | 0.72 | 0.004 |
| CA4 | 3.93 | 0.003 | 2.20 | 0.000 |
| CD177 | 3.77 | 0.023 | 0.41 | 0.050 |
| SFN | 3.39 | 0.020 | 0.60 | 0.021 |
| LEFTY1 | 3.18 | 0.008 | 0.45 | 0.096 |
| ADORA2B | 3.10 | 0.033 | 0.72 | 0.000 |
| CA12 | 3.07 | 0.015 | 1.08 | 0.000 |
| SLC16A1 | 2.79 | 0.018 | 0.38 | 0.011 |
| FXYD3 | 2.53 | 0.025 | 0.48 | 0.063 |
| b. Ileal biomarkers | ||||
|---|---|---|---|---|
| Symbols | logFC# | adj.P.Val# | logFC* | adj.P.Val* |
| SERPINA1 | −2.43 | 0.028 | −0.47 | 0.012 |
| SLC7A7 | −2.60 | 0.043 | −0.65 | 0.002 |
| SFXN3 | −2.73 | 0.020 | −0.66 | 0.000 |
| PDZK1 | −3.11 | 0.016 | −1.01 | 0.001 |
| ACE2 | −3.39 | 0.024 | −0.86 | 0.000 |
| CCL25 | −3.53 | 0.005 | −0.61 | 0.011 |
| CHRFAM7A | −3.54 | 0.034 | −0.81 | 0.000 |
| SLC5A1 | −3.58 | 0.018 | −0.55 | 0.010 |
| SI | −3.69 | 0.014 | −0.43 | 0.087 |
| ABCG5 | −3.92 | 0.041 | −1.28 | 0.000 |
| SLC6A20 | −4.21 | 0.018 | −0.47 | 0.015 |
| GPD1 | −4.35 | 0.001 | −0.60 | 0.001 |
| SLC7A9 | −4.39 | 0.002 | −0.83 | 0.002 |
| KCNJ13 | −4.52 | 0.018 | −0.60 | 0.045 |
| TM4SF4 | −4.70 | 0.047 | −2.18 | 0.000 |
| DPP4 | −4.82 | 0.030 | −0.96 | 0.000 |
| APOB | −4.90 | 0.011 | −1.41 | 0.000 |
| ENPEP | −4.95 | 0.017 | −0.91 | 0.003 |
| MAOB | −5.01 | 0.011 | −1.27 | 0.000 |
| SLC2A2 | −5.07 | 0.015 | −0.95 | 0.001 |
| AADAC | −5.27 | 0.005 | −1.85 | 0.000 |
| SLC15A1 | −5.42 | 0.019 | −0.80 | 0.000 |
| MTTP | −5.53 | 0.002 | −0.72 | 0.002 |
| APOC3 | −5.59 | 0.027 | −1.35 | 0.000 |
| CYP3A4 | −5.72 | 0.018 | −2.01 | 0.000 |
| DNASE1 | −5.76 | 0.001 | −0.60 | 0.026 |
| CYBRD1 | −5.88 | 0.020 | −0.67 | 0.004 |
| MME | −6.03 | 0.003 | −1.50 | 0.000 |
| ALDOB | −6.24 | 0.005 | −0.70 | 0.008 |
| APOA1 | −6.59 | 0.002 | −2.26 | 0.000 |
| ALPI | −6.60 | 0.000 | −0.72 | 0.005 |
| APOA4 | −6.71 | 0.003 | −1.49 | 0.000 |
Colonic biomarkers (Table 2A) or ileal biomarkers (Table 2B) were assembled from Quadrant-1 or 3 genes, respectively in Figure 3A & 3B. Fold changes were determined by normal Colon/Ileum in Comelli’s microarray data
or Pouch/pre-pouch in our RNA-seq data
Adjusted p-value were computed using R/Bioconductor package 'limma'.
Figure 4. RT qPCR validation of selected biomarkers on paired pouch and pre-pouch samples from an independent UC cohort.
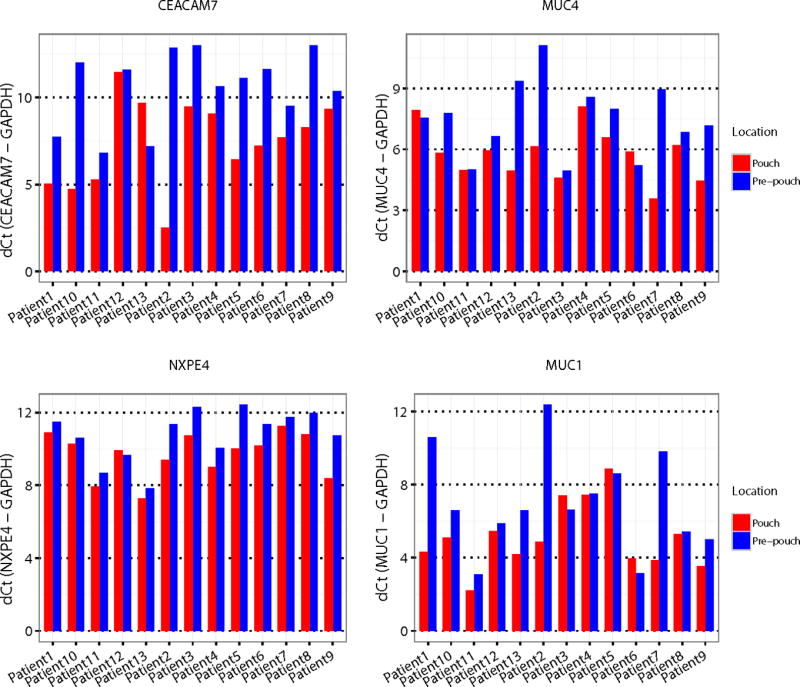
We validated four colonic biomarkers by RT qPCR on paired pouch and pre-pouch samples derived from an independent cohort of 13 UC patients. The expression of each candidate gene was calculated relative to GAPDH. The p value based on paired t-test is shown for corresponding gene. Note that lower dCt value indicates higher expression value.
Gain of colonic gene expression and loss of ileal gene expression were not found in the pouches of our FAP patients, because there were no significant DEGs between pouch and pre-pouch in FAP in our RNA-seq data. In addition, our re-analysis of microarray expression data 27 revealed that 76% (35/46) of these markers were differentially expressed (adjusted p<0.05) between the ileal pouches of UC pouchitis and FAP patients, while only 5/46 markers displayed differential expression (adjusted p<0.05) between FAP pouch and pre-pouch ileum. Thus, we conclude that maturation of the UC ileal pouch involves transcriptomic remodeling with increased colonic transcript expression and decreased ileal transcript expression, and that many of these changes must be driven by some unique biological feature of the UC pouch environment.
We further estimated the colonic shift of the UC pouch transcriptome using published microarray data of global gene expression from the normal ileum, ascending, descending and sigmoid colon.30 Differentially expressed UC pouch transcripts were examined for their expression levels along the intestine using self-organizing mapping (SOM), which groups transcripts based on similarity of their regional expression pattern (Figure 5). Among transcripts up-regulated in the UC pouch compared to pre-pouch, the largest gene cluster of regional expression (954 genes) displayed higher expression levels in colon than ileum (Figure 5A, cluster highlighted with red *). Among transcripts down-regulated in the pouch, the largest gene cluster showed decreased expression pattern along the colon vs. ileum ((Figure 5B, cluster with 1065 genes highlighted with green #). Similar results were obtained using DEGs between pouch and pre-pouch from pouchitis microarray expression data 27 as the inputs for SOM analysis (Figures 5C–D). However, SOM analysis of up-regulated pouch genes in this dataset also revealed a large cluster of genes whose expression levels along the intestinal axis decreased from ileum to colon (Figure 5C, top right highlighted with green #). The appearance of this cluster may reflect a more complex reprogramming of the transcriptome influenced by active pouch inflammation. Overall, we take these findings as further confirmation that the UC pouch transcriptome gains colonic features and loses ileal features as it undergoes reprogramming following IPAA.
Figure 5. Self-organizing map (SOM) clustering of pouch DEGs along the proximal-distal axis of normal intestine.
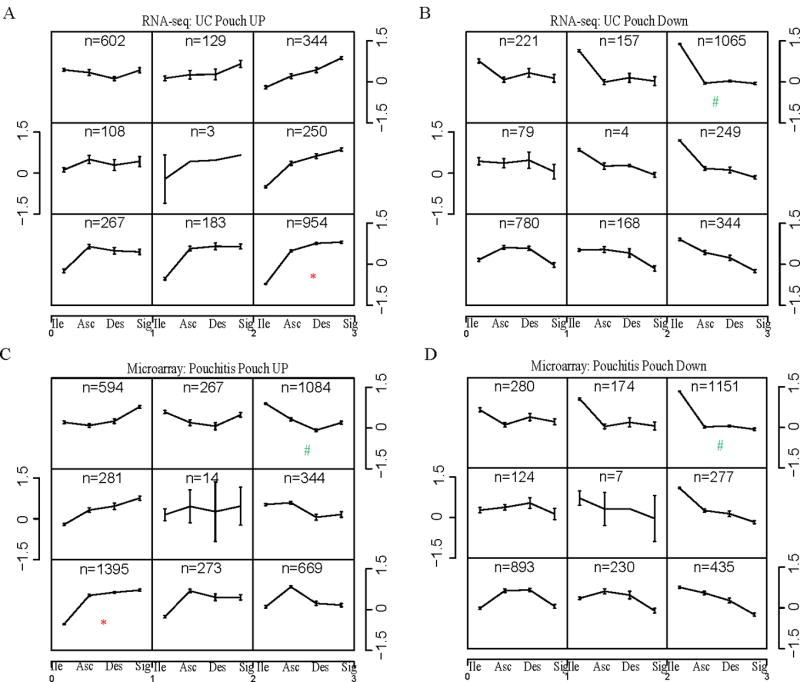
DEGs between pouch and pre-pouch ileum in our UC cohort (A–B) and from Morgan et al. (2015) (C–D) were mapped to published microarray expression data for normal human ileum and ascending, descending and sigmoid colon (30). The mapped up- or down-regulated genes in pouch were partitioned into 3×3 SOM clusters. Location within the intestine (as defined in ref. 30) is shown on the x-axis. Gene expression levels were standardized with mean of 0 and variance of 1, and shown on the y-axis. The number of up- or down-regulated genes in pouch whose expression pattern along the normal intestine matches the given pattern is shown at the top of each box. * or # indicates the largest incline or decline gene cluster in the corresponding SOM, and thus represents the major pattern of gene expression levels along the normal intestine.
Transcripts in the largest SOM gene clusters in our UC pouch cohort (Figure 5A, bottom right; and Figure 5B, top right) were submitted to Ingenuity Pathway Analysis software to identify enriched canonical pathways and networks. Interestingly, the genes in both clusters were involved in “Cell Morphology, Cellular Assembly and Organization” (Supplemental Figure S4).
Concordant gene expression profiles across longitudinal transcriptomic assay
We identified 762 or 679 DEGs between UC pouch and pre-pouch ileum at 4 months or 12 months post ileostomy closure, respectively with criterion of adjusted p<0.01 and fold change >1.5. The 374 up- or 388-down-regulated genes at 12mo were used to generate gene expression heat maps for the pouch and pre-pouch ileum samples collected 4 months postoperatively (Supplemental Figure S5). Notably, all significantly up- or down-regulated genes in the pouch over pre-pouch ileum at 12mo were also consistently increased or decreased in the 4mo pouch over 4mo pre-pouch ileum, respectively. (Supplemental Figure S5A or S5B). Similarly, all of the 328 up- or 351 down-regulated genes at 4mo pouch were also increased or decreased in the 12mo pouch compared to 12 mo pre-pouch ileum, respectively (Supplemental Figure S6A or S6B). These findings further confirmed that the transcriptomic alterations in UC pouch developed at a very early stage after ileostomy closure well before any changes in states of health and persisted.
Concordant gene expression alterations in UC pouch and in inflamed UC
The 746 DEGs (378 up- and 368 down-regulated genes) in UC pouch compared to pre-pouch ileum were mapped to the published microarray dataset GSE10191 31 containing 8 inflamed UC colon and 15 healthy colon samples. 556/746 DEGs, including 237 up- and 319 down-regulated genes in UC pouch could be mapped to the GSE10191 microarray platform. These genes were clustered based on their expression correlations in the GSE10191 dataset (Figure 6A & 6B). Of the 237 up-regulated pouch genes, 166 (70%) were higher in inflamed mucosa than normal colon (Figure 6A, blue sub-cluster of genes on left side). Similarly, 232/319 (73%) down-regulated pouch genes were decreased during inflammation compared to the normal colon (Figure 6B, blue sub-cluster of genes on left side). Gene Ontology analysis showed that the 166 mutually up-regulated genes in early stage pouch and inflamed UC were overrepresented in the biological processes involved in inflammatory response, extracellular matrix disassembly, cell matrix chemotaxis and maintenance of gastrointestinal epithelium (Supplemental Table S4). The 232 mutually down-regulated genes were overrepresented in xenobiotic metabolism, CYP450 pathway, polysaccharide digestion, sodium ion transport, vitamin K catabolism and intestinal cholesterol absorption (Supplemental Table S5).
Figure 6. Concordant transcriptome alterations in the UC pouch and UC colon.
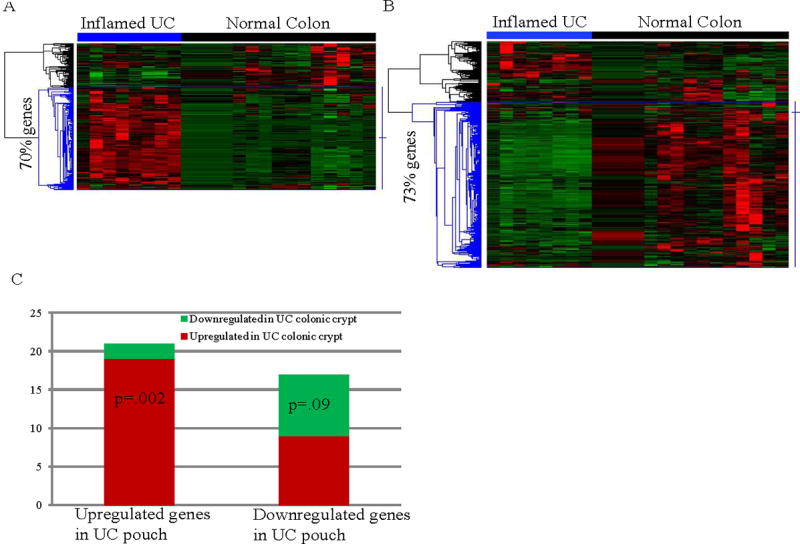
Up-regulated (A) and down-regulated genes (B) in UC pouch were mapped to the published UC colon microarray dataset GSE10191 (33), which contains 8 inflamed UC and 15 normal colon biopsy samples. Hierarchical clustering of the mapped genes was based on their correlation across all samples in GSE10191, as shown on the left dendrogram of the heatmap. Note that 70% of the up-regulated genes or 79% of the down-regulated genes in pouches were also increased or decreased in inflamed UC colons in (A) or (B) (blue highlighted dendrogram), respectively. Relative expression levels for each gene are indicated by color (black, mean level across all samples; green, below the mean; red, above the mean). (C) 49 up- and 28 down-regulated genes in UC colonic crypts compared to normal colonic crypts were retrieved from the microarray study by Kim et al. (33). The 77 genes were mapped to the up- or down-regulated genes in the UC pouch. Red or green bar color denotes up- or down-regulated genes in the UC colonic crypt, respectively. Significant overlap of up-regulated (or down-regulated) genes between UC colonic crypts and UC pouches was determined using hypergeometric distribution, p-value shown in the corresponding section of each bar.
We also mapped the DEGs in our UC pouch to a microarray dataset (GSE20881) with Crohn’s disease and healthy controls.32 The analysis did not show correspondence between gene expression profiles in Crohn’s disease and in the UC pouch (Supplemental Figure S7). These findings indicate that the pattern of transcriptomic alterations in the early stages of the pouch is consistent with that in active UC, but not with Crohn’s disease.
Enrichment analysis of UC colonic crypt genes for pouch DEGs
To further investigate similarities between the early UC pouch transcriptome and gene expression patterns associated with active UC in the colon, we retrieved the 49 up- and 28 down-regulated gene in UC colonic crypt compared to normal healthy colonic crypt from the microarray data published by Kim et al 33 (Figure 5C). Hypergeometric distribution testing revealed significant enrichment of the UC colonic crypt genes among pouch up-regulated genes (p=2.1×10−4, Supplemental Table S6). Notably, 79% (15/19) of the UC colonic crypt genes that displayed up-regulation in our UC pouch were also up-regulated in the active pouchitis pouch (12) (Supplemental Table S6). These findings highlight shared molecular features between UC ileal pouch and UC colonic crypt pathogenesis.
Discussion
This study, the first longitudinal analysis of transcriptomic changes in the ileal pouch that occur during the first year after IPAA, provides several novel insights into the pathogenesis of UC pouchitis. In UC patients, the pouch undergoes a unique shift in its global transcriptional program soon after functionalization. As a result of this transcriptomic reprogramming, many genes typically expressed in the colon become activated in the mucosa of the ileal pouch. At the same time, many ileum-associated transcripts also become less abundant. We showed that the colonic shift of transcriptome is a typical and dominant profile unique to the early stage of the UC pouch. This transcriptomic phenomenon occurs in the absence of detectable histological changes, showing that levels of specific RNAs are highly sensitive markers of tissue transformation. Many of the affected transcripts are found in pathways associated with inflammation and extracellular matrix remodeling. These changes are highly correlated with transcriptomic data from a recent cross-sectional study of pouchitis patients 27, and also partially resemble the molecular pathogenesis of UC.31,33
The significant shifts that occur in the UC pouch transcriptome appear to provide unique insights into why this tissue becomes susceptible to the development of inflammation. Canonical pathways enriched for up-regulated genes in the UC pouch included "IL17 signaling", "Communication between Innate and Adaptive Immune Cells", "Primary Immunodeficiency Signaling and Acute Phase Response Signaling” (Supplemental Table S2). This analysis supports proposed roles for aberrant immune function in pouchitis etiology.4,34,35 Down-regulated genes in the UC pouch are enriched for multiple lipid metabolism and signaling pathways, including "LXR/RXR activation" and "FXR/RXR activation" (Supplemental Table S2 & S3). "LXR/RXR activation" is involved in the regulation of lipid metabolism, inflammation, and cholesterol to bile acid catabolism.36 Activation of LXRs strongly suppresses the expression of inflammatory mediators, such as TNFα.37 "FXR/RXR activation" plays a crucial role in linking bile acid regulation with lipoprotein, lipid and glucose metabolism.38 Nicotine suppresses the production of pro-inflammatory cytokines, attenuates inflammation and improves gut function in patients with active colitis.39 Other studies have also suggested that active smokers have fewer episodes of UC pouchitis after proctocolectomy.40 Consistent with this notion, our findings of the suppression of nicotine degradation pathway in the ileal pouch (Figure 2B) may be relevant and underlie the interesting epidemiological relationship between UC and smoking.41 Finally, altered mucosal barrier function might serve as an additional contributor to disease susceptibility, given that 39 genes encoding tight junction components are differentially expressed between the UC pouch and pre-pouch ileum in both of our RNA-seq UC cohort and Morgan’s microarray pouchitis cohort (Supplemental Figure S3). In summary, our functional pathway analysis uncovered several molecular mechanisms present in early stage pouch tissues that may promote the development of pouchitis.
The colon-like shift that the ileal tissue undergoes after IPAA may increase disease susceptibility. The colonic biomarker CEACAM-7 (carcinoembryonic antigen-related cell adhesion molecule 7) was among the most highly up-regulated genes in the UC pouch in this study and also in the previous study of Morgan et al. 27 Moreover, CEACAM-7 was previously known to be overexpressed in the histologically normal crypts micro-dissected from formalin-fixed biopsies of early stage ulcerative colitis before active inflammation is initiated, suggesting that this gene is relevant to the pathogenesis of ulcerative colitis (Supplemental Table S6).33 CEACAM7 is part of an immunoglobulin superfamily of genes that have numerous functions including cell adhesion and signaling.42 Like other members of the family, CEACAM-7 is GPI-linked and, in epithelium, is sorted to the apical membrane. However, very little is known about its functions, except it lacks a cytoplasmic tail that usually is needed for cell signaling.42 CEACAM-7 has been proposed to modify the function of other CEACAM isoforms through protein-protein interactions.43 Its up-regulation in ileal pouch as colonic biomarker, and in colonic crypt epithelia of early stage UC illustrates mutual molecular pathogenesis for pouchitis and UC.
We hypothesize that the gene expression changes that render previously normal ileal tissue susceptible to the possible development of pouchitis may resemble the original disease promoting gene expression changes in UC. The expression of MMPs in the ileal pouch shares characteristics with inflamed IBD tissues.44 MMP1, MMP2, and MMP3 are increased in both pouchitis and UC.45–49 Degradation of extracellular matrix and tissue remodeling are achieved through the concerted action of diverse extracellular proteases. Among these, MMPs and their physiological counter-parts, tissue inhibitors of metalloproteinases (TIMPs) are of central importance50 MMPs are involved in mucosal destruction and crypt hyperplasia in pouchitis.45 Our pathway analysis of transcriptomic profile in early stage pouch revealed an increased tissue remodeling activity associated with up-regulation of MMP1, MMP3, MMP10, MMP12 and TIMP1 (Supplemental Table S2). These findings point to shared transcriptional regulation for extracellular matrix remodeling as a key feature of pathogenesis in both pouchitis and UC. Importantly, the patients in this study were sampled before the onset of any disease. Thus, the similarity in gene expression between the non-inflamed ileal pouch and actively inflamed UC tissue suggests that these ileal pouch gene expression changes may be a precondition for the development of inflammation triggered by other environmental (possibly microbial) factors. In this regard, our group recently reported the presence of unique Bacteroides capsular polysaccharide (CPS) adaptations between luminal and mucosal strains within different UC pouches. Based on the known virulence potential of CPS genes to stimulate, suppress, and/or evade host-specific immune and epithelial responses, we speculate that genetic elements transferred horizontally may impart to microbial strains the ability to become causative agents of disease on a background of host genetic susceptibility (Vineis et al. in press MBio).
Although this study provides important insights into the molecular processes associated with early stage pouch progression, there are some limitations. Our patient cohort was relatively small especially with respect to FAP patients. As a result, a gender bias was observed in our UC cohort in that more female patients developed pouchitis (chi-sqr test p=0.0133). This is in concordance with a published report about an increased risk for the development of pouchitis in female patients 3. While the gender bias cannot be fully avoided in prospective enrollment of patients, a larger cohort of patients could help minimize such bias. Because the FAP sample size is small in our current RNA-seq study, we performed a meta-analysis to validate the larger effect size of the pouch in our RNA-seq UC cohort as compared to Morgan’s larger microarray analyzed FAP cohort. (Supplemental Figure S2B). Due to the early time points chosen for this study and the small numbers of patients, distinguishing the noted gene expression changes by pouchitis phenotype was not possible in this study. However, future studies that follow patients for a longer time period may be better able to characterize antibiotic refractory patients that may more closely resemble UC patients.
Our data provide new insights into why the previously normal ileal tissue of UC patients becomes susceptible to the development of inflammation after creation of an ileal pouch. Furthermore, FAP patients rarely develop pouchitis despite undergoing the same procedure. We propose that the unique shift toward a colon-like transcriptome in the UC patient pouch (Figures 3 and 5) renders the mucosa susceptible to UC-related processes that potentiate the development of inflammation. Moreover, a large set of up- or down-regulated genes (Figure 6), and activated or suppressed biological processes are shared between early stage ileal pouch and inflamed UC (Supplemental Tables S4 and S5). Given that both patients that did and did not develop pouchitis exhibited ileal pouch gene expression changes as compared to the pre pouch ileum, these changes likely set the preconditions for inflammation, and other environmental factors are likely needed to ultimately trigger inflammation. Onset of disease likely requires an interaction between the pro-inflammatory pouch tissue and other yet unknown factors, including the pouch microbiota.51
Supplementary Material
Acknowledgments
The authors would like to thank Dr. John Kwon and Dr. Gautham Reddy for performing pouchoscopy procedures, Amy Duong and Kristi Kearney for patient recruitment, Nate Hubert for sample collection and RNA isolation, and Julia Tao for RT-PCR. The authors thanks Mike Jarsulic and the University of Chicago Center for Research Informatics (https://cri.uchicago.edu) for their computing resources.
Funding: The authors received funding from the Leona M. And Harry B. Helmsley Charitable Trust (SHARE), the NIH grants P30 DK42086, DK97268, DK47722, UH2/UH3DK083993, T32 DK07074, and the Gastrointestinal Research Foundation of Chicago.
Footnotes
Competing interests: None
References
- 1.Bernstein CN, Ng SC, Lakatos PL, Moum B, Loftus EVJ. A review of mortality and surgery in ulcerative colitis: milestones of the seriousness of the disease. Inflamm Bowel Dis. 2013;19(9):2001–2010. doi: 10.1097/MIB.0b013e318281f3bb. [DOI] [PubMed] [Google Scholar]
- 2.Hoda KM, Collins JF, Knigge KL, Deveney KE. Predictors of pouchitis after ileal pouch-anal anastomosis: a retrospective review. Dis Colon Rectum. 2008;51(5):554–560. doi: 10.1007/s10350-008-9194-7. [DOI] [PubMed] [Google Scholar]
- 3.Simchuk EJ, Thirlby RC. Risk Factors and True Incidence of Pouchitis in Patients after Ileal Pouch-Anal Anastomoses. World J Surg. 2000;24(7):851–856. doi: 10.1007/s002680010136. [DOI] [PubMed] [Google Scholar]
- 4.Hurst RD, Molinari M, Chung TP, Rubin M, Michelassi F. Prospective study of the incidence, timing and treatment of pouchitis in 104 consecutive patients after restorative proctocolectomy. Arch Surg. 1996;131(5):492–497. doi: 10.1001/archsurg.1996.01430170043007. [DOI] [PubMed] [Google Scholar]
- 5.Kartheuser A, Stangherlin P, Brandt D, Remue C, Sempoux C. Restorative proctocolectomy and ileal pouch-anal anastomosis for familial adenomatous polyposis revisited. Fam Cancer. 2006;5(3):241–260. doi: 10.1007/s10689-005-5672-4. [DOI] [PubMed] [Google Scholar]
- 6.Lovegrove RE, Tilney HS, Heriot AG, et al. A comparison of adverse events and functional outcomes after restorative proctocolectomy for familial adenomatous polyposis and ulcerative colitis. Dis Colon Rectum. 2006;49(9):1293–1306. doi: 10.1007/s10350-006-0608-0. [DOI] [PubMed] [Google Scholar]
- 7.Trovato C, Sonzogni A, Fiori G, et al. Confocal laser endomicroscopy for the detection of mucosal changes in ileal pouch after restorative proctocolectomy. Dig Liver Dis. 2009;41(8):578–585. doi: 10.1016/j.dld.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Arashiro RT, de G, Teixeira MG, Rawet V, et al. Histopathological evaluation and risk factors related to the development of pouchitis in patients with ileal pouches for ulcerative colitis. Clinics (Sao Paulo) 2012;67(7):705–710. doi: 10.6061/clinics/2012(07)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merrett MN. Ileal pouches: adaptation and inflammation. Baillieres Clin Gastroenterol. 1997;11(1):175–193. doi: 10.1016/s0950-3528(97)90060-9. http://www.ncbi.nlm.nih.gov/pubmed/9192067. [DOI] [PubMed] [Google Scholar]
- 10.Fruin AB, El-Zammer O, Stucchi AF, et al. Colonic metaplasia in the ileal pouch is associated with inflammation and is not the result of long-term adaptation. J Gastrointest Surg. 2003;7(2):246–254. doi: 10.1016/S1091-255X(02)00191-9. [DOI] [PubMed] [Google Scholar]
- 11.Bambury N, Coffey JC, Burke J, Redmond HP, Kirwan WO. Sulphomucin expression in ileal pouches: emerging differences between ulcerative colitis and familial adenomatous polyposis pouches. Dis Colon Rectum. 2008;51(5):561–567. doi: 10.1007/s10350-008-9200-0. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Shachar S, Yanai H, Baram L, et al. Gene expression profiles of ileal inflammatory bowel disease correlate with disease phenotype and advance understanding of its immunopathogenesis. Inflamm Bowel Dis. 2013;19(12):2509–2521. doi: 10.1097/01.MIB.0000437045.26036.00. [DOI] [PubMed] [Google Scholar]
- 13.Paziewska A, Horbacka K, Goryca K, et al. Transcriptional changes between unin fl amed ulcerative colitis and familial adenomatous polyposis pouch mucosa can be attributed to an altered immune response. 2015;62(1):69–75. doi: 10.18388/abp.2014_778. [DOI] [PubMed] [Google Scholar]
- 14.Kabakchiev B, Tyler A, Stempak JM, Milgrom R, Silverberg MS. Downregulation of expression of xenobiotic efflux genes is associated with pelvic pouch inflammation in ulcerative colitis. Inflamm Bowel Dis. 2014;20(7):1157–1164. doi: 10.1097/MIB.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 15.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao Y, Smyth GK, Shi W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013;41(10):e108. doi: 10.1093/nar/gkt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smyth G. Limma: linear models for microarray data. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. 2005:397–420. [Google Scholar]
- 19.Law CW, Chen Y, Shi W, Smyth GK. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15(2):R29. doi: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28(6):882–883. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57(1):289–300. doi: 10.2307/2346101. [DOI] [Google Scholar]
- 23.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 24.Alexa A, Rahnenfuhrer J, Lengauer T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics. 2006;22(13):1600–1607. doi: 10.1093/bioinformatics/btl140. [DOI] [PubMed] [Google Scholar]
- 25.Li C. Automating dChip: toward reproducible sharing of microarray data analysis. BMC Bioinformatics. 2008;9:231. doi: 10.1186/1471-2105-9-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamayo P, Slonim D, Mesirov J, et al. Interpreting patterns of gene expression with self-organizing maps: methods and application to hematopoietic differentiation. [Accessed March 24, 2016];Proc Natl Acad Sci U S A. 1999 96(6):2907–2912. doi: 10.1073/pnas.96.6.2907. http://www.ncbi.nlm.nih.gov/pubmed/10077610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan XC, Kabakchiev B, Waldron L, et al. Associations between host gene expression, the mucosal microbiome, and clinical outcome in the pelvic pouch of patients with inflammatory bowel disease. Genome Biol. 2015;16(1):67. doi: 10.1186/s13059-015-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGuckin MA, Eri R, Simms LA, Florin THJ, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15(1):100–113. doi: 10.1002/ibd.20539. [DOI] [PubMed] [Google Scholar]
- 29.Comelli EM, Lariani S, Zwahlen M-C, et al. Biomarkers of human gastrointestinal tract regions. Mamm Genome. 2009;20(8):516–527. doi: 10.1007/s00335-009-9212-7. [DOI] [PubMed] [Google Scholar]
- 30.Noble CL, Abbas a R, Cornelius J, et al. Regional variation in gene expression in the healthy colon is dysregulated in ulcerative colitis. Gut. 2008;57(10):1398–1405. doi: 10.1136/gut.2008.148395. [DOI] [PubMed] [Google Scholar]
- 31.Ahrens R, Waddell A, Seidu L, et al. Intestinal macrophage/epithelial cell-derived CCL11/eotaxin-1 mediates eosinophil recruitment and function in pediatric ulcerative colitis. [Accessed March 24, 2016];J Immunol. 2008 181(10):7390–7399. doi: 10.4049/jimmunol.181.10.7390. http://www.ncbi.nlm.nih.gov/pubmed/18981162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noble CL, Abbas AR, Lees CW, et al. Characterization of intestinal gene expression profiles in Crohn’s disease by genome-wide microarray analysis. Inflamm Bowel Dis. 2010;16(10):1717–1728. doi: 10.1002/ibd.21263. [DOI] [PubMed] [Google Scholar]
- 33.Kim M, Lee S, Yang SK, Song K, Lee I. Differential expression in histologically normal crypts of ulcerative colitis suggests primary crypt disorder. Oncol Rep. 2006;16(4):663–670. [PubMed] [Google Scholar]
- 34.Penna C, Tiret E, Kartheuser A, Hannoun L, Nordlinger B, Parc R. Function of ileal J pouch-anal anastomosis in patients with familial adenomatous polyposis. [Accessed March 24, 2016];Br J Surg. 1993 80(6):765–767. doi: 10.1002/bjs.1800800638. http://www.ncbi.nlm.nih.gov/pubmed/8392425. [DOI] [PubMed] [Google Scholar]
- 35.Tjandra JJ, Fazio VW, Church JM, Oakley JR, Milsom JW, Lavery IC. Similar functional results after restorative proctocolectomy in patients with familial adenomatous polyposis and mucosal ulcerative colitis. [Accessed March 24, 2016];Am J Surg. 1993 165(3):322–325. doi: 10.1016/s0002-9610(05)80834-7. http://www.ncbi.nlm.nih.gov/pubmed/8383471. [DOI] [PubMed] [Google Scholar]
- 36.Edwards PA, Kennedy MA, Mak PA. LXRs; oxysterol-activated nuclear receptors that regulate genes controlling lipid homeostasis. [Accessed March 24, 2016];Vascul Pharmacol. 2002 38(4):249–256. doi: 10.1016/s1537-1891(02)00175-1. http://www.ncbi.nlm.nih.gov/pubmed/12449021. [DOI] [PubMed] [Google Scholar]
- 37.Jakobsson T, Vedin L-L, Hassan T, et al. The oxysterol receptor LXRβ protects against DSS- and TNBS-induced colitis in mice. Mucosal Immunol. 2014;7(6):1416–1428. doi: 10.1038/mi.2014.31. [DOI] [PubMed] [Google Scholar]
- 38.Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. [Accessed March 24, 2016];Science. 1999 284(5418):1362–1365. doi: 10.1126/science.284.5418.1362. http://www.ncbi.nlm.nih.gov/pubmed/10334992. [DOI] [PubMed] [Google Scholar]
- 39.McGrath J, McDonald JWD, Macdonald JK. Transdermal nicotine for induction of remission in ulcerative colitis. Cochrane database Syst Rev. 2004;(4):CD004722. doi: 10.1002/14651858.CD004722.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Merrett MN, Mortensen N, Kettlewell M, Jewell DO. Smoking may prevent pouchitis in patients with restorative proctocolectomy for ulcerative colitis. Gut. 1996;38(3):362–364. doi: 10.1136/gut.38.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motley RJ, Rhodes J, Ford GA, et al. Time relationships between cessation of smoking and onset of ulcerative colitis. Digestion. 1987;37(2):125–127. doi: 10.1159/000199478. [DOI] [PubMed] [Google Scholar]
- 42.Tchoupa AK, Schuhmacher T, Hauck CR. Signaling by epithelial members of the CEACAM family - mucosal docking sites for pathogenic bacteria. Cell Commun Signal. 2014;12:27. doi: 10.1186/1478-811X-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonsor DA, Beckett D, Sundberg EJ. Structure of the N-terminal dimerization domain of CEACAM7. Acta Crystallogr Sect F, Struct Biol Commun. 2015;71(Pt 9):1169–1175. doi: 10.1107/S2053230×15013576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mäkitalo L, Piekkala M, Ashorn M, et al. Matrix metalloproteinases in the restorative proctocolectomy pouch of pediatric ulcerative colitis. World J Gastroenterol. 2012;18(30):4028–4036. doi: 10.3748/wjg.v18.i30.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stallmach A, Chan CC, Ecker KW, et al. Comparable expression of matrix metalloproteinases 1 and 2 in pouchitis and ulcerative colitis. [Accessed March 24, 2016];Gut. 2000 47(3):415–422. doi: 10.1136/gut.47.3.415. http://www.ncbi.nlm.nih.gov/pubmed/10940281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ulisse S, Gionchetti P, D’Alò S, et al. Expression of cytokines, inducible nitric oxide synthase, and matrix metalloproteinases in pouchitis: effects of probiotic treatment. Am J Gastroenterol. 2001;96(9):2691–2699. doi: 10.1111/j.1572-0241.2001.04139.x. [DOI] [PubMed] [Google Scholar]
- 47.Bailey CJ, Hembry RM, Alexander A, Irving MH, Grant ME, Shuttleworth CA. Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn’s disease and normal intestine. [Accessed March 24, 2016];J Clin Pathol. 1994 47(2):113–116. doi: 10.1136/jcp.47.2.113. http://www.ncbi.nlm.nih.gov/pubmed/8132824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matthes H, Stallmach A, Matthes B, Herbst H, Schuppan D, Riecken EO. [Indications for different collagen metabolism in Crohn disease and ulcerative colitis] [Accessed March 24, 2016];Med Klin (Munich) 1993 88(4):185–192. http://www.ncbi.nlm.nih.gov/pubmed/8492773. [PubMed] [Google Scholar]
- 49.Günther U, Matthes H, Herbst H, Stallmach A, Riecken EO, Schuppan D. Phenotype of cells expressing matrix metalloproteinase-3 in ulcerative colitis. [Accessed March 24, 2016];Ann N Y Acad Sci. 1998 859:237–240. doi: 10.1111/j.1749-6632.1998.tb11137.x. http://www.ncbi.nlm.nih.gov/pubmed/9928396. [DOI] [PubMed] [Google Scholar]
- 50.Lakatos G, Hritz I, Varga MZ, et al. The impact of matrix metalloproteinases and their tissue inhibitors in inflammatory bowel diseases. Dig Dis. 2012;30(3):289–295. doi: 10.1159/000336995. [DOI] [PubMed] [Google Scholar]
- 51.Young VB, Raffals LH, Huse SM, et al. Multiphasic analysis of the temporal development of the distal gut microbiota in patients following ileal pouch anal anastomosis. Microbiome. 2013;1(1):9. doi: 10.1186/2049-2618-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


