Abstract
For decades the brain was erroneously considered an insulin insensitive organ. Although gaps in our knowledge base remain, conceptual frameworks are starting to emerge to provide insight into the mechanisms through which insulin facilitates critical brain functions like metabolism, cognition, and motivated behaviors. These diverse physiological and behavioral activities highlight the region-specific activities of insulin in the CNS; that is, there is an anatomical context to the activities of insulin in the CNS. Similarly, there is also a temporal context to the activities of insulin in the CNS. Indeed, brain insulin receptor activity can be conceptualized as a continuum in which insulin promotes neuroplasticity from development into adulthood where it is an integral part of healthy brain function. Unfortunately, brain insulin resistance likely contributes to neuroplasticity deficits in obesity and type 2 diabetes mellitus (T2DM). This neuroplasticity continuum can be conceptualized by the mechanisms through which insulin promotes cognitive function through its actions in brain regions like the hippocampus, as well as the ability of insulin to modulate motivated behaviors through actions in brain regions like the nucleus accumbens and the ventral tegmental area. Thus, the goals of this review are to highlight these anatomical, temporal, and functional contexts of insulin activity in these brain regions, and to identify potentially critical time points along this continuum where the transition from enhancement of neuroplasticity to impairment may take place.
Keywords: Glutamate, Nucleus accumbens, Ventral tegmental area, Hippocampus, Cognition, Motivation
1. Introduction
Insulin is synthesized in pancreatic β cells and increases in plasma glucose levels stimulate the release of insulin from the pancreas into the circulation. Once released into circulation, insulin binds to and activates insulin receptors in the periphery and thereby stimulates several signaling cascades, including the PI-3 kinase/Akt pathway and the MEK pathway, the former of which is responsible for insulin-stimulated translocation of glucose transporter 4 (GLUT4) to the plasma membrane. Through the activation of these pathways, insulin stimulates glucose uptake from blood into peripheral tissues like muscle and fat. For many years it was assumed that the activities of insulin were restricted to the periphery, and as such it was erroneously assumed that the brain should be considered an ‘insulin insensitive’ organ. The initial acceptance that insulin mediates activities in the CNS came from studies demonstrating that insulin plays important roles in homeostatic regulation mediated by the hypothalamus (Schwartz et al., 2000). However, more recently it has become clear that the actions of insulin are more widespread in the CNS, and are a critical part of normal development (Chiu and Cline, 2010), as well as plasticity throughout adulthood (Park, 2001).
In addition to peripheral effects, insulin crosses the blood-brain barrier via a saturable transport system (Banks et al., 2012). The current consensus in the field is that the insulin gene is not expressed in adult brain, and thus neural actions of insulin are mediated by active transport of peripheral insulin into the CNS (Banks, 2004; Schwartz et al., 2010). However, debate remains in part because of changes in insulin polypeptide genes have been observed in disease states including Alzheimer's Disease (Steen et al., 2005). While insulin receptors are expressed in the CNS and stimulate GLUT4 translocation in the brain (Grillo et al., 2009), it is very likely that the majority of glucose uptake in the brain is insulin-independent (McEwen and Reagan, 2004). Instead of regulating glucose metabolism, the current evidence supports the concept that insulin facilitates critical brain functions ranging from metabolism and feeding, to cognition and motivation. These diverse physiological and behavioral activities highlight the region-specific activities of insulin in the CNS; that is, there is an anatomical context to the role of insulin in the brain. This feature is shared by many peptides acting in the CNS, particularly peripherally-derived peptides like insulin, GLP-1, leptin and ghrelin (among others). Similarly, there is also a temporal context to the activities of insulin in the CNS. For example, acute administration of exogenous insulin rapidly facilitates synaptic plasticity, whereas more gradual and prolonged physiological changes in insulin tone create an environment upon which neuroplasticity occurs.
Any discussion of the temporal context of insulin activity must also include insulin resistance, which ultimately contributes to neuroplasticity deficits in metabolic disorders like obesity and type 2 diabetes mellitus (T2DM). The progressive dysregulation of insulin is one of the primary features of obesity and T2DM, leading researchers to examine the effects of insulin on the function of hippocampal and mesocorticolimibic circuits that underlie cognitive and motivational processes. In this framework, the CNS activities of insulin could be conceptualized as a continuum in which insulin promotes neuroplasticity as an integral part of healthy brain function which then becomes dysregulated alongside the development of obesity, T2DM and associated complications such as neuroinflammation. Indeed, the clinical and preclinical data to date suggest that the ability of insulin to influence neural activity breaks down as a result of metabolic stress and the physiological alterations that accompany obesity (see Fig. 1).
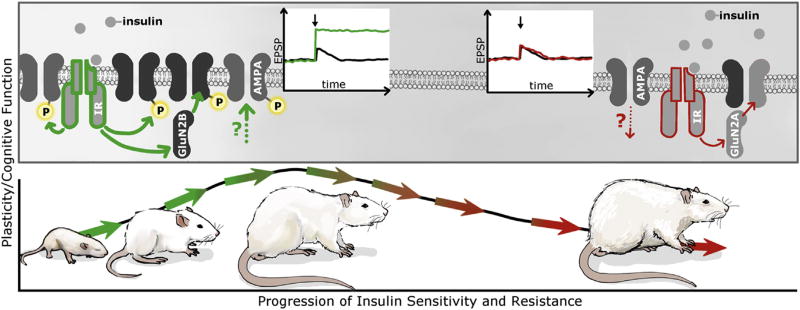
Fig. 1. Continuum of insulin effects on neuronal plasticity across a temporal/developmental context.
While glucose utilization in the CNS is likely to be largely insulin independent, the brain is not an insulin insensitive organ. Rather, insulin promotes neuroplasticity across development in the CNS in a variety of ways ranging from neuronal maturation to behavior. As depicted by the green insulin receptors and arrows in the upper panel, insulin facilitates plasticity at the synaptic level by regulating the expression and phosphorylation of glutamate receptors in the hippocampus and also coordinates the activity of mesolimbic networks. These activities of insulin are proposed to result in enhancement of synaptic transmission, as depicted by the green traces indicating enhancement of excitatory post-synaptic potentials (EPSPs). These activities provide examples of the anatomical and temporal contexts of insulin signaling activity in the CNS. However, insulin resistance will result in decreases in the phosphorylation state of glutamate receptors, as well as decreases in EPSPs, as depicted in red insulin receptors and arrows. Under these conditions, insulin resistance associated with obesity and T2DM impairs the substrate on which synaptic plasticity takes place. In this way, insulin activity in the CNS can be thought of a continuum beginning with the facilitation of neuroplasticity during development into adulthood (as depicted by the green arrows in the bottom panel), which then may be followed by reductions in neuroplasticity resulting from insulin resistance (as depicted in the red arrows in the bottom panel). See text for details.
In view of these observations, the goals of this review are to provide an overview of our current understanding of the role insulin plays in neural plasticity, cognition, and motivation from a temporal, anatomical, and functional perspective. We begin by discussing the neural and behavioral actions of insulin in the hippocampus and the role of hippocampal insulin resistance in cognitive deficits associated with obesity. We then discuss the emerging literature examining insulin activity in “motivation” centers like the nucleus accumbens (NAc) and ventral tegmental area (VTA).
2. Anatomical and temporal context of insulin activity in the CNS: hippocampal-dependent behaviors
Molecular and pharmacological approaches have determined that insulin receptor mRNA and protein are widely expressed in the CNS (Kar et al., 1993; Hill et al., 1986; Havrankova et al., 1981; Unger et al., 1989; Marks et al., 1990). From a temporal perspective, the highest expression of CNS insulin receptors occurs during the early stages of development. During these periods insulin receptors are proposed to play in critical roles in neuronal maturation, neurogenesis and synaptogenesis (for review see (Chiu and Cline, 2010)). From an anatomical perspective, insulin regulates very different physiological and behavioral responses depending upon which receptor populations are activated. For example, one of the first reports describing the activities of insulin in the CNS came from Woods and coworkers indicating that intracerebral administration of insulin decreased food intake and body weight in baboons (Woods et al., 1979). Subsequent studies by many investigators determined that these effects of insulin on metabolism and body weight were mediated by insulin receptors expressed in the hypothalamus [for review see (Schwartz et al., 2000)] and more recent analyses have focused on the hypothalamic circuitry involved in these processes (Woods and Begg, 2016).
Interestingly, hippocampal insulin receptors have been shown to have distinct functional roles vis-à-vis hypothalamic receptors; namely, hippocampal insulin receptors are known to regulate structural and functional plasticity, and to enhance cognition (Fadel and Reagan, 2016). For example, while an early study reported that insulin impaired behavior in the passive avoidance test (Schwarzberg et al., 1989), subsequent studies reported that icv insulin administration enhanced behavior in this test (Park et al., 2000). Closer examination of these disparate results provide an example of the temporal context of insulin activity in that administration of insulin preceding retention testing reduced avoidance latency (Schwarzberg et al., 1989), while administration prior to training enhanced avoidance latency (Park et al., 2000). More recent studies demonstrated that intra-hippocampal administration of insulin enhances spatial learning in a dose-dependent manner (McNay et al., 2010; Moosavi et al., 2006, 2007). Consistent with the clinical literature [see (Benedict et al., 2004; Craft et al., 2012) for examples] intranasal insulin enhances behavioral performance in aged F344 rats, effects that were dependent upon both insulin formulation (i.e. short-acting versus long-acting) and dose (Maimaiti et al., 2016). Additionally, it is interesting to note the bidirectional relationship between learning and memory and insulin receptor signaling. For example, studies by Alkon and coworkers have shown that spatial training in the water maze increases hippocampal insulin receptor expression and signaling (Zhao et al., 1999). From an anatomical and temporal context at the synaptic level, insulin may mediate these pro-cognitive activities through modulation of the expression and activity of classic neurotransmitter systems, in particular the glutamatergic system Fig. 1).
2.1. Temporal context of insulin activity: facilitation of synaptic plasticity at hippocampal synapses
One of the first examples of insulin-mediated potentiation of glutamatergic neurotransmission examined the ability of insulin to facilitate NMDA receptor-mediated transmission in oocyte expression systems, as well as in hippocampal slice preparations. In this regard, brief application of insulin transiently potentiated NMDA currents, responses that were attenuated by the PKC inhibitor staurosporine (Liu et al., 1995). Subsequent studies by these same investigators revealed that insulin elicits robust and transient increases in tyrosine phosphorylation of NR2A and NR2B subunits (Christie et al., 1999). Other studies similarly reported that insulin enhanced NMDA-mediated currents in a dose- and time-dependent manner, and that insulin facilitation of NMDA currents was attenuated by tyrosine kinase inhibitors (Chen and Leonard, 1996). At the synaptic level, these insulin-mediated effects likely result from exocytotic delivery of NMDA receptors to the membrane surface (Skeberdis et al., 2001). Collectively these studies supported the concept that insulin receptor signaling enhances NMDA-mediated glutamatergic neurotransmission in the hippocampus.
Studies have also examined the effects of exogenous insulin administration on AMPA-receptor (AMPAR) mediated glutamatergic transmission, and interestingly these studies identified different functional outcomes when compared to studies that examined insulin/NMDA receptors relationships. For example, insulin stimulated internalization of GluA2-containing AMPARs in hippocampal primary cultures (Beattie et al., 2000; Man et al., 2000). These results are consistent with an earlier report that insulin dose-dependently decreased the spontaneous firing rates of hippocampal pyramidal neurons (Palovcik et al., 1984). Subsequent studies determined that insulin-induced internalization of hippocampal AMPARs was tyrosine kinase dependent and identified the tyrosine residues required for insulin-stimulated endocytosis (Ahmadian et al., 2004; Lin et al., 2000). Additional studies examined the contribution of cytoskeletal changes to AMPAR subunit internalization (Zhou et al., 2001) and identified the subcellular compartmentalization of GluA1 and GluA2 subunits in response to insulin treatment in hippocampal primary neuronal cultures (Lin et al., 2000). From a functional perspective, these synaptic changes elicited by insulin administration were associated with induction of LTD in the CA1 region of the hippocampus (Ahmadian et al., 2004; Man et al., 2000). At the time, rather little was known about how AMPAR subunit composition affected the trafficking of the receptors, and these studies provided evidence for differential regulation of GluA1/2 vs GluA2/3-containing AMPARs and the role of AMPAR subunit phosphorylation in trafficking of the receptor. Thus, in these early studies, insulin was predominantly used as a tool to understand the mechanisms of AMPAR trafficking and plasticity. However, the physiological relevance of the results was only cautiously interpreted in the context of neuronal development and maturation.
The results described above provide seemingly dissimilar views of the effects of insulin on glutamatergic function in hippocampal synapses. Specifically, some studies reported that insulin induces LTD and produces AMPAR endocytosis (Beattie et al., 2000; Lin et al., 2000; Man et al., 2000; Ahmadian et al., 2004; Huang et al., 2004), while other studies reported that insulin elicits LTP and enhances membrane trafficking of glutamate receptor subunits (Chen and Leonard, 1996; Liu et al., 1995; Skeberdis et al., 2001). However, the differential trafficking and plasticity rules governing AMPA receptor sub-types may account for these differences. Specifically, AMPARs comprised of GluA1/GluA2 subunits move in and out of the synapse in an activity dependent manner that relies in part on phosphorylation of serine residues on the intracellular tail of GluA1, while GluA2/GluA3 containing AMPA receptors constitutively recycle in and out of the synapse (Huganir and Nicoll, 2013). In addition, a third population of GluA2-lacking AMPA receptors (GluA1/GluA1 or GluA1/GluA3) is also subject to activity-dependent synaptic insertion and removal. Although less well-understood, these receptors are abundant during early development and are up-regulated in response to consumption of sugary, fatty foods as well as food restriction in adults (Carr, 2016; Ferrario, 2017; Oginsky et al., 2016; Peng et al., 2011). In the hippocampal studies described above, insulin-induced reductions in AMPAR surface expression were predominantly mediated by the removal of GluA2-containing AMPARs, whereas insulin-induced increases in AMPAR surface expression were predominantly mediated by the addition of GluA1-containing AMPARs (GluA1/GluA2 or GluA1/GluA1). Thus, it is possible that insulin may have distinct effects on GluA1-vs. GluA2-containing AMPA receptors, although this has not been directly tested.
Another consideration is how experimental context may influence insulin-induced glutamatergic plasticity. For example, a study by Ramakers and coworkers determined that insulin tone can also influence subsequent hippocampal synaptic plasticity (van der Heide et al., 2005). In this study, bath application of insulin produced a rightward shift in the frequency response curve, such that a reduced stimulus intensity was required for the induction of LTP in the CA1 region of the hippocampus. This study also determined that these effects of insulin were NMDA dependent. When placed in the broader functional context, these data illustrate that insulin elicits dynamic effects on glutamatergic synaptic transmission that can be dependent on the insulin tone present.
Beyond excitatory neurotransmission, a more limited number of studies have examined the ability of insulin to modulate the GABAergic system. Similar to observations in NMDA and AMPARs, insulin stimulates the trafficking of GABAA receptor subunits from an intracellular compartment to the membrane surface in transfected HEK cells and in primary hippocampal neuronal cultures, resulting in an increase in mIPSCs (Wan et al., 1997). More recent studies reported that insulin shifts tonic GABAergic currents in the CA1 region of the hippocampus, an effect that was suggested to result from the recruitment of high affinity GABAA receptors (Jin et al., 2011). The ability of insulin to alter excitatory and inhibitory neurotransmission has important functional implications for hippocampal transmission and plasticity. However, understanding the net effect of insulin on neurotransmission is challenging, as the studies described above require use of receptor antagonists in order to isolate the activity of the receptor of interest (i.e. bath application of the GABAA receptor antagonist bicuculline to measure EPSPs). When placed in the anatomical context of the in vivo synapse, it is interesting to speculate that insulin facilitates excitatory neurotransmission by decreasing the threshold required for induction of LTP and by stimulating changes in NMDA and AMPAR subunit composition and activity, while effects on GABAA receptors may assist in the coordination of glutamatergic transmission in excitatory pyramidal neurons.
2.2. Insights from molecular approaches to induce brain insulin resistance
Studies from experimental models of diabetes and obesity support the concept that insulin facilitates hippocampal synaptic plasticity (Biessels and Reagan, 2015). For example, deficits in hippocampal neuroplasticity that include impairments in learning and memory are observed in streptozotocin (STZ)-treated rodents, rodents provided a high fat diet, and rodents with genetic mutations that result in an obesity/T2DM phenotype such as ob/ob mice, db/db mice and Zucker rats (Fadel and Reagan, 2016). However, one of the major limitations of these studies is that these rodents do not exclusively exhibit brain insulin resistance, but rather exhibit all the hallmark features of metabolic disorders, including peripheral insulin resistance and glucose intolerance. Even studies employing molecular approaches to selectively target brain insulin receptor signaling have suffered from these limitations. In this regard, while insulin receptor substrate 2 knockout mice exhibit reductions in glutamatergic neurotransmission in the CA1 region of the hippocampus (Martin et al., 2012), these mice also exhibit metabolic abnormalities that include peripheral insulin resistance and impaired glucose tolerance (Withers et al., 1998). Similarly, mice with neuron-specific deletion of the insulin receptor (NIRKO) exhibit endocrine and metabolic deficits that include increases in body weight and body adiposity, as well as increases in plasma leptin and triglyceride levels (Bruning et al., 2000). Collectively, these studies emphasize the challenges to disentangling the specific role of brain insulin receptor activity in synaptic plasticity independent of peripheral insulin activity.
Fortunately, a number of recent studies have examined how brain insulin resistance impacts hippocampal synaptic plasticity independent of peripheral metabolic and endocrine changes. In this regard, heterozygous insulin receptor knockout mice, which clear glucose as effectively as wild type mice in response to an oral glucose tolerance test (Accili et al., 1996), exhibit deficits in high frequency stimulation-induced LTP in the hippocampus, as well as behavioral deficits as assessed in the novel object recognition test (Nistico et al., 2012). More recently, we have shown that hippocampal-specific insulin resistance induced through selective antisense-mediated knock down of hippocampal insulin receptors impairs hippocampal synaptic plasticity in the absence of peripheral metabolic or endocrine impairments (Grillo et al., 2015). Specifically, rats with hippocampal-specific insulin resistance exhibited impairments in high frequency stimulated LTP that were associated with decreases in the expression and phosphorylation of glutamate receptor subunits. Additionally, rats with hippocampal-specific insulin resistance exhibited spatial learning and memory deficits, as assessed in the water maze. The results from this study support the concept that hippocampal insulin resistance can occur independently of peripheral insulin resistance and thereby contribute to neuroplasticity deficits in metabolic disorders. In addition, these data suggest that the hippocampus may be a locus responsible for the pro-cognitive effects of intranasal insulin administration.
3. Actions of insulin in mesocorticolimbic systems
As stated in the introduction, the unabated growth of the obesity epidemic and the revelation that cognitive decline, depression, and anxiety can all be exacerbated, and likely induced, by obesity have invigorated studies of the role of insulin in adult brain. It is well established that mesolimbic circuits act as an interface between emotional and motivational states to direct behavior towards and away from primary rewards like food (Castro et al., 2015; Kelley, 2004; Kelley et al., 2005; Sesack and Grace, 2010). Thus, mesolimbic circuits are involved in a wide array of complex behaviors from Pavlovian and instrumental learning, to decision making and the encoding of value. For the purposes of this review, we will focus on the neural and behavioral effects of insulin administration in the nucleus accubmens (NAc) and ventral tegmental area (VTA). Insulin receptors are expressed in the NAc and VTA, and insulin from the periphery readily reaches striatal regions (Banks and Kastin, 1998). However, to date only a handful of studies have examined the effects of insulin on neurotransmission in these regions (see also Table 1).
Table 1.
Summary of effects of insulin on NAc and VTA excitatory transmission.
| Reference | Region | Measure | Direction of change | Ins Con | Timing |
|---|---|---|---|---|---|
| Liu et al., 2013 | VTA | eEPSC amp | ↓ | 500 nM | immediate onset, long-lasting |
|
| |||||
| Labouebe et al., 2013 | VTA | eEPSC amp | ↓ | 1, 10, 100, 500 nM; IC50 = 17 | immediate onset, long-lasting |
| mEPSC freq | ↓ | 500 nM | not reported | ||
| PPR | ↑ | 500 nM | not reported | ||
| mEPSC amp | no change | 500 nM | not reported | ||
|
|
|
||||
| Stouffer et al., 2015 | NAc Core | evoked [DA]o | ↑ | EC50 = 2 nM | delayed onset (20 min) maximal at ~40–50 min |
| NAc Shell | evoked [DA]o | ↑ | EC50 = 5 nM | not reported | |
| CPu | evoked [DA]o | ↑ | EC50 = 2.4 nM | not reported | |
| NAc all regions | evoked [DA]o | no change | >100 nM | immediate onset, reversible | |
|
| |||||
| Oginsky and Ferrario, 2016 | NAc core | eEPSC amp | ↑ | 10, 30 nM | immediate onset, reversible |
| mEPSC freq | ↑ | 30 nM | |||
| PPR | ↓ | 30 nM | |||
| mEPSC amp | no change | 30, 100 nM | |||
| eEPSC amp | ↓ | 100–500 nM | immediate onset, reversible | ||
| mEPSC freq | ↓ | 500 nM | |||
| PPR | ↑ | 500 nM | |||
| mEPSC amp | no change | 500 nM | |||
3.1. Anatomical context of insulin in the NAc
Insulin receptors are moderately abundant in the NAc compared to other brain regions (Kar et al., 1993). Within the NAc, 85–90% of neurons are GABAergic medium spiny projection neurons (MSNs), while the remaining 10–15% of neurons are comprised cholinergic, parvalbumin fast-spiking GABAergic, and somatostatin and nitric oxide synthetase-containing GABAergic interneurons that regulate presynaptic transmitter release as well as MSN excitability (Wilson, 2007). The NAc can be further divided into core and shell subregions that have distinct anatomical inputs and roles in feeding-related behaviors (Kravitz and Kreitzer, 2012; Yager et al., 2015). However, given the limited number of studies that have been conducted, we will not discuss core/shell differences below, but will note when studies have differentiated between these subregions.
Insulin receptors are located on cholinergic interneurons and on MSNs, but are not thought to be located on other cells within the NAc, or on presynaptic terminals within the region (Figlewicz et al., 2003). Insulin receptor function has been extensively reviewed (Fernandez and Torres-Aleman, 2012) and thus detailed information will not be reiterated here. However, it is worthwhile to note that insulin can be promiscuous, binding to and activating insulin-like growth factor receptors (IGFR), as well as hybrid receptors comprised of insulin receptor subunits and IGFR subunits (Vigneri et al., 2010). The degree of activation varies as a function of insulin concentration, with ~100 fold difference in sensitivity of IRs vs. IGFRs to insulin (Schumacher et al., 1991). Both receptor populations are expressed within the NAc and VTA; thus, careful pharmacological studies that include receptor specific antagonists are needed to confirm that effects of insulin are due to insulin receptor vs. IGFR activation (Saperstein et al., 1989).
3.2. Temporal context: effects of insulin on synaptic transmission in the NAc
Some recent studies by our group have examined the effect of insulin on excitatory transmission in the NAc (Oginsky and Ferrario, 2016). For these studies acute brain slices containing the striatum were prepared from adult, male rats and whole-cell patch-clamp recordings of evoked EPSCs (eEPSCs) were made from MSNs before and after bath application of insulin (1–500 nM). Similar to some results in hippocampus, insulin receptor activation enhanced excitatory transmission in the NAc core (10, 30 nM). This effect was blocked by co-application of an insulin receptor antagonist and was due to an increase in presynaptic glutamate release as measured by paired pulse facilitation and increases in mEPSC frequency. Furthermore, blockade of insulin receptor signaling only within the recorded MSN was sufficient to prevent insulin's effects on excitatory transmission, suggesting that there is a retrograde feedback signal to reduce glutamate release. However, unlike the hippocampus (above) and VTA (see below), where effects of insulin are long-lasting, effects of insulin on excitatory transmission within the NAc were rapidly and completely reversed by insulin wash out. In addition, increasing the concentration of insulin to 100 nM recruited activation of IGFRs and produced an opposing decrease in excitatory transmission. This dynamic and bidirectional response to insulin may enable the NAc to be particularly sensitive to shifts in insulin levels and allow for the rapid regulation of excitatory transmission (see below on NAc insulin and behavior).
One caveat to the bidirectional actions of insulin described above is that physiological concentrations of insulin are thought to remain below 30 nM. However, this idea is based on a limited number of studies measuring insulin levels in CSF, plasma, or brain homogenates (Banks and Kastin, 1998; Havrankova et al., 1978; Strubbe et al., 1988; Wallum et al., 1987). In addition, no direct studies of NAc insulin levels, particularly after a high-sugar/high-fat meal or chronic hyperinsulinemic states, have been conducted. Thus, the true dynamic range of insulin in non-obese, and obese states remains to be be determined. However, it is clear that alterations in peripheral insulin levels, either through acute or chronic diet manipulation, affect the ability of insulin to modulate neuronal function in several brain regions including the NAc. For example, we also found that free access to a 60% high-fat diet for 8 weeks induced obesity that was accompanied by elevations in fasted insulin levels. When recordings were made from MSNs in the NAc core of these rats, insulin-induced increases in excitatory transmission following 30 nM insulin were completely lost. This was accompanied by a decrease in insulin receptor surface expression in the NAc. These data are consistent with deficits in hippocampal neuroplasticity after diet-induced obesity discussed above. However, whether brain insulin resistance in the NAc occurs prior to peripheral insulin resistance, as has been established for the hippocampus, is unknown.
In addition to effects on glutamatergic transmission described above, one recent study showed that insulin also indirectly alters dopamine transmission within the NAc core and shell (Stouffer et al., 2015). Specifically, Stouffer et al., 2015 used whole-cell recordings and voltammetry in acute brain slices to examine alterations in cholinergic interneuron activity and evoked dopamine release following bath application of insulin. They found that insulin receptor activation enhanced activity of cholinergic interneurons, thereby enhancing dopamine release at the level of presynaptic terminals within the NAc. Interestingly, this effect was seen at concentrations of insulin up to 30 nM, but was absent at higher concentrations. This is consistent with the sensitivity of insulin receptors to insulin, and suggests that basal insulin tone in the brain may alter insulin's effects on striatal dopamine.
The ability of insulin receptor activation to enhance dopamine release was somewhat surprising given that insulin treatment in ex vivo slices has also been found to enhance dopamine transporter function in the NAc (Daws et al., 2011; Speed et al., 2011), and to reduce excitatory drive to VTA dopamine neurons that terminate in the NAc (Labouebe et al., 2013; see also below). However, data from behavioral studies are consistent with an overall facilitation of NAc activity by insulin, and with a role for NAc insulin in enhanced reinforcement (see section 3.3). Thus, although insulin may have varying and potentially competing effects within mesolimbic circuits and micro-domains within the NAc, data to date suggest that the net effect of insulin receptor activation in the NAc is to enhance both dopaminergic and glutamatergic transmission (see (Stouffer et al., 2015) for additional discussion).
When trying to integrate the temporal context of insulin's effects on NAc dopamine vs. pre-synaptic glutamate release it's important to consider the timescales on which these effects occur. Effects of insulin on local dopamine release emerge ~20 min after insulin application, whereas effects on MSN excitatory transmission are immediately apparent and reach a maximum within ~7 min. Thus, the temporal profile of increases in insulin, as well as the concentration of insulin achieved, may dramatically influence the net effect of insulin on transmission within the NAc (see also Table 1).
3.3. Effects of NAc insulin on feeding-related behavior
While it's well-accepted that central actions of insulin influence feeding behavior, the nature of this effect and the underlying mechanisms are poorly understood (Figlewicz et al., 2008). In two elegant studies, Carr and colleagues have shown that insulin administered directly into the NAc shell reduces preference for glucose containing solutions (Stouffer et al., 2015; Woods et al., 2016). For these studies they used two different flavor-nutrient preference learning procedures in which distinct flavors were paired with glucose solution. Consumption of glucose results in a rapid rise in peripheral and central insulin. Thus, they reasoned that glucose consumption itself would enhance endogenous insulin brain levels, and that affects downstream of this increase could be prevented by pairing a given solution with blockade of insulin signaling in the NAc shell. In their first study, two different flavors were paired with the same glucose concentration (0.8%; Stouffer et al., 2015). Controls received mock infusions or vehicle prior to exposure to each solution; this resulted in no preference between the two flavors when sucrose was omitted from the solution. In experimental animals, an antibody against insulin was infused directly into the NAc shell just prior to consumption of one flavored 0.8% glucose solution, while a distinct flavor was paired with mock infusion and the same 0.8% glucose. Under these conditions, rats consumed less of the flavored solution that was paired with insulin antibody infusion, showing that blockade of insulin signaling within the shell was sufficient to reduce preference for the insulin-antibody paired solution (Stouffer et al., 2015). In a follow-up study, they took a slightly different approach in which rats were trained to associate one flavor with low glucose (1%), and another flavor with high glucose (6.1%). In controls, this resulted in a preference for the flavor that was previously paired with high glucose. However, when the high glucose solution was paired with intra-NAc shell insulin antibody infusion during initial training, the subsequent expression of a preference for the high glucose paired flavor was abolished (Woods et al., 2016). Together these data suggest that actions of insulin within the NAc shell influence the reinforcing properties of nutritive value. Importantly, glucose consumption increased insulin receptor phosphorylation, indicative of insulin receptor activation, and infusion of the insulin receptor antibody prevented this change (Woods et al., 2016). Interestingly, the reduction in preference for the insulin antibody paired flavors was apparent both during initial training and during flavor preference testing in which glucose was omitted. Thus, manipulation of insulin affected both the acquisition of the initial preference, as well as its expression. Furthermore, the magnitude of insulin receptor phosphorylation in the NAc (core and shell combined) was greater following consumption of high vs. low sucrose-containing solutions. This change occurred within 7 min of sucrose consumption and provides a potential mechanism by which the NAc may “sense” sugar consumption.
The results described above are from ad lib fed male rats. Although comparable studies of the effect of intra-NAc insulin blockade were not conducted in obese rats, flavor preference for high vs. low sucrose-paired flavors was lost following diet-induced obesity (Woods et al., 2016). This is consistent with a loss of neural insulin sensitivity following high-fat diet consumption described for the hippocampus and NAc, and led Woods and colleagues to conclude that, “obese individuals, with insulin insensitivity, may make food choice based on inaccurate nutritive assessments.” (Woods et al., 2016; pg 61). While it's unclear how this would explain a continued preference for high sugar containing foods observed in many obese individuals, it may help explain in part the initial establishment of preferences for sweeter foods and for the escalation in consumption of increasingly sweeter foods in obesity. However, for the latter to be true one would have to posit that neural insulin insensitivity could be over-come by consumption of sufficiently high concentrations of glucose. To date no such evidence suggests that this is the case. Another possibility is that insulin in the NAc plays a role in establishing nutritive value, particularly in relation to the sensory properties of foods that are paired with glucose. However, once neural insulin resistance develops the ability to shift these established preferences and conditioned responses may be reduced, thereby perpetuating unhealthy food choices. Of course additional studies are needed to determine if this is the case.
3.4. What are the potential mechanisms underlying behavioral effects of intra-NAc insulin?
While the specific mechanism underlying the role of NAc insulin in flavor nutrient learning is not known, the ability of insulin to enhance striatal dopamine and glutamate likely play a role (see also Woods et al., 2016 for discussion). As stated above, application of insulin in the NAc enhances local dopamine release in the NAc core and shell (Stouffer et al., 2015), and data from our lab show that insulin receptor activation increases excitatory transmission onto MSNs in the NAc core (Oginsky and Ferrario, 2016). Thus, it is tempting to speculate that elevations in insulin result in coordinated actions of dopamine and glutamate to promote plasticity that underlies the reinforcing properties of foods high in sugar. Consistent with this idea, intra-gastric infusion or oral consumption of glucose (16%) is sufficient to enhance GluA1s845 phosphorylation in the NAc within 7 min (Woods et al., 2016). Phosphorylation of this serine residue results in the priming of GluA1-containing AMPARs for synaptic insertion, enhances single-channel conductance, and is mediated by PKA activity (Lee and Kirkwood, 2011; Song and Huganir, 2002). Furthermore, activation of D1-type dopamine receptors increases AMPAR surface expression in NAc MSNs via activation of PKA and phosphorylation of s845 on GluA1 (Sun et al., 2008; Wolf et al., 2003). Thus, enhanced GluA1s845 phosphorylation following glucose consumption may be mediated in part by insulin-induced increases in dopamine. One caveat to this interpretation is that the time course of insulin's effects on dopamine release and GluA1s845 phosphorylation differs. Specifically, insulin-induced increases in local dopamine release in acute brain slices occur ~20 min after insulin application, whereas increases in GluA1s845 phosphorylation were found within 7, but not 17, minutes after in vivo glucose consumption. However, increases in glutamatergic transmission following insulin application were immediately apparent, supporting the idea that insulin is tapping into plasticity mechanisms. Of course, these temporal differences in the response to insulin could reflect differences in physiological vs. pharmacological manipulation of insulin signaling, and caution must be taken when attempting to integrate data from ex vivo and in vivo approaches. None-the-less, given the prominent role of striatal dopamine and glutamate in reinforcement and motivation for food, the data available provide strong support of the regulation of striatal motivational circuits by insulin.
3.5. Effects of insulin on synaptic transmission within the VTA
Only two studies have examined effects of insulin on excitatory neurotransmission within the VTA. This work from Borgland and colleagues using acute slices in young male mice showed that bath application of insulin reduces presynaptic glutamate release and leads to a long-lasting depression of excitatory transmission onto VTA dopamine neurons (Labouebe et al., 2013; Liu et al., 2013). This effect of insulin was concentration dependent, with modest but appreciable reductions following 1 or 10 nM insulin and more pronounced reductions following 100 or 500 nM insulin (~40% of baseline). This LTD was due to retrograde endocannabinoid feedback, and was blocked by insulin receptor, but not IGFR antagonists. The latter is important because these studies predominantly used 100 nM insulin, a concentration that can produce IGFR activation. Thus, although IGFRs are located in the VTA, effects of insulin on excitatory transmission in this brain region appear to be limited to insulin receptor activation, unlike in the NAc.
The onset of insulin's effects in VTA were fairly rapid, consistent with effects on excitatory transmission in the NAc, and with time scales needed to regulate ongoing food intake (see below for VTA-insulin and behavior). While insulin induced LTD may seem at odds with the increases in local NAc dopamine release following insulin discussed above, it's unlikely that the effects of insulin on VTA dopamine neurons represent a simple all or none change in firing. Instead, Borgland and colleagues suggest that reductions in excitatory drive induced by insulin may reduce the probability of burst-firing, and thereby influence feeding behavior (Mebel et al., 2012); see also Fig. 2.
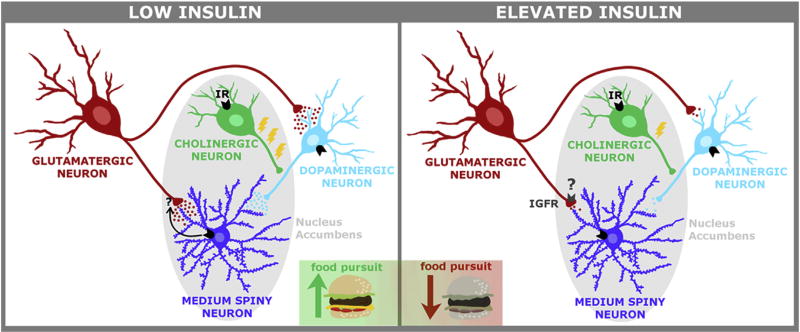
Fig. 2. Proposed VTA-NAc circuit for insulin regulation of food pursuit.
Relatively low concentrations of insulin produce increased glutamate release in the NAc and enhance dopamine release in this region through actions on cholinergic interneurons. As insulin concentrations increase, activation of IGFRs is recruited, causing a reduction in glutamate release. In addition, at these higher concentrations, local dopamine release within the NAc is no longer enhanced, and instead insulin results in LTD of VTA-dopamine neurons. Thus, small anticipatory increases in insulin in response to stimuli associated with food may activate local NAc circuits to promote food pursuit, while larger post-prandial increases in insulin may reduce activity in the VTA and NAc to reduce the pursuit of food and promote feeding cessation.
The effect of acute food consumption, which is expected to increase endogenous insulin, on subsequent neural responses to insulin application were also examined in the studies described above. Specifically, when mice were allowed to eat sweetened high fat (SHF) food immediately prior to slice preparation, insulin-induced reductions in excitatory transmission were completely absent (Liu et al., 2013). Although indirect, this suggests that physiological increases in insulin induced by SHF food consumption occluded the effect of subsequent bath application of insulin. To date, no studies have determined how diet-induced obesity or chronic consumption of SHF foods may alter insulin's ability to influence excitatory transmission in the VTA. However, in BTBR Tþ Itpr3tf/J (BTBR) mice (which are hyperinsulinemic) insulin's capacity to induce LTD in VTA dopamine neurons was reduced (Liu et al., 2013). Importantly, both low-frequency stimulation- and cannabinoid-induced LTD were normal, suggesting that the blunted response to insulin was not due to overall deficits in VTA plasticity. Although this mutation causes a number of alterations to peripheral metabolism, the data are none-the-less consistent with the loss of insulin sensitivity associated with diet-induced obesity in other brain regions described above.
3.6. How does intra-VTA insulin affect feeding behavior?
Effects of intra VTA insulin on instrumental responding for food, and on the reinforcing properties of food using conditioned place preference (CPP) have been examined (Labouebe et al., 2013; Figlewicz et al., 2008). Interestingly, intra-VTA insulin did not alter break point (i.e., the maximum number of responses an animal is willing to make to receive a given reinforcer) for either liquid sucrose or sweetened condensed milk, but did prevent the expression of CPP for a sweet treat [Froot Loops (Labouebe et al., 2013)]. The latter is consistent with reductions in food CPP following i.c.v. insulin (Figlewicz et al., 2004), and may reflect reductions in homoestatic drive where insulin may act more as a satiety signal. However, if this is the case, it is unclear why intra-VTA insulin did not also reduce motivation for food during progressive ratio testing, even when similar concentrations of insulin were used (2 µM). In addition, because insulin was only given during the final test for CPP or motivation, and not during initial acquisition of either task, the potential ability of insulin in the VTA to modulate initial learning of nutritive value remains unclear.
In hungry male mice, intra-VTA administration of insulin did not alter consumption of standard lab chow within a 4 h period, but did reduce consumption of a sweetened high-fat food (Mebel et al., 2012). In this set of studies, mice were only allowed to eat their daily chow within one 4 h period outside the home cage each day. Thus, the absence of effects of insulin on chow consumption within that 4 h period may be influenced by this feeding schedule. For example, patterned feeding of this kind can induce conditioned anticipatory increases in peripheral insulin that may have occluded additional effects of intra-VTA insulin (Simon et al., 1986; Teff, 2011). Furthermore, the effect of insulin on consumption of sweetened high-fat food was measured after a 4 h chow feeding period. Thus, the authors concluded that insulin-induced reductions in the amount of sweetened high-fat food consumed reflected a blunting of hedonic feeding, as mice had already been sated on chow. These data are generally consistent with the ability of insulin to reduce excitatory drive to the VTA (see above), and with the generally anorectic effects of insulin in other brain regions. However, one additional caveat to interpretation is the fairly wide range of insulin concentrations used (e.g., 2 µM for break point for sucrose, 100 µM for break point for sweetened condensed milk). In sum, although the physiological role of insulin's actions in the VTA are not yet clear, studies to date do support a role for VTA insulin in feeding, particularly of sugary, calorie dense foods.
4. Summary, perspectives and gaps in our understanding
Our understanding of the actions of insulin in the CNS has progressed rapidly from the notion that the brain is an insulin-insensitive organ to our current appreciation that insulin promotes neuroplasticity and is integral to learning, cognition, and motivation. Although substantial progress has been made, there are many gaps in our knowledge base. In this regard, while studies have shown that intravenously administered insulin is detected in the brain parenchyma (Banks et al., 1999) and regional brain analysis of the distribution of radiolabeled insulin identified high levels of blood-borne insulin in the hippocampus and striatum (Banks and Kastin, 1998), critical questions remain to be answered (Gray et al., 2014). For example, what are the in vivo synaptic concentrations of insulin under physiological conditions both before and after a meal? Are chronic elevations in peripheral insulin, such as those occurring during obesity, directly reflected in brain insulin levels? What is the temporal relationship between fluctuations in peripheral and central insulin? How does degree and duration of peripheral insulin dysregulation affect actions of insulin on neural plasticity?
Gaining insight into synaptic concentrations of insulin under different conditions has important functional implications. For example, effects of insulin on mesolimbic systems are concentration dependent. This may allow for the tuning of activity in these circuits such that relatively small increases in insulin in anticipation of a meal may enhance activity in striatal regions to facilitate food-seeking, while larger post-prandial elevations may reduce dopamine activity in VTA to reduce feeding (Fig. 2). Moreover, while the studies described above clearly demonstrate that insulin rapidly alters glutamatergic transmission, the neuronal localization of insulin receptors vis-à -vis NMDA and AMPA receptors remains to be equivocally identified.
Beyond these questions related to anatomical context, questions of temporal context regarding insulin activity across the lifespan remain to be fully addressed and understood. Indeed, insulin enhancement of plasticity likely begins during development, as autoradiographic studies of insulin receptor binding indicate that insulin receptor expression is higher in neonates and gradually decreases into adulthood (Kar et al., 1993), and insulin is involved in neuronal differentiation and development (Chiu and Cline, 2010). Furthermore, while none of the studies above examined effects of insulin in females, substantial evidence from clinical and preclinical studies of polycystic ovary syndrome and the effects of gestational diabetes on metabolism and development strongly suggest that there are likely sex differences in the physiological role of insulin in neural plasticity.
While the current review focused on the actions of insulin on neuronal activity, insulin activity has also been described in astrocytes. For example, from a developmental perspective insulin stimulates the differentiation of fetal hypothalamic stem cells towards an astrocytic phenotype (Desai et al., 2011). From a functional perspective, an early study reported that insulin receptors are expressed in rat primary glial cells and function to regulate glucose uptake (Clarke et al., 1984). More recent studies suggest that insulin does not regulate glucose uptake, but rather stimulates glycogen production in cultured human astrocytes (Heni et al., 2011). Given the functional relationships between insulin and IGF-I described above, it is interesting to note that insulin combined with IGF-I increases glucose uptake in hippocampal/cortical primary astroglial cultures (Fernandez et al., 2017). While all these studies and others (Hamai et al., 1999; Kum et al., 1992) suggest important roles for insulin in astrocytic metabolic function, one caveat associated with these studies is that they were performed in primary cultures. As such, an important future direction will be to replicate these provocative findings to the in vivo setting.
With regard to decreases in insulin receptor activity, insulin resistance, and the progression of age-related cognitive decline (Biessels and Reagan, 2015), the success of intranasal insulin administration to enhance cognitive function in patients with Alzheimer's disease (AD) (Craft et al., 2012) indicate that insulin resistance is perhaps a reversible pathophysiological feature of AD. Such results suggest that interventions to enhance brain insulin activity may be beneficial at many different time points along the continuum from normal brain aging to AD. Furthermore, while it's clear that insulin plays important roles in feeding, the nature and extent of this role, as well as how it is affected by obesity and T2DM is poorly understood. This is a particularly pressing problem given the potentially synergistic relationships between obesity and cognitive decline. Interestingly, one recent study in humans found that intra-nasal insulin was sufficient to reduce ratings of food-preference and this behavioral effect was associated with reduced activations in the NAc and VTA of people with normal insulin sensitivity. In contrast, intra-nasal insulin produced an increase in activations of these regions in subjects identified as overweight and insulin resistant. In these same individuals, intra-nasal insulin produced trends for increased food-preference scores (p = 0.09); (Tiedemann et al., 2017). While it is difficult to directly determine causal relationships in studies like these, the data clearly indicate that intra-nasal insulin modulates food preference and activations of mesolimbic regions, and that this regulation is disrupted at the behavioral and neural level in insulin-resistant states. Thus, continuing to examine the effects of insulin on neural function and behavior in model systems and human studies will be critical to forming a complete understanding of the role of central insulin in physiological and pathophysiological states.
Finally, it is also possible that there may be two modes of insulin action that might differentially impact these brain regions: one that's circadian that may act more as a modulator of meta-plasticity, and another where larger more rapid changes occurring post-prandially drive traditional plasticity involved in learning and memory. Of course, only through continued examination of insulin's function in normal and disease states will we begin to understand its physiological role, as well as how we can harness its actions for potential therapeutic benefit.
Acknowledgments
Supported by the Department of Veterans Affairs (IO1BX001804 and I21 BX002085 to LPR), and NIDDK R01DK106188 and the Brain and Behavior Research Foundation N018940 to CRF. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the above stated funding agencies.
The authors would like to thank Victoria Macht for the excellent artwork and creative input for the figures in this review. The authors would also like to thank Dr. Marina Wolf for helpful discussions.
Footnotes
The authors have no conflicts of interest to declare.
References
- Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, Salvatore P, Asico LD, Jose PA, Taylor SI, Westphal H. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat. Genet. 1996;12:106–109. doi: 10.1038/ng0196-106. [DOI] [PubMed] [Google Scholar]
- Ahmadian G, Ju W, Liu L, Wyszynski M, Lee SH, Dunah AW, Taghibiglou C, Wang Y, Lu J, Wong TP, Sheng M, Wang YT. Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD. EMBO J. 2004;23:1040–1050. doi: 10.1038/sj.emboj.7600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA. The source of cerebral insulin. Eur. J. Pharmacol. 2004;490:5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Banks WA, DiPalma CR, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides. 1999;20:1341–1345. doi: 10.1016/s0196-9781(99)00139-4. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides. 1998;19:883–889. doi: 10.1016/s0196-9781(98)00018-7. [DOI] [PubMed] [Google Scholar]
- Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol. Ther. 2012;136:82–93. doi: 10.1016/j.pharmthera.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat. Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Reagan LP. Hippocampal insulin resistance and cognitive dysfunction. Nat. Rev. Neurosci. 2015;16:660–671. doi: 10.1038/nrn4019. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Carr KD. Nucleus accumbens AMPA receptor trafficking upregulated by food restriction: an unintended target for drugs of abuse and forbidden foods. Curr. Opin. Behav. Sci. 2016;9:32–39. doi: 10.1016/j.cobeha.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, Cole SL, Berridge KC. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front. Syst. Neurosci. 2015;9:90. doi: 10.3389/fnsys.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Leonard JP. Protein tyrosine kinase-mediated potentiation of currents from cloned NMDA receptors. J. Neurochem. 1996;67:194–200. doi: 10.1046/j.1471-4159.1996.67010194.x. [DOI] [PubMed] [Google Scholar]
- Chiu SL, Cline HT. Insulin receptor signaling in the development of neuronal structure and function. Neural Dev. 2010;5:7. doi: 10.1186/1749-8104-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Wenthold RJ, Monaghan DT. Insulin causes a transient tyrosine phosphorylation of NR2A and NR2B NMDA receptor subunits in rat hippocampus. J. Neurochem. 1999;72:1523–1528. doi: 10.1046/j.1471-4159.1999.721523.x. [DOI] [PubMed] [Google Scholar]
- Clarke DW, Boyd FT, Kappy MS, Raizada MK. Insulin binds to specific receptors and stimulates 2-deoxy-D-glucose uptake in cultured glial-cells from rat-brain. J. Biol. Chem. 1984;259:1672–1675. [PubMed] [Google Scholar]
- Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch. Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Avison MJ, Robertson SD, Niswender KD, Galli A, Saunders C. Insulin signaling and addiction. Neuropharm. 2011;61:1123–1128. doi: 10.1016/j.neuropharm.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, Li T, Ross MG. Fetal hypothalamic neuroprogenitor cell culture: preferential differentiation paths induced by leptin and insulin. Endocrinology. 2011;152:3192–3201. doi: 10.1210/en.2010-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel JR, Reagan LP. Stop signs in hippocampal insulin signaling: the role of insulin resistance in structural, functional and behavioral deficits. Curr. Opin. Behav. Sci. 2016;9:47–54. doi: 10.1016/j.cobeha.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez AM, Hernandez-Garzon E, Perez-Domper P, Perez-Alvarez A, Mederos S, Matsui T, Santi A, Trueba-Saiz A, Garcia-Guerra L, Pose-Utrilla J, Fielitz J, Olson EN, Fernandez dlR, Garcia GL, Pozo MA, Iglesias T, Araque A, Soya H, Perea G, Martin ED, Torres AI. Insulin regulates astrocytic glucose handling through cooperation with IGF-I. Diabetes. 2017;66:64–74. doi: 10.2337/db16-0861. [DOI] [PubMed] [Google Scholar]
- Fernandez AM, Torres-Aleman I. The many faces of insulin-like peptide signalling in the brain. Nat. Rev. Neurosci. 2012;13:225–239. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- Ferrario CR. Food addiction and obesity. Neuropsychopharmacology. 2017;42:361. doi: 10.1038/npp.2016.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav. Neurosci. 2004;118:479–487. doi: 10.1037/0735-7044.118.3.479. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett JL, Aliakbari S, Zavosh A, Sipols AJ. Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R388–R394. doi: 10.1152/ajpregu.90334.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964:107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- Gray SM, Meijer RI, Barrett EJ. Insulin regulates brain function, but how does it get there? Diabetes. 2014;63:3992–3997. doi: 10.2337/db14-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Hendry RM, Reagan LP. Insulin-stimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Res. 2009;1296:35–45. doi: 10.1016/j.brainres.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Lawrence RC, Wrighten SA, Green AJ, Wilson SP, Sakai RR, Kelly SJ, Wilson MA, Mott DD, Reagan LP. Hippocampal insulin resistance impairs spatial learning and synaptic plasticity. Diabetes. 2015;64:3927–3936. doi: 10.2337/db15-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamai M, Minokoshi Y, Shimazu T. L-Glutamate and insulin enhance glycogen synthesis in cultured astrocytes from the rat brain through different intracellular mechanisms. J. Neurochem. 1999;73:400–407. doi: 10.1046/j.1471-4159.1999.0730400.x. [DOI] [PubMed] [Google Scholar]
- Havrankova J, Brownstein M, Roth J. Insulin and insulin receptors in rodent brain. Diabetologia. 1981;20(Suppl):268–273. [PubMed] [Google Scholar]
- Havrankova J, Schmechel D, Roth J, Brownstein M. Identification of insulin in rat brain. Proc. Natl. Acad. Sci. U. S. A. 1978;75:5737–5741. doi: 10.1073/pnas.75.11.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heni M, Hennige AM, Peter A, Siegel-Axel D, Ordelheide AM, Krebs N, Machicao F, Fritsche A, Haring HU, Staiger H. Insulin promotes glycogen storage and cell proliferation in primary human astrocytes. PLoS One. 2011;6:e21594. doi: 10.1371/journal.pone.0021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM, Lesniak MA, Pert CB, Roth J. Autoradiographic localization of insulin receptors in rat brain: prominence in olfactory and limbic areas. Neuroscience. 1986;17:1127–1138. doi: 10.1016/0306-4522(86)90082-5. [DOI] [PubMed] [Google Scholar]
- Huang CC, Lee CC, Hsu KS. An investigation into signal transduction mechanisms involved in insulin-induced long-term depression in the CA1 region of the hippocampus. J. Neurochem. 2004;89:217–231. doi: 10.1111/j.1471-4159.2003.02307.x. [DOI] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Jin Y, Kumar-Mendu S, Degerman E, Groop L, Birnir B. Insulin reduces neuronal excitability by turning on GABA(A) channels that generate tonic current. PLoS One. 2011;6:e16188. doi: 10.1371/journal.pone.0016188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar S, Chabot J-G, Quirion R. Quantitative autoradiographic localization of [125I]Insulin-like growth factor I, [125I]Insulin-like growth factor II and [125I] Insulin binding sites in developing and adult rat brain. J. Comp. Neurol. 1993;333:375–397. doi: 10.1002/cne.903330306. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci. Biobehav. Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol. Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Kreitzer AC. Striatal mechanisms underlying movement, reinforcement, and punishment. Physiol. (Bethesda) 2012;27:167–177. doi: 10.1152/physiol.00004.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kum W, Zhu SQ, Ho SK, Young JD, Cockram CS. Effect of insulin on glucose and glycogen metabolism and leucine incorporation into protein in cultured mouse astrocytes. Glia. 1992;6:264–268. doi: 10.1002/glia.440060404. [DOI] [PubMed] [Google Scholar]
- Labouebe G, Liu S, Dias C, Zou H, Wong JC, Karunakaran S, Clee SM, Phillips AG, Boutrel B, Borgland SL. Insulin induces long-term depression of ventral tegmental area dopamine neurons via endocannabinoids. Nat. Neurosci. 2013;16:300–308. doi: 10.1038/nn.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Kirkwood A. AMPA receptor regulation during synaptic plasticity in hippocampus and neocortex. Semin. Cell Dev. Biol. 2011;22:514–520. doi: 10.1016/j.semcdb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat. Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- Liu L, Brown JC, III, Webster WW, Morrisett RA, Monaghan DT. Insulin potentiates N-methyl-D-aspartate receptor activity in Xenopus oocytes and rat hippocampus. Neurosci. Lett. 1995;192:5–8. doi: 10.1016/0304-3940(95)11593-l. [DOI] [PubMed] [Google Scholar]
- Liu S, Labouebe G, Karunakaran S, Clee SM, Borgland SL. Effect of insulin on excitatory synaptic transmission onto dopamine neurons of the ventral tegmental area in a mouse model of hyperinsulinemia. Nutr. Diabetes. 2013;3:e97. doi: 10.1038/nutd.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimaiti S, Anderson KL, DeMoll C, Brewer LD, Rauh BA, Gant JC, Blalock EM, Porter NM, Thibault O. Intranasal insulin improves age-related cognitive deficits and reverses electrophysiological correlates of brain aging. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:30–39. doi: 10.1093/gerona/glu314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- Marks JL, Porte D, Jr, Stahl WL, Baskin DG. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology. 1990;127:3234–3236. doi: 10.1210/endo-127-6-3234. [DOI] [PubMed] [Google Scholar]
- Martin ED, Sanchez-Perez A, Trejo JL, Martin-Aldana JA, Cano JM, Pons S, Acosta UC, Menes L, White MF, Burks DJ. IRS-2 Deficiency impairs NMDA receptor-dependent long-term potentiation. Cereb. Cortex. 2012;22:1717–1727. doi: 10.1093/cercor/bhr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur. J. Pharmacol. 2004;490:13–24. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol. Learn. Mem. 2010;93:546–553. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebel DM, Wong JC, Dong YJ, Borgland SL. Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake. Eur. J. Neurosci. 2012;36:2336–2346. doi: 10.1111/j.1460-9568.2012.08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosavi M, Naghdi N, Choopani S. Intra CA1 insulin microinjection improves memory consolidation and retrieval. Peptides. 2007;28:1029–1034. doi: 10.1016/j.peptides.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Moosavi M, Naghdi N, Maghsoudi N, Zahedi AS. The effect of intra-hippocampal insulin microinjection on spatial learning and memory. Horm. Behav. 2006;50:748–752. doi: 10.1016/j.yhbeh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Nistico R, Cavallucci V, Piccinin S, Macri S, Pignatelli M, Mehdawy B, Blandini F, Laviola G, Lauro D, Mercuri NB, D'Amelio M. Insulin receptor beta-subunit haploinsufficiency impairs hippocampal late-phase LTP and recognition memory. Neuromolecular. Med. 2012 Dec;14(4):262–269. doi: 10.1007/s12017-012-8184-z. [DOI] [PubMed] [Google Scholar]
- Oginsky MF, Ferrario CR. Effects of insulin on excitatory transmission in the nucleus accumbens of non-obese and obese rats. Soc. Neurosci. Abstr 2016 [Google Scholar]
- Oginsky MF, Goforth PB, Nobile CW, Lopez-Santiago LF, Ferrario CR. Eating 'Junk-Food' produces rapid and long-lasting increases in NAc CP-AMPA receptors: implications for enhanced cue-induced motivation and food addiction. Neuropsychopharmacology. 2016;41:2977–2986. doi: 10.1038/npp.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palovcik RA, Phillips MI, Kappy MS, Raizada MK. Insulin inhibits pyramidal neurons in hippocampal slices. Brain Res. 1984;309:187–191. doi: 10.1016/0006-8993(84)91028-x. [DOI] [PubMed] [Google Scholar]
- Park CR. Cognitive effects of insulin in the central nervous system. Neurosci. Biobehav. Rev. 2001;25:311–323. doi: 10.1016/s0149-7634(01)00016-1. [DOI] [PubMed] [Google Scholar]
- Park CR, Seely RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol. Behav. 2000;68:509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- Peng XX, Ziff EB, Carr KD. Effects of food restriction and sucrose intake on synaptic delivery of AMPA receptors in nucleus accumbens. Synapse. 2011;65:1024–1031. doi: 10.1002/syn.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saperstein R, Vicario PP, Strout HV, Brady E, Slater EE, Greenlee WJ, Ondeyka DL, Patchett AA, Hangauer DG. Design of a selective insulin receptor tyrosine kinase inhibitor and its effect on glucose uptake and metabolism in intact cells. Biochemistry. 1989;28:5694–5701. doi: 10.1021/bi00439a053. [DOI] [PubMed] [Google Scholar]
- Schumacher R, Mosthaf L, Schlessinger J, Brandenburg D, Ullrich A. Insulin and insulin-like growth factor-1 binding specificity is determined by distinct regions of their cognate receptors. J. Biol. Chem. 1991;266:19288–19295. [PubMed] [Google Scholar]
- Schwartz MW, Guyenet SJ, Cirulli V. The hypothalamus and ss-cell connection in the gene-targeting era. Diabetes. 2010;59:2991–2993. doi: 10.2337/db10-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Schwarzberg H, Bernstein HG, Reiser M, Gunther O. Intracerebroventricular administration of insulin attenuates retrieval of a passive avoidance response in rats. Neuropeptides. 1989;13:79–81. doi: 10.1016/0143-4179(89)90002-4. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Schlienger JL, Sapin R, Imler M. Cephalic phase insulin secretion in relation to food presentation in normal and overweight subjects. Physiol. Behav. 1986;36:465–469. doi: 10.1016/0031-9384(86)90316-1. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV. Insulin promotes rapid delivery of N-methyl-D- aspartate receptors to the cell surface by exocytosis. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3561–3566. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Speed N, Saunders C, Davis AR, Owens WA, Matthies HJ, Saadat S, Kennedy JP, Vaughan RA, Neve RL, Lindsley CW, Russo SJ, Daws LC, Niswender KD, Galli A. Impaired striatal Akt signaling disrupts dopamine homeostasis and increases feeding. PLoS One. 2011;6:e25169. doi: 10.1371/journal.pone.0025169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease–is this type 3 diabetes? J. Alzheimers. Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L, Machold RP, Jones KT, de Vaca SC, Reith ME, Carr KD, Rice ME. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat. Commun. 2015;6:8543. doi: 10.1038/ncomms9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strubbe JH, Porte D, Jr, Woods SC. Insulin responses and glucose levels in plasma and cerebrospinal fluid during fasting and refeeding in the rat. Physiol. Behav. 1988;44:205–208. doi: 10.1016/0031-9384(88)90139-4. [DOI] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J. Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teff KL. How neural mediation of anticipatory and compensatory insulin release helps us tolerate food. Physiol. Behav. 2011;103:44–50. doi: 10.1016/j.physbeh.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedemann LJ, Schmid SM, Hettel J, Giesen K, Francke P, Buchel C, Brassen S. Central insulin modulates food valuation via mesolimbic pathways. Nat. Commun. 2017;8:16052. doi: 10.1038/ncomms16052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger J, McNeill TH, Moxley RT, III, White M, Moss A, Livingston JN. Distribution of insulin receptor-like immunoreactivity in the rat forebrain. Neuroscience. 1989;31:143–157. doi: 10.1016/0306-4522(89)90036-5. [DOI] [PubMed] [Google Scholar]
- van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GM. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyld-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J. Neurochem. 2005;94:1158–1166. doi: 10.1111/j.1471-4159.2005.03269.x. [DOI] [PubMed] [Google Scholar]
- Vigneri R, Squatrito S, Sciacca L. Insulin and its analogs: actions via insulin and IGF receptors. Acta Diabetol. 2010;47:271–278. doi: 10.1007/s00592-010-0215-3. [DOI] [PubMed] [Google Scholar]
- Wallum BJ, Taborsky GJ, Jr, Porte D, Jr, Figlewicz DP, Jacobson L, Beard JC, Ward WK, Dorsa D. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J. Clin. Endocrinol. Metab. 1987;64:190–194. doi: 10.1210/jcem-64-1-190. [DOI] [PubMed] [Google Scholar]
- Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature. 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. GABAergic inhibition in the neostriatum. Prog. Brain Res. 2007;160:91–110. doi: 10.1016/S0079-6123(06)60006-X. [DOI] [PubMed] [Google Scholar]
- Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Mangiavacchi S, Sun X. Mechanisms by which dopamine receptors may influence synaptic plasticity. Ann. N. Y. Acad. Sci. 2003;1003:241–249. doi: 10.1196/annals.1300.015. [DOI] [PubMed] [Google Scholar]
- Woods CA, Guttman ZR, Huang D, Kolaric RA, Rabinowitsch AI, Jones KT, Cabeza dV, Sclafani A, Carr KD. Insulin receptor activation in the nucleus accumbens reflects nutritive value of a recently ingested meal. Physiol. Behav. 2016;159:52–63. doi: 10.1016/j.physbeh.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, Begg DP. Regulation of the motivation to eat. Curr. Top. Behav. Neurosci. 2016;27:15–34. doi: 10.1007/7854_2015_381. [DOI] [PubMed] [Google Scholar]
- Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- Yager LM, Garcia AF, Wunsch AM, Ferguson SM. The ins and outs of the striatum: role in drug addiction. Neuroscience. 2015;301:529–541. doi: 10.1016/j.neuroscience.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Chen H, Xu H, Moore E, Meiri N, Quon MJ, Alkon DL. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J. Biol. Chem. 1999;274:34893–34902. doi: 10.1074/jbc.274.49.34893. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Xiao M, Nicoll RA. Contribution of cytoskeleton to the internalization of AMPA receptors. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1261–1266. doi: 10.1073/pnas.031573798. [DOI] [PMC free article] [PubMed] [Google Scholar]