Abstract
Acoustofluidic devices have continuously demonstrated their potential to impact medical diagnostics and lab-on-a-chip applications. To bring these technologies to real-world applications, they must be made more accessible to end users. Herein, we report on the effort to provide an easy-to-use and portable system for controlling sharp-edge based acoustofluidic devices. With the use of a cell phone and modified Bluetooth® speaker, on-demand and hands-free pumping and mixing are achieved. Additionally, a novel design for a sharp-edge based acoustofluidic device is proposed that combines both pumping and mixing functions into a single device, thus removing the need for external equipment typically needed to accomplish these two tasks. These applications serve to demonstrate the potential function that acoustofluidic devices can provide in point-of-care platforms. To further this point-of-care goal, we also design a portable microscope that combines with the cell phone and Bluetooth® power supply, providing a completely transportable acoustofluidic testing station. This work serves to bolster the promising position that acoustofluidic devices have within the rapidly changing research and diagnostics fields.
Graphic Content Entry

A portable control system consisting of a cell phone and a portable Bluetooth® speaker is presented to wirelessly control sharp-edge based acoustofluidic devices.
Introduction
Recently, there has been an increased focus on how to solve problems imposed by the ever rising costs for medical research, development, and care. One pathway that has presented great promise is the use of Point-of-Care (POC) technology, which has the capability of lowering cost through simplifying operations and placing more of a focus on rapid results and preventative care.1–6 While there has been extensive discussion of the promise POC technology offers, and many platforms have been developed that move towards the POC goal, many advancements remain dependent on laboratory equipment in one way or another.7–13
Acoustofluidics – the fusion of acoustics and microfluidics – has recently been recognized for its robustness, simplicity, and non-invasive nature that enables versatile applications, including but not limited to, cell/particle and fluid manipulation.14–25 It is our belief that acoustofluidic technology has the potential to meet the needs that many POC systems hope to address. Current acoustofluidics advancements, however, remain constrained to the laboratory setting, due to their need for bulky secondary equipment and their accompanying power requirements. Nonetheless, over the past several years, we have developed acoustofluidic tools that have the potential to be integrated into much more portable designs than previously feasible. For example, we have developed sharp-edge based acoustofluidic devices26–32 (acoustically oscillated solid structures that protrude from the channel wall) that can accomplish a wide range of laboratory tasks: pumping,26 mixing,27 gradient generation,29 sputum liquefaction,30 etc.
In this work, we are devoted to developing a system that can transfer our previously established sharp-edge based acoustofluidic technology from the laboratory setting into a more accessible and adaptable form. We sought to accomplish this transition through the development of a portable control system that could relieve the constraints associated with typical operation. When developing a portable system, the first device that comes to mind is the cell phone: one of the most common electronics devices. The cell phone is readily available to, and understood by, most users, and provides the ability to customize functionality and accessibility (through applications development). Using a cellular phone as the control center of our portable design, we developed a system to control and power our sharp-edge based acoustofluidics technology using solely battery power.
As a demonstration, we used the newly designed portable power system to operate both our sharp-edge based micropump and micromixer, as well as a newly designed chip which combines both the pumping and mixing function onto a single device. Successfully combing the micropump and micromixer onto a single chip serves to simplify and miniaturize two critical microfluidics components. Finally, as a further progression towards portability, we demonstrated a simple portable microscope station capable of utilizing the camera on the cell phone to monitor the operation of our sharp-edge based acoustofluidic devices. Both the acoustofluidic and microscopic aspect of these designs are battery powered, providing a significant improvement in mobility over current acoustofludic device operation and monitoring. In combination, the removal of the need for a syringe pump to mix two fluids together, as well as the powering and control of sharp edge designs with a portable system represents a significant progression in the acoustofluidic platform. We believe that this system can serve as a starting point for the development of more highly integrated, portable, and functional acoustofluidic technologies for the rapidly evolving medical testing landscape.
Experimental Design
Portable Control Platform: Design and Operation
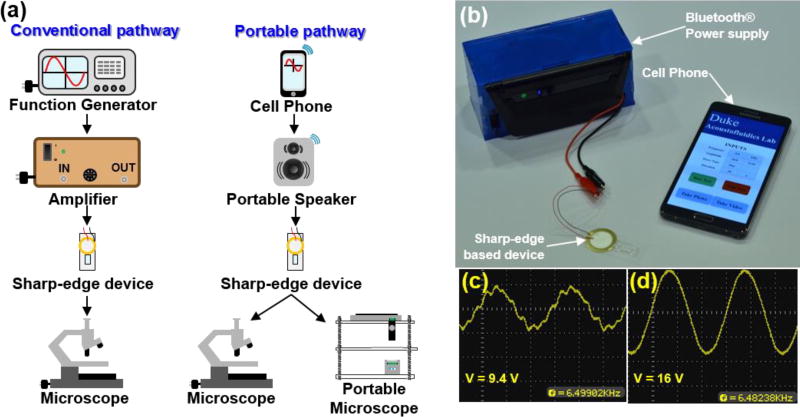
Conventionally, the operation of acoustofluidic devices relies on the use of a plug-in function generator and power amplifier to provide the necessary signal to activate the piezoelectric transducer which transfers its energy into the chip.26–32 In this work, we sought to find an alternative route to powering our acoustofluidic platforms (Fig. 1a). To this end, we established a completely portable control system for our sharp-edge based acoustofluidic devices. Conveniently, the driving frequency of the piezoelectric transducers used in the sharp-edge devices lie within the audible frequency range, allowing for commercial audio equipment to provide the power source. This concept provided us with our starting point for developing our alternative route to acoustofluidic control. A Bluetooth® speaker (Klipsch® Groove®, Klipsch®) was deconstructed and the speaker was detached from the control board. The leftover components from the Bluetooth® device were mounted into an acrylic box shown in Fig. 1b. Within the box, the outputs from the control board were connected to a Resistor-Inductor-Capacitor (RLC) resonant circuit. The RLC circuit serves to amplify the voltage of the AC signal across the capacitor and inductor in the circuit. This amplification occurs at a specific resonance frequency where the impedances of the inductor and capacitor cancel each other. The PZTs used in sharp-edge based acoustofluidic devices mimic a traditional capacitor, and thus only an appropriate inductor is needed to create resonance at the operational frequency of the device. Fig. 1c–d provides oscilloscope images with and without the resonant circuit, respectively. A significant increase in voltage can be seen, as well as an improvement in the shape of the signal exiting the Bluetooth® control board. The use of a Bluetooth® speaker as the power source of our devices not only provides numerous control possibilities, most notably, the connection to a cellular phone, but also demonstrate the potential that our devices can be powered by widely used, cheaply available audio equipment. Pure tone sound files were created using an audio editing software (Audacity®) and loaded onto a smartphone (Samsung Note4). The use of audio editing software allows users to create any possible combination of sound signals (e.g., frequency changes, pauses, and volume changes) to fit their device needs. From this point, the cell phone and Bluetooth® control board were paired and formed the connection to pass audio signals to the acoustofluidic device. It is important to note that the maximum output signal from the current cell phone platform is limited to below 20 Vpp with slight variations due to the influence of the RLC circuit. Throughout testing, the voltage was varied using the volume control on the cell phone. Lowering the phones volume from 100% to 40% transitioned the voltage incrementally from its maximum down to roughly 3 Vpp. While this output is not as high as the function generator and amplifier setup, different commercial speakers, or custom electronics could easily yield higher signals. A video that demonstrates the use of the device to power a sharp-edge based micropump can be found in the ESI (Video 1). We then used the compact, portable system to operate both our acoustofluidic micropump and micromixer as a demonstration of its adaptability and functionality. Additionally, we used the system to showcase a new design in which the pumping and mixing devices are combined onto a single chip that can accomplish both functions simultaneously.
Fig. 1.
(a) Comparison between conventional acoustofluidic device operation and portable operation. The portable control platform can be used with either a traditional benchtop microscope, or a portable microscope based on the cell phone camera. Not to scale. (b) Photo of the cell phone, modified speaker and acoustofluidic device. Signal measured from the speaker (c) before and (d) after passing through the RLC filter circuit to increase the voltage.
Results and Discussion
Cell Phone Controlled Pumping
With the development of more complex electronics systems, researchers have striven to automate systems that can be used for conducting common biological testing. Systems have been created to perform on chip PCR, ELISA and numerous bio-assays.7,33–35 However, the majority of these platforms do not take advantage of the wide variety of microscale pumping devices that have been developed, and rely heavily on bulky and expensive external syringe pumps.7 Some researchers have taken steps towards removing the need for bulky equipment, but have lost the benefit of automation.36–38 Many microfluidic methods for fluid pumping at microscale have been reported, and these pumping methods are classically divided into two regimes: passive pumping39–41 and active pumping.42–44 While both of these methods allow for a great reduction in size, there are distinct benefits and downsides to either mechanism.26 Previously, we have developed a sharp-edge based acoustofluidic micropump that relies on the acoustic streaming effect induced by acoustically oscillated sharp-edge structures.26 Our micropump can achieve precise, tunable pumping with no moving parts and minimum hardware. In this work, we aim to combining the benefits of both electronic control and our micropump to provide a portable, inexpensive, hands-free yet precise, controllable, and reliable microfluidic pump.
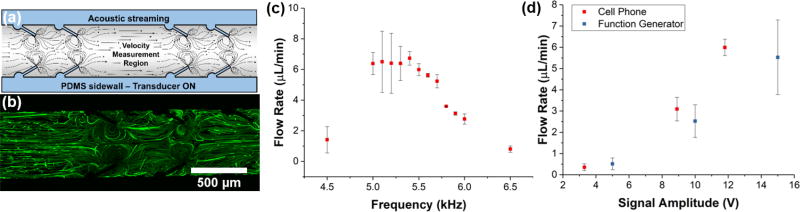
Design protocol for a typical sharp-edge based acoustofluidic pumping device can be found in our previous work (Fig. S2 in the ESI provides a detailed pumping device design).26 Briefly, a single-layer polydimethylsiloane (PDMS) channel with 30-degree tilted sharp-edge pumping structures was first created by photolithography, deep-ion-reactive etching (DRIE) and soft lithography, followed by surface modification via plasma oxidation before being bonded onto a glass coverslip (CAT. No. 48404-455, VWR). To visualize and characterize the pumping, we designed the PDMS channel to have a velocity measurement region as shown in Fig. 2a, where five pairs of sharp-edge structures were constructed on both sides. The channel had inlet and outlet ports punched with a 3.0 mm biopsy punch. A piezoelectric transducer (part no. 7BB-27-4L0, Murata Electronics) was attached adjacent to the PDMS channel onto the coverslip using an epoxy (PermaPoxy™ 5 Minute General Purpose, Permatex). Upon the activation of the PZT, the vibration of the PZT brings the glass slide and PDMS channel into oscillation, which in turn acoustically oscillates the sharp-edge structures. The oscillation of sharp-edge structures then induce the acoustic streaming effect that pumps fluids and particles in the channel. Fig. 2b provides the trajectories of 1.9 µm fluorescent beads that are being pumped by the acoustic streaming. To quantify the functionality of our cell phone controlled system, we characterized the flow rate that could be generated within the channel by tracing the movement of 6 µm polystyrene (PS) particles with ImageJ. The fluid velocity was calculated as an average of 25 randomly-selected PS particles for each frequency. To validate the reliability and reproducibility of our pumping device, the velocity from three devices was used to determine an overall average for a given frequency. A plot of the fluid velocity in the channel versus the frequency of the applied signal is provided in Fig. 2c. The presence of a peak in the pumping velocity along the frequency spectrum is characteristic of the sharp-edge based devices, and indicates the resonant frequency of the PZT-glass slide-PDMS chip system. The plot shown in Fig. 2d provides a comparison between the pumping performances when powered by the cell phone and Bluetooth® speaker or designated function generator. The volume control on the cell phone provides a simple method for modulating the signal amplitude passed to the acoustofluidic device, and the data points shown in the plot represent the 60, 80, and 100 percent volume increments on the cell phone. The peak volume of the cell phone was able to produce a flow rate of 5.99 µL/min with a measured input of 11.8 Vpp, whereas the function generator generated a flow rate of 5.53 µL/min with an input of 15 Vpp. The slight variation in the flow rates produced by the cell phone and function generator, while within statistical reason, could be caused by differences in the waveform shape produced by the two methods. The function generator produces a perfect sine wave, whereas the output from the Bluetooth® speaker could be slightly different (i.e., Fig. 1c). The energy density of this waveform could vary from the perfect sine wave (we have seen this effect when comparing square waves to sine waves generated from the function generator). Nonetheless, both of these plots serve to demonstrate the capabilities of the cell phone and Bluetooth® design to perform comparably to the traditional function generator method when controlling our sharp-edge based micropump.
Fig. 2.
(a) Schematic of the pumping mechanism via acoustic streaming. (b) Fluorescent green tracing of particles being pumped. (c) Flow rate versus frequency for the acoustofluidic pump powered by the cell phone. (d) Flow rate versus applied voltage for both the function generator and cell phone. Voltage values for the cell phone correspond to the 60, 80, and 100% volume on the phone. Data represents average of n = 3 independent devices ± standard deviation.
Cell Phone Controlled Mixing
Complete mixing is difficult to achieve on a micro-scale due to viscous domination in laminar flows.45–49 Several methods have been developed to mix fluids and reagents on a microscale, such as magnetic,50,51 optical,52 thermal,53 acoustic,54–56 and electrokinetic57–62 methods. The development of acoustic methods, particularly, the sharp-edge based acoustofluidic mixer, requires no moving/mechanical part, providing an excellent platform for efficiently and uniformly mixing fluids, even highly viscous fluids.27 Combining the sharp-edge based micromixer with a portable power and control system provides a cheaper and more compact alternative to traditional mixing strategies, and demonstrates a feature that can be extremely useful to the development of point-of-care platforms.
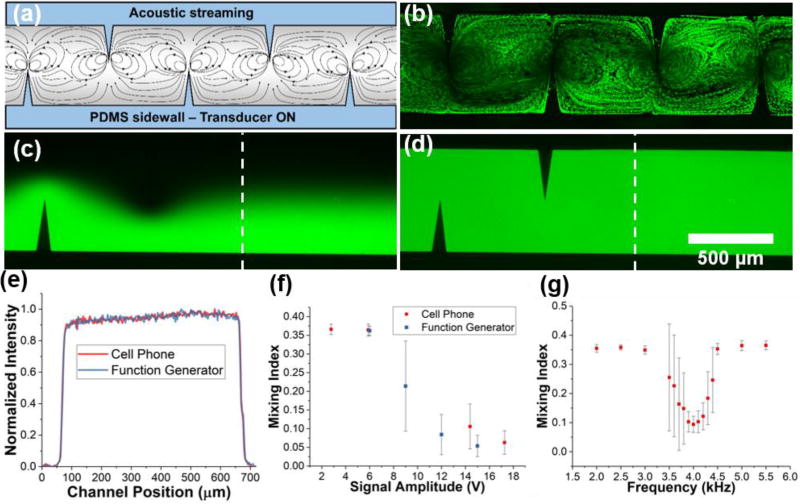
The acoustofluidic micromixer was fabricated in a similar manner to the pumping device; however, a different PZT (part no. AB2720B-LW100-R, PUI Audio) was used to achieve better mixing (Fig. S3 in the ESI provides a detailed mixing device design). An external syringe pump was used to deliver both fluorescein and DI water to separate inlets of the PDMS device (flow rate = 2.0 µL/min from each inlet). Upon activation of the PZT, the 90-degree sharp-edge structures create a strong streaming pattern that act to uniformly mix the two fluids together (Fig. 3a–b). Photos of the difference between the fluorescent distributions with and without mixing are provided in Fig. 3c–d; a comparison of the fluorescent intensity profile after mixing with the cell phone or function generator is provided in Fig. 3e. The mixing index, defined as the standard deviation of the normalized fluorescent intensity across the channel profile, enables characterization of the mixing performance; a lower mixing index indicates a more thorough mixing, with the value of 0.1 being used to define complete mixing. As a further comparison between the function generator and cell phone powered design, the mixing index versus applied voltage was plotted. Once again, the data points for the cell phone correspond to the 40, 60, 80, and 100 percent volume levels on the phone.
Fig. 3.
(a) Schematic of mixing induced by acoustic streaming in the channel. (b) Fluorescent green particle tracing to visualize the streaming patterns. Fluorescein and DI water (c) before and (d) after being mixed by the acoustofluidic device controlled with a cell phone. (e) Normalized intensity profiles across the channel width (dashed lines in Fig. 3c–d) indicate the location at which the intensity profile was measured). (f) Mixing index versus signal voltage from the cell phone and function generator. Voltage value for the cell phone correspond to the 40, 60, 80, and 100% volume levels. (g) Mixing index versus frequency of applied signal from the cell phone. Q = 2.0 µL/min from each inlet for mixing experiments. Data represents average of n = 3 independent devices ± standard deviation.
As shown in Fig. 3f, the cell phone functions adequately when compared to the mixing performance achieved by the function generator. As the voltage applied to the transducer is increased, the strength of the acoustic streaming generated by the oscillating sharp-edge structures increases and, therefore, improves the mixing performance. At the higher range of the cell phone system’s voltage output, the mixing index falls below 0.1, indicating complete mixing. The cell phone was able to produce an input voltage of 17.3 Vpp at its maximum volume, which yielded a mixing index of 0.063. The traditional setup produced a mixing index of 0.054 with an input voltage of 15 Vpp. Once again, the disparity in performance could be due to different shapes of the voltage signal, and a resultant change in power density. Additionally, the increase in maximum voltage from the pumping to mixing experiments should be noted, and is the result of the change in capacitance of the transducers used in the pumping and mixing devices interacting with the inductor in the RLC circuit. Fig. 3g provides a plot of the average mixing index of three devices across a wide driving-frequency spectrum. A characteristic dip near the resonant frequency of the device can be seen, where the mixing index dips below 0.1, thus indicating complete mixing of the two components. The larger standard deviation in points surrounding the dip in mixing index is due to the slight variation in resonant frequency across each device, which can be explained by slight variations in PZT alignment, epoxy volume, PDMS size, etc. Nonetheless, each of the devices exhibited acceptable mixing performance near 4.0 kHz range, thus establishing a safe average resonant frequency at that point. The results, once again, demonstrate that the wireless control platform, as an alternative power source to a function generator, operates sharp-edge based devices with comparable performance in a portable manner.
Combined Pumping and Mixing on a Single Chip
As explained previously, micropumping and micromixing both enable precise, controlled, and rapid reactions, assays, and diagnostics tests.8,37,63,64 While each of these functions is critical for many microfluidic applications, they remain discrete innovations. Having developed the sharp-edge based technology necessary to achieve both functions separately, herein we also demonstrated that by combining the design of sharp-edge structures for micropumping and micromixing together, concurrent pumping and mixing can be achieved on a single acoustofluidic chip. Thus, the device can achieve thorough mixing without the need for an external syringe pump to deliver the fluids. Successfully integrating these devices hinged on properly integrating the structures so that flow would be developed and mixed using only a single transducer.
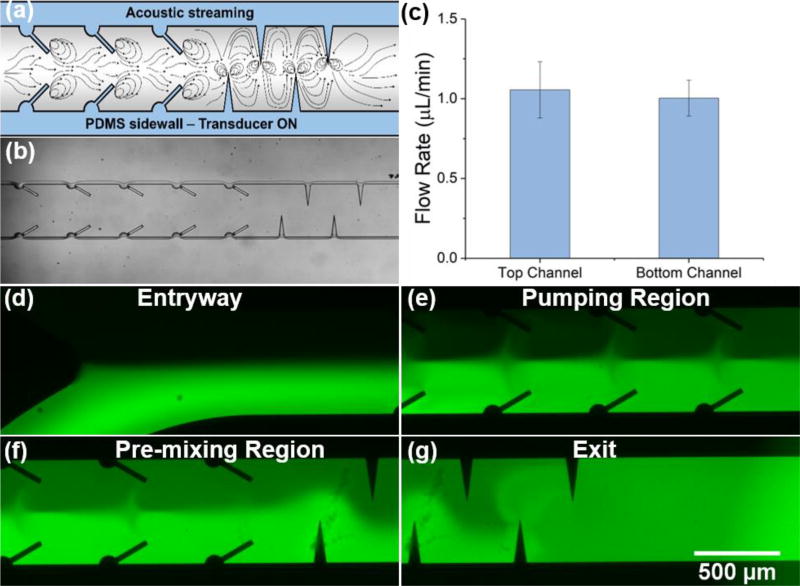
The chip was designed using the same processes as our other sharp-edge based devices, but contains both the tilted (pumping) and vertical (mixing) sharp-edge structures (Fig. S4 in the ESI provides a detailed design for the combined pumping and mixing device). Two inlet chambers allow for the storage and loading of two separate reagents, and a single outlet provides a collection port. The entrance region (where the two inlet channels meet) was carefully designed to facilitate an even flow from each channel as fluid is pumped from the inlets. The tilted sharp-edge structures serve to propel the fluid through the channel, whereas the vertical structures induce vortices spanning the full channel width to mix the two streams. Fig. 4a–b provide a schematic of the channel with the pathlines acoustic streaming would develop, and a tracing of fluorescent beads, respectively. A quantitative comparison of the flow rates generated from each inlet was conducted in order to assure that equal volumes of the fluids would be introduced from either channel (Fig. 4c). The smooth entrance of the two inlet channels facilitates an even flow from either inlet. As a demonstration, DI water and fluorescein were placed in either inlet and the device was activated via the Bluetooth® power supply. Fluorescent images at different location throughout the channel can be found in Fig. 4d–g. The fluorescent images confirm the streaming patterns proposed in Fig. 4a, where vortices remain isolated to either half of the channel within the pumping region (creating a uniform solution in the bottom and top half of the channel), and encompass the entire channel within the mixing region (providing a fully mixed solution after the last sharp-edge structure). This result demonstrates the functionality of this all-in-one acoustofluidic chip that can concurrently achieve two vital microfluidic functions in a single device.
Fig. 4.
(a) Schematic of the combined pumping and mixing sharp-edged structures and the streamlines created via acoustic streaming. (b) Microscope image of the PDMS channel bonded to a glass slide. Two sets of mixing structures were preceded by seven pumping structures. (c) Comparison of the flow rates of reagents from the top and bottom inlets. (d–g) Fluorescent images of concurrently pumped DI water and fluorescein in the entryway, pumping region, pre-mixing region, and exit, respectively. Data represents average of n = 3 independent devices ± standard deviation.
Portable Microscope Station
When discussing a POC device, one must consider all aspects of the analysis needed to accomplish the “care” being provided. The cellular phone provides an excellent platform for controlling the Bluetooth® device used in our design. It also has the added benefit of a high-resolution camera, creating the possibility for an even more functional portable device. Many recent research ventures have begun to exploit the cell phone for its imaging technologies and potential benefits to POC designs.7,35,65–70
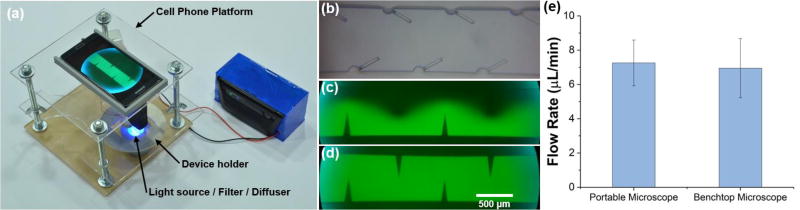
In this work, after demonstrating the control of sharp-edge based devices using cell phone, we further used the cell phone as the base to create a simplified, portable microscopy system that is capable of viewing microstructures and even analyzing fluorescent samples. The system was constructed using laser-cut acrylic sheets with nuts and bolts providing the structural support system; this laboratory-made system also allows for a moveable sample platform (Fig. 5a). A portable cell phone microscope (CML-60X-100X-BL-01, Efanr®) was modified and fixed into the top platform of the design (Fig. 5a). The cell phone was placed above the microscope, with a 3D printed holder used to align the lens of the phone camera with the lens of the portable microscope. White (7000K) and blue (470 nm) LED lights were mounted beneath the sample platform to provide both a bright-field and fluorescent light source, respectively. A variable resistance potentiometer allows the user to adjust the LED’s intensity, allowing for a tunable light source. A diffuser (part no. 43-723, Edmonds Optics) was placed in between the light source and the sample to smooth out the light field.
Fig. 5.
(a) Photo of the portable cell phone based microscope station. (b) Bright field image of the pumping channel taken with the portable microscope. Fluorescent images of (c) Un-mixed and (d) Mixed DI water and fluorescein taken with the portable microscope. (e) Graph comparing the flow rates calculated using video from the portable microscope and the traditional benchtop microscope. Data represents average of n = 3 independent devices ± standard deviation.
For fluorescent detection, an excitation (AT480/30x, Chroma) filter was placed in between the light source and sample. An emissions filter (part no. 86-939, Edmonds Optics) between the sample and cell phone was implemented to isolate the fluorescent signal emitted by the sample. The image shown in Fig. 5b provides a bright-field image of an empty channel with tilted sharp-edge structures used for pumping. Fig. 5c–d serve to demonstrate the capability of the simplified microscope for fluorescence detection, providing images of unmixed and mixed DI water and fluorescein within the channel, respectively. This simple fluorescence image could be useful in simple assays, including direct fluorescent antibody testing; this test requires only a positive fluorescent signal detection to identify specific bacterial or viral targets.
As a further display of the use of inexpensive, portable microscope equipment for research (and eventually medical) investigations, we used video recorded from the cell phone to quantify the pumping velocity. A video of pumping in the channel recorded using the cell phone camera and portable microscope can be found in the ESI (Video 2). For the same three pumping devices, we used both the traditional benchtop microscope and the portable microscope to characterize the velocity of 6 µm particles within the channel. The results taken from the cell phone correlate very well with the results from the benchtop microscope and fast camera, proving the functionality of the cell-phone based simplified microscope.
While the images may be limited in their detail, they actively demonstrate the potential that a cheap imaging system, paired with a low-cost acoustofluidic approach could have for portable diagnostics and testing. Paired with the acoustofluidic micromixer and micropump, a baseline detection could be made, with a portable, inexpensive system.
Conclusions
With our sharp–edge based acoustofludic technology, and cell phone control, we have removed the need for much of the bulky and expensive equipment typically associated with acoustofluidics research and biological assays. Additionally, we demonstrated a functional, portable microscope that allowed for fluorescent detection, thus providing a completely portable system that can be taken anywhere with ease. The battery powered nature of the cell phone and Bluetooth® speaker used in our design eliminated the requirement of a wall based power source, making the system capable of traveling wherever researchers need to go. While this system is only a first step towards POC detection, it is a true demonstration of the potential that acoutsofluidics technologies have in this field. The ability to remotely operate microfluidic devices using cost-effective, compact, and commercial components has immeasurable benefits to the medical and diagnostics community. The characteristics of microscale reactions, including diminished reagent volumes and lessened reaction times are well known as benefits towards lowering cost, and our system capitalizes on this advantage. We believe that this system can act as a starting point for further development of a capable and portable diagnostic tool that takes full advantage of the benefits of acoustofluidics and other lab-on-a-chip technologies.
Supplementary Material
Acknowledgments
We acknowledge support from the National Institutes of Health (R01 GM112048, R33 EB019785, R44HL126441, and R01 HD086325) and National Science Foundation (CBET-1438126 and IDBR-1455658).
Footnotes
Electronic Supplementary Information (ESI) available: Video of sharp-edge based device operation via portable power supply, and video of sharp-edge pumping recorded via cell phone. See DOI: 10.1039/x0xx00000x
References
- 1.Mao X, Huang TJ. Lab Chip. 2012;12:1412. doi: 10.1039/c2lc90022j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bigelow MEG, Jamieson BG, Chui CO, Mao Y, Shin KS, Huang TJ, Huang PH, Ren L, Adhikari B, Chen J, Iturriaga E. IEEE J. Transl. Eng. Heal. Med. doi: 10.1109/JTEHM.2016.2593920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeo LY, Chang HC, Chan PPY, Friend JR. Small. 2011;7:12–48. doi: 10.1002/smll.201000946. [DOI] [PubMed] [Google Scholar]
- 4.Liao SC, Peng J, Mauk MG, Awasthi S, Song J, Friedman H, Bau HH, Liu C. Sensors Actuators, B Chem. 2016;229:232–238. doi: 10.1016/j.snb.2016.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Lillehoj PB. Lab Chip. 2016;16:2093–2098. doi: 10.1039/c6lc00307a. [DOI] [PubMed] [Google Scholar]
- 6.Song J, Mauk MG, Hackett BA, Cherry S, Bau HH, Liu C. Anal. Chem. 2016;88:7289–7294. doi: 10.1021/acs.analchem.6b01632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu H, Mavandadi S, Coskun AF, Yaglidere O, Ozcan A. Anal. Chem. 2011;83:6641–6647. doi: 10.1021/ac201587a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin YH, Wang CC, Lei KF. Biomed. Microdevices. 2014;16:199–207. doi: 10.1007/s10544-013-9822-4. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Zapatero-Rodríguez J, Estrela P, O’Kennedy R. Biosensors. 2015;5:577–601. doi: 10.3390/bios5030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers FB, Lee LP. Lab Chip. 2008;8:2015. doi: 10.1039/b812343h. [DOI] [PubMed] [Google Scholar]
- 11.Moon S, Keles H. Biosens. 2009;24:3208–3214. doi: 10.1016/j.bios.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gervais L, Delamarche E. Lab Chip. 2009;9:3330. doi: 10.1039/b906523g. [DOI] [PubMed] [Google Scholar]
- 13.Chin CD, Linder V, Sia SK. Lab Chip. 2012;12:2118. doi: 10.1039/c2lc21204h. [DOI] [PubMed] [Google Scholar]
- 14.Guo F, Mao Z, Chen Y, Xie Z, Lata JP, Li P, Ren L, Liu J, Yang J, Dao M, Suresh S, Huang TJ. Proc. Natl. Acad. Sci. 2016;113:1522–1527. doi: 10.1073/pnas.1524813113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi J, Ahmed D, Mao S-CS, Lin X, Lawit A, Huang TJ. Lab Chip. 2009;9:2890. doi: 10.1039/b910595f. [DOI] [PubMed] [Google Scholar]
- 16.Guo F, Li P, French JB, Mao Z, Zhao H, Li S, Nama N, Fick JR, Benkovic SJ, Huang TJ. Proc. Natl. Acad. Sci. 2015;112:43–48. doi: 10.1073/pnas.1422068112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding X, Peng S-CS, Lin Z, Geri M, Li S, Li P, Chen Y, Dao M, Suresh S, Huang TJ. Proc. Natl. Acad. Sci. 2014;111:12992–12997. doi: 10.1073/pnas.1413325111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding X, Li S-CS, Lin P, Stratton ZS, Nama N, Guo F, Slotcavage D, Mao X, Shi J, Costanzo F, Huang TJ. Lab Chip. 2013;13:3626. doi: 10.1039/c3lc50361e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding S-CS, Lin X, Kiraly B, Yue H, Li I-K, Chiang S, Shi J, Benkovic SJ, Huang TJ. Proc. Natl. Acad. Sci. 2012;109:11105–11109. doi: 10.1073/pnas.1209288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xuan W, He X, Chen J, Wang W, Wang X, Xu Y, Xu Z, Fu YQ, Luo JK. Nanoscale. 2015;7:7430–7436. doi: 10.1039/c5nr00040h. [DOI] [PubMed] [Google Scholar]
- 21.De Vellis A, Gritsenko D, Lin Y, Wu Z, Zhang X, Pan Y, Xue W, Xu J. Sensors Actuators, B Chem. 2017;243:298–302. [Google Scholar]
- 22.Yeo LY, Friend JR. Biomicrofluidics. doi: 10.1063/1.3056040. [DOI] [Google Scholar]
- 23.Wu M, Ouyang Y, Wang Z, Zhang P-H, Huang R, Chen C, Li H, Li P, Quinn D, Dao M, Suresh S, Sadovsky Y, Huang TJ. Proc. Natl. Acad. Sci. 2017;114:10584–10589. doi: 10.1073/pnas.1709210114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed D, Ozcelik A, Bojanala N, Nama N, Upadhyay A, Chen Y, Hanna-Rose W, Huang TJ. Nat. Commun. 2016;7:1–11. doi: 10.1038/ncomms11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed D, Chan S-CS, Lin CY, Muddana HS, Nama N, Benkovic SJ, Jun Huang T. Lab Chip. 2013;13:328–331. doi: 10.1039/c2lc40923b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang P-H, Nama N, Mao Z, Li P, Rufo J, Chen Y, Xie C-H, Wei Y, Wang L, Huang TJ. Lab Chip. 2014;14:4319–4323. doi: 10.1039/c4lc00806e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang P-H, Xie Y, Ahmed D, Rufo J, Nama N, Chen Y, Chan CY, Huang TJ. Lab Chip. 2013;13:3847. doi: 10.1039/c3lc50568e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nama P-H, Huang N, Huang TJ, Costanzo F. Lab Chip. 2014;14:2824–2836. doi: 10.1039/c4lc00191e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang P-H, Chan CY, Li P, Nama N, Xie C-H, Wei Y, Chen Y, Ahmed D, Huang TJ. Lab Chip. 2015;15:4166–4176. doi: 10.1039/c5lc00868a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang P-H, Ren L, Nama N, Li S, Li P, Yao X, Cuento C-H, Wei RA, Chen Y, Xie Y, Nawaz AA, Alevy YG, Holtzman MJ, McCoy JP, Levine SJ, Huang TJ. Lab Chip. 2015;15:3125–3131. doi: 10.1039/c5lc00539f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nama N, Huang PH, Huang TJ, Costanzo F. Biomicrofluidics. doi: 10.1063/1.4946875. [DOI] [Google Scholar]
- 32.Ozcelik A, Nama N, Huang PH, Kaynak M, McReynolds MR, Hanna-Rose W, Huang TJ. Small. 2016;12:5230. doi: 10.1002/smll.201601760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chin CD, Laksanasopin T, Cheung YK, Steinmiller D, Linder V, Parsa H, Wang J, Moore H, Rouse R, Umviligihozo G, Karita E, Mwambarangwe L, Braunstein SL, van de Wijgert J, Sahabo R, Justman JE, El-Sadr W, Sia SK. Nat. Med. 2011;17:1015–1019. doi: 10.1038/nm.2408. [DOI] [PubMed] [Google Scholar]
- 34.Yeh E, Fu C, Hu L, Thakur R, Feng J, Lee LP. 2017:1–12. doi: 10.1126/sciadv.1501645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez AW, Phillips ST, Carrilho E, Thomas SW, Sindi H, Whitesides GM. Anal. Chem. 2008;80:3699–3707. doi: 10.1021/ac800112r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong MM, MacDonald BD, Vu Nguyen T, Sinton D. Biomicrofluidics. doi: 10.1063/1.4762851. [DOI] [Google Scholar]
- 37.Kim S, Kwon S, Cho CH, Park J-K. Lab Chip. 2017;17:702–709. doi: 10.1039/c6lc01495j. [DOI] [PubMed] [Google Scholar]
- 38.Weibel DB, Siegel AC, Lee A, George AH, Whitesides GM. Lab Chip. 2007;7:1832. doi: 10.1039/b714664g. [DOI] [PubMed] [Google Scholar]
- 39.Chao S-H, Meldrum DR. Lab Chip. 2009;9:867–869. doi: 10.1039/b819887j. [DOI] [PubMed] [Google Scholar]
- 40.Walker GM, Beebe DJ. Lab Chip. 2002;2:131. doi: 10.1039/b202473j. [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann M, Hunziker P, Delamarche E. Microfluid. Nanofluidics. 2008;5:395–402. [Google Scholar]
- 42.Unger MA. Science (80-.) 2000;288:113–116. doi: 10.1126/science.288.5463.113. [DOI] [PubMed] [Google Scholar]
- 43.Brask A, Snakenborg D, Kutter JP, Bruus H. Lab Chip. 2006;6:280–288. doi: 10.1039/b509997h. [DOI] [PubMed] [Google Scholar]
- 44.Huang S, Li C, Lin B, Qin J. Lab Chip. 2010;10:2925. doi: 10.1039/c005227b. [DOI] [PubMed] [Google Scholar]
- 45.Mao S-CS, Lin X, Dong C, Huang TJ. Lab Chip. 2009;9:1583. doi: 10.1039/b820138b. [DOI] [PubMed] [Google Scholar]
- 46.Zhang C, Van Noort D. Cells in microfluidics. 2011;304 doi: 10.1007/128_2011_147. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen N-T, Wu Z. J. Micromechanics Microengineering. 2005;15:R1–R16. [Google Scholar]
- 48.Mao X, Waldeisen JR, Huang TJ. Lab Chip. 2007;7:1260–1262. doi: 10.1039/b711155j. [DOI] [PubMed] [Google Scholar]
- 49.Whitesides GM. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 50.Zhu G-P, Nguyen N-T. Lab Chip. 2012;12:4772. doi: 10.1039/c2lc40818j. [DOI] [PubMed] [Google Scholar]
- 51.Ryu KS, Shaikh K, Goluch E, Fan Z, Liu C. Lab Chip. 2004;4:608–13. doi: 10.1039/b403305a. [DOI] [PubMed] [Google Scholar]
- 52.Hellman AN, Rau KR, Yoon HH, Bae S, Palmer JF, Phillips KS, Allbritton NL, Venugopalan V. Anal. Chem. 2007;79:4484–4492. doi: 10.1021/ac070081i. [DOI] [PubMed] [Google Scholar]
- 53.Tsai J, Lin L. Sensors Actuators A Phys. 2002;97–98:665–671. [Google Scholar]
- 54.Tovar AR, Lee AP. Lab Chip. 2009;9:41–43. doi: 10.1039/b812435c. [DOI] [PubMed] [Google Scholar]
- 55.Destgeer G, Im S, Hang Ha B, Ho Jung J, Ahmad Ansari M, Jin Sung H. Appl. Phys. Lett. 2014;104:10–15. [Google Scholar]
- 56.Huang PH, Ian Lapsley M, Ahmed D, Chen Y, Wang L, Jun Huang T. Appl. Phys. Lett. 2012;101:141101. doi: 10.1063/1.4742864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang RJ, Wu CH, Tseng TI, Huang SB, Lee GB. Japanese J. Appl. Physics, Part 1 Regul. Pap. Short Notes Rev. Pap. 2005;44:7634–7642. [Google Scholar]
- 58.Sigurdson M, Wang D, Meinhart CD. Lab Chip. 2005;5:1366. doi: 10.1039/b508224b. [DOI] [PubMed] [Google Scholar]
- 59.Ng WY, Goh S, Lam YC, Yang C, Rodríguez I. Lab Chip. 2009;9:802–809. doi: 10.1039/b813639d. [DOI] [PubMed] [Google Scholar]
- 60.Lee C-Y, Lee G-B, Fu L-M, Lee K-H, Yang R-J. J. Micromechanics Microengineering. 2004;14:1390–1398. [Google Scholar]
- 61.Harnett CK, Templeton J, a Dunphy-Guzman K, Senousy YM, Kanouff MP. Lab Chip. 2008;8:565–572. doi: 10.1039/b717416k. [DOI] [PubMed] [Google Scholar]
- 62.Coleman JT, McKechnie J, Sinton D. Lab Chip. 2006;6:1033–1039. doi: 10.1039/b602085b. [DOI] [PubMed] [Google Scholar]
- 63.Pollack L, Tate MW, Darnton NC, Knight JB, Gruner SM, a Eaton W, Austin RH. Proc. Natl. Acad. Sci. 1999;96:10115–10117. doi: 10.1073/pnas.96.18.10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie Y, Ahmed D, Lapsley MI, Lin SCS, Nawaz AA, Wang L, Huang TJ. Anal. Chem. 2012;84:7495–7501. doi: 10.1021/ac301590y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D’Ambrosio MV, Bakalar M, Bennuru S, Reber C, Skandarajah A, Nilsson L, Switz N, Kamgno J, Pion S, Boussinesq M, Nutman TB, Fletcher DA. Sci. Transl. Med. 2015;7:286re4–286re4. doi: 10.1126/scitranslmed.aaa3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ozcan A. Lab Chip. 2014;14:3187–3194. doi: 10.1039/c4lc00010b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen L, Hagen JA, Papautsky I. Lab Chip. 2012;12:4240. doi: 10.1039/c2lc40741h. [DOI] [PubMed] [Google Scholar]
- 68.Vashist SK, Mudanyali O, Schneider EM, Zengerle R, Ozcan A. Anal. Bioanal. Chem. 2014;406:3263–3277. doi: 10.1007/s00216-013-7473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang S, Zhao X, Khimji I, Akbas R, Qiu W, Edwards D, Cramer DW, Ye B, Demirci U. Lab Chip. 2011;11:3411. doi: 10.1039/c1lc20479c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu H, Isikman SO, Mudanyali O, Greenbaum A, Ozcan A. Lab Chip. 2013;13:51–67. doi: 10.1039/c2lc40864c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.