Abstract
Background
The clinical significance of pneumonia visualized on CT scan in the setting of a normal chest radiograph is uncertain.
Methods
In a multicenter prospective surveillance study of adults hospitalized with community-acquired pneumonia (CAP), we compared the presenting clinical features, pathogens present, and outcomes of patients with pneumonia visualized on a CT scan but not on a concurrent chest radiograph (CT-only pneumonia) and those with pneumonia visualized on a chest radiograph. All patients underwent chest radiography; the decision to obtain CT imaging was determined by the treating clinicians. Chest radiographs and CT images were interpreted by study-dedicated thoracic radiologists blinded to the clinical data.
Results
The study population included 2,251 adults with CAP; 2,185 patients (97%) had pneumonia visualized on chest radiography, whereas 66 patients (3%) had pneumonia visualized on CT scan but not on concurrent chest radiography. Overall, these patients with CT-only pneumonia had a clinical profile similar to those with pneumonia visualized on chest radiography, including comorbidities, vital signs, hospital length of stay, prevalence of viral (30% vs 26%) and bacterial (12% vs 14%) pathogens, ICU admission (23% vs 21%), use of mechanical ventilation (6% vs 5%), septic shock (5% vs 4%), and inhospital mortality (0 vs 2%).
Conclusions
Adults hospitalized with CAP who had radiological evidence of pneumonia on CT scan but not on concurrent chest radiograph had pathogens, disease severity, and outcomes similar to patients who had signs of pneumonia on chest radiography. These findings support using the same management principles for patients with CT-only pneumonia and those with pneumonia seen on chest radiography.
Key Words: chest radiography, CT scan, pneumonia
Abbreviations: CAP, community-acquired pneumonia; CDC, Centers for Disease Control and Prevention; EPIC, Etiology of Pneumonia in the Community; IQR, interquartile range; RT-PCR, real-time polymerase chain reaction
FOR EDITORIAL COMMENT, SEE PAGE 583
Pneumonia continues to be a major cause of morbidity and mortality in adults, accounting for approximately 55,000 deaths, 1.2 million hospitalizations, and 1.7 million ED visits annually in the United States.1, 2, 3 The diagnosis of pneumonia is based on clinical features plus radiological findings consistent with pulmonary infection.4 Traditionally, chest radiography has been the primary radiographic test used to evaluate for community-acquired pneumonia (CAP).4 However, the use of chest CT imaging to evaluate patients with acute respiratory symptoms has markedly increased during the past 2 decades as clinical practice has evolved to more commonly assess for noninfectious conditions, such as pulmonary embolism and aortic dissection, and to more thoroughly image the lungs for signs of pneumonia.5, 6 CT imaging is more sensitive than chest radiography for identifying radiological signs of pneumonia, resulting in some patients having pneumonia visualized on CT scans but not on concurrent chest radiographs (CT-only pneumonia).7, 8, 9, 10, 11
The clinical significance of radiological signs of pneumonia visualized only on CT imaging is largely unknown, creating uncertainty about whether these patients should be managed according to the same principles as those with pneumonia identified on chest radiography or whether CT-only pneumonia is a distinct, less severe disease.12 The purpose of this study was to compare the pathogens detected, illness severity, and clinical outcomes in patients with CT-only pneumonia and those with pneumonia visualized on chest radiography among adults hospitalized with CAP.
Methods
This analysis was nested within the Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study, a multicenter prospective active surveillance study of patients hospitalized with CAP.13 Adults were enrolled from January 1, 2010 to June 30, 2012 at five hospitals, including three in Chicago, Illinois and two in Nashville, Tennessee. Institutional review boards at the CDC and each enrolling hospital approved the study (Vanderbilt University IRB No. 091422). Informed consent for study participation was obtained from all enrolled patients or their representatives.
Study Population
The EPIC study population of adults hospitalized with CAP has been previously described.13 In brief, adults (≥ 18 years old) were eligible if they were admitted to a study hospital, lived in a study catchment area surrounding each participating hospital, and had clinical evidence of an acute respiratory infection and radiological signs of pneumonia as interpreted by a study-dedicated thoracic radiologist. Exclusion criteria included patients with a recent hospitalization (within the previous 28 days for immunocompetent patients and within 90 days for immunosuppressed patients), nursing home residents who were not functionally independent, patients who had undergone tracheostomy or gastrostomy, patients with cystic fibrosis, cancer with neutropenia, solid organ or hematopoietic stem cell transplantation within the previous 90 days, active graft-vs-host disease, bronchiolitis obliterans, HIV infection with a CD4 cell count < 200 mm3, and those with a nonpneumonia alternative diagnosis for the cause of acute respiratory symptoms. The study population for the current analysis included adults in the EPIC study who had adequate etiology testing (one test or more for bacteria and one test or more for viruses) and a chest radiograph obtained within 48 hours of enrollment.
Radiological Evidence of Pneumonia
Inclusion in the EPIC study required a chest radiograph or CT scan within 48 hours before or after hospital admission demonstrating signs of pneumonia.13 The choice of chest imaging (ie, whether to obtain a chest CT scan in addition to or in lieu of a chest radiograph) was made by the treating clinician independent of the study protocol. Initially, patients were enrolled based on interpretation of chest imaging as consistent with acute pneumonia by the clinicians or radiologists interpreting images for routine clinical care. Subsequently, all chest radiographs and CT scans obtained within 48 hours of admission were independently reviewed by a board-certified, study-dedicated thoracic radiologist blinded to the clinical data. The study included two thoracic radiologists (E. M. H. and F. C.), with one reading images from the three Chicago hospitals and the other reading images from the two Nashville hospitals. These radiologists used the same protocol and data collection form when interpreting images and recording their findings. Available chest imaging prior to the current hospitalization was used to help distinguish between acute and chronic findings.
Only patients with radiological evidence of pneumonia on chest radiography or CT imaging, or both, as interpreted by the study-dedicated radiologists were included in the final EPIC study population. Patients were classified as having chest radiographic evidence of pneumonia if the radiologist identified a new consolidation (dense or fluffy opacity with or without air bronchograms), other infiltrate (linear or patchy alveolar or interstitial densities), or pleural effusion on any chest radiograph completed within 48 hours of hospital presentation.13, 14 When interpreting CT scans, radiologists noted their global impression of whether the images were consistent with acute pneumonia by selecting one of four options: (1) no pneumonia, (2) possible pneumonia, (3) probable pneumonia, or (4) definite pneumonia. Patients were classified as having CT scan evidence of pneumonia if the radiologist indicated possible, probable, or definite pneumonia.
Study Groups for Comparison
Patients with evidence of pneumonia on CT scan but not chest radiograph were classified as having CT-only pneumonia. Patients with chest radiograph evidence of pneumonia were classified as having pneumonia on chest radiography regardless of whether CT scan imaging was completed. Patients who were included in the EPIC study based on CT findings without a chest radiograph completed within 48 hours of admission were excluded from the current analysis, because the goal was to evaluate patients with pneumonia visualized on CT imaging but not on concurrent chest radiography.
The primary analysis involved a comparison between patients with CT-only pneumonia and those with pneumonia on chest radiography. In secondary analyses, which are reported in e-Tables 1-8, the pneumonia on chest radiography group was divided into three subcategories—pneumonia on chest radiography/CT imaging not performed; pneumonia on chest radiography/CT pneumonia; and pneumonia on chest radiography/no pneumonia on CT scan.
Outcomes
Patients with CT-only pneumonia and pneumonia on chest radiography were compared based on three categories: initial clinical characteristics and antibiotics received, pathogens detected, and short-term clinical outcomes.
Initial clinical characteristics and antibiotics
Patient characteristics were systematically ascertained through patient interview and medical record abstraction.13 Obesity was defined as a BMI ≥ 30 kg/m2.15 Pneumonia severity scores, including the Pneumonia Severity Index,16 CURB-65 (confusion, urea nitrogen, respiratory rate, blood pressure, 65 years of age and older),17 and American Thoracic Society Minor Criteria for Severe CAP,4 were calculated to summarize each patient’s condition at the time of initial hospital presentation. Patients with residual serum collected at hospital presentation also had serum procalcitonin (PCT) measured for research purposes using the VIDAS BRAHMS PCT immunoassay kit (BioMerieux, Inc.).18, 19 The lower limit of detection for this assay was 0.05 ng/mL. PCT results were not available to clinicians and were not used for patient management. Prior research suggests a PCT threshold of 0.25 ng/mL can help distinguish between viral and bacterial infection20, 21; therefore, PCT was analyzed on a continuous scale and also dichotomized as < 0.25 ng/mL (low) vs ≥ 0.25 ng/mL (high). We also reported the proportion of patients who received antibiotics within 6 and 12 hours of hospital presentation and the specific antibiotics used for initial empirical treatment, defined as antibiotics delivered on the first day of hospitalization, including those given in the ED.
Pathogens
Biological specimens for pathogen detection were systematically collected from each patient as soon as possible after hospital presentation.13 Viral testing as part of the study protocol included real-time polymerase chain reaction (RT-PCR) of nasopharyngeal/oropharyngeal swabs and paired acute and convalescent serologic tests for respiratory viruses, including adenovirus, coronaviruses (RT-PCR only), human metapneumovirus, human rhinovirus (RT-PCR only), influenza, parainfluenza, and respiratory syncytial virus.22, 23, 24, 25 Bacterial testing as part of the study protocol included blood cultures, sputum cultures (limited to high-quality samples26), urinary antigen tests for Streptococcus pneumoniae and Legionella pneumophila,27, 28 RT-PCR of nasopharyngeal/oropharyngeal swabs for Chlamydophila pneumoniae and Mycoplasma pneumoniae and of sputum for Legionella.29 Other respiratory samples, including endotracheal aspirates, pleural fluid, and BAL, obtained for routine clinical care also underwent bacterial culture.
Clinical outcomes
Clinical outcomes during the index hospitalization were ascertained by medical record abstraction.13 These outcomes included hospital length-of-stay, ICU admission, invasive mechanical ventilation, vasopressor-dependent septic shock, moderate or severe ARDS, and inhospital mortality. Invasive mechanical ventilation was defined as new assisted ventilation through an endotracheal tube or tracheostomy. Vasopressor-dependent septic shock was defined as vasopressor administration by continuous infusion at any time during the first 72 hours of the hospitalization for pneumonia. Moderate or severe ARDS was defined as meeting all the following criteria within 72 hours of hospitalization: bilateral pulmonary opacities, Pao2/Fio2 < 200 mm Hg, and respiratory failure not fully explained by cardiac failure.30
Statistical Analysis
Outcomes were compared between patients with CT-only pneumonia and those with pneumonia on chest radiograph with the Wilcoxon rank-sum test for continuous variables and the χ2 or Fisher exact test for categorical variables, as appropriate. Analyses were performed with Stata, version 12.1; (StataCorp LLC).
Results
Study Population
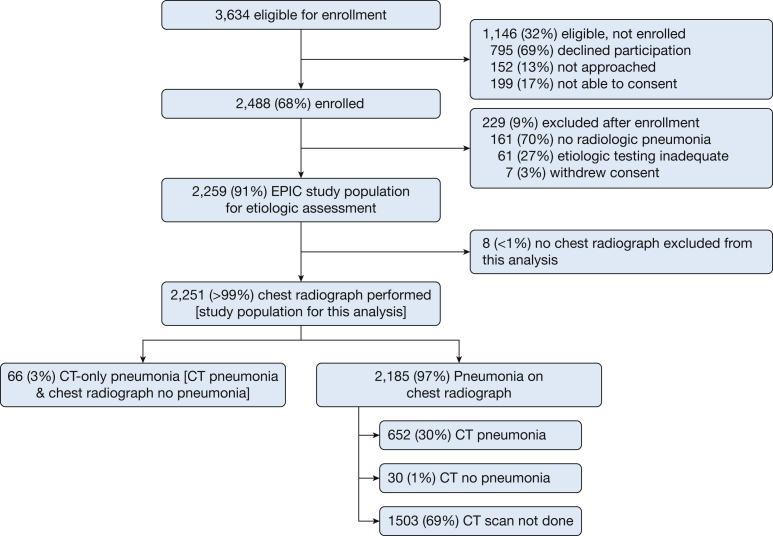
During the 2½-year study period, 3,634 eligible patients were identified through prospective active surveillance; 2,251 of these patients (62%) were enrolled, had radiological pneumonia confirmed by a study radiologist, underwent adequate testing for the cause of pneumonia, and had chest radiography performed (study population for the current analysis) (Fig 1). All 2,251 included patients underwent chest radiography, and 748 (33%) had concurrent CT imaging. Compared with patients who did not undergo CT imaging, those with a CT scan had similar vital signs but fewer comorbidities, were more likely to present with hemoptysis, shortness of breath, and chest pain, and had somewhat lower pneumonia severity scores (e-Tables 1-3).
Figure 1.
Flow diagram for generation of the study population.
Sixty-six (3%) of the 2,251 included patients had CT-only pneumonia. All 66 of these patients had CT imaging within 1 calendar day of undergoing chest radiography that showed no signs of pneumonia, including 43 patients (65%) with CT imaging and chest radiography on the same day and 23 patients (35%) with CT imaging completed 1 calendar day later than chest radiography. Among the 66 cases of CT-only pneumonia, the radiologist interpreted CT images as definite pneumonia in 34 patients (52%), probable pneumonia in 14 patients (21%), and possible pneumonia in 18 (27%) patients.
Of the 2,251 included patients, 2,185 (97%) had pneumonia on chest radiography. Among those with pneumonia on chest radiography, 1,503 (69%) did not have a concurrent CT scan, 652 (30%) had pneumonia confirmed on CT scan, and 30 (1%) had a CT scan without evidence of pneumonia (e-Tables 4-6).
Initial Clinical Characteristics and Antibiotics
Overall, the presenting clinical profiles of patients with CT-only pneumonia and pneumonia on chest radiography were similar, including comorbidities, vital signs, and laboratory test results (Table 1). However, compared with the pneumonia on chest radiography patients, the patients with CT-only pneumonia were younger and more likely to present with chest pain, wheezing, and low-risk Pneumonia Severity Index categories I and II. BMI was also higher in the CT-only pneumonia group (median, 30 kg/m2) compared with the pneumonia on chest radiography group (median, 27 kg/m2; P = .02), thus leading to a higher proportion of patients in the CT-only pneumonia group meeting the definition for obesity (49% vs 36%; P = .04). Serum PCT concentrations were lower in the CT-only pneumonia group (median, < 0.05 ng/mL; interquartile range [IQR], < 0.05-0.11 ng/mL) compared with the pneumonia on chest radiography group (median, 0.16 ng/mL; IQR, < 0.05-0.85 ng/mL); 18% of the patients with CT-only pneumonia had a PCT level > 0.25 ng/mL compared with 41% of the patients with pneumonia on chest radiography (P < .01).
Table 1.
Clinical Characteristics at Hospital Presentation
| Variable | CT-Only Pneumonia (n = 66) | Pneumonia on Chest Radiography (n = 2,185) | P Value |
|---|---|---|---|
| Demographics | |||
| Age, median (IQR), y | 53 (40-63) | 58 (47-71) | < .01 |
| Female sex, No. (%) | 39 (59.1) | 1,111 (50.9) | .18 |
| Vital signs, median (IQR) | |||
| Heart rate, beats/min | 100 (88-118) | 100 (87-114) | .51 |
| Systolic blood pressure, mm Hg | 140 (126-157) | 131 (114-148) | < .01 |
| Respiratory rate, breaths/min | 20 (18-24) | 20 (18-24) | .62 |
| Temperature, °C | 36.9 (36.3-37.9) | 36.9 (36.2-37.8) | .73 |
| Oxygen saturation, % | 96 (94-98) | 95 (92-97) | .07 |
| Signs and symptoms, No. (%) | |||
| Cough with sputum production | 39 (59.1) | 1,206 (55.2) | .53 |
| Hemoptysis | 5 (7.6) | 192 (8.8) | .73 |
| Shortness of breath | 49 (74.2) | 1,703 (77.9) | .48 |
| Chest pain | 44 (66.7) | 1,065 (48.7) | < .01 |
| Confusion | 11 (16.7) | 438 (20.1) | .50 |
| Wheezing | 37 (56.1) | 937 (42.9) | .03 |
| Comorbidities, No. (%) | |||
| Asthma | 21 (31.8) | 561 (25.7) | .26 |
| COPD | 14 (21.2) | 505 (23.1) | .72 |
| Chronic heart failure | 8 (12.1) | 422 (19.3) | .14 |
| Diabetes mellitus | 21 (31.9) | 563 (25.8) | .27 |
| Chronic kidney disease | 9 (13.6) | 344 (15.7) | .64 |
| Chronic liver disease | 3 (4.6) | 122 (5.6) | .72 |
| Obesity (BMI ≥ 30 kg/m2) | 32 (48.5) | 789 (36.1) | .04 |
| Current tobacco smoker | 17 (25.8) | 577 (26.4) | .91 |
| Laboratory values, median (IQR) | |||
| WBC count, cells/μL | 11,050 (6,900-14,200) | 11,400 (8,000-14,900) | .13 |
| Blood urea nitrogen, mg/dL | 12 (9-19) | 15 (10-24) | < .01 |
| Blood glucose, mg/dL | 120 (102-144) | 115 (99-145) | .46 |
| Procalcitonin, ng/mLa | < 0.05 (<0.05-0.11) | 0.16 (< 0.05-0.85) | < .01 |
| Pneumonia severity | |||
| Index risk class, No. (%) | |||
| I-II (low risk) | 41 (62.1) | 979 (44.8) | .02 |
| III (moderate risk) | 11 (16.7) | 439 (20.1) | |
| IV-V (high risk) | 14 (21.2) | 767 (35.1) | |
| CURB-65 score, No. (%) | |||
| 0-1 (low risk) | 53 (80.3) | 1,517 (69.4) | .14 |
| 2 (moderate risk) | 7 (10.6) | 422 (19.3) | |
| 3-5 (high risk) | 6 (9.1) | 246 (11.3) | |
| ATS severe pneumonia criteria, No. (%) | |||
| < 3 criteria (not severe) | 63 (95.5) | 2,028 (92.8) | .62 |
| ≥ 3 criteria (severe) | 3 (4.5) | 157 (7.2) |
ATS = American Thoracic Society; CURB-65 = confusion, uremia, respiratory rate, blood pressure, age ≥ 65 years; IQR = interquartile range.
Five hundred twenty patients did not have PCT measured, including 17 (25.8%) in the CT-only pneumonia group and 503 (23.0%) in the pneumonia seen on chest radiography group.
A smaller proportion of patients with CT-only pneumonia received antibiotics within 6 hours of hospital presentation than did those with pneumonia on chest radiography (59% vs 83%; P < .01), but results were similar by 24 hours (97% vs 96%; P = .80) (e-Table 7). Specific antibiotic regimens were quite similar for patients with CT-only pneumonia and those with pneumonia on chest radiography, with ß-lactam plus macrolide and fluoroquinolone monotherapy regimens the most common in both groups (Table 2).
Table 2.
Antibiotics Administered on the First Day of Hospitalization
| Antibiotic Regimen on Hospital Day 1 | CT-Only Pneumonia (n = 66) No. (%) | Pneumonia on Chest Radiography (n = 2,185) No. (%) |
|---|---|---|
| ß-lactam + macrolide | 12 (18.2) | 488 (22.3) |
| Respiratory fluoroquinolonea alone | 12 (18.2) | 470 (21.5) |
| Respiratory fluoroquinolonea + ß-lactam | 6 (9.1) | 113 (5.2) |
| ß-lactam + macrolide + glycopeptide | 5 (7.6) | 151 (6.9) |
| Respiratory fluoroquinolonea + ß-lactam + macrolide | 3 (4.6) | 202 (9.2) |
| Respiratory fluoroquinolonea + ß-lactam + glycopeptide | 3 (4.6) | 92 (4.2) |
| ß-lactam alone | 2 (3.0) | 65 (3.0) |
| Respiratory fluoroquinolonea + ß-lactam + macrolide + glycopeptide | 2 (3.0) | 52 (2.4) |
| ß-lactam + glycopeptide | 2 (3.0) | 50 (2.3) |
| Respiratory fluoroquinolonea + macrolide | 1 (1.5) | 42 (1.9) |
| Respiratory fluoroquinolonea + glycopeptide | 2 (3.0) | 32 (1.5) |
| Macrolide alone | 3 (4.6) | 22 (1.0) |
| Respiratory fluoroquinolonea + macrolide + glycopeptide | 0 (0) | 5 (0.2) |
| Glycopeptide alone | 1 (1.5) | 2 (0.1) |
| Other regimens | 11 (16.7) | 349 (16.0) |
Respiratory fluoroquinolones: gemifloxacin, levofloxacin, and moxifloxacin.
Pathogens
Pathogen detection was similar in the CT-only pneumonia group and the pneumonia on chest radiography group, with viruses being more commonly detected than bacteria in both groups (Table 3). The prevalence of major CAP pathogens, including S pneumoniae, M pneumoniae, Staphylococcus aureus, and influenza, were also similar between groups. However, the detection of human rhinovirus was more common in the CT-only pneumonia group than in the pneumonia on chest radiography group (17% vs 8%; P = .02). Interestingly, two of the eight patients in the CT-only pneumonia group with bacterial pathogens detected had PCT < 0.25 ng/mL. Both of these patients had S. pneumoniae detected by a urinary antigen test (e-Table 8).
Table 3.
Pathogens Detected
| Pathogens Detected, No. (%) | CT-Only Pneumonia (n = 66) | Pneumonia on Chest Radiography (n = 2,185) | P Value |
|---|---|---|---|
| Any pathogen | 27 (40.9) | 822 (37.6) | .59 |
| Codetection of ≥ 1 pathogen | 2 (3.0) | 108 (4.9) | .48 |
| Any bacteria | 8 (12.1) | 297 (13.6) | .86 |
| Streptococcus pneumoniae | 3 (4.6) | 111 (5.1) | .00 |
| Group A streptococcus | 0 (0) | 7 (0.32) | .65 |
| Viridans group streptococci | 0 (0) | 8 (0.37) | .62 |
| Other streptococcal species | 0 (0) | 8 (0.4) | .62 |
| Staphylococcus aureus | 0 (0) | 37 (1.7) | .63 |
| Haemophilus influenzae | 0 (0) | 13 (0.6) | .00 |
| Escherichia coli | 1 (1.5) | 16 (0.7) | .47 |
| Pseudomonas aeruginosa | 1 (1.5) | 7 (0.3) | .11 |
| Klebsiella pneumoniae | 2 (3.0) | 10 (0.5) | .01 |
| Mycoplasma pneumoniae | 0 (0) | 43 (2.0) | .64 |
| Chlamydia pneumoniae | 1 (1.5) | 8 (0.4) | .24 |
| Legionella pneumophila | 0 (0) | 32 (1.5) | .62 |
| Other bacteriaa | 0 | 9 (0.4) | .60 |
| Any virus | 20 (30.3) | 570 (26.1) | .44 |
| Influenza | 4 (6.1) | 126 (5.8) | .92 |
| Parainfluenza virus | 1 (1.5) | 66 (3.0) | .72 |
| Coronavirus | 0 (0) | 53 (2.4) | .41 |
| Human metapneumovirus | 4 (6.1) | 84 (3.8) | .36 |
| Respiratory syncytial virus | 0 (0) | 68 (3.1) | .26 |
| Human rhinovirus | 11 (16.7) | 182 (8.3) | .02 |
| Adenovirus | 1 (1.5) | 31 (1.4) | .62 |
Other bacteria included Fusobacteria (3 cases), Enterobacter (2 cases), Neisseria, Bacteroides, Pasteurella, and Proteus.
Clinical Outcomes
Clinical outcomes, including hospital length of stay, ICU admission, mechanical ventilation, and shock, were similar between patients with CT-only pneumonia and patients with pneumonia on chest radiography (Table 4). Inhospital death was rare in both the CT-only and pneumonia on chest radiography groups (0% vs 2%).
Table 4.
Clinical Outcomes
| Clinical Outcome | CT-Only Pneumonia (n = 66) | Pneumonia on Chest Radiography (n = 2,185) | P Value |
|---|---|---|---|
| In-hospital death, No. (%) | 0 (0) | 49 (2.2) | .40 |
| Hospital length-of-stay among survivors, median (IQR), d | 3.5 (2-5) | 3 (2-6) | .90 |
| ICU admission, No. (%) | 15 (22.7) | 467 (21.4) | .80 |
| Invasive mechanical ventilation, No. (%) | 4 (6.1) | 113 (5.2) | .76 |
| Vasopressor-dependent septic shock, No. (%) | 3 (4.6) | 84 (3.8) | .74 |
| Moderate-severe ARDS, No. (%) | 1 (1.5) | 89 (4.1) | .52 |
See Table 1 legend for expansion of abbreviation.
Description of Patients With Pneumonia on Chest Radiography and No Pneumonia on CT Scan
Thirty patients (1.3% of the full study population and 4.0% of patients who had CT imaging completed) had a chest radiograph interpreted as consistent with pneumonia and a CT scan interpreted as not consistent with pneumonia. Nonpneumonia abnormalities were identified on CT scans in each of these patients that explained why a chest radiograph may have been interpreted as consistent with pneumonia; these findings included lymphadenopathy (13 cases), pulmonary edema (five cases), atelectasis (three cases), pulmonary fibrosis (three cases), emphysema (three cases), pulmonary infarct (two cases), and postsurgical changes (one patient).
Compared with the 652 patients who had both a chest radiograph and CT scan interpreted as pneumonia, these 30 patients with pneumonia on chest radiography and no pneumonia on CT imaging were older, were more likely to have asthma or COPD, and had lower WBC counts (median, 8,900 vs 11,300 cells/μL) and lower PCT concentrations (median, < 0.05 vs 0.15 ng/mL) (e-Table 2). Bacterial pathogens and severe infection-related outcomes were rare in these 30 patients with pneumonia on chest radiography but no pneumonia on CT imaging. Only four of these patients (13.3%) had a pathogen detected, including two with parainfluenza virus, one with rhinovirus, and one with Fusobacterium necrophorum (e-Table 5). The patient with F necrophorum bacteremia presented with a 2-day history of sore throat and was found to have a pulmonary infarct on CT scanning, suggestive of Lemierre’s syndrome (bacterial pharyngitis, usually caused by F necrophorum, associated thrombophlebitis of the internal jugular vein)31; this patient recovered with antibiotic treatment and was discharged. None of these 30 patients with pneumonia on chest radiography but no pneumonia on CT scanning had respiratory failure, septic shock, or ARDS (e-Table 4). One of these patients died—a patient with advanced liver disease and metastatic hepatocellular carcinoma who had a pulmonary infarct visualized on CT imaging.
Discussion
In this cohort of 2,251 adults hospitalized with CAP, 66 had radiological evidence of pneumonia on CT imaging but not on concurrent chest radiography. Detailed evaluation of these 66 patients with CT-only pneumonia revealed illness severity, pathogens, and clinical outcomes similar to those of patients with pneumonia evident on chest radiography. These findings support managing patients with CT-only pneumonia according to the same principles used for those with pneumonia on chest radiography, including the selection of empirical antibiotics and site of care. Additionally, these results suggest that pneumonia that is visible only on CT scan can be associated with significant physiological abnormalities and morbidity, and hence, support using CT imaging to evaluate for pneumonia when the clinical presentation is suggestive of pneumonia but an initial chest radiograph does not demonstrate radiological signs of pneumonia and identification of pneumonia as the cause of clinical symptoms is important. Examples of such a scenario may include patients with septic shock or fever with altered mental status but no clear source of infection after initial evaluation with a history, physical examination, laboratory work, and chest radiography.32, 33, 34
Currently, nearly all adults hospitalized with CAP in the United States are treated with antibiotics.13 However, only a minority of these patients have detectable bacterial pathogens.13 Hence, many antibiotic stewardship strategies are under development to identify patients who historically have been treated with antibiotics but are unlikely to benefit from them.20, 35 Our results suggest that stewardship strategies are also needed for CT-only pneumonia. We found that 12% of patients with CT-only pneumonia had a bacterial pathogen; this prevalence is too high to recommend withholding antibiotics for all patients with CT-only pneumonia. However, similar to pneumonia on chest radiography, a substantial portion of patients with CT-only pneumonia appear to have nonbacterial disease. Although more research in this area is needed, stewardship strategies developed for pneumonia on chest radiography, such as PCT-based algorithms for guiding antibiotic prescribing,20 may also be useful for CT-only pneumonia.
Although patients with CT-only pneumonia and patients with pneumonia on chest radiography were largely similar, our analysis identified some potential differences that will be important to explore in future studies. For example, CT-only pneumonia appeared to be more common in obese patients, perhaps due to lower chest radiography sensitivity associated with x-ray beam attenuation by adipose tissues in obese patients.36, 37 Also, patients with CT-only pneumonia as a group appeared to have lower PCT levels and a higher prevalence of human rhinovirus detection, suggesting that viral pathogens may be more common in CT-only pneumonia than in pneumonia seen on chest radiography. However, it is important to note that 12% of patients with CT-only pneumonia had a bacterial pathogen detected, which was similar to the incidence observed in the pneumonia on chest radiography group. Additionally, two patients with CT-only pneumonia and a serum PCT concentration < 0.25 ng/mL had pneumococcal infection, highlighting that the combination of chest radiography and PCT determination does not identify all bacterial pneumonia.
Claessens et al7 recently published a study evaluating the effect of routine CT scan use for patients with suspected CAP in the ED. While we focused on identifying the pathogens and clinical outcomes of patients with CT-only pneumonia, Claessens et al7 focused on understanding how CT scans performed in patients with suspected pneumonia changed clinical management. Among the 308 patients with clinically suspected pneumonia in the Claessens et al study, 40 (13%) had CT-only pneumonia, and clinicians typically responded to a positive CT scan for pneumonia after a normal chest radiograph by administering antibiotics. Our results complement those of Claessens et al by suggesting that the pathogens and clinical outcomes associated with CT-only pneumonia do indeed warrant consideration for antibiotic therapy. Claessens et al7 also found that 18% of patients in their study had a chest radiograph suggestive of pneumonia but a CT scan without evidence of pneumonia, and clinicians were likely to discontinue antibiotics and change the disposition from inpatient to outpatient based on results of the CT scan demonstrating no pneumonia. Our data also support a clinical decision to use normal CT scans to de-escalate care based on a chest radiograph suggestive of pneumonia. In our study, only one of 30 patients with a chest radiograph suggestive of pneumonia and a CT scan without evidence of pneumonia had a bacterial pathogen detected, and CT images often clarified the abnormality seen on chest radiograph as a nonpneumonia finding.
There were limitations to our study. First, the use of CT imaging was determined by treating clinicians independent of the study protocol; clinicians elected to perform CT imaging in approximately one-third of the patients in the cohort. As noted in e-Table 1, patients with chest pain, shortness of breath, and hemoptysis were more likely to undergo CT imaging, likely due to clinicians’ concerns for pulmonary embolism or cancer in patients with these symptoms; therefore, study results may not be generalizable to patients whose clinicians are not concerned enough to order chest CT imaging. Second, because radiological evidence of pneumonia on chest radiography or CT scanning was required for cohort inclusion, we did not evaluate patients with clinically suspected pneumonia who had a chest radiograph without evidence of pneumonia and no CT imaging performed. If CT imaging was systematically completed on these patients, additional cases of CT-only pneumonia would likely have been discovered. Third, patients were considered to have CT-only pneumonia if a CT scan showing evidence of pneumonia was completed on the same day or the day following a chest radiograph that showed no signs of pneumonia, and both imaging studies were completed within 2 days of hospital admission. Only including patients who had a CT scan obtained immediately after a chest radiograph (within minutes or hours) may have decreased the discordance between chest radiography and CT imaging; however, considering a CT scan and chest radiograph performed within 1 calendar day of each other as concurrent imaging is consistent with clinical practice in which routine chest imaging more frequently than once per day is not recommended.4 Fourth, multiple comparisons between groups were performed, and differences observed should be considered hypothesis generating. Fifth, although this is the largest study to date assessing clinical outcomes of patients with CT-only pneumonia, our sample size was somewhat limited, precluding multivariable analyses and robust comparisons of rare outcomes, such as mortality. Sixth, nearly all patients were treated with antibiotics; hence, the impact of antibiotics on clinical outcomes could not be evaluated. Finally, only hospitalized patients were included in this study, and generalizability to the outpatient setting is unknown.
Conclusions
In this large prospective cohort study of adults hospitalized with CAP who underwent systematic testing for the cause of the pneumonia, patients with pneumonia visualized on CT scans but not on chest radiographs had pathogens and clinical outcomes similar to those of patients who had pneumonia visualized on chest radiographs. These findings support the management of patients with pneumonia visualized only on CT scans according to the same principles as those with pneumonia visualized on chest radiographs.
Acknowledgments
Author contributions: W. H. S. had full access to the data and takes responsibility for the integrity of the data and accuracy of the analysis. C. P. U., C. G. G., R. G. W., S. J., K. M. E., and W. H. S. contributed to the study concept and design. R. G. W., D. J. W., E. J. A., E. J. H., F. C., A. M. B., S. J., K. M. E., and W. H. S. contributed to the acquisition of data. C. P. U., C. G. G., Y. Z., A. M. B., and W. H. S. contributed to the statistical analysis. C. P. U., C. G. G., R. J. W., D. J. W., G. W. W., E. J. A., S. J., K. M. E., and W. H. S. contributed to interpretation of the data. C. P. U. and W. H. S. contributed to drafting of the initial manuscript. All authors contributed to the critical revision of the manuscript. K. M. E. and R. G. W. obtained funding. R. G. W., S. J., K. M. E., and W. H. S. contributed to study supervision.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: C. G. G. reports serving as a consultant for Pfizer, Inc. R. G. W. reports receiving grants from the Centers for Disease Control, funds to conduct clinical research from BioMerieux and Vertex Pharmaceuticals, and personal fees from BioMerieux and Visterra, Inc. E. J. A. reports receiving funds to conduct clinical research from MedImmune and NovaVax, personal fees from AbbVie, and nonfinancial support from Roche. D. J. W. reports receiving grants from the Centers for Disease Control. K. M. E. reports receiving grants from the Centers for Disease Control and Novartis, and consultant fees from Novartis. W. H. S. reports receiving grants from the Centers for Disease Control and personal fees from BioFire Diagnostics, Venaxis, Abbott Point of Care, Cempra Pharmaceuticals, and Ferring Pharmaceuticals. None declared (C. P. U., G. W. W., Y. Z., E. M. H., F. C., A. M. B., S. J.).
Role of Sponsors: Personnel from CDC participated in this study as investigators and authors. NIH had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Additional information: The e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work was supported by a cooperative agreement with the Centers for Disease Control and Prevention [Grant U18 IP000299]. C. G. G. was supported in part by the National Institute on Aging [Grant R01AG043471]. W. H. S. was supported in part by the National Institute of General Medical Sciences [Grant K23GM110469].
Supplementary Data
References
- 1.Kochanek K.D., Murphy S.L., Xu J., Tejada-Vera B. Death: Final data for 2014. Natl Vital Stat Rep. 2016;64(2):1–119. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Number and rate of discharges from short-stay hospitals and days of care, with average length of stay and standard error, by selected first-listed diagnostic categories: United States, 2010. https://www.cdc.gov/nchs/data/nhds/2average/2010ave2_firstlist.pdf. Accessed July 27, 2017.
- 3.Self W.H., Grijalva C.G., Zhu Y. Rates of emergency department visits due to pneumonia in the United States, July 2006-June 2009. Acad Emerg Med. 2013;20(9):957–960. doi: 10.1111/acem.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandell L.A., Wunderink R.G., Anzueto A. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kocher K.E., Meurer W.J., Fazel R., Scott P.A., Krumholz H.M., Nallamothu B.K. National trends in use of computed tomography in the emergency department. Ann Emerg Med. 2011;58(5):452–462. doi: 10.1016/j.annemergmed.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Larson D.B., Johnson L.W., Schnell B.M., Salisbury S.R., Forman H.P. National trends in CT use in the emergency department: 1995-2007. Radiology. 2011;258(1):164–173. doi: 10.1148/radiol.10100640. [DOI] [PubMed] [Google Scholar]
- 7.Claessens Y.E., Debray M.P., Tubach F. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192(8):974–982. doi: 10.1164/rccm.201501-0017OC. [DOI] [PubMed] [Google Scholar]
- 8.Haga T., Fukuoka M., Morita M., Cho K., Tatsumi K. Computed tomography for the diagnosis and evaluation of the severity of community-acquired pneumonia in the elderly. Intern Med. 2016;55(5):437–441. doi: 10.2169/internalmedicine.55.5556. [DOI] [PubMed] [Google Scholar]
- 9.Self W.H., Courtney D.M., McNaughton C.D., Wunderink R.G., Kline J.A. High discordance of chest x-ray and CT for detection of pulmonary opacities in ED patients: implications for diagnosing pneumonia. Am J Emerg Med. 2013;31(2):401–405. doi: 10.1016/j.ajem.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esayag Y., Nikitin I., Bar-Ziv J. Diagnostic value of chest radiographs in bedridden patients suspected of having pneumonia. Am J Med. 2012;123:88.e1–88.e6. doi: 10.1016/j.amjmed.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Syrjala H., Broas M., Suramo I. High resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis. 1998;27:358–363. doi: 10.1086/514675. [DOI] [PubMed] [Google Scholar]
- 12.Waterer G.W. The diagnosis of community-acquired pneumonia. Do we need to take a big step backward? Am J Respir Crit Care Med. 2015;192(8):912–913. doi: 10.1164/rccm.201507-1460ED. [DOI] [PubMed] [Google Scholar]
- 13.Jain S., Self W.H., Wunderink R.G. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherian T., Mulholland E.K., Carlin J.B. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Org. 2005;83:353–359. [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Defining adult overweight and obesity. Available at: https://www.cdc.gov/obesity/adult/defining.html. Accessed August 17, 2016.
- 16.Fine M.J., Auble T.E., Yealy D.M. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 17.Lim W.S., van der Eerden M.M., Laing R. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Self W.H., Grijalva C.G., Williams D.J. Procalcitonin as an early marker of the need for invasive respiratory or vasopressor support in adults with community-acquired pneumonia. Chest. 2016;150(4):819–828. doi: 10.1016/j.chest.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.BioMerieux, Inc. VIDAS BRAHMS PCT. http://www.biomerieux-diagnostics.com/vidas-brahms-pct. Accessed July 15, 2016.
- 20.Schuetz P., Christ-Crain M., Thomann R. Effect of PCT-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302:1059–1066. doi: 10.1001/jama.2009.1297. [DOI] [PubMed] [Google Scholar]
- 21.Self WH, Balk RA, Grijalva CG, et al. Procalcitonin as a marker of etiology in adults hospitalized with community-acquired pneumonia [published online ahead of print April 12, 2017]. Clin Infect Dis. http://dx.doi.org/10.1093/cid/cix317. [DOI] [PMC free article] [PubMed]
- 22.Weinberg G.A., Schnabel K.C., Erdman D.D. Field evaluation of TaqMan Array Card (TAC) for the simultaneous detection of multiple respiratory viruses in children with acute respiratory infection. J Clin Virol. 2013;57:254–260. doi: 10.1016/j.jcv.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dare R.K., Fry A.M., Chittaganpitch M., Sawanpanyalert P., Olsen S.J., Erdman D.D. Human coronavirus infections in rural Thailand: a comprehensive study using real-time reverse-transcription polymerase chain reaction assays. J Infect Dis. 2007;196:1321–1328. doi: 10.1086/521308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawatwong P., Chittaganpitch M., Hall H. Serology as an adjunct to polymerase chain reaction assays for surveillance of acute respiratory virus infections. Clin Infect Dis. 2012;54:445–446. doi: 10.1093/cid/cir710. [DOI] [PubMed] [Google Scholar]
- 25.Feikin D.R., Njenga M.K., Bigogo G. Additional diagnostic yield of adding serology to PCR in diagnosing viral acute respiratory infections in Kenyan patients 5 years of age and older. Clin Vaccine Immunol. 2013;20:113–114. doi: 10.1128/CVI.00325-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartlett R.C. John Wiley; New York: 1974. Medical Microbiology: Quality, Cost and Clinical Relevance; pp. 24–31. [Google Scholar]
- 27.Murdoch D.R., Laing R.T.R., Mills G.D. Evaluation of a rapid immunochromatographic test for detection of Streptococcus pneumoniae antigen in urine samples from adults with community-acquired pneumonia. J Clin Microbiol. 2001;39:3495–3498. doi: 10.1128/JCM.39.10.3495-3498.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murdoch D.R. Diagnosis of Legionella infection. Clin Infect Dis. 2003;36:64–69. doi: 10.1086/345529. [DOI] [PubMed] [Google Scholar]
- 29.Thurman K.A., Warner A.K., Cowart K.C., Benitez A.J., Winchell J.M. Detection of Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella spp. in clinical specimens using a single-tube multiplex real-time PCR assay. Diagn Microbiol Infect Dis. 2011;70:1–9. doi: 10.1016/j.diagmicrobio.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ARDS Definition Task Force Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 31.Karkos P.D., Asrani S., Karkos C.D. Lemierre’s syndrome: a systematic review. Laryngoscope. 2009;119(8):1552–1559. doi: 10.1002/lary.20542. [DOI] [PubMed] [Google Scholar]
- 32.Heussel C.P., Kauczor H.U., Heussel G., Fischer B., Mildenberger P., Thelen M. Early detection of pneumonia in febrile neutropenic patients: use of thin-section CT. AJR Am J Roentgenol. 1997;169:1347–1353. doi: 10.2214/ajr.169.5.9353456. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed W.A., de Heredia L.L., Hughes R.J., Belci M., Meagher T.M. Outcomes of whole-body computed tomography in spinal cord patients with sepsis. Spinal Cord. 2014;52:536–540. doi: 10.1038/sc.2014.42. [DOI] [PubMed] [Google Scholar]
- 34.Karhu J.M., Ala-Kokko T.I., Ahvenjarvi L.K., Rauvala E., Ohtonen P., Syrjala H.P. Early chest computed tomography in adult acute severe community-acquired pneumonia patients treated in the intensive care unit. Acta Anesthesiol Scand. 2016;60(8):1102–1110. doi: 10.1111/aas.12749. [DOI] [PubMed] [Google Scholar]
- 35.Dellit T.H., Owens R.C., McGowan J.E., Jr. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 36.Uppot R.N. Impact of obesity on radiology. Radiol Clin North Am. 2007;45:231–246. doi: 10.1016/j.rcl.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Uppot R.N., Sahani D.V., Hahn P.F., Kalra M.K., Saini S.S., Mueller P.R. Effect of obesity on image quality: fifteen-year longitudinal study for evaluation of dictated radiology reports. Radiology. 2006;240(2):435–439. doi: 10.1148/radiol.2402051110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.